ABSTRACT
Globally, enteropathogenic bacteria are a major cause of morbidity and mortality.1-3 Campylobacter, Salmonella, Shiga-toxin-producing Escherichia coli, and Listeria are among the top five most commonly reported zoonotic pathogens in the European Union.4 However, not all individuals naturally exposed to enteropathogens go on to develop disease. This protection is attributable to colonization resistance (CR) conferred by the gut microbiota, as well as an array of physical, chemical, and immunological barriers that limit infection. Despite their importance for human health, a detailed understanding of gastrointestinal barriers to infection is lacking, and further research is required to investigate the mechanisms that underpin inter-individual differences in resistance to gastrointestinal infection. Here, we discuss the current mouse models available to study infections by non-typhoidal Salmonella strains, Citrobacter rodentium (as a model for enteropathogenic and enterohemorrhagic E. coli), Listeria monocytogenes, and Campylobacter jejuni. Clostridioides difficile is included as another important cause of enteric disease in which resistance is dependent upon CR. We outline which parameters of human infection are recapitulated in these mouse models, including the impact of CR, disease pathology, disease progression, and mucosal immune response. This will showcase common virulence strategies, highlight mechanistic differences, and help researchers from microbiology, infectiology, microbiome research, and mucosal immunology to select the optimal mouse model.
Introduction
The intestine plays a key role in the digestion of food and absorption of nutrients and is the location of a significant proportion of the immune system in higher animals.1, 2, 3, 4, 5,6 It is directly exposed to the external environment and is therefore at significant risk of infection. The gastrointestinal tract houses a very dense microbial community, the gut microbiota, which aids digestion, immune conditioning, and host defense.6–8 At higher taxonomic levels, the microbiota community structure is similar between different mammalian species9,10 as they are generally composed of 4–6 major phyla (most prevalently Bacteroidetes, Firmicutes, Proteobacteria, Verrucomicrobiota, and Actinobacteria), suggesting that animal models are generally suitable for investigating mechanisms that control microbial infection in mammals.10 Moreover, the adult microbiota community structure is relatively stable over time,11 indicating that most microbial species continuously ingested via food or water are prevented from being established in the gut.
The gut is the site of infection for a diverse range of enteropathogenic bacteria, including Gram-negative Enterobacteriaceae such as Salmonella enterica, enteropathogenic Escherichia coli (EPEC), enterohemorrhagic E. coli (EHEC), or Citrobacter rodentium, the Gram-negative pathogen Campylobacter jejuni as well as Gram-positive pathogens such as Listeria monocytogenes or Clostridioides difficile.12–15 In this review, we discuss the similarities and differences between these pathogens and how their infection biology can be studied in murine infection models. We review their control by chemical and physical barriers, the role of colonization resistance (CR), as well as the immunological defense mechanisms, which are mounted by the intestinal mucosa.
Barriers against enteric infections
Upon ingestion, the acidity of the stomach is a formidable barrier that eliminates the majority of incoming bacteria. Within the intestine, anaerobiosis, antimicrobial peptides, and nutrient scarcity also influence luminal growth of the pathogen. The physical protection provided by the mucus layer covering the gut epithelium16,17 and fast gut transit times further reduce the local proliferation of the pathogen.18–21
The protection conferred by the resident microbiota, referred to as “colonization resistance” (CR), is the result of multiple factors including the production of antimicrobial substances, nutrient competition, and bacteriophage activity (see Figure 1).22–24 It is estimated that about 25% of bacteria in the human colon carry genes for the production of type VI secretion systems (T6SS) which they may use for the direct killing of invading pathogens.25 Antimicrobial products such as bacteriocins are produced by certain bacteria (Gram-positive and Gram-negative species alike) to limit the growth of competitors.26 Bacteriocins act by inhibiting cell wall biosynthesis, transcription, translation, DNA replication, and outer membrane biogenesis, as well as by disrupting cell membranes.22,26 In addition, nutrient competition is regarded as one of the most important factors when it comes to the establishment of a new bacterium in the gut.27,28 Microorganisms in the gut compete for macro- and micronutrients provided by the host diet, intestinal epithelial cells, and the resident microbiota (cross-feeding).28–30 Finally, bacteriophages are highly specific bacterial viruses which often target a limited number of bacterial strains of given species.31 However, the human phageome is highly diverse and stably covers a wide range of bacterial species and has been shown to contribute to the exclusion of incoming microorganisms in the gut.32 Together, these mechanisms determine if an incoming pathogen will prevail, bloom, and eventually cause disease.
Figure 1.
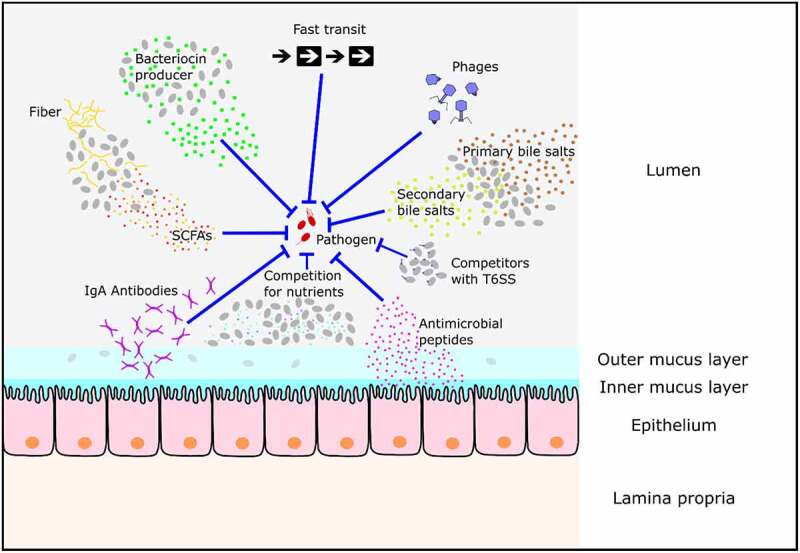
Known mechanisms inhibiting enteropathogen growth in the gut. The resident microbiota in the gut of mammals protects their host from pathogens via mechanisms including the production of short-chain fatty acids (SCFAs), the secretion of bacteriocins, the conversion of primary into secondary bile salts, and the competition for nutrients from the host diet. In addition, phages restrict the colonization of invading bacteria. Finally, a fast transit time makes it challenging for a pathogen to colonize the gut and intestinal epithelial cells (IECs) secrete antimicrobial peptides and IgA antibodies. It is not known if all of the mechanisms indicated are relevant to every enteropathogen.
In addition to overcoming CR, an invading pathogen must avoid, escape, or endure the adaptive and innate immune responses of the host. The mucosal immune system faces a challenge to distinguish invading pathogens from commensal organisms while maintaining homeostasis, promoting appropriate immune responses, and limiting immune-mediated damage. IECs play a vital role not only as a physical barrier but also as a means of communication between the microbiota and the host.33,34 They secrete mucins, antimicrobial peptides, and hormones into the gut lumen and communicate with immune cells on the basolateral side.34 Furthermore, neutrophilic granulocytes can transmigrate into the gut lumen and attack bacteria by phagocytosis, release of antimicrobial substances, and the formation of neutrophil extracellular traps (NETs).35–38 Intestinal antibodies (mainly of the IgA isotype) specifically restrict the colonization of certain bacterial strains by blocking their access to epithelial receptors, entrapping them in mucus, and facilitating their removal by peristaltic activity,39,40 or by selectively clumping rapidly dividing bacteria.39
These barriers are so effective that many hosts will remain healthy upon exposure to an enteropathogenic bacterium. However, some individuals will develop enteric disease. It is currently unclear which mechanisms define a successful barrier to infection though significant progress has been made in the investigation of individual responses to several pathogens, most notably C. difficile.22,41–43 CR can be disrupted by various drugs such as antibiotics, proton pump inhibitors, antidiabetics, and antipsychotics as well as by dietary shifts.22,23,44,45 Some of these substances can be utilized in mouse models to break CR and enable researchers to reproducibly study mechanistic factors which influence enteric diseases. Since efficient therapies or vaccines against most enteric bacterial infections are still lacking, research into the disease mechanisms and the mechanisms that prevent acute infections is of great importance.
Why do we still need animal infection models?
In the intestine, enteropathogenic bacteria engage in complex and incompletely understood interactions with the microbiota, food and its digestion products, the intestinal mucosa, and the host’s immune system. The in vitro models available today insufficiently recapitulate this complexity of interactions. Thus, animal models remain necessary for studying host–enteropathogen infections. Advantages and limitations of mouse models in research on the gastrointestinal tract and the microbiome as a proxy for its human counterpart have been extensively reviewed in Nguyen et al. 2015 and Hugenholtz et al. 201810,46 Our review will summarize and compare the specific features of several available mouse gut infection models. We will cover mouse models for enteric disease triggered by C. jejuni, C. difficile, EPEC, EHEC, L. monocytogenes, and S. enterica serovar Typhimurium (S. Tm) to foster research on filling in the gaps in our knowledge about enteric infections.
Mouse models for enteropathogen infection
Non-typhoidal Salmonella enterica
S. enterica causes 180 million cases of diarrheal disease globally each year.47 The most prevalent non-typhoidal S. enterica serovars diagnosed in human diarrhea are Enteritidis and Typhimurium (S. Tm).1 Humans are commonly infected by contaminated foods, most notably eggs, pork, poultry meat, and dairy products.1 Disease symptoms usually begin 7–132 h after the ingestion of contaminated food48 and include abdominal pain (gastroenteritis), diarrhea, nausea, sometimes vomiting, and transient fever.1 In healthy individuals, the infection is self-limiting, acute diarrhea ends after 3–5 d, and pathogen-shedding in the stool will last for a few more weeks. Even though systematic data are scarce, the infection is typically associated with intestinal inflammation and increases the risk of irritable or inflammatory bowel disease.49 Systemic spread associated with fever can occur in the young, the elderly, and immunocompromised people. The incidence of the disease is 10- to 100-fold lower than the rate of exposure,23 but the risk of infection increases after an antibiotic treatment.50,51 In combination with our knowledge from mouse models (discussed below), this suggests the importance of protection by CR.
Mouse infection models have revealed a key protective role of the gut microbiota. Experimental mice with a complex (but specific pathogen-free, so-called “SPF”) microbiome are in most cases resistant against S. Tm infection.44,52 The CR of SPF mice can be alleviated by antibiotics such as streptomycin, ampicillin, or ciprofloxacin.52–55 SPF mice pretreated with 20 mg streptomycin 24 h before infection with 5 × 107 CFU S. Tm develop gut inflammation with very little mouse-to-mouse variability. The CFU/g feces reach 107 as early as 8 h postinfection (p.i.), rise to 109 CFU/g by 24 h p.i., and stay at that level for weeks to months.23,52,56,57 Gnotobiotic mice associated with up to 12 different microbiota strains (OligoMM12 mice) show partial CR.58 Infection kinetics are different compared to the streptomycin pretreatment model and gut inflammation appears only after 2–3 d of infection.23,59 In the OligoMM12 model, colonization resistance against S. Tm can be fully restored by adding three additional anaerobic strains to the microbiome.60 Moreover, this model offers a unique opportunity to analyze the entire microbiome throughout the infection as all strains are culturable, genetically accessible, and genome sequenced.60,61 Mouse-to-mouse variation in the gnotobiotic mouse models is moderate compared to streptomycin-pretreated or germ-free mice.60,62 Germ-free mice are fully susceptible to S. Tm infection and develop symptoms in the initial 10 h after infection, with a rapid increase in CFU/g feces.62 Admittedly, germ-free mice differ from all the above-mentioned mouse models not only in the absence of microbiota but also in the accompanying immaturity of the immune system, which might in part explain the rapid disease progression.63–65 However, the mechanisms of S. Tm pathogenicity appear to be similar in germ-free and streptomycin-pretreated mice.62 Recently, it has been found that dietary composition has pronounced effects on CR against S. Tm.44 As long as mice harboring a complex SPF microbiota are kept on a standard plant-based mouse chow, they typically exhibit a high degree of CR.44 This CR is strongly impaired, if the mice are shifted for as little as 1 d to a western-style diet (high-fat, low-fiber) or a low-fat plus low-fiber diet.44 This dietary shift alone (without antibiotic treatment) is sufficient to trigger the disease in most mice after inoculation with the same inoculum size (5 x 107 CFU).44 Evidence suggests that altered food composition creates a transient niche for colonization. In the case of a high-fat diet, the compromised CR was traced back to enhanced bile salt secretion.44 This physiological response aids fat digestion, but high primary bile salt concentrations inhibit microbiota growth, while S. Tm can tolerate up to 10-fold higher bile salt concentrations than other members of the microbiota.44 Altered enterocyte physiology, as indicated by changes in mucosal gene expression and Enterobacteriaceae colonization experiments, may further contribute to reduced CR.66,67 In the high-fat diet model for S. Tm gut infection, the microbiota is only mildly suppressed (compared to antibiotic-pretreated mice) and overt enteropathy takes at least 48 h to develop.23,44 The mouse-to-mouse variation with this model is rather high, but in most mice, Salmonella stool densities reach 109 CFU/g after 72 h of infection.23,44 Finally, in other studies, newborn mice were found to lack CR and permit-efficient gut luminal growth of S. Tm and pathogen spread to systemic sites.68
Despite the availability of the above-mentioned mouse models with various levels of CR and decades of research in the microbiota field,69 further work is necessary to identify the precise constituents of a functional microbiome needed to protect against Salmonella spp. infections. Nevertheless, these mouse models have facilitated important insights into the mechanisms of gut colonization by S. Tm and have consolidated the fundamental concept of CR against Salmonella spp.
S. Tm infections in microbiota-colonized mice have shown that the pathogen initially grows by anaerobic hydrogen/fumarate respiration, thus utilizing leftover food molecules and intermediates from anaerobic microbiota metabolism.28,59,70 The pathogen employs flagella to penetrate through gaps in the mucus layer16 and cheY, motA, fliC, and fliB mutants of S. Tm show delayed disease kinetics in streptomycin-pretreated mice.71,72 Upon arrival at the epithelial surface, S. Tm uses adhesins and the TTSS-1, a syringe-like protein injection system to trigger invasion into the gut epithelium.52,73–75 Furthermore, it employs TTSS-2 to traverse the epithelium and proliferate in the lamina propria and organized tissues of the gut-associated immune system (Figure 2).76 The tissue-lodged bacteria and their products (such as LPS77) trigger further innate immune responses. Gut inflammation has both a detrimental and a beneficial effect on the colonization of S. Tm in the gut.35 Severe forms of gut inflammation, as observed in the streptomycin mouse model, can eradicate as much as 99.999% of the gut luminal S. Tm population. However, inflammation also provides the surviving pathogens with a relative growth advantage against the resident microbiota.35,56 Factors that contribute to this selective advantage include the availability of host-derived oxygen and other respiratory electron acceptors, such as tetrathionate and nitrate.78–81 129SvEv mice (expressing a functional natural resistance-associated macrophage protein (NRAMP) which enables them to remove divalent metal ions from the pathogen-containing vacuole) can control systemic infection and can be used to study long-term infections with wild-type Salmonella spp.57,82,83 As C57BL/6 and Balb/c mice carry an NRAMP mutation, they cannot efficiently control systemic pathogen spread.57,82 To prevent life-threatening stages of systemic disease, gut infection assays with wild-type S. Tm in these mice are typically limited to 4 d. Despite this limitation, the extensive set of knockout lines, analytic tools, and the large body of immunological literature on C57BL/6 mice has driven the discovery of mechanisms using this model, in particular in streptomycin-pretreated C57BL/6 mice. Here, gut-luminal growth is associated with invasion into the gut epithelium, as well as proliferation in the gut-associated lymphatic tissue and the lamina propria. This tissue invasion triggers innate immune responses, including the epithelial NAIP/NLRC4 inflammasome,75,84–88 Interleukin (IL)-18 release by the gut epithelium,89 Tumor Necrosis Factor (TNF)-production by lamina propria cells,77,85 Interferon gamma (IFNγ)-elicited triggering of mucus secretion by goblet cells90 and expulsion of infected enterocytes into the gut lumen.84 Despite differences in the way that CR is alleviated, S. Tm infections in adult mice appear to elicit quite similar enteropathy. This is in striking contrast to infections in newborn mice, where the pathogen invades the gut tissue, but fails to trigger overt enteropathy or the expulsion of infected enterocytes.68 The molecular and cellular differences between the disease in newborn and adult mice are still not fully understood.
Figure 2.
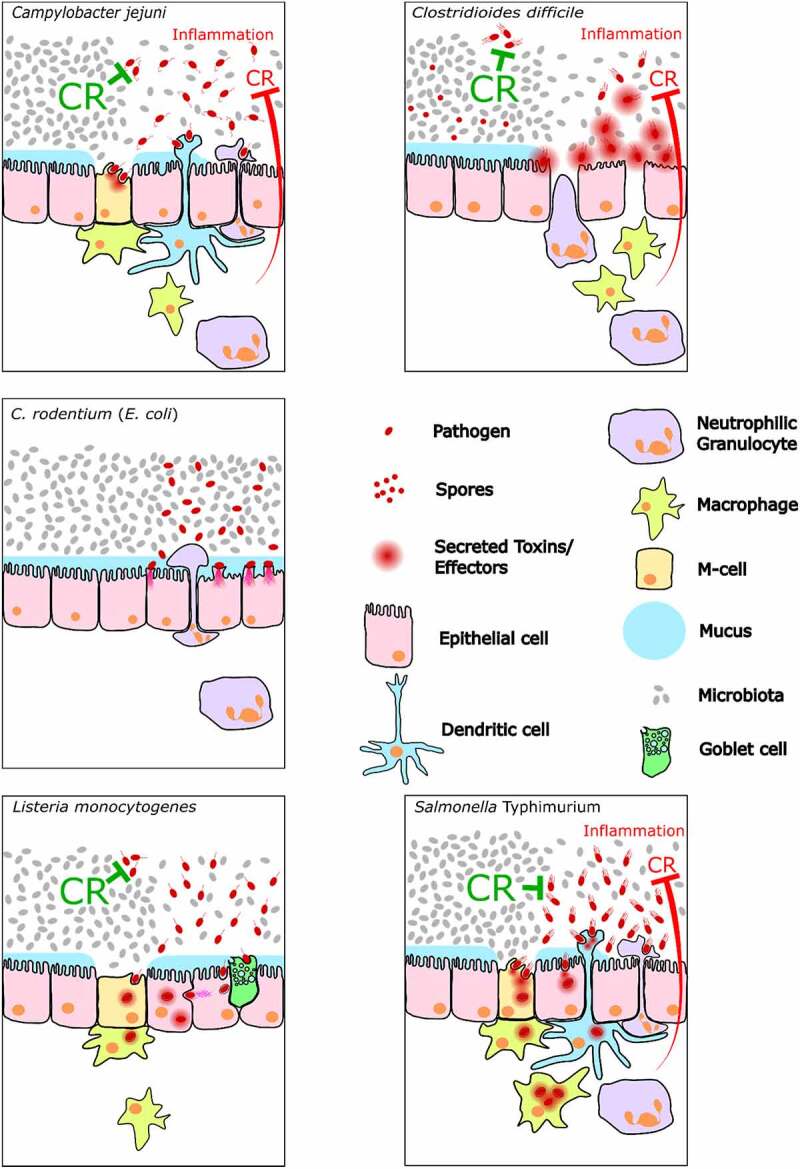
Comparison of the infection process at the intestinal epithelium between the five enteropathogens. The red inhibition arrow indicates the reduction in CR against the pathogen if the pathogen can trigger gut inflammation.
