Abstract
Recombinant adeno-associated viruses (AAVs) have emerged as promising gene delivery vehicles resulting in three US Food and Drug Administration (FDA) and one European Medicines Agency (EMA)-approved AAV-based gene therapies. Despite being a leading platform for therapeutic gene transfer in several clinical trials, host immune responses against the AAV vector and transgene have hampered their widespread application. Multiple factors, including vector design, dose, and route of administration, contribute to the overall immunogenicity of AAVs. The immune responses against the AAV capsid and transgene involve an initial innate sensing. The innate immune response subsequently triggers an adaptive immune response to elicit a robust and specific response against the AAV vector. AAV gene therapy clinical trials and preclinical studies provide important information about the immune-mediated toxicities associated with AAV, yet studies suggest preclinical models fail to precisely predict the outcome of gene delivery in humans. This review discusses the contribution of the innate and adaptive immune response against AAVs, highlighting the challenges and potential strategies to mitigate these responses, thereby enhancing the therapeutic potential of AAV gene therapy.
Key Points
| Adeno-associated virus (AAV) capsid, vector DNA, and transgene products induce innate and adaptive immune responses. |
| Several strategies such as broad immunosuppression, capsid engineering, induction of tolerance, etc. are being developed to circumvent immune-mediated toxicities associated with AAVs. |
Introduction
Gene therapy using recombinant adeno-associated viral (AAV) vectors have yielded encouraging results over recent years, in addition to the three US Food and Drug Administration (FDA)-approved AAV gene therapy products, Luxturna, Zolgensma, and HEMGENIX, for treating Leber’s congenital amaurosis, spinal muscular atrophy (SMA), and hemophilia B, respectively [1, 2], the European Medicines Agency (EMA) has recently approved an AAV5-Factor 8 vector (Roctavian) for patients with hemophilia A [3]. There are substantial ongoing AAV gene therapy clinical trials for several diseases, including hemophilia, neurodegenerative diseases, and muscular dystrophies [4], yet one of the factors that hinders the clinical success of these trials is AAV immunogenicity, which remains an unmet challenge in the field.
Thirteen different wild-type AAV serotypes and more than 108 capsid variants have been identified and classified [5, 6]. Originally discovered in 1965 [7] as contaminants in adenovirus (Ad) preparations, wild-type AAVs are parvoviruses that are naturally found in multiple vertebrate species, including humans and non-human primates (NHPs) [8], but were never associated with any known disease. AAVs have rapidly emerged as the vehicle of choice for in vivo gene delivery and carry a transgene expression cassette, flanked by inverted terminal repeats (ITRs), instead of the viral protein coding sequences. The ITRs, derived from the wild-type AAV2, are the only cis-acting sequences required for replication and packaging but are non-coding regions. AAVs persist as extrachromosomal DNA, enabling stable transgene expression in non-replicating cells [9]. Although AAV is mildly immunogenic, immune responses observed in AAV gene therapy clinical trials have limited the therapeutic application. Since AAV vectors do not contain any expressed viral genome, the only sources of foreign antigens brought in during gene transfer are derived from the viral capsid and the transgene cassette. However, host immune responses against AAV vectors and delivered transgene impact effective and stable transgene expression [10, 11].
Significant research efforts have been made to evaluate immune-mediated toxicities associated with AAVs. The immune system's main role is to recognize self- from non-self to protect against pathogens. The innate immune response, including the complement system, can be triggered by the AAV capsid and AAV genome along with other host-specific factors. Subsequent activation signals help to recruit antigen-presenting cells (APCs), T cells, and B cells, which are responsible for mediating adaptive immunity. Numerous reports have dissected the role of the adaptive immune response against AAVs. Due to wide dissemination of wild-type AAVs among humans, pre-existing neutralizing antibodies (NAbs) against AAV capsids are common in many populations [12]. These antibodies can prevent transduction of target cells with AAV. Moreover, antibodies can also be developed against the delivered transgene, which may neutralize soluble transgene products and interfere with successful gene therapy [13]. Additionally, capsid-specific cell-mediated cytotoxic T-cell responses affect transgene expression and result in clearance of transduced cells hindering therapeutic efficacy [14]. Patients receiving high systemic doses of AAVs for SMA type 1 (SMA1), Duchenne muscular dystrophy (DMD), and X-linked myotubular myopathy (XLMTM) experienced adverse effects of varying severity over recent years. These outcomes could largely be attributed to innate and adaptive immune responses to the vector. Thus, understanding the immune system response against AAVs is pivotal for improving the safety and therapeutic efficacy of AAV gene therapy.
Activation of host immune responses by AAV vectors depends on factors including the route and dose of administration, which host cells and body fluids encounter the vector, the vector components, and transgene products. Direct injection into the subretinal space, as was the route for the first FDA-approved AAV vector, results in less exposure to plasma proteins, circulating antibodies, blood cells, and tissue-resident phagocytic cells [15]. Injection into other sites such as the central nervous system (CNS) may partially restrict the exposure to serum antibodies prior to target cell entry [16, 17]. In contrast, intravenous infusion of AAV enables widespread exposure of vector to blood cells, plasma proteins, including antibodies, as well as a broad range of tissue-resident cells, such as Kupffer cells within the liver. Maximizing the potential vector-antibody interaction results in reducing efficacy or safety of the gene therapy [18]. Widespread biodistribution of AAV vector genomes to the liver is observed with essentially all capsid types and routes, depending on the dose administered. Thus, while direct injection routes may largely bypass preformed antibodies, de novo immune responses to vector components may still be robust. Finally, certain host responses have only been observed with very high doses of intravenous AAV, suggesting a threshold effect for these host reactions [19–21]. This suggests numerous factors need to be taken into consideration when evaluating immune responses to AAV gene therapy beyond the considerations of the components of the AAV vector.
In this review, we provide an overview of the innate and adaptive immune responses directed against the AAV capsid and transgene and discuss strategies to circumvent this major roadblock to the development of a successful AAV-based therapeutic gene delivery platform.
Immune Responses to Adeno-Associated Viruses (AAVs)
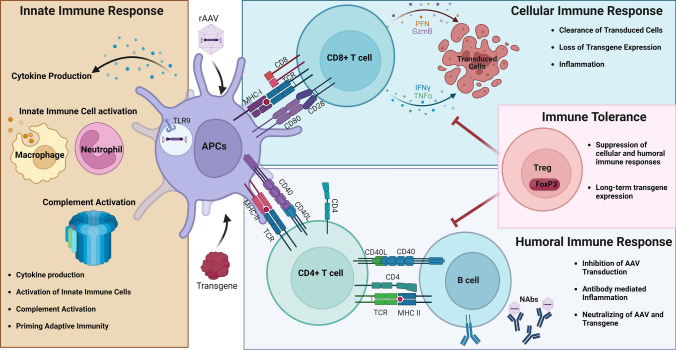
Immune responses can be divided into innate and adaptive immune responses. In the following sections, we give a brief overview of the innate and adaptive immune pathways that are stimulated by AAVs and discuss the mechanisms that are activated in response to AAV infection, leading to suppression of transgene expression (Fig. 1).
Fig. 1.
Immune responses in AAV gene therapy. AAV capsid or introduced transgene can be recognized by innate immunity which results in cytokine production, activation of innate immune cells, complement activation, and priming of adaptive immunity. Presentation of capsid peptides or delivered transgene on MHC I molecules results in activation of CD8+ T cells and clearance of transgene expression. Antigen presentation on MHC II activates CD4+ T cells, which leads to production of cytokines and further induces humoral or cell-mediated immune responses and creates immunological memory. Activation of Tregs results in suppression of cellular and humoral immune responses and long-term transgene expression. AAV adeno-associated virus, APCs antigen-presenting cells, FoxP3 forkhead box protein 3, GzmB granzyme B, IFNγ interferon gamma, MHC major histocompatibility complex, NAbs neutralizing antibodies, PFN perforin, rAAV recombinant adeno-associated virus, TCR T-cell receptor, TLR9 toll-like receptor, TNFα tumor necrosis factor alpha, Tregs regulatory T cells
Innate Immune Response to AAV
Upon exposure to pathogens, the innate immune system is triggered in a nonspecific manner, and subsequently stimulates the activation of the more specific adaptive immune responses [22]. In this review, we briefly discuss the key players involved in sensing AAV vector elements, resulting in activation of innate immune signaling cascades.
Pathogen-Associated Molecular Patterns and Pattern Recognition Receptors
Foreign pathogens exhibiting unique pathogen-associated molecular patterns (PAMPs) are recognized by pattern recognition receptors (PRRs) that are highly expressed by innate immune cells, such as macrophages and dendritic cells (DCs). PRRs have been classified into different families, namely toll-like receptors (TLRs), NOD-like receptors (NLRs), C-type lectin receptors (CLRs), and DNA sensors [23]. Recognition of the PAMPs by the respective PRRs primarily activates signaling pathways, such as NF-ĸB, via adaptor proteins, such as MyD88. These signaling cascades induce the expression of major histocompatibility complex (MHC) gene expression and pro-inflammatory cytokines or type I and III interferon (IFN) secretion [24, 25]. Secreted IFNs and cytokines in turn mediate expression of genes that limit viral replication, as well as stimulate adaptive and memory immune responses [26]. Adaptive anti-capsid humoral [27] and cytotoxic [28, 29] immune responses have been shown to be restricted in vivo by inhibiting innate immune activation pathways.
TLR-mediated signaling, triggering innate immunity and subsequent activation of adaptive immune response, is an important AAV sensing mechanism [30]. TLRs are primarily expressed on immune cells, such as DCs and macrophages, and some non-immune cells, such as epithelial cells and fibroblasts [31]. Cell surface TLRs recognize membrane components of pathogens, such as lipoproteins and lipids, and intracellular TLRs recognize pathogen-derived nucleic acids [32, 33]. Studies have shown that intracellular TLR9 contributes to immune responses against the transgene and capsid [30, 34, 35]. In addition to ITRs, inserted transgenes, promoter sequences, and vector-expressed RNAs may contain inflammatory signals that TLRs recognize. Endosomal TLR9, present in Kupffer cells or DCs, particularly binds to unmethylated CpG dinucleotides in the double-stranded DNA (dsDNA) vector genomes [36]. Studies suggest codon optimization, a technique to improve transgene expression, may increase CpG motifs in AAV vectors [37, 38]. TLR9 involvement favors activation of AAV-specific cytotoxic CD8+ T-cell response due to increased antigen presentation on MHC I [39]. Immune responses mounted by IFN induction resulted in a loss of transgene expression delivered by AAV in mice [34, 40]. Furthermore, the TLR9/MyD88 pathway is not only vital for mounting a CD8+ T-cell response against the AAV capsid but also liver and muscle-directed transgenes [28, 34, 35, 41]. Studies have shown that empty AAV capsids alone can also trigger innate immune responses, potentially mediated via cell surface TLR2 in non-parenchymal liver cells [42]. Alternatively, some studies have reported that anti-AAV capsid immunoglobulin (Ig) G2 antibody production is not dependent on any specific TLR alone but on the intrinsic MyD88 signaling in B cells [30, 43]. In addition, other studies have shown that self-complementary AAV (scAAV) vectors exhibited an increased innate immune response to the transgene compared with single-stranded AAVs (ssAAVs), possibly due to enhanced activation of the TLR9/MyD88 signaling cascade [30, 44, 45].
Cytosolic DNA sensors, such as cyclic GMP–AMP synthase (cGAS), are activated by the AAV genome [46]. AAV transduction may cause the cell to release its mitochondrial DNA into the cytoplasm, thereby activating the cytosolic DNA sensors [47]. An alternative possibility is that proteasomal degradation of the AAV capsid upon its entry results in exposure of the genome, which may then be sensed by DNA sensors [48]. Recent studies have pointed out the involvement of dsRNA sensors in innate sensing of AAVs. The intrinsic promoter activity of the ITRs can generate a dsRNA intermediate in the cytoplasm, which could stimulate the melanoma differentiation-associated protein 5 (MDA5) and retinoic acid-inducible gene (RIG) sensors [49]. Following AAV transduction of human hepatocytes, upregulation of MDA5 and RIG-1 was observed in some of the human subjects, triggering IFN-β production [49]. High levels of MDA5 and RIG-1 were also observed post AAV transduction in primate retina [50]. Further investigation is required to delineate the role that dsRNA formation plays in priming adaptive immune responses in AAV-transduced cells.
AAV and the Complement System
The complement system consists of several soluble and membrane-bound proteins that constitute part of the cell-free innate immune system. It can be activated by three different pathways—classical, lectin, and alternative. While the classical pathway works in concert with antibody bound to an antigen target, the alternative pathway is activated by interaction of foreign macromolecules with circulating complement components and thus does not require preformed antibodies. These three pathways converge at the complement component C3 convertase, which cleaves the C3 protein into the functional subunits C3a and C3b, eventually forming the terminal membrane attack complex [51].
