Abstract
Objectives
This study aimed to describe the actions taken to implement a telepharmacy programme with home medication dispensing and informed delivery in an outpatient pharmaceutical care unit of a tertiary hospital, where approximately 5000 patients are treated per year. It also aimed to substantiate the applicability and benefits of the programme through analysing the findings and measuring patient satisfaction.
Methods
We identified the operational, logistical, technological and legal needs, as well as the need for training, information and coordination with the care team and patient associations. A standard operating procedure was developed which described the home dispensing model and the profile of patients eligible for telepharmacy. Care activity was evaluated, between the months of July 2020 and January 2021; and a survey was conducted to measure patient satisfaction based on the Enopex project, a cross-sectional observational study of patients who used telepharmacy services during the COVID-19 lockdown period in Spain.
Results
A total of 2536 medication deliveries were made over 144 working days, with a mean of 18 (standard deviation (SD): 6) deliveries per day, and a total of 2854 dispensings (1.1 drugs per delivery). In total, 197 different types of pharmaceutical formulations were delivered, corresponding to 123 active ingredients. The distance and time avoided during the study period totalled 1 05 624 km and 1 09 452 min (76 days), whereby the median distance and time saved per patient were 66 (interquartile range (IQR):122 km and 90 (IQR:90) minutes, which represents an approximate carbon footprint reduction of 25 kg of CO2 per patient and 16.5 tonnes in total. The satisfaction survey conducted, completed by 134 patients, revealed high satisfaction with the pharmacy service of 9.88 points out of 10.
Conclusions
The SARS-CoV-2 pandemic (COVID-19) has provided the pharmacy service with an opportunity to develop and implement a telepharmacy programme that benefits patients, which has enabled better organisation of the unit and greater accessibility for patients attending in person. It is a replicable method that is applicable in other pharmacy services with similar characteristics and requirements.
Keywords: PHARMACY SERVICE, HOSPITAL; Hospital Distribution Systems; COVID-19; MEDICATION SYSTEMS, HOSPITAL; Health Care Economics and Organizations
Introduction
The pandemic caused by coronavirus SARS-CoV-2 (COVID-19) in March 2020 forced the Spanish authorities to place the entire population into lockdown. This historic event increased the pressure on Spanish healthcare services and forced new organisational measures to be implemented in hospitals to ensure the care of all patients. In addition to the general dispensing of medication undertaken by pharmacies in Spain, hospital pharmacy services (PS) also dispense some specific medications to outpatients. Ensuring treatment continuity and avoiding the risks associated with hospital visits became fundamental objectives for PS, as well as providing an opportunity to promote the development of telepharmacy (online pharmaceutical assistance) and new ways of remotely dispensing hospital medications, such as delivery to community pharmacies, health centres or patients’ homes.
As regards the legal framework, with the declaration of the state of alarm in Spain based on Royal Decree 463/2020 of 14 March for the management of the health crisis situation, provisions for dispensing and administering medicines within the scope of the Spanish National Health System (SNS) were established nationally for the first time.1 Under Article 4.3 of the Royal Decree, the Spanish Ministry of Health passed Order SND/293/2020 of 25 March (Official State Gazette, BOE, of 27 March), authorising the competent body for pharmaceutical provision in each Autonomous Community to establish appropriate measures to ensure the dispensing of these medications outside hospital premises (point 3).2
Then, in May 2020, the Spanish Society of Hospital Pharmacy (SEFH) published its position on telepharmacy, the definition of which includes a remote pharmacy practice approach through the use of information and communication technologies (ICT). Telepharmacy has been incorporated as a strategic line of care in the Spanish healthcare system. However, despite its enormous potential, there are serious limitations in its development and application, especially in terms of the regulations at a national level on remote medication dispensing and informed delivery. Accordingly, the document ‘Strategic Framework in Telepharmacy’ from the MAPEX (Strategic Outpatient Pharmaceutical Care Map) project by SEFH has established the objectives and methodology for successful implementation in the different PS.3 This document also outlines the strengths of telepharmacy, as well as its limitations: risk of excluding certain patient profiles, guarantee of confidentiality and data protection, and coordination and alignment problems with other healthcare professionals. Therefore, equal access must be guaranteed by avoiding discrimination against patients on the basis of pathologies, age or socio-economic circumstances; by ensuring remote assistance through an adequate legal framework; and by carrying out educational work in search of synergies between patient associations, public administrations and other healthcare professionals.