In adult mice, expulsion of infected enterocytes and infiltration by neutrophilic granulocytes pose a strong defense mechanism against S. Tm. The neutrophilic granulocytes transmigrate into the gut lumen and substantially diminish the pathogen population in the gut during inflammation.35,91,92 Attenuated S. Tm mutants defective in systemic spread in C57BL/6 mice (and 129SvEv mice) permit the analysis of the adaptive immune response by d 10–30 p.i.93,94 This includes the development of protective antibody responses against flagellins, outer membrane porins, and the lipopolysaccharide (LPS) O-antigen.95–97 O-antigen-specific secretory IgA, “enchains” gut luminal Salmonella cells (leading to the formation of monoclonal pathogen clumps), which prevents further tissue invasion and accelerates pathogen elimination from the gut.39,94 While a complex microbiota that lack E. coli strains can still confer substantial CR (e.g. by propionate-mediated disruption of the pathogen’s pH homeostasis98), E. coli strains have been identified as important in outcompeting S. Tm from the diseased gut.30,44,99,100 Competition for micronutrients, such as iron ions, terminal electron acceptors like oxygen or nitrate and bacteriocin production, is thought to contribute to this E. coli-mediated CR against Salmonella infection.30,78,101,102
Campylobacter jejuni
The Gram-negative, spirally curved C. jejuni belongs to the four key global causes of food-borne diarrheal infections.103 Among other bacterial species within the genus Campylobacter, C. jejuni (and less frequently Campylobacter coli, Campylobacter lari, and Campylobacter upsaliensis) are the most common causative agents of campylobacteriosis. The manifestations of intestinal enteritis range from mild to severe symptoms, which include, but are not limited to, 1–3 d of fever, vomiting, and malaise followed by 3–7 d of abdominal cramps and bloody diarrhea.104,105 The initial enteritis is induced by a low dose of C. jejuni bacteria that are mainly transmitted by the consumption of raw or undercooked poultry meat, unpasteurized milk, contaminated surface water, as well as cross-contaminated food that is consumed uncooked, such as fruits and vegetables.106 With a lower incidence, other non-gastrointestinal sequelae are associated with campylobacteriosis. Systemic manifestations include infectious complications such as bacteremia and post-infectious immune disorders such as reactive arthritis, Guillain–Barré syndrome, and myocarditis.107,108 In a small but relevant subpopulation of infected individuals, C. jejuni infection triggers the onset of chronic intestinal diseases, such as ulcerative colitis, Crohn´s disease, or irritable bowel syndrome.109,110 Clinical studies revealed that it is fundamental to the understanding of pathogenesisinduced by C. jejuni infection that both the severity of initial enteritis and the risk for the development of post-infectious syndromes are dependent on the molecular structure of the surface endotoxin lipooligosaccharide (LOS), which is highly variable among individual C. jejuni strains.111–113 Thus, endotoxin-induced inflammation initiated by innate immune responses determines the severity of symptoms and the subsequent complications of campylobacteriosis.114
The commensal murine microbiota provides full CR against C. jejuni, thus preventing colonization of the gastrointestinal tract of conventional laboratory mice. For many years, this precluded the development of proper murine infection models for campylobacteriosis. Effective colonization of the murine intestinal tract by C. jejuni requires modifications to the composition of the gut microbiota. This can be accomplished by aggressive antibiotic treatment that yields so-called “secondary abiotic” (SAB) wild-type mice, which can be colonized by C. jejuni but lack overt clinical signs of infection and campylobacteriosis symptoms.108 In the gut of C3H mice colonized by a limited defined enteric microbiota consisting of nonpathogenic Clostridial species, Lactobacillus, and Acinetobacter, C. jejuni was able to colonize at high loads reaching concentrations of 108 to 109 CFU/g of feces after 1 week of infection. Infection doses as little as 200 bacteria were sufficient for intestinal colonization, while the individual immune responses of mice were highly variable.115 Infant mice constitute another murine campylobacteriosis model.116–120 Immediately after weaning, conventional 3-week-old wild-type mice develop self-limited enterocolitis characterized by bloody diarrhea and colonic epithelial cell apoptosis within 1 week following C. jejuni infection and recovered thereafter, thus mimicking the time course of human campylobacteriosis.119,120 Gut microbiota analyses revealed that infant mice harbored higher commensal E. coli but lower Lactobacilli numbers in their large intestines as compared to adult mice.120 The elevated colonic E. coli loads might explain the susceptibility of infant mice toward C. jejuni colonization given that exogenous application of commensal E. coli to conventional adult wild-type mice was shown to abrogate the CR against C. jejuni.120
Interestingly, SAB wild-type mice reconstituted with human gut microbiota derived from healthy donors left these mice susceptible to C. jejuni infection. This was not the case with SAB mice recolonized with murine gut microbiota. C. jejuni loads in mice associated with human gut microbiota reached high levels of 108 CFU/g of feces in the 1st d after infection which remained constant for weeks. However, C. jejuni colonization was effectively cleared during the first 2–3 d after infection in mice associated with murine gut microbiota.104 These studies demonstrate that CR depends on distinct microbial communities that exist in the murine intestinal microbiota. Therefore, SAB mice and other murine models of infection, wherein antibiotics are used to perturb the gut microbiota and abolish CR, have been efficiently used for the study of intestinal colonization by C. jejuni. Oral vancomycin treatment, which promotes S. Tm colonization in a similar fashion to streptomycin pretreatment,121 was shown to deplete bacteroidetes and clostridia and to increase Lactobacillus populations.122 In vancomycin-pretreated mice, C. jejuni was able to establish intestinal colonization at high levels,123 and this was also the case upon pretreatment of mice with ciprofloxacin or penicillin at therapeutic dosages.124 Similarly, ampicillin-pretreated CBA/J mice were shown to be susceptible to C. jejuni colonization.125
As a consequence of antibiotic treatment, SAB wild-type mice can be colonized by C. jejuni but do not develop infectious enteritis, mainly because the murine innate immune system does not react against the LOS of C. jejuni.126 This was shown by Mansfield et al., who successfully developed a murine campylobacteriosis model, in which the absence of IL-10 drastically increases the LOS sensitivity of mice and impairs the immune system’s capacity to resolve inflammation.127 However, this work was done in mice that overexpressed innate immune receptors.126 Finally, the SAB IL-10-deficient mice were confirmed to reproducibly develop typical symptoms of acute campylobacteriosis upon C. jejuni infection. These mice were successfully used to study the immunopathological response against defined C. jejuni virulence factors during disease initiation and progression.128–131 Moreover, further standardization of C. jejuni infection in SAB IL-10-deficient mice allowed for preclinical studies testing the efficacy of drugs affecting the innate immune response against campylobacteriosis.109
Compared to other enteric bacterial pathogens, C. jejuni does not rely on conventional exotoxins that are typically utilized to infect the host. Consequently, campylobacteriosis constitutes an endotoxin-mediated inflammatory disease induced by the contact of C. jejuni-LOS and other endotoxins with innate immune cells, such as dendritic cells, macrophages, monocytes, and neutrophilic granulocytes. However, the physical contact between live C. jejuni and host immune cells depends on the ability of the pathogen to move into the mucus layer, adherence to epithelial cells, and subsequent invasion to subepithelial tissue sites. This is accomplished by the flagellum present at one or at both ends of the cell,132 by adhesins, and by invasins (Figure 2).133 In addition to their function in bacterial motility, C. jejuni utilizes flagella for protein secretion, biofilm formation, and adhesion.134,135 Flagellar motility of C. jejuni is associated with a chemotaxis system, which is essential for effective colonization in the avian and mammalian guts. C. jejuni displays a chemotactic response to amino acids and organic acids originating from either the host or the residual gut microbiota, in addition to distinct constituents of bile and mucus.136
Another important virulence factor for C. jejuni is the capsular polysaccharide (CPS), which plays a major role in systemic infection. The structure of the CPS is variable among C. jejuni strains and may differ in sugar composition and linkage.137,138 CPS also plays an immunomodulatory role by preventing excessive production of cytokines by the host immune system.139,140 Moreover, lipid A in LOS helps C. jejuni to survive hostile environments, to evade the host immune system, and to adhere to and invade epithelial cells.141,142 Interestingly, LOS is not only a major factor in C. jejuni-induced intestinal inflammation but is also essential for triggering post-infectious sequelae, such as Guillain–Barré syndrome caused by cross-reactive LOS-specific antibodies as a result of structural mimicry of pathogenic LOS and the surface of neuronal gangliosides.143 The use of genetically modified SAB mice as a preclinical model for severe human campylobacteriosis constitutes a major advance for investigating C. jejuni virulence factors in vivo. As highlighted in the previous paragraph, IL-10-deficient mice provide a valuable tool to investigate differences between LOS variants in the pathogenesis of C. jejuni. In addition, the SAB IL-10-deficient mouse model was successfully used to explore the role of the flagella during campylobacteriosis, whereby C. jejuni lacking the flagella genes flaA and flaB were unable to trigger enteric disease despite colonizing the murine colon at high loads.131 This model was also applied to investigate the function of other virulence factors, such as the serine protease HtrA and the outer membrane adhesin Cj0268, in C. jejuni pathogenesis.129,144,145 Moreover, it is valuable for preclinical intervention studies and has already been applied to investigate antibiotic-independent therapeutic approaches, such as using the phenolic compounds carvacrol, curcumin, and resveratrol, as well as vitamin C and D, urolithin A, and activated charcoal, to combat C. jejuni or to ameliorate disease manifestations and progression.146–151
Citrobacter rodentium as a model for enteropathogenic Escherichia coli and enterohaemorrhagic Escherichia coli
Human gastrointestinal pathogens EPEC and EHEC remain a major global health problem. While EPEC causes diarrhea in children in low- and middle-income countries, EHEC is mainly found in industrial countries and can lead to hemorrhagic colitis and hemolytic–uremic syndrome (HUS).152,153 Several animal models have been used to study EPEC and EHEC in vivo.154 EPEC has been shown to infect rabbits 155 and pigs,155,156 and studies of EHEC pathogenesis in vivo have involved rabbits,157 chickens,158 gnotobiotic piglets,159,160 and calves.161 However, small animal models, and particularly mouse models, exhibit many advantages, including low relative costs of maintenance and the possibility to manipulate host genetics.162 Thus, various mouse models were also proposed to study EPEC163–165 and EHEC166–169 infections. Although there have been some exceptions,163,169 studies have mostly shown that EPEC and EHEC do not colonize the mouse intestine in the presence of an intact commensal microbiota.170 This is similar to S. Typhimurium and C. jejuni, as discussed above. In the case of EPEC and EHEC, gnotobiotic,168 antibiotic-pretreated,165–167 or young mice harboring immature microbiota164 are used, as they present a reduced CR against these pathogens. Nevertheless, there is an inherent limitation in these models, as they do not reflect natural and physiological host–pathogen–microbiota interactions.171 C. jejuni can colonize mice associated with human (but not mouse) microbiotas, as discussed above. Since commensals play a dual role in C. rodentium infection, both assisting and repelling the pathogen, it might therefore be of interest to test susceptibility to EPEC/EHEC infection in mice reconstituted with a human microbiota.
Citrobacter rodentium is a mouse-adapted pathogen and the etiologic agent of transmissible murine colonic crypt hyperplasia, which causes epithelial cell hyperproliferation and colonic crypt elongation in laboratory mice.172 Genomic analysis revealed around 32% of the C. rodentium genome is not shared with EPEC and EHEC. Nonetheless, C. rodentium, EPEC, and EHEC share a similar infection strategy and virulence genes.173 In fact, like EPEC and EHEC infections, colonization of the gastrointestinal tract by C. rodentium relies on attaching and effacing (A/E) lesions. These are characterized by intimate attachment of the bacteria to the apical surface of epithelial cells, effacement of the brush border, and formation of actin pedestals beneath the adherent bacteria (Figure 2).172,174 The ability of these pathogens to form A/E lesions relies on the locus of enterocyte effacement (LEE) pathogenicity island, which encodes gene regulators, the outer membrane bacterial adhesin intimin, a T3SS and several effector proteins.175–177 Additional T3SS effectors are encoded on prophages and insertion sequences. Intimate attachment is mediated by avid interactions between the intimin and the translocated receptor Tir, a pathogen-encoded protein, which is transported into the host cell via the T3SS.178–181 Attachment to the IECs triggers a type 3 immune response in the lamina propria.174,182,183 During the early stages of infection, C. rodentium is recognized by myeloid differentiation primary-response protein 88 (MYD88)-dependent Toll-like receptor (TLR) signaling, mediated by TLR2 and TLR4 at the surface of epithelial cells.182 Dendritic cells (DCs) produce IL-23, which induces secretion of IL-22 and IL-17 by type 3 innate lymphoid cells (ILC3s). IL-22 induces IECs to express and secrete antimicrobial peptides, like REG3β and REG3γ, and nutritional immunity proteins, such as calprotectin and lipocalin 2 (LCN2).174,182 Consistently, KO mice lacking IL-22, NLRC4, INFγ, TLRs, or Nfil3 (leading to diminished mucosal ILC3) and RAG-1-deficient mice either succumb to C. rodentium infection or exhibit severe pathological mucosal damage.184,185 As a natural A/E mouse pathogen, C. rodentium colonizes mice with an intact microbiota,170 and therefore provides an ideal model for studying A/E pathogens in vivo.162,171,174,182,186
The severity of C. rodentium infections is dependent on the genetic background of the host. While mouse strains like C57BL/6, BALB/c, 129S1/SvImJ, or NIH Swiss present a mild, self-limiting infection, susceptible strains like C3H/HeJ or C3H/HeOuJ succumb to infection 6 to 12 d after C. rodentium inoculation.187 Although genetic factors such as the expression of Rspo2 can have a direct influence on strain susceptibility to infection,188,189 these do not completely explain the observed differences.187 The genetic background can also indirectly influence the intestinal bacterial community,190 affecting susceptibility or resistance to colonization and infection.191–193 In fact, fecal transplantations of resistant NIH Swiss or C57BL/6 mice into antibiotic-pretreated (thus susceptible) C3H/HeJ or C3H/HeOuJ mice, respectively,191,192 reverted susceptibility phenotypes, leading to delayed colonization192 and survival rates varying from 70%192 to 100%.191 Knockout of the vitamin D receptor in mice has also been shown to lead to altered microbiota conferring CR against C. rodentium, even when the dysbiotic microbiota was transferred into germ-free mice.194 Consistent with the major role of the microbiota in host protection, germ-free mice or mice presenting a simplified microbiota like in the neonatal or the OligoMM12 model do not seem to be able to clear C. rodentium infection and present with high bacterial loads in feces up to 42 d p.i.195–200 When co-housing adult mice with a neonatal microbiota with conventional adult mice, CR is restored and the infection is rapidly cleared,197 showing that a fully developed microbiome is essential for pathogen clearance by outcompeting C. rodentium.195,196
Diet has also been shown to play a major role in shaping the microbiota and thus, enhancing or impairing C. rodentium infection. For example, a reduced-fat diet or the addition of dairy products to normal diet-induced protective effects against C. rodentium and ameliorated associated pathology.201,202 In contrast, a fiber-free diet leads to the bloom of mucus-layer degrading commensals and thereby increases susceptibility to C. rodentium infection.203 While treatment of mice with fermented dairy products did not affect C. rodentium colonization, organ specificity, or A/E lesion formation, it reduced colonic hyperplasia and it prevented the decrease of Ruminococcus and increased Turicibacteraceae (Turicibacter) abundance,202 whose decrease has been associated with susceptibility to dextran sodium sulfate-induced colitis.204
Microbial diversity in the gut makes it difficult to disentangle individual contributions, meaning that the mechanisms by which CR is achieved are not yet completely understood. However, several commensals have been reported to play a role in CR against C. rodentium. When administered to mice prior to C. rodentium inoculation, various Lactobacillus strains such as Lactobacillus rhamnosus, Lactobacillus acidophilus, or Lactobacillus helveticus were able to modulate the immune response during C. rodentium infection, thereby reducing immune cell infiltration of the lamina propria and colonic crypt hyperplasia.205–207 In neonatal mice, treatment with L. helveticus and L. rhamnosus reduced colonic crypt hyperplasia and mortality,208,209 induced anti-inflammatory pathways, and reduced C. rodentium attachment to colonic cells.209 Other Lactobacillus strains were also shown to protect against A/E pathogens in vitro by upregulating secretion of the mucin MUC3 and thereby reducing EPEC adherence.210 Preventive treatment with Bifidobacterium breve in mice also reduced crypt hyperplasia even if it did not reduce colonization or A/E lesion formation.211 However, another report showed a reduction in C. rodentium loads in the gut when treating mice with B. breve through a mechanism involving production of exopolysaccharide.212 Germ-free mice colonized with neonatal microbiota are unable to clear C. rodentium infection, but administration of Clostridia commensals allowed establishment of CR and clearance of the infection.197 Moreover, segmented filamentous bacteria (SFB) have been shown to confer protection against C. rodentium infection via promoting protective immune responses in the gut213 and stimulating retinoic acid responses in intestinal epithelial cells.214
Although many mechanisms conferring CR through the microbiota involve modulation of the immune response,174,191,206–209,212,213 microbiota can also have direct effects on C. rodentium. L. rhamnosus, L. acidophilus, and Citrobacter amalonaticus showed inhibitory effects on C. rodentium growth in vitro.206,217 C. amalonaticus also impaired the growth of C. rodentium in vivo.215 Butyrate, a metabolite produced in the gut by some commensals, inhibited C. rodentium growth in vitro and reduced colonization in vivo.193 Treatment of infected mice with Saccharomyces boulardii 2 d after C. rodentium inoculation, resulted in inhibition of expression and secretion of EspB and inhibition of secretion and translocation of Tir, thereby reducing the number of mucosal adherent C. rodentium and ensuing pathology.216
Interestingly, although microbiota can play a key role in CR against C. rodentium and in clearance of infection, it is also needed for C. rodentium virulence.198 Following kanamycin-induced dysbiosis in mice infected with KanR C. rodentium, the pathogen was displaced to the cecal lumen and was able to persist avirulently.198,215 This was not observed after vancomycin or metronidazole treatment, suggesting C. rodentium relies on specific commensals for colonization.198 Bacteroides thetaiotaomicron, a major constituent of the microbiota, was also shown to enhance the expression of C. rodentium virulence genes during infection.217 B. thetaiotaomicron also contributed to the loss of the mucosal layer during C. rodentium infection,217 which leads to increased colonization and mortality in mice.218
The use of C. rodentium, a natural mouse pathogen, as a model for A/E pathogens has given important insights into the complex interactions that occur between the pathogen, the microbiota, and the host in the context of infection.162,171,174,182,186
Given the essential role that the microbiota plays in gastrointestinal infection and disease, moving forward it will be key to consider the factors that affect the composition of the microbial community in the gut in order to study the colonization andvirulence mechanisms of A/E pathogens and ensure reproducibility between facilities.
