Abstract
Untreated chronic hyperkalemia is associated with an increased risk of mortality. Novel potassium binders (e.g., patiromer) are new additions to the clinician’s armamentarium. Prior to their approval, clinicians often considered trialing sodium polystyrene sulfonate. The study objective was to assess patiromer utilization and associated changes in serum potassium (K+) in US veterans with prior sodium polystyrene sulfonate exposure. This was a real-world observational study of US veterans with chronic kidney disease and a baseline K+ ≥ 5.1 mEq/L, initiated on patiromer between January 1, 2016, and February 28, 2021. The primary endpoints were patiromer utilization (dispensations and treatment courses), and K+ change at 30-, 91-, and 182-day follow-up (FU) intervals. Patiromer utilization was described using Kaplan–Meier probabilities and the proportion of days covered. Descriptive changes in population average K+ were obtained from a pre-post design using single-arm within-patient pre-post lab pairs and paired t tests. Two hundred five veterans met the study criteria. We observed an average of 1.25 (95% CI, 1.19–1.31) treatment courses and a median treatment duration of 64 days. Fifty veterans (24.4%) had >1 course, and 17.6% of patients remained on their initial patiromer treatment course until the end of the 180-day FU. The mean K+ value was 5.73 mEq/L (5.66–5.79) at baseline, 4.95 mEq/L (95% CI, 4.86–5.05) at the 30-day interval, 4.93 mEq/L (95% CI, 4.84–5.03) at the 91-day interval, and 4.9 mEq/L (95% CI, 4.8–4.99) at the 182-day interval. Novel potassium binders (e.g., patiromer) are newer chronic hyperkalemia management tools for clinicians. The average population K+ decreased to <5.1 mEq/L at all follow-up intervals. Patiromer appeared to be well tolerated with nearly 18% of patients remaining on their initial treatment course during the entire 180-day FU period. The median treatment duration was 64 days and approximately 24% of patients initiated a second course during FU.
Keywords: chronic kidney disease, hyperkalemia, patiromer, Potassium-binding polymer, sodium polystyrene sulfonate
1. Introduction
Clinical management of hyperkalemia is differentiated by acute or chronic hyperkalemic episodes.[1] Until recently, chronic hyperkalemia treatment options were limited to implementing a low potassium diet, stopping or reducing the dose of hyperkalemia-inducing medications (e.g., RAASi [renin-angiotensin-aldosterone system inhibitors]), trialing sodium polystyrene sulfonate (SPS) and/or a loop or thiazide diuretic.[1–4] The guidance to trial SPS for chronic management had several limitations. First, SPS is primarily used as an acute agent and there is limited evidence supporting SPS as a long-term option for hyperkalemia management.[4,5] Second, SPS has a concerning safety profile, most noticeably an increased risk for gastrointestinal necrosis and high sodium content with potential to exacerbate heart failure (HF) symptoms and hypertension.[4] The implications of high sodium content and fluid overload are an important consideration given that 2 common hyperkalemia etiologies are chronic kidney disease (CKD) and HF, both requiring careful attention to fluid status.[4,6]
In 2015, the Food & Drug Administration approved patiromer, a novel potassium-binding agent, for the treatment of non-emergent or life-threatening hyperkalemia.[3] Patiromer safety, effectiveness, and added benefit for RAASi continuity has been established in 5 major phase II and phase III trials: PEARL-HF,[7] AMETHYST-DN,[8] AMBER,[9] OPAL-HK,[10] and DIAMOND.[11] In CKD, the irreversible nature of impaired mechanisms for potassium homeostasis may reflect a need for long-term hyperkalemia treatment. Hyperkalemia treatment algorithms have been proposed, however, there is minimal real-world evidence reporting long-term treatment patterns in a CKD population.[2,4] One observational study evaluated serum potassium (K+) changes up to 180 days following patiromer initiation but included a broader population of CKD, HF, and diabetes type II patients.[12] The paucity of real-world long-term data places clinicians in a difficult position for determining the role of patiromer in chronic hyperkalemia management and its optimal use in CKD. The purpose of this study was to describe real-world patiromer utilization patterns and corresponding changes in K+ in a CKD population.
2. Methods
2.1. Population, data sources, and study design
This is a national real-world observational study using US veterans healthcare data who received an outpatient dispensing of patiromer between the dates of January 1, 2016, and February 28, 2021 (i.e., eligibility period). The study cohort was established using the following inclusion criteria: any outpatient SPS prescription dispensing within 3 months prior to the patiromer index date; any CKD diagnostic code recorded within 1 year prior to the patiromer index date (see Supplemental Table S1, Supplemental Digital Content, http://links.lww.com/MD/I589 for International Classification of Diseases [ICD]-9 and ICD-10 codes used); and at least 1 K+ laboratory event ≥ 5.1 mEq/L within 3 months prior to the index date. Outpatient SPS dispensing within 3 months prior to initiating patiromer was included since we were specifically interested in identifying CKD patients with prior experience with K+ management, reflecting the need for recurrent or chronic hyperkalemia management. Patients with a diagnostic code for end-stage kidney disease recorded within 1 year prior to the index date were excluded (see Supplemental Table S1, Supplemental Digital Content, http://links.lww.com/MD/I589 for ICD-9 and ICD-10 codes used). The primary analysis was restricted to patients with a baseline K+ (last value within 3 months of the index date, see Supplemental Table S2, Supplemental Digital Content, http://links.lww.com/MD/I590 for Logical Observation Identifiers Names and Codes [LOINC] used) ≥ 5.1 mEq/L to reflect patients who had persistent hyperkalemia at the time of patiromer initiation. Secondary analyses for the full study population and patients with a baseline K+ ≥ 5.5 mEq/L were conducted.
