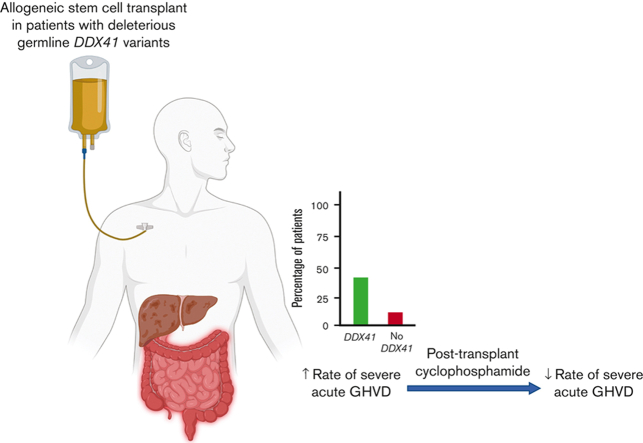
Key Points
-
•
Patients with deleterious germ line DDX41 variants experience higher rates of severe acute GVHD after allogeneic stem cell transplant.
-
•
Posttransplant cyclophosphamide reduces GVHD rates in patients with deleterious germ line DDX41 variants.
Visual Abstract
Abstract
There is increasing recognition that pathogenic germ line variants drive the development of hematopoietic cancers in many individuals. Currently, patients with hereditary hematologic malignancies (HHMs) receive similar standard therapies and hematopoietic stem cell transplant (HSCT) approaches as those with sporadic disease. We hypothesize that patients with myeloid malignancies and deleterious germ line predisposition variants have different posttransplant outcomes than those without such alleles. We studied 472 patients with myeloid neoplasms, of whom 26% had deleterious germ line variants and 34% underwent HSCT. Deleterious germ line variants in CHEK2 and DDX41 were most commonly seen in American and Australian cohorts, respectively. Patients with deleterious germ line DDX41 variants had a higher incidence of severe (stage 3-4) acute graft-versus-host disease (GVHD) (38%) than recipients with deleterious CHEK2 variants (0%), other HHM variants (12%), or patients without such germ line variants (9%) (P = .002). Importantly, the use of posttransplant cyclophosphamide reduced the risk of severe acute GVHD in patients receiving HSCT for deleterious germ line DDX41-associated myeloid neoplasms (0% vs 53%, P = .03). Based on these results, we advocate the use of posttransplant cyclophosphamide when individuals with deleterious germ line DDX41 variants undergo allogeneic HSCT for myeloid malignancies, even when transplantation has been performed using wild-type donors.
Introduction
Recognition of hereditary hematopoietic malignancies (HHMs) has accelerated recently,1 with deleterious variants in >20 genes identified as myeloid and lymphoid malignancy drivers.2, 3, 4 In those with myelodysplastic syndrome, pathogenic/likely pathogenic (P/LP) germ line variants are found in 25% of pediatric patients5 and in 19% of those aged between 18 and 40 years.6 Moreover, 20% of individuals with ≥2 cancers, 1 being an HM, have a P/LP germ line variant.7 Causative genes vary across the age spectrum, with older adults commonly having such alleles in DDX41, challenging the prevailing ideology that individuals with germ line predisposition always present young.8,9
Individuals with HHMs receive similar treatments as those with de novo disease, often because the hereditary nature of the malignancy is not recognized until after treatment initiation. Hematopoietic stem cell transplantation (HSCT) is often used as a curative approach for myeloid malignancies, and in those with germ line P/LP predisposition variants, related donors who lack the familial variant are preferred. However, there are limited data on the outcomes of patients with HHMs after HSCT. We used comprehensive clinical and genomic data to investigate the allogeneic transplant–related outcomes in a large international myeloid HHM cohort.
