Abstract
The comorbid mechanism of depression and chronic pain has been a research hotspot in recent years. Until now, the role of purinergic signals in the comorbid mechanism of depression and chronic pain has not been fully understood. This review mainly summarizes the research results published in PubMed during the past 5 years and concludes that purinergic signaling is a potential therapeutic target for comorbid depression and chronic pain, and the purinergic receptors A1, A2A, P2X3, P2X4, and P2X7and P2Y6, P2Y1, and P2Y12 may be important factors. The main potential pathways are as follows: A1 receptor-related G protein-dependent activation of introverted K+ channels (GIRKs), A2A receptor-related effects on the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) and MAPK/nuclear factor-κB (NF-κB) pathways, P2X3 receptor-related effects on dorsal root ganglia (DRG) excitability, P2X4 receptor-related effects on proinflammatory cytokines and inflammasome activation, P2X7 receptor-related effects on ion channels, the NLRP3 inflammasome and brain-derived neurotrophic factor (BDNF), and P2Y receptor-related effects on the phospholipase C (PLC)/inositol triphosphate (IP3)/Ca2+ signaling pathway. We hope that the conclusions of this review will provide key ideas for future research on the role of purinergic signaling in the comorbid mechanism of depression and chronic pain.
Keywords: P2X receptor, P2Y receptor, Depression, Chronic pain, Glial cells
Introduction
As stated in the Fifth Edition of the Diagnostic and Statistical Manual of Mental Disorders, depression, as the second-most common cause of disability in the world [1], is also known as major depressive disorder (MDD). MDD is caused by various etiologies and is characterized as causing mood disorders that last for 2 weeks or longer, and depressive symptoms are the main clinical manifestations. As the world’s second-most common disease, the incidence of depression is increasing each year and seriously endangers the physical and mental health of the public. According to statistics from the World Health Organization (WHO), by the end of 2019, more than 350 million people in the world suffered from depression. According to data by Huang Y et al. [2], the lifetime prevalence rate of depression in China is 6.9%.. The International Association for the Study of Pain (IASP) defines chronic pain as “pain that exceeds the healing time of normal tissues (usually 3 months)” [3]. According to a recent survey, Chinese women (39.92%) and men (32.17%) have high prevalences of chronic pain [4]. Neuropathic pain manifests as spontaneous pain, hyperalgesia, allodynia, and paresthesias. Patients with chronic pain are prone to MDD, and patients with depression have more chronic pain symptoms than normal individuals [5]. Chronic pain with depression comorbid has been increased [6]. Since these two pathological conditions often coexist, understanding the potential common therapeutic targets for depression and chronic pain is important for formulating effective treatment strategies.
As early as 1970, Burnstock proposed the term “purinergic” [7]. The purinergic signal, that is, a nucleotide acting as an extracellular signaling molecule, was proposed in 1972 [8]. Medical terms such as purinergic receptor did not formally appear until 1978. Purinoceptors are divided into P1 and P2 receptors. P1 receptors include four types: adenosine (A)1, A2A, A2B, and A3. The P2 receptor group includes two categories: P2X and P2Y. Among them, there are 7 types of P2X receptors (P2X1–7) and 8 types of P2Y receptors (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11-14). Adenosine triphosphate (ATP) binds to these receptors to induce cell-to-cell communication and inflammation propagation [9]. ATP binds to P2X receptors, which are ligand-gated ion channels (Na+, K+, and Ca2+) that regulate rapid responses; ATP and adenosine diphosphate (ADP) activate P2Y receptors, which are second messenger systems that act through the G protein, thereby regulating the release of various neurotransmitters and hormones [10].
The relationship between purinergic signals and depression was first proposed a century ago. Robin Ortiz proposed that purinergic signals, especially the regulation of P2 receptor subtypes, affected the level of upstream receptors and caused certain mood disorders through downstream behavioral effects [11]. Furthermore, purinergic signals, as important pharmacological targets for chronic pain treatment, could also change the qualities and quantities of downstream neurotransmitters through the mechanism similar to that of depression mentioned above [12]. A2A and P2X7R antagonists were reported to have antidepressant activity [9]. Therefore, we deduced that purinergic receptors could be used as targets for the treatment of chronic pain and depression comorbid diseases.
Glial cells and neurons are important participants in the transmission of purinergic signaling in depression and chronic pain. Among them, the reported receptors expressed by microglia are the P2X4 receptor (R), P2X7R [13], P2Y1R, P2Y2R, P2Y2/4R, P2Y6R, P2Y12R, and P2Y13R [14]. The receptors expressed by astrocytes are P2X1R [15], P2X3R [5], P2X1/5R [15], P2X4R, P2X7R [16], P2Y1R[15], P2Y4R [17], P2Y11R [18], and P2Y12R [19]. There is also a high level of P1R and P2R expression on neurons. Activating the above purine receptors on glial cells (microglia and astrocytes) and neurons can produce tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-1β [9], ATP, and other purines. Inflammatory factors can act as signaling molecules and bind to the corresponding receptors [20], causing depression [21] or chronic pain [22]. Previous studies have shown that purinergic signaling in glial cells and neurons is a key link in the molecular mechanism of depression and chronic pain [9]. Therefore, this review mainly explores the molecular mechanism of purinergic signaling as a potential therapeutic target for depression and chronic pain.
Adenosine receptors in depression and chronic pain comorbidity
Among the P1 receptors, A1R and A2AR are mainly involved in the mechanism of depression and chronic pain comorbid [9]. Adenosine, a natural by-product that accumulates during cellular respiration, is a neuromodulator and can bind to two G protein-coupled receptors with opposite functions in the central nervous system (CNS): Gi/o-coupled A1 receptors (A1Rs) and Gs-coupled A2A receptors (A2ARs). Activation of A1Rs and A2ARs inhibits or promotes the release of neurotransmitters associated with glial cells and neurons, respectively.
