Key Points
-
•
HSCBs for persistent neutropenia showed recovery or sustained neutrophil improvement in 84% of patients.
-
•
HSCBs given early and even for moderate neutropenia but high risk for infection showed excellent outcome.
Abstract
Hematotoxicity after chimeric antigen receptor (CAR) T-cell therapy is associated with infection and death but management remains unclear. We report results of 31 patients receiving hematopoietic stem cell boost (HSCB; 30 autologous, 1 allogeneic) for either sustained severe neutropenia of grade 4 (<0.5 × 109/L), sustained moderate neutropenia (≤1.5 × 109/L) and high risk of infection, or neutrophil count ≤2.0 × 109/L and active infection. Median time from CAR T-cell therapy to HSCB was 43 days and median absolute neutrophil count at time of HSCB was 0.2. Median duration of neutropenia before HSCB was 38 days (range, 7-151). Overall neutrophil response rate (recovery or improvement) was observed in 26 patients (84%) within a median of 9 days (95% confidence interval, 7-14). Time to response was significantly associated with the duration of prior neutropenia (P = .007). All nonresponders died within the first year after HSCB. One-year overall survival for all patients was 59% and significantly different for neutropenia (≤38 days; 85%) vs neutropenia >38 days before HSCB (44%; P = .029). In conclusion, early or prophylactic HSCB showed quick response and improved outcomes for sustained moderate to severe neutropenia after CAR-T.
Introduction
Although the emergence of chimeric antigen receptor (CAR) T-cell therapy has improved response rates and overall outcomes significantly in patients with relapsed or refractory B-cell malignancies, it is associated with a unique toxicity profile.1 Besides cytokine release syndrome (CRS) and neurotoxicity (ICANS), hematotoxicity involving all 3 cell lineages is associated with infection and mortality, representing a major challenge in the clinical setting.2, 3, 4 Although recent improvements may help to stratify patients according to risk for severe hematotoxicity,5 its precise mechanisms and the management of persistent neutropenia remain unclear, with current focus on supportive care measures. A few cases describing the use of cryopreserved autologous hematopoietic stem cells as a boost after CAR-T have been published, signaling potential.6,7 However, no sufficient evidence exists regarding the general role, response, and timing of hematopoietic stem cell boosts (HSCBs). We report the first large series of patients receiving HSCBs for persistent neutropenia after CAR-T.
Methods
This is a multicenter cohort study on behalf of the German Lymphoma Alliance and the German National Registry for Stem Cell Transplants. We included patients from 8 centers who received HSCBs (autologous or allogeneic) after CAR-T. Patients included had received HSCBs between 2018 and January 2022 for the following indications: first, sustained severe neutropenia (<0.5 neutrophils × 109/L); second, sustained moderate neutropenia (≤1.5 neutrophils × 109/L) and high risk of infection; or third, active infection and neutrophil count ≤2 × 109/L.5,8 In case of a biphasic course after CAR T-cell therapy, duration of neutropenia was calculated from the time neutrophils dipped the second time.5 The University Medical Center Hamburg-Eppendorf institutional review board approved the study, which was conducted in accordance with the Declaration of Helsinki.
Blood samples were collected longitudinally. The temporal analysis included all patients until censoring (for relapse or initiation of cytotoxic treatment including allogeneic transplant) or until day 250. The main objective was to investigate the incidence of and the time until neutrophil response, defined as either recovery with absolute count >0.5 × 109/L for 3 consecutive test days (irrespective of growth factor [granulocyte colony-stimulating factor; GCSF] administration) for patients receiving HSCB for severe neutropenia, or sustained improvement >1.5 × 109/L for patients receiving the HSCB for moderate neutropenia (despite GCSF administration) or infection.8
Cumulative incidence of response was estimated using the Kalbfleisch and Prentice method.9 Overall survival (OS) and progression-free survival were estimated using Kaplan-Meier estimators. Associations between continuous variables were analyzed using the Spearman correlation coefficient (r). Analysis was performed with R (version 4.0.5).
Results and Discussion
We included 31 patients who received axicabtagene ciloleucel (n = 20), tisagenlecleucel (n = 7), allogeneic CAR-T cells (n = 3; NCT04035434), or brexucabtagene autoleucel (n = 1). The conditioning scheme before CAR-T consisted of fludarabine and cyclophosphamide. Diffuse large B-cell lymphoma was the most frequent indication for CAR-T (n = 25, 81%). Median age at time of CAR-T infusion was 62 years. Twenty-four patients (77%) received bridging therapy (Table 1; supplemental Material).