Methods
We performed a retrospective analysis of all patients with myeloid malignancies who underwent germ line predisposition testing at The University of Chicago (U of C) or the Central Adelaide Health Network between 2010 and 2021 or who were known to have deleterious germ line DDX41 variants from the University of Pennsylvania and Moffitt Cancer Center. Detailed information on methods regarding the identification of germ line predisposition alleles and statistical analysis is provided in supplemental Methods.7,10,11
Results
Among 472 patients with myeloid malignancies who underwent germ line testing, 28% (80 of 289) in the U of C cohort and 20% (35 of 175) in the Adelaide cohort had a deleterious germ line variant (supplemental Table 1), with P/LP CHEK2 variants most common in the U of C cohort and P/LP DDX41 variants in the Adelaide cohort (supplemental Figures 2 and 3). Patients with P/LP germ line DDX41 variants presented at an older age than patients with P/LP germ line CHEK2 variants, other P/LP germ line variants, or those without such variants (median, 67 vs 62, 61, and 66 years, respectively; P = .002), as expected (supplemental Table 2).9,12 Of the patients with P/LP germ line DDX41 variants, 53% had a first-degree relative with an HM, which was higher than those of other groups (41%, 16%, 19%, respectively; P = .001) (supplemental Table 2).
A total of 161 patients (34% of the entire cohort) underwent allogeneic HSCT: 21 of 35 patients (60%) with P/LP germ line DDX41 variants, 7 of 22 patients (32%) with germ line P/LP CHEK2 variants, 26 of 63 patients (41%) with other P/LP germ line variants, and 107 of 351 patients (31%) without a P/LP germ line variant. The distribution of diagnoses, donor types, disease status at the time of HSCT (remission vs active disease), conditioning regimens, and conditioning intensity are summarized in Table 1. All donor cells were mobilized from peripheral blood. Only 1 donor shared a deleterious familial variant (in DDX41) with the HSCT recipient. We did not observe delayed engraftment in HHM vs non-HHM groups (Table 1).
Table 1.
Characteristics of patients who underwent allogeneic hematopoietic cell transplant
| Germ line DDX41 (n = 21) | Germ line CHEK2 (n = 7) | Other HHM (n = 26) | No HHM (n = 107) | |
|---|---|---|---|---|
| Age at HSCT, y, median (range) | 65 (51-73) | 57 (44-69) | 48 (19-71) | 57 (18-74) |
| Female, n (%) | 6 (28.5) | 5 (71.4) | 8 (30.8) | 47 (43.9) |
| Year of HSCT | ||||
| 2017-2021 | 16 (76.2) | 5 (71.4) | 13 (50) | 83 (77.6) |
| 2012-2016 | 3 (14.3) | 2 (28.6) | 6 (23.1) | 20 (18.7) |
| Before 2011 | 2 (9.5) | 0 | 7 (26.9) | 4 (3.7) |
| DRI group, n (%) | ||||
| Low | 0 | 0 | 2 (7.8) | 23 (21.5) |
| Intermediate | 10 (47.6) | 2 (28.5) | 5 (19.2) | 24 (22.4) |
| High | 9 (42.9) | 3 (42.9) | 13 (50) | 41 (38.3) |
