Abstract
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection activates mast cells and induces a cytokine storm, leading to severe Coronavirus disease in 2019 (COVID-19). SARS-CoV-2 employs angiotensin-converting enzyme 2 (ACE2) for cell entry. In the present study, the expression of ACE2 and its mechanism in activated mast cells were studied utilizing the human mast cell line, HMC-1 cells and it was elucidated whether dexamethasone used as a treatment for COVID-19 could regulate ACE2 expression. Here we documented for the first time that levels of ACE2 were increased by stimulation of phorbol 12-myristate 13-acetate and A23187 (PMACI) in HMC-1 cells. Increased levels of ACE2 were significantly diminished by treatment with Wortmannin, SP600125, SB203580, PD98059, or SR11302. The expression of ACE2 was most significantly reduced by the activating protein (AP)-1 inhibitor SR11302. PMACI stimulation enhanced the expression of the transcription factor AP-1 for ACE2. In addition, levels of transmembrane protease/serine subfamily member 2 (TMPRSS2) and tryptase were increased in PMACI-stimulated HMC-1 cells. However, dexamethasone significantly lowered levels of ACE2, TMPRSS2, and tryptase generated by PMACI. Treatment with dexamethasone also reduced activation of signaling molecules linked to ACE2 expression. According to these findings, levels of ACE2 were up-regulated through activation of AP-1 in mast cells, suggesting that suppressing ACE2 levels in mast cells would be a therapeutic approach to lessen the harm caused by COVID-19.
Keywords: SARS-CoV-2, Mast cell, ACE2, AP-1, Dexamethasone
1. Introduction
Coronavirus disease in 2019 (COVID-19) is mostly caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) that invades the respiratory tract. As of right now, COVID-19 is in a pandemic state due to asymptomatic infection, virus mutation, and reinfection by breaching the immune system [1]. Since the COVID-19 outbreak, a variety of vaccinations and antiviral medications have been utilized, however allergic inflammatory reactions have been demonstrated to be major adverse effects [2].
The principal entry receptor for SARS-CoV-2 is angiotensin-converting enzyme 2 (ACE2), which the spike protein on the surface of the virus binds to, and when priming occurs by trans-membrane protease serine 2 (TMPRSS2), fusion with the cell membrane is promoted and intracellular infection occurs [3]. Therefore, as a result of the expression of ACE2 and TMPRSS2 in a variety of tissues, including the nasal cavity, oral cavity, ear, respiratory tract, and lung, these organs can become infected with SARS-CoV-2 [4]. Mast cells are present in almost all tissues of the body, and are particularly concentrated in the whole respiratory tract and the nasal cavity, and are the main causative cells of allergic inflammatory reactions [5]. They can act as hosts for SARS-CoV-2 because they express the enzymes ACE2 and tryptase, which can facilitate the virus's infection [2]. In addition, mast cells are activated through the retinoic acid-inducible gene I, toll-like receptors (TLR)3, TLR7, and TLR8 by RNA viruses such as SARS-CoV-2 [6]. Cytokine storm induced by infection of SARS-CoV-2 is mediated by hyperinflammation via increasing inflammatory cytokine from mast cells in severe COVID-19 [7]. The COVID-19 postmortem lung biopsies in SARS-CoV-2 infections patients exhibit an enormously elevated number of mast cells, indicating that SARS-CoV-2 infection may have attracted mast cells to the alveolar septa [8]. Post-COVID syndrome may progress in association with the development of mast cell activation syndrome (MCAS) [9].
A glucocorticoid drug called dexamethasone is used to treat rheumatic conditions, a number of severe allergies, asthma, brain edema, chronic obstructive pulmonary disease, skin disorders, eye pain, and croup [10]. It has a mast cell-stabilizing effects and inhibits degranulation of mast cells [5]. Thus, corticosteroid therapy interferes with mast cell activity in relation to inflammation [11].
Based on the above theories, it can be seen that mast cell is an important cell in COVID-19. In the present study, the expression of ACE2 and its mechanism in activated mast cells were investigated using the human mast cell line, HMC-1 and it was elucidated whether dexamethasone could regulate ACE2 expression.
2. Materials and methods
2.1. HMC-1 cells culture
HMC-1 cell (Cat# SCC067, RRID:CVCL_H206) was kindly provided by Eichi Morri (Osaka University, Osaka, Japan). HMC-1 cells were grown in IMDM supplemented with antibiotics (100 U/ml penicillin/100 μg/ml streptomycin) and 10% heat inactivated fetal bovine serum (FBS) at 37 °C, 5% CO2 and 95% humidity. HMC-1 cells were stimulated with 5 μM phorbol 12-myristate 13-acetate and 100 nM A23187 (PMACI, Sigma-Aldrich, St. Louis, Mo, USA) on 24 or 6 well plate. The following drugs were utilized to target phosphatidylinositide-3-kinase (PI3K), c-Jun NH2-terminal kinases (JNK), p38, extracellular signal-regulated kinase (ERK), activating protein (AP)-1, and nuclear factor (NF)-κB: Wortmannin, SP600125, SB203580, PD98059, SR11302, and pyrrolidine dithiocarbamate (PDTC). These drugs were obtained from Sigma-Aldrich. This study utilized 500 ng/ml of dexamethasone (Sigma-Aldrich) in accordance with the prior study [5].