A1 receptor
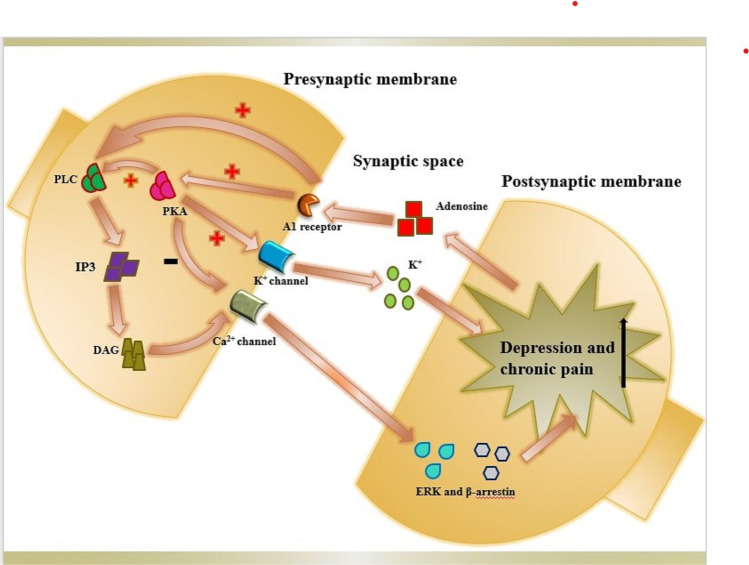
Fritz BM et al. [22] used the in vitro whole-cell patch-clamp slice electrophysiological records of stinging neurons from the medial dorsal striatum (DMS) and lateral dorsal striatum (DLS) of C57BL/6 J mice and optogenetic methods to prove A1R-mediated spreading of the cortex and thalamic striatum was inhibited by regulating excitatory glutamate transmission. In addition, conditional A1R-KO mice lacking A1Rs at the input of the DMS and DLS were generated to directly determine the effect of these presynaptic A1Rs on the measured electrophysiological response, and it was concluded that the activation of presynaptic A1Rs produced significant synaptic inhibition of prolonged excitatory transmission, which may implicate their potential contribution to neuropsychiatric diseases [23]. Moreover, adenosine can bind to A1R, activate protein kinase A (PKA), inhibit calcium ion channels, activate K+ currents, and interact with phospholipase C (PLC), inositol triphosphate (IP3), and diacylglycerol (DAG), while extracellular signal-regulated kinase (ERK) and the β-arrestin pathway (β-arrestin) interact to alleviate chronic pain (Fig. 1) [24]. Based on this theory, MRS5474, a potent and selective A1AR agonist, has been put into use as an antidepressant without cardiovascular side effects [25]. The selective A1AR antagonist DPCPX [24] reversed the analgesic effect of local and systemic paracetamol or tramadol administration in the formalin test, which confirmed the contribution of glial cells and peripheral A1R to the analgesic effect (Table 1).
Fig. 1.
Potential mechanism by which A1 adenosine receptors are involved in the comorbidity of chronic pain and depression. (
 ) on behalf of activation. (
) on behalf of activation. (
 ) and (
) and (
 ) on behalf of inhibition
) on behalf of inhibition
Table 1.
The mechanism of depression and chronic pain comorbidity mediated by P1 receptors
| P1 receptor subtype | Molecular mechanism | Method/experimental models | Type of disease | Treatment |
|---|---|---|---|---|
| A1R | Coupled with Gi/o; activates presynaptic A1R to produce synaptic inhibition and prolong the duration of excitatory transmission [23] | In vitro whole-cell patch-clamp slice electrophysiological records, optogenetic methods/C57BL/6 J mice, A1R-KO mice [23] | Depression | MRS5474 [25] |
| Adenosine can bind to A1R, activate PKA, inhibit calcium channels, activate K+ currents, and interact with PLC, IP3, and diacylglycerol [24] | Immunohistochemistries/a rat model of unilateral lingual nerve crush [24] | Chronic pain | DPCPX [25] | |
| ERK and β-arrestin pathway [24] | - | Chronic pain | DPCPX [25] | |
| A2AR | ATP and the tyrosine kinase FGF receptor simultaneously activate A2AR and the MAPK/ERK pathway [27] | The yeast two-hybrid method/E. coli[27] | Depression | - |
| Activates the secretion of proinflammatory cytokines [29] | Photochemical method/a novel isomer of curcumin (cis–trans curcumin or CTCUR) [29] | Chronic pain | Cis-curcumin [29] | |
| Phosphorylation of MAPK/NF-κB pathway factors to promote the expression of a variety of proinflammatory cytokines [28] | Cyclophosphamide-induced interstitial cystitis rats [28] | Depression and chronic pain | - |
Abbreviations: A1R, A1 receptor; PKA, protein kinase A; PLC, phospholipase C; IP3, inositol triphosphate; ERK, extracellular signal-regulated kinase; ATP, adenosine triphosphate; A2AR, A2A receptor; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor-κB
A2A receptor
A2AR in the striatum is activated by adenosine produced by extracellular nucleotidase-mediated degradation of ATP released by neurons and astrocytes [26]. Flajolet M pointed out that A2AR must be activated at the same time as the tyrosine kinase FGF receptor to cause strong activation of the mitogen-activated protein kinase (MAPK)/ERK pathway, which is associated with depression [27]. However, A2AR mainly plays a role in alleviating chronic pain by inhibiting the secretion of proinflammatory cytokines after the inflammatory response is activated [28]. Ko IG proposed that A2AR, as an important neuroregulator, may be involved in the activation of the MAPK/nuclear factor-kappa B (NF-κB) pathway: MAPK and NF-κB are stimulated by many factors, such as cytokine and cellular stress, and activation of MAPK/NF-κB (phosphorylation) can promote the expression of a variety of proinflammatory cytokines, thereby producing inflammation, which is one of the comorbid mechanisms of depression and pain [28]. Hamilton LJ et al. developed a new curcumin analog (cis-curcumin) as a ligand for A2AR that has the potential to treat chronic pain [29] (Table 1).
