Abstract
Introduction:
Sex hormones are hypothesized to drive sex-specific health disparities. Here, we study the association between sex steroid hormones and DNA methylation-based (DNAm) biomarkers of age and mortality risk including Pheno Age Acceleration (AA), Grim AA, and DNAm-based estimators of Plasminogen Activator Inhibitor 1 (PAI1), and leptin concentrations.
Methods:
We pooled data from three population-based cohorts, the Framingham Heart Study Offspring Cohort (FHS), the Baltimore Longitudinal Study of Aging (BLSA), and the InCHIANTI Study, including 1,062 postmenopausal women without hormone therapy and 1,612 men of European descent. Sex hormone concentrations were standardized with mean 0 and standard deviation of 1, for each study and sex separately. Sex-stratified analyses using a linear mixed regression were performed, with a Benjamini-Hochberg (BH) adjustment for multiple testing. Sensitivity analysis was performed excluding the previously used training-set for the development of Pheno and Grim age.
Results:
Sex Hormone Binding Globulin (SHBG) is associated with a decrease in DNAm PAI1 among men (per 1 standard deviation (SD): −478 pg/mL; 95%CI: −614 to −343; P:1e-11; BH-P: 1e-10), and women (−434 pg/mL; 95%CI: −589 to −279; P:1e-7; BH-P:2e-6). The testosterone/estradiol (TE) ratio was associated with a decrease in Pheno AA (−0.41 years; 95%CI: −0.70 to −0.12; P:0.01; BH-P: 0.04), and DNAm PAI1 (−351 pg/mL; 95%CI: −486 to −217; P:4e-7; BH-P:3e-6) among men. In men, 1 SD increase in total testosterone was associated with a decrease in DNAm PAI1 (−481 pg/mL; 95%CI: −613 to −349; P:2e-12; BH-P:6e-11).
Conclusion:
SHBG was associated with lower DNAm PAI1 among men and women. Higher testosterone and testosterone/estradiol ratio were associated with lower DNAm PAI and a younger epigenetic age in men. A decrease in DNAm PAI1 is associated with lower mortality and morbidity risk indicating a potential protective effect of testosterone on lifespan and conceivably cardiovascular health via DNAm PAI1.
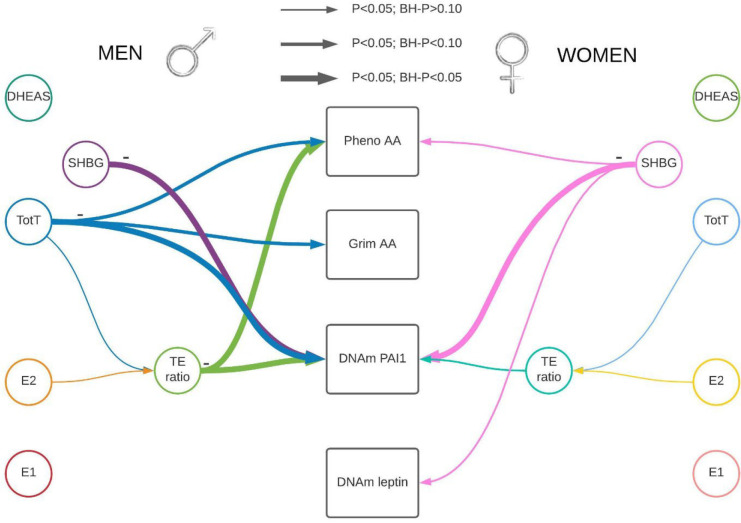
Visual representation of our main results stratified by sex.
There were four outcomes of interest in the rectangular shapes in the middle of this figure, Pheno-Age Acceleration (AA), Grim AA, DNAm-based PAI1, and DNAm-based leptin. We measured five hormone concentrations (testosterone, estrone, estradiol, DHEAS, and Sex Hormone Binding Globulin (SHBG)). In addition, one hormone level ratio (testosterone / estradiol) was estimated. Associations were calculated by linear mixed regression models between sex hormones and the outcomes of interests. The associations are represented by colored arrows with the lines’ thickness representing the strength of the association. As the association was measured mainly cross-sectional, the directionality of the association cannot be established. Hormone levels were inversely associated with epigenetic estimators of mortality risk.
Abbreviations: E1: total estrone; E2: total estradiol; SHBG: Sex Hormone Binding Globulin; TotT: total testosterone; TE ratio: Total testosterone divided by total estradiol concentration
Introduction
Sex hormones are hypothesized to drive sex-specific health disparities.1 Females are overrepresented among the elderly population, and experience lower mortality rates and cardiovascular disease (CVD) risk.2,3 Prior studies suggest women may also have a higher risk of some neurodegenerative diseases.3 A higher concentration of various sex hormones concentrations has been hypothesized to decrease mortality and morbidity.1,4
Steroid sex hormones are a group of hormones that play a role in sexual development and reproduction, although they have many additional functions. Three main categories are estrogens, progesterone, and androgens. Estradiol and estrone are estrogens and female-dominated sex hormones while androsterone sulfate, androstenedione, dehydroepiandrosterone sulfate (DHEAS), and testosterone are androgens and considered male-dominated sex hormones. A testosterone/estradiol (TE) ratio can be used as a proxy for hormonal balance between androgens and estrogens. This ratio has also been associated with various health outcomes, including cerebrovascular disease, metabolic disorders and CVD.5–7 Sex hormone binding globulin (SHBG) is a protein that transports sex hormones, binds estrone, estradiol and testosterone, and influences bioavailability of these hormones.8
The sex steroid hormones have time-trends. Among adult women, major changes of sex hormones concentrations occur during menopause. Around menopause, estradiol (and estrone to a more limited degree) decreases rapidly over 6 months, then slowly decreases over time with the largest decrease in the first 3 years. After menopause, estrone become the major estrogen.9 Among postmenopausal women, testosterone and androsterone also decrease slowly with age, while SHBG appears to change with age in a U-shaped form, with lowest concentrations around 60–70 years of age, followed by an increase with age.9–12 For men, SHBG increases over time,11–13 and estradiol remains stable or decreases slightly,14,15 and testosterone decrease over time.12,13,15 DHEAS peaks around 20–24 years of age among men and women and then decrease with age.13,16–18
Animal studies indicate that higher estrogen concentrations are associated with a longer lifespan among male mice, but not in females.19–21 Observational human studies suggest that low androgen levels among men, and possibly postmenopausal women, are associated with CVD and their risk factors.22,23 Among women, a majority of the studies have used female reproductive characteristics as a proxy for hormone levels, as sex hormones increase after menarche and estradiol, a strong estrogen, decreases after menopause. Several studies have indicated that women who gave birth later in life (>40 years), had high parity, or late menopause with longer exposure to higher levels of estrogens had lower all-cause mortality rates,24–31 while in contrast high levels of postmenopausal estradiol concentrations have been associated with an increased mortality.32
Biomarkers of aging that are strongly associated with increased morbidity and mortality can serve as surrogate endpoints for these outcomes. Specifically, these indicators of biological age can be used to investigate whether an exposure of interest might be associated with morbidity and mortality risk, though causality cannot be established. Recently epigenetic age has become a widely used indicator of biological age, as it has been shown to be strongly associated with morbidity and mortality.33–35 Interestingly, among centenarians, epigenetic (and hence biologic) age has been shown to be lower.36 There are some indications that sex hormones influence epigenetic age and that lower sex hormone concentrations (due to earlier age of menopause, ovariectomy, or lower ovarian reserve etc.) could accelerate epigenetic aging.37–40 Among the various epigenetic age measures, GrimAge accelerations (AA) and Pheno AA are strongly associated with mortality and morbidity, especially CVD.35 GrimAge is established by a two-step approach that combines DNA methylation (DNAm) based estimates of plasma protein levels, DNAm based estimates of smoking pack years, age and sex into a mortality risk estimate. Of these DNAm based estimates of protein biomarkers, plasminogen activator inhibitor 1 (PAI1) is most strongly associated with morbidity and leptin shows sex differential effects.35
PAI1 is a protein that is involved in tissue hemostasis and an increase in PAI1 is associated with metabolic syndrome, lipid metabolism, and cardiovascular health.41–45 Genetic mutations in the SERPINE1 mutation, associated with PAI-1 concentrations, have been associated with cellular senescence, leukocyte telomere length, and longevity.46 Previously, studies that assessed associations between sex hormones and PAI1 protein concentrations in blood reported conflicting results. Among men, several small studies indicated that higher testosterone and SHBG concentrations are associated with lower PAI1 concentrations.47,48 SHBG is also inversely correlated with PAI1 concentrations among women.49 Conversely, there is some indication that higher testosterone and DHEAS in women may be associated with higher PAI1 concentrations, though results from two studies were not consistent.49,50
Leptin is a peptide hormone and is associated with regulation of food intake and energy balance. Leptin also influences inflammatory processes, angiogenesis, lipolysis, and neuroplasticity.51–53 For women, several studies indicated positive correlations between estrone, estradiol and testosterone and leptin concentrations,54–58 whereas reported correlations of leptin with SHBG and DHEAS were inconsistent.55–58 Body mass index (BMI) is strongly positively associated with leptin concentration as leptin is secreted by adipocytes.15 In addition, adipose tissue can convert estrone to estradiol.15,59 Hence, BMI could be a strong confounder. Adjusting for BMI attenuated the correlations between sex hormones (estradiol, testosterone, SHBG and DHEAS) and leptin concentrations in some studies.56,57 Among men, higher testosterone concentrations are correlated with lower leptin concentrations.57,58,60–62 Two studies among men indicated a negative association between SHBG and leptin concentration,58,61 one no association,60 and yet another study a positive association.57 These inconsistencies might be due to sample size, differences in population characteristics (age, ethnicity etc.), or due to methodological differences.
