Abstract
Many primary tumors as well as transformed cell lines display high sensitivity to chemotherapeutic drugs and radiation. The molecular mechanisms that underlie this sensitivity are largely unknown. Here we show that the sensitization of transformed cells to stress stimuli is due to the potentiation of the c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase pathways. Activation of these pathways by the antitumor drug cis-platin (CDDP) and by other stress agents is markedly enhanced and is induced by lower stress doses in NIH 3T3 cells overexpressing epidermal growth factor receptor, HER1–2 kinase, or oncogenic Ras than in nontransformed NIH 3T3 cells. Inhibition of stress kinase activity by specific inhibitors reduces CDDP-mediated cell death in transformed cells, whereas overactivation of stress kinase pathways augments cells death. Potentiation of stress kinases is a common feature of cells transformed by different oncogenes, including cells derived from human tumors, and is shown here to be independent of the activity of the particular transforming oncoprotein. We further show that the mechanism that underlies potentiation of stress kinases in transformed cells involves reactive oxygen species (ROS), whose production is elevated in these cells. JNK/p38 activation is inhibited by antioxidants and in particular by inhibitors of the mitochondrial respiratory chain and NADPH oxidase. Conversely, by artificially elevating ROS levels in nontransformed NIH 3T3 cells we were able to induce potentiation of JNK/p38 activation. Taken together, our findings suggest that ROS-dependent potentiation of stress kinase pathways accounts for the sensitization of transformed cells to stress and anticancer drugs.
The expression of oncogenes or the enhanced activity of proto-oncogenes induces unregulated mitosis and cell proliferation in most cell types. Conversely, oncogenic transformation often results in the apparently contradictory phenomenon of sensitization to stress-induced apoptosis. For example, rat embryo fibroblasts transformed by the oncogenic forms of Myc, Mos, Src, or Ras are sensitized to induction of apoptosis by the antitumor drug cis-platin (CDDP) (4). Similarly, transformation of NIH 3T3 cells by oncogenic Src, Ras, or Raf potentiates apoptosis in response to treatment with etoposide (11). In several cases, overexpression of the epidermal growth factor receptor (EGFR) or the related HER-2/neu receptor was found to be associated with enhanced sensitivity to CDDP (3, 19). In fact, the enhanced sensitivity of oncogenically transformed cells to these agents forms the basis of present cytotoxic chemotherapy. However, although the enhanced sensitivity of transformed cells is well documented and is of great advantage in the clinic, its molecular basis is not clear. Elucidation of the basis for sensitization of transformed cells to stress is important, since it may further promote our understanding as to why cells from advanced tumors or from tumors that were exposed to chronic chemotherapy often lose this sensitivity and become resistant to apoptotic signals.
The mitogen-activated protein kinase (MAPK) family of proteins belongs to distinct and evolutionarily conserved signal transduction pathways that are activated by extracellular stimuli (33, 37, 49). In particular, c-Jun N-terminal kinase (JNK) and p38 MAPK (also referred to as stress-activated protein kinases) pathways are activated by stress agents, including tumor necrosis factor alpha, interleukin 1, heat shock, UV light, gamma radiation, and chemotherapeutic drugs (27, 31, 34, 42, 47, 49). This activation has been shown to correlate with induction of apoptosis by these agents. Studies using dominant negative mutants of JNK and p38 and specific pharmacological inhibitors have shown that JNK and/or p38 activation is necessary for UV-, cytokine-, chemotherapy-, ceramide-, and serum deprivation-induced apoptosis (6, 7, 13, 14, 32, 53, 57). Also, studies on fibroblasts with targeted disruptions of all the functional Jnk genes established an essential role for JNK in UV-induced and other stress-induced apoptosis (50). However, it is not known how oncogenic transformation affects the stress kinase pathways. In view of the important role of JNK and p38 MAPKs in the cellular response to stress, we sought to compare the activation and regulation of these pathways in transformed cells and in nontransformed cells.
Here we show that JNK and p38 expression and activity are similar in transformed and nontransformed cell lines under normal growth conditions. Under stress, however, JNK and p38 activation are greatly enhanced in transformed NIH 3T3 cells. For transformed cells, low doses of stress stimuli are sufficient to induce JNK/p38 activation and consequently cell death. We further show that stress kinase potentiation in transformed cells is due to an elevated rate of production of reactive oxygen species (ROS). We propose that the ROS-mediated JNK and p38 activation play a key role in the sensitization of oncogenically transformed cells to stress signals and to anticancer drugs.
MATERIALS AND METHODS
Antibodies and other reagents.
Antibodies were obtained as follows: anti-JNK1, anti-p38, anti-SEK1 (MEK4), anti-ERK2, and antiactin from Santa Cruz Biotechnology; anti-phospho-p38, anti-phospho-JNK, anti-phospho-SEK1, anti-phospho-MKK3/6, anti-phospho-ATF2, and anti-phospho-c-Jun from New England BioLabs; anti-phospho-ERK from Sigma; and antihemagglutinin (anti-HA; high affinity) from Roche. SB203580, SB202190, and PD98059 were purchased from Calbiochem. AG1478 was synthesized by Aviv Gazit in our laboratory. CDDP was obtained from ABIC Ltd., Netanya, Israel. All other chemicals were purchased from Sigma.
Cell culture and treatment.
NIH 3T3 cells transformed with either the EGFR (DHER14 cells), with the HER1-HER2 chimera (CSH12 cells), or with myristylated Ras (NIH 3T3/Ras cells) have been described (17, 26, 35). These and A431 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, penicillin, and streptomycin and were incubated at 37°C in 5% CO2. HT29 cells were grown in McCoy medium with 10% serum and antibiotics. Unless otherwise stated, cells (106) were seeded in a 100-mm-diameter petri dish with 10 ml of growth medium and were treated on the 3rd day as indicated. Stock solutions of kinase inhibitors (PD98059, SB203580, SB202190, and AG1478) in dimethyl sulfoxide (DMSO) were diluted 1:1,000 prior to use in Dulbecco's modified Eagle's medium which contained 10% fetal calf serum. The concentration of DMSO in the controls was equal to the concentration of DMSO in inhibitor-containing media and did not exceed 0.05%. UV irradiation was conducted with a germicidal 254-nm UV lamp at the rate of 2 J/m2/s. Prior to UV irradiation, the entire medium was removed and replaced immediately after treatment.
Survival and apoptosis assays. (i) Survival analysis.
Cells were seeded at 3,000 cells per well in 96-microculture-well plates. After 2 days, cells were treated as indicated. The fraction of surviving cells was measured after the indicated times using the automated microculture methylene blue assay (23). Briefly, cells were fixed in 0.05% glutaraldehyde for 10 min at room temperature. After being washed, the microplates were stained with 0.1% methylene blue in 0.1 M borate buffer (pH 8.5) for 60 min at room temperature. Thereafter, the plates were thoroughly washed to remove excess dye and then dried. The dye absorbed by the cells was eluted in 0.1 M HCl for 60 min at 37°C and read at 630 nm.
(ii) Apoptosis assays.
DNA fragmentation was visualized by incorporation of fluorescent oligonucleotides by terminal deoxynucleotidyltransferase with a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) kit (Roche). Cells were grown on glass coverslips, and staining of apoptotic cells was performed as instructed by the manufacturer 24 h after CDDP or UV treatment.
Apoptosis was quantified by fluorescence-activated cell sorter (FACS) analysis. Cells were grown in 60-mm-diameter plates and were treated as indicated. After 48 h, attached and floating cells were pooled, pelleted by centrifugation, washed in phosphate-buffered saline, and fixed with cold 70% ethanol for 1 h. Cells were repelleted and resuspended in 500 μl of propidium iodide solution containing 0.1% sodium citrate, 0.1% Triton X-100, 100 μg of RNase A/ml, and 50 μg of propidium iodide/ml. FACS analysis was performed with a Becton Dickinson FACScan machine. The left edge of the control cell profile was taken as the border separating normal, diploid cells (to the right) from apoptotic, hypodiploid cells (to the left). The percentage of apoptotic cells was calculated as the ratio of events on the left side to events from the whole population.
Plasmids and transfections.
HA-JNK was the gift of M. Karin (University of California, San Diego, Calif.), FLAG-ASK1 was the gift of K. Matsumoto (Nagoya University, Nagoya, Japan), and pEBG-SEK1 was the gift of L. Zon (Children's Hospital, Howard Hughes Medical Institute, Harvard Medical School, Boston, Mass.). Stable transfection of SEK1 was performed with Fugene 6 transfection reagent (Roche) according to the manufacturer's instructions. DHER14 cells were cotransfected with SEK1 and pEFr-PGKpuropAv18 (puromycin resistance)-expressing plasmids. Forty-eight hours after transfection, cells were selected in the presence of puromycin (2 μg/ml). After 2 weeks colonies were pooled.
Transient-transfection death assay.
