Abstract
Despite the advances in research and treatment of human breast cancer, its incidence rate continues to increase by 0.5% per year, and the discovery of novel therapeutic strategies for specific subtypes of human breast cancer remains challenging. Traditional laboratory mouse models have contributed tremendously to human breast cancer research. However, mice do not develop tumors spontaneously; consequently, genetically engineered mouse models or patient-derived xenograft models are often relied upon for more sophisticated human breast cancer studies. Since human breast cancer develops spontaneously, there is a need for alternative, yet complementary, models that can better recapitulate the features of human breast cancer to better understand the molecular and clinical complexities of the disease in developing new therapeutic strategies. Canine mammary tumors are one such alternative model that share features with human breast cancer, including prevalence rate, subtype classification, treatment, and mutational profiles, all of which are described in this review.
Keywords: Canine Mammary Tumor, Canine Somatic and Germline Mutation Profiles, Canine-Patient Derived Xenograft, Comparative Oncology Model
INTRODUCTION
To help us better understand human mammary tumors, both mammalian and non-mammalian animals have been used as model organisms. Among mammals, cancer scientists most frequently use mice, rats, and tree shrews to investigate human mammary tumor biology [1]. In addition, canines have become increasingly recognized as important translational models for human mammary tumors, particularly in the past two decades [2,3]. Canines are considered attractive model organisms because they spontaneously develop numerous tumor types, including lymphoma or leukemia, osteosarcoma, melanoma, and mammary tumors [2,4]. Similarly, tree shrews spontaneously develop tumors, but mice and rats require chemical induction or genetic engineering to develop tumors [1]. Given the spontaneous course of malignant transformation, including initiation, promotion, and progression, these naturally occurring canine cancers share similar aspects with human cancers, including epidemiology, histopathology, tumor biology, and response to therapy [5,6,7,8].
One fascinating feature of the canine model is that certain breeds are known to develop specific types of cancer with higher incidence rates [9]. The breed-specific development of certain cancer types has also been observed in canine mammary tumors (CMTs) [9]. Thus, the identification of conserved genomic signatures or variants that are shared among CMT-predisposed dog breeds, but not found in non-predisposed breeds, could reveal potentially undiscovered and new germline candidates that contribute to breast cancer etiopathogenesis, initiation, and progression. This review article aimed to describe the important characteristics of CMTs and compare them with those of human breast cancers.
PREVALENCE OF CMTs
Estradiol, a hormone produced primarily in the ovaries, increases the risk of mammary tumor development in humans and other mammalian species. Thus, it is not surprising that in unspayed (i.e., those having intact ovaries) female dogs, CMTs are the most common tumor type diagnosed worldwide [10,11]. Conversely, spaying (ovariohysterectomy) at an early age can significantly decrease the risk of developing CMT [12]. In the United States, the incidence rate of CMT in female dogs spayed before their first estrus cycle is 0.05%. If spaying is delayed after the first or second estrus cycle, the CMT incidence rates in female dogs increase to 8% and 26%, respectively [13]. Overall, dog breeds including Dachshund, Cavalier King Charles Spaniel, Papillon, Pomeranian, Yorkshire terrier, and Maltese [14] have been reported as being most predisposed to CMT (Table 1, Figure 1A) [10,14,15,16] and CMTs have been observed more frequently in purebred dogs (62%) than in mixed-breed dogs (38%) [17]. Therefore, it is plausible to believe that germline variants in predisposed dogs might contribute significantly to the etiology of CMTs.
Table 1. Dog breeds that are highly predisposed to developing CMT.
| Dog breed | CMT prevalence (%) | No. of CMT cases | No. of dogs | Population from which cases | References |
|---|---|---|---|---|---|
| Dachshund | 41.5% | NA | 407 | MFT | [14] |
| 0.3% | NA | 311 | F | [15] | |
| Cavalier King Charles Spaniel | 41.2% | NA | 34 | MFT | [14] |
| Papillon | 38.0% | NA | 79 | MFT | [14] |
| Pomeranian | 36.4% | NA | 33 | MFT | [14] |
| 0.4% | NA | 169 | F | [15] | |
| Yorkshire Terrier | 33.9% | NA | 112 | MFT | [14] |
| 14.7% | 99 | 672 | FCMT | [16] | |
| 1.0% | NA | 373 | F | [15] | |
| Maltese | 33.7% | NA | 92 | MFT | [14] |
| 22.0% | 148 | 672 | FCMT | [16] | |
| 2.8% | NA | 151 | F | [15] | |
| Poodle-Miniature | 28.6% | NA | 63 | MFT | [14] |
| 9.4% | 63 | 672 | FCMT | [16] | |
| 1.6% | NA | 99 | F | [15] | |
| Chihuahua | 24.3% | NA | 70 | MFT | [14] |
| 2.5% | NA | 587 | F | [15] | |
| Welsh Corgi | 17.8% | NA | 185 | MFT | [14] |
| Shih Tzu | 14.9% | NA | 281 | MFT | [14] |
| 10.7% | 72 | 672 | FCMT | [16] | |
| 1.1% | NA | 216 | F | [15] | |
| Beagle | 13.7% | NA | 139 | MFT | [14] |
| Labrador Retriever | 12.8% | NA | 304 | MFT | [14] |
| 1.5% | NA | 838 | F | [15] | |
| Schnauzer | 12.3% | NA | 65 | MFT | [14] |
| English Springer Spaniel | 11.8% | NA | 103 | F | [15] |
| Siba | 10.8% | NA | 204 | MFT | [14] |
| Cocker Spaniel | 7.9% | 53 | 672 | FCMT | [16] |
| 3.6% | NA | 188 | F | [15] | |
| Pug | 7.1% | NA | 84 | MFT | [14] |
| Shetland Sheepdog | 7.0% | NA | 171 | MFT | [14] |
| Golden Retriever | 6.4% | NA | 388 | MFT | [14] |
| 3.0% | NA | 564 | F | [15] | |
| Rottweiler | 6.1% | NA | 382 | F | [15] |
| Australian Cattle Dog | 6.0% | NA | 118 | F | [15] |
| German Shepherd Dog | 5.6% | NA | 471 | F | [15] |
| Australian Shepherd | 5.1% | NA | 212 | F | [15] |
| French Bulldog | 4.3% | NA | 23 | MFT | [14] |
| Poodle-Standard | 4.0% | NA | 140 | F | [15] |
| Boxer | 3.5% | 101 | 2,847 | F | [10] |
| Corgi | 3.3% | NA | 120 | F | [15] |
| Doberman Pinscher | 3.3% | NA | 161 | F | [15] |
| Bichon Frise | 1.8% | 106 | 5,992 | F | [10] |
| Bulldog | 1.6% | NA | 204 | F | [15] |
| Jack Russell Terrier | 1.7% | NA | 197 | F | [15] |
| Collie | 1.6% | NA | 61 | F | [15] |
| Great Dane | 0.9% | NA | 160 | F | [15] |
| Poodle-Toy | 0.9% | NA | 136 | F | [15] |
| Boston Terrier | 0.7% | NA | 105 | F | [15] |
| Border Collie | 0.4% | NA | 209 | F | [15] |
| Bernese Mountain Dog | 0.4% | 7 | 1,808 | F | [10] |
CMT = canine mammary tumor; NA = not available; MFT = male and female dogs with any type of tumor; F = female dogs; FCMT = female dogs with CMT.