Clostridioides difficile
The endospore-forming bacterium C. difficile (formerly Clostridium difficile219) is a leading cause of nosocomial antibiotic therapy-associated diarrhea. In the 2000s, its incidence was rising in Europe and North America, concomitant with increases in disease severity.220,221 More recent data revealed currently decreasing numbers in the USA,221 while the situation in Europe remains less clear because of national differences in detecting and reporting C. difficile infection (CDI) cases.222 Still, C. difficile remains a formidable pathogen burdening the health-care services of Europe and the USA with annual costs of $3 223 and 4.8 billion,224 respectively. Annually, the ECDC reports over a hundred thousand cases in Europe,225 while the CDC reports just under half a million cases in North America, both with mortality rates of 3–17%.224,226 Infection occurs via the fecal-to-oral route through endospores and is facilitated by low hygiene standards. The disease is not only transmitted in health-care facilities but also commonly acquired in community settings and may arise from strains present in the patient’s own microbiome.227,228 The role of environmental reservoirs and contaminated food as sources of infection remains elusive. In addition to general risk factors for nosocomial infections, including extended hospitalization, advanced age, and comorbidities, CDI is strongly associated with antibiotic therapy that lowers microbiota-mediated CR.229 Symptoms of human CDI ranges from asymptomatic carrier status (approximately 4–15% of healthy individuals were estimated to be asymptomatic carriers230), fever, abdominal pain, and watery or bloody diarrhea to life-threatening pseudomembranous colitis.231 Asymptotic carriers colonized with toxigenic C. difficile are 6 times more likely to experience an episode of CDI than non-colonized individuals.232 C. difficile pathogenicity depends on its main virulence factors, toxins A and B.233 A third toxin, the so-called binary toxin, can exacerbate disease symptoms, but its precise function is still unclear.234 Antibiotic therapy with vancomycin or preferably fidaxomicin is the first-line treatment against primary CDI, which most commonly resolves the infection, but may also perpetuate intestinal dysbiosis, leading to recurrent CDI in about ~16% to 25% of cases.235–239
Healthy gut microbiota of mice usually provides highly protective CR against infection with C. difficile (Figure 2). Therefore, in the past, pathogens have been studied in inherently more susceptible host species, such as hamsters, guinea pigs, and rabbits, as well as germ-free rats and mice.240–243 For nearly 3 decades, the main CDI model has been the Syrian hamster, although the severity and lethality of the induced disease are drastically increased compared to the situation in humans. More recently, a more representative conventional mouse model has been established in which mice are pretreated with a cocktail of multiple antibiotics to alleviate CR.244 The subsequent infection with C. difficile induces strong colitis and high lethality in a dose-dependent manner. Strain-level genetic variabilities in C. difficile are responsible for variability in symptom severity in mice. This was shown in a study in which manifestations of diarrhea and weight loss depended on the C. difficile strain (R20291, VPI 10463 (ATCC 43255), or 630 (ATCC BAA-1382)) and the type of antibiotic pretreatment.245 The SPF mouse model has been used more frequently since and has been further refined by reducing the number of administered antibiotics to break CR. Thus, robust infection models could be established by pretreating mice with either cefoperazone in the drinking water,246 streptomycin by gavage247 (the same pretreatment as in the streptomycin-pretreated S. Tm model), or clindamycin by intraperitoneal injection.248 Standard infection models are usually inoculated with C. difficile spores, the relevant main transmissive form, but mice may also be infected directly with high doses (107 CFU) of vegetative cells, such as sporulation-deficient spo0A mutants to study the role of spores.249–251 The high variability of antibiotic pretreatment procedures as well as the genetic differences between C. difficile strains counter-intuitively do not affect the number of vegetative cells found in the cecum and the amount of toxins that are produced 18 h p.i.245,252 However, each antibiotic shapes the community differently after administration and therefore creates a distinct ecological niche. Hence, differential gene expression, especially in catabolic pathways used for simple carbohydrate-molecule acquisition/uptake, was observed in C. difficile.253 Vegetative cells can be detected after 6 h p.i. in the distal intestine at low concentrations (102–103 CFU/g). After 18 h, colonization expands to the proximal intestine including the stomach and the maximum concentration of 108 CFU/g is reached in the cecum. Despite colonization of the entire gastrointestinal tract, pathology is limited to the cecum and colon.254
Germ-free mice can be infected with low-to-moderate doses of spores (103–106 CFU) without inducing lethality,242 although this strongly depends on the virulence of the particular C. difficile strain and on the susceptibility of the mouse model.255–257 In germ-free mice, the C. difficile sporulation (and hence transmission) and toxin production are generally higher than in SPF mouse models.252 Therefore, germ-free mice can be useful to study the effect of protective microbial species or other factors against CDI.258 Mono-association with these species revealed niche competition with C. difficile, especially for nutrients, which is also supported by in vitro studies.257,259,260 Increased sialic acid levels observed in gnotobiotic mice mono-colonized with Bacteroides thetaiotaomicron led to increased fecal C. difficile densities, demonstrating that not all species of the gut microbiota may be inherently protective.261 Further, the addition of the bile acid 7α-dehydroxylating (deoxycholic and lithocholic acid producing) bacterium Clostridium scindens to gnotobiotic OligoMM12 mice60 showed protective effects against C. difficile in early-phase colonization.247 The strong correlative data obtained in the antibiotic treated SPF mouse model further support the hypothesis that the main contribution of C. scindens to CR is causally related to the conversion of primary bile acids, which enhance C. difficile spore germination, into 7α-dehydroxylated secondary bile acids, which inhibit vegetative cells of C. difficile.262 Secondary bile acids were shown to have not only direct negative impacts on vegetative cells but also synergize with C. scindens-secreted antimicrobials that inhibit C. difficile.263 However, the importance of secondary bile acids was challenged in a recent study using Cyp8b1−/− (cholic acid deficient) mutant mice colonized with C. scindens that suggested a bile acid-independent antagonistic effect of C. scindens toward C. difficile.256 Competition for Stickland metabolites may explain most of the protective effect of C. scindens in this mouse strain, which previously was mainly, but not exclusively,264 attributed to its production of 7α-dehydroxylated bile acids.256 This finding remains to be corroborated by in vivo studies of bai gene C. scindens mutants (deficient for bile acid 7α-dehydroxylation), which so far have been unavailable.
Fecal microbiota transplantation (FMT) is already commonly used in patients suffering from multiple episodes of recurrent CDI (rCDI) to restore the host–microbiota-mediated colonization resistance with high success rates. In a rCDI mouse model, it was demonstrated that FMT successfully reduces C. difficile to undetectable levels.265 The restoration of bile acid homeostasis, especially the conversion of primary bile acids to secondary bile acids, seems an important function to restore microbiota mediated CR.265–267 Elevated levels of short-chain fatty acids (SCFA) also correlate with enhanced CR against C. difficile268 and it was shown that FMT fosters SCFA-producers269 implying that reintroduction of SCFA-production is an important feature of colonization resistance restoration.270
Despite its remarkable efficacy and the recent FDA approval of a first FMT preparation as therapeutic against C. difficile infection,271 FMT lacks standardization and carries the risk of introducing undetected opportunistic pathogens as well as other unwanted microbiota-related effects272 including possible long-term effects on immunity or development of adiposity. However, there is little solid evidence for negative long-term effects to date.273–276 On the contrary, most studies report beneficial outcomes. In order to minimize FMT-related risks, efforts to develop defined microbial therapy have been made both in humans277 and mice278 where C. difficile was successfully inhibited with consortia of 6 to 33 species. A rationally designed bacterial consortium of five mucosal sugar utilizers was also able to limit C. difficile expansion in the gut of C57BL/6 mice.279
Listeria monocytogenes
L. monocytogenes is a Gram-positive foodborne pathogen that is widely distributed in nature and is readily isolated from soil, water, silage, and vegetation.280 This wide ecological distribution reflects the ability to adapt to a range of environmental stress conditions, such as low pH, variations in temperature, and elevated osmolarity that are encountered in the natural environment and in the host.281 L. monocytogenes is the causative agent of listeriosis, an invasive and potentially fatal infection in susceptible animals and humans. Listeriosis is still considered relatively rare; however, its mortality rate (20–30%) is high relative to other food-borne pathogens,282 and it is considered a significant public health concern.283 The virulence potential of the bacterial strain and the immune status of the host determines the severity of L. monocytogenes infection. The most at-risk groups are those with compromised immune systems including pregnant women (leading to spontaneous miscarriage), infants, older adults, and immunocompromised individuals (leading to meningitis or meningoencephalitis).284 Combined epidemiological, clinical, and genomic analysis has identified hypervirulent and hypovirulent clonal clusters of L. monocytogenes, with CC1, CC4, and CC6 being demonstrably hypervirulent in mouse models of the disease.285
L. monocytogenes enters the host after ingestion of contaminated food. Pathogenesis involves systemic disease and the crossing of various epithelial barriers, initially the gastrointestinal barrier and subsequently the blood–brain barrier (to cause meningitis) or the feto-placental barrier in pregnant individuals. Entry into gut epithelial cells occurs through the expression of bacterial internal A (InlA) that engages E-cadherin, an eukaryotic cell membrane receptor that ultimately triggers bacterial internalization (Figure 2).286 In particular, goblet cells which express exposed E-cadherin represent a targeted site for initial infection and can directly facilitate gastrointestinal transcytosis.287 As established primarily using in vitro studies, L. monocytogenes is internalized into the vacuole in epithelial cells and expression of the microbial pore-forming toxin Listeriolysin O (LLO) and phospholipase A and PlcB rupture the vacuolar membrane to release bacteria into the host cell cytoplasm where they divide rapidly and can move from cell to cell using a mechanism that involves host actin-based motility.286 Upon entry into the host gastrointestinal tract, L. monocytogenes senses the local environment to regulate virulence gene expression, thereby switching between saprophytic and infectious gene expression patterns. The major regulator of virulence gene expression is positive regulatory factor A (PrfA) which is regulated at the transcriptional and post-transcriptional levels by different environmental signals which include temperature, carbon-sources transported via phosphoenolpyruvate (PEP), carbohydrate phosphotransferase system (PTS) and stress response regulatory proteins such as Sigma.288–291 In particular, non-PTS carbohydrates, such as glycerol, enhance virulence gene expression.292 Evidence suggests that nutrient metabolism and virulence are tightly co-regulated to control niche-specific virulence in response to environmental nutritional content.293
Germ-free mice are more sensitive to L. monocytogenes infection than SPF mice, indicating a role for the microbiota in resistance to infection.294–296 Mono-colonization of germ-free mice with Lactobacillus casei, paracasei, or saki strains resulted in increased survival of mice in the probiotic-treated group after 6 d p.i., suggesting enhanced CR.295,296 Archambaud et al.296 determined that monocolonization by Lactobacillus casei and paracasei strains in transgenic ECadhum mice (which express the human E-Cadherin as receptor for Listeria monocytogenes internalization; see below) provided an environmental signal which promoted adaptation of the pathogen to the gut. However, colonization with Lactobacillus strains also enhanced the anti-Listerial interferon response and reduced dissemination of the pathogen in the host. Other mechanisms by which the gut microbiota may protect against Listeria infection include local production of bacteriocins297 or the triggering of host expression of anti-Listerial defensins such as RegIIIγ.298 Recent studies demonstrated that a high fat diet reduced RegIIIγ expression and increased goblet cell number in normal mice infected with murinized L. monocytogenes (which binds to the murine version of the cadherin receptor; see below) and concomitantly increased severity of infection.299 Interestingly, whilst RegIIIγ is seen to express high activity against L. monocytogenes, RegIIIβ is active against Salmonella strains but not L. monocytogenes indicating potential nuances in responses to different pathogens.300
Evidence suggests that L. monocytogenes strains may produce bacteriocins locally in the gut to alter microbiota community structure and favor disease progression. Lmo2776 is a bacteriocin produced by L. monocytogenes that targets commensal Prevotella copri to enhance infection by the pathogen.301 Listeriolysin S (LLS) is a protein with characteristics of both hemolysins and bacteriocins which is produced by epidemic strains of L. monocytogenes.302 It showed bactericidal activity against Alloprevotella, Allobaculum, and Streptococcus.303 LLS is not cytotoxic and acts locally in the gut as a bacteriocin to modify the microbiota and favor gastrointestinal colonization by the pathogen.304
Recent work is beginning to determine the key taxa within the microbiota that provide CR against L. monocytogenes. By inference from the studies above Prevotella copri may be a key contributor to CR as specific targeting of this species enhances colonization by the pathogen.301 Feeding mice, a high-fat diet alters the microbiota and enhances Listeria colonization, however further work is necessary to determine whether species that are reduced by high-fat diet and known to influence barrier functions (such as Akkermansia muciniphila) are important for protection against the pathogen.299 Studies have used streptomycin pretreatment in conventionally raised mice to dramatically reduce CR with subsequent analyses of taxa that contribute to reestablishing resistance to L. monocytogenes.305 Rational selection and subsequent administration of Clostridium saccharogumia, Clostridium ramosum, Clostridium hathewayi, and Blautia producta prevented a systemic infection with L. monocytogenes in germ-free mice.305
Information derived from studies investigating the role of microbiota in CR may give rise to novel methods of treating or protecting against infection. A number of novel approaches have included the identification of potential next-generation probiotic strains,305 development of engineered probiotic strains that produce bacteriocins306 and the development of engineered probiotics that express InlA, InlB, or other Listeria adherence proteins (LAPs) to directly compete with the pathogen.307,308 Drolia et al. 308 showed that the expression of LAP on the surface of a Lactobacillus casei strain reduced intestinal colonization of L. monocytogenes and protected mice from lethal infection.
There are several animal models that can be used to investigate the role of microbiota in L. monocytogenes infection. Normal inbred laboratory mice are relatively resistant to oral infection with L. monocytogenes 309,310 due to the significantly reduced affinity of InlA for the murine E-cad receptor. However, the efficiency of infection can be increased by transgenic expression of human E-cad in enterocytes of the small intestine in mice311,312 or mutagenesis of InlA in L. monocytogenes to create engineered (murinized) strains.313,314 Nevertheless, a high dose of administered L. monocytogenes is still necessary to study the in vivo behavior of L. monocytogenes.309,311 In addition, murinization of the inlA gene in Listeria promotes targeting of another receptor, M-cadherin, in mice which alters the local inflammatory response relative to the humanized mouse model system.315 Other small animal models such as guinea pigs express E-cadherin that resembles human E-cadherin and may represent an effective animal model for oral infection, albeit they are limited by cost.316,317 Some studies utilize high-dose Listeria infection in regular laboratory mouse strains.305 It should be noted that common mouse strains can differ in ability to mount inflammatory responses or to fix complement and in particular A/J and BALB/c/By/J are more susceptible to intravenous and intraperitoneal Listeria infection than C57BL/6 or C57BL/10 mice.318 Finally, future studies should examine the role of the microbiota in aged or pregnant models in order to more closely mimic those groups of individuals that are more likely to develop human disease. Pregnant mouse or guinea pig models319 or aged mouse colonies320 have been examined as models for basic L. monocytogenes infection.
Concluding remarks
Our review highlights the importance of an intact and diverse microbiome for protection against enteric disease. Germ-free and antibiotic pretreated mice are susceptible to colonization by all the pathogens mentioned above. But even more subtle approaches, like switching normal mouse diet to western-style diet, allows colonization of conventional mice with at least two (very distinct) pathogens: the Gram-positive Listeria monocytogenes and the Gram-negative S. Tm. However, to trigger disease symptoms in mice that are similar to those found in humans, it is often necessary to change certain properties of the mouse immune system and/or the surface receptors of the intestinal epithelial cells. (A comprehensive comparison of the mentioned pathogens can be found in Table 1.) We expect that the diversity offered by this set of murine infection models (Table 2) will provide unique opportunities to demonstrate generalizable principles underlying CR and to discover species-specific adaptations. This tool-box is still expanding as indicated by the recent discovery of Shigella flexneri infection models in inflammasome-deficient mice.321 First attempts to apply the same principles for therapy of one pathogen to another by restoring CR have already been made.322 We hope that this review will further accelerate progress toward a better understanding of gastrointestinal infections in order to harness the protective mechanisms to develop preventive measures and new therapies.
Table 1.
Key features of the pathogens relevant for enteric infection.
| Pathogen | Key virulence factors of pathogen | CR (complex microbiota) | Protection by mucus layer | Innate immune responses | Adaptive immune responses | References |
|---|---|---|---|---|---|---|
| Salmonella Typhimurium | flagella, TTSS-1, TTSS-2, sii adhesin | Strong | ≈10-fold | NLRC4 inflammasome, TLR, MyD88, TNF, IFN | sIgA against O-antigen | Hapfelmeier (2005); Fattinger (2021); Hausmann et al. (2020); Hausmann (2021); Sellin (2014); Barthel (2003) |
| Listeria monocytogens | InlA, InlB, LAP, lmo1413, LLO, PlcA, PlcB, ActA, | Medium to strong | Evidence for mucous-binding | MyD88, NOD2, NLRC4/AIM2/NLRP3 inflammasome, NF-κB, RegIIIγ | CD8 & CD4 (TH1) T cell response | Brandl, K. (2007); Drolia & Bhunia, (2019); Pizarro-Cerda & Cossart (2018), PMID: 30,523,778 |
| Campylobacter jejuni | flagella, LOS, HtrA, Cj0268, adhesins, invasins | Strong (only in mice) | No protection provided (mucus can be a chemoattractant) | NLRP3 inflammasome, TLR, MyD88, TRIF, NF-κB, mTOR | Th1 and Th17 lymphocytes responses, IgA and IgG which can be cross reactive to human gangliosides in neuerons | Heimesaat, M. M. et al. (2014); Kim, S. et al. (2018); Schmidt, A. M. et al. (2019); Mansfield, L. S. et al. (2007); Tetmeyer, N. et al. (2021); Sun, X. et al. (2012) |
| Escherichia coli/ C. rodentium | LEE encoded genes, particularly a type III secretion system (T3SS), translocators, intimin and translocated intimin receptor (Tir); non-LEE encoded effectors + Shiga toxin (Stx) for EHEC | Strong for EPEC and EHEC, no CR in inbred mice for C. rodentium | 10–100 fold reduction of C. rodentium burdens in feces and reduction of mortality compared to mice lacking a mucus layer (Muc2-/- mice) | Type 3 immunity, IL-22 and IL-17 secretion, antimicrobial peptide secretion | Type 3 immunity, IgG opsonization | Luperchio and Schauer (2001); Mullineaux-Sanders et al. (2019); Mundy et al. (2006); Vallance et al. (2003); Bergstrom et al. (2010); Silberger et al. (2017) |
| Clostridoides difficile | flagella, TcdA and TcdB, binary toxin CDT, Spo0A | Strong | Unknown | NLRP3 inflammasome, TLR 4/5, MyD88, NOD1, IFNγ, antimicrobials, ROS, RNS | sIgA against TcdA and TcdBHM | Smits, W. K. (2016); Johnson, S. (1992); Abt, M. C., et al,(2016) |
Table 2.
