Abstract
Epidural stimulation of the motor cortex (eMCS) was devised in the 1990’s, and has now largely supplanted thalamic stimulation for neuropathic pain relief. Its mechanisms of action involve activation of multiple cortico-subcortical areas initiated in the thalamus, with involvement of endogenous opioids and descending inhibition toward the spinal cord. Evidence for clinical efficacy is now supported by at least seven RCTs; benefits may persist up to 10 years, and can be reasonably predicted by preoperative use of non-invasive repetitive magnetic stimulation (rTMS). rTMS first developed as a means of predicting the efficacy of epidural procedures, then as an analgesic method on its own right. Reasonable evidence from at least six well-conducted RCTs favors a significant analgesic effect of high-frequency rTMS of the motor cortex in neuropathic pain (NP), and less consistently in widespread/fibromyalgic pain. Stimulation of the dorsolateral frontal cortex (DLPFC) has not proven efficacious for pain, so far. The posterior operculo-insular cortex is a new and attractive target but evidence remains inconsistent. Transcranial direct current stimulation (tDCS) is applied upon similar targets as rTMS and eMCS; it does not elicit action potentials but modulates the neuronal resting membrane state. tDCS presents practical advantages including low cost, few safety issues, and possibility of home-based protocols; however, the limited quality of most published reports entails a low level of evidence. Patients responsive to tDCS may differ from those improved by rTMS, and in both cases repeated sessions over a long time may be required to achieve clinically significant relief. Both invasive and non-invasive procedures exert their effects through multiple distributed brain networks influencing the sensory, affective and cognitive aspects of chronic pain. Their effects are mainly exerted upon abnormally sensitized pathways, rather than on acute physiological pain. Extending the duration of long-term benefits remains a challenge, for which different strategies are discussed in this review.
Key words: Transcutaneous electric nerve stimulation, Pain, Motor cortex, Transcranial magnetic stimulation, Neuralgia
Stimulating the motor cortex for pain relief: historical background
Reports on possible descending nociceptive controls activated by stimulation of the motor cortex date back to the middle of the last Century. In 1957, Lindblom et al.1 described in cats the inhibition of dorsal horn spinal neurons during electrical stimulation of the pyramidal tract or motor cortex, and very shortly after it was suggested that this effect involved descending presynaptic inhibition.2 Early attempts to apply these discoveries to the control of pain in humans via stimulation of the internal capsule obtained a relative success3-5 but were discontinued due to surgical-related morbidity. In 1991, Tsubokawa et al.6 described a relatively simple and safe technique of motor cortex stimulation using epidural plate electrodes (eMCS), and succeeded in alleviating central post-stroke pain in eight of 12 patients with thalamic or supra-thalamic lesions, with one year follow-up. The epidural technique was swiftly adopted by different neurosurgeons around the world, and applied to different neuropathic pain (NP) conditions.7-9 In parallel, non-invasive modes of cortical stimulation were rapidly developed, first with the aim of optimizing the selection of candidates to the epidural procedure, then gradually as analgesic techniques in their own right. In what follows, we will discuss the progression from anecdotal reports to evidence-based data, in relation to both neurosurgical approaches and non-invasive techniques.
Neurosurgically-implanted motor cortex stimulation (eMCS)
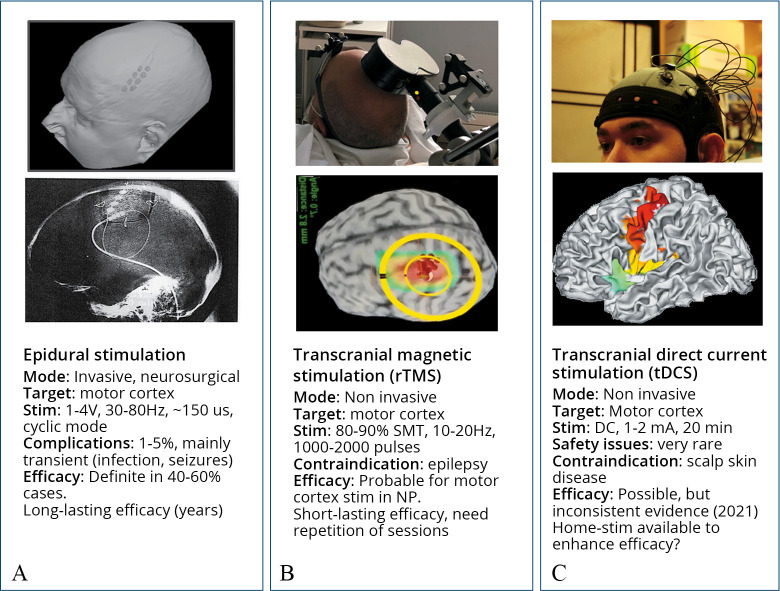
Although the stimulating devices and localization techniques have considerably evolved, the surgical procedure remains largely comparable to that described by Tsubokawa 30 years ago.6, 10 After anesthesia and craniotomy, the stimulating electrodes are placed overlying the motor strip contralateral to the pain side, either parallel or orthogonal to and crossing the central sulcus (Figure 1A).
Figure 1.
—Summary of key-features of epidural, transcranial magnetic and direct-current procedures for cortical stimulation, as currently used in the treatment of chronic pain.
The electrode wires are then tunneled under the skin down to the lateral neck and connected to the system antenna (a receiver activated by external radiofrequencies) in sub-clavicular or latero-abdominal position. Somatotopic concordance between the electrode position and the painful territory appears important for clinical effect,6, 11, 12 therefore the stimulating electrodes are placed over the cortical convexity for upper limb/facial pain, and over its medial aspect for lower limb pain. Epidural is preferred to subdural position because of shorter operative time, and because subdural stimulation increases the risk of epileptic seizures and intracranial haematoma13-16 without evidence of better results.17 However, the subdural position is still preferred by some teams16 and may be useful when the electrode needs to be positioned in the interhemispheric fissure.
The localization of the central sulcus is performed using MRI-based neuronavigation and neurophysiological testing with somatosensory evoked potentials, and its position compared with that suggested by MRI neuronavigation. Recording of motor responses can be also performed to ensure the optimal position of the electrodes. Correct determination of the Rolandic sulcus is crucial to avoid stimulation over the somatosensory cortex, which may enhance painful symptoms.10, 18 Stimulation frequency is commonly set at 30–80 Hz, amplitude at 80% of the motor threshold to avoid contractions and seizures, and one week of testing under hospitalization is required to optimize the stimulation parameters.
Mechanisms of action of eMCS
Studies in humans
Early hypotheses linking eMCS effects to activation of cortico-cortical fibers or interneurons within the motor cortex10, 15, 19 were contradicted by the lack of metabolic changes within the motor cortex underneath the stimulating electrodes.18, 20-23 Instead, a prominent metabolic enhancement during eMCS is found within the thalamus ipsilateral to the stimulated cortex,18, 21, 23-25 and occasionally contralateral to it,26 suggesting descending cortico-thalamic activation.27, 28
Thalamic activation is followed by activity changes in numerous cortico-subcortical areas, including the premotor, prefrontal and orbitofrontal cortices, perigenual cingulate, basal ganglia and periaqueductal grey matter (PAG).23 High-order areas are thought to modulate the affective/motivational appraisal of pain, while activation of the PAG can trigger descending inhibition toward the spinal cord, and explain the attenuation of spinal nociceptive reflexes.21 Most of these structures remain activated for hours after eMCS is discontinued,20 which may explain the persistence of clinical effects beyond the stimulation periods. Long-lasting changes in neurotransmitters were also demonstrated using PET-scan, with potential secretion of endogenous opioids and a positive correlation between opioid receptors availability and clinical efficacy.29, 30 Since endo-opioidergic changes and local CBF increase involved the same regions, both mechanisms may be related and concur to eMCS efficacy.
Studies in animals
MCS consistently alleviates neuropathic hypersensitivity in rodents and cats,31-39 and also tonic pain in one study.40 Most animal studies have confirmed the general mechanistic hypotheses driven from human data, with functional changes being reported in thalamus, cingulate, striatum, PAG and dorsal horn.41 In convergence with human studies, descending inhibition and early thalamic involvement are the most reproducible results obtained in animals. Abnormal thalamic bursting during neuropathic pain is associated with a hypometabolic state,42, 43 and MCS can both decrease thalamic bursting and increase thalamic metabolism.34, 36, 44 These effects may be driven in part by a GABAergic pathway from the subthalamic Zona incerta.33, 37, 38 Spread of thalamic activation and of c-Fos expression changes progressively appear in rodents after chronic stimulation,45, 46 suggesting a time-dependent neural plasticity that may contribute to long-term efficacy.
MCS-related descending inhibition in rodents and cats is reflected by depressed activity in the superficial dorsal horn together with enhanced c-Fos reactivity in ACC and PAG.2, 34, 36, 47, 48 It may involve different neurotransmitters and receptors, including endogenous opioids,49, 50 catecholamines,51 serotonin and its spinal 5-HT1A receptor,32, 36, 52 dopamine through its D2 receptor,53 and cannabinoids via the CB2 receptor.50 Contradictory results also exist, including reports of either decrease or increase of GABAergic activity in the PAG,39, 44 involvement versus lack of involvement of glutamate signaling in the same region,39, 54 and activation versus lack of activation of locus coeruleus during MCS.32, 52
Clinical efficacy of epidural MCS
Loss of eMCS efficacy following battery exhaustion or broken wires were helpful anecdotes to establish confidence in the technique,18, 55, 56 but definite clinical efficacy can only be established from controlled studies (RCTs). Pooled data from the literature indicates a 45-50% average success rate of eMCS in patients with drug-resistant, central or peripheral NP.57-59 However, most clinical reports are subject to multiple bias such as lack of blinding, small sample size, heterogeneity of assessment tools, imprecision in reporting, and limited follow-up, which makes the evidence methodologically weak.60 Very few randomized or blinded studies with >10 patients have been reported. While one of them was halted because of limited efficacy and adverse events (infections, panic attack),61 six other trials reported positive results: Rasche et al.62 and André-Obadia et al.63 used blinded procedures to detect responders (>50% pain decrease or >30% decrease plus medication reduction), and reported good results in 47-50% of a total of 37 operated patients, with up to 10 years’ follow-up. Other groups reported 40-60% success rate in randomized cross-over trials, with reversible pain increase when the stimulator was turned “off” and “on” in double-blinded conditions.64-67 Even allowing for some decline of efficacy with time,17, 63 data obtained under blinded and randomized conditions support the real efficacy of eMCS in a rough half of the patients with drug-resistant NP.
eMCS often displays delayed and fluctuating effects which can be underestimated in randomised trials. In one series, almost 20% of patients were considered as non-responders during the first month, but were relieved at 1 year.66 Also, a number of patients with “insignificant” VAS changes noted an increase in pain after battery depletion and requested replacement of their device,61 or declared themselves favorable to re-intervention for the same outcome.63, 68 These discordances, which have been also reported for spinal cord stimulation,69 suggest that high values and preferences for neuromodulation therapy may be dissociated from quantitative VAS scales.
Prediction of eMCS clinical effects
Demographic, clinical, anatomical or pharmacological pre-operative data have not proven useful so far to predict the long-term efficacy of eMCS.68, 70 Suggested but unconfirmed predictors include preservation of corticospinal function,71 normal thermal thresholds,72 relief of burning pain,73 stimulation intensity,74 susceptibility to ketamine,75 and availability of brain opioid receptors.30 Good efficacy at first month post-implantation predicted long-term efficacy in two independent studies.68, 76 Small sample size, heterogeneity of evaluation methods and short follow-up probably explain the lack of reproducibility of such putative predictors, some of which might be confirmed in the future.
The only procedure consistently predicting the eMCS clinical effect is the response to transcranial repetitive magnetic stimulation (rTMS). A successful rTMS predicted subsequent efficacy of eMCS with ~90% accuracy.17, 63, 70, 77-80 Although its negative predictive value is lesser, it increases with the length of follow-up and may approach 70% at 2 or more years.63, 70 Since in most previous reports the patients were operated without consideration of predicting procedures, the clinical effect size of eMCS should increase significantly with a better selection of patients via preoperative rTMS assessment.
Take-home messages: eMCS
Implanted epidural stimulation is the original neurosurgical technique for motor cortex stimulation, which has now largely supplanted thalamic procedures. Its mechanisms of action involve activation of multiple cortico-subcortical areas, secretion of endogenous opioids, and descending inhibition toward the spinal cord. Clinical efficacy, including “on-off” effects, is supported by at least seven RCTs in more than 100 operated patients. Efficacy can persist up to 10 years, and can be reasonably predicted by preoperative use of rTMS.