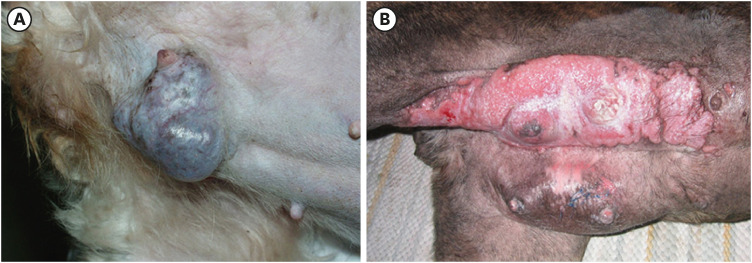
Figure 1. Predisposed and non-predisposed canine breeds with phenotypically different mammary gland carcinomas. Predisposed breed: (A) A 9-year-old, intact female Maltese dog with large, multilobulated, and well-vascularized solid mammary gland carcinoma involving the right fifth mammary gland. (B) Geriatric female boxer dog with extensive and advanced-stage inflammatory mammary carcinoma affecting the right and left caudal mammary glands. Photo courtesy of Drs. Louis-Philippe de Lorimier (Centre Veterinaire Rive-Sud) and Nick Dervisis (Virginia-Maryland College of Veterinary Medicine).
CLASSIFICATION AND GRADING OF CMTs USING HISTOLOGICAL AND MORPHOLOGICAL FEATURES
Mammary cancers exhibit a wide scope of morphological features, different immunohistochemical profiles, and unique histopathological subtypes that have specific clinical progressions and outcomes. For human breast cancer, the histopathological subtype classification was determined based on the World Health Organization (WHO) tumor classification [18]. Similarly, Beveridge and Misdorp developed the original WHO classification for mammary tumors in dogs and cats [19,20]. In 2011, Goldschmidt et al. modified the WHO classification for mammary tumors in dogs and cats by including additional histological subtypes [21], which is now the global standard criteria for determining the diagnosis and prognosis of CMT [22,23]. In 2018, Al-Mansour et al. [24] compared the histological and morphological characteristics of CMTs with those of human mammary tumors. They found that both species exhibited infiltrated ductal cells in malignant tumor types. In addition, humans and canines share similar morphological features, particularly for certain rare mammary tumor types such as invasive micropapillary carcinoma, mucinous carcinoma, and invasive comedocarcinoma. Taken together, the cellular similarities between human and canine mammary tumors make CMT models attractive for human breast cancer research.
Histological grading, in addition to the histopathological classification system, is used to rate the tumor aggressiveness. For human breast cancers, the Bloom and Richardson grading method was developed in 1957, and tubule formation, nuclear pleomorphism, and mitotic activity were assessed to provide independent prognostic information [25]. Elston and Ellis [26] added semiquantitative criteria to the Bloom and Richardson grading method to improve its objectivity and reproducibility. The Elston and Ellis grading method (also known as the Nottingham method) is the global standard for breast cancer classification [27]. In general, each of the three elements (tubule formation, nuclear pleomorphism, and mitotic activity) is rated from 1 to 3 (i.e., 1 being the best and 3 the worst), and the scores of all three components are summed up to determine the disease grade—i.e., low grade (I), intermediate grade (II), and high grade (III). No specific grading methods are used for assessing CMTs; therefore, the Elston and Ellis grading method for human breast cancer has been adopted for grading CMTs. Using the 2-year follow-up criteria, Karayannopoulou et al. [28] found that dogs with Elston and Ellis high-grade (grade III) CMTs had worse survival compared with dogs with low-grade (grade I or grade II) CMTs, as would be expected for humans. This finding suggests that the human grading method can accurately classify and predict the prognosis of CMTs.
Although assessment of the histologic grade contributes to the determination of its biological behavior, the prognosis for human and canine patients diagnosed with mammary gland tumors is better predicted through integration and compilation of multiple variables. For CMT, several studies have reported additional variables, including tumor size, histological type, proliferative index, and clinical stage (regional and/or distant metastases), which are important for accurately predicting the clinical behavior of mammary gland neoplasia [29,30,31,32,33]. As such, a whole organism approach should be adopted to improve the forecasting of expected biological behavior and clinical outcomes (Figure 2).
