ABSTRACT
The nuclear pore complex (NPC) has long been assumed to be the sole route across the nuclear envelope, and under normal homeostatic conditions it is indeed the main mechanism of nucleo-cytoplasmic transport. However, it has also been known that e.g. herpesviruses cross the nuclear envelope utilizing a pathway entitled nuclear egress or envelopment/de-envelopment. Despite this, a thread of observations suggests that mechanisms similar to viral egress may be transiently used also in healthy cells. It has since been proposed that mechanisms like nuclear envelope budding (NEB) can facilitate the transport of RNA granules, aggregated proteins, inner nuclear membrane proteins, and mis-assembled NPCs. Herein, we will summarize the known roles of NEB as a physiological and intrinsic cellular feature and highlight the many unanswered questions surrounding these intriguing nuclear events.
KEYWORDS: Nuclear import, nuclear export, nuclear envelope budding
Introduction
Before the appearance of eukaryotic cells, membranes were present only in the form of the plasma membrane that defined the cell’s borders, whereas its internal environment lacked compartmentalization. About 1.5 billion years ago, one of the most defining evolutionary steps occurred, leading to the formation of an endomembrane system and a sophisticated cellular compartmentalization. Eukaryotic cells benefit from this compartmentalization that, among other things, enables them to maintain incompatible biochemical reactions simultaneously through division into distinct membrane-delineated environments.
The nucleus is one of the largest organelles of the cell [1-5], carrying out many different cellular functions. It contains nuclear DNA and is the site for DNA replication, transcription, and post-transcriptional modification of mRNA [6]. Until recently, nuclear pore complexes (NPCs) were considered the only route in and out of the nucleus for endogenous proteins and RNA. This mode of nuclear export is highly regulated, intensely studied, and indeed a major player in nucleo-cytoplasmic transport [7]. Another pathway, known as nuclear egress or envelopment/de-envelopment has long been accepted as a route of herpesviral escape from the nucleus [8,9] but was proposed to be specific to herpesviruses and not an endogenous cellular transport mechanism. However, over the past 70 years of research, there have been hints of similar mechanisms being utilized in non-infected cells [10–26], but these studies were mainly at the observational level and lacked mechanistic insights. More recently, nuclear envelope budding (NEB) was proposed to be an endogenous mechanism of nuclear transport and shown to be present in all organisms investigated under normal growth conditions [27], suggesting that NEB-like mechanisms were likely co-opted by herpesviruses for nuclear egress. Recent studies have suggested that NEB can serve to transport ribonucleoproteins (RNPs) [28,29], protein aggregates [27] and inner nuclear membrane components [30] (also reviewed in [31]). Discovery and characterization of NEB has until now occurred via ultrastructural analysis. The unavailability of a NEB-specific fluorescent marker has prevented the unequivocal visualization of transport across the nuclear envelope in a time-lapse movie. This lack of live imaging is currently the biggest obstacle to the progression of the investigation of NEB events. In this review, we will summarize the state of knowledge around these mostly overlooked but potential nucleo-cytoplasmic transport pathways and discuss the known and potential implications of them in aging, cancer, and neurodegenerative diseases.
A neglected child with many names?
It is important to note that the terminology for NEB-like nuclear envelope dynamics observed over the years is quite diverse, which can make it hard when performing literature searches to uncover previous observations. Moreover, the determination of common nuclear envelope dynamics for pathways that seem to have distinct molecular functions is complex. While not an exhaustive list, dynamics of the nuclear envelope similar to NEB have been referred to as nuclear blebbing [11], nuclear or nucleolar – where presumably the nucleolus itself also protrudes into the cytosol – extrusion [10,32], nucleo-cytoplasmic relations [21,33], nucleo-cytoplasmic interactions [18,20], nuclear envelope (NE) budding [28,34], outpocketing of the nuclear envelope [21], and nuclear envelope herniations [35]. As it is still the early days of mechanistically dissecting these pathways, it should be cautioned that it is not clear how all these various nuclear envelope dynamics interrelate and whether they represent pathways that have overlapping morphological features but distinct molecular functions. Definitive experiments to unequivocally prove that all NEB and NEB-like pathways represent a mechanism to transfer material over the nuclear envelope have not been conducted. That said, the existing evidence strongly suggests that multiple alternative mechanisms of nucleo-cytoplasmic transfer exist. Indeed, it is almost certain that NPC-mediated transport accounts for the vast majority of nuclear transport in the cell [36–38] (and reviewed in [39]) and that these alternative modes of transport are more utilized during distinct development stages and under pathological conditions. However, evidence from the herpesviral egress field alone is enough to make a strong argument that alternative endogenous modes of nuclear export exist. Herein, we define NEB events as a visible deformation and separation of nuclear membranes, with cargo usually surrounded by membranes, contained within the perinuclear space. As the field moves forward, we can hopefully start to parse out distinct molecular pathways and assign clear nomenclature to the various pathways that are emerging.
The nuclear pore complex: the only route of transport across the nuclear envelope?
A vital component of the nucleus is the nuclear envelope, which separates its contents from the rest of the cell. Both the inner (INM) and outer (ONM) nuclear membranes consist of a phospholipid bilayer that contains a plethora of membrane proteins [40,41], making it a dynamic and crowded environment. The space between the INM and ONM is referred to as the perinuclear space. This nuclear enclosure provides isolation of the genome from many sources of damage. However, due to these separating membranes, the existence of mechanisms that ensure an immaculate flux of metabolites, RNA, and proteins is required.
NPCs have long been assumed to be the sole means of transport of molecules across the nuclear envelope [36,38]. Central to all eukaryotic life, NPCs mediate bidirectional communication between the nucleoplasm and the cytoplasm. They maintain the integrity of the nuclear compartment by preventing macromolecules from diffusing freely in and out of the nucleus [42]. When macromolecules are translocated through the NPCs, it is a very rapid process, occurring at a rate of about 1000 translocations per second [43].
NPCs are among the largest protein complexes found in cells, with an estimated mass of 60 MDa in yeast and 120 MDa in vertebrates [44–47]. They consist of multiple copies of over 30 unique proteins, termed nucleoporins (Nups), with the exact number of Nups being species-dependent [48–50]. With their eightfold symmetry, NPCs consist of a core ring embedded in the nuclear envelope, two outer rings, one cytoplasmic ring, and eight attached nuclear filaments [51]. The nuclear filaments are joined in a distal ring assembling as the nuclear basket [44,51,52].
Each NPC resides at and stabilizes an ~80 nm-wide pore with an inner diameter of ~40 nm that is generated by fusion of the INM and ONM [44,53]. Due to the channel size, NPCs can transport small molecules in their natively folded state, which means that these molecules remain functional [44]. While small molecules such as metabolites and ions pass freely through the NPC, the diffusion of larger molecules is restricted and governed by their size and surface properties [44]. This size limit is not a hard cutoff, rather the energetic barrier for passive diffusion increases rapidly for molecules larger than 40 kDa, but molecules as large as 60–100 kDa can pass through the NPC [54–56]. However, large macromolecular assemblies that greatly exceed the size limit of the 40 nm pore, such as whole virions (100–300 nm), RNA granules (average of 200 nm), or large protein aggregates (variable but up to several hundred nm) have been proposed to be transported across the nuclear envelope and thus the idea of an alternative nuclear export pathway needs to be considered.
The slow discovery of nuclear envelope budding pathways
Numerous electron microscopy studies, some dating back to the early 1950s, have described NEB events under various names [10–12,14–21,23] (Figure 1a-g). NEB-like events have now been seen in about 20 different species within all kingdoms of eukaryotic life. Although most events are protrusions of the ONM into the cytoplasm (Figure 1a-c, e and f), some variations of this structure include a protrusion of the INM into the nucleoplasm (Figure 1d) [23]. The shape of these NEB events can differ, ranging from spherical (Figure 1b and d) to elongated (Figure 1a, c and e). Occasionally, the perinuclear content is also exactly between the INM and ONM, giving it the appearance of an eye (Figure 1g). NEB events also vary in electron density and appearance. The cargo contained in the perinuclear space is often surrounded by a membrane and may be of similar texture to the nucleoplasm. Nevertheless, NEB events occasionally contain material that resembles cytosol, including ribosomes [27], suggesting bidirectionality of this transport route across the nuclear envelope.
Figure 1.
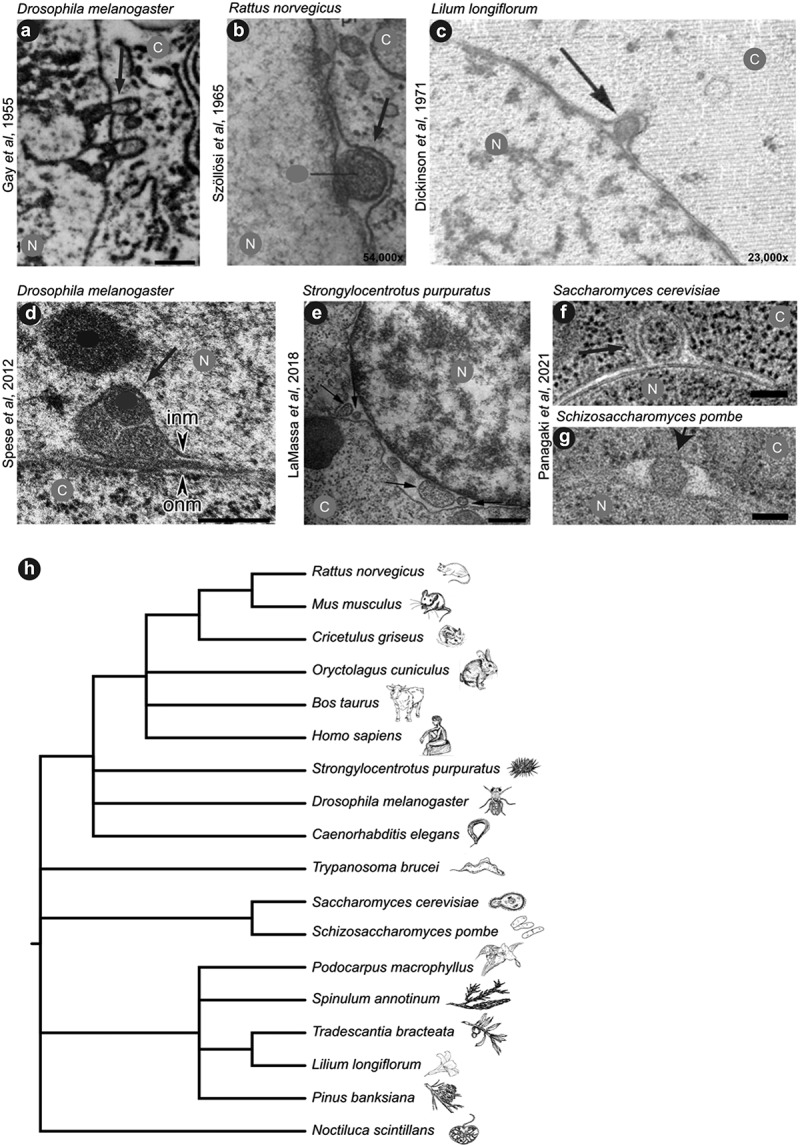
A selection of NEB events from different species found in literature over the past 70 years. a) Electron micrograph of a fruit fly cell [21]. b) Rat oocytes 54,000x magnification [10]. c) Microspores from an Easter lily. 23,000x [20]. d) Drosophila larval muscle nuclei with arrowheads pointing to inner nuclear membrane (INM) and outer nuclear membrane (ONM) [28]. e) Sea urchin gastrula [13]. f) Slice from an electron tomogram of a NEB event in budding yeast. g) Thin section of a fission yeast cell [27]. Scale bars in panels a, d, and e are 500 nm and in panels f and g are 100 nm, scale bars were unavailable in the original publications of panels b and c; N: nucleus, C: cytosol. Arrows are pointing to nuclear budding events, other labels from the original publications were hidden (grey and black dots) to ease comprehension of the adapted figure. (h) Phylogenetic tree of species in which NEB and NEB-like mechanisms has been observed, adapted from [27].