This study aims to describe the actions taken to implement a telepharmacy programme with home medication dispensing and informed delivery in an outpatient pharmaceutical care unit (OPCU) of a tertiary hospital, where approximately 5000 patients are treated per year. It also aims to substantiate the applicability and benefits of the programme by analysing the findings and measuring patient satisfaction.
Methods
Literature search methodology
References were searched using the terms “Telepharmacy”, “Telemedicine”, “Home delivery” and “Hospital pharmacy” to select successful experiences, to design a pharmaceutical care model on-site/distance with dispensing and delivery of medicines from a distance. The PubMed database was searched without restrictions; in addition, the reference lists of important studies and reviews were hand searched. Available abstracts and oral communications from the conferences of the European Journal of Hospital Pharmacy (EJHP) were also reviewed.4–6 Recommendations from different scientific societies were included.3 7 8
Description of the home delivery programme
Operational and logistical elements
A standard operating procedure (SOP) was developed, which was approved by the centre’s management. The need for human resources was defined. The physical spaces required to ensure proper remote pharmaceutical care, as well as for medication preparation and storage, were determined. The time at which the patient would receive in-person care was defined. The different actions carried out were recorded in the patient’s clinical history.
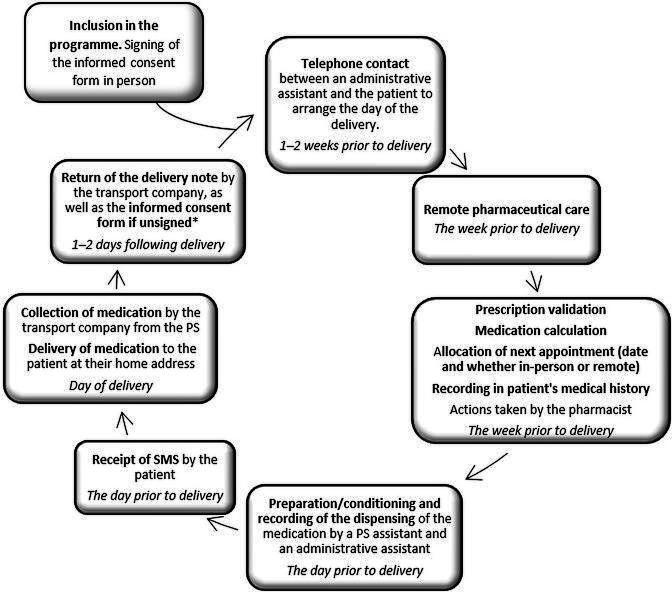
The periodicity of deliveries was determined, as well as the location, based on the requirements established by the logistics provider. A workflow adapted to the care environment was drawn up based on this analysis for telematic patient care (figure 1).
Figure 1.
Workflow for medication dispensing and informed delivery for telepharmacy assistance. PS: pharmacy service; SMS: short message service/text.
Incidents identified in the process by the logistics operator were communicated to the responsible pharmacist by telephone at the time. In the same way, the patient contacted the PS if an issue occurred.
In light of the pandemic situation, a patient home delivery model was chosen. Transport services were provided by an external provider. This transport model was determined by the financing model, which was chosen by the hospital centre’s management.
Technological aspects
Patients were contacted by telephone as it was the most widely installed means used by all patients.
A Microsoft Access 2010 database was created in the PS for recording and planning hospital medication deliveries, as well as for scheduling the pharmacotherapeutic follow-up. The programme included patient data: name, age, sex, address, telephone number, Spanish Population Information System (SIP) number, medication and dosage regimen. A master drug list was included alongside an appointment schedule that enabled the units being dispensed to be calculated and successive medication deliveries to be planned. In addition, this application made it possible to record incidents and extract activity indicators, as well as information on the profile of the patients (demographic data, type of medication, distance to the hospital) and the characteristics of the deliveries (thermolability). These data and the Google Maps application were used to estimate the distance and mean time between the locality of each patient and the hospital PS if they had travelled in their own vehicle.