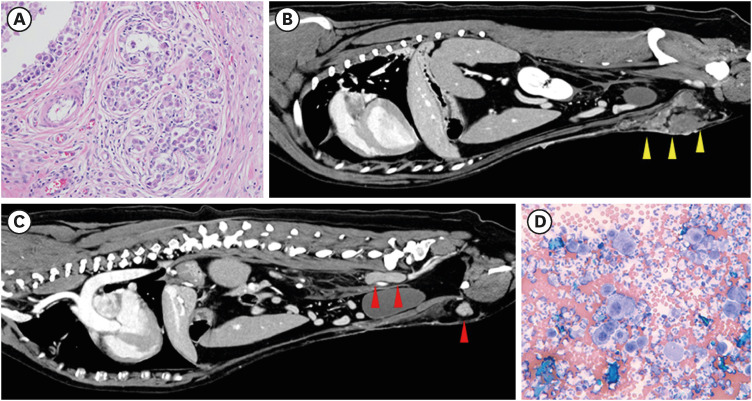
Figure 2. Multiple host and tumor variables contributing to the biologic behavior. A 10-year-old, spayed female Golden Retriever with (A) histologic grade II mammary gland carcinoma surgically removed by regional excision of the left mammary glands 4 and 5 with incomplete margins. (B) Rapidly growing locally recurrent disease (yellow arrowheads) and (C) regional lymph node metastases (red arrowheads; left inguinal [single arrowhead] and left medial iliac lymph nodes [double arrowheads]) identified on computed tomography scan. (D) Serosanguinous abdominal fluid cytologically consistent with a malignant effusive process suggesting distant mammary carcinoma metastases within the peritoneal cavity. Magnification (A) and (D) 200×. Histology courtesy of Dr. Jonathan Samuelson (University of Illinois at Urbana-Champaign).
CLASSIFICATION OF CMTs USING MOLECULAR FEATURES
Although the histopathological and morphological data can help classify human breast cancers, the addition of molecular information obtained through immunohistochemistry provides more accurate diagnostic and prognostic information. The biomarkers for molecular subtype classification of human breast cancers include estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor (HER-2), and Ki-67 [34]. The molecular subtypes of human breast cancers are classified according to the expression levels of the four above mentioned biomarkers: luminal A (ER/PR+, HER-2−, and Ki-67 low), luminal B (ER/PR+, HER-2− or +, and Ki-67 high or any), HER-2 overexpression (ER/PR−, HER-2+, and Ki-67 high), and triple negative (ER−, PR−, HER-2−, and Ki-67 high) [35]. Patients with luminal A, luminal B, and HER-2-overexpressed types show favorable outcomes, whereas those with triple-negative types have a poor prognosis [36,37].
Unlike human breast cancers, the molecular subtypes of CMTs have not yet been well established. However, efforts have been made to apply the current molecular classification of human breast cancer in classifying CMTs. For example, in a study by Gama et al. [38], CMTs were classified according to the human breast cancer molecular classification method; dogs with luminal A type tumors (44.8%) had low histologic grade and low proliferation rates, whereas dogs with basal-type tumors (29.2%, a subtype of triple-negative tumors) had mostly high histologic grade and high proliferation rates. In addition, the basal subtype was associated with shorter disease-free intervals and overall survival rates compared with the luminal A type, similar to that observed in humans. A similar study by Abadie et al. [39] classified 350 CMTs using the human molecular classification method and found that 14.3% of the CMTs were luminal A type, 9.4% were luminal B type, 0% were HER-2-overexpressing type, and 76.3% were triple-negative type. Dogs with luminal A tumors showed significantly longer survival times compared with dogs with luminal B or triple-negative tumors. Another study of 110 CMTs classified tumor histology as luminal A type (38.1%), luminal B type (15.4%), HER-2-overexpressing type (9%), and triple-negative type (15.4%) [40]. The luminal A and B phenotypes were associated with improved prognosis, whereas HER-2-overexpressing and triple-negative tumors were more aggressive and exhibited a significant association with the occurrence of metastasis and significantly shorter survival time. Although the results of individual studies showed some variability, these findings reported in dogs with mammary gland neoplasia are comparable to those observed in human breast cancers. These results indicate that the molecular subtypes of CMTs, classified based on the Elston and Ellis criteria for human mammary tumors, also have a prognostic association in canines, similar to that observed in humans. In summary, the significant degree of histological and molecular resemblance between CMTs and human breast cancers further substantiates the usefulness of CMTs as comparative models for human breast cancer research.
TREATMENT OF CMTs
Surgery is the primary treatment of CMTs, although additional adjuvant chemotherapy and radiotherapy can also be administered postoperatively to reduce the incidence of local tumor recurrence and delay metastatic progression, respectively [41]. Currently, the standard procedures for adjuvant therapy in CMTs have not yet been established, but diverse therapeutic approaches have been employed such as chemotherapy, radiation therapy, hormonal therapy (tamoxifen), receptor tyrosine kinase inhibitor therapy (toceranib phosphate), non-steroidal anti-inflammatory drug (NSAID) therapy that block the cyclooxygenase activities (piroxicam), and immunotherapies (anti-PD-1 antibody) [42] Although diverse adjuvant treatment options exist for improving the management of CMT postoperatively, only a limited number of minimally powered clinical trials in dogs have been conducted as an initial step to establish standard procedures for CMT-associated adjuvant therapies. For example, a group of patients with CMTs treated with a combination of 5-fluorouracil and cyclophosphamide after surgery showed a 2-year survival rate of 100% without developing distant metastases, while those with CMTs treated with surgical excision alone had a 2-year survival rate of 28.6% [43]. For canine inflammatory mammary carcinomas (Figure 1B), seven dogs were treated with piroxicam (an NSAID and a cyclooxygenase inhibitor) and were found to have a longer survival time (median, 185 days) compared with the three dogs treated with doxorubicin-based protocols (median, 7 days) [44]. With regard to the effect of piroxicam on human breast cancers, a few in vitro studies were conducted and showed the ability of piroxicam to suppress the progression of breast cancer [45,46]. Meanwhile, in vivo and other clinical investigations on the efficacy of piroxicam in human breast cancer have not been performed. For other chemotherapeutic agents including paclitaxel [47], gemcitabine [48], a combination of doxorubicin and docetaxel [49], and a combination of mitoxantrone and carboplatin [50], no significant improvement was observed in dogs with CMTs treated with these drugs (Table 2) [43,44,47,48,49,50,51].