Table 1.
Patient characteristics
| Characteristic | Total cohort (n = 31) |
|---|---|
| Age, median (range), y | 61 (23-79) |
| Female, n (%) | 11 (36) |
| Diagnosis, n (%) | |
| DLBCL | 25 (81) |
| PMBCL | 2 (7) |
| PCNS lymphoma | 1 (3) |
| Burkitt | 1 (3) |
| cBALL | 1 (3) |
| MCL | 1 (3) |
| CAR-T cell product, n (%) | |
| Axicabtagene ciloleucel | 20 (64) |
| Tisagenlecleucel | 7 (23) |
| Allogeneic | 3 (10) |
| Brexucabtagene autoleucel | 1 (3) |
| Bridging therapy, n (%) | |
| No | 7 (23) |
| Yes | 24 (77) |
| CRS grade, n (%) | |
| 0 | 1 (3) |
| 1-2 | 25 (81) |
| 3-4 | 5 (16) |
| Neurotoxicity grade, n (%) | |
| 0 | 10 (32) |
| 1-2 | 13 (42) |
| 3-4 | 8 (26) |
| Time to boost in months, median (range) | 1.45 (0.2-14.6) |
| CD34+ progenitors × 106/kg BW, median (range) | 3.6 (1.1-11.5) |
| ANC at time of lymphodepletion × 109/L, median (range) | 2.9 (1.8-9.5) |
| ANC at time of boost × 109/L, median (range) | 0.2 (0-2.0) |
| Platelets at time of boost × 109/L, median (range) | 12 (0-30) |
| Hemoglobin at time of boost × g/dL, median (range) | 9.0 (6.1-11.8) |
| Transfusion dependence at time of boost, n (%) | 12 (39) |
Abbreviations: ANC, absolute neutrophile count; BW, body weight; CAR-T, chimeric antigen receptor T-cell therapy; cBALL, common B acute lymphoblastic leukemia; CRS, cytokine release syndrome; DLBCL, diffuse large B-cell lymphoma; MCL, mantle cell lymphoma; PCNS, primary central nervous system; PMBCL, primary mediastinal B-cell lymphoma.
HSCBs were given within a median of 43 days (range, 7-442) for sustained severe neutropenia (n = 23), sustained moderate neutropenia (n = 3), or infection (n = 5). The median absolute neutrophil count at the time of HSCB was 0.2 × 109/L (range, 0-2.0) and the median duration of neutropenia was 38 days (range, 7-151). Absolute neutrophil count significantly correlated with severity of thrombocytopenia at time of HSCB (P = .03). For 5 patients with severe neutropenia of grade 4, bone marrow biopsy information before HSCB (obtained between days 26 and 52) was available. Aplasia or severe hypocellularity was reported in all biopsies, affecting all cell lineages, which is in line with a previous single-center experience.10
Time from CAR-T infusion until HSCB and duration of neutropenia were significantly correlated (r = 0.843, P < .001). Most HSCBs were cryopreserved autologous products (n = 30) harvested either for intended autologous transplant before the indication for CAR-T was made (n = 29) or prophylactically (n = 1) for potential boost. Median time of cryopreservation was 17.9 months (95% confidence interval [CI], 13.7-31.8). One patient received allogeneic CD34-selected stem cells from a matched related donor after having received an allogeneic transplant one year before CAR-T. The median number of CD34+ progenitors was 3.6 × 106/kg body weight (range, 1.1-11.5). Before HSCB, patients received a median of 10 consecutive applications of GCSF (range, 0-51) within a median of 13 days (range, 1-55) after CAR-T cell infusion.