One of the first studies that described NEB events was performed in third instar larvae of the fruit fly (Drosophila melanogaster) in 1955 [21]. In disbelief of their initial observations, several attempts were made to improve the sample preparation protocol, without successfully eliminating the presence of such nuclear blebs. Instead, it was hypothesized that these ‘outpocketings’ were probably a manifestation of normal cellular function with two main theories around their existence. First, the authors suggested the cargo of these events to be strongly associated with the transport of some sort of genetic material from the nucleus to the cytoplasm. Second, they proposed that the protruding membrane could serve as a new membrane component of the endoplasmic reticulum (ER). Common to both theories was the assumption that the shape and composition of these blebs would rapidly change upon release into the cytoplasm.
Several other studies then have made similar observations as well as theories behind these events [13,25,28,29,57–60]. These studies were also performed in several different organisms, implying an evolutionarily conserved nature of this process (Figure 1h). The first publication that reached beyond the observational stage and described the nature of the cargo and the physiological occurrence of these events, was performed in D. melanogaster by Speese et al. in 2012 [28]. In their study, the authors proposed that the transported cargo contained large ribonucleoprotein (RNP) granules, which, after exiting the nucleus through NEB, translocated to specific sites where synapse formation normally takes place. This translocation step has yet to be directly demonstrated. Importantly, later findings from the Budnik lab provided first insights into a mechanism that connects NEB with muscular dystrophies and related disorders, which we will discuss further in the disease-related chapter below [57,58]. To date, several reports of NEB events occurring during normal cellular physiological pathways have been made [27,28,59]. In support of these results, we recently showed that NEB events occur during normal growth conditions, by examining five different organisms across the eukaryotic domain: the protist Trypanosoma brucei, the two yeast species Saccharomyces cerevisiae and Schizosaccharomyces pombe, the nematode Caenorhabditis elegans, and a human mast cell line (HMC-1) [27]. This strengthens the conclusion that budding of the nuclear envelope represents a normal cellular function conserved across species barriers, but also invokes the possibility that morphologically similar events in the nuclear membrane could be transporting different types of cargo and thus could represent distinct molecular pathways that just happen to share overlapping nuclear envelope dynamics.
To date, these observations have been completely dependent on the use of electron microscopy as a means of capturing snapshots of this transport process. More recently, different molecular markers have also been used to study NEB in light microscopy. These markers include nuclear localization signals fused to a fluorophore, other proteins found at the nuclear surface, or viral nuclear egress proteins [28,30,34,61–64]. However, only a few studies have thoroughly correlated their light microscopy markers, for example, nuclear lamins in D. melanogaster [28], Atg39 in S. cerevisiae [30], or viral proteins [63,65,66] with NEB events within the perinuclear space using electron microscopy, which is needed to definitively identify NEB events. Optimizing markers to study NEB and the use of super-resolution light microscopy will be essential to progress and provide a mechanistic understanding of this exciting research field.
Herpesviral egress from the nucleus
Most DNA viruses and a set of RNA viruses exploit the host cell’s nuclear DNA replication and transcription machinery for their own transcription and genome replication (reviewed in [67–71]). To make use of these cellular machineries, the nuclear envelope needs to be crossed twice, once for import of the viral DNA and once for exit of newly assembled virions [68]. Here, we focus on how newly formed herpesviruses leave the nucleus after viral replication, as this occurs in a manner similar to NEB (Figure 2) [72–74].
Figure 2.
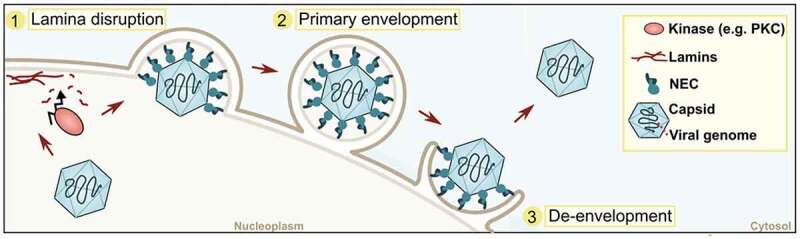
Scheme of herpesviral egress. Three main steps during the envelopment/de-envelopment process of Herpesviridae are depicted. NEC: nuclear egress complex, PKC: protein kinase C.
The herpesvirus family consists of numerous different viruses infecting vertebrates and invertebrates, with the common morphology of an icosahedral capsid enclosing linear double-stranded DNA that is surrounded by a membrane [75]. After DNA replication, transcription and translation, new capsids containing the viral genome are assembled in the nucleus and then employ a specialized mechanism for nuclear and cellular egress. Nuclear egress requires specific viral proteins (extensively reviewed in [76–78]). In short, two conserved viral proteins (e.g., UL31 and UL34 for HSV-1) form a heterodimer that together with host-specific proteins [65] builds a nuclear egress complex (NEC) ([79–81] and reviewed in [82]). The NEC controls capsid envelopment at the INM. To that end, the nuclear lamina, a dense filamentous protein network underneath the INM that provides shape and mechanical stability (reviewed in [83]), requires (partial) disruption of the viral capsid to gain access to the NEC and to bud at the INM (Figure 2). The nuclear lamina is disrupted [84] by recruitment of host Protein Kinase C (PKC)/viral kinases (e.g., US3 for HSV-1) which locally phosphorylate components of the nuclear lamina, resulting in alterations that enable the capsid to access the INM [85–88]. This mimics the host cell’s kinase activity that occurs during nuclear envelope breakdown in the cell cycle and thus promotes nuclear egress of viral capsids [89]. The NEC not only governs recruitment of capsid proteins to the INM but also facilitates membrane curvature during viral budding [66,90–92]. Host ESCRT-III (endosomal sorting complex required transport III) machinery is proposed to be required for vesicular scission, ensuring the budding off of membranes containing capsids into the perinuclear space (primary envelopment) [93].
These perinuclear enveloped viruses then fuse with the ONM to release naked viral capsids into the cytosol – a process called de-envelopment (Figure 2). It is assumed that, among other viral and host factors, viral kinases (US5 for HSV-1) play an important role during this process [65,94,95]. However, the fusogen that drives fusion of the INM and ONM has remained elusive. This mechanism of crossing the nuclear envelope via envelopment/de-envelopment steps has long been assumed to be unique to herpesvirus trafficking. However, as viruses are known to highjack normal cellular functions to their own advantage, it is not surprising that cells are using this route to transport their own cargo and that NEB represents a physiological and intrinsic feature of all cells.
The nature of NEB cargo
A key question, of course, is: what is possibly being transported across the nuclear envelope in these NEB events in a healthy growing cell? In the following sections, we will elaborate on the different cargoes so far identified that are all products the nucleus needs to dispose of. This list will likely grow as the field progresses.
Transport of RNA granules
Due to ‘the intimate association between chromosomal material and membrane outpocketings revealed in these electron micrographs’ [21] (Figure 1a), Gay first suggested that the NEB events might be transporting a nucleic acid from the nucleus to the cytoplasm. A little over 30 years later, Hochstrasser and Sedat [12] made similar observations and suggested the cargo might be aggregates of RNPs [12]. The first publication positively identifying the cargo being transported through this pathway was then published about 60 years after the first observation [28] (Figure 1d). When studying synaptic signaling in D. melanogaster, Speese et al. observed that the C-terminus of the protein Frizzled-2 (DFz2C) forms foci at the periphery of the nucleus (Figure 3a). These foci are surrounded by A- and B-type lamins (LamC and LamDm0, respectively). Just like in the process of herpesviral egress via NEB, PKC is also required for the formation of these NEB events, as it causes the partial dissolution of the filamentous lamin network. This is a prerequisite to move large cargo through the otherwise small fenestrations in the nuclear lamina.
Figure 3.
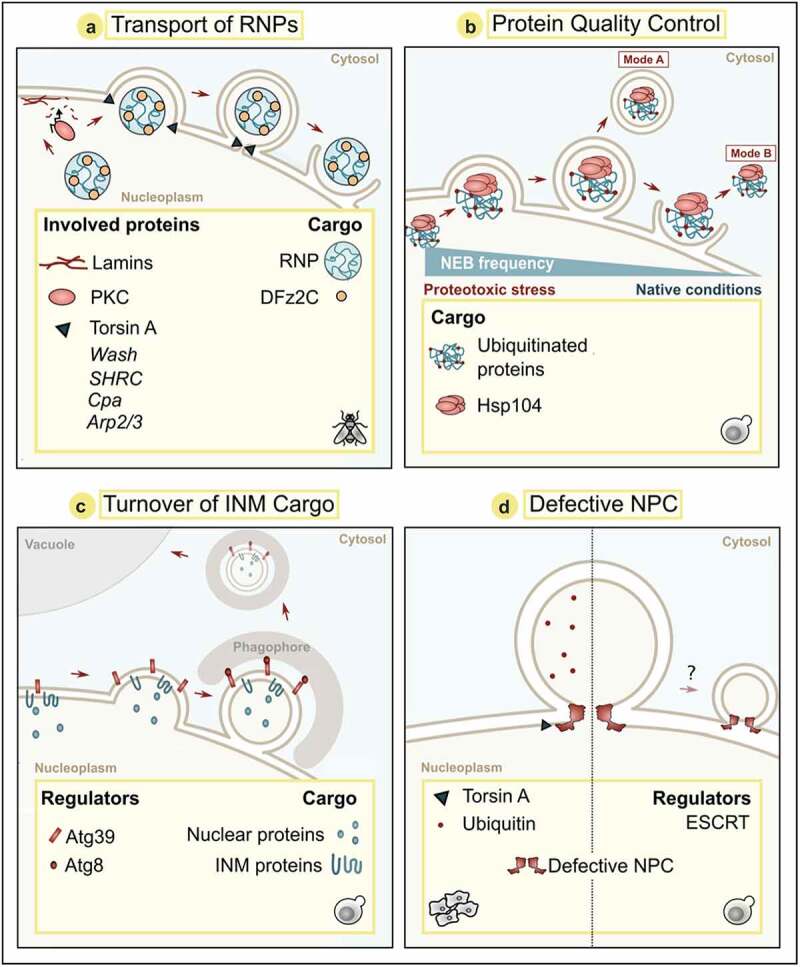
The nature of NEB cargo. Schemes of four modes of nuclear envelope budding events with known cargos and regulators. Panel b shows two modes: (Mode a) Double-membrane vesicle carrying cargo after budding event; (Mode b) Cargo released without membrane enclosure. Organisms, where these modes of actions were described are panel a: Drosophila melanogaster; panels b, c, d (right): Saccharomyces cerevisiae; panel d (left): Mammals. NPC: Nuclear pore complex, PKC: Protein Kinase C.