Mouse gut infection models.
| Model | Microbiota | Alleviation of CR | Mouse strain | Pathogen density in stool | Onset of enteric disease | Histopathology | Method of infection | Volume and CFU for infection | Pathogen strain | References |
|---|---|---|---|---|---|---|---|---|---|---|
| SalmonellaTyphimurium | ||||||||||
| Streptomycin model | Complex, gnotobiotic | Streptomycin (20 mg p.o.; 24 h before infection) | C57BL/6, Balb/c, 129, any other | 108–1010 CFU/g | 10–12 h p.i. | Epithelium erosion, mucus release by goblet cells, granulocyte infiltration, submucosa edema | Oral gavage | 50 µL of 5 × 107 CFU | SL1344 | Barthel et al. (2003) |
| Gnotobiotic microbiota model | OligoMM12, LCM | Not needed | C57BL/6 (others not tested) | 106 CFU/g at d 1; 1010 CFU/g by d 4 | 2–4 d p.i. | Epithelium erosion, mucus release by goblet cells, granulocyte infiltration, submucosa edema | Oral gavage | 50 µL of 5 × 107 CFU | SL1344 | Brugiroux et al. (2016); Maier et al. (2013) |
| Germ-free model | None | Not needed | C57BL/6 | 108 cfu/g 8 h p.i., 109 at 24 h and 1010 at 48 h | 8–12 h p.i. | Extreme epithelium erosion, due to slow onset of regeneration; mucus release by goblet cells, granulocyte infiltration, submucosa edema | Oral gavage | 50 µL of 5 × 107 CFU | SL1344 | Lima-Filho et al. (2004) |
| Western-diet model | Complex | Switch to WD 24 h before infection | C57BL/6 (others not tested) | 106 cfu/g at d 1 and 2, 107 to 108 at d 2 and 109 at d 4 | 3–4 d pi. (high animal to animal variation) | Epithelium erosion, mucus release by goblet cells, granulocyte infiltration, submucosa edema; high mouse-to-mouse variability | Oral gavage | 50 µL of 5 × 107 CFU | SL1344 | Wotzka et al. (2019) |
| Clostridioides difficile | ||||||||||
| Antibiotic cocktail model | Complex | Kanamycin (0.4 mg/mL), gentamicin (0.035 mg/mL), colistin (850 U/mL), metronidazole (0.215 mg/mL), and vancomycin (0.045 mg/mL) for three d via drinking water. 2 d pause. clindamycin (10 mg/kg) intraperitoneally one d prior to infection | C57BL/6 | 108–109 CFU/g | 24 h p.i. | Epithelium erosion, forming ofpseudomembranes, neutrophil and macrophage exsudation, mucosal injury | Oral gavage | 103 CFU | VPI 10463 (ATCC 43255) | Chen et al. (2008) |
| Cefoperazone model | Complex | Cefoperazone (0.5 mg/ml) for five d via drinking water. 2 d pause prior to challenge. Optionally, clindamycin (10 mg/kg) intraperitoneally one d prior to infection | C57BL/6 | 107 CFU/g | 24 h p.i. | Epithelium erosion, forming of pseudomembranes, neutrophil and macrophage exsudation, mucosal injury | Oral gavage | 25 µl with 105 CFUor 6*107 CFU | VPI 10463 (ATCC 43255), R20291 or 630 (ATCC BAA-1382) | Castro-Cordova et al. (2016) |
| Clindamycin model | Complex | Clindamycin (200 μg) by intraperitoneal injection on d −1 | C57BL/6 | 107 cfu/g at d 1; 107–108 cfu/g by d 2,3 | 24 h p.i. | Epithelium erosion, forming of pseudomembranes, neutrophil and macrophage exsudation, mucosal injury | Oral gavage | 103 CFU | VPI 10463 (ATCC 43255) | Buffie et al. (2012) |
| Streptomycin model | Complex | Streptomycin 100 μL by gavage, 200 mg/mL by gavage on d −1 or 5.0 mg/ml via drinking water for 5 d with 2 d pause prior to infection | C57BL/6 | 107–108 CFU/g | 18 h – 24 h p.i. | Epithelium erosion, forming of pseudomembranes, neutrophil and macrophage exsudation, mucosal injury | Oral gavage | 100 µl 103 CFU | DH1916 or 630 (ATCC BAA-1382) | Studer et al. (2016); Jenior et al. (2017) |
| Gnotobiotic microbiota model | OligoMM12 | Not needed | C57BL/6 | 107 cfu/g at d 1; 107–108 cfu/g by d 3 | 24 h p.i. | Epithelium erosion, forming of pseudomembranes, neutrophil and macrophage exsudation, mucosal injury | Oral gavage | 100 µl with 103 CFU | DH1916 | Studer et al. (2016) |
| Germ-free model | None | Not needed | CD-1, Swiss Webster, C57BL/6 | 109 CFU/g | 18 h − 24 h p.i. | Epithelium erosion, forming of pseudomembranes, neutrophil and macrophage exsudation, mucosal injury | Oral gavage | 100 µl with 109 CFU(vegetative cells) or 102 CFU(spores) | HUC2-4, VPI 10463 (ATCC 43255) or 630 (ATCC BAA-1382) | Onderdonk et al. (1980); Reeves et al. (2012) |
| Listeria monocytogenes | ||||||||||
| Western-diet model | Complex | Western Diet High fat (45% calories from fat) for 13 d prior to infection | C57Bl/6 J | 109 CFU/g at d 1; 107 at d 2: 106 at d 3 | 24 h.p.i. | Increase of goblet cell numbers by high fat prior to infection; reduced inflammatory response in HF diet post-infection | Oral gavage | 200 µl with 5 × 109 CFU | EGDm (murinized) | Las Heras et al. (2019) |
| Germ-free model andmonocolonized mice with Lactobacillus sakei 2a | None | Not needed | germ-free NIH mice | 1x108 cfu/g at d 1 in both germ-free and monocolonized mice | ND | Increased survival of monocolonized mice; greater evidence of inflammatory lesions in the ileum, cecum and liver of Listeria infected germ-free mice relative to infected monocolonized mice | Oral gavage | 100 µl with 1 × 108 CFU | Scott A (wild-type) | Bambirra et al. (2007) |
| Streptomycin model | Complex | Streptomycin (20 mg p.o.; 24 h before infection) | C57BL/6, Rag1−/−, Ifng−/− and Il17−/− | 1 CFU/g at d 1, 104 at d 6 and 102 d 10 | 3 d p.i. | Iinfection, edema, inflammatory cell infiltration, and epithelial cell shedding | Oral gavage | 1 x 108 CFU (volume unclear) | 10403s (wild-type) | Becattini et al. (2017) |
| Mono-colonized model (Lactobacillus) | A single strain: Lactobacillus | Germ-free mice or mice monocolonized with either Lactobacillus paracasei or Lb. casei | germ-free humanized E-Cad-hum mice | 106 at d 1 for germ-free; 105 at d 1monocolonized | ND | Enhancement of host micro-RNA expression and downregulation of IL-2 and IL-10 by Lactobacillus monocolonoization during Listeria infection | Oral gavage | 200ul with 5 × 109 CFU | EGDe (wild-type) | Archambaud et al. (2012) |
| Campylobacter jejuni | ||||||||||
| Hhumanized FMT model | Human gut microbiota from healthy donors | Not needed | C57BL/6 C57BL/10 IL-10 KO | 108 cfu/g | 2 d p.i. only in IL-10 KO | Moderate to severe hyperplasia, elevated levels of apoptotic cells, moderate inflammatory cell infiltration into the mucosa, moderate goblet cell loss high mouse-to-mouse variability | Oral gavage | 300 µl of 109 CFU | ATCC 43431; 81–176; B2 | Mansfield et al. (2007); Bereswill et al. (2011); Heimesaat et al. (2019) |
| Secondary abiotic model | None | Not needed | C57BL/6 C57BL/10 IL-10 KO | 108–109 CFU/g | 2 d p.i. only in IL-10 KO | Severe hyperplasia, elevated levels of apoptotic cells, marked inflammatory cell infiltration into the mucosa and submucosa, marked goblet cell loss, multiple crypt abscesses, and crypt loss | Oral gavage | 300 µl of 109 CFU | ATCC 43431; 81–176; B2 | Mansfield (2007); Bereswill (2011); Heimesaat (2022) |
| Infant model | Complex; higherEnterobacteriaceae loads | Not needed | C57BL/6 | 107 CFU/g at d 1 p.i. 104–105 CFU/g by d 3 p.i. | 6–7 d. p.i. | Mild to moderate inflammatory cell infiltration into the mucosa and submucosa, mild to moderate hyperplasia, mild to moderate goblet cell loss long-term tissue damage is also observed | Oral gavage | 300 µl of 109 CFU | ATCC 43431; B2 | Haag et al. (2012), Haag et al. (2012) |
| Defined microbiota model | Nonpathogenic Clostridial species, Lactobacillus, Acinetobacter | Not needed | C3H (others not tested) | 108–109 CFU/g | No clinical signs of disease | Mild inflammation in the lamina propria, with preservation of the normal tissue architecture. | Oral gavage | 200 µl of 5 × 108 CFU | 81–176; NCTC 11168 | Chang & Miller (2006) |
| EPEC/EHEC | ||||||||||
| Germ-free mice (EHEC) | None | Not needed | Swiss-Webster | 109 cfu/g by d 1 and until death | 4–7 d p.i. | Renal tubular necrosis, necrosis of colonic epithelial cell, neurologic signs or lesions, luminal fluid accumulation in the cecum | Oral gavage | 100 µL with 102 to 106 CFU | EHEC and STEC 86–24, EDL933, DEC8B, DEC10B, TW14359, TW04863, MI02-102, MI04-43, MI06-31, and Sakai | Eaton et al. (2008) |
| antibiotic pretreated mice (EPEC and EHEC) | complex, gnotobiotic | streptomycin (24 h prior to infection); antibiotic cocktail of gentamicin, vancomycin, metronidazole and colistin (for 3 d prior to infection) | C57BL/6, CD-1 | 107–1010 cfu/g | 2–3 d p.i. | Loss of epithelial integrity, moderate edema in the submucosa, infiltration of inflammatory cells into the lamina propria in the ileum and the colon for EPEC | Oral gavage | 100 µL with 109 CFU | EPEC E2348/69 (serotype O127:H6) and EPEC E2348/69 DescN CVD425 | Ledwaba et al. (2020);Wadolkowski et al. (1990) |
| antibiotic and mitomycin C pretreated mice (EHEC) | complex, gnotobiotic | streptomycin (continuous treatment) and mitomycin C treatment (3 times every 3 hours after infection) | BALB/c | 109 cfu/g by d 1 and until d 7 | 1–2 d p.i. depending on mitomycin C injections | Apoptotic injury in the cryptic area of the intestinal mucosa, injuries in the bone marrow and mesenteric lymph nodes, toxic tubular necrosis | Oral gavage | 100 µL with 2–6 × 103 CFU | STEC 89020087, V354, V406, CDC EDL933, V356, TT-18, V20, V50, TB226A, EDL931 and O-1 | Shimizu et al. (2003) |
| Young mice (EPEC) | Complex, probably immature | Not needed | C57BL/6 N | 103–108 CFU/g of colon at 4 d p.i. and for 12 d, age-dependent | Not described | Not described | Oral gavage | 1 µL with 0.5–1x105 CFU in young mice and 100 µL with 0.5–1x108 CFU in adult mice | EPEC E2348/69 | Dupont et al. (2016) |
| Citrobacter rodentium | ||||||||||
| Resistant mouse strain (e.g. C57BL/6) | Complex | Not needed | C57BL/6, BALB/c, 129S1/SvImJ, NIH Swiss | 108 CFU/g 1–8 d p.i.; 109 cfu/g 8–12 d p.i. and ddecrease until clearance | Not specified | Cell proliferation and increase in colonic crypt lengths, mucosal inflammatory response | Oral gavage | 100 µL with 2.5 × 108 CFU | DBS100 | Vallance et al. (2003); Crepin et al. (2016) |
| Susceptible mouse strain (e.g. C3H/HeJ) | Complex | Not needed | C3H/HeJ | 109 CFU counts in the colon at d 4 p.i. | Not specified, death by d 6–10 p.i. | Fulid and mucus accumulation in the colonic lumen, increase in colon weights and crypt lengths, mucosal inflammatory response with immune cell infiltration, submucosal hyperemia and mucosal ulceration, enlargement of the distal colon | Oral gavage | 100 µL with 2.5 × 108 CFU or 200 µL with 1–2 × 109 CFU | DBS100; ICC169 | Vallance et al. (2003); Mundy et al. (2006) |
| Germ-free mice | None | Not needed | C57BL/6 | 109 CFU/g from d 2 p.i. and up to 42 d p.i. | Not specified | A/E lesions | Oral gavage | 200 µL with 109–1010 CFU | ATCC51459; ICC169; DBS120 | Buschor et al. (2017);Mullineaux-Sanders et al. (2017); Kamada et al. (2012); Kamada et al. (2015) |
Funding Statement
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No 956279 to C.G.M.G., G.F., M.M.H., S.B., and W.-D.H. Additional funding is acknowledged from the Swiss National Science Foundation’s NCCR Microbiomes to S.H. and W.-D.H.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
M.K.-M.H, W.-D.H., S.H., M.M.H., S.B., G.F., C.G.M.G. developed the concept of this review. M.K.-M.H. coordinated the writing process and designed the figures. M.K.-M.H., W.-D.H., and C.G.M.G. developed the first outline. M.K.-M.H., M.C., A.P., N.S., L.B., and W.-D.H. contributed to the first draft of the manuscript. All authors edited the final manuscript.
References
- 1.The burden of foodborne diseases in the WHO European Region . (WHO Regional Office for Europe, Copenhagen, Denmark, 2017).
- 2.CDC . Antibiotic resistance threats in the United States, 2019. (2019).
- 3.Organization, W. H . World health statistics 2021: monitoring health for the SDGs, sustainable development goals. (WHO, 2021).
- 4.ECDC), E. F. S. A. a. E. C. f. D. P. a. C. E. a . The European Union one health 2020 zoonoses report. EFSA Journal, doi: 10.2903/j.efsa.2021.6971 (2021). [DOI] [PMC free article] [PubMed]
- 5.Mowat AM, Agace WW.. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14(667–685). doi: 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- 6.Torow N, Marsland BJ, Hornef MW, Gollwitzer ES. Neonatal mucosal immunology. Mucosal Immunol. 2017;10(5–17). doi: 10.1038/mi.2016.81. [DOI] [PubMed] [Google Scholar]
- 7.Schirmer M, Smeekens, S. P., Vlamakis, H., Jaeger, M., Oosting, M., Franzosa, E. A. & Xavier, R. J. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. 2016;167:1125–36. doi: 10.1016/j.cell.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walter J, Ley R. The human gut microbiome: ecology and recent evolutionary changes. Annu Rev Microbiol. 2011;65(411–429). doi: 10.1146/annurev-micro-090110-102830. [DOI] [PubMed] [Google Scholar]
- 9.Rojas CA, Ramirez-Barahona S, Holekamp KE, Theis KR. Host phylogeny and host ecology structure the mammalian gut microbiota at different taxonomic scales. Anim Microbiome. 2021;3(33). doi: 10.1186/s42523-021-00094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen TL, Vieira-Silva S, Liston A, Raes J. How informative is the mouse for human gut microbiota research? Dis Model Mech. 2015;8(1):1–16. doi: 10.1242/dmm.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajilic-Stojanovic M, Heilig HG, Tims S, Zoetendal EG, de Vos WM. Long-term monitoring of the human intestinal microbiota composition. Environ Microbiol. 2012. doi: 10.1111/1462-2920.12023. [DOI] [PubMed] [Google Scholar]
- 12.Kirk MD, Pires, S. M., Black, R. E., Caipo, M., Crump, J. A., Devleesschauwer, B. & Angulo, F. J.. World health organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med. 2015;12(e1001921). doi: 10.1371/journal.pmed.1001921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Havelaar AH, Kirk, MD, Torgerson, PR, Gibb, HJ, Hald, T, Lake, RJ, Praet, N, Bellinger, DC, de Silva, NR, Gargouri, N, Speybroeck, N, Cawthorne, A, Mathers, C, Stein, C, Angulo, FJ, Devleesschauwer, B. Vol. 12 (Public Library of Science; 2015). doi: 10.1371/journal.pmed.1001923. [DOI] [Google Scholar]
- 14.Abt MC, McKenney PT, Pamer EG. Clostridium difficile colitis: pathogenesis and host defence. Nat Rev Microbiol. 2016;14(609–620). doi: 10.1038/nrmicro.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim SC, Knight DR, Riley TV. Clostridium difficile and One health. Clin Microbiol Infect. 2020;26(857–863). doi: 10.1016/j.cmi.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Furter M, Sellin ME, Hansson GC, Hardt WD. Mucus architecture and near-surface swimming affect distinct salmonella typhimurium infection patterns along the murine intestinal tract. Cell Rep. 2019;27:2665–2678. doi: 10.1016/j.celrep.2019.04.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ermund A, Schutte A, Johansson ME, Gustafsson JK, Hansson GC. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am J Physiol Gastrointest Liver Physiol. 2013;305:G341–347. doi: 10.1152/ajpgi.00046.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roager HM, Hansen, L., Bahl, M. I., Frandsen, H. L., Carvalho, V., Gøbel, R. J. & Licht, T. R. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat Microbiol. 2016;1(16093). doi: 10.1038/nmicrobiol.2016.93 [DOI] [PubMed] [Google Scholar]
- 19.Arnoldini M, Cremer J, Hwa T. Bacterial growth, flow, and mixing shape human gut microbiota density and composition. Gut Microbes. 2018;9(559–566). doi: 10.1080/19490976.2018.1448741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han S, Lu, Y., Xie, J., Fei, Y., Zheng, G., Wang, Z. & Li, L.. Probiotic gastrointestinal transit and colonization after oral administration: a long journey. Front Cell Infect Microbiol. 2021;11(609722). doi: 10.3389/fcimb.2021.609722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takiishi T, Fenero CIM, Camara NOS. Intestinal barrier and gut microbiota: shaping our immune responses throughout life. Tissue Barriers. 2017;5(e1373208). doi: 10.1080/21688370.2017.1373208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ducarmon QR, Zwittink, R. D., Hornung, B. V. H., Van Schaik, W., Young, V. B., & Kuijper, E. J. Gut microbiota and colonization resistance against bacterial enteric infection. Microbiol Mol Biol Rev. 2019;83. doi: 10.1128/MMBR.00007-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreuzer M, Hardt WD. How Food affects colonization resistance against enteropathogenic bacteria. Annu Rev Microbiol. 2020;74(787–813). doi: 10.1146/annurev-micro-020420-013457. [DOI] [PubMed] [Google Scholar]
- 24.Stecher B, Berry D, Loy A. Colonization resistance and microbial ecophysiology: using gnotobiotic mouse models and single-cell technology to explore the intestinal jungle. FEMS Microbiology Reviews. 2013. doi: 10.1111/1574-6976.12024. [DOI] [PubMed] [Google Scholar]
- 25.Chen C, Yang X, Shen X. Confirmed and potential roles of bacterial t6SSS in the intestinal ecosystem. Front Microbiol. 2019;10(1484). doi: 10.3389/fmicb.2019.01484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heilbronner S, Krismer B, Brotz-Oesterhelt H, Peschel A. The microbiome-shaping roles of bacteriocins. Nat Rev Microbiol. 2021;19(726–739). doi: 10.1038/s41579-021-00569-w. [DOI] [PubMed] [Google Scholar]
- 27.Rogers AWL, Tsolis RM, Baumler AJ. Salmonella versus the Microbiome. Microbiol Mol Biol Rev. 2021:85. doi: 10.1128/MMBR.00027-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen BD, Cuenca, M., Hartl, J., Gül, E., Bauer, R., Meile, S. & Hardt, W. D. Import of aspartate and malate by DcuABC drives H2/fumarate respiration to promote initial salmonella gut-lumen colonization in mice. Cell Host Microbe. 2020;27:922–936. doi: 10.1016/j.chom.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Celis AI, Relman DA. Competitors versus collaborators: micronutrient processing by pathogenic and commensal human-associated gut bacteria. Mol Cell. 2020;78(570–576). doi: 10.1016/j.molcel.2020.03.032. [DOI] [PubMed] [Google Scholar]
- 30.Liou MJ, Miller, B. M., Litvak, Y., Nguyen, H., Natwick, D. E., Savage, H. P. & Bäumler, A. J. Host cells subdivide nutrient niches into discrete biogeographical microhabitats for gut microbes. Cell Host Microbe. 2022;30:836–847. doi: 10.1016/j.chom.2022.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wittebole X, De Roock S, Opal SM. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence. 2014;5(226–235). doi: 10.4161/viru.25991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shkoporov AN, Clooney, A. G., Sutton, T. D., Ryan, F. J., Daly, K. M., Nolan, J. A. & Hill, C. The human gut virome is highly diverse, stable, and individual specific. Cell Host Microbe. 2019;26:527–541. doi: 10.1016/j.chom.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Zhou A, Yuan, Y., Yang, M., Huang, Y., Li, X., Li, S. & Tang, B. Crosstalk Between the gut microbiota and epithelial cells under physiological and infectious conditions. Frontiers in Cellular and Infection Microbiology. 2022;12:832672. doi: 10.3389/fcimb.2022.832672 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14(141–153). doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 35.Maier L, et al. Granulocytes impose a tight bottleneck upon the gut luminal pathogen population during Salmonella typhimurium colitis. PLoS Pathog. 2014;10(e1004557). doi: 10.1371/journal.ppat.1004557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaiser P, Diard M, Stecher B, Hardt W-D. The streptomycin mouse model for Salmonella diarrhea: functional analysis of the microbiota, the pathogen’s virulence factors, and the host’s mucosal immune response. Immunological Reviews. 2012;245:56–83. doi: 10.1111/j.1600-065X.2011.01070.x. [DOI] [PubMed] [Google Scholar]
