Summary
Background
Polycystic ovary syndrome (PCOS) is one of the most common diseases with the coexistence of reproductive malfunction and metabolic disorders. Previous studies have found increased branched chain amino acid (BCAA) levels in women with PCOS. However, it remains unclear whether BCAA metabolism is causally associated with the risk of PCOS.
Methods
The changes of BCAA levels in the plasma and follicular fluids of PCOS women were detected. Mendelian randomization (MR) approaches were used to explore the potential causal association between BCAA levels and the risk of PCOS. The function of the gene coding the protein phosphatase Mg2+/Mn2+-dependent 1K (PPM1K) was further explored by using Ppm1k-deficient mouse model and PPM1K down-regulated human ovarian granulosa cells.
Findings
BCAA levels were significantly elevated in both plasma and follicular fluids of PCOS women. Based on MR, a potential direct, causal role for BCAA metabolism was revealed in the pathogenesis of PCOS, and PPM1K was detected as a vital driver. Ppm1k-deficient female mice had increased BCAA levels and exhibited PCOS-like traits, including hyperandrogenemia and abnormal follicle development. A reduction in dietary BCAA intake significantly improved the endocrine and ovarian dysfunction of Ppm1k−/− female mice. Knockdown of PPM1K promoted the conversion of glycolysis to pentose phosphate pathway and inhibited mitochondrial oxidative phosphorylation in human granulosa cells.
Interpretation
Ppm1k deficiency-impaired BCAA catabolism causes the occurrence and development of PCOS. PPM1K suppression disturbed energy metabolism homeostasis in the follicular microenvironment, which provided an underlying mechanism of abnormal follicle development.
Funding
This study was supported by the National Key Research and Development Program of China (2021YFC2700402, 2019YFA0802503), the National Natural Science Foundation of China (81871139, 82001503, 92057107), the CAMS Innovation Fund for Medical Sciences (2019-I2M-5-001), Key Clinical Projects of Peking University Third Hospital (BYSY2022043), the China Postdoctoral Science Foundation (2021T140600), and the Collaborative Innovation Program of Shanghai Municipal Health Commission (2020CXJQ01).
Keywords: PCOS, BCAA Catabolism, PPM1K, Mendelian randomization
Research in context.
Evidence before this study
Some metabolomics studies, including the authors’ previous reports have indicated the imbalance of amino acids metabolism in PCOS, especially significantly increased levels of BCAAs in PCOS. However, it remains unknown whether BCAA metabolism causally affects the risk of PCOS or in versa.
Added value of this study
-
1.
Based on MR approaches coupled with lab research, BCAA metabolism was determined to have causal association with increased risk of PCOS, and the ringleader was PPM1K gene.
-
2.
Ppm1k-deficient female mice and high BCAA diet-induced female mice exhibited PCOS-like endocrine and reproductive traits.
-
3.
Reducing dietary BCAAs improved the endocrine and ovarian dysfunction of Ppm1k-deficient female mice.
-
4.
PPM1K knockdown in human ovarian granulosa cells disturbed the energy metabolism homeostasis in the follicular microenvironment.
Implications of all the available evidence
Both genetic determinants and dietary induction of BCAA catabolism cause the occurrence and development of PCOS. PPM1K can function as a vital BCAA catabolism-related genetic driver for PCOS.
Introduction
Polycystic ovary syndrome (PCOS) is a common reproductive and metabolic disorder that affects 4–20% of women of reproductive age worldwide.1 PCOS is characterized by menstrual irregularity (oligo-ovulation or anovulation), hyperandrogenism (clinical or biochemical) and polycystic ovarian morphology.2 PCOS is the main cause of female infertility and, in many cases, encompasses a wide range of cardiometabolic disorders, such as insulin resistance, obesity, type 2 diabetes (T2D), hypertension, and cardiovascular disease.3 The aetiology of PCOS remains largely unknown, but growing evidence suggests that PCOS may be a complex multigenic disorder that is influenced by both genetic and environmental factors, including diet and other lifestyle factors.2
Branched-chain amino acids (BCAAs) are members of a unique category of essential amino acids, including valine, leucine, and isoleucine. Previous studies have found that a BCAA-abundant diet positively affects glucose homeostasis, muscular protein synthesis, and body weight regulation.4 Despite these beneficial effects on metabolic health, recent studies have highlighted that elevated circulating levels of BCAAs are associated with insulin resistance, T2D, obesity and cardiovascular diseases.5, 6, 7 Circulating levels of BCAAs are regulated by both the dietary intake of BCAAs and the catabolism of BCAAs in the body.8 The BCAA catabolic pathway widely regulates the metabolism of glucose9 and lipid synthesis,10 and defects in BCAA catabolism promote insulin resistance, diabetes and obesity.4,11
In previous research, we observed elevated plasma levels of BCAAs in PCOS patients through metabolomics analysis,12 and this finding has been replicated in other studies.13,14 We further determined that the increased levels of BCAAs were significantly associated with insulin resistance and metabolic syndrome in women with PCOS.15 However, it remains unclear whether abnormal BCAA metabolism is causally associated with the risk of PCOS or vice versa.
In the present study, we used Mendelian randomization (MR) to explore the potential causal association between elevated levels of BCAAs and an increased risk of PCOS. Our analysis revealed that the causal gene is the protein phosphatase Mg2+/Mn2+-dependent 1K (PPM1K) gene, which encodes the enzyme that dephosphorylates and activates the mitochondrial branched-chain a-ketoacid dehydrogenase (BCKD) that controls BCAA catabolism.16 Moreover, we utilized Ppm1k knockout female mice to elucidate whether defects in BCAA catabolism could lead to PCOS-like phenotypes. Then, we performed a BCAA-restricting dietary intervention study to evaluate the effect of BCAA level manipulation on the reproductive and metabolic dysfunction of Ppm1k knockout female mice. Furthermore, we examined the impact of PPM1K knockdown in human ovarian granulosa cells on energy metabolism homeostasis in the follicular microenvironment.
Methods
GWAS data source
The current three BCAA GWAS datasets were extracted from a GWAS meta-analysis by Lotta, Pietzner et al.17 Two different PCOS GWAS datasets were obtained from the UK BioBank18 derived from the Neale laboratory and the study by Day F et al.19 The quality control details for GWASs for analysis are presented in Supplementary Table S1. These original studies were approved by the local ethical committee of the institutional review board, and all participants signed informed consent forms.
SNPs with genome-wide associations with BCAAs were extracted through publicly available meta-GWAS summary statistics from the study of Lotta, Pietzner et al.17 (meta-analyzed p < 5 × 10−8), which examined up to 174 metabolites among 86,507 European participants. SNPs with low minor allele frequency (MAF) or imputed rate were excluded (criteria: in the Fenland and EPIC-Norfolk study, MAF <2% or imputation quality score <0.3; in the INTERVAL study, MAF ≤1% and imputed variants info score of ≤0.4). The Hardy–Weinberg equilibrium in the whole meta-GWAS was checked for p ≥ 1 × 10−6. Variants with an absolute effect size value > 5 and a standard error > 10 or <0 were excluded, as were insertions and deletions. Age-, sex-, and study-specific covariates were adjusted for in mixed linear models before examining genome-wide associations (Supplementary Table S1). Since the authors did not provide a BCAA phenotype GWAS for female patients, we used the BCAA GWAS for both sexes instead.
Two PCOS GWAS were extracted to estimate the effect of BCAA genetic determinants (Supplementary Table S1). One from the UK BioBank imputed v3, which is a large prospective cohort, includes data from up to 361,194 European women with 194,153 participants, including 436 PCOS patients and 193,717 controls.18 Phenotypes were collected from self-reports. Data from another GWAS for PCOS were derived from a meta-analysis by Day F et al.19 (not containing data from 23andMe), including a European population of 4138 PCOS patients and 20,109 controls. Two diagnostic criteria were utilized in this meta-GWAS: hyperandrogenism and ovulatory dysfunction are required by the National Institutes of Health criteria, while polycystic ovarian morphology is required by Rotterdam with at least two of the traits above.
Detailed phenotypic information of PCOS was further analysed to understand the relationship between BCAAs and PCOS. Based on the limited data, 4 available phenotypic GWAS data that often correlate with abnormal hormone levels were used to examine PCOS-related phenotypes: bioavailable testosterone levels, total testosterone levels, and sex hormone-binding globulin (SHBG) with/without adjustment for BMI. Ruth et al.20 identified the genetic determinants of these traits among 425,097 UK Biobank study participants; the GWAS details for each phenotype are shown in Supplementary Table S1.
MR analysis
Preprocessing before performing MR: GWAS of BCAAs
The BCAA meta-GWAS was based on a weighted z score approach. To obtain the effect estimates and standard error, we converted z scores for further MR analysis using the method provided by Kho et al.21
Where N is the sample size of all participants matched for each genetic association, eaf represents the effect allele frequency, and SE is the standard error for converted beta.
Two-sample Mendelian randomization
We performed two-sample MR22 with three BCAAs as exposures and PCOS and its related traits as outcomes. All analyses were conducted using the “TwoSampleMR” package in R.23 We screened for instrumental variables based on the top genetic determinant SNP of BCAAs (p < 5 × 10−8). The estimated model effects are presented with ORs and 95% CIs, and two-tailed p < 0.05 was considered statistically significant. To assess weak instrumental variable effects, the R2 and F-statistics were also calculated, and an SNP with an F-statistic value greater than 10 reflects a strong instrument.24 We performed multiple models including the inverse variance weighted (IVW), weighted median, weighted modes and MR-Egger to obtain effect estimates because these methods rely on different assumptions for valid causal inference. We primarily used IVW approach for two-sample MR analyses, as this is considered the most reliable when there is no evidence of directional pleiotropy. IVW meta-analysis is a straightforward way of obtaining an MR estimate for multiple SNPs, which is equivalent to a weighted regression of gene–outcome association where the intercept is limited to zero.23 However, since directional pleiotropy exists, this estimation can be highly biased, particularly with an increasing sample size. A more robust method in this situation is MR‒Egger regression, as it has less pleiotropic bias but at the expense of statistical power, and the slope coefficient provides the causal effect estimation.25
Heterogeneity test and sensitivity analysis
Sensitivity analysis is often used to control the pleiotropic effects of SNPs in instrumental variables to improve the reliability of the MR results.22 When determining the reliability of our results, the heterogeneity test was performed by using Cochran Q as an indicator of possible horizontal pleiotropy, and an MR Egger intercept was performed to determine potential directional pleiotropy. Funnel plots displayed the individual Wald ratios for each SNP, and the graphical asymmetry suggested outliers that may cause directional pleiotropy. Furthermore, we conducted a "leave-one-out" analysis to assess whether causal effects were driven by one single SNP by removing each potentially pleiotropic SNP at a time and re-estimating the effects.26 We also applied the Mendelian Randomization Pleiotropy RESidual Sum and Outlier test (MR-PRESSO) to detect the pleiotropy caused by outlier variables in IVW analysis and correct horizontal pleiotropy via outlier removal. Lastly, we applied MR Steiger filtering as implemented in the “TwoSampleMR” R package to calculate the variance explained in the exposure and the outcome by the instrument SNPs and test whether causality was in the expected direction.27 MR Steiger test with p < 0.05 was considered statistically significant, supporting the validity of causality direction.