The Veterans Health Administration (VHA) corporate data warehouse, a repository of all clinical, laboratory, pharmacy, and administrative records from the VHA’s electronic health record and purchased care data sources, was used to identify the study population and cohort of interest.[13] This study was approved by the University of Utah Institutional Review Board (IRB_00107072) and the Veterans Affairs Salt Lake City Healthcare System Research Service.
2.2. Measurement
2.2.1. Patiromer utilization.
Patiromer utilization was evaluated in the 180-day post-index window using outpatient prescription dispensing data. The analytic workflow, including model input and output, was guided by the Medication History Estimator.[14] We defined a patiromer treatment course as starting with an initial patiromer dispensation and ending when a treatment gap exceeded 30 days. For example, if the initial patiromer dispensation was for a 30-day supply and there is no re-dispensation within 30 days of the calculated supply end date, the treatment course is classified as terminated. In that same case, if a patiromer dispensation occurs 45 days after the calculated supply end date, this constitutes a new (second) patiromer treatment course. Patients could have multiple treatment courses during the follow-up period.
Basic measures of patiromer dispensing data included the number of dispensations, cumulative dosage, and days supplied. Measures of patiromer utilization included the number of drug courses, course duration (i.e., persistence), prescribed and observed daily dose, adherence (medication possession ratio and proportion of days covered [PDC]), active courses at the end of the study, and discontinuation frequency. Formulas used to calculate adherence and persistence measures were previously reported.[14] Censoring of treatment courses occurred if any of the criteria were met; patient death date recorded in between the treatment course start and end date, or active patiromer course at the end of the study period.
2.2.2. K+.
Baseline K+ was defined as the laboratory event closest to the index date, within a 3-month pre-index window. K+ values collected in the 180-day post-index window were sub-divided into 3 discrete follow-up (FU) intervals: days 1 to 30 (labeled 30-day follow-up or 30-day FU), days 31 to 91 (91-day FU) and days 92 to 182 (182-day FU). The K+ measure within the interval and closest to the interval end date was selected to represent a surveillance measure for the interval. K+ laboratory events were identified using a 2-step approach and harmonizing results. First, laboratory events mapped to LOINC codes of interest (see Supplemental Table S2, Supplemental Digital Content, http://links.lww.com/MD/I590 for LOINC codes used) were extracted. Second, a text search for laboratory test names indicative of K+ was conducted, in order to identify unmapped or mismapped LOINC codes. The approach to cleaning and standardizing these data involved removing events with topographies not of interest, non-numeric laboratory result values, or grossly hemolyzed specimens (as listed in the laboratory comments). K+ measures associated with hospitalizations were not included. In patterns of repeated K+ labs during hospitalization, the first chronological laboratory event value in the sequence was considered since it was thought to represent their potassium status upon admission.
Measures used to describe K+ included mean laboratory value, the mean difference in FU laboratory value compared to baseline (using within-person laboratory pairs), and the distribution of FU laboratory values by clinically meaningful ranges: <3.5 mEq/L, 3.5–5.0 mEq/L, 5.1 to 5.4 mEq/L, 5.5 to 5.9 mEq/L, and >6.0 mEq/L. Each measure was calculated using baseline and the 3 FU intervals.
2.2.3. Sensitivity analysis.
A sensitivity analysis was conducted to assess the potential impact of selecting the K+ laboratory event closest to the end of the interval to represent that measurement period. The mean K+ of all laboratory events measured within a given interval was calculated to determine if our findings were sensitive to how we selected potassium measures to represent each interval.
2.2.4. Secondary analysis.
Secondary analyses describing patiromer utilization and changes in K+ were conducted for the full study population (those who met study inclusion criteria of K+ ≥ 5.1 mEq/L during the 3 months prior to the patiromer index date), and patients with a baseline K+ ≥ 5.5 mEq/L.
2.2.5. Statistical methods.
For non-normal baseline characteristics and changes in K+ values, the median and interquartile range were also calculated. Sample paired t tests were used to calculate differences in baseline and FU K+ concentrations. Kaplan–Meier curves were used to describe the duration of patiromer treatment courses (i.e., persistence). Data extraction, processing, and management were conducted using Microsoft SQL Server Management Studio 17.4 (Microsoft Corporation, Redmond, WA). Frequency and percentages were computed using SAS 9.4 (SAS Institute, Cary, NC) and Enterprise Guide 7.1 (SAS Institute, Cary, NC).
3. Results
3.1. Study population
This study identified 3419 veterans initiated on outpatient patiromer in the VHA during the eligibility period. The study population was comprised of patients with SPS exposure (at least 1 outpatient dispensing) within 3 months of patiromer index date (n = 601); CKD diagnosis (stage 1 to 4 or unspecified) recorded within 1 year prior to patiromer index date and no end-stage kidney disease or CKD Stage 5 diagnosis (n = 313); and at least 1 K+ laboratory event within 3 months before index date ≥ 5.1 mEq/L (n = 270). See Supplemental Figure S1, Supplemental Digital Content, http://links.lww.com/MD/I591, which describes attrition from study criteria to identify the study population. Two hundred five veterans had a baseline K+ ≥ 5.1 mEq/L and were included in the primary analysis.