Table 2. Drug efficacy in mammary tumors of humans and dogs.
| Drug name | Subtype of canine mammary tumor | Clinical trial results for dogs | Use in human breast cancer or clinical trial results | References |
|---|---|---|---|---|
| 5-fluoreouracial and cyclophosphamide | Tubular adenocarcinoma, papillary | Improvement of disease-free interval and the survival time | FDA approved | [43] |
| Adenocarcinoma, solid carcinoma, sarcoma | ||||
| Piroxicam | Inflammatory mammary carcinoma | Improvement in clinical condition and disease stability | NA | [44] |
| Paclitaxel | Metastatic mammary adenocarcinoma | Partial response but unacceptable toxicity | FDA approved | [47] |
| Gemcitabine | Aggressive mammary carcinoma | No statistically significant difference in time to local recurrence, time to distant metastases, and overall survival | FDA approved | [48] |
| Doxorubicin or docetaxel | Malignant mammary tumors with histologic II and III (vascular or lymphatic invasion, regional lymph node metastasis, or distant metastasis) | No statistically significant benefit in duration of the recurrence-free interval, time to metastasis or overall survival | FDA approved | [49] |
| Mitoxantrone and/or carboplatin | NA | No statistically significant improvement | A phase II study with a vinorelbine/mitoxantrone/carboplatin combination showed complete or partial responses in 56% of metastatic breast cancer patients (n = 50) | [50,51] |
NA = not available.
Unfortunately, there are limited data on the efficacy of available drugs for CMTs as pet owners are generally not motivated to enroll their dogs in such clinical trials, pay the high costs associated with a given chemotherapeutic agent, and/or endure the long duration of treatment. Hence, another method of obtaining the predictive drug efficacy information for CMTs is to use patient-derived xenograft (PDX) models [52]. We established a biorepository of canine PDX models by implanting tumor tissues in immunodeficient mice at the Tallwood Canine Cancer Research Initiative in the Jackson Laboratory (Table 3). Well-characterized canine tumors can be grown and hosted in immunodeficient mice for drug efficacy testing (Figure 3) [53]. Few studies have been conducted to examine CMT PDX models. However, one study used a spontaneous inflammatory mammary carcinoma PDX to study the effect of indole-3-carbinol, a natural phytochemical derived from cruciferous vegetables, and observed the suppression of tumor proliferation and increased apoptosis in mice bearing the inflammatory mammary carcinoma canine PDX [54].
Table 3. Established cPDX models through the Tallwood Canine Cancer Research Initiative at The Jackson Laboratory.
| Tumor type | No. of cPDX established |
|---|---|
| Apocrine gland adenocarcinoma | 2 |
| B cell lymphoma | 4 |
| Hemangiosarcoma | 1 |
| Liver tumor | 1 |
| Lung tumor | 2 |
| Mammary carcinoma | 1 |
| Mast cell tumor | 2 |
| Penile tumor | 1 |
| Soft tissue sarcoma | 2 |
| T cell lymphoma | 1 |
| Total | 17 |
cPDX = canine patient-derived xenograft.
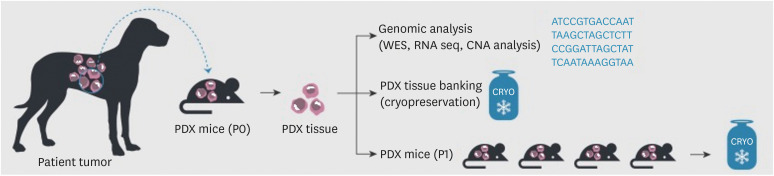
Figure 3. Establishing a biorepository of canine PDX models in immunodeficient mice. Surgical specimens from canine patients were divided into small pieces and transplanted into immunodeficient mice (P0 group). When tumors are grown in P0 mice, the xenografts are used for genomic analysis (e.g., WES, RNA seq, and CNA analysis) and then maintained in cryo-banks for preservation. After expanding and cryopreserving the tumor xenografts in immunodeficient mice (P1), they can be used for in vivo drug responsiveness screening.
PDX = patient-derived xenograft; WES = whole exome sequencing; RNA seq = RNA sequencing; CNA = copy number alteration.
GERMLINE AND SOMATIC MUTATION PROFILES OF CMT GENOME
Germline mutations (contained within the heritable genome) and somatic mutations (acquired de novo by cancer cells) contribute to the formation of tumors [55]. Consequently, genetic testing for germline and somatic mutations has become standard practice in the diagnosis of human cancer [56]. BRCA1 and/or BRCA2 mutations are well-known germline biomarkers that can increase the incidence of human breast and ovarian cancers [57]. Interestingly, a recent large-scale CMT cohort study (n = 183) found that among dogs with CMTs, 5.5% had cancer-predisposing BRCA1/2 germline variants [58], similar to that observed in human breast cancers (i.e., 5%–10%) [59]. Furthermore, the association between germline mutations in BRCA1/2 and an increased risk of CMTs was reported by Rivera et al. in 2009. They reported that germline mutations in both BRCA1 and BRCA2 genes increased the risk of CMTs by approximately four-fold [60]. Similarly, 72% of women with BRCA1 mutations and 69% of women with BRCA2 mutations are expected to develop breast cancer [35]. Table 4 contains information on the additional germline gene variants that predispose canines and, in some cases, humans to developing mammary tumors [58,61,62,63,64,65,66].