Cells were plated in six-well plates 24 h before transfection at a density of 2 × 105 cells/well. Cells were cotransfected with green fluorescent protein (GFP) plasmid (1 μg) and empty vector or plasmids expressing a kinase (2 μg) as indicated. The total DNA concentration was kept constant by including empty vector. The polyethyleneimine transfection protocol was performed as described previously (5). Twenty-four hours after transfection, cells were treated with 30 μM CDDP. Thirty hours after treatment, floating and attached cells were pooled and analyzed by FACS. Survival was expressed as follows: (the number of GFP-expressing cells in the CDDP-treated group/the number of GFP-expressing cells in the nontreated group) × 100%.
Immunoblotting.
Cells were lysed in 0.3 ml of lysis buffer (20 mM Tris, pH 7.5, 250 mM NaCl, 0.5% NP-40, 3 mM EDTA, 3 mM EGTA, 10% glycerol, 20 mM β-glycerolphosphate, 1 mM of p-nitrophenyl phosphate, 0.5 mM Na3VO4, 1 mM dithiothreitol, 2 μg of leupeptin/ml, 2 μg of aprotonin/ml, and 1 mM AEBSF) for 15 min on ice. Cell debris was removed by centrifugation at 20,000 × g for 15 min at 4°C. Protein concentration was determined by a modification of the Bradford method (59). Thirty micrograms of protein lysate was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After electrophoresis, proteins were transferred to a nitrocellulose membrane. After incubation of the membrane with the appropriate antibodies, specific proteins were visualized using an enhanced chemiluminescence detection reagent. For quantitation purposes, several exposures were done for each experiment, and only subsaturation exposures were further analyzed. Densitometry of immunoblots was performed with NIH Image 1.61.
JNK kinase assay.
For immunoprecipitation, 100 μg of protein was incubated with 4 μg of goat polyclonal anti-JNK1 (C17) for at least 3 h at 4°C in a rotating wheel. The immune complex formed was then precipitated by adding 40 μl of 50% protein G-Sepharose beads in the lysis buffer and incubating for an additional 1 h at 4°C. The beads were pelleted by centrifugation and were washed once in lysis buffer and three times in assay buffer (25 mM HEPES, pH 7.5, 20 mM MgCl2, 20 mM β-glycerolphosphate, 10 mM p-nitrophenyl phosphate, 0.5 mM Na3VO4, and 1 mM dithiothreitol). Finally the beads were suspended in 30 μl of assay buffer to which 3 μg of glutathione transferase (GST)–c-Jun was added. The kinase reaction was initiated by the addition of [γ-32P]ATP solution (20 μM, 5 μCi per reaction) and was then carried out at 30°C for 20 min. Reaction mixes were spotted on Whatman 3-mm filter paper squares and were immersed immediately in wash solution (10% trichloroacetic acid and 1% sodium pyrophosphate). Filters were washed at room temperature overnight, and radioactivity was counted with a β-counter (1600CA; Packard) using the Cerenkov program. JNK activity was calculated as phosphate incorporated to c-Jun in femtomoles per minute normalized to 1 mg of lysate. Measurement of the activity of transfected HA-JNK was performed by immunoprecipitation of HA-JNK from 300 μg of lysate with anti-HA antibodies. All other assay conditions were as described for endogenous JNK1. The reaction mixture was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and quantification of phosphorylated c-Jun was performed by using a phosphorimager (FLA 300; Fujifilm).
Determination of ROS.
Production of ROS was detected using a dichlorodihydrofluorescein diacetate (DCDHF) analog (C2938) fluorescent probe obtained from Molecular Probes. This compound is an uncharged cell-permeable molecule. Inside cells, this probe is cleaved by nonspecific esterases, forming carboxydichlorofluoroscein, which is oxidized in the presence of ROS. Cells were loaded with 10 μM C2938 in serum-free medium for 30 min at 37°C. Cells were washed and incubated in phenol-red-free medium and were treated as indicated in the figure legends. Fluorescence was monitored using a microplate fluorometer (FLUOstar; BMG Lab Technologies), using wavelengths of 485 and 538 nm for excitation and emission, respectively.
RESULTS
Stress-induced apoptosis is enhanced in NIH 3T3 cells that overexpress the EGFR.
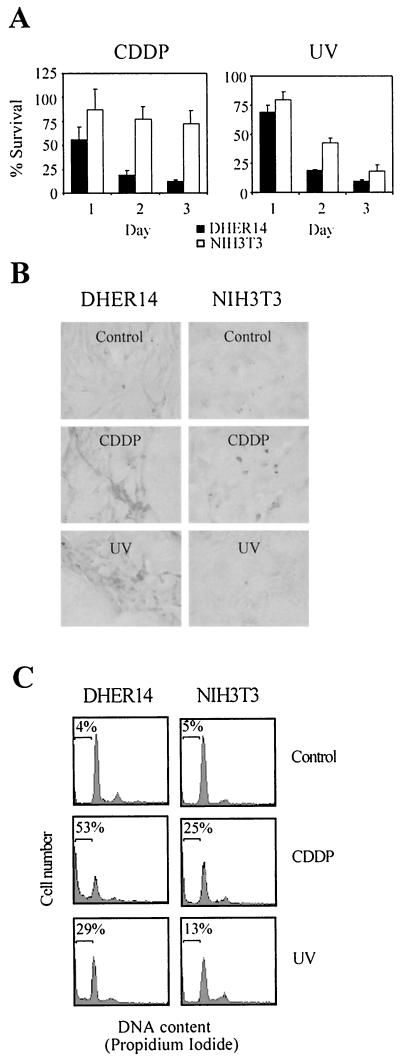
To investigate the stress response in oncogenically transformed cells, we compared NIH 3T3 cells that overexpress the EGFR (DHER14 cells) with their parental NIH 3T3 cells. To examine sensitivity to stress, cultures of the two cell lines were exposed to either CDDP treatment or UV irradiation. DHER14 cells were significantly more sensitive than NIH 3T3 cells to both CDDP and UV (Fig. 1). The differences were more pronounced when cells were treated with CDDP (Fig. 1A). About 75% of NIH 3T3 cells survived CDDP treatment, while only 10% of DHER14 cells were still viable after 3 days. DHER14 cells were also at least as twice as sensitive than NIH 3T3 cells to UV treatment. CDDP and UV are known inducers of apoptotic cell death in various cell types. To test whether they induce apoptosis in DHER14 and NIH 3T3 cells, TUNEL and FACS analysis was performed. As shown, 24 h after CDDP treatment or UV irradiation, a large proportion of DHER14 cells was positive for TUNEL staining (Fig. 1B). In contrast, only a small fraction of NIH 3T3 cells was TUNEL positive after CDDP treatment, almost none after UV irradiation. Similar results were obtained with FACS analysis. In this method, cellular DNA is stained with propidium iodide following cell permeabilization; apoptotic cells can be distinguished by virtue of their subdiploid (“sub-G1”) DNA content. Forty-eight hours after CDDP treatment, 53% of DHER14 cells but only 25% of NIH 3T3 cells were in the sub-G1 population. After UV irradiation, 29% of DHER14 cells but only 13% of NIH 3T3 cells were in the sub-G1 population (Fig. 1C). DHER14 cells were also found to be more sensitive to oxidative stress (induced by H2O2) or to methyl methanesulfonate (MMS)-mediated cell death (data not shown). These results demonstrate that DHER14 cells are significantly more sensitive than NIH 3T3 cells to stress-induced apoptosis.
FIG. 1.
Potentiation of cell death induced by CDDP and UV in NIH 3T3 cells that overexpress the EGFR. (A) Survival of DHER14 and NIH 3T3 cells following treatment with CDDP (30 μM) or UV irradiation (40 J/m2). The fraction of surviving cells was determined by the automated microculture methylene blue assay (as described in Materials and Methods). (B) TUNEL analysis of DHER14 and NIH 3T3 cells exposed to CDDP (30 μM) or UV irradiation (80 J/m2). Cell cultures were treated as indicated and subjected to TUNEL analysis 24 h after treatment. (C) FACS analysis of cells 48 h after CDDP (30 μM) or UV treatment (80 J/m2). The horizontal bar denotes the position of cells with sub-G1 DNA content, indicative of apoptosis. The percentage of such cells out of the total population is listed for each culture.
Potentiation of JNK and p38 activation in NIH 3T3 cells that overexpress EGFR.