P2X receptors in depression and chronic pain comorbidity
P2X3 receptor
Chen Y et al. suggested that P2X3R was only expressed in small- and medium-diameter neurons in the dorsal root ganglia (DRG) [30]. However, in severe injury, an increase in ATP can increase the excitability of the DRG and promote the expression of P2X3R by activating TNF-α [31]. Neuronal P2X3R hyperexpression is induced by enhanced TNF-α signaling in the trigeminal ganglion (TG) [32]. The excitability of DRG neurons plays an important role in the neuroinflammatory pathway and participates in the production of depressive symptoms [33, 34]. P2X3 antagonists are also believed to control chronic pain from neuropathic pain sources, such as pain associated with overactive bladder and endometriosis [35]. P2X3R antagonists may be used to control chronic pain caused by inflammation or neuropathic pain. For example, the compound Gefapixant, which was named after Geoff (Gef = Geoff; pixant = P2X receptor antagonist), is expected to become the first P2X3 antagonist to be approved [36]. In addition, some articles suggest that as long-term depression was impaired in the P2X3 KO mice, it may play a role in the performance of the visible platform training (VPT). It is also possible that P2X3 receptor plays roles in anxiety and motivation, which could influence performance on the VPT [37]. However, in clinical studies, anxiety and depression are difficult to distinguish and mostly exist in combination. Systematic summary information is shown in Table 2.
Table 2.
The mechanism of depression and chronic pain comorbidity mediated by P2X receptors
| P2X receptor subtype | Molecular mechanism | Method/experimental models | Type of disease | Treatment |
|---|---|---|---|---|
| P2X3R | P2X3 receptors play roles in anxiety and motivation, which could influence performance on the VPT, which may leads to depression [37] | P2X3 KO mice [37] | Depression | - |
| Neuropathic pain or inflammation pathway [33, 34] | Quantitative real-time PCR, Western blotting, and double immunofluorescence/comorbid diabetic neuropathic pain and major depressive disorder rat model [33] | Chronic pain | Gefapixant [36] | |
| P2X4R | Binding with ATP induces activation of microglia, releases proinflammatory cytokines, mainly IL-1β, and induces neuronal necrosis [40] | Morris water maze test/a natural aging rat model [40] | Depression and chronic pain | - |
| Inflammasome NLRP3, pro-caspase-1 and the ASC increase the secretion of IL-1β and induce neuronal pyroptosis [41] | Sucrose preference test, forced swimming test, and open field test/chronic unpredictable mild stress-induced rats [41] | Depression and chronic pain | Hesperidin [41] | |
| P2X7R | Activates the TLR4 signaling pathway and promotes the activation of NF-κB to mediate the production of IL-1β and IL-18 precursors, activate NLRP3, and thereby activate P2X7R [42] | Enzyme-linked immunosorbent assay, immunofluorescence staining, flow cytometry assay/chronic unpredictable mild stress-induced rats [42] | Depression and chronic pain | - |
| The signal activated by P2X4R promotes the assembly of the NLRP3/CARD (ASC)/caspase-1 preprotein complex. Activated caspase-1 releases IL-1β and IL-18 to the extracellular space, thereby activating P2X7R [42] | Same as above | Depression and chronic pain | - | |
| Activating P2X7R, the outflow of K+ ions is compensated for by the influx of Ca2+ ions, leading to the loss of K+ in the cell or an increase in cytoplasmic Ca2+, thereby activating caspase-1, causing the proinflammatory cytokine IL-1β to activate, be released, trigger inflammation and induce other inflammatory mediators [46] | P2X7 receptor expressing gene knockout phenotypes [46] | Depression and chronic pain | - | |
| P2X7R activates the TRKB receptor in the ventral hippocampus and inhibits the level of BDNF-AKT-p70 S6 kinase [47] | Forced swim test/an animal model of depression based on selective breeding [47] | Depression | - | |
| CPSP increases P2X7R, ATP activates P2X7R, and Ca2+ promotes the release of glutamate and excessively enhances the thalamic γ-aminobutyric acid system, leading to rapid proinflammatory microglia maturation and the release of IL-1β [50] | Animal models for spinal cord injury [50] | Chronic pain | - |
Abbreviations: P2X3R, P2X3 receptor; ATP, adenosine triphosphate; TNF, tumor necrosis factor; DRG, dorsal root ganglion; PCR, polymerase chain reaction; IL, interleukin; NLRP3, NOD-like receptor protein 3; ASC, apoptosis-related connexin dot-like protein including CARD; TLR4, Toll-like receptor 4; NF-κB, nuclear factor kappa B; TRKB, tropomyosin-related kinase B; BDNF, brain-derived neurotrophic factor; CPSP, central post stroke pain
P2X4 receptor
The activation of P2X4R on microglia is associated with depression [21], and neuroinflammation and inflammasomes, which are driven by the activation of P2X4R in microglia, are also closely associated with the development of chronic pain [38]. A study by Li L et al. [39] confirmed that P2X4R activation by ATP may induce microglial activation and proinflammatory cytokines, such as IL-1β, to induce hippocampal neuronal inflammatory changes [40]. Subsequent NOD-like receptor protein 3 (NLRP3) inflammasome activation also increases the secretion of IL-1β, but this pathway induces neuronal pyroptosis [41]. The NLRP3 inflammasome includes NLRP3, pro-caspase-1, and the adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC) (Fig. 2). Wang H et al. [42] concluded that IL-1β released by microglia via P2X4R also causes astrocyte activation but does not result in the synthesis or release of IL-1α and IL-1β. In summary, P2X4R may participate in the comorbid mechanism of depression and chronic pain by mediating hippocampal neuroinflammation (Table 2).
Fig. 2.
