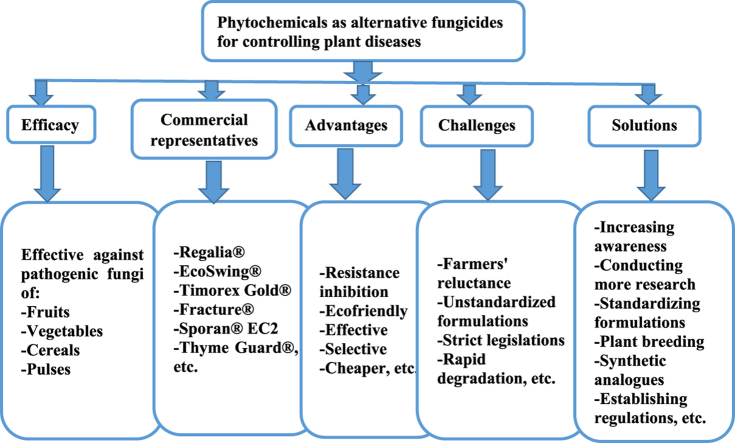
Abstract
Fungal infections are responsible for about 70–80% of the losses in agricultural production brought on by microbial diseases. Synthetic fungicides have been employed to manage plant diseases caused by phytopathogenic fungi but their use has been criticized due to unfavorable side effects. As alternative strategies, botanical fungicides have caught the interest of many researchers in recent years. There are numerous experimental studies on the fungicidal activities of phytochemicals against phytopathogenic fungi, but there is not a thorough review article that summarizes these experimental studies. The purpose of this review is therefore to consolidate data from in vitro and in vivo studies on the antifungal activity of phytochemicals reported by various researchers. This paper describes antifungal activities of plant extracts and compounds against phytopathogenic fungi, approved botanical fungicides, their benefits, obstacles and mitigation strategies. Relevant sources were collected using online data bases such as Google Scholar, PubMed and Science Direct, and comprehensively reviewed for preparation of this manuscript. This review revealed that phytochemicals are effective to manage plant diseases caused by phytopathogenic fungi. Botanical fungicides are endowed with benefits such as resistance inhibition, being ecofriendly, effective, selective, and more affordable compared to synthetic fungicides. However, there are only small number of approved botanical fungicides due to the many challenges that hinder their adoption and utilization for a wider scale production. Farmers' reluctance, lack of standardized formulation techniques, strict legislation, rapid degradation, and other factors hinder their adoption and utilization. The ways to address these challenges include increasing awareness among farmers, conducting more research to identify potential plants with fungicidal properties, standardizing extraction and formulation techniques, implementing the idea of plant breeding to increase bioactive agents, identifying favorable environments for site-specific plant species production, discovering synthetic analogues of the active ingredient to maintain quality standards, establishing reasonable regulation procedures and price points for a quicker market introduction. To put all these into practice, we recommend collaboration of regulatory agencies and researchers from a variety of fields.
Keywords: Phytochemicals, Botanical fungicides, Synthetic fungicides, Phytopathogenic fungi, Plant diseases
Graphical abstract
Highlights
-
•
Phytochemicals are effective antifungal agents that can be used as an alternative to synthetic fungicides.
-
•
Botanical fungicides inhibit resistance, are ecofriendly, effective, selective, and cheaper than synthetic fungicides.
-
•
There are only a few botanical fungicides that are commercially approved for treating plant diseases.
-
•
Lack of standardized formulation, strict legislation, rapid degradation, etc. hinder their adoption and utilization.
-
•
To address these issues, we recommend collaboration between regulatory agencies and researchers from various fields.
1. Introduction
The eukaryotic organism fungi have numerous uses in the agricultural, medical, and industrial fields, from the generation of life-saving drugs to food supplements, but they are also responsible for significant crop losses around the world each year, which has a negative impact on the economy [1]. Fungal infections are responsible for about 70–80% of the losses in agricultural production brought on by microbial diseases. About 8000 different fungal species were known to cause around 100,000 different diseases of plants [2]. In recent years, the number of fungi known to cause plant diseases worldwide has been increased to over 19,000 [3].
One of the main infectious agents that affect plants, causing changes during the various stages of plant growth on the field, post-harvest, and even during storage, is phytopathogenic fungi. These fungi cause quality problems in cereals, fruits and vegetables, affecting their nutritional value, organoleptic characteristics and half-life [4]. They cause the death and extinction of the crop species by affecting various plant parts (roots, stems, leaves, fruits, tubers, etc.). They also secrete various types of toxic chemicals collectively called mycotoxins such as aflatoxins, ochratoxins, patulin, fumonisin, zearalenone, deoxynivalenol, and so on in the stored food products, resulting in postharvest losses of cereals, pulses, dry fruits, and spices [5]. Globally, mycotoxin contamination is a severe issue for the security and safety of food. These pollutants are responsible for significant economic losses in commerce and agricultural output, which are particularly pronounced in underdeveloped and developing nations. According to estimates, mycotoxins can infect between 60 and 80% of crops globally, causing huge economic losses [6]. Mycotoxins are also powerful disease causing chemicals in humans that can cause cancer, liver damage, kidney failure, and paralysis in addition to spoiling food [5].
For a long time, synthetic fungicides have been used to manage plant illnesses brought on by phytopathogenic fungus, although their usage has drawn criticism for many reasons. Continuous use leads to resistance, excessive use and improper handling of synthetic fungicides can have detrimental impacts on people, the environment, and non-target organisms, which has a negative influence on biodiversity. Due to their low biodegradability and high tendency to accumulate in the environment, constituent molecules of synthetic fungicides have been linked to chronic human illnesses in either intake or exposure scenarios in addition to ozone layer depletion [1,6,7].