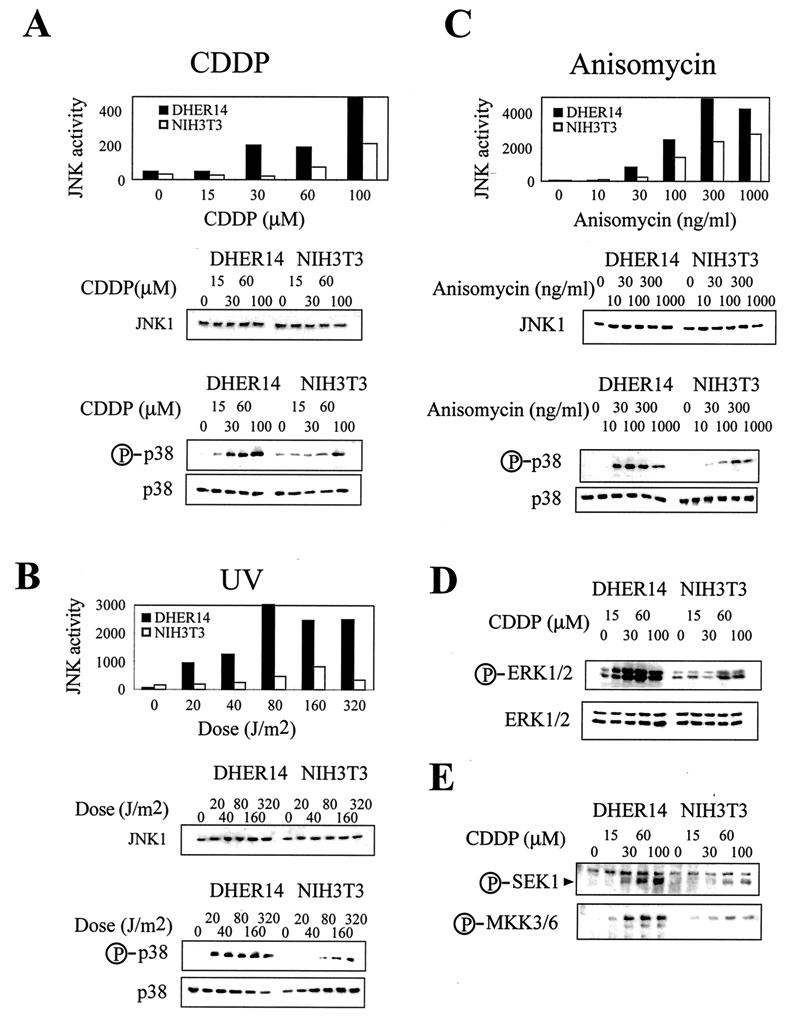
As JNK and p38 MAPKs have been implicated as key regulators of stress-induced apoptosis in different cell types, we postulated that their regulation in transformed cells could be altered, affecting the sensitivity of these cells to apoptotic signals. Therefore, we compared the patterns of activation of the JNK/p38 pathways in DHER14 cells and in their parental NIH 3T3 cells. We found that JNK1 and p38 were expressed at similar levels in NIH 3T3 and DHER14 cells. Also, activation of these kinases was not induced in either cell line under normal growing conditions (Fig. 2). However, the patterns of stress kinase activation induced by treatment with CDDP (Fig. 2A), UV (Fig. 2B), and another stress-inducing agent, anisomycin (Fig. 2C), were dramatically different in the two cell lines. JNK1 enzymatic activity induced by these stress agents was at least twofold higher in DHER14 cells than in NIH 3T3 cells. Similarly, levels of activated p38 were significantly higher in stimulated DHER14 cells than in NIH 3T3 cells. Importantly, JNK1 and p38 activation in DHER14 cells required lower stress doses than did activation in the parental NIH 3T3 cells. For example, while exposure of NIH 3T3 cells to 30 μM CDDP had no effect on JNK1 activity, exposure of DHER14 cells to the same treatment resulted in approximately fivefold activation of JNK1 (Fig. 2A). This phenomenon was even more pronounced for p38 activation: 100 μM CDDP was required to induce significant levels of activated p38 in NIH 3T3 cells, whereas in DHER14 cells, 30 μM CDDP was sufficient to induce very pronounced p38 activation (Fig. 2A). Time course experiments showed that the kinetics of JNK1 and p38 activation are similar in NIH 3T3 and DHER14 cells and that the stronger activation of these MAPKs in DHER14 is sustained over time (data not shown). A similar pattern of JNK and p38 activation was observed when DHER14 cells and NIH 3T3 cells were exposed to other stress-inducing agents, such as MMS, cycloheximide, or osmotic shock (data not shown).
FIG. 2.
Potentiation of JNK1 and p38 activation in DHER14 cells. DHER14 or NIH 3T3 cells were treated with CDDP for 16 h (A), UV radiation (analysis 1 h after irradiation) (B), or anisomycin for 1 h (C). JNK1 activity was measured by immunocomplex kinase assay, using GST–c-Jun as a substrate. The amount of JNK1 in cell lysates was measured by immunoblotting using anti-JNK1 antibodies. The activation of p38 was measured by immunoblotting, using antibodies that recognize the doubly phosphorylated (activated) form of p38. Identical blots run in parallel were reacted with anti-p38 antibodies. (D) Activation of ERK1/2. Cells were treated with CDDP for 16 h at the doses indicated. The activation of ERK1 and ERK2 was measured by immunoblotting, using antibodies that recognize the doubly phosphorylated (activated) forms of ERK1 and ERK2. Identical blots run in parallel were reacted with anti-ERK2 antibodies. Due to cross-reactivity the anti-ERK2 antibodies recognize both ERK isoforms: ERK2 (p42, lower band) and ERK1 (p44, upper band). (E) Activation of SEK1 and MKK3/6 was measured by immunoblotting, using antibodies that recognize the phosphorylated (activated) forms of SEK1 and MKK3/6.
Activation of ERK1/2 in DHER14 cells.
We also examined the activation of the ERK subgroup of MAPKs. In many cell types, ERK1 and ERK2 (ERK1/2) are activated most prominently by mitogenic stimuli and only weakly in response to stress (37). In DHER14 cells, however, ERK1/2 behaved similarly to JNK and p38 and were markedly activated by stress signals (Fig. 2D). Moreover, ERK1/2 activation was achieved at lower CDDP doses and reached higher maximal levels in DHER14 cells than in NIH 3T3 cells. Similar results were observed with other stress stimuli, including UV radiation and MMS (data not shown). Stress had no effect on the levels of JNK1, p38, or ERK1/2 proteins (Fig. 2). In summary, while the basal activity of MAPKs is similar in DHER14 and parental NIH 3T3 cells, their activation under stress is significantly augmented in DHER14 cells.
Activation of SEK1 and MKK3/6 in DHER14 cells.
To test whether it is only MAPKs themselves that are sensitized in DHER14 cells or whether the entire MAPK cascade is sensitized, we analyzed the activation of SEK1 and MKK3/6, activators of JNK and p38, respectively. Activation of these MAPK kinases in response to CDDP treatment was detected using antibodies that recognize the phosphorylated (activated) forms of these enzymes. These experiments showed that, like JNK and p38, SEK1 and MKK3/6 activation is augmented in CDDP-treated DHER14 cells in comparison to parental NIH 3T3 cells (Fig. 2E). Similar results were obtained when cells were exposed to UV radiation (data not shown). These results indicate that potentiation of the stress response in DHER14 cells involves the upstream regulators of JNK and p38.
JNK and p38 MAPKs activation by CDDP leads to cell death in DHER14 cells.
The above results show that DHER14 cells are more susceptible than their parental NIH 3T3 cells to apoptosis induction, as well as to activation of the JNK and p38 MAPKs when subjected to stress.
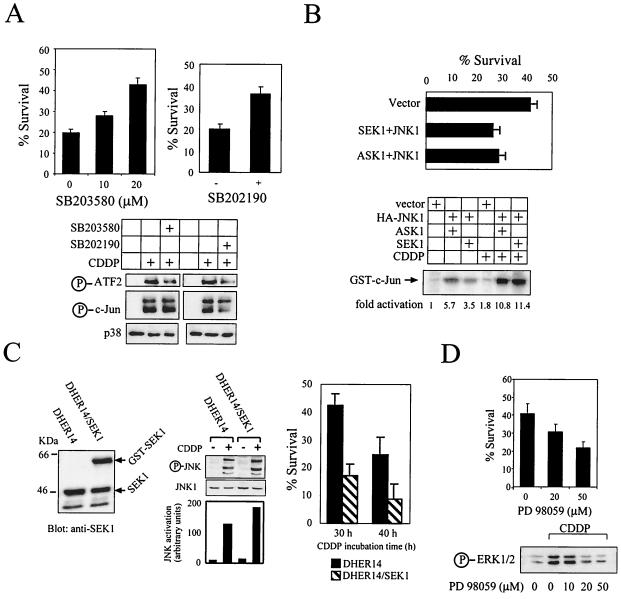
To examine whether activation of the p38 MAPK affects survival of DHER14 cells treated with CDDP, the specific p38 inhibitor SB203580 (15) was utilized. DHER14 cells were pretreated for 1 h with a 10 or 20 μM concentration of the inhibitor before treatment with 30 μM CDDP. As shown, SB203580 inhibited CDDP-induced cell death in a dose-dependent manner (Fig. 3A). Pretreatment with 20 μM SB203580 led to about a twofold increase in cell survival. These data show that p38 activation plays a direct role in inducing cell death in DHER14 cells. Similar experiments were carried out with an additional p38 inhibitor, SB202190. This inhibitor was reported to inhibit both JNK and p38 kinase (10, 46), as measured by the inhibition of the phosphorylation of c-Jun (a direct target for JNK but not for p38). Treatment with 20 μM SB202190 markedly attenuated the phosphorylation of c-Jun induced by CDDP (Fig. 3A). Survival measurements revealed that the addition of SB202190 led to a significant increase in cell survival (Fig. 3A). Immunoblot analysis, which showed a decrease in ATF2 (p38 substrate) phosphorylation (Fig 3A), confirmed that SB203580 inhibits CDDP-induced p38 activity. SB202190 treatment inhibited CDDP-induced ATF2 and c-Jun phosphorylation, confirming that this compound inhibits the activity of both p38 and JNK in DHER14 dells. Collectively, these data suggest that the activation of stress kinases mediates CDDP-induced cell death in DHER14 cells.