| Very high | 0 | 1 (14.3) | 1 (3.8) | 4 (3.7) |
| Unknown | 2 (9.5) | 1 (14.3) | 5 (19.2) | 15 (14.1) |
| Diagnosis, n (%) | ||||
| AML | 14 (66.7) | 5 (71.4) | 13 (50) | 64 (59.8) |
| MDS | 6 (28.5) | 1 (14.3) | 12 (46.2) | 34 (31.8) |
| MDS/MPN | 0 | 0 | 1 (3.8) | 9 (8.4) |
| MPN | 1 (4.8) | 1 (14.3) | 0 | 0 |
| Donor type, n (%) | ||||
| MUD | 13 (62) | 7 (100) | 12 (46.2) | 60 (56.1) |
| MRD | 4 (19) | 0 | 9 (34.6) | 23 (21.5) |
| Haplo/cord | 2 (9.5) | 0 | 2 (7.7) | 12 (11.2) |
| Haploidentical | 2 (9.5) | 0 | 0 | 1 (0.9) |
| Cord | 0 | 0 | 2 (7.7) | 4 (3.7) |
| Unknown | 0 | 0 | 1 (3.8) | 7 (6.6) |
| Related donor carrying germ line mutation, n (%) | 1 (4.8) | 0 | 1 (3.8) | 0 |
| Graft type, n (%) | ||||
| Peripheral blood | 21 (100) | 7 (100) | 26 (100) | 107 (100) |
| Disease status, n (%) | ||||
| CR1 | 12 (57.1) | 3 (42.9) | 11 (42.4) | 55 (51.4) |
| CR2 | 4 (19) | 4 (57.1) | 4 (15.3) | 16 (14.9) |
| Active disease | 3 (14.4) | 0 | 8 (30.8) | 28 (26.2) |
| Unknown | 2 (9.5) | 0 | 3 (11.5) | 8 (7.5) |
| Conditioning regimen, n (%) | ||||
| Flu/Mel | 6 (28.5) | 4 (57.1) | 11 (42.4) | 47 (43.9) |
| Flu/Bu | 8 (38.2) | 2 (28.6) | 6 (23.1) | 32 (29.9) |
| Bu/Cy | 0 | 1 (14.3) | 1 (3.8) | 9 (8.4) |
| Flu/Cy/TBI | 4 (19) | 0 | 2 (7.7) | 4 (3.7) |
| Flu/Mel/TBI | 2 (9.5) | 0 | 0 | 7 (6.6) |
| Cy/TBI | 0 | 0 | 2 (7.7) | 0 |
| Flu/Cy | 0 | 0 | 0 | 1 (0.9) |
| Clo/Mel | 0 | 0 | 1 (3.8) | 0 |
| Unknown | 1 (4.8) | 0 | 3 (11.5) | 7 (6.6) |
| Conditioning intensity | ||||
| Myeloablative | 6 (28.5) | 3 (42.9) | 11 (42.4) | 46 (43) |
| Reduced intensity | 14 (66.7) | 4 (57.1) | 12 (46.1) | 54 (50.5) |
| Unknown | 1 (4.8) | 0 | 3 (11.5) | 7 (6.5) |
| GVHD prophylaxis, n (%) | ||||
| CNI + methotrexate | 7 (33.3) | 3 (42.9) | 4 (15.3) | 26 (24.3) |
| CNI + alemtuzumab | 5 (23.8) | 3 (42.9) | 9 (34.7) | 23 (21.5) |
| CNI + methotrexate + ATG | 0 | 1 (14.2) | 6 (23.1) | 22 (20.6) |
| CNI + ATG | 2 (9.5) | 0 | 2 (7.7) | 17 (15.9) |
| CNI + post-HSCT Cy | 5 (23.8) | 0 | 0 | 6 (5.6) |
| CNI + MMF | 0 | 0 | 2 (7.7) | 4 (3.7) |
| CNI + Tregs | 1 (4.8) | 0 | 0 | 0 |
| Unknown | 1 (4.8) | 0 | 3 (11.5) | 9 (8.4) |
| Day of engraftment, median (range) | 15.5 (10-23) | 11 (10-20) | 14 (8-16) | 12 (9-19) |
AML, acute myeloid leukemia; ATG, antithymocyte globulin; Bu, busulfan; Clo, clofarabine; CNI, calcineurin inhibitor; CR, complete remission; Cy, cyclophosphamide; DRI, disease related index; Flu, fludarabine; GI, gastrointestinal; GVHD, graft-versus-host disease; haplo/cord, haplo-identical/umbilical cord hematopoietic cell transplant; MDS, myelodysplastic syndrome; Mel, melphalan; MMF, mycophenolate mofetil; MPN, myeloproliferative neoplasm; MRD, matched related donor; MUD, matched unrelated donor; TBI, total body irradiation; Tregs, T-regulatory cells.