Potential mechanisms by which P2X4, P2X7, and P2Y11 receptors are involved in the comorbidity of chronic pain and depression. (
 ) on behalf of activation
) on behalf of activation
P2X7 receptor
P2X7R is an important target for the comorbid mechanism and treatment of depression and chronic pain [43]. Chronic unpredictable stress (CUS) leads to increases in extracellular ATP, caspase-1, and IL-1β and causes the activation of P2X7R. P2X7Rs are mainly expressed in microglia and participate in the association between microglia and neurons. The rapid opening of potassium-selective channels leads to a sudden drop in intracellular potassium levels and the release of inflammatory cytokines, which mediate depression-like behavior [44]. However, with the accumulation of CUS, extracellular ATP, caspase-1, and IL-1β in the hippocampus were significantly increased, while this increase did not occur in P2X7-null mice [45]. In addition, the NLRP3 inflammasome can be activated by two signals. The first signal activates the Toll-like receptor 4 (TLR4) signaling pathway and promotes the activation of NF-κB to mediate IL-1β and IL-18 precursor production [38]. The second signal (described in the section on P2X4R) promotes the assembly of the NLRP3/ASC/caspase-1 complex, resulting in the release of IL-1β and IL-18 through activated caspase-1 to the extracellular space. Moreover, NLRP3 can activate P2X7R [41]. In the maintenance of neuropathic pain, the activation of P2X7 is also associated with the opening of ion channels and makes cells permeable to monovalent and divalent cations (Na+, K+, Ca2+). The outflow of K+ ions is compensated for by the influx of Ca2+ ions, which causes a loss of K+ ions in the cell induced by P2X7R or an increase in cytoplasmic Ca2+ ions to activate caspase-1. This activated caspase-1 further causes the rapid activation and release of the proinflammatory cytokine IL-1β from its inactive form. After that, the increased concentration of active IL-1β triggers inflammation and may also induce other inflammatory mediators, such as pro-caspase-1, nitric oxide synthase (NOS), cyclooxygenase-2 (COX-2), TNF-α, phospholipase D (PLD), phospholipase A2 (PLA2), NF-κB, and MAPK [46]. In short, P2X7R is related not only to depression but also to chronic pain (Fig. 2).
In addition, another mediator associated with P2X7R is brain-derived neurotrophic factor (BDNF). Ribeiro DE et al. [47] used a carrier or the P2X7R antagonist A-804598 (3, 10, or 30 mg/kg/day) to study BDNF signaling in the frontal cortex and ventral and dorsal hippocampus of rats for 1 or 7 days. The results showed that antagonizing P2X7R may block tropomyosin-related kinase B (TRKB) receptor activation and mediate an increase in BDNF-AKT-p70 S6 kinase levels in the ventral hippocampus, which produces related antidepressant effects. Central post stroke pain (CPSP) was used to examine continuous sensitization behavior [48]. The rat model showed that the expression of P2X7R in the surrounding areas of CPSP lesions was increased, and P2X7R was activated by ATP to elevate Ca2+. Ca2+ promotes the release of glutamate [49] and excessive enhancement of the thalamic γ-aminobutyric acid system [50], which leads to the rapid maturation of proinflammatory microglia and the release of IL-1β, resulting in chronic pain. Therefore, BDNF is also a potential target of P2X7R-mediated depression and chronic pain comorbidity via purinergic signaling. Systematic summary information is shown in Table 2.
P2Y receptors in depression and chronic pain comorbidity
P2Y1R and P2Y12R have been found in satellite glial cells (SGCs) of the trigeminal ganglia (TG) [48]. P2Y1R, P2Y2R, P2Y4R, P2Y5R, P2Y13R, and P2Y14R have been found in the SGCs of the DRG [51]. According to the G protein-coupled characteristics of P2YRs, these receptors can be divided into two categories: P2Y1R, P2Y2R, and P2Y4R are coupled to Gq/G11 to activate the phospholipase C (PLC)/IP3/Ca2+ signaling pathway, while P2Y12R, P2Y13R, and P2Y14R are coupled to Gi/Go and inhibit the synthesis of adenylate cyclase and cyclic adenosine monophosphate (cAMP) [52]. P2Y1R is involved in the alleviation of depression [53]. Therefore, P2YRs are closely associated with depression [54]. In addition, P2Y11R can be coupled to Gq/G11 and Gs to activate adenylate cyclase [52]. The activation of Gi/Go-coupled P2YRs can reduce behavioral hyperalgesia, while Gq/G11-coupled P2YRs can promote hyperalgesia [52]. Moreover, the activation of Gq/G11-coupled P2YRs is not necessarily excitatory. P2Y1R activation reduces the expression and activity of P2X3R in DRG neurons [54, 55]. Therefore, we concluded that activated P2YRs regulate the activity of other channels or receptors through second messengers and participate in SGC and neuronal communication, which leads to chronic pain [56]. In addition, P2YRs in microglia play important roles in inflammation [57]. Activated P2Y11R in microglia is coupled to Gs, leading to cAMP accumulation and PKA activation, which can alleviate depression (Fig. 2). P2Y11R on microglia can mobilize intracellular calcium and activate Ca2+/calmodulin-dependent kinase (CaM kinase). P2Y11R is coupled with Gs and Gq, leading to Ca2+mobilization in the inositol 1,4,5-triphosphate-sensitive reservoir [18]. In addition, the binding of ATP to P2Y6R or P2Y12R is associated with chronic pain mediated by spinal cord microglia. According to reports, P2Y6R is a key receptor that activates the phagocytic function of microglia, and P2Y12R is a key molecule that induces microglial chemotaxis and is associated with chronic pain [58]. G protein-coupled receptor kinase (GRK)2 can induce and regulate the intensity and duration of inflammation, leading to the transition from stress to depression and chronic pain [59]. In short, the role of P2YRs in the molecular pathways of depression and chronic pain cannot be ignored (Table 3).
Table 3.