AAV-mediated complement activation has been the focus of many recent studies. One study suggested that AAV capsid interacts with C3, resulting in a higher AAV2 uptake in macrophages and a subsequent upregulation of cytokines, such as IL-8, IL-1β, and macrophage inflammatory protein [52]. Mice lacking the complement proteins CR1/2 or C3 did not exhibit a robust humoral immune response to AAV, suggesting that the complement system plays a role in the production of AAV-specific antibodies [53].
In clinical trials for DMD and SMA1, patients who received intravenous AAV doses > 1×1014 vector genomes (vg)/kg presented with symptoms of complement activation and a thrombotic microangiopathy (TMA) [54–56]. In addition, TMA was recently reported in a press release in two patients with methylmalonic acidemia (MMA) who received 5×1013 vg/kg of AAV-LK03 [57]. Activation of the alternative complement pathway in these patients appears to cause platelet activation and binding to leukocytes and endothelial cells, thus triggering a consumptive thrombocytopenia, microangiopathic anemia, and disseminated intravascular coagulation, as evidenced by elevated D-dimer and fibrin split products. In some patients, these mechanisms can progress to a range of symptoms of varying severity, such as vomiting, acute liver failure, and cardiac and kidney injury (atypical hemolytic uremic syndrome [aHUS]) after AAV administration (NCT03362502 and NCT03368742) [44]. This syndrome has typically developed 5–10 days after infusion. Therapy with eculizumab has been used in AAV recipients with TMA syndrome in order to interrupt the TMA cascade. However, results from these trials are preliminary and further investigation is required.
Adaptive Immune Response
Beyond initiating primary immune responses against AAV, innate immunity primes adaptive responses. Adaptive immune responses induce antigen-specific responses while creating an immunological memory. Adaptive immunity is composed of two main mechanisms: humoral immunity, through B cells and antibodies, and cellular immunity, through cytotoxic CD8+ T cells. CD4+ helper T cells are key mediators in both humoral and cellular immunity by activating B cells and cytotoxic T cells [58].
Following initial exposure to AAV, innate immune cells are broadly activated through expression of PRRs and the release of cytokines. Upon prolonged exposure to AAV, APCs such as DCs take up AAV particles either through transduction or phagocytosis and present the processed antigens for activation of B or T cells [59–61]. B cells recognize soluble or presented antigen and produce antibodies. Activation of T cells involves helper CD4+ cells which further induce an inflammatory response and the activation of CD8+ cytotoxic T cells resulting in removal of AAV-infected cells. Upon resolution of inflammatory signal, regulatory T cells suppress the immune response to return to homeostasis. The following sections will highlight the role of the adaptive immune response in AAV gene therapy [61–63].
Humoral Immune Responses
Humoral immunity is mediated by the production of antibodies against specific antigens. Antibodies function through multiple mechanisms: NAbs prevent pathogens from entering cells, antibodies coating the surface of the pathogen marking them for phagocytosis, or antibody binding resulting in initiation of the classical complement pathway activation [64].
The humoral immune response, specifically the production of NAbs, imposes one of the most effective barriers against successful gene delivery of AAV. Following AAV transduction, APCs present antigens to CD4+ helper T cells, which results in B-cell maturation, expansion, class switching, and an increase in antibody production against AAV capsid [65, 66].
In humans, anti-AAV NAbs, which are characterized by their ability to impair AAV infection or pathogenesis, form through natural exposure to wild-type AAVs. Depending on the AAV capsid variant, 2–60% of human sera contains antibodies that limit the application of AAV gene therapy [67, 68]. Of these, anti-AAV2 and AAV1 NAbs are the most prevalent NAbs among AAV capsid variants [69]. However, anti-AAV NAbs are often highly cross-reactive across multiple capsid variants, likely due to high homology among AAV capsid variants [70]. Several studies have shown that following AAV administration, antibodies produced in response were cross-reactive against other non-administered AAV capsid variants [71–73]. Characterization of anti-AAV NAbs showed IgG1 is the dominant subclass among Nabs, followed by IgG2 and IgM [74]. Patients post AAV gene therapy will also develop AAV-specific antibodies that prevent subsequent redosing with AAV. In one study, re-administration of AAV8 in NHP did not result in increased transgene expression, but increased anti-AAV antibody levels were observed [75]. However, current therapeutic approaches suggest that repeated dosing of AAV may be necessary in neonates, children, and adult patients with degenerative disorders due to vector dilution or loss [76]. Binding antibodies (BAbs) are involved in recruitment of immune cells and triggering antibody-dependent cellular cytotoxicity (ADCC) [67]. One study reported increased capsid internalization in the presence of BAbs in vitro. Moreover, mice immunized with BAbs showed a more efficient liver transduction compared with mice immunized with NAbs [18]. The role of BAbs in AAV immune responses is currently being further investigated and may highlight the possible role for BAbs and the need to reconsider screening techniques.
Most clinical trials of systemic (intravenous) AAV gene therapy have excluded patients with significant anti-capsid antibodies directed to the serotype being used. For example, the pivotal trials of the BioMarin AAV5-FVIII vector (Roctavian) excluded patients who had detectable anti-AAV5 NAb titers and anti-AAV5 total binding antibody (TAb) titers [77]. Current assays to measure anti-capsid antibodies include TAb assays and NAb assays, but both NAb assays and TAb assays are difficult to standardize. NAb assays are either cell-based or mouse-based, and are hence expensive, time-consuming, and have a higher risk of underestimating TAb titer. Since TAb assays screen out all antibodies against AAV capsid, neutralizing and non-neutralizing, the resulting titer is more conservative and safer [78]. Moreover, different capsid serotypes have been approved by the FDA and EMA and each product requires developing a different anti-AAV antibody assay. For instance, companion diagnostic tests available for AAV9 are not applicable for AAV1, AAV2 or AAV5 [79, 80]. A recent study established a Meso Scale Discovery (MSD)-based assay for the quantification of BAbs and Nabs, which allows for rapid and quantitative assessment of AAV-specific antibodies [81].
In addition to a humoral immune response, the T-cell-mediated response plays an important role in limiting AAV gene therapy as highlighted in the following section.
Capsid-Specific T-Cell Response
The T-cell response is mediated by multiple subsets of T cells. Effector CD4+ T cells induce inflammation and immune activation through cytokine production. Whereas cytotoxic CD8+ T cells mediate targeted killing of infected cells by secreting granzyme, perforin, and inflammatory cytokines. Furthermore, Tregs prevent hyperimmune activation and return the immune response to homeostasis by direct interaction with immune cells or producing immunosuppressive cytokines [82].
Cell-mediated immune response against AAV resulting in loss of transgene expression was not widely observed in animal studies [31, 83]. Initiation of a T-cell response requires antigen presentation through APCs. Endogenous or intracellular antigens are presented on MHC I, whereas exogenous or extracellular antigens are presented on MHC II [84]. In certain APCs, cross-presentation allows exogenous antigens typically exhibited on MHC II to be presented on MHC I. Studies suggest that cooperation between plasmacytoid DCs, conventional DCs, and CD4+ T cells results in cross-presentation of AAV particles on MHC I, and, subsequently, activation of CD8+ T cells [28, 29]. A cell-mediated immune response was first observed in a hemophilia clinical trial where liver delivery of AAV2 expressing human coagulation factor 9 (hFIX) resulted in liver transaminase elevation and loss of transgene expression. Further studies confirmed the presence of capsid-specific CD8+ T cells [14]. Capsid-specific CD8+ T cells expand and potentially clear AAV transduced cells, which results in loss of transgene expression and inflammation of target organs [59, 60]. In addition, APCs present capsid peptides on MHC II, which leads to CD4+ T-cell activation. The activation of CD4+ T cells results in production of cytokines further activating humoral or cell-mediated immune responses and creating immunological memory [62, 63, 85].
Capsid-specific T-cell responses were observed in a subsequent study in human subjects who received high-dose AAV8-hFIX, resulting in elevation of liver enzymes and decreased hFIX expression. Treatment with steroids reduced liver enzymes and partially rescued hFIX expression. Moreover, administration of intermediate doses of AAV resulted in detectable circulating capsid-specific T cells but transaminase elevation and loss of transgene expression were not observed [21]. Further studies confirmed that the capsid-specific immune response is dose-dependent and administration of low vector doses of AAV are more likely to induce mild inflammation that is manageable by steroids [86–88]. Additional characterization showed capsid-specific CD8+ T cells eliminate human hepatocytes after cross-presentation of capsid peptides on MHC I in vitro [89].
In contrast, other trials have observed T-cell immune responses that did not clear transgene expression, particularly muscle-directed trials [86, 90, 91]. Several factors including utilizing non-secreted transgenes in most of the muscle delivery trials, and apoptosis of reactive T cells, has complicated analyzing these results. Infiltration of CD4+ forkhead box P3+ (FoxP3) T cells, in addition to CD8+ T cells, was reported in α1-antitrypsin (AAT) deficiency and Glybera studies [92, 93]. Similarly, capsid-specific T-cell responses were limited or not observed in DMD clinical trials [94, 95]. Tolerance induction and long-term expression of transgene in local muscle-directed AAV gene therapy was attributed to Tregs due to their ability to suppress immune responses [92]. Nonetheless, AAV gene therapy in limb-girdle muscular dystrophy resulted in a capsid-specific T-cell response [96].
Additional studies are necessary to further characterize the T-cell immune response and the role of Tregs in inducing immune tolerance, which requires suitable animal models. Several studies have focused on mimicking the observed human immune responses in animals [97, 98]. Introduction of ovalbumin immunodominant epitope to AAV capsid was not able to trigger specific killing [99]. However, transfer of expanded and activated capsid-specific T cells to mice resulted in reduced transgene expression and loss of transduced cells [100, 101]. Moreover, immunization of mice with AAV or adenoviruses expressing VP1 capsid protein resulted in induction of capsid-specific T cells [102, 103]. Another study utilized chimeric antigen receptor (CAR) technology to create capsid-specific T cells. AAV-CAR T cells were reactive against multiple AAV capsid variants in vitro and reduced transgene expression in vivo [104]. However, AAV tropism and immune responses are not identical in humans and mice [83, 105]. Further studies are required to create animal models that accurately mimic human AAV-specific T-cell responses.
In addition to the cell-mediated responses observed upon introduction of AAV, immunological memory forms after the first encounter to AAV, either through AAV gene therapy or exposure to wild-type AAV. Activation of this memory response does not require an innate immune response [62]. Similar to humoral immunity, capsid-specific T-cell responses are less frequent in younger children and likely arise during infancy after infection with wild-type AAV. Studies suggest that following secondary AAV exposure, capsid-specific memory T cells produce cytokines and acquire a cytotoxic phenotype [27, 106–108]. Several studies characterized T-cell responses in healthy human subjects and reported 10–100% of human subjects have functional circulating capsid-specific T cells against multiple AAV serotypes [109–111]. Further analysis confirmed the majority of AAV-specific CD8+ and/or CD4+ T cells belong to central memory subsets [108]. Although AAV-specific immune responses are critical for gene therapy and redosing studies, delivered transgene can elicit immune responses. The following section will discuss the immune responses against the transgene.
Immune Responses to Transgene
Delivered transgene products often contain foreign antigens, therefore they may induce humoral or cellular immune responses that neutralize secreted transgenes and clear transduced cells [112]. Upon exposure to the AAV-delivered transgene, APCs acquire protein product through transduction or phagocytosis and present processed antigen on either MHC I or MHC II molecules, resulting in cellular or humoral immune responses, respectively [113]. Immune responses against the transgene continue until clearance of the transduced cells, or suppression of the immune response by Tregs or T-cell exhaustion [62]. However, when compared with adenoviral and lentiviral vectors, AAVs have a lower risk of inducing an immune response against the transgene due to the low transduction efficiency of APCs by AAVs [113, 114]. In this review, we summarize and discuss the complexity of transgene immune responses.
To date, transgene immune responses in AAV gene therapy have been mostly reported in muscle-directed clinical trials [94, 115–119]. In one study, muscle delivery of AAV resulted in formation of anti-transgene antibodies in some patients, which was predicted by NHP studies [120–123]. Furthermore, studies show higher risk of transgene immune response for gene transfer in human subjects with mutations resulting in no residual protein expression or large deletions [124–126], whereas gene delivery in subjects with missense mutations is more tolerated. Some clinical trials have controlled immune responses to transgene by simply not enrolling patients who were at higher risk to develop responses. In most liver-directed clinical trials, human subjects with a history of immune response to protein replacement therapy or null mutations were excluded from these studies [127, 128]. However, in Pompe clinical trials, where AAV was administered intramuscularly, immune responses were observed in patients who lacked functional enzyme expression or very low levels, similar to what is observed in protein replacement therapy [119, 129]. In an AAT clinical trial with muscle delivery of AAV, a transgene immune response was only observed in one human subject with a common non-pathogenic polymorphism and a less common HLA-haplotype [115]. Moreover, when patients with missense mutations were included in muscle delivery of hFIX, no transgene immune response was observed [130]. A recent abstract suggests DMD patients with genomic deletions including N-terminal epitopes that are present in the transgene protein are at a higher risk for transgene immune responses [131]. These observations suggest the level of residual protein expression and patient genotype have more critical roles influencing an immune response against the transgene. Furthermore, in the clinical setting, ocular and intrathecal AAV gene delivery have shown limited transgene-related immune responses, possibly due to their compartmentation and reduced antigen presentation [16, 132, 133]. AAV capsid variants are another contributing factor to generate an immune response to delivered transgene. Capsids with less transduction efficiency in APCs potentially have lower risk of induction of an immune response against the transgene [134–136].