- 37.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30(459–489). doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 38.Molloy MJ, Bouladoux, N., Hand, T. W., Koo, L. Y., Naik, S. & Belkaid, Y. Intraluminal containment of commensal outgrowth in the gut during infection-induced dysbiosis. Cell Host Microbe. 2013;14(318–328). doi: 10.1016/j.chom.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moor K, Diard, M., Sellin, M. E., Felmy, B., Wotzka, S. Y., Toska, A. & Slack, E. High-avidity IgA protects the intestine by enchaining growing bacteria. Nature. 2017;544(498–502). doi: 10.1038/nature22058 [DOI] [PubMed] [Google Scholar]
- 40.Mantis NJ, Rol N, Secretory Iga’s CB. complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4(603–611). doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pike CM, Theriot CM. Mechanisms of colonization resistance against clostridioides difficile. The Journal of Infectious Diseases. 2020;223:S194–S200. doi: 10.1093/infdis/jiaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mizrahi A, Bruxelle JF, Pechine S, Le Monnier A. Prospective evaluation of the adaptive immune response to SlpA in Clostridium difficile infection. Anaerobe. 2018;54(164–168). doi: 10.1016/j.anaerobe.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Nagao-Kitamoto H, Nagao-Kitamoto, H., Leslie, J. L., Kitamoto, S., Jin, C., Thomsson, K. A., Gillilland III, M. G. & Kamada, N. Interleukin-22-mediated host glycosylation prevents Clostridioides difficile infection by modulating the metabolic activity of the gut microbiota. Nat Med. 2020;26(608–617). doi: 10.1038/s41591-020-0764-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wotzka SY, Kreuzer, M., Maier, L., Arnoldini, M., Nguyen, B. D., Brachmann, A. O. & Hardt, W. D. Escherichia coli limits Salmonella Typhimurium infections after diet shifts and fat-mediated microbiota perturbation in mice. Nat Microbiol. 2019. doi: 10.1038/s41564-019-0568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bohnhoff M, Drake BL, Miller CP. Effect of streptomycin on susceptibility of intestinal tract to experimental Salmonella infection. Proc Soc Exp Biol Med. 1954;86(132–137). doi: 10.3181/00379727-86-21030. [DOI] [PubMed] [Google Scholar]
- 46.Hugenholtz F, de Vos WM. Mouse models for human intestinal microbiota research: a critical evaluation. Cell Mol Life Sci. 2018;75(149–160). doi: 10.1007/s00018-017-2693-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Besser JM. Salmonella epidemiology: a whirlwind of change. Food Microbiol. 2018;71(55–59). doi: 10.1016/j.fm.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 48.Chai SJ, Gu W, O’Connor KA, Richardson LC, Tauxe RV. Incubation periods of enteric illnesses in foodborne outbreaks, United States, 1998-2013. Epidemiol Infect. 2019;147(e285). doi: 10.1017/S0950268819001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Axelrad JE, Olén, O., Askling, J., Lebwohl, B., Khalili, H., Sachs, M. C., & Ludvigsson, J. F.. Gastrointestinal infection increases odds of inflammatory bowel disease in a nationwide case-control study. Clin Gastroenterol Hepatol. 2019;17:1311–1322. doi: 10.1016/j.cgh.2018.09.034. [DOI] [PubMed] [Google Scholar]
- 50.Doorduyn Y, Van Den Brandhof WE, Van Duynhoven YT, Wannet WJ, Van Pelt W. Risk factors for Salmonella Enteritidis and Typhimurium (DT104 and non-DT104) infections in The Netherlands: predominant roles for raw eggs in Enteritidis and sandboxes in Typhimurium infections. Epidemiol Infect. 2006;134(617–626). doi: 10.1017/S0950268805005406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pavia AT, Shipman, L. D., Wells, J. G., Puhr, N. D., Smith, J. D., McKinley, T. W., & Tauxe, R. V.. Epidemiologic evidence that prior antimicrobial exposure decreases resistance to infection by antimicrobial-sensitive Salmonella. J Infect Dis. 1990;161(255–260). doi: 10.1093/infdis/161.2.255 [DOI] [PubMed] [Google Scholar]
- 52.Barthel M, Hapfelmeier, S., Quintanilla-Martínez, L., Kremer, M., Rohde, M., Hogardt, M. & Hardt, W. D. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun. 2003;71 2839–2858). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bakkeren E, Gül, E., Huisman, J. S., Steiger, Y., Rocker, A., Hardt, W. D., & Diard, M. Impact of horizontal gene transfer on emergence and stability of cooperative virulence in Salmonella Typhimurium. Nat Commun. 2022;13(1939). doi: 10.1038/s41467-022-29597-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bakkeren E, Huisman, J. S., Fattinger, S. A., Hausmann, A., Furter, M., Egli, A. & Hardt, W. D. Salmonella persisters promote the spread of antibiotic resistance plasmids in the gut. Nature. 2019;573(276–280). doi: 10.1038/s41586-019-1521-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bakkeren E, Diard M, Hardt W-D-D. Evolutionary causes and consequences of bacterial antibiotic persistence. Nature Reviews. Microbiology. 2020;18:479–490. doi: 10.1038/s41579-020-0378-z. [DOI] [PubMed] [Google Scholar]
- 56.Stecher B, Robbiani, R., Walker, A. W., Westendorf, A. M., Barthel, M., Kremer, M. & Hardt, W. D. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5:2177–2189. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stecher B, Paesold, G., Barthel, M., Kremer, M., Jantsch, J., Stallmach, T. & Hardt, W. D. Chronic Salmonella enterica serovar Typhimurium-induced colitis and cholangitis in streptomycin-pretreated Nramp1+/+ mice. Infect Immun. 2006;74(5047–5057). doi: 10.1128/IAI.00072-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stecher B, Chaffron, S., Käppeli, R., Hapfelmeier, S., Freedrich, S., Weber, T. C. & Hardt, W. D. Like will to like: abundances of closely related species can predict susceptibility to intestinal colonization by pathogenic and commensal bacteria. PLoS Pathog. 2010;6(e1000711). doi: 10.1371/journal.ppat.1000711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maier L, Vyas, R., Cordova, C. D., Lindsay, H., Schmidt, T. S. B., Brugiroux, S. & Hardt, W. D. Microbiota-derived hydrogen fuels Salmonella typhimurium invasion of the gut ecosystem. Cell Host Microbe. 2013;14(641–651). doi: 10.1016/j.chom.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 60.Brugiroux S, Beutler, M., Pfann, C., Garzetti, D., Ruscheweyh, H. J., Ring, D. & Stecher, B. Genome-guided design of a defined mouse microbiota that confers colonization resistance against Salmonella enterica serovar Typhimurium. Nat Microbiol. 2016;2(16215). doi: 10.1038/nmicrobiol.2016.215 [DOI] [PubMed] [Google Scholar]
- 61.Garzetti D, Brugiroux, S., Bunk, B., Pukall, R., McCoy, K. D., Macpherson, A. J., & Stecher, B. High-quality whole-genome sequences of the oligo-mouse-microbiota bacterial community. Genome Announc. 2017;5. doi: 10.1128/genomeA.00758-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stecher B, Macpherson, A. J., Hapfelmeier, S., Kremer, M., Stallmach, T., & Hardt, W. D. Comparison of Salmonella enterica serovar Typhimurium colitis in germfree mice and mice pretreated with streptomycin. Infect Immun. 2005;73(3228–3241). doi: 10.1128/IAI.73.6.3228-3241.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chung H, Pamp, S. J., Hill, J. A., Surana, N. K., Edelman, S. M., Troy, E. B. & Kasper, D. L. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149(1578–1593). doi: 10.1016/j.cell.2012.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4(478–485). doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 65.Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol. 2007;19(59–69). doi: 10.1016/j.smim.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Yoo W, Zieba, J. K., Foegeding, N. J., Torres, T. P., Shelton, C. D., Shealy, N. G. & Byndloss, M. X. High-fat diet-induced colonocyte dysfunction escalates microbiota-derived trimethylamine N-oxide. Science. 2021;373(813–818). doi: 10.1126/science.aba3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee JY, Cevallos, S. A., Byndloss, M. X., Tiffany, C. R., Olsan, E. E., Butler, B. P. & Bäumler, A. J. High-fat diet and antibiotics cooperatively impair mitochondrial bioenergetics to trigger dysbiosis that exacerbates pre-inflammatory bowel disease. Cell Host Microbe. 2020;28:273–284. doi: 10.1016/j.chom.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang K, Riba, A, Nietschke, M, Torow, N, Repnik, U, Pütz, A. Minimal SPI1-T3SS effector requirement for Salmonella enterocyte invasion and intracellular proliferation in vivo. PLoS Pathog. 2018;14:e1006925. doi: 10.1371/journal.ppat.1006925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Relman D (Anand Jagatia ed) (Nature Portfolio, 2019).
- 70.Maier L, Barthel, M, Stecher, B, Maier, RJ, Gunn, JS, et al. Salmonella Typhimurium strain ATCC14028 requires H2-hydrogenases for growth in the gut, but not at systemic sites. PLoS One. 2014;9(e110187). doi: 10.1371/journal.pone.0110187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stecher B, Barthel, M, Schlumberger, MC, Haberli, L, Rabsch, W, Kremer, M, Hardt, WD, et al. Motility allows S. Typhimurium to benefit from the mucosal defence. Cell Microbiol. 2008;10:1166–1180. doi: 10.1111/j.1462-5822.2008.01118.x. [DOI] [PubMed] [Google Scholar]
- 72.Stecher B, Hapfelmeier, S, Muller, C, Kremer, M, Stallmach, T, Hardt, WD, et al. Flagella and chemotaxis are required for efficient induction of Salmonella enterica serovar Typhimurium colitis in streptomycin-pretreated mice. Infect Immun. 2004;72(7):4138–50. doi: 10.1128/IAI.72.7.4138-4150.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hapfelmeier S, Ehrbar, K, Stecher, B, Barthel, M, Kremer, M, Hardt, WD, et al. Role of the Salmonella pathogenicity Island 1 effector proteins SipA, SopB, SopE, and SopE2 in Salmonella enterica subspecies 1 serovar Typhimurium colitis in streptomycin-pretreated mice. Infect Immun. 2004;72:795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hapfelmeier S, Stecher, B, Barthel, M, Kremer, M, Müller, AJ, Heikenwalder, M, Stallmach, T, Hensel, M, Pfeffer, K, Akira, S, Hardt, WD, et al. The Salmonella pathogenicity Island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J Immunol. 2005;174:16–1685. [DOI] [PubMed] [Google Scholar]
- 75.Fattinger SA, Bock, D, Di Martino, ML, Deuring, S, Ventayol, PS, Ek, V, et al. Salmonella Typhimurium discreet-invasion of the murine gut absorptive epithelium. PLoS Pathog. 2020;16(e1008503). doi: 10.1371/journal.ppat.1008503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Müller AJ, Kaiser, P, Dittmar, KE, Weber, TC, Haueter, S, Endt, K, et al. Salmonella gut invasion involves TTSS-2-dependent epithelial traversal, basolateral exit, and uptake by epithelium-sampling lamina propria phagocytes. Cell Host & Microbe. 2012;11(1):19–32. [DOI] [PubMed] [Google Scholar]
- 77.Hausmann A, Felmy, B, Kunz, L, Kroon, S, Berthold, DL, Ganz, G, et al. Intercrypt sentinel macrophages tune antibacterial NF-kappaB responses in gut epithelial cells via TNF. J Exp Med. 2021;218:11. doi: 10.1084/jem.20210862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Litvak Y, Mon, KKZ, Nguyen, H, Chanthavixay, G, Liou, M, Velazquez, EM. Commensal Enterobacteriaceae protect against salmonella colonization through oxygen competition. Cell Host Microbe. 2019;25(1):128–139. doi: 10.1016/j.chom.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rivera-Chavez F, Zhang, LF, Faber, F, Lopez, CA, Byndloss, MX, Olsan, EE, et al. Depletion of butyrate-producing clostridia from the gut microbiota drives an aerobic luminal expansion of salmonella. Cell Host Microbe. 2016;19(4):443–454. doi: 10.1016/j.chom.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Winter SE, Thiennimitr, P, Winter, MG, Butler, BP, Huseby, DL, Crawford, RW. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467(7314):426–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rivera-Chavez F, Lopez, CA, Zhang, LF, Cracia-Pastor, L, Chavez-Arroyo, A, Lokken, KL, Tsolis, RM, Winter, S, Bäumler, AJ, et al. Energy taxis toward host-derived nitrate supports a salmonella pathogenicity island 1-independent mechanism of invasion. mBio. 2016;7:4. doi: 10.1128/mBio.00960-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zaharik ML, Vallance BA, Puente JL, Gros P, Finlay BB. Host-pathogen interactions: host resistance factor Nramp1 up-regulates the expression of Salmonella pathogenicity Island-2 virulence genes. Proc Natl Acad Sci U S A. 2002;99(15705–15710). doi: 10.1073/pnas.252415599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Govoni G, Macrophage GP. NRAMP1 and its role in resistance to microbial infections. Inflamm Res. 1998;47(277–284). doi: 10.1007/s000110050330. [DOI] [PubMed] [Google Scholar]
- 84.Sellin ME, Muller, AA, Felmy, B, Dolowschiak, T, Diard, M, Tardivel, A, et al. Epithelium-intrinsic NAIP/NLRC4 inflammasome drives infected enterocyte expulsion to restrict Salmonella replication in the intestinal mucosa. Cell Host Microbe. 2014;16(2):237–248. doi: 10.1016/j.chom.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 85.Fattinger SA, Geiser, P, Samperio Ventayol, P, Di Martino, ML, Furter, M, Felmy, B, et al. Epithelium-autonomous NAIP/NLRC4 prevents TNF-driven inflammatory destruction of the gut epithelial barrier in Salmonella-infected mice. Mucosal Immunol. 2021;14(3):615–629. doi: 10.1038/s41385-021-00381-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fattinger SA, Sellin ME, Hardt WD Vol. 59 86‐94 (Elsevier Ltd, 2021). [DOI] [PubMed] [Google Scholar]
- 87.Hausmann A, Bock, D, Geiser, P, Berthold, DL, Fattinger, SA, Furter, M. Intestinal epithelial NAIP/NLRC4 restricts systemic dissemination of the adapted pathogen Salmonella Typhimurium due to site-specific bacterial PAMP expression. Mucosal Immunology. 2020;13(3)530–544. doi: 10.1038/s41385-019-0247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hausmann A, Russo, G, Grossmann, J, Zünd, M, Schwank, G, Aebersold, R, et al. Germ-free and microbiota-associated mice yield small intestinal epithelial organoids with equivalent and robust transcriptome/proteome expression phenotypes. Cellular Microbiology. 2020;22(6):e13191. doi: 10.1111/cmi.13191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Müller AA, Dolowschiak, T, Sellin, ME, Felmy, B, Verbree, C, Gadient, S, et al. An NK cell perforin response elicited via IL-18 controls mucosal inflammation kinetics during Salmonella gut infection. PLoS Pathogens. 2016;12(6):e1005723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Songhet P, Barthel, M, Stecher, B, Müller, AJ, Kremer, M, Hansson, GC, et al. Stromal IFN-γR-signaling modulates goblet cell function during Salmonella Typhimurium infection. PloS one. 2011;6(7):e22459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Agbor TA, Demma, Z, Mrsny, RJ, Castillo, A, Boll, EJ, McCormick, BA, et al. The oxido-reductase enzyme glutathione peroxidase 4 (GPX4) governs Salmonella Typhimurium-induced neutrophil transepithelial migration. Cell Microbiol. 2014;16(9):1339–1353. doi: 10.1111/cmi.12290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Loetscher Y, Wieser, A, Lengefeld, J, Kaiser, P, Schubert, S, Heikenwalder, M, et al. Salmonella transiently reside in luminal neutrophils in the inflamed gut. PLoS One. 2012;7(4):e34812. doi: 10.1371/journal.pone.0034812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Endt K, Stecher, B, Chaffron, S, Slack, E, Tchitchek, N, Benecke, A, et al. The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal salmonella diarrhea. PLoS Pathogens. 2010;6:9. doi: 10.1371/journal.ppat.1001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Diard M, Bakkeren, E, Lentsch, V, Rocker, A, Bekele, NA, Hoces, D, et al. A rationally designed oral vaccine induces immunoglobulin A in the murine gut that directs the evolution of attenuated Salmonella variants. Nat Microbiol. 2021;6(7):830–841. doi: 10.1038/s41564-021-00911-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Salazar-Gonzalez RM, McSorley SJ. Salmonella flagellin, a microbial target of the innate and adaptive immune system. Immunol Lett. 2005;101:117–122. doi: 10.1016/j.imlet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 96.Sztein MB. Cell-mediated immunity and antibody responses elicited by attenuated Salmonella enterica Serovar Typhi strains used as live oral vaccines in humans. Clin Infect Dis. 2007;45(Suppl 1):S15–19. doi: 10.1086/518140. [DOI] [PubMed] [Google Scholar]
- 97.Murphy JR, Baqar, S, Munoz, C, Schlesinger, L, Ferreccio, C, Lindberg, AA, et al. Characteristics of humoral and cellular immunity to Salmonella typhi in residents of typhoid-endemic and typhoid-free regions. J Infect Dis. 1987;156(6):1005–1009. doi: 10.1093/infdis/156.6.1005 [DOI] [PubMed] [Google Scholar]
- 98.Jacobson A, Lam L, Rajendram M, Tamburini F, Honeycutt J, Pham T, Van Treuren W, Pruss K, Stabler SR, Lugo K, et al. A gut commensal-produced metabolite mediates colonization resistance to salmonella infection. Cell Host Microbe. 2018;24(2):296–307. doi: 10.1016/j.chom.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stecher B, Denzler, R, Maier, L, Bernet, F, Sanders, MJ, Pickard, DJ, et al. Gut inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae PNAS. 2012; 109(4):1269–1274. doi: 10.1073/pnas.1113246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Velazquez EM, Nguyen, H, Heasley, KT, Seachao, CH, Gil, LM, Rogers, AWL, et al. Endogenous Enterobacteriaceae underlie variation in susceptibility to Salmonella infection. Nat Microbiol. 2019;4:1057–1064. doi: 10.1038/s41564-019-0407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Palmer JD, Mortzfeld, BM, Piattelli, E, Silby, MW, McCormick, BA, Bucci, V, et al. Microcin H47: a class IIb microcin with potent activity against multidrug resistant enterobacteriaceae. ACS Infect Dis. 2020;6(4):672–679. doi: 10.1021/acsinfecdis.9b00302 [DOI] [PubMed] [Google Scholar]
- 102.Sassone-Corsi M, Nuccio, SP, Liu, H, Hernandez, D, Vu, CT, Takahashi, AA, et al. Microcins mediate competition among Enterobacteriaceae in the inflamed gut. Nature. 2016;540(7632): 280–283. doi: 10.1038/nature20557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.WHO . Campylobacter, https://www.who.int/news-room/fact-sheets/detail/campylobacter Accessed 4 November 2022. (2020).