The mechanism of depression and chronic pain comorbidity mediated by P2YRs
| P2Y receptor subtype | Molecular mechanism | Method/experimental models | Type of disease | Treatment |
|---|---|---|---|---|
| P2Y1R, P2Y2R, P2Y4R, P2Y11R | Coupled with Gq/G11 to activate the PLC/IP3/Ca2+ signaling pathway [52] | P2Y1 knockout mice [52] | Depression and chronic pain | - |
| P2Y11R | Coupled with Gs, mobilizes Ca2+ in the inositol 1,4,5-trisphosphate-sensitive reservoir, and activates Ca2+/CaM kinase, leading to cAMP accumulation and PKA activation [18, 56] | Astrocyte-selective VNUT-knockout mice [18], symptomatic/end stage SOD1-G93A ALS mice [56] | Depression | - |
| P2Y12R, P2Y13R, and P2Y14R | Coupled with Gi/Go and inhibits the synthesis of adenylate cyclase and cAMP [52] | Immunohistochemical analysis, ratio metric calcium imaging [52] | Reduce chronic pain | - |
| P2Y6R, P2Y12R | Activates the phagocytic function of microglia, induces microglial chemotaxis, and is associated with chronic pain [57] | - | Chronic pain | - |
Abbreviations: P2Y1R, P2Y1 receptor; PLC, phospholipase C; CaM kinase, calmodulin-dependent kinase; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; VNUT, vesicular nucleotide transporter; ALS, amyotrophic lateral sclerosis
Conclusion and prospects
Based on purinergic signaling, this review summarizes the possible molecular mechanisms mediated by different purinergic receptors and explores the idea of purinergic signaling as a new potential drug target. A1R, A2AR, P2X3R, P2X4R, P2X7R, P2Y6R, P2Y11R, or P2Y12R and purinergic signaling are potential common therapeutic targets in the comorbid mechanism of depression and chronic pain. Based on these findings, if future studies can truly verify that the purinergic signaling pathways mediated by these receptors exist in both depression and chronic pain, it will not be necessary to use traditional antidepressants and analgesics separately. Therefore, we infer that in the near future, the relevant targets described in this review will have common therapeutic effects on depression and chronic pain, such as inhibitors of the purinergic modulator xanthine oxidase (XO) [11], the P2X7R antagonists OxATP and BBG [16], the selective p38 MAPK inhibitor SB-681323, the p38 kinase inhibitor SCIO-469, and the NF-κB inhibitor parthenolide [60]. Although the combination of low-dose triptolide (T10), an HSP90 inhibitor, and fluoxetine (FLX), which act on P2Y11 [18], is already a commonly used antidepressant, this combination may also be a more effective strategy for the treatment of depression and chronic pain comorbidity [61]. This mechanism may be closely associated with inhibiting the activation of microglia in the dorsal hippocampus, thereby reducing the inflammatory response in the hippocampus [62]. Wu-Tou decoction can have a synergistic therapeutic effect on pain and depression by inhibiting the activation of hippocampal microglia [63]. Purinergic receptors are involved in cellular neuroinflammatory responses, depression, and chronic pain in CNS [64, 65]. Purinergic signaling is related to brain circuits for pain and depression in CNS [66, 67]. This strategy requires further research to verify.
This review not only provides a basis and direction for how to better treat depression and chronic pain by changing the molecular mechanisms associated with purinergic signaling in the future but also facilitates a deeper understanding of purinergic receptors.
Yuting Zou
(drzytpeace@163.com; Tel: +86 13665715675), Undergraduate student. First Clinical Medical College of Nanchang University, No 461, BaYi Street, Donghu District, Nanchang 330000, Jiangxi, People’s Republic of China.
Funding
This work was supported by grants (Nos. 81861138042, 81870574, 81570735, and 31560276) from the National Natural Science Foundation of China.
Data availability
Not applicable.
Declarations
Ethical approval
Not applicable.
Informed consent
Not applicable.
Conflicts of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuting Zou and Runan Yang contributed equally to this work.
References
- 1.Suleman R, Tucker BV, Dursun SM, Demas ML. The neurostimulation of the brain in depression trial: protocol for a randomized controlled trial of transcranial direct current stimulation in treatment-resistant depression. JMIR Res Protoc. 2021;10(3):e22805. doi: 10.2196/22805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J, Yu Y, Kou C, Xu X, Lu J, Wang Z, He S, Xu Y, He Y, Li T, Guo W, Tian H, Xu G, Xu X, Ma Y, Wang L, Wang L, Yan Y, Wang B, Xiao S, Zhou L, Li L, Tan L, Zhang T, Ma C, Li Q, Ding H, Geng H, Jia F, Shi J, Wang S, Zhang N, Du X, Du X, Wu Y. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2019;6(3):211–224. doi: 10.1016/S2215-0366(18)30511-X. [DOI] [PubMed] [Google Scholar]
- 3.Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavand'homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11) Pain. 2019;160(1):19–27. doi: 10.1097/j.pain.0000000000001384. [DOI] [PubMed] [Google Scholar]
- 4.Wohleb ES. Neuron-microglia interactions in mental health disorders: "For Better, and For Worse". Front Immunol. 2016;7:544. doi: 10.3389/fimmu.2016.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J, Bae JY, Lee CJ, Bae YC. Electrophysiological evidence for functional astrocytic P2X3 receptors in the mouse trigeminal caudal nucleus. Exp Neurobiol. 2018;27(2):88–93. doi: 10.5607/en.2018.27.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mills SEE, Nicolson KP, Smith BH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth. 2019;123(2):e273–e283. doi: 10.1016/j.bja.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbracchio MP. The history of the Purine Club: a tribute to Prof. Geoffrey Burnstock. Purinergic Signal. 2021;17(1):127–134. doi: 10.1007/s11302-020-09749-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24(3):509–581. [PubMed] [Google Scholar]