Ultrastructural analyses revealed that these foci correspond to NEB events where the INM invaginates into the nucleoplasm, thus creating an enlarged perinuclear space that the RNA granules can bud into. It has later been proposed that the AAA+ ATPase Torsin A found in the perinuclear space is required for membrane scission [60]. The cargo within these invaginations consists of RNA granules of around 200 nm in diameter that are bound by a membrane [28]. RNA granules contain transcripts encoding for various scaffolding proteins required for assembly of the postsynaptic domain of the neuromuscular junction synapse [28,96]. It is still unclear whether these granules contain multiple copies of single or several species of RNA, but it is postulated that RNA granules allow for localized translation of their mRNAs, e.g., at postsynaptic sites. The RNA granules contained within the NEB events were hypothesized to be delivered to other parts of the cell via fusion with the ONM, as shown by their occurrence in the cytoplasm; however, it cannot be ruled out that the transcripts contained are bound for degradation.
Using Fz2C foci as a marker for nuclear envelope buds containing RNA granules, the Drosophila washout (WASH) protein, its regulatory complex (SHRC), capping protein Cpa and the Arp2/3 protein complex were identified as players of the molecular machinery underlying NEB in salivary glands of D. melanogaster by Verboon et al. [34] (Figure 3a). WASH belongs to the Wiskott-Aldrich Syndrome Protein (WASP) family [97] that all have well-established roles in cytoskeleton reorganization. While most members of this protein family function in the cytoplasm, WASH has also been shown to be present in the nucleus, where it interacts with B-type lamins [34]. Genetic modifications that interfere with the function of WASH, SHRC subunits, or Arp2/3 decrease NEB frequency, which was associated with disrupted integrity of the neuromuscular junction and decreased mitochondrial activity [34]. Verboon et al. suggest that WASH and SHRC act early in the NEB pathway, upstream of Arp2/3 and Torsin A. Interestingly, a point mutation in WASH that precludes its interaction with Arp2/3 (but does not affect other WASH functions) results in reduced NEB frequency. As Arp2/3 is critical for actin nucleation activity of WASH, the authors propose that WASH might contribute to NEB via modulation of the cortical actin filaments [34]. How exactly these regulatory complexes may be functioning to drive membrane or cortical cytoskeleton remodeling during NEB is yet to be understood. It will also be interesting to see if the function of these complexes in NEB and other nuclear envelope remodeling events is conserved in other species.
NEB to transport protein aggregates
Rose et al. [98] proposed already in 2012 that NEB-like mechanisms may also be used as a pathway to clear the nucleus of aggregated proteins. Misfolded proteins are marked with ubiquitin as a flag for the proteasome to degrade it, as reviewed in [99]. When the removal of misfolded proteins via the ubiquitin-proteasome system fails, terminally damaged and aggregated proteins can be sequestered into insoluble inclusions, reviewed in [100]. This is called spatial protein quality control and represents an intrinsic feature of the cellular stress response, preventing proteotoxicity of accumulating misfolded proteins [101–103]. There are several locations where cells sequester protein aggregates, e.g., the intranuclear and/or juxtanuclear quality control compartments (INQ/JUNQ), the vacuole-associated insoluble protein deposit (IPOD), and the cytoplasmic CytoQ in yeast or the aggresome in mammalian cells [102–104]. Thus, a damaged and misfolded protein within the nucleoplasm can either be removed via the proteasome or, if this fails, be terminally sequestered into INQ (reviewed in [105]). New evidence suggests that upon proteotoxic stress or proteasome inactivation, aggregated proteins can also be transported across the nuclear membrane via NEB [27]. Our research groups investigated the presence and frequency of NEB events under different cellular stress conditions in the budding yeast S.cerevisiae (Figure 3b). Interestingly, different stress conditions, such as heat shock, arsenite, hydrogen peroxide, proteasomal inhibition, and treatment with the proline analog azetidine-2-carboxylic acid, which promotes protein misfolding, significantly increase the frequency of NEB events. These observations, in combination with the presence of ubiquitin and the protein disaggregase Hsp104 inside the forming buds, established a connection between NEB and the protein quality control system.
NEB to sequester INM cargo
Recent evidence suggests that NEB-like mechanisms also contribute to the turnover of damaged INM components via a selective mode of autophagy termed nucleophagy [30, 107] (reviewed in [106]). In yeast, autophagy can be induced when cells are starved of nitrogen or treated with the drug rapamycin. Both treatments resulted in nuclear envelope protrusions [107] and NEB [30] followed by vacuolar removal of INM and nuclear cargo (Figure 3c). The autophagy receptor Atg39 is a transmembrane protein that spans the ONM [108] and connects to the luminal leaflet of the INM via membrane-binding amphipathic helices [30,107]. Atg39 contributes to membrane bending and capturing of INM cargo and nuclear material into NEBs. Interestingly, NPCs seem to be excluded from the regions of NEB in this pathway, and thus this likely represents a distinct selective quality control pathway [109]. Ultrastructural investigation suggests that this process might occur with two mechanistic variations [30,107]. Upon accumulation of Atg39 at the ONM, a simultaneous protrusion of the INM and ONM outwards has been observed, releasing a nuclear envelope-vesicle with a double bilayer in a single scission event [107]. Alternatively, Atg39-captured INM cargo can be sequestered into an INM vesicle that is first released into the perinuclear space, requiring a first membrane scission event at the INM. This is followed by a protrusion outwards and a second scission event at the ONM that also releases a double bilayer vesicle for subsequent autophagic removal [30]. Whether these nuclear envelope remodeling events observed during nucleophagy correspond to those formed upon stress and proteasomal inhibition [27] remains to be shown, but such double bilayer vesicles have not been observed in the cytoplasm of stressed cells. On the other hand, as Atg39 is expressed specifically upon cell stress [110] and overexpression of Atg39 is sufficient to induce INM cargo sequestration and NEB formation [30], a connection seems likely.
Nuclear envelope herniations in response to defective NPC assembly
Transportation through the NPC is a highly efficient and regulated mechanism for nucleo-cytoplasmic communication, and so it is not surprising that malfunction of this process is fatal for the cell. S. cerevisiae for example has dedicated protein quality control mechanisms, one of them being the strategy of keeping dysfunctional NPCs in the mother cells via asymmetric segregation of cell material during budding [35,109]. This ensures that daughter cells begin their lives with only fully functional, recently synthesized proteins. This is achieved by only passing short-lived basket Nups but not long-lived core Nups to the offspring [111]. The protein Nsp1 represents a crucial node in this NPC quality control pathway as it senses their functionality and regulates their transmission into progeny, most likely via a bud neck diffusion barrier [112–115]. Nuclear envelope herniations very similar to NEB events have been observed in response to compromised NPCs. These herniations form upon deletion or mutation of genes encoding Nups or NPC-associated factors [109,116–122], as comprehensively summarized and discussed in a recent review [31].
Assembly of the NPC in the NE occurs stepwise. Starting at the INM, the different subcomplexes are sequentially recruited, the INM is protruding into the perinuclear space, the INM and ONM fuse and the cytosolic filaments are added (reviewed in [123]). Malfunctioning of this assembly process drives the formation of hernia in the INM. These omega-shaped structures cause the ONM to protrude toward the cytosol while often remaining associated with the INM. Here, partially assembled NPCs are found (reviewed in [31]), as recently confirmed by cryo-ET followed by sub-tomogram averaging in yeast, revealing its structure (Figure 3d) [124].
It remains to be explored whether these nuclear envelope herniations that occur in response to defects in NPC assembly are formed by similar mechanisms and are functionally connected to the NEB events containing other cargoes. Interestingly, the nuclear egress of herpesvirus, the proposed nucleo-cytoplasmic shuttling of RNA granules via NEB in Drosophila muscle and salivary gland, as well as NPC-containing herniations share Torsin A as common molecular determinant [60,125,126]. In human cell lines, the lack of Torsin A or its cofactors has been shown to induce herniations that contain NPC assembly intermediates at their neck [127–129]. Those herniations carry ubiquitin, just as the stress-induced NEB events in yeast [27]. How exactly the loss of Torsin A disrupts NPC assembly, thereby leading to herniation formation, is not yet known.
Nuclear envelope herniations have also been linked to the quality control of already fully assembled but damaged NPCs (reviewed in [31]). Formation of herniations in this context seems to involve membrane remodeling by the ESCRT machinery and INM proteins of the Lap2-emerin-MAN1 (LEM) family [109,122,130,131]. In a yeast mutant lacking Nup116, phosphatidic acid-rich membrane stretches and an interaction with LEM proteins recruits ESCRT components to the nuclear envelope herniations [122,132], but the precise role of these proteins at the herniations remains to be explored.
The fate of the nuclear envelope herniations containing defective NPCs remains unclear. One could imagine it as either a mere storage site for a damaged NPC, or a first step in a degradation pathway. Two lines of evidence suggest that this might be a matter of storage. First, the degradation of NPCs upon starvation-induced autophagy was reduced in the herniation-forming mutant nup116Δ [124]. Second, NPCs have even been shown to be excluded from Atg39-associated NEB events that facilitate the autophagic turnover of INM cargo [30]. So, what about the nuclear herniations being a first step in a degradation pathway? The partial NPC intermediates within membrane-concealed herniations are not accessible to the autophagy machinery as they are not exposed to the cytoplasm [124]. Such a degradation mechanism would therefore require further steps for example, releasing the herniation as a vesicle between the INM and ONM, a vesicle that is later released into the cytoplasm. Such vesicles trapped between the INM and ONM have occasionally been seen in NPC assembly-defective yeast cells lacking the ESCRT component Vps4 [109]. However, to determine if these vesicles are the subsequent steps of an NPC degradation pathway, one would ideally see the partially assembled NPC or localize its proteins to the same structure. Therefore, future research will have to settle what the function and fate of these nuclear herniations are.
