Abstract
Purpose
Breast cancer-related lymphedema (BCRL) is a chronic, progressive side effect of breast cancer treatment, occurring in one-third of patients treated with axillary lymph node dissection and nodal radiotherapy. Cluster of differentiation 4-positive (CD4+) cells plays a key role in BCRL by facilitating inflammation and inhibiting lymphangiogenesis. Tacrolimus is an anti-inflammatory and immunosuppressive macrolide that targets CD4+ cells. Treatment of lymphedema with topical tacrolimus has revealed promising results in preclinical trials. This clinical trial was aimed at evaluating the feasibility, safety, and effect of tacrolimus in women with stage I or II BCRL, according to the International Society of Lymphology.
Methods
We conducted this open-label, single-arm, phase II pilot trial from September 2020 to April 2021. Eighteen women with BCRL stage I or II BCRL were treated with topical tacrolimus for 6 months and followed up at 3 and 6 months. The primary outcome was arm volume, and secondary outcomes were the lymphedema index (L-Dex), health-related quality of life (HRQoL), lymph flow and function, and safety and feasibility of the trial design.
Results
The mean lymphedema arm volume and L-Dex reduced significantly by 130.44 ± 210.13 mL (p < 0.05; relative reduction: 3.6%) and 3.54 ± 4.98 (p < 0.05), respectively, and health-related quality of life scores was improved significantly (p < 0.05). According to the MD Anderson scale, in terms of lymph flow and function, three patients (16.7%) showed improvement, while none showed worsening. Lymph flow or function showed no change according to the Arm Dermal Backflow scale.
Conclusion
In this trial, treatment with tacrolimus was safe and feasible in women with stage I or II BCRL. Tacrolimus alleviated BCRL in terms of improved arm volume, L-Dex, and HRQoL. Assessments of lymph flow and function were positive, although inconclusive. Larger randomized controlled trials are required to verify these findings.
Trial Registration
ClinicalTrials.gov Identifier: NCT04541290
Keywords: Breast Neoplasms, Breast Cancer Lymphedema, Lymphedema, Quality of Life
INTRODUCTION
Breast cancer is highly prevalent worldwide, with an incidence of 2.3 million new cases in 2020 [1,2]. Breast cancer-related lymphedema (BCRL) is a side effect of breast cancer treatment that decreases the health-related quality of life (HRQoL) [3,4,5,6] and occurs in one-third of patients with breast cancer following axillary lymph node dissection (ALND) and nodal radiotherapy [3,5,7,8,9]. BCRL results from of an impaired function of the lymphatics. This leads to excessive retention of protein-rich lymphatic fluid in the interstitium, resulting in localized swelling in the axilla, arms and hands [10]. Excessive fluid is primarily seen in the early stages of BCRL and is potentially reversible. The formation of fibroadipose tissue due to chronic inflammation makes BCRL irreversible in later stages [10]. Symptoms include limb swelling, pain, a feeling of heaviness and numbness, skin changes, recurring infections (e.g., cellulitis, erysipelas), and emotional distress.
Conservative management of lymphedema with physiotherapy, skincare, and complete decongestive therapy is a cornerstone of BCRL therapy [11]. No definitive curative treatment for BCRL is currently available.
Cluster of differentiation 4-positive (CD4+) T-cells plays a key role in the development of lymphedema through various mechanisms, including the regulation and promotion of fibrosis through inflammation [12,13]. Animal showed a correlation between the infiltration of CD4+ cells on histopathology and the degree of fibrosis and severity of lymphedema [14,15]. CD4+ T-cells inhibit lymphangiogenesis by producing interferon-gamma (IFN-γ), interleukin (IL)-4, and transforming growth factor β-1which have anti lymphangiogenic effects [16,17].
Preclinical trials of topical therapy for lymphedema with growth factors and anti-inflammatory drugs have shown positive results. Tacrolimus is an immunosuppressive and anti-inflammatory macrolide that inhibits CD4+ T-cells [18,19]. It exerts anti-inflammatory action by inhibiting phosphatase activity of calcineurin, which is responsible for IL-2 transcription in T-cells. IL-2 in T-cells is essential for T-cell activation, proliferation, and differentiation, and its level and activity are reduced in tissues administered with topical tacrolimus [20]. A mouse model of tail lymphedema showed reduced soft tissue thickness, inflammatory infiltration, inflammatory cytokine expression, and degree of fibrosis after treatment with topical tacrolimus. Further, lymphangiogenesis increased, lymphatic function recovered [21]. The results of this preclinical trial of topical tacrolimus for lymphedema are promising; however, clinical studies are lacking [19,21]. The application of topical tacrolimus has demonstrated both short- and long-term safety and efficacy in atopic dermatitis [22]. The study was aimed at evaluating the feasibility, safety, and effect of tacrolimus in women with stage I or II BCRL according to the International Society of Lymphology (ISL). We hypothesized that treatment with topical tacrolimus would reduce the arm volume and symptoms of BCRL, resulting in an improved HRQoL.
METHODS
Trial design and registration
This prospective, open-label, single-arm, phase II pilot trial was conducted at the Department of Plastic Surgery at Odense University Hospital, Odense, Denmark, from September 2020 to April 2021. The patients were evaluated at baseline and after 3 and 6 months of treatment.
Data were stored in a REDCap database via the Open Patient data Explorative Network [23]. Participants received oral and written communication regarding the study procedures and signed informed consent forms before inclusion in the study. The trial was registered with the Danish Medicinal Agency (EudraCT No. 2020-000877-25), the Regional Committees on Health Research Ethics of Southern Denmark (S-20200032), and at www.ClinicalTrials.gov (NCT04541290), and completed according to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use-Good Clinical Practice guidelines, current legislation, and regulatory requirements.