In summary, the transgene immune response is mostly observed in patients with no residual protein expression; therefore, clinical studies preferably choose subjects with missense mutation or subjects with no response in protein replacement therapy [21, 24]. However, this approach eliminates target populations where the need is the greatest. To address this unmet need, several strategies have been employed that will be described in Sect. 5.3.
Immune Responses to AAV Vectors Post Regulatory Approval
Clinical trials are conducted with small homogeneous populations under standardized conditions that differ from post-approval patient selection and treatment conditions. Therefore, discrepancies in safety profile and responses may be observed [137]. Thus far, three AAV-based drugs (Luxturna, Zolgensma, and HEMGENIX) have been approved by the FDA, providing a considerably larger data set for post-licensure studies. In this section, we briefly discuss the overall safety profile and immune responses linked to Luxturna and Zolgensma.
Luxturna
Luxturna (voretigene neparvovec) is the first FDA-approved ocular AAV gene therapy for treating biallelic retinal pigmented epithelium-specific 65 kilodalton (RPE65) mutation-associated retinal dystrophy. A functional copy of the RPE65 gene is delivered directly to the retinal cells using a single AAV gene delivery, restoring the patient’s vision [134]. No deleterious immune responses have been observed either to the AAV2 capsid or transgene. Mild immune responses to Luxturna were reported that were controlled by systemic corticosteroid treatment prior to and post the subretinal delivery of Luxturna [135]. Similarly, humoral and cellular immune responses were limited in patients with leber hereditary optic neuropathy (LHON) treated with intravitreal injection of lenadogene nolparvovec (AAV2-ND4) [138]. Mild immune responses observed in Luxturna and LHON patients can be attributed to local injection into a compartmentalized environment containing elevated concentrations of immunomodulatory neuropeptides and cytokines [139].
Zolgensma
Zolgensma (onasemnogene abeparvovec) is the first AAV-based gene replacement therapy that has been approved by the FDA for treating SMA. Zolgensma is composed of a self-complementary, replication-deficient AAV9, encoding a functional SMN protein, and is administered via a single intravenous injection at a dose of 1.1×1014 vg/kg body weight [140].
Zolgensma was able to achieve clinically relevant therapeutic outcomes and has attained promising results in reducing premature death in SMA patients [141, 142]. Data from clinical trials and postmarketing surveillance reveal that there are safety risks such as liver toxicity, thrombocytopenia, TMA, cardiac events, and ganglionopathy [55, 143–145]. Transient elevations in liver transaminases were reported in SMA post-licensure that were resolved with prednisolone treatment. A prophylactic dose of prednisolone was administered to all SMA patients, but the dosing and duration of steroid treatment was variable. Tapering began only when the transaminase levels were less than two times the upper limit of normal. Patients who exhibited acute liver failure were administered a transient high corticosteroid dose. For patients receiving prolonged steroid treatment, it is recommended to consult an endocrinologist for assessing adrenal insufficiency. The hepatotoxicity may be attributed to capsid-specific cell-mediated immune response. In the event of acute illness, even after prolonged corticosteroid treatment, stress dosing with hydrocortisone may be considered [143]. Thus far, prednisolone has proved effective in restoring liver transaminase levels in SMA clinical trials and post-licensure experience. However, there is evidence of acute hepatotoxicity in SMA patients despite steroid treatment. Novartis recently reported the death of two pediatric SMA patients from acute liver failure. Liver function was severely impaired and rapid progression to hepatic encephalopathy and multi-organ failure was observed in these patients. The deaths occurred 6–7 weeks post Zolgensma treatment, after the initiation of prednisolone taper [146].
In addition to hepatotoxicity, other adverse effects included transient thrombocytopenia. In one patient, late onset of thrombocytopenia and multiorgan failure was observed, however the mechanism remained unknown [143]. Of more than 1400 patients treated with Zolgensma, nine cases of TMA have been reported by the FDA [54]. The EMA has reported five cases of TMA among the 800 patients who received Zolgensma [147]. One of the patients diagnosed with TMA at day 12 after gene therapy faced a fatal outcome due to a multitude of factors. Interestingly, increased levels of soluble C5b9 complex (sC5b9), a biomarker of complement activation, was reported in this patient. The patient progressively worsened and the symptoms were characteristic of aHUS. Based on the treatment recommendations for pediatric aHUS, the patient was treated with eculizumab [148], a monoclonal antibody known to inhibit the cleavage of complement factor C5. Eculizumab injection resulted in EsC5b9 C3 levels being restored to normal, with a decrease in C3, normal C4, and a mild increase in platelet count, suggesting TMA recovery after 1 month. However, at day 40, TMA relapsed due to the onset of Staphylococcus epidermidis infection, and unfortunately the patient suffered cardiac arrest [54]. Another study reported that three patients also developed TMA after 1 week of Zolgensma treatment, suggestive of an immune-mediated etiology. Other factors, such as concurrent bacterial infection and recent vaccine exposure, also contributed to TMA. Activation of the complement system via the alternate pathway was reported in two of the cases, and one patient who received eculizumab did not show significant clinical improvement [55]. Further investigation is required to assess the efficacy of eculizumab in TMA, induced by AAV gene therapy. Notably, TMA with complement activation has been reported in other AAV9-based gene therapies in DMD and Danon disease [149, 150]. Future studies are needed to delineate the role of complement-mediated factors in TMA for SMA patients.
A recent study reported hemophagocytic lymphohistiocytosis (HLH) in an SMA patient treated with Zolgensma. High doses of methylprednisolone for 3 days, with a progressive tapering in the following days, resulted in clinical improvement. Therefore, careful monitoring of inflammatory markers is needed to avoid fatality due to HLH [151].
Strategies to Mitigate Immune Responses in AAV Gene Therapy
In this section, we discuss possible strategies to circumvent the deleterious effects of the immune responses to AAV capsids and transgene, thereby enabling successful transgene delivery and expression (Table 1).
Table 1.
Immune responses in AAV gene therapy and possible strategies to modulate these responses
| Immune responses | Mechanism | Strategy | Clinical status |
|---|---|---|---|
| Innate immunity | Evading TLR activation and DNA sensing | CpG depletion, CpG free ITRs | Preclinical |
| TLR9-inhibitory DNA sequences | Preclinical | ||
| Hydroxychloroquine | Preclinical | ||
| DNA and RNA modification to ameliorate dsRNA and dsDNA | Preclinical | ||
| Evading complement system | APL-9 | Clinical trial | |
| Eculizumab | Clinical trial | ||
| Reducing transgene expression in APCs | Proteasome inhibitor | Preclinical | |
| miRNA | Preclinical | ||
| Humoral immunity | Evading NAb formation | Excluding patients with pre-existing NAbs | Clinical trial |
| Immunosuppressive drugs | Clinical trial | ||
| ImmTOR | Clinical trial | ||
| Antibody degrading enzymes | Preclinical | ||
| Plasmapheresis | Clinical trial | ||
| Saline flushing with or without balloon catheter | Preclinical | ||
| Altering the route of administration | Clinical trial | ||
| AAV-based strategies | Empty capsids | Preclinical | |
| Capsid engineering and modification | Preclinical | ||
| Switching capsid variant | Preclinical | ||
| Cell-mediated immune responses | Systemic immune suppression | Immunosuppressive drugs | Clinical trial |
| Reducing capsid presentation | Mutation of surface tyrosine | Preclinical | |
| Reducing therapeutic dose | Clinical trial | ||
| Removing empty capsid | Preclinical | ||
| Treg-based strategy | Tregitope | Preclinical | |
| ImmTOR | Clinical trial | ||
| AAV-specific CAR Treg | Preclinical | ||
| Transgene immune responses | Systemic immune suppression | Immunosuppressive drugs | Clinical trial |
| Tissue targeting | Tissue specific promoters | Preclinical | |
| Treg-based strategies | Polyclonal Tregs | Preclinical | |
| Transgene-specific CAR Treg | Preclinical | ||
| Transgene-specific TRuC Treg | Preclinical | ||
| BAR Treg | Preclinical | ||
| Evading antibody-producing cells | BAR T cell | Preclinical |
AAV adeno-associated virus, APCs antigen-presenting cells, BAR B-cell antibody receptor, CAR chimeric antigen receptor, ds double-stranded, ITRs inverted terminal repeats, miRNA microRNA, NAbs neutralizing antibodies, TLR toll-like receptor, Treg regulatory T cells
Strategies to Mitigate Innate Immune Responses to AAVs
Immunomodulatory techniques are actively being developed to reduce AAV vector immunogenicity and allow for safe and repeated interventions. While corticosteroids administered in most trials have helped to modulate immune-mediated toxicities and achieve long-term transgene expression, some cases require more complex steroid regimens and additional approaches to potentially reduce vector and transgene immunogenicity.
Overcoming Toll-Like Receptor Activation and DNA Sensing
TLR activation plays a pivotal role in triggering innate immune responses to AAV vectors. Strategies to circumvent TLR activation aim to mitigate these responses. TLR9-deficient mice display high levels of transgene expression [152], an observation that prompted several groups to modify the transgene sequence to interfere with TLR9-mediated recognition. Preventing TLR9 signaling by depleting CpG dinucleotides in the AAV genome has been shown to enhance AAV-mediated gene expression. Deletion of the CpG dinucleotides exhibited stable transgene expression with reduced infiltration of effector T cells (Teff) when packaged in the immunogenic AAVrh32.33 vector [40]. Moreover, CpG deletion resulted in markedly reduced CD8+ T-cell infiltration upon intramuscular injection in hemophilia B mice but did not impact antibody levels [153]. The ITR region also contains high CpG content, therefore one study generated functional CpG-free ITRs. However, the vector yield was decreased by approximately threefold due to ITRs’ crucial role in encapsidation [154]. In another study, it has been reported that the incorporation of TLR9-inhibitory (TLR9i) DNA sequences in the AAV genome can reduce immune responses in mice and pigs [155]. TLR9i is composed of multiple copies of TTAGGG, which can also block DNA sensors, such as cGAS [156, 157]. Additionally, the antimalarial drug hydroxychloroquine (HCQ) may act as a TLR9 inhibitor [158]. In mouse and human tissues, HCQ administration 1 h prior to AAV delivery enhanced transduction efficiency [46]. Other modifications to ameliorate dsDNA- and dsRNA-sensing mechanisms include engineering the expression cassette such that transcription is blocked from the 3′ ITR. As a result, dsRNA will not be generated and innate immune responses that stem from binding to the cytosolic dsDNA and dsRNA can be avoided [159]. Introduction of short hairpin RNAs designed against dsRNA sensors or their downstream signaling pathways into the AAV expression cassette may be used to circumvent dsRNA-mediated innate immune responses. Adopting a multidisciplinary approach to block TLR9 activation and DNA sensing to circumvent innate immune responses may improve clinical responses.
Evading Complement System Activation
Restricting complement activation may prevent inflammatory responses and the ensuing tissue damage. Apellis Pharmaceuticals has proposed the use of a PEGylated synthetic cyclic peptide called APL-9 that inhibits all three pathways of complement activation to prevent complement activation in response to AAV. In phase I testing, a single dose of APL-9 administered intravenously was able to restrict complement activation, lasting up to 12 h. No serious adverse events have been reported and APL-9 treatment was able to suppress AH50 hemolytic activity completely [160]. In a recent study, APL-9 reduced CD86 levels on APCs, AAV uptake, and cytokine/chemokine secretion in response to AAV in vitro [161]. Since the involvement of classical pathway was confirmed by the loss of complement activation in IgG-depleted serum [52], treatments that reduce the interaction between AAVs and antibodies could be effective in preventing complement activation. A plethora of complement inhibitors, ranging from small molecule inhibitors, siRNAs, aptamers, antisense oligonucleotides, inhibitory peptides, pharmacological agents to block antibodies, etc., are being tested for suppressing complement activation [162]. One monoclonal antibody that is used against complement hyperactivation and functions by inhibiting C5 and MAC formation is eculizumab [163]. Eculizumab has been used successfully to limit complement activation in AAV gene therapy for SMA and DMD (NCT03362502, NCT03368742, NCT03381729, NCT02122952) [164]. Further studies that inhibit complement system activation by AAVs need to be conducted in the future.