Imbalances in nuclear-cytoplasmic transport in human disease
One of the most unfailing ways to render a cell dysfunctional is to hinder proper NPC function, a strategy executed by caspases during apoptosis and by viral proteins during infection [133–137]. Thus, NPC building blocks require sophisticated surveillance, sequestration, and removal strategies to ensure cell survival [35,109,113–115,138–142]. Many factors lead to NPC malfunctions and eventual degradation, including the misassembly of newly synthesized NPCs, aggregation of Nups, as well as cellular stress and aging-related diseases (neurodegeneration, cardiovascular diseases, and cancer) [143–148]. Thus, relying solely on the NPC-mediated route for a process as important as the nuclear import and export of proteins, RNA, and ribosomes seems rather risky for the cell. The existence of an alternative nucleo-cytoplasmic gateway makes sense.
NPC-linked health problems are broad and have been reviewed extensively [138,146,149–152]. For example, impaired nucleo-cytoplasmic transport is linked to amyotrophic lateral sclerosis [153,154], frontotemporal dementia, and Huntington’s disease [155]. In Huntington’s disease, NPCs were found decorating the disease-linked poly Q aggregates [156]. In Alzheimer’s disease it was recently reported that Nup98 directly interacts with tau, leading to tau aggregation and Nup sequestration into cytoplasmic neurofibrillary tangles, followed by deterioration of NPCs [157]. Overall, impaired function of NPCs has been strongly connected to neuronal aging and neurodegeneration.
In addition, cardiovascular diseases are also linked to NPC malfunction, as first shown by Zhang et al. where a mutation in Nup155 led to atrial fibrillation in humans and mice [158]. Patients who have suffered a heart failure have increased levels of several Nups [159], and in ischemic cardiomyocytes Nup35 level is reduced [160]. Over the years, Nups have also been found to build oncogenic fusion proteins with different partners [161]. For example, Nup98 fusions to Hox9, a family of transcription factors commonly mutated in cancer, were shown to induce leukemic transformations [162–164]. Nup88 is elevated in many cancer lines and tumors [165,166] and overexpression causes intestinal tumors in mice, without affecting nucleo-cytoplasmic transport [167].
If NEB indeed represents an alternative route of nucleo-cytoplasmic transport, its frequency likely increases in cells afflicted by the aforementioned diseases with malfunctioning NPC-mediated transport. Although a truly systemic study is yet to be conducted, many indications listed in the next section are pointing that way.
NEB in aging and disease
The implications of NEB playing a role in physiological cellular functions are just starting to be unveiled. Below we outline several lines of evidence suggesting that the NEB pathway is involved in cellular aging and age-related diseases.
As yeast cells grow old, nuclear envelope herniations that very much resemble NEB events increase in frequency. In fact, these herniations increased 7.5 times in old cells compared to young controls [35]. The authors associated frequent herniations with damaged NPCs, which may likely be the case. However, further studies are needed to clarify whether some of these age-associated herniations may correspond to NEB events whose task is to clear other material from the aging nuclei.
In humans, a group of diseases commonly associated with aging arise from mutations in genes coding for proteins of the nuclear lamina, collectively referred to as laminopathies. In a D. melanogaster model for laminopathies based on lamin C mutations (A-type lamin), the NEB pathway has been shown to be affected [59]. The authors propose that the impaired lamina network upon lamin C mutation prevents RNA granules containing mitochondria-related mRNAs from exiting the nucleus through NEB. In consequence, this causes a deterioration of mitochondrial function in muscle tissue [59]. Overall, the compromised integrity of the lamina network in these flies results in disrupted nuclear envelope morphology, mitochondrial defects, and impaired flight behavior. The incorrect cellular localization of RNA granules has also been connected to neurodegeneration [168]. Both insufficient transport of RNA granules to their final destination in the cell periphery and overaccumulation of RNA granules in the nucleus have been observed in neurodegenerative diseases [168–171]. In samples from patients with the neurodegenerative disorder fragile X tremor ataxia syndrome (FXTAS), nuclear RNA granules have been found to co-localize with lamin A and C [172], resembling the structures shown in Speese et al. [28]. How NEB contributes to RNA granule transport and assembly, disassembly, or clearance in neurodegenerative diseases still needs to be elucidated.
Torsin A links NEB to another neurological disease. In humans, mutations of the torsin A gene cause DYT1 dystonia – a disease where the synapses between muscle and neuronal cells do not develop properly, leading to uncontrolled muscular twitching. Interestingly, when the torsin A gene is silenced in D. melanogaster muscle cells, RNA granule-containing NEB events accumulate in the perinuclear space [60].
The overexpression of a pathogenic torsin A variant in transgenic mice also resulted in movement defects and perinuclear inclusions (or vesicular structures) which contained ubiquitin, lamin A and Torsin A, very similar to NEB events [24–26,173]. Subsequently, it was shown that loss of torsin A in mice also results in accumulation of NEB events in the perinuclear space [24]. Nuclear buds were observed in all brain regions examined but were enriched in a particular neurodevelopmental window [25].
Not only aged cells rely heavily on protein quality control pathways. Due to rapid proliferation and high protein turnover, cancer cells are sensitized to proteotoxic stress. Thus, the heat shock response is often activated in cancer cells, and some chemotherapies target the proteostasis system [174]. Interestingly, the proteasome inhibitor MG132, also known to induce apoptosis in tumor cells [175], significantly increased the frequency of NEB events in yeast [27]. In human breast cancer cells, the receptor SRC-3/AIB1 (amplified in Breast Cancer-1) was extruded by some form of nuclear budding under normal growth conditions [61]. This indicates that the NEB pathway might also be more active in cancer cells, but ultra-structural investigation is needed to confirm that these events have the same morphology as NEB events outlined above. The deletion of WASH inhibits NEB events in Drosophila [34] and the human WASH complex is strongly associated with both cancers and neurodegenerative disorders [176–178], but if or how this is in relation to NEB remains to be investigated.
In summary, alterations in NEB and NEB-like mechanisms are associated with several human diseases, many of which are connected to aging. A better understanding of NEB and NEB-like mechanisms and their respective roles in nucleo-cytoplasmic transport is relevant for the development of innovative therapies.
Discussion
Evolution often equips cells with redundant systems for important processes to ensure their survival, as is the case for DNA repair and cellular metabolism [179,180]. It is therefore not surprising that nucleo-cytoplasmic transport, an essential function of eukaryotes, would have more than one route. We argue that NEB and NEB-like pathways are hidden in plain sight. They are frequently compared to viral egress, which has long been assumed to be a virus-specific trafficking route, even though viruses usually highjack already existing cellular functions for their replication and infection of other cells, e.g., as the vaccinia virus does [181,182]. We hypothesize that the collective NEB pathways represent a heavily understudied means of endogenous nucleo-cytoplasmic transport and conclude that many questions remain unanswered (Text box 1).
It is without doubt that to help answer at least some of the questions, live cell imaging techniques are required. This will be a difficult task due to the level of spatial and temporal resolution needed. Super-resolution light microscopy techniques for example, using a single-molecule approach, can provide answers if the temporal resolution is high enough and appropriate markers for cargo and membranes can be used. Correlation with an ultrastructural method after live imaging could then finally provide confirmation of bud morphology. Finally, an additional difficulty is that the cargo of these NEB pathways may only transiently exist in the cytoplasm before being degraded, further complicating the experimental design.
Unanswered NEB questions:
Is NEB truly a transport of molecules across the nuclear envelope? How can this be experimentally verified?
Do all NEB events transport cargo via the same mechanism, or are there different transport pathways that have overlapping nuclear envelope dynamics?
Are multiple cargoes transported in the same NEB pathway or are they selective for certain substrates?
Is the transport uni- or bi-directional?
What is the destination of cargo that utilizes these various NEB pathways?
All NEB events have certain features in common, such as deformation of one or both nuclear membranes and the fact that they contain cargo. Yet, there are ultrastructural differences between them. RNA granules found in D. melanogaster protrude into the cell nucleus and contain multiple, electron-dense granules. Those individual granules are surrounded by a single membrane, possibly INM [28]. NEB events in the yeast S. cerevisiae, on the other hand, point outward of the nucleus, protruding into the cytoplasm. The cargo is not always membrane-encapsulated and has different electron densities. Sometimes the appearance of cargo on micrographs resembles that of the nucleoplasm, and other times that of the cytoplasm [27]. In the same organism, when the transport of cargo is mediated by Atg39 under autophagy-inducing conditions, multiple vesicles consisting of INM and nuclear material accumulate. Together, they form a network covered in ONM, adjacent to the nuclear envelope. Lastly, nuclear envelope herniations that transport defective NPC units, such as in the NPC mutant nup116∆, have yet another morphology [121]. Those buddings have a partial NPC at their base, connecting it to the nucleoplasm, in contrast to NEB formed during cellular stress, which are often detached from the INM. These nuclear envelope herniations also appear rather elongated, compared to those observed containing other cargoes such as aggregated proteins. Additionally, immuno-EM on healthy cells shows only partial labeling of Nups on NEB events [27]. This indicates that not all NEB events are formed around a partially assembled and/or defective NPC.
The fact that there is variety in the appearance of NEB events poses the question of whether all observations fall under the same cellular pathway. The membrane surrounding the cargo could be less visible due to technical reasons. For example, the position where the NEB event was sectioned could obscure a membrane. However, the fact that NEB events without visible membrane have been observed using electron tomography [27] increases the probability that membrane-less cargo can accumulate between the nuclear membranes.
It is also unclear whether NEB events are transporting a single cargo with its own NEB pathway or a mixture of different cargoes in the same event. It should be noted that ubiquitin, although observed both in S. cerevisiae and human NEB events [27,127–129], does not localize to the NEB events in D. melanogaster muscle nuclei (Speese – unpublished observations), which is consistent with the idea that different types of cargo can be ferried across the nuclear envelope by NEB.