To cope up with the mentioned problems of synthetic fungicides, a number of alternative techniques have been tried. Botanical fungicides are one of these methods and can be a viable and sustainable alternative to synthetic fungicides. Numerous studies have demonstrated that phytochemicals derived from plants have fungicidal effects [7]. Plants can be considered as a perfect laboratory with potential to supply organic substances which can be classified as primary metabolites (proteins, carbohydrates, and fats) or secondary metabolites (terpenes, steroids, anthocyanins, anthraquinones, phenols, alkaloids etc.) [8]. Due to a number of factors, the study of medicinal plants as potential natural sources of active compounds against phytopathogens has gained increased attention in recent years [9]. There are numerous experimental studies on the fungicidal activities of phytochemicals against phytopathogenic fungi, but there is not a thorough review article that summarizes these experimental studies. The aim of this review is therefore to compile information on the use of phytochemicals as substitutes for synthetic fungicides in the management of fungi-caused plant diseases. It includes investigations on the antifungal activity of crude extracts of plants and isolated compounds carried out in in vitro and in vivo models by various researchers. The review also discusses some representative commercial botanical fungicides, obstacles to the use of botanicals for managing plant diseases sustainably, and potential mitigating strategies. For simplicity and readers convenience, informations gathered from literatures were organized in tabular form with brief description of the scientific name of plants, plant parts used, extraction solvent and methods employed, fungi species tested along with the host plant disease, bioassay methods used and antifungal efficacy observed.
2. The review methodology
The relevant sources for this study were retrieved utilizing search engines including Google Scholar, PubMed, and Science Direct. For the purpose of finding relevant sources, several combinations of the terms and phrases such as antifungal phytochemicals, plant extracts, natural products, secondary metabolites, compounds, plant diseases, phytopathogenic fungi, and botanical fungicides were utilized. This review covered studies showing both in vitro and in vivo antifungal activity of plant extracts and compounds against pathogenic fungi that cause plant illnesses, but it excluded studies reporting such activities against pathogenic fungi that cause human diseases. Reports on antifungal effects of other derivatives, such as nanoparticles made from plant crude extracts or compounds were also disregarded. Studies that were published in languages other than English were not at all taken into account in this study. Following the collection of all sources, a rapid study of the sources' titles, abstracts, and conclusions was done to determine which ones met the qualifying requirements. The chosen sources were then carefully examined in order to prepare this review paper. The chemical structures of compounds were depicted using ChemDraw Ultra 8.0 software, while citations and references were provided using Mendeley Desktop software.
3. Crude extracts from plants as fungicides against phytopathogenic fungi
Crude extracts from numerous plant species have been discovered to be efficacious against a variety of phytopathogenic fungi without causing negative side effects, according to research being conducted worldwide to use botanicals in plant disease control [10]. There are a large number of papers published on an in vitro antifungal activity of crude extracts obtained from plants. Plant extracts have the benefit that they typically include a combination of compounds that may combine to suppress the growth of phytopathogenic fungus. Additionally, many plant extracts include many antifungal substances and a reduction in the emergence of resistance may result from the varied modes of action of these substances. Therefore, the usage of plant extracts may prevent the emergence of antimicrobial chemical resistance [2].
Plant extracts are substances obtained from the roots, barks, seeds, shoots, leaves, fruits, flowers, cloves, rhizomes, or stems of plants which have a long therapeutic history and chosen for their natural defense mechanisms. The process of obtaining plant extracts typically entails macerating the plant material with various organic solvents, and may be followed by the purification of the resulting crude extracts using chromatographic techniques to acquire specific chemicals, which ultimately results in the isolation of the metabolites in pure form. Additionally, it has been noted that the method and solvent used to get the final material (extract) of this procedure affect the quantity and variety of chemicals or secondary metabolites thought to have antifungal properties. As a result, the extracts' antifungal effects may be influenced by different compounds present in the extracts or by the same compounds present in varied concentrations [4]. The intended bioactive chemicals and their concentration within the subject plant part will determine which plant part is employed. The allelopathic effect of botanical fungicides on crops varies depending on the source plant and the amounts utilized. Their effectiveness depends on the type of the source plant, whether it is dried or fresh, the extraction solvents and the extraction techniques employed. The common bioactive compounds in botanical pesticides are majorly secondary metabolites that possess fungicidal and many other biological activities [11].
A given plant species are efficient against a particular class of pests because of the specific chemicals found in those species. Botanical fungicides contain secondary metabolites that are poisonous to the cell membranes, organelles, and walls of fungi. These metabolites prevent the germination of spores, the growth of mycelium, the lengthening of germ tubes, delayed sporulation, as well as the production of critical enzymes, DNA, and proteins. Additionally, they cause structural changes in the hypha and mycelia, which prevent some fungi such as Aspergillus spp. and Fusarium spp. from producing toxic compounds like aflatoxin and fumonisin respectively. As a result, mycotoxin-producing fungal infections are less pathogenic [11].
3.1. Crude extracts from plants as fungicides against phytopathogenic fungi that affect fruits and vegetables
Many plant extracts have been extensively studied for controlling fruits and vegetable diseases including blueberry dieback, okra seed rot and seedlings death, banana anthracnose, citrus fruit decay, tea leaf disease, black molds in tomato ripe fruits and blight of pepper crops, early and late blight of tomato, wilt disease of tomato, sugar beet damping-off, white yam anthracnose, murcott tangor fruits brown spot disease, tomato damping-off diseases, carrot leaf blight and black rot, root rot of tomato, late blight disease of potato, artichoke diseases, pepper phytophthora blight, and mango anthracnose which are caused by pythopathogenic fungi as indicated in Table 1. Among these, the fungal diseases of tomato are the most widely studied one. Most of the tested plant extracts showed promising antifungal activity in in vitro and in vivo assays in controlling the mentioned fungal diseases of fruits and vegetables as described in Table 1.
Table 1.
Antifungal activities of crude extracts from plants against phytopathogenic fungi that affect fruits and vegetables.