Training, information and coordination with the care team and patient associations
Training sessions were held for PS staff. Information on the telepharmacy programme was disseminated through different communication channels, such as the corporate website, social media (Twitter) and conventional media (television, radio and newspapers), as well as to prescribing doctors and the Patient Association of Multiple Sclerosis (AEMC) of the hospital area. Prior to the inclusion of the programme, the pharmacist trained the patient during a pharmaceutical care consultation. A leaflet was prepared to inform patients about telepharmacy, how it works and the technological requirements, as well as the programme inclusion criteria (online supplemental 1).
ejhpharm-2021-003194supp001.pdf (1.8MB, pdf)
Description of the study
To verify the model’s usability and implementation, an observational, prospective and descriptive study was undertaken, which included the patients treated by this remote model between the months of July 2020 and January 2021.
Patient profile
The following inclusion criteria were considered: the chronicity of treatments (>6 months), adherence (an adherent patient was considered: adherence >80% in the last 6 months, measured by the dispensing record), as well as the proper understanding of the information on the telepharmacy programme and signing an informed consent form.7
Based on the human and economic resources available, at the beginning of the project, priority was given to older patients (>65 years), distance to the hospital centre (patients not residing in the hospital’s municipality), disability or dependency. Neither pathology nor medication were taken into consideration. Likewise, exclusion criteria were established: non-compliance with remote consultations, not being at home to receive the medication and individualised master formulas.
Assessment of patient satisfaction
To assess patient satisfaction, a telephone survey was conducted in January 2021 which included 134 randomly selected patients. The survey was carried out when contacting the patient to arrange for a new medication delivery. The survey was based on the Enopex questionnaire.9
Legal aspects
The study was approved by the local Clinical Research Ethics Committee, in accordance with the principles of the Declaration of Helsinki. All patients with inclusion criteria who agreed to take part in the telepharmacy programme were required to sign an informed consent form authorising the use of their personal data, both for remote care and for medication dispensing and informed delivery. This consent form was drafted by the PS and endorsed by the hospital’s legal department. This document was digitised and included in each patient’s medical record.
The confidentiality of the treatment was guaranteed at every stage of the process, in compliance with Spanish Organic Law 3/2018 of 5 December on the Protection of Personal Data and Guarantee of Digital Rights.
Statistical analysis
Quantitative variables were expressed as mean and SD when they followed a normal distribution, and if not, median and interquartile range (IQR) were used. Categorical variables were shown with frequency and percentage. Statistical analysis was performed using Stata 14.2 software.
Results
Of the 91 citations obtained from PubMed, 19 fulfilled were included and four citations were hand searched.
One pharmacist, one administrative assistant and one pharmacy assistant was established as telepharmacy personnel. Pharmaceutical care (face to face and telepharmacy) was done in the pharmacist’s office and medication preparation and storage at the OPCU store. A remote appointment schedule was created. The time at which the patient would receive in-person care coincided with their in-person medical consultation at the same hospital centre. Daily deliveries were set up based on the healthcare area.
The workflow defined was the following: 1–2 weeks before delivery an administrative assistant contacts the patient by telephone to arrange the day of the delivery. Next, the pharmacist does remote pharmaceutical care (resolves any doubts about the medication, identifies potential interactions, etc.) and validates the prescription and drug delivery. One day before the delivery, a PS assistant and an administrative assistant prepare and record the dispensing of the medication and an automatic short messaging service or text (SMS) is sent to the patient as a reminder. Finally, the transport company collects and delivers medication to the patient and returns the delivery note signed by the patient to the PS (figure 1).
We created two documents: the first for medication preparation, which included Wepatient data, in addition to the medication, units and date of the next delivery. The second document detailed delivery destinations and was sent via email to the logistics provider.
Individualised magistral formulas were excluded from the service due to the complexity of their preparation and the close clinical follow-up required. Both proper medicine storage and confidentiality were ensured throughout the process. To this end, medicines requiring storage at room temperature were packaged in opaque white envelopes, and thermolabile drugs, in opaque white insulated bags. Labels were designed for medicine identification which included the minimum patient information required for delivery. Furthermore, delivery notes were signed by the patients and returned to the PS by the logistics provider.
During the period described, 912 patients received the home delivery service, of which 472 (52%) were women, with a mean age of 63 years (SD: 17) (table 1).
Table 1.