The mean age in the study cohort was 71 years and the majority were male. The distribution of race and ethnicity with 130 (63.4%) White, 60 (29.3%) Black or African American, 1 (0.5%) American Indian or Alaska Native, and 14 (6.8%) unknowns. There was 1 (<1%) patient in CKD stage 1, 3 (1.5%) in stage 2, 84 (41.0%) in stage 3, 103 (50.2%) in stage 4, 0 in stage 5, and 14 (6.8%) with CKD stage unspecified. Of note, 168 (82.0%) patients had a co-diagnosis of diabetes type II. The mean baseline K+ lab value was 5.73 (5.66–5.79). Additional demographic data and baseline medication use for pertinent drug classes are provided in Table 1.
Table 1.
Cohort demographics.
| Study cohort n = 205 | |||
|---|---|---|---|
| Demographic data (as of index date) | Mean (95% CI) | Med (IQR) | |
| Age | 71.7 (70.4–73.0) | 71 (66–77) | |
| Age categories | n | % | |
| <35 yr | 1 | 0.5 | |
| 35–50 yr | 1 | 0.5 | |
| 51–64 yr | 29 | 14.2 | |
| 65–74 yr | 111 | 54.2 | |
| ≥75 yr | 63 | 30.7 | |
| Sex | |||
| Male | 201 | 98.1 | |
| Female | 4 | 2 | |
| Race/Ethnicity | |||
| Caucasian Hispanic | 9 | 4.4 | |
| Caucasian Non-Hispanic | 121 | 59.0 | |
| African American Hispanic | |||
| African American Non-Hispanic | 60 | 29.3 | |
| Asian Hispanic | 0 | ||
| Asian Non-Hispanic | 0 | ||
| American Indian or Alaska Native Hispanic | 0 | ||
| American Indian or Alaska Native Non-Hispanic | 1 | 0.5 | |
| Unknown | 14 | 6.8 | |
| Chronic kidney disease (CKD) stage | |||
| Stage 1 | 1 | 0.5 | |
| Stage 2 | 3 | 1.5 | |
| Stage 3 | 84 | 41.0 | |
| Stage 4 | 103 | 50.2 | |
| Stage 5 | 0 | 0 | |
| ESKD | 0 | 0 | |
| Stage unspecified | 14 | 6.8 | |
| Comorbidities (within 365 d prior to the index date) | |||
| Cancer | 91 | 44.4 | |
| Cardiac dysrhythmias | 46 | 22.4 | |
| Cerebrovascular disease | 40 | 19.5 | |
| Chronic pulmonary disease | 51 | 24.9 | |
| Congestive heart failure | 60 | 29.3 | |
| Coronary artery disease | 84 | 41.0 | |
| Diabetes type II | 168 | 82.0 | |
| Liver disease | 33 | 16.1 | |
| Myocardial infarction | 10 | 4.9 | |
| Peptic ulcer disease | 5 | 2.4 | |
| Peripheral vascular disease | 43 | 21.0 | |
| Medications | |||
| Amiodarone* (past 365 d) | 6 | 2.9 | |
| Recent amiodarone† | 1 | 0.5 | |
| Active amiodarone ‡ | 1 | 0.5 | |
| Beta-blocker (past 365 d) | 135 | 65.9 | |
| Recent beta blocker | 52 | 25.4 | |
| Active beta blocker | 86 | 42.0 | |
| Combination of sacubitril and valsartan (Entresto) | 2 | 1.0 | |
| Recent combination of sacubitril and valsartan (Entresto) | 0 | 0 | |
| Active combination of sacubitril and valsartan (Entresto) | 2 | 1.0 | |
| Cyclosporine/tacrolimus (past 365 d) | 18 | 8. 8 | |
| Recent cyclosporine/tacrolimus | 7 | 3.4 | |
| Active cyclosporine/tacrolimus | 13 | 6.3 | |
| Digoxin (past 365 d) | 7 | 3.4 | |
| Recent digoxin | 3 | 1.5 | |
| Active digoxin | 3 | 1.5 | |
| Diuretic | |||
| Loop (past 365 d) | 113 | 55.1 | |
| Recent loop | 43 | 21.0 | |
| Active loop | 71 | 34.6 | |
| Potassium-sparing (past 365 d) | 4 | 2.0 | |
| Recent potassium sparing | 3 | 1.5 | |
| Active potassium sparing | 1 | 0.5 | |
| Thiazide (past 365 d) | 59 | 28.8 | |
| Recent thiazide | 16 | 7.8 | |
| Active thiazide | 27 | 13.2 | |
| Insulin (past 365 d) | 114 | 55.6 | |
| Recent insulin | 46 | 22.4 | |
| Active insulin | 70 | 34.2 | |
| NSAID (past 365 d) | 18 | 8. 8 | |
| Recent NSAID | 4 | 2.0 | |
| Active NSAID | 2 | 1.0 | |
| RAASi | |||
| ACE inhibitor (past 365 d) | 84 | 41.0 | |
| Recent ACE inhibitor | 30 | 14.6 | |
| Active ACE inhibitor | 34 | 16.6 | |
| ARB (past 365 d) | 28 | 13.7 | |
| Recent ARB | 11 | 5.4 | |
| Active ARB | 13 | 6.3 | |
| Direct renin inhibitor (DRI) (past 365 d) | 0 | 0 | |