FIG. 3.
JNK and p38 activation is involved in CDDP-induced cell death in DHER14 cells. (A) Cells were pretreated for 1 h with SB203580 at the doses indicated or with 20 μM SB202190 before addition of 30 μM CDDP. Survival was determined after 48 h by the automated microculture methylene blue assay (as described in Materials and Methods). In addition, the effect of SB203580 and SB202190 on ATF2 and c-Jun phosphorylation was measured (16 h after addition of the inhibitor) by immunoblotting, using antibodies which recognize the phosphorylated form of ATF2 and c-Jun. (B) Upper panel: DHER14 cells were cotransfected with GFP plasmid (1 μg) and empty vector or plasmids expressing kinases (2 μg each) as indicated. Twenty-four hours after transfection, cells were treated with 30 μM CDDP. Thirty hours after treatment, floating and attached cells were pooled and analyzed by FACS. Percent survival was defined as follows: (number of GFP-expressing cells in the CDDP-treated group/number of GFP-expressing cells in the nontreated group) × 100. Lower panel: DHER14 cells were transfected with empty vector, HA-JNK1, SEK1, and ASK1 as indicated. Twenty-four hours posttransfection, cells were treated with CDDP (100 μM) for 16 h. HA-JNK activity was determined by immunocomplex kinase assay. (C) Expression of GST-SEK1 in DHER14/SEK1 cells was detected by immunoblotting using anti-SEK1 antibodies. Stable transfectants of DHER14 cells expressing SEK1 (DHER14/SEK1) and DHER14 cells were exposed to 100 μM CDDP. JNK activation (16 h posttreatment) was measured by immunoblotting using anti-phospho-JNK antibodies. DHER14/SEK1 and DHER14 cells were exposed to CDDP (30 μM). Survival was measured as described above at the times indicated. (D) DHER14 cells were pretreated with PD98059 for 1 h before addition of 30 μM CDDP. Survival after 30 h was determined as above. Bottom panel: DHER14 cells were pretreated with PD98059 for 1 h before the addition of 100 μM CDDP. Cells were lysed 16 h after treatment, and ERK1 and ERK2 activation was determined as described for Fig. 2.
To further assess the role of JNK in cell death, a transient-transfection death assay was employed. DHER14 cells were cotransfected with empty vector or with HA-tagged JNK1, ASK1, or SEK1 as well as with GFP. The survival of transfected cells was determined 30 h after CDDP treatment by FACS analysis using GFP as a marker of transfected cells. A combination of transfected SEK1 and JNK1 enhanced CDDP-mediated cell death compared to transfection of vector alone, from 59 to 74%, respectively (Fig. 3B). Similarly, when cells were transfected with a combination of ASK1 and JNK1, the extent of cell death increased from 59 to 72%. Expression of JNK1 with SEK1 or ASK1 in the absence of CDDP resulted in a low level of cell death (less than 15%; data not shown). The activity of transfected HA-JNK1 was measured by immunoprecipitation of HA-JNK1 coupled to in vitro phosphorylation of c-Jun (see Materials and Methods). As shown, expression of JNK1 with its upstream kinases resulted in some basal activity that was markedly increased upon CDDP treatment (Fig. 3B). Thus, expression and activation of exogenous JNK1 enhance cell death in DHER14 cells. In addition, stable transfection of a SEK1 construct was performed to generate DHER14 cells that constitutively express high levels of SEK1. Expression of the exogenous SEK1 was confirmed by immunoblotting. The SEK1 construct was expressed as a GST fusion protein, with the expected molecular mass of ∼64 kDa (Fig. 3C). The stable transfectants (designated DHER14/SEK1) were then exposed to CDDP, and JNK1 activation was measured. As shown, JNK1 activation was enhanced in DHER14/SEK1 cells compared to DHER14 cells (Fig. 3C). Analysis of CDDP-induced cell death showed that DHER14/SEK1 cells are significantly more sensitive to CDDP in comparison to DHER14 cells (Fig. 3C). Under normal growth conditions (in the absence of CDDP), the DHER14/SEK1 growth rate was similar to that of DHER14 cells (data not shown). Taken together, these results suggest that activation of the JNK pathway promotes cell death in DHER14 cells.
In order to examine the role of ERK activation in CDDP-induced cell death in DHER14 cells, we utilized the MEK inhibitor PD98059 (2, 21). As shown, pretreatment of DHER14 cells with 20 or 50 μM PD98059 for 1 h prior to CDDP treatment significantly inhibited ERK1/2 activation (Fig. 3D). A survival assay done in a similar manner revealed that the addition of the MEK inhibitor led to a significant decrease in cell survival. JNK and p38 activation was not altered under such conditions, and PD98059 alone did not have any toxic effects on these cells (data not shown). These findings suggest that CDDP-induced activation of ERK1/2 in DHER14 cells stimulates survival signals in these cells.
Potentiation of stress kinase activation in NIH 3T3 cells that overexpress the HER1–2 receptor or oncogenic Ras.
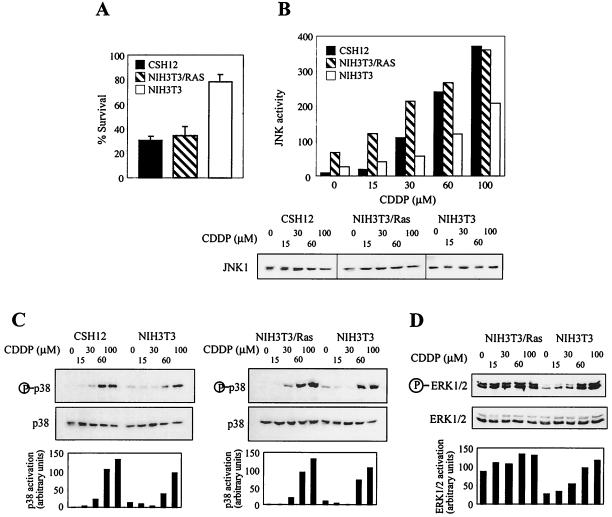
The potentiation of JNK/p38 activation in DHER14 cells prompted us to explore whether stress kinase activation is also potentiated in NIH 3T3 cells transformed by other oncogenes. To this end, we compared CDDP-induced cell death and JNK/p38 activation in parental NIH 3T3 cells, in NIH 3T3 cells that overexpress the chimeric receptor HER1–2 (CSH12 cells), and in NIH 3T3 cells that overexpress myristylated Ras (NIH 3T3/Ras cells). As shown, CSH12 cells and NIH 3T3/Ras cells, like DHER14 cells, displayed higher sensitivity to CDDP, manifested by their low survival rate in comparison to parental NIH 3T3 cells (Fig. 4A). CDDP also induced stronger activation of JNK1 and p38 MAPKs in CSH12 cells and in NIH 3T3/Ras than in NIH 3T3 cells, as shown by higher levels of active JNK and p38 and lower CDDP doses required for activation of these kinases (Fig. 4B and C). These results show that the association between sensitization to stress stimuli and potentiation of JNK/p38 activation is a common feature of NIH 3T3 cells transformed by several different oncogenes.
FIG. 4.
Analysis of CDDP-induced cell death and JNK/p38 activation in CSH12 and NIH 3T3/Ras cells. (A) Survival of CSH12, NIH 3T3/Ras, and NIH 3T3 cells following treatment with CDDP (30 μM). Survival after 2 days was determined by the automated microculture methylene blue assay. (B and C) CSH12, NIH 3T3/Ras, or NIH 3T3 cells were treated with CDDP for 16 h at the indicated doses. The activation of JNK1 and p38 was determined as done for Fig. 2. Graphs show the data as quantified by densitometry of immunoblots using NIH image 1.61. Activation was calculated by dividing the densitometry values for the phosphoprotein by that of the total protein. (D) NIH 3T3/Ras or NIH 3T3 cells were treated with CDDP for 16 h at the indicated doses. The activation of ERK1/2 was determined as for Fig. 2.
We also examined the activation of ERK1/2 in NIH 3T3/Ras cells. In these cells, ERK1/2 is highly activated under normal growth conditions, and unlike the situation in DHER14 cells, CDDP treatment triggers only a small increase of ERK1/2 activation (Fig. 4D). These findings indicate that ERK1/2 activation is subject to regulation in EGFR-transformed cells different from that in Ras-transformed cells.
EGFR does not mediate CDDP-induced activation of JNK or p38 MAPKs in DHER14 cells.
The results described so far show that DHER14 and other transformed NIH 3T3 cells are highly susceptible to stress stimuli and that potentiation of JNK/p38 activation is important in mediating stress-induced cell death in these cells. The ensuing experiments were carried out to clarify the molecular mechanism underlying the potentiation of JNK/p38 pathways associated with oncogenic transformation.