- 9.Burnstock G. Purinergic signalling: therapeutic developments. Front Pharmacol. 2017;8:661. doi: 10.3389/fphar.2017.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alves M, Smith J, Engel T. Differential expression of the metabotropic P2Y receptor family in the cortex following status epilepticus and neuroprotection via P2Y1 antagonism in mice. Front Pharmacol. 2019;10:1558. doi: 10.3389/fphar.2019.01558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortiz R, Ulrich H, Zarate CA, Jr, Machado-Vieira R. Purinergic system dysfunction in mood disorders: a key target for developing improved therapeutics. Prog Neuropsychopharmacol Biol Psychiatry. 2015;57:117–31. doi: 10.1016/j.pnpbp.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang, W.J., H.L. Luo, and Z.M. Zhu (2020) The role of P2X4 receptors in chronic pain: a potential pharmacological target. Biomed Pharmacother 129: 110447. 10.1016/j.biopha.2020.110447 [DOI] [PubMed]
- 13.Trang, M., G. Schmalzing, C.E. Muller, and F. Markwardt (2020) Dissection of P2X4 and P2X7 receptor current components in BV-2 microglia. Int J Mol Sci 21 (22). 10.3390/ijms21228489 [DOI] [PMC free article] [PubMed]
- 14.Kyrargyri V, Madry C, Rifat A, Arancibia-Carcamo IL, Jones SP, Chan VTT, Xu Y, Robaye B, Attwell D. P2Y13 receptors regulate microglial morphology, surveillance, and resting levels of interleukin 1beta release. Glia. 2020;68(2):328–344. doi: 10.1002/glia.23719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lalo U, Bogdanov A, Pankratov Y. Age- and experience-related plasticity of ATP-mediated signaling in the neocortex. Front Cell Neurosci. 2019;13:242. doi: 10.3389/fncel.2019.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, D.S. and J.E. Kim (2020) P2 x 7 Receptor inhibits astroglial autophagy via regulating FAK- and PHLPP1/2-mediated AKT-S473 phosphorylation following kainic acid-induced seizures. Int J Mol Sci 21 (18). 10.3390/ijms21186476 [DOI] [PMC free article] [PubMed]
- 17.Zhou F, Liu X, Gao L, Zhou X, Cao Q, Niu L, Wang J, Zuo D, Li X, Yang Y, Hu M, Yu Y, Tang R, Lee BH, Choi BW, Wang Y, Izumiya Y, Xue M, Zheng K, Gao D. HIV-1 Tat enhances purinergic P2Y4 receptor signaling to mediate inflammatory cytokine production and neuronal damage via PI3K/Akt and ERK MAPK pathways. J Neuroinflammation. 2019;16(1):71. doi: 10.1186/s12974-019-1466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinoshita M, Hirayama Y, Fujishita K, Shibata K, Shinozaki Y, Shigetomi E, Takeda A, Le HPN, Hayashi H, Hiasa M, Moriyama Y, Ikenaka K, Tanaka KF, Koizumi S. Anti-depressant fluoxetine reveals its therapeutic effect via astrocytes. EBioMedicine. 2018;32:72–83. doi: 10.1016/j.ebiom.2018.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quintas C, Vale N, Goncalves J, Queiroz G. Microglia P2Y13 receptors prevent astrocyte proliferation mediated by P2Y1 receptors. Front Pharmacol. 2018;9:418. doi: 10.3389/fphar.2018.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campos ACP, Antunes GF, Matsumoto M, Pagano RL, Martinez RCR. Neuroinflammation, pain and depression: an overview of the main findings. Front Psychol. 2020;11:1825. doi: 10.3389/fpsyg.2020.01825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yirmiya R, Rimmerman N, Reshef R. Depression as a microglial disease. Trends Neurosci. 2015;38(10):637–658. doi: 10.1016/j.tins.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13(7):533–48. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fritz BM, Yin F, Atwood BK. Input-selective adenosine A1 receptor-mediated synaptic depression of excitatory transmission in dorsal striatum. Sci Rep. 2021;11(1):6345. doi: 10.1038/s41598-021-85513-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borea PA, Gessi S, Merighi S, Varani K. Adenosine as a multi-signalling guardian angel in human diseases: when, where and how does it exert its protective effects? Trends Pharmacol Sci. 2016;37(6):419–434. doi: 10.1016/j.tips.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Serchov T, Clement HW, Schwarz MK, Iasevoli F, Tosh DK, Idzko M, Jacobson KA, de Bartolomeis A, Normann C, Biber K, van Calker D. Increased signaling via adenosine A1 receptors, sleep deprivation, imipramine, and ketamine inhibit depressive-like behavior via induction of Homer1a. Neuron. 2015;87(3):549–62. doi: 10.1016/j.neuron.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akiyama Y, Luo Y, Hanno PM, Maeda D, Homma Y. Interstitial cystitis/bladder pain syndrome: the evolving landscape, animal models and future perspectives. Int J Urol. 2020;27(6):491–503. doi: 10.1111/iju.14229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flajolet M, Wang Z, Futter M, Shen W, Nuangchamnong N, Bendor J, Wallach I, Nairn AC, Surmeier DJ, Greengard P. FGF acts as a co-transmitter through adenosine A(2A) receptor to regulate synaptic plasticity. Nat Neurosci. 2008;11(12):1402–9. doi: 10.1038/nn.