Abstract
INTRODUCTION
The primary aim of this work was to summarize and compare the effects of active rehabilitation assisted by new technologies (virtual reality [VR], robot-assisted therapy [RAT] and telerehabilitation [TR)) on upper limb motor function and everyday living activity during the subacute and chronic phases of stroke. The secondary aims were to compare the effects of these technologies according to the intervention design (in addition to or in substitution of conventional therapy), the duration of active rehabilitation and the severity of patients’ motor impairments.
EVIDENCE ACQUISITION
Several databases, namely PubMed, Scopus, Embase and Cochrane Library, were searched. Studies were included if they were meta-analyses with a moderate to high level of confidence (assessed with AMSTAR-2) that compared the effects of a new technology promoting active rehabilitation to that of a conventional therapy program among patients with stroke. Network meta-analyses were conducted to compare the effects of the new technologies.
EVIDENCE SYNTHESIS
Eighteen different meta-analyses were selected and fifteen included in the quantitative analysis. In total these 15 meta-analyses were based on 189 different randomized controlled trials. VR (SMD≥0.25; P<0.05), RAT (SMD≥0.29; P≤0.29) and TR (SMD≥-0.08; P≤0.64) were found to be at least as effective as conventional therapy. During the subacute phase, RAT’s greatest effect was observed for patients with severe-moderate impairments whereas VR and TR’s greatest effects were observed for patients with mild impairments. During the chronic phase, the highest effects were observed for patients with mild impairments, for all studies technologies. Network meta-analyses showed that VR and RAT were both significantly superior to TR in improving motor function during the chronic phase but revealed no significant difference between VR, RAT and TR effectiveness on both motor function (during the subacute phase) and activity (during both chronic and subacute phase).
CONCLUSIONS
This overview provides low-to-moderate evidence that rehabilitation assisted with technologies are at least as effective as conventional therapy for patients with stroke. While VR and RAT seem to be more efficient during the subacute phase, all technologies seem to be as efficient as one another in the chronic phase.
Key words: Stroke, Robotics, Virtual reality, Telerehabilitation
Introduction
Each year, 1.1 million people suffer from a stroke in Europe.1 By 2025, this number is expected to rise to 1.5 million, leading to an increased demand for neurorehabilitation. Despite rehabilitation, almost 50% of the patients remain with upper limb (UL) motor impairments which limits their capacity to perform activities of daily living and diminishes their quality of life.2 In this context, rehabilitation therefore needs to be early, intensive, and functional.3, 4
To answer these needs, the development of new technologies, which provide cost-effective rehabilitation, has substantially expanded in recent years. In this review, we considered new technologies as any recent technological device that assists rehabilitation.5 These new rehabilitation means aim to increase treatment intensity and offer patients the possibility to perform autonomous rehabilitation-exercises.6, 7 This includes all interactive systems or serious games displayed in virtual reality (VR), robot-assisted therapy (RAT) and telerehabilitation (TR).5, 8 Rapid and constant evolution of such technologies has enabled these rehabilitation tools to become increasingly user-friendly.9 The effectiveness of UL rehabilitation after stroke is expected to continue to improve as their evolution keep progressing.10 Any other recent passive or invasive technological means such as transcranial direct current stimulation, deep brain stimulation, transcutaneous nerve stimulation and functional electrical stimulation were not considered in this work as they rely on neuromodulation technics and require much input from the therapist.
VR is a promising computer-based technology that allows users to interact with a simulated multisensory environment.11 It aims to enhance the impact of rehabilitation and to provide feedbacks on performance. VR may be described as immersive or non-immersive. In non-immersive VR, subjects are facing, and interacting with a digital environment through a simple screen, which allows the awareness of reality. Immersive VR uses different systems such as VR helmets, motion detection devices and VR caves in order to fully project patients into a virtual environment.12 Recent meta-analyses have demonstrated a superior effect of VR when compared to conventional therapy (CT) regarding rehabilitation of UL motor function and activity.13, 14
A robot is defined as “a re-programmable, multi-functional manipulator designed to move material, parts, or specialized devices through variable programmed motions to accomplish and assist a task”.15 RAT comprises different types of devices with different technical specifications such as end-effectors or exoskeletons acting proximally or distally, unilaterally or bilaterally.16 The main purpose of RAT in neurorehabilitation is to increase treatment intensity and rehabilitate patients with moderate to strong upper-limb dysfunctions by producing repetitive passive and active-assisted movement through motor relearning exercises. With the assistance of the machine, the doses increase gradually. Therefore, personal time of therapists is not permanently required leading to a more autonomous rehabilitation.17 RAT can also provide bimanual task training by reproducing the mirror action of the unaffected limb.18 While rehabilitation robots and electromechanical devices share common characteristics in neurorehabilitation, several factors and functional modalities may differ between these devices. A recent work issued from the Italian Consensus Conference Cicerone proposes to classify and report the use of these devices under clinical, technical and regulatory perspectives.19 Numerous systematic reviews have found that RAT can improve activity and arm function of patients after stroke, irrespective of the type of device used.16, 20-23
Lastly, TR is a technological mean that aims to increase treatment-intensity by providing rehabilitation in addition to conventional therapy. TR is also a treatment solution for patients who cannot attend in-hospital therapy sessions. TR has been defined as “the delivery of rehabilitation services via information and communication technologies”.24 This rehabilitation method uses telecommunication devices such as smartphones, tablets, and video cameras to provide home-based rehabilitation.25 The safety and effectiveness of TR have been demonstrated.26 According to several randomized controlled trials (RCTs), TR seems to offer similar results as clinical rehabilitation regarding post-stroke motor recovery.27-29
There have been several systematic reviews regarding the effectiveness of these new technologies on poststroke motor function and activity.13, 14, 20-23, 30 However, to our knowledge, little comparison has been drawn between them. In fact, to date, there is no existing study or network meta-analysis that compares the effectiveness of these technologies according to the patient’s stage (acute/subacute vs chronic stroke), the intervention design (in addition vs in substitution of CT), the patient’s severity of motor impairments and the treatment duration. This overview’s primary aim is to summarize the effect of new technologies (comprising VR, RAT and TR) on upper-limb motor function and everyday living activity in a high number of patients with stroke according to data issued from the highest level of evidence. Secondary objectives were to compare the effectiveness of these technologies to one another according to patient’s time since stroke onset by conducting a network meta-analysis and to assess the influence of the rehabilitation design (in addition to vs in substitution of CT), the severity of motor impairments and the treatment duration.
Evidence acquisition
This overview of meta-analyses was performed according to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) as presented in Supplementary Digital Material 1: Supplementary Text File 1.
Search strategy
We conducted literature searches through PubMed, Scopus, Embase and Cochrane Library. Keywords related to stroke and the investigated therapies (namely VR, RAT, TR) were found using Embase’s explode function. They were then plugged into PubMed, Scopus, Embase and Cochrane library to conduct the literary search. Detailed search strategies of all databases are presented in Supplementary Digital Material 2: Supplementary Text File 2.
Eligibility criteria
The PICOS criteria was used to define eligibility. Articles were eligible if (P) the population assessed was composed of adults with a first-ever stroke; (I) the intervention involved the use of a new technology (including VR, RAT, smartphones, tablets, computers or any other device specifically developed for TR) and was compared (C) to a conventional rehabilitation; (O) relevant outcomes were measured to assess the ICF-WHO motor function or activity components and; (S) the study followed a meta-analysis design, was written in English or French, was published before February 2022 and had a moderate to high level of confidence in the results rated on A MeaSurement Tool to Assess systematic Reviews (AMSTAR) tool 2. Systematic reviews comparing the effect of a new technology to sham or placebo interventions, old versions of updated articles, conference abstracts and non-available full-text papers were excluded.
Study selection
All references issued from the search strategies were exported into the EndNote X9 reference management software. After removal of duplicates, two researchers (SL and GB) independently selected the articles based on titles and abstracts reading first. Articles were included according to the eligibility criteria. A full-text screening was then performed following the same procedure. After the second selection, a meeting was organized between the researchers. Any conflicting decisions were solved through discussion by involving a third researcher (GE) in the decision-making process.
Quality appraisal
The confidence in the results of articles was assessed by the two researchers (SL and GB) independently by using the AMSTAR-2.31 This tool is made up of 16 items in total, and each item is rated as being present (“yes” or “partial yes”) or being absent (“no”). Its validity has been demonstrated by strong correlations between AMSTAR-2 and AMSTAR (r=0.91) as well as between AMSTAR-2 and Risk of Bias in Systematic Reviews tool (ROBIS)32 (r=0.84).33 The AMSTAR-2 has a moderate inter-rater reliability (median kappa-value=0.51).33
The AMSTAR network provides an open access program (https://amstar.ca/Amstar_Checklist.php), which allows to plug in the item-answers and automatically rates the articles as a high, moderate, low or critically low quality. Two researchers (SL and GB) independently entered their evaluation on amstar.ca. Any conflicting results were solved by reaching a consensus with a third and fourth researcher (GE and LD).
Data extraction
For each included meta-analysis, two researchers (SL and GB) undertook independent data extraction of the following data: number of studies, number of participants, patient’s mean age, time since stroke, type of intervention, type of control group, time-match intervention with control group, outcomes, follow-up assessment and main findings. A third researcher (GE) extracted the number of participants, the duration of the intervention, the relevant effect sizes and their standard deviations from all randomized controlled trials assessed by the meta-analysis included in this work. For each RCT, participants’ mean Upper-Extremity Fugl-Meyer Assessment (UE-FMA) score was also extracted to assess the severity of motor impairments. Studies were then classified into three categories: severe impairments (UE-FMA: 0 to 14), severe-moderate impairments (UE-FMA: 15 to 34) and mild to moderate impairments (UE-FMA>34).34
Statistical analysis
Statistical analysis was performed using Review Manager software. Given that some of the meta-analyses included in this review assessed the same randomized controlled trials, standardized mean differences (SMD) were computed from the effect sizes of the randomized trials themselves directly, and not by calculating a resultant weighted SMD from the SMD of the meta-analyses. The SMD represents the ratio between the difference of mean effect between groups and the pooled standard deviation of the effect among participants. According to Cohen, an SMD≥0.2 is associated with a small effect size, when 0.2<SMD<0.8, the effect size is medium and if SMD≥0.8, the effects size is considered as large.35 The heterogeneity of the different included studies was assessed using I2 statistics. Based on the Cochrane Handbook, if I2<30%, heterogeneity might not be significant, when 30%<I2<50%, moderate heterogeneity may be present, when 50%<I2<75% substantial heterogeneity may be present and if I2>75% considerable heterogeneity is present. In case of heterogeneity, a random model effect was used and when heterogeneity was not present, the fixed-effect model was used. Certainty of evidence regarding forest-plots results was assessed using the GRADE approach.
Publication bias and outliers were assessed through funnel plot asymmetry and a sensitivity analysis was systematically conducted before removing outliers. We conducted subgroup analyses to assess the influence of the intervention design (in addition to vs in substitution of CT), the time since stroke onset (subacute vs chronic phase), the treatment duration (<15 hours, 15-30 hours and>30 hours) and patients’ motor impairments severity (severe, severe-moderate and moderate to mild) on the effect of new-technologies. Other sensitivity analyses were conducted to verify if the potential inclusion of non-time matched interventions influenced these results.