In this study, we are focusing on the association between five sex steroid hormones (estrone, estradiol, SHBG, testosterone, DHEAS), the testosterone/estradiol (TE) ratio, and epigenetic age acceleration for older men and postmenopausal women. We chose to restrict the study to postmenopausal women without hormone therapy as female sex hormones stabilize after menopause,9 and assessment of sex hormones is not influenced by timing within the menstrual cycle. We combined data from three large studies that have collected DNAm as well as measured sex hormones in the blood of men and women. No previous studies have reviewed whether sex hormones are also associated with the epigenetic biomarkers for leptin (DNAm Leptin) and PAI1 (DNAm PAI1). In addition, no previous studies on sex hormone concentrations and epigenetic based age acceleration have been performed. We hypothesize that higher concentrations of sex hormones are associated with lower epigenetic age.
Main results
Our study included 1,612 men and 1,062 postmenopausal women of European descent, who were not using hormone therapy at the time of blood draw, with at least one sex hormone measurement and genome-wide DNA methylation (DNAm) array data. Estrone was only available in the Framingham Heart Study (FHS), while DHEAS was available in the Baltimore Longitudinal Study of Aging (BLSA) and the InCHIANTI.
The average age at sex hormones assay was 66.5 years (standard deviation [SD]: 9.8) for women and 62.4 years for men (SD: 12.2). The time between the sex hormone measurement and subsequent blood collection for DNAm analysis among women and men of the FHS study was 6.6 and 6.6 years (SD: 0.6, SD: 0.7, for women and men respectively). For subjects from the InCHIANTI and BLSA, sex hormones and DNAm were measured during the same visit. Sex hormones for women were measured on average 17.4 years (SD: 10.7) after their menopause with an average age of menopause of 49.0 years (SD: 5.9). See more details on the characteristics in Table 1, and the characteristics stratified by study including the sex hormone concentrations in supplemental Table 1. The average concentrations for total sex hormones were comparable with previous studies of individuals of similar age.63–65 Due to differences in sex hormone concentrations and differences in characteristics between the three studies, as well as different methods used to estimate sex hormones concentration, the sex hormones were standardized (mean 0; standard deviation 1) for each sex within their study, followed by winsorization of the top and bottom 1% (Supplemental Table 1). See supplemental figure 1 for scatterplots of the sex hormone concentration by age and study.
Table 1.
Overview of the characteristics of the study population
| Women | Men | ||||||
|---|---|---|---|---|---|---|---|
| Mean / N | SD / % | N | Mean / N | SD / % | N | ||
| Characteristics | |||||||
| Study | FHS | 665 | 62.6 | 1062 | 1,088 | 67.5 | 1612 |
| InCHIANTI | 193 | 18.2 | 222 | 13.8 | |||
| BLSA | 204 | 19.2 | 302 | 18.7 | |||
| Age at sex hormone measurement | years | 66.5 | 9.8 | 1062 | 62.4 | 12.2 | 1612 |
| Age at DNA methylation | years | 70.6 | 8.9 | 1062 | 66.9 | 11.6 | 1612 |
| Time between visits among FHS | years | 6.6 | 0.6 | 665 | 6.6 | 0.7 | 1088 |
| Age of menopause | years | 48.6 | 6.0 | 991 | NA | ||
| Time since menopause at sex hormone measurements | years | 17.3 | 11.0 | 991 | NA | ||
| Smoking status at sex hormone measurements | never | 437 | 40.7 | 1075 | 485 | 29.9 | 1624 |
| Former/current | 638 | 59.3 | 1,139 | 70.1 | |||
| Packyears at sex hormone measurements among smokers | packyears | 17.8 | 18.4 | 638 | 24.1 | 20.8 | 1139 |
| Alcohol intake | Drinks/weeks | 7.0 | 9.2 | 1015 | 19.4 | 24.2 | 1488 |
| BMI at sex hormone measurement | kg/m2 | 27.5 | 5.2 | 1073 | 28.3 | 4.4 | 1622 |
| Hormone levels | |||||||
| Total estrone | pg/ml | 32.2 | 20.9 | 634 | 50.6 | 17.7 | 1037 |
| Total estradiol | pg/ml | 11.0 | 19.6 | 984 | 23.9 | 10.1 | 1519 |
| SHBG | nmol/L | 83.3 | 49.4 | 832 | 61.7 | 31.5 | 1260 |
| Total testosterone | ng/dl | 35.4 | 28.0 | 1017 | 541.3 | 226.6 | 1533 |
| DHEAS | ug/dl | 86.2 | 71.4 | 192 | 143.1 | 115.6 | 221 |
| Bioavailable sex hormones | |||||||
| Bioavailable estrone | pg/ml | 1.0 | 0.7 | 633 | 1.8 | 0.6 | 1037 |
| Bioavailable estradiol | pg/ml | 0.2 | 0.3 | 812 | 0.5 | 0.2 | 1250 |
| Bioavailable testosterone | pg/ml | 4.0 | 3.0 | 829 | 82.7 | 35.0 | 1259 |
| Main outcome | |||||||
| Pheno AA | years | −0.7 | 6.4 | 1075 | 0.8 | 6.1 | 1624 |
| Grim AA | years | −1.6 | 4.0 | 1075 | 1.7 | 4.5 | 1624 |
| DNAm PAI1 | pg/ml | 19021 | 2862 | 1075 | 20865 | 3117 | 1624 |
| DNAm Leptin | pg/ml | 12026 | 2839 | 1075 | 4769 | 1648 | 1624 |
Abbreviations: N: Number; SD: Standard Deviation; years: years; NA: Not applicable; FHS: Framingham Heart Study; BLSA: Baltimore Longitudinal Study of Aging
Correlations
Pairwise correlations were calculated and represented in supplemental figure 2. The correlations between the five sex hormones (estrone, estradiol, SHBG, total testosterone, and DHEAS) varied between 0.01 and 0.66. While the correlation between total estrone and SHBG was relatively weak (r=0.10 in men; r=0.01 in women), the correlation between estrone and estradiol was relatively high (r=0.59 in men; r=0.66 in women). The correlations between Pheno AA, Grim AA, DNAm PAI1, and DNAm leptin, i.e., the outcomes we will focus on as the strongest surrogate markers for morbidity and mortality, were weak to moderate in size, i.e., between 0.06 and 0.45 (supplemental figure 3).
Sex hormones and main outcomes (Grim AA, Pheno AA, DNAm PAI1 and DNAm leptin)
Each sex hormone concentrations were standardized within sex and study group. The standardized sex hormone concentrations were pooled over the three studies for males and females separately. We used linear mixed regression models to analyze the association between sex hormones and the epigenetic clocks/DNAm proteins to take family relatedness within the FHS into account. We also adjusted for age, BMI, average alcohol intake, smoking packyears, physical activity, time between visits, cohort study, and blood cell composition based on DNAm. For all postmenopausal women, we also included time since menopause at the time sex hormones were measured. All analyses were performed sex stratified and we adjusted for multiple testing (Number of tests in the Benjamini Hochberg analysis: 24). Our main findings for the association between sex hormones and epigenetic clocks/DNAm proteins are summarized in table 2, figures 1 & 2, with the precursors for total and bioavailable sex hormones displayed in Figure 2.
Table 2.