2.2. Extraction of total RNA and analysis of mRNA expression using quantitative real-time polymerase chain reaction (qRT-PCR)
The total RNA was extracted from HMC-1 cells using an easy-BLUE RNA extraction kit (iNtRON Biotechnology, Kyunggi-do, Korea). The concentration of total RNA was analyzed by Nanodrop spectrophotometry (Thermo fisher scientific Inc., Waltham, MA, USA). Total RNA sample (2.5 µg) was reverse-transcribed to cDNA using a power cDNA synthesis kit (iNtRON Biotechnology, Sungnam, Republic of Korea). For human ACE2, TMPRSS2, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), qRT-PCR was performed using a SYBR Green master mix with primers (ACE2: forward 5′-GGG ATC AGA GAT CGG AAG AAG AAA-3′, reverse 5′- AGG AGG TCT GAA CAT CAT CAG TG-3′, TMPRSS2: forward 5′-ACT CTG GAA GTT CAT GGG CAG-3′, reverse 5′-TGA AGT TTG GTC CGT AGA GGC-3′, GAPDH: forward 5′- CCA AAG GGT CAT CAT CTC TG-3′, reverse 5′-CCT GCT TCA CCA CCT TCT TG-3′) and expression of mRNA was analyzed using an ABI StepOne real-time PCR System (Applied Biosystems, Foster City, CA, USA). All data were analyzed using the ΔΔCT method.
2.3. Western blot analysis
Collected cell was rinsed twice with phosphate buffered saline (PBS) and then lysed in ice-cold RIPA lysis buffer containing 1% protease inhibitors and phosphatase inhibitor). Total extracted proteins were separated by electrophoresis, and protein bands were transferred to nitrocellulose membranes by transfer. After blocking in 6% bovine serum albumin for 2 h, membranes were reacted with an ACE2, TMPRSS2, PI3K, phosphorylated (p)AKT, AKT, p-mitogen-activated protein kinases (p-MAPKs; pJNK, p-p38, pERK), MAPKs (JNK, p38, ERK), c-jun, c-fos, or GAPDH antibodies (Santa Cruz, CA, USA) diluted 1: 500 in PBS containing Tween-20 overnight at room temperature. Membranes were washed and reacted for 30 min with horse radish peroxidase-conjugated secondary antibody. Proteins were visualized using an enhanced chemiluminescence kit (Amersham Corp. Newark, NJ, USA).
2.4. Fluorescence microscopy
Collected were fixed with 10% formaldehyde. Sections were blocked with 10% FBS and then reacted with an anti-mouse ACE2 or TMPRSS2 antibody diluted 1: 200 in 10% FBS. After washing, tetramethylrhodamin (TRITC)-conjugated secondary antibody (Invitrogen Co., Carlsbad, USA) was reacted for 30 min. 4′, 6-diamidino-2-phenylinodole contained mounting medium (Vector Laboratories, Burlingame, CA, USA) was used to counterstain DNA. All stained sections were analyzed under a fluorescence microscopy.
2.5. Tryptase assay
Tryptase from HMC-1 cells were measured using a Human Mast Cell Tryptase ELISA Kits according to manufacturer’s protocols (MyBiosource Co., San Diego, California, USA). Optical density (OD) was read at 450 nm using an ELISA reader.
2.6. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) tetrazolium assay
An evaluation of cell viability was attained by the MTT (Sigma Chemical Co., St. Louis, MO, USA) assay where a tetrazolium reagent is reduced to insoluble purple formazan crystals by mitochondria of viable cells. Absorbance was measured at OD 540 nm.
2.7. Statistical analysis
For the statistical analysis, SPSS statistics software (SPSS Inc., Chicago, IL, USA) was utilized. Each experiment was performed at least three times independently. The mean ± standard deviations (SDs) was used to express data. Independent t-test was used when there were 2 groups to compare (Blank and PMACI or PMACI and PMACI + dexamethasone). Each dataset as P < 0.05 was considered statistically significant result.
3. Results
3.1. Up-regulation of ACE2 in activated HMC-1 cells
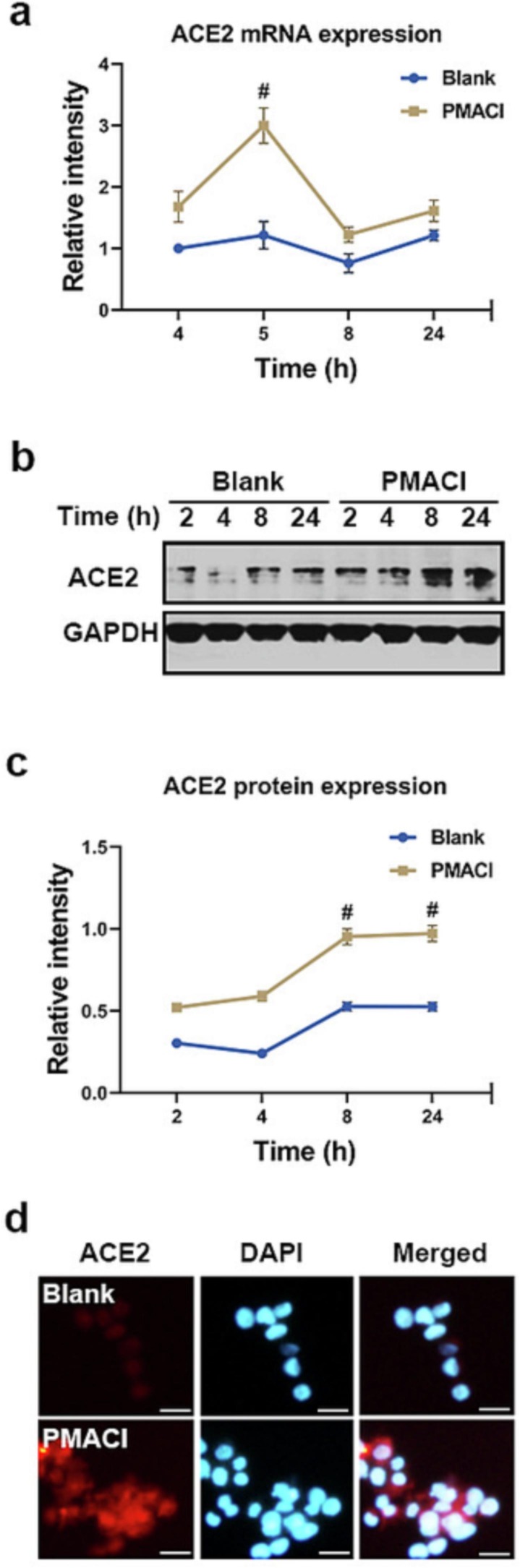
SARS-CoV-2 hit the innate immune system in order to cause infection, through TLR7 and TLR8, and induces the degranulation of mast cells [6]. Mast cell is used as a host cell for SARS-CoV-2 [2]. Resiquimod is a small agonist of the endosome-located TLR7/8. PMACI is a mast cell stimulator that activates the signaling process involved in mast cell degranulation and inflammatory response [12]. In order to find out whether ACE2 is expressed in activated mast cells, we treated a resiquimod or PMACI. First, to check if ACE2 mRNA was expressed in activated HMC-1 cells, we applied qRT-PCR. As a result, it was discovered that ACE2 mRNA expression peaked at 5 h after PMACI activation and thereafter dropped (Fig. 1 a). For a variety of time periods, resiquimod did not boost the expression of ACE mRNA (data not shown).