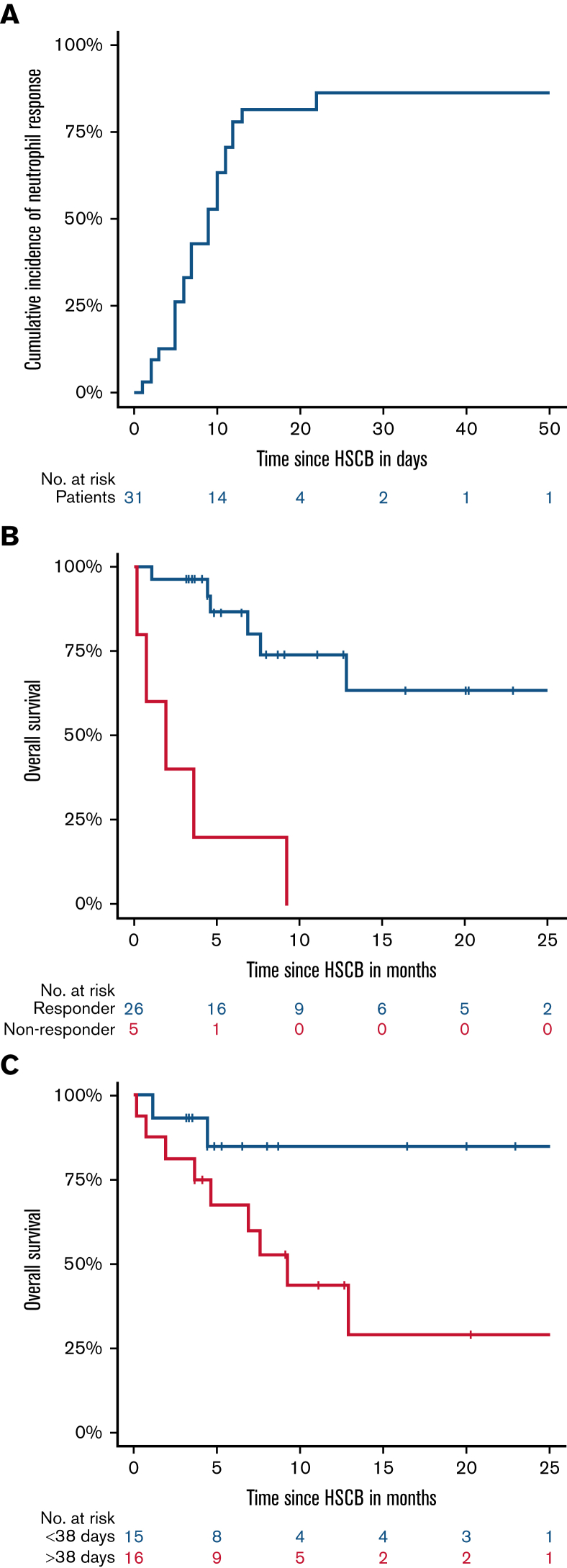
A response was observed in 26 patients (84%) and was associated with the indication (P < .001). Twenty-two of 23 patients receiving HSCB for sustained severe neutropenia and 3/3 patients for moderate neutropenia showed a response, whereas 4/5 patients who received HSCBs for active infection and neutrophil count ≤2 × 109/L showed no response (P < .001). The median time to response after the HSCB was 9 days (95% CI, 7-14) in all patients (Figure 1A). The length of time between HSCB and response was significantly associated with the duration of prior neutropenia (r = 0.475; P = .007) and the length of time between neutropenia and HSCB (r = 0.488, P = .005). A shorter duration of neutropenia (≤38 days) before HSCB was associated with an earlier response (median 7 vs 11 days; P = .029); and early application of HSCB (≤43 days after CAR-T infusion) was associated with significantly earlier (median, 6 vs 11 day; P = .022) and more stable responses (supplemental Material). No correlation was observed with prior GCSF application (P = .672).
Figure 1.
(A) Cumulative incidence of response (recovery or improvement), overall survival according to response (B), and duration of neutropenia before hematopoietic stem cell boost (C). A response was observed in 26 patients (84%), and the median time to response after the boost was 9 days (95% CI, 7-14) in all patients. All nonresponders died within the first year after HSCB, showing 1-year OS of 0% vs a 1-year OS of 74% (95% CI, 54-94; P < .001) for responders. The 1-year OS for neutropenia ≤38 days was 85% (95% CI, 65-100) vs 44% (95% CI, 27-61; P = .029) for neutropenia >38 days before HSCB.
Median time from HSCB to follow-up was 9.1 months (95% CI, 4.9-13.1). In total, 12 (39%) patients died. The 1-year OS after HSCB was 59% (95% CI, 38-80) and the 1-year progression-free survival was 52% (95% CI, 33-71). Relapse was the cause of death in 8 patients, 3 of whom died after salvage allogeneic transplant. Four patients died from sepsis after having received an HSCB for active infection, 2 of whom had moderate to severe neutropenia lasting 80 and 148 days before the HSCB. All 3 patients who received the HSCB for moderate neutropenia to prevent infection were alive at last follow-up.