Linear mixed regression analysis with random intercept were performed to assess the effect of sex hormones on Pheno Age, GrimAge, DNAm-based PAI1, and DNAm-based leptin. Sex hormones were standardized and winsorized (highest and lowest 1%) by study. The analyses were performed stratified by sex, and adjusted for age, body mass index (BMI), alcohol intake, smoking packyears, physical activity, the time between visits, cohort study, and blood cell composition based on DNAm. Among women, we also adjusted for time since menopause.
| Men | Females | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Sex Hormones | Est. | 95% LL | 95% UL | P | BH-P | Est. | 95% LL | 95% UL | P | BH-P |
| Pheno AA | E1 | 0.26 | −0.09 | 0.61 | 0.14 | 0.31 | 0.06 | −0.42 | 0.54 | 0.79 | 0.96 |
| E2 | 0.13 | −0.15 | 0.40 | 0.37 | 0.67 | 0.05 | −0.32 | 0.41 | 0.81 | 0.96 | |
| SHBG | −0.11 | −0.41 | 0.19 | 0.47 | 0.67 | −0.40 | −0.75 | −0.04 | 0.03 | 0.22 | |
| TotT | −0.35 | −0.64 | −0.06 | 0.02 | 0.08 | 0.11 | −0.23 | 0.44 | 0.54 | 0.92 | |
| TE ratio | −0.41 | −0.70 | −0.12 | 0.01 | 0.04 | −0.12 | −0.48 | 0.23 | 0.49 | 0.92 | |
| DHEAS | −0.02 | −0.62 | 0.57 | 0.94 | 0.98 | 0.38 | −0.12 | 0.87 | 0.14 | 0.47 | |
| Grim AA | E1 | 0.19 | −0.02 | 0.39 | 0.07 | 0.22 | 0.03 | −0.22 | 0.29 | 0.80 | 0.96 |
| E2 | −0.04 | −0.21 | 0.12 | 0.59 | 0.71 | 0.04 | −0.15 | 0.23 | 0.66 | 0.96 | |
| SHBG | 0.05 | −0.13 | 0.22 | 0.59 | 0.71 | −0.10 | −0.29 | 0.08 | 0.28 | 0.76 | |
| TotT | −0.22 | −0.39 | −0.05 | 0.01 | 0.06 | 0.00 | −0.18 | 0.18 | 0.99 | 0.99 | |
| TE ratio | −0.16 | −0.33 | 0.01 | 0.07 | 0.22 | −0.14 | −0.32 | 0.05 | 0.16 | 0.48 | |
| DHEAS | 0.28 | −0.08 | 0.64 | 0.12 | 0.30 | 0.09 | −0.16 | 0.34 | 0.50 | 0.92 | |
| DNAm PAI1 | E1 | 61 | −100 | 222 | 0.46 | 0.67 | −67 | −270 | 136 | 0.52 | 0.92 |
| E2 | −103 | −230 | 24 | 0.11 | 0.30 | 139 | −22 | 299 | 0.09 | 0.37 | |
| SHBG | −478 | −614 | −343 | 1E-11 | 1E-10 | −434 | −589 | −279 | 1E-07 | 2E-06 | |
| TotT | −481 | −613 | −349 | 2E-12 | 6E-11 | −134 | −284 | 15 | 0.08 | 0.37 | |
| TE ratio | −351 | −486 | −217 | 4E-07 | 3E-06 | −197 | −356 | −39 | 0.02 | 0.18 | |
| DHEAS | 68 | −200 | 335 | 0.62 | 0.71 | −21 | −264 | 222 | 0.86 | 0.96 | |
| DNAm LEPTIN | E1 | 0 | −90 | 89 | 1.00 | 1.00 | 13 | −162 | 187 | 0.89 | 0.96 |
| E2 | −28 | −101 | 45 | 0.44 | 0.67 | 28 | −106 | 163 | 0.68 | 0.96 | |
| SHBG | −30 | −109 | 50 | 0.46 | 0.67 | −140 | −271 | −10 | 0.04 | 0.22 | |
| TotT | −26 | −103 | 52 | 0.52 | 0.69 | 42 | −82 | 166 | 0.50 | 0.92 | |
| TE ratio | 18 | −60 | 95 | 0.66 | 0.72 | −26 | −159 | 107 | 0.70 | 0.96 | |
| DHEAS | −75 | −253 | 104 | 0.41 | 0.67 | 9 | −174 | 193 | 0.92 | 0.96 | |
Abbreviations: Est: Effect estimate; LL: Lower Limit; UL: Upper Limit; P: P-value; BH-P: Benjamini-Hochberg adjusted P-value; AA: Age Acceleration; DNAm: DNA-methylation; E1: total estrone; E2: total estradiol; SHBG: Sex Hormone Binding Globulin; TotT: total testosterone; TE ratio: Total testosterone divided by total estradiol concentration
Figure 1. Visual representation of our main results stratified by sex.
There were four outcomes of interest in the rectangular shapes in the middle of this figure, Pheno-Age Acceleration (AA), Grim AA, and DNAm-based PAI1 and DNAm-based leptin. We measured five hormone concentrations (testosterone, estrone, estradiol, DHEAS, and Sex Hormone Binding Globulin (SHBG)). In addition, one hormone level ratio (testosterone / estradiol) was estimated. Associations identified in a linear mixed regression model are represented by colored arrows between sex hormones and the outcomes of interests, with the lines’ thickness representing the strength of the association. Hormone levels were inversely associated with epigenetic estimators of mortality risk.
Abbreviations: E1: total estrone; E2: total estradiol; SHBG: Sex Hormone Binding Globulin; TotT: total testosterone; TE ratio: Total testosterone divided by total estradiol concentration
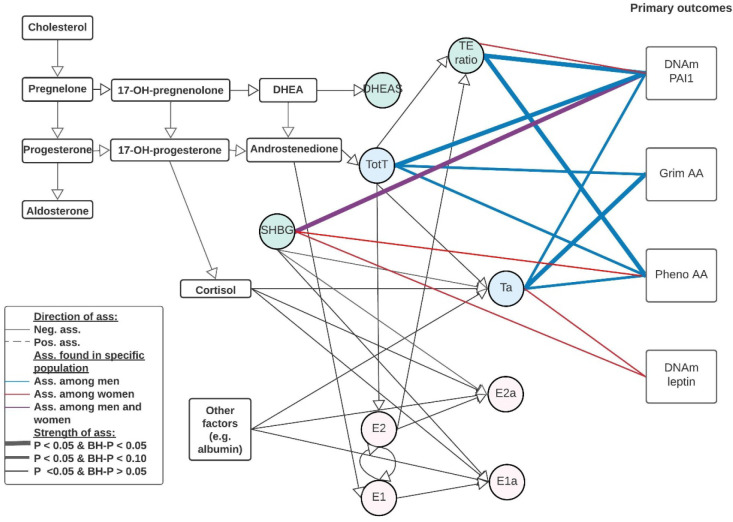
Figure 2. Visual representation of our main results including bioavailable testosterone and precursors.
There were 4 outcomes of interest in the rectangle shapes on the right, Grim Age Acceleration (AA), Pheno AA, and DNAm-based PAI1, and DNAm-based leptin. There were 8 various hormone levels, 5 total concentrations of testosterone, estrone, estradiol, DHEAS, and SHBG, and 3 bioavailable concentrations of testosterone, estrone and estradiol. In addition, there was one hormone level ratio (testosterone / estradiol) estimated. Associations were estimated using a linear mixed regression model, please note that these are not causal estimates but associations only.
Abbreviations: E1: total estrone; E2: total estradiol; SHBG: Sex Hormone Binding Globulin; TotT: total testosterone; TE ratio: Total testosterone divided by total estradiol concentration; E1a: bioavailable estrone; E2a: bioavailable estradiol; Ta: bioavailable testosterone
In line with our hypothesis that high sex hormone concentrations are associated with better epigenetic age or epigenetic profile, among males, we identified four significant associations all indicating a negative association. None of these associations significantly differed by cohort (Supplemental table 2). Three of these associations were with DNAm PAI1, namely SHBG, total testosterone, and the TE ratio. The fourth was an association between the TE ratio and Pheno AA. A one SD increase in SHBG was associated with lower DNAm PAI1 by −478 pg/mL, which is equivalent to approximately 0.17 SD of DNAm PAI1 (95%CI: −614 to −343; P-value: 1e-11; Benjamini Hochberg adjusted P-value (BH-P): 1e-10). One SD increase in total testosterone was associated with a similar decrease in DNAm PAI1 (−481 pg/mL; 95%CI: −6213 to −349; P-value: 2e-12; BH-P: 6e-11), and one SD increase in the TE ratio decreased DNAm PAI1 by 351 pg/ml (95%CI: −620 to −355; P-value: 2e-12; BH-P: 4e-11). An increase of 1 SD in TE ratio was associated with a decrease in Pheno AA with 0.41 years (95%CI: −0.70 to −0.12; P-value: 0.01; BH-P: 0.04); i.e., suggesting a “younger” DNAm Pheno age compared to chronological age. For two additional hormones there was a decrease in epigenetic age acceleration suggested (P-value < 0.05 or BH-P < 0.10); specifically, an increase in total testosterone appeared to decrease both Grim AA and Pheno AA. With each increase of 1 SD in testosterone Grim AA decreases by 0.22 years (95%CI: −0.39 to −0.05; P-value: 0.01; BH-P: 0.06), and Pheno AA by 0.35 years (95%CI: −0.64 to −0.06 years; P-value: 0.02; BH-P: 0.08). When adjusting for DNAm PAI1 in the model for the effect of testosterone on Grim AA, the association between testosterone and Grim AA attenuated (0.03 yrs; 95%CI: −0.13 to 0.19 years; P-value: 0.71), whereas adjusting for Grim AA in the model between testosterone and DNAm PAI1, the estimated effect remained the same (−413 pg/ml; 95%CI: −534 to −291; P-value: 6e-11).