Fig. 1.
Up-regulation of ACE2 in activated HMC-1 cells. (a) HMC-1 cells were treated with PMACI for various times. The levels of ACE2 mRNA expression were analyzed by qRT-PCR. (b) HMC-1 cells were treated with PMACI for various times. ACE2 protein expression was analyzed by Western blotting. Results are representative of three independent experiments. (c) The relative densities were quantified by densitometry means ACE2/GAPDH. Values are expressed as the means ± SD (n = 3 experiments). (d) HMC-1 cells were treated with PMACI for 24 h. ACE2 was stained by a primary antibody for 1 h and then incubated with secondary TRITC-conjugated IgG for 30 min. Results are representative of three independent experiments (Original magnification × 400, scale bar = 10 μm). #P < 0.05: significantly different from unstimulated cells.
Next, whether the protein levels of ACE2 were increased by PMACI stimulation, Western blotting was performed for ACE2. After being stimulated with PMACI for 8 h, ACE2 expression started to rise and persisted for 24 h (Fig. 1b-c). Additionally, it was demonstrated by fluorescence microscopy that PMACI stimulation increased the amounts of ACE2 protein (Fig. 1d).
3.2. Involvement of PI3K, MAPKs, AP-1, and NF-κB in ACE2 expression
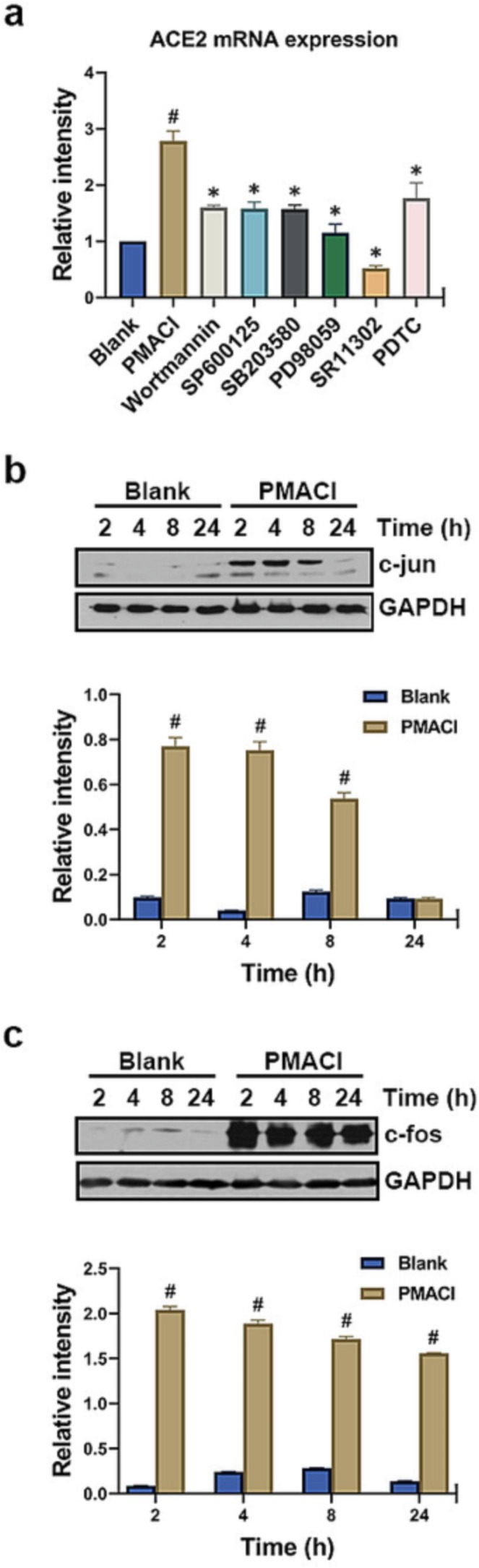
To identify the signaling mechanisms that contribute to the expression of ACE2, we used specific pharmacological inhibitors (Wortmannin, SP600125, SB203580, PD98059, SR11302, and PDTC) targeting PI3K, JNK, p38, ERK, AP-1 and NF-κB. The rise in ACE2 mRNA expression caused by PMACI was considerably suppressed by Wortmannin (about 66% inhibition), SP600125 (about 67% inhibition), SB203580 (about 68% inhibition), PD98059 (about 91% inhibition), SR11302 (about 126% inhibition) or PDTC (about 57% inhibition) (Fig. 2 a, P < 0.05). Notably, AP-1 inhibitor SR11302 showed the best effect in the suppression of ACE2 mRNA expression (Fig. 2a).
Fig. 2.
Involvement of PI3K, MAPKs, AP-1, and NF-κB in ACE2 expression. (a) HMC-1 cells were pre-treated with 1 µM Wortmann, 10 µM SP600125, 0.2 µM SB203580, 1 µM PD98059, 10 µM SR11302, or 10 µM PDTC for 1 h, then stimulated with PMACI for 5 h. The levels of ACE2 mRNA expression were analyzed by qRT-PCR. (b-c) HMC-1 cells were treated with PMACI for various times. The protein expression of c-jun and c-fos was analyzed by Western blotting. (upper) Results are representative of three independent experiments. (lower) The relative densities were quantified by densitometry means c-jun or c-fos/GAPDH. Values are expressed as the means ± SD (n = 3 experiments). #P < 0.05; significantly different from unstimulated cells, *P < 0.05, significantly different from PMACI-stimulated cells.