Participants
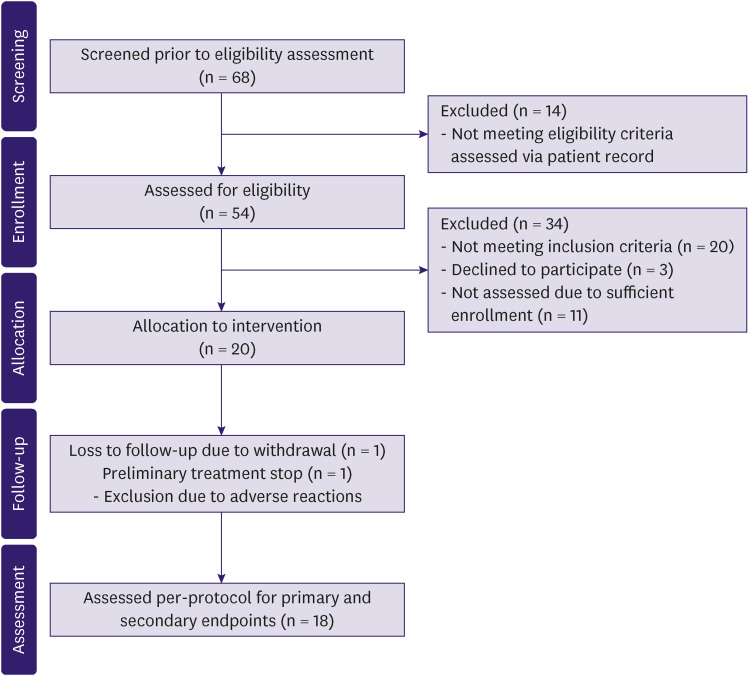
Patients were recruited from a waitlist for participating in experimental trials of lymphedema treatment. Inclusion criteria were: female sex; the age of 30–65 years; BCRL stage I or II according to ISL; ipsilateral ALND; no bilateral breast cancer; no allergy to tacrolimus or other macrolides; no diagnosed immunodeficiency; no psychiatric illness; and no reduced kidney or liver function. Further, at the outpatient clinic before enrollment, the following inclusion criteria were applied: significant pitting edema; postmenopausal status or current use of contraceptive drugs; good general condition; consent to participate; comprehension of Danish; no lymphedema in the opposite arm; not pregnant or breastfeeding or aiming to conceive within the next year; and no defect in the skin barrier (Figure 1).
Figure 1. Flow diagram of the progress through the phases of the study (i.e., screening, enrolment, intervention allocation, follow-up, and assessment) including exclusions and reasons for exclusion.
Intervention
The participants were administered 13 tubes of Protopic (hereinafter referred to as “tacrolimus”; 0.1% tacrolimus; Leo Pharma, Ballerup, Denmark) at baseline. They were instructed to apply the ointment in a thin layer covering the armpit, entire arm, and hand once daily for 6 months, similar to the current treatment regime for atopic dermatitis. The tacrolimus ointment dose (amount applied in grams) was calculated after weighing the tubes of tacrolimus before and after use, using the following formula:
| Amount of Ointment Used (Total) = Total Weight of Sealed Tubes Pre-intervention − Total Weight of Tubes Post-intervention (Sum of Sealed and Used Tubes). |
The participants were instructed not to discard the used tubes. They were informed of the side effects of tacrolimus according to the product resumé: mild skin irritation, erythema, warm sensation, pain, paresthesia, -rash at the application site, and alcohol intolerance (flushing or skin irritation). They were advised to stop alcohol consumption if any side effects occurred upon alcohol consumption.
Safety
All adverse effects reported by the patients were recorded in the REDCap database at each consultation to evaluate the safety of topical tacrolimus. Pain and infection at the application site were focused on. Adverse reactions to tacrolimus (e.g., mild rash, itching or burning sensations and flushing) were registered. The product resumé for Protopic (0.1% tacrolimus) was used as a reference for all reported adverse effects. The participants were able to report adverse events and reactions throughout the project. A senior consultant evaluated all adverse events. Adverse events assessed to contraindicate continuation led to exclusion from the study.
Baseline characteristics
Baseline characteristics included patient (age, height, weight, dominant side, relationship status, smoking status, alcohol consumption, lymphedema duration, and current lymphedema treatment) and treatment (type of surgery, mastectomy or lumpectomy, laterality of surgery, number of lymph nodes removed, and type of oncologic treatment) characteristics.
Water displacement volumetry (WDV)
The arm volume was measured with WDV, a precise and validated method [24] following the Archimedes’ principle, a physical law of buoyancy, using the Bravometer (Novuqare BV, PJ Horst, Nederland) and the technique described in Damstra et al. [24]. In brief, the patient lowered the arm in a basin filled with water, which caused an equal volume of water to displace into another basin. The amount of displaced water was measured in grams and converted to milliliters. Volumes of both arms were measured at baseline and at 3- and 6-month follow-ups. The edema volume was estimated by subtracting the volume of the healthy arm from that of the BCRL arm.
Bioimpedance spectroscopy (BIS)
BIS was performed as previously described [25,26] using SOZO® (ImpediMed, Brisbane, Australia) along with the manufacturer’s software to measure the impedance of each arm’s extracellular fluid. The device measured how the body impeded current flow through electrode pads placed on patients’ hands and feet. The measured outcome was the lymphedema index (L-Dex). L-Dex > 10 was defined as lymphedema. The following variables were measured using the device: extracellular fluid, intracellular fluid, total body water, fat mass, active tissue mass, extracellular mass, and skeletal muscle mass. The variables were reported as percentages (%) of total body water or body weight, respectively. BIS was measured at baseline and at 3- and 6-month follow-ups.