| Recent DRI | 0 | 0 | |
| Active DRI | 0 | 0 | |
| Mineralocorticoid receptor antagonist (MRA) (past 365 d) | 21 | 10.2 | |
| Recent MRA | 8 | 3.9 | |
| Active MRA | 4 | 2.0 | |
| SPS (past 365 d) | 205 | 100 | |
| Recent SPS | 134 | 65.4 | |
| Active SPS | 51 | 24.9 | |
| SGLT2 inhibitor (past 365 d) | |||
| Recent | 2 | 1.0 | |
| Active | 1 | 0.5 | |
| Baseline serum potassium | Mean (95% CI) | Med (IQR) | |
| K+ value | 5.73 (5.66–5.79) | 5.6 (5.4–5.9) | |
| K+ categories | n | % | |
| K+ < 3.5 | 0 | 0 | |
| K+ 3.5–5.0 | 0 | 0 | |
| K+ 5.1–5.4 | 64 | 31.2 | |
| K+ 5.5–5.9 | 91 | 44.4 | |
| K+ ≥6.0 | 50 | 24.4 | |
| Estimated GFR (eGFR) using the VA MDRD variable | Mean (95% CI) | Med (IQR) | |
| eGFR value | 30.9 (29.1–32.7) | 28 (21–38) | |
| eGFR categories | n | % | |
| eGFR ≥90 | 0 | 0 | |
| eGFR 60–89 | 10 | 4.9 | |
| eGFR 30–59 | 80 | 39.4 | |
| eGFR 15–29 | 104 | 51.2 | |
| eGFR <15 | 9 | 4.4 | |
| Unable to calculate | 0 | 0 | |
| Hospitalizations (past 365 d) | n | % | |
| Patients with any | 89 | 43.4 | |
| Mean (95% CI) | Med (IQR) | ||
| # of hospitalizations per patient | 2.45 (1.98–2.92) | 2 (1–3) | |
| Length of stay in days (in pts with ≥1 hospitalization) | 4.87 (3.68–6.05) | 3 (2–6) | |
| Emergency department visits (past 365 d) | n | % | |
| Patients with any | 130 | 63.4 | |
| Mean (95% CI) | Med (IQR) | ||
| # of ED visits per patient | 3.98 (3.30–4.65) | 3 (1–5) | |
| Outpatient visits (past 365 d) | n | % | |
| Patients with any | 205 | 100 | |
| Mean (95% CI) | Med (IQR) | ||
| # of outpatients visits per patient | 47.42 (43.31–51.52) | 40 (26–58) | |
ACE = angiotensin-converting-enzyme, ARB = angiotensin receptor blocker, CI = confidence interval, CKD = chronic kidney disease, DRI = direct renin inhibitor, ED = emergency department, eGFR = estimated glomerular filtration rate, ESKD = end-stage kidney disease, IQR = interquartile range, MDRD = modification of diet in renal disease, MRA = mineralocorticoid receptor antagonists, NSAD = nonsteroidal anti-inflammatory drug, SGLT2 = sodium-glucose transport protein 2, SPS = sodium polystyrene sulfonate, VA = Veterans Affairs.
* Patients with medication dispensing during the 365-day baseline period. † Patients who had exposure within 30 d of patiromer index but were not active on medication during the index. ‡ Patients who were active on medication during the index.
3.2. Patiromer utilization
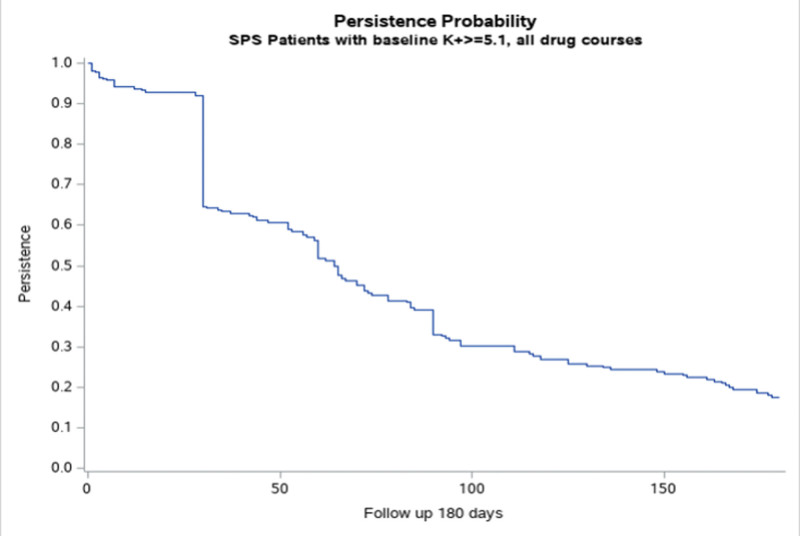
In the 180-day follow-up period, we observed the following patiromer dispensing characteristics: mean number of patiromer dispensations was 2.8 (95% CI, 2.5–3.0), mean cumulative dose dispensed was 1003.7 mg (95% CI, 864.1–1143.4), and the mean day supply per dispensing was 38.5 days (95% CI, 35.4–41.5). The mean medication possession ratio for the entire 180-day follow-up period was 0.53 (95% CI, 0.48–0.58). Two hundred fifty-six patiromer treatment courses were observed in the follow-up period (Table 2). The mean number of treatment courses per patient was 1.25 (95% CI, 1.19–1.31). The number of patients with only 1 treatment course was 155 (75.6%), 2 courses were 49 (23.9%), and 3 courses were 1 (0.5%). The mean prescribed daily dose was 9.2 g (95% CI, 8.7–9.7) and the mean observed daily dose was 9.5 g (95% CI, 8.8–10.2). The median course duration was 64 days (95% CI, 59–72) and the average course PDC was 0.95 (95% CI, 0.94–0.96). Patiromer persistence probability is shown in Figure 1. A detailed description of patiromer utilization for the first patiromer course is provided in Table 3. The number of patients with an active first course who remained on treatment at the end of the follow-up period was 36 (17.6%).
Table 2.