The directionality of NEB transport is hard to establish in static imaging studies. In Speese et al. [28], it was hypothesized that NEB was proceeding out of the nucleus due to the nature of the RNA content, but this remains unclear in electron microscopic studies when only a snapshot in time can be visualized. A vesicle about to fuse with the nuclear envelope and one about to bud off would look very similar. Since fusion of membranes is a faster process than that of forming a bud [28,30,183–185], one would be more likely to observe the latter event. This implies that a large fraction of the visible buds would be in the process of forming and not fusing. NEB events observed within the perinuclear space could in principle be transported either way. Thus, only the appearance of the cargo gives some sort of indication of where the cargo originates from. A small subset of NEB events in S. cerevisiae contained ribosomes and cargo with the texture of the cytoplasm [27]. This could indicate that the NEB transport route also allows a way into the nucleus. As a portion of cellular proteasomes is localized within the nucleus, this is an important site for proteasomal removal of damaged proteins. Cytoplasmic proteins can be shuttled to the nucleus for proteolytic breakdown [186–188]. Whether these proteins are potentially transported to the nucleus already as aggregates is unclear, but large protein aggregates would struggle to traverse the NPC due to their size restrictions.
In the case of cargo being transported out of the nucleus, the most elusive step of the NEB pathway is the final budding event across the ONM into the cytoplasm. It is not clear whether the cargo will be released inside a vesicle or membrane-free into the cytoplasm via a fusion event, like in the viral egress of herpes viral nucleocapsids. Various flavors of NEB pathways may solve this last step differently, and it is thus important to remain open-minded as to what the final destination of the cargo moving through the various NEB pathways may be. The fate of the cargo once in the cytoplasm also remains elusive, but it might be removed via autophagy or fed into proteasomes for degradation. NEB events formed upon nutritional stress to aid the turnover of INM are engulfed into autophagosomes and shuttled to the vacuole for autophagic degradation [30,107]. The proteome of the nuclear envelope is safeguarded by dedicated degradation machineries, including the INM-associated degradation (INMAD) as well its equivalent at the ONM and the continuous ER membrane, the ER-associated degradation (ERAD). These machineries retrotranslocate misfolded or orphan proteins for subsequent removal by the proteasome [189–191]. Here, NEB seems to contribute to supporting the INM integrity by sequestering its damaged parts for turnover via autophagy. How the NEB pathway is embedded into a broad spectrum of protein quality control systems that maintain the integrity and function of the nucleus and the nuclear envelope remains to be fully understood. However, NEB pathways seem to represent an important mode of nuclear protein quality control that safeguard proteostasis when one subsystem fails for instance, upon insufficient function of the proteasome [27].
With links to several diseases proposed, such as laminopathies, cancer, and neurodegenerative disorders, the acceptance of NEB pathways as an alternative route across the nuclear envelope will open up a whole range of new important questions concerning potential cargoes, molecular components, mechanisms, and cellular destinations.
Acknowledgments
We would like to thank Jacomina Krijsne Locker and Martin Beck for their expert comments on virology and NPCs, respectively. This work was supported by the Austrian Science Fund FWF (J4342-B21 to VK), the Knut and Alice Wallenberg foundation (2017.009 to SB and JLH), the Swedish Research Council VR (2019-05249 and 2019-04004 to SB and JLH, respectively), Stiftelsen Olle Engkvist Byggmästare (207-0527 to SB), and Cancerfonden (21 1865 Pj 01 H and 22 2488 Pj to JLH and SB, respectively).
Funding Statement
This work was supported by the Austrian Science Fund (FWF) [J4342-B21]; Cancerfonden [21 1865 Pj 01 H]; Cancerfonden [22 2488 Pj]; Knut och Alice Wallenbergs Stiftelse [2017.009]; Stiftelsen Olle Engkvist Byggmästare [207-0527]; Vetenskapsrådet [2019-04004]; Vetenskapsrådet [2019-05249]; Vetenskapsrådet [2015-00560].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Keuenhof KS, Larsson Berglund L, Malmgren Hill S, et al. Large organellar changes occur during mild heat shock in yeast. J Cell Sci. 2022;135(5):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Austin CR, Braden AWH.. Observations on nuclear size and form in living rat and mouse eggs. Exp Cell Res. 1955;8(1):163–172. [DOI] [PubMed] [Google Scholar]
- [3].Lambert RA. Comparative studies upon cancer cells and normal cells: II. the character of growth in vitro with special reference to cell division. J Exp Med. 1913;17(5):499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jorgensen P, Edgington NP, Schneider BL, et al. The Size of the Nucleus Increases as Yeast Cells Grow. Mol Biol Cell. 2007. Sep;18(9):3523–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wilson EB. The Cell in Development and Heredity. 3rd ed. New York: The Macmillan Company; 1925. [Google Scholar]
- [6].Zidovska A. The rich inner life of the cell nucleus: dynamic organization, active flows, and emergent rheology. Biophys Rev. 2020. Oct;12(5):1093–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Beck M, Hurt E. The nuclear pore complex: understanding its function through structural insight. Nat Rev Mol Cell Biol. 2017. Feb;18(2):73–89. [DOI] [PubMed] [Google Scholar]
- [8].Lusk CP, King MC. The nucleus: keeping it together by keeping it apart. Curr Opin Cell Biol. 2017. Feb;44(1):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lammerding J. Comprehensive Physiology. In: Terjung R, editor. Mechanics of the Nucleus. Wiley; 2011. p. 783–807. DOI: 10.1002/cphy.c100038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Szollosi D. Extrusion of nucleoli from pronuclei of the rat. J Cell Biol. 1965. Jun;25(3):545–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Szollosi MS, Szollosi D. ‘Blebbing’ of the nuclear envelope of mouse zygotes, early embryos and hybrid cells. J Cell Sci. 1988. Oct;91(2):257–267. [DOI] [PubMed] [Google Scholar]
- [12].Hochstrasser M, Sedat JW. Three-dimensional organization of Drosophila melanogaster interphase nuclei. II. Chromosome spatial organization and gene regulation. J Cell Biol. 1987. Jun;104(6):1471–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].LaMassa N, Arenas-Mena C, Phillips GR. Electron microscopic characterization of nuclear egress in the sea urchin gastrula. J Morphol. 2018. May;279(5):609–615. [DOI] [PubMed] [Google Scholar]
- [14].Aldrich HC, Vasil IK. Ultrastructure of the postmeiotic nuclear envelope in microspores of Podocarpus macrophyllus. J Ultrastruct Res. 1970. Aug;32(3–4):307–315. [DOI] [PubMed] [Google Scholar]
- [15].Gullvåg BM. Release of Nuclear Material During the Development of Lycopodium Annotinum L. Spores. Grana. 1970. Jan;10(1):31–34. [Google Scholar]
- [16].Afzelius BA. The nucleus of Noctiluca scintillans. J Cell Biol. 1963. Oct;19(1):229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Elston RN, Stenkvist B. Quantitative estimation of nuclear buds and micronuclei in bovine cells transformed by Rous sarcoma and SV 40 viruses. Zeitschrift für Zellforschung und Mikroskopische Anatomie. 1965;68(4):543–549. [DOI] [PubMed] [Google Scholar]
- [18].Dickinson HG, Bell PR. Nucleocytoplasmic interaction at the nuclear envelope in post meiotic microspores of Pinus banksiana. J Ultrastruct Res. 1970. Nov;33(3–4):356–359. [DOI] [PubMed] [Google Scholar]
- [19].Mepham RH, Lane GR. Observations on the fine structure of developing microspores of Tradescantia bracteata. Protoplasma. 1970;70(1):1–20. [Google Scholar]
- [20].Dickinson HG. Nucleo-cytoplasmic interaction following meiosis in the young microspores of Lilium longiflorum; events at the nuclear envelope. Grana. 1971;11(2):117–127. [Google Scholar]
- [21].Gay H. Nucleo-cytoplasmic relations in salivary-gland cells of Drosophila. Proceedings of the National Academy of Sciences. Jun 1955;41(6):370–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Romanauska A, Köhler A. The Inner Nuclear Membrane Is a Metabolically Active Territory that Generates Nuclear Lipid Droplets. Cell. 2018. Jul;174(3):700–715.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Longwell AC, Yerganian G. Some Observations on Nuclear Budding and Nuclear Extrusions in a Chinese Hamster Cell Culture. Jnci. 1965. Jan;34(1):53–69. [PubMed] [Google Scholar]
- [24].Goodchild RE, Kim CE, Dauer WT. Loss of the dystonia-associated protein torsinA selectively disrupts the neuronal nuclear envelope. Neuron. 2005;48(6):923–932. [DOI] [PubMed] [Google Scholar]
- [25].Tanabe LM, Liang CC, Dauer WT. Neuronal Nuclear Membrane Budding Occurs during a Developmental Window Modulated by Torsin Paralogs. Cell Rep. 2016;16(12):3322–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tanabe LM, Martin C, Dauer WT. Genetic background modulates the phenotype of a mouse model of dyt1 dystonia. PLoS One. 2012;7(2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Panagaki D, Croft JT, Keuenhof K, et al. Nuclear envelope budding is a response to cellular stress. 2021;118(30). DOI: 10.1073/pnas.2020997118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Speese SD, Ashley J, Jokhi V, et al. Nuclear Envelope Budding Enables Large Ribonucleoprotein Particle Export during Synaptic Wnt Signaling. Cell. 2012. May;149(4):832–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ding B, Mirza AM, Ashley J, et al. “Nuclear Export Through Nuclear Envelope Remodeling in Saccharomyces cerevisiae. bioRxiv. 2017;224055. [Google Scholar]
- [30].Chandra S, Mannino P, Thaller D, et al. Atg39 selectively captures inner nuclear membrane into lumenal vesicles for delivery to the autophagosome. J Cell Bio. 2021. Dec;220(12):2021.02.22.432332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Thaller DJ, Patrick Lusk C. Fantastic nuclear envelope herniations and where to find them. Biochem Soc Trans. 2018. Aug;46(4):877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hadek R, Swift H. Nuclear extrusion and intracisternal inclusions in the rabbit blastocyst. J Cell Biol. 1962. Jun;13(3):445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gay H. Chromosome-nuclear membrane-cytoplasmic interrelations in Drosophila. J Biophys Biochem Cytol. 1956. Jul;2(4):407–414. [PMC free article] [PubMed] [Google Scholar]