Table 4. Genes with germline or somatic mutations in canine and human mammary tumors.
| Genes with germline mutations | Genes with somatic mutations |
|---|---|
| B2M [61]* | AKT1† |
| BRCA1† | BRCA1 [62]† |
| BRCA2† | BRCA2 [62,63]† |
| CDK5RAP2 [64]* | CLHC1 [65]* |
| ESR1 [66]* | KRAS [58]† |
| NBN [58]† | MKI67 [58]* |
| NSMCE1 [58]* | NF1 [58]* |
| POLD1 [58]* | PIK3CA† |
| RECQL4 [58]* | PIK3R1† |
| RMI1 [58]* | PTEN† |
| RTEL1 [58]* | SCRN1 [65]* |
| SLX4 [58]† | SF3B1 [58]† |
| SMC5 [58]† | TP53† |
| TOP3B [58]* | |
| XRCC3 [58]† |
*Genes with germline or somatic mutations found exclusively in canine mammary tumors; †Genes with germline or somatic mutations found in both human and canine mammary tumors.
As mentioned above, breed predisposition to cancer is a powerful advantage of canine models in uncovering the underlying genomic influences in cancer etiopathogenesis. This allows investigation of heritable genetic contributors responsible for cancer initiation by analyzing germline mutations. Our laboratory has sequenced and analyzed the whole genome DNA from blood samples of 11 purebred dogs. Maltese, Shih-Tzu, and Beagle are dog breeds that are highly predisposed to developing CMTs. All three dog breeds shared 1,035,815 single nucleotide polymorphisms (SNPs) and 14,696 structural variants (SVs). When the same SNPs and SVs were assessed in other dog breeds (i.e., dog breeds [n = 8] that were not predisposed to CMTs), 200 SNPs and 3 SVs were identified, overlapping three protein-coding genes. Strikingly, these genes have not yet been reported as human breast cancer susceptibility genes, suggesting that these canine germline variants could be the underlying genomic drivers associated with breast cancer.
Among the known genes with somatic mutations in human breast cancer, TP53 is a well-known tumor suppressor gene associated with cancer initiation and progression. TP53 mutations are linked to several cancer types, including human breast cancer, and were initially linked with CMT in the 1990s. These studies observed mutations in the TP53 gene in patients with benign [67,68] and malignant tumors [69,70]. Furthermore, Wakui et al. [71] found that tumor recurrence and death risk increased in 17% of dogs with canine mammary carcinomas harboring TP53 somatic mutations.
Somatic mutations in genes within the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway are also significantly associated with the development of human mammary tumors. The most well-studied gene in this pathway is PIK3CA, which is a mutational hotspot for human breast cancer. Somatic mutations in this gene have also been observed in CMTs [58,65,72]. Mutations in the PTEN, PIK3R1, and AKT1 genes, all in the PI3K/Akt pathway, have also been found in CMTs [58]. Table 4 lists the other somatic variants observed in CMTs.
CONCLUSIONS AND FUTURE DIRECTIONS
Over the last two decades, naturally occurring CMTs have proven their value as alternative translational models for human breast cancer. CMT and human breast cancer share similar aspects, including high prevalence, molecular subtypes, and mutation profiles. Human and dog mammary tumors have similar histological traits and biomarkers. However, there are also some limitations of CMTs as a model for studying human breast cancer. As the information on the efficacy of therapeutics in dogs remains limited, additional clinical trials or preclinical studies using canine patient-derived xenograft models are required. In addition, the mutational burden in CMTs is substantially lower than in human breast cancers, indeed, the mutational composition of breast cancer development might be different across species [58,73]. Nevertheless, comparative genomic approaches have the potential to identify new genomic variants associated with mammary tumors and therefore should be encouraged. Increasing our knowledge of the genomic landscape of CMTs should make it an important complementary resource for human breast cancer research.
ACKNOWLEDGEMENTS
The authors would like to thank The Tallwood Canine Cancer Research Initiative at The Jackson Laboratory for providing support in establishing a biorepository for canine PDX models. We would also like to thank Ms. Jane Cha for creating the illustration in Figure 3, Ms. Leigh Maher for establishing the canine PDX models, and Dr. Feyza Yilmaz for performing the genomic analysis of canine models.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
- Supervision: Lee C.
- Writing - original draft: Kwon JY.
- Writing - review & editing: Kwon JY, Moskwa N, Kang W, Fan TM, Lee C.