Overall survival was significantly associated with the response status, duration of neutropenia, and the length of time between neutropenia and HSCB. All nonresponders died within the first year after HSCB, showing a 1-year OS of 0% vs a 1-year OS of 74% (54%-94%; P < .001) for responders (Figure 1B). The 1-year OS for neutropenia ≤38 days was 85% (65%-100%) vs 44% (27%-61%; P = .029) for neutropenia >38 days before HSCB (Figure 1C). Continuous variable analysis did not show a significant impact of duration of neutropenia (P = .193). Rather, spline analysis showed a benefit for patients with shorter duration of neutropenia (≤38 days) and for patients receiving early HSCB (supplemental Material).
The rate of HSCB for severe neutropenia relative to total number of patients was 20% in our study since 2018, which is in line with previous findings on rates for severe neutropenia,4,5 suggesting evaluation of this option for every fifth CAR-T patient. However, this needs to be interpreted with caution in view of the sample size in this study and its retrospective nature, as experiences may have grown over time and patient selection may have been refined. Several characteristics may affect hematopoietic recovery. Previous studies have shown an association of severe CRS or ICANS on hematotoxicity. Markers of acute inflammation, particularly the nonrecovery of these markers within 1 month after CAR-T infusion, have also been shown to affect hematotoxicity.3,11,12 In our cohort, rates of higher grade CRS or ICANS were in accordance with previous reports and did not affect recovery or overall outcomes. However, other reports suggest that inflammation might only be associated with a delay in hematopoietic recovery early after CAR-T.13 In this regard, different cytokines as well as bone marrow microenvironment changes may contribute to the recovery of hematopoietic progenitor cells.12 However, the high response rates and rapid recovery dynamics after HSCB observed here do not support a major contribution of immunological or environmental factors to CAR-T cell-associated hematotoxicity.
Different CAR T-cell products may exhibit individual safety profiles4 and may show differences in peak, expansion, and persistence of CAR constructs, which may suggest differences in outcomes. Furthermore, other treatment-related factors, such as lymphodepletion chemotherapy, extensive cytotoxic treatments, transplants, or clonal hematopoiesis before CAR-T, may influence the development of cytopenia.2,14, 15, 16 A limitation of the study is the heterogeneity in indications and CAR-T products, which is mainly from its retrospective real-practice nature. Most frequent indication for HSCB was sustained severe neutropenia (72%) and the most frequently used CAR-T product was axicabtagene ciloleucel (65%). Outcome of patients for these groups showed at least similar results in comparison with recently published real-practice outcome data (supplemental Material).4,17 However, such indirect comparisons need to be interpreted with caution and can only be resolved by controlled studies. Last, given that the total cohort consisted of several small samples from different centers, despite finding no significant difference in outcome (P = .16), a center-effect cannot be ruled out completely. Follow-up studies are therefore needed to control for attrition bias and to identify multivariable effects on outcome after HSCB.
This multicenter cohort study is the first to demonstrate that (cryopreserved autologous) HSCB generally result in prompt resolution of neutropenia, especially when given early after CAR-T. This provides evidence that persistent neutropenia may be due to dysfunction of hematopoietic stem or progenitor cells rather than to immunologic or microenvironment factors. Current practice guidelines in Germany recommend collection for autologous transplant for fit relapsed patients after initial therapy, whereas older unfit patients may not be considered suitable for autograft but potential candidates for CAR-T cell therapy after second relapse.18 Given its potential benefit for approximately every fifth patient in view of serious morbidity and mortality risks from severe hematotoxicity and taking into account an increase in number of CAR-T patients after most recent publication of CAR-T trials for second-line therapy, prophylactic collection of autologous stem cells may be considered for patients that are both fit and unfit for second-line autograft. This would provide options for patients that directly underwent CAR-T as second-line treatment but developed severe hematotoxicity; it could reduce the burden of prolonged hospitalization, from which especially older patients may benefit. In fact, some of our centers have already started prophylactic collection even for patients who did not receive prior collection for an autologous transplant. This strategy may be broadly discussed in light of CAR-T entering second-line treatment algorithms.
Conflict-of-interest disclosure: U.H. declares having received honoraria from BMS, Kite Gilead, Miltenyi Biotech, and Novartis. B.v.T. is an advisor or consultant for Allogene, BMS/Celgene, Cerus, Incyte, Miltenyi, Novartis, Pentixafarm, Roche, Amgen, Pfizer, Takeda, Merck Sharp & Dohme, and Gilead Kite. The remaining authors declare no competing financial interests.