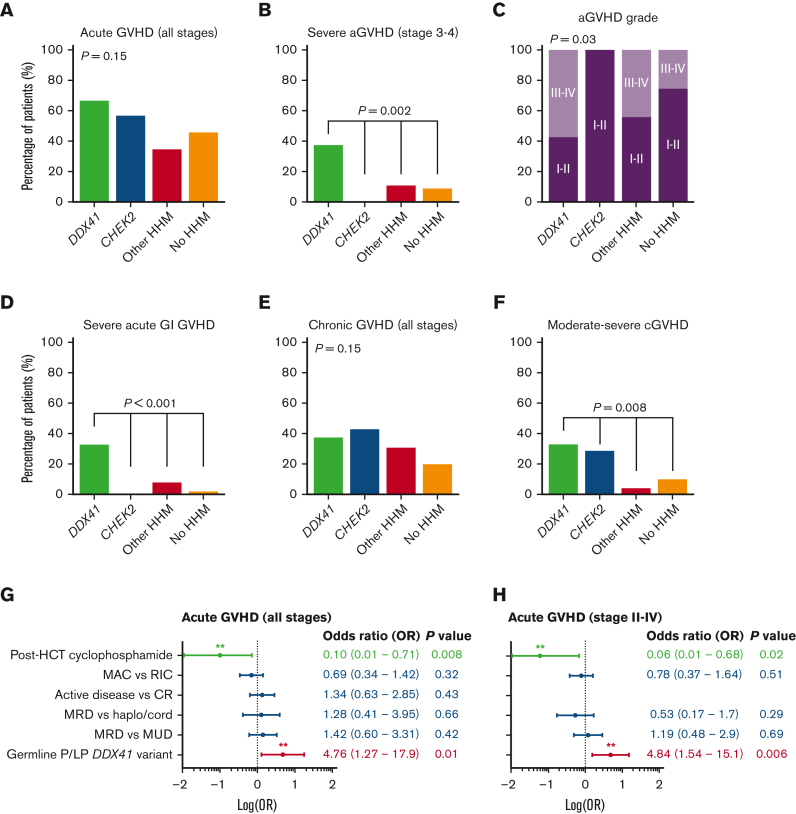
When we compared the rates of acute GVHD (aGVHD) globally among the 4 groups, patients with P/LP germ line DDX41 variants had higher rates of severe (stage 3-4) aGVHD than patients with P/LP CHEK2 variants, those with other germ line variants, or those without such variants (38% vs 0%, 12%, and 9%, respectively; P = .002, Figure 1A-C). Notably, severe gastrointestinal GVHD was seen in one-third of patients with P/LP germ line DDX41 variants vs <10% in other groups (P < .001, Figure 1D). Similarly, 33% of patients with P/LP germ line DDX41 variants experienced moderate to severe chronic GVHD (cGVHD), in contrast to 29% of patients with P/LP germ line CHEK2 variants, 4% of patients with other P/LP germ line variants, and 10% of patients without such variants (P = .008; Figure 1E-F, supplemental Figure 4).
Figure 1.
Rates of aGVHD and cGVHD among patients with or without HHMs. (A-F) The rates of GVHD are given for (A) aGVHD of all stages, (B) severe aGVHD, (C) aGVHD grades, (D) severe acute gastrointestinal (GI) GVHD, (E) cGVHD of all stages, and (F) moderate to severe cGVHD. Severe acute GVHD is defined as GVHD stage 3 to 4.13 Chronic GVHD is staged based on 2014 NIH criteria.14 (G-H) Multivariable logistic regression analysis of predictors for (G) aGVHD of all stages, and (H) for stages II-IV. CR, complete remission; haplo/cord: haplo-identical/umbilical cord HCT; HCT, hematopoietic cell transplant; LP, likely pathogenic; MAC, myeloablative conditioning; MRD, matched related donor; MUD, matched unrelated donor; OR, odds ratio; RIC, reduced-intensity conditioning.
GVHD prophylaxis varied according to standard institutional practices (Table 1). Among patients with P/LP germ line DDX41 variants, none of the 5 patients who received post-HSCT cyclophosphamide had severe aGVHD, whereas 53% (8 of 15) of those who received other GVHD prophylaxis regimens had severe aGVHD (P = .03). To adjust for the effects of known GVHD risk factors, we performed multivariable logistic regression analysis (Figure 1G-H). Germ line DDX41 status remained an independent predictor of aGVHD of all stages (OR: 4.76, range: 1.27-17.9; P = .01) and stage II-IV aGVHD (OR: 4.84, range: 1.54-15.1; P = .006). Importantly, post-HSCT cyclophosphamide use was associated with less aGHVD when adjusted for other variables. Among 15 patients with P/LP germ line DDX41 variants who underwent HSCT but did not receive post-HSCT cyclophosphamide, 7 deaths occurred, 4 of which were due to severe GVHD, and 1 was due to sepsis (transplant-related mortality of 33%). All 5 patients with P/LP germ line DDX41 variants who received post-HSCT cyclophosphamide were alive and free of GVHD (transplant-related mortality of 0%). One patient relapsed after transplant. The median follow-up time for patients with and without P/LP germ line DDX41 variants was 30 and 18 months, respectively.