We also conducted network meta-analyses (according to the Cochrane Handbook) to undertake an undirect comparison between the effectiveness of the different technologies (RAT, VR, TR) according to the intervention design (in addition vs in substitution of CT) and the stroke stage (subacute vs. chronic). This method enables to estimate a SMDAC and 95% confidence interval (CI) comparing a treatment A to a treatment C by calculating the difference between the SMDAB comparing treatment A to B and SMDBC comparing treatment B to C. Resultant 95%CI of SMDAC can be estimated using this formula:
| 95%CI of SMDAC=[SMDAC - 1.96 √ (varSMDAB+ |
| var SMDBC); SMDAC+1.96 √ (varSMDAB+var SMDBC)] |
Network geometry
Network diagrams were plotted to characterize the relations and risk of bias between the different interventions (RAT, VR, TR and CT). To perform these graphs, the Github R software “netmeta” package was used (https://github.com/gertvv/gemtc).36 In these diagrams, each node represents an intervention and each line a direct or indirect comparisons between two interventions. The thickness of direct comparison was correlated with the number of RCTs comparing the two interventions connected by the line. Moreover, the color of the line corresponds to the global risk of bias (red: high, yellow: moderate, green: low). Indirect comparisons were also represented through a blue line.
Evidence synthesis
Selection and quality appraisal
A total of 2808 articles were identified in the searched databases. After duplicates were removed, 1993 articles were screened by titles and abstracts. A total of 219 articles were assessed for eligibility by retrieving and reading the full text. Thirty-three reviews were then selected. The remaining articles were excluded as they presented exclusion criteria. In total, 45% (n=15) of these selected meta-analyses were considered to be at low or critically low level of confidence and were therefore removed from the selection. The remaining eighteen full-text articles were included in the qualitative synthesis (Supplementary Digital Material 3: Material 3: Supplementary Table I)13, 14, 16, 21-23, 30, 37-47 and 15 of these articles were included in quantitative analysis since 3 articles could not provide reliable data (Figure 1).
Figure 1.
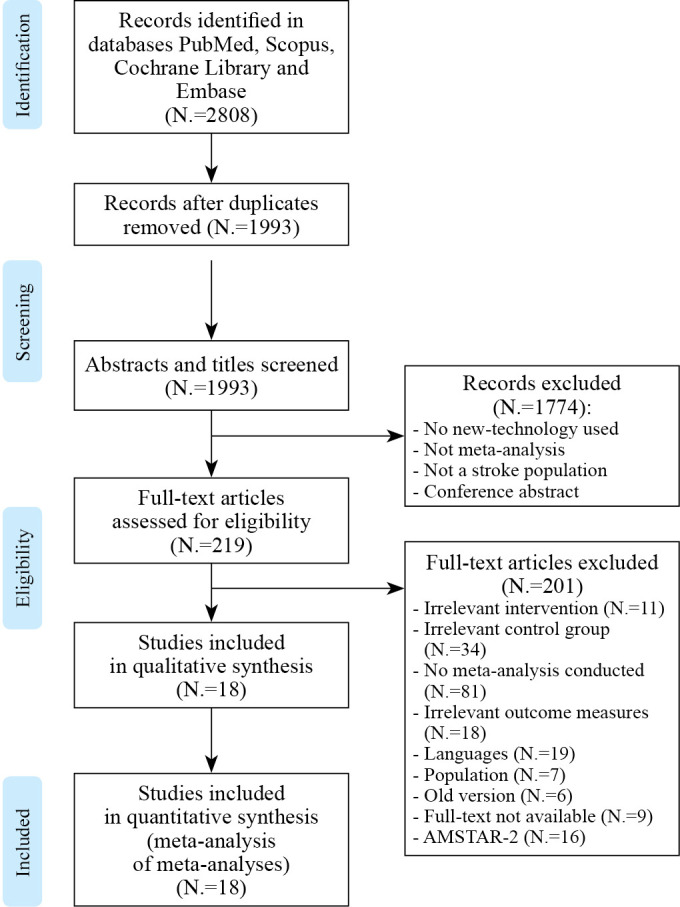
—Flow chart (PRISMA) of the selection process.
These eighteen studies were rated with a moderate N.=9, 50%) to high quality (N.=9, 50%). All reviews used appropriate statistical methods to synthesize results, eleven (61%) evaluated between-studies heterogeneity and sixteen (89%) assessed the potential impact of risk of bias in the results (Supplementary Table I).
Characteristics of the included studies
The 18 reviews included in the qualitative synthesis were based on 407 individual clinical trials. However, of these trials, 197 were comprised in two, three or even four reviews meaning that the included reviews represented 210 different randomized controlled trials. These 210 individual clinical trials assessed a total of 8265 different patients with stroke. Of these reviews, 99 RCTs (47%) focused on VR and involved 3539 patients, 92 (44%) focused on RAT and involved 3146 patients and the final 19 (9%) studied TR among a sample of 1580 patients.
For each included meta-analysis, the number of studies included in the review, the number of participants, the mean age range and the stage of stroke are presented in Table I.13, 14, 16, 21, 23, 37-47 The total mean age of participants ranged from 41 to 79.5 years old though 2 reviews did not report this data.14, 37
Table I. —Characteristics of the included studies.13, 14, 16, 21, 23, 37-47.
| Meta-analysis | Number of studies in the MA | Number of participants in the MA | Mean age (year) in the MA | Stage of stroke in the MA |
|---|---|---|---|---|
| Ahn et al. (2019)37 | 9 RCTs | 698 | - | - |
| Aminov et al. (2018)38 | 31 RCTs 28 for UL 2 for UL+cognition 1 for cognition |
971 | M=60.0 (range 48.2-74.1) | 1.9-427.8 weeks 7 studies: sub-acute (range 1.9-10.3) 24 studies: chronic (range 17.2-427.8) |
| Chien et al. (2020)21 | 11 RCTs | 493 | M range=54-71 | 9 days-4 months (M=6 weeks) |
| Coupar et al. (2012)39 | 4 RCTs | 166 | M range=53-70.2 | 56-412 days |
| Doumas et al. (2021)46 | 51 RCTs | 2083 | M range=49.3 - 76 | 31% trials: subacute 69% trials: chronic |
| Ferreira et al. (2018)22 | 38 RCTs | 1174 | M range=51.2-57.8 | 2 weeks-24 months 24 studies: chronic |
| Karamians et al. (2020)14 | 38 RCTs | 1198 | - | Acute to chronic |
| Kwakkel et al. (2008)40 | 10 RCTs | 218 | M range=55.6-67 2 missing data |
1 week ->6 months |
| Laver et al. (2017)41 | 72 RCTs 35 RCTs for UL |
2470 | M range=46-75 | 2-12 months |
| Laver et al. (2020)41 | 14 RCTs | 1748 | M range=50’s-70’s | 3 studies: acute 5 studies: sub-acute 5 studies: chronic 2 missing data |
| Lee et al. (2019)42 | 21 9 for UL 12 for LL |
562 | M range=47.55-71.35 2 missing data |
M range=8-73.5 months |
| Lo et al. (2017)43 | 51 30 for UL 21 for LL |
1798 | M=60 | 27 studies: acute/sub-acute 24 studies: chronic |
| Maier et al. (2019)13 | 30 RCTs 22 SVR 8 NSVR |
2287 | M range=49.3-73.7 | Acute: 3 studies Sub-acute: 10 studies Chronic: 18 studies 1 missing data |
| Mehrholz et al. (2020)16 | 55 53 RCTs 2 randomized cross-over trials |
2654 | M range=44-76 | M range=14 days-4 years |
| Norouzi-Gheidari et al. (2012)23 | 12 11 RCTs 1 follow-up study |
425 | M range=54-79.5 | M range=1 week-112.4 months |
| Prange et al. (2006)44 | 8 RCTs | 228 | M range=41.0-65.9 | Sub-acute: 76 patients Chronic: 152 patients |
| Rintala et al. (2019)45 | 13 RCTs -6 for UL -5 for LL |
336 | M=65.6 (range 59-76) | Missing data |
| Zhao et al. (2022)47 | 22 RCTs | 758 | M range=50-73 | M range=15.2 days – 47.9 months |
LL: lower limb; M: mean; MA: meta-analysis; NSVR: non-specific virtual reality; RCT: randomized control trial; SVR: specific virtual reality; UL: upper limb.
Characteristics of intervention and control groups as well as the time-match intervention with the control intervention (when reported by the study) are presented in Table II.13, 14, 16, 21-23, 30, 37-47 The majority of control groups received CT comprising of conventional physiotherapy, occupational and recreational therapy.
Table II. —Characteristics of intervention and control groups, outcome measures, follow-up and main findings of the included studies.13, 14, 16, 21-23, 30, 37-47.