Delivery information according to patient characteristics, destination locality and medication type
| Total (n=912) |
|
| Sex, n (%) | |
| Female | 472 (52) |
| Age, n (%) | |
| <65 years | 470 (52) |
| ≥65 years | 442 (48) |
| Inclusion request, n (%) | |
| Pharmacist | 773 (85) |
| Patient | 127 (14) |
| Doctor | 7 (<1) |
| Patient association | 5 (<1) |
| Locality, n (%) | |
| Rest of municipalities | 613 (67) |
| Hospital municipality | 299 (33) |
| Deliveries, n (%) | 2536 |
| Thermolabile | 1408 (56) |
| Room temperature | 1128 (44) |
A total of 2536 deliveries were made over 144 working days, with a mean of 18 (SD: 6) deliveries per day, and a total of 2854 dispensings (1.1 drugs per delivery) (figure 2).
Figure 2.
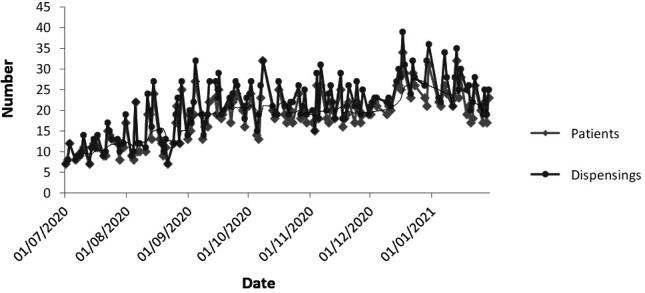
Deliveries per patient and number of dispensings during the study period. The black line corresponds to the rolling average of the 7 days prior to dispensings. Each different medication delivered is considered a medication dispensing.
According to the Anatomical Therapeutic Chemical (ATC) classification, the highest percentage of dispensed drugs belonged to group L (antineoplastic and immunomodulating agents), followed by group J (general antiinfectives for systemic use) and group B (blood and blood forming organs) (figure 3). In total, 197 different types of pharmaceutical formulations, with 123 different active ingredients, were delivered.
Figure 3.
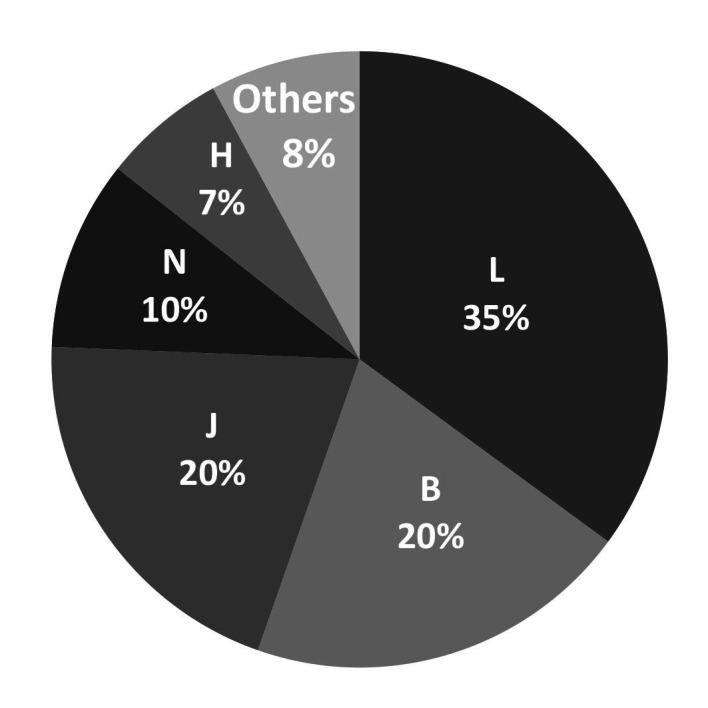
Distribution of dispensed medications per Anatomical Therapeutic Chemical (ATC) classification.
Of the total number of patients, 613 (67%) resided in localities surrounding the hospital. The median distance (round trip) between the patient’s residence and the hospital was 22 km with a median time of 30 min per patient, covering an interval between 10 and 244 km, and 20 and 200 min (table 2). The distance and time avoided during the study period totalled 1 05 624 km and 1 09 452 min (76 days), whereby the median distance and time per patient were 66 (IQR:122) km and 90 (IQR:90) minutes, resulting in an approximate reduction in CO2 of 25 kg per patient and a total range of 15.3–30.5 tonnes.10
Table 2.