From the result of Fig. 2a, it was found that ACE2 mRNA expression was mainly regulated by AP-1. So, we performed Western blotting on the AP-1 components (c-jun and c-fos) to analyze whether the expression of AP-1 is up-regulated in activated HMC-1 cells. At 2–4 h after PMACI stimulation, the expression of c-jun was dramatically increased. However, the expression of c-jun decreased after 8 h of PMACI stimulation (Fig. 2b). In addition, it was found that expression of c-fos peak at 2 h after PMACI stimulation and tended to decreased after 4 h (Fig. 2c).
3.3. Up-regulation of TMPRSS2 and tryptase in activated HMC-1 cells
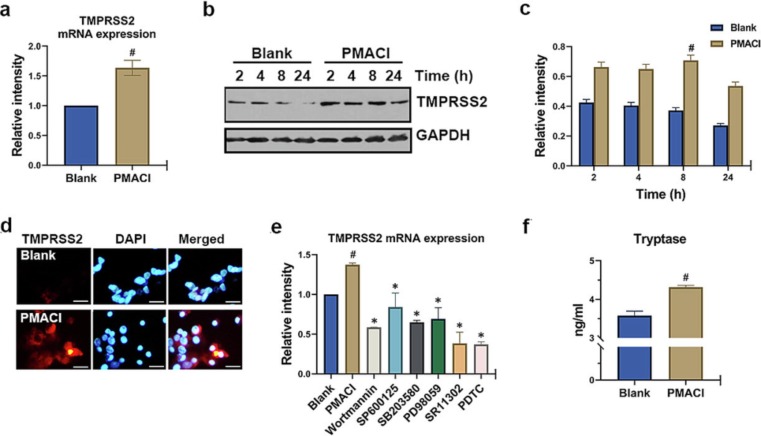
The engagement of the viral spike protein with ACE2 and the proteolytic effect of a TMPRSS2 on the viral spike protein permit SARS-CoV-2 to enter the target [1]. So, we used qRT-PCR and Western blotting to investigate if PMACI activation raised the TMPRSS2 mRNA and protein levels. As shown in Fig. 3 a, PMACI stimulation significantly increased the mRNA levels of TMPRSS2 (P < 0.05). Also, protein levels of TMPRSS2 increased by PMACI stimulation persisted for up to 24 h (Fig. 3b-c). At 8 h after PMACI stimulation, the protein expression of TMPRSS2 was significantly increased compared with the unstimulated cells (Fig. 3c, P < 0.05). It was discovered using immunocytochemistry that the mast cell surface had elevated TMPRSS2 expression (Fig. 3d). Additionally, particular pharmacological inhibitors were employed in order to identify the signaling pathways involved in TMPRSS2 expression (Wortmannin, SP600125, SB203580, PD98059, SR11302, and PDTC). As a result, Wortmannin, SP600125, SB203580, PD98059, SR11302, or PDTC greatly reduced the rise in TMPRSS2 mRNA expression brought on by PMACI (Fig. 3e, P < 0.05).
Fig. 3.
Up-regulation of TMPRSS2 and tryptase in activated HMC-1 cells. (a) HMC-1 cells were treated with PMACI for 5 h. The levels of TMPRSS2 mRNA expression were analyzed by qRT-PCR. (b) HMC-1 cells were treated with PMACI for various times. TMPRSS2 protein expression was analyzed by Western blotting. Results are representative of three independent experiments. (c) The relative densities were quantified by densitometry means TMPRSS2/GAPDH. Values are expressed as the means ± SD (n = 3 experiments). (d) HMC-1 cells were treated with PMACI for 24 h. TMPRSS2 was stained by a primary antibody for 1 h and then incubated with secondary TRITC-conjugated IgG for 30 min. Results are representative of three independent experiments (Original magnification × 400, scale bar = 10 μm). (e) HMC-1 cells were pre-treated with 1 µM Wortmann, 10 µM SP600125, 0.2 µM SB203580, 1 µM PD98059, 10 µM SR11302, or 10 µM PDTC for 1 h, then stimulated with PMACI for 5 h. The levels of TMPRSS2 mRNA expression were analyzed by qRT-PCR. (f) HMC-1 cells were treated with PMACI for 24 h. Levels of tryptase were analyzed by Tryptase assay kit. #P < 0.05: significantly different from unstimulated cells.
Tryptase can encourage SARS-CoV-2 infection, and when spike protein binds to ACE2, it causes a fast degranulation of mast cells [13]. Tryptase is one of the most abundant serine proteases produced by degranulation of mast cells. In the current investigation, PMACI stimulation significantly boosted release of tryptase (Fig. 3f, P < 0.05).
3.4. Down-regulation of ACE2, TMPRSS2, and tryptase by dexamethasone
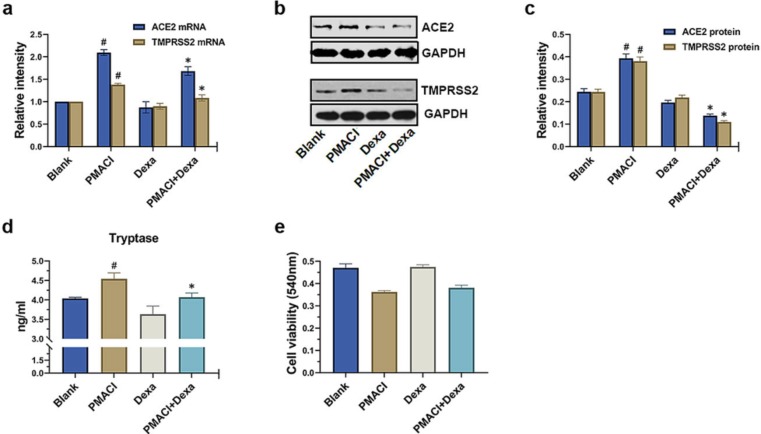
The ACE2, TMPRSS2, and tryptase are important targets for SARS-CoV-2 entry inhibitors. Dexamethasone is an inhibitor of mast cell degranulation and has been shown to be helpful in a randomized clinical trial with COVID-19 patients [11]. Thus, it was investigated whether dexamethasone could control the expression of ACE2 and TMPRSS2 and secretion of tryptase on PMACI-stimulated HMC-1 cells. As a result, it was found that dexamethasone was shown to significantly lower the protein levels of ACE2 and TMPRSS2 as well as the mRNA levels of ACE2 and TMPRSS2 (Fig. 4 a-c, P < 0.05). Tryptase levels that were elevated by PMACI stimulation were significantly diminished by dexamethasone (Fig. 4d, P < 0.05). The MTT assay was used to evaluate whether dexamethasone affects cytotoxicity. As shown in Fig. 4e, dexamethasone had no discernible impact on cytotoxicity.