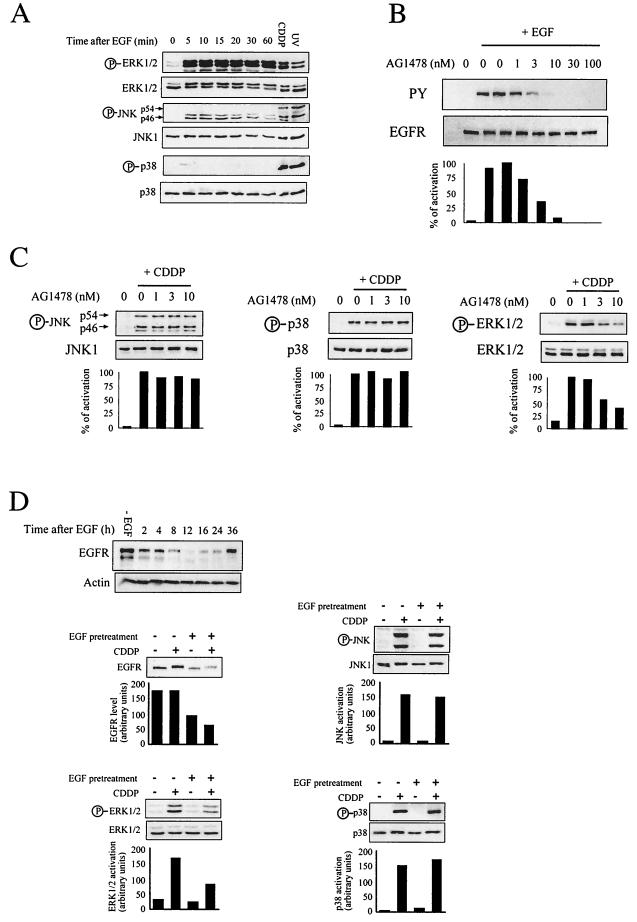
As DHER14 cells are transformed as a result of overexpression of EGFR, we reasoned that high EGFR expression and/or activity could underlie the potentiation of stress kinase activation in these cells. To evaluate whether the EGFR can signal through the different MAPK pathways, we treated DHER14 cells with EGF and examined the activation of MAPKs over time. As shown, EGF stimulation induced strong ERK1/2 activation, which was sustained over time (Fig. 5A). EGF also stimulated moderate and transient JNK activation. In contrast, EGF did not induce p38 activation. These data demonstrate that in DHER14 cells, the EGFR signals predominantly via the ERK pathway, signals to a much lesser degree through the JNK pathway, and does not signal at all via the p38 pathway.
FIG. 5.
JNK and p38 activation is largely independent of the EGFR in DHER14 cells. (A) DHER14 cells were stimulated with 10 nM EGF for the indicated times. Activation of MAPKs was measured by immunoblotting. (B) DHER14 cells were pretreated with AG1478 (EGFR kinase inhibitor) at the indicated concentrations for 1 h before addition of EGF (10 nM) for 10 min. Cells were lysed and subjected to Western blot analysis using antiphosphotyrosine (PY) or anti-EGFR specific antibodies. (C) Cells were treated with CDDP (100 μM) for 16 h. During the last hour of incubation, AG1478 was added at the indicated concentrations. Cells were lysed and subjected to Western blot analysis, using anti-phospho-JNK, anti-phospho-p38, or anti-phospho-ERK1/2 specific antibodies. Parallel blots were reacted with anti-JNK1, anti-p38, or anti-ERK2 antibodies. Anti-phospho-JNK antibodies recognize the phosphorylated form of the p46 (JNK1) and p54 (JNK2) isoforms. Graphs show the percent activation as quantified by densitometry of immunoblots using NIH image 1.61. The phosphorylation observed in cells treated with CDDP in the absence of AG1478 was defined as 100%. Results are representative of three independent experiments. (D) Upper panel: DHER14 cells were pretreated with 1 μg of EGF per ml for 1 h. The EGF-containing medium was then removed, and cells were kept in regular medium for the times indicated. EGFR expression was measured by immunoblotting. Bottom panels: DHER14 cells were pretreated with 1 μg of EGF per ml for 1 h. The EGF-containing medium was then removed, and cells were kept in regular medium for 12 h prior to treatment with CDDP (100 μM) for an additional 12 h. Cells were lysed, and EGFR expression or MAPK activation was analyzed by immunoblotting. Graphs show the data as quantified by densitometry of immunoblots using NIH image 1.61.
To evaluate whether EGFR activity is needed during CDDP-induced MAPK activation, we utilized the selective EGFR kinase inhibitor AG1478 (36, 45). We first determined the concentration of AG1478 needed to inhibit EGF-dependent EGFR autophosphorylation in intact DHER14 cells. EGFR autophosphorylation inhibition was already detected with 1 nM AG1478, and over 90% inhibition was observed with a 10 nM concentration of the inhibitor (Fig. 5B). Treatment with AG1478 did not significantly affect JNK or p38 activation triggered by CDDP stimulation (Fig. 5C), but a significant reduction in ERK1/2 activation was observed in cells exposed to 3 to 10 nM AG1478 (Fig. 5C). These results suggest that in DHER14 cells, EGFR activity is required for ERK1/2 activation but not for JNK or p38 activation, in response to CDDP treatment.
The continued presence of a growth factor such as EGF in the culture medium often results in decreased receptor levels on the cell surface due to internalization and proteolysis (8, 12). To further evaluate the role of EGFR in the activation of MAPKs following stress stimuli, we examined the effect of down-modulating the EGFR on this response. Initially, the time course and degree of receptor down-regulation were determined. As shown, EGF treatment led to a significant decrease in EGFR levels. The lowest level of EGFR was detected 12 h after EGF treatment, and by 36 h the amount of EGFR was restored to the level seen in untreated cells (Fig. 5D). We next examined the effect of the decreased EGFR level on MAPK activation. DHER14 cells were pretreated with 1 μg of EGF per ml for 1 h. The EGF-containing medium was then removed, and the cells were kept in regular medium for 12 h, prior to treatment with CDDP for an additional 12 h. As shown, under this protocol, the EGFR level decreased approximately twofold (Fig. 5D). Concomitantly with EGFR down-regulation, ERK1/2 activation decreased twofold. In contrast, JNK and p38 activation was similar in cells that express high levels of EGFR (without EGF pretreatment) or reduced levels of EGFR (with EGF pretreatment). EGF pretreatment had no significant effect on CDDP-induced cell death (data not shown). Taken together, these findings support the notion that EGFR does not play a direct role in CDDP-induced JNK/38 activation, even though the initial transformation event created the setting for potentiation of these kinases (see Discussion).
Enhanced production of ROS results in potentiation of stress kinase activation in transformed cells.
Our findings hitherto strongly suggest that the potentiation of JNK/p38 activation in transformed cells is largely independent of EGFR activity and occurs with other oncogenes. It appears, therefore, that the molecular mechanism responsible for potentiation of stress kinase activation in transformed cells is universal, presumably independent of the particular biochemical activity of the transforming oncogene. We considered the possible involvement of ROS, which have been recognized as important regulators of the stress response in many cell types and have also been implicated in MAPK activation (1, 30, 54).
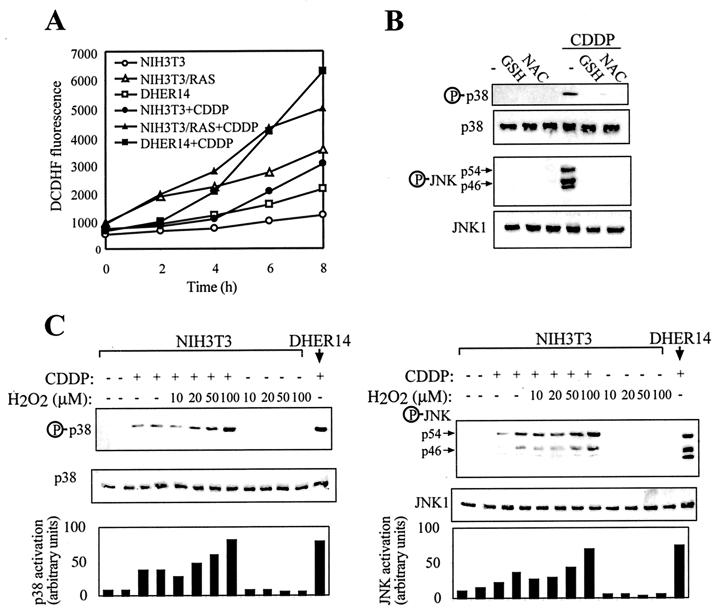
We therefore measured the rate of ROS production in parental NIH 3T3 cells, DHER14 cells, and NIH 3T3/Ras cells under normal growth conditions as well as after exposure to CDDP. The rate of ROS production measured was markedly increased in transformed NIH 3T3 cells compared to nontransformed cells, with or without CDDP treatment (Fig. 6A). Thus, increased generation of ROS is intrinsically different in transformed and nontransformed cells, where CDDP treatment leads to an increase in ROS production in all three cell lines. Moreover, CDDP-induced JNK and p38 activation was blocked by treatment with the antioxidants glutathione (GSH) or N-acetyl-cysteine (NAC). These findings suggest that the activation of stress kinase pathways is regulated in a ROS-dependent manner (see Discussion).