Summary of patiromer dispensing events and drug courses.
| Study cohort (n = 205) | |||
|---|---|---|---|
| Mean | 95% CI | ||
| Patiromer dispensing events | 2.78 | 2.52–3.03 | |
| Cumulative dosage dispensed (g) | 1003.74 | 864.11–1143.37 | |
| Day supply per dispensing | 38.48 | 35.41–41.54 | |
| Patiromer treatment courses (c = 256) |
|||
| Mean | 95% CI | ||
| Number of drug courses | 1.25 | 1.19–1.31 | |
| n | % | ||
| Patients with 1 course | 155 | 75.6 | |
| Patients with >1 course | 50 | 24.4 | |
| Mean | 95% CI | ||
| Course duration (d) | 73.48 | 66.34–80.62 | |
| Average prescribed daily dose (g) | 9.22 | 8.72–9.72 | |
| Average observed daily dose (g) | 9.47 | 8.75–10.19 | |
| Course PDC | 0.95 | 0.94–0.96 | |
| n | % | ||
| Censored course at the end of follow-up time* | 68 | 26.6 | |
| Median course duration (d) | 64 | 59–72 | |
CI = confidence interval, PDC = proportion of days covered.
* Including active courses and courses with patient death date during drug course.
Figure 1.
Persistence probability for all patiromer treatment courses.
Table 3.
Summary of first patiromer course.
| First patiromer course (c = 205) | |||
|---|---|---|---|
| Mean | 95% CI | ||
| Course duration (d) | 82.41 | 60.5–74.08 | |
| Average prescribed daily dose (g) | 9.04 | 3.65–8.54 | |
| Average observed daily dose (g) | 9.49 | 5.99–8.67 | |
| Course PDC | 0.95 | 0.08–0.94 | |
| n | % | ||
| Active course at the end of follow-up time | 36 | 17.6 | |
| Courses with patient death date during course | 3 | 1.5 | |
| Censored course at the end of follow-up time* | 39 | 19 | |
| Median course duration (d) | 65 | 60–78 | |
PDC = proportion of days covered.
* Including active courses and courses with patient death date during drug course.
3.3. K+
The mean baseline K+ value for the study cohort was 5.73 mEq/L (95% CI, 5.66–5.79). The mean K+ value was 4.95 mEq/L (n = 146, 95% CI, 4.86–5.05) in the follow-up (FU) 1 to 30 days interval, 4.93 mEq/L (n = 150, 95% CI, 4.84–5.03) in the FU 31 to 91 days interval, and 4.9 mEq/L (n = 166, 95% CI, 4.8–4.99) in the FU 92 to 182 days interval. Compared to baseline, this reflected a mean change in K+ of −0.81 mEq/L (95% CI, 0.70–0.93, P < .01) at FU 1 to 30 days, −0.81 mEq/L (95% CI, 0.70–0.93, P < .01) at FU 31 to 91 days, and −0.84 mEq/L (95% CI, 0.72–0.96, P < .01) at FU 92 to 182 days. The proportion of study patients with a K+ value < 5.1 mEq/L was 40.5% at FU 1 to 30 days intervals, 45.4% at FU 31 to 91 days intervals, and 51.7% at FU 92 to 182 days. The proportion of study patients with a K+ value < 3.5 mEq/L was <1% at each follow-up interval. A summary of K+ measures for the entire study period is shown in Table 4.
Table 4.
Summary of serum potassium values during study period.
| Study cohort (n = 205) | ||||
|---|---|---|---|---|
| Baseline | Mean (95% CI) | Median (IQR) | P value | |
| K+ value * | 5.73 (5.66–5.79) | 5.6 (5.4–5.9) | ||
| K+ categories | n | % | ||
| K+ 5.1–5.4 | 64 | 31.2 | ||
| K+ 5.5–5.9 | 91 | 44.4 | ||
| K+ ≥6.0 | 50 | 24.4 | ||
| K+ missing | 0 | 0 | ||
| Follow-up 1–30 d. | ||||
| K+ value* | 4.95 (4.86–5.05) | 4.9 (4.6–5.4) | ||
| K+ change from baseline† | −0.81 (0.70–0.93) | <.01 | ||
| K+ categories | n | % | ||
| K+ <3.5 | 1 | 0.5 | ||
| K+ 3.5–5.0 | 82 | 40.0 | ||
| K+ 5.1–5.4 | 32 | 15.6 | ||
| K+ 5.5–5.9 | 28 | 13.7 | ||
| K+ ≥6.0 | 3 | 1.5 | ||
| K+ missing | 59 | 28.8 | ||
| Follow-up 31–91 d. | ||||
| K+ value | 4.93 (4.84–5.03) | 4.9 (4.6–5.3) | ||
| K+ change from baseline | −0.81 (0.70–0.93) | <.01 | ||
| K+ categories | n | % | ||
| K+ <3.5 | 1 | 0.5 | ||
| K+ 3.5–5.0 | 92 | 44.9 | ||
| K+ 5.1–5.4 | 31 | 15.1 | ||
| K+ 5.5–5.9 | 17 | 8.3 | ||
| K+ ≥6.0 | 9 | 4.4 | ||
| K+ missing | 55 | 26.8 | ||
| Follow-up 92–182 d. | ||||
| K+ value | 4.9 (4.8–4.99) | 4.9 (4.5–5.3) | ||
| K+ change from baseline | −0.84 (0.72–0.96) | <.01 | ||
| K+ categories | n | % | ||
| K+ <3.5 | 2 | 1.0 | ||
| K+ 3.5–5.0 | 104 | 50.7 | ||
| K+ 5.1–5.4 | 31 | 15.1 | ||
| K+ 5.5–5.9 | 19 | 9.3 | ||
| K+ ≥6.0 | 10 | 4.9 | ||
| K+ missing | 39 | 19.0 | ||
IQR = interquartile range, K+ = serum potassium.