Among females, only SHBG was statistically significantly associated with DNAm PAI1, and three suggestive associations were detected, that is even though the P-value was less than 0.05, the BH-P was above 0.10. One SD increase of SHBG was associated with a decrease of DNAm PAI1 (−434; 95%CI: 589 to −279; P-value: 1e-7; BH-P: 2e-6). There was indication for heterogeneity based on the likelihood ratio test adding the interaction term for SHBG and study (P:4e-3). In addition, an increase in the TE ratio was associated with a decrease in the DNAm PAI1 (−197 (95%CI: −356 to −39; P-value: 0.02; BH-P: 0.18), and an increase in SHBG was associated with a decrease in Pheno AA by 0.40 years (95%CI: −0.75 to −0.04; P-value 0.03; BH-P: 0.22). Finally, SHBG suggestively decreased DNAm leptin by approximately 140 pg/ml per SD increase of SHBG (95%CI: −271 to −10; P-value: 0.04; BH-P: 0.22).
Combined outcomes
We combined our four main outcomes (Grim AA, Pheno AA, DNAm PAI1 and DNAm leptin) into one summarized outcome by normalizing (mean:0, and SD: 1) each outcome and averaging the overall outcome (see table 3). This analysis indicated that an increase in SHBG, testosterone, and TE-ratio were associated with a decreased summarized outcome (SHBG: −0.05 SD; 95%CI: −0.08 to −0.02; P-value: 2e-3; BH-P: 4e-3; testosterone: −0.07 SD; 95%CI: −0.10 to −0.04; P-value: 4e-6; BH-P: 3e-5; and TE-ratio: −0.05 SD; 95%CI: −0.08 to −0.02; P-value: 1e-3; BH-P: 4e-3). Among females, an increase in SHBG was associated with a decrease in our overall outcome (−0.08 SD; 95%CI: −0.11 to −0.04; P-value: 9e-5; BH-P: 5e-4). We also created a latent variable using PCA, and the results were almost identical (see supplemental table 3).
Table 3.
Association between sex hormones and an aggregate outcome. We normalized each outcome first (mean zero; standard deviation one) followed by summarization of the four outcomes (Pheno AA, Grim AA, DNAm PAI1, and DNAm Leptin) into one aggregate. Associations were calculated using a linear mixed model adjusting for familial relationships.
| Men | Females | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Exposure | Estimate | 95%LL | 95%UL | P-value | BH-P | Estimate | 95%LL | 95%UL | P-value | BH-P |
|
| ||||||||||
| E1 | 0.03 | −0.01 | 0.06 | 0.17 | 0.25 | 0.00 | −0.04 | 0.05 | 1.00 | 1.00 |
| E2 | −0.01 | −0.04 | 0.02 | 0.42 | 0.50 | 0.03 | −0.01 | 0.07 | 0.11 | 0.23 |
| SHBG | −0.05 | −0.08 | −0.02 | 2E-03 | 4E-03 | −0.08 | −0.11 | −0.04 | 9E-05 | 5E-04 |
| TotT | −0.07 | −0.10 | −0.04 | 4E-06 | 3E-05 | 0.00 | −0.04 | 0.03 | 0.96 | 1.00 |
| TE ratio | −0.05 | −0.08 | −0.02 | 1E-03 | 4E-03 | −0.04 | −0.07 | 0.00 | 0.05 | 0.16 |
| DHEAS | 0.02 | −0.05 | 0.09 | 0.59 | 0.59 | 0.03 | −0.04 | 0.10 | 0.37 | 0.55 |
Bioavailable sex hormones
While our main analysis focused on the total concentration of estrone, estradiol and testosterone, we also calculated the bioavailable concentrations of these sex hormones relying on the mass action law calculation.66 Bioavailable sex hormones are the concentration of the sex hormone that are considered bioactive and have the possibility to be utilized in biologic processes. The bioavailable and total concentrations of sex hormones were strongly correlated, and were strongly correlated with SHBG, which is one of the main binding proteins of sex hormones. The correlations between the total and bioavailable sex hormone concentrations ranged between 0.68 and 0.98 (see supplemental figure 2, supplemental table 4).
Results for all sex hormones, including bioavailable sex hormones, are available in supplemental table 5. Most of the results for bioavailable sex hormones were very similar to those that were seen for the total concentration of the specific sex hormone. This was expected due to the high correlation between total and bioavailable sex hormone concentration.
However, some differences were observed, bioavailable estradiol was suggestively associated with an increase in DNAm PAI1 in men and women (men: 110; 95%CI: −21 to 240; P-value: 0.10; BH-P: 0.28 / women: 220; 95%CI: 57 to 382; P-value: 0.01; BH-P: 0.15). Among men, a slightly stronger association, compared to total testosterone, between bioavailable testosterone and Grim AA was identified (1 SD increase in bioavailable testosterone was associated with a decrease of 0.28 years in Grim AA (95%CI: −0.46 to −0.11; P-value: 2e-3; BH-P: 0.01)). On the contrary, the effect for DNAm PAI1 was attenuated for bioavailable versus total testosterone (−160; 95%CI: −299 to −22; P-value: 0.02; BH-P: 0.09) among men, and appeared to be attenuated or even change directions among women (−134; 95%CI: −284 to 15; P-value: 0.08, BH-P: 0.31; and 142; 95%CI: −10 to 294; P-value: 0.07, BH-P: 0.31, for total and bioavailable testosterone, respectively). Finally, there appears to be a positive association between bioavailable testosterone and DNAm leptin among females, though this was not statistically significant after correction for multiple testing (134; 95%CI: 8 to 260; P-value: 0.04, BH-P: 0.23).
Other epigenetic AA and sex hormones
We additionally studied the acceleration measures from 6 epigenetic clocks (IEAA, EEAA, Horvath AA, Hannum AA, Skin-blood clock AA, and Dunedin PoAM; see supplemental table 6).67–70 There were no statistically significant associations between epigenetic age acceleration and sex hormones after adjusting for multiple testing. The strongest associations were seen among men for the TE ratio, with a decrease in IEAA (−0.35; 95%CI: −0.60 to −0.11; P-value: 5e-3, BH-P: 0.11) and Horvath AA (−0.37; 95%CI: −0.61 to −0.12; P-value: 3e-3, BH-P: 0.11).
Sensitivity analysis
Using non-winsorized data instead of winsorized data, most of the effect estimates remained very similar (see supplemental table 7). InCHIANTIThe cross-sectional (BLSA & InCHIANTI) and prospective (FHS) effect estimates were comparable among males (supplemental table 8). Among females, there appeared to be some differences. Cross-sectionally, estradiol was associated with an increase in DNAm PAI1, while no association was seen in the longitudinal, prospective FHS study. SHBG and testosterone were associated with a more strongly negative association (decrease) in DNAm PAI1 in the FHS study, while the analysis based on the cross-sectional studies indicated a smaller effect size estimate for SHBG, and possibly a null or positive association for total testosterone.
A subset of FHS was used for the creation of the original GrimAge clock, as well as the DNAm biomarkers used for the GrimAge clock,35 while InCHIANTI was used for the creation of Pheno age.33 We expect the DNAm-based estimates to have a much stronger correlation with their estimand in the training set, allowing us to capture more of the effect of the estimand (e.g., plasma PAI1) on sex hormone levels. There was however no indication for heterogeneity by study. When excluding the training sets (so excluding InCHIANTI for Pheno AA and excluding the individuals from the training set in the FHS for Grim AA, DNAm PAI1, and DNAm leptin), the sample size reduced considerably to 869 females and 1390 males for the Pheno AA; and 569 females and 793 males for the other outcomes. Among this subset, our primary findings among males remained significant, with some associations slightly attenuated (supplemental table 9).
We also gathered a group of 90 male and 86 female individuals of Black race/ethnicity (BLSA) for our sensitivity analysis. We performed the same analysis for this subgroup (see supplemental table 10) and we found similar effect estimates. Nevertheless, studies with larger sample sizes are needed to replicate our findings and ensure that they are indeed valid for Black and other race/ethnicities.
Discussion
We found that an increase in testosterone and the TE ratio in men was associated with a decreased epigenetic age acceleration, suggesting that higher testosterone levels are associated with biologically younger age and a lower DNAm-based estimate of PAI1 concentration. In both men and women, an increase in SHBG was associated with a lower DNAm-based PAI1 concentration. These findings may indicate that SHBG in both sexes and testosterone in men influence the morbidity, especially cardiovascular risks, and mortality risk in members of these population-based cohorts (BLSA, FHS, and InCHIANTI).