FIG. 6.
Involvement of ROS in potentiation of JNK/p38 activation in transformed NIH 3T3 cells. (A) Kinetics of ROS production in nonstimulated and CDDP-treated cells. Cells were labeled with the fluorescent dye DCDHF, treated as indicated, and analyzed as described in Material and Methods. Points on the graph are the average values from duplicate samples. Data are from a representative experiment, which was repeated three times with comparable results. (B) DHER14 cells were treated with 5 mM reduced GSH or NAC for 1 h, followed by treatment with 100 μM CDDP. After 16 h lysates were prepared and assayed for JNK or p38 activation by Western blot analysis as done for Fig. 5. (C) NIH 3T3 cells were treated once with H2O2 and/or CDDP at the doses indicated. After 16 h cells were lysed, and JNK or p38 activation was determined by immunoblotting.
To further test the hypothesis that increased rates of ROS production potentiate stress kinase activation, we treated NIH 3T3 cells with H2O2 together with CDDP. H2O2 alone did not activate JNK or p38 in NIH 3T3 cells but potentiated JNK and p38 activation in response to CDDP, resulting in activation levels similar to those observed in DHER14 cells (Fig. 6C).
Implication of mitochondria and NADPH oxidase in CDDP-induced ROS generation in DHER14 cells.
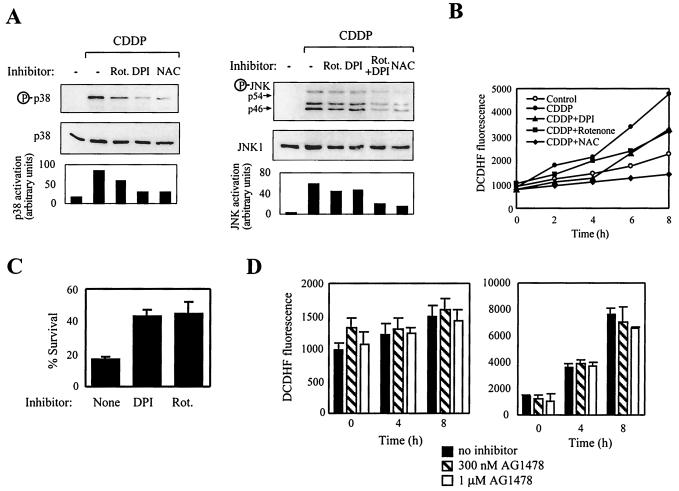
ROS emanate from mitochondrial and nonmitochondrial enzymes (30). To gain insight into the sources of ROS that are involved in stress kinase activation, cells were treated with rotenone, an inhibitor of complex I of the mitochondrial respiratory chain, or with diphenilene iodonium (DPI), a specific flavoprotein inhibitor that blocks ROS generation by NADPH oxidase. Both rotenone and DPI significantly attenuated the activation of JNK and p38 following CDDP treatment (Fig. 7A). Additional experiments showed that inhibitors of cyclooxygenase (indomethacin), lipooxygenase (nordihydroguaiaretic acid), and xanthine oxidase (allopurinol) did not inhibit JNK or p38 activation (data not shown). Treatment with rotenone or DPI reduced the rate of ROS production induced by CDDP approximately twofold (Fig. 7B). Our findings implicate both mitochondria and NADPH oxidase in ROS generation upstream of stress kinase pathways. Furthermore, rotenone or DPI treatment reduced the killing effect of CDDP, resulting in about a twofold increase in cell survival, when compared to cells exposed to CDDP alone (Fig. 7C). Collectively, these data suggest that enhanced production of ROS in transformed cells which originate from the mitochondria and NADPH oxidase enhances the activation of JNK and p38, which in turn augment cell death. We also examined the possibility that ROS production is dependent on EGFR activity in DHER14 cells. As shown, AG1478 had no significant effect on the basal rate of ROS production (Fig. 7D, left panel) or on the rate of ROS production in the presence of CDDP (Fig. 7D, right panel). These data suggest that, similar to stress kinase potentiation, enhanced ROS production in DHER14 cells is independent of the activity of the oncogene utilized to transform the cells (EGFR).
FIG. 7.
Implication of mitochondria and NADPH oxidase in CDDP-induced ROS generation in DHER14 cells. (A) DHER14 cells were incubated with CDDP (100 μM) for 16 h in the absence or presence of rotenone (Rot., 100 μM), DPI (10 μM), or NAC (1 mM). Cell lysates were assayed for JNK or p38 activation by Western blot analysis. (B) DHER14 cells were labeled with the fluorescent dye DCDHF and were treated with CDDP (100 μM) together with rotenone (Rot., 100 μM), DPI (10 μM), or NAC (1 mM) as indicated. The production of ROS was monitored as described in Materials and Methods. (C) Survival of DHER14 cells following CDDP treatment (30 μM) in the presence of rotenone (Rot., 100 μM) or DPI (10 μM). The fraction of surviving cells was determined by the automated microculture methylene blue assay (as described in Materials and Methods). (D) DHER14 cells were treated for 1 h with vehicle (no inhibitor), 300 nM AG1478, or 1 μM AG1478. ROS production was measured during the following 8 h in the absence of CDDP (left panel) or in the presence of 100 μM CDDP (right panel).
Activation of JNK and p38 is correlated with cell death induction in A431 and HT29 cells.
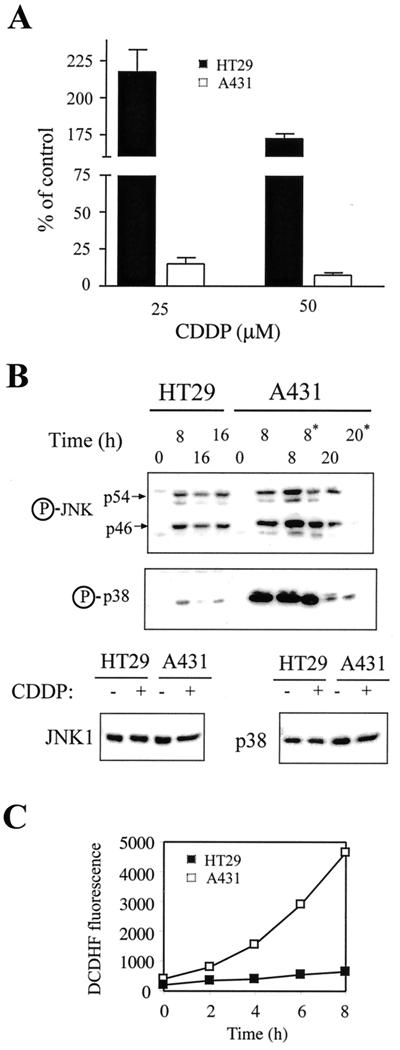
Many types of tumor cells display a wide range of sensitivity to physical and chemical stresses. In our experimental system, the enhanced sensitivity of transformed cells to stress stimuli could be attributed to the potentiation of stress kinase activation. The question remains, what is the status of these kinases in tumor cells vis-à-vis the sensitivity of these cells to stress. To address this question, we examined JNK and p38 activation and its relationship to cell death in two human tumor cell lines: A431 epidermoid carcinoma cells and HT29 colon cancer cells. Treatment of these cells with CDDP revealed that A431 cells were very sensitive to CDDP, whereas HT29 cells were remarkably resistant (Fig. 8A). For example, a 72-h treatment with 25 μM CDDP killed ∼80% of A431 cells, whereas HT29 cells were only growth inhibited. Analysis of CDDP-induced stress kinase activation showed that JNK and particularly p38 were activated more strongly in A431 cells than in HT29 cells (Fig. 8B). This correlates with the higher sensitivity to CDDP displayed by A431 cells. Moreover, ROS measurements revealed that the rate of ROS production is pronouncedly elevated in A431 cells compared to HT29 cells (Fig. 8C). These results suggest that the direct linkage between sensitization to apoptosis induction, elevated levels of ROS, and potentiation of stress kinase activation observed in our model system may be a general situation that exists in apoptotically sensitized cancer cells. Thus, ROS-dependent JNK and p38 activation may be potentiated in some cancerous cells, rendering them sensitive to cytotoxic agents, while ROS levels and stress kinase activation may be suppressed in other cancerous cells (presumably originating from more advanced tumors), rendering them resistant to such agents.
FIG. 8.
Analysis of CDDP-induced cell death, JNK/p38 activation, and ROS production in human tumor cells. (A) Survival of A431 and HT29 cells 72 h following treatment with CDDP. The fraction of surviving cells was determined by the automated microculture methylene blue assay (as described in Materials and Methods). (B) A431 or HT29 cells were treated with 100 μM CDDP. The activation of JNK1 and p38 was measured by immunoblotting, using anti-phospho-JNK or anti-phospho-p38 specific antibodies. For comparison, levels of JNK and p38 in HT29 and A431 cells before or 8 h after CDDP treatment are shown. Two 16-h samples of HT29 cells and two 8-h samples of A431 cells were included in the analysis. Due to massive apoptosis in A431 cells, floating cells were collected and treated as a separate sample (marked by an asterisk). (C) Kinetics of ROS production in A431 and HT29 cells. Cells were labeled with the fluorescent dye DCDHF and were analyzed for ROS generation as described in Materials and Methods.