* Average of K+ values that were closest to the end of the baseline period/index date or closest to the end of the follow-up interval window. † Change of K+ values closest to the end of the follow-up interval and K+ values closest to the end of baseline period/index date.
3.4. Sensitivity analysis
Sensitivity analysis findings are provided in Supplemental Figure S2, Supplemental Digital Content, http://links.lww.com/MD/I592. Changes in mean potassium values from baseline were robust and insensitive to whether we used a single potassium measure to represent the FU interval or included all measures during the FU interval.
3.5. Secondary analysis
Secondary analyses that included the full study population and the subpopulation restricted to patients with a baseline K+ ≥ 5.5 mEq/L are available in the Supplemental File. For the full study population, see Supplemental Table S3, Supplemental Digital Content, http://links.lww.com/MD/I593 for a summary of patiromer dispensing and drug courses, see Supplemental Figure S3, Supplemental Digital Content, http://links.lww.com/MD/I594 for patiromer persistence probability, and see Supplemental Table S4, Supplemental Digital Content, http://links.lww.com/MD/I595 for K+ distributions by category and study interval. For the baseline K+ ≥ 5.5 mEq/L subpopulation, see Supplemental Table S5, Supplemental Digital Content, http://links.lww.com/MD/I596 for a summary of patiromer dispensing and drug courses, see Supplemental Figure S4, Supplemental Digital Content, http://links.lww.com/MD/I597 for patiromer persistence probability, and see Supplemental Table S6, Supplemental Digital Content, http://links.lww.com/MD/I598 for K+ distributions by category and study interval. Patiromer utilization was similar across the 3 populations and not sensitive to how the study population was defined. Changes in K+ were also similar but were sensitive to how we defined these populations since potassium criteria affected average baseline potassium values. For example, we observed a greater average treatment effect in the baseline K+ ≥ 5.5 mEq/L population (mean baseline = 5.94 mEq/L) compared to the baseline K+ ≥ 5.1 mEq/L population (mean baseline = 5.73 mEq/L).
4. Discussion
This study evaluated patiromer utilization and changes in K+ among US veterans with CKD and previous SPS exposure. The effectiveness of patiromer on reducing K+ in non-acute or non-emergent settings, as well as enabling continued RAASi utilization has been studied in 5 key clinical trials, to date.[7–11] However, long-term real-world evidence is limited. As such, we conducted this study to evaluate prescription patterns, treatment duration, and the effect of patiromer on potassium levels for 182 days from initial treatment. Our findings suggest that patiromer is an important component in obtaining and maintaining normal K+ levels in a real-world setting. In terms of real-world evidence, Kovesdy et al evaluated K+ values in an observational study up to 180 days following patiromer initiation in hyperkalemic patients with CKD, HF, or diabetes mellitus type II.[12] Though our study methods differed, we found similar results for patiromer course duration (including the proportion of patients remaining on treatment at the end of follow-up) and changes in K+.
We observed similar changes in K+ across subpopulations in the secondary analyses. Larger treatment effects were seen in the baseline ≥ 5.5 mEq/L subpopulation due to the higher baseline K+ criteria. This resulted in larger differences between the baseline and each FU interval. Our rationale for conducting a full population analysis (in addition to the baseline ≥ 5.1 mEq/L primary analysis) was to assess patients who were not baseline hyperkalemia at the time of patiromer initiation, yet was started due to other prescriber justification. The sensitivity analysis was conducted to inform our approach for measurement selection to best reflect K+ at pre-specified time points. Similarities in K+ values across both approaches support our rationale for choosing the last value to represent the interval.
Our study required at least 1 SPS dispensing in the 3-month pre-index period to reflect our understanding of clinical practice during the study period. In VHA, patiromer is a formulary agent restricted by criteria-for-use (CFU). From March 2016 to January 2019, the patiromer CFU required clinicians to trial SPS prior to patiromer dispensation. This criterion was downgraded to a “consideration” in February 2019 and ultimately removed from the CFU in March 2021. Our initial assumption was that patiromer patients with previous SPS use reflected SPS ineffectiveness or intolerance. However, chart review revealed a myriad of clinical and administrative reasons for transitioning from SPS to patiromer, including potential SPS safety concerns and inventory shortages, in light of a newer agent available for use.[15] Prior to the availability of novel potassium binders, treating clinicians were limited to SPS, an agent primarily used for acute hyperkalemia without rigorous evaluation for long-term use.[5,16]
The availability of novel potassium binders reflects an important pharmacotherapy addition to the chronic hyperkalemia armamentarium. Clinical trials have demonstrated the long-term value of patiromer in reducing K+ and enabling RAASi use. The latter is of extreme importance in both HF and CKD populations given clinicians' conundrum of reducing RAASi dose to manage hyperkalemia at the expense of cardio-renal benefits of these medications. Consensus papers, based on either CKD or HF etiology have taken note of the value of novel potassium binders (such as patiromer), and recommend them as first-line agents when pharmacotherapy is indicated.[4,6] One panel based their preference for novel potassium binders over SPS on the rationale of unfavorable safety and effectiveness profile, poor tolerability, and unpalatable taste.[4]
Our study provides real-world experience with patiromer use in a CKD population. Perhaps most revealing was the observation of treatment patterns. Specifically, the median patiromer course duration was 64 days, approximately 1 in 5 patients remained on their first course of treatment until the end of the study period, and approximately 1 in 5 patients had multiple treatment courses. This challenged our initial assumption of continuous treatment given the chronic nature of CKD. These and other real-world data[12] are necessary to understand chronic hyperkalemia treatment patterns in the era of novel potassium binders aimed at long-term use and to further validate their suggested role as first-line line agents for chronic hyperkalemia management in CKD and HF.