Multivariable Mendelian Randomization Analysis (MVMR)
MVMR is an extension of MR that takes into account the multiplicity between traits. In this study, we used MVMR to assess whether an outcome (PCOS and PCOS-related traits) was related to exposure (BCAAs) as well as “independent” causality between each type of BCAA. Our MVMR was performed using the “MendelianRandomization” R package.28 In the setting of MVMR, we included most GWAS-associated SNPs for all traits in the model. However, rs4253272 in the test conducted by the UK BioBank PCOS GWAS was removed prematurely due to the missing exposure data.
Phenome-Wide Association Study (PheWAS)
We applied PheWAS to further detect the pleiotropic effect of the top SNPs that were associated at genome-wide significance with BCAAs and located in the PPM1K gene from publicly available genetic association studies29 based on the GWAS ATLAS database, using the GWAS summary statistics of 3302 complex traits from 28 domains. Given that the phenotypes investigated were not completely independent, correction was made for multiple testing by the Benjamini & Hochberg method with a 5% FDR threshold.
Study population
This study was approved by the Ethics Committee of Peking University Third Hospital (No. 2016SZ-027), and informed consent was obtained from all participants.
Chinese women with PCOS and healthy women as controls were recruited from the Reproductive Medicine Center of Peking University Third Hospital from March 2017 to June 2019. PCOS was diagnosed according to the Rotterdam criteria, in which two or more of the following traits are required for diagnosis: (1) hyperandrogenism (clinical and/or biochemical signs); (2) oligo-ovulation and/or anovulation; and (3) polycystic ovaries. The exclusion criterion was the presence of any endocrine- or metabolism-related disorder (congenital adrenal hyperplasia, androgen-secreting tumour, hypogonadotropic hypogonadism, thyroid dysfunction, Cushing syndrome, hyperprolactinemia and premature ovarian failure). The control group included women attending the clinic owing to male azoospermia or tubal occlusion who were matched for age and BMI. All the controls had regular menstrual cycles and normal ovarian morphology and did not exhibit clinical or biochemical hyperandrogenism. None of the women used medications known to affect metabolic function or reproductive function within 3 months before enrollment.
Clinical sample collection and biochemical measurement
Peripheral blood samples were collected, and biochemical parameters were determined for all participants as described previously.30 Patients underwent in vitro fertilization with the same stimulation protocol as previously described.31 Follicular fluid samples were obtained from the largest follicle aspirated from each ovary. Mural granulosa cells were isolated from the follicular fluids aspirated during oocyte retrieval by Ficoll density gradient centrifugation.32
Analysis of plasma and follicular fluid BCAA levels
BCAA concentrations in plasma and follicular fluids were determined by the liquid chromatography-tandem mass spectrometric method using a 3200 Q TRAP triple-quadrupole linear ion trap mass spectrometer (Applied Biosystems, Darmstadt, Germany) and a high-performance liquid chromatography system (Dionex, Sunnyvale, CA, USA).33
Animal studies
All animal experimental procedures were approved by the Animal Care and Use Committee of Peking University Health Science Center according to the national legislation for animal care. Female C57Bl/6J mice were raised in the Animal Center of Peking University Health Science Center in an SPF environment. Ppm1k−/− mice, a generous gift from Professor Haipeng Sun, Tianjin Medical University, have also been previously described.16 To assess the effect of low dietary BCAAs on the phenotype of Ppm1k−/− female mice, mice at 4 weeks were divided into three groups as follows: wild-type females were fed a chow diet, while Ppm1k−/− mice were fed either a chow diet or a low-BCAA diet. The low-BCAA diet contained one-fifth of the standard content of BCAAs as described previously,34 specifically including 7.8% BCAAs (3.4% Leu + 1.9% Ile + 2.5% Val, and the Leu:Ile:Val ratio was 2:1.1:1.5). The Low-BCAA diet was isocaloric based on the formula reported.34 When BCAA level was reduced, other amino acids were supplemented in proportion to casein, and the deficient BCAA was supplemented with glutamic acid. The female mice were analysed after dietary intervention for 8 weeks.
To evaluate the influence of higher BCAA intake on the phenotype of female mice, normal females at 4 weeks of age were fed either a chow diet or a high BCAA diet. High BCAA diets contained twice the standard content of BCAAs as described previously,34 specifically including 78.7% BCAAs (35.6% Leu + 17.9% Ile + 25.2% Val, and a Leu:Ile:Val ratio of 2:1:1.4). The female mice were analyzed after dietary intervention for 8 weeks.
Hormone and biochemical index determination in mice
Blood was collected from the retro-orbital plexus after the mice were fasted for 8 h. The serum was immediately and stored at −80 °C. The levels of total testosterone were detected with an ELISA kit (Demeditec Diagnostics, GmnH, DEV9911) according to the manufacturers' instructions. The levels of luteinizing hormone (LH) were analysed using an ELISA kit (E-EL-M3053; Elabscience) according to the manufacturers’ instruction. The levels of follicle stimulating hormone (FSH), estradiol and insulin were measured by radioimmunoassay kits (Beijing North Institute of Biological Technology, Beijing, China). The glycerol phosphate oxidase-catalase method was used to measure the serum levels of total cholesterol (total cholesterol assay kit 211016, WEIYIBIO) and triglyceride (triglyceride assay kit 211016, WEIYIBIO), and direct surfactant method was applied to examine the serum levels of high density lipoprotein (HDL) cholesterol (direct HDL-cholesterol kit, 210926, WEIYIBIO) and low density lipoprotein (LDL) cholesterol (direct LDL-cholesterol kit, 211017, WEIYIBIO).
Estrous cycle determination
For at least two estrus cycles, vaginal smears were performed at 09:00 every day. The stage of the estrus cycle was determined by microscopic analysis of the main cell types in vaginal smears stained with the Shorr method. Proestrus was composed of round nucleated epithelial cells; estrus was composed of keratinized squamous epithelial cells; metestrus was composed of epithelial cells and leukocytes; and diestrus was composed of nucleated epithelial cells and a predominance of leukocytes.
Ovarian morphology and immunohistochemistry
The ovaries were quickly collected, cleaned of fat, fixed in 4% paraformaldehyde, dehydrated in 70% ethanol and embedded in paraffin. Then, the ovaries were serially sectioned into 5 μm thick sections (LEICA CM1850, Germany) and stained with hematoxylin and eosin (Beisuo Biotech Company, China). Follicles were counted in every fifth section and categorized into different developmental stages based on standards of classification.35 Immunohistochemistry was performed as described previously.36 The primary antibodies included anti-CYP19A1 (ab18995; Abcam) and anti-3β-HSD (sc-515120; Santa Cruz).
Fertility assessment of mice
Eight-week-old WT and Ppm1k−/− female mice were mated to WT male mice 1:2 until the first litter of pups was born. The number of pups in the first litter were quantified per pairing.
Knockdown of PPM1K in human granulosa cells
The human ovarian granulosa tumour cell line COV434 was kindly provided by Professor Yimin Zhu, Women's Hospital School of Medicine Zhejiang University. For PPM1K-shRNA transduction, a lentiviral vector plasmid pLV3(H1/GFP&Puro) (GenePharma Inc, Shanghai, China) was used to construct the stable clones. Lentiviral particles were produced by cotransfection of HEK293T cells with the target plasmid pLV3-shRNA and lentiviral packaging plasmid (pGag/Pol, pRev, pVSV-G). The target gene shRNA sequence, target location and sequence information were detailed in Supplementary Table S2.
Seahorse Extracellular Respiratory Flux Test
The experiment was performed as described previously.37 Cells were counted and plated at 1.5 × 105 cells per well in a Seahorse XF96 Cell Culture Microplate for all experiments (Agilent). According to the manufacturer's instructions, OCR was analysed using an XF96 Extracellular Flux Analyzer (Seahorse Bioscience). Cells were cultured in RPMI 1640 media and measured after the addition of 2 μM oligomycin and 100 mM 2-DG. The consumption rate was measured under basal conditions.
Metabolic flux analysis
COV434 cells were grown on 10-cm plates (Corning). U–13C-glucose (11 mM)-labelled medium was replaced 24 h before metabolic analysis. An Agilent 1290/6460 (Agilent) triple quadrupole mass spectrometer was used to collect positive ionization mode data. The cell extract (10 μL) was injected onto a 100 × 2.1 mm 1.9 μm Hypersil GOLD aQ (Thermo). The [M + H] of the analyte was chosen as the precursor ion. The quantitative mode was a multiple reaction monitoring (MRM) mode using mass conversion (precursor ion/product ion). Quantitative Analysis Version B.04.00 (Agilent) was used for acquisition and processing. The details of the LC-MS method were described previously.37
Statistical analysis
SPSS 26.0 and GraphPad Prism version 8.0 were used for statistical analysis. The sample distribution was determined by the Kolmogorov–Smirnov normality test. Statistical comparisons of patients with PCOS and the controls were made using Student's t test or nonparametric test when appropriate. The data are presented as the median (interquartile range). Spearman's rank correlation was used to evaluate the correlation between BCAA levels and clinical indicators. Binary logistic regression was performed to evaluate the correlation between BCAA levels and the risk for PCOS before and after adjustment for baseline age and BMI, and BCAA levels were scaled to standard deviation (SD) units. We categorized testosterone levels and menstrual cycle intervals into high and low levels according to the median values of PCOS patients and then performed logistic regression to assess the relation of BCAA levels to the risk for the clinical phenotype of PCOS. All reported confidence interval (CI) values were calculated at the 95% level. The significance tests were two-sided, and a p value < 0.05 was considered a significant difference.
Role of the funding source
All of the funders (National Key Research and Development Program of China/National Natural Science Foundation of China/the CAMS Innovation Fund for Medical Sciences/Key Clinical Projects of Peking University Third Hospital/the China Postdoctoral Science Foundation/the China Postdoctoral Science Foundation) had no role in the study design, sample collection and examination, animal experiments, data analysis and interpretation, or writing of the manuscript. The corresponding authors had full access to all the data and final responsibility for the decision to submit for publication.