Median time and distance between the place of residence and the hospital pharmacy service according to Google Maps
| Time (min) | Patients n (%) | Distance (km) | Patients n (%) |
| (1—40) | 636 (70) | (1—50) | 675 (74) |
| (41—80) | 195 (21) | (51—100) | 171 (19) |
| (81—120) | 53 (6) | (101—150) | 36 (4) |
| (121—160) | 22 (2) | (151—200) | 24 (2) |
| (160—200) | 6 (1) | (200—250) | 6 (1) |
min, minute.
During the programme, 24 incidents were reported, of which 12 were related to the patient’s absence at the time of delivery, eight to the PS and four incidents were linked to the transport company. All incidents were resolved and represented an improvement in the work circuit. Half of the incidents were the result of the patient not being at home at the time of delivery, in these cases the patient’s medication was returned to the PS and the patients attended in person and were excluded from the programme. The delivery incident rate was 0.9%.
The satisfaction survey was conducted on a sample of 134 patients. The results described in table 3 show patients’ high acceptance of the home delivery service.
Table 3.
Patient satisfaction questionnaire on home medication delivery
| Questions | Yes, n (%) | No, n (%) |
| Have you received the correct medication? | 134 (100) | 0 (0) |
| Have you received your medication in proper conditions relating to | ||
| Temperature? | 134 (100) | 0 (0) |
| Packaging? | 134 (100) | 0 (0) |
| Confidentiality? | 134 (100) | 0 (0) |
| Has avoiding a trip to the hospital increased your comfort levels? | 134 (100) | 0 (0) |
| What aspects would you improve? | ||
| A more specific delivery time | 28 (21) | 106 (79) |
| The quality of the transport company | 0 (0) | 134 (100) |
| Other | 5 (4) | 129 (96) |
| Qestions | Average | |
| Are you satisfied with the transport service? Rate on a scale of 1 to 10, with one being totally dissatisfied and 10 being totally satisfied | 9.75 | |
| What is your opinion of the pharmacy service in terms of home medication delivery? Rate on a scale of 1 to 10, with one being totally dissatisfied and 10 being totally satisfied | 9.88 | |
Discussion
In Spain, telepharmacy has reduced the necessity for patients to travel to hospital centres during the COVID-19 pandemic, as well as ensuring treatment continuity with hospital-dispensed medication. In a survey of 185 Spanish public hospitals, 83.2% of PS did not include medication delivery as part of their remote pharmaceutical care service prior to the health crisis.11 The declaration of the state of alarm on 14 March 2020 presented PS with an opportunity to develop and implement new models of remote care and remote medication dispensing.12–15
Nevertheless, the telepharmacy experiences described are limited and include different dispensing and informed delivery models (community pharmacy or home).15–23 Furthermore, most of them present descriptive results in small patient groups selected according to pathology,17 18 24–26 with certain drugs, such as thermolabile or narcotic drugs, excluded in many instances.1 13 Moreover, the degree of implementation is heterogeneous; in the USA, for example, in a study carried out by the American Society of Health-System Pharmacists (ASHP) on pharmaceutical practice in hospital settings, only a quarter of the 265 hospitals surveyed performed telepharmacy.27 These data reveal the huge margin for improvement presented by this pharmaceutical care model.28
In the hospital PS where this study was conducted, around 4700 patients were treated during 2020. There was a total of 26 000 dispensings. Therefore, approximately one fifth of the patients treated were able to benefit from telepharmacy during the study period. Likewise, the programme has made it possible to reduce in-person healthcare activity and reorganise workflows and agendas. The findings indicate that an average of 20 medications were delivered per day, the majority of which were drugs belonging to the groups of antineoplastic and immunomodulating agents, anti-infectives for systemic use, and blood and haematopoietic organs. This same profile of medication dispensed is seen in other studies, such as Zozaya et al, 13 in which antineoplastic agents and immunomodulators accounted for two-thirds of the medication, this is also seen in a study by Peláez et al. 15
In light of the urgency of implementation and the novelty of the project, the PS primarily selected the patients to be included in the programme due to the exceptional circumstances of the pandemic and patients’ and doctors’ lack of knowledge about the service. Indeed, 67% of patients included did not reside in the same municipality as the hospital centre; the potential benefit was considered due to certain patients not having their own vehicle, or because no public transport with an adequate frequency was available, or the cost of transportation was not affordable for the patient. In this regard, we can highlight how the model reduces the carbon footprint by lowering the number of trips to the hospital. It also saves time for patients, which provides them with a better work-life balance, and therefore leads to improved quality of life, together with economic savings both directly, through avoiding travelling expenses, and indirectly, through the time spent travelling and loss of labour productivity. Other studies corroborate these benefits; in this way, Zozaya et al estimate that a total of 1939 shipments in 2 months had avoided trips associated with a total saved time of 1374 hours and had saved the patient a total of €23,309, including the costs of avoided trips and avoided productivity losses.13