Management of electrolyte abnormalities in a CKD population represents a clinically complex therapeutic challenge given the multitude of intrinsic and extrinsic contributing factors.[17] Clinical management for certain electrolyte abnormalities in CKD, such as initiating phosphate binders for treating hyperphosphatemia, is mature.[18] Others, such as utilizing novel potassium binders for chronic hyperkalemia management, are rapidly maturing with increasing evidence of safety, effectiveness, tolerability, and continuity of use.[4,6] A parallel to highlight the general sense of emerging maturity is that the observed adherence and treatment duration data from real-world patiromer studies are beginning to near similar results in real-world phosphate binder studies.[19] This represents a significant improvement for chronic hyperkalemia management, compared to when novel potassium binders were unavailable, and the primary treatment option was SPS. Future real-world studies will further mature the clinical practice of novel potassium binder initiation as a primary treatment option for chronic hyperkalemia.
Our research sheds light on the ambiguity of chronic hyperkalemia management as both a research and clinical care concept and demonstrates the need for a more detailed classification schema, all of which has been previously discussed.[4] Further investigation to validate and classify treatment patterns as acute, episodic, or chronic is also needed. One proposed definition for classifying hyperkalemia treatment as chronic is the presence of multiple refills, days supplied ≥ 30 days and PDC 0.47 to 0.97.[12] The use of validated classification rules to differentiate treatment patterns may reveal intricacies in clinical decision-making for chronic hyperkalemia management. Studying chronic hyperkalemia requires a nuanced approach, given the multi-modal management model, which may include co-prescribed direct pharmacotherapy (e.g., potassium binders), dietary potassium reduction, and decreased doses of hyperkalemia-inducing pharmacotherapy.[2] As previously mentioned, a carefully constructed causal model is needed to isolate the contribution of patiromer in chronic hyperkalemia management.[12]
4.1. Limitations
Our study cohort consisted of US veterans who are mostly male and White. This may not represent the broader population of patients treated for chronic hyperkalemia, which may limit the generalizability of study findings. Furthermore, since the corporate data warehouse was the sole data source for this project, the study investigators are unable to identify veterans initiated and continued on patiromer outside the VHA (e.g., private insurance and Medicaid/Medicare). Nevertheless, we do not expect many veterans to continue on patiromer outside VHA based on the likelihood of potentially higher copays. Our study is observational in nature and, even though we selected intervals to reflect a prospective study, there was variability in time to K+ measurement. The sensitivity analysis that included all K+ results during follow-up, nevertheless, did not affect our conclusions. The change from baseline analysis does not consider whether patients remained on patiromer at the time of the K+ measurement or other confounding treatments (e.g., low potassium diet, loop diuretics, or decreased RAASi dosage) may have occurred that affected potassium status. The study did not assess patiromer adverse events or reasons for patiromer discontinuation.
5. Conclusion
Uncontrolled chronic hyperkalemia is an indicator of poor prognosis. Patiromer is a novel potassium-binding agent, with evidence for long-term use.[10,11] Our study assessed patiromer utilization and changes in K+ when a new pharmacotherapy option was available in VHA. Patiromer appeared to be well tolerated based on an observed median course duration of 64 days. Approximately 20% of patients experienced >1 patiromer course during the follow-up period, and 17.6% of patients remained on their first course of patiromer at the end of the 180-day FU period. Furthermore, patiromer appeared to be an effective component of potassium management. The average population K+ was reduced to < 5.1 mEq/L during each follow-up interval. Collectively, these findings highlight the important contribution of patiromer in the management of hyperkalemia.
Author contributions
Conceptualization: Shardool Patel, Derek Pinnell, Joshua Qualls, Sylvie Boutin, Steven D. Woods, Brian C. Sauer.
Data curation: Anitha Rathod.
Formal analysis: Shardool Patel, Derek Pinnell, Joshua Qualls, Wei Chen, Sylvie Boutin, Steven D. Woods, Brian C. Sauer.
Investigation: Shardool Patel, Derek Pinnell, Joshua Qualls, Brian C. Sauer.
Methodology: Shardool Patel, Derek Pinnell, Joshua Qualls, Wei Chen, Sylvie Boutin, Steven D. Woods, Brian C. Sauer.
Supervision: Shardool Patel, Brian C. Sauer.
Validation: Wei Chen.
Visualization: Wei Chen.
Writing – original draft: Shardool Patel, Brian C. Sauer.
Writing – review & editing: Shardool Patel, Derek Pinnell, Joshua Qualls, Sylvie Boutin, Steven D. Woods, Csaba P. Kovesdy, Navdeep Tangri, Brian C. Sauer.
Supplementary Material
Abbreviations:
- CFU
- criteria-for-use
- CKD
- chronic kidney disease
- FU
- follow-up
- HF
- heart failure
- ICD
- International Classification of Diseases
- K+
- serum potassium
- LOINC
- Logical Observation Identifiers Names and Codes
- PDC
- proportion of days covered
- RAASi
- renin-angiotensin-aldosterone system inhibitor
- SPS
- sodium polystyrene sulfonate
- VHA
- Veterans Health Administration
Supplemental Digital Content is available for this article.