| Meta-analysis | Intervention group | Control group | Frequency of intervention group | Time-match intervention with control group | Outcomes | Follow-up | Main findings |
|---|---|---|---|---|---|---|---|
| Ahn et al. (2019)37 | VR VR+ CT Commercial VR rTMS+VR training |
CT Sham rTMS+VR training |
- | 8 studies out of 9 | Activity: BI and FIM | - | VR improves UL function and activities. |
| Aminov et al. (2018)38 | VE interventions+CT CG: Wii, Xavix, EyeToy, IREX system, Xbox Kinect, or a combination of systems+CT |
CT: PT, OT | Duration: M=18 sessions (range 4-36) Intensity: M=153.9 min/week (range 60-180) Frequency: mean 3 sessions/week (range 1-5) |
21 studies out of 33 (=active control group) | Body function: FMA-UE Activity: BBT, FIM, BI Participation: MAL |
6 studies: 4-6 weeks follow-up 6 studies: 8-26 follow-up |
VR intervention as an adjunct improves UL body structure and activity. |
| Chien et al. (2020)21 | RAT+CT RAT alone (of the UL) Devices: SMART Arm, Armeo Spring, REAplan robot, NeReBot training, MIT-MANUS, Gloreha, Hand Mentor Pro |
CT=PT, OT, task-training program, daily rehabilitation treatment, intermittent cutaneous electric stimulation, and home exercise program | Duration: M=5.6 weeks and 25 sessions (range 2-12 weeks and 9-40 sessions) Intensity: M=75min (range 30-120) Frequency: 5 sessions/week |
8 studies out of 11 | Body function: FMA-UE, MAS Activity: FIM, BI, Activlim questionnaire, ARAT, WMFT, QuickDASH, SIS |
- | RAT=CT for function and disability |
| Coupar et al. (2012)39 | Home therapy program: functional exercises, assistive/resistive exercises with proprioceptive neuromuscular facilitation and resistive exercises VR intervention with telerehabilitation |
Usual care VR intervention with a therapist present |
- | Not mentioned | Body function: FMA-UE Activity: BI, JTHF, WMFT |
1 study: 1 month follow-up 1 study: 6 months follow-up |
Insufficient evidence to determine if home therapy program is more or less effective than CT in hospital. |
| Doumas et al. (2021)46 | -Serious games alone -Serious games+CT Devices: end-effectors, motion capture gloves, exoskeletons, immersive VR, smartphones, tablets, EMG-controlled sensor, arm support system |
CT: OT, PT | Duration: M=5 weeks (range 2-12 weeks) Intensity: M range=30-225 min |
44 studies out of 51 | -Body function: FMA-UE -Activity: ARAT, WFMT, BBT -Participation: SIS scale |
50% of trials M=2.3 months (range 1-6) |
Serious games (displayed with RAT, VR or TR devices) showed superior results to CT for UL motor function, activity and participation |
| Ferreira et al. (2018)22 | RAT alone RAT added to CT, standard therapy, motor learning, repetitive task-specific practice, an arm-hand training program and functional task practice Devices: MIT-MANUS, Haptic Knob, ARMinIII, MIME, UL-EXO7, InMotion2, Bi-Manu Track, ARM Guide |
MI: sham RAT, no intervention, placebo intervention OI=CT=usual care, repetitive task practice, intensive conventional arm exercise program, physical therapy, electrical stimulation |
Duration: M=8 weeks (range 2-20) Intensity: 0.2-2 hours/session Frequency: M=3 sessions/week (range 2-6) |
22 studies RAT alone vs OI | Body function: FMA-UE, CMSA, MAS, MRC, hand-held dynamometer, MPS, MMT | Short term:<3 months Medium term:>3 months and<12 months Long term:>12 months |
RAT has small effects on motor control and medium effects on strength. |
| Karamians et al. (2020)14 | VR/gaming | CT=Bobath, NDT, stretching, strengthening, and ADL training | - | Not mentioned | Body function: FMA-UE Activity: ARAT, WMFT |
- | VR is more effective than CT. |
| Kwakkel et al. (2008)40 | RAT Devices: MIT-MANUS, MIME, ARM Guide, Bi-Manu-Track, InMotion Shoulder-Elbow Robot |
NDT CT Electrical stimulation |
Frequency: M=48.3 min/day | No | Body function: FMA-UE, CMSA Activity: FIM |
- | RAT=CT RAT improves motor function |
| Laver et al. (2017)41 | 5 intervention approaches including VR in UL training (35 studies) Devices: commercially available gaming consoles, CAREN system, Customised VR programs |
Recreational therapy CIMT No intervention Usual care CT |
Duration: 5 ->21 hours | Not mentioned | Body function: FMA-UE Activity: WMFT, MAL, ARAT, BI |
Short term follow-up:<3 months | VR and video gaming=CT but VR used as an adjunct to CT may be effective in UL function and ADL. |
| Laver et al. (2020)30 | Telerehabilitation at home, in a long-term care facility or a separated local healthcare centre goal-setting, education, family therapy, and case management UL physical function OT+PT Devices: telephone, videoconferencing hardware and software, desktop videophones |
In-person rehabilitation No rehabilitation Usual care |
- | Not mentioned | Body function: FMA-UE, ARAT, NHPT Activity: BI |
- | Telerehabilitation=usual care and in person therapy. |
| Lee et al. (2019)42 | VR unilateral UL: Nintendo VR bilateral UL: Wii sport, Xbox Kinect Robotic/virtually stimulated Some studies add CT |
CT No treatment (1 study) |
- | All studies except for one (no treatment) | Body function: FMA-UE, BBT | - | VR improves motor function in chronic stroke patients. |
| Lo et al. (2017)43 | RAT (TR=0) RAT+CT (TR=0.2-0.6) Devices: unilateral and bilateral arm robotics |
CT | Duration: total hours=4-300 | TR=0 | Body function: FMA-UE, CMSA, WMFT-FAS Activity: FIM, BI, SIS, CAFE40, MAL-QOM, AMAT-F |
20 studies:<3 months 16 studies:>3 months |
RAT=CT for UL movement and ADL. |
| Maier et al. (2019)13 | SVR: alone or with CT Devices: Microsoft Kinect, data gloves, computer vision, sensors, video camera, webcam, light-emitting diodes, hand-held sensors NSVR Devices: Nintendo Wii, Microsoft Xbox kinect, Sony PlayStation EyeToy Some studies add CT |
CT OT PT Recreational therapy |
Duration: M=4.3-4.4 weeks (range 2-12) with M=23.9 and 21.9 of total hours Intensity: range 20-158.3 minutes/session |
Not for all studies | Body function: FMA-UE, MI, SIS (hand), Brunnstrom Motor Recovery Stage Activity: BBT, FIM, BI, ARAT, WMFT |
- | SVR is more beneficial than CT in UL recovery. NSVR is not more beneficial than CT. |
| Mehrholz et al. (2020)16 | RAT: UDFHT, EPAHT, UPAHT, EXAHT, DGFHT, EBAHT Devices: MIT Manus/InMotion, Bi-Manu-Track, Amadeo |
CT | - | Yes | Body function: FMA-UE, SIS (hand function), Activity: WMFT, BI, FIM |
- | RAT=CT in UL function and ADL. Any device is better or worse than another one. |
| Norouzi-Gheidari et al. (2012)23 | RAT Devices: REHAROB, T-WREX, ARM-Guide, MIME, NeReBot, and MIT-Manus. |
CT | - | Yes, statistical analysis separated time-match and additional RT | Body function: FMA-UE, MSS, MPS Activity: FIM |
7 studies follow-up (3; 6; 8 months and 3 years) | Intensive CT=RAT for motor recovery, ADL, strength and motor control. |
| Prange et al. (2006)44 | RAT=repetitive, goal-directed forward-reaching movements (active movement) RAT+CT Devices: MIT-Manus, MIME, ARM-Guide |
CT | - | Not mentioned | Body function: FMA-UE | - | RAT improve motor control more than CT. |
| Rintala et al. (2019)45 | Home based training non-supervised or tele-supervised for UL motor function Devices: Video, audiovidual DVD Online web-based telerehabilitation program VR with game play (Nintendo Wii) |
- UL exercises in VE at home non-supervised - Telephone Calls - Usual care |
Frequency: 3-5 sessions/week | Not mentioned | Activity: MBI, BI, FONEFIM, MRS, SIS (ADL) | - | Technology-based distance physical rehabilitation=traditional treatment in UL function and ADL. |
| Zhao et al. (2022)47 | RAT Devices: AMADEO, InMotion3.0 WRIST, Gloreha, Bi-Manu-Track, Rehapticknob |
Typical treatment: -therapist-assisted training -passive range of motion exercices |
Duration: M range=30-120 min Frequency: M=5 times/week Intensity: M=30 min |
Not mentionned | -Body Function: FMA-UE -Activity: NHPT, BBT, MAS, MBI, SIS |
- | RAT improves UL motor function, strength, spasticity and dexterity |
ADL: activity of daily living; AMAT-F: Arm Motor Ability Test Function; ARAT: Action Research Arm Test; ARM Guide: Assisted Rehabilitation and Measurement Guide; BBT: Box and Block Test; BI: Barthel Index; CAFE 40: California Functional Evaluation 40; CG: Commercial Gaming; CIMT: Constraint Induced Movement Therapy; CT: Conventional Therapy; CMSA: Chedoke McMaster Stroke Assessment; DASH: Disabilities of Arm Shoulder and Hand; DGFHT: unilateral Distal Glove-based Finger/Hand Training; EBAHT: end effector assisted distal and bilateral arm/hand training; EPAHT: end effector-assisted proximal emphasised unilateral arm/hand training; EXAHT: exoskeleton assisted unilateral arm/hand training; FIM: Function Independence Scale; FMA-UE: Fugl-Mayer Assessment of the Upper Extremity; FONEFIM: telephone version of FIM; JTHF: Jebsen Test of Hand Function; MAL: Motor Activity Log; MAL-QOM: motor activity log-quality of movement; MAS: Modified Ashworth Scale; MBI: Modified Barthel Index; MI: minimal intervention; MIME: mirror image movement enabler; MMT: Manual Muscle Testing; MPS: Motor Power Scale; MRC: Medical Research Council; MRS: Modified Rankin Scale; MSS: Motor Statue Scale; NDT: Neurodevelopmental Treatment; NHPT: Nine Hole Peg Test; NSVR: non-specific virtual reality; OI: other intervention; OT: occupational therapy; PT: physiotherapy; rTMS: repetitive transcranial magnetic Stimulation; RAT: Robot Assisted Therapy; SIS: Stroke Impact Scale; SVR: specific virtual reality; TR=0: RAT alone vs CT alone; UDFHT: unilateral distally emphasized finger/hand training; UPAHT: unilaterally proximal emphasized arm/ hand training; UL: upper limb; VE: virtual environments; VR: virtual reality; WFMT: Wolf Motor Function Test; WFMT-FAS: Wolf Motor Function Test-Functional Ability Score.
Table II also provides a list of outcomes and follow-up included in the studied meta-analyses. Of these, 16 (89%) analyzed data on body function and 15 (83%) assessed data on activity limitation. Main outcomes were Fugl-Meyer Upper Extremity for body function, and, Functional Independence Measure, Barthel Index, Action Research Arm Test (ARAT), Wolf Motor Function Test for activity. Eight reviews (50%) used follow-up data ranging from post-treatment to 3 years for statistical analyses.
Network geography
Regarding motor function, the network diagram presented in Figure 2 illustrates that the majority of RCTs (19; 54%) that assessed patients in the subacute phase compared RAT to CT. Regarding the post-stroke chronic phase, a majority of trials compared RAT to CT (28; 47%) and VR to CT (28; 47%). VR was provided in addition to CT in 25 RCTs (58%), RAT in 17 RCTs (40%) and TR in one RCT (2%). Thirteen RCTs provided VR (27%), 30 RAT (63%) and 5 TR (10%) in substitution to CT.
Figure 2.
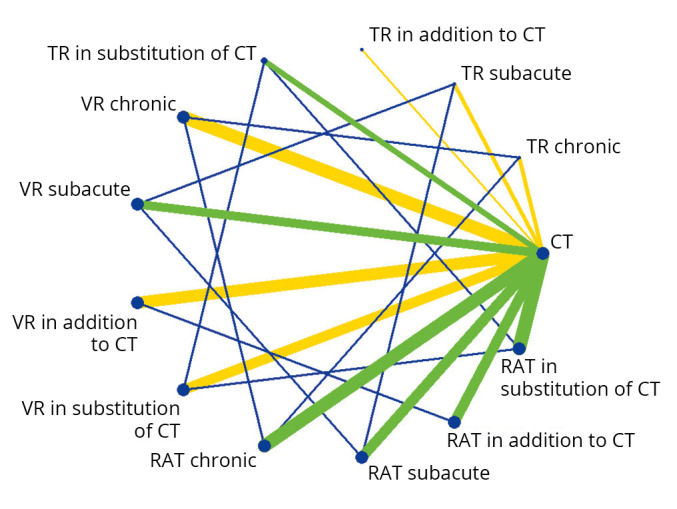
—Network diagram representing the direct and indirect comparisons between new technologies and conventional therapy regarding post-stroke upper limb motor function. Each node represents an intervention. The green and yellow lines (online version), connecting two nodes, represent direct comparisons. The thickness of the lines is proportional to the number of RCTs taken into account in the comparison. The green line (online version) represents low global risk of bias, while the yellow lines represent moderate risk of bias. The blue lines (online version), connecting two nodes, represent indirect comparisons.
Regarding activity, the network diagram presented in Figure 3 illustrates that the majority of RCTs assessing patients in the subacute and chronic phases, compared VR to CT (respectively 62% and 82%). VR was provided in addition to CT in 34 RCTs (74%), RAT in 10 RCTs (22%) and TR in 2 RCTs (4%). Twenty-one RCTs provided VR (62%), 9 RAT (26%) and 4 TR (12%) in substitution to CT.