Reducing Transgene Expression in Antigen-Presenting Cells
Strategies to limit immune cell activation are being developed. For example, TLR9 inhibitory oligonucleotides restored the increased macrophage infiltration, triggered by scAAV administration [44]. Proteasome inhibitors that restrict ubiquitination and evade proteasome-mediated degradation can reduce the presentation of capsid peptide fragments on MHC I molecules [165, 166]. MicroRNA (miRNA)-mediated detargeting is another strategy that enables target tissue and cell-specific expression by blocking unwanted expression from other cells [167, 168]. This approach can mitigate the antigen presentation by innate immune cells and subsequently block adaptive immune responses to transgene delivered by AAVs. Additional methods to limit adaptive immune responses to AAV capsid and transgene are discussed below.
Mitigating Adaptive Immune Response to AAV Capsid
Induction of adaptive immune response following AAV gene delivery limits transgene expression and generates immunological memory which inhibits repeated dosing of AAV. Therefore, several strategies are being employed to limit adaptive capsid-specific immune response. In the following sections, strategies to overcome humoral and T-cell-mediated immune responses against AAV capsid are discussed.
Strategies to Overcome Humoral Immune Response
AAV gene therapy is hindered by the formation of NAbs, therefore multiple strategies are employed to overcome the humoral immune response. Patients who require systemic administration of AAVs are particularly at a greater disadvantage to NAbs. One approach is to dose subjects who lack pre-existing immunity to AAV, which is more common for younger patients but considerably less for adolescents and adults [31].
Due to the high prevalence of NAbs, several strategies have been employed to suppress or ablate the humoral immune response in gene therapy subjects. The primary approach to inhibit humoral immunity is the use of immunosuppressive drugs (IS). A broadly applicable strategy is to use B-cell-depleting monoclonal antibodies. Studies have shown promising results of B-cell depletion with decreased NAb titers after utilizing rituximab, an anti-CD20 antibody [118, 169]. The combination of rituximab with rapamycin, mTOR inhibitor, or with rapamycin and corticosteroids were successful in inducing tolerance and preventing antibody formation in clinical trials [16, 170]. Belimumab, an anti-B-cell-activating factor (BAFF), is another B-cell-depleting antibody that has shown promising results in controlling autoimmune diseases [171, 172]. Furthermore, utilizing ibrutinib, a B-cell inhibitor, reduced primary antibody responses against AAV in a murine model [173]. However, systemic IS may increase the risk of infection and is not effective in complete remission of high titer NAbs [107, 169]. In addition to conventional IS, coadministration of AAV vectors and synthetic vaccine encapsulating rapamycin (SV[rapa]), ImmTOR, is another encouraging approach. ImmTOR treatment resulted in inhibition of AAV-specific antibody formation and reduced B- and T-cell activation [103, 174]. A recent abstract showed coadministration of ImmTOR with belimumab reduced anti-AAV IgM antibodies, provided more durable suppression of anti-AAV IgG antibodies, and allowed for multiple re-administrations of an AAV8 vector in mice. A reduced effect was observed when ImmTOR was combined with ibrutinib [131]. However, coadministration of ImmTOR with empty AAV8 in healthy human subjects only delayed the formation of NAbs [175].
Several approaches focus on evading NAbs directly. One approach utilizes bacterial-derived antibody-degrading enzymes [176–179]. An alternate approach is plasmapheresis, which is used to reduce circulating antibodies but may not completely eradicate NAbs or requires multiple cycles [180]. Moreover, plasmapheresis removes total antibody content and therefore increases the risk of infectious diseases. To overcome this obstacle, one study used an AAV-specific plasmapheresis column to deplete anti-AAV NAbs from the plasma in NHPs, without affecting total IgG levels [181]. Another study showed AAV9 particles coupled to Sepharose beads selectively depleted anti-AAV antibodies in rats [182]. This approach has a limited therapeutic window since plasmapheresis does not remove antibody-producing cells. In another study, saline flushing with or without balloon catheter was used to isolate the target tissue from systemic circulation. This strategy is relatively invasive and may not be feasible for all target tissues and systemic delivery of AAV. Moreover, in this study, NHPs with low NAbs were used and the efficacy of this method in animals with high NAbs needs further evaluation [183, 184]. Utilizing routes with minimal exposure to NAbs has been suggested. In a hemophilia clinical trial, muscle delivery of AAV was less susceptible to pre-existing NAbs compared with the systemic delivery route [130]. In addition, direct delivery of AAV to the CNS was well tolerated in animal models [185, 186]. However, altering the route of administration can potentially attenuate transduction efficiency and change the biodistribution of the AAV vector [67].
Additional approaches focus on AAV capsid to modulate the humoral immune response. One approach is to use decoy empty capsids to absorb circulating NAbs [187]. The addition of empty capsids is often not effective and may exacerbate immunotoxicity of AAV and reduce transduction efficiency [188, 189]. Moreover, production of large amounts of empty capsids in addition to the capsids with transgene adds additional complications and associated costs to the process [189].
Capsid engineering is another approach to generate recombinant AAVs with desired tropism and reduced immunogenicity. Modifications in the NAb recognition sites can enhance transduction efficiency [190–193]. Alternatively, capsids generated by directed evolution can escape NAb sensing. Compared with natural variants, the engineered AAV-DJ variant, consisting of residues from AAV2, AAV8, and AAV9 variants, has been shown to transduce hepatocytes in the presence of human intravenous IgG [194, 195]. In addition, AAV3b isolated from humanized mouse models is not sensitive to NAb recognition and has higher transduction efficiency in human hepatocytes [196]. Nonetheless, engineering AAV capsid instead of utilizing currently available and well-studied capsids is time-consuming, labor intensive, and requires identification of NAb recognition sites [70, 197]. To avoid complication of engineering AAV capsid, chemical modification of AAV capsid was suggested, including PEGylation and polymer encapsulation. However, these chemical modifications can potentially decrease transduction efficiency, lower production yield, alter biodistribution of AAV vectors, and result in anti-polymer antibody formation [198, 199]. In addition to chemically modified capsids, multiple studies showed exosome-enveloped AAVs can transduce the target cells in the presence of pre-existing immunity, induce tolerance, and confer altered tropism to AAV. However, encapsulating AAVs with exosomes may further complicate the manufacturing process and introduce nucleic acid or protein contaminations [200–202].
Often, a single treatment of AAV gene therapy may not be sufficient. In addition to immune responses following AAV gene therapy, exposure to wild-type AAV results in pre-existing immunity that impedes AAV redosing. Multiple studies showed CD28/B7 and CD40/CD40L blockade resulted in mitigation of inflammatory response and allowed for vector re-administration [203–205]. In this strategy, CTLA4 fusion protein interacts with B7 with higher affinity than CD28, resulting in downregulation of immune responses. This approach was combined with an anti-CD40 ligand antibody that blocks stimulatory signals for B cells [206]. Despite the promising result, CD28/B7 and CD40/CD40L blockade broadly inhibits NAbs and requires further investigation. Furthermore, mitigation strategies such as plasmapheresis, capsid decoy, and switching capsid variant are not effective. Moreover, immune suppression and utilizing antibody degrading enzymes do not target AAV-specific immune responses. Further studies are needed to develop more specific and effective approaches. Switching capsid variants is another alternative to allow for re-administration of AAV but is limited by varying tropism of AAV capsids, cross-reactivity of NAbs, and complexity of developing different products [207]. Evading the humoral immune response to allow for gene therapy remains elusive. Several variables, including route of administration, required dose, and target tissue play critical roles in choosing the appropriate strategy. In addition, utilizing other strategies beyond B-cell depletion such as antibodies against IL-1β and IL-6, etanercept (TNFα inhibitor) or anakinra (IL-1R blocker) may prove beneficial in regulating humoral immune reposes in AAV gene therapy. Utilizing one or a combination of strategies may allow for AAV gene therapy in patients who would otherwise not be eligible.
Strategies to Overcome Cell-Mediated Immune Response
Unlike humoral immune responses where patients’ responses can be predicted, cell-mediated responses to AAV have been largely variable across patients and clinical trials. Since first observed in hemophilia clinical trials, capsid-specific T-cell responses have been widely inhibited through immunosuppression regimens. IS treatments used in AAV clinical trials vary from steroids to monoclonal antibodies and proteasome inhibitors. Steroids are commonly used in clinical trials to reduce T-cell activation and cytokine production by affecting T-cell receptor signaling. Moreover, they increase anti-inflammatory cytokine production and Treg proliferation [208, 209]. Steroids inhibit inflammatory response by inhibiting vascularization, leukocyte migration to inflamed sites, and alter immune cell death and trafficking [210]. As previously described, glucocorticoids were used in all human subjects to inhibit capsid-specific T-cell response in Zolgensma trials, yet two patients died from acute liver failure during steroid taper [142]. Multiple clinical studies showed that using steroids did not prevent the gradual loss of transgene [211–213]. Therefore, several studies have focused on utilizing combinations of immunosuppressant drugs and optimization of the timing of treatment [214–216]. Cyclosporine and tacrolimus downregulate IL-2 transcription, thereby inhibiting T-cell proliferation and activation. Daily administration of tacrolimus allowed for prolonged expression of AAV-delivered transgene in NHP [217]. Mycophenolate mofetil (MMF) depletes guanosine nucleotides preferentially in T and B lymphocytes and inhibits their proliferation [218]. A combination of cyclosporine and MMF was used in Glybera clinical trials but did not inhibit the immune responses in all human subjects. The follow-up studies showed commencing immunosuppression before AAV dosing and additional corticosteroid treatment is beneficial [93, 219, 220]. In vitro studies showed Treg proliferation inhibition by tacrolimus and cyclosporine [221]. One study showed treatment with tacrolimus impaired Treg functionality in human subjects with kidney and liver transplants, while rapamycin had a beneficial effect on Tregs [222]. Thus, rapamycin in combination with rituximab or prednisone has been frequently used to induce tolerance in AAV clinical trials [16, 119, 223]. In addition, cyclosporine and non-depleting CD4 receptor antibody induced Tregs and allowed for redosing in mice [65]. Utilizing proteasome inhibitors such as bortezomib has been suggested. In vitro and preclinical animal studies have shown administration of bortezomib prevents degradation of internalized AAVs, reduces capsid presentation, and increases transduction efficiency by enhancing nuclear translocation of AAV [165, 224, 225]. Despite the wide application, systemic immune suppression may cause Treg suppression and multiple adverse effects, including intestinal complications, muscle pain ,and headaches, which has brought other strategies to light [107, 226–228].
During AAV trafficking, tyrosine residues on the surface of AAV capsid can be phosphorylated and subsequently ubiquitinated, which leads to capsid degradation and presentation on the surface of transduced cells. Therefore, mutation of surface tyrosine has been suggested, which resulted in reduced presentation of AAV capsid, decreased T-cell toxicity, and required therapeutic dose [100, 225, 229]. Multiple studies reduced the therapeutic dose of AAV and utilized hyperactive variants of delivered transgene [88, 193, 230]. The hyperactive version of a transgene is not available for every transgene and may not always result in reduced T-cell responses [62, 188]. In addition to previously discussed strategies, lowering administration dose and removing empty capsids has been proposed. This approach attempts to reduce the availability of AAV capsid for antigen presentation. However, lowering the dose of AAV may reduce transduction efficiency and result in neutralization of AAVs by NAbs [63, 112].
Infiltration of Tregs and long-term expression of transgene in muscle-directed AAV gene therapy trials emphasized Treg roles in inducing immune tolerance. Therefore, Treg induction epitopes, Tregitope, were incorporated into AAV capsid structure, which successfully expanded Treg and inhibited CD8 T cells [102]. In addition to engineering AAV capsid, ImmTOR particles showed promising results in capsid-specific T cell and humoral immune responses [103]. However, these novel strategies need further efficacy and safety investigation since they are non-antigen-specific treatments.
To induce localized and specific tolerance, another study combined Tregs’ ability to suppress immune response and antigen specificity of CAR. In this study, AAV-specific CAR Tregs and polyclonal Tregs were used to allow for transgene expression despite a capsid-specific T-cell response [104].
Clinically, capsid-specific T-cell responses have mostly been controlled utilizing steroids. Immunosuppression increases the risk of infection and is less effective when high doses of AAVs are required. Therefore, developing a novel and antigen-specific strategy seems inevitable.
Mitigating Transgene Immune Response
To avoid immune response against delivered transgene, several approaches have been suggested, including limiting target population, immunosuppression, utilizing tissue-specific promoters, miR technology, and immune cell therapy.