Results
BCAA levels are associated with the clinical features of PCOS
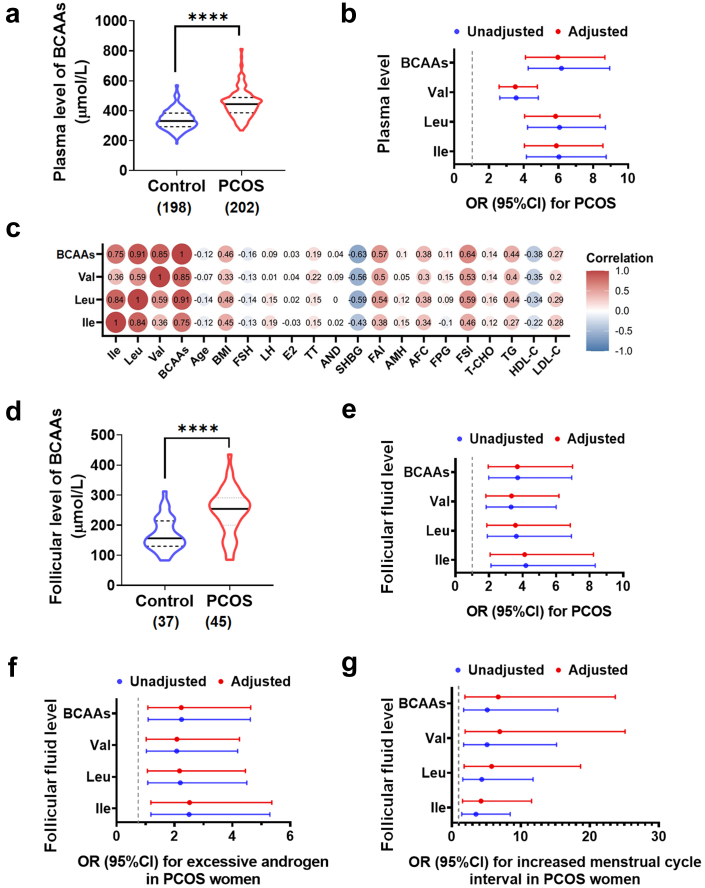
First, we explored the association of plasma BCAA levels and PCOS clinical characteristics in a cross-sectional study including 202 PCOS patients and 198 control women (Supplementary Table S3). Total BCAA levels were significantly higher in the PCOS group than in the control group (Fig. 1a). Adjusting for potential confounding factors (age and BMI), the levels of total BCAAs and each BCAA were all still positively associated with PCOS (Fig. 1b). Additionally, plasma BCAA levels were closely associated with the endocrine and metabolic indicators of PCOS, including positive correlations with BMI, serum total testosterone, free androgen index, fasting serum insulin and antral follicle count and a negative association with the serum level of SHBG (Fig. 1c). BCAA levels were found to be associated with the increased odds of insulin resistance with and without BMI adjustment (Supplementary Figure S1). Moreover, we detected elevated BCAA levels in the follicular fluids of women with PCOS compared with control women (Fig. 1d; Supplementary Table S4) and determined the positive association of increased BCAA levels in the follicular fluids with the risk for PCOS (Fig. 1e). In addition, the effects of high BCAA levels on the typical clinical features of women with PCOS were further evaluated, and the elevations of three BCAAs in the follicular fluids were significantly correlated with excessive testosterone levels (Fig. 1f) and increased menstrual cycle intervals (Fig. 1g) in women with PCOS with and without age and BMI adjustment, suggesting that increased BCAA levels were closely related to the pathogenesis of PCOS.
Fig. 1.
BCAA levels associate with clinical features of PCOS women. (a) The plasma levels of BCAAs in PCOS (n = 202) and control (n = 198) groups. (b) The odds ratios (95% CIs) for PCOS per 1-SD increase in plasma abundance of BCAAs with and without age and BMI adjustment. (c) The correlations between the plasma levels of BCAAs and the endocrine and metabolic parameters of PCOS. (d) The follicular fluid levels of BCAAs in PCOS (n = 45) and control (n = 37) groups. (e) The odds ratios (95% CIs) for PCOS per 1-SD increase in BCAA levels in follicular fluids with and without age and BMI adjustment. (f and g) The odds ratios (95% CIs) for excessive androgen level (f) or increased menstrual cycle interval (g) in PCOS women per 1-SD increase in BCAA levels in follicular fluids with and without age and BMI adjustment. P values were determined by two-tailed Mann–Whitney U-test. ∗∗∗∗, p < 0.0001.
MR of BCAAs and PCOS
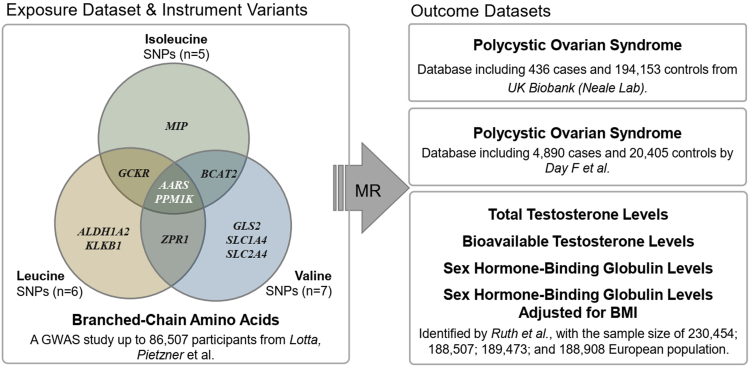
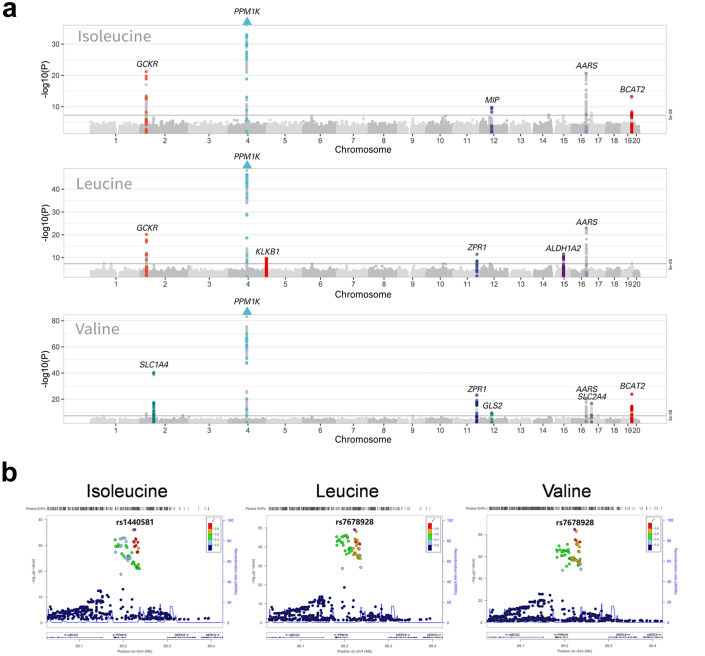
We explored the association between BCAAs and PCOS by performing a two-sample-based MR (Fig. 2). A total of 13 unique loci were obtained from a previous genome-wide association study (GWAS) by Lotta et al.17 and used as the instrumental variables of BCAAs, including 5 loci for isoleucine, 6 for leucine, and 7 for valine (Supplementary Table S5). Fig. 3a shows 11 genes associated with any of these BCAAs in these 13 loci, including AARS, ALDH1A2, BCAT2, GCKR, GLS2, KLKB1, MIP, PPM1K, SLC1A4, SLC2A4, and ZPR1, of which AARS and PPM1K are shared in isoleucine, leucine, and valine (Fig. 3a; Supplementary Figure S2). Notably, PPM1K is the common gene with the strongest signal (p Ile = 7.69 × 10−37, p Leu = 8.25 × 10−50, p Val = 2.51 × 10−85) (Fig. 3b) and has been widely reported to be associated with many diseases, such as cardiovascular disease38 and T2D.39
Fig. 2.
Flow diagram of Mendelian randomization analysis. We used two-sample MR with summary statistics from 3 GWAS genetic datasets to determine the causal relationship between BCAAs and PCOS (with related traits). 18 robust variants were included as instruments, AARS and PPM1K were the shared gene in all BCAAs. “TwoSampleMR” R package was used in the analysis.
Fig. 3.
The Manhattan plot of BCAAs and Locus Zoom plots for PPM1K. (a) Genes associated with exposure are annotated the MR analysis annotates the genes associated with exposure. SNPs above the threshold (in black line) were considered significant (p < 5 × 10−8). Where 11 genes are highlighted (AARS, ALDH1A2, BCAT2, GCKR, GLS2, KLKB1, MIP, PPM1K, SLC1A4, SLC2A4, ZPR1). The triangle indicated the −log10(P) value of SNPs in the corresponding gene has exceeded the drawn region. (b) The Locus Zoom plots for PPM1K. Abbreviations: PPM1K, the protein phosphatase Mg2+/Mn2+ dependent 1K.
The effect of the top SNPs of BCAAs on PCOS was obtained from a previous GWAS of PCOS data in the UK Biobank, a fully independent cohort and the source of BCAA SNPs.18 The MR results showed significant associations for all BCAAs and PCOS using IVW method (beta Ile-PCOS (UKB) = 0.003, p = 0.043, IVW; beta Leu-PCOS (UKB) = 0.003, p = 0.020, IVW; beta Val-PCOS (UKB) = 0.002, p = 0.004, IVW; Table 1, Supplementary Figure S3). These directions of effects were also consistent with other methods (weighted median, weighted mode, and MR‒Egger; Supplementary Table S6). No significant heterogeneity or pleiotropic effect was found to drive the causal link (Supplementary Table S6; Supplementary Figure S3). A sensitivity analysis was performed using another PCOS GWAS from a previous study by Day F et al.19 The MR results were consistent in the direction of effect estimates, although the effect estimates in the study of Day F et al. study did not reach a significant level (beta Leu-PCOS (Day F et al.) = 0.296, p = 0.130, IVW; beta Val-PCOS (Day F et al.) = 0.105, p = 0.573, IVW; Supplementary Table S7). The MR Steiger test also indicated the accuracy of our estimate of the causal directionality (Table 1). Overall, our MR results suggest that BCAAs contribute to an increased risk of PCOS, and this was also consistent with our observational study.
Table 1.
Mendelian randomization of branched chain amino acids (isoleucine, leucine, valine) and risk of polycystic ovary syndrome (PCOS) by UK BioBank GWAS.
| Exposure | N | F | Method | beta | se | p | Heterogeneity test | Pleiotropic test | MR Steiger test |
|---|---|---|---|---|---|---|---|---|---|
| Univariable MR | |||||||||
| Isoleucine | 5 | 92 | IVW | 0.003 | 0.001 | 0.043a | 0.19 | 0.57 | 1.40E-54 |
| Leucine | 6 | 95 | IVW | 0.003 | 0.001 | 0.020a | 0.23 | 0.47 | 7.25E-68 |
| Valine | 7 | 148 | IVW | 0.002 | 0.001 | 0.004a | 0.61 | 0.68 | 1.49E-128 |
| Multivariable MR | |||||||||
| Isoleucine | 12 | Multivariable IVW | −0.004 | 0.007 | 0.507 | 0.02 | |||
| Isoleucine | 12 | Multivariable MR Egger | −0.003 | 0.008 | 0.681 | 0.01 | |||
| Leucine | 12 | Multivariable IVW | 0.003 | 0.008 | 0.723 | 0.02 | |||
| Leucine | 12 | Multivariable MR Egger | 0.002 | 0.008 | 0.763 | 0.01 | |||
| Valine | 12 | Multivariable IVW | 0.002 | 0.003 | 0.372 | 0.02 | |||
| Valine | 12 | Multivariable MR Egger | 0.003 | 0.003 | 0.371 | 0.01 |
N number of SNPs, F F-statistic, IVW Inverse variance weighted, OR odds ratio, CI confidence interval, p p-value.