Increases in PAI1 concentration have been positively associated with risk for CVD, ischemic heart disease and stroke, with metabolic abnormalities such as diabetes and metabolic syndrome, and increased inflammation.45,71 While the correlation between plasma PAI-1 and DNAm PAI-1 was only moderate (r=0.36), DNAm PAI-1 was shown to be a better surrogate for lifespan than the actual plasma measure, and performs better than Grim AA regarding associations with the comorbidity-index.35 Another potential benefit of using DNAm-based biomarkers instead of plasma biomarkers is that the DNAm-based biomarkers are representing a longer average estimate of the biomarker concentration and are not as affected by day-to-day variations that could bias the results.72–74 In the study by Lu et al, a higher concentration of DNAm PAI-1 was strongly associated with coronary heart disease, hypertension, type 2 diabetes, computed tomography based measurements of adiposity, and early age of menopause for women, while lower DNAm PAI-1 was associated with disease free status and better physical functioning.35 In our study, we identified a negative association of DNAm PAI1 with total testosterone, TE ratio (men only), and SHBG. The association between testosterone with plasma PAI1 concentration has been previously described for measured (not DNAm based) PAI1 protein concentrations.47,48 A recent review has described the current state of research findings on testosterone concentrations and CVD and vascular aging.75 Though PAI1 was not specifically reviewed, previously studies appeared to provide contradictory results for testosterone and CVD among women, while among men low testosterone concentration was associated with an increase in CVD and vascular aging.75 This is in line with our findings, where testosterone is associated with lower DNAm-based concentrations of PAI1, suggestive of a more protective cardiovascular profile. As the TE ratio is composed of testosterone and estradiol, it is not surprising that results are highly correlated with the total testosterone concentration. However, the balance represented by the TE ratio has been shown to have an influence on many health outcomes, and were found to be associated with libido, cognitive function, well-being and mental state, increased muscle mass, loss of fat mass, increased basal metabolic rate, and reduced cardiovascular markers.76
With regards to the association between SHBG and DNAm PAI1 concentration, an increase in SHBG concentration was associated with an on average decrease in bioavailable hormone concentrations (estrone, estradiol, testosterone). Total testosterone and SHBG were both associated with a decrease in DNAm PAI1 concentration. This could indicate a direct effect of SHBG acting as a hormone, or be due to the fact that both SHBG and PAI1 are synthesized within hepatocytes.8,77–80 One of the major factors that influence SHBG levels is high body mass index, or obesity, and even though we adjusted for this in our analysis, we cannot rule out residual confounding. In addition, other factors that influence SHBG, such as chronic infections, thyroid disease, and certain medications81,82 could partially explain this finding. However, our findings do indicate that an increase in SHBG, and among men also total testosterone, is independently associated with a decrease in DNAm PAI1. Hence, SHBG, as well as testosterone among men may decrease PAI1 levels, and thereby positively influence the risk of developing CVD, metabolic disorders, and inflammation.
Among men only, testosterone and the TE ratio are both associated with a decrease in epigenetic age acceleration. Slightly stronger associations were found for Pheno AA, while the association of testosterone with Grim AA disappeared after taking DNAm PAI1 into account, suggesting that the effect of testosterone on Grim AA was driven by changes in the DNAm PAI1 concentrations. Previous studies that investigated the associations of testosterone with mortality or CVD reported contradictory and inconclusive results. Some early studies of testosterone replacement treatment and/or higher testosterone concentrations among men and possibly women suggested an increased risk for mortality or CVD.83–89 Other clinical trials and observational studies found a decreased mortality or morbidity when testosterone was increased.90–94 Two more recent, large studies reported testosterone to be associated with a decrease in overall mortality and cardiovascular-related deaths among men, but possibly an increase in women.95,96 Thus, the increased mortality and CVD risk may be specific to men with low testosterone levels and these studies also suggested that the effect might not be linear. Yet, our own findings among men were not driven by those with low testosterone levels and we saw a linear effect for our measures. While our study did not review the association between testosterone and mortality directly, our results indicate that testosterone and the balance between testosterone and estradiol (TE ratio) is associated with an epigenetic profile predictive of lower mortality and lower risk for CVD among men. In addition, we also found some evidence for an association between the TE ratio and other epigenetic age acceleration clocks, e.g., for Horvath AA. Since the Horvath pan tissue clock was designed as an age estimator it is referred to as a first-generation clock. By contrast, GrimAge and PhenoAge are referred to as second generation clocks since they were designed as mortality risk predictors. Using this terminology, we find that the beneficial effect of the TE ratio in men can be detected using both first- and second-generation epigenetic clocks. As such, we consider it a highly robust finding. We did not find an association between sex hormones and epigenetic age acceleration among women. This could be due to multiple reasons. First, it could reflect that epigenetic clocks perform differently across the sexes. We think this explanation is unlikely since both GrimAge and PhenoAge predict mortality risk in both sexes. Second, it might be due to the relatively smaller sample size for women as we had to restrict our sample to postmenopausal women without hormone therapy to avoid confounding due to menopausal stage or hormone treatment. Third, it might be that sex hormone concentrations among postmenopausal women, except for SHBG, tend to generally be very low and their variability too limited to estimate effects reducing the power to identify associations in women.
Two of the three cohorts we used provided cross-sectional data, while among participants of the FHS, there was a significant time delay between the measurement of the sex hormones and the blood draw used for DNA methylation analyses. Most sex hormone concentrations decline with age, and it is possible that this resulted in an underestimation of effects in the FHS. However, most of the associations were consistent in the FHS and BLSA, especially for DNAm PAI1, while effect estimation in the InCHIANTI study alone appeared attenuated or null. Differences in estimated effect size across studies may also have been caused by differences in measurement techniques, relatively lower concentrations of sex hormones in the study, or by residual confounding from differences in lifestyle across the cohorts. The BLSA cohort is a multi-ethnic population among a highly educated population in an urban location. This population was relatively older than the other two at time of the assessment. The InCHIANTI study consists of relatively younger men (average age 61 years) at DNA methylation assessment and is a longitudinal study among subjects of Italian descent. As such, this population has a very different lifestyle then the other two studies. We adjusted for many potential confounders (including blood cell types, body mass index, smoking packyears, alcohol intake, physical activity, age at sex hormone assessment, time between measurements, study and for women time since menopause). Effect estimates varied slightly depending on the selection of confounders, with the strongest attenuation observed when we adjusted for BMI, which was expected to be a major confounder. However, given the differences in population characteristics, it is still possible that differences in lifestyle characteristics resulted in residual confounding.
The FHS was used in the original development of GrimAge (and hence also DNAm PAI1 and DNAm Leptin) and the InCHIANTI was used for the development of PhenoAge. As such, using individuals who were used in the training set for the development of the biomarkers could potentially introduce stronger correlation with the outcome (measured biomarkers and time to death). Our sensitivity analysis excluding these individuals indicated no significant difference in the effect estimates and association, suggesting that this potential bias did not change our overall results.
Our primary analysis consisted of men and women of Caucasian or European descent in order to avoid confounding by race/ethnicity. Thus, it is plausible that the results cannot be generalized to other races/ethnicities. However, our findings for a subgroup with Black ancestry subjects indicate that the association between total testosterone and epigenetic age accelerations as well as with DNAm PAI1 are similar at least among men (see also supplemental table 10). Further evaluation in larger minority populations or populations with different lifestyles, should be undertaken to confirm or refute our findings.
Conclusion
A higher testosterone and a higher TE ratio, among men are associated with a decreased epigenetic age acceleration, suggesting a protective effect of testosterone. In addition, we found a strong effect of testosterone and TE ratio on the DNAm PAI1 concentration among men, and between SHBG and DNAm PAI1 concentration independent of sex, suggesting that both SHBG and testosterone, as well as the TE ratio (testosterone and TE ratio among men only) is associated with DNAm PAI1 and therefore potentially with cardiovascular health and overall mortality.
Methods:
Study population
We used data from three population-based cohorts, the Framingham Heart Study (FHS), the Baltimore Longitudinal Study of Aging (BLSA) and the InCHIANTI Study. For more details regarding the study populations, see the supplemental table 1 and the supplemental note. Both FHS and InCHIANTI consist of subjects with European descent, while the BLSA has a more diverse population with 69.8% of European descent, 26.3% of African American or Black ancestry, and 3.9% of other descent. Most female individuals were postmenopausal (N: 1,504, 84.6%). In our main analysis, we restricted to postmenopausal women without hormone therapy and men of European descent.
Sex hormones
For specific details about the measurement of sex hormones, we refer to the supplemental note. For sensitivity analysis, we calculated bioavailable estrone, estradiol and testosterone by using the mass action law when estimates of the total sex hormone (estrone, estradiol or testosterone) and SHBG were available.66 (Bioavailable) estrone was only available in the FHS, and DHEAS was only available in the InCHIANTI and BLSA.
Sex hormone concentrations were standardized with mean 0 and standard deviation of 1, for each study, sex, and postmenopausal status separately. We winsorized the outliers for sex hormone measurements by replacing the top and bottom outliers with the 1st and 99th percentile estimates, respectively. The winsorization was done stratified by sex, and study. Sex hormones were measured at the same time as blood DNA methylation for individuals in the BLSA and InCHIANTI study. For participants of the FHS, sex hormones were measured one visit prior to DNA methylation (average difference between visits: 6.6 years), the length of time between the measurements was added as a covariate in the model to take timing into account. For analysis, we removed postmenopausal women who reported that they were using hormone therapy at either the time of sex hormone or DNAm assessment (N=421), leaving 1,062 postmenopausal women without HRT.