Transcranial magnetic repetitive stimulation (rTMS)
High-voltage electrical pulses applied to the scalp can activate the motor cortex and thus mimic eMCS, but they are also very painful. This difficulty can be surmounted by activating the motor cortex via magnetic pulses. Short-lasting currents in a coil applied on the scalp (1000 A, 1 ms) create a magnetic field of ~1 T, which painlessly generates a secondary current in the brain via electromagnetic induction, according to Faraday’s law. This secondary current has a magnitude similar to that used in direct cortical studies, and allows activation of the underlying motor cortex. These non-invasive techniques were initially intended to predict the effectiveness of epidural procedures, but their potential value as a pain therapy in their own right was soon envisaged. “Figure-of-eight” coils ensure precise millimetric cortical stimulation and are most widely used,81, 82 and the technical settings to implement rTMS in clinical practice are discussed in Lefaucheur and Nguyen.82
rTMS mechanisms
Although rTMS stimulates cortical interneurons83, 84 it remains unclear whether it entails sizeable changes in intracortical motor circuits under the conditions used to treat pain. Indeed, rTMS for pain relief threshold is applied at lower levels than the motor threshold, in conditions where local motor metabolic activation subsides or disappears.85-87 Although a correlation was initially reported between intracortical motor inhibition (ICI) and rTMS-induced pain relief,88 later studies failed to reproduce such effects,89 perhaps because ICI changes in chronic pain depend on non-pain pathways.90 Also, rTMS effects could be blocked pharmacologically in the absence of cortical excitability changes91 and GABAergic drugs that modify intracortical inhibition92 are not effective in neuropathic pain. Cortical motor inhibition appeared unrelated to pain relief in post-amputation or spinal cord injury pain,93, 94 and in general the relevance of motor cortex excitability for rTMS analgesic effects remains largely unconfirmed.95, 96
In contrast, the activation of structures distant from the motor cortex has received consistent support. Subthreshold rTMS activates multiple areas that overlap the network activated during epidural MCS,23 including the anterior cingulate (ACC), operculo-insular and dorsolateral prefrontal cortices (DLPFC), striatum and brainstem.85-87 rTMS-induced input into the insula, operculum and ACC has been suggested by causal modelling studies97 and enhancement of functional connectivity between these areas after rTMS has been shown in both human patients98 and a nonhuman primate model of central pain.99 rTMS in rats and mice also induced c-fos neural activation in regions distant from the stimulation including thalamus, ACC, striatum and hippocampus.100-102 The clinical relevance of such multifocal changes is supported by the predictive value of preserved thalamo-cortical and corticofugal motor tracts on rTMS clinical effects.103, 104
Contribution of endogenous opioids to these effects is supported by enhancement of serum beta-endorphin after successful rTMS,105 naloxone blockade of rTMS analgesia,106 and rTMS-induced increase in opioid receptor occupancy.107 Enhanced dopamine striatal secretion was also described in rodents108 and some human PET-scan studies109 but not in others.107 Since stimulus-related dopamine release returns rapidly to baseline, its secretion may be too short-lasting to account for clinical effects, but could have an indirect effect by its synergy with opioid-related activity. The potential contribution of NMDA glutamate receptors has also received indirect support from two studies in humans,91, 110 while in rats rTMS effects were not blocked by NMDA antagonists.101
rTMS appears to influence abnormally hyperactive states, rather than physiological pain. Markers of acute nociception such as heat-pain detection and pain-evoked potentials, did not change after rTMS in healthy subjects,107, 111-113 although cold pain was reported to be attenuated.106, 114 Contrary to epidural stimulation, rTMS has not been shown to attenuate spinal nociceptive reflexes.114, 115 This suggests a superior capacity of implanted eMCS to trigger descending mechanisms influencing spinal nociception, and would be in accordance with the enhanced degree of pain relief achieved with eMCS, relative to non-invasive procedures.63, 79, 80
rTMS as a predictive factor of epidural MCS
The suggestion that rTMS could be predictive of eMCS efficacy came very shortly after the first neurosurgical reports, but controlled studies on this matter were not available until 10 years later.78, 79 Cumulative evidence from seven studies in 150 patients consistently indicates that a positive result of preoperative rTMS may be associated in ~90% of cases with satisfactory pain relief after epidural implantation.17, 63, 70, 77-80 The negative predictive value, i.e. the probability that eMCS fails if rTMS is negative, was low at 6-12 months (~30-40%78, 79), but increased with longer follow-ups to reach ~70% or more in studies with 2-10 years follow-up.63, 70, 80 A 70% chance of eMCS failure if preoperative rTMS is negative is often a reason to withhold operation.
rTMS as a pain-relieving procedure on its own right
A number of systematic reviews concluded to a statistical superiority of motor cortex rTMS relative to placebo to improve chronic neuropathic pain in the short or mid-term.116-122 Other assessments were however much less optimistic, in particular in regard to the low quality of evidence included in some reviews due to low patient samples, absent blinding, defective handling, lack of follow-up, no report on withdrawals, etc.60, 123, 124 In 2018, an influential Cochrane analysis examined the use of rTMS for chronic pain in 42 studies.123 While underscoring the multiple biases due to the above-mentioned flaws, it also recognized “low-quality evidence” of rTMS effects on chronic pain and quality of life up to 6 weeks post-intervention. Since this account, at least six large and well-conducted studies (single/double-blinded, >20 patients in active group) have been reported on motor rTMS in chronic NP, with positive results in all but one of them.96, 125-129 One further study reported significant effects in parkinsonian pain130 and another negative results in fibromyalgia.131 A recent report of the US Department of Veterans Affairs119 using a “best-evidence approach” concluded that rTMS may reduce symptoms in NP, while in fibromyalgia it may not be better than sham interventions. Although the level of evidence was (again) limited by methodological drawbacks, this influential report concluded that rTMS, which has fewer side effects compared to most approved pharmaceuticals for NP, “could be a treatment option for patients who have exhausted other available options for treatment of chronic pain.”119
Specific aspects of rTMS stimulation procedures.
Many practical details of rTMS remain controversial and are seldom analyzed specifically, although they can impact on the efficacy of rTMS procedures. Some of the effects of these variables are summarized in what follows, in the hope that they may serve to establish a minimum technical core set to be applied in a rehabilitation setting.
Stimulus frequency
Beneficial effects have been reported using “high frequencies” of 5, 10 or 20 Hz (commonly labelled “HF-rTMS”) while low frequencies of 0.5 or 1 Hz were found useless in both patients and animal models.78, 132, 133 Superiority of HF-rTMS was initially considered to depend on its ability to induce long-term potentiation (LTP) in the cortex; however, enhancement of LTP capacities with “theta burst” rTMS did not enhance analgesia.96, 134, 135 A potentially important feature of rTMS is its relation with the neuronal oscillations of the underlying cortex. The transmission efficiency of neural networks increases when the stimuli match their intrinsic oscillatory frequency.136, 137 Since rTMS synchronises oscillatory activity in the underlying cortex,138 and since human sensorimotor networks oscillate at around 10 and 20 Hz, this might underlie the superior efficacy of rTMS at these frequencies.
Stimulus intensity and number of pulses
Stimulus intensity is universally set at 80-90% of motor threshold; conversely, the optimal number of pulses per session has variously considered to be 1000,60 1200,88 or even 3000 pulses.82 One single comparative study reported that significant analgesia in NP was obtained with 2000 pulses, but not with 500.139 An excessive number of stimuli, however, may reverse the effects of rTMS in humans,140 and trigger allodynia in rodents,141 hence more than 3000 stimuli per session are not advised. Independent of the number of stimuli, shortening the stimulation time from 20 to 10 min was reported to decrease analgesia.142
Somatotopy
Somatotopic match between the cortical stimulus and the painful region may be critical for epidural MCS,12, 143 but appears much less relevant for rTMS. The pain-relieving effects of rTMS seem independent of any strict relation between pain location and rTMS placement over the motor homunculus.144, 145 In patients with facial or leg pains the stimulation of the hand area proved as efficacious, or better, than that of the somatotopically corresponding area.95, 139, 146, 147
Alternative cortical targets
So far, only stimulation of the primary motor area (M1) has received consensus as to its efficacy in neuropathic pain (much less consistently in widespread pain/fibromyalgia). Stimulation of the postcentral gyrus (S1), premotor area (preM), or supplementary motor area (SMA) did not provide effective pain relief in comparative studies,77, 148, 149 and rTMS over the posterior parietal cortex failed to perform beyond sham in experimental hyperalgesia.150
Stimulation of the left dorsolateral prefrontal cortex (DLPFC) has yielded controversial but overall disappointing results. Initial reports suggesting a decrease in postoperative morphine use151 were later contradicted in large-scale studies.152 Similarly, initially positive results of DLPFC stimulation in small series of NP patients153 failed to be confirmed in larger samples,129, 149 and had no effect on human models of neuropathic hyperalgesia.112 In fibromyalgia/widespread pain two studies reported positive results154, 155 while no difference from placebo was reported in four other studies of similar sample size.156-160 M1 also showed superiority over DLPFC stimulation in opioid-resistant back pain161 and peripheral neuropathic pain,129 and only one small-sample study suggested the reverse in non-specific back/neck pain.162 Recent systematic reviews have considered DLPFC rTMS as either ineffective versus sham,119, 123, 163 less effective than M1 stimulation121 or mildly effective in the short-term.164 Other forms of DLPFC stimulation (bilateral, low-frequency)165, 166 remain anecdotal and do not allow any conclusion. Caution is advised when dealing with high-order cortices such as DLPFC, whose stimulation may give rise to a wide array of unpredictable cognitive and emotional effects, including changes in sexual arousal or in craving for drugs.167-169
The posterior operculo-insular cortex may represent a unique area for pain modulation, as it receives a majority of ascending spinothalamic afferents in primates,170 but reports on its stimulation are scarce. One sham-controlled study involving 17 patients with visceral pain was reported positive,110 but has not been replicated. In neuropathic pain, positive results of S2 or posterior insula stimulation have been reported in small to medium-sized studies (15-31 patients),171-173 while no significant relief beyond sham was obtained in a larger RCT recruiting 98 patients with central pain.174 Despite its inherent relevance as a target, the multimodal nature of the insula makes this region more susceptible than M1 to adverse effects from stimulation. Two cases of epileptic seizures were reported during theta burst stimulation of the posterior operculo-insular cortex175 perhaps due to current spread toward the anterior insula.176
Timing and repetition of sessions
The pain-relieving effects of a single rTMS session develop 1-3 days after the stimulation and fade away in less than 10 days. Repeated sessions over 5-10 days allow expanding their effect to up to one month,82, 177 but this remains insufficient for chronic syndromes that persist for years. An initial series of 5-10 daily sessions followed by progressively spaced “maintenance” sessions achieved sustained efficacy for 6 months.129, 142, 178 Other groups reported long-lasting efficacy of rTMS sessions repeated at long intervals of 2-4 weeks, without the need of an initial series of daily stimulation,80, 125, 179-182 making of such “slow-pace” rTMS a potential avenue allowing long term efficacy with limited burden for patients and doctors. Solutions to implement rTMS at home are under study using modified coils adapted for home use. Although some preliminary data have been published182, 183 clinical systems are not yet operational to our knowledge.
Take-home messages: rTMS
rTMS can be used to predict the efficacy of implanted neurostimulation, but also as an analgesic procedure in its own right, with effects over both the sensory and affective pain domains. Reasonable evidence supports a significant analgesic effect of motor cortex HF-rTMS in neuropathic pain, and less consistently in widespread/fibromyalgic pain. Dorsolateral frontal stimulation has not proven efficacious so far, and the posterior operculo-insular cortex is an attractive target but evidence remains insufficient. rTMS acts preferentially upon abnormal hyperexcitable states rather than experimental pain. Short-term efficacy of rTMS in NP can be achieved with NNT ~2-3, but ensuring long-lasting efficacy remains a challenge.
Transcranial direct current stimulation (tDCS)
Direct current (galvanic) stimulation, i.e. a flow of electric charge that does not change direction, was empirically applied for medical purposes since the Roman antiquity,184 then used for research and therapy in psychiatry during the 19th to early 20th centuries, until it was abandoned with the advent of electroconvulsive therapy.185 Experimental studies in the second part of the 20th century showed that DC stimulation over the cortex influenced spontaneous neural firing in rodents and humans,186-188 with surface anodal polarization increasing spontaneous unit discharges, and cathodal polarization decreasing them.187, 189, 190 The use of transcranial DC stimulation appeared therefore as a promising tool to modulate cerebral excitability in a safe, painless, reversible and selective way, hence mimicking the analgesic effects of motor cortex stimulation.
Conventional tDCS procedures use a pair of surface electrodes (4 to 30 cm2) connected to a stimulator delivering electrical direct current at 1-2 mA (Figure 1). Higher focalization of stimulation (“High-definition” tDCS, or HD-tDCS) can be achieved with one electrode surrounded by four others of opposite polarity.191, 192
Mechanisms of action
Although tDCS modifies the excitability of the underlying cortex, it also induces widespread metabolic alterations much beyond local motor excitability. Indeed, activity changes in cortical and subcortical regions have been documented in humans during or following tDCS,193-195 and tDCS-induced analgesia was associated with distributed metabolic changes in a large array of brain areas,194 while it was dissociated from motor excitability.196-198 Changes in functional connectivity have been described between regions beneath the stimulation and distant areas including thalamus, striatum and parietal association cortices, but results are somewhat inconsistent and sometimes contradictory.199-201 Although the notion of distributed activity has received robust evidence, the precise causal relation between such changes and the clinical effects should be considered cautiously in view of the inconsistencies in different reports. At a difference from both eMCS and rTMS, distributed neural activation in tDCS may come not only from neural connections between brain areas, but also from the direct current spread in distant brain structures, in particular when using widely separated anode and cathode.202 In this respect, note that the terms “anodal” and “cathodal” do not capture the whole picture of tDCS, since both anodal and cathodal currents are in fact delivered,203 and the effects should be understood as a compound of both.
In humans, neurotransmitter studies have reported GABA and glutamate concentration changes as well as a possible secretion of endogenous opioids during tDCS, mainly in the insula and ACC.204, 205 In rodent models of neuropathic pain tDCS effects have been associated to virtually all neurotransmitter systems,206 but the literature in this domain is confusing, rarely reproduced and sometimes contradictory. There remain significant unknowns about the influence on biochemical and behavioural effects of many tDCS parameters, including polarity, number, size and position of electrodes, duration of stimulation, etc. As in other forms of neurostimulation, part of the effects from tDCS might involve descending inhibitory mechanisms, since tDCS decreased spinal nociceptive reflexes in models of experimental hyperalgesia207 and normalized BOLD responses in pain modulatory networks.208 Evidence for descending modulation was also reported after motor anodal tDCS on a rodent model of neuropathic pain.209
tDCS has often failed to decrease experimental pain in healthy individuals,191, 198, 210-212 whereas it reduced abnormal sensations (allodynia, hyperalgesia) induced by capsaicin,213 suggesting that the procedure acts on abnormally sensitized pathways, rather than on physiological pain.198 Similar conclusions have been proposed regarding rTMS.113 On these premises, experimental studies in healthy subjects should rather involve human models of neuropathic hyperalgesia (capsaicin, high-frequency stimulation, etc.) rather than simple acute physiological pain.214
tDCS clinical efficacy
From 2015 to date, no less than 120 articles, including 25 systematic reviews and meta-analyses have been proposed on the use of tDCS for pain, using diverse grading systems and providing inconsistent results. Because of the limited quality of many published reports, the level of evidence for pain remains low despite such a large number of studies.