References
- 1.Zeng L, Li W, Chen CS. Breast cancer animal models and applications. Zool Res. 2020;41:477–494. doi: 10.24272/j.issn.2095-8137.2020.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vail DM, MacEwen EG. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 2000;18:781–792. doi: 10.3109/07357900009012210. [DOI] [PubMed] [Google Scholar]
- 3.Gray M, Meehan J, Martínez-Pérez C, Kay C, Turnbull AK, Morrison LR, et al. Naturally-occurring canine mammary tumors as a translational model for human breast cancer. Front Oncol. 2020;10:617. doi: 10.3389/fonc.2020.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdelmegeed SM, Mohammed S. Canine mammary tumors as a model for human disease. Oncol Lett. 2018;15:8195–8205. doi: 10.3892/ol.2018.8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8:147–156. doi: 10.1038/nrc2273. [DOI] [PubMed] [Google Scholar]
- 6.Rowell JL, McCarthy DO, Alvarez CE. Dog models of naturally occurring cancer. Trends Mol Med. 2011;17:380–388. doi: 10.1016/j.molmed.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon I, Paoloni M, Mazcko C, Khanna C The Comparative Oncology Trials Consortium. The Comparative Oncology Trials Consortium: using spontaneously occurring cancers in dogs to inform the cancer drug development pathway. PLoS Med. 2009;6:e1000161. doi: 10.1371/journal.pmed.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeBlanc AK, Mazcko CN. Improving human cancer therapy through the evaluation of pet dogs. Nat Rev Cancer. 2020;20:727–742. doi: 10.1038/s41568-020-0297-3. [DOI] [PubMed] [Google Scholar]
- 9.Dobson JM. Breed-predispositions to cancer in pedigree dogs. ISRN Vet Sci. 2013;2013:941275. doi: 10.1155/2013/941275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moe L. Population-based incidence of mammary tumours in some dog breeds. J Reprod Fertil Suppl. 2001;57:439–443. [PubMed] [Google Scholar]
- 11.Salas Y, Márquez A, Diaz D, Romero L. Epidemiological study of mammary tumors in female dogs diagnosed during the period 2002–2012: a growing animal health problem. PLoS One. 2015;10:e0127381. doi: 10.1371/journal.pone.0127381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorenmo KU, Shofer FS, Goldschmidt MH. Effect of spaying and timing of spaying on survival of dogs with mammary carcinoma. J Vet Intern Med. 2000;14:266–270. doi: 10.1892/0891-6640(2000)014<0266:eosato>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Schneider R, Dorn CR, Taylor DO. Factors influencing canine mammary cancer development and postsurgical survival. J Natl Cancer Inst. 1969;43:1249–1261. [PubMed] [Google Scholar]
- 14.Komazawa S, Sakai H, Itoh Y, Kawabe M, Murakami M, Mori T, et al. Canine tumor development and crude incidence of tumors by breed based on domestic dogs in Gifu prefecture. J Vet Med Sci. 2016;78:1269–1275. doi: 10.1292/jvms.15-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart BL, Hart LA, Thigpen AP, Willits NH. Assisting decision-making on age of neutering for 35 breeds of dogs: associated joint disorders, cancers, and urinary incontinence. Front Vet Sci. 2020;7:388. doi: 10.3389/fvets.2020.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HW, Lim HY, Shin JI, Seung BJ, Ju JH, Sur JH. Breed- and age-related differences in canine mammary tumors. Can J Vet Res. 2016;80:146–155. [PMC free article] [PubMed] [Google Scholar]
- 17.Vascellari M, Capello K, Carminato A, Zanardello C, Baioni E, Mutinelli F. Incidence of mammary tumors in the canine population living in the Veneto region (Northeastern Italy): risk factors and similarities to human breast cancer. Prev Vet Med. 2016;126:183–189. doi: 10.1016/j.prevetmed.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Sinn HP, Kreipe H. A brief overview of the WHO classification of breast tumors, 4th edition, focusing on issues and updates from the 3rd edition. Breast Care (Basel) 2013;8:149–154. doi: 10.1159/000350774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beveridge WI, Sobin LH. International histological classification of tumours of domestic animals. Introduction. Bull World Health Organ. 1974;50:1–6. [PubMed] [Google Scholar]
- 20.Misdorp W, Else RW, Hellmén E, Lipscomb TP. 1999 [Google Scholar]
- 21.Goldschmidt M, Peña L, Rasotto R, Zappulli V. Classification and grading of canine mammary tumors. Vet Pathol. 2011;48:117–131. doi: 10.1177/0300985810393258. [DOI] [PubMed] [Google Scholar]
- 22.Im KS, Kim NH, Lim HY, Kim HW, Shin JI, Sur JH. Analysis of a new histological and molecular-based classification of canine mammary neoplasia. Vet Pathol. 2014;51:549–559. doi: 10.1177/0300985813498780. [DOI] [PubMed] [Google Scholar]
- 23.Tricarico D, Convertino AS, Mehmeti I, Ranieri G, Leonetti F, Laface C, et al. Inflammatory related reactions in humans and in canine breast cancers, a spontaneous animal model of disease. Front Pharmacol. 2022;13:752098. doi: 10.3389/fphar.2022.752098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Mansour MA, Kubba MA, Al-Azreg SA, Dribika SA. Comparative histopathology and immunohistochemistry of human and canine mammary tumors. Open Vet J. 2018;8:243–249. doi: 10.4314/ovj.v8i3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frkovic-Grazio S, Bracko M. Long term prognostic value of Nottingham histological grade and its components in early (pT1N0M0) breast carcinoma. J Clin Pathol. 2002;55:88–92. doi: 10.1136/jcp.55.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 27.van Dooijeweert C, van Diest PJ, Ellis IO. Grading of invasive breast carcinoma: the way forward. Virchows Arch. 2022;480:33–43. doi: 10.1007/s00428-021-03141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karayannopoulou M, Kaldrymidou E, Constantinidis TC, Dessiris A. Histological grading and prognosis in dogs with mammary carcinomas: application of a human grading method. J Comp Pathol. 2005;133:246–252. doi: 10.1016/j.jcpa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Ferreira E, Bertagnolli AC, Cavalcanti MF, Schmitt FC, Cassali GD. The relationship between tumour size and expression of prognostic markers in benign and malignant canine mammary tumours. Vet Comp Oncol. 2009;7:230–235. doi: 10.1111/j.1476-5829.2009.00193.x. [DOI] [PubMed] [Google Scholar]