Fig. 4.
Down-regulation of ACE2, TMPRSS2, and tryptase by dexamethasone. (a) HMC-1 cells were pretreated with dexamethasone (500 ng/ml) for 1 h and then stimulated with PMACI for 5 h. The levels of ACE2 and TMPRSS2 mRNA expression were analyzed by qRT-PCR. (b) HMC-1 cells were pretreated with dexamethasone (500 ng/ml) for 1 h and then stimulated with PMACI for 24 h. The protein expression of ACE2 and TMPRSS2 was analyzed by Western blotting. Results are representative of three independent experiments. (c) The relative densities were quantified by densitometry means ACE2/GAPDH and TMPRSS2/GAPDH. Values are expressed as the means ± SD (n = 3 experiments). HMC-1 cells were pretreated with dexamethasone (500 ng/ml) for 1 h and then stimulated with PMACI for 24 h. (d) Levels of tryptase were analyzed by Tryptase assay kit. (e) Cell viability was evaluated by MTT assay. #P < 0.05; significantly different from unstimulated cells, *P < 0.05, significantly different from PMACI-stimulated cells. Dexa, dexamethasone.
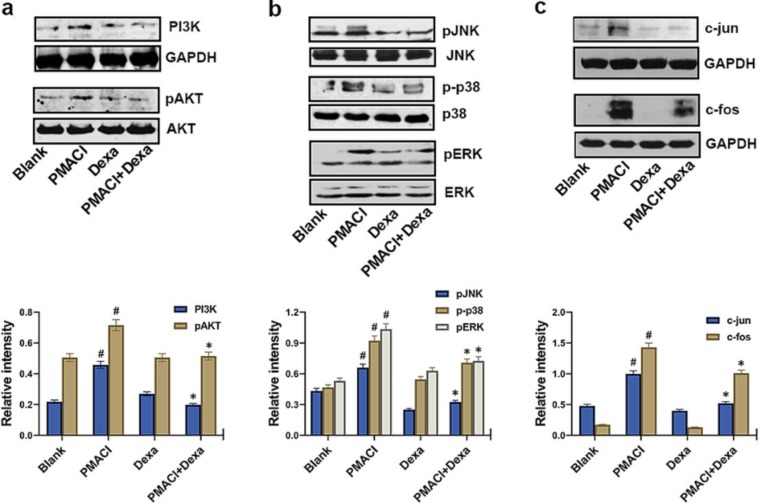
3.5. Blocking of PI3K, MAPKs, and AP-1 by dexamethasone
Finally, Western blotting was done on PI3K, pAKT, pJNK, p-p38, pERK, c-fos, and c-jun to demonstrate the suppressive action of dexamethasone in the signaling pathways linked to the expression of ACE2. The findings demonstrated showed that PMACI massively raised the activation of PI3K, pAKT, pJNK, p-p38, pERK, c-fos, and c-jun, while dexamethasone considerably suppressed the activation of PI3K, pAKT, pJNK, p-p38, pERK, c-fos and c-jun in the PMACI-stimulated HMC-1 cells (Fig. 5 ).
Fig. 5.
Blocking of PI3K, MAPKs, and AP-1 by dexamethasone. (a) HMC-1 cells were pretreated with dexamethasone (500 ng/ml) for 1 h and then stimulated with PMACI for 30 min. The protein expression of PI3K and pAKT was analyzed by Western blotting. (upper) Results are representative of three independent experiments. (lower) The relative densities were quantified by densitometry means PI3K/GAPDH and pAKT/AKT. (b) HMC-1 cells were pretreated with dexamethasone (500 ng/ml) for 1 h and then stimulated with PMACI for 30 min. The phosphorylation of MAPKs was analyzed by Western blotting. (upper) Results are representative of three independent experiments. (lower) The relative densities were quantified by densitometry means pJNK/JNK, p-p38/p38, and pERK/ERK. (c) HMC-1 cells were pretreated with dexamethasone (500 ng/ml) for 1 h and then stimulated with PMACI for 2 h. The protein expression of c-jun and c-fos was analyzed by Western blotting. (upper) Results are representative of three independent experiments. (lower) The relative densities were quantified by densitometry means c-jun or c-fos/GAPDH. Values are expressed as the means ± SD (n = 3 experiments). #P < 0.05; significantly different from unstimulated cells, *P < 0.05, significantly different from PMACI-stimulated cells. Dexa, dexamethasone.
4. Discussion
The study's findings demonstrated that expression of ACE2 gene was up-regulated by activation of PI3K/MAPK/AP-1 signaling pathway in the stimulated HMC-1 cells. Mast cell activation caused by PMACI stimulation enhanced TMPRSS2 expression and tryptase secretion as well. Dexamethasone, a mast cell stabilizer, prevented the PI3K/MAPK/AP-1 signaling pathway from functioning in the activated HMC-1 cells, hence reducing the expression of ACE2 and TMPRSS2 and production of tryptase.