In the study period, 24 incidents with the delivery service were recorded, half of them occurred because the patient was not at home. This highlights one of the limitations of the home delivery model. Most of the published studies do not describe the incidents that occurred. However, Peláez et al identified 10 incidents related to no drug delivery, dosing error, wrong or unnecessary drug, wrong formulation or wrong patient. All of these incidents were resolved, as in our study.15
The use of telephones for patient contact is a safe system that most patients prefer to videoconferencing,29 therefore the majority of studies use this tool to contact patients.13 15 The main problem in the study is the ability to guarantee confidentiality during shipment. This is related to pathologies with greater social stigma (HIV and disabling diseases such as multiple sclerosis or amyotrophic lateral sclerosis). In our study, two of the recorded incidents were related to the non-confidential delivery of medication. To improve this aspect, an additional question was added in the initial patient interview regarding the person authorised to receive the shipment.
This study is one of the first to describe a telepharmacy model with direct home delivery of hospital medication and to offer an analysis of the long-term model, in addition to including chronic patients without discrimination on the basis of pathology or medication. Currently, there are studies limited to the first months of the pandemic that proved the viability of telepharmacy;5 6 11–16 however, they do not specify whether this service continued after this period. In the study hospital, however, the service is currently available to all patients likely to benefit from the process, in line with the principle of equity.
In the same way, this analysis shows the potential benefit that telepharmacy with home medication dispensing and delivery presents in terms of patient satisfaction, similar to that of other published studies such as Peláez et al,15 which shows an overall average of satisfaction of 9.83 on a 10-point scale, Margusino-Framiñán et al report a score of 9.7.14 In our study, avoiding travel to the hospital was the aspect patients valued most, as reported by Álvarez et al,12 while the main inconvenience reported by 21% of our patients was not having a fixed delivery slot. In relation to the referenced OPCU, implementing the model has made it possible to reduce the in-person care burden and to reorganise work flows more efficiently.
However, this study has certain limitations. First, inorder to estimate the distance and the mean time saved between each patient’s locality and the hospital, the authors assume patients travel to the PS in their own vehicles, other modes of travel such as public transportation are not considered. Second, the satisfaction survey was conducted over the telephone by PS staff, preventing the anonymisation of patients, which could generate a bias when reporting negative aspects. Finally, the remote pharmaceutical care given to these patients was not monitored, which would have enabled the impact of telepharmacy on aspects such as improving adherence, detecting adverse reactions and incorrect administration of medications to be analysed. It would be interesting to conduct long-term studies to determine telepharmacy’s potential in these pharmaceutical care units in hospital PS.
It is clear that the health crisis caused by the pandemic has been the ideal time for PS to implement different models of telepharmacy and remote medication delivery. The benefits for both professionals and patients are also apparent, giving rise to an essential service that is in demand. However, different informed delivery models for hospital medication have been developed (in community pharmacies, health centres or at the patient’s home),12–14 17 18 and therefore the most appropriate model for each patient has yet to be defined, as well as for the health system in terms of efficiency. In this sense, more long-term studies are needed that evaluate the impact of these programmes on patients and analyse the associated costs. Furthermore, it would be helpful to stratify the potential beneficiaries of these programmes.3
Conclusion
The COVID-19 pandemic has provided the PS with an opportunity to develop and implement a telepharmacy programme with home medication dispensing and informed