Significance at p < 0.05.
MVMR was further performed to explore whether any particular BCAA drove the MR. We did not find any BCAAs that appeared alone to significantly influence PCOS, suggesting that the three BCAAs may contribute to the occurrence of PCOS jointly with shared pathways (p > 0.05, Multivariable IVW; Table 1).
Association of BCAAs and PCOS-related traits from MR
To better understand the phenotypic association of BCAAs with PCOS, we further used the MR approach to test the association of the genetic predisposition of the three BCAAs and PCOS-related traits, including bioavailable testosterone, total testosterone, and SHBG (Table 2). MR-Egger intercepts were not indicative of directional pleiotropy (Table 2), but there were potential outliers present on visual inspection in both scatter and leave-one-out plots (Supplementary Figure S4). When corrected for the outliers detected by MR-PRESSO, the re-estimated effects were consistent with the significant associations (Table 2, Supplementary Table S8). The results show that the genetically predicted isoleucine and leucine was associated with higher levels of bioavailable testosterone (beta Ile- bioavailable testosterone = 0.095, p = 0.01, IVW; beta Leu- bioavailable testosterone = 0.088, p = 0.02, IVW), and the genetically predicted isoleucine and leucine were associated with lower levels of SHBG (beta Ile-SHBG = −0.085, p = 0.01, IVW; beta Ile-SHBG adjusted for BMI = −0.123, p = 6.9 × 10−6, IVW; beta Leu-SHBG = −0.048, p = 1.6 × 10−4, IVW; beta Leu-SHBG adjusted for BMI = −0.034, p = 0.01, IVW) (Table 2; Supplementary Table S8). The MVMR did not detect a significant effect of any genetically predicted BCAAs on PCOS-related traits independently (Supplementary Table S9).
Table 2.
Mendelian randomization of branched chain amino acids (isoleucine, leucine, valine) and related traits of polycystic ovary syndrome.
| Exposure | Outcome | N | F | IVW OR (95% CI) | beta | se | p | Heterogeneity test | Pleiotropic test | MR Steiger test |
|---|---|---|---|---|---|---|---|---|---|---|
| Isoleucine | BT Levels | 4 | 90 | 1.100 (1.021, 1.185) | 0.095 | 0.038 | 0.01a | 0.01a | 0.76 | 2.28E-33 |
| SHBG Levels | 1 | 44 | 0.918 (0.863, 0.977) | −0.085 | 0.032 | 0.01a | NA | NA | 1.20E-04 | |
| SHBG Levels Adjusted for BMI | 1 | 44 | 0.884 (0.838, 0.933) | −0.123 | 0.027 | 6.9E-06a | NA | NA | 4.35E-03 | |
| TT Levels | 2 | 102 | 1.043 (0.973, 1.118) | 0.042 | 0.035 | 0.24 | 0.22 | NA | 2.54E-28 | |
| Leucine | BT Levels | 5 | 91 | 1.092 (1.013, 1.178) | 0.088 | 0.038 | 0.02a | 7.1E-04a | 0.91 | 3.47E-42 |
| SHBG Levels | 2 | 135 | 0.953 (0.929, 0.977) | −0.048 | 0.013 | 1.6E-04a | 0.34 | NA | 1.48E-29 | |
| SHBG Levels Adjusted for BMI | 1 | 220 | 0.967 (0.944, 0.991) | −0.034 | 0.012 | 0.01a | NA | NA | 5.72E-27 | |
| TT Levels | 3 | 61 | 0.812 (0.499, 1.320) | −0.208 | 0.248 | 0.40 | 1.3E-29a | 0.81 | 1.52E-05 | |
| Valine | BT Levels | 6 | 162 | 1.020 (0.976, 1.066) | 0.020 | 0.023 | 0.37 | 3.4E-03a | 0.93 | 2.72E-114 |
| SHBG Levels | 5 | 168 | 0.979 (0.953, 1.007) | −0.021 | 0.014 | 0.13 | 4.8E-03a | 0.95 | 5.14E-97 | |
| SHBG Levels Adjusted for BMI | 3 | 117 | 1.002 (0.935, 1.074) | 0.002 | 0.035 | 0.96 | 2.0E-06a | 0.32 | 6.13E-35 | |
| TT Levels | 7 | 148 | 1.035 (0.996, 1.077) | 0.035 | 0.020 | 0.08 | 2.9E-02a | 0.95 | 2.05E-130 |
BT bioavailable testosterone, SHBG sex hormone-binding globulin, TT total testosterone, N number of SNPs, IVW inverse variance weighted, OR odds ratio, CI confidence interval, p p-value, NA not applicable.
Significance at p < 0.05.
Phenotypic pleiotropy of the PPM1K gene
PheWAS can explore associations between specific genetic variants and various phenotypes and are increasingly used in identifying pleiotropy between SNPs and other phenotypes except for the one of interest.40 PPM1K is the representative gene of BCAAs with the strongest signal and was verified as a gene related to PCOS in MR analysis (Fig. 3; Supplementary Tables S5, S6, and S8). To explore the potential effects, we conducted a PheWAS of the top two genetic variants of the PPM1K gene (rs1440581, rs7678928; Supplementary Figures S5 and S6). Both were found to be significantly (false discovery rate (FDR) < 0.05) and extensively associated with BCAA traits in the metabolic domain. The next highly related traits are usual walking pace and blood pressure medication in the activity domain. Other highly significant related traits included diabetes, osteoarthritis, Crohn's disease, etc. These results indicate that rs1440581 and rs7678928 contribute to metabolic disorders and inflammation that are common in PCOS.
Ppm1k deficiency causes endocrine and reproductive dysfunction in female mice
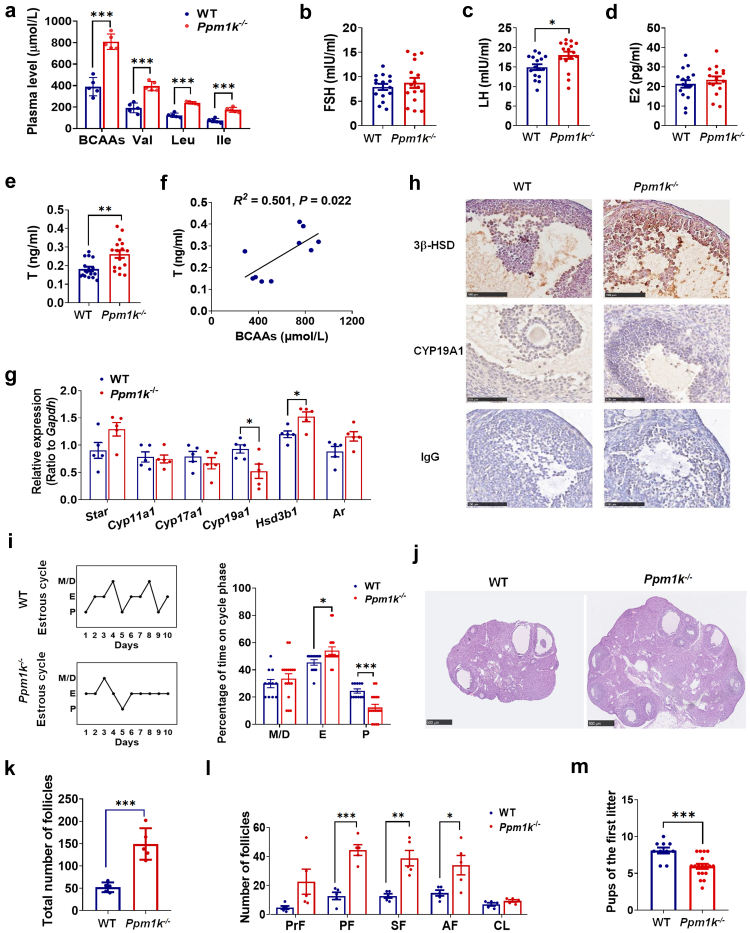
To determine the effect of impaired BCAA catabolism on the occurrence of PCOS, we characterized the phenotypes of BCAA catabolism-deficient female mice, in which Ppm1k has been genetically disrupted.16 Ppm1k−/− female mice indeed exhibited significantly increased plasma levels of valine, leucine and isoleucine compared with wild-type controls (Fig. 4a). Moreover, Ppm1k−/− female mice showed obvious alterations in whole-body metabolism, including enhanced glucose clearance, insulin sensitivity and the respiratory exchange ratio, which were consistent with previous findings (Supplementary Figure S7a–d). In addition, the percentage of body fat was increased and percentage of lean mass was decreased in Ppm1k−/− female mice (Supplementary Figure S7e–h). The level of serum triglycerides in Ppm1k−/− female mice were higher than those in wild-type mice, although the body weight and serum levels of total cholesterol, LDL-cholesterol and HDL-cholesterol were unaffected (Supplementary Figure S7i).
Fig. 4.
Ppm1k-deficient female mice have a PCOS-like phenotype. (a) The plasma levels of BCAAs in wild-type (WT) and Ppm1k−/− female mice (n = 5 mice per group). (b–e) The changes of serum levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2) and testosterone (T) in WT and Ppm1k−/− female mice (n = 16 mice per group). (f) The correlation analysis between serum BCAAs and total testosterone levels (n = 10 mice per group). (g) The mRNA expression of androgen synthesis-related genes in the ovarian tissue of WT and Ppm1k−/− female mice (n = 5 mice per group). (h) Immunohistochemical analysis of the protein expression levels of CYP19A1 and 3β-HSD in ovarian tissues of WT and Ppm1k−/− mice, scale bar = 100 μm. (i) Representative and quantitative analysis of estrous cycles (WT, n = 11; Ppm1k−/−female mice, n = 17). (j) Representative ovarian morphology of WT and Ppm1k−/− mice by hematoxylin and eosin staining, scale bar = 500 μm, images are representative of three independent experiments with similar results. (k) The total number of follicles in the entire ovary of WT and Ppm1k−/− mice (n = 5 mice per group). (l) Differential follicle counts of primordial (PrF), primary (PF), secondary follicles (SF), antral follicles (AF) and corpus luteum (CL) (n = 5 mice per group). (m) Fertility test of female mice (WT, n = 6; Ppm1k−/−female mice, n = 18). Data are represented as means ± SEM. P values were determined by Student's t-test. ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001.