| Plant species (part used) | Fungi species (disease caused) | Efficacy observed | References |
|---|---|---|---|
|
|
|
[12] |
|
|
|
[13] |
|
|
|
[14] |
|
|
|
[15] |
|
|
|
[16] |
|
|
|
[17] |
|
|
|
[18] |
|
|
|
[19] |
|
|
|
[20] |
|
|
|
[21] |
|
|
|
[22] |
|
|
|
[23] |
|
|
|
[24] |
|
|
|
[25] |
|
|
|
[26] |
|
|
|
[27] |
|
|
|
[28] |
|
|
|
[29] |
|
|
|
[30] |
|
|
|
[31] |
|
|
|
[32] |
|
|
|
[33] |
|
|
|
[34] |
|
|
|
[35] |
|
|
|
[36] |
|
|
|
[37] |
|
|
|
[38] |
|
|
|
[39] |
As it can be seen from Table 1, different parts of plants including roots, rhizomes, bulbs, stem, barks, leaves, flowers, fruits, peels, and seeds, were studied on different phytopathogenic fungi in in vitro and in vivo models and the leaf part is the most frequently studied plant part. The method of extraction used for obtaining the crude extract involves maceration, steam distillation, soxhlet extraction, liquid carbon dioxide subcritical extraction, and hydrodistillation, maceration being the most frequently used one. In most cases, the crude extract were used directly for antifungal activity study, while partitioning into different fractions using solvents of different polarity is also applied in some cases. The solvents employed for extraction involve ethyl acetate, ethanol, methanol, water (aqueous), chloroform, liquid carbon dioxide, and dichloromethane: methanol (1:1 v/v) as presented in Table 1. Among these solvents, ethanol, methanol and water constitute the three most commonly used extractants. Majority of the studies presented in Table 1 involve in vitro assays while in vivo models (including both greenhouse and field trials) were rarely used. The antifungal assays used generally involve inhibition of mycelial growth, radial growth, spore germination, conidial germination, germ tube elongation, and sporangial germination, the first being the most frequently applied approach.
In some in vivo studies under greenhouse and/or field conditions, the tested extracts showed no phytotoxicity to the host plant [20,29], and increased fruit yield after treatment [22,24,29,34]. In some of the studies presented in Table 1, identification of phytochemical constituents of the crude extract was carried out using GC-MS analysis [12,20,27,35,39], and phytochemical screening tests [22,[30], [31], [32], [33], [34]]. However, they did not involve identification of the active compound in the crude extract. All studies also lack mechanisms by which the crude extract showed its antifungal activity against phytopathogenic fungi.
3.2. Crude extracts from plants as fungicides against phytopathogenic fungi that affect cereals and pulses
Different solvent extracts of many plants were also studied for their antifungal activity against pathogenic fungi that cause diseases of cereals and pulses including wheat blast disease, rice blast, rice sheath blight, wheat leaf rust, sorghum grains disease, barley seeds disease, maize seeds spoilage, milkvetch yellow dwarf and root-rot, chocolate spot of broad bean, rust and anthracnose of soybean leaf, bean and cowpea anthracnose, peanut rust, and so on in in vitro and in vivo assays and promising efficacy were observed as indicated in Table 2.
Table 2.
Antifungal activities of crude extracts of plants against phytopathogenic fungi affecting cereals and pulses.
| Plant species (part used) | Fungi species (disease caused) | Efficacy observed | References |
|---|---|---|---|
|
|
|
[40] |
|
|
|
[41] |
|
|
|
[42] |
|
|
|
[43] |
|
|
|
[44] |
|
|
|
[45] |
|
|
|
[46] |
|
|
|
[47] |
|
|
|
[48] |
|
|
|
[49] |
|
|
|
[33] |
|
|
|
[50] |
|
|
|
[51] |
|
Puccinia arachidis (peanut rust disease) |
|
[52] |
|
|
|
[53] |
4. Compounds isolated from plants as fungicides against phytopathogenic fungi
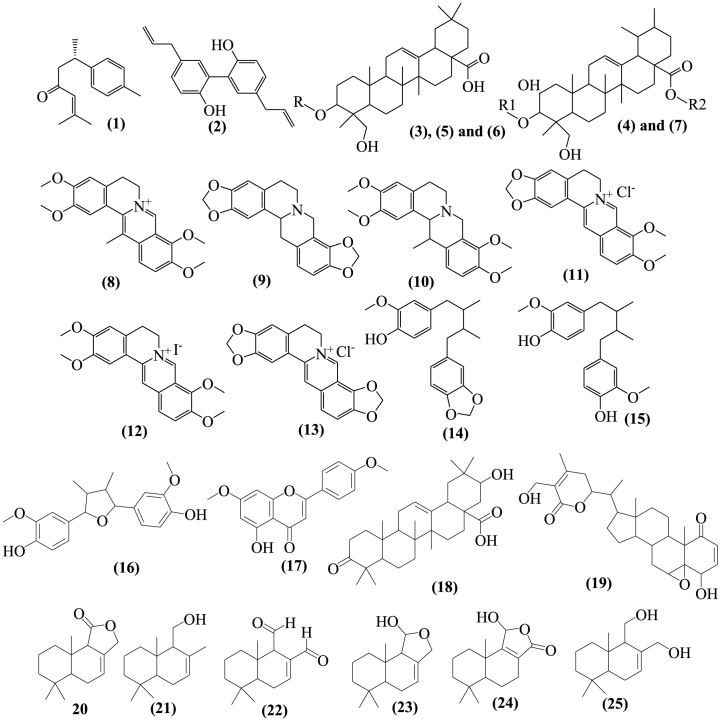
There are numerous reports of plant compounds with antifungal properties. Higher plants provide an abundant source of bioactive secondary metabolites that have been shown to have antifungal effects in in vitro assay. In order to achieve a sustainable control of phytopathogenic fungi and to lessen the heavy reliance on synthetic fungicides used to control them, secondary metabolites with antifungal activity constitute an alternative mechanisms. These compounds can be utilized directly or as a starting point for developing more effective fungicidal chemicals [54]. Numerous plant secondary metabolites have been examined for their antifungal properties [55]. Table 3, below describes in vitro and in vivo studies conducted on antifungal activity of compounds isolated from plants (structures given on Fig. 1) in controlling plant diseases caused by phytopathogenic fungi. The tested compounds belong to different classes of secondary metabolites including sesquiterpenoids (1, 20–25), triterpenoid (18), triterpene glycosides or triterpenoid saponins (3–7), isoquinoline alkaloids (8–13), lignans (2, 14-16), flavone (17), and steroidal lactone (19) as shown on Fig. 1.
Table 3.
Activity of compounds isolated from plants against phytopathogenic fungi of fruits, vegetable, and cereals.
| Plant species (part used) | Fungi species (disease caused) | Efficacy observed | References |
|---|---|---|---|
| Efficacy against pathogenic fungi of fruits and vegetables | |||
|
|
|
[56] |
|
|
|
[57] |
|
|
|
[58] |
|
|
|
[59] |
|
|
|
[60] |
|
|
|
[61] |
| Efficacy against pathogenic fungi of cereals | |||
|
|
|
[62] |
|
|
|
[63] |
|
|
|
[58]. |
|
|
|
[59] |
|
|
|
[60] |
|
|
|
[61] |
Fig. 1.