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ko IG, Jin JJ, Hwang L, Kim SH, Kim CJ, Won KY, Na YG, Kim KH, Kim SJ. Adenosine A2A receptor agonist polydeoxyribonucleotide alleviates interstitial cystitis-induced voiding dysfunction by suppressing inflammation and apoptosis in rats. J Inflamm Res. 2021;14:367–378. doi: 10.2147/JIR.S287346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamilton LJ, Walker M, Pattabiraman M, Zhong HA, Luedtke B, Chandra S. Novel curcumin analog (cis-trans curcumin) as ligand to adenosine receptors A2A and A2B: potential for therapeutics. Pharmacol Res. 2021;165:105410. doi: 10.1016/j.phrs.2020.105410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guida F, Luongo L, Marmo F, Romano R, Iannotta M, Napolitano F, Belardo C, Marabese I, D'Aniello A, De Gregorio D, Rossi F, Piscitelli F, Lattanzi R, de Bartolomeis A, Usiello A, Di Marzo V, de Novellis V, Maione S. Palmitoylethanolamide reduces pain-related behaviors and restores glutamatergic synapses homeostasis in the medial prefrontal cortex of neuropathic mice. Mol Brain. 2015;8:47. doi: 10.1186/s13041-015-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohtori, S., K. Takahashi, H. Moriya, and R.R. Myers (2004) TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury: studies in murine DRG and spinal cord. Spine (Phila Pa 1976) 29 (10): 1082–8. 10.1097/00007632-200405150-00006 [DOI] [PubMed]
- 32.Koizumi M, Asano S, Furukawa A, Hayashi Y, Hitomi S, Shibuta I, Hayashi K, Kato F, Iwata K, Shinoda M. P2X3 receptor upregulation in trigeminal ganglion neurons through TNFalpha production in macrophages contributes to trigeminal neuropathic pain in rats. J Headache Pain. 2021;22(1):31. doi: 10.1186/s10194-021-01244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan S, Shen Y, Ge H, Xiong W, He L, Liu L, Yin C, Wei X, Gao Y. Dihydromyricetin alleviates diabetic neuropathic pain and depression comorbidity symptoms by inhibiting P2X7 receptor. Front Psychiatry. 2019;10:770. doi: 10.3389/fpsyt.2019.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci U S A. 2007;104(23):9864–9. doi: 10.1073/pnas.0611048104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ford AP. In pursuit of P2X3 antagonists: novel therapeutics for chronic pain and afferent sensitization. Purinergic Signal. 2012;8(Suppl 1):3–26. doi: 10.1007/s11302-011-9271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morice, A.H., M.M. Kitt, A.P. Ford, A.M. Tershakovec, W.C. Wu, K. Brindle, R. Thompson, S. Thackray-Nocera, and C. Wright (2019) The effect of gefapixant, a P2X3 antagonist, on cough reflex sensitivity: a randomised placebo-controlled study. Eur Respir J 54 (1). 10.1183/13993003.00439-2019 [DOI] [PubMed]
- 37.Wang Y, Mackes J, Chan S, Haughey NJ, Guo Z, Ouyang X, Furukawa K, Ingram DK, Mattson MP. Impaired long-term depression in P2X3 deficient mice is not associated with a spatial learning deficit. J Neurochem. 2006;99(5):1425–34. doi: 10.1111/j.1471-4159.2006.04198.x. [DOI] [PubMed] [Google Scholar]
- 38.Wang M, Cai X, Wang Y, Li S, Wang N, Sun R, Xing J, Liang S, Liu S. Astragalin alleviates neuropathic pain by suppressing P2X4-mediated signaling in the dorsal root ganglia of rats. Front Neurosci. 2020;14:570831. doi: 10.3389/fnins.2020.570831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L, Zou Y, Liu B, Yang R, Yang J, Sun M, Li Z, Xu X, Li G, Liu S, Greffrath W, Treede RD, Li G, Liang S. Contribution of the P2X4 receptor in rat hippocampus to the comorbidity of chronic pain and depression. ACS Chem Neurosci. 2020;11(24):4387–4397. doi: 10.1021/acschemneuro.0c00623. [DOI] [PubMed] [Google Scholar]
- 40.Yu Y, Feng L, Li J, Lan X, L. A, X. Lv, M. Zhang, and L. Chen, The alteration of autophagy and apoptosis in the hippocampus of rats with natural aging-dependent cognitive deficits. Behav Brain Res. 2017;334:155–162. doi: 10.1016/j.bbr.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Xie L, Gu Z, Liu H, Jia B, Wang Y, Cao M, Song R, Zhang Z, Bian Y. The anti-depressive effects of hesperidin and the relative mechanisms based on the NLRP3 inflammatory signaling pathway. Front Pharmacol. 2020;11:1251. doi: 10.3389/fphar.2020.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H, Guo W, Liu H, Zeng R, Lu M, Chen Z, Xiao Q. Inhibition of inflammatory mediator release from microglia can treat ischemic/hypoxic brain injury. Neural Regen Res. 2013;8(13):1157–68. doi: 10.3969/j.issn.1673-5374.2013.13.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen Y, Guan S, Ge H, Xiong W, He L, Liu L, Yin C, Liu H, Li G, Xu C, Xu H, Liu S, Li G, Liang S, Gao Y. Effects of palmatine on rats with comorbidity of diabetic neuropathic pain and depression. Brain Res Bull. 2018;139:56–66. doi: 10.1016/j.brainresbull.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Chu YX, Zhang Y, Zhang YQ, Zhao ZQ. Involvement of microglial P2X7 receptors and downstream signaling pathways in long-term potentiation of spinal nociceptive responses. Brain Behav Immun. 2010;24(7):1176–89. doi: 10.1016/j.bbi.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Yue N, Huang H, Zhu X, Han Q, Wang Y, Li B, Liu Q, Wu G, Zhang Y, Yu J. Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. J Neuroinflammation. 2017;14(1):102. doi: 10.1186/s12974-017-0865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehta N, Kaur M, Singh M, Chand S, Vyas B, Silakari P, Bahia MS, Silakari O. Purinergic receptor P2X(7): a novel target for anti-inflammatory therapy. Bioorg Med Chem. 2014;22(1):54–88. doi: 10.1016/j.bmc.2013.10.054. [DOI] [PubMed] [Google Scholar]
- 47.Ribeiro DE, Muller HK, Elfving B, Eskelund A, Joca SR, Wegener G. Antidepressant-like effect induced by P2X7 receptor blockade in FSL rats is associated with BDNF signalling activation. J Psychopharmacol. 2019;33(11):1436–1446. doi: 10.1177/0269881119872173. [DOI] [PubMed] [Google Scholar]