- 104.Bereswill S, Fischer, A, Plickert, R, Haag, LM, Otto, B, Kühl, AA, Dasti, JI, et al. Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS One. 2011;6:e20953. doi: 10.1371/journal.pone.0020953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heimesaat MM, Backert S, Alter T, Human Campylobacteriosis-A BS. Serious Infectious threat in a one health perspective. Curr Top Microbiol Immunol. 2021;431:51–23. doi: 10.1007/978-3-030-65481-8_1. [DOI] [PubMed] [Google Scholar]
- 106.Kaakoush NO, Castano-Rodriguez N, Mitchell HM, Man SM. Global epidemiology of campylobacter infection. Clin Microbiol Rev. 2015;28(687–720). doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peterson MC. Clinical aspects of Campylobacter jejuni infections in adults. West J Med. 1994;161:148–152. [PMC free article] [PubMed] [Google Scholar]
- 108.Mousavi S, Bereswill S, Heimesaat MM. Murine models for the investigation of colonization resistance and innate immune responses in campylobacter jejuni infections. Curr Top Microbiol Immunol. 2021;431(233–263). doi: 10.1007/978-3-030-65481-8_9. [DOI] [PubMed] [Google Scholar]
- 109.Gradel KO, Nielsen, HL, Schonheyder, HC, Ejlertsen, T, Kristensen, B, Nielsen, H, et al. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology. 2009;137 2 :495–501. doi: 10.1053/j.gastro.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 110.Peters S, Pascoe, B, Wu, Z, Bayliss, SC, Zeng, X, Edwinson, A, et al. Campylobacter jejuni genotypes are associated with post-infection irritable bowel syndrome in humans. Commun Biol. 2021;4:1015. doi: 10.1038/s42003-021-02554-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mortensen NP, Kuijf, ML, Wim Ang, C, Schiellerup, P, Krogfelt, KA, Jacobs, BC, van Belkum, A, Ph Endtz, H, Bergman, MP, et al. Sialylation of Campylobacter jejuni lipo-oligosaccharides is associated with severe gastro-enteritis and reactive arthritis. Microbes Infect. 2009;11:12 988–994 . doi: 10.1016/j.micinf.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 112.Huizinga R, van den Berg, B, van Rijs, W, Tio-Gillen, A, Fokkink, WJR, Bakker-Jonges, LE, Geleijns, Karin, Samsom, JN, van Doorn, PA, Laman, JD, Jacobs, BC, et al. Innate immunity to campylobacter jejuni in guillain-barre syndrome. Ann Neurol. 2015;78(3): 343–354. doi: 10.1002/ana.24442 [DOI] [PubMed] [Google Scholar]
- 113.Bucker R, Krug, SM, Moos, V, Bojarski, C, Schweiger, MR, Kerick, M, Fromm, A, et al. Campylobacter jejuni impairs sodium transport and epithelial barrier function via cytokine release in human colon. Mucosal Immunol. 2018;11(2):474–485. doi: 10.1038/mi.2017.66 [DOI] [PubMed] [Google Scholar]
- 114.Mousavi S, Bereswill S, Heimesaat MM. Novel clinical campylobacter jejuni infection models based on sensitization of mice to lipooligosaccharide, a major bacterial factor triggering innate immune responses in human campylobacteriosis. Microorganisms. 2020:8. doi: 10.3390/microorganisms8040482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chang C, Miller JF. Campylobacter jejuni colonization of mice with limited enteric flora. Infect Immun. 2006;74(5261–5271). doi: 10.1128/IAI.01094-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Newell DG, Pearson A. The invasion of epithelial cell lines and the intestinal epithelium of infant mice by Campylobacter jejuni/coli. J Diarrhoeal Dis Res. 1984;2:19–26. [PubMed] [Google Scholar]
- 117.Newell DG, McBride H, Dolby JM. Investigations on the role of flagella in the colonization of infant mice with Campylobacter jejuni and attachment of Campylobacter jejuni to human epithelial cell lines. J Hyg (Lond). 1985;95(217–227). doi: 10.1017/s0022172400062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Newell DG. Monoclonal antibodies directed against the flagella of Campylobacter jejuni: cross-reacting and serotypic specificity and potential use in diagnosis. J Hyg (Lond). 1986;96(377–384). doi: 10.1017/s0022172400066134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Haag LM, Fischer, A, Otto, B, Grundmann, U, Kühl, AA, Goebel, UB, Bereswill, S, Heimesaat, MM , et al. Campylobacter jejuni infection of infant mice: acute enterocolitis is followed by asymptomatic intestinal and extra-intestinal immune responses. Eur J Microbiol Immunol (Bp). 2012;2:2–11. doi: 10.1556/EuJMI.2.2012.1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Haag LM, Fischer, A, Otto, B, Plickert, R, Kühl, AA, Goebel, UB, Bereswill, S, Heimesaat, MM, et al. Intestinal microbiota shifts towards elevated commensal Escherichia coli loads abrogate colonization resistance against Campylobacter jejuni in mice. PLoS One. 2012;7 5 :e35988. doi: 10.1371/journal.pone.0035988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ferreira RBR, Gill, N, Willing, BP, Antunes, LCM, Russell, SL, Croxen, MA, Finlay, BB, et al. The intestinal microbiota plays a role in Salmonella-induced colitis independent of pathogen colonization. PLoS One. 2011;6 5 :e20338. doi: 10.1371/journal.pone.0020338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Russell SL, Gold, MJ, Hartmann, M, Willing, BP, Thorson, L, Wlodarska, M, Gill, N, et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012;13 5 :440–447. doi: 10.1038/embor.2012.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stahl M, Ries, J, Vermeulen, J, Yang, H, Sham, HP, Crowley, SM, Badayeva, Y, et al. A novel mouse model of Campylobacter jejuni gastroenteritis reveals key pro-inflammatory and tissue protective roles for Toll-like receptor signaling during infection. PLoS Pathog. 2014;10 7 :e1004264. doi: 10.1371/journal.ppat.1004264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Iizumi T, Taniguchi, T, Yamazaki, W, Vilmen, G, Alekseyenko, AV, Gao, Z, Perez, GIP, Blaser, MJ, et al. Effect of antibiotic pre-treatment and pathogen challenge on the intestinal microbiota in mice. Gut Pathog. 2016;8(60). doi: 10.1186/s13099-016-0143-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.O’Loughlin JL, Samuelson, DR, Braundmeier-Fleming, AG, White, BA, Haldorson, GJ, Stone, JB, Lessmann, JJ, Eucker, Tyson, et al. The Intestinal microbiota influences campylobacter jejuni colonization and extraintestinal dissemination in mice. Appl Environ Microbiol. 2015;81 14 :4642–4650. doi: 10.1128/AEM.00281-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stahl M, Vallance BA. Insights into Campylobacter jejuni colonization of the mammalian intestinal tract using a novel mouse model of infection. Gut Microbes. 2015;6(143–148). doi: 10.1080/19490976.2015.1016691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mansfield LS, Bell, JA, Wilson, DL, Murphy, AJ, Elsheikha, HM, Rathinam, VAK, Fierro, BR, Linz, JE, Young, VB, et al. C57BL/6 and congenic interleukin-10-deficient mice can serve as models of Campylobacter jejuni colonization and enteritis. Infect Immun. 2007;75(3): 1099–1115. doi: 10.1128/IAI.00833-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Masanta WO, Heimesaat, MM, Bereswill, S, Tareen, AM, Lugert, R, Gross, U, Zautner, AE, et al. Modification of intestinal microbiota and its consequences for innate immune response in the pathogenesis of campylobacteriosis. Clin Dev Immunol. 2013;2013(526860). doi: 10.1155/2013/526860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Heimesaat MM, Alutis, M, Grundmann, U, Fischer, A, Tegtmeyer, N, Boehm, M, Kühl, AA, Goebel, UB, Backert, S, Bereswill, S, et al. The role of serine protease HtrA in acute ulcerative enterocolitis and extra-intestinal immune responses during Campylobacter jejuni infection of gnotobiotic IL-10 deficient mice. Front Cell Infect Microbiol. 2014;4(77). doi: 10.3389/fcimb.2014.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Heimesaat MM, Bereswill S. Murine infection models for the investigation of Campylobacter jejuni–host interactions and pathogenicity. Berl Munch Tierarztl Wochenschr. 2015;128(3–4):98–103. [PubMed] [Google Scholar]
- 131.Schmidt AM, Escher, U, Mousavi, S, Tegtmeyer, N, Boehm, M, Backert, S, Bereswill, S, Heimesaat, MM, et al. Immunopathological properties of the Campylobacter jejuni flagellins and the adhesin CadF as assessed in a clinical murine infection model. Gut Pathog. 2019;11(24). doi: 10.1186/s13099-019-0306-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Debruyne L, Gevers D, Vandamme P. in Campylobacter 1-25 (2008).
- 133.Tegtmeyer N, Sharafutdinov, I, Harrer, A, Esmaeili, DS, Linz, B, Backert, S, et al. Campylobacter Virulence Factors and Molecular Host-Pathogen Interactions. Curr Top Microbiol Immunol. 2021;431: 169–202. doi: 10.1007/978-3-030-65481-8_7 [DOI] [PubMed] [Google Scholar]
- 134.Li J, Gulbronson CJ, Bogacz M, Hendrixson DR, Thompson SA. FliW controls growth-phase expression of Campylobacter jejuni flagellar and non-flagellar proteins via the post-transcriptional regulator CsrA. Microbiology (Reading). 2018;164(1308–1319). doi: 10.1099/mic.0.000704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ren F, Li, X, Tang, H, Jiang, Q, Yun, X, Fang, L, Huang, P, Tang, Y, Li, Q, Huang, J, Jiao, X, et al. Insights into the impact of flhF inactivation on Campylobacter jejuni colonization of chick and mice gut. BMC Microbiol. 2018;18(1):149. doi: 10.1186/s12866-018-1318-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Burnham PM, Hendrixson DR. Campylobacter jejuni: collective components promoting a successful enteric lifestyle. Nat Rev Microbiol. 2018;16(551–565). doi: 10.1038/s41579-018-0037-9. [DOI] [PubMed] [Google Scholar]
- 137.St Michael F, Szymanski, CM, Li, J, Chan, KH, Khieu, NH, Larocque, S, Wakarchuk, WW, Brisson, JR, Monteiro, MA, et al. The structures of the lipooligosaccharide and capsule polysaccharide of Campylobacter jejuni genome sequenced strain NCTC 11168. Eur J Biochem. 2002;269(21):5119–5136. doi: 10.1046/j.1432-1033.2002.03201.x [DOI] [PubMed] [Google Scholar]
- 138.Guerry P, Poly, F, Riddle, M, Maue, AC, Chen, Yu-Han, Monteiro, MA, et al. Campylobacter polysaccharide capsules: virulence and vaccines. Front Cell Infect Microbiol. 2012;2(7). doi: 10.3389/fcimb.2012.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rose A, Kay E, Wren BW, Dallman MJ. The Campylobacter jejuni NCTC11168 capsule prevents excessive cytokine production by dendritic cells. Med Microbiol Immunol. 2012;201(137–144). doi: 10.1007/s00430-011-0214-1. [DOI] [PubMed] [Google Scholar]
- 140.Kim S, Vela, A, Clohisey, SM, Athanasiadou, S, Kaiser, P, Stevens, MP, Vervelde, L, et al. Host-specific differences in the response of cultured macrophages to Campylobacter jejuni capsule and O-methyl phosphoramidate mutants. Vet Res. 2018;49(1):3. doi: 10.1186/s13567-017-0501-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Karlyshev AV, Ketley JM, Wren BW. The Campylobacter jejuni glycome. FEMS Microbiol Rev. 2005;29(377–390). doi: 10.1016/j.femsre.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 142.Hameed A, Woodacre A, Machado LR, Marsden GL. An updated classification system and review of the lipooligosaccharide biosynthesis gene locus in campylobacter jejuni. Front Microbiol. 2020;11(677). doi: 10.3389/fmicb.2020.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Godschalk PC, Heikema, A. P., Gilbert, M., Komagamine, T. The crucial role of Campylobacter jejuni genes in anti-ganglioside antibody induction in Guillain-Barre syndrome. J Clin Invest. 2004;114(1659–1665). doi: 10.1172/JCI15707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Heimesaat MM, Lugert, R., Fischer, A., Alutis, M. Impact of Campylobacter jejuni cj0268c knockout mutation on intestinal colonization, translocation, and induction of immunopathology in gnotobiotic IL-10 deficient mice. PLoS One. 2014;9(e90148). doi: 10.1371/journal.pone.0090148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schmidt AM, Escher, U., Mousavi, S., Boehm, M. Protease activity of campylobacter jejuni HtrA modulates distinct intestinal and systemic immune responses in infected secondary abiotic IL-10 deficient mice. Front Cell Infect Microbiol. 2019;9(79). doi: 10.3389/fcimb.2019.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lobo de Sá FD, Butkevych, E., Nattramilarasu, P. K. Curcumin mitigates immune-induced epithelial barrier dysfunction by campylobacter jejuni. Int J Mol Sci. 2019;20(4830). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mousavi S, Lobo de Sá, F. D., Schulzke, J. D., Bücker, R. Vitamin D in Acute campylobacteriosis-results from an intervention study applying a clinical campylobacter jejuni induced enterocolitis model. Front Immunol. 2019;10(2094). doi: 10.3389/fimmu.2019.02094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mousavi S, Escher, U., Thunhorst, E., Kittler, S. Vitamin C alleviates acute enterocolitis in Campylobacter jejuni infected mice. Sci Rep. 2020;10(2921). doi: 10.1038/s41598-020-59890-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mousavi S, Schmidt, A. M., Escher, U., Kittler, S. Carvacrol ameliorates acute campylobacteriosis in a clinical murine infection model. Gut Pathog. 2020;12(2). doi: 10.1186/s13099-019-0343-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mousavi S, Weschka D, Bereswill S, Heimesaat MM. Preclinical evaluation of oral urolithin-A for the treatment of acute campylobacteriosis in campylobacter jejuni infected microbiota-depleted IL-10(-/-) Mice. Pathogens. 2020:10. doi: 10.3390/pathogens10010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bereswill S, Mousavi S, Weschka D, Heimesaat MM. Disease-alleviating effects of peroral activated charcoal treatment in acute murine campylobacteriosis. Microorganisms. 2021:9. doi: 10.3390/microorganisms9071424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11(142–201). doi: 10.1128/CMR.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hartland EL, Leong JM. Enteropathogenic and enterohemorrhagic E. coli: ecology, pathogenesis, and evolution. Front Cell Infect Microbiol. 2013;3:15. doi: 10.3389/fcimb.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wales AD, Woodward MJ, Pearson GR. Attaching-effacing bacteria in animals. J Comp Pathol. 2005;132(1–26). doi: 10.1016/j.jcpa.2004.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Moon HW, Whipp SC, Argenzio RA, Levine MM, Giannella RA. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41(1340–1351). doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cantey JR, Blake RK. Diarrhea due to Escherichia coli in the rabbit: a novel mechanism. J Infect Dis. 1977;135(454–462). doi: 10.1093/infdis/135.3.454. [DOI] [PubMed] [Google Scholar]
- 157.Garcia A, Bosques, C. J., Wishnok, J. S., Feng, Y. Renal injury is a consistent finding in Dutch Belted rabbits experimentally infected with enterohemorrhagic Escherichia coli. J Infect Dis. 2006;193(1125–1134). doi: 10.1086/501364 [DOI] [PubMed] [Google Scholar]
- 158.Sueyoshi M, Nakazawa M. Experimental infection of young chicks with attaching and effacing Escherichia coli. Infect Immun. 1994;62(4066–4071). doi: 10.1128/iai.62.9.4066-4071.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tzipori S, Wachsmuth, I. K., Chapman, C., Birner, R., Brittingham, J., Jackson, C. The pathogenesis of hemorrhagic colitis caused by Escherichia coli O157: h7in gnotobiotic piglets. J Infect Dis. 1986;154(712–716). doi: 10.1093/infdis/154.4.712 [DOI] [PubMed] [Google Scholar]
- 160.Gunzer F, Hennig-Pauka, I., Waldmann, K. H., Sandhoff, R. Gnotobiotic piglets develop thrombotic microangiopathy after oral infection with enterohemorrhagic Escherichia coli. Am J Clin Pathol. 2002;118(364–375). doi: 10.1309/UMW9-D06Q-M94Q-JGH2 [DOI] [PubMed] [Google Scholar]
- 161.Dean-Nystrom EA, Stoffregen WC, Bosworth BT, Moon HW, Pohlenz JF. Early attachment sites for Shiga-toxigenic Escherichia coli O157: h7in experimentally inoculated weaned calves. Appl Environ Microbiol. 2008;74(6378–6384). doi: 10.1128/AEM.00636-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mohawk KL, O’Brien AD. Mouse models of Escherichia coli O157: h7infection and Shiga toxin injection. J Biomed Biotechnol. 2011;2011(258185). doi: 10.1155/2011/258185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Savkovic SD, Villanueva J, Turner JR, Matkowskyj KA, Hecht G. Mouse model of enteropathogenic Escherichia coli infection. Infect Immun. 2005;73(1161–1170). doi: 10.1128/IAI.73.2.1161-1170.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dupont A, Sommer, F., Zhang, K., Repnik, U. Age-dependent susceptibility to enteropathogenic Escherichia coli (EPEC) infection in mice. PLoS Pathog. 2016;12(e1005616). doi: 10.1371/journal.ppat.1005616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ledwaba SE, Costa, D. V., Bolick, D. T., Giallourou, N. Enteropathogenic Escherichia coli infection induces diarrhea, intestinal damage, metabolic alterations, and increased intestinal permeability in a murine model. Front Cell Infect Microbiol. 2020;10(595266). doi: 10.3389/fcimb.2020.595266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wadolkowski EA, Burris JA, O’Brien AD. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 1990;58(2438–2445). doi: 10.1128/iai.58.8.2438-2445.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shimizu K, Asahara, T., Nomoto, K., Tanaka, R., Hamabata, T., Ozawa, A., & Takeda, Y. Development of a lethal Shiga toxin-producing Escherichia coli-infection mouse model using multiple mitomycin C treatment. Microb Pathog. 2003;35(1–9). doi: 10.1016/s0882-4010(03)00065-2 [DOI] [PubMed] [Google Scholar]
- 168.Eaton KA, Friedman, D. I., Francis, G. J. Pathogenesis of renal disease due to enterohemorrhagic Escherichia coli in germ-free mice. Infect Immun. 2008;76(3054–3063). doi: 10.1128/IAI.01626-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mohawk KL, Melton-Celsa AR, Zangari T, Carroll EE, O’Brien AD. Pathogenesis of Escherichia coli O157: h7strain 86-24 following oral infection of BALB/c mice with an intact commensal flora. Microb Pathog. 2010;48(131–142). doi: 10.1016/j.micpath.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mundy R, Girard F, FitzGerald AJ, Frankel G. Comparison of colonization dynamics and pathology of mice infected with enteropathogenic Escherichia coli, enterohaemorrhagic E. coli and Citrobacter rodentium. FEMS Microbiol Lett. 2006;265:126–132. doi: 10.1111/j.1574-6968.2006.00481.x. [DOI] [PubMed] [Google Scholar]
- 171.Mullineaux-Sanders C, Suez J, Elinav E, Frankel G. Sieving through gut models of colonization resistance. Nat Microbiol. 2018;3(132–140). doi: 10.1038/s41564-017-0095-1. [DOI] [PubMed] [Google Scholar]
- 172.Luperchio SA, Schauer DB. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect. 2001;3(333–340). doi: 10.1016/s1286-4579(01)01387-9. [DOI] [PubMed] [Google Scholar]
- 173.Petty NK, Bulgin, R., Crepin, V. F. The Citrobacter rodentium genome sequence reveals convergent evolution with human pathogenic Escherichia coli. J Bacteriol. 2010;192(525–538). doi: 10.1128/JB.01144-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mullineaux-Sanders C, Sanchez-Garrido, J., Hopkins, E. G. Citrobacter rodentium-host-microbiota interactions: immunity, bioenergetics and metabolism. Nat Rev Microbiol. 2019;17(701–715). doi: 10.1038/s41579-019-0252-z [DOI] [PubMed] [Google Scholar]
- 175.McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92(1664–1668). doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.McDaniel TK, Kaper JB. A cloned pathogenicity Island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol Microbiol. 1997;23:399–407. doi: 10.1046/j.1365-2958.1997.2311591.x. [DOI] [PubMed] [Google Scholar]
- 177.Deng W, Li Y, Vallance BA, Finlay BB. Locus of enterocyte effacement from Citrobacter rodentium: sequence analysis and evidence for horizontal transfer among attaching and effacing pathogens. Infect Immun. 2001;69(6323–6335). doi: 10.1128/IAI.69.10.6323-6335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Frankel G, Phillips, A. D., Novakova, M. Intimin from enteropathogenic Escherichia coli restores murine virulence to a Citrobacter rodentium eaeA mutant: induction of an immunoglobulin A response to intimin and EspB. Infect Immun. 1996;64(5315–5325). doi: 10.1128/iai.64.12.5315-5325.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Higgins LM, Frankel, G., Connerton, I., Gonçalves, N. S. Role of bacterial intimin in colonic hyperplasia and inflammation. Science. 1999;285(588–591). doi: 10.1126/science.285.5427.588 [DOI] [PubMed] [Google Scholar]
- 180.Frankel G, Phillips, A. D., Trabulsi, L. R.. Intimin and the host cell–is it bound to end in Tir(s)? Trends Microbiol. 2001;9:214–218. doi: 10.1016/s0966-842x(01)02016-9. [DOI] [PubMed] [Google Scholar]
- 181.Deng W, Vallance BA, Li Y, Puente JL, Finlay BB. Citrobacter rodentium translocated intimin receptor (Tir) is an essential virulence factor needed for actin condensation, intestinal colonization and colonic hyperplasia in mice. Mol Microbiol. 2003;48(95–115). doi: 10.1046/j.1365-2958.2003.03429.x. [DOI] [PubMed] [Google Scholar]
- 182.Collins JW, Keeney, K. M., Crepin, V. F., Rathinam, V. A. Citrobacter rodentium: infection, inflammation and the microbiota. Nat Rev Microbiol. 2014;12(612–623). doi: 10.1038/nrmicro3315 [DOI] [PubMed] [Google Scholar]
- 183.Silberger DJ, Zindl CL, Weaver CT. Citrobacter rodentium: a model enteropathogen for understanding the interplay of innate and adaptive components of type 3 immunity. Mucosal Immunol. 2017;10(1108–1117). doi: 10.1038/mi.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Geiger TL, Abt, M. C., Gasteiger, G. Nfil3 is crucial for development of innate lymphoid cells and host protection against intestinal pathogens. J Exp Med. 2014;211(1723–1731). doi: 10.1084/jem.20140212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nordlander S, Pott J, Maloy KJ. NLRC4 expression in intestinal epithelial cells mediates protection against an enteric pathogen. Mucosal Immunol. 2014;7(775–785). doi: 10.1038/mi.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hopkins EGD, Frankel G. Overview of the effect of citrobacter rodentium infection on host metabolism and the microbiota. Methods Mol Biol. 2021;2291(399–418). doi: 10.1007/978-1-0716-1339-9_20. [DOI] [PubMed] [Google Scholar]