Chemical structures of antifungal compounds isolated from plants. In structures 3–7, R, R1 & R2 stands for the following substituents. (3). R = β-d-glucopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside. (4). R1 = α-l-rhamnopyranosyl, R2 = H. (5). R = β-d-glucopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside. (6). R = α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside. (7). R1 = α-l-arabinopyranosyl, R2 = β-d-glucopyranosyl
5. Commercialized botanical fungicides
New classes of natural plant protection products have recently been developed, approved, and successfully integrated into agricultural practice with the help of organizations empowered to market these products and this has been a real success for commerce. Some examples are jojoba essential oil (commercial names: Detur, E-Rasem, Eco E-Rase, Permatrol, Erase™), rosemary essential oil (commercial names: Ecotrol™, Sporan™, Ecosmart), and others [8]. Table 4, below lists a few cutting-edge plant products that have been successfully marketed as fungicides to treat plant diseases.
Table 4.
Plant products commercialized as effective fungicides for controlling plant diseases.
| Trade names | Descriptions on botanical sources, uses, efficacy, application method and mechanism of action | References |
|---|---|---|
|
|
[64] |
|
|
[65] |
|
|
[66] |
|
|
[67] |
|
|
[68] |
|
|
[69] |
6. Advantages of using botanical fungicides for controlling plant diseases
Botanical fungicide development could lessen the drawbacks of synthetic fungicides, such as resistance and environmental contamination. Botanical fungicides may be less hazardous to the environment, effective, selective, and biodegradable in this regard [70]. The finest defense against any form of infection, pathogenesis, or disease protection issues is a product from nature. They are the primary alternatives that agriculturalists and plant biologists utilize to prevent fungal disease due to their degradability in nature [1]. Plant extracts have benefits like multiple action mechanisms because there are so many active ingredients in each mixture, low toxicity to non-target organisms, including humans, relatively straightforward and inexpensive production processes, and reduced health risks during application because of low residue toxicity [8]. Botanicals are more affordable and more environmentally friendly than synthetic fungicides in agriculture [71]. They are enzymatically biodegradable with, typically, short half-lives, they have selectivity and specificity in how they affect the target species, they can be combined in ways that reduce the amount of active ingredients needed to achieve an effect, they come from a variety of chemical families, and by expanding the range of molecules that are available, they help to diversify the biochemical and molecular targets that are directed at fungi and thus limit or delay the resistance phenomenon [72].
7. Challenges in adoption and utilization of botanical fungicides for controlling plant diseases
Despite the fact that plant products are effective substitutes for synthetic fungicides and have a strong track record, their extensive practical applicability is still constrained by farmers' resistance to using natural products as biofungicides and the paucity of research in this field. Developing efficient stabilization processes (such as microencapsulation), simplifying complicated and expensive authorization requirements for the use of natural plant protection products, and optimizing plant growth conditions and extraction processes leading to a homogenous chemical composition are the main challenges for future research, according to an analysis of the main strengths and weaknesses that arise from the use of plant extracts as natural plant protection products [8].
The main causes of their low adoption for production at a commercial scale are a lack of adequate information and extension services at the farmer's level and sluggish results compared to synthetic fungicides. Farmers are discouraged from using botanical fungicides since they are less effective than chemical fungicides and are not readily available on the market when needed. Farmers themselves can make botanical fungicides but they tend to choose chemical fungicides since the creation of botanical fungicides necessitates the use of specialized plants and takes a lot of time and effort. Furthermore, the widespread use of botanical fungicides is hindered by rigorous regulations, less lasting or quick degradation, and variations in the active ingredient composition with plants growing in various climatic situations. Large biomass of chosen plants is needed for the commercial manufacture of botanical fungicides. Their manufacturing and adoption are hindered by the bulkiness issue during collection, production, and application. Plant products for the production of fungicides have limited market range. Less market demand of such products and increasing demand of food crops hinder the commercial production of botanical fungicides [7].
The need to develop formulations, the presence of some chemical compounds that are harmful to people and plants, the lack of standardized extraction methods, rapid degradation, inadequate in vivo studies, less effectiveness, and limited availability of formulations are additional barriers to the use of botanicals in the management of plant diseases [71].
Additionally, there are a number of variables that affect the industrial development of formulations incorporating plant components, including the accessibility and availability of the raw material. The supply of vegetable biomass must be constant, abundant, and easily renewable, which excludes species with slow development, like the woody species, if they are not cultivated. Other considerations include the standardization and refinement of the plant commercial product, which is susceptible to variation in its chemistry due to geographic, genetic, and climatic factors; the products put on the market must be of uniform and consistent quality; the difference in procedures of regulatory approval processes in different countries, as well as the protection of technologies and formulations, which will ensure companies exclusivity in the market through the protection granted by patents. Due to toxicologic and ecotoxicologic standards, fungicide registration is extremely expensive for businesses planning to market their products in industrialized nations. The current issue is that few appropriate and acceptable test procedures have been created for biofungicides and regulators are unsure of the best course of action as a result [72].
8. Possible solutions for mitigating the challenges of adoption and utilization of botanical fungicides
For the production on a small scale, farmers need to have access to extension services about botanical fungicide identification, preparation methods, and application. They should get subsidies in order to promote the creation and use of botanical fungicides. It is important to raise awareness about the advantages of natural fungicides over synthetic ones. Focus should be placed on sustainable agriculture and organic farming because these concepts can draw customers to such goods. None of the producers are willing to take risk in their production and thus, market for the botanical fungicides should be made secured. It would be preferable if the government set the prices in accordance with the goods' quality. Legislation regarding their import and export must be made simple. Taxes on these goods ought to be decreased, although they might be raised on chemical fungicides. For large-scale production, the government must make loans with low interest rates available to the producers. It is important to investigate prospective plants with fungicidal qualities. By using plant breeding methods, the percentage of bioactive compounds in such plants can be raised. In order to retain high quality, extraction and processing techniques should be improved. By undertaking a number of studies, particular climatic requirements for the site-specific production of plant species should be discovered [7].