Figure 3.
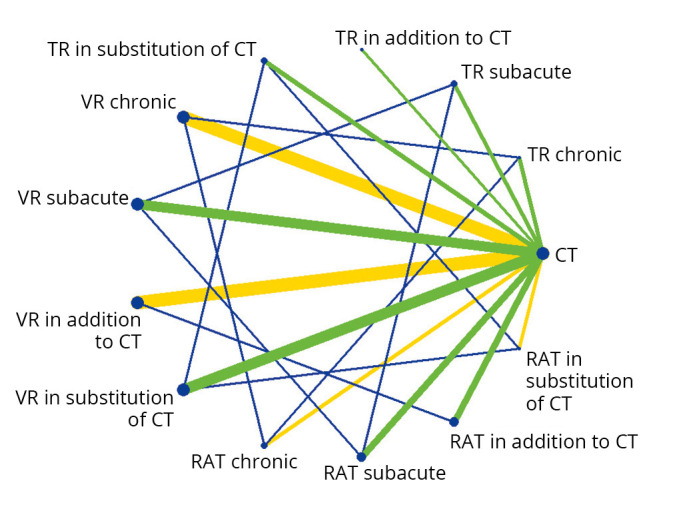
—Network diagram representing the direct and indirect comparisons between new technologies and conventional therapy regarding poststroke activity. Legend: Each node represents an intervention. The green and yellow lines (online version), connecting two nodes, represent direct comparisons. The thickness of the lines is proportional to the number of RCTs taken into account in the comparison. The green line (online version) represents low global risk of bias, while the yellow lines in the online version represent moderate risk of bias. The blue lines (online version) connecting two nodes, represent indirect comparisons.
Effect of new technologies on motor function
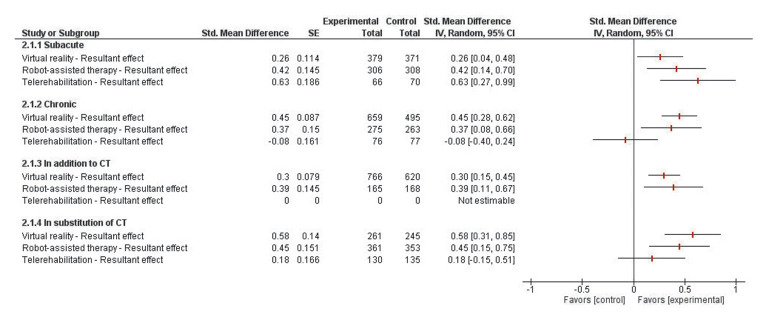
Outlier RCTs assessing RAT, VR or TR effect were excluded after performing a sensitivity analysis (Supplementary Digital Material 4: Supplementary Table II, Supplementary Figure 1, Supplementary Table III, Supplementary Figure 2, Supplementary Table IV, Supplementary Figure 3, Supplementary Table V, Supplementary Figure 4, Supplementary Table VI, Supplementary Figure 5, Supplementary Table VII, Supplementary Figure 6, Supplementary Table VIII, Supplementary Table IX, Supplementary Table X). As presented in Figure 4, during the subacute phase, effect of VR-based rehabilitation (SMD=0.26 [0.04 to 0.48]; P=0.03), RAT (SMD=0.42 [0.14 to 0.70]; P=0.004) and TR (SMD=0.63 [0.27 to 0.99]; P<0.001) on poststroke arm motor function were significantly superior to CT.
Figure 4.
—Forest-plot representing the effect of new-technologies, whether provided in addition or substitution of conventional therapy, on poststroke motor function in subacute and chronic patients.
The effect of RAT was the greatest for patients with severe-moderate motor impairments (SMD=0.54 [0.16 to 0.92]; P=0.005) and when provided in a treatment comprising 6 to 15 hours of intervention (SMD=0.93 [0.06 to 1.8]; P=0.04) (Figure 5). The greatest effect of VR was observed for patients with moderate to mild impairments (SMD=0.32 [0.03 to 0.61]; P=0.03) and when delivered during in a treatment of 15 to 30 hours (SMD=0.5 [0.1 to 0.9]; P=0.01). The effect of TR was found to be superior to CT for patients with moderate to mild impairments and when provided for more than 30 hours (SMD=0.51 [0.14 to 0.88]; P=0.008).
Figure 5.
—Forest-plot representing the effect of new-technologies, whether provided in addition or substitution of conventional therapy, on poststroke motor function in subacute and chronic patients.
Regarding the chronic phase, both VR (SMD=0.45 [0.28 to 0.62]; P<0.001) and RAT (SMD=0.37 [0.08 to 0.66]; P=0.01) showed superior effect to conventional rehabilitation for post-stroke motor function (Figure 4). No significant difference was found between TR and CT (SMD=-0.08 [-0.40 to 0.24]; P=0.61). During this phase, RAT (SMD=0.54 [0.26 to 0.82]; P<0.001) and VR effect (SMD=0.35 [0.11 to 0.59], P=0.002) were greater when provided for patients with moderate to mild motor impairments (Figure 5). The effect of TR was equivalent to CT when provided to patients with this same degree of severity (SMD=-0.07 [-0.4 to 0.26]; P=0.65). In terms of duration, the greatest effect of RAT (SMD=0.25 [0.06 to 0.44], P=0.01) and VR (SMD=0.46 [0.26 to 0.66]; P<0.001) were observed when provided during a treatment of 15 to 30 hours. No significant difference was found between TR and CT when delivered during 15 to 30 hours (SMD=-0.08 [-0.42 to 0.26]; P=0.64).
When provided in addition to CT, VR (SMD=0.30 [0.15 to 0.45]; P<0.001) and RAT effects (SMD=0.39 [0.11 to 0.67]; P<0.006) were found to be superior to CT. Since only one study was found regarding TR effect when provided in addition to CT, no meta-analysis was conducted.
When provided in substitution of CT, both VR (SMD=0.58 [0.31 to 0.85]; P<0.001) and RAT (SMD=0.45 [0.15 to 0.75]; p =0.003) showed superior effects to CT. No significant difference was identified between TR and CT effects (SMD=0.18 [-0.15 to 0.51]; P=0.29).
According to the Grade approach, the relative certainty of evidence regarding VR, RAT and TR effect on motor function was considered as very low to moderate depending on the post-stroke phase and intervention design (Supplementary Digital Material 5: Supplementary Table XI, Supplementary Table XII). Between-studies heterogeneities were non-significant to substantial (0%≤I2≤70%) and effect sizes ranged from small to medium (0.08≤SMD≤0.63). The detailed forest-plots comprising all the randomized controlled trials used to compute the resultant effect of new technologies on motor function are available in Supplementary Digital Material 6 (Supplementary Tables XIII-XXIX).
Effect of new technologies on activity
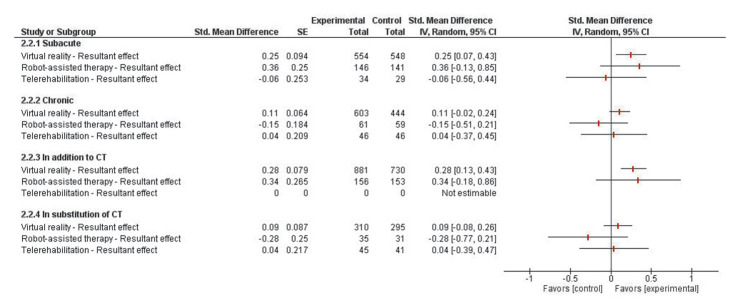
Outlier RCTs assessing RAT, VR or TR effect were excluded after performing a sensitivity analysis (Supplementary Material 4). As presented in Figure 6, during the subacute phase, the effect of VR on post-stroke activity (SMD=0.25 [0.07 to 0.43]; P=0.008) was found to be significantly superior to CT. RAT (SMD=0.36 [-0.13 to 0.85]; P=0.15) and TR (SMD=-0.06 [-0.56 to 0.44]; P=0.82) were similarly effective to CT.
Figure 6.
—Forest-plot representing the network meta-analysis indirectly assessing the effect comparison between the new technologies on poststroke activity.
According to Figure 7, the effect of RAT (SMD=0.47 [0.11 to 0.83]; P=0.01) was greater when administered in patients with severe-moderate impairments and the effect of VR (SMD=0.46 [0.01 to 0.91]; P<0.001) when administered in patients with moderate to mild impairments.
Figure 7.
—Forest-plot representing the network meta-analysis indirectly assessing the effect comparison between the new technologies on poststroke activity.
No significant difference was found between TR and CT effect when delivered in patients with mild to moderate impairments (SMD=0.91 [-0.91 to 2.73]; P=0.33). During the subacute phase, RAT was found to be equivalent to CT irrespective of the rehabilitation duration (P>0.05) whereas the greatest effect of VR was observed when the treatment was delivered during more than 30 hours (SMD=0.5 [0.15 to 0.85]; P=0.006). Regarding TR, no significant difference was found with CT for a treatment with such duration (SMD=0.61 [-0.72 to 1.94]; P=0.37).
For patients in the chronic phase, VR demonstrated no superior effect to CT (SMD=0.11 [-0.02 to 0.24]; P=0.07) (Figure 6). No significant difference was found between RAT and CT (SMD=-0.15 [-0.51 to 0.21]; P=0.41) and neither between TR nor CT regarding post-stroke activity (SMD=0.04 [-0.37 to 0.45]; P=0.86). For the chronic phase, RAT (SMD=0.07 [-0.47 to 1.43]; P=0.79) and TR (SMD=0.08 [-0.53 to 0.69]; P=0.81) were found to be equivalent to CT for patients with moderate to mild impairments whereas VR effect was found to be superior (SMD=0.31 [0.06 to 0.56]; P=0.01). Regarding the duration, the greatest effect of VR was observed when the treatment ranged from 15 to 30 hours (SMD=0.41 [0.09 to 0.73]; P=0.01). RAT effect was equivalent to CT for such duration (SMD=-0.24 [-0.85 to 0.37]; P=0.43) and no sufficient data was found for TR.
When provided in addition to CT, VR effect was found to be superior to CT (SMD=0.28 [0.13 to 0.43]; P<0.001) whereas RAT (SMD=0.34 [-0.18 to 0.86]; P=0.19) showed an equivalent effect to CT. Since only one study was found regarding TR effect when provided in addition to CT, no meta-analysis was conducted.
When provided in substitution of CT, all technologies VR (SMD=0.09 [-0.08 to 0.26]; P=0.30), RAT (SMD=-0.28 [-0.77 to 0.21]; P=0.26) and TR (SMD=0.04 [-0,39 to 0.4]; P=0.87) showed equivalent effects to CT.
The certainty of evidence regarding VR, TR and RAT effects on activity ranged from very low to moderate depending on the post-stroke phase and intervention design (Supplementary Digital Material 7: Supplementary Tables XXX, XXXI). Heterogeneity between studies were low to considerable (0%≤I2≤78%) and effect-sizes small to low (0.04 ≤ SMD≤0.36). The detailed forest-plots comprising all the randomized controlled trials used to compute the resultant effect of new technologies on activity are available in Supplementary Digital Material 8 (Supplementary Tables XXXII-XLVIII).