- [34].Verboon JM, Nakamura M, Davidson KA, et al. Drosophila Wash and the Wash regulatory complex function in nuclear envelope budding. J Cell Sci. 2020. Jan;130(13):jcs243576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rempel IL, Crane M, Thaller DJ, et al. Age-dependent deterioration of nuclear pore assembly in mitotic cells decreases transport dynamics. Elife. 2019Jun;8:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Feldherr CM. The nuclear annuli as pathways for nucleocytoplasmic exchanges. J Cell Biol. 1962. Jul;14(1):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Richardson WD, Mills AD, Dilworth SM, et al. Nuclear protein migration involves two steps: rapid binding at the nuclear envelope followed by slower translocation through nuclear pores. Cell. 1988. Mar;52(5):655–664. [DOI] [PubMed] [Google Scholar]
- [38].Dworetzky SI, Feldherr CM. Translocation of RNA-coated gold particles through the nuclear pores of oocytes. J Cell Biol. 1988;106(3):575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Görlich D, Kutay U. Transport Between the Cell Nucleus and the Cytoplasm. Annu Rev Cell Dev Biol. 1999. Nov;15(1):607–660. [DOI] [PubMed] [Google Scholar]
- [40].Zetka M, Paouneskou D, Jantsch V. ‘The nuclear envelope, a meiotic jack-of-all-trades,’. Curr Opin Cell Biol. 2020;64:34–42. [DOI] [PubMed] [Google Scholar]
- [41].de Magistris P, Antonin W. The Dynamic Nature of the Nuclear Envelope. Curr Biol. 2018. Apr;28(8):R487–R497. [DOI] [PubMed] [Google Scholar]
- [42].Knockenhauer KE, Schwartz TU. The Nuclear Pore Complex as a Flexible and Dynamic Gate. Cell. 2016;164(6):1162–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kabachinski G, Schwartz TU. The nuclear pore complex - Structure and function at a glance. J Cell Sci. 2015;128(3):423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lin DH, Hoelz A. The structure of the nuclear pore complex (An Update). Annu Rev Biochem. 2019;88:725–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Reichelt R, Holzenburg A, Buhle EL, et al. Correlation between structure and mass distribution of the nuclear pore complex and of distinct pore complex components. J Cell Biol. 1990;110(4):883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yang Q, Rout MP, Akey CW. Three-dimensional architecture of the isolated yeast nuclear pore complex: functional and evolutionary implications. Mol Cell. 1998;1(2):223–234. [DOI] [PubMed] [Google Scholar]
- [47].Rout MP, Aitchison JD, Suprapto A, et al. The yeast nuclear pore complex: composition, architecture, transport mechanism. J Cell Biol. 2000;148(4):635–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tamura K, Fukao Y, Iwamoto M, et al. Identification and Characterization of Nuclear Pore Complex Components in Arabidopsis thaliana. Plant Cell. 2011. Jan;22(12):4084–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].DeGrasse JA, Dubois KN, Devos D, et al. Evidence for a shared nuclear pore complex architecture that is conserved from the last common eukaryotic ancestor. Molecular and Cellular Proteomics. 2009;8(9):2119–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cronshaw JM, Krutchinsky AN, Zhang W, et al. Proteomic analysis of the mammalian nuclear pore complex. J Cell Biol. 2002;158(5):915–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hampoelz B, Andres-Pons A, Kastritis P, et al. Structure and Assembly of the Nuclear Pore Complex. Annu Rev Biophys. 2019;48:515–536. [DOI] [PubMed] [Google Scholar]
- [52].Akey CW, Singh D, Ouch C, et al. Comprehensive structure and functional adaptations of the yeast nuclear pore complex. Cell. 2022;185(2):361–378.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Watson ML. Further Observations on the Nuclear Envelope of the Animal Cell. J Biophys Biochem Cytol. 1959. Oct;6(2):147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Naim B, Zbaida D, Dagan S, et al. Cargo surface hydrophobicity is sufficient to overcome the nuclear pore complex selectivity barrier. EMBO J. 2009;28(18):2697–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Frey S, Rees R, Schünemann J, et al. Surface Properties Determining Passage Rates of Proteins through Nuclear Pores. Cell. 2018;174(1):202–217.e9. [DOI] [PubMed] [Google Scholar]
- [56].Wang R, Brattain MG. The maximal size of protein to diffuse through the nuclear pore is larger than 60 kDa. FEBS Lett. 2007;581(17):3164–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Fradkin LG, Budnik V. This bud’s for you: mechanisms of cellular nucleocytoplasmic trafficking via nuclear envelope budding. Curr Opin Cell Biol. 2016. Aug;41(3):125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Parchure A, Munson M, Budnik V. Getting mRNA-Containing Ribonucleoprotein Granules Out of a Nuclear Back Door. Neuron. 2017. Nov;96(3):604–615. [DOI] [PubMed] [Google Scholar]
- [59].Li Y, Hassinger L, Thomson L, et al. Lamin Mutations Accelerate Aging via Defective Export of Mitochondrial mRNAs through Nuclear Envelope Budding. Curr Biol. 2016;26(15):2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Jokhi V, Ashley J, Nunnari J, et al. Torsin Mediates Primary Envelopment of Large Ribonucleoprotein Granules at the Nuclear Envelope. Cell Rep. 2013;3(4):988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cabrita MA, Renart LI, Lau R, et al. Intrinsically disordered SRC-3/AIB1 protein undergoes homeostatic nuclear extrusion by nuclear budding while ectopic expression induces nucleophagy. Cells. 2019;8(10):Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Otto FB, Thumm M. Mechanistic dissection of macro- and micronucleophagy. Autophagy. 2021. Mar;17(3):626–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hagen C, Dent KC, Zeev-Ben-Mordehai T, et al. Structural Basis of Vesicle Formation at the Inner Nuclear Membrane. Cell. 2015. Dec;163(7):1692–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lorenz M, Vollmer B, Unsay JD, et al. A single herpesvirus protein can mediate vesicle formation in the nuclear envelope. J Biol Chem. 2015;290(11):6962–6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Liu Z, Kato A, Oyama M, et al. Role of Host Cell p32 in Herpes Simplex Virus 1 De-Envelopment during Viral Nuclear Egress. J Virol. 2015;89(17):8982–8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Roller RJ, Bjerke SL, Haugo AC, et al. Analysis of a Charge Cluster Mutation of Herpes Simplex Virus Type 1 UL34 and Its Extragenic Suppressor Suggests a Novel Interaction between pUL34 and pUL31 That Is Necessary for Membrane Curvature around Capsids. J Virol. 2010;84(8):3921–3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Smith AE, Helenius A. How Viruses Enter Animal Cells. Sci (1979). 2004;304(5668):237–242. [DOI] [PubMed] [Google Scholar]
- [68].Molenberghs F, Bogers JJ, de Vos WH. Confined no more: viral mechanisms of nuclear entry and egress. Int J Biochem Cell Biol. 2020Dec;129:105875. [DOI] [PubMed] [Google Scholar]
- [69].de Castro IJ, Lusic M. Navigating through the nucleus with a virus. Curr Opin Genet Dev. 2019;55:100–105. [DOI] [PubMed] [Google Scholar]
- [70].Lucifora J, Delphin M. Current knowledge on Hepatitis Delta Virus replication. Antiviral Res. 2020;179:104812. [DOI] [PubMed] [Google Scholar]
- [71].Lucic B, de Castro IJ, Lusic M. Viruses in the Nucleus. Cold Spring Harb Perspect Biol. 2021. Aug;13(8):a039446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Mäntylä E, Niskanen EA, Ihalainen TO, et al. Reorganization of Nuclear Pore Complexes and the Lamina in Late-Stage Parvovirus Infection. J Virol. 2015;89(22):11706–11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Giorda KM, Raghava S, Hebert DN. The Simian Virus 40 Late Viral Protein VP4 Disrupts the Nuclear Envelope for Viral Release. J Virol. 2012;86(6):3180–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Raghava S, Giorda KM, Romano FB, et al. SV40 late protein VP4 forms toroidal pores to disrupt membranes for viral release. Biochemistry. 2013;52(22):3939–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Davison AJ, Eberle R, Ehlers B, et al. The order Herpesvirales. Arch Virol. 2009;154(1):171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Marschall M, Häge S, Conrad M, et al. Nuclear Egress Complexes of HCMV and Other Herpesviruses: solving the Puzzle of Sequence Coevolution, Conserved Structures and Subfamily-Spanning Binding Properties. Viruses. 2020;12(6): 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Roller RJ, Baines JD. Herpesvirus Nuclear Egress. In: Osterrieder K, editor. Cell Biology of Herpes Viruses. Vol. 223. Springer; 2017. p. 143–169. [DOI] [PubMed] [Google Scholar]
- [78].Ahmad I, Wilson DW. HSV-1 Cytoplasmic Envelopment and Egress. Int J Mol Sci. 2020. Aug;21(17):5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Shiba C, Daikoku T, Goshima F, et al. The UL34 gene product of herpes simplex virus type 2 is a tail-anchored type II membrane protein that is significant for virus envelopment. J Gen Virol. 2000;81(10):2397–2405. [DOI] [PubMed] [Google Scholar]
- [80].Chang YE, Roizman B. The product of the UL31 gene of herpes simplex virus 1 is a nuclear phosphoprotein which partitions with the nuclear matrix. J Virol. 1993;67(11):6348–6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Reynolds AE, Wills EG, Roller RJ, et al. Ultrastructural Localization of the Herpes Simplex Virus Type 1 UL31, UL34, and US3 Proteins Suggests Specific Roles in Primary Envelopment and Egress of Nucleocapsids. J Virol. 2002. Sep;76(17):8939–8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Bigalke JM, Heldwein EE. The Great (Nuclear) Escape: new Insights into the Role of the Nuclear Egress Complex of Herpesviruses. J Virol. 2015;89(18):9150–9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wong X, Melendez-Perez AJ, Reddy KL. The Nuclear Lamina. Cold Spring Harb Perspect Biol. 2022. Feb;14(2):a040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Reynolds AE, Liang L, Baines JD. Conformational Changes in the Nuclear Lamina Induced by Herpes Simplex Virus Type 1 Require Genes UL31 and UL34. J Virol. 2004. Jun;78(11):5564–5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Park R, Baines JD. Herpes Simplex Virus Type 1 Infection Induces Activation and Recruitment of Protein Kinase C to the Nuclear Membrane and Increased Phosphorylation of Lamin B. J Virol. 2006;80(1):494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Morris JB, Hofemeister H, O’Hare P. Herpes Simplex Virus Infection Induces Phosphorylation and Delocalization of Emerin, a Key Inner Nuclear Membrane Protein. J Virol. 2007;81(9):4429–4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Mou F, Forest T, Baines JD. US3 of Herpes Simplex Virus Type 1 Encodes a Promiscuous Protein Kinase That Phosphorylates and Alters Localization of Lamin A/C in Infected Cells. J Virol. 