The SARS-CoV-2 is to blame for the COVID-19 pandemic, which has been quickly spreading throughout many parts of the world since December 2019 [14]. COVID-19 is mostly spread from person to person by intimate interaction and respiratory secretions infected with SARS-CoV-2 [15]. The spike protein of the coronavirus and the host cellular ACE2 facilitate the infection and cellular entrance of SARS-CoV-2 [16]. TMPRSS2 is necessary to encourage SARS-CoV entrance by membrane fusion in addition to ACE2 [17]. Lung epithelial carcinoma cells, epidermal keratinocytes, and endothelial cells all consistently express the genes ACE2 and TMPRSS2, suggesting that the blood capillaries, the skin, and the lungs are probable sites of SARS-CoV-2 infection [18]. Roughly 15% to 20% of COVID-19 patients who are infected through these receptors develop a hyper-inflammatory cytokine storm and undergo a severe acute infection [19]. In comparison to direct viral cytotoxicity, the mortality and morbidity rate caused by a cytokine storm is higher [14]. The COVID-19 cytokine storm is defined by chemical mediators and inflammatory cytokines generated by immune/nonimmune cells, as well as hyperactivation and accelerated proliferation of T cells, natural killer cells, and macrophages [20]. Mast cells may be one of the key players in this group of inflammatory cells since they are activated and produce a variety of inflammatory mediators when they identify viral particles [21]. Mast cell-induced proinflammatory cytokines promote the lung inflammation and cause fever [7]. The hallmark of COVID-19 pathogenesis is the cytokine storm with elevated levels of inflammatory cytokines including tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, IL-1β, and TSLP [7], [22], [23], [24]. TNF-α is released by mast cells [25], [26] and considered one of the leading mediators of acute inflammation, the activation of which can also trigger IL-1 and IL-6 production [27]. High concentrations of TNF-α were reported in both plasma and tissues of patients with COVID-19 [22]. Mast cells produce IL-6 in response to viruses, and increased IL-6 is a biomarker for COVID-19 [23]. Moreover, mast cells secrete IL-1 and TSLP, which cause mast cells to release cytokines and chemokines that are inflammatory chemical mediators [26], [28], [29]. Additionally, tryptase released by mast cells causes vascular barrier failure, lung fibrosis, and airway inflammation and encourages the spread of SARS-CoV-2 infection [21]. MCAS caused by SARS-CoV-2 may elevate the incidence of developing severe acute COVID-19 and chronic post-COVID-19 diseases [9]. Afrin et al. [14] reported that MCAS targeted therapy can lessen the severity of the disease as soon as the development of a COVID-19 disease is identified or suspected. Theoharides [2] reported that mast cells have various mechanisms of recognizing the virus, but they can also become hosts for this virus by expressing ACE2, which has now been identified as the major receptor for SARS-CoV-2. Mast cells express a variety of serine proteases, including chymase and tryptase, which are essential for SARS-CoV-2 infection [2]. Until far, several therapies have been created to limit mast cell activation as a means of limiting the inflammatory mediators generated by virus-induced mast cells or of managing cytokine storm and lung injury. In this study, we showed for the first time that activated HMC-1 cells express ACE2 and TMPRSS2. Therefore, we suggest that knowing how ACE2 and TMPRSS2 co-express on mast cells can help one forecast whether or not the human body will become infected with SARS-CoV-2.
Mast cells are activated by infections, antigen, medications, insect or reptile venoms and produce inflammatory mediators through the release of calcium and the activation of the protein kinase C (PKC), PI3K, MAPK, or NF-κB signaling pathway [5]. Alveolar epithelia inflammation and lung damage are brought on by SARS-CoV-2-triggered mast cell activation and degranulation [13]. PMA is a specific activator of PKC. The calcium ionophore (A23187) is an extremely selective calcium ionophore that can pass freely through cell membranes and form stable complexes with Ca2+, effectively raising intracellular Ca2+ levels. PMACI induces the degranulation of mast cells and increases expression of various inflammatory mediator genes by the activation of PI3K/MAPK/AP-1/NF-κB signaling pathways [30], [31] In this study, PMACI increased the expression of ACE2, whereas Wortmannin, SP600125, SB203580, PD98059, SR11302, or PDTC reduced the PMACI-induced ACE2 mRNA expression. Subsequently, it was found that PI3K/MAPK/NF-κB play an imperative part within the expression of ACE2. Notably, an AP-1 inhibitor (SR11302) in particular virtually completely reduced ACE2 expression. According to Richard et al. [32], motif enrichments for the AP-1 transcription factor binding site were found at the ACE2 locus, indicating that AP-1 is a transcription factor that controls the expression of ACE2. Therefore, these results suggest that expression of ACE2 was regulated by PI3K/MAPK/AP-1/NF-κB signaling pathways in activated mast cells.
A broad-spectrum immunosuppressant, dexamethasone is a synthetic corticosteroid that the FDA approved in 1958. It alleviates the generation and negative effects of inflammatory cytokines. Dexamethasone also acted as a mast cell stabilizer, controlling mast cell degranulation and activation [5]. According to a study, dexamethasone prevents COVID-19 patients from experiencing a mast cell-mediated cytokine storm [33]. In patients with established moderate-to-severe acute respiratory distress syndrome, early dexamethasone medication may shorten the time spent on mechanical ventilation and general death rate [11]. According to numerous earlier research, dexamethasone is thought to reduce levels of inflammatory cytokines by blocking the PI3K/MAPK/AP-1/NF-κB signaling pathways in activated mast cells [5], [34], [35]. In the present study, dexamethasone regulated the expression of ACE2 via regulating PI3K/MAPK/AP-1 signaling pathways in activated mast cells. Therefore, it is clear that dexamethasone beneficial to COVID-19 through reducing ACE2 expression and stabilizing mast cells.