Acknowledgments
Authorship
Contribution: N.G. and N.K. designed the study, collected and analyzed data, interpreted results, and wrote the first draft of the manuscript; P.D. reviewed the study design, collected data, interpreted results, and wrote the manuscript; G.G.W., J.D., B.G., P.v.H., B.v.T., M.F., O.P., F.A., H.E., U.H., and J.T collected data, interpreted results, and wrote the manuscript; and all authors approved the final version of the manuscript.
Footnotes
Data are available on request from the corresponding author, Nicolaus Kröger (nkroeger@uke.uni-hamburg.de).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. doi: 10.1016/j.blre.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried S, Avigdor A, Bielorai B, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transplant. 2019;54(10):1643–1650. doi: 10.1038/s41409-019-0487-3. [DOI] [PubMed] [Google Scholar]
- 3.Jain T, Knezevic A, Pennisi M, et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 2020;4(15):3776–3787. doi: 10.1182/bloodadvances.2020002509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bethge WA, Martus P, Schmitt M, et al. GLA/DRST real-world outcome analysis of CAR-T cell therapies for large B-cell lymphoma in Germany. Blood. 2022 doi: 10.1182/blood.2021015209. blood.2021015209. [DOI] [PubMed] [Google Scholar]
- 5.Rejeski K, Perez A, Sesques P, et al. CAR-HEMATOTOX: a model for CAR T-cell-related hematologic toxicity in relapsed/refractory large B-cell lymphoma. Blood. 2021;138(24):2499–2513. doi: 10.1182/blood.2020010543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan L, Shang J, Shi X, et al. Successful treatment of marrow failure after CARTs for myeloma by the infusion of cryopreserved stem cells. Am J Hematol. 2020;95(1):E20–E23. doi: 10.1002/ajh.25664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashmi H, Shockley A, Davis JA. ‘Save the day with a stem cell rescue’: use of autologous hematopoietic stem cell boost for hematopoietic recovery after CAR T cell therapy. Bone Marrow Transplant. 2022;57(3):504–506. doi: 10.1038/s41409-022-01570-4. [DOI] [PubMed] [Google Scholar]
- 8.Bader P, Carreras E, Dufour C, Mohty M, Kröger N, editors. The EBMT handbook, Hematopoietic stem cell transplantation and cellular therapies. 7th ed. Springer Open; Leiden, Munich, Cham: 2019. [PubMed] [Google Scholar]
- 9.Kalbfleisch JD. 2nd ed. J. Wiley; Hoboken, NJ: 2002. The statistical analysis of failure time data. [Google Scholar]
- 10.Nagle SJ, Murphree C, Raess PW, et al. Prolonged hematologic toxicity following treatment with chimeric antigen receptor T cells in patients with hematologic malignancies. Am J Hematol. 2021;96(4):455–461. doi: 10.1002/ajh.26113. [DOI] [PubMed] [Google Scholar]
- 11.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juluri KR, Wu QV, Voutsinas J, et al. Severe cytokine release syndrome is associated with hematologic toxicity following CD19 CAR T-cell therapy. Blood Adv. 2022;6(7):2055–2068. doi: 10.1182/bloodadvances.2020004142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hay KA, Hanafi L-A, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130(21):2295–2306. doi: 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cordeiro A, Bezerra ED, Hirayama AV, et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transplant. 2020;26(1):26–33. doi: 10.1016/j.bbmt.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schubert M-L, Dietrich S, Stilgenbauer S, et al. Feasibility and safety of CD19 chimeric antigen receptor T cell treatment for B cell lymphoma relapse after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2020;26(9):1575–1580. doi: 10.1016/j.bbmt.2020.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Hamadani M, Gopal AK, Pasquini M, et al. Allogeneic transplant and CAR-T therapy after autologous transplant failure in DLBCL: a noncomparative cohort analysis. Blood Adv. 2022;6(2):486–494. doi: 10.1182/bloodadvances.2021005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenz G, Chapuy B, Glass B, et al. Diffuse large B-cell lymphoma. https://www.onkopedia.com/de/onkopedia/guidelines/diffuses-grosszelliges-b-zell-lymphom/@@guideline/html/index.html#ID0EUDAE
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.