2007;81(12):6459–6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Bjerke SL, Roller RJ. Roles for herpes simplex virus type 1 UL34 and US3 proteins in disrupting the nuclear lamina during herpes simplex virus type 1 egress. Virology. 2006. Apr;347(2):261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hamirally S, Kamil JP, Ndassa-Colday YM, et al. Viral mimicry of Cdc2/cyclin-dependent kinase 1 mediates disruption of nuclear lamina during human cytomegalovirus nuclear egress. PLoS Pathog. 2009;5(1). DOI: 10.1371/journal.ppat.1000275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Chang YE, van Sant C, Krug PW, et al. The null mutant of the UL31 gene of herpes simplex virus 1: construction and phenotype in infected cells. J Virol. 1997. Nov;71(11):8307–8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Klupp BG, Granzow H, Mettenleiter TC. Primary Envelopment of Pseudorabies Virus at the Nuclear Membrane Requires the UL34 Gene Product. J Virol. 2000;74(21):10063–10073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Roller RJ, Zhou Y, Schnetzer R, et al. Herpes Simplex Virus Type 1 UL34 Gene Product Is Required for Viral Envelopment. J Virol. 2000. Jan;74(1):117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Arii J, Watanabe M, Maeda F, et al. ESCRT-III mediates budding across the inner nuclear membrane and regulates its integrity. Nat Commun. 2018;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Wagenaar F, Pol JMA, Peeters B, et al. The US3-encoded protein kinase from pseudorabies virus affects egress of virions from the nucleus. J Gen Virol. 1995;76(7):1851–1859. [DOI] [PubMed] [Google Scholar]
- [95].Liu Z, Kato A, Shindo K, et al. Herpes Simplex Virus 1 UL47 Interacts with Viral Nuclear Egress Factors UL31, UL34, and Us3 and Regulates Viral Nuclear Egress. J Virol. 2014. May;88(9):4657–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Sigrist SJ, Thiel PR, Reiff DF, et al. Postsynaptic translation affects the efficacy and morphology of neuromuscular junctions. Nature. 2000. Jun;405(6790):1062–1065. [DOI] [PubMed] [Google Scholar]
- [97].Linardopoulou EV, Parghi SS, Friedman C, et al. Human subtelomeric WASH genes encode a new subclass of the WASP family. PLoS Genet. 2007;3(12):2477–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Rose A, Schlieker C. Alternative nuclear transport for cellular protein quality control. Trends Cell Biol. 2012. Oct;22(10):509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 1998. Dec;17(24):7151–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Chen B, Retzlaff M, Roos T, et al., “Cellular Strategies of Protein Quality Control”, doi: 10.1101/cshperspect.a004374. [DOI] [PMC free article] [PubMed]
- [101].Escusa-Toret S, Vonk WIM, Frydman J. Spatial sequestration of misfolded proteins by a dynamic chaperone pathway enhances cellular fitness during stress. Nat Cell Biol. 2013. Oct;15(10):1231–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Hill SM, Hanzén S, Nyström T. Restricted access: spatial sequestration of damaged proteins during stress and aging. EMBO Rep. 2017;18(3):377–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Sontag EM, Samant RS, Frydman J. Mechanisms and Functions of Spatial Protein Quality Control. Annu Rev Biochem. 2017. Jun;86(1):97–122. [DOI] [PubMed] [Google Scholar]
- [104].Schneider KL, Nyström T, Widlund PO. Studying Spatial Protein Quality Control, Proteopathies, and Aging Using Different Model Misfolding Proteins in S. Cerevisiae. Front Mol Neurosci. 2018;11:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kumar A, Mathew V, Stirling PC. Nuclear protein quality control in yeast: the latest INQuiries. J Biol Chem. 2022;298(8):102199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Mochida K, Otani T, Katsuma Y, et al. Atg39 links and deforms the outer and inner nuclear membranes in selective autophagy of the nucleus. J Cell Biol. 2022Feb;221(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Mannino PJ, Lusk CP. Quality control mechanisms that protect nuclear envelope identity and function. J Cell Biol. 2022. Sep;221(9):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Mochida K, Oikawa Y, Kimura Y, et al. Receptor-mediated selective autophagy degrades the endoplasmic reticulum and the nucleus. Nature. 2015;522(7556):359–362. [DOI] [PubMed] [Google Scholar]
- [109].Webster BM, Colombi P, Jäger J, et al. Surveillance of nuclear pore complex assembly by ESCRT-III/Vps4. Cell. 2014;159(2):388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Mizuno T, Irie K. Msn2/4 transcription factors positively regulate expression of Atg39 ER-phagy receptor. Sci Rep. 2021;11(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].King GA, Goodman JS, Schick JG, et al. Meiotic cellular rejuvenation is coupled to nuclear remodeling in budding yeast. Elife. 2019Aug;8:1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Denoth Lippuner A, Julou T, Barral Y. Budding yeast as a model organism to study the effects of age. FEMS Microbiol Rev. 2014. Mar;38(2):300–325. [DOI] [PubMed] [Google Scholar]
- [113].Lusk CP, Colombi P. Toward a consensus on the mechanism of nuclear pore complex inheritance. Nucleus. 2014. Mar;5(2):97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Makio T, Lapetina DL, Wozniak RW. Inheritance of yeast nuclear pore complexes requires the Nsp1p subcomplex. J Cell Biol. 2013. Oct;203(2):187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Colombi P, Webster BM, Fröhlich F, et al. The transmission of nuclear pore complexes to daughter cells requires a cytoplasmic pool of Nsp1. J Cell Biol. 2013. Oct;203(2):215–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Bucci M, Wente SR. A Novel Fluorescence-based Genetic Strategy Identifies Mutants of Saccharomyces cerevisiae Defective for Nuclear Pore Complex Assembly. Mol Biol Cell. 1998. Sep;9(9):2439–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Siniossoglou S, Wimmer C, Rieger M, et al. A novel complex of nucleoporins, which includes Sec13p and a Sec13p homolog, is essential for normal nuclear pores. Cell. 1996;84(2):265–275. [DOI] [PubMed] [Google Scholar]
- [118].Onischenko E, Tang JH, Andersen KR, et al. Natively Unfolded FG Repeats Stabilize the Structure of the Nuclear Pore Complex. Cell. 2017;171(4):904–917.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Gigliotti S, Callaini G, Andone S, et al. Nup154, a new Drosophila gene essential for male and female gametogenesis is related to the Nup155 vertebrate nucleoporin gene. J Cell Biol. 1998;142(5):1195–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Schneiter R, Hitomi M, Ivessa AS, et al. A yeast acetyl coenzyme A carboxylase mutant links very-long-chain fatty acid synthesis to the structure and function of the nuclear membrane-pore complex. Mol Cell Biol. 1996. Dec;16(12):7161–7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Wente SR, Blobel G. A temperature-sensitive NUP116 null mutant forms a nuclear envelope seal over the yeast nuclear pore complex thereby blocking nucleocytoplasmic traffic. J Cell Biol. 1993. Oct;123(2):275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Webster BM, Thaller DJ, Jäger J, et al. Chm7 and Heh1 collaborate to link nuclear pore complex quality control with nuclear envelope sealing. EMBO J. 2016;35(22):2447–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Fernandez-Martinez J, Rout MP. Nuclear pore complex biogenesis. Curr Opin Cell Biol. 2009;21(4):603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Allegretti M, Zimmerli CE, Rantos V, et al. In-cell architecture of the nuclear pore and snapshots of its turnover. Nature. 2020. Oct;586(7831):796–800. [DOI] [PubMed] [Google Scholar]
- [125].Turner EM, Brown RSH, Laudermilch E, et al. The Torsin Activator LULL1 Is Required for Efficient Growth of Herpes Simplex Virus 1. J Virol. 2015;89(16):8444–8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Maric M, Shao J, Ryan RJ, et al. A Functional Role for TorsinA in Herpes Simplex Virus 1 Nuclear Egress. J Virol. 2011;85(19):9667–9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Laudermilch E, Tsai PL, Graham M, et al. Dissecting Torsin/cofactor function at the nuclear envelope: a genetic study. Mol Biol Cell. 2016;27(25):3964–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Rampello AJ, Laudermilch E, Vishnoi N, et al. Torsin ATPase deficiency leads to defects in nuclear pore biogenesis and sequestration of MLF2. J Cell Biol. 2020;6:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Pappas SS, Liang C-C, Kim S, et al. TorsinA dysfunction causes persistent neuronal nuclear pore defects. Hum Mol Genet. 2018. Feb;27(3):407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].von Appen A, LaJoie D, Johnson IE, et al. LEM2 phase separation promotes ESCRT-mediated nuclear envelope reformation. Nature. 2020;582(7810):115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Koch BA, Staley E, Jin H, et al. The ESCRT-III complex is required for nuclear pore complex sequestration and regulates gamete replicative lifespan in budding yeast meiosis. Nucleus. 2020;11(1):219–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Thaller DJ, Tong D, Marklew CJ, et al. Direct binding of ESCRT protein Chm7 to phosphatidic acid-rich membranes at nuclear envelope herniations. J Cell Biol. 2021;3:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Faleiro L, Lazebnik Y. Caspases disrupt the nuclear-cytoplasmic barrier. J Cell Biol. 2000;151(5):951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Ferrando-May E, Cordes V, Biller-Ckovric I, et al. Caspases mediate nucleoporin cleavage, but not early redistribution of nuclear transport factors and modulation of nuclear permeability in apoptosis. Cell Death Differ. 2001;8(5):495–505. [DOI] [PubMed] [Google Scholar]
- [135].Gomez GN, Abrar F, Dodhia MP, et al. SARS coronavirus protein nsp1 disrupts localization of Nup93 from the nuclear pore complex. Biochem Cell Biol. 2019. Dec;97(6):758–766. [DOI] [PubMed] [Google Scholar]
- [136].Patre M, Tabbert A, Hermann D, et al. Caspases target only two architectural components within the core structure of the nuclear pore complex. J Biol Chem. 2006;281(2):1296–1304. [DOI] [PubMed] [Google Scholar]
- [137].Yarbrough ML, Mata MA, Sakthivel R, et al. Viral Subversion of Nucleocytoplasmic Trafficking. Traffic. 2014. Feb;15(2):127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Liu J, Hetzer MW. Nuclear pore complex maintenance and implications for age-related diseases. Trends Cell Biol. 2022. Mar;32(3):216–227. [DOI] [PubMed] [Google Scholar]