Patient reported outcome measures (PROMs)
PROMs are obtained using questionaries to measure the patient’s view of their health status. We used the following PROM questionnaires, validated and translated to Danish, to assess HRQoL: Lymphoedema Functioning, Disability and Health (LYMPH-ICF); Disabilities of the Arm, Shoulder, and Hand (DASH); and the 36-Item Short Form Health Survey (SF-36) [27,28,29].
The LYMPH-ICF is a disease-specific questionnaire for patients with lymphedema. The questionnaire contains five domains: physical function, mental function, household activities, mobility activities, and life and social activities. Twenty-nine questions across five domains are answered on a scale of 0 to 10.
DASH is developed for patients with disabilities of the arm, shoulder, or hand. It includes 38 questions about the patient’s ability to perform different movements. The questions are answered on a five-step scale from “not difficult” to “impossible”.
SF-36 is a generic quality-of-life questionnaire that includes eight domains on general health: physical functioning, body pain, role limitations due to physical health problems, role limitations due to personal or emotional problems, emotional well-being, social functioning, energy or fatigue, and general health perceptions. It includes 36 questions regarding the patient’s general quality of life. The questions are answered on three, five, and six-step scales and with binary (yes/no) answers depending on the question. All questionnaires were filled out in the clinic at baseline and the 3- and 6-month follow-ups.
Indocyanine green lymphography (ICG-L)
ICG emits near-infrared fluorescence and allows real-time imaging of lymphatic function and flow using a near-infrared camera. ICG-L was performed as described previously [30]. In brief, we injected 0.1 mL of ICG (2.5 mg/mL Verdye; Diagnostic Green, Ascheim, Germany) subcutaneously in the web space between the thumb and the index finger, between the middle and ring fingers, and at the ulnar border of the palmaris longus tendon at the wrist level. Fluorescent imaging of the lymph vessels and flow was performed using the HyperEye Medical System (MNIRC-501, HEMS; Mitzuho Co., Tokyo, Japan). ICG-L scans were obtained at 0, 10, and 60 minutes following the injection. Two senior consultants individually graded the ICG-L according to MD Anderson (MDA) and Arm Dermal Backflow (ADB) [30]. Discrepancies were resolved by consensus after reevaluation. ICG-L was performed at baseline and 6-month follow-up.
Multiple circumference measurements (MCM)
MCMs were obtained in both arms using a measuring tape. Arm volumes were calculated with the formula to calculate the volume of a truncated cone as described by Brorson and Höijer [31]. Arm circumferences were measured at baseline and at 3- and 6-month follow-ups.
Outcomes
The primary outcome was the change in arm volume measured with WDV. Secondary outcomes were changes in L-Dex, HRQoL, and lymph flow and function.
Statistical analysis
Statistical analyses were performed using STATA 16 (StataCorp 2021 Stata Statistical Software, Release 16; StataCorp LLC, College Station, TX, USA) with a two-tailed significance level of 0.05. Among baseline characteristics, continuous parametric variables are expressed as mean ± standard deviation, and categorical variables are expressed as frequency and percentage (%). Data were analyzed per protocol and tested for normality using skewness, kurtosis and visual assessment using Q–Q plot. The means of parametric variables were analyzed with the paired sample t-test. LYMPH-ICF and DASH raw scores were transformed into scores ranging from 0 (best) to 100 (worst), and SF-36 scores were transformed into scores ranging from 0 (worst) to 100 (best) using the original scoring keys.
Ethics approval and consent to participate
The study was conducted with approval by the Danish Medicines Agency (2020101617) and the Regional Committees on Health Research Ethics for Southern Denmark (S-20200032). Informed consent was obtained from study participants.
RESULTS
A total of 68 patients were screened for eligibility, and 20 patients were included in the trial (Figure 1). One patient withdrew from the trial citing personal reasons, and one patient was excluded because of the onset of adverse reactions contraindicating the treatment. Finally, 18 of 20 patients were treated per protocol for 6 months. Fourteen patients (77.8%) reported over 97% compliance after < 6 days without the treatment. Among the remaining four patients, the compliance rate was 78% in one patient and 92.8% in three patients. The mean amount of ointment used per application was 3.22 ± 1.00 (range: 2–5) g.
Table 1 lists the baseline characteristics. The mean age was 55.28 ± 4.60 years, and the mean body mass index was 27.24 ± 5.78 kg/m2. For breast cancer, 10 patients (55.6%) were treated with mastectomy, and eight patients (44.4%) were treated with lumpectomy. The mean number of lymph nodes removed with ALND was 19 ± 7.13. All patients (100.0%) had undergone both chemotherapy and radiotherapy. The mean difference between the BCRL and the healthy arms was 491.5 ± 227.55 mL. The mean time since the lymphedema diagnosis was 7.33 ± 5.13 years, and 16 patients (88.9%) had used an arm compression sleeve daily (Supplementary Figure 1). Ten patients (55.6%) reported a previous episode of skin infection. Table 2 lists the main findings of the trial.
Table 1. Baseline characteristics of the 18 patients treated per protocol.
| Variables | All patients (n = 18) | |
|---|---|---|
| Age | 55.28 ± 4.60 | |
| In relationship (yes) | 14 (77.8) | |
| Smoking (yes) | 1 (5.6) | |
| Alcohol consumption (units per week) | 2.78 ± 2.78 | |
| BMI (kg/m2) | 27.24 ± 5.78 | |
| Surgical treatment | ||
| Lumpectomy | 8 (44.4) | |
| Mastectomy | 10 (55.6) | |
| Number of lymph nodes resected | 19 ± 7.13 | |
| Oncologic treatment | ||
| Radiotherapy (yes) | 18 (100.0) | |
| Chemotherapy (yes) | 18 (100.0) | |
| Endocrine therapy (yes) | 13 (72.0) | |
| Biological therapy (yes) | 5 (27.8) | |
| Lymphedema volume (mL) | 512.22 ± 228.71 | |
| Lymphedema volume (%) | 19.65 ± 9.24 | |
| Lymphedema duration (yr) | 7.33 ± 5.13 | |
| Lymphedema in dominant side (yes) | 9 (50.0) | |
| Current lymphedema treatment | ||
| Compression arm sleeve (yes) | 16 (88.9) | |
| Compression gauntlet (yes) | 9 (50.0) | |
| Manual drainage (yes) | 7 (38.9) | |
| Previous physiotherapeutic bandaging (yes) | 14 (77.8) | |
| Pneumatic compression device (yes) | 2 (11.1) | |
| Night compression (yes) | 2 (11.1) | |
| Breast compression (yes) | 1 (5.6) | |
| Other (yes) | 3 (16.7) | |
| Previous episode of skin infections (yes) | 10 (55.6) | |
Values are presented as mean±standard deviation or number (%).