5. Conclusions
In COVID-19, directly/indirectly activated mast cells not only accelerate the inflammatory response through a cytokine storm, but are also usefully used for SARS-CoV-2 infection by increasing the expression of ACE2. In the present study, these results suggest that activated mast cells increase the expression of ACE2 through activating PI3K/MAPK/AP-1, whereas dexamethasone inhibits the up-regulation of ACE2 by inhibiting activation of mast cells. As a result of the findings of this investigation, it is anticipated that a medication that reduces mast cell activity and lowers the expression of ACE2 may significantly aid in the treatment and prevention of COVID-19 by reducing SARS-CoV-2 infection and inflammatory response. However, there are limitations in interpreting the results of ACE2 expression and its mechanism in mast cells activated by SARS-CoV-2 because this study was only in vitro without direct infection with SARS-CoV-2. So, further investigation is necessary to determine whether mast cell stabilizers/regulators have anti-inflammatory and anti-infective effects in animal and in vitro models of SARS-CoV-2 infection.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2022R1F1A1063893).
CRediT authorship contribution statement
Hee-Yun Kim: Data curation, Formal analysis, Methodology, Writing – original draft. Ho-Geun Kang: Investigation, Resources, Software. Hyung-Min Kim: Conceptualization, Project administration. Hyun-Ja Jeong: Supervision, Validation, Visualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- 1.Lin H., Cherukupalli S., Feng D., Gao S., Kang D., Zhan P., Liu X. SARS-CoV-2 Entry Inhibitors Targeting Virus-ACE2 or Virus-TMPRSS2 Interactions. Curr. Med. Chem. 2022;29:682–699. doi: 10.2174/0929867328666210420103021. [DOI] [PubMed] [Google Scholar]
- 2.Theoharides T.C. COVID-19, pulmonary mast cells, cytokine storms, and beneficial actions of luteolin. Biofactors. 2020;46:306–308. doi: 10.1002/biof.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senapati S., Banerjee P., Bhagavatula S., Kushwaha P.P., Kumar S. Contributions of human ACE2 and TMPRSS2 in determining host-pathogen interaction of COVID-19. J. Genet. 2021;100:12. doi: 10.1007/s12041-021-01262-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ortiz M.E., Thurman A., Pezzulo A.A., Leidinger M.R., Klesney-Tait J.A., Karp P.H., Tan P., Wohlford-Lenane C., McCray P.B., Jr., Meyerholz D.K. Heterogeneous expression of the SARS-Coronavirus-2 receptor ACE2 in the human respiratory tract. EBioMedicine. 2020;60 doi: 10.1016/j.ebiom.2020.102976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nam S.Y., Kim H.Y., Min J.Y., Kim H.M., Jeong H.J. An osteoclastogenesis system, the RANKL/RANK signalling pathway, contributes to aggravated allergic inflammation. Br. J. Pharmacol. 2019;176:1664–1679. doi: 10.1111/bph.14615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gebremeskel S., Schanin J., Coyle K.M., Butuci M., Luu T., Brock E.C., Xu A., Wong A., Leung J., Korver W., Morin R.D., Schleimer R.P., Bochner B.S., Youngblood B.A. Mast Cell and Eosinophil Activation Are Associated With COVID-19 and TLR-Mediated Viral Inflammation: Implications for an Anti-Siglec-8 Antibody. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.650331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinstock L.B., Brook J.B., Walters A.S., Goris A., Afrin L.B., Molderings G.J. Mast cell activation symptoms are prevalent in Long-COVID. Int. J. Infect. Dis. 2021;112:217–226. doi: 10.1016/j.ijid.2021.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.J.D.S. Motta Junior, A. Miggiolaro, S. Nagashima, C.B.V. de Paula, C.P. Baena, J. Scharfstein, L. de Noronha, Mast Cells in Alveolar Septa of COVID-19 Patients: A Pathogenic Pathway That May Link Interstitial Edema to Immunothrombosis, Front. Immunol. 11(2020) 574862. 10.3389/fimmu.2020.574862. [DOI] [PMC free article] [PubMed]
- 9.Batiha G.E., Al-Kuraishy H.M., Al-Gareeb A.I., Welson N.N. Pathophysiology of Post-COVID syndromes: a new perspective. Virol. J. 2022;19:158. doi: 10.1186/s12985-022-01891-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciapponi A., Klein K., Colaci D., Althabe F., Belizán J.M., Deegan A., Veroniki A.A., Florez I.D. Dexamethasone versus betamethasone for preterm birth: a systematic review and network meta-analysis. Am. J. Obstet. Gynecol. MFM. 2021;3 doi: 10.1016/j.ajogmf.2021.100312. [DOI] [PubMed] [Google Scholar]
- 11.Villar J., Ferrando C., Martínez D., Ambrós A., Muñoz T., Soler J.A., Aguilar G., Alba F., González-Higueras E., Conesa L.A., Martín-Rodríguez C., Díaz-Domínguez F.J., Serna-Grande P., Rivas R., Ferreres J., Belda J., Capilla L., Tallet A., Añón J.M., Fernández R.L., González-Martín J.M. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir. Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 12.Jeong H.J., Kim H.Y., Kim H.M. Molecular mechanisms of anti-inflammatory effect of chrysophanol, an active component of AST2017-01 on atopic dermatitis in vitro models. Int. Immunopharmacol. 2018;54:238–244. doi: 10.1016/j.intimp.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Wu M.L., Liu F.L., Sun J., Li X., He X.Y., Zheng H.Y., Zhou Y.H., Yan Q., Chen L., Yu G.Y., Chang J., Jin X., Zhao J., Chen X.W., Zheng Y.T., Wang J.H. SARS-CoV-2-triggered mast cell rapid degranulation induces alveolar epithelial inflammation and lung injury. Signal. Transduct. Target. Ther. 2021;6:428. doi: 10.1038/s41392-021-00849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afrin L.B., Weinstock L.B., Molderings G.J. Covid-19 hyperinflammation and post-Covid-19 illness may be rooted in mast cell activation syndrome. Int. J. Infect. Dis. 2020;100:327–332. doi: 10.1016/j.ijid.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.J.F. Chan, S. Yuan, K.H. Kok, K.K. To, H. Chu, J. Yang, F. Xing, J. Liu, C.C. Yip, R.W. Poon, H.W. Tsoi, S.K. Lo, K.H. Chan, V.K. Poon, W.M. Chan, J.D. Ip, J.P. Cai, V.C. Cheng, H. Chen, C.K. Hui, K.Y. Yuen, A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster, Lancet 395(2020) 514-523. 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed]