- [139].Savas JN, Toyama BH, Xu T, et al. Extremely long-lived nuclear pore proteins in the rat brain. Sci (1979). 2012;335(6071):942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Lee CW, Wilfling F, Ronchi P, et al. Selective autophagy degrades nuclear pore complexes. Nat Cell Biol. 2020;22(2):159–166. [DOI] [PubMed] [Google Scholar]
- [141].Shcheprova Z, Baldi S, Frei SB, et al. A mechanism for asymmetric segregation of age during yeast budding. Nature. 2008;454(7205):728–734. [DOI] [PubMed] [Google Scholar]
- [142].Toyama BH, Savas JN, Park SK, et al. Identification of Long-Lived Proteins Reveals Exceptional Stability of Essential Cellular Structures. Cell. 2013. Aug;154(5):971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].D’Angelo MA, Raices M, Panowski SH, et al. Age-Dependent Deterioration of Nuclear Pore Complexes Causes a Loss of Nuclear Integrity in Postmitotic Cells. Cell. 2009. Jan;136(2):284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Rempel IL, Steen A, Veenhoff LM. Poor old pores—The challenge of making and maintaining nuclear pore complexes in aging. FEBS J. 2020. Mar;287(6):1058–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Gross AS, Graef M. Stress eating: autophagy targets nuclear pore complexes. J Cell Biol. 2020. Jul;219(7):7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Sakuma S, D’Angelo MA. The roles of the nuclear pore complex in cellular dysfunction, aging and disease. Semin Cell Dev Biol. 2017. Aug;68(10):72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Bussolati G, Maletta F, Asioli S, et al. Advances in Experimental Medicine and Biology. In: To Be or Not to Be in a Good Shape’: diagnostic and Clinical Value of Nuclear Shape Irregularities in Thyroid and Breast Cancer. Vol. 773. New York, NY: Springer;2014. p.101–121. [DOI] [PubMed] [Google Scholar]
- [148].Ding B, Tang Y, Ma S, et al. Disease modeling with human neurons reveals lmnb1 dysregulation underlying dyt1 dystonia. J Neurosci. 2021;41(9):2024–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Li N, Lagier-Tourenne C. Nuclear pores: the gate to neurodegeneration. Nat Neurosci. 2018. Feb;21(2):156–158. [DOI] [PubMed] [Google Scholar]
- [150].Hachiya N, Sochocka M, Brzecka A, et al. Nuclear Envelope and Nuclear Pore Complexes in Neurodegenerative Diseases—New Perspectives for Therapeutic Interventions. Mol Neurobiol. 2021;58(3):983–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Nofrini V, Di Giacomo D, Mecucci C. Nucleoporin genes in human diseases. Eur J Hum Genet. 2016;24(10):1388–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Xu S, Powers MA. Nuclear pore proteins and cancer. Semin Cell Dev Biol. 2009;20(5):620–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Freibaum BD, Lu Y, Lopez-Gonzalez R, et al. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature. 2015. Sep;525(7567):129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Zhang K, Donnelly CJ, Haeusler AR, et al. The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature. 2015. Sep;525(7567):56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Grima JC, Daigle JG, Arbez N, et al. Mutant Huntingtin Disrupts the Nuclear Pore Complex. Neuron. 2017;94(1):93–107.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Liu K-Y, Shyu Y, Barbaro BA,et al. Disruption of the nuclear membrane by perinuclear inclusions of mutant huntingtin causes cell-cycle re-entry and striatal cell death in mouse and cell models of Huntington’s disease. Hum Mol Genet. 2015. Mar;24(6):1602–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Eftekharzadeh B, Daigle JG, Kapinos LE, et al. Tau Protein Disrupts Nucleocytoplasmic Transport in Alzheimer’s Disease. Neuron. 2018;99(5):925–940.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Zhang X, Chen S, Yoo S, et al. Mutation in Nuclear Pore Component NUP155 Leads to Atrial Fibrillation and Early Sudden Cardiac Death. Cell. 2008;135(6):1017–1027. [DOI] [PubMed] [Google Scholar]
- [159].Tarazó E, Rivera N,M, Roselló-Lletí E, et al. Heart Failure Induces Significant Changes in Nuclear Pore Complex of. Cardiomyocytes,” H PLoS One. 2012;7(11):48957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Xu L, Pan L, Li J, et al. Nucleoporin 35 regulates cardiomyocyte pH homeostasis by controlling Na + -H + exchanger-1 expression. J Mol Cell Biol. 2015. Oct;7(5):476–485. [DOI] [PubMed] [Google Scholar]
- [161].Legrand JMD, Hobbs RM. RNA processing in the male germline: mechanisms and implications for fertility. Semin Cell Dev Biol. 2018Jul;79:80–91. [DOI] [PubMed] [Google Scholar]
- [162].Kroon E, Thorsteinsdottir U, Mayotte N, et al. NUP98-HOXA9 expression in hemopoietic stem cells induces chronic and acute myeloid leukemias in mice. EMBO J. 2001;20(3):350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Borrow J, Shearman AM, Stanton VP, et al. The t(7;11)(p15;p15) translocation in acute myeloid leukaemia fuses the genes for nucleoporin NUP96 and class I homeoprotein HOXA9. Nat Genet. 1996. Feb;12(2):159–167. [DOI] [PubMed] [Google Scholar]
- [164].Nakamura T, Largaespada DA, Lee MP, et al. Fusion of the nucleoporin gene NUP98 to HOXA9 by the chromosome translocation t(7;11)(p15;p15) in human myeloid leukaemia. Nat Genet. 1996. Feb;12(2):154–158. [DOI] [PubMed] [Google Scholar]
- [165].Martínez N, Alons A, Moragues MD, et al. The Nuclear Pore Complex Protein Nup88 Is Overexpressed in Tumor Cells1. Cancer Res. 1999. Nov;59(21):5408–5411. [PubMed] [Google Scholar]
- [166].Li J, Zhao J, Li Y. Multiple biological processes may be associated with tumorigenesis under NUP88-overexpressed condition. Genes Chromosomes Cancer. 2017. Feb;56(2):117–127. [DOI] [PubMed] [Google Scholar]
- [167].Naylor RM, Jeganathan KB, Cao X, et al. Nuclear pore protein NUP88 activates anaphase-promoting complex to promote aneuploidy. J Clin Investig. 2016;126(2):543–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Knowles RB, Sabry JH, Martone ME, et al. Translocation of RNA granules in living neurons. J Neurosci. 1996;16(24):7812–7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Fernandopulle MS, Lippincott-Schwartz J, Ward ME. RNA transport and local translation in neurodevelopmental and neurodegenerative disease. Nat Neurosci. 2021;24(5):622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Alami NH, Smith RB, Carrasco MA, et al. Axonal Transport of TDP-43 mRNA Granules Is Impaired by ALS-Causing Mutations. Neuron. 2014;81(3):536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Fallini C, Donlin-Asp PG, Rouanet JP, et al. Deficiency of the Survival of Motor Neuron Protein Impairs mRNA Localization and Local Translation in the Growth Cone of Motor Neurons. J Neurosci. 2016. Mar;36(13):3811–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Iwahashi CK, Yasui DH, An H-J, et al. Protein composition of the intranuclear inclusions of FXTAS. Brain. 2006;129(1):256–271. [DOI] [PubMed] [Google Scholar]
- [173].Shashidharan P, Sandu D, Potla U, et al. Transgenic mouse model of early-onset DYT1 dystonia. Hum Mol Genet. 2005;14(1):125–133. [DOI] [PubMed] [Google Scholar]
- [174].Balch WE, Morimoto RI, Dillin A, et al. Adapting proteostasis for disease intervention. Sci (1979). 2008;319(5865):916–919. [DOI] [PubMed] [Google Scholar]
- [175].Guo N, Peng Z. MG132, a proteasome inhibitor, induces apoptosis in tumor cells. Asia Pac J Clin Oncol. 2013. Mar;9(1):6–11. [DOI] [PubMed] [Google Scholar]
- [176].Zavodszky E, Seaman MN, Moreau K, et al. Mutation in VPS35 associated with Parkinson’s disease impairs WASH complex association and inhibits autophagy. Nat Commun. 2014. May;5(1):3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].McGough IJ, Steinberg F, Jia D, et al. Retromer binding to FAM21 and the WASH complex is perturbed by the Parkinson disease-linked VPS35(D620N) mutation. Curr Biol. 2014;24(14):1670–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Nordgard SH, Johansen FE, Alnaes GIG, et al. Genome-wide analysis identifies 16q deletion associated with survival, molecular subtypes, mRNA expression, and germline haplotypes in breast cancer patients. Genes Chromosomes Cancer. 2008. Aug;47(8):680–696. [DOI] [PubMed] [Google Scholar]
- [179].Boiteux S, Jinks-Robertson S. DNA repair mechanisms and the bypass of DNA damage in Saccharomyces cerevisiae. Genetics. 2013;193(4):1025–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Wang Z, Zhang J. Abundant Indispensable Redundancies in Cellular Metabolic Networks. Genome Biol Evol. 2009;1:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Postigo A, Ramsden AE, Howell M, et al. Cytoplasmic ATR Activation Promotes Vaccinia Virus Genome Replication. Cell Rep. 2017. May;19(5):1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Snetkov X, Weisswange I, Pfanzelter J, et al. NPF motifs in the vaccinia virus protein A36 recruit intersectin-1 to promote Cdc42: n-WASP-mediated viral release from infected cells. Nat Microbiol. 2016. Aug;1(10):16141. [DOI] [PubMed] [Google Scholar]
- [183].Spruce AE, Breckenridge LJ, Lee AK, et al. Properties of the fusion pore that forms during exocytosis of a mast cell secretory vesicle. Neuron. 1990. May;4(5):643–654. [DOI] [PubMed] [Google Scholar]
- [184].Breckenridge LJ, Almers W. Currents through the fusion pore that forms during exocytosis of a secretory vesicle. Nature. 1987. Aug;328(6133):814–817. [DOI] [PubMed] [Google Scholar]
- [185].Zhang P. Rumschitzki D, Edwards RH. High-speed imaging reveals the bimodal nature of dense core vesicle exocytosis. Proceedings of the National Academy of Sciences. Jan. 2023;120(1):2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Russell SJ, Steger KA, Johnston SA. Subcellular localization, stoichiometry, and protein levels of 26S proteasome subunits in yeast. J Biol Chem. 1999;274(31):21943–21952. [DOI] [PubMed] [Google Scholar]
- [187].Reits EAJ, Benham AM, Plougastel B, et al. Dynamics of proteasome distribution in living cells. EMBO J. 1997;16(20):6087–6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Enenkel C, Kang RW, Wilfling F, et al. Intracellular localization of the proteasome in response to stress conditions. J Biol Chem. 2022;298(7):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Carvalho P, Goder V, Rapoport TA. Distinct Ubiquitin-Ligase Complexes Define Convergent Pathways for the Degradation of ER Proteins. Cell. 2006;126(2):361–373. [DOI] [PubMed] [Google Scholar]
- [190].Smoyer CJ, Jaspersen SL. Patrolling the nucleus: inner nuclear membrane-associated degradation. Curr Genet. 2019;65(5):1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Franić D, Zubčić K, Boban M. Nuclear ubiquitin-proteasome pathways in proteostasis maintenance. Biomolecules. 2021;11(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]