Short-term treatment with cyclophosphamide, an alkylating agent causing a decrease in proinflammatory cytokine production, led to successful gene expression in mice and canine models [124, 125]. Moreover, transient immunosuppression with cyclosporine, anti-thymocyte globulin, and MMF prevented the immune responses and achieved transgene expression in a canine study [231]. In addition to systemic immune suppression, preclinical studies pointed out the role of tissue-specific promoters in reducing immune responses against delivered transgene [232, 233]. Lower levels of transgene expression and strong humoral and cellular immune responses were observed when the transgene was driven by a ubiquitous promoter compared with a muscle-specific promoter [232]. Furthermore, utilizing a muscle-specific promoter resulted in stable expression of the transgene without immune responses in a canine model [233]. In addition, a study in NHP reported that liver-directed expression may help to limit anti-transgene antibodies, induce tolerance, and allow for re-administration of a different capsid variant [122].
Other studies utilized Tregs to suppress transgene-related immune responses. One study used polyclonal Tregs to suppress immune responses against factor 8 and 9 in protein replacement therapy [234]. However, low frequency of Tregs and potential nonspecific immune suppression have limited the application of polyclonal Tregs. Therefore, multiple studies generated transgene-specific CAR Tregs, which showed promising results in suppression of transgene-induced immune responses [235–237]. TRuC Tregs were developed by fusion of anti-human coagulation factor VIII (hFVIII) single chain variable fragment and T-cell receptor. This study showed superior suppressive function of F8-TRuC Treg compared with FVIII-CAR Treg [172]. B-cell antibody receptor (BAR) was generated by combining factor 8 antigen and T-cell stimulatory signaling domains. BAR T cells successfully eliminated FVIII reactive B cells in vitro and in vivo [238]. Subsequently BAR Tregs were created by transducing Tregs with BAR construct and showed promising results in suppression of transgene-specific B cells [239, 240]. Despite the promising results of immune cell therapy in suppression of transgene immune responses, their clinical safety and efficacy need to be investigated.
Currently, subjects with potentially lower risk of transgene immune response are enrolled in the clinical studies. Utilizing newly developed approaches enables us to include a greater number of representative study subjects.
Conclusion
Still much is unknown about the immune response to AAV gene therapy in patients. However growing evidence is mounting based on ongoing clinical trials and post-licensure data of treating thousands rather than a small number of patients in clinical trials. To further understand the development of immune responses to AAV gene therapy, further immunomonitoring of enrolled subjects is necessary, including strategies for monitoring of serum factors, characterization of immune cells in peripheral blood, and tissue analysis. However, the lack of standard protocols for immune assays as well as the inability to collect relevant samples results in variability among studies and further complicates the interpretation of findings. The development of standard protocols to monitor subjects in AAV clinical trials would be beneficial for the field for better comparison. However, the variability that exists in dose, route of administration, and use of multiple capsid serotypes within current trials may further make comparison and interpretation of immune responses to AAV gene therapy more complicated.
In most trials, systemic immunosuppression is broadly administered to modulate immune responses in AAV gene therapy clinical trials, despite some significant adverse effects. However, immunosuppression regimens, dosage, and timing are also all highly variable and there is no consensus in the field. Appropriate animal models to test current or new drugs are not readily available, making it difficult to standardize protocols. Applying newly developed strategies that are more targeted as compared with broad, nonspecific immune modulation may prove beneficial in improving outcomes and reducing complications.
Since the first AAV clinical trial in 1995, significant clinical data regarding AAV safety and immune responses have been observed, however the immune responses remain a barrier for successful AAV gene therapy. Emerging strategies to overcome the immune responses will allow for the full potential of AAV gene therapy to positively impact thousands of lives.
Acknowledgments
The authors thank Ashley L. Harkins and Katelyn Sylvia for insightful comments. Graphical schematics were created using BioRender.com
Declarations
Funding
Funding was provided by National Heart, Lung and Blood Institute grant number NHLBI-P01 HL158506 to AMK.
Conflicts of Interest
Terence R. Flotte serves as a paid consultant for Ferring Ventures that is unrelated to the work described here. Motahareh Arjomandnejad, Terence R. Flotte, and Allison M. Keeler are inventors on patent US2020029527. Ishani Dasgupta was an employee at UMMS at the time the initial draft of this manuscript was written but is currently an employee of AbbVie.
Ethics Approval
Not applicable.
Consent to Participate/Publish
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Author Contributions
Conceptualization: MA, ID, AMK. Writing—original draft preparation: MA, ID, AMK. Writing—review and editing: MA, ID, TRF, AMK. Visualization: MA and AMK. All authors have read and agreed to the published version of the manuscript. MA and ID contributed equally to this work.
Footnotes
Motahareh Arjomandnejad and Ishani Dasgupta contributed equally to this work.
References
- 1.FDA approves hereditary blindness gene therapy. Nat Biotechnol. 2018;36(1):6. [DOI] [PubMed]
- 2.Gene therapy’s next installment. Nat Biotechnol. 2019;37(7):697. [DOI] [PubMed]
- 3.Nathwani AC, McIntosh J, Sheridan R. Liver Gene Therapy. Hum Gene Ther. 2022;33(17–18):879–888. doi: 10.1089/hum.2022.169. [DOI] [PubMed] [Google Scholar]
- 4.Kotterman MA, Chalberg TW, Schaffer DV. Viral vectors for gene therapy: translational and clinical outlook. Annu Rev Biomed Eng. 2015;17:63–89. doi: 10.1146/annurev-bioeng-071813-104938. [DOI] [PubMed] [Google Scholar]
- 5.Pipe S, et al. Clinical considerations for capsid choice in the development of liver-targeted AAV-based gene transfer. Mol Ther Methods Clin Dev. 2019;15:170–178. doi: 10.1016/j.omtm.2019.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viney L, et al. Adeno-associated virus (AAV) capsid chimeras with enhanced infectivity reveal a core element in the AAV genome critical for both cell transduction and capsid assembly. J Virol. 2021;95(7):e02023–e2120. doi: 10.1128/JVI.02023-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atchison RW, Casto BC, Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149(3685):754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- 8.Gao G, et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78(12):6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mingozzi F. KA High, Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12(5):341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 10.Li C, Samulski RJ. Engineering adeno-associated virus vectors for gene therapy. Nat Rev Genet. 2020;21(4):255–272. doi: 10.1038/s41576-019-0205-4. [DOI] [PubMed] [Google Scholar]
- 11.Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019;18(5):358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louis Jeune V, et al. Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum Gene Ther Methods. 2013;24(2):59–67. doi: 10.1089/hgtb.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samelson-Jones BJ, et al. Timing of intensive immunosuppression impacts risk of transgene antibodies after AAV gene therapy in nonhuman primates. Mol Ther Methods Clin Dev. 2020;17:1129–1138. doi: 10.1016/j.omtm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manno CS, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12(3):342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 15.Bainbridge JW, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358(21):2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 16.Flotte TR, et al. AAV gene therapy for Tay-Sachs disease. Nat Med. 2022;28(2):251–259. doi: 10.1038/s41591-021-01664-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samaranch L, et al. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum Gene Ther. 2012;23(4):382–389. doi: 10.1089/hum.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzpatrick Z, et al. Influence of pre-existing anti-capsid neutralizing and binding antibodies on AAV vector transduction. Mol Ther Methods Clin Dev. 2018;9:119–129. doi: 10.1016/j.omtm.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinderer C, et al. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum Gene Ther. 2018;29(3):285–298. doi: 10.1089/hum.2018.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson JM, Flotte TR. Moving forward after two deaths in a gene therapy trial of myotubular myopathy. Hum Gene Ther. 2020;31(13–14):695–696. doi: 10.1089/hum.2020.182. [DOI] [PubMed] [Google Scholar]
- 21.Nathwani AC, et al. Adenovirus-associated virus vector–mediated gene transfer in hemophilia B. N Engl J Med. 2011;365(25):2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hensley SE, et al. Type I interferon inhibits antibody responses induced by a chimpanzee adenovirus vector. Mol Ther. 2007;15(2):393–403. doi: 10.1038/sj.mt.6300024. [DOI] [PubMed] [Google Scholar]
- 23.Goubau D, Deddouche S, Reis e Sousa C. Cytosolic sensing of viruses. Immunity. 2013;38(5):855–869. doi: 10.1016/j.immuni.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 25.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7(3):179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 26.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14(1):36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuranda K, et al. Exposure to wild-type AAV drives distinct capsid immunity profiles in humans. J Clin Invest. 2018;128(12):5267–5279. doi: 10.1172/JCI122372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers GL, et al. Plasmacytoid and conventional dendritic cells cooperate in crosspriming AAV capsid-specific CD8(+) T cells. Blood. 2017;129(24):3184–3195. doi: 10.1182/blood-2016-11-751040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shirley JL, et al. Type I IFN sensing by cDCs and CD4(+) T cell help are both requisite for cross-priming of AAV capsid-specific CD8(+) T cells. Mol Ther. 2020;28(3):758–770. doi: 10.1016/j.ymthe.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers GL, et al. Unique roles of TLR9- and MyD88-dependent and -independent pathways in adaptive immune responses to AAV-mediated gene transfer. J Innate Immun. 2015;7(3):302–314. doi: 10.1159/000369273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ronzitti G, Gross DA, Mingozzi F. Human immune responses to adeno-associated virus (AAV) vectors. Front Immunol. 2020;11:670. doi: 10.3389/fimmu.2020.00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 33.Satoh T, S Akira. Toll-like receptor signaling and its inducible proteins. Microbiol Spectr. 2016;4(6). 10.1128/microbiolspec.MCHD-0040-2016. [DOI] [PubMed]
- 34.Zhu J, Huang X, Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J Clin Invest. 2009;119(8):2388–2398. doi: 10.1172/JCI37607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashley SN, et al. TLR9 signaling mediates adaptive immunity following systemic AAV gene therapy. Cell Immunol. 2019;346:103997. doi: 10.1016/j.cellimm.2019.103997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rabinowitz, J., Y.K. Chan, and R.J. Samulski, Adeno-associated Virus (AAV) versus Immune Response. Viruses. 2019;11(2). 102; 10.3390/v11020102. [DOI] [PMC free article] [PubMed]
- 37.Wright JF. Codon modification and PAMPs in clinical AAV vectors: the tortoise or the hare? Mol Ther. 2020;28(3):701–703. doi: 10.1016/j.ymthe.2020.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gustafsson C, Govindarajan S, Minshull J. Codon bias and heterologous protein expression. Trends Biotechnol. 2004;22(7):346–353. doi: 10.1016/j.tibtech.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Huang X, Yang Y. Targeting the TLR9-MyD88 pathway in the regulation of adaptive immune responses. Expert Opin Ther Targets. 2010;14(8):787–796. doi: 10.1517/14728222.2010.501333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faust SM, et al. CpG-depleted adeno-associated virus vectors evade immune detection. J Clin Invest. 2013;123(7):2994–3001. doi: 10.1172/JCI68205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butterfield JSS, et al. TLR9-activating CpG-B ODN but Not TLR7 agonists triggers antibody formation to factor IX in muscle gene transfer. Hum Gene Ther Methods. 2019;30(3):81–92. doi: 10.1089/hgtb.2019.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hosel M, et al. Toll-like receptor 2-mediated innate immune response in human nonparenchymal liver cells toward adeno-associated viral vectors. Hepatology. 2012;55(1):287–297. doi: 10.1002/hep.24625. [DOI] [PubMed] [Google Scholar]