Epigenetic age accelerations and DNAm-based biomarkers
DNA methylation was performed using the Illumina 450k array on whole blood of all individuals in this study. For details regarding DNA methylation, we refer to the supplemental note. The epigenetic age accelerations and DNAm-based biomarkers were calculated using the DNAmage-website (https://dnamage.genetics.ucla.edu). We winsorized the top and bottom outliers (1%) for epigenetic age-accelerations and DNAm biomarkers, stratified by sex, and study. For this specific analysis, we focused on Pheno Age Acceleration (Pheno AA), Grim Age Acceleration (Grim AA), DNAm Plasminogen Activator Inhibitor-1 (PAI1) and DNAm leptin, as these have been shown to be the strongest epigenetic biomarker predictors for mortality and morbidity.35
Statistical analysis
We performed our analysis, stratified by sex, among postmenopausal women without hormone therapy and all men. We analyzed the associations between various sex hormones and our outcomes using linear mixed regression analysis with a random intercept term to account for familial relationships from pedigrees in the FHS. We adjusted for age, body mass index (BMI), average alcohol intake, and smoking packyears estimated at the visit when sex hormones were assessed. We also adjusted for physical activity (in quartiles), the time between visits, cohort study, and blood cell composition based on DNAm. The estimated cell types (plasma blasts, exhausted T cells, naïve CD8 cells, CD4 T-cells, Natural Killer (NK) cells, monocytes, and granulocytes) were calculated using the Houseman and Horvath methods.67,97 For all postmenopausal women, we also included time since menopause at the time sex hormones were measured. If confounders were missing (0.1% missing BMI, 5.3% missing physical activity, 7.3% missing alcohol intake), they were imputed using the MICE R-package. We adjusted for multiple testing by calculating the Benjamini-Hochberg adjusted P-value (BH-P) for both men and women separately (total of 24 tests per sex).
Sensitivity analysis
Various sensitivity analyses were performed. These included analyzing bioavailable sex hormones with the outcomes, using non-winsorized data, stratifying by study, removing individuals from the original training set for the epigenetic outcomes, and analyzing the effect estimates among African American/Black participants only. Furthermore, we reviewed the influence of confounder selection. In addition to our main outcomes, we also performed linear mixed regression analysis for the association between the various sex hormones and six additional epigenetic age accelerations clocks.67–70
Supplementary Material
Acknowledgments/Funding sources:
This study was funded by NIH grants F32AG063442 (CK); K01AG072044 (KCP); U01AG060908 (CK; BR). This study was supported in part by the Intramural Research Program of the National Institute on Aging, NIH. The InCHIANTI study was supported as a “targeted project” (ICS 110.1/RS97.71) by the Italian Ministry of Health and by the U.S. National Institute on Aging (contracts N01-AG-916413, N01-AG-5-0002, and N01-AG-821336, and grant R01-AG-027012). AMB was supported by the National Cancer Institute of the National Institutes of Health under Award Number K07CA225856. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest/Disclosure statement:
The Regents of the University of California is the sole owner of a patent application directed on the GrimAge clock and other epigenetic clocks invention for which Ake T. Lu and Steve Horvath are named inventors. Steve Horvath is a founder and paid consultant of the nonprofit Epigenetic Clock Development Foundation that licenses this patent. AMB is a scientific advisor to the Epigenetic Clock Development Foundation.
Bibliography
- 1.Baggio G, Corsini A, Floreani A, Giannini S, Zagonel V. Gender medicine: a task for the third millennium. Clin Chem Lab Med. 2013;51(4):713–727. doi: 10.1515/CCLM-2012-0849 [DOI] [PubMed] [Google Scholar]
- 2.Marais GABB, Gaillard J-M, Vieira C, et al. Sex gap in aging and longevity: can sex chromosomes play a role ? Biol Sex Differ. 2018;9(1):33. doi: 10.1186/s13293-018-0181-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sampathkumar NK, Bravo JI, Chen Y, et al. Widespread sex dimorphism in aging and age-related diseases. Hum Genet. 2020;139(3):333–356. doi: 10.1007/s00439-019-02082-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blenck CL, Harvey PA, Reckelhoff JF, Leinwand LA. The importance of biological sex and estrogen in rodent models of cardiovascular health and disease. Circ Res. 2016;118(8):1294–1312. doi: 10.1161/CIRCRESAHA.116.307509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong Y, Xiao H, Li C, et al. Elevated t/e2 ratio is associated with an increased risk of cerebrovascular disease in elderly men. PLoS One. 2013;8(4). doi: 10.1371/JOURNAL.PONE.0061598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng HY, Li Y, Dai W, Wei CD, Sun KS, Tong YQ. Imbalance of testosterone/estradiol promotes male CHD development. Biomed Mater Eng. 2012;22(1–3):179–185. doi: 10.3233/BME-2012-0705 [DOI] [PubMed] [Google Scholar]
- 7.Cadegiani F, Luiz P, Da Silva H, Abrao TPC, Kater CE, Sathavarodom N. Reproductive Endocrinology. Male reproductive health- from hormones to gametes. The Testosterone-to-Estradiol ratio, rather than testosterone or estradiol alone, is a more precise marker of metabolic-related outcomes in males: insights from a systematic r. J Endocr Soc. 2020;4:A1157. doi: 10.1210/jendso/bvaa046 [DOI] [Google Scholar]
- 8.Lakshman KM, Bhasin S, Araujo AB. Sex hormone-binding globulin as an independent predictor of incident type 2 diabetes mellitus in men. Journals Gerontol - Ser A Biol Sci Med Sci. 2010;65 A(5):503–509. doi: 10.1093/gerona/glq002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas. 1995;21(2):103–113. doi: 10.1016/0378-5122(94)00869-9 [DOI] [PubMed] [Google Scholar]
- 10.Maggio M, Lauretani F, Basaria S, et al. Sex hormone binding globulin levels across the adult lifespan in women--the role of body mass index and fasting insulin. J Endocrinol Invest. 2008;31(7):597–601. doi: 10.1007/BF03345608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aribas E, Kavousi M, Laven JSE, Ikram MA, Roeters Van Lennep JE. Aging, Cardiovascular Risk, and SHBG Levels in Men and Women From the General Population. J Clin Endocrinol Metab. 2021;106(10):2890–2900. doi: 10.1210/CLINEM/DGAB470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabbri E, An Y, Gonzalez-Freire M, et al. Bioavailable testosterone linearly declines over a wide age spectrum in men and women from the Baltimore longitudinal study of aging. Journals Gerontol - Ser A Biol Sci Med Sci. 2016;71(9):1202–1209. doi: 10.1093/gerona/glw021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez A, Muller DC, Metter EJ, et al. Aging, androgens, and the metabolic syndrome in a longitudinal study of aging. J Clin Endocrinol Metab. 2007;92(9):3568–3572. doi: 10.1210/jc.2006-2764 [DOI] [PubMed] [Google Scholar]
- 14.Maggio M, Lauretani F, Ceda GP, et al. Estradiol and metabolic syndrome in older Italian men: The InCHIANTI study. J Androl. 2010;31(2):155–162. doi: 10.2164/jandrol.108.006098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decaroli MC, Rochira V. Aging and sex hormones in males. Virulence. 2017;8(5):545. doi: 10.1080/21505594.2016.1259053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59(3):551–555. doi: 10.1210/JCEM-59-3-551 [DOI] [PubMed] [Google Scholar]
- 17.Nafziger AN, Bowlin SJ, Jenkins PL, Pearson TA. Longitudinal changes in dehydroepiandrosterone concentrations in men and women. J Lab Clin Med. 1998;131(4):316–323. doi: 10.1016/S0022-2143(98)90181-0 [DOI] [PubMed] [Google Scholar]
- 18.Valenti G, Denti L, Maggio M, et al. Effect of DHEAS on skeletal muscle over the life span: The InCHIANTI study. Journals Gerontol - Ser A Biol Sci Med Sci. 2004;59(5):466–472. doi: 10.1093/gerona/59.5.m466 [DOI] [PubMed] [Google Scholar]
- 19.Garratt M, Lagerborg KA, Tsai YM, Galecki A, Jain M, Miller RA. Male lifespan extension with 17-α estradiol is linked to a sex-specific metabolomic response modulated by gonadal hormones in mice. Aging Cell. 2018;17(4):e12786. doi: 10.1111/acel.12786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strong R, Miller RA, Antebi A, et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15(5):872–884. doi: 10.1111/acel.12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison DE, Strong R, Allison DB, et al. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13(2):273–282. doi: 10.1111/acel.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abi-Ghanem C, Robison LS, Zuloaga KL. Androgens’ effects on cerebrovascular function in health and disease. Biol Sex Differ. 2020;11(1). doi: 10.1186/s13293-020-00309-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bianchi VE, Bresciani E, Meanti R, Rizzi L, Omeljaniuk RJ, Torsello A. The role of androgens in women’s health and wellbeing. Pharmacol Res. 2021;171. doi: 10.1016/J.PHRS.2021.105758 [DOI] [PubMed] [Google Scholar]
- 24.Helle S, Lummaa V, Jokela J. Are reproductive and somatic senescence coupled in humans? Late, but not early, reproduction correlated with longevity in historical Sami women. Proc R Soc B Biol Sci. 2005;272(1558):29–37. doi: 10.1098/rspb.2004.2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doblhammer G, Oeppen J. Reproduction and longevity among the British peerage: The effect of frailty and health selection. Proc R Soc B Biol Sci. 2003;270(1524):1541–1547. doi: 10.1098/rspb.2003.2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larke A, Crews DE. Parental investment, late reproduction, and increased reserve capacity are associated with longevity in humans. J Physiol Anthropol. 2006;25(1):119–131. doi: 10.2114/jpa2.25.119 [DOI] [PubMed] [Google Scholar]