Discussion
Inherited myeloid malignancies have historically been considered uncommon. However, recognition of HHMs has increased with new classification schemes and more testing.15,16 In this international cohort, we observed a lower frequency of P/LP germ line CHEK2 variants in the Adelaide cohort, likely underscoring the influence of genetic isolation within that group, and the documented enrichment of CHEK2 variants in those of Eastern European descent.17 When compared with a recently published cohort of 391 unselected patients with AML where P/LP CHEK2 and DDX41 variants were discovered in 8 of 391 (2.0%) and 7 of 391 (1.8%) individuals, respectively, our germ line variant positivity rate was enriched by the clinical suspicion of providers and a low threshold for pursuing germ line workup.18
With data available from 54 patients with HHM who received HSCT, to our knowledge, our analysis is the largest to date to describe allogeneic transplant outcomes in patients with HHM, revealing an increased risk for severe aGVHD and cGVHD after transplant in patients with P/LP germ line DDX41 variants, which can potentially be alleviated by using posttransplant cyclophosphamide for GVHD prophylaxis. Given the role of DDX41 in immune activation through STING signaling, these results suggest that recipient cells with the germ line DDX41 variant may present an inflammatory milieu that causes aberrant immune activation after transplant.19, 20, 21
A limitation of this study is the lack of uniform conditioning and GVHD prophylaxis regimens used in patients undergoing HSCT. However, the uniformity in diagnosis and stem cell source reduced other confounders in our retrospective HSCT analyses.
Clinical equipoise currently surrounds treatment decisions for HHMs, as morbidity associated with curative-intent HSCT is weighed against the risk of recurrent leukemic episodes if an HSC pool harboring pathogenic germ line variants is not replaced.22,23 Our data establish severe GVHD as a relevant posttransplant outcome after HSCT for germ line DDX41–associated HHMs. Post-HSCT cyclophosphamide was associated with a lower incidence of severe GVHD in these patients, similar to its GVHD protective effects in allogeneic HSCT recipients without HHM. Broader confirmation of these results in other cohorts will expand the spectrum of known pathogenic variants causing HHMs and support the development of targeted interventions. Further mechanistic studies may reveal the molecular pathways underlying our observations.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgments
The authors are grateful to their patients and their families.
C.S. is supported by the Leukemia Lymphoma Society Special Fellow Award. D.H. was supported by Investigator Grant, NHMRC/MRFF and Cancer Australia.
Authorship
Contribution: C.S., D.H., and L.A.G. designed the study; C.S., G.R., C.N.H., R.C., S.G., H.E., C.T., E.N., A.K., S.D., D.S., P.V., C.C.H., A.B., and D.H. collected the data; C.S., G.R., and G.G. analyzed the data; and all authors edited the manuscript and agreed with the final version.