Network meta-analysis – motor function
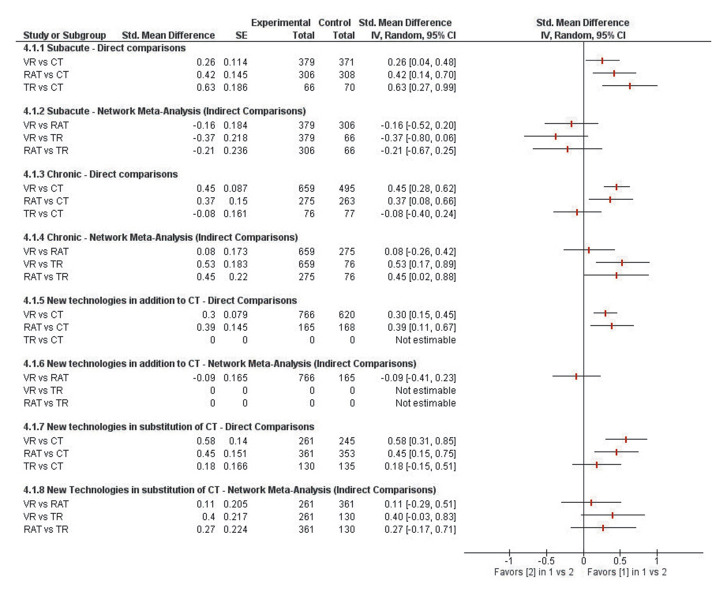
Regarding post-stroke motor function, results of these network analyses revealed that there was no significant difference between VR, RAT and TR during the subacute phase (P>0.05) (Figure 8).
Figure 8.
—Results of these network analyses showing no significant difference between VR, RAT and TR during the subacute phase.
However, during the chronic phase, both VR (SMD=0.53 [0.17 to 0.89]; P=0.004) and RAT effects (SMD=0.45 [0.02 to 0.88]; P=0.04) were found to be significantly superior to TR. During this phase, VR and RAT were found to be equally effective to one another (SMD=0.08 [-0.26 to 0.42]; P=0.64). Whether provided in addition to or in substitution of CT, network comparison revealed no significant difference between VR, RAT and TR (P>0.05).
Network meta-analysis – activity
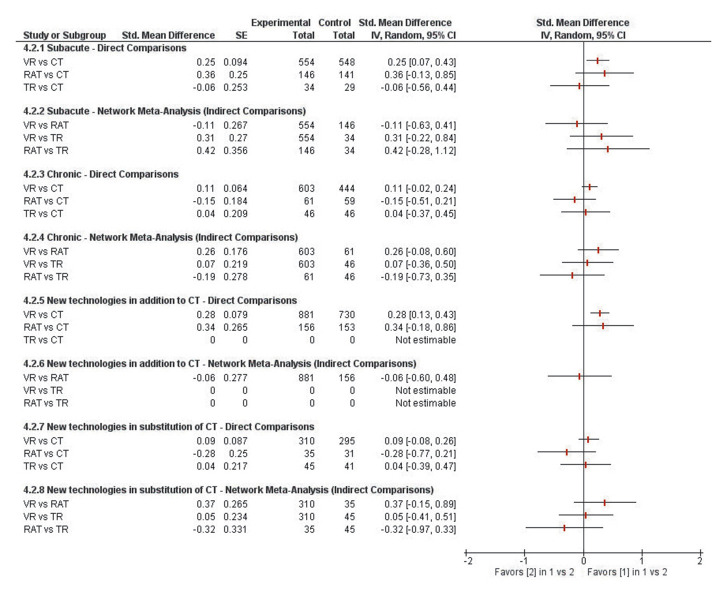
Regarding activity, results of these network analyses revealed that there was no significant effect difference between VR, RAT and TR during both the subacute and chronic phase (P>0.05) (Figure 9).
Figure 9.
—Results of these network analyses revealing no significant effect difference between VR, RAT and TR during both the subacute and chronic phase.
When provided in addition to CT, network comparison between VR and RAT effects showed no significant difference (SMD=-0.06 [-0.60 to 0.48; P=0.83). Results also showed that, when provided in substitution of CT, there was no significant differences between VR and RAT effects (SMD=0.37 [-0.15 to 0.89; P=0.16), VR and TR effects (SMD=0.05 [-0.41 to 0.51]; P=0.83) and RAT and TR effects (SMD=-0.32 [-0.97 to 0.33]; P=0.33).
Discussion
This overview and network meta-analysis provides low to moderate evidence that new technologies comprising VR, RAT and TR are more effective, or at least as effective, as CT to rehabilitate post-stroke motor function and activity (irrespective of whether provided in addition to or in substitution of CT). During the subacute phase, RAT’s greatest effect was observed for patients with severe-moderate impairments whereas VR and TR’s greatest effects for patients with mild impairments. During the chronic phase, all technologies’ highest effects were observed for patients with mild impairments. Duration analyses suggested that TR be used for a minimum of 30 hours and VR for a minimum of 15 hours. However, these analyses remained inconsistent for RAT. Results of network meta-analyses showed no difference regarding the effectiveness of VR, RAT and TR during the subacute phase but suggest that RAT and VR effect on post-stroke motor function are superior to TR during the chronic phase. When provided in addition or in substitution to CT, no difference was found between the effects of these technologies on both motor function and activity.
VR
According to our results, VR is more effective than CT at rehabilitating motor function and activity during both subacute and chronic phases of stroke. This may be explained by the fact that VR systems are often combined with serious games that allow to apply neurorehabilitation principles. These principles are, among others, providing early, intensive, progressive and functional rehabilitation, giving performance feedbacks and promoting the use of the affected limb.48 Indeed, previous research has demonstrated that respecting these principles increased the effect of serious games on motor function and activity.46 A second explanation may be that this technology provides a certain level of motivation to patients.49 In fact, according to the self-determination theory, it can be hypothesized that gaming motivation is determined by the level of autonomy, competence and playfulness experienced by players.49, 50 In virtual reality, players can experience all these factors due to the display of rewards and positive feedbacks, the immersion in a realistic environment and the provision of an optimal level of difficulty.50 Virtual reality motivation is therefore generally increased through the utilization of serious games.51 Lastly, the computerization of games may help to tailor rehabilitation to patients’ abilities and necessities which could increase the effect on both motor function and activity.
VR’s degree of immersion may also have an influence on patients’ outcomes. In fact, on one hand, immersive VR may offer better results thanks to the provisioning of more realistic environments, potentially leading to better motivation and adherence.52 On the other hand, non-immersive VR may also increase patients’ adherence, especially when provided autonomously, as these devices may be more usable.53 A recent meta-analysis has focused on comparing the effects of these interventions on upper-limb motor function.54 Results revealed that, when provided in addition to CT, immersive VR was superior to CT alone whereas non-immersive VR was equivalent to CT alone. However, up to now, the number of RCTs using an immersive intervention remains low. For instance, in another recent review that compared immersive and non-immersive VR effects on balance function among patients with stroke, only 17% of the studies included in the work used an immersive device.55 This low number of studies makes it difficult to currently make reliable comparisons.
Regarding impairments severity, we found that VR effect was greater when delivered among patients with moderate to mild motor impairments. These findings are not surprising as VR devices do not allow to assist movements and therefore implies having a minimum of upper limb (UL) motricity. Therefore, VR does not seem to be the technology of choice for rehabilitation of patients with a low UE-FMA score. Nevertheless, VR could still have an interest for these patients as it has showed potential to provide effective mirror therapy and cognitive rehabilitation.38, 56
RAT
Regarding RAT, the forest-plots computed in our study demonstrated it is superior to CT for post-stroke motor function, but not for activity. A previous review has also highlighted that the motor learning experience in addition to the high intensity and repetition level provided by upper-limb rehabilitation with robots was responsible for the increased motor function in patients with stroke.23 However, these results are not supported by a recent multicenter RCT (RATULS) that showed no significant effect difference between a time-matched RAT and CT regarding upper-limb motor function.57 This discordance may be explained by the fact that patients included in RATULS presented a bad prognosis of recovery since their mean UE-FMA score was of 18/66 and a wide range of delay since stroke (22%:<3 months, 41% between 3 months and a year and 37% >1 year). Moreover, there was a certain heterogeneity present across studies included in the present meta-analysis of meta-analyses, regarding patients’ stage of recovery and RAT effectiveness is likely to be impacted by patients’ stage of recovery.
Nevertheless, concerning RAT’s comparable effect to CT on activity, our results are in line with previous studies.16, 23, 57 Several factors can explain this lack of superiority between the new technology and conventional therapy. First, rehabilitation robots are often developed for arms and shoulders whereas activity rehabilitation requires to target all upper-limb segments, including the hands. Moreover, robots’ tasks are often bidimensional and do not always involve meaningful goal-oriented actions (such as hand pronation, lifting, pinching, etc). Yet, to rehabilitate patients’ activity, it is recommended to work on tridimensional functional specific tasks which may involve objects as this more closely resembles activities performed in daily life.
RAT comprises different types of devices with different technical specifications such as unilateral distal and proximal end-effectors, bilateral end-effectors, and exoskeletons.16 A recently published network meta-analysis demonstrated no significant effect difference between all the types of robots suggesting that there is no evidence in favor of one device over another in arm rehabilitation after stroke.16
Regarding impairments severity, we found that, during the subacute phase, RAT effect was the greatest when provided among patients with severe-moderate impairments. It can be hypothesized that, the assistance delivered by robots would induce greater benefits among patients with a low UE-FMA than patients who already recovered some motricity as severe motor impairments may prevent patients to perform movements autonomously. Conversely, during the chronic phase, RAT seems to offer better results when provided to patients with moderate to mild impairments. This effect might be attributed to the fact that the assistance delivered by robots is better potentialized during spontaneous recovery. However, no conclusion can be drawn as the quantity of available data regarding RAT effect according to the impairment severity during the chronic phase remains limited.
TR
Results regarding TR effect are globally equivalent to CT. Findings of this review are consistent with previous works.30, 45 However, to date, there remains a lack of studies of good quality comparing TR to CT and more importantly, TR programs are currently very heterogeneous.
It could be hypothesized that the effects of TR are equivalent to CT due to treatment adherence. Indeed, TR is much more unsupervised than CT which could be responsible for a lack of adherence. A previous work reported that in several studies, 15% to 40% of patients with stroke who followed a self-rehabilitation program did not reach the total amount session.7 Moreover, difficulties with equipment setup, the limited scope of exercises and connectivity issues are other factors identified as barriers to TR by patients and therapists that may explain TR’s lack of superiority.58
TR effect was found to be at least equivalent to CT when delivered to patients with mild to moderate impairments. However, no studies assess its effect on patients with severe impairments. This lack of studies may be explained by the fact that TR requires a certain amount of functional independency and motor recovery. Therefore, patients with severe impairments might not take sufficient advantage of such intervention.
Program parameters
Results of this work suggest that, when provided in addition to CT, VR is more effective than CT and RAT equivalent to CT. When provided in substitution to CT, RAT and VR’s effects on motor function appear to be superior to CT, and TR seems to be equivalent to CT. However, although literature results seem similar regarding VR and TR effects,30, 41 several studies have shown that RAT was not superior to CT when provided in substitution.23, 57 This discordance regarding RAT effect is not surprising as, in our meta-analysis, the level of heterogeneity between studies comparing RAT effect (when provided in substitution to CT) to CT alone was considerable (I2>80%).