While initial reviews cautiously concluded to a “possible pain-relieving efficacy,”215, 216 more recent ones have uncritically stated that tDCS “successfully relieves NP.”122, 217-219 Other meta-analyses, however, concluded that tDCS had no effect in NP beyond sham stimulation,124, 220 or found limited and conflicting evidence precluding reliable interpretations.60, 221-224 Similar inconsistencies are found regarding widespread pain syndromes. While some systematic reviews concluded to “probable” or even “definite” efficacy in fibromyalgia,215, 225-228 others found “tentative,” “inconclusive,” or simply absent evidence of pain reduction when compared to sham stimulation.60, 123, 229 Such discrepancies are undoubtedly driven by the heterogeneity of reports in terms of sample size, randomization, procedures of stimulation, quality of blinding, control of bias, statistical thresholds, and so forth. Thus, the magnitude of clinical effects in fibromyalgia dramatically decreased with increased sample size, to become very often clinically insignificant or not better than sham.230-233 As with every neuromodulation procedure, some subsets of patients may be more receptive to tDCS than others,234 which underscores the importance of reporting precisely the percentage and clinical characteristics of responding subjects, along with numbers needed to treat (NNTs), rather than simply providing statistical group analyses.
tDCS addressed to the frontal cortex has not proved better than standard M1 stimulation, and in most cases, was inferior to it, both in NP145, 235 and fibromyalgia.226 There is a lack of head-to-head prospective studies comparing tDCS with conventional rTMS in neuropathic pain. One study contrasting their effects in patients with lumbosacral radiculopathy reported rTMS superiority;236 however, patients unresponsive to conventional rTMS can also be alleviated by subsequent tDCS,237 and two recent studies in different NP conditions reported similar global efficacy but a different subset of responding patients to each technique.238 The use of tDCS as add-on therapy to invasive procedures was recently reported in a small randomised study, where combining tDCS with dorsal root ganglion (DRG) stimulation provided better results than DRG alone.239
A critical advantage of tDCS is the possibility of performing home-based therapy, hence allowing long-lasting maintenance of effects in responding patients. Although the development of systems for self-stimulation is technically simple, inadequate choice of targets, stimulation mode, electrode contact, or stimulus intensity can create significant harm240, 241 including cognitive impairment,242 which has lead major authors to issue notices of caution.243 Remotely supervised systems can circumvent most of these problems by allowing online monitoring and control of the stimulation by clinical personnel. Such systems ensure that tDCS cannot be performed unless authorized by clinical staff, who follows in real time the procedure, can detect faulty or incomplete sessions, and communicate with patients in case of problems. Supporting clinical evidence remains weak, but real-life feasibility has been reported in a few sham-controlled pilot studies on small samples of NP patients, where about half of the patients were significantly improved with follow-up up to 6 months.244, 245
Take-home messages: tDCS
tDCS modulates the neuronal resting membrane state of the underlying cortex, induces activity changes in distributed brain networks, and can influence both cognitive-emotional and sensory aspects of pain. Lower cost relative to rTMS, few safety issues, and availability of home-based protocols are practical advantages; however, the limited quality of most published reports greatly lowers the level of evidence regarding its effects in chronic pain. Limited evidence suggests that: 1) M1 is superior to DLPFC stimulation for chronic pain; 2) repeated sessions over a long time may be necessary for clinically significant pain relief; 3) patients responsive to tDCS may differ from those improved by rTMS. Well-conducted RCTs are needed to gather conclusive evidence of its possible clinical relevance and NNTs.
Conclusions
Invasive and non-invasive cortical stimulation can be of significant benefit to patients with drug-resistant chronic pain. Noninvasive procedures are extremely safe when conducted by well-trained practitioners; they are being increasingly used as either ancillary or last-resort treatments mainly in neuropathic pain, and may be successfully combined with rehabilitation. Although the potentialities are huge, evidence for successful clinical use in the long term remains low, in particular for tDCS, and extending the duration of beneficial effects beyond the first weeks post-treatment remains a challenge. Different strategies are being currently under investigation, and Figure 2 proposes an algorithm with pathways and options for long-term use of cortical stimulation in patients with chronic neuropathic pain.
Figure 2.
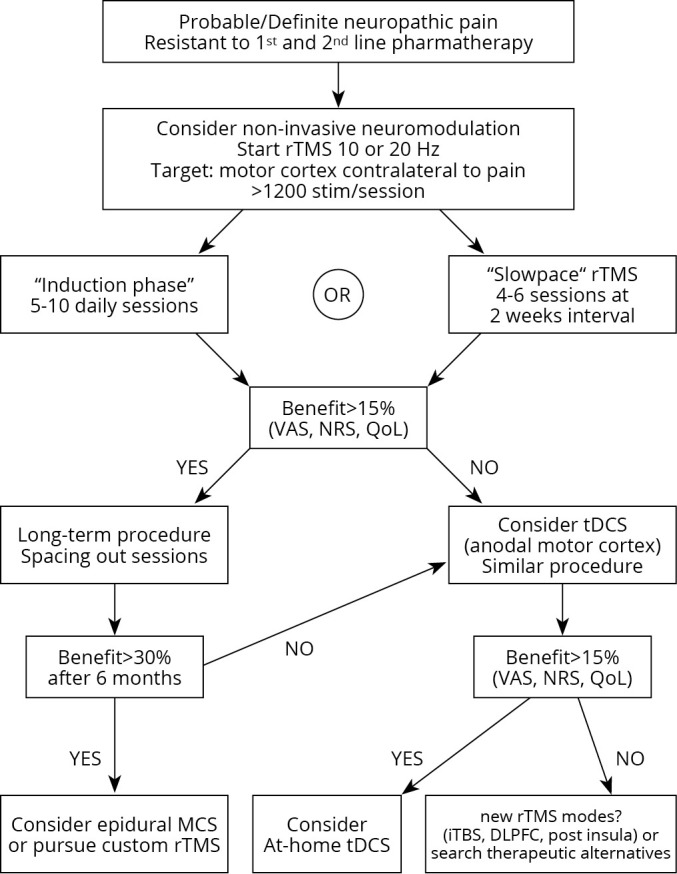
—A proposed therapeutic algorithm with options and paths for non-invasive cortical stimulation in chronic neuropathic pain patients.
References
- 1.Lindblom UF, Ottosson JO. Influence of pyramidal stimulation upon the relay of coarse cutaneous afferents in the dorsal horn. Acta Physiol Scand 1957;38:309–18. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=13410629&dopt=Abstract 10.1111/j.1748-1716.1957.tb01394.x [DOI] [PubMed] [Google Scholar]
- 2.Andersen P, Eccles JC, Sears TA. Presynaptic inhibitory action of cerebral cortex on the spinal cord. Nature 1962;194:740–1. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=13861184&dopt=Abstract 10.1038/194740a0 [DOI] [PubMed] [Google Scholar]
- 3.Fields HL, Adams JE. Pain after cortical injury relieved by electrical stimulation of the internal capsule. Brain 1974;97:169–78. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=4434168&dopt=Abstract 10.1093/brain/97.1.169 [DOI] [PubMed] [Google Scholar]
- 4.Hosobuchi Y, Adams JE, Rutkin B. Chronic thalamic and internal capsule stimulation for the control of central pain. Surg Neurol 1975;4:91–2. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1080907&dopt=Abstract [PubMed] [Google Scholar]
- 5.Ohmoto T, Nakao Y, Sakurai M, Asano T, Matsumoto Y, Namba S, et al. [Electrical stimulation of the posterior limb of the internal capsule for treatment of thalamic pain]. No To Shinkei 1983;35:1009–16. [Japanese]. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=6606434&dopt=Abstract [PubMed] [Google Scholar]
- 6.Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl (Wien) 1991;52:137–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1792954&dopt=Abstract 10.1007/978-3-7091-9160-6_37 [DOI] [PubMed] [Google Scholar]
- 7.Meyerson BA, Lindblom U, Linderoth B, Lind G, Herregodts P. Motor cortex stimulation as treatment of trigeminal neuropathic pain. Acta Neurochir Suppl (Wien) 1993;58:150–3. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8109279&dopt=Abstract 10.1007/978-3-7091-9297-9_34 [DOI] [PubMed] [Google Scholar]
- 8.Nguyen JP, Keravel Y, Feve A, Uchiyama T, Cesaro P, Le Guerinel C, et al. Treatment of deafferentation pain by chronic stimulation of the motor cortex: report of a series of 20 cases. Acta Neurochir Suppl (Wien) 1997;68:54–60. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9233414&dopt=Abstract 10.1007/978-3-7091-6513-3_10 [DOI] [PubMed] [Google Scholar]
- 9.Mertens P, Nuti C, Sindou M, Guenot M, Peyron R, Garcia-Larrea L, et al. Precentral cortex stimulation for the treatment of central neuropathic pain: results of a prospective study in a 20-patient series. Stereotact Funct Neurosurg 1999;73:122–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10853116&dopt=Abstract 10.1159/000029769 [DOI] [PubMed] [Google Scholar]
- 10.Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation in patients with thalamic pain. J Neurosurg 1993;78:393–401. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8433140&dopt=Abstract 10.3171/jns.1993.78.3.0393 [DOI] [PubMed] [Google Scholar]
- 11.Nguyen JP, Lefaucheur JP, Decq P, Uchiyama T, Carpentier A, Fontaine D, et al. Chronic motor cortex stimulation in the treatment of central and neuropathic pain. Correlations between clinical, electrophysiological and anatomical data. Pain 1999;82:245–51. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10488675&dopt=Abstract 10.1016/S0304-3959(99)00062-7 [DOI] [PubMed] [Google Scholar]
- 12.Afif A, Garcia-Larrea L, Mertens P. Stimulation of the motor cerebral cortex in chronic neuropathic pain: the role of electrode localization over motor somatotopy. J Neurosurg Sci 2020. [Epub ahead of print]. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32951416&dopt=Abstract 10.23736/S0390-5616.20.04991-7 [DOI] [PubMed]
- 13.Bezard E, Boraud T, Nguyen JP, Velasco F, Keravel Y, Gross C. Cortical stimulation and epileptic seizure: a study of the potential risk in primates. Neurosurgery 1999;45:346–50. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10449080&dopt=Abstract 10.1097/00006123-199908000-00030 [DOI] [PubMed] [Google Scholar]
- 14.Saitoh Y, Kato A, Ninomiya H, Baba T, Shibata M, Mashimo T, et al. Primary motor cortex stimulation within the central sulcus for treating deafferentation pain. Acta Neurochir Suppl (Wien) 2003;87:149–52. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14518543&dopt=Abstract 10.1007/978-3-7091-6081-7_31 [DOI] [PubMed] [Google Scholar]
- 15.Nguyen JP, Nizard J, Keravel Y, Lefaucheur JP. Invasive brain stimulation for the treatment of neuropathic pain. Nat Rev Neurol 2011;7:699–709. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21931348&dopt=Abstract 10.1038/nrneurol.2011.138 [DOI] [PubMed] [Google Scholar]
- 16.Lavrov I, Latypov T, Mukhametova E, Lundstrom B, Sandroni P, Lee K, et al. Pre-motor versus motor cerebral cortex neuromodulation for chronic neuropathic pain. Sci Rep 2021;11:12688. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34135363&dopt=Abstract 10.1038/s41598-021-91872-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosomi K, Saitoh Y, Kishima H, Oshino S, Hirata M, Tani N, et al. Electrical stimulation of primary motor cortex within the central sulcus for intractable neuropathic pain. Clin Neurophysiol 2008;119:993–1001. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18329955&dopt=Abstract 10.1016/j.clinph.2007.12.022 [DOI] [PubMed] [Google Scholar]
- 18.Peyron R, Garcia-Larrea L, Deiber MP, Cinotti L, Convers P, Sindou M, et al. Electrical stimulation of precentral cortical area in the treatment of central pain: electrophysiological and PET study. Pain 1995;62:275–86. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8657427&dopt=Abstract 10.1016/0304-3959(94)00211-V [DOI] [PubMed] [Google Scholar]
- 19.Lefaucheur JP, Holsheimer J, Goujon C, Keravel Y, Nguyen JP. Descending volleys generated by efficacious epidural motor cortex stimulation in patients with chronic neuropathic pain. Exp Neurol 2010;223:609–14. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20188091&dopt=Abstract 10.1016/j.expneurol.2010.02.008 [DOI] [PubMed] [Google Scholar]
- 20.Peyron R, Faillenot I, Mertens P, Laurent B, Garcia-Larrea L. Motor cortex stimulation in neuropathic pain. Correlations between analgesic effect and hemodynamic changes in the brain. A PET study. Neuroimage 2007;34:310–21. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17055297&dopt=Abstract 10.1016/j.neuroimage.2006.08.037 [DOI] [PubMed] [Google Scholar]
- 21.García-Larrea L, Peyron R, Mertens P, Grégoire MC, Lavenne F, Le Bars D, et al. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain 1999;83:259–73. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10534598&dopt=Abstract 10.1016/S0304-3959(99)00114-1 [DOI] [PubMed] [Google Scholar]
- 22.Roux FE, Ibarrola D, Lazorthes Y, Berry I. Chronic motor cortex stimulation for phantom limb pain: a functional magnetic resonance imaging study: technical case report. Neurosurgery 2001;48:681–7, discussion 687–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11270562&dopt=Abstract 10.1097/00006123-200103000-00050 [DOI] [PubMed] [Google Scholar]
- 23.Volkers R, Giesen E, van der Heiden M, Kerperien M, Lange S, Kurt E, et al. Invasive Motor Cortex Stimulation Influences Intracerebral Structures in Patients With Neuropathic Pain: An Activation Likelihood Estimation Meta-Analysis of Imaging Data. Neuromodulation 2020;23:436–43. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32030854&dopt=Abstract 10.1111/ner.13119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saitoh Y, Osaki Y, Nishimura H, Hirano S, Kato A, Hashikawa K, et al. Increased regional cerebral blood flow in the contralateral thalamus after successful motor cortex stimulation in a patient with poststroke pain. J Neurosurg 2004;100:935–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15137612&dopt=Abstract 10.3171/jns.2004.100.5.0935 [DOI] [PubMed] [Google Scholar]
- 25.Ito M, Kuroda S, Shiga T, Tamaki N, Iwasaki Y. Motor cortex stimulation improves local cerebral glucose metabolism in the ipsilateral thalamus in patients with poststroke pain: case report. Neurosurgery 2011;69:E462–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21792141&dopt=Abstract 10.1227/NEU.0b013e318218cfa0 [DOI] [PubMed] [Google Scholar]
- 26.Kishima H, Saitoh Y, Osaki Y, Nishimura H, Kato A, Hatazawa J, et al. Motor cortex stimulation in patients with deafferentation pain: activation of the posterior insula and thalamus. J Neurosurg 2007;107:43–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17639872&dopt=Abstract 10.3171/JNS-07/07/0043 [DOI] [PubMed] [Google Scholar]
- 27.Nowak LG, Bullier J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. II. Evidence from selective inactivation of cell bodies and axon initial segments. Exp Brain Res 1998;118:489–500. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9504844&dopt=Abstract 10.1007/s002210050305 [DOI] [PubMed] [Google Scholar]
- 28.Katayama Y, Tsubokawa T, Maejima S, Hirayama T, Yamamoto T. Corticospinal direct response in humans: identification of the motor cortex during intracranial surgery under general anaesthesia. J Neurol Neurosurg Psychiatry 1988;51:50–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2832547&dopt=Abstract 10.1136/jnnp.51.1.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M, et al. Motor cortex stimulation for pain control induces changes in the endogenous opioid system. Neurology 2007;69:827–34. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17724284&dopt=Abstract 10.1212/01.wnl.0000269783.86997.37 [DOI] [PubMed] [Google Scholar]