DISCUSSION
The unusual sensitivity of many primary tumors as well as of cells that have been transformed in vitro to chemotherapeutic drugs and radiation was recognized long ago, yet the molecular mechanisms that underlie this sensitivity have not been established. Our study shows that the sensitization of oncogenically transformed cells to genotoxic stress is largely due to potentiation of the JNK and p38 pathways. This potentiation appears to be independent of the overexpressed proto-oncogene that initially induced the transformed phenotype and the sensitized state but seems to be mechanistically related to the increased rate of ROS generation.
The main issue addressed in this study, namely, how oncogenic transformation affects the stress kinase pathways, has not been systematically investigated until now. We show here that the sensitization of transformed cells to stress signals is due to the potentiation of the stress kinase pathways. Low-dose stress stimuli, which do not trigger activation of JNK/p38 in nontransformed cells, are sufficient to elicit activation of these kinases in transformed NIH 3T3 cells (Fig. 2 and 4). This is the case not only in cells transformed in vitro, such as DHER14, CSH12, or Ras-transformed NIH 3T3 cells but also in tumor-derived A431 cells. Transformation of NIH 3T3 cells with oncogenic Src or Raf also potentiates activation of JNK by etoposide (11). Collectively, these observations suggest that potentiation of JNK and p38 activation is a common feature of oncogenically transformed cells and is probably relevant to understanding the unusual sensitivity of primary tumors to cytotoxic agents.
Tumor cells originating from more advanced tumors seem to acquire additional lesions, which render them resistant to stress stimuli. Inactivation of the tumor suppressor p53 and amplification of the antiapoptotic proteins of the Bcl-2 family are two prevalent mechanisms that render tumor cells refractory to death signaling (48). Our results with HT29 cells (Fig. 8) suggest that another mechanism that contributes to the resistance of advanced tumors to cancer therapy is the suppression of stress kinase activation. Recent reports describing the overexpression of the MAPK phosphatase 1, a negative regulator of the JNK and p38 pathways, in prostate and colon and several other types of tumor cells (39, 40), support this notion.
Mechanistic aspects of stress kinase potentiation.
MAPK cascades are regulated by a complicated network of upstream kinases and small G proteins (27, 37). Our results show that potentiation of stress kinase pathways in DHER14 cells occurs at or upstream of the level of MAPK kinase activation (Fig. 2). Since we found potentiation of MAPK activation in cells that overexpress EGFR, we examined the role of EGFR signaling in the activation of JNK/p38 and ERK MAPKs. Interestingly, EGFR signaling appears to have a limited role in the activation of JNK or p38, as down-modulation of EGFR level or activity does not inhibit activation of JNK or p38 (Fig. 5). Since EGFR is involved in the activation of ERK but not of JNK/p38, our findings may explain why inhibition of EGFR signaling in several cell types induces apoptosis or potentiates the cytotoxic effects of CDDP and other stress-inducing agents (41, 44).
Since potentiation of JNK and p38 activation was found to be independent of the particular oncogene or stress agent (Fig. 2, 4, and 5), we looked for a general mechanism that may account for this phenomenon. In this regard, we show that ROS play an important role in stress signaling in transformed cells via potentiation of JNK/p38 activation. ROS have been shown to be involved in diverse aspects of the cellular stress response, including induction of programmed cell death (9, 25, 29, 30, 43, 56). Moreover, ROS have been implicated in JNK activation induced by stress agents and cytokines (1, 38, 55). Interestingly, tumor cells are known to contain elevated levels of ROS, as measured by H2O2 levels or by enhanced oxidative damage (20, 51). Also, the Ras oncoprotein was shown to induce cellular pathways that lead to the production of superoxides (28). Here we show that ROS production is enhanced in oncogene-expressing NIH 3T3 cells when compared to parental NIH 3T3 cells, both in nonstressed cells as well as following CDDP treatment (Fig. 6). CDDP induces ROS production, and the activation of JNK and p38 is effectively inhibited by the antioxidants GSH and NAC, as well as by more specific inhibitors of mitochondria and NADPH oxidase (Fig. 6 and 7). Conversely, when ROS levels are elevated in NIH 3T3 cells by addition of H2O2, activation of JNK/p38 is potentiated, reaching levels similar to those observed in DHER14 cells treated by CDDP alone (Fig. 6C). Collectively, these findings suggest that increased ROS production in transformed cells results in the potentiation of JNK/p38 activation.
We further show that ROS formation largely comes from the mitochondria and NADPH oxidase (Fig. 7). Mitochondria play a critical role in the execution of apoptosis (24). Our data also implicate the mitochondria, as well as NADPH oxidase, in the early signaling events prior to the execution of the apoptotic process, by enhancing stress signaling via the JNK/p38 cascades. Little is known of the function of the NADPH oxidase complex in nonphagocytic cells, but a few studies have implicated this enzyme in the activation of stress kinases (16, 38, 52).
The association between oncogenic transformation and up-regulation of cellular pathways that promote cell death is intriguing. In some cases (e.g., Myc and E1A), it is the activity of the oncoprotein itself that is required for promoting apoptosis (18, 58). In other cases (as shown is this study), in the transformed state, sensitization to apoptosis is independent of the direct activity of the oncoprotein. The emerging view is that the basic mechanisms of cellular proliferation and transformation are tied to the process of apoptosis (22). According to this notion, one can propose the following model: JNK and p38 activation is induced as a part of the normal cellular response to the deregulated activation of an oncoprotein. The biological rationale of such a response is to induce apoptosis and eliminate the affected cell. As a result of a secondary event, survival of the defective cell and the establishment of the transformed state are attained, in spite of the potentiated state of stress kinases. Since in these cells stress kinase pathways remain potentiated, the cells remain hypersensitive to stress signals such as genotoxic agents.
ACKNOWLEDGMENTS
We thank Aviv de-Morgan for technical assistance and Shoshana Klein for her help in preparing the manuscript. This study was partially supported by the James S. McDonnel Foundation and the Konover Fund of the Hebrew University.
REFERENCES
- 1.Adler V, Yin Z, Tew K D, Ronai Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999;18:6104–6111. doi: 10.1038/sj.onc.1203128. [DOI] [PubMed] [Google Scholar]
- 2.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 3.Arteaga C L, Winnier A R, Poirier M C, Lopez-Larraza D M, Shawver L K, Hurd S D, Stewart S J. p185c-erbB-2 signal enhances cisplatin-induced cytotoxicity in human breast carcinoma cells: association between an oncogenic receptor tyrosine kinase and drug-induced DNA repair. Cancer Res. 1994;54:3758–3765. [PubMed] [Google Scholar]
- 4.Basu A, Cline J S. Oncogenic transformation alters cisplatin-induced apoptosis in rat embryo fibroblasts. Int J Cancer. 1995;63:597–603. doi: 10.1002/ijc.2910630422. [DOI] [PubMed] [Google Scholar]
- 5.Boussif O, Lezoualc'h F, Zanta M A, Mergny M D, Scherman D, Demeneix B, Behr J P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner B, Koppenhoefer U, Weinstock C, Linderkamp O, Lang F, Gulbins E. Fas- or ceramide-induced apoptosis is mediated by a Rac1-regulated activation of Jun N-terminal kinase/p38 kinases and GADD153. J Biol Chem. 1997;272:22173–22181. doi: 10.1074/jbc.272.35.22173. [DOI] [PubMed] [Google Scholar]
- 7.Butterfield L, Storey B, Maas L, Heasley L E. c-Jun NH2-terminal kinase regulation of the apoptotic response of small cell lung cancer cells to ultraviolet radiation. J Biol Chem. 1997;272:10110–10116. doi: 10.1074/jbc.272.15.10110. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter G, Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976;71:159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan W H, Yu J S. Inhibition of UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermal carcinoma A431 cells by genistein. J Cell Biochem. 2000;78:73–84. doi: 10.1002/(sici)1097-4644(20000701)78:1<73::aid-jcb7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 10.Chen C Y, Del Gatto-Konczak F, Wu Z, Karin M. Stabilization of interleukin-2 mRNA by the c-Jun NH2-terminal kinase pathway. Science. 1998;280:1945–1949. doi: 10.1126/science.280.5371.1945. [DOI] [PubMed] [Google Scholar]