Notably, serum levels of testosterone and luteinizing hormone were significantly elevated in Ppm1k−/− female mice, while no differences in follicle stimulating hormone or estradiol levels were observed when compared with healthy controls (Fig. 4b–e). Interestingly, BCAA concentrations were positively correlated with testosterone levels in female mice (Fig. 4f). To explore the potential mechanism of androgen excess induced by Ppm1k defects, we detected the expression of enzymes involved in androgen synthesis and regulation. Both the mRNA and protein levels of 3β-hydroxysteroid dehydrogenase (3β-HSD) were significantly increased, and aromatase (CYP19A1) expression was reduced in the ovaries of Ppm1k−/− mice compared to those in the wild-type group (Fig. 4g and h), implying enhanced androgen synthesis and suppressed conversion of androgens to estrogens in Ppm1k−/− female mice. In addition, the estrous cycles were disturbed, and the ovarian volume was enlarged in Ppm1k−/− mice compared to controls (Fig. 4i and j). An analysis of follicular development at different stages showed increased numbers of growing follicles (primary, secondary, and antral follicles) in the ovaries of Ppm1k−/− mice, while the numbers of primordial follicles and corpus lutea did not differ between the two groups (Fig. 4k and l). Furthermore, the number of offspring in the first litter in Ppm1k−/− mice was decreased compared with that of wild-type mice (Fig. 4m), indicating the reduced fertility of Ppm1k−/− female mice. Taken together, these data demonstrate that Ppm1k−/− female mice exhibit PCOS-like reproductive traits.
The impact of increasing BCAA intake on the reproductive and metabolic phenotypes of female mice
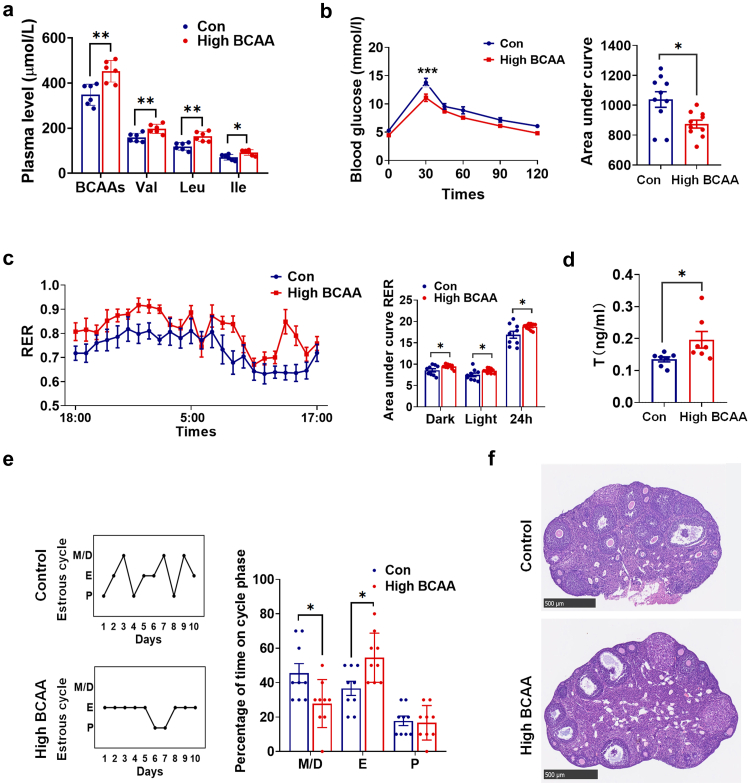
Previous studies have demonstrated that dietary intake of BCAAs regulates metabolic health, and high dietary BCAAs promote adiposity and insulin resistance in rodents.5,41 We further examined the impact of a high BCAA diet containing twice the BCAAs of a normal diet on the reproductive and metabolic phenotypes of female mice. Circulating levels of BCAAs were significantly elevated (Fig. 5a) with BCAA supplementation. Similar to Ppm1k−/− females, mice fed a high BCAA diet showed altered glucose tolerance (Fig. 5b), an increased respiratory exchange ratio (Fig. 5c), excessive serum testosterone (Fig. 5d), irregular menstrual cycles (Fig. 5e) and abnormal follicular development (Fig. 5f).
Fig. 5.
The effects of high dietary BCAAs on the reproductive and metabolic phenotypes of female mice. (a) The plasma levels of BCAAs (n = 6 mice per group). (b) The glucose tolerance test (n = 10 mice per group). (c) Respiratory exchange ratios (RER) and the area under curve of RER during light and dark cycles (n = 10 mice per group). (d) The serum levels of testosterone (n = 7 mice per group). (e) Representative and quantitative analysis of estrous cycles (n = 9 mice per group). (f) Representative ovarian morphology by hematoxylin and eosin staining, scale bar = 500 μm. Data are presented as means ± SEM. P values were determined by Student's t-test.∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001.
Restricting dietary BCAAs improves the endocrine and ovarian dysfunction induced by Ppm1k defects
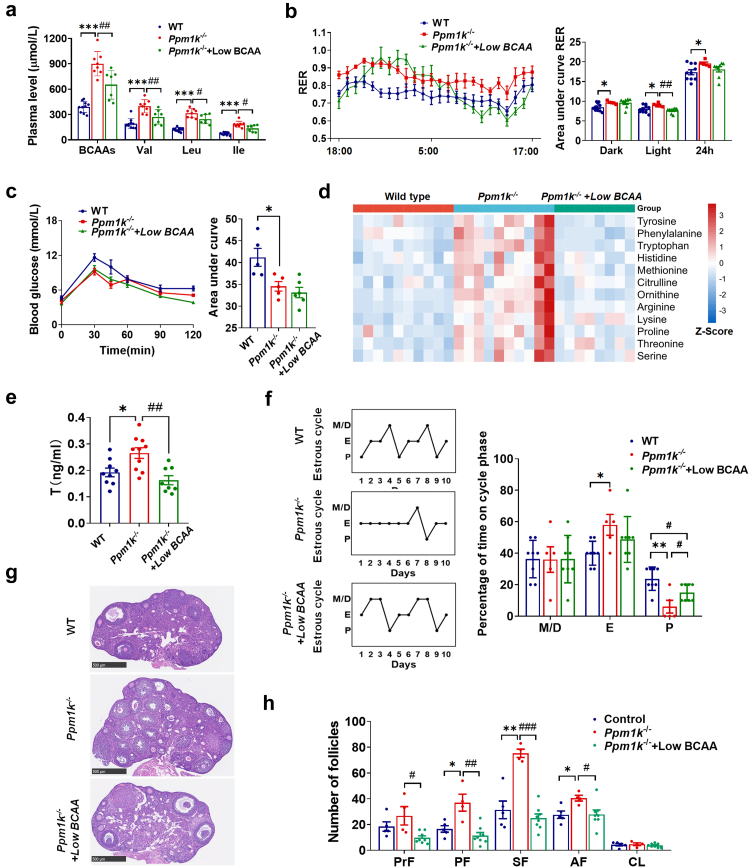
Previous reports have shown that the limitation of dietary BCAAs could promote metabolic health in rodents.41,42 Dietary intervention is also the first-line treatment for women with PCOS.43 In this study, Ppm1k deficiency led to the elevation of BCAA levels; thus, we further investigated the effects of reducing dietary BCAAs on the reproductive and metabolic abnormalities of Ppm1k−/− female mice. When fed a low BCAA diet containing one-fifth the BCAAs of a normal diet,34 Ppm1k−/− female mice displayed significantly decreased plasma levels of BCAAs (Fig. 6a). A low BCAA diet had no effect on the glucose tolerance of Ppm1k−/− female mice but induced a decrease in the respiratory exchange ratio at night (Fig. 6b and c), indicating an enhanced propensity to oxidize fat relative to glucose for energy production. In addition, Ppm1k defect-regulated impaired catabolism of BCAAs affected the levels of other amino acids, especially aromatic amino acids, histidine, methionine, etc., which were increased. A low BCAA diet decreased the concentrations of these affected amino acids to normal levels (Fig. 6d). Intriguingly, the endocrine and ovarian dysfunction of Ppm1k−/− mice was significantly ameliorated after the restriction of dietary BCAAs, as evidenced by the reduction in serum testosterone levels (Fig. 6e), the recovery of a regular estrous cycle (Fig. 6f) and normal ovarian morphology (Fig. 6g and h). These results demonstrate the contribution of BCAA limitation to improvements in PCOS-like pathological phenotypes in Ppm1k−/− mice.
Fig. 6.
Reduction of dietary BCAAs improves the abnormal reproductive and metabolic phenotypes of Ppm1k-deficient female mice. (a) The plasma levels of BCAAs (wild-type, n = 10; Ppm1k−/− female mice, n = 8; Ppm1k−/− mice with Low-BCAA diet, n = 7). (b) Respiratory exchange ratios (RER) and the area under curve of RER during light and dark cycles (wild-type, n = 10; Ppm1k−/− female mice, n = 5; Ppm1k−/− mice with Low BCAA diet, n = 8). (c) The glucose tolerance test (wild-type, n = 5; Ppm1k−/− female mice, n = 5; Ppm1k−/− mice with Low-BCAA diet, n = 6). (d) The plasma levels of other amino acids (wild-type, n = 10; Ppm1k−/− female mice, n = 10; Ppm1k−/− mice with Low-BCAA diet, n = 8) (e) The serum levels of testosterone (wild-type, n = 9; Ppm1k−/− female mice, n = 10; Ppm1k−/− mice with Low BCAA diet, n = 8). (f) Representative and quantitative analysis of estrous cycles (wild-type, n = 8; Ppm1k−/− female mice, n = 5; Ppm1k−/− mice with Low-BCAA diet, n = 8). (g) Representative ovarian morphology by hematoxylin and eosin staining, scale bar = 500 μm. (h) Differential follicle counts of primordial (PrF), primary (PF), secondary follicles (SF), antral follicles (AF) and corpus luteum (CL) (wild-type, n = 5; Ppm1k−/− female mice, n = 4; Ppm1k−/− mice with Low-BCAA diet, n = 8). Data are presented as means ± SEM. P values were determined by one-way ANOVA with Tukey's multiple comparison post-hoc test. Wild-type versus Ppm1k−/− female mice, ∗p < 0.05, ∗∗∗p < 0.001; Ppm1k−/− versus Ppm1k−/− mice with Low-BCAA diet, #p < 0.05, ##p < 0.01, ###p < 0.001.