- 30.Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M, et al. Brain opioid receptor density predicts motor cortex stimulation efficacy for chronic pain. Pain 2013;154:2563–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23900133&dopt=Abstract 10.1016/j.pain.2013.07.042 [DOI] [PubMed] [Google Scholar]
- 31.Vaculín S, Franek M, Yamamotová A, Rokyta R. Motor cortex stimulation in rats with chronic constriction injury. Exp Brain Res 2008;185:331–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17940756&dopt=Abstract 10.1007/s00221-007-1158-y [DOI] [PubMed] [Google Scholar]
- 32.Viisanen H, Pertovaara A. Roles of the rostroventromedial medulla and the spinal 5-HT(1A) receptor in descending antinociception induced by motor cortex stimulation in the neuropathic rat. Neurosci Lett 2010;476:133–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20398735&dopt=Abstract 10.1016/j.neulet.2010.04.014 [DOI] [PubMed] [Google Scholar]
- 33.Lucas JM, Ji Y, Masri R. Motor cortex stimulation reduces hyperalgesia in an animal model of central pain. Pain 2011;152:1398–407. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21396776&dopt=Abstract 10.1016/j.pain.2011.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pagano RL, Assis DV, Clara JA, Alves AS, Dale CS, Teixeira MJ, et al. Transdural motor cortex stimulation reverses neuropathic pain in rats: a profile of neuronal activation. Eur J Pain 2011;15:268.e1–14. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20817578&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 35.Jiang L, Ji Y, Voulalas PJ, Keaser M, Xu S, Gullapalli RP, et al. Motor cortex stimulation suppresses cortical responses to noxious hindpaw stimulation after spinal cord lesion in rats. Brain Stimul 2014;7:182–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24468093&dopt=Abstract 10.1016/j.brs.2013.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J, Ryu SB, Lee SE, Shin J, Jung HH, Kim SJ, et al. Motor cortex stimulation and neuropathic pain: how does motor cortex stimulation affect pain-signaling pathways? J Neurosurg 2016;124:866–76. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26274988&dopt=Abstract 10.3171/2015.1.JNS14891 [DOI] [PubMed] [Google Scholar]
- 37.Cha M, Ji Y, Masri R. Motor cortex stimulation activates the incertothalamic pathway in an animal model of spinal cord injury. J Pain 2013;14:260–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23332495&dopt=Abstract 10.1016/j.jpain.2012.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cha M, Lee KH, Lee BH. Astroglial changes in the zona incerta in response to motor cortex stimulation in a rat model of chronic neuropathy. Sci Rep 2020;10:943. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31969638&dopt=Abstract 10.1038/s41598-020-57797-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Andrade EM, Martinez RC, Pagano RL, Lopes PS, Auada AV, Gouveia FV, et al. Neurochemical effects of motor cortex stimulation in the periaqueductal gray during neuropathic pain. J Neurosurg 2019;132:239–51. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30611141&dopt=Abstract 10.3171/2018.7.JNS173239 [DOI] [PubMed] [Google Scholar]
- 40.Davoody L, Quiton RL, Lucas JM, Ji Y, Keller A, Masri R. Conditioned place preference reveals tonic pain in an animal model of central pain. J Pain 2011;12:868–74. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21515090&dopt=Abstract 10.1016/j.jpain.2011.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henssen D, Giesen E, van der Heiden M, Kerperien M, Lange S, van Cappellen van Walsum AM, et al. A systematic review of the proposed mechanisms underpinning pain relief by primary motor cortex stimulation in animals. Neurosci Lett 2020;719:134489. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31518678&dopt=Abstract 10.1016/j.neulet.2019.134489 [DOI] [PubMed] [Google Scholar]
- 42.Hirato M, Watanabe K, Takahashi A, Hayase N, Horikoshi S, Shibasaki T, et al. Pathophysiology of central (thalamic) pain: combined change of sensory thalamus with cerebral cortex around central sulcus. Stereotact Funct Neurosurg 1994;62:300–3. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7631086&dopt=Abstract 10.1159/000098636 [DOI] [PubMed] [Google Scholar]
- 43.Magnin M, Morel A, Jeanmonod D. [Toward a unified theory of positive symptoms]. Neurophysiol Clin 2005;35:154–61. [French]. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16530132&dopt=Abstract 10.1016/j.neucli.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 44.Pagano RL, Fonoff ET, Dale CS, Ballester G, Teixeira MJ, Britto LR. Motor cortex stimulation inhibits thalamic sensory neurons and enhances activity of PAG neurons: possible pathways for antinociception. Pain 2012;153:2359–69. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23017297&dopt=Abstract 10.1016/j.pain.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 45.Shijo K, Katayama Y, Yamashita A, Kobayashi K, Oshima H, Fukaya C, et al. c-Fos Expression After Chronic Electrical Stimulation of Sensorimotor Cortex in Rats. Neuromodulation 2008;11:187–95. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22151095&dopt=Abstract 10.1111/j.1525-1403.2008.00165.x [DOI] [PubMed] [Google Scholar]
- 46.Morishita T, Yamashita A, Katayama Y, Oshima H, Nishizaki Y, Shijo K, et al. Chronological changes in astrocytes induced by chronic electrical sensorimotor cortex stimulation in rats. Neurol Med Chir (Tokyo) 2011;51:496–502. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21785243&dopt=Abstract 10.2176/nmc.51.496 [DOI] [PubMed] [Google Scholar]
- 47.Senapati AK, Huntington PJ, Peng YB. Spinal dorsal horn neuron response to mechanical stimuli is decreased by electrical stimulation of the primary motor cortex. Brain Res 2005;1036:173–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15725415&dopt=Abstract 10.1016/j.brainres.2004.12.043 [DOI] [PubMed] [Google Scholar]
- 48.França NR, Toniolo EF, Franciosi AC, Alves AS, de Andrade DC, Fonoff ET, et al. Antinociception induced by motor cortex stimulation: somatotopy of behavioral response and profile of neuronal activation. Behav Brain Res 2013;250:211–21. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23692698&dopt=Abstract 10.1016/j.bbr.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 49.Fonoff ET, Dale CS, Pagano RL, Paccola CC, Ballester G, Teixeira MJ, et al. Antinociception induced by epidural motor cortex stimulation in naive conscious rats is mediated by the opioid system. Behav Brain Res 2009;196:63–70. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18718490&dopt=Abstract 10.1016/j.bbr.2008.07.027 [DOI] [PubMed] [Google Scholar]
- 50.Silva GD, Lopes PS, Fonoff ET, Pagano RL. The spinal anti-inflammatory mechanism of motor cortex stimulation: cause of success and refractoriness in neuropathic pain? J Neuroinflammation 2015;12:10. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25600429&dopt=Abstract 10.1186/s12974-014-0216-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Viltart O, Sequeira H. Induction of c-Fos-like protein in bulbar catecholaminergic neurones by electrical stimulation of the sensorimotor cortex in the rat. Neurosci Lett 1999;260:65–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10027701&dopt=Abstract 10.1016/S0304-3940(98)00907-0 [DOI] [PubMed] [Google Scholar]
- 52.Lopes PS, Campos AC, Fonoff ET, Britto LR, Pagano RL. Motor cortex and pain control: exploring the descending relay analgesic pathways and spinal nociceptive neurons in healthy conscious rats. Behav Brain Funct 2019;15:5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30909927&dopt=Abstract 10.1186/s12993-019-0156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viisanen H, Ansah OB, Pertovaara A. The role of the dopamine D2 receptor in descending control of pain induced by motor cortex stimulation in the neuropathic rat. Brain Res Bull 2012;89:133–43. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22902996&dopt=Abstract 10.1016/j.brainresbull.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 54.Negrini-Ferrari SE, Medeiros P, Malvestio RB, de Oliveira Silva M, Medeiros AC, Coimbra NC, et al. The primary motor cortex electrical and chemical stimulation attenuates the chronic neuropathic pain by activation of the periaqueductal grey matter: the role of NMDA receptors. Behav Brain Res 2021;415:113522. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34391797&dopt=Abstract 10.1016/j.bbr.2021.113522 [DOI] [PubMed] [Google Scholar]
- 55.Velasco F, Carrillo-Ruiz JD, Castro G, Argüelles C, Velasco AL, Kassian A, et al. Motor cortex electrical stimulation applied to patients with complex regional pain syndrome. Pain 2009;147:91–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19793621&dopt=Abstract 10.1016/j.pain.2009.08.024 [DOI] [PubMed] [Google Scholar]
- 56.Sokal P, Harat M, Zieliński P, Furtak J, Paczkowski D, Rusinek M. Motor cortex stimulation in patients with chronic central pain. Adv Clin Exp Med 2015;24:289–96. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25931362&dopt=Abstract 10.17219/acem/40452 [DOI] [PubMed] [Google Scholar]
- 57.Fontaine D, Hamani C, Lozano A. Efficacy and safety of motor cortex stimulation for chronic neuropathic pain: critical review of the literature. J Neurosurg 2009;110:251–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18991496&dopt=Abstract 10.3171/2008.6.17602 [DOI] [PubMed] [Google Scholar]
- 58.Hosomi K, Seymour B, Saitoh Y. Modulating the pain network—neurostimulation for central poststroke pain. Nat Rev Neurol 2015;11:290–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25896085&dopt=Abstract 10.1038/nrneurol.2015.58 [DOI] [PubMed] [Google Scholar]
- 59.Henssen D, Kurt E, van Walsum AV, Kozicz T, van Dongen R, Bartels R. Motor cortex stimulation in chronic neuropathic orofacial pain syndromes: a systematic review and meta-analysis. Sci Rep 2020;10:7195. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32346080&dopt=Abstract 10.1038/s41598-020-64177-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cruccu G, Garcia-Larrea L, Hansson P, Keindl M, Lefaucheur JP, Paulus W, et al. EAN guidelines on central neurostimulation therapy in chronic pain conditions. Eur J Neurol 2016;23:1489–99. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27511815&dopt=Abstract 10.1111/ene.13103 [DOI] [PubMed] [Google Scholar]
- 61.Radic JA, Beauprie I, Chiasson P, Kiss ZH, Brownstone RM. Motor Cortex Stimulation for Neuropathic Pain: A Randomized Cross-over Trial. Can J Neurol Sci 2015;42:401–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26324857&dopt=Abstract 10.1017/cjn.2015.292 [DOI] [PubMed] [Google Scholar]
- 62.Rasche D, Ruppolt M, Stippich C, Unterberg A, Tronnier VM. Motor cortex stimulation for long-term relief of chronic neuropathic pain: a 10 year experience. Pain 2006;121:43–52. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16480828&dopt=Abstract 10.1016/j.pain.2005.12.006 [DOI] [PubMed] [Google Scholar]
- 63.André-Obadia N, Mertens P, Lelekov-Boissard T, Afif A, Magnin M, Garcia-Larrea L. Is Life better after motor cortex stimulation for pain control? Results at long-term and their prediction by preoperative rTMS. Pain Physician 2014;17:53–62. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24452645&dopt=Abstract 10.36076/ppj.2014/17/53 [DOI] [PubMed] [Google Scholar]
- 64.Nguyen JP, Velasco F, Brugières P, Velasco M, Keravel Y, Boleaga B, et al. Treatment of chronic neuropathic pain by motor cortex stimulation: results of a bicentric controlled crossover trial. Brain Stimul 2008;1:89–96. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20633375&dopt=Abstract 10.1016/j.brs.2008.03.007 [DOI] [PubMed] [Google Scholar]
- 65.Velasco F, Argüelles C, Carrillo-Ruiz JD, Castro G, Velasco AL, Jiménez F, et al. Efficacy of motor cortex stimulation in the treatment of neuropathic pain: a randomized double-blind trial. J Neurosurg 2008;108:698–706. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18377249&dopt=Abstract 10.3171/JNS/2008/108/4/0698 [DOI] [PubMed] [Google Scholar]
- 66.Lefaucheur JP, Drouot X, Cunin P, Bruckert R, Lepetit H, Créange A, et al. Motor cortex stimulation for the treatment of refractory peripheral neuropathic pain. Brain 2009;132:1463–71. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19336459&dopt=Abstract 10.1093/brain/awp035 [DOI] [PubMed] [Google Scholar]
- 67.Hamani C, Fonoff ET, Parravano DC, Silva VA, Galhardoni R, Monaco BA, et al. Motor cortex stimulation for chronic neuropathic pain: results of a double-blind randomized study. Brain 2021;144:2994–3004. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34373901&dopt=Abstract 10.1093/brain/awab189 [DOI] [PubMed] [Google Scholar]
- 68.Nuti C, Peyron R, Garcia-Larrea L, Brunon J, Laurent B, Sindou M, et al. Motor cortex stimulation for refractory neuropathic pain: four year outcome and predictors of efficacy. Pain 2005;118:43–52. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16214292&dopt=Abstract 10.1016/j.pain.2005.07.020 [DOI] [PubMed] [Google Scholar]
- 69.Sears NC, Machado AG, Nagel SJ, Deogaonkar M, Stanton-Hicks M, Rezai AR, et al. Long-term outcomes of spinal cord stimulation with paddle leads in the treatment of complex regional pain syndrome and failed back surgery syndrome. Neuromodulation 2011;14:312–8, discussion 318. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21992424&dopt=Abstract 10.1111/j.1525-1403.2011.00372.x [DOI] [PubMed] [Google Scholar]
- 70.Zhang X, Hu Y, Tao W, Zhu H, Xiao D, Li Y. The Effect of Motor Cortex Stimulation on Central Poststroke Pain in a Series of 16 Patients With a Mean Follow-Up of 28 Months. Neuromodulation 2017;20:492–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28102925&dopt=Abstract 10.1111/ner.12547 [DOI] [PubMed] [Google Scholar]
- 71.Katayama Y, Fukaya C, Yamamoto T. Poststroke pain control by chronic motor cortex stimulation: neurological characteristics predicting a favorable response. J Neurosurg 1998;89:585–91. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9761052&dopt=Abstract 10.3171/jns.1998.89.4.0585 [DOI] [PubMed] [Google Scholar]
- 72.Drouot X, Nguyen JP, Peschanski M, Lefaucheur JP. The antalgic efficacy of chronic motor cortex stimulation is related to sensory changes in the painful zone. Brain 2002;125:1660–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12077014&dopt=Abstract 10.1093/brain/awf161 [DOI] [PubMed] [Google Scholar]
- 73.Zhang X, Zhu H, Tao W, Li Y, Hu Y. Motor Cortex Stimulation Therapy for Relief of Central Post-Stroke Pain: A Retrospective Study with Neuropathic Pain Symptom Inventory. Stereotact Funct Neurosurg 2018;96:239–43. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30125888&dopt=Abstract 10.1159/000492056 [DOI] [PubMed] [Google Scholar]