BMI = body mass index.
Table 2. Primary and secondary outcomes of the 18 patients treated per protocol.
| Outcome | Mean change ± SD | 95% CI | p-value | |
|---|---|---|---|---|
| BCRL arm volume (WDV) | ||||
| 3 months | −42.89 ± 124.52 mL | −104.81 to 19.03 | 0.16 | |
| 6 months | −130.44 ± 210.13 mL | −234.94 to −25.95 | < 0.05 | |
| BCRL arm volume (WDV) | ||||
| 3 months | −1.31% ± 3.53% | −0.45 to 3.06 | 0.13 | |
| 6 months | −3.65% ± 5.47% | −6.37 to −0.93 | < 0.05 | |
| Healthy arm volume (WDV) | ||||
| 3 months | −13.44 ± 85.28 mL | −55.85 to 28.96 | 0.51 | |
| 6 months | −60.44 ± 164.48 mL | −142.24 to 21.35 | 0.14 | |
| Edema volume | ||||
| 3 months | −29.44 ± 213.70 mL | −90.96 to 32.07 | 0.33 | |
| 6 months | −70 ± 151.82 mL | −145.5 to 5.50 | 0.067 | |
| BCRL arm volume (MCM) | ||||
| 3 months | −52.09 ± 97.84 mL | −100.75 to −3.43 | < 0.05 | |
| 6 months | −65.65 ± 111.45 mL | −121.07 to −10.23 | < 0.05 | |
| L-Dex | ||||
| 3 months | −3.23 ± 4.60 | −5.59 to −0.86 | < 0.01 | |
| 6 months | −3.54 ± 4.98 | −6.02 to −1.06 | < 0.01 | |
| LYMPH-ICF | ||||
| 3 months | −2.72 ± 10.32 | −7.85 to 2.41 | 0.28 | |
| 6 months | −7.73 ± 9.55 | −11.97 to −2.48 | < 0.01 | |
| DASH | ||||
| 3 months | −4.22 ± 5.53 | −6.97 to −1.48 | < 0.005 | |
| 6 months | −4.68 ± 6.61 | −7.96 to −1.39 | < 0.01 | |
| SF-36 | ||||
| 3 months | 1.96 ± 6.28 | −1.17 to 5.08 | 0.2 | |
| 6 months | 4.12 ± 6.97 | 0.65 to 7.58 | < 0.05 | |
SD = standard deviation; CI = confidence interval; BCRL = breast cancer-related lymphedema; WDV = water displacement volumetry; MCM = multiple circumference measurements; L-Dex = lymphedema index; LYMPH-ICF = Lymphoedema Functioning, Disability and Health; DASH = Disabilities of the Arm, Shoulder, and Hand; SF-36 = 36-Item Short Form Health Survey.
Arm volume
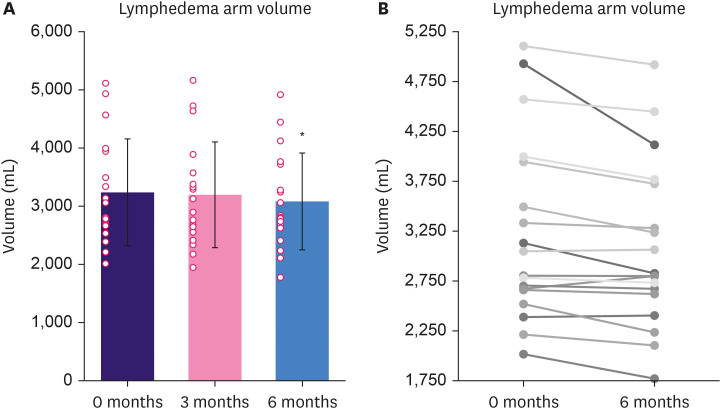
In WDV, the BCRL arm volume reduced by 42.89 ± 124.52 mL (p = 0.16) at 3 months and 130.44 ± 210.13 mL (p < 0.05) at 6 months (Figure 2), corresponding reductions in the total arm volume of 1.31% at 3 months and 3.6% at 6 months. The mean volume of the healthy arm was reduced by 60.44 ± 164.48 mL (p = 0.14).
Figure 2. Change in arm volume from baseline to 6-month follow-up. Results are expressed as mean ± standard deviation and as individual datapoints. (A) Change in the mean volume of the BCRL arm in percent (%) from baseline (100.0%). (B) Individual change in BCRL arm volumes from baseline to 6-month follow-up.
BCRL = breast cancer-related lymphedema.
*p < 0.05 compared to baseline.
The mean edema volume reduced by 29.44 ± 123.70 mL (p = 0.33) at 3 months and 70 ± 35.79 mL (p = 0.067) at 6 months, equivalent to a reduction of 11.7%. In circumference measurements, the BCRL arm volume decreased by 52.09 ± 97.84 mL (p < 0.05) at 3 months and 65.65 ± 26.27 mL (p < 0.05) at 6 months.