These factors are particularly significant for the industrial future of plant-derived products. In order to meet the quality requirements of the marketed products, companies that produce botanical fungicides strive to improve formulations by stabilizing extracts and maintaining a consistent chemical compositions. These requirements could be resolved with the marketing of synthetic molecules that are exact replicas of natural molecules in all aspects (i.e., organoleptic properties, degree of purity, and absence of residual solvents) [72].
The data needed for the biocontrol agents, such as plant extracts and allelochemicals, should be decided by expert committees. To construct reasonable regulatory procedures and speed up market introduction, regulators, industrial, and academic employees should come together to develop suggestions that take the risk assessment into account [72]. Therefore, it is now more important than ever for academics, decision-makers, business people, and farmers to work together to explore, legalize, properly market, and widely utilize botanical fungicides. Focus should be placed on botanical fungicides if methods like integrated pest management (IPM), organic farming, and sustainable agriculture need to be expanded [7].
9. Conclusion
Phytochemicals are effective fungicides against a wide range of fungal species that cause pre- and post-harvest illnesses of plants. Reviewing available literatures on efficacy of plant products against phytopathogenic fungi causing diseases of economically important crops such as cereal grains, pulses, fruits and vegetables revealed that plant extracts and isolated compounds have significant antifungal activity in in vitro and in vivo assays. Botanical fungicides inhibit development of resistance, are ecofriendly, effective, selective, and more affordable compared to synthetic fungicides. However, their number in the market is very small because of many factors that hinder wide scale production at commercial scale. Challenges of adoption and utilization of botanical fungicides at commercial level for wide scale production may be caused by a variety of issues, including farmers' resistance to using natural products as biofungicides, lack of standardized extraction and formulation techniques, slow results in comparison to chemical fungicides, strict legislation, rapid degradation, and variations in the active ingredient composition with plants grown in various climatic conditions. The ways to address these challenges include increasing awareness among farmers, conducting more research to identify potential plants with fungicidal properties, standardizing extraction and formulation techniques, implementing the idea of plant breeding to increase bioactive agents, identifying favorable environments for site-specific plant species production, discovering synthetic analogues of the active ingredient to maintain quality standards, establishing reasonable regulation procedures and price points for a quicker market introduction. Numerous researchers have recommended isolating and characterizing the active antifungal compounds in the crude extract, conducting in vivo experiments in controlled greenhouse settings and open fields to practically evaluate the use of these extract in the context of an Integrated Pest Management system, determining the precise mechanism of action by which these extracts work, the use of multiple plant extracts in combination to increase effectiveness, conducting phytotoxicity research, analyzing the number and timing of applications to determine the efficacy of the extract to prevent disease in the field, investigating potential toxicity on humans or livestock, as well as the stability of the extracts during grain storage treatment, and the use of plant extracts in conjunction with other well-established disease control practices for effective control. In addition to these recommendations, the authors of this article suggest collaboration of regulatory agencies and researchers from a variety of fields, including chemistry, biology, agriculture, environmental science, engineering, and so on in order to put all these plans into practice.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interest's statement
The authors declare no competing interests.
Additional information
No additional information is available for this paper.
Contributor Information
Ebisa Mirete Deresa, Email: mirete.deresa@ju.edu.et.
Tamiru Fayisa Diriba, Email: fayisa.tamiru@ju.edu.et.
References
- 1.Santra H.K., Banerjee D. Natural products as fungicide and their role in crop protection. Natural . Products in Sustainable Agric. 2020:131–219. doi: 10.1007/978-981-15-3024-1_9. [DOI] [Google Scholar]
- 2.Shuping D.S.S., Eloff J.N. The use of plants to protect plants and food against fungal pathogens : a review afr J tradit complement altern med. Afr. J. Tradit., Complementary Altern. Med. 2017;14(4):120–127. doi: 10.21010/ajtcam.v14i4.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain A., Sarsaiya S., Wu Q., Lu Y., Shi J. A review of plant leaf fungal diseases and its environment speciation. Bioengineered. 2019;10(1):409–424. doi: 10.1080/21655979.2019.1649520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiménez-Reyes M.F., Carrasco H., Olea A.F., Silva-Moreno E. Natural compounds: a sustainable alternative to the phytopathogens control. J. Chil. Chem. Soc. 2019;64(2):4459–4465. doi: 10.4067/S0717-97072019000204459. [DOI] [Google Scholar]
- 5.Shabana Y.M., Abdalla M.E., Shahin A.A., El-Sawy M.M., Draz I.S., Youssif A.W. Efficacy of plant extracts in controlling wheat leaf rust disease caused by Puccinia triticina. Egypt. J. Basic Appl. Sci. 2017;4(1):67–73. doi: 10.1016/j.ejbas.2016.09.002. [DOI] [Google Scholar]