PPM1K suppression affects follicle development by disturbing energy metabolism in human granulosa cells
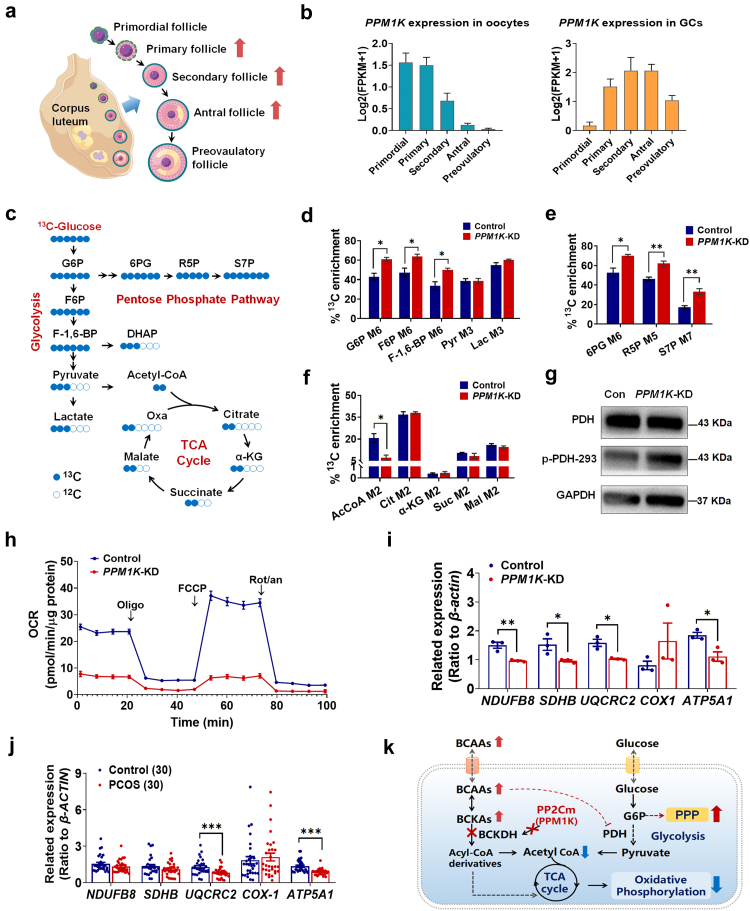
To determine the impact of PPM1K suppression on human follicle development, we analysed the PPM1K expression changes in the follicles at different developmental stages using RNA-sequencing data of human oocytes and corresponding granulosa cells (GCs) in folliculogenesis.36 The transcription level of PPM1K was profoundly decreased in oocytes with follicle growth and was extremely low in the oocytes of preovulatory follicles (Fig. 7b). Conversely, PPM1K was upregulated in the GCs of growing follicles (primary, secondary and antral follicles) and then declined in the GCs of preovulatory follicles (Fig. 7b). Based on the data in mice, a loss of Ppm1k induces the alteration of growing follicles. Therefore, we speculated that PPM1K deficiency might affect human follicle growth mainly by disturbing the function of GCs.
Fig. 7.
Effects of PPM1K knockdown on the energy metabolism homeostasis of human granulosa cells. (a) Schematic diagram of follicle development (By Figdraw). Red arrows indicate abnormal follicle development in Ppm1k−/− female mice. (b) The changes of PPM1K gene expression in oocytes and granulosa cells in human follicles at different developmental stages. (c) Schematic map of 13C-glucose incorporation from glucose into TCA cycle in human ovarian granulosa cells. G6P, glucose 6-phosphate; F6P, fructose 6-phosphate; F-1,6-BP, fructose 1,6-bisphosphate; DHAP, dihydroxyacetone phosphate; 6 PG, glucose-6-phosphate; R5P, ribose 5-phosphate; S7P, sedoheptulose 7-phosphate; α-KG, α-Ketoglutarate; Oxa, oxaloacetic acid. (d–f) Relative levels of glycolysis (d), pentose phosphate pathway (e) and TCA cycle (f) determined by 13C-labeled metabolites in control and PPM1K knocked-down (PPM1K-KD) groups (n = 3). (g) Analysis of phosphorylation levels changes of pyruvate dehydrogenase (PDH) protein. (h) Real-time changes of the O2 consumption rate (OCR) in control and PPM1K-KD human ovarian granulosa cells. Cells were treated with 2 μM oligomycin (Oligo), 1 μM carbonyl cyanide-ptrifluoromethoxyphenylhydrazone (FCCP) and 1 μM rotenone and antimycin A (Rot/an), as indicated by the three black arrows. (i) The expressions of mitochondrial oxidative phosphorylation-related genes in control and PPM1K-KD granulosa cells (n = 3). (j) The expressions of mitochondrial oxidative phosphorylation-related genes in the primary granulosa cells of PCOS and non-PCOS control individuals (n = 30). (k) Summary of PPM1K knockdown inducing the conversion of glycolysis to pentose phosphate pathway and the suppression of oxidative phosphorylation in human ovarian GCs. Data are represented as means ± SEM. For d, e, f and i, p values were determined by Student's t-test. For j, p values were determined by two-tailed Mann–Whitney U-test. ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001.
Mitochondrial energy metabolism is essential for follicle development, so we further investigated the impact of PPM1K suppression on the metabolic homeostasis of human GCs. 13C-glucose-tracing analysis in the human ovarian granulosa tumour cell line COV434 showed that PPM1K knockdown (Supplementary Figure S8a and b) significantly enhanced the labelling of glucose 6-phosphate (G6P), fructose 6-phosphate (F6P), and fructose-1,6-bisphosphate (F-1,6-P) in glycolysis and 6-phosphogluconic acid (6PG), ribose 5-phosphate (R5P), and sedoheptulose 7-phosphate (S7P) in the pentose phosphate pathway, whereas no changes were detected in pyruvate and lactate (Fig. 7d and e), indicating enhanced glucose catabolism via the pentose phosphate pathway. A previous report showed that the phosphorylation activation of AKT enhanced the transcription of glucose-6-phosphate dehydrogenase by activating mTORC1 signaling,44 modulating the shunt of glycolysis to the pentose phosphate pathway. We further detected phospho-AKT activation in PPM1K knockdown GCs (Supplementary Figure S8c). Moreover, pyruvate is an essential metabolite that connects glycolysis and the TCA cycle, and pyruvate dehydrogenase (PDH) is responsible for catalysing the irreversible reaction of pyruvate decarboxylation to produce acetyl coenzyme A (acetyl-CoA). We found an augmented phosphorylation level of PDH in PPM1K knockdown GCs (Fig. 7g), demonstrating the inhibition of pyruvate catabolism and thus its entry into the TCA cycle. Consistent with this finding, PPM1K knockdown decreased the level of 13C-labeled acetyl coenzyme A (acetyl-CoA) (Fig. 7f). Furthermore, PPM1K inhibition significantly reduced the oxygen consumption rate (OCR) of human GCs (Fig. 7h; Supplementary Figure S8d). In addition, the mitochondrial oxidative phosphorylation-related genes coding complexes I, II, III and V were significantly downregulated in PPM1K knockdown GCs (Fig. 7i). In particular, as the core subunits of complexes III and V, the expression of ubiquinol-cytochrome C reductase core protein 2 (UQCRC2) and ATP synthase α subunit (ATP5A1) was also obviously decreased in the primary granulosa cells of PCOS patients compared with controls (Fig. 7j). Taken together, these results indicate that PPM1K suppression promotes the conversion from glycolysis to the pentose phosphate pathway and inhibits oxidative phosphorylation in human ovarian GCs, which aggravates the burden of mitochondrial energy metabolism and interferes with follicular environmental homeostasis.
Discussion
The present study systematically explored the associations of BCAA catabolism with PCOS using observational and causal analyses and experimental approaches. We found that increased plasma levels of BCAAs were positively associated with androgen levels, the number of antral follicles and irregular menstrual cycles in women with PCOS. MR analyses revealed causal associations between the elevation of BCAAs and a higher risk of PCOS and found that the causal gene was the PPM1K gene. In addition, Ppm1k knockout female mice manifested PCOS-like traits such as hyperandrogenemia and abnormal follicle development. A reduction in dietary BCAA intake relieved the abnormal reproductive and metabolic phenotypes of Ppm1k-deficient female mice. Finally, we provided evidence that PPM1K deficiency in human ovarian granulosa cells led to the conversion from glycolysis to the pentose phosphate pathway and the inhibition of mitochondrial oxidative phosphorylation.
Due to the heritability of PCOS, the MR method has recently been gradually used to assess causal associations between PCOS and etiological risk factors.45 Based on MR approaches, we identified that BCAA metabolism was likely to have a causal association with an increased risk of PCOS. Among the genomic loci associated with BCAA levels, the strongest signal was located 21 kb upstream of the PPM1K gene. The knockout of Ppm1k in mice46,47 or loss-of-function mutations of PPM1K in humans48 led to impaired catabolism of BCAAs and increased levels of BCAAs, a pattern that resembles that observed for common PPM1K genetic variants in our study and in the study of Lotta et al.17 Interestingly, in addition to PPM1K, rs12149660 at the AARS locus was positively associated with three BCAAs. Aminoacyl-tRNA synthetases (AaRSs) encoded by AARS genes are essential enzymes in translation that charge amino acids onto their cognate tRNAs during protein synthesis. Mutations in AARS have been implicated in a plethora of diseases, including neurological conditions, metabolic disorders and cancer.49 Two SNPs at BCAT2 loci were also positively associated with the levels of isoleucine and valine. Mice with adipose tissue knockout of Bcat2, which converts BCAAs to branched-chain keto acids (BCKAs), are resistant to high-fat diet-induced obesity due to increased inguinal white adipose tissue browning and thermogenesis. These findings suggest that BCAA-raising polymorphisms affect BCAA levels and might be implicated in PCOS.
Consistent with the MR results, Ppm1k defect-induced impaired BCAA catabolism results in PCOS-like endocrine and ovarian dysfunction in female mice. Hyperandrogenism is a typical clinical feature in PCOS patients, playing a pathologic role in the development and progression of PCOS. In the ovary, androgen is mostly synthesized from cholesterol and converted into bioactive types by a series of steroidogenesis-related enzymes. In this study, the elevated testosterone levels might be due to enhanced androgen synthesis mediated by increased 3β-HSD expression in Ppm1k−/− female mice. Reports have shown that the transcriptional regulation of 3β-HSD involves several different factors, especially via the cyclic adenosine monophosphate/protein kinase-C (cAMP/PKC)50 and mitogen-activated protein kinase (MAPK) pathways.51 BCAAs can serve as nutrient signals and play critical roles in regulating signalling networks.52 The elevation of BCAAs induced by Ppm1k deficiency might modulate specific signalling pathways or transcription factors to affect 3β-HSD expression, yet the molecular mechanism still needs further clarification.
On the other hand, our findings indicate that BCAA-raising polymorphisms are significantly associated with SHBG levels. In particular, isoleucine and leucine are related to decreased SHBG levels, while isoleucine is associated with elevated bioactive testosterone levels. These results are consistent with data from observational studies, in that the plasma levels of BCAAs are negatively associated with SHBG levels in individuals with PCOS. SHBG is often used as an indicator of hyperandrogenism in women with PCOS, acting as a sex hormone transporter that binds androgens and affects their bioavailability. The synthesis and secretion of SHBG occur mainly in the liver, and hepatocyte nuclear factor 4α (HNF-4α) is the most important transcription factor activating SHBG expression. The level of HNF-4α is negatively correlated with lipogenesis, and the conversion of monosaccharides to palmitate inhibits HNF-4α expression and SHBG levels.53 A previous report showed that hepatic overexpression of BCKD kinase (BCKDK, a suppressor of BCKD) and the repression of PPM1K activated de novo lipogenesis,54 which might be the underlying mechanism of the downregulation of HNF-4α by enhanced BCAAs and subsequent inhibition of SHBG synthesis. Further investigation is required to confirm this cascade regulatory effect in women with PCOS.