Secondary outcomes
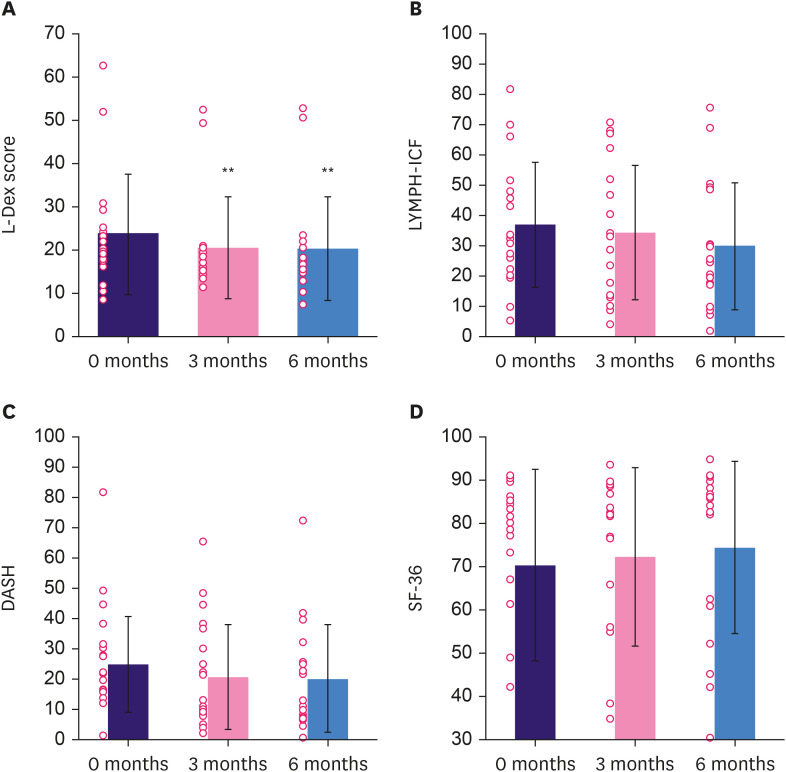
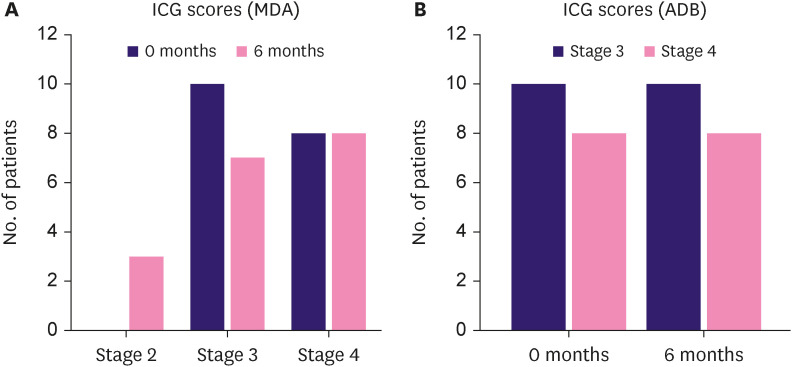
The mean L-Dex scores were reduced by 3.23 ± 4.60 (p < 0.05) at 3 months and by 3.54 ± 4.98 (p < 0.05) at 6 months (Figure 3). Extra-cellular fluid, intracellular fluid, total body water, fat mass, active tissue mass, extracellular mass, or skeletal muscle mass showed no changes (p > 0.05) (Supplementary Table 1). PROMs showed significantly improved HRQoL in the intervention group: mean LYMPH-ICF scores improved by 7.23 ± 9.55 (p < 0.05); mean DASH scores improved by 4.68 ± 1.56 (p < 0.05); and mean SF-36 scores improved by 4.12 ± 1.64 (p < 0.05; Figure 3). The physical and social function domains improved significantly in the LYMPH-ICF, and role limitations due to physical health problems and body pain improved significantly in the SF-36. Figure 4 and Supplementary Table 2 show the distribution of ICG-L scores. In lymph flow and function according to the MDA scale, three patients (16.7%) showed improvement, while none showed worsening. The lymph flow and function showed no changes according to the ADB scale.
Figure 3. Secondary outcomes. Results are expressed as mean ± standard deviation and as individual datapoints. (A) Change in mean L-Dex scores. (B) Change in mean of HRQoL assessed with the LYMPH-ICF questionnaire ranging from 0 (best) to 100 (worst). (C) Change in mean of HRQoL assessed with the DASH questionnaire ranging from 0 (best) to 100 (worst). (D) Change in mean of HRQoL assessed with the SF-36 questionnaire ranging from 100 (best) to 0 (worst).
L-Dex = lymphedema index; HRQoL = health-related quality of life; LYMPH-ICF = Lymphoedema Functioning, Disability and Heath; DASH = Disability of the Arm Shoulder and Hand; SF-36 = 36-Item Short Form Health Survey.
*p < 0.05 compared to baseline; **p < 0.001 compared to baseline.
Figure 4. This figure presents the distribution of MDA scores (A) and ADB (B) scores at baseline (0 months) and after 6 months of treatment.
MDA = MD Anderson; ADB = Arm Dermal Backflow; ICG = Indocyanine green.
Safety
Five patients (27.8%) experienced serious adverse events during the trial, all of which were skin infections. One patient experienced this infection for the first time. No serious adverse reactions occurred in this trial. However, other adverse reactions were registered occurred. Six patients (33.3%) experienced flushing related to alcohol intake. They were advised to not consume alcohol for the rest of the trial. One patient (5.6%) reported tingling, burning, and itching sensations in the fingers. One patient (5.6%) experienced general malaise, including dizziness, a tingling sensation in the arm, and pain in the arm. These symptoms occurred daily for 3–5 hours after the application of the ointment. The treatment was paused for 14 days, and whether the symptoms recurred when the treatment was restarted was assessed. The symptoms recurred on the same day as the ointment was reapplied, indicating a contraindication continuation of the treatment. Therefore, the patient was excluded from the trial after receiving treatment for 110 of 180.