- 27.Shadyab AH, LaMonte MJ, Kooperberg C, et al. Leisure-time physical activity and leukocyte telomere length among older women. Exp Gerontol. 2017;95:141–147. doi: 10.1016/j.exger.2017.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Liu Y, Sun X, et al. Age at menarche and risk of all-cause and cardiovascular mortality: A systematic review and dose-response meta-analysis. Menopause. 2019;26(6):670–676. doi: 10.1097/GME.0000000000001289 [DOI] [PubMed] [Google Scholar]
- 29.Charalampopoulos D, McLoughlin A, Elks CE, Ong KK. Age at menarche and risks of all-cause and cardiovascular death: A systematic review and meta-analysis. Am J Epidemiol. 2014;180(1):29–40. doi: 10.1093/aje/kwu113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mishra SR, Chung HF, Waller M, et al. Association between Reproductive Life Span and Incident Nonfatal Cardiovascular Disease: A Pooled Analysis of Individual Patient Data from 12 Studies. JAMA Cardiol. 2020;5(12):1410–1418. doi: 10.1001/jamacardio.2020.4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okoth K, Chandan JS, Marshall T, et al. Association between the reproductive health of young women and cardiovascular disease in later life: Umbrella review. BMJ. 2020;371. doi: 10.1136/bmj.m3502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maggio M, Ceda G, Lauretani F, et al. Relationship between higher estradiol levels and 9-year mortality in older women: the Invecchiare in Chianti study. J Am Geriatr Soc. 2009;57(10):1810–1815. doi: 10.1111/J.1532-5415.2009.02464.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018;10(4):573–591. doi: 10.18632/aging.101414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marioni RE, Shah S, McRae AF, et al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015;16(1):25. doi: 10.1186/s13059-015-0584-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu AT, Quach A, Wilson JG, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 2019;11(2):303–327. doi: 10.18632/aging.101684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horvath S, Pirazzini C, Bacalini MG, et al. Decreased epigenetic age of PBMCs from Italian semi-supercentenarians and their offspring. Aging (Albany NY). 2015;7(12):1159–1170. doi: 10.18632/aging.100861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine ME, Lu AT, Chen BH, et al. Menopause accelerates biological aging. Proc Natl Acad Sci. 2016;113(33):9327–9332. doi: 10.1073/pnas.1604558113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryan CP, Hayes MG, Lee NR, et al. Reproduction predicts shorter telomeres and epigenetic age acceleration among young adult women. Sci Rep. 2018;8(1). doi: 10.1038/s41598-018-29486-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morin SJ, Tao X, Marin D, et al. DNA methylation-based age prediction and telomere length in white blood cells and cumulus cells of infertile women with normal or poor response to ovarian stimulation. Aging (Albany NY). 2018;10(12):3761–3773. doi: 10.18632/aging.101670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monseur B, Murugappan G, Bentley J, Teng N, Westphal L. Epigenetic clock measuring age acceleration via DNA methylation levels in blood is associated with decreased oocyte yield. J Assist Reprod Genet. 2020;37(5):1097–1103. doi: 10.1007/s10815-020-01763-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levine JA, Oleaga C, Eren M, et al. Role of PAI-1 in hepatic steatosis and dyslipidemia. Sci Reports 2021 111. 2021;11(1):1–13. doi: 10.1038/s41598-020-79948-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katsiki N, Mikhailidis DP, Banach M. Leptin, cardiovascular diseases and type 2 diabetes mellitus review-article. Acta Pharmacol Sin. 2018;39(7):1176–1188. doi: 10.1038/aps.2018.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghadge AA, Khaire AA. Leptin as a predictive marker for metabolic syndrome. Cytokine. 2019;121. doi: 10.1016/j.cyto.2019.154735 [DOI] [PubMed] [Google Scholar]
- 44.Raiko JRH, Oikonen M, Wendelin-Saarenhovi M, et al. Plasminogen activator inhitor-1 associates with cardiovascular risk factors in healthy young adults in the Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2012;224(1):208–212. doi: 10.1016/j.atherosclerosis.2012.06.062 [DOI] [PubMed] [Google Scholar]
- 45.Sillen M, Declerck PJ. A narrative review on plasminogen activator inhibitor-1 and its (Patho)physiological role: To target or not to target? Int J Mol Sci. 2021;22(5):1–16. doi: 10.3390/ijms22052721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan SS, Shah SJ, Klyachko E, et al. A null mutation in SERPINE1 protects against biological aging in humans. Sci Adv. 2017;3(11). doi: 10.1126/SCIADV.AAO1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang XC, Jing TY, Resnick LM, Phillips GB. Relation of hemostatic risk factors to other risk factors for coronary heart disease and to sex hormones in men. Arterioscler Thromb. 1993;13(4):467–471. doi: 10.1161/01.atv.13.4.467 [DOI] [PubMed] [Google Scholar]
- 48.De Pergola G, De Mitrio V, Sciaraffia M, et al. Lower androgenicity is associated with higher plasma levels of prothrombotic factors irrespective of age, obesity, body fat distribution, and related metabolic parameters in men. Metabolism. 1997;46(11):1287–1293. doi: 10.1016/S0026-0495(97)90232-8 [DOI] [PubMed] [Google Scholar]
- 49.Williams MS, Cushman M, Ouyang P, Heckbert SR, Kalyani RR, Vaidya D. Association of serum sex hormones with hemostatic factors in women on and off hormone therapy: The multiethnic study of atherosclerosis. J Women’s Heal. 2016;25(2):166–172. doi: 10.1089/jwh.2015.5465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sowers MR, Jannausch M, Randolph JF, et al. Androgens are associated with hemostatic and inflammatory factors among women at the Mid-life. J Clin Endocrinol Metab. 2005;90(11):6064–6071. [DOI] [PubMed] [Google Scholar]
- 51.Obradovic M, Sudar-Milovanovic E, Soskic S, et al. Leptin and Obesity: Role and Clinical Implication. Front Endocrinol (Lausanne). 2021;12:563. doi: 10.3389/FENDO.2021.585887/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghosh-Swaby OR, Reichelt AC, Sheppard PAS, Davies J, Bussey TJ, Saksida LM. Metabolic hormones mediate cognition. Front Neuroendocrinol. 2022;66. doi: 10.1016/J.YFRNE.2022.101009 [DOI] [PubMed] [Google Scholar]
- 53.Stieg MR, Sievers C, Farr O, Stalla GK, Mantzoros CS. Leptin: A hormone linking activation of neuroendocrine axes with neuropathology. Psychoneuroendocrinology. 2015;51:47–57. doi: 10.1016/J.PSYNEUEN.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 54.Castracane VD, Kraemer GR, Ogden BW, Kraemer RR. Interrelationships of serum estradiol, estrone, and estrone sulfate, adiposity, biochemical bone markers, and leptin in post-menopausal women. Maturitas. 2006;53(2):217–225. doi: 10.1016/j.maturitas.2005.04.007 [DOI] [PubMed] [Google Scholar]
- 55.Alexander C, Cochran CJ, Gallicchio L, Miller SR, Flaws JA, Zacur H. Serum leptin levels, hormone levels, and hot flashes in midlife women. Fertil Steril. 2010;94(3):1037–1043. doi: 10.1016/j.fertnstert.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wildman RP, Wang D, Fernandez I, et al. Associations of testosterone and sex hormone binding globulin with adipose tissue hormones in midlife women. Obesity. 2013;21(3):629–636. doi: 10.1002/oby.20256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Isidori AM, Strollo F, Moré M, et al. Leptin and aging: Correlation with endocrine changes in male and female healthy adult populations of different body weights. J Clin Endocrinol Metab. 2000;85(5):1954–1962. doi: 10.1210/jcem.85.5.6572 [DOI] [PubMed] [Google Scholar]
- 58.Seyfart T, Friedrich N, Kische H, et al. Association of sex hormones with physical, laboratory, and imaging markers of anthropometry in men and women from the general population. PLoS One. 2018;13(1). doi: 10.1371/journal.pone.0189042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hetemäki N, Savolainen-Peltonen H, Tikkanen MJ, et al. Estrogen Metabolism in Abdominal Subcutaneous and Visceral Adipose Tissue in Postmenopausal Women. J Clin Endocrinol Metab. 2017;102(12):4588–4595. doi: 10.1210/JC.2017-01474 [DOI] [PubMed] [Google Scholar]
- 60.Behre HM, Simoni M, Nieschlag E. Strong association between serum levels of leptin and testosterone in men. Clin Endocrinol (Oxf). 1997;47(2):237–240. doi: 10.1046/j.1365-2265.1997.2681067.x [DOI] [PubMed] [Google Scholar]
- 61.Liu C-CC, Huang S-PP, Cheng K-HH, et al. Lower SHBG Level Is Associated with Higher Leptin and Lower Adiponectin Levels as Well as Metabolic Syndrome, Independent of Testosterone. Vol 7.; 2017. doi: 10.1038/s41598-017-03078-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lagiou P, Signorello LB, Mantzoros CS, Trichopoulos D, Hsieh CC, Trichopoulou A. Hormonal, lifestyle, and dietary factors in relation to leptin among elderly men. Ann Nutr Metab. 1999;43(1):23–29. doi: 10.1159/000012763 [DOI] [PubMed] [Google Scholar]
- 63.Bae YJ, Zeidler R, Baber R, et al. Reference intervals of nine steroid hormones over the life-span analyzed by LC-MS/MS: Effect of age, gender, puberty, and oral contraceptives. J Steroid Biochem Mol Biol. 2019;193(May). doi: 10.1016/j.jsbmb.2019.105409 [DOI] [PubMed] [Google Scholar]
- 64.Milewicz A, Miazgowski T, Arkowska A, Mieszczanowicz U, Bar-andziak E. The reference values of sex hormones and SHBG serum levels in subjects over 65 years old — The PolSenior Study Wartości referencyjne stężenia hormonów płciowych oraz SHBG w surowicy. Endokrynol Pol. 2013;64(2). [PubMed] [Google Scholar]
- 65.Reference levels sex hormone Mayo clinic. https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/84230.