- 30.Peña LL, Nieto AI, Pérez-Alenza D, Cuesta P, Castaño M. Immunohistochemical detection of Ki-67 and PCNA in canine mammary tumors: relationship to clinical and pathologic variables. J Vet Diagn Invest. 1998;10:237–246. doi: 10.1177/104063879801000303. [DOI] [PubMed] [Google Scholar]
- 31.Sarli G, Preziosi R, Benazzi C, Castellani G, Marcato PS. Prognostic value of histologic stage and proliferative activity in canine malignant mammary tumors. J Vet Diagn Invest. 2002;14:25–34. doi: 10.1177/104063870201400106. [DOI] [PubMed] [Google Scholar]
- 32.Yamagami T, Kobayashi T, Takahashi K, Sugiyama M. Prognosis for canine malignant mammary tumors based on TNM and histologic classification. J Vet Med Sci. 1996;58:1079–1083. doi: 10.1292/jvms.58.11_1079. [DOI] [PubMed] [Google Scholar]
- 33.Gundim LF, de Araújo CP, Blanca WT, Guimarães EC, Medeiros AA. Clinical staging in bitches with mammary tumors: Influence of type and histological grade. Can J Vet Res. 2016;80:318–322. [PMC free article] [PubMed] [Google Scholar]
- 34.Payne SJ, Bowen RL, Jones JL, Wells CA. Predictive markers in breast cancer--the present. Histopathology. 2008;52:82–90. doi: 10.1111/j.1365-2559.2007.02897.x. [DOI] [PubMed] [Google Scholar]
- 35.Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Primers. 2019;5:66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 36.Hennigs A, Riedel F, Gondos A, Sinn P, Schirmacher P, Marmé F, et al. Prognosis of breast cancer molecular subtypes in routine clinical care: a large prospective cohort study. BMC Cancer. 2016;16:734. doi: 10.1186/s12885-016-2766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inwald EC, Koller M, Klinkhammer-Schalke M, Zeman F, Hofstädter F, Gerstenhauer M, et al. 4-IHC classification of breast cancer subtypes in a large cohort of a clinical cancer registry: use in clinical routine for therapeutic decisions and its effect on survival. Breast Cancer Res Treat. 2015;153:647–658. doi: 10.1007/s10549-015-3572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gama A, Alves A, Schmitt F. Identification of molecular phenotypes in canine mammary carcinomas with clinical implications: application of the human classification. Virchows Arch. 2008;453:123–132. doi: 10.1007/s00428-008-0644-3. [DOI] [PubMed] [Google Scholar]
- 39.Abadie J, Nguyen F, Loussouarn D, Peña L, Gama A, Rieder N, et al. Canine invasive mammary carcinomas as models of human breast cancer. Part 2: immunophenotypes and prognostic significance. Breast Cancer Res Treat. 2018;167:459–468. doi: 10.1007/s10549-017-4542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varallo GR, Gelaleti GB, Maschio-Signorini LB, Moschetta MG, Lopes JR, De Nardi AB, et al. Prognostic phenotypic classification for canine mammary tumors. Oncol Lett. 2019;18:6545–6553. doi: 10.3892/ol.2019.11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorenmo K, Worley D, Goldschmidt M. Withrow & MacEwen's Small Animal Clinical Oncology. Elsevier; 2013. [Google Scholar]
- 42.Valdivia G, Alonso-Diez Á, Pérez-Alenza D, Peña L. From conventional to precision therapy in canine mammary cancer: a comprehensive review. Front Vet Sci. 2021;8:623800. doi: 10.3389/fvets.2021.623800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karayannopoulou M, Kaldrymidou E, Constantinidis TC, Dessiris A. Adjuvant post-operative chemotherapy in bitches with mammary cancer. J Vet Med A Physiol Pathol Clin Med. 2001;48:85–96. doi: 10.1046/j.1439-0442.2001.00336.x. [DOI] [PubMed] [Google Scholar]
- 44.de M Souza CH, Toledo-Piza E, Amorin R, Barboza A, Tobias KM de MSCH. Inflammatory mammary carcinoma in 12 dogs: clinical features, cyclooxygenase-2 expression, and response to piroxicam treatment. Can Vet J. 2009;50:506–510. [PMC free article] [PubMed] [Google Scholar]
- 45.Thabet NA, El-Guendy N, Mohamed MM, Shouman SA. Suppression of macrophages-induced inflammation via targeting RAS and PAR-4 signaling in breast cancer cell lines. Toxicol Appl Pharmacol. 2019;385:114773. doi: 10.1016/j.taap.2019.114773. [DOI] [PubMed] [Google Scholar]
- 46.Noguchi M, Earashi M, Minami M, Miyazaki I, Tanaka M, Sasaki T. Effects of piroxicam and esculetin on the MDA-MB-231 human breast cancer cell line. Prostaglandins Leukot Essent Fatty Acids. 1995;53:325–329. doi: 10.1016/0952-3278(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 47.Poirier VJ, Hershey AE, Burgess KE, Phillips B, Turek MM, Forrest LJ, et al. Efficacy and toxicity of paclitaxel (Taxol) for the treatment of canine malignant tumors. J Vet Intern Med. 2004;18:219–222. doi: 10.1892/0891-6640(2004)18<219:eatopt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 48.Marconato L, Lorenzo RM, Abramo F, Ratto A, Zini E. Adjuvant gemcitabine after surgical removal of aggressive malignant mammary tumours in dogs. Vet Comp Oncol. 2008;6:90–101. doi: 10.1111/j.1476-5829.2007.00143.x. [DOI] [PubMed] [Google Scholar]
- 49.Simon D, Schoenrock D, Baumgärtner W, Nolte I. Postoperative adjuvant treatment of invasive malignant mammary gland tumors in dogs with doxorubicin and docetaxel. J Vet Intern Med. 2006;20:1184–1190. doi: 10.1892/0891-6640(2006)20[1184:patimm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 50.Tran CM, Moore AS, Frimberger AE. Surgical treatment of mammary carcinomas in dogs with or without postoperative chemotherapy. Vet Comp Oncol. 2016;14:252–262. doi: 10.1111/vco.12092. [DOI] [PubMed] [Google Scholar]