DISCUSSION
This clinical trial of tacrolimus for BCRL showed significantly reduced BCRL arm volume and L-Dex and improved HRQoL, particularly concerning arm function. The volume reduction in the healthy arm of the patients was not significant. The visual ICG-L assessments were slightly discrepant between the two grading systems. According to the MDA scale, three patients (16.7%) showed improvement, while none showed worsening. According to the ADB scale, the lymph flow and function showed no change.
This was the first study to investigate the effect of topical tacrolimus on women with BCRL stage I or II according to ISL. This trial was strengthened by the high compliance of the patients. Reporting bias in this trial was minimized owing to the use of a complete dataset without missing data according to baseline, subjective, and objective measures.
This study has some limitations. First, it was an unblinded pilot trial with no control group. Second, the sample size was small. Third, no long-term follow-up was performed. Consequently, the findings should be interpreted with caution. Fourth, power estimation and sample size calculation were because of the shortage of literature on the treatment of BCRL with tacrolimus. We leaned toward the recommendations provided by Whitehead et al. [32] and enrolled 20 patients in this trial. The small cohort (n = 18) analyzed per protocol increased the risk of chance in our observations and limit the presentation of definitive conclusions. Fifth, the lack of included men in the trial weakened its generalizability. Men account for 1% of all cases of breast cancer, making it difficult to achieve a representative sample [33]. Future studies should be performed using a control group that includes both men and women.
Gardenier et al. [21] investigated the effect of tacrolimus on mice with induced tail lymphedema. The mice were treated with topical tacrolimus twice daily for 3 or 4 weeks and showed a decrease in lymphedema limb tail volume of 95%. Similarly, we found a smaller decrease in the volume of the lymphedema arm and a trend in a decrease in edema volume. The effect sizes differed between the studies because of the different test subjects (mice vs. humans). In general, the treatment effect is assumed to be overestimated by 30% in animal studies because of publication bias and differences in complexity and regulation between species [34,35]. The different treatment regimens (once vs. twice daily) might influence the magnitude of the effect in the two trials. The induced lymphedema resulted in an increase in limb volume of 100% in mice. In our patients, the lymphedema arms were 19.65% larger than the healthy arm at baseline. This difference is expected to reflect itself in the following volume reduction as well.
The literature has no comprehensive studies describing the rate of spontaneous remission of secondary lymphedema. However, lymphedema in principle is stable or worsens with time, and spontaneous remission in stages II or III is rare [36]. Assuming tacrolimus has the hypothesized effect on lymphedema (i.e., decreased fibrosis, enhanced lymphangiogenesis and better lymph flow and function, resulting in decreased arm volume and excess water), other treatments, such as compression sleeves, may contribute to the improvement or maintenance of this effect. Compression treatment is essential for maintaining an acceptable result in liposuction [37]. The lack of a control group in this trial weakens the hypothesis that the changes in this study were due to tacrolimus. Spontaneous remission is a possibility, primarily in patients with stage I lymphedema.
HRQoL scores were potentially biased because of the lack of blinding. This bias was minimized by blinding the patients to the results of the objective assessments. Nevertheless, the improvement in HRQoL may be due more to the diligent and frequent follow-up and attention given to patients and their diseases, than to the treatment itself. The change in HRQoL correlates well with the rest of the results in this trial, and the specific domains of improvement support the indication of physical improvement of BCRL symptoms.
The daily application of the ointment potentially mimics manual lymphatic drainage (MLD), a type of massage therapy performed by lymphedema therapists [38]. The effect could potentially be confounded by MLD. However, current evidence suggests that MLD alone does not reduce the volume of BCRL [3,39], thereby making the risk of confounding minimal.
The ICG-L assessments in this trial made according to MDA and ADB were slightly discrepant. An obvious explanation for this could be the different approaches in the grading of lymphedema (anatomical localization vs. lymphatic vessels and contractility). We previously validated the ICG-L in 237 patients using the MDA and ADB scales and found a similar discrepancy [30]. Currently, no consensus exists on the gold-standard visual assessment of ICG-L, and a definitive conclusion cannot be made based on these findings [40].
The rationale behind the inclusion of patients in ISL stages I and II only was the expectation that tacrolimus would affect excessive fluid and fibrosis in lymphedema due to CD4+ T-cell inhibition rather than adipose tissue deposition. Adipose hypertrophy is responsible for a significant amount of volume increase in BCRL, particularly in the later stages, and we do not expect adipocytes to respond to tacrolimus [41].
Five patients (27.8%) were treated for skin infections during this trial. Four of them had a history of skin infections. This is consistent with what Moffatt et al. [42] found in a study investigating 228 patients with lymphedema. No association between topical tacrolimus and skin infections has been described in the literature. Therefore, we do not expect the treatment to have caused the infections in this trial. The remaining adverse events registered in this trial correlated well with the product resumé for Protopic and were evaluated as mild, implying that tacrolimus is safe and generally well tolerated in women with BCRL stage I or II according to ISL.
This clinical trial investigating the effect of tacrolimus on BCRL stages I or II according to ISL in women proved tacrolimus to be feasible and safe. The findings suggest that topical tacrolimus may alleviate BCRL stage I or II according to ISL in women; however, other contributing factors cannot be ruled out. Blinded randomized, placebo-controlled trials should be performed to confirm the findings of this trial before evaluating the role of tacrolimus in treating BCRL.
ACKNOWLEDGMENTS
We thank Open Patient data Explorative Network (OPEN), Odense University Hospital for statistical assistance and data storage.
Footnotes
Funding: This trial was funded by Danish Cancer Society (R288-A16159), Dagmar Marshalls Fond (1022-2993-01), Fonden af 1870, Guldsmed A L Rasmussens mindefond and Grosserer A. V. Lykfeldts og Hustrus legat.