- 43.Sudres M, et al. MyD88 signaling in B cells regulates the production of Th1-dependent antibodies to AAV. Mol Ther. 2012;20(8):1571–1581. doi: 10.1038/mt.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martino AT, et al. The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver. Blood. 2011;117(24):6459–6468. doi: 10.1182/blood-2010-10-314518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu T, et al. Self-complementary AAVs induce more potent transgene product-specific immune responses compared to a single-stranded genome. Mol Ther. 2012;20(3):572–579. doi: 10.1038/mt.2011.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chandler LC, et al. Enhancement of adeno-associated virus-mediated gene therapy using hydroxychloroquine in murine and human tissues. Mol Ther Methods Clin Dev. 2019;14:77–89. doi: 10.1016/j.omtm.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding W, et al. Intracellular trafficking of adeno-associated viral vectors. Gene Ther. 2005;12(11):873–880. doi: 10.1038/sj.gt.3302527. [DOI] [PubMed] [Google Scholar]
- 48.Huang LS, et al. mtDNA activates cGAS signaling and suppresses the YAP-mediated endothelial cell proliferation program to promote inflammatory injury. Immunity. 2020;52(3):475–486e5. doi: 10.1016/j.immuni.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shao W, et al. Double-stranded RNA innate immune response activation from long-term adeno-associated virus vector transduction. JCI Insight. 2018;3(12). e120474. 10.1172/jci.insight.120474. [DOI] [PMC free article] [PubMed]
- 50.Reichel FF, et al. AAV8 can induce innate and adaptive immune response in the primate eye. Mol Ther. 2017;25(12):2648–2660. doi: 10.1016/j.ymthe.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Merle NS, et al. Complement system part I—molecular mechanisms of activation and regulation. Front Immunol. 2015;6:262. doi: 10.3389/fimmu.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaiss AK, et al. Complement is an essential component of the immune response to adeno-associated virus vectors. J Virol. 2008;82(6):2727–2740. doi: 10.1128/JVI.01990-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5(10):981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 54.Guillou J, et al. Fatal thrombotic microangiopathy case following adeno-associated viral SMN gene therapy. Blood Adv. 2022;6(14):4266–4270. doi: 10.1182/bloodadvances.2021006419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chand DH, et al. Thrombotic microangiopathy following onasemnogene abeparvovec for spinal muscular atrophy: a case Series. J Pediatr. 2021;231:265–268. doi: 10.1016/j.jpeds.2020.11.054. [DOI] [PubMed] [Google Scholar]
- 56.Mullard A. Gene therapy community grapples with toxicity issues, as pipeline matures. Nat Rev Drug Discov. 2021;20(11):804–805. doi: 10.1038/d41573-021-00164-x. [DOI] [PubMed] [Google Scholar]
- 57.LogicBio . LogicBio therapeutics reports second quarter 2022 FINANCIAL RESULTS AND PROVIDES CORPORATE UPDATE. LogicBio; 2022. [Google Scholar]
- 58.Bonilla FA. HC Oettgen. Adaptive immunity. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S33–40. doi: 10.1016/j.jaci.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 59.Shirley JL, et al. Type I IFN sensing by cDCs and CD4+ T cell help are both requisite for cross-priming of AAV capsid-specific CD8+ T cells. Mol Ther. 2020;28(3):758–770. doi: 10.1016/j.ymthe.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rogers GL, et al. Plasmacytoid and conventional dendritic cells cooperate in crosspriming AAV capsid-specific CD8+ T cells. Blood. 2017;129(24):3184–3195. doi: 10.1182/blood-2016-11-751040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ertl HCJ. Immunogenicity and toxicity of AAV gene therapy. Front Immunol. 2022;13:975803. doi: 10.3389/fimmu.2022.975803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ertl HCJ. T cell-mediated immune responses to AAV and AAV vectors. Front Immunol. 2021;12. 10.3389/fimmu.2021.666666. [DOI] [PMC free article] [PubMed]
- 63.Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122(1):23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sebina I, Pepper M. Humoral immune responses to infection: common mechanisms and unique strategies to combat pathogen immune evasion tactics. Curr Opin Immunol. 2018;51:46–54. doi: 10.1016/j.coi.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McIntosh JH, et al. Successful attenuation of humoral immunity to viral capsid and transgenic protein following AAV-mediated gene transfer with a non-depleting CD4 antibody and cyclosporine. Gene Ther. 2012;19(1):78–85. doi: 10.1038/gt.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chirmule N, et al. Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle. J Virol. 2000;74(5):2420–2425. doi: 10.1128/JVI.74.5.2420-2425.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whitehead M, et al. Humoral immune responses to AAV gene therapy in the ocular compartment. Biol Rev. 2021;96(4):1616–1644. doi: 10.1111/brv.12718. [DOI] [PubMed] [Google Scholar]
- 68.Stolte B, et al. Prevalence of anti-AAV9 antibodies in adult patients with spinal muscular atrophy. Hum Gene Ther. 2022;33(17–18):968–976. doi: 10.1089/hum.2022.054. [DOI] [PubMed] [Google Scholar]
- 69.Calcedo R, et al. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199(3):381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Calcedo R, Wilson JM. Humoral immune response to AAV. Front Immunol. 2013;4:341. doi: 10.3389/fimmu.2013.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gardner MR, et al. High concordance of ELISA and neutralization assays allows for the detection of antibodies to individual AAV serotypes. Mol Ther Methods Clin Dev. 2022;24:199–206. doi: 10.1016/j.omtm.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kruzik A, et al. Prevalence of anti-adeno-associated virus immune responses in international cohorts of healthy donors. Mol Ther Methods Clin Dev. 2019;14:126–133. doi: 10.1016/j.omtm.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harbison CE, et al. Examining the cross-reactivity and neutralization mechanisms of a panel of mAbs against adeno-associated virus serotypes 1 and 5. J Gen Virol. 2012;93(Pt 2):347–355. doi: 10.1099/vir.0.035113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murphy SL, et al. Diverse IgG subclass responses to adeno-associated virus infection and vector administration. J Med Virol. 2009;81(1):65–74. doi: 10.1002/jmv.21360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Welles HC, et al. Vectored delivery of anti-SIV envelope targeting mAb via AAV8 protects rhesus macaques from repeated limiting dose intrarectal swarm SIVsmE660 challenge. PLoS Pathog. 2018;14(12):e1007395. doi: 10.1371/journal.ppat.1007395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tse LV, Moller-Tank S, Asokan A. Strategies to circumvent humoral immunity to adeno-associated viral vectors. Expert Opin Biol Ther. 2015;15(6):845–855. doi: 10.1517/14712598.2015.1035645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Falese L, et al. Strategy to detect pre-existing immunity to AAV gene therapy. Gene Ther. 2017;24(12):768–778. doi: 10.1038/gt.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mendell JR, et al. Testing preexisting antibodies prior to AAV gene transfer therapy: rationale, lessons and future considerations. Mol Ther Methods Clin Dev. 2022;25:74–83. doi: 10.1016/j.omtm.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gorovits B, et al. Recommendations for the development of cell-based anti-viral vector neutralizing antibody assays. Aaps j. 2020;22(2):24. doi: 10.1208/s12248-019-0403-1. [DOI] [PubMed] [Google Scholar]
- 80.Gorovits B, et al. Evaluation of the humoral response to adeno-associated virus-based gene therapy modalities using total antibody assays. AAPS J. 2021;23(6):108. doi: 10.1208/s12248-021-00628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haar J, et al. MSD-based assays facilitate a rapid and quantitative serostatus profiling for the presence of anti-AAV antibodies. Mol Ther Methods Clin Dev. 2022;25:360–369. doi: 10.1016/j.omtm.2022.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Santana MA, Esquivel-Guadarrama F. Cell biology of T cell activation and differentiation. Int Rev Cytol. 2006;250:217–274. doi: 10.1016/S0074-7696(06)50006-3. [DOI] [PubMed] [Google Scholar]
- 83.Martino AT, Markusic DM. Immune response mechanisms against AAV vectors in animal models. Mol Ther Methods Clin Dev. 2020;17:198–208. doi: 10.1016/j.omtm.2019.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Embgenbroich M, S Burgdorf. Current concepts of antigen cross-presentation. Front Immunol. 2018;9. [DOI] [PMC free article] [PubMed]
- 85.Li C, et al. Adeno-associated virus capsid antigen presentation is dependent on endosomal escape. J Clin Invest. 2013;123(3):1390–1401. doi: 10.1172/JCI66611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mingozzi F, et al. AAV-1–mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114(10):2077–2086. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nathwani AC, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371(21):1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.George LA, et al. Hemophilia B Gene Therapy with a High-Specific-Activity Factor IX Variant. N Engl J Med. 2017;377(23):2215–2227. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pien GC, et al. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J Clin Investig. 2009;119(6):1688–1695. doi: 10.1172/JCI36891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brantly ML, et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci U S A. 2009;106(38):16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Flotte TR, et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-antitrypsin: interim results. Hum Gene Ther. 2011;22(10):1239–1247. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mueller C, et al. Human Treg responses allow sustained recombinant adeno-associated virus–mediated transgene expression. J Clin Investig. 2013;123(12):5310–5318. doi: 10.1172/JCI70314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ferreira V, H Petry, F Salmon. Immune responses to AAV-vectors, the Glybera example from bench to bedside. Front Immunol. 2014;5:82. 10.3389/fimmu.2014.00082. [DOI] [PMC free article] [PubMed]
- 94.Mendell JR, et al. Dystrophin immunity in Duchenne's muscular dystrophy. N Engl J Med. 2010;363(15):1429–1437. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bowles DE, et al. Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol Ther. 2012;20(2):443–455. doi: 10.1038/mt.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mendell JR, et al. Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann Neurol. 2010;68(5):629–638. doi: 10.1002/ana.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Greig JA, et al. Non-clinical study examining AAV8.TBG.hLDLR vector-associated toxicity in chow-fed wild-type and LDLR+/− rhesus macaques. Human Gene Ther Clin Dev. 2017;28(1):39–50. doi: 10.1089/humc.2017.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun J, et al. An observational study from long-term AAV re-administration in two hemophilia dogs. Mol Ther Methods Clin Dev. 2018;10:257–267. doi: 10.1016/j.omtm.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiang Z, et al. The effect of CpG sequences on capsid-specific CD8+ T cell responses to AAV vector gene transfer. Mol Ther. 2020;28(3):771–783. doi: 10.1016/j.ymthe.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martino AT, et al. Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells. Blood. 2013;121(12):2224–2233. doi: 10.1182/blood-2012-10-460733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Palaschak B, et al. An immune-competent murine model to study elimination of AAV-transduced hepatocytes by capsid-specific CD8+ T cells. Mol Ther Methods Clin Dev. 2017;5:142–152. doi: 10.1016/j.omtm.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hui DJ, et al. Modulation of CD8+ T cell responses to AAV vectors with IgG-derived MHC class II epitopes. Mol Ther. 2013;21(9):1727–1737. doi: 10.1038/mt.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Meliani A, et al. Antigen-selective modulation of AAV immunogenicity with tolerogenic rapamycin nanoparticles enables successful vector re-administration. Nat Commun. 2018;9(1):1–13. doi: 10.1038/s41467-018-06621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arjomandnejad M, et al. Modulating immune responses to AAV by expanded polyclonal T-regs and capsid specific chimeric antigen receptor T-regulatory cells. Mol Ther Methods Clin Dev. 2021;23:490–506. doi: 10.1016/j.omtm.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vercauteren K, et al. Superior In vivo transduction of human hepatocytes using engineered AAV3 capsid. Mol Ther. 2016;24(6):1042–1049. doi: 10.1038/mt.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hui DJ, et al. AAV capsid CD8+ T-cell epitopes are highly conserved across AAV serotypes. Mol Ther-Methods Clin Dev. 2015;2:15029. doi: 10.1038/mtm.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mingozzi F, et al. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110(7):2334–2341. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li H, et al. Capsid-specific T-cell responses to natural infections with adeno-associated viruses in humans differ from those of nonhuman primates. Mol Ther. 2011;19(11):2021–2030. doi: 10.1038/mt.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chirmule N, et al. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6(9):1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 110.Veron P, et al. Humoral and cellular capsid-specific immune responses to adeno-associated virus type 1 in randomized healthy donors. J Immunol. 2012;188(12):6418–6424. doi: 10.4049/jimmunol.1200620. [DOI] [PubMed] [Google Scholar]