- 6.Makhuvele R., Naidu K., Gbashi S., Thipe V.C., Adebo O.A., Njobeh P.B. The use of plant extracts and their phytochemicals for control of toxigenic fungi and mycotoxins. Heliyon. 2020;6(10) doi: 10.1016/j.heliyon.2020.e05291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhandari S., Yadav P.K., T Sarhan A. Botanical fungicides; current status, fungicidal properties and challenges for wide scale adoption: a review. Rev. Food Agric. 2021;2(2):63–68. doi: 10.26480/rfna.02.2021.63.68. [DOI] [Google Scholar]
- 8.Suteu D., Rusu L., Zaharia C., Badeanu M., Daraban G.M. Challenge of utilization vegetal extracts as natural plant protection products. Appl. Sci. 2020;10(24):1–21. doi: 10.3390/app10248913. [DOI] [Google Scholar]
- 9.Ramírez-Gómez S., Jiménez-García S., Beltrán Campos V., Lourdes García Campos Ma. Plant Pathology and Management of Plant Diseases [Working Title] 2019. Plant metabolites in plant defense against pathogens. [DOI] [Google Scholar]
- 10.Okwute S.K. 2012. Plants as Potential Sources of Pesticidal Agents: A Review. Pesticides - Advances in Chemical and Botanical Pesticides. [DOI] [Google Scholar]
- 11.Lengai G.M.W., Muthomi J.W., Mbega E.R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. African. 2020;7 doi: 10.1016/j.sciaf.2019.e00239. [DOI] [Google Scholar]
- 12.Hernández-Ceja A., Loeza-Lara P.D., Espinosa-García F.J., García-Rodríguez Y.M., Medina-Medrano J.R., Gutiérrez-Hernández G.F., Ceja-Torres L.F. In vitro antifungal activity of plant extracts on pathogenic fungi of blueberry (Vaccinium sp.) Plants. 2021;10(5):1–12. doi: 10.3390/plants10050852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdulabbar Abdulhassan H. The efficiency of some plant extracts on the fungus Macrophomina phaseolina, the causes agent of seed rot and the Okra seedlings death. IOP Conf. Ser. Earth Environ. Sci. 2020;553(1) doi: 10.1088/1755-1315/553/1/012046. [DOI] [Google Scholar]
- 14.Kebede Woldetsadik, mare A. Antifungal activity of some plant extracts against (colletotrichum musae) the cause of postharvest banana anthracnose. J. Plant Pathol. Microbiol. 2014;5(2):2–5. doi: 10.4172/2157-7471.1000226. [DOI] [Google Scholar]
- 15.Ameziane N., Boubaker H., Boudyach H., Msanda F., Jilal A., Ait Benaoumar A. Antifungal activity of Moroccan plants against citrus fruit pathogens. Agron. Sustain. Dev. 2007;27(3):273–277. doi: 10.1051/agro:2007022. [DOI] [Google Scholar]
- 16.Saha D., Dasgupta S., Saha A. Antifungal activity of some plant extracts against fungal pathogens of tea (Camellia sinensis) Pharmaceut. Biol. 2005;43(1):87–91. doi: 10.1080/13880200590903426. [DOI] [Google Scholar]
- 17.Lira-de León K.I., Ramírez-Mares M.V., Sánchez-López V., Ramírez-Lepe M., Salas-Coronado R., Santos-Sánchez N.F., Valadez-Blanco R., Hernández-Carlos B. Effect of crude plant extracts from some Oaxacan flora on two deleterious fungal phytopathogens and extract compatibility with a biofertilizer strain. Front. Microbiol. 2014;5(AUG):1–10. doi: 10.3389/fmicb.2014.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mugao L.G., Muturi P.W., Gichimu B.M., Njoroge E.K. In vitro control of phytophthora infestans and Alternaria solani using crude extracts and essential oils from selected plants. Int. J. Agronomy. 2020 doi: 10.1155/2020/8845692. [DOI] [Google Scholar]
- 19.Kumar Ramaiah A., Kumar Garampalli R.H. Vitro antifungal activity of some plant extracts against Fusarium oxysporum f. sp. lycopersici. Pelagia Res. Library Asian J. Plant Sci. Res. 2015;5(1):22–27. www.pelagiaresearchlibrary.com [Google Scholar]
- 20.Derbalah A.S., Dewir Y.H., El-Sayed A.E.N.B. Antifungal activity of some plant extracts against sugar beet damping-off caused by Sclerotium rolfsii. Ann. Microbiol. 2012;62(3):1021–1029. doi: 10.1007/s13213-011-0342-2. [DOI] [Google Scholar]
- 21.Pwakem D.B., Sowley E.N.K., Kankam F. Evaluation of efficacy of some plant extracts for the control of anthracnose (colletotrichum gloeosporioides) of white yam (Dioscorea rotundata poir) Agric. Food Sci. J. Ghana. 2020;13(October):1268–1281. [Google Scholar]
- 22.Baka Z.A.M., Rashad Y.M. Alternative control of early blight of tomato using plant extracts from Acacia nilotica, Achillea fragrantissima and Calotropis procera. Phytopathol. Mediterr. 2016;55(1):121–129. doi: 10.14601/Phytopathol_Mediterr-17161. [DOI] [Google Scholar]
- 23.Osman A., Osman A., Mohamed I.S. Antifungal evaluation of some leaves extracts and fungicide against Fusarium oxysporum f . sp . lycopersici causal agent wilt of tomato. Bioteknologi. 2017;14(1):1–8. doi: 10.13057/biotek/c140101. [DOI] [Google Scholar]
- 24.Nashwa S.M.A., Abo-Elyou K.A.M. Evaluation of various plant extracts against the early blight disease of tomato plants under greenhouse and field conditions. Plant Protect. Sci. 2012;48(2):74–79. doi: 10.17221/14/2011-pps. [DOI] [Google Scholar]
- 25.Carvalho D.D.C., Alves E., Barbosa Camargos R., Ferreira Oliveira D., Soares Scolforo J.R., de Carvalho D.A., Sâmia Batista T.R. Plant extracts to control Alternaria alternata in Murcott tangor fruits. Rev. Iberoam. De. Micol. 2011;28(4):173–178. doi: 10.1016/j.riam.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Darapanit A., Boonyuen N., Leesutthiphonchai W., Nuankaew S., Piasai O. Identification, pathogenicity and effects of plant extracts on Neopestalotiopsis and Pseudopestalotiopsis causing fruit diseases. Sci. Rep. 2021;11(1):1–11. doi: 10.1038/s41598-021-02113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Rahman a N., Mostafa a a, Abdel-Megeed a, Yakout S.M., Hussein S.a. Fungicidal activities of certain methanolic plant extracts against tomato phytopathogenic fungi. Afr. J. Microbiol. Res. 2013;7(6):517–524. doi: 10.5897/AJMR12.1902. [DOI] [Google Scholar]