- 16.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2014;88:1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma D., Chen C.B., Jhanji V., Xu C., Yuan X.L., Liang J.J., Huang Y., Cen L.P., Ng T.K. Expression of SARS-CoV-2 receptor ACE2 and TMPRSS2 in human primary conjunctival and pterygium cell lines and in mouse cornea. Eye (Lond.) 2020;34:1212–1219. doi: 10.1038/s41433-020-0939-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., Cereda D., Coluccello A., Foti G., Fumagalli R., Iotti G., Latronico N., Lorini L., Merler S., Natalini G., Piatti A., Ranieri M.V., Scandroglio A.M., Storti E., Cecconi M., Pesenti A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region. Italy, Jama. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun X., Wang T., Cai D., Hu Z., Chen J., Liao H., Zhi L., Wei H., Zhang Z., Qiu Y., Wang J., Wang A. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020;53:38–42. doi: 10.1016/j.cytogfr.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam H.Y., Tergaonkar V., Kumar A.P., Ahn K.S. Mast cells: Therapeutic targets for COVID-19 and beyond. IUBMB Life. 2021;73:1278–1292. doi: 10.1002/iub.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.L. Wang, W. He, X. Yu, D. Hu, M. Bao, H. Liu, J. Zhou, H. Jiang, Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up, J. Infect. 80(2020) 639–645. 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed]
- 23.Chen L.Y.C., Hoiland R.L., Stukas S., Wellington C.L., Sekhon M.S. Assessing the importance of interleukin-6 in COVID-19. Lancet. Respir. Med. 2021;9:e13. doi: 10.1016/S2213-2600(20)30600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerla L., Moitra S., Pink D., Govindasamy N., Duchesne M., Reklow E., Hillaby A., May A., Lewis J.D., Melenka L., Hobman T.C., Mayers I., Lacy P. SARS-CoV-2-Induced TSLP Is Associated with Duration of Hospital Stay in COVID-19 Patients. Viruses. 2023;15:556. doi: 10.3390/v15020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.A. Taracanova, M. Alevizos, A. Karagkouni, Z. Weng, E. Norwitz, P. Conti, S.E. Leeman, T.C. Theoharides, SP and IL-33 together markedly enhance TNF synthesis and secretion from human mast cells mediated by the interaction of their receptors, Proc. Natl. Acad. Sci. USA. 114(2017) E4002–E4009. 10.1073/pnas.1524845114. [DOI] [PMC free article] [PubMed]
- 26.Kim H.Y., Kang H.G., Choi Y.J., Kim H.M., Jeong H.J. Caudatin attenuates inflammatory reaction by suppressing JNK/AP-1/NF-κB/caspase-1 pathways in activated HMC-1 cells. Food. Sci. Biotechnol. 2023;17:1–9. doi: 10.1007/s10068-023-01251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lefebvre A.L., McAuliffe L. Targeted Immunomodulatory Therapy: An Overview, R. I. Med. J. 2016;99:19–22. [PubMed] [Google Scholar]
- 28.Krystel-Whittemore M., Dileepan K.N., Wood J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2015;6:620. doi: 10.3389/fimmu.2015.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallenga C.E., Pandolfi F., Caraffa A., Kritas S.K., Ronconi G., Toniato E., Martinotti S., Conti P. Interleukin-1 family cytokines and mast cells: Activation and inhibition. J. Biol. Regul. Homeost. Agents. 2019;33:1–6. [PubMed] [Google Scholar]
- 30.Lu Y., Suh S.J., Li X., Liang J.L., Chi M., Hwangbo K., Kwon O., Chung T.W., Kwak C.H., Kwon K.M., Murakami M., Jahng Y., Kim C.H., Son J.K., Chang H.W. Citreorosein inhibits production of proinflammatory cytokines by blocking mitogen activated protein kinases, nuclear factor-κB and activator protein-1 activation in mouse bone marrow-derived mast cells. Biol. Pharm. Bull. 2012;35:938–945. doi: 10.1248/bpb.35.938. [DOI] [PubMed] [Google Scholar]
- 31.Ramesh T.P., Kim Y.D., Kwon M.S., Jun C.D., Kim S.W. Swiprosin-1 regulates cytokine expression of human mast cell line HMC-1 through actin remodeling. Immune. Netw. 2009;9:274–284. doi: 10.4110/in.2009.9.6.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D. Richard, P. Muthuirulan, J. Aguiar, A.C. Doxey, A. Banerjee, K. Mossman, J. Hirota, T.D. Capellini, Intronic regulation of SARS-CoV-2 receptor (ACE2) expression mediated by immune signaling and oxidative stress pathways, iScience 25(2022) 104614. 10.1016/j.isci.2022.104614. [DOI] [PMC free article] [PubMed]
- 33.Kazama I. Stabilizing mast cells by commonly used drugs: a novel therapeutic target to relieve post-COVID syndrome? Drug Discov. Ther. 2020;14:259–261. doi: 10.5582/ddt.2020.03095. [DOI] [PubMed] [Google Scholar]
- 34.Han N.R., Ko S.G., Moon P.D., Park H.J. Chloroquine attenuates thymic stromal lymphopoietin production via suppressing caspase-1 signaling in mast cells. Biomed. Pharmacother. 2021;141 doi: 10.1016/j.biopha.2021.111835. [DOI] [PubMed] [Google Scholar]
- 35.Jeong H.J., Ryu K.J., Kim H.M. Anticancer agent ABT-737 possesses anti-atopic dermatitis activity via blockade of caspase-1 in atopic dermatitis in vitro and in vivo models. Immunopharmacol. Immunotoxicol. 2018;40:319–326. doi: 10.1080/08923973.2018.1482497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.