Circulating levels of BCAAs are regulated by the balance between the catabolic pathway and the dietary intake of BCAAs. There is increasing evidence that a high BCAA diet aggravates glucose and lipid metabolic abnormalities,34,41,55 whereas the limitation of dietary BCAAs alleviates obesity, abnormal glucose tolerance and insulin resistance in rodents.42,56,57 In our study, high dietary BCAAs enhanced serum testosterone levels and impaired the reproductive function of female mice, while a selective reduction in dietary BCAAs ameliorated PCOS-like reproductive traits of Ppm1k−/− female mice, emphasizing the crucial role of elevated BCAAs (induced by PPM1K genetic deficiency and high dietary BCAAs) in regulating the occurrence of PCOS.
Diet as a main lifestyle intervention is recommended as a first-line treatment for women with PCOS.43 A recent study elucidated the impact of dietary macronutrients on the development of PCOS using a hyperandrogenic mouse model and identified that a low protein, medium carbohydrate and fat diet could ameliorate key PCOS reproductive defects.58 Strikingly, we observed that plasma BCAA levels were markedly increased in a dehydroepiandrosterone (DHEA)-induced PCOS mouse model, demonstrating that excessive androgen could conversely interfere with the BCAA catabolism process. Restricting dietary BCAAs significantly improved the metabolic, endocrine and reproductive disorders in DHEA-treated female mice (Supplementary Figure S9). Overall, these findings provide evidence to support an intervention strategy of a low protein diet, especially a low BCAA diet, for the treatment of PCOS. Additionally, previous randomized trials have indicated an effect of the PPM1K rs1440581 genetic variant on metabolic changes in obese individuals in response to an energy-restricted diet with different nutrient ratios.59,60 Thus, it is necessary to investigate the effects of genetic determinants of BCAA catabolism on improving the metabolic and reproductive abnormalities of women with PCOS. In the future, the assessment of BCAA levels and BCAA catabolism-related genetic variants may be profound for the development of more accurate typing and dietary intervention strategies for PCOS.
Apart from dietary restriction of BCAAs, some reports have clarified the therapeutic validity of targeting BCAA catabolism. 3,6-Dichlorobenzo[b]thiophene-2-carboxylic acid (BT2), a small-molecule pharmacological inhibitor of BCKDK, can accelerate BCAA catabolic flux and reduce the abundance of BCAAs.61 BT2 treatment markedly attenuated insulin resistance and improved glucose tolerance in obese and diabetes rodent models.11,54 In addition, sodium phenylbutyrate (NaPB), an aromatic fatty acid, can also enhance BCAA catabolism by preventing BCKDK phosphorylation. NaPB administration was effective in improving glucose metabolism in a diabetic mouse model62 and attenuating lipid-induced insulin resistance in obese individuals.63 Future studies will be required to investigate whether pharmacologically modulating BCAA catabolism helps to improve metabolic and reproductive disorders of PCOS.
Based on our findings, PPM1K deficiency could affect follicle development by disturbing the glucose metabolism and mitochondrial function of GCs. With the progress of sequencing technology, researchers have made more in-depth study of genes expression pattern in a variety of cell types of ovary in recent years. According to the single-cell RNA-sequencing data of human ovarian cortex in caesarean section and gender reassignment patients,64 we found that PPM1K is widely expressed in human ovarian tissue, not only in granulosa cells and oocytes, but also in stromal cells, immune cells, perivascular cells and epithelial cells. However, there is still lack of dynamic change of gene expression in other ovarian cell types outside the follicles during periodic changes of ovary. It needs to further explore the physiological role of PPM1K in different cell types of ovary during follicle development.
There are several limitations of the current study. First, for MR analysis, we included two different PCOS GWASs. Although the PCOS GWAS by Day F et al.19 has a larger sample size than the GWAS using UK Biobank data, we did not find a significant relationship with BCAAs. Even though GWAS by Day F et al.19 has larger sample size, it was subject to multiple sources, and the use of different measurements and diagnostic criteria might lead to inaccurate results. Second, residual pleiotropy and bias due to invalid instruments are common concerns for MR study. We assessed limited potential pleiotropy using the MR-Egger intercept, MR-PRESSO method and MR Steiger filtering test. In our analysis, we primarily focused on the results of the IVW method for its higher statistic power compared to other methods. Although part of the results was not showed significant across several methods and different PCOS GWAS source, their consistency in the direction of effect estimates is complementary evidence for our study. We also applied outlier removal process for testing SNPs to minimize pleiotropy and confounding bias for MR analysis of BCAA and PCOS-related traits, which may lead to a limited number of instrumental variables. But we obtained directionally consistent results with the same significance in most sensitivity approaches, which may add to the robustness of the conclusions. Third, it was valuable but challenging to identify independent instrumental variable for the lack of individual-level data, and considering that rs1440581 and rs7678928 were located in the same coding region and were both significantly associated with BCAA levels, we included both in our PheWAS analysis. The PheWAS results also supported the multiple functions of the PPM1K gene in other metabolic disorders, such as diabetes. Therefore, larger-scale genetic studies and randomized controlled trials will help to further qualify the causal nature of the relationship between BCAA metabolism and PCOS risk. Lastly, we have not yet assessed the significant independent effect of a specific type of BCAAs on PCOS through MVMR analysis. Our study revealed the association between BCAAs and PCOS, but failed to disentangle the independent relationship caused by a specific type of amino acid, which could be partially explained by shared genetic risks and potential common biological mechanisms between BCAAs. The molecular mechanism by which the impaired BCAA catabolism induced by PPM1K deficiency regulates the occurrence of PCOS needs to be further elucidated.
In conclusion, the findings of our study suggest that impaired BCAA catabolism causes the occurrence and development of PCOS and that PPM1K could be used as a molecular target for regulating BCAA catabolism to develop a potential treatment strategy for PCOS. Furthermore, there is a need to perform randomized controlled trials in humans to further evaluate the improvement effect of proposed nutritional strategy on PCOS patients.
Contributors
All authors were involved in the development of the primary manuscript and have read and approved the final version. JQ, YZ, LSM, and JL designed the study. LSM, JL, JHH, and WAM performed the MR analyses. HPS provided the Ppm1k−/− mice and the guidance to animal experiments. ZHY, YZ, KC, and YRZ performed the animal studies. RL, XYL, CMZ, and YCL collected samples and clinical information in individual studies. ZHY and YZ performed the in vitro experiments in human granulosa cells. YZ, JL, LSM, and ZHY verified the underlying data. YZ, JQ, JL, LSM, and ZHY wrote the manuscript. All authors reviewed and revised the manuscript and approved the final version, and are fully responsible for all content and editorial decisions.
Data sharing statement
All summary statistics for the analysis are available in the Supplemental Tables. The BCAA GWAS can be downloaded from the corresponding public browser (https://omicscience.org/apps/crossplatform). The GWAS for PCOS was derived from the UK BioBank imputed v3 provided by the Neale laboratory (http://www.nealelab.is/uk-BioBank; code “20002_1350”) and the GWAS from Day F et al. (https://www.repository.cam.ac.uk/handle/1810/283491). Please follow the instructions in the original article provided by the authors for the rest of the GWAS data. LDlink is a publicly available online website (https://analysistools.cancer.gov/LDlink). The Locus Zoom plot was drawn through its official website (http://locuszoom.org).
Declaration of interests
The authors declare that they have no competing interests.
Acknowledgements
We thanks all the sample donors and the doctors and nurses of the Reproductive Medical Center of Peking University Third Hospital for their excellent assistance. This study was supported by the National Key Research and Development Program of China (2021YFC2700402, 2019YFA0802503), the National Natural Science Foundation of China (81871139, 82001503, 92057107), the CAMS Innovation Fund for Medical Sciences (2019-I2M-5-001), Key Clinical Projects of Peking University Third Hospital (BYSY2022043), the China Postdoctoral Science Foundation (2021T140600), and the Collaborative Innovation Program of Shanghai Municipal Health Commission (2020CXJQ01).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104492.
Contributor Information
Jun Liu, Email: Jun.liu@ndph.ox.ac.uk.
Yue Zhao, Email: zhaoyue0630@163.com.
Jie Qiao, Email: jie.qiao@263.net.