- 28.Chrapačienė S., Rasiukevičiūtė N., Valiuškaitė A. Plant extracts as biofungicides against soil-borne pathogen alternaria spp. Rural Dev. 2022;2021(1):15–18. doi: 10.15544/rd.2021.003. [DOI] [Google Scholar]
- 29.Mekam P.N., Martini S., Nguefack J., Tagliazucchi D., Mangoumou G.N., Stefani E. Activity of extracts from three tropical plants towards fungi pathogenic to tomato (Solanum lycopersicum) Phytopathol. Mediterr. 2019;58(3):573–586. doi: 10.14601/Phyto-10891. [DOI] [Google Scholar]
- 30.Chohan S., Perveen R. Phytochemical analysis and antifungal efficacy of rhizome extracts of various plants against fusarium wilt and root rot of tomato. Int. J. Agric. Biol. 2015;17(6):1193–1199. doi: 10.17957/IJAB/15.0055. [DOI] [Google Scholar]
- 31.Olakunle O., Deborah J., Irene O. Antifungal activity and phytochemical analysis of selected fruit peels. J. Bio. Med. 2019;3(1):40–43. doi: 10.17352/jbm.000013. [DOI] [Google Scholar]
- 32.Yamdeu G., Hubert J., Julienne N., Charles D.D., Sandrine P.T., Romain F.F., Zollo A., Henry P. Antifungal potential and phytochemical analysis of extracts from seven Cameroonian plants against late blight pathogen Phytophthora infestans. Int. J. Current Microbiol. Appl. Sci. 2013;2(5):140–154. [Google Scholar]
- 33.Elsherbiny A Elsherbiny A.Y.E.-K. Phytochemical analysis and antifungal activity of fruit leaves extracts on the mycelial growth of fungal plant pathogens. J. Plant Pathol. Microbiol. 2013;4(9) doi: 10.4172/2157-7471.1000199. [DOI] [Google Scholar]
- 34.Chohan S., Perveen R., Anees M., Azeem M., Abid M. Estimation of secondary metabolites of indigenous medicinal plant extracts and their in vitro and in vivo efficacy against tomato early blight disease in Pakistan. J. Plant Dis. Prot. 2019;126(6):553–563. doi: 10.1007/s41348-019-00252-6. [DOI] [Google Scholar]
- 35.Soylu E.M., Yiğitbaş H., Tok F.M., Soylu S., Kurt Ş., Baysal Ö., Kaya A.D. Chemical composition and antifungal activity of the essential oil of Artemisia annua L. against foliar and soil-borne fungal pathogens/Die chemische Zusammensetzung und antimikrobielle Aktivität das ätherischen Öls von Artemisia annua L. gegen blatt- und bodenbürtige pilzliche Krankheitserreger. Z. für Pflanzenkrankh. Pflanzenschutz/J. Plant Dis. Prot. 2005;112(3):229–239. https://www.jstor.org/stable/45154906 [Google Scholar]
- 36.Liao M., Ren X., Gao Q., Liu N., Tang F., Wang G., Cao H. Anti-fungal activity of moso bamboo (Phyllostachys pubescens) leaf extract and its development into a botanical fungicide to control pepper phytophthora blight. Sci. Rep. 2021;11(1):1–10. doi: 10.1038/s41598-021-83598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goufo P., Teugwa Mofor C., Fontem D.A., Ngnokam D. High efficacy of extracts of Cameroon plants against tomato late blight disease. Agron. Sustain. Dev. 2008;28(4):567–573. doi: 10.1051/agro:2008036. [DOI] [Google Scholar]
- 38.Nahunnaro H., Bayaso I. Inhibitory activity of plant extracts on the early blight pathogen Alternaria solani</i< growth. Global J. Agric. Sci. 2012;11(1):57–62. doi: 10.4314/gjass.v11i1.10. [DOI] [Google Scholar]
- 39.Bashir S., Jabeen K., Iqbal S., Javed S., Naeem A. Lantana camara: phytochemical analysis and antifungal prospective. Planta Daninha. 2019;37 doi: 10.1590/s0100-83582019370100137. [DOI] [Google Scholar]
- 40.Shamim A.H.M. Medicinal plants, A promising source of natural fungicides against Magnaporthe oryzae Triticum, causal Agent of wheat blast. Am. J. Plant Sci. 2021;12(5):748–758. doi: 10.4236/ajps.2021.125051. [DOI] [Google Scholar]
- 41.Salhi N., Mohammed Saghir S.A., Terzi V., Brahmi I., Ghedairi N., Bissati S. Antifungal activity of aqueous extracts of some dominant Algerian medicinal plants. BioMed Res. Int. 2017 doi: 10.1155/2017/7526291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olufolaji D., Adeosun B., Onasanya R. In vitro investigation on antifungal activity of some plant extracts against Pyricularia oryzae. Nigerian J. Biotechnol. 2015;29(1):38. doi: 10.4314/njb.v29i1.6. [DOI] [Google Scholar]
- 43.Durgeshlal C., Sahroj Khan M., Prabhat S.A., Aaditya Prasad Y. Antifungal activity of three different ethanolic extract against isolates from diseased rice plant. J. Anal. Tech. Res. 2019;1(1):47–63. doi: 10.26502/jatri.007. [DOI] [Google Scholar]
- 44.Elkhwaga A., Elzaawely A., Draz I., Ismail A., El-Zahaby H. Potential of some plant extracts in controlling wheat leaf rust caused by Puccinia triticina Eriks. Environ. Bio. Soil Sec. 2018;2(1):66–67. doi: 10.21608/jenvbs.2018.4421.1031. [DOI] [Google Scholar]
- 45.Geta K. 2019. Efficiency of Medicinal Plants to Control Seed Borne Fungi of Sorghum Grains; pp. 2017–2019. [Google Scholar]
- 46.Ahmad L., Pathak N. Antifungal potential of plant extracts against seed-borne fungi isolated from barley seeds (hordeum vulgare L.) J. Plant Pathol. Microbiol. 2016;7(5):5–8. doi: 10.4172/2157-7471.1000350. [DOI] [Google Scholar]
- 47.Nabila N.H., Ramli N.K.C.M., Yunus N.Y., Md Latip S.N.H. Evaluation of selected herbs for biocontrol of rice blast disease. IOP Conf. Ser. Earth Environ. Sci. 2021;685(1) doi: 10.1088/1755-1315/685/1/012026. [DOI] [Google Scholar]
- 48.Seepe H.A., Lodama K.E., Sutherland R., Nxumalo W., Amoo S.O. In vivo antifungal activity of south african medicinal plant extracts against fusarium pathogens and their phytotoxicity evaluation. Plants. 2020;9(12):1–21. doi: 10.3390/plants9121668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng C., Zhu X., Cui Z., Li Y. Antifungal activity of plant extracts against Embellisia astragali, the fungal causal agent of yellow dwarf and root-rot disease of standing milkvetch. Crop Pasture Sci. 2015;66(7):735–739. doi: 10.1071/CP15012. [DOI] [Google Scholar]