- 187.Vallance BA, Deng W, Jacobson K, Finlay BB. Host susceptibility to the attaching and effacing bacterial pathogen Citrobacter rodentium. Infect Immun. 2003;71(3443–3453). doi: 10.1128/IAI.71.6.3443-3453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Itoh K, Matsui T, Tsuji K, Mitsuoka T, Ueda K. Genetic control in the susceptibility of germfree inbred mice to infection by Escherichia coli O115a,c:K(B). Infect Immun. 1988;56(930–935). doi: 10.1128/iai.56.4.930-935.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Papapietro O, Teatero, S., Thanabalasuriar, A. R-spondin 2 signalling mediates susceptibility to fatal infectious diarrhoea. Nat Commun. 2013;4(1898). doi: 10.1038/ncomms2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vaahtovuo J, Toivanen P, Eerola E. Bacterial composition of murine fecal microflora is indigenous and genetically guided. FEMS Microbiol Ecol. 2003;44(131–136). doi: 10.1016/S0168-6496(02)00460-9. [DOI] [PubMed] [Google Scholar]
- 191.Ghosh S, Dai, C., Brown, K., Rajendiran, E. Colonic microbiota alters host susceptibility to infectious colitis by modulating inflammation, redox status, and ion transporter gene expression. Am J Physiol Gastrointest Liver Physiol. 2011;301(G39–49). doi: 10.1152/ajpgi.00509.2010 [DOI] [PubMed] [Google Scholar]
- 192.Willing BP, Vacharaksa A, Croxen M, Thanachayanont T, Finlay BB. Altering host resistance to infections through microbial transplantation. PLoS One. 2011;6(e26988). doi: 10.1371/journal.pone.0026988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Osbelt L, Thiemann, S., Smit, N., Lesker, T. R. Variations in microbiota composition of laboratory mice influence Citrobacter rodentium infection via variable short-chain fatty acid production. PLoS Pathog. 2020;16. e1008448. doi: 10.1371/journal.ppat.1008448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chen J, Waddell A, Lin YD, Cantorna MT. Dysbiosis caused by vitamin D receptor deficiency confers colonization resistance to Citrobacter rodentium through modulation of innate lymphoid cells. Mucosal Immunol. 2015;8(618–626). doi: 10.1038/mi.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kamada N, Kim, Y. G., Sham, H. P., Vallance, B. A. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science. 2012;336(1325–1329). doi: 10.1126/science.1222195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kamada N, Sakamoto, K., Seo, S. U., Zeng, M. Y. Humoral immunity in the gut selectively targets phenotypically virulent attaching-and-effacing bacteria for intraluminal elimination. Cell Host Microbe. 2015;17(617–627). doi: 10.1016/j.chom.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kim YG, Sakamoto, K., Seo, S. U., Pickard, J. M. Neonatal acquisition of Clostridia species protects against colonization by bacterial pathogens. Science. 2017;356(315–319). doi: 10.1126/science.aag2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mullineaux-Sanders C, Collins, J. W., Ruano-Gallego, D., Levy, M. Citrobacter rodentium Relies on commensals for colonization of the colonic mucosa. Cell Rep. 2017;21(3381–3389). doi: 10.1016/j.celrep.2017.11.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Herp S, Brugiroux, S., Garzetti, D., Ring, D. Mucispirillum schaedleri Antagonizes Salmonella Virulence to Protect Mice against Colitis. Cell Host Microbe. 2019;25:681–694. doi: 10.1016/j.chom.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 200.Buschor S, Cuenca, M., Uster, S. S., Schären, O. P. Innate immunity restricts Citrobacter rodentium A/E pathogenesis initiation to an early window of opportunity. PLoS Pathog. 2017;13(e1006476). doi: 10.1371/journal.ppat.1006476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Maattanen P, Lurz, E., Botts, S. R., Wu, R. Y., Yeung, C. W., Li, B. & Sherman, P. M. Ground flaxseed reverses protection of a reduced-fat diet against Citrobacter rodentium-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2018;315:G788–G798. doi: 10.1152/ajpgi.00101.2018. [DOI] [PubMed] [Google Scholar]
- 202.Collins JW, Chervaux, C., Raymond, B., Derrien, M. Fermented dairy products modulate Citrobacter rodentium-induced colonic hyperplasia. J Infect Dis. 2014;210(1029–1041). doi: 10.1093/infdis/jiu205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Desai MS, Seekatz, A. M., Koropatkin, N. M., Kamada, N. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167:1339–1353. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zenewicz LA, Yin, X., Wang, G., Elinav, E. IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. J Immunol. 2013;190:5306–5312. doi: 10.4049/jimmunol.1300016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Varcoe JJ, Krejcarek G, Busta F, Brady L. Prophylactic feeding of Lactobacillus acidophilus NCFM to mice attenuates overt colonic hyperplasia. J Food Prot. 2003;66(457–465). doi: 10.4315/0362-028x-66.3.457. [DOI] [PubMed] [Google Scholar]
- 206.Johnson-Henry KC, Nadjafi, M., Avitzur, Y., Mitchell, D. J. Amelioration of the effects of Citrobacter rodentium infection in mice by pretreatment with probiotics. J Infect Dis. 2005;191(2106–2117). doi: 10.1086/430318 [DOI] [PubMed] [Google Scholar]
- 207.Chen CC, Chiu CH, Lin TY, Shi HN, Walker WA. Effect of probiotics Lactobacillus acidophilus on Citrobacter rodentium colitis: the role of dendritic cells. Pediatr Res. 2009;65(169–175). doi: 10.1203/PDR.0b013e31818d5a06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gareau MG, Wine E, Reardon C, Sherman PM. Probiotics prevent death caused by Citrobacter rodentium infection in neonatal mice. J Infect Dis. 2010;201(81–91). doi: 10.1086/648614. [DOI] [PubMed] [Google Scholar]
- 209.Rodrigues DM, Sousa AJ, Johnson-Henry KC, Sherman PM, Gareau MG. Probiotics are effective for the prevention and treatment of Citrobacter rodentium-induced colitis in mice. J Infect Dis. 2012;206(99–109). doi: 10.1093/infdis/jis177. [DOI] [PubMed] [Google Scholar]
- 210.Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut. 2003;52(827–833). doi: 10.1136/gut.52.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Collins JW, Akin, A. R., Kosta, A., Zhang, N., Tangney, M., Francis, K. P., & Frankel, G. Pre-treatment with Bifidobacterium breve UCC2003 modulates Citrobacter rodentium-induced colonic inflammation and organ specificity. Microbiology (Reading). 2012;158(2826–2834). doi: 10.1099/mic.0.060830-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Fanning S, Hall, L. J., Cronin, M., Zomer, A., MacSharry, J., Goulding, D. & van Sinderen, D. Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection. Proc Natl Acad Sci USA. 2012;109(2108–2113). doi: 10.1073/pnas.1115621109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ivanov II, Atarashi, K., Manel, N., Brodie, E. L. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(485–498). doi: 10.1016/j.cell.2009.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Woo V, Eshleman, E. M., Hashimoto-Hill, S., Whitt, J. Commensal segmented filamentous bacteria-derived retinoic acid primes host defense to intestinal infection. Cell Host Microbe. 2021;29:1744–1756. doi: 10.1016/j.chom.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mullineaux-Sanders C, Carson, D., Hopkins, E. G., Glegola-Madejska, I. Citrobacter amalonaticus inhibits the growth of citrobacter rodentium in the gut lumen. mBio. 2021;12(e0241021). doi: 10.1128/mBio.02410-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wu X, Vallance, B. A., Boyer, L., Bergstrom, K. S. Saccharomyces boulardii ameliorates Citrobacter rodentium-induced colitis through actions on bacterial virulence factors. Am J Physiol Gastrointest Liver Physiol. 2008;294(G295–306). doi: 10.1152/ajpgi.00173.2007 [DOI] [PubMed] [Google Scholar]
- 217.Curtis MM, Hu, Z., Klimko, C., Narayanan, S. The gut commensal Bacteroides thetaiotaomicron exacerbates enteric infection through modification of the metabolic landscape. Cell Host Microbe. 2014;16(759–769). doi: 10.1016/j.chom.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bergstrom KS, Kissoon-Singh, V., Gibson, D. L., Ma, C. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6(e1000902). doi: 10.1371/journal.ppat.1000902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lawson PA, Citron DM, Tyrrell KL, Finegold SM. Reclassification of clostridium difficile as clostridioides difficile (Hall and O’Toole 1935) Prevot 1938. Anaerobe. 2016;40(95–99). doi: 10.1016/j.anaerobe.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 220.Bauer MP, et al. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011;377(63–73). doi: 10.1016/S0140-6736(10)61266-4 [DOI] [PubMed] [Google Scholar]
- 221.Guh AY, Mu, Y., Winston, L. G., Johnston, H. Trends in U.S. Burden of clostridioides difficile infection and outcomes. N Engl J Med. 2020;382:1320–1330. doi: 10.1056/NEJMoa1910215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kampouri E, Croxatto A, Prod’hom G, Guery B. Clostridioides difficile infection, still a long way to go. J Clin Med. 2021:10. doi: 10.3390/jcm10030389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Reigadas Ramirez E, Bouza ES. Economic burden of clostridium difficile infection in European Countries. Adv Exp Med Biol. 2018;1050(1–12). doi: 10.1007/978-3-319-72799-8_1. [DOI] [PubMed] [Google Scholar]
- 224.Fu Y, Luo Y, Grinspan AM. Epidemiology of community-acquired and recurrent Clostridioides difficile infection. Therap Adv Gastroenterol. 2021;14(17562848211016248). doi: 10.1177/17562848211016248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals. 207 (European Centre for Disease Prevention and Control, Stockholm, 2013). [Google Scholar]
- 226.Lessa FC, Mu, Y., Bamberg, W. M., Beldavs, Z. G. Burden of clostridium difficile infection in the United States. N Engl J Med. 2015;372(825–834). doi: 10.1056/NEJMoa1408913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Penit A, Bemer, P., Besson, J., Cazet, L. Community-acquired Clostridium difficile infections. Med Mal Infect. 2016;46(131–139). doi: 10.1016/j.medmal.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 228.Warriner K, Xu C, Habash M, Sultan S, Weese SJ. Dissemination of Clostridium difficile in food and the environment: significant sources of C. difficile community-acquired infection? J Appl Microbiol. 2017;122:542–553. doi: 10.1111/jam.13338. [DOI] [PubMed] [Google Scholar]
- 229.Czepiel J, Dróżdż, M., Pituch, H., Kuijper, E. J. Clostridium difficile infection: review. Eur J Clin Microbiol Infect Dis. 2019;38(1211–1221). doi: 10.1007/s10096-019-03539-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Crobach MJT, Vernon, J. J., Loo, V. G., Kong, L. Y. Understanding clostridium difficile colonization. Clin Microbiol Rev. 2018;31. doi: 10.1128/CMR.00021-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Smits WK, Lyras D, Lacy DB, Wilcox MH, Kuijper EJ. Clostridium difficile infection. Nat Rev Dis Primers. 2016;2:16020. doi: 10.1038/nrdp.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zacharioudakis IM, Zervou FN, Pliakos EE, Ziakas PD, Mylonakis E. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110:381–390. doi: 10.1038/ajg.2015.22. [DOI] [PubMed] [Google Scholar]
- 233.Kuehne SA, Cartman, S. T., Heap, J. T., Kelly, M. L. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010;467(711–713). doi: 10.1038/nature09397 [DOI] [PubMed] [Google Scholar]
- 234.Chandrasekaran R, Lacy DB. The role of toxins in Clostridium difficile infection. FEMS Microbiol Rev. 2017;41(723–750). doi: 10.1093/femsre/fux048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Louie TJ, Miller, M. A., Mullane, K. M., Weiss, K., Lentnek, A., Golan, Y. & Shue, Y. K. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(422–431). doi: 10.1056/NEJMoa0910812 [DOI] [PubMed] [Google Scholar]
- 236.Johnson S, Louie, T. J., Gerding, D. N., Cornely, O. A., Chasan-Taber, S., Fitts, D. & Polymer Alternative for CDI Treatment (PACT) investigators . Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59(345–354). doi: 10.1093/cid/ciu313 [DOI] [PubMed] [Google Scholar]
- 237.Oksi J, Anttila VJ, Mattila E. Treatment of Clostridioides (Clostridium) difficile infection. Ann Med. 2020;52(12–20). doi: 10.1080/07853890.2019.1701703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lee C, Louie, T. J., Weiss, K., Valiquette, L., Gerson, M., Arnott, W., & Gorbach, S. L. Fidaxomicin versus Vancomycin in the Treatment of Clostridium difficile Infection: Canadian Outcomes. Can J Infect Dis Med Microbiol. 2016;2016(8048757). doi: 10.1155/2016/8048757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.van Prehn J, Reigadas, E., Vogelzang, E. H., Bouza, E., Hristea, A., Guery, B. & Kuijper, E. J. European society of clinical microbiology and infectious diseases: 2021 update on the treatment guidance document for clostridioides difficile infection in adults. Clin Microbiol Infect. 2021;27(Suppl 2):S1–S21. doi: 10.1016/j.cmi.2021.09.038. [DOI] [PubMed] [Google Scholar]
- 240.Fekety R, Silva, J., Toshniwal, R., Allo, M., Armstrong, J., Browne, R. & Rifkin, G. Antibiotic-associated colitis: effects of antibiotics on Clostridium difficile and the disease in hamsters. Rev Infect Dis. 1979;1(386–397). doi: 10.1093/clinids/1.2.386 [DOI] [PubMed] [Google Scholar]
- 241.Knoop FC. Clindamycin-associated enterocolitis in Guinea pigs: evidence for a bacterial toxin. Infect Immun. 1979;23(31–33). doi: 10.1128/iai.23.1.31-33.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Onderdonk AB, Cisneros RL, Bartlett JG. Clostridium difficile in gnotobiotic mice. Infect Immun. 1980;28(277–282). doi: 10.1128/iai.28.1.277-282.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Czuprynski CJ, Johnson WJ, Balish E, Wilkins T. Pseudomembranous colitis in Clostridium difficile-monoassociated rats. Infect Immun. 1983;39(1368–1376). doi: 10.1128/iai.39.3.1368-1376.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Chen X, Katchar, K., Goldsmith, J. D., Nanthakumar, N., Cheknis, A., Gerding, D. N., & Kelly, C. P. A mouse model of Clostridium difficile-associated disease. Gastroenterology. 2008;135(1984–1992). doi: 10.1053/j.gastro.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 245.Castro-Cordova P, Diaz-Yanez F, Munoz-Miralles J, Gil F, Paredes-Sabja D. Effect of antibiotic to induce Clostridioides difficile-susceptibility and infectious strain in a mouse model of Clostridioides difficile infection and recurrence. Anaerobe. 2020;62(102149). doi: 10.1016/j.anaerobe.2020.102149. [DOI] [PubMed] [Google Scholar]
- 246.Winston JA, Thanissery R, Montgomery SA, Theriot CM. Cefoperazone-treated mouse model of clinically-relevant clostridium difficile strain R20291. J Vis Exp. 2016. doi: 10.3791/54850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Studer N, Desharnais, L., Beutler, M., Brugiroux, S., Terrazos, M. A., Menin, L. & Hapfelmeier, S. Functional intestinal bile acid 7alpha-dehydroxylation by clostridium scindens associated with protection from clostridium difficile infection in a gnotobiotic mouse model. Front Cell Infect Microbiol. 2016;6(191). doi: 10.3389/fcimb.2016.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Yamaguchi T, Konishi, H., Aoki, K., Ishii, Y., Chono, K., & Tateda, K. The gut microbiome diversity of Clostridioides difficile-inoculated mice treated with vancomycin and fidaxomicin. J Infect Chemother. 2020;26(483–491). doi: 10.1016/j.jiac.2019.12.020 [DOI] [PubMed] [Google Scholar]
- 249.Lai YH, Tsai, B. Y., Hsu, C. Y., Chen, Y. H., Chou, P. H., Chen, Y. L. & Hung, Y. P. The role of toll-like receptor-2 in clostridioides difficile infection: evidence from a mouse model and clinical patients. Front Immunol. 2021;12(691039). doi: 10.3389/fimmu.2021.691039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Pruss KM, Sonnenburg JL. C. difficile exploits a host metabolite produced during toxin-mediated disease. Nature. 2021;593:261–265. doi: 10.1038/s41586-021-03502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Deakin LJ, Clare, S., Fagan, R. P., Dawson, L. F., Pickard, D. J., West, M. R. & Lawley, T. D. The Clostridium difficile spo0A gene is a persistence and transmission factor. Infect Immun. 2012;80(2704–2711). doi: 10.1128/IAI.00147-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Jenior ML, Leslie JL, Young VB, Schloss PD. Clostridium difficile colonizes alternative nutrient niches during infection across distinct murine gut microbiomes. mSystems. 2017:2. doi: 10.1128/mSystems.00063-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Jenior ML, Leslie JL, Young VB, Schloss PD. Clostridium difficile Alters the structure and metabolism of distinct cecal microbiomes during initial infection to promote sustained colonization. mSphere. 2018:3. doi: 10.1128/mSphere.00261-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Koenigsknecht MJ, Theriot, C. M., Bergin, I. L., Schumacher, C. A., Schloss, P. D., & Young, V. B. Dynamics and establishment of Clostridium difficile infection in the murine gastrointestinal tract. Infect Immun. 2015;83(934–941). doi: 10.1128/IAI.02768-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Corthier G, Muller MC, Elmer GW, Lucas F, Dubos-Ramare F. Interrelationships between digestive proteolytic activities and production and quantitation of toxins in pseudomembranous colitis induced by Clostridium difficile in gnotobiotic mice. Infect Immun. 1989;57(3922–3927). doi: 10.1128/iai.57.12.3922-3927.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Aguirre AM, Yalcinkaya, N., Wu, Q., Swennes, A., Tessier, M. E., Roberts, P. & Sorg, J. A. Bile acid-independent protection against Clostridioides difficile infection. PLoS Pathog. 2021;17(e1010015). doi: 10.1371/journal.ppat.1010015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Girinathan BP, DiBenedetto, N., Worley, J. N., Peltier, J., Arrieta-Ortiz, M. L., Immanuel, S. R. C. & Bry, L. In vivo commensal control of Clostridioides difficile virulence. Cell Host Microbe. 2021;29:1693–1708. doi: 10.1016/j.chom.2021.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Corthier G, Muller MC, Wilkins TD, Lyerly D, L’Haridon R. Protection against experimental pseudomembranous colitis in gnotobiotic mice by use of monoclonal antibodies against Clostridium difficile toxin A. Infect Immun. 1991;59(1192–1195). doi: 10.1128/iai.59.3.1192-1195.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Bouillaut L, Self WT, Sonenshein AL. Proline-dependent regulation of Clostridium difficile Stickland metabolism. J Bacteriol. 2013;195(844–854). doi: 10.1128/JB.01492-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lopez CA, McNeely TP, Nurmakova K, Beavers WN, Skaar EP. Clostridioides difficile proline fermentation in response to commensal clostridia. Anaerobe. 2020;63(102210). doi: 10.1016/j.anaerobe.2020.102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ng KM, Ferreyra, J. A., Higginbottom, S. K., Lynch, J. B., Kashyap, P. C., Gopinath, S. & Sonnenburg, J. L. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature. 2013;502:96–99. doi: 10.1038/nature12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Buffie CG, Bucci, V., Stein, R. R., McKenney, P. T., Ling, L., Gobourne, A. & Pamer, E. G. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015;517(205–208). doi: 10.1038/nature13828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kang JD, Myers, C. J., Harris, S. C., Kakiyama, G., Lee, I. K., Yun, B. S. & Hylemon, P. B. Bile acid 7alpha-dehydroxylating gut bacteria secrete antibiotics that inhibit clostridium difficile: role of secondary bile acids. Cell Chem Biol. 2019;26:27–34. doi: 10.1016/j.chembiol.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Jukes CA, Ijaz, U. Z., Buckley, A., Spencer, J., Irvine, J., Candlish, D. & Douce, G. Bile salt metabolism is not the only factor contributing to Clostridioides (Clostridium) difficile disease severity in the murine model of disease. Gut Microbes. 2020;11(481–496). doi: 10.1080/19490976.2019.1678996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Seekatz AM, Theriot, C. M., Molloy, C. T., Wozniak, K. L., Bergin, I. L., & Young, V. B. Fecal microbiota transplantation eliminates clostridium difficile in a murine model of relapsing disease. Infect Immun. 2015;83(3838–3846). doi: 10.1128/IAI.00459-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Mullish BH, McDonald, J. A., Pechlivanis, A., Allegretti, J. R., Kao, D., Barker, G. F. & Marchesi, J. R. Microbial bile salt hydrolases mediate the efficacy of faecal microbiota transplant in the treatment of recurrent Clostridioides difficile infection. Gut. 2019;68(1791–1800). doi: 10.1136/gutjnl-2018-317842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Li X, Kang, Y., Huang, Y., Xiao, Y., Song, L., Lu, S., & Ren, Z. A strain of Bacteroides thetaiotaomicron attenuates colonization of Clostridioides difficile and affects intestinal microbiota and bile acids profile in a mouse model. Biomed Pharmacother. 2021;137(111290). doi: 10.1016/j.biopha.2021.111290 [DOI] [PubMed] [Google Scholar]
- 268.Hryckowian AJ, Van Treuren, W., Smits, S. A., Davis, N. M., Gardner, J. O., Bouley, D. M., & Sonnenburg, J. L. Microbiota-accessible carbohydrates suppress Clostridium difficile infection in a murine model. Nat Microbiol. 2018;3(662–669). doi: 10.1038/s41564-018-0150-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Zhang W, Zou, G., Li, B., Du, X., Sun, Z., Sun, Y., & Jiang, X. Fecal microbiota transplantation (FMT) alleviates experimental colitis in mice by gut microbiota regulation. J Microbiol Biotechnol. 2020;30(1132–1141). doi: 10.4014/jmb.2002.02044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Varga A, Kocsis, B., Sipos, D., Kása, P., Vigvári, S., Pál, S. & Péterfi, Z. How to apply FMT more effectively, conveniently and flexible - a comparison of FMT methods. Front Cell Infect Microbiol. 2021;11(657320). doi: 10.3389/fcimb.2021.657320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.(USA), F. a. D. A. REBYOTA, https://www.fda.gov/vaccines-blood-biologics/vaccines/rebyota (2022).