- 51.Kakolyris S, Kourousis C, Koukourakis M, Androulakis N, Vamvakas L, Agelaki S, et al. First-line treatment of metastatic breast cancer with mitoxantrone, vinorelbine, and carboplatin. Am J Clin Oncol. 1999;22:568–572. doi: 10.1097/00000421-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 52.National Cancer Policy Forum; Board on Health Care Services, Institute of Medicine, National Academies of Sciences, Engineering, and Medicine. The Role of Clinical Studies for Pets with Naturally Occurring Tumors in Translational Cancer Research. Workshop Summary. Washington, D.C.: National Academies Press; 2015. [PubMed] [Google Scholar]
- 53.Na D, Moon HG. Patient-derived xenograft models in breast cancer research. Adv Exp Med Biol. 2021;1187:283–301. doi: 10.1007/978-981-32-9620-6_14. [DOI] [PubMed] [Google Scholar]
- 54.Martín-Ruiz A, Peña L, González-Gil A, Díez-Córdova LT, Cáceres S, Illera JC. Effects of indole-3-carbinol on steroid hormone profile and tumor progression in a mice model of canine inflammatory mammarycancer. BMC Cancer. 2018;18:626. doi: 10.1186/s12885-018-4518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramroop JR, Gerber MM, Toland AE. Germline variants impact somatic events during tumorigenesis. Trends Genet. 2019;35:515–526. doi: 10.1016/j.tig.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tischler J, Crew KD, Chung WK. Cases in precision medicine: the role of tumor and germline genetic testing in breast cancer management. Ann Intern Med. 2019;171:925–930. doi: 10.7326/M18-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toland AE, Forman A, Couch FJ, Culver JO, Eccles DM, Foulkes WD, et al. Clinical testing of BRCA1 and BRCA2: a worldwide snapshot of technological practices. NPJ Genom Med. 2018;3:7. doi: 10.1038/s41525-018-0046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim TM, Yang IS, Seung BJ, Lee S, Kim D, Ha YJ, et al. Cross-species oncogenic signatures of breast cancer in canine mammary tumors. Nat Commun. 2020;11:3616. doi: 10.1038/s41467-020-17458-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larsen MJ, Thomassen M, Gerdes AM, Kruse TA. Hereditary breast cancer: clinical, pathological and molecular characteristics. Breast Cancer (Auckl) 2014;8:145–155. doi: 10.4137/BCBCR.S18715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rivera P, Melin M, Biagi T, Fall T, Häggström J, Lindblad-Toh K, et al. Mammary tumor development in dogs is associated with BRCA1 and BRCA2. Cancer Res. 2009;69:8770–8774. doi: 10.1158/0008-5472.CAN-09-1725. [DOI] [PubMed] [Google Scholar]
- 61.Tanaka T, Shimada T, Akiyoshi H, Zheng C, Mie K, Yijyun L, et al. Germline polymorphism at the β2-microglobulin exon 1/intron 1 splice site in canine mammary gland simple and complex carcinomas. Vet Rec. 2013;172:529. doi: 10.1136/vr.101238. [DOI] [PubMed] [Google Scholar]
- 62.Di Giacomo D, Di Domenico M, Defourny SV, Malatesta D, Di Teodoro G, Martino M, et al. Validation of AmpliSeq NGS panel for BRCA1 and BRCA2 variant detection in canine formalin-fixed paraffin-embedded mammary tumors. Life (Basel) 2022;12:851. doi: 10.3390/life12060851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsu WL, Huang YH, Chang TJ, Wong ML, Chang SC. Single nucleotide variation in exon 11 of canine BRCA2 in healthy and cancerous mammary tissue. Vet J. 2010;184:351–356. doi: 10.1016/j.tvjl.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 64.Melin M, Rivera P, Arendt M, Elvers I, Murén E, Gustafson U, et al. Genome-wide analysis identifies germ-line risk factors associated with canine mammary tumours. PLoS Genet. 2016;12:e1006029. doi: 10.1371/journal.pgen.1006029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee KH, Hwang HJ, Noh HJ, Shin TJ, Cho JY. Somatic mutation of PIK3CA (H1047R) is a common driver mutation hotspot in canine mammary tumors as well as human breast cancers. Cancers (Basel) 2019;11:2006. doi: 10.3390/cancers11122006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Borge KS, Melin M, Rivera P, Thoresen SI, Webster MT, von Euler H, et al. The ESR1 gene is associated with risk for canine mammary tumours. BMC Vet Res. 2013;9:69. doi: 10.1186/1746-6148-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee CH, Kweon OK. Mutations of p53 tumor suppressor gene in spontaneous canine mammary tumors. J Vet Sci. 2002;3:321–325. [PubMed] [Google Scholar]
- 68.Muto T, Wakui S, Takahashi H, Maekawa S, Masaoka T, Ushigome S, et al. p53 gene mutations occurring in spontaneous benign and malignant mammary tumors of the dog. Vet Pathol. 2000;37:248–253. doi: 10.1354/vp.37-3-248. [DOI] [PubMed] [Google Scholar]
- 69.Mayr B, Reifinger M, Alton K. Novel canine tumour suppressor gene p53 mutations in cases of skin and mammary neoplasms. Vet Res Commun. 1999;23:285–291. doi: 10.1023/a:1006314903272. [DOI] [PubMed] [Google Scholar]
- 70.Van Leeuwen IS, Hellmèn E, Cornelisse CJ, Van den Burgh B, Rutteman GR. P53 mutations in mammary tumor cell lines and corresponding tumor tissues in the dog. Anticancer Res. 1996;16:3737–3744. [PubMed] [Google Scholar]
- 71.Wakui S, Muto T, Yokoo K, Yokoo R, Takahashi H, Masaoka T, et al. Prognostic status of p53 gene mutation in canine mammary carcinoma. Anticancer Res. 2001;21:611–616. [PubMed] [Google Scholar]
- 72.Alsaihati BA, Ho KL, Watson J, Feng Y, Wang T, Dobbin KK, et al. Canine tumor mutational burden is correlated with TP53 mutation across tumor types and breeds. Nat Commun. 2021;12:4670. doi: 10.1038/s41467-021-24836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Inglebert M, Dettwiler M, Hahn K, Letko A, Drogemuller C, Doench J, et al. A living biobank of canine mammary tumor organoids as a comparative model for human breast cancer. Sci Rep. 2022;12:18051. doi: 10.1038/s41598-022-21706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]