Appendix A. Supplementary data
References
- 1.Skiba M.A., Islam R.M., Bell R.J., Davis S.R. Understanding variation in prevalence estimates of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2018;24(6):694–709. doi: 10.1093/humupd/dmy022. [DOI] [PubMed] [Google Scholar]
- 2.Escobar-Morreale H.F. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270–284. doi: 10.1038/nrendo.2018.24. [DOI] [PubMed] [Google Scholar]
- 3.Dumesic D.A., Oberfield S.E., Stener-Victorin E., Marshall J.C., Laven J.S., Legro R.S. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):487–525. doi: 10.1210/er.2015-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch C.J., Adams S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10(12):723–736. doi: 10.1038/nrendo.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newgard C.B., An J., Bain J.R., et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahola-Olli A.V., Mustelin L., Kalimeri M., et al. Circulating metabolites and the risk of type 2 diabetes: a prospective study of 11,896 young adults from four Finnish cohorts. Diabetologia. 2019;62(12):2298–2309. doi: 10.1007/s00125-019-05001-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruiz-Canela M., Toledo E., Clish C.B., et al. Plasma branched-chain amino acids and incident cardiovascular disease in the PREDIMED trial. Clin Chem. 2016;62(4):582–592. doi: 10.1373/clinchem.2015.251710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neinast M.D., Jang C., Hui S., et al. Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell Metab. 2019;29(2):417–429.e4. doi: 10.1016/j.cmet.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li T., Zhang Z., Kolwicz S.C., Jr., et al. Defective branched-chain amino acid catabolism disrupts glucose metabolism and sensitizes the heart to ischemia-reperfusion injury. Cell Metab. 2017;25(2):374–385. doi: 10.1016/j.cmet.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sánchez-González C., Nuevo-Tapioles C., Herrero Martín J.C., et al. Dysfunctional oxidative phosphorylation shunts branched-chain amino acid catabolism onto lipogenesis in skeletal muscle. EMBO J. 2020;39(14) doi: 10.15252/embj.2019103812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou M., Shao J., Wu C.Y., et al. Targeting BCAA catabolism to treat obesity-associated insulin resistance. Diabetes. 2019;68(9):1730–1746. doi: 10.2337/db18-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y., Fu L., Li R., et al. Metabolic profiles characterizing different phenotypes of polycystic ovary syndrome: plasma metabolomics analysis. BMC Med. 2012;10:153. doi: 10.1186/1741-7015-10-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang A.Y., Lalia A.Z., Jenkins G.D., et al. Combining a nontargeted and targeted metabolomics approach to identify metabolic pathways significantly altered in polycystic ovary syndrome. Metabolism. 2017;71:52–63. doi: 10.1016/j.metabol.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halama A., Aye M.M., Dargham S.R., Kulinski M., Suhre K., Atkin S.L. Metabolomics of dynamic changes in insulin resistance before and after exercise in PCOS. Front Endocrinol. 2019;10:116. doi: 10.3389/fendo.2019.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye Z., Zhang C., Wang S., et al. Amino acid signatures in relation to polycystic ovary syndrome and increased risk of different metabolic disturbances. Reprod Biomed Online. 2022;44(4):737–746. doi: 10.1016/j.rbmo.2021.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Lu G., Sun H., She P., et al. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J Clin Invest. 2009;119(6):1678–1687. doi: 10.1172/JCI38151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lotta L.A., Pietzner M., Stewart I.D., et al. A cross-platform approach identifies genetic regulators of human metabolism and health. Nat Genet. 2021;53(1):54–64. doi: 10.1038/s41588-020-00751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudlow C., Gallacher J., Allen N., et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3) doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Day F., Karaderi T., Jones M.R., et al. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 2018;14(12) doi: 10.1371/journal.pgen.1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruth K.S., Day F.R., Tyrrell J., et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26(2):252–258. doi: 10.1038/s41591-020-0751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kho P.F., Glubb D.M., Thompson D.J., Spurdle A.B., O'Mara T.A. Assessing the role of selenium in endometrial cancer risk: a Mendelian randomization study. Front Oncol. 2019;9:182. doi: 10.3389/fonc.2019.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawlor D.A., Harbord R.M., Sterne J.A., Timpson N., Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 23.Hemani G., Zheng J., Elsworth B., et al. The MR-base platform supports systematic causal inference across the human phenome. Elife. 2018;7 doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierce B.L., Ahsan H., Vanderweele T.J. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. 2011;40(3):740–752. doi: 10.1093/ije/dyq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowden J., Davey Smith G., Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verbanck M., Chen C.Y., Neale B., Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemani G., Tilling K., Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017;13(11) doi: 10.1371/journal.pgen.1007081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yavorska O.O., Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46(6):1734–1739. doi: 10.1093/ije/dyx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe K., Stringer S., Frei O., et al. A global overview of pleiotropy and genetic architecture in complex traits. Nat Genet. 2019;51(9):1339–1348. doi: 10.1038/s41588-019-0481-0. [DOI] [PubMed] [Google Scholar]
- 30.Ye Z., Zhang C., Zhao Y. Potential effects of adropin on systemic metabolic and hormonal abnormalities in polycystic ovary syndrome. Reprod Biomed Online. 2021;42(5):1007–1014. doi: 10.1016/j.rbmo.2021.01.020. [DOI] [PubMed] [Google Scholar]
- 31.Zhang C.M., Zhao Y., Li R., et al. Metabolic heterogeneity of follicular amino acids in polycystic ovary syndrome is affected by obesity and related to pregnancy outcome. BMC Pregnancy Childbirth. 2014;14:11. doi: 10.1186/1471-2393-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y., Zhang C., Huang Y., et al. Up-regulated expression of WNT5a increases inflammation and oxidative stress via PI3K/AKT/NF-κB signaling in the granulosa cells of PCOS patients. J Clin Endocrinol Metab. 2015;100(1):201–211. doi: 10.1210/jc.2014-2419. [DOI] [PubMed] [Google Scholar]
- 33.Hemmings K.E., Maruthini D., Vyjayanthi S., et al. Amino acid turnover by human oocytes is influenced by gamete developmental competence, patient characteristics and gonadotrophin treatment. Hum Reprod. 2013;28(4):1031–1044. doi: 10.1093/humrep/des458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solon-Biet S.M., Cogger V.C., Pulpitel T., et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat Metab. 2019;1(5):532–545. doi: 10.1038/s42255-019-0059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon S.Y., Yoon J.A., Park M., et al. Recovery of ovarian function by human embryonic stem cell-derived mesenchymal stem cells in cisplatin-induced premature ovarian failure in mice. Stem Cell Res Ther. 2020;11(1):255. doi: 10.1186/s13287-020-01769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y., Yan Z., Qin Q., et al. Transcriptome landscape of human folliculogenesis reveals oocyte and granulosa cell interactions. Mol Cell. 2018;72(6):1021–1034.e4. doi: 10.1016/j.molcel.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 37.Li G., Wu J., Li L., Jiang P. p53 deficiency induces MTHFD2 transcription to promote cell proliferation and restrain DNA damage. Proc Natl Acad Sci U S A. 2021;118(28) doi: 10.1073/pnas.2019822118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu W., Liu Z., Yu W., et al. Effects of PPM1K rs1440581 and rs7678928 on serum branched-chain amino acid levels and risk of cardiovascular disease. Ann Med. 2021;53(1):1316–1326. doi: 10.1080/07853890.2021.1965204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White P.J., McGarrah R.W., Herman M.A., Bain J.R., Shah S.H., Newgard C.B. Insulin action, type 2 diabetes, and branched-chain amino acids: a two-way street. Mol Metab. 2021;52 doi: 10.1016/j.molmet.2021.101261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Denny J.C., Ritchie M.D., Basford M.A., et al. PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics. 2010;26(9):1205–1210. doi: 10.1093/bioinformatics/btq126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu D., Richardson N.E., Green C.L., et al. The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metab. 2021;33(5):905–922.e6. doi: 10.1016/j.cmet.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cummings N.E., Williams E.M., Kasza I., et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J Physiol. 2018;596(4):623–645. doi: 10.1113/JP275075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teede H.J., Misso M.L., Costello M.F., et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110(3):364–379. doi: 10.1016/j.fertnstert.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Düvel K., Yecies J.L., Menon S., et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39(2):171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang C., Wu W., Yang H., et al. Mendelian randomization analyses for PCOS: evidence, opportunities, and challenges. Trends Genet. 2022;38(5):468–482. doi: 10.1016/j.tig.2022.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Liu X., Zhang F., Zhang Y., et al. PPM1K regulates hematopoiesis and leukemogenesis through CDC20-mediated ubiquitination of MEIS1 and p21. Cell Rep. 2018;23(5):1461–1475. doi: 10.1016/j.celrep.2018.03.140. [DOI] [PubMed] [Google Scholar]
- 47.Xu Y., Jiang H., Li L., et al. Branched-chain amino acid catabolism promotes thrombosis risk by enhancing tropomodulin-3 propionylation in platelets. Circulation. 2020;142(1):49–64. doi: 10.1161/CIRCULATIONAHA.119.043581. [DOI] [PubMed] [Google Scholar]
- 48.Oyarzabal A., Martínez-Pardo M., Merinero B., et al. A novel regulatory defect in the branched-chain α-keto acid dehydrogenase complex due to a mutation in the PPM1K gene causes a mild variant phenotype of maple syrup urine disease. Hum Mutat. 2013;34(2):355–362. doi: 10.1002/humu.22242. [DOI] [PubMed] [Google Scholar]
- 49.Datt M., Sharma A. Evolutionary and structural annotation of disease-associated mutations in human aminoacyl-tRNA synthetases. BMC Genom. 2014;15(1):1063. doi: 10.1186/1471-2164-15-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rasmussen M.K., Ekstrand B., Zamaratskaia G. Regulation of 3β-hydroxysteroid dehydrogenase/Δ⁵-Δ⁴ isomerase: a review. Int J Mol Sci. 2013;14(9):17926–17942. doi: 10.3390/ijms140917926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ye W., Xie T., Song Y., Zhou L. The role of androgen and its related signals in PCOS. J Cell Mol Med. 2021;25(4):1825–1837. doi: 10.1111/jcmm.16205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nie C., He T., Zhang W., Zhang G., Ma X. Branched chain amino acids: beyond nutrition metabolism. Int J Mol Sci. 2018;19(4) doi: 10.3390/ijms19040954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xing C., Zhang J., Zhao H., He B. Effect of sex hormone-binding globulin on polycystic ovary syndrome: mechanisms, manifestations, genetics, and treatment. Int J Womens Health. 2022;14:91–105. doi: 10.2147/IJWH.S344542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White P.J., McGarrah R.W., Grimsrud P.A., et al. The BCKDH kinase and phosphatase integrate BCAA and lipid metabolism via regulation of ATP-citrate lyase. Cell Metab. 2018;27(6):1281–1293.e7. doi: 10.1016/j.cmet.2018.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee J., Vijayakumar A., White P.J., et al. BCAA supplementation in mice with diet-induced obesity alters the metabolome without impairing glucose homeostasis. Endocrinology. 2021;162(7) doi: 10.1210/endocr/bqab062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karusheva Y., Koessler T., Strassburger K., et al. Short-term dietary reduction of branched-chain amino acids reduces meal-induced insulin secretion and modifies microbiome composition in type 2 diabetes: a randomized controlled crossover trial. Am J Clin Nutr. 2019;110(5):1098–1107. doi: 10.1093/ajcn/nqz191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fontana L., Cummings N.E., Arriola Apelo S.I., et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 2016;16(2):520–530. doi: 10.1016/j.celrep.2016.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodriguez Paris V., Solon-Biet S.M., Senior A.M., et al. Defining the impact of dietary macronutrient balance on PCOS traits. Nat Commun. 2020;11(1):5262. doi: 10.1038/s41467-020-19003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu M., Qi Q., Liang J., et al. Genetic determinant for amino acid metabolites and changes in body weight and insulin resistance in response to weight-loss diets: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation. 2013;127(12):1283–1289. doi: 10.1161/CIRCULATIONAHA.112.000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goni L., Qi L., Cuervo M., et al. Effect of the interaction between diet composition and the PPM1K genetic variant on insulin resistance and β cell function markers during weight loss: results from the Nutrient Gene Interactions in Human Obesity: implications for dietary guidelines (NUGENOB) randomized trial. Am J Clin Nutr. 2017;106(3):902–908. doi: 10.3945/ajcn.117.156281. [DOI] [PubMed] [Google Scholar]
- 61.Vanweert F., Schrauwen P., Phielix E. Role of branched-chain amino acid metabolism in the pathogenesis of obesity and type 2 diabetes-related metabolic disturbances BCAA metabolism in type 2 diabetes. Nutr Diabetes. 2022;12(1):35. doi: 10.1038/s41387-022-00213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ozcan U., Yilmaz E., Ozcan L., et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao C., Giacca A., Lewis G.F. Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and beta-cell dysfunction in humans. Diabetes. 2011;60(3):918–924. doi: 10.2337/db10-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wagner M., Yoshihara M., Douagi I., et al. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat Commun. 2020;11(1):1147. doi: 10.1038/s41467-020-14936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.