- 11.Chen G, Shu J, Stacey D W. Oncogenic transformation potentiates apoptosis, S-phase arrest and stress-kinase activation by etoposide. Oncogene. 1997;15:1643–1651. doi: 10.1038/sj.onc.1201347. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Martindale J L, Holbrook N J, Liu Y. Tumor promoter arsenite activates extracellular signal-regulated kinase through a signaling pathway mediated by epidermal growth factor receptor and Shc. Mol Cell Biol. 1998;18:5178–5188. doi: 10.1128/mcb.18.9.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y R, Wang X, Templeton D, Davis R J, Tan T H. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J Biol Chem. 1996;271:31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z, Seimiya H, Naito M, Mashima T, Kizaki A, Dan S, Imaizumi M, Ichijo H, Miyazono K, Tsuruo T. ASK1 mediates apoptotic cell death induced by genotoxic stress. Oncogene. 1999;18:173–180. doi: 10.1038/sj.onc.1202276. [DOI] [PubMed] [Google Scholar]
- 15.Cuenda A, Rouse J, Doza Y N, Meier R, Cohen P, Gallagher T F, Young P R, Lee J C. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 16.Cui X L, Douglas J G. Arachidonic acid activates c-jun N-terminal kinase through NADPH oxidase in rabbit proximal tubular epithelial cells. Proc Natl Acad Sci USA. 1997;94:3771–3776. doi: 10.1073/pnas.94.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeClue J E, Vass W C, Papageorge A G, Lowy D R, Willumsen B M. Inhibition of cell growth by lovastatin is independent of ras function. Cancer Res. 1991;51:712–717. [PubMed] [Google Scholar]
- 18.de Stanchina E, McCurrach M E, Zindy F, Shieh S Y, Ferbeyre G, Samuelson A V, Prives C, Roussel M F, Sherr C J, Lowe S W. E1A signaling to p53 involves the p19(ARF) tumor suppressor. Genes Dev. 1998;12:2434–2442. doi: 10.1101/gad.12.15.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixit M, Yang J L, Poirier M C, Price J O, Andrews P A, Arteaga C L. Abrogation of cisplatin-induced programmed cell death in human breast cancer cells by epidermal growth factor antisense RNA. J Natl Cancer Inst. 1997;89:365–373. doi: 10.1093/jnci/89.5.365. [DOI] [PubMed] [Google Scholar]
- 20.Dorward A, Sweet S, Moorehead R, Singh G. Mitochondrial contributions to cancer cell physiology: redox balance, cell cycle, and drug resistance. J Bioenerg Biomembr. 1997;29:385–392. doi: 10.1023/a:1022454932269. [DOI] [PubMed] [Google Scholar]
- 21.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evan G, Littlewood T. A matter of life and cell death. Science. 1998;281:1317–1322. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- 23.Goldman R, Bar-Shavit Z. Dual effect of normal and stimulated macrophages and their conditioned media on target cell proliferation. JNCI. 1979;63:1009–1016. [PubMed] [Google Scholar]
- 24.Green D R, Reed J C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 25.Herrlich P, Bohmer F D. Redox regulation of signal transduction in mammalian cells. Biochem Pharmacol. 2000;59:35–41. doi: 10.1016/s0006-2952(99)00298-1. [DOI] [PubMed] [Google Scholar]
- 26.Honegger A, Dull T J, Szapary D, Komoriya A, Kris R, Ullrich A, Schlessinger J. Kinetic parameters of the protein tyrosine kinase activity of EGF-receptor mutants with individually altered autophosphorylation sites. EMBO J. 1988;7:3053–3060. doi: 10.1002/j.1460-2075.1988.tb03170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ip Y T, Davis R J. Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 28.Irani K, Xia Y, Zweier J L, Sollott S J, Der C J, Fearon E R, Sundaresan M, Finkel T, Goldschmidt-Clermont P J. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson M D. Reactive oxygen species and programmed cell death. Trends Biochem Sci. 1996;21:83–86. [PubMed] [Google Scholar]
- 30.Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 31.Karin M. Mitogen-activated protein kinase cascades as regulators of stress responses. Ann N Y Acad Sci. 1998;851:139–146. doi: 10.1111/j.1749-6632.1998.tb08987.x. [DOI] [PubMed] [Google Scholar]
- 32.Kim R, Ohi Y, Inoue H, Toge T. Enhancement of chemotherapeutic agents induced-apoptosis associated with activation of c-Jun N-terminal kinase 1 and caspase 3 (CPP32) in bax-transfected gastric cancer cells. Anticancer Res. 2000;20:439–444. [PubMed] [Google Scholar]
- 33.Kyriakis J M, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- 34.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 35.Lee J, Dull T J, Lax I, Schlessinger J, Ullrich A. HER2 cytoplasmic domain generates normal mitogenic and transforming signals in a chimeric receptor. EMBO J. 1989;8:167–173. doi: 10.1002/j.1460-2075.1989.tb03361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- 37.Lewis T S, Shapiro P S, Ahn N G. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 38.Lo Y Y C, Wong J M S, Cruz T F. Reactive oxygen species mediate cytokine activation of c-Jun NH2-terminal kinases. J Biol Chem. 1996;271:15703–15707. doi: 10.1074/jbc.271.26.15703. [DOI] [PubMed] [Google Scholar]
- 39.Loda M, Capodieci P, Mishra R, Yao H, Corless C, Grigioni W, Wang Y, Magi-Galluzzi C, Stork P J. Expression of mitogen-activated protein kinase phosphatase-1 in the early phases of human epithelial carcinogenesis. Am J Pathol. 1996;149:1553–1564. [PMC free article] [PubMed] [Google Scholar]
- 40.Magi-Galluzzi C, Mishra R, Fiorentino M, Montironi R, Yao H, Capodieci P, Wishnow K, Kaplan I, Stork P J, Loda M. Mitogen-activated protein kinase phosphatase 1 is overexpressed in prostate cancers and is inversely related to apoptosis. Lab Investig. 1997;76:37–51. [PubMed] [Google Scholar]
- 41.Mendelsohn J, Fan Z. Epidermal growth factor receptor family and chemosensitization. J Natl Cancer Inst. 1997;89:341–343. doi: 10.1093/jnci/89.5.341. [DOI] [PubMed] [Google Scholar]
- 42.Minden A, Karin M. Regulation and function of the JNK subgroup of MAP kinases. Biochim Biophys Acta. 1997;1333:F85–F104. doi: 10.1016/s0304-419x(97)00018-8. [DOI] [PubMed] [Google Scholar]
- 43.Miyajima A, Nakashima J, Yoshioka K, Tachibana M, Tazaki H, Murai M. Role of reactive oxygen species in cis-dichlorodiammineplatinum-induced cytotoxicity on bladder cancer cells. Br J Cancer. 1997;76:206–210. doi: 10.1038/bjc.1997.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagane M, Levitzki A, Gazit A, Cavenee W K, Huang H J. Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proc Natl Acad Sci USA. 1998;95:5724–5729. doi: 10.1073/pnas.95.10.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osherov N, Levitzki A. Epidermal-growth-factor-dependent activation of the src-family kinases. Eur J Biochem. 1994;225:1047–1053. doi: 10.1111/j.1432-1033.1994.1047b.x. [DOI] [PubMed] [Google Scholar]
- 46.Pages G, Berra E, Milanini J, Levy A P, Pouyssegur J. Stress-activated protein kinases (JNK and p38/HOG) are essential for vascular endothelial growth factor mRNA stability. J Biol Chem. 2000;275:26484–26491. doi: 10.1074/jbc.M002104200. [DOI] [PubMed] [Google Scholar]
- 47.Raingeaud J, Gupta S, Rogers J S, Dickens M, Han J, Ulevitch R J, Davis R J. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 48.Reed J C. Mechanisms of apoptosis avoidance in cancer. Curr Opin Oncol. 1999;11:68–75. doi: 10.1097/00001622-199901000-00014. [DOI] [PubMed] [Google Scholar]
- 49.Robinson M J, Cobb M H. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 50.Tournier C, Hess P, Yang D D, Xu J, Turner T K, Nimnual A, Bar-Sagi D, Jones S N, Flavell R A, Davis R J. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 51.Toyokuni S, Okamoto K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- 52.Ushio-Fukai M, Alexander R W, Akers M, Griendling K K. p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J Biol Chem. 1998;273:15022–15029. doi: 10.1074/jbc.273.24.15022. [DOI] [PubMed] [Google Scholar]
- 53.Verheij M, Bose R, Lin X H, Yao B, Jarvis W D, Grant S, Birrer M J, Szabo E, Zon L I, Kyriakis J M, Haimovitz-Friedman A, Fuks Z, Kolesnick R N. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Martindale J L, Liu Y, Holbrook N J. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J. 1998;333:291–300. doi: 10.1042/bj3330291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilhelm D, Bender K, Knebel A, Angel P. The level of intracellular glutathione is a key regulator for the induction of stress-activated signal transduction pathways including Jun N-terminal protein kinases and p38 kinase by alkylating agents. Mol Cell Biol. 1997;17:4792–4800. doi: 10.1128/mcb.17.8.4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong T W, Yu H Y, Kong S K, Fung K P, Kwok T T. The decrease of mitochondrial NADH dehydrogenase and drug induced apoptosis in doxorubicin resistant A431 cells. Life Sci. 2000;67:1111–1118. doi: 10.1016/s0024-3205(00)00699-8. [DOI] [PubMed] [Google Scholar]
- 57.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 58.Zindy F, Eischen C M, Randle D H, Kamijo T, Cleveland J L, Sherr C J, Roussel M F. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zor T, Selinger Z. Linearization of the Bradford protein assay increases its sensitivity: theoretical and experimental studies. Anal Biochem. 1996;236:302–308. doi: 10.1006/abio.1996.0171. [DOI] [PubMed] [Google Scholar]