- 272.Khoruts A, Staley C, Sadowsky MJ. Faecal microbiota transplantation for Clostridioides difficile: mechanisms and pharmacology. Nat Rev Gastroenterol Hepatol. 2021;18(67–80). doi: 10.1038/s41575-020-0350-4. [DOI] [PubMed] [Google Scholar]
- 273.Nooij S, Ducarmon, Q. R., Laros, J. F., Zwittink, R. D., Norman, J. M., Smits, W. K. & Kuijper, E. J. Fecal microbiota transplantation influences procarcinogenic Escherichia coli in Recipient recurrent clostridioides difficile patients. Gastroenterology. 2021;161:1218–1228. doi: 10.1053/j.gastro.2021.06.009. [DOI] [PubMed] [Google Scholar]
- 274.Brandt LJ, Aroniadis, O. C., Mellow, M., Kanatzar, A., Kelly, C., Park, T. & Surawicz, C. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107(1079–1087). doi: 10.1038/ajg.2012.60 [DOI] [PubMed] [Google Scholar]
- 275.van Beurden YH, de Groot, P. F., van Nood, E., Nieuwdorp, M., Keller, J. J., & Goorhuis, A. Complications, effectiveness, and long term follow-up of fecal microbiota transfer by nasoduodenal tube for treatment of recurrent Clostridium difficile infection. United European Gastroenterol J. 2017;5(868–879). doi: 10.1177/2050640616678099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Jalanka J, Satokari Hillamaa, R., Mattila E., Anttila V‐J., and Arkkila P.. The long-term effects of faecal microbiota transplantation for gastrointestinal symptoms and general health in patients with recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2018;47(371–379). doi: 10.1111/apt.14443 [DOI] [PubMed] [Google Scholar]
- 277.Petrof EO, Gloor, G. B., Vanner, S. J., Weese, S. J., Carter, D., Daigneault, M. C. & Allen-Vercoe, E. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome. 2013;1(3). doi: 10.1186/2049-2618-1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Lawley TD, Clare, S., Walker, A. W., Stares, M. D., Connor, T. R., Raisen, C. & Dougan, G. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 2012;8(e1002995). doi: 10.1371/journal.ppat.1002995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Pereira FC, Wasmund, K., Cobankovic, I., Jehmlich, N., Herbold, C. W., Lee, K. S. & Berry, D. Rational design of a microbial consortium of mucosal sugar utilizers reduces Clostridiodes difficile colonization. Nat Commun. 2020;11(5104). doi: 10.1038/s41467-020-18928-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Nightingale KK, Schukken, Y. H., Nightingale, C. R., Fortes, E. D., Ho, A. J., Her, Z. & Wiedmann, M. Ecology and transmission of Listeria monocytogenes infecting ruminants and in the farm environment. Appl Environ Microbiol. 2004;70(4458–4467). doi: 10.1128/AEM.70.8.4458-4467.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Bucur FI, Grigore-Gurgu L, Crauwels P, Riedel CU, Nicolau AI. Resistance of Listeria monocytogenes to stress conditions encountered in food and food processing environments. Front Microbiol. 2018;9(2700). doi: 10.3389/fmicb.2018.02700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Silk BJ, Date, K. A., Jackson, K. A., Pouillot, R., Holt, K. G., Graves, L. M. & Mahon, B. E.. Invasive listeriosis in the foodborne diseases active surveillance network (FoodNet), 2004-2009: further targeted prevention needed for higher-risk groups. Clin Infect Dis. 2012;54(Suppl 5):S396–404. doi: 10.1093/cid/cis268. [DOI] [PubMed] [Google Scholar]
- 283.Churchill KJ, Sargeant JM, Farber JM, O’Connor AM. Prevalence of listeria monocytogenes in select ready-to-eat foods-deli meat, soft cheese, and packaged salad: a systematic review and meta-analysis. J Food Prot. 2019;82(344–357). doi: 10.4315/0362-028X.JFP-18-158. [DOI] [PubMed] [Google Scholar]
- 284.Swaminathan B, Gerner-Smidt P. The epidemiology of human listeriosis. Microbes Infect. 2007;9(1236–1243). doi: 10.1016/j.micinf.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 285.Maury MM, Tsai, Y. H., Charlier, C., Touchon, M., Chenal-Francisque, V., Leclercq, A. & Lecuit, M. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat Genet. 2016;48(308–313). doi: 10.1038/ng.3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Radoshevich L, Cossart P. Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat Rev Microbiol. 2018;16(32–46). doi: 10.1038/nrmicro.2017.126. [DOI] [PubMed] [Google Scholar]
- 287.Nikitas G, Deschamps, C., Disson, O., Niault, T., Cossart, P., & Lecuit, M. Transcytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin. J Exp Med. 2011;208(2263–2277). doi: 10.1084/jem.20110560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Sheehan B, Klarsfeld A, Msadek T, Cossart P. Differential activation of virulence gene expression by PrfA, the Listeria monocytogenes virulence regulator. J Bacteriol. 1995;177(6469–6476). doi: 10.1128/jb.177.22.6469-6476.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Ollinger J, Bowen B, Wiedmann M, Boor KJ, Bergholz TM. Listeria monocytogenes sigmaB modulates PrfA-mediated virulence factor expression. Infect Immun. 2009;77(2113–2124). doi: 10.1128/IAI.01205-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Gahan CG, Hill C. Listeria monocytogenes: survival and adaptation in the gastrointestinal tract. Front Cell Infect Microbiol. 2014;4(9). doi: 10.3389/fcimb.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Sauer JD, Herskovits AA, O’Riordan MXD. Metabolism of the gram-positive bacterial pathogen listeria monocytogenes. Microbiol Spectr. 2019:7. doi: 10.1128/microbiolspec.GPP3-0066-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Joseph B, Mertins, S., Stoll, R., Schar, J., Umesha, K. R., Luo, Q. & Goebel, W. Glycerol metabolism and PrfA activity in Listeria monocytogenes. J Bacteriol. 2008;190(5412–5430). doi: 10.1128/JB.00259-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Tiensuu T, Guerreiro DN, Oliveira AH, O’Byrne C, Johansson J. Flick of a switch: regulatory mechanisms allowing Listeria monocytogenes to transition from a saprophyte to a killer. Microbiology (Reading). 2019;165(819–833). doi: 10.1099/mic.0.000808. [DOI] [PubMed] [Google Scholar]
- 294.Czuprynski CJ, Balish E. Pathogenesis of Listeria monocytogenes for gnotobiotic rats. Infect Immun. 1981;32(323–331). doi: 10.1128/iai.32.1.323-331.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Bambirra FH, Lima, K. G. C., Franco, B. D. G. D. M., Cara, D. C., Nardi, R. M. D., Barbosa, F. H. F., & Nicoli, J. R. Protective effect of Lactobacillus sakei 2a against experimental challenge with Listeria monocytogenes in gnotobiotic mice. Lett Appl Microbiol. 2007;45(663–667). doi: 10.1111/j.1472-765X.2007.02250.x [DOI] [PubMed] [Google Scholar]
- 296.Archambaud C, Nahori, M. A., Soubigou, G., Bécavin, C., Laval, L., Lechat, P., & Cossart, P. Impact of lactobacilli on orally acquired listeriosis. Proc Natl Acad Sci U S A. 2012;109:16684–16689. doi: 10.1073/pnas.1212809109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Corr SC, Hill C, Gahan CG. Understanding the mechanisms by which probiotics inhibit gastrointestinal pathogens. Adv Food Nutr Res. 2009;56(1–15). doi: 10.1016/S1043-4526(08)00601-3. [DOI] [PubMed] [Google Scholar]
- 298.Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204(1891–1900). doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Las Heras V, Clooney, A. G., Ryan, F. J., Cabrera-Rubio, R., Casey, P. G., Hueston, C. M. & Gahan, C. G. Short-term consumption of a high-fat diet increases host susceptibility to Listeria monocytogenes infection. Microbiome. 2019;7(7). doi: 10.1186/s40168-019-0621-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.van Ampting MT, Loonen, L. M., Schonewille, A. J., Konings, I., Vink, C., Iovanna, J. & Bovee-Oudenhoven, I. M. Intestinally secreted C-type lectin Reg3b attenuates salmonellosis but not listeriosis in mice. Infect Immun. 2012;80(1115–1120). doi: 10.1128/IAI.06165-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Rolhion N, Chassaing, B., Nahori, M. A., De Bodt, J., Moura, A., Lecuit, M. & Cossart, P. A listeria monocytogenes bacteriocin can target the commensal prevotella copri and modulate intestinal infection. Cell Host Microbe. 2019;26:691–701. doi: 10.1016/j.chom.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Cotter PD, Draper, L. A., Lawton, E. M., Daly, K. M., Groeger, D. S., Casey, P. G. & Hill, C. Listeriolysin S, a novel peptide haemolysin associated with a subset of lineage I Listeria monocytogenes. PLoS Pathog. 2008;4(e1000144). doi: 10.1371/journal.ppat.1000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Quereda JJ, Meza-Torres J, Cossart P, Listeriolysin P-CJ. S: a bacteriocin from epidemic Listeria monocytogenes strains that targets the gut microbiota. Gut Microbes. 2017;8(384–391). doi: 10.1080/19490976.2017.1290759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Quereda JJ, Dussurget, O., Nahori, M. A., Ghozlane, A., Volant, S., Dillies, M. A. & Pizarro-Cerda, J. Bacteriocin from epidemic Listeria strains alters the host intestinal microbiota to favor infection. Proc Natl Acad Sci U S A. 2016;113(5706–5711). doi: 10.1073/pnas.1523899113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Becattini S, Littmann, E. R., Carter, R. A., Kim, S. G., Morjaria, S. M., Ling, L. & Pamer, E. G. Commensal microbes provide first line defense against Listeria monocytogenes infection. J Exp Med. 2017;214(1973–1989). doi: 10.1084/jem.20170495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Corr SC, Gahan CG, Hill C. Impact of selected Lactobacillus and Bifidobacterium species on Listeria monocytogenes infection and the mucosal immune response. FEMS Immunol Med Microbiol. 2007;50(380–388). doi: 10.1111/j.1574-695X.2007.00264.x. [DOI] [PubMed] [Google Scholar]
- 307.Mathipa MG, Bhunia AK, Thantsha MS. Internalin AB-expressing recombinant Lactobacillus casei protects Caco-2 cells from Listeria monocytogenes-induced damages under simulated intestinal conditions. PLoS One. 2019;14(e0220321). doi: 10.1371/journal.pone.0220321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Drolia R, Amalaradjou, M. A. R., Ryan, V., Tenguria, S., Liu, D., Bai, X. & Bhunia, A. K. Receptor-targeted engineered probiotics mitigate lethal Listeria infection. Nat Commun. 2020;11(6344). doi: 10.1038/s41467-020-20200-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Bou Ghanem EN, Jones, G. S., Myers-Morales, T., Patil, P. D., Hidayatullah, A. N., & D'Orazio, S. E. InlA promotes dissemination of Listeria monocytogenes to the mesenteric lymph nodes during food borne infection of mice. PLoS Pathog. 2012;8(e1003015). doi: 10.1371/journal.ppat.1003015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Hoelzer K, Pouillot R, Dennis S. Animal models of listeriosis: a comparative review of the current state of the art and lessons learned. Vet Res. 2012;43(18). doi: 10.1186/1297-9716-43-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Lecuit M, Vandormael-Pournin, S., Lefort, J., Huerre, M., Gounon, P., Dupuy, C. & Cossart, P. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science. 2001;292(1722–1725). doi: 10.1126/science.1059852 [DOI] [PubMed] [Google Scholar]
- 312.Disson O, Grayo, S., Huillet, E., Nikitas, G., Langa-Vives, F., Dussurget, O. & Lecuit, M.. Conjugated action of two species-specific invasion proteins for fetoplacental listeriosis. Nature. 2008;455(1114–1118). doi: 10.1038/nature07303 [DOI] [PubMed] [Google Scholar]
- 313.Schubert WD, Urbanke, C., Ziehm, T., Beier, V., Machner, M. P., Domann, E. & Heinz, D. W. Structure of internalin, a major invasion protein of Listeria monocytogenes, in complex with its human receptor E-cadherin. Cell. 2002;111(825–836). doi: 10.1016/s0092-8674(02)01136-4 [DOI] [PubMed] [Google Scholar]
- 314.Monk IR, Casey PG, Hill C, Gahan CG. Directed evolution and targeted mutagenesis to murinize Listeria monocytogenes internalin A for enhanced infectivity in the murine oral infection model. BMC Microbiol. 2010;10(318). doi: 10.1186/1471-2180-10-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Tsai YH, Disson O, Bierne H, Lecuit M. Murinization of internalin extends its receptor repertoire, altering Listeria monocytogenes cell tropism and host responses. PLoS Pathog. 2013;9(e1003381). doi: 10.1371/journal.ppat.1003381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Cossart P. Molecular and cellular basis of the infection by Listeria monocytogenes: an overview. International Journal of Medical Microbiology. 2001;291(401–409). doi: 10.1078/1438-4221-00146. [DOI] [PubMed] [Google Scholar]
- 317.Roldgaard BB, Andersen JB, Hansen TB, Christensen BB, Licht TR. Comparison of three Listeria monocytogenes strains in a Guinea-pig model simulating food-borne exposure. FEMS Microbiol Lett. 2009;291(88–94). doi: 10.1111/j.1574-6968.2008.01439.x. [DOI] [PubMed] [Google Scholar]
- 318.Pitts MG, D’Orazio SEF. A comparison of oral and intravenous mouse models of listeriosis. Pathogens. 2018:7. doi: 10.3390/pathogens7010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Holch A, Ingmer H, Licht TR, Gram L. Listeria monocytogenes strains encoding premature stop codons in inlA invade mice and Guinea pig fetuses in orally dosed dams. J Med Microbiol. 2013;62(1799–1806). doi: 10.1099/jmm.0.057505-0. [DOI] [PubMed] [Google Scholar]
- 320.Alam MS, Costales, M., Cavanaugh, C., Pereira, M., Gaines, D., & Williams, K. Oral exposure to Listeria monocytogenes in aged IL-17RKO mice: a possible murine model to study listeriosis in susceptible populations. Microb Pathog. 2016;99(236–246). doi: 10.1016/j.micpath.2016.08.035 [DOI] [PubMed] [Google Scholar]
- 321.Mitchell PS, Roncaioli, J. L., Turcotte, E. A., Goers, L., Chavez, R. A., Lee, A. Y. & Vance, R. E. NAIP–NLRC4-deficient mice are susceptible to shigellosis. eLife. 2020;9(e59022). doi: 10.7554/eLife.59022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Martz SL, McDonald, J. A., Sun, J., Zhang, Y. G., Gloor, G. B., Noordhof, C. & Petrof, E. O. Administration of defined microbiota is protective in a murine Salmonella infection model. Sci Rep. 2015;5(16094). doi: 10.1038/srep16094 [DOI] [PMC free article] [PubMed] [Google Scholar]