- 111.Vandamme C, et al. Tetramer-based enrichment of preexisting Anti-AAV8 CD8+ T cells in human donors allows the detection of a TEMRA subpopulation. Front Immunol. 2020;10:3110. doi: 10.3389/fimmu.2019.03110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nidetz NF, et al. Adeno-associated viral vector-mediated immune responses: Understanding barriers to gene delivery. Pharmacol Ther. 2020;207:107453. doi: 10.1016/j.pharmthera.2019.107453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Herzog RW. Complexity of immune responses to AAV transgene products—example of factor IX. Cell Immunol. 2019;342:103658. doi: 10.1016/j.cellimm.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jooss K, et al. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol. 1998;72(5):4212–4223. doi: 10.1128/JVI.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Calcedo R, et al. Class I-restricted T-cell responses to a polymorphic peptide in a gene therapy clinical trial for α-1-antitrypsin deficiency. Proc Natl Acad Sci U S A. 2017;114(7):1655–1659. doi: 10.1073/pnas.1617726114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tardieu M, et al. Intracerebral gene therapy in children with mucopolysaccharidosis type IIIB syndrome: an uncontrolled phase 1/2 clinical trial. Lancet Neurol. 2017;16(9):712–720. doi: 10.1016/S1474-4422(17)30169-2. [DOI] [PubMed] [Google Scholar]
- 117.Le Meur G, et al. Safety and long-term efficacy of AAV4 gene therapy in patients with RPE65 Leber congenital amaurosis. Mol Ther. 2018;26(1):256–268. doi: 10.1016/j.ymthe.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Corti M, et al. B-cell depletion is protective against anti-AAV capsid immune response: a human subject case study. Mol Ther-Methods Clin Devt. 2014;1:14033. doi: 10.1038/mtm.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Corti M, et al. Safety of Intradiaphragmatic delivery of adeno-associated virus-mediated alpha-glucosidase (rAAV1-CMV-hGAA) gene therapy in children affected by Pompe disease. Hum Gene Ther Clin Dev. 2017;28(4):208–218. doi: 10.1089/humc.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Casazza JP, et al. Safety and tolerability of AAV8 delivery of a broadly neutralizing antibody in adults living with HIV: a phase 1, dose-escalation trial. Nat Med. 2022;28(5):1022–1030. doi: 10.1038/s41591-022-01762-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Priddy FH, et al. Adeno-associated virus vectored immunoprophylaxis to prevent HIV in healthy adults: a phase 1 randomised controlled trial. Lancet HIV. 2019;6(4):e230–e239. doi: 10.1016/S2352-3018(19)30003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fuchs SP, et al. Liver-directed but not muscle-directed AAV-antibody gene transfer limits humoral immune responses in rhesus monkeys. Mol Ther Methods Clin Dev. 2020;16:94–102. doi: 10.1016/j.omtm.2019.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gardner MR, et al. Anti-drug Antibody Responses Impair Prophylaxis Mediated by AAV-Delivered HIV-1 Broadly Neutralizing Antibodies. Mol Ther. 2019;27(3):650–660. doi: 10.1016/j.ymthe.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fields PA, et al. Risk and prevention of anti-factor IX formation in AAV-mediated gene transfer in the context of a large deletion of F9. Mol Ther. 2001;4(3):201–210. doi: 10.1006/mthe.2001.0441. [DOI] [PubMed] [Google Scholar]
- 125.Herzog RW, et al. Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther. 2001;4(3):192–200. doi: 10.1006/mthe.2001.0442. [DOI] [PubMed] [Google Scholar]
- 126.Cao O, et al. Impact of the underlying mutation and the route of vector administration on immune responses to factor IX in gene therapy for hemophilia B. Mol Ther. 2009;17(10):1733–1742. doi: 10.1038/mt.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Herzog RW. A cure for hemophilia: the promise becomes a reality. Mol Ther. 2016;24(9):1503–1504. doi: 10.1038/mt.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dolgin E. Early clinical data raise the bar for hemophilia gene therapies. Nat Biotechnol. 2016;34(10):999–1001. doi: 10.1038/nbt1016-999. [DOI] [PubMed] [Google Scholar]
- 129.Smith BK, et al. Phase I/II trial of adeno-associated virus-mediated alpha-glucosidase gene therapy to the diaphragm for chronic respiratory failure in Pompe disease: initial safety and ventilatory outcomes. Hum Gene Ther. 2013;24(6):630–640. doi: 10.1089/hum.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Manno CS, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101(8):2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 131.2022 ASGCT Annual Meeting Abstracts. Molecular Therapy. 2022. 30(4 Suppl 1):1-592. [DOI] [PMC free article] [PubMed]
- 132.Bennett J, et al. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med. 2012;4(120):120ra15. doi: 10.1126/scitranslmed.3002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bennett J, et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet. 2016;388(10045):661–672. doi: 10.1016/S0140-6736(16)30371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xin KQ, et al. Induction of robust immune responses against human immunodeficiency virus is supported by the inherent tropism of adeno-associated virus type 5 for dendritic cells. J Virol. 2006;80(24):11899–11910. doi: 10.1128/JVI.00890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lin SW, et al. Recombinant adeno-associated virus vectors induce functionally impaired transgene product-specific CD8+ T cells in mice. J Clin Invest. 2007;117(12):3958–3970. doi: 10.1172/JCI33138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lin J, et al. Vaccines based on novel adeno-associated virus vectors elicit aberrant CD8+ T-cell responses in mice. J Virol. 2007;81(21):11840–11849. doi: 10.1128/JVI.01253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Martin K, et al. Differences between clinical trials and postmarketing use. Br J Clin Pharmacol. 2004;57(1):86–92. doi: 10.1046/j.1365-2125.2003.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vignal-Clermo ḤC, et al. Safety of lenadogene nolparvovec gene therapy over 5 years in 189 patients with Leber hereditary optic neuropathy. Am J Ophthalmol. 2022 doi: 10.1016/j.ajo.2022.11.026. [DOI] [PubMed] [Google Scholar]
- 139.Bucher K, et al. Immune responses to retinal gene therapy using adeno-associated viral vectors – Implications for treatment success and safety. Prog Retin Eye Res. 2021;83:100915. doi: 10.1016/j.preteyeres.2020.100915. [DOI] [PubMed] [Google Scholar]
- 140.Center for Biologics Evaluation and Research. (n.d.). Zolgensma. U.S. Food and Drug Administration. Retrieved February 23, 2023, from https://www.fda.gov/vaccines-blood-biologics/zolgensma
- 141.Day JW, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20(4):284–293. doi: 10.1016/S1474-4422(21)00001-6. [DOI] [PubMed] [Google Scholar]
- 142.Mendell JR, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 143.Day JW, et al. Clinical trial and postmarketing safety of onasemnogene abeparvovec therapy. Drug Saf. 2021;44(10):1109–1119. doi: 10.1007/s40264-021-01107-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chand D, et al. Hepatotoxicity following administration of onasemnogene abeparvovec (AVXS-101) for the treatment of spinal muscular atrophy. J Hepatol. 2021;74(3):560–566. doi: 10.1016/j.jhep.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 145.Zolgensma 2 x 10exp13 vector genomes/ML solution for infusion - summary of product characteristics (SmPC) - (EMC). (n.d.). Retrieved February 23, 2023, from https://www.medicines.org.uk/emc/product/11572#gref
- 146.Novartis Confirms Deaths of Two Patients Treated with Gene Therapy Zolgensma. Human Gene Therapy. 2022;33(17-18):842-844. [DOI] [PubMed]
- 147.Ema. (2022, August 10). Direct Healthcare Professional Communications. European Medicines Agency. Retrieved February 23, 2023, from https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/direct-healthcare-professional-communications
- 148.Kaplan BS, et al. Current treatment of atypical hemolytic uremic syndrome. Intractable Rare Dis Res. 2014;3(2):34–45. doi: 10.5582/irdr.2014.01001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Svetkey LP, et al. Double-blind, placebo-controlled trial of twice-daily nifedipine as a step-2 agent in mild essential hypertension. J Clin Hypertens. 1987;3(4):579–588. [PubMed] [Google Scholar]
- 150.Cruz PE, Mueller C, Flotte TR. The promise of gene therapy for the treatment of alpha-1 antitrypsin deficiency. Pharmacogenomics. 2007;8(9):1191–1198. doi: 10.2217/14622416.8.9.1191. [DOI] [PubMed] [Google Scholar]
- 151.Galletta F, et al. Hemophagocytic lymphohistiocytosis following gene replacement therapy in a child with type 1 spinal muscular atrophy. J Clin Pharm Ther. 2022;47(9):1478–1481. doi: 10.1111/jcpt.13733. [DOI] [PubMed] [Google Scholar]
- 152.Li N, Bertolini T, Herzog RW. AAV vector dose determines TLR9 dependence of CD8+ T Cell response to transgene product. Blood. 2020;136:3. doi: 10.1182/blood-2020-141054. [DOI] [Google Scholar]
- 153.Bertolini TB, et al. Effect of CpG depletion of vector genome on CD8(+) T cell responses in AAV gene therapy. Front Immunol. 2021;12:672449. doi: 10.3389/fimmu.2021.672449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pan X, et al. Rational engineering of a functional CpG-free ITR for AAV gene therapy. Gene Ther. 2022;29(6):333–345. doi: 10.1038/s41434-021-00296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chan YK et al. Engineering adeno-associated viral vectors to evade innate immune and inflammatory responses. Sci Transl Med. 2021;13(580). 10.1126/scitranslmed.abd3438. [DOI] [PMC free article] [PubMed]
- 156.Kaminski JJ, et al. Synthetic oligodeoxynucleotides containing suppressive TTAGGG motifs inhibit AIM2 inflammasome activation. J Immunol. 2013;191(7):3876–3883. doi: 10.4049/jimmunol.1300530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Steinhagen F, et al. Suppressive oligodeoxynucleotides containing TTAGGG motifs inhibit cGAS activation in human monocytes. Eur J Immunol. 2018;48(4):605–611. doi: 10.1002/eji.201747338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kuznik A, et al. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol. 2011;186(8):4794–4804. doi: 10.4049/jimmunol.1000702. [DOI] [PubMed] [Google Scholar]
- 159.Haberman RP, McCown TJ, Samulski RJ. Novel transcriptional regulatory signals in the adeno-associated virus terminal repeat A/D junction element. J Virol. 2000;74(18):8732–8739. doi: 10.1128/JVI.74.18.8732-8739.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sullivan L. Apellis pharmaceuticals will commence APL-9 program to control the complement system in host responses to AAV vector administration for gene Therapies. Appellis Pharmaceuticals; 2019. [Google Scholar]
- 161.Smith CJ, et al. Pre-existing humoral immunity and complement pathway contribute to immunogenicity of adeno-associated virus (AAV) vector in human blood. Front Immunol. 2022;13. 10.3389/fimmu.2022.999021. [DOI] [PMC free article] [PubMed]
- 162.Trouw LA, Pickering MC, Blom AM. The complement system as a potential therapeutic target in rheumatic disease. Nat Rev Rheumatol. 2017;13(9):538–547. doi: 10.1038/nrrheum.2017.125. [DOI] [PubMed] [Google Scholar]
- 163.Legendre CM, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368(23):2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 164.Li, X., et al. A versatile toolkit for overcoming AAV immunity. Front Immunol. 2022;13. [DOI] [PMC free article] [PubMed]
- 165.Finn JD, et al. Proteasome inhibitors decrease AAV2 capsid derived peptide epitope presentation on MHC class I following transduction. Mol Ther. 2010;18(1):135–142. doi: 10.1038/mt.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Monahan PE, et al. Proteasome inhibitors enhance gene delivery by AAV virus vectors expressing large genomes in hemophilia mouse and dog models: a strategy for broad clinical application. Mol Ther. 2010;18(11):1907–1916. doi: 10.1038/mt.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Muhuri M, et al. Novel combinatorial MicroRNA-binding sites in AAV VECTORS SYNERGISTICALLY DIMINISH ANTIGEN PRESENTATION AND TRANSGENE IMMUNITY FOR EFFICIENT AND STABLE TRANSDUCTION. Front Immunol. 2021;12:674242. doi: 10.3389/fimmu.2021.674242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xiao Y, et al. Circumventing cellular immunity by miR142-mediated regulation sufficiently supports rAAV-delivered OVA expression without activating humoral immunity. JCI Insight. 2019;5.(13):e99052. 10.1172/jci.insight.99052. [DOI] [PMC free article] [PubMed]
- 169.Mingozzi F, et al. Prevalence and pharmacological modulation of humoral immunity to AAV vectors in gene transfer to synovial tissue. Gene Ther. 2013;20(4):417–424. doi: 10.1038/gt.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Byrne BJ, et al. Pompe disease gene therapy: neural manifestations require consideration of CNS directed therapy. Ann Transl Med. 2019;7(13):290. doi: 10.21037/atm.2019.05.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Furie R, et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med. 2020;383(12):1117–1128. doi: 10.1056/NEJMoa2001180. [DOI] [PubMed] [Google Scholar]
- 172.Rana J, et al. CAR- and TRuC-redirected regulatory T cells differ in capacity to control adaptive immunity to FVIII. Mol Ther. 2021;29(9):2660–2676. doi: 10.1016/j.ymthe.2021.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xiang Z, et al. The EFFECT OF RAPAMYCIN AND IBRUTINIB ON ANTIBODY RESPONSES TO ADENO-ASSOCIATED VIRUS VECTOR-MEDIATED GENE TRANSFER. Hum Gene Ther. 2022;33(11–12):614–624. doi: 10.1089/hum.2021.258. [DOI] [PubMed] [Google Scholar]
- 174.Ilyinskii PO, et al. ImmTOR nanoparticles enhance AAV transgene expression after initial and repeat dosing in a mouse model of methylmalonic acidemia. Mol Ther Methods Clin Dev. 2021;22:279–292. doi: 10.1016/j.omtm.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schroeder, H., et al. Functional assessment of T cell responses to AAV8 empty capsids in healthy volunteers. Molecular Therapy. 2022;30(4):19-20.