Presentation: The manuscript has previously been submitted as a brief abstract at the International Lymphedema Framework Conference 2021 in Copenhagen.
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Gulmark Hansen FC, Jørgensen MG, Sørensen JA.
- Formal analysis: Gulmark Hansen FC, Jørgensen MG, Sørensen JA.
- Funding acquisition: Gulmark Hansen FC.
- Investigation: Gulmark Hansen FC.
- Methodology: Gulmark Hansen FC, Sørensen JA.
- Supervision: Jørgensen MG, Sørensen JA.
- Validation: Jørgensen MG.
- Writing - original draft: Gulmark Hansen FC, Jørgensen MG, Sørensen JA.
- Writing - review & editing: Gulmark Hansen FC, Jørgensen MG, Sørensen JA.
SUPPLEMENTARY MATERIALS
Body composition variables measured with BIS
Distribution of ICG-L scores according to the MDA and the ADB scores
Overview of the patients’ lymphedema treatment other than tacrolimus before and during the trial
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Breast cancer. 2021. [Accessed November 1, 2022]. https://www.who.int/news-room/fact-sheets/detail/breast-cancer .
- 3.Armer JM, Ballman KV, McCall L, Armer NC, Sun Y, Udmuangpia T, et al. Lymphedema symptoms and limb measurement changes in breast cancer survivors treated with neoadjuvant chemotherapy and axillary dissection: results of American College of Surgeons Oncology Group (ACOSOG) Z1071 (Alliance) substudy. Support Care Cancer. 2019;27:495–503. doi: 10.1007/s00520-018-4334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teo I, Novy DM, Chang DW, Cox MG, Fingeret MC. Examining pain, body image, and depressive symptoms in patients with lymphedema secondary to breast cancer. Psychooncology. 2015;24:1377–1383. doi: 10.1002/pon.3745. [DOI] [PubMed] [Google Scholar]
- 5.Hayes SC, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema after breast cancer: incidence, risk factors, and effect on upper body function. J Clin Oncol. 2008;26:3536–3542. doi: 10.1200/JCO.2007.14.4899. [DOI] [PubMed] [Google Scholar]
- 6.Jørgensen MG, Toyserkani NM, Hansen FG, Bygum A, Sørensen JA. The impact of lymphedema on health-related quality of life up to 10 years after breast cancer treatment. NPJ Breast Cancer. 2021;7:70. doi: 10.1038/s41523-021-00276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribeiro Pereira AC, Koifman RJ, Bergmann A. Incidence and risk factors of lymphedema after breast cancer treatment: 10 years of follow-up. Breast. 2017;36:67–73. doi: 10.1016/j.breast.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 8.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:500–515. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 9.Erickson VS, Pearson ML, Ganz PA, Adams J, Kahn KL. Arm edema in breast cancer patients. J Natl Cancer Inst. 2001;93:96–111. doi: 10.1093/jnci/93.2.96. [DOI] [PubMed] [Google Scholar]
- 10.Lawenda BD, Mondry TE, Johnstone PA. Lymphedema: a primer on the identification and management of a chronic condition in oncologic treatment. CA Cancer J Clin. 2009;59:8–24. doi: 10.3322/caac.20001. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin SA, DeSnyder SM, Klimberg S, Alatriste M, Boccardo F, Smith ML, et al. Considerations for clinicians in the diagnosis, prevention, and treatment of breast cancer-related lymphedema, recommendations from an expert panel: Part 2: Preventive and therapeutic options. Ann Surg Oncol. 2017;24:2827–2835. doi: 10.1245/s10434-017-5964-6. [DOI] [PubMed] [Google Scholar]
- 12.Ogata F, Fujiu K, Matsumoto S, Nakayama Y, Shibata M, Oike Y, et al. Excess lymphangiogenesis cooperatively induced by macrophages and CD4+ T cells drives the pathogenesis of lymphedema. J Invest Dermatol. 2016;136:706–714. doi: 10.1016/j.jid.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Ghanta S, Cuzzone DA, Torrisi JS, Albano NJ, Joseph WJ, Savetsky IL, et al. Regulation of inflammation and fibrosis by macrophages in lymphedema. Am J Physiol Heart Circ Physiol. 2015;308:H1065–H1077. doi: 10.1152/ajpheart.00598.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avraham T, Zampell JC, Yan A, Elhadad S, Weitman ES, Rockson SG, et al. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. 2013;27:1114–1126. doi: 10.1096/fj.12-222695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zampell JC, Yan A, Elhadad S, Avraham T, Weitman E, Mehrara BJ. CD4+ cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS One. 2012;7:e49940. doi: 10.1371/journal.pone.0049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin K, Kataru RP, Park HJ, Kwon BI, Kim TW, Hong YK, et al. TH2 cells and their cytokines regulate formation and function of lymphatic vessels. Nat Commun. 2015;6:6196. doi: 10.1038/ncomms7196. [DOI] [PubMed] [Google Scholar]
- 17.Shao X, Liu C. Influence of IFN-alpha and IFN-gamma on lymphangiogenesis. J Interferon Cytokine Res. 2006;26:568–574. doi: 10.1089/jir.2006.26.568. [DOI] [PubMed] [Google Scholar]