Footnotes
Data are available on request from the corresponding author, Lucy A. Godley (lgodley@medicine.bsd.uchicago.edu).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Roloff GW, Drazer MW, Godley LA. Inherited susceptibility to hematopoietic malignancies in the era of precision oncology. JCO Precis Oncol. 2021;5:107–122. doi: 10.1200/PO.20.00387. [DOI] [PubMed] [Google Scholar]
- 2.Klco JM, Mullighan CG. Advances in germline predisposition to acute leukaemias and myeloid neoplasms. Nat Rev Cancer. 2021;21(2):122–137. doi: 10.1038/s41568-020-00315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraft IL, Godley LA. Identifying potential germline variants from sequencing hematopoietic malignancies. Blood. 2020;136(22):2498–2506. doi: 10.1182/blood.2020006910. [DOI] [PubMed] [Google Scholar]
- 4.Godley LA. Inherited predisposition to myeloid malignancies. Blood Adv. 2019;3(17):2688. doi: 10.1182/bloodadvances.2019000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz JR, Ma J, Lamprecht T, et al. The genomic landscape of pediatric myelodysplastic syndromes. Nat Commun. 2017;8(1):1557. doi: 10.1038/s41467-017-01590-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feurstein S, Churpek JE, Walsh T, et al. Germline variants drive myelodysplastic syndrome in young adults. Leukemia. 2021;35(8):2439–2444. doi: 10.1038/s41375-021-01137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singhal D, Hahn CN, Feurstein S, et al. Targeted gene panels identify a high frequency of pathogenic germline variants in patients diagnosed with a hematological malignancy and at least one other independent cancer. Leukemia. 2021;35(11):3245–3256. doi: 10.1038/s41375-021-01246-w. [DOI] [PubMed] [Google Scholar]
- 8.Li P, White T, Xie W, et al. AML with germline DDX41 variants is a clinicopathologically distinct entity with an indolent clinical course and favorable outcome. Leukemia. 2022;36(3):664–674. doi: 10.1038/s41375-021-01404-0. [DOI] [PubMed] [Google Scholar]
- 9.Sebert M, Passet M, Raimbault A, et al. Germline DDX41 mutations define a significant entity within adult MDS/AML patients. Blood. 2019;134(17):1441–1444. doi: 10.1182/blood.2019000909. [DOI] [PubMed] [Google Scholar]
- 10.Guidugli L, Johnson AK, Alkorta-Aranburu G, et al. Clinical utility of gene panel-based testing for hereditary myelodysplastic syndrome/acute leukemia predisposition syndromes. Leukemia. 2017;31(5):1226–1229. doi: 10.1038/leu.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singhal D, Wee LYA, Kutyna MM, et al. The mutational burden of therapy-related myeloid neoplasms is similar to primary myelodysplastic syndrome but has a distinctive distribution. Leukemia. 2019;33(12):2842–2853. doi: 10.1038/s41375-019-0479-8. [DOI] [PubMed] [Google Scholar]
- 12.Quesada AE, Routbort MJ, DiNardo CD, et al. DDX41 mutations in myeloid neoplasms are associated with male gender, TP53 mutations and high-risk disease. Am J Hematol. 2019;94(7):757–766. doi: 10.1002/ajh.25486. [DOI] [PubMed] [Google Scholar]
- 13.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 14.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389–401.e381. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 16.University of Chicago Hematopoietic Malignancies Cancer Risk Team How I diagnose and manage individuals at risk for inherited myeloid malignancies. Blood. 2016;128(14):1800–1813. doi: 10.1182/blood-2016-05-670240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stolarova L, Kleiblova P, Janatova M, et al. CHEK2 germline variants in cancer predisposition: stalemate rather than checkmate. Cells. 2020;9(12):2675. doi: 10.3390/cells9122675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang F, Long N, Anekpuritanang T, et al. Identification and prioritization of myeloid malignancy germline variants in a large cohort of adult patients with AML. Blood. 2022;139(8):1208–1221. doi: 10.1182/blood.2021011354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barreyro L, Chlon TM, Starczynowski DT. Chronic immune response dysregulation in MDS pathogenesis. Blood. 2018;132(15):1553–1560. doi: 10.1182/blood-2018-03-784116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chlon TM, Stepanchick E, Hershberger CE, et al. Germline DDX41 mutations cause ineffective hematopoiesis and myelodysplasia. Cell Stem Cell. 2021;28(11):1966–1981.e1966. doi: 10.1016/j.stem.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z, Yuan B, Bao M, Lu N, Kim T, Liu YJ. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol. 2011;12(10):959–965. doi: 10.1038/ni.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton KV, Maese L, Marron JM, Pulsipher MA, Porter CC, Nichols KE. Stopping leukemia in its tracks: should preemptive hematopoietic stem-cell transplantation be offered to patients at increased genetic risk for acute myeloid leukemia? J Clin Oncol. 2019;37(24):2098–2104. doi: 10.1200/JCO.19.00181. [DOI] [PubMed] [Google Scholar]
- 23.Tawana K, Wang J, Renneville A, et al. Disease evolution and outcomes in familial AML with germline CEBPA mutations. Blood. 2015;126(10):1214–1223. doi: 10.1182/blood-2015-05-647172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.