This review also suggests that VR and RAT’s effects on motor function are superior to CT, irrespective of whether patients are in the subacute or chronic phase. However, regarding VR, these results contradict other findings. A Cochrane review showed that VR’s effect on upper-limb motor function was equivalent to CT when rehabilitating patients in both subacute and chronic phase.41 This may be attributed to the patient’s level of functional recovery included in the RCTs of this review. In fact, patients with important impairments (such as severe hemiparesis or hemiplegia), whatever their stroke stage, may not benefit from virtual reality intervention as it requires a minimum of reaching function and ability to initiate movement. This hypothesis is also true for patients with severe aphasia and apraxia who may not be able to understand the function of the helmet and controllers during a virtual reality intervention.
Furthermore, our network meta-analysis suggests that VR and RAT’s effects on motor function are superior to TR during the chronic phase. This may be explained by the fact that patients are generally more impaired during the first 6 months after a stroke than after (on both motor and cognitive levels). Therefore, the patients may benefit more from an intensive, gamified and supervised therapy, such as VR and RAT, than from TR.
Adverse effects of rehabilitation technologies
According to the literature and reviews included in this work, few adverse effects are related to the use of rehabilitation technologies. Regarding RAT, a review of 2006 reported that no adverse events occurred in any of the studies included.44 In 2017, a second review indicated that the global attrition rate of RAT intervention was of 10% suggesting a good acceptability and safety.21 However, one study of 2014 reported that during the experiment, out of 24 patients with stroke, nine experienced discomfort and one suffered from blisters after using the robot.59
A recent Cochrane review assessing VR effect after stroke revealed that out of 23 studies, nineteen reported no serious adverse events.41 The adverse effects mentioned by the other studies were headache, dizziness, an increased tonicity and pain. Nevertheless, these were not specifically attributed to the intervention as patients in the control group also experienced these undesired events.60-62
Lastly, a Cochrane review assessing TR effect after a stroke reported that no adverse events occurred in any of the included studies.39 More recently, a RCT of 2019 indicated that 5 out of 62 patients who followed a TR program suffered from arm pain.28 However, in the same study, 5 patients who followed the control intervention also suffered from shoulder pain.
Implication for clinical practice
This review indicated that new technologies (RAT, VR and TR) promoting active upper-limb rehabilitation are more effective than, or equivalent to, CT for both motor function and activity. These technology-based rehabilitation programs should be considered as a complement to CT as patients benefit from both new technologies and CT at subacute and chronic stage.63 Moreover, RAT, VR and TR are complementary rehabilitation means. According to the results of this work, we suggest that, during the subacute phase, RAT be used for patients with severe-moderate to moderate motor impairments whereas VR and TR should primarily be used for patients with moderate to mild impairments. During the chronic phase, RAT, VR and TR are recommended for patients with mild to moderate impairments. RAT can be used to intensify treatment and provide patients with assistance-as-needed whereas VR enables to work goal-oriented and functional tasks. Moreover, TR offers patients the opportunity to rehabilitate at home at a reasonable cost. Such programs should be designed according to the neurorehabilitation principles and tailored to the patients’ needs and ability. Regarding treatment duration, results seem to favor a minimum of 30 hours for TR and a minimum of 15 hours for VR whereas the ideal duration of interventions comprising RAT remains unclear. Further trials would be needed to confirm these trends.
To measure upper-limb recovery before and after these interventions, we encourage therapist to operate the UE-FMA for measuring motor function and the ARAT to evaluate activity. We also recommend taking the opportunity of using technologies to objectively measure and better understand patients’ recovery.64, 65 For instance, these technologies could be used to provide kinematics and actimetry measures.
Strengths and limitations of the study
This overview and network meta-analysis is the first to compare the effect of 3 new-technologies on ICF-WHO post-stroke outcomes according to the stroke stage (chronic vs subacute) and patients’ impairments severity. Another strength of this work is it covers a high number total of different RCTs (189 involving 7524 patients) issued from 18 meta-analyses.
The main limitation of this work is that some high-quality RCTs may have been missed in the statistical analysis since we decided to only include trials issued from meta-analyses. A second limitation is that consistency of network meta-analysis could not be assessed due to the fact that there were no existing direct comparisons between new technologies. Lastly, some analyses suffered from an important level of between-studies heterogeneity which may decrease the certainty of the evidence.
Conclusions
Active rehabilitation using new-technologies effects on post-stroke outcomes are at least equivalent to CT. Regarding post-stroke motor function, VR and TR are more effective than CT in both subacute and chronic patients whereas TR is equally effective as CT. For activity outcomes, VR is superior to CT in subacute and chronic patients whereas TR and RAT are equally effective. In addition, network meta-analyses showed that VR and RAT were both significantly superior to RAT in improving motor function during the chronic phase. Lastly, during the subacute phase, severely impaired patients seem to obtain greater benefits from RAT, while VR and TR seem to be a more adequate therapy for patients with moderate to low impairments. During the chronic phase, all these technologies seem to be more adequate for patients with mild impairments.
Supplementary Digital Material 1
Supplementary Text File 1
PRISMA-P 2015 Checklist.
Supplementary Digital Material 2
Supplementary Text File 2
Search strategies.
Supplementary Digital Material 3
Supplementary Table I
AMSTAR assessment.
Supplementary Digital Material 4
Supplementary Table II
4.1. RAT effect on motor function during subacute phase.
Supplementary Figure 1
Distribution.
Supplementary Table III
RAT effect on motor function during chronic phase.
Supplementary Figure 2
Distribution.
Supplementary Table IV
VR effect on motor function during chronic phase.
Supplementary Figure 3
Distribution.
Supplementary Table V
TR effect on activity during subacute phase.
Supplementary Figure 4
Distribution.
Supplementary Table VI
RAT effect on activity during chronic phase.
Supplementary Figure 5
Distribution.
Supplementary Table VII
VR effect on activity during chronic phase.
Supplementary Figure 6
Distribution.
Supplementary Table VIII
New technologies effects on motor function when provided in addition (all studies vs only time-matches interventions).
Supplementary Table IX
New technologies effects on motor function when provided in substitution (all studies vs only time-matches interventions).
Supplementary Table X
New technologies effects on activity when provided in addition (all studies vs only time-matches interventions).
Supplementary Digital Material 5
Supplementary Table XI
During subacute and chronic phase.
Supplementary Table XII
When provided in addition and in substitution to conventional therapy.
Supplementary Digital Material 6
Supplementary Table XIII
Forest-plot regarding the effect of new-technologies on motor function in patients with subacute stroke.
Supplementary Table XIV
Forest-plot regarding the effect of new-technologies on motor function in patients with chronic stroke.
Supplementary Table XV
Forest-plot regarding the effect of new-technologies on motor function in patients with stroke when provided in addition vs in substitution of conventional therapy.
Supplementary Table XVI
Robot-assisted therapy therapy.
Supplementary Table XVII
Telerehabilitation.
Supplementary Table XVIII
Forest-plot regarding the effect of new-technologies on motor function in patients with subacute stroke according to the impairment severity - robot-assisted therapy.
Supplementary Table XIX
Virtual reality.
Supplementary Table XX
Telerehabilitation.
Supplementary Table XXI
Forest-plot regarding the effect of new-technologies on motor function in patients with chronic stroke according to the impairment severity - robot-assisted therapy.
Supplementary Table XXII
Virtual reality.
Supplementary Table XXIII
Telerehabilitation.
Supplementary Table XXIV
Forest-plot regarding the effect of new-technologies on motor function in patients with subacute stroke according to the treatment intensity - robot-assisted therapy.
Supplementary Table XXV
Virtual reality.
Supplementary Table XXVI
Telerehabilitation.
Supplementary Table XXVII
Forest-plot regarding the effect of new-technologies on motor function in patients with chronic stroke according to the treatment intensity - robot-assisted therapy.
Supplementary Table XXVIII
Virtual reality.
Supplementary Table XXIX
Telerehabilitation.
Supplementary Digital Material 7
Supplementary Table XXX
GRADE Approach regarding activity outcome during chronic and subacute phase.
Supplementary Table XXXI
GRADE Approach regarding activity outcome when provided in addition to or in substitution of conventional therapy.
Supplementary Digital Material 8
Supplementary Table XXXII
Forest-plot regarding the effect of new-technologies on activity in patients with subacute stroke.
Supplementary Table XXXIII
Forest-plot regarding the effect of new-technologies on activity in patients with chronic stroke.
Supplementary Table XXXIV
Forest-plot regarding the effect of new-technologies on activity in patients with chronic stroke when provided in addition vs in substitution of conventional therapy - virtual reality.
Supplementary Table XXXV
Robot-assisted therapy.
Supplementary Table XXXVI
Telerehabilitation.
Supplementary Table XXXVII
Forest-plot regarding the effect of new-technologies on activity in patients with subacute stroke according to the impairment severity - robot-assisted therapy.
Supplementary Table XXXVIII
Virtual reality.
Supplementary Table XXXI
Telerehabilitation.
Supplementary Table XL
Forest-plot regarding the effect of new-technologies on activity in patients with chronic stroke according to the impairment severity - robot-assisted therapy.
Supplementary Table XLI
Virtual reality.
Supplementary Table XLII
Telerehabilitation.
Supplementary Table XLIII
Forest-plot regarding the effect of new-technologies on activity in patients with subacute stroke according to the treatment intensity - robot-assisted therapy.
Supplementary Table XLIV
Virtual reality.
Supplementary Table XLV
Telerehabilitation.
Supplementary Table XLVI
Forest-plot regarding the effect of new-technologies on activity in patients with chronic stroke according to the treatment intensity - robot-assisted therapy.
Supplementary Table XLVII
Virtual reality.
Supplementary Table XLVIII
Telerehabilitation.