- 74.Slotty PJ, Chang S, Honey CR. Motor Threshold: A Possible Guide to Optimizing Stimulation Parameters for Motor Cortex Stimulation. Neuromodulation 2015;18:566–71, discussion 571–3. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26245728&dopt=Abstract 10.1111/ner.12336 [DOI] [PubMed] [Google Scholar]
- 75.Yamamoto T, Katayama Y, Hirayama T, Tsubokawa T. Pharmacological classification of central post-stroke pain: comparison with the results of chronic motor cortex stimulation therapy. Pain 1997;72:5–12. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9272782&dopt=Abstract 10.1016/S0304-3959(97)00028-6 [DOI] [PubMed] [Google Scholar]
- 76.Tanei T, Kajita Y, Maesawa S, Nakatsubo D, Aoki K, Noda H, et al. Long-term Effect and Predictive Factors of Motor Cortex and Spinal Cord Stimulation for Chronic Neuropathic Pain. Neurol Med Chir (Tokyo) 2018;58:422–34. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30158352&dopt=Abstract 10.2176/nmc.oa.2018-0106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saitoh Y, Hirayama A, Kishima H, Oshino S, Hirata M, Kato A, et al. Stimulation of primary motor cortex for intractable deafferentation pain. Acta Neurochir Suppl (Wien) 2006;99:57–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17370765&dopt=Abstract 10.1007/978-3-211-35205-2_11 [DOI] [PubMed] [Google Scholar]
- 78.André-Obadia N, Peyron R, Mertens P, Mauguière F, Laurent B, Garcia-Larrea L. Transcranial magnetic stimulation for pain control. Double-blind study of different frequencies against placebo, and correlation with motor cortex stimulation efficacy. Clin Neurophysiol 2006;117:1536–44. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16753335&dopt=Abstract 10.1016/j.clinph.2006.03.025 [DOI] [PubMed] [Google Scholar]
- 79.Lefaucheur JP, Ménard-Lefaucheur I, Goujon C, Keravel Y, Nguyen JP. Predictive value of rTMS in the identification of responders to epidural motor cortex stimulation therapy for pain. J Pain 2011;12:1102–11. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21807565&dopt=Abstract 10.1016/j.jpain.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 80.Pommier B, Quesada C, Fauchon C, Nuti C, Vassal F, Peyron R. Added value of multiple versus single sessions of repetitive transcranial magnetic stimulation in predicting motor cortex stimulation efficacy for refractory neuropathic pain. J Neurosurg 2018;1:1–12. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29775149&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 81.André-Obadia N, Mertens P, Gueguen A, Peyron R, Garcia-Larrea L. Pain relief by rTMS: differential effect of current flow but no specific action on pain subtypes. Neurology 2008;71:833–40. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18779511&dopt=Abstract 10.1212/01.wnl.0000325481.61471.f0 [DOI] [PubMed] [Google Scholar]
- 82.Lefaucheur JP, Nguyen JP. A practical algorithm for using rTMS to treat patients with chronic pain. Neurophysiol Clin 2019;49:301–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31375381&dopt=Abstract 10.1016/j.neucli.2019.07.014 [DOI] [PubMed] [Google Scholar]
- 83.Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul 2010;3:95–118. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20633438&dopt=Abstract 10.1016/j.brs.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 84.Opie GM, Semmler JG. Preferential Activation of Unique Motor Cortical Networks With Transcranial Magnetic Stimulation: A Review of the Physiological, Functional, and Clinical Evidence. Neuromodulation 2021;24:813–28. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33295685&dopt=Abstract 10.1111/ner.13314 [DOI] [PubMed] [Google Scholar]
- 85.Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Dose-dependent reduction of cerebral blood flow during rapid-rate transcranial magnetic stimulation of the human sensorimotor cortex. J Neurophysiol 1998;79:1102–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9463466&dopt=Abstract 10.1152/jn.1998.79.2.1102 [DOI] [PubMed] [Google Scholar]
- 86.Siebner HR, Peller M, Willoch F, Minoshima S, Boecker H, Auer C, et al. Lasting cortical activation after repetitive TMS of the motor cortex: a glucose metabolic study. Neurology 2000;54:956–63. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10690992&dopt=Abstract 10.1212/WNL.54.4.956 [DOI] [PubMed] [Google Scholar]
- 87.Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci 2004;19:1950–62. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15078569&dopt=Abstract 10.1111/j.1460-9568.2004.03277.x [DOI] [PubMed] [Google Scholar]
- 88.Lefaucheur JP, Drouot X, Ménard-Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology 2006;67:1568–74. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17101886&dopt=Abstract 10.1212/01.wnl.0000242731.10074.3c [DOI] [PubMed] [Google Scholar]
- 89.Hosomi K, Kishima H, Oshino S, Hirata M, Tani N, Maruo T, et al. Cortical excitability changes after high-frequency repetitive transcranial magnetic stimulation for central poststroke pain. Pain 2013;154:1352–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23707310&dopt=Abstract 10.1016/j.pain.2013.04.017 [DOI] [PubMed] [Google Scholar]
- 90.Tang SC, Lee LJ, Jeng JS, Hsieh ST, Chiang MC, Yeh SJ, et al. Pathophysiology of Central Poststroke Pain: Motor Cortex Disinhibition and Its Clinical and Sensory Correlates. Stroke 2019;50:2851–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31500556&dopt=Abstract 10.1161/STROKEAHA.119.025692 [DOI] [PubMed] [Google Scholar]
- 91.Ciampi de Andrade D, Mhalla A, Adam F, Texeira MJ, Bouhassira D. Repetitive transcranial magnetic stimulation induced analgesia depends on N-methyl-D-aspartate glutamate receptors. Pain 2014;155:598–605. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24342462&dopt=Abstract 10.1016/j.pain.2013.12.022 [DOI] [PubMed] [Google Scholar]
- 92.Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, et al. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol 2000;111:794–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10802448&dopt=Abstract 10.1016/S1388-2457(99)00314-4 [DOI] [PubMed] [Google Scholar]
- 93.Pacheco-Barrios K, Pinto CB, Saleh Velez FG, Duarte D, Gunduz ME, Simis M, et al. Structural and functional motor cortex asymmetry in unilateral lower limb amputation with phantom limb pain. Clin Neurophysiol 2020;131:2375–82. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32828040&dopt=Abstract 10.1016/j.clinph.2020.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Candido Santos L, Gushken F, Gadotti GM, Dias BF, Marinelli Pedrini S, Barreto ME, et al. Intracortical Inhibition in the Affected Hemisphere in Limb Amputation. Front Neurol 2020;11:720. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32849197&dopt=Abstract 10.3389/fneur.2020.00720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jetté F, Côté I, Meziane HB, Mercier C. Effect of single-session repetitive transcranial magnetic stimulation applied over the hand versus leg motor area on pain after spinal cord injury. Neurorehabil Neural Repair 2013;27:636–43. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23579183&dopt=Abstract 10.1177/1545968313484810 [DOI] [PubMed] [Google Scholar]
- 96.André-Obadia N, Magnin M, Garcia-Larrea L. Theta-burst versus 20 Hz repetitive transcranial magnetic stimulation in neuropathic pain: A head-to-head comparison. Clin Neurophysiol 2021;132:2702–10. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34217600&dopt=Abstract 10.1016/j.clinph.2021.05.022 [DOI] [PubMed] [Google Scholar]
- 97.Hodkinson DJ, Bungert A, Bowtell R, Jackson SR, Jung J. Operculo-insular and anterior cingulate plasticity induced by transcranial magnetic stimulation in the human motor cortex: a dynamic casual modeling study. J Neurophysiol 2021;125:1180–90. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33625934&dopt=Abstract 10.1152/jn.00670.2020 [DOI] [PubMed] [Google Scholar]
- 98.Pei Q, Zhuo Z, Jing B, Meng Q, Ma X, Mo X, et al. The effects of repetitive transcranial magnetic stimulation on the whole-brain functional network of postherpetic neuralgia patients. Medicine (Baltimore) 2019;98:e16105. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31232955&dopt=Abstract 10.1097/MD.0000000000016105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kadono Y, Koguchi K, Okada KI, Hosomi K, Hiraishi M, Ueguchi T, et al. Repetitive transcranial magnetic stimulation restores altered functional connectivity of central poststroke pain model monkeys. Sci Rep 2021;11:6126. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33731766&dopt=Abstract 10.1038/s41598-021-85409-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ji RR, Schlaepfer TE, Aizenman CD, Epstein CM, Qiu D, Huang JC, et al. Repetitive transcranial magnetic stimulation activates specific regions in rat brain. Proc Natl Acad Sci USA 1998;95:15635–40. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9861022&dopt=Abstract 10.1073/pnas.95.26.15635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hausmann A, Weis C, Marksteiner J, Hinterhuber H, Humpel C. Chronic repetitive transcranial magnetic stimulation enhances c-fos in the parietal cortex and hippocampus. Brain Res Mol Brain Res 2000;76:355–62. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10762712&dopt=Abstract 10.1016/S0169-328X(00)00024-3 [DOI] [PubMed] [Google Scholar]
- 102.Doi W, Sato D, Fukuzako H, Takigawa M. c-Fos expression in rat brain after repetitive transcranial magnetic stimulation. Neuroreport 2001;12:1307–10. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11338212&dopt=Abstract 10.1097/00001756-200105080-00050 [DOI] [PubMed] [Google Scholar]
- 103.Ohn SH, Chang WH, Park CH, Kim ST, Lee JI, Pascual-Leone A, et al. Neural correlates of the antinociceptive effects of repetitive transcranial magnetic stimulation on central pain after stroke. Neurorehabil Neural Repair 2012;26:344–52. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21980153&dopt=Abstract 10.1177/1545968311423110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goto T, Saitoh Y, Hashimoto N, Hirata M, Kishima H, Oshino S, et al. Diffusion tensor fiber tracking in patients with central post-stroke pain; correlation with efficacy of repetitive transcranial magnetic stimulation. Pain 2008;140:509–18. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19004554&dopt=Abstract 10.1016/j.pain.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 105.Ahmed MA, Mohamed SA, Sayed D. Long-term antalgic effects of repetitive transcranial magnetic stimulation of motor cortex and serum beta-endorphin in patients with phantom pain. Neurol Res 2011;33:953–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22080997&dopt=Abstract 10.1179/1743132811Y.0000000045 [DOI] [PubMed] [Google Scholar]
- 106.de Andrade DC, Mhalla A, Adam F, Texeira MJ, Bouhassira D. Neuropharmacological basis of rTMS-induced analgesia: the role of endogenous opioids. Pain 2011;152:320–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21146300&dopt=Abstract 10.1016/j.pain.2010.10.032 [DOI] [PubMed] [Google Scholar]
- 107.Lamusuo S, Hirvonen J, Lindholm P, Martikainen IK, Hagelberg N, Parkkola R, et al. Neurotransmitters behind pain relief with transcranial magnetic stimulation - positron emission tomography evidence for release of endogenous opioids. Eur J Pain 2017;21:1505–15. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28493519&dopt=Abstract 10.1002/ejp.1052 [DOI] [PubMed] [Google Scholar]
- 108.Keck ME, Welt T, Müller MB, Erhardt A, Ohl F, Toschi N, et al. Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and mesostriatal system. Neuropharmacology 2002;43:101–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12213264&dopt=Abstract 10.1016/S0028-3908(02)00069-2 [DOI] [PubMed] [Google Scholar]
- 109.Strafella AP, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain 2003;126:2609–15. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12937078&dopt=Abstract 10.1093/brain/awg268 [DOI] [PubMed] [Google Scholar]
- 110.Fregni F, Potvin K, Dasilva D, Wang X, Lenkinski RE, Freedman SD, et al. Clinical effects and brain metabolic correlates in non-invasive cortical neuromodulation for visceral pain. Eur J Pain 2011;15:53–60. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20822942&dopt=Abstract 10.1016/j.ejpain.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Valmunen T, Pertovaara A, Taiminen T, Virtanen A, Parkkola R, Jääskeläinen SK. Modulation of facial sensitivity by navigated rTMS in healthy subjects. Pain 2009;142:149–58. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19201092&dopt=Abstract 10.1016/j.pain.2008.12.031 [DOI] [PubMed] [Google Scholar]
- 112.Sacco P, Prior M, Poole H, Nurmikko T. Repetitive transcranial magnetic stimulation over primary motor vs non-motor cortical targets; effects on experimental hyperalgesia in healthy subjects. BMC Neurol 2014;14:166. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25182028&dopt=Abstract 10.1186/s12883-014-0166-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bradley C, Perchet C, Lelekov-Boissard T, Magnin M, Garcia-Larrea L. Not an Aspirin: No Evidence for Acute Anti-Nociception to Laser-Evoked Pain After Motor Cortex rTMS in Healthy Humans. Brain Stimul 2016;9:48–57. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26433607&dopt=Abstract 10.1016/j.brs.2015.08.015 [DOI] [PubMed] [Google Scholar]
- 114.Nahmias F, Debes C, de Andrade DC, Mhalla A, Bouhassira D. Diffuse analgesic effects of unilateral repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers. Pain 2009;147:224–32. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19822394&dopt=Abstract 10.1016/j.pain.2009.09.016 [DOI] [PubMed] [Google Scholar]
- 115.Mylius V, Reis J, Knaack A, Haag A, Oertel WH, Rosenow F, et al. High-frequency rTMS of the motor cortex does not influence the nociceptive flexion reflex but increases the unpleasantness of electrically induced pain. Neurosci Lett 2007;415:49–54. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17258394&dopt=Abstract 10.1016/j.neulet.2006.12.042 [DOI] [PubMed] [Google Scholar]
- 116.Leung A, Donohue M, Xu R, Lee R, Lefaucheur JP, Khedr EM, et al. rTMS for suppressing neuropathic pain: a meta-analysis. J Pain 2009;10:1205–16. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19464959&dopt=Abstract 10.1016/j.jpain.2009.03.010 [DOI] [PubMed] [Google Scholar]
- 117.Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014-2018). Clin Neurophysiol 2020;131:474–528. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31901449&dopt=Abstract 10.1016/j.clinph.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 118.Jin Y, Xing G, Li G, Wang A, Feng S, Tang Q, et al. High Frequency Repetitive Transcranial Magnetic Stimulation Therapy For Chronic Neuropathic Pain: A Meta-analysis. Pain Physician 2015;18:E1029–46. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26606017&dopt=Abstract [PubMed] [Google Scholar]