- 66.Södergard R, Bäckström T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17β to human plasma proteins at body temperature. J Steroid Biochem. 1982;16(6):801–810. doi: 10.1016/0022-4731(82)90038-3 [DOI] [PubMed] [Google Scholar]
- 67.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. doi: 10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Belsky DW, Caspi A, Arseneault L, et al. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. Elife. 2020;9:1–56. doi: 10.7554/ELIFE.54870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Horvath S, Gurven M, Levine ME, et al. An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol. 2016;17(1):0–22. doi: 10.1186/s13059-016-1030-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hannum G, Guinney J, Zhao L, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49(2):359–367. doi: 10.1016/j.molcel.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cesari M, Pahor M, Incalzi RA. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc Ther. 2010;28(5). doi: 10.1111/J.1755-5922.2010.00171.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Conole ELS, Stevenson AJ, Maniega SM, et al. DNA Methylation and Protein Markers of Chronic Inflammation and Their Associations With Brain and Cognitive Aging. Neurology. 2021;97(23):e2340–e2352. doi: 10.1212/WNL.0000000000012997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stevenson AJ, Gadd DA, Hillary RF, et al. Creating and Validating a DNA Methylation-Based Proxy for Interleukin-6. Journals Gerontol Ser A. 2021;76(12):2284–2292. doi: 10.1093/GERONA/GLAB046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gadd DA, Hillary RF, McCartney DL, et al. Epigenetic scores for the circulating proteome as tools for disease prediction. Elife. 2022;11:15. doi: 10.7554/ELIFE.71802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moreau KL, Babcock MC, Hildreth KL. Sex differences in vascular aging in response to testosterone. Biol Sex Differ. 2020;11(1):1–14. doi: 10.1186/s13293-020-00294-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cadegiani F, Luiz P, Da Silva H, Abrao TPC, Kater CE, Sathavarodom N. The Testosterone-To-Estradiol Ratio, Rather Than Testosterone or Estradiol Alone, Is a More Precise Marker of Metabolic-Related Outcomes in Males: Insights From a Systematic Review. J Endocr Soc. 2020;4(Supplement_1). doi: 10.1210/JENDSO/BVAA046.2293 [DOI] [Google Scholar]
- 77.Haffner SM, Katz MS, Stern MP, Dunn JF. The relationship of sex hormones to hyperinsulinemia and hyperglycemia. Metabolism. 1988;37(7):683–688. doi: 10.1016/0026-0495(88)90091-1 [DOI] [PubMed] [Google Scholar]
- 78.Bhasin S, Jasjua GK, Pencina M, et al. Sex hormone-binding globulin, but not testosterone, is associated prospectively and independently with incident metabolic syndrome in men: The framingham heart study. Diabetes Care. 2011;34(11):2464–2470. doi: 10.2337/dc11-0888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosner W, Hryb DJ, Khan MS, Nakhla AM, Romas NA. Androgens, estrogens, and second messengers. In: Steroids. Vol 63. Steroids; 1998:278–281. doi: 10.1016/S0039-128X(98)00017-8 [DOI] [PubMed] [Google Scholar]
- 80.Wiman B. Plasminogen activator inhibitor 1 (PAI-1) in plasma: Its role in thrombotic disease. In: Thrombosis and Haemostasis. Vol 74. Schattauer GmbH; 1995:71–76. doi: 10.1055/s-0038-1642655 [DOI] [PubMed] [Google Scholar]
- 81.Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A reappraisal of testosterone’s binding in circulation: Physiological and clinical implications. Endocr Rev. 2017;38(4):302–324. doi: 10.1210/ER.2017-00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arathimos R, Millard LACC, Bell JA, Relton CL, Suderman M. Impact of sex hormone-binding globulin on the human phenome. Hum Mol Genet. 2020;29(11):1824–1832. doi: 10.1093/hmg/ddz269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA - J Am Med Assoc. 2013;310(17):1829–1836. doi: 10.1001/jama.2013.280386 [DOI] [PubMed] [Google Scholar]
- 84.Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9(1). doi: 10.1371/journal.pone.0085805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McCarthy M. Testosterone therapy is associated with raised risk of myocardial infarction, US study finds. BMJ. 2014;348. doi: 10.1136/bmj.g1297 [DOI] [PubMed] [Google Scholar]
- 86.Budoff MJ, Ellenberg SS, Lewis CE, et al. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA - J Am Med Assoc. 2017;317(7):708–716. doi: 10.1001/jama.2016.21043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: A systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11(1). doi: 10.1186/1741-7015-11-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gyawali P, Martin SA, Heilbronn LK, et al. Higher Serum Sex Hormone-Binding Globulin Levels Are Associated with Incident Cardiovascular Disease in Men. J Clin Endocrinol Metab. 2019;104(12):6301–6315. doi: 10.1210/jc.2019-01317 [DOI] [PubMed] [Google Scholar]
- 89.Penn CA, Chan J, Mesaros C, et al. Association of serum androgens and coronary artery calcium scores in women. Fertil Steril. 2019;112(3):586–593. doi: 10.1016/j.fertnstert.2019.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li H, Mitchell L, Zhang X, Heiselman D, Motsko S. Testosterone Therapy and Risk of Acute Myocardial Infarction in Hypogonadal Men: An Administrative Health Care Claims Study. In: Journal of Sexual Medicine. Vol 14. Elsevier B.V.; 2017:1307–1317. doi: 10.1016/j.jsxm.2017.09.010 [DOI] [PubMed] [Google Scholar]
- 91.Basaria S, Harman SM, Travison TG, et al. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with lowor low-normal testosterone levels: A randomized clinical trial. JAMA - J Am Med Assoc. 2015;314(6):570–581. doi: 10.1001/jama.2015.8881 [DOI] [PubMed] [Google Scholar]
- 92.Hildreth KL, Barry DW, Moreau KL, et al. Effects of testosterone and progressive resistance exercise in healthy, highly functioning older men with low-normal testosterone levels. J Clin Endocrinol Metab. 2013;98(5):1891–1900. doi: 10.1210/jc.2012-3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yeap BB, Alfonso H, Paul Chubb SA, et al. In older men an optimal plasma testosterone is associated with reduced all-cause mortality and higher dihydrotestosterone with reduced ischemic heart disease mortality, while estradiol levels do not predict mortality. J Clin Endocrinol Metab. 2014;99(1). doi: 10.1210/jc.2013-3272 [DOI] [PubMed] [Google Scholar]
- 94.Haddad RM, Kennedy CC, Caples SM, et al. Testosterone and cardiovascular risk in men: A systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82(1):29–39. doi: 10.4065/82.1.29 [DOI] [PubMed] [Google Scholar]
- 95.Belladelli F, Del Giudice F, Kasman A, Salonia A, Eisenberg ML. The Association between Testosterone, Estradiol and Their Ratio and Mortality among US Men. Vol 53. Blackwell Publishing Ltd; 2021. doi: 10.1111/and.13993 [DOI] [PubMed] [Google Scholar]
- 96.Wang J, Fan X, Yang M, et al. Sex-specific associations of circulating testosterone levels with all-cause and cause-specific mortality. Eur J Endocrinol. 2021;184(5):723–732. doi: 10.1530/EJE-20-1253 [DOI] [PubMed] [Google Scholar]
- 97.Houseman EA, Accomando WP, Koestler DC, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics. 2012;13(1):86. doi: 10.1186/1471-2105-13-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.