- 48.Kuan YH, Shih HC, Shyu BC. Involvement of P2X7 receptors and BDNF in the pathogenesis of central poststroke pain. Adv Exp Med Biol. 2018;1099:211–227. doi: 10.1007/978-981-13-1756-9_18. [DOI] [PubMed] [Google Scholar]
- 49.Glaser T, Andrejew R, Oliveira-Giacomelli A, Ribeiro DE, Bonfim Marques L, Ye Q, Ren WJ, Semyanov A, Illes P, Tang Y, Ulrich H. Purinergic receptors in basal ganglia diseases: shared molecular mechanisms between Huntington's and Parkinson's disease. Neurosci Bull. 2020;36(11):1299–1314. doi: 10.1007/s12264-020-00582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iqubal A, Ahmed M, Iqubal MK, Pottoo FH, Haque SE. Polyphenols as potential therapeutics for pain and inflammation in spinal cord injury. Curr Mol Pharmacol. 2020 doi: 10.2174/1874467213666201223111743. [DOI] [PubMed] [Google Scholar]
- 51.Magni G, Ceruti S. P2Y purinergic receptors: new targets for analgesic and antimigraine drugs. Biochem Pharmacol. 2013;85(4):466–77. doi: 10.1016/j.bcp.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 52.Malin SA, Molliver DC. Gi- and Gq-coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior. Mol Pain. 2010;6:21. doi: 10.1186/1744-8069-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rajani V, Zhang Y, Jalubula V, Rancic V, SheikhBahaei S, Zwicker JD, Pagliardini S, Dickson CT, Ballanyi K, Kasparov S, Gourine AV, Funk GD. Release of ATP by pre-Botzinger complex astrocytes contributes to the hypoxic ventilatory response via a Ca(2+) -dependent P2Y1 receptor mechanism. J Physiol. 2018;596(15):3245–3269. doi: 10.1113/JP274727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Y, Zhang X, Wang C, Li G, Gu Y, Huang LY. Activation of P2X7 receptors in glial satellite cells reduces pain through downregulation of P2X3 receptors in nociceptive neurons. Proc Natl Acad Sci U S A. 2008;105(43):16773–8. doi: 10.1073/pnas.0801793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu X, Liu B, Yang J, Zou Y, Sun M, Li Z, Li L, Yang R, Zou L, Li G, Liu S, Li G, Liang S. Glucokinase in stellate ganglia cooperates with P2X3 receptor to develop cardiac sympathetic neuropathy in type 2 diabetes rats. Brain Res Bull. 2020;165:290–297. doi: 10.1016/j.brainresbull.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 56.Mikuzuki L, Saito H, Katagiri A, Okada S, Sugawara S, Kubo A, Ohara K, Lee J, Toyofuku A, Iwata K. Phenotypic change in trigeminal ganglion neurons associated with satellite cell activation via extracellular signal-regulated kinase phosphorylation is involved in lingual neuropathic pain. Eur J Neurosci. 2017;46(6):2190–2202. doi: 10.1111/ejn.13667. [DOI] [PubMed] [Google Scholar]
- 57.Amadio S, Parisi C, Montilli C, Carrubba AS, Apolloni S, Volonte C. P2Y(12) receptor on the verge of a neuroinflammatory breakdown. Mediators Inflamm. 2014;2014:975849. doi: 10.1155/2014/975849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inoue K. Purinergic signaling in microglia in the pathogenesis of neuropathic pain. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(4):174–182. doi: 10.2183/pjab.93.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walker AK, Kavelaars A, Heijnen CJ, Dantzer R. Neuroinflammation and comorbidity of pain and depression. Pharmacol Rev. 2014;66(1):80–101. doi: 10.1124/pr.113.008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Popiolek-Barczyk K, Mika J. Targeting the microglial signaling pathways: new insights in the modulation of neuropathic pain. Curr Med Chem. 2016;23(26):2908–2928. doi: 10.2174/0929867323666160607120124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Long T, He W, Pan Q, Zhang S, Zhang D, Qin G, Chen L, Zhou J. Microglia P2X4R-BDNF signalling contributes to central sensitization in a recurrent nitroglycerin-induced chronic migraine model. J Headache Pain. 2020;21(1):4. doi: 10.1186/s10194-019-1070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu X, Dong Y, Jin X, Zhang C, Zhang T, Zhao J, Shi J, Li J. The novel and potent anti-depressive action of triptolide and its influences on hippocampal neuroinflammation in a rat model of depression comorbidity of chronic pain. Brain Behav Immun. 2017;64:180–194. doi: 10.1016/j.bbi.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 63.Zhu C, Xu Q, Mao Z, Lin N. The Chinese medicine Wu-Tou decoction relieves neuropathic pain by inhibiting hippocampal microglia activation. Sci Rep. 2018;8(1):12292. doi: 10.1038/s41598-018-30006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang Z, Xie N, Illes P, Di Virgilio F, Ulrich H, Semyanov A, Verkhratsky A, Sperlagh B, Yu SG, Huang C, Tang Y. From purines to purinergic signalling: molecular functions and human diseases. Signal Transduct Target Ther. 2021;6(1):162. doi: 10.1038/s41392-021-00553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zarrinmayeh H, Territo PR. Purinergic receptors of the central nervous system: biology, PET ligands, and their applications. Mol Imaging. 2020;19:1536012120927609. doi: 10.1177/1536012120927609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Illes P, Verkhratsky A, Tang Y. Pathological ATPergic signaling in major depression and bipolar disorder. Front Mol Neurosci. 2019;12:331. doi: 10.3389/fnmol.2019.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ribeiro, D.E., A.L. Roncalho, T. Glaser, H. Ulrich, G. Wegener, and S. Joca (2019) P2X7 receptor signaling in stress and depression. Int J Mol Sci 20 (11). 10.3390/ijms20112778 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.