- 119.Anderson J, Parr NJ, Vela K. Evidence Brief: Transcranial Magnetic Stimulation (TMS) for Chronic Pain, PTSD, TBI, Opioid Addiction, and Sexual Trauma. Washington (DC): Department of Veterans Affairs (US); 2020. [PubMed] [Google Scholar]
- 120.Gatzinsky K, Bergh C, Liljegren A, Silander H, Samuelsson J, Svanberg T, et al. Repetitive transcranial magnetic stimulation of the primary motor cortex in management of chronic neuropathic pain: a systematic review. Scand J Pain 2020;21:8–21. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32892189&dopt=Abstract 10.1515/sjpain-2020-0054 [DOI] [PubMed] [Google Scholar]
- 121.Attia M, McCarthy D, Abdelghani M. Repetitive Transcranial Magnetic Stimulation for Treating Chronic Neuropathic Pain: a Systematic Review. Curr Pain Headache Rep 2021;25:48. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33978846&dopt=Abstract 10.1007/s11916-021-00960-5 [DOI] [PubMed] [Google Scholar]
- 122.Zhang KL, Yuan H, Wu FF, Pu XY, Liu BZ, Li Z, et al. Analgesic Effect of Noninvasive Brain Stimulation for Neuropathic Pain Patients: A Systematic Review. Pain Ther 2021;10:315–32. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33751453&dopt=Abstract 10.1007/s40122-021-00252-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.O’Connell NE, Marston L, Spencer S, DeSouza LH, Wand BM. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev 2018;3:CD008208. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29547226&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 124.Knotkova H, Hamani C, Sivanesan E, Le Beuffe MF, Moon JY, Cohen SP, et al. Neuromodulation for chronic pain. Lancet 2021;397:2111–24. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34062145&dopt=Abstract 10.1016/S0140-6736(21)00794-7 [DOI] [PubMed] [Google Scholar]
- 125.Quesada C, Pommier B, Fauchon C, Bradley C, Créac’h C, Murat M, et al. New procedure of high-frequency repetitive transcranial magnetic stimulation for central neuropathic pain: a placebo-controlled randomized crossover study. Pain 2020;161:718–28. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31764387&dopt=Abstract 10.1097/j.pain.0000000000001760 [DOI] [PubMed] [Google Scholar]
- 126.Hosomi K, Sugiyama K, Nakamura Y, Shimokawa T, Oshino S, Goto Y, et al. TEN-P11-01 investigators . A randomized controlled trial of 5 daily sessions and continuous trial of 4 weekly sessions of repetitive transcranial magnetic stimulation for neuropathic pain. Pain 2020;161:351–60. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31593002&dopt=Abstract 10.1097/j.pain.0000000000001712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao CG, Sun W, Ju F, Wang H, Sun XL, Mou X, et al. Analgesic Effects of Directed Repetitive Transcranial Magnetic Stimulation in Acute Neuropathic Pain After Spinal Cord Injury. Pain Med 2020;21:1216–23. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31722404&dopt=Abstract 10.1093/pm/pnz290 [DOI] [PubMed] [Google Scholar]
- 128.Zhao CG, Sun W, Ju F, Jiang S, Wang H, Sun XL, et al. Analgesic Effects of Navigated Repetitive Transcranial Magnetic Stimulation in Patients With Acute Central Poststroke Pain. Pain Ther 2021;10:1085–100. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33866522&dopt=Abstract 10.1007/s40122-021-00270-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Attal N, Poindessous-Jazat F, De Chauvigny E, Quesada C, Mhalla A, Ayache SS, et al. Repetitive transcranial magnetic stimulation for neuropathic pain: a randomized multicentre sham-controlled trial. Brain 2021;144:3328–39. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34196698&dopt=Abstract 10.1093/brain/awab208 [DOI] [PubMed] [Google Scholar]
- 130.Li J, Mi TM, Zhu BF, Ma JH, Han C, Li Y, et al. High-frequency repetitive transcranial magnetic stimulation over the primary motor cortex relieves musculoskeletal pain in patients with Parkinson’s disease: A randomized controlled trial. Parkinsonism Relat Disord 2020;80:113–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32980772&dopt=Abstract 10.1016/j.parkreldis.2020.07.006 [DOI] [PubMed] [Google Scholar]
- 131.Guinot M, Maindet C, Hodaj H, Hodaj E, Bachasson D, Baillieul S, et al. Effects of Repetitive Transcranial Magnetic Stimulation and Multicomponent Therapy in Patients With Fibromyalgia: A Randomized Controlled Trial. Arthritis Care Res (Hoboken) 2021;73:449–58. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31785190&dopt=Abstract 10.1002/acr.24118 [DOI] [PubMed] [Google Scholar]
- 132.Lefaucheur JP, Drouot X, Keravel Y, Nguyen JP. Pain relief induced by repetitive transcranial magnetic stimulation of precentral cortex. Neuroreport 2001;12:2963–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11588611&dopt=Abstract 10.1097/00001756-200109170-00041 [DOI] [PubMed] [Google Scholar]
- 133.Yang L, Wang SH, Hu Y, Sui YF, Peng T, Guo TC. Effects of Repetitive Transcranial Magnetic Stimulation on Astrocytes Proliferation and nNOS Expression in Neuropathic Pain Rats. Curr Med Sci 2018;38:482–90. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30074216&dopt=Abstract 10.1007/s11596-018-1904-3 [DOI] [PubMed] [Google Scholar]
- 134.Houzé B, Bradley C, Magnin M, Garcia-Larrea L. Changes in sensory hand representation and pain thresholds induced by motor cortex stimulation in humans. Cereb Cortex 2013;23:2667–76. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22918979&dopt=Abstract 10.1093/cercor/bhs255 [DOI] [PubMed] [Google Scholar]
- 135.Lefaucheur JP, Ayache SS, Sorel M, Farhat WH, Zouari HG, Ciampi de Andrade D, et al. Analgesic effects of repetitive transcranial magnetic stimulation of the motor cortex in neuropathic pain: influence of theta burst stimulation priming. Eur J Pain 2012;16:1403–13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22508405&dopt=Abstract 10.1002/j.1532-2149.2012.00150.x [DOI] [PubMed] [Google Scholar]
- 136.Scarpetta S, Zhaoping L, Hertz J. Hebbian imprinting and retrieval in oscillatory neural networks. Neural Comput 2002;14:2371–96. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12396567&dopt=Abstract 10.1162/08997660260293265 [DOI] [PubMed] [Google Scholar]
- 137.Axmacher N, Mormann F, Fernández G, Elger CE, Fell J. Memory formation by neuronal synchronization. Brain Res Brain Res Rev 2006;52:170–82. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16545463&dopt=Abstract 10.1016/j.brainresrev.2006.01.007 [DOI] [PubMed] [Google Scholar]
- 138.Paus T, Sipila PK, Strafella AP. Synchronization of neuronal activity in the human primary motor cortex by transcranial magnetic stimulation: an EEG study. J Neurophysiol 2001;86:1983–90. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11600655&dopt=Abstract 10.1152/jn.2001.86.4.1983 [DOI] [PubMed] [Google Scholar]
- 139.Mori N, Hosomi K, Nishi A, Matsugi A, Dong D, Oshino S, et al. Exploratory study of optimal parameters of repetitive transcranial magnetic stimulation for neuropathic pain in the lower extremities. Pain Rep 2021;6:e964. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34667918&dopt=Abstract 10.1097/PR9.0000000000000964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gamboa OL, Antal A, Moliadze V, Paulus W. Simply longer is not better: reversal of theta burst after-effect with prolonged stimulation. Exp Brain Res 2010;204:181–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20567808&dopt=Abstract 10.1007/s00221-010-2293-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ambriz-Tututi M, Sánchez-González V, Drucker-Colín R. Transcranial magnetic stimulation reduces nociceptive threshold in rats. J Neurosci Res 2012;90:1085–95. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22315163&dopt=Abstract 10.1002/jnr.22785 [DOI] [PubMed] [Google Scholar]
- 142.Hodaj H, Alibeu JP, Payen JF, Lefaucheur JP. Treatment of chronic facial pain including cluster headache by repetitive transcranial magnetic stimulation of the motor cortex with maintenance sessions: a naturalistic study. Brain Stimul 2015;8:801–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25979838&dopt=Abstract 10.1016/j.brs.2015.01.416 [DOI] [PubMed] [Google Scholar]
- 143.Pommier B, Quesada C, Nuti C, Peyron R, Vassal F. Is the analgesic effect of motor cortex stimulation somatotopically driven or not? Neurophysiol Clin 2020;50:195–203. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32434693&dopt=Abstract 10.1016/j.neucli.2020.04.002 [DOI] [PubMed] [Google Scholar]
- 144.Lefaucheur JP. Cortical neurostimulation for neuropathic pain: state of the art and perspectives. Pain 2016;157(Suppl 1):S81–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26785160&dopt=Abstract 10.1097/j.pain.0000000000000401 [DOI] [PubMed] [Google Scholar]
- 145.Ayache SS, Ahdab R, Chalah MA, Farhat WH, Mylius V, Goujon C, et al. Analgesic effects of navigated motor cortex rTMS in patients with chronic neuropathic pain. Eur J Pain 2016;20:1413–22. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27061948&dopt=Abstract 10.1002/ejp.864 [DOI] [PubMed] [Google Scholar]
- 146.Andre-Obadia N, Magnin M, Simon E, Garcia-Larrea L. Somatotopic effects of rTMS in neuropathic pain? A comparison between stimulation over hand and face motor areas. Eur J Pain 2018;22:707–15. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29194849&dopt=Abstract 10.1002/ejp.1156 [DOI] [PubMed] [Google Scholar]
- 147.Sun X, Long H, Zhao C, Duan Q, Zhu H, Chen C, et al. Analgesia-enhancing effects of repetitive transcranial magnetic stimulation on neuropathic pain after spinal cord injury:An fNIRS study. Restor Neurol Neurosci 2019;37:497–507. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31381538&dopt=Abstract 10.3233/RNN-190934 [DOI] [PubMed] [Google Scholar]
- 148.Hirayama A, Saitoh Y, Kishima H, Shimokawa T, Oshino S, Hirata M, et al. Reduction of intractable deafferentation pain by navigation-guided repetitive transcranial magnetic stimulation of the primary motor cortex. Pain 2006;122:22–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16495011&dopt=Abstract 10.1016/j.pain.2005.12.001 [DOI] [PubMed] [Google Scholar]
- 149.de Oliveira RA, de Andrade DC, Mendonça M, Barros R, Luvisoto T, Myczkowski ML, et al. Repetitive transcranial magnetic stimulation of the left premotor/dorsolateral prefrontal cortex does not have analgesic effect on central poststroke pain. J Pain 2014;15:1271–81. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25267523&dopt=Abstract 10.1016/j.jpain.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 150.Seifert F, Fuchs O, Nickel FT, Garcia M, Dörfler A, Schaller G, et al. A functional magnetic resonance imaging navigated repetitive transcranial magnetic stimulation study of the posterior parietal cortex in normal pain and hyperalgesia. Neuroscience 2010;170:670–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20643193&dopt=Abstract 10.1016/j.neuroscience.2010.07.024 [DOI] [PubMed] [Google Scholar]
- 151.Borckardt JJ, Weinstein M, Reeves ST, Kozel FA, Nahas Z, Smith AR, et al. Postoperative left prefrontal repetitive transcranial magnetic stimulation reduces patient-controlled analgesia use. Anesthesiology 2006;105:557–62. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16931989&dopt=Abstract 10.1097/00000542-200609000-00020 [DOI] [PubMed] [Google Scholar]
- 152.Borckardt JJ, Reeves ST, Kotlowski P, Abernathy JH, Field LC, Dong L, et al. Fast left prefrontal rTMS reduces post-gastric bypass surgery pain: findings from a large-scale, double-blind, sham-controlled clinical trial. Brain Stimul 2014;7:42–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24527503&dopt=Abstract 10.1016/j.brs.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 153.Nardone R, Höller Y, Langthaler PB, Lochner P, Golaszewski S, Schwenker K, et al. rTMS of the prefrontal cortex has analgesic effects on neuropathic pain in subjects with spinal cord injury. Spinal Cord 2017;55:20–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27241450&dopt=Abstract 10.1038/sc.2016.87 [DOI] [PubMed] [Google Scholar]
- 154.Cheng CM, Wang SJ, Su TP, Chen MH, Hsieh JC, Ho ST, et al. Analgesic effects of repetitive transcranial magnetic stimulation on modified 2010 criteria-diagnosed fibromyalgia: pilot study. Psychiatry Clin Neurosci 2019;73:187–93. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30588705&dopt=Abstract 10.1111/pcn.12812 [DOI] [PubMed] [Google Scholar]
- 155.Forogh B, Haqiqatshenas H, Ahadi T, Ebadi S, Alishahi V, Sajadi S. Repetitive transcranial magnetic stimulation (rTMS) versus transcranial direct current stimulation (tDCS) in the management of patients with fibromyalgia: A randomized controlled trial. Neurophysiol Clin 2021;51:339–47. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33814258&dopt=Abstract 10.1016/j.neucli.2021.03.002 [DOI] [PubMed] [Google Scholar]
- 156.Short BE, Borckardt JJ, Anderson BS, Frohman H, Beam W, Reeves ST, et al. Ten sessions of adjunctive left prefrontal rTMS significantly reduces fibromyalgia pain: a randomized, controlled pilot study. Pain 2011;152:2477–84. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21764215&dopt=Abstract 10.1016/j.pain.2011.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Avery DH, Zarkowski P, Krashin D, Rho WK, Wajdik C, Joesch JM, et al. Transcranial magnetic stimulation in the treatment of chronic widespread pain: a randomized controlled study. J ECT 2015;31:57–66. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24755729&dopt=Abstract 10.1097/YCT.0000000000000125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fitzgibbon BM, Hoy KE, Knox LA, Guymer EK, Littlejohn G, Elliot D, et al. Evidence for the improvement of fatigue in fibromyalgia: A 4-week left dorsolateral prefrontal cortex repetitive transcranial magnetic stimulation randomized-controlled trial. Eur J Pain 2018;22:1255–67. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29542208&dopt=Abstract 10.1002/ejp.1213 [DOI] [PubMed] [Google Scholar]
- 159.Bilir I, Askin A, Sengul I, Tosun A. Effects of High-Frequency Neuronavigated Repetitive Transcranial Magnetic Stimulation in Fibromyalgia Syndrome: A Double-Blinded, Randomized Controlled Study. Am J Phys Med Rehabil 2021;100:138–46. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32701637&dopt=Abstract 10.1097/PHM.0000000000001536 [DOI] [PubMed] [Google Scholar]
- 160.Altas EU, Askin A, Beşiroğlu L, Tosun A. Is high-frequency repetitive transcranial magnetic stimulation of the left primary motor cortex superior to the stimulation of the left dorsolateral prefrontal cortex in fibromyalgia syndrome? Somatosens Mot Res 2019;36:56–62. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30955403&dopt=Abstract 10.1080/08990220.2019.1587400 [DOI] [PubMed] [Google Scholar]