- 50.Dalcin M.S., Dias B.L., Osorio P.R.A., Cardoso V.D., Ferreira T.P. de S., Tschoeke P.H., Alves M.V.G., Dos Santos G.R. Botanical fungicides in the control of soybean leaf diseases/Fungicidas botânicos no controle de doenças foliares na soja. Brazilian J. Dev. 2021;7(4):37715–37733. doi: 10.34117/bjdv7n4-303. [DOI] [Google Scholar]
- 51.Masangwa J.I.G., Aveling T.A.S., Kritzinger Q. Screening of plant extracts for antifungal activities against Colletotrichum species of common bean (Phaseolus vulgaris L.) and cowpea (Vigna unguiculata (L.) Walp) J. Agric. Sci. 2013;151(4):482–491. doi: 10.1017/S0021859612000524. [DOI] [Google Scholar]
- 52.Yusnawan E., Inayati A. Methanolic extracts ofthreeweeds as botanical fungicides to controlpeanut rust disease. Nusantara Bio. 1970;8(1):117–122. doi: 10.13057/nusbiosci/n080120. [DOI] [Google Scholar]
- 53.Kareem K.T., Afolabi Q.O., Shorinmade A.Y., Akinbode O.A. Management of seed-borne fungi in cowpea using leaf extracts and sodium bicarbonate. J. Appl. Sci. Environ. Manag. 2018;22(4):565. doi: 10.4314/jasem.v22i4.23. [DOI] [Google Scholar]
- 54.Ribera A.E., Zuñiga G. Induced plant secondary metabolites for phytopatogenic fungi control: a review. J. Soil Sci. Plant Nutr. 2012 doi: 10.4067/s0718-95162012005000040. [DOI] [Google Scholar]
- 55.Sukrasno S. Fusarium - Plant Diseases, Pathogen Diversity, Genetic Diversity, Resistance and Molecular Markers. 2018. Plant secondary metabolites for antifusarium and antiphytophthora. [DOI] [Google Scholar]
- 56.Fu W.J., Liu J., Zhang M., Li J.Q., Hu J.F., Xu L.R., Dai G.H. Isolation, purification and identification of the active compound of turmeric and its potential application to control cucumber powdery mildew. J. Agric. Sci. 2018;156(3):358–366. doi: 10.1017/S0021859618000345. [DOI] [Google Scholar]
- 57.Kim M.Y., Han J.W., Dang Q., Le Kim J.C., Kim H., Choi G.J. Characterization of Alternaria porri causing onion purple blotch and its antifungal compound magnolol identified from Caryodaphnopsis baviensis. PLoS One. 2022;17(1 January):1–17. doi: 10.1371/journal.pone.0262836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim B., Han J.W., Thi Ngo M., Le Dang Q., Kim J.C., Kim H., Choi G.J. Identification of novel compounds, oleanane- and ursane-type triterpene glycosides, from Trevesia palmata: their biocontrol activity against phytopathogenic fungi. Sci. Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-32956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han J.W., Shim S.H., Jang K.S., Choi Y.H., Kim H., Choi G.J. In vivo disease control efficacy of isoquinoline alkaloids isolated from Corydalis ternata against wheat leaf rust and pepper anthracnose. J. Microbiol. Biotechnol. 2018;28(2):262–266. doi: 10.4014/jmb.1707.07009. [DOI] [PubMed] [Google Scholar]
- 60.Lee C.H., Lee H.J., Jeon J.H., Lee H.S. In vivo antifungal effects of coptis japonica root-derived isoquinoline alkaloids against phytopathogenic fungi. J. Microbiol. Biotechnol. 2005;15(6):1402–1407. https://agris.fao.org/agris-search/search.do?recordID=KR2006015352 [Google Scholar]
- 61.Cho J.Y., Choi G.J., Son S.W., Jang K.S., Lim H.K., Lee S.O., Sung N.D., Cho K.Y., Kim J.-C. Isolation and antifungal activity of lignans from Myristica fragrans against various plant pathogenic fungi. Pest Manag. Sci. 2007;63(9):935–940. doi: 10.1002/ps.1420. [DOI] [PubMed] [Google Scholar]
- 62.Seepe H.A., Ramakadi T.G., Lebepe C.M., Amoo S.O., Nxumalo W. Antifungal activity of isolated compounds from the leaves of combretum erythrophyllum (burch.) sond. And withania somnifera (L.) dunal against Fusarium pathogens. Molecules. 2021;26(16):4732. doi: 10.3390/molecules26164732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paz C., Viscardi S., Iturra A., Marin V., Miranda F., Barra P.J., Mendez I., Duran P. Antifungal effects of drimane sesquiterpenoids isolated from drimys winteri against gaeumannomyces graminis var. tritici. Appl. Environ. Microbiol. 2020;86(24) doi: 10.1128/aem.01834-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su H., Blair R., Johnson T., Marrone P. Regalia® bioprotectant in plant disease management. Outlooks Pest Manag. 2012;23(1):30–34. doi: 10.1564/23feb09. [DOI] [Google Scholar]
- 65.Riesland M. Office of chemical safety and pollution prevention; 2019. United States Environmental Protection Agency Washington, D.C. 20460.https://www3.epa.gov/pesticides/chem_search/ppls/010163-00357-20190614.pdf [Google Scholar]
- 66.Pehrson C. Office of chemical safety and pollution prevention; 2018. United States Environmental Protection Agency Washington, D.C. 20460.https://www3.epa.gov/pesticides/chem_search/ppls/086182-00001-20180531.pdf [Google Scholar]
- 67.FMC launches Fracture fungicide. (n.d.). Fruit Growers News. https://fruitgrowersnews.com/news/fmc-launches-fracture-fungicide/.
- 68.Sporan EC2. (n.d.). KeyPlex Plant Nutrition. https://www.keyplex.com/product/sporan-ec2/.
- 69.Thyme Guard® ARBICO organics. https://www.arbico-organics.com/product/thyme-guard-pesticide-fungicide-bactericide/natural-organic-plant-disease-control n.d.
- 70.Yoon M.Y., Cha B., Kim J.C. Recent trends in studies on botanical fungicides in agriculture. Plant Pathol. J. 2013;29(1):1–9. doi: 10.5423/PPJ.RW.05.2012.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gurjar M.S., Ali S., Akhtar M., Singh K.S. Efficacy of plant extracts in plant disease management. Agric. Sci. 2012;3(3):425–433. doi: 10.4236/as.2012.33050. [DOI] [Google Scholar]
- 72.Regnault-Roger C., Philogène B.J.R. Past and current prospects for the use of botanicals and plant allelochemicals in integrated pest management. Pharmaceut. Biol. 2008;46(1–2):41–52. doi: 10.1080/13880200701729794. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.