References
- 1.Béjot Y, Bailly H, Durier J, Giroud M. Epidemiology of stroke in Europe and trends for the 21st century. Presse Med 2016;45:e391–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27816343&dopt=Abstract 10.1016/j.lpm.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 2.Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke 1997;28:2518–27. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9412643&dopt=Abstract 10.1161/01.STR.28.12.2518 [DOI] [PubMed] [Google Scholar]
- 3.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research . Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2016;47:e98–169. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27145936&dopt=Abstract 10.1161/STR.0000000000000098 [DOI] [PubMed] [Google Scholar]
- 4.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet 2011;377:1693–702. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21571152&dopt=Abstract 10.1016/S0140-6736(11)60325-5 [DOI] [PubMed] [Google Scholar]
- 5.Amir M, Saeed B, Mahmoud Saadat F. Robotics and Tele-Rehabilitation: Recent Advancements, Future Trends. Int J Reliab Qual E-Healthc 2013;2:1–13. [IJRQEH] 10.4018/ijrqeh.2013100101 [DOI] [Google Scholar]
- 6.Duret C, Grosmaire AG, Krebs HI. Robot-Assisted Therapy in Upper Extremity Hemiparesis: Overview of an Evidence-Based Approach. Front Neurol 2019;10:412. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31068898&dopt=Abstract 10.3389/fneur.2019.00412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everard G, Luc A, Doumas I, Ajana K, Stoquart G, Edwards MG, et al. Self-Rehabilitation for Post-Stroke Motor Function and Activity-A Systematic Review and Meta-Analysis. Neurorehabil Neural Repair 2021;35:1043–58. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34696645&dopt=Abstract 10.1177/15459683211048773 [DOI] [PubMed] [Google Scholar]
- 8.Iosa M, Hesse S, Oliviero A, Paolucci S. New technologies for stroke rehabilitation. Stroke Res Treat 2013;2013:815814. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23401849&dopt=Abstract 10.1155/2013/815814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasr N, Leon B, Mountain G, Nijenhuis SM, Prange G, Sale P, et al. The experience of living with stroke and using technology: opportunities to engage and co-design with end users. Disabil Rehabil Assist Technol 2016;11:653–60. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25879304&dopt=Abstract 10.3109/17483107.2015.1036469 [DOI] [PubMed] [Google Scholar]
- 10.Winstein C, Requejo P. Innovative technologies for rehabilitation and health promotion: what is the evidence? Phys Ther 2015;95:294–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25734191&dopt=Abstract 10.2522/ptj.2015.95.2.294 [DOI] [PubMed] [Google Scholar]
- 11.Saposnik G, Levin M, Outcome Research Canada (SORCan) Working Group. Virtual reality in stroke rehabilitation: a meta-analysis and implications for clinicians. Stroke 2011;42:1380–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21474804&dopt=Abstract 10.1161/STROKEAHA.110.605451 [DOI] [PubMed] [Google Scholar]
- 12.Domínguez-Téllez P, Moral-Muñoz JA, Salazar A, Casado-Fernández E, Lucena-Antón D. Game-Based Virtual Reality Interventions to Improve Upper Limb Motor Function and Quality of Life After Stroke: Systematic Review and Meta-analysis. Games Health J 2020;9:1–10. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32027185&dopt=Abstract 10.1089/g4h.2019.0043 [DOI] [PubMed] [Google Scholar]
- 13.Maier M, Rubio Ballester B, Duff A, Duarte Oller E, Verschure PF. Effect of Specific Over Nonspecific VR-Based Rehabilitation on Poststroke Motor Recovery: A Systematic Meta-analysis. Neurorehabil Neural Repair 2019;33:112–29. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30700224&dopt=Abstract 10.1177/1545968318820169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karamians R, Proffitt R, Kline D, Gauthier LV. Effectiveness of Virtual Reality- and Gaming-Based Interventions for Upper Extremity Rehabilitation Poststroke: A Meta-analysis. Arch Phys Med Rehabil 2020;101:885–96. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31821799&dopt=Abstract 10.1016/j.apmr.2019.10.195 [DOI] [PubMed] [Google Scholar]
- 15.Chang WH, Kim YH. Robot-assisted Therapy in Stroke Rehabilitation. J Stroke 2013;15:174–81. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24396811&dopt=Abstract 10.5853/jos.2013.15.3.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehrholz J, Pollock A, Pohl M, Kugler J, Elsner B. Systematic review with network meta-analysis of randomized controlled trials of robotic-assisted arm training for improving activities of daily living and upper limb function after stroke. J Neuroeng Rehabil 2020;17:83. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32605587&dopt=Abstract 10.1186/s12984-020-00715-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keeling AB, Piitz M, Semrau JA, Hill MD, Scott SH, Dukelow SP. Robot enhanced stroke therapy optimizes rehabilitation (RESTORE): a pilot study. J Neuroeng Rehabil 2021;18:10. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33478563&dopt=Abstract 10.1186/s12984-021-00804-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu CY, Yang CL, Chen MD, Lin KC, Wu LL. Unilateral versus bilateral robot-assisted rehabilitation on arm-trunk control and functions post stroke: a randomized controlled trial. J Neuroeng Rehabil 2013;10:35. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23587106&dopt=Abstract 10.1186/1743-0003-10-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandolfi M, Valè N, Posteraro F, Morone G, Dell’orco A, Botticelli A, et al. Italian Consensus Conference on Robotics in Neurorehabilitation (CICERONE) . State of the art and challenges for the classification of studies on electromechanical and robotic devices in neurorehabilitation: a scoping review. Eur J Phys Rehabil Med 2021;57:831–40. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34042413&dopt=Abstract 10.23736/S1973-9087.21.06922-7 [DOI] [PubMed] [Google Scholar]
- 20.Mehrholz J, Pohl M, Platz T, Kugler J, Elsner B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst Rev 2018;9:CD006876. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30175845&dopt=Abstract 10.1002/14651858.CD006876.pub5 [DOI] [PMC free article] [PubMed]
- 21.Chien WT, Chong YY, Tse MK, Chien CW, Cheng HY. Robot-assisted therapy for upper-limb rehabilitation in subacute stroke patients: A systematic review and meta-analysis. Brain Behav 2020;10:e01742. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32592282&dopt=Abstract 10.1002/brb3.1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira FM, Chaves ME, Oliveira VC, Van Petten AM, Vimieiro CB. Effectiveness of robot therapy on body function and structure in people with limited upper limb function: A systematic review and meta-analysis. PLoS One 2018;13:e0200330. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30001417&dopt=Abstract 10.1371/journal.pone.0200330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norouzi-Gheidari N, Archambault PS, Fung J. Effects of robot-assisted therapy on stroke rehabilitation in upper limbs: systematic review and meta-analysis of the literature. J Rehabil Res Dev 2012;49:479–96. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22773253&dopt=Abstract 10.1682/JRRD.2010.10.0210 [DOI] [PubMed] [Google Scholar]
- 24.Cramer S. Interventions to Improve Recovery after Stroke. In:2016:972-980. [Google Scholar]
- 25.Schwamm LH, Holloway RG, Amarenco P, Audebert HJ, Bakas T, Chumbler NR, et al. American Heart Association Stroke Council ; Interdisciplinary Council on Peripheral Vascular Disease. A review of the evidence for the use of telemedicine within stroke systems of care: a scientific statement from the American Heart Association/American Stroke Association. Stroke 2009;40:2616–34. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19423852&dopt=Abstract 10.1161/STROKEAHA.109.192360 [DOI] [PubMed] [Google Scholar]
- 26.Redzuan NS, Engkasan JP, Mazlan M, Freddy Abdullah SJ. Effectiveness of a video-based therapy program at home after acute stroke: a randomized controlled trial. Arch Phys Med Rehabil 2012;93:2177–83. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22789773&dopt=Abstract 10.1016/j.apmr.2012.06.025 [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Jin W, Dong WS, Jin Y, Qiao FL, Zhou YF, et al. Effects of Home-based Telesupervising Rehabilitation on Physical Function for Stroke Survivors with Hemiplegia: A Randomized Controlled Trial. Am J Phys Med Rehabil 2017;96:152–60. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27386808&dopt=Abstract 10.1097/PHM.0000000000000559 [DOI] [PubMed] [Google Scholar]
- 28.Cramer SC, Dodakian L, Le V, See J, Augsburger R, McKenzie A, et al. ; National Institutes of Health StrokeNet Telerehab Investigators. Efficacy of Home-Based Telerehabilitation vs In-Clinic Therapy for Adults After Stroke: A Randomized Clinical Trial. JAMA Neurol 2019;76:1079–87. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31233135&dopt=Abstract 10.1001/jamaneurol.2019.1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lloréns R, Noé E, Colomer C, Alcañiz M. Effectiveness, usability, and cost-benefit of a virtual reality-based telerehabilitation program for balance recovery after stroke: a randomized controlled trial. Arch Phys Med Rehabil 2015;96:418–425.e2. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25448245&dopt=Abstract 10.1016/j.apmr.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 30.Laver KE, Adey-Wakeling Z, Crotty M, Lannin NA, George S, Sherrington C. Telerehabilitation services for stroke. Cochrane Database Syst Rev 2020;1:CD010255. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32002991&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 31.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28935701&dopt=Abstract 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whiting P, Savović J, Higgins JP, Caldwell DM, Reeves BC, Shea B, et al. ROBIS group . ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol 2016;69:225–34. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26092286&dopt=Abstract 10.1016/j.jclinepi.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorenz RC, Matthias K, Pieper D, Wegewitz U, Morche J, Nocon M, et al. A psychometric study found AMSTAR 2 to be a valid and moderately reliable appraisal tool. J Clin Epidemiol 2019;114:133–40. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31152864&dopt=Abstract 10.1016/j.jclinepi.2019.05.028 [DOI] [PubMed] [Google Scholar]
- 34.Woytowicz EJ, Rietschel JC, Goodman RN, Conroy SS, Sorkin JD, Whitall J, et al. Determining Levels of Upper Extremity Movement Impairment by Applying a Cluster Analysis to the Fugl-Meyer Assessment of the Upper Extremity in Chronic Stroke. Arch Phys Med Rehabil 2017;98:456–62. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27519928&dopt=Abstract 10.1016/j.apmr.2016.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen J. Statistical power analysis for the behavioral sciences. Academic press; 2013. [Google Scholar]
- 36.Shim SR, Kim SJ, Lee J, Rücker G. Network meta-analysis: application and practice using R software. Epidemiol Health 2019;41:e2019013. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30999733&dopt=Abstract 10.4178/epih.e2019013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahn S, Hwang S. Virtual rehabilitation of upper extremity function and independence for stoke: a meta-analysis. J Exerc Rehabil 2019;15:358–69. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31316927&dopt=Abstract 10.12965/jer.1938174.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aminov A, Rogers JM, Middleton S, Caeyenberghs K, Wilson PH. What do randomized controlled trials say about virtual rehabilitation in stroke? A systematic literature review and meta-analysis of upper-limb and cognitive outcomes. J Neuroeng Rehabil 2018;15:29. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29587853&dopt=Abstract 10.1186/s12984-018-0370-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coupar F, Pollock A, Legg LA, Sackley C, van Vliet P. Home-based therapy programmes for upper limb functional recovery following stroke. Cochrane Database Syst Rev 2012;(5):CD006755. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22592715&dopt=Abstract 10.1002/14651858.CD006755.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwakkel G, Kollen BJ, Krebs HI. Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabil Neural Repair 2008;22:111–21. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17876068&dopt=Abstract 10.1177/1545968307305457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laver KE, Lange B, George S, Deutsch JE, Saposnik G, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database Syst Rev 2017;11:CD008349. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29156493&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 42.Lee HS, Park YJ, Park SW. The effects of virtual reality training on function in chronic stroke patients: A systematic review and meta-analysis. BioMed Res Int 2019;2019:7595639. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31317037&dopt=Abstract 10.1155/2019/7595639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lo K, Stephenson M, Lockwood C. Effectiveness of robotic assisted rehabilitation for mobility and functional ability in adult stroke patients: a systematic review. JBI Database Syst Rev Implement Reports 2017;15:3049–91. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29219877&dopt=Abstract 10.11124/JBISRIR-2017-003456 [DOI] [PubMed] [Google Scholar]