- 18.Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 19.Forte AJ, Boczar D, Huayllani MT, McLaughlin SA, Bagaria S. Topical approach to delivering targeted therapies in lymphedema treatment: a systematic review. Cureus. 2019;11:e6269. doi: 10.7759/cureus.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuda K, Yamanaka K, Kitagawa H, Akeda T, Naka M, Niwa K, et al. Calcineurin inhibitors suppress cytokine production from memory T cells and differentiation of naïve T cells into cytokine-producing mature T cells. PLoS One. 2012;7:e31465. doi: 10.1371/journal.pone.0031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardenier JC, Kataru RP, Hespe GE, Savetsky IL, Torrisi JS, Nores GD, et al. Topical tacrolimus for the treatment of secondary lymphedema. Nat Commun. 2017;8:14345. doi: 10.1038/ncomms14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cury Martins J, Martins C, Aoki V, Gois AF, Ishii HA, da Silva EM. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst Rev. 2015;2015:CD009864. doi: 10.1002/14651858.CD009864.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey LA. REDCap: web-based software for all types of data storage and collection. Spinal Cord. 2018;56:625. doi: 10.1038/s41393-018-0169-9. [DOI] [PubMed] [Google Scholar]
- 24.Damstra RJ, Glazenburg EJ, Hop WC. Validation of the inverse water volumetry method: a new gold standard for arm volume measurements. Breast Cancer Res Treat. 2006;99:267–273. doi: 10.1007/s10549-006-9213-0. [DOI] [PubMed] [Google Scholar]
- 25.Cornish BH, Chapman M, Hirst C, Mirolo B, Bunce IH, Ward LC, et al. Early diagnosis of lymphedema using multiple frequency bioimpedance. Lymphology. 2001;34:2–11. [PubMed] [Google Scholar]
- 26.Cornish BH, Ward LC, Thomas BJ, Bunce IH. Quantification of lymphoedema using multi-frequency bioimpedance. Appl Radiat Isot. 1998;49:651–652. doi: 10.1016/s0969-8043(97)00266-2. [DOI] [PubMed] [Google Scholar]
- 27.Grarup KR, Devoogdt N, Strand LI. The Danish version of Lymphoedema Functioning, Disability and Health questionnaire (LYMPH-ICF) for breast cancer survivors: Translation and cultural adaptation followed by validity and reliability testing. Physiother Theory Pract. 2019;35:327–340. doi: 10.1080/09593985.2018.1443186. [DOI] [PubMed] [Google Scholar]
- 28.Park JE, Jang HJ, Seo KS. Quality of life, upper extremity function and the effect of lymphedema treatment in breast cancer related lymphedema patients. Ann Rehabil Med. 2012;36:240–247. doi: 10.5535/arm.2012.36.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treanor C, Donnelly M. A methodological review of the Short Form Health Survey 36 (SF-36) and its derivatives among breast cancer survivors. Qual Life Res. 2015;24:339–362. doi: 10.1007/s11136-014-0785-6. [DOI] [PubMed] [Google Scholar]
- 30.Jørgensen MG, Toyserkani NM, Hansen FCG, Thomsen JB, Sørensen JA. Prospective validation of indocyanine green lymphangiography staging of breast cancer-related lymphedema. Cancers (Basel) 2021;13:1540. doi: 10.3390/cancers13071540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brorson H, Höijer P. Standardised measurements used to order compression garments can be used to calculate arm volumes to evaluate lymphoedema treatment. J Plast Surg Hand Surg. 2012;46:410–415. doi: 10.3109/2000656X.2012.714785. [DOI] [PubMed] [Google Scholar]
- 32.Whitehead AL, Julious SA, Cooper CL, Campbell MJ. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res. 2016;25:1057–1073. doi: 10.1177/0962280215588241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Cancer Society. Breast Cancer Facts & Figures 2022-2024. Atlanta: American Cancer Society; 2022. [Google Scholar]
- 34.Brubaker DK, Lauffenburger DA. Translating preclinical models to humans. Science. 2020;367:742–743. doi: 10.1126/science.aay8086. [DOI] [PubMed] [Google Scholar]
- 35.Sena ES, van der Worp HB, Bath PM, Howells DW, Macleod MR. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 2010;8:e1000344. doi: 10.1371/journal.pbio.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ochalek K, Gradalski T, Szygula Z. Five-year assessment of maintenance combined physical therapy in postmastectomy lymphedema. Lymphat Res Biol. 2015;13:54–58. doi: 10.1089/lrb.2014.0027. [DOI] [PubMed] [Google Scholar]
- 37.Schaverien MV, Munnoch DA, Brorson H. Liposuction treatment of lymphedema. Semin Plast Surg. 2018;32:42–47. doi: 10.1055/s-0038-1635116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ezzo J, Manheimer E, McNeely ML, Howell DM, Weiss R, Johansson KI, et al. Manual lymphatic drainage for lymphedema following breast cancer treatment. Cochrane Database Syst Rev. 2015;(5):CD003475. doi: 10.1002/14651858.CD003475.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang TW, Tseng SH, Lin CC, Bai CH, Chen CS, Hung CS, et al. Effects of manual lymphatic drainage on breast cancer-related lymphedema: a systematic review and meta-analysis of randomized controlled trials. World J Surg Oncol. 2013;11:15. doi: 10.1186/1477-7819-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aso K, Tsukuura R. Universal ICG lymphography stage for reproducible severity evaluation of extremity lymphedema. J Plast Reconstr Aesthet Surg. 2021;74:1633–1701. doi: 10.1016/j.bjps.2020.12.106. [DOI] [PubMed] [Google Scholar]
- 41.Brorson H, Ohlin K, Olsson G, Nilsson M. Adipose tissue dominates chronic arm lymphedema following breast cancer: an analysis using volume rendered CT images. Lymphat Res Biol. 2006;4:199–210. doi: 10.1089/lrb.2006.4404. [DOI] [PubMed] [Google Scholar]
- 42.Moffatt CJ, Franks PJ, Doherty DC, Williams AF, Badger C, Jeffs E, et al. Lymphoedema: an underestimated health problem. QJM. 2003;96:731–738. doi: 10.1093/qjmed/hcg126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Body composition variables measured with BIS
Distribution of ICG-L scores according to the MDA and the ADB scores
Overview of the patients’ lymphedema treatment other than tacrolimus before and during the trial