- 176.Leborgne C, et al. IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat Med. 2020;26(7):1096–1101. doi: 10.1038/s41591-020-0911-7. [DOI] [PubMed] [Google Scholar]
- 177.Elmore ZC, et al. Rescuing AAV gene transfer from neutralizing antibodies with an IgG-degrading enzyme. JCI insight. 2020;5(19). e139881. 10.1172/jci.insight.139881. [DOI] [PMC free article] [PubMed]
- 178.Jordan SC, et al. IgG endopeptidase in highly sensitized patients undergoing transplantation. N Engl J Med. 2017;377(5):442–453. doi: 10.1056/NEJMoa1612567. [DOI] [PubMed] [Google Scholar]
- 179.Winstedt L, et al. Complete removal of extracellular IgG antibodies in a randomized dose-escalation phase I study with the bacterial enzyme IdeS–a novel therapeutic opportunity. PLoS ONE. 2015;10(7):e0132011. doi: 10.1371/journal.pone.0132011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Monteilhet V, et al. A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol Ther. 2011;19(11):2084–2091. doi: 10.1038/mt.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bertin B, et al. Capsid-specific removal of circulating antibodies to adeno-associated virus vectors. Sci Rep. 2020;10(1):1–11. doi: 10.1038/s41598-020-57893-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Orlowski A, et al. Successful transduction with AAV vectors after selective depletion of anti-AAV antibodies by immunoadsorption. Mol Ther Methods Clin Dev. 2020;16:192–203. doi: 10.1016/j.omtm.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Raper SE, Wilson JM, Nunes FA. Flushing out antibodies to make AAV gene therapy available to more patients. Mol Ther. 2013;21(2):269–271. doi: 10.1038/mt.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mimuro J, et al. Minimizing the inhibitory effect of neutralizing antibody for efficient gene expression in the liver with adeno-associated virus 8 vectors. Mol Ther. 2013;21(2):318–323. doi: 10.1038/mt.2012.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Haurigot V, et al. Whole body correction of mucopolysaccharidosis IIIA by intracerebrospinal fluid gene therapy. J Clin Invest. 2013;123(8):3254–3271. doi: 10.1172/JCI66778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jackson KL, Dayton RD, Klein RL. AAV9 supports wide-scale transduction of the CNS and TDP-43 disease modeling in adult rats. Mol Ther-Methods Clin Dev. 2015;2:15036. doi: 10.1038/mtm.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mingozzi F, et al. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci Transl Med. 2013;5(194):194ra92. doi: 10.1126/scitranslmed.3005795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Monahan PE, et al. Employing a gain-of-function factor IX variant R338L to advance the efficacy and safety of hemophilia B human gene therapy: preclinical evaluation supporting an ongoing adeno-associated virus clinical trial. Hum Gene Ther. 2014;26(2):69–81. doi: 10.1089/hum.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gao K, et al. Empty virions in AAV8 vector preparations reduce transduction efficiency and may cause total viral particle dose-limiting side effects. Molecular Therapy - Methods & Clinical Development. 2014;1:9. doi: 10.1038/mtm.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dalkara D, et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med. 2013;5(189):189ra76. doi: 10.1126/scitranslmed.3005708. [DOI] [PubMed] [Google Scholar]
- 191.Tse LV, et al. Structure-guided evolution of antigenically distinct adeno-associated virus variants for immune evasion. Proc Natl Acad Sci U S A. 2017;114(24):E4812–e4821. doi: 10.1073/pnas.1704766114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wobus CE, et al. Monoclonal antibodies against the adeno-associated virus type 2 (AAV-2) capsid: epitope mapping and identification of capsid domains involved in AAV-2-cell interaction and neutralization of AAV-2 infection. J Virol. 2000;74(19):9281–9293. doi: 10.1128/JVI.74.19.9281-9293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cantore A, et al. Hyperfunctional coagulation factor IX improves the efficacy of gene therapy in hemophilic mice. Blood. 2012;120(23):4517–4520. doi: 10.1182/blood-2012-05-432591. [DOI] [PubMed] [Google Scholar]
- 194.Maheshri N, et al. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat Biotechnol. 2006;24(2):198–204. doi: 10.1038/nbt1182. [DOI] [PubMed] [Google Scholar]
- 195.Grimm D, et al. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J Virol. 2008;82(12):5887–5911. doi: 10.1128/JVI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Paulk NK, et al. Bioengineered AAV capsids with combined high human liver transduction in vivo and unique humoral seroreactivity. Mol Ther. 2018;26(1):289–303. doi: 10.1016/j.ymthe.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hamilton BA, JF Wright. Challenges posed by immune responses to AAV vectors: addressing root causes. Front Immunol. 2021;12. 10.3389/fimmu.2021.675897. [DOI] [PMC free article] [PubMed]
- 198.Lee GK, et al. PEG conjugation moderately protects adeno-associated viral vectors against antibody neutralization. Biotechnol Bioeng. 2005;92(1):24–34. doi: 10.1002/bit.20562. [DOI] [PubMed] [Google Scholar]
- 199.Thi TH, Pilkington E, Nguyen D. The importance of Poly (ethylene glycol) alternatives for overcoming PEG immunogenicity in drug delivery and bioconjugation. Polymers (Basel). 2020;12(2):298. doi: 10.3390/polym12020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Meliani A, et al. Enhanced liver gene transfer and evasion of preexisting humoral immunity with exosome-enveloped AAV vectors. Blood Adv. 2017;1(23):2019–2031. doi: 10.1182/bloodadvances.2017010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hudry E, et al. Exosome-associated AAV vector as a robust and convenient neuroscience tool. Gene Ther. 2016;23(4):380–392. doi: 10.1038/gt.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.György B, et al. Naturally enveloped AAV vectors for shielding neutralizing antibodies and robust gene delivery in vivo. Biomaterials. 2014;35(26):7598–7609. doi: 10.1016/j.biomaterials.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhong C, et al. Repeated systemic dosing of adeno-associated virus vectors in immunocompetent mice after blockade of T cell costimulatory pathways. Hum Gene Ther. 2021;33(5–6):290–300. doi: 10.1089/hum.2021.129. [DOI] [PubMed] [Google Scholar]
- 204.Lorain S, et al. Transient immunomodulation allows repeated injections of AAV1 and correction of muscular dystrophy in multiple muscles. Mol Ther. 2008;16(3):541–547. doi: 10.1038/sj.mt.6300377. [DOI] [PubMed] [Google Scholar]
- 205.Frentsch M, et al. Blockade of the costimulatory CD28-B7 family signal axis enables repeated application of AAV8 gene vectors. J Thromb Haemost. 2020;18(5):1075–1080. doi: 10.1111/jth.14757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Arjomandnejad M, Keeler AM. Evaluating readministration of adeno-associated virus for gene therapy. Hum Gene Ther. 2022;33(5–6):218–220. doi: 10.1089/hum.2022.29204.mar. [DOI] [PubMed] [Google Scholar]
- 207.Shirley JL, et al. Immune responses to viral gene therapy vectors. Mol Ther. 2020;28(3):709–722. doi: 10.1016/j.ymthe.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bereshchenko O, et al. GILZ promotes production of peripherally induced Treg cells and mediates the crosstalk between glucocorticoids and TGF-β signaling. Cell Rep. 2014;7(2):464–475. doi: 10.1016/j.celrep.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 209.Van Laethem F, et al. Glucocorticoids attenuate T cell receptor signaling. J Exp Med. 2001;193(7):803–814. doi: 10.1084/jem.193.7.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Monahan P, et al. Update on a phase 1/2 open-label trial of BAX335, an adeno-associated virus 8 (AAV8) vector-based gene therapy program for hemophilia B. J Thromb Haemost. 2015;13:87. [Google Scholar]
- 212.Calcedo R, et al. Immune responses in 101HEMB01, a Phase 1/2 open-Label, single ascending dose-finding trial of DTX101 (AAVrh10FIX) in patients with severe hemophilia B. Blood. 2017;130:3333. [Google Scholar]
- 213.High KA, et al. A phase 1/2 trial of investigational Spk-8011 in hemophilia a demonstrates durable expression and prevention of bleeds. Washington, DC: American Society of Hematology; 2018. [Google Scholar]
- 214.Ferreira V, et al. Immune responses to intramuscular administration of alipogene tiparvovec (AAV1-LPLS447X) in a phase II clinical trial of lipoprotein lipase deficiency gene therapy. Hum Gene Ther. 2014;25(3):180–188. doi: 10.1089/hum.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Samelson-Jones BJ, et al. Timing of intensive immunosuppression impacts risk of transgene antibodies after AAV gene therapy in nonhuman primates. Mol Ther-Methods Clin Dev. 2020;17:1129–1138. doi: 10.1016/j.omtm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fanigliulo D, et al. Clinically-relevant cyclosporin and rapamycin concentrations enhance regulatory T cell function to a similar extent but with different mechanisms: an in-vitro study in healthy humans. Int Immunopharmacol. 2015;24(2):276–284. doi: 10.1016/j.intimp.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 217.Ishii A, et al. rAAV8 and rAAV9-mediated long-term muscle transduction with tacrolimus (FK506) in non-human primates. Mol Ther Methods Clin Dev. 2020;18:44–49. doi: 10.1016/j.omtm.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47(2–3):85–118. doi: 10.1016/S0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 219.Gaudet D, et al. Long-term retrospective analysis of gene therapy with alipogene tiparvovec and its effect on lipoprotein lipase deficiency-induced pancreatitis. Hum Gene Ther. 2016;27(11):916–925. doi: 10.1089/hum.2015.158. [DOI] [PubMed] [Google Scholar]
- 220.Gaudet D, et al. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther. 2013;20(4):361–369. doi: 10.1038/gt.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Miroux C, et al. In vitro effects of cyclosporine A and tacrolimus on regulatory T-cell proliferation and function. Transplantation. 2012;94(2):123–131. doi: 10.1097/TP.0b013e3182590d8f. [DOI] [PubMed] [Google Scholar]
- 222.Akimova T, et al. Differing effects of rapamycin or calcineurin inhibitor on T-regulatory cells in pediatric liver and kidney transplant recipients. Am J Transplant. 2012;12(12):3449–3461. doi: 10.1111/j.1600-6143.2012.04269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mueller C, et al. SOD1 Suppression with adeno-associated virus and MicroRNA in familial ALS. N Engl J Med. 2020;383(2):151–158. doi: 10.1056/NEJMoa2005056. [DOI] [PubMed] [Google Scholar]
- 224.Duan D, et al. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Investig. 2000;105(11):1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Li C, et al. Adeno-associated virus capsid antigen presentation is dependent on endosomal escape. J Clin Investig. 2013;123(3):1390–1401. doi: 10.1172/JCI66611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Narum S, Westergren T, Klemp M. Corticosteroids and risk of gastrointestinal bleeding: a systematic review and meta-analysis. BMJ Open. 2014;4(5):e004587. doi: 10.1136/bmjopen-2013-004587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tomizawa M, et al. Immunosuppressive agents are associated with peptic ulcer bleeding. Exp Ther Med. 2017;13(5):1927–1931. doi: 10.3892/etm.2017.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Campagne O, et al. The impact of tacrolimus exposure on extrarenal adverse effects in adult renal transplant recipients. Br J Clin Pharmacol. 2019;85(3):516–529. doi: 10.1111/bcp.13811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhong L, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci. 2008;105(22):7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Finn JD, et al. The efficacy and the risk of immunogenicity of FIX Padua (R338L) in hemophilia B dogs treated by AAV muscle gene therapy. Blood. 2012;120(23):4521–4523. doi: 10.1182/blood-2012-06-440123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wang Z, et al. Successful regional delivery and long-term expression of a dystrophin gene in canine muscular dystrophy: a preclinical model for human therapies. Mol Ther. 2012;20(8):1501–1507. doi: 10.1038/mt.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cordier L, et al. Muscle-specific promoters may be necessary for adeno-associated virus-mediated gene transfer in the treatment of muscular dystrophies. Hum Gene Ther. 2001;12(2):205–215. doi: 10.1089/104303401750061267. [DOI] [PubMed] [Google Scholar]
- 233.Koo T, et al. Long-term functional adeno-associated virus-microdystrophin expression in the dystrophic CXMDj dog. J Gene Med. 2011;13(9):497–506. doi: 10.1002/jgm.1602. [DOI] [PubMed] [Google Scholar]
- 234.Sarkar D, et al. Ex vivo expanded autologous polyclonal regulatory T cells suppress inhibitor formation in hemophilia. Mol Ther Methods Clin Dev. 2014;1:14030. doi: 10.1038/mtm.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yoon J, et al. FVIII-specific human chimeric antigen receptor T-regulatory cells suppress T- and B-cell responses to FVIII. Blood. 2017;129(2):238–245. doi: 10.1182/blood-2016-07-727834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Herzog RW, et al. Reprogrammed CD4+ T cells that express FoxP3+ control inhibitory antibody formation in hemophilia A mice. Front Immunol. 2019;10:274. doi: 10.3389/fimmu.2019.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Fu RY, et al. CD4(+) T cells engineered with FVIII-CAR and murine Foxp3 suppress anti-factor VIII immune responses in hemophilia a mice. Cell Immunol. 2020;358:104216. doi: 10.1016/j.cellimm.2020.104216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Parvathaneni K, Scott DW. Engineered FVIII-expressing cytotoxic T cells target and kill FVIII-specific B cells in vitro and in vivo. Blood Adv. 2018;2(18):2332–2340. doi: 10.1182/bloodadvances.2018018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhang A-H, et al. Targeting Antigen-Specific B Cells Using Antigen-Expressing Transduced Regulatory T Cells. J Immunol. 2018;201(5):1434–1441. doi: 10.4049/jimmunol.1701800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Pohl AP, et al. Suppression of FVIII-specific memory B cells by chimeric BAR receptor-engineered natural regulatory T cells. Front Immunol. 2020;11:693. doi: 10.3389/fimmu.2020.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]