This work was funded through a partnered research mechanism. Otsuka Canada Pharmaceutical Inc. was involved in the review of this manuscript. Shardool Patel and Brian C. Sauer received funding from Otsuka Canada Pharmaceutical Inc. to study the use of patiromer in the Veterans Health Administration. This material is the result of work supported with resources and the use of facilities at the Veterans Affairs Salt Lake City Health Care System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government. Joshua Qualls and Derek Pinnell are supported by the Veterans Affairs Advanced Fellowship Program in Medical Informatics with the Office of Academic Affiliations.
Shardool Patel and Brian C. Sauer received research funding from Otsuka Canada Pharmaceutical Inc. Csaba Kovesdy reports consultancy agreements with and receiving honoraria from Abbott, Akebia, AstraZeneca, Bayer, Boehringer-Ingelheim, Cara, CSL Behring, GlaxoSmithKline, Rockwell, and Vifor; and receiving royalties from Springer and UpToDate. Navdeep Tangri reports consultancy agreements with and receiving honoraria from Tricada, PulseData, Mesentech, Renibus, Marizyme, Otsuka, Astra Zenica, BI-Lilly, Janssen, Pfizer, and Bayer; and receiving royalties from Renibus and Marizyme. Sylvie Boutin is an employee of Otsuka Canada Pharmaceutical Inc. Steven D. Woods is a fomer employee of Vifor Pharma. Derek Pinnell, Joshua Qualls, Anitha Rathod and Wei Chen declare that they have no relevant financial interests.The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
How to cite this article: Patel S, Pinnell D, Qualls J, Rathod A, Chen W, Boutin S, Woods SD, Kovesdy CP, Tangri N, Sauer BC. Assessing patiromer utilization and associated serum potassium changes in US veterans with prior sodium polystyrene sulfonate exposure. Medicine 2023;102:9(e33134).
Contributor Information
Derek Pinnell, Email: derek.pinnell@utah.edu.
Joshua Qualls, Email: joshua.qualls@utah.edu.
Anitha Rathod, Email: anitha.rathod@utah.edu.
Wei Chen, Email: wei.chen@utah.edu.
Sylvie Boutin, Email: Sylvie.Boutin@otsuka-ca.com.
Steven D. Woods, Email: swoods0703@gmail.com.
Csaba P. Kovesdy, Email: ckovesdy@uthsc.edu.
Navdeep Tangri, Email: ntangri@sogh.mb.ca.
Brian C. Sauer, Email: brian.sauer@utah.edu.
References
- [1].Clase CM, Carrero JJ, Ellison DH, et al. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2020;97:42–61. [DOI] [PubMed] [Google Scholar]
- [2].Palmer BF, Carrero JJ, Clegg DJ, et al. Clinical management of hyperkalemia. Mayo Clin Proc. 2021;96:744–62. [DOI] [PubMed] [Google Scholar]
- [3].Fried L, Kovesdy CP, Palmer BF. New options for the management of chronic hyperkalemia. Kidney Int Suppl (2011). 2017;7:164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rafique Z, Weir MR, Onuigbo M, et al. Expert panel recommendations for the identification and management of hyperkalemia and role of patiromer in patients with chronic kidney disease and heart failure. J Manag Care Spec Pharm. 2017;23(4-a Suppl):S10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hunt TV, DeMott JM, Ackerbauer KA, et al. Single-dose sodium polystyrene sulfonate for hyperkalemia in chronic kidney disease or end-stage renal disease. Clin Kidney J. 2019;12:408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ferreira JP, Butler J, Rossignol P, et al. Abnormalities of potassium in heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2836–50. [DOI] [PubMed] [Google Scholar]
- [7].Pitt B, Anker SD, Bushinsky DA, et al. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32:820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bakris GL, Pitt B, Weir MR, et al. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA. 2015;314:151–61. [DOI] [PubMed] [Google Scholar]
- [9].Agarwal R, Rossignol P, Romero A, et al. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2019;394:1540–50. [DOI] [PubMed] [Google Scholar]
- [10].Weir MR, Bakris GL, Bushinsky DA, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372:211–21. [DOI] [PubMed] [Google Scholar]
- [11].Butler J, Anker SD, Lund LH, et al. Patiromer for the management of hyperkalemia in heart failure with reduced ejection fraction: the DIAMOND trial. Eur Heart J. 2022;43:4362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kovesdy CP, Gosmanova EO, Woods SD, et al. Real-world management of hyperkalemia with patiromer among United States veterans. Postgrad Med. 2020;132:176–83. [DOI] [PubMed] [Google Scholar]
- [13].Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33:1203–11. [DOI] [PubMed] [Google Scholar]
- [14].Sauer BC, He T, Nebeker JR. SAS® Tools for Transparent and Reproducible Research: Medication History Estimator. 2013. May 1;2013(169). [Google Scholar]
- [15].Patel S, Qualls J, Pinnell D, et al. Justification for initiating patiromer when restricted by prior authorization and clinical guidance in a US health care system. 2022 [DOI] [PMC free article] [PubMed]
- [16].Palmer BF, Clegg DJ. Hyperkalemia across the continuum of kidney function. Clin J Am Soc Nephrol. 2018;13:155–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dhondup T, Qian Q. Acid-base and electrolyte disorders in patients with and without chronic kidney disease: an update. Kidney Dis (Basel). 2017;3:136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Scialla JJ, Kendrick J, Uribarri J, et al. State-of-the-art management of hyperphosphatemia in patients with CKD: an NKF-KDOQI controversies perspective. Am J Kidney Dis. 2021;77:132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ferro C, Dieguez G, Metz S, et al. Real-world adherence and persistence on phosphate binders among dialysis-dependent patients with chronic kidney disease. Am J Kidney Dis. 2022;79:S18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.