- 44.Prange GB, Jannink MJ, Groothuis-Oudshoorn CG, Hermens HJ, Ijzerman MJ. Systematic review of the effect of robot-aided therapy on recovery of the hemiparetic arm after stroke. J Rehabil Res Dev 2006;43:171–84. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16847784&dopt=Abstract 10.1682/JRRD.2005.04.0076 [DOI] [PubMed] [Google Scholar]
- 45.Rintala A, Päivärinne V, Hakala S, Paltamaa J, Heinonen A, Karvanen J, et al. Effectiveness of Technology-Based Distance Physical Rehabilitation Interventions for Improving Physical Functioning in Stroke: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Arch Phys Med Rehabil 2019;100:1339–58. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30529323&dopt=Abstract 10.1016/j.apmr.2018.11.007 [DOI] [PubMed] [Google Scholar]
- 46.Doumas I, Everard G, Dehem S, Lejeune T. Serious games for upper limb rehabilitation after stroke: a meta-analysis. J Neuroeng Rehabil 2021;18:100. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34130713&dopt=Abstract 10.1186/s12984-021-00889-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao M, Wang G, Wang A, Cheng LJ, Lau Y. Robot-assisted distal training improves upper limb dexterity and function after stroke: a systematic review and meta-regression. Neurol Sci 2022;43:1641–57. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35089447&dopt=Abstract 10.1007/s10072-022-05913-3 [DOI] [PubMed] [Google Scholar]
- 48.Maier M, Ballester BR, Verschure PF. Principles of Neurorehabilitation After Stroke Based on Motor Learning and Brain Plasticity Mechanisms. Front Syst Neurosci 2019;13:74. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31920570&dopt=Abstract 10.3389/fnsys.2019.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Epure P, Holte M. Analysis of Motivation in Virtual Reality Stroke Rehabilitation. In 2018:282-93. 10.1007/978-3-319-76908-0_27 [DOI]
- 50.Ryan RM, Rigby CS, Przybylski A. The Motivational Pull of Video Games: A Self-Determination Theory Approach. Motiv Emot 2006;30:344–60. 10.1007/s11031-006-9051-8 [DOI] [Google Scholar]
- 51.De Mauro A. Virtual Reality Based Rehabilitation and Game Technology. In: 2011;71:341-342. [Google Scholar]
- 52.Henderson A, Korner-Bitensky N, Levin M. Virtual reality in stroke rehabilitation: a systematic review of its effectiveness for upper limb motor recovery. Top Stroke Rehabil 2007;14:52–61. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17517575&dopt=Abstract 10.1310/tsr1402-52 [DOI] [PubMed] [Google Scholar]
- 53.Santos B, Dias P, Pimentel A, et al. Head-mounted display versus desktop for 3D navigation in virtual reality: A user study. Multimedia Tools Appl 2009;41:161–81. 10.1007/s11042-008-0223-2 [DOI] [Google Scholar]
- 54.Fang Z, Wu T, Lv M, Chen M, Zeng Z, Qian J, et al. Effect of Traditional Plus Virtual Reality Rehabilitation on Prognosis of Stroke Survivors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am J Phys Med Rehabil 2022;101:217–28. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33929347&dopt=Abstract 10.1097/PHM.0000000000001775 [DOI] [PubMed] [Google Scholar]
- 55.Garay-Sánchez A, Suarez-Serrano C, Ferrando-Margelí M, Jimenez-Rejano JJ, Marcén-Román Y. Effects of Immersive and Non-Immersive Virtual Reality on the Static and Dynamic Balance of Stroke Patients: A Systematic Review and Meta-Analysis. J Clin Med 2021;10:4473. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34640491&dopt=Abstract 10.3390/jcm10194473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mekbib DB, Debeli DK, Zhang L, Fang S, Shao Y, Yang W, et al. A novel fully immersive virtual reality environment for upper extremity rehabilitation in patients with stroke. Ann N Y Acad Sci 2021;1493:75–89. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33442915&dopt=Abstract 10.1111/nyas.14554 [DOI] [PubMed] [Google Scholar]
- 57.Rodgers H, Bosomworth H, Krebs HI, van Wijck F, Howel D, Wilson N, et al. Robot assisted training for the upper limb after stroke (RATULS): a multicentre randomised controlled trial. Lancet 2019;394:51–62. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31128926&dopt=Abstract 10.1016/S0140-6736(19)31055-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tyagi S, Lim DS, Ho WH, Koh YQ, Cai V, Koh GC, et al. Acceptance of Tele-Rehabilitation by Stroke Patients: Perceived Barriers and Facilitators. Arch Phys Med Rehabil 2018;99:2472–2477.e2. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29902469&dopt=Abstract 10.1016/j.apmr.2018.04.033 [DOI] [PubMed] [Google Scholar]
- 59.Hesse S, Heß A, Werner C C, Kabbert N, Buschfort R. Effect on arm function and cost of robot-assisted group therapy in subacute patients with stroke and a moderately to severely affected arm: a randomized controlled trial. Clin Rehabil 2014;28:637–47. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24452706&dopt=Abstract 10.1177/0269215513516967 [DOI] [PubMed] [Google Scholar]
- 60.Crosbie JH, Lennon S, McGoldrick MC, McNeill MD, McDonough SM. Virtual reality in the rehabilitation of the arm after hemiplegic stroke: a randomized controlled pilot study. Clin Rehabil 2012;26:798–806. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22275463&dopt=Abstract 10.1177/0269215511434575 [DOI] [PubMed] [Google Scholar]
- 61.Sucar LE, Leder R, Hernandez J, Sanchez I, Azcarate G. Clinical evaluation of a low-cost alternative for stroke rehabilitation. Paper presented at: 2009 IEEE International Conference on Rehabilitation Robotics; 23-26 June 2009, 2009. [Google Scholar]
- 62.Hung JW, Chou CX, Hsieh YW, Wu WC, Yu MY, Chen PC, et al. Randomized comparison trial of balance training by using exergaming and conventional weight-shift therapy in patients with chronic stroke. Arch Phys Med Rehabil 2014;95:1629–37. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24862764&dopt=Abstract 10.1016/j.apmr.2014.04.029 [DOI] [PubMed] [Google Scholar]
- 63.Stinear CM, Lang CE, Zeiler S, Byblow WD. Advances and challenges in stroke rehabilitation. Lancet Neurol 2020;19:348–60. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32004440&dopt=Abstract 10.1016/S1474-4422(19)30415-6 [DOI] [PubMed] [Google Scholar]
- 64.Everard G, Otmane-Tolba Y, Rosselli Z, Pellissier T, Ajana K, Dehem S, et al. Concurrent validity of an immersive virtual reality version of the Box and Block Test to assess manual dexterity among patients with stroke. J Neuroeng Rehabil 2022;19:7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35065678&dopt=Abstract 10.1186/s12984-022-00981-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deblock-Bellamy A, Batcho CS, Mercier C, Blanchette AK. Quantification of upper limb position sense using an exoskeleton and a virtual reality display. J Neuroeng Rehabil 2018;15:24. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29548326&dopt=Abstract 10.1186/s12984-018-0367-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text File 1
PRISMA-P 2015 Checklist.
Supplementary Text File 2
Search strategies.
Supplementary Table I
AMSTAR assessment.
Supplementary Table II
4.1. RAT effect on motor function during subacute phase.
Supplementary Figure 1
Distribution.
Supplementary Table III
RAT effect on motor function during chronic phase.
Supplementary Figure 2
Distribution.
Supplementary Table IV
VR effect on motor function during chronic phase.
Supplementary Figure 3
Distribution.
Supplementary Table V
TR effect on activity during subacute phase.
Supplementary Figure 4
Distribution.
Supplementary Table VI
RAT effect on activity during chronic phase.
Supplementary Figure 5
Distribution.
Supplementary Table VII
VR effect on activity during chronic phase.
Supplementary Figure 6
Distribution.
Supplementary Table VIII
New technologies effects on motor function when provided in addition (all studies vs only time-matches interventions).
Supplementary Table IX
New technologies effects on motor function when provided in substitution (all studies vs only time-matches interventions).
Supplementary Table X
New technologies effects on activity when provided in addition (all studies vs only time-matches interventions).
Supplementary Table XI
During subacute and chronic phase.
Supplementary Table XII
When provided in addition and in substitution to conventional therapy.
Supplementary Table XIII
Forest-plot regarding the effect of new-technologies on motor function in patients with subacute stroke.
Supplementary Table XIV
Forest-plot regarding the effect of new-technologies on motor function in patients with chronic stroke.
Supplementary Table XV
Forest-plot regarding the effect of new-technologies on motor function in patients with stroke when provided in addition vs in substitution of conventional therapy.
Supplementary Table XVI
Robot-assisted therapy therapy.
Supplementary Table XVII
Telerehabilitation.
Supplementary Table XVIII
Forest-plot regarding the effect of new-technologies on motor function in patients with subacute stroke according to the impairment severity - robot-assisted therapy.
Supplementary Table XIX
Virtual reality.
Supplementary Table XX
Telerehabilitation.
Supplementary Table XXI
Forest-plot regarding the effect of new-technologies on motor function in patients with chronic stroke according to the impairment severity - robot-assisted therapy.
Supplementary Table XXII
Virtual reality.
Supplementary Table XXIII
Telerehabilitation.
Supplementary Table XXIV
Forest-plot regarding the effect of new-technologies on motor function in patients with subacute stroke according to the treatment intensity - robot-assisted therapy.
Supplementary Table XXV
Virtual reality.
Supplementary Table XXVI
Telerehabilitation.
Supplementary Table XXVII
Forest-plot regarding the effect of new-technologies on motor function in patients with chronic stroke according to the treatment intensity - robot-assisted therapy.
Supplementary Table XXVIII
Virtual reality.
Supplementary Table XXIX
Telerehabilitation.
Supplementary Table XXX
GRADE Approach regarding activity outcome during chronic and subacute phase.
Supplementary Table XXXI
GRADE Approach regarding activity outcome when provided in addition to or in substitution of conventional therapy.
Supplementary Table XXXII
Forest-plot regarding the effect of new-technologies on activity in patients with subacute stroke.
Supplementary Table XXXIII
Forest-plot regarding the effect of new-technologies on activity in patients with chronic stroke.
Supplementary Table XXXIV
Forest-plot regarding the effect of new-technologies on activity in patients with chronic stroke when provided in addition vs in substitution of conventional therapy - virtual reality.
Supplementary Table XXXV
Robot-assisted therapy.
Supplementary Table XXXVI
Telerehabilitation.
Supplementary Table XXXVII
Forest-plot regarding the effect of new-technologies on activity in patients with subacute stroke according to the impairment severity - robot-assisted therapy.
Supplementary Table XXXVIII
Virtual reality.
Supplementary Table XXXI
Telerehabilitation.
Supplementary Table XL
Forest-plot regarding the effect of new-technologies on activity in patients with chronic stroke according to the impairment severity - robot-assisted therapy.
Supplementary Table XLI
Virtual reality.
Supplementary Table XLII
Telerehabilitation.
Supplementary Table XLIII
Forest-plot regarding the effect of new-technologies on activity in patients with subacute stroke according to the treatment intensity - robot-assisted therapy.
Supplementary Table XLIV
Virtual reality.
Supplementary Table XLV
Telerehabilitation.
Supplementary Table XLVI
Forest-plot regarding the effect of new-technologies on activity in patients with chronic stroke according to the treatment intensity - robot-assisted therapy.
Supplementary Table XLVII
Virtual reality.
Supplementary Table XLVIII
Telerehabilitation.