- 161.Imperatore JP, McCalley DM, Borckardt JJ, Brady KT, Hanlon CA. Non-invasive brain stimulation as a tool to decrease chronic pain in current opiate users: A parametric evaluation of two promising cortical targets. Drug Alcohol Depend 2021;218:108409. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33250384&dopt=Abstract 10.1016/j.drugalcdep.2020.108409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Freigang S, Lehner C, Fresnoza SM, Mahdy Ali K, Hlavka E, Eitler A, et al. Comparing the Impact of Multi-Session Left Dorsolateral Prefrontal and Primary Motor Cortex Neuronavigated Repetitive Transcranial Magnetic Stimulation (nrTMS) on Chronic Pain Patients. Brain Sci 2021;11:961. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34439580&dopt=Abstract 10.3390/brainsci11080961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hamid P, Malik BH, Hussain ML. Noninvasive Transcranial Magnetic Stimulation (TMS) in Chronic Refractory Pain: A Systematic Review. Cureus 2019;11:e6019. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31824787&dopt=Abstract 10.7759/cureus.6019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Che X, Cash RF, Luo X, Luo H, Lu X, Xu F, et al. High-frequency rTMS over the dorsolateral prefrontal cortex on chronic and provoked pain: A systematic review and meta-analysis. Brain Stimul 2021;14:1135–46. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34280583&dopt=Abstract 10.1016/j.brs.2021.07.004 [DOI] [PubMed] [Google Scholar]
- 165.Tanwar S, Mattoo B, Kumar U, Bhatia R. Repetitive transcranial magnetic stimulation of the prefrontal cortex for fibromyalgia syndrome: a randomised controlled trial with 6-months follow up. Adv Rheumatol 2020;60:34. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32600394&dopt=Abstract 10.1186/s42358-020-00135-7 [DOI] [PubMed] [Google Scholar]
- 166.Nizard J, Esnault J, Bouche B, Suarez Moreno A, Lefaucheur JP, Nguyen JP. Long-Term Relief of Painful Bladder Syndrome by High-Intensity, Low-Frequency Repetitive Transcranial Magnetic Stimulation of the Right and Left Dorsolateral Prefrontal Cortices. Front Neurosci 2018;12:925. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30618554&dopt=Abstract 10.3389/fnins.2018.00925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schecklmann M, Sakreida K, Oblinger B, Langguth B, Poeppl TB. Repetitive Transcranial Magnetic Stimulation as a Potential Tool to Reduce Sexual Arousal: A Proof of Concept Study. J Sex Med 2020;17:1553–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32580914&dopt=Abstract 10.1016/j.jsxm.2020.05.002 [DOI] [PubMed] [Google Scholar]
- 168.Li X, Hartwell KJ, Owens M, Lematty T, Borckardt JJ, Hanlon CA, et al. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol Psychiatry 2013;73:714–20. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23485014&dopt=Abstract 10.1016/j.biopsych.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.McNeill AM, Monk RL, Qureshi AW, Makris S, Cazzato V, Heim D. Elevated ad libitum alcohol consumption following continuous theta burst stimulation to the left-dorsolateral prefrontal cortex is partially mediated by changes in craving. Cogn Affect Behav Neurosci 2022;22:160–70. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34410618&dopt=Abstract 10.3758/s13415-021-00940-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dum RP, Levinthal DJ, Strick PL. The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. J Neurosci 2009;29:14223–35. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19906970&dopt=Abstract 10.1523/JNEUROSCI.3398-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lindholm P, Lamusuo S, Taiminen T, Pesonen U, Lahti A, Virtanen A, et al. Right secondary somatosensory cortex-a promising novel target for the treatment of drug-resistant neuropathic orofacial pain with repetitive transcranial magnetic stimulation. Pain 2015;156:1276–83. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25830924&dopt=Abstract 10.1097/j.pain.0000000000000175 [DOI] [PubMed] [Google Scholar]
- 172.Dongyang L, Fernandes AM, da Cunha PH, Tibes R, Sato J, Listik C, et al. Posterior-superior insular deep transcranial magnetic stimulation alleviates peripheral neuropathic pain - A pilot double-blind, randomized cross-over study. Neurophysiol Clin 2021;51:291–302. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34175192&dopt=Abstract 10.1016/j.neucli.2021.06.003 [DOI] [PubMed] [Google Scholar]
- 173.Ojala J, Vanhanen J, Harno H, Lioumis P, Vaalto S, Kaunisto MA, et al. A Randomized, Sham-Controlled Trial of Repetitive Transcranial Magnetic Stimulation Targeting M1 and S2 in Central Poststroke Pain: A Pilot Trial. Neuromodulation 2021. [Epub ahead of print]. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34370364&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 174.Galhardoni R, Aparecida da Silva V, García-Larrea L, Dale C, Baptista AF, Barbosa LM, et al. Insular and anterior cingulate cortex deep stimulation for central neuropathic pain: disassembling the percept of pain. Neurology 2019;92:e2165–75. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30952795&dopt=Abstract 10.1212/WNL.0000000000007396 [DOI] [PubMed] [Google Scholar]
- 175.Lenoir C, Algoet M, Vanderclausen C, Peeters A, Santos SF, Mouraux A. Report of one confirmed generalized seizure and one suspected partial seizure induced by deep continuous theta burst stimulation of the right operculo-insular cortex. Brain Stimul 2018;11:1187–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29805097&dopt=Abstract 10.1016/j.brs.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hagiwara K, Isnard J, Peyron R, Garcia-Larrea L. Theta-burst-induced seizures reported by Lenoir et al.: anterior or posterior insular seizures? Brain Stimul 2019;12:200–1. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30377087&dopt=Abstract 10.1016/j.brs.2018.10.013 [DOI] [PubMed] [Google Scholar]
- 177.Khedr EM, Kotb H, Kamel NF, Ahmed MA, Sadek R, Rothwell JC. Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry 2005;76:833–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15897507&dopt=Abstract 10.1136/jnnp.2004.055806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mhalla A, Baudic S, de Andrade DC, Gautron M, Perrot S, Teixeira MJ, et al. Long-term maintenance of the analgesic effects of transcranial magnetic stimulation in fibromyalgia. Pain 2011;152:1478–85. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21397400&dopt=Abstract 10.1016/j.pain.2011.01.034 [DOI] [PubMed] [Google Scholar]
- 179.Lefaucheur JP, Drouot X, Ménard-Lefaucheur I, Nguyen JP. Neuropathic pain controlled for more than a year by monthly sessions of repetitive transcranial magnetic stimulation of the motor cortex. Neurophysiol Clin 2004;34:91–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15130555&dopt=Abstract 10.1016/j.neucli.2004.02.001 [DOI] [PubMed] [Google Scholar]
- 180.Kobayashi M, Fujimaki T, Mihara B, Ohira T. Repetitive transcranial magnetic stimulation once a week induces sustainable long-term relief of central poststroke pain. Neuromodulation 2015;18:249–54. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25906811&dopt=Abstract 10.1111/ner.12301 [DOI] [PubMed] [Google Scholar]
- 181.Quesada C, Pommier B, Fauchon C, Bradley C, CréacCh C, Vassal F, et al. Robot-guided neuronavigated repetitive transcranial magnetic stimulation (rTMS) in central neuropathic pain. Arch Phys Med Rehabil 2018;99:2203–2215.e1. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29750900&dopt=Abstract 10.1016/j.apmr.2018.04.013 [DOI] [PubMed]
- 182.Okada A, Nishikawa A, Fukushima T, Taniguchi K, Miyazaki F, Sekino M, et al. Magnetic navigation system for home use of repetitive transcranial magnetic stimulation (rTMS) 2012 ICME International Conference on Complex Medical Engineering (CME); 2012. [Google Scholar]
- 183.Kawasaki Y, Yamamoto K, Hosomi K, Saitoh Y, Sekino M. Development of double-D coils for Transcranial Magnetic Stimulation treatment at home. 8th International IEEE/EMBS Conference on Neural Engineering (NER); 2017. [Google Scholar]
- 184.Jocks IT. Scribonius Largus’ Compounding of Drugs (Compositiones medicamentorum): introduction, translation, and medico-historical comments. PhD thesis, Vol. II: Translation with explanatory notes and medico-historical comments; 2020 [Internet]. Available from: http://theses.gla.ac.uk/82178/ [cited 2022, Mar 22].
- 185.Priori A. Brain polarization in humans: a reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin Neurophysiol 2003;114:589–95. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12686266&dopt=Abstract 10.1016/S1388-2457(02)00437-6 [DOI] [PubMed] [Google Scholar]
- 186.Terzuolo CA, Bullock TH. Measurement of imposed voltage gradient adequate to modulate neuronal firing. Proc Natl Acad Sci USA 1956;42:687–94. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16589932&dopt=Abstract 10.1073/pnas.42.9.687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bindman LJ, Lippoldand OC, Redfearn JW. The action of brief polarizing currents on the cerebralcortex of the rat. J Physiol 1964;172:369–82. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14199369&dopt=Abstract 10.1113/jphysiol.1964.sp007425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dymond AM, Coger RW, Serafetinides EA. Intracerebral current levels in man during electrosleep therapy. Biol Psychiatry 1975;10:101–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1120172&dopt=Abstract [PubMed] [Google Scholar]
- 189.Creutzfeldt OD, Fromm GH, Kapp H. Influence of transcortical d-c currents on cortical neuronal activity. Exp Neurol 1962;5:436–52. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=13882165&dopt=Abstract 10.1016/0014-4886(62)90056-0 [DOI] [PubMed] [Google Scholar]
- 190.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000;527:633–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10990547&dopt=Abstract 10.1111/j.1469-7793.2000.t01-1-00633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Borckardt JJ, Bikson M, Frohman H, Reeves ST, Datta A, Bansal V, et al. A pilot study of the tolerability and effects of high-definition transcranial direct current stimulation (HD-tDCS) on pain perception. J Pain 2012;13:112–20. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22104190&dopt=Abstract 10.1016/j.jpain.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 192.Solomons CD, Shanmugasundaram V. Transcranial direct current stimulation: A review of electrode characteristics and materials. Med Eng Phys 2020;85:63–74. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33081965&dopt=Abstract 10.1016/j.medengphy.2020.09.015 [DOI] [PubMed] [Google Scholar]
- 193.Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, et al. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci 2005;22:495–504. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16045502&dopt=Abstract 10.1111/j.1460-9568.2005.04233.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yoon EJ, Kim YK, Kim HR, Kim SE, Lee Y, Shin HI. Transcranial direct current stimulation to lessen neuropathic pain after spinal cord injury: a mechanistic PET study. Neurorehabil Neural Repair 2014;28:250–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24213958&dopt=Abstract 10.1177/1545968313507632 [DOI] [PubMed] [Google Scholar]
- 195.Naegel S, Biermann J, Theysohn N, Kleinschnitz C, Diener HC, Katsarava Z, et al. Polarity- specific modulation of pain processing by transcranial direct current stimulation –a blinded longitudinal fMRI study. J Headache Pain 2019;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Antal A, Terney D, Kühnl S, Paulus W. Anodal transcranial direct current stimulation of the motor cortex ameliorates chronic pain and reduces short intracortical inhibition. J Pain Symptom Manage 2010;39:890–903. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20471549&dopt=Abstract 10.1016/j.jpainsymman.2009.09.023 [DOI] [PubMed] [Google Scholar]
- 197.Fischer DB, Fried PJ, Ruffini G, Ripolles O, Salvador R, Banus J, et al. Multifocal tDCS targeting the resting state motor network increases cortical excitability beyond traditional tDCS targeting unilateral motor cortex. Neuroimage 2017;157:34–44. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28572060&dopt=Abstract 10.1016/j.neuroimage.2017.05.060 [DOI] [PubMed] [Google Scholar]
- 198.Gregoret L, Zamorano AM, Graven-Nielsen T. Effects of multifocal transcranial direct current stimulation targeting the motor network during prolonged experimental pain. Eur J Pain 2021;25:1241–53. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33539582&dopt=Abstract 10.1002/ejp.1743 [DOI] [PubMed] [Google Scholar]
- 199.Polanía R, Paulus W, Nitsche MA. Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum Brain Mapp 2012;33:2499–508. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21922602&dopt=Abstract 10.1002/hbm.21380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Luft CD, Pereda E, Banissy MJ, Bhattacharya J. Best of both worlds: promise of combining brain stimulation and brain connectome. Front Syst Neurosci 2014;8:132. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25126060&dopt=Abstract 10.3389/fnsys.2014.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cummiford CM, Nascimento TD, Foerster BR, Clauw DJ, Zubieta JK, Harris RE, et al. Changes in resting state functional connectivity after repetitive transcranial direct current stimulation applied to motor cortex in fibromyalgia patients. Arthritis Res Ther 2016;18:40. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26842987&dopt=Abstract 10.1186/s13075-016-0934-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.DaSilva AF, Truong DQ, DosSantos MF, Toback RL, Datta A, Bikson M. State-of-art neuroanatomical target analysis of high-definition and conventional tDCS montages used for migraine and pain control. Front Neuroanat 2015;9:89. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26236199&dopt=Abstract 10.3389/fnana.2015.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Garnett EO, Malyutina S, Datta A, den Ouden DB. On the Use of the Terms Anodal and Cathodal in High-Definition Transcranial Direct Current Stimulation: A Technical Note. Neuromodulation 2015;18:705–13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26076228&dopt=Abstract 10.1111/ner.12320 [DOI] [PubMed] [Google Scholar]
- 204.Foerster BR, Nascimento TD, DeBoer M, Bender MA, Rice IC, Truong DQ, et al. Excitatory and inhibitory brain metabolites as targets of motor cortex transcranial direct current stimulation therapy and predictors of its efficacy in fibromyalgia. Arthritis Rheumatol 2015;67:576–81. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25371383&dopt=Abstract 10.1002/art.38945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.DosSantos MF, Love TM, Martikainen IK, Nascimento TD, Fregni F, Cummiford C, et al. Immediate effects of tDCS on the μ-opioid system of a chronic pain patient. Front Psychiatry 2012;3:93. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23130002&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Souza A, Martins DF, Medeiros LF, Nucci-Martins C, Martins TC, Siteneski A, et al. Neurobiological mechanisms of antiallodynic effect of transcranial direct current stimulation (tDCS) in a mice model of neuropathic pain. Brain Res 2018;1682:14–23. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29274881&dopt=Abstract 10.1016/j.brainres.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 207.Hughes S, Grimsey S, Strutton PH. Primary motor cortex transcranial direct current stimulation modulates temporal summation of the nociceptive withdrawal reflex in healthy subjects. Pain Med 2019;20:1156–65. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30535165&dopt=Abstract 10.1093/pm/pny200 [DOI] [PubMed] [Google Scholar]
- 208.Meeker TJ, Keaser ML, Khan SA, Gullapalli RP, Seminowicz DA, Greenspan JD. Non- invasive motor cortex neuromodulation reduces secondary hyperalgesia and enhances activation of the descending pain modulatory network. Front Neurosci 2019;13:467. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31139047&dopt=Abstract 10.3389/fnins.2019.00467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gan Z, Li H, Naser PV, Oswald MJ, Kuner R. Suppression of neuropathic pain and comorbidities by recurrent cycles of repetitive transcranial direct current motor cortex stimulation in mice. Sci Rep 2021;11:9735. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33958647&dopt=Abstract 10.1038/s41598-021-89122-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Grundmann L, Rolke R, Nitsche MA, Pavlakovic G, Happe S, Treede RD, et al. Effects of transcranial direct current stimulation of the primary sensory cortex on somatosensory perception. Brain Stimul 2011;4:253–60. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22032740&dopt=Abstract 10.1016/j.brs.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 211.Jürgens TP, Schulte A, Klein T, May A. Transcranial direct current stimulation does neither modulate results of a quantitative sensory testing protocol nor ratings of suprathreshold heat stimuli in healthy volunteers. Eur J Pain 2012;16:1251–63. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22416036&dopt=Abstract 10.1002/j.1532-2149.2012.00135.x [DOI] [PubMed] [Google Scholar]
- 212.Kold S, Graven-Nielsen T. Effect of anodal high-definition transcranial direct current stimulation on the pain sensitivity in a healthy population: a double-blind, sham-controlled study. Pain 2021;162:1659–68. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33449508&dopt=Abstract 10.1097/j.pain.0000000000002187 [DOI] [PubMed] [Google Scholar]
- 213.Hughes SW, Ward G, Strutton PH. Anodal transcranial direct current stimulation over the primary motor cortex attenuates capsaicin-induced dynamic mechanical allodynia and mechanical pain sensitivity in humans. Eur J Pain 2020;24:1130–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32170802&dopt=Abstract 10.1002/ejp.1557 [DOI] [PubMed] [Google Scholar]
- 214.Quesada C, Kostenko A, Ho I, Leone C, Nochi Z, Stouffs A, et al. Human surrogate models of central sensitization: A critical review and practical guide. Eur J Pain 2021;25:1389–428. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33759294&dopt=Abstract 10.1002/ejp.1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mehta S, McIntyre A, Guy S, Teasell RW, Loh E. Effectiveness of transcranial direct current stimulation for the management of neuropathic pain after spinal cord injury: a meta-analysis. Spinal Cord 2015;53:780–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26193817&dopt=Abstract 10.1038/sc.2015.118 [DOI] [PubMed] [Google Scholar]
- 216.Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol 2017;128:56–92. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27866120&dopt=Abstract 10.1016/j.clinph.2016.10.087 [DOI] [PubMed] [Google Scholar]
- 217.Xu XM, Luo H, Rong BB, Zheng XM, Wang FT, Zhang SJ, et al. Nonpharmacological therapies for central poststroke pain: A systematic review. Medicine (Baltimore) 2020;99:e22611. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33080696&dopt=Abstract 10.1097/MD.0000000000022611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Antal A. Transcranial Direct Current Stimulation in the Treatment of Facial Pain. Prog Neurol Surg 2020;35:116–24. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32694260&dopt=Abstract 10.1159/000509655 [DOI] [PubMed] [Google Scholar]
- 219.Yang S, Chang MC. Transcranial Direct Current Stimulation for the Management of Neuropathic Pain: A Narrative Review. Pain Physician 2021;24:E771–81. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34554695&dopt=Abstract 10.36076/ppj.2021.24.E771 [DOI] [PubMed] [Google Scholar]
- 220.Yu B, Qiu H, Li J, Zhong C, Li J. Noninvasive Brain Stimulation Does Not Improve Neuropathic Pain in Individuals With Spinal Cord Injury: Evidence From a Meta-Analysis of 11 Randomized Controlled Trials. Am J Phys Med Rehabil 2020;99:811–20. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32175926&dopt=Abstract 10.1097/PHM.0000000000001421 [DOI] [PubMed] [Google Scholar]
- 221.Pessoa BL, Escudeiro G, Nascimento OJ. Emerging Treatments for Neuropathic Pain. Curr Pain Headache Rep 2015;19:56. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26530058&dopt=Abstract 10.1007/s11916-015-0530-z [DOI] [PubMed] [Google Scholar]
- 222.David MC, Moraes AA, Costa ML, Franco CI. Transcranial direct current stimulation in the modulation of neuropathic pain: a systematic review. Neurol Res 2018;40:555–63. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29600889&dopt=Abstract 10.1080/01616412.2018.1453190 [DOI] [PubMed] [Google Scholar]
- 223.Shen Z, Li Z, Ke J, He C, Liu Z, Zhang D, et al. Effect of non-invasive brain stimulation on neuropathic pain following spinal cord injury: A systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e21507. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32846761&dopt=Abstract 10.1097/MD.0000000000021507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Li C, Jirachaipitak S, Wrigley P, Xu H, Euasobhon P. Transcranial direct current stimulation for spinal cord injury-associated neuropathic pain. Korean J Pain 2021;34:156–64. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33785667&dopt=Abstract 10.3344/kjp.2021.34.2.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Baptista AF, Fernandes AM, Sá KN, Okano AH, Brunoni AR, Lara-Solares A, et al. Latin American and Caribbean consensus on noninvasive central nervous system neuromodulation for chronic pain management (LAC2-NIN-CP). Pain Rep 2019;4:e692. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30801041&dopt=Abstract 10.1097/PR9.0000000000000692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Brighina F, Curatolo M, Cosentino G, De Tommaso M, Battaglia G, Sarzi-Puttini PC, et al. Brain Modulation by Electric Currents in Fibromyalgia: A Structured Review on Non-invasive Approach With Transcranial Electrical Stimulation. Front Hum Neurosci 2019;13:40. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30804771&dopt=Abstract 10.3389/fnhum.2019.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Teixeira PE, Alawdah L, Alhassan HA, Guidetti M, Priori A, Papatheodorou S, et al. The Analgesic Effect of Transcranial Direct Current Stimulation (tDCS) combined with Physical Therapy on Common Musculoskeletal Conditions: A Systematic Review and Meta-Analysis. Princ Pract Clin Res 2020;6:23–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32766451&dopt=Abstract 10.21801/ppcrj.2020.61.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Conde-Antón Á, Hernando-Garijo I, Jiménez-Del-Barrio S, Mingo-Gómez MT, Medrano-de-la-Fuente R, Ceballos-Laita L. Effects of transcranial direct current stimulation and transcranial magnetic stimulation in patients with fibromyalgia. A systematic review. Neurologia (Engl Ed) 2020. [Spanish] [Epub ahead of print]. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33071017&dopt=Abstract 10.1016/j.nrl.2020.07.024 [DOI] [PubMed]
- 229.Lloyd DM, Wittkopf PG, Arendsen LJ, Jones AK. Is Transcranial Direct Current Stimulation (tDCS) Effective for the Treatment of Pain in Fibromyalgia? A Systematic Review and Meta-Analysis. J Pain 2020;21:1085–100. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31982685&dopt=Abstract 10.1016/j.jpain.2020.01.003 [DOI] [PubMed] [Google Scholar]
- 230.Riberto M, Marcon Alfieri F, Monteiro de Benedetto Pacheco K, Dini Leite V, Nemoto Kaihami H, Fregni F, et al. Efficacy of transcranial direct current stimulation coupled with a multidisciplinary rehabilitation program for the treatment of fibromyalgia. Open Rheumatol J 2011;5:45–50. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22046206&dopt=Abstract 10.2174/1874312901105010045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Fagerlund AJ, Hansen OA, Aslaksen PM. Transcranial direct current stimulation as a treatment for patients with fibromyalgia: a randomized controlled trial. Pain 2015;156:62–71. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25599302&dopt=Abstract 10.1016/j.pain.0000000000000006 [DOI] [PubMed] [Google Scholar]
- 232.Villamar MF, Wivatvongvana P, Patumanond J, Bikson M, Truong DQ, Datta A, et al. Focal modulation of the primary motor cortex in fibromyalgia using 4×1-ring high-definition transcranial direct current stimulation (HD-tDCS): immediate and delayed analgesic effects of cathodal and anodal stimulation. J Pain 2013;14:371–83. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23415877&dopt=Abstract 10.1016/j.jpain.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 233.Samartin-Veiga N, Pidal-Miranda M, González-Villar AJ, Bradley C, Garcia-Larrea L, O’Brien AT, et al. Transcranial direct current stimulation of three cortical targets is no more effective than placebo as treatment for fibromyalgia: a double-blind sham-controlled clinical trial. Pain 2021. [Epub ahead of print]. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34561393&dopt=Abstract 10.1097/j.pain.0000000000002493 [DOI] [PubMed]
- 234.Fregni F, Gimenes R, Valle AC, Ferreira MJ, Rocha RR, Natalle L, et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum 2006;54:3988–98. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17133529&dopt=Abstract 10.1002/art.22195 [DOI] [PubMed] [Google Scholar]
- 235.Kim YJ, Ku J, Kim HJ, Im DJ, Lee HS, Han KA, et al. Randomized, sham controlled trial of transcranial direct current stimulation for painful diabetic polyneuropathy. Ann Rehabil Med 2013;37:766–76. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24466511&dopt=Abstract 10.5535/arm.2013.37.6.766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Attal N, Ayache SS, Ciampi De Andrade D, Mhalla A, Baudic S, Jazat F, et al. Repetitive transcranial magnetic stimulation and transcranial direct-current stimulation in neuropathic pain due to radiculopathy: a randomized sham-controlled comparative study. Pain 2016;157:1224–31. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26845524&dopt=Abstract 10.1097/j.pain.0000000000000510 [DOI] [PubMed] [Google Scholar]
- 237.Hodaj H, Payen JF, Lefaucheur JP. A Case of Long-Term Treatment of Chronic Pain Syndrome by Anodal tDCS of the Motor Cortex, Previously Resistant to High-Frequency rTMS and Implanted Spinal Cord Stimulation. Brain Stimul 2016;9:618–20. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27101725&dopt=Abstract 10.1016/j.brs.2016.02.008 [DOI] [PubMed] [Google Scholar]
- 238.Bonifácio de Assis ED, Martins WK, de Carvalho CD, Ferreira CM, Gomes R, de Almeida Rodrigues ET, et al. Effects of rTMS and tDCS on neuropathic pain after brachial plexus injury: a randomized placebo-controlled pilot study. Sci Rep 2022;12:1440. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35087138&dopt=Abstract 10.1038/s41598-022-05254-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Parker T, Raghu A, Huang Y, Gillies MJ, FitzGerald JJ, Aziz T, et al. Paired Acute Invasive/Non-invasive Stimulation (PAINS) study: A phase I/II randomized, sham-controlled crossover trial in chronic neuropathic pain. Brain Stimul 2021;14:1576–85. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34673258&dopt=Abstract 10.1016/j.brs.2021.10.384 [DOI] [PubMed] [Google Scholar]
- 240.Frank E, Wilfurth S, Landgrebe M, Eichhammer P, Hajak G, Langguth B. Anodal skin lesions after treatment with transcranial direct current stimulation. Brain Stimul 2010;3:58–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20633432&dopt=Abstract 10.1016/j.brs.2009.04.002 [DOI] [PubMed] [Google Scholar]
- 241.Loo CK, Martin DM, Alonzo A, Gandevia S, Mitchell PB, Sachdev P. Avoiding skin burns with transcranial direct current stimulation: preliminary considerations. Int J Neuropsychopharmacol 2011;14:425–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20923600&dopt=Abstract 10.1017/S1461145710001197 [DOI] [PubMed] [Google Scholar]
- 242.Steenbergen L, Sellaro R, Hommel B, Lindenberger U, Kühn S, Colzato LS. “Unfocus” on foc.us: commercial tDCS headset impairs working memory. Exp Brain Res 2016;234:637–43. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26280313&dopt=Abstract 10.1007/s00221-015-4391-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wurzman R, Hamilton RH, Pascual-Leone A, Fox MD. An open letter concerning do-it-yourself users of transcranial direct current stimulation. Ann Neurol 2016;80:1–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27216434&dopt=Abstract 10.1002/ana.24689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Garcia-Larrea L, Perchet C, Hagiwara K, André-Obadia N. At-Home Cortical Stimulation for Neuropathic Pain: a Feasibility Study with Initial Clinical Results. Neurotherapeutics 2019;16:1198–209. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31062295&dopt=Abstract 10.1007/s13311-019-00734-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Brietzke AP, Zortea M, Carvalho F, Sanches PR, Silva DP, Torres IL, et al. Large Treatment Effect With Extended Home-Based Transcranial Direct Current Stimulation Over Dorsolateral Prefrontal Cortex in Fibromyalgia: A Proof of Concept Sham-Randomized Clinical Study. J Pain 2020;21:212–24. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31356985&dopt=Abstract 10.1016/j.jpain.2019.06.013 [DOI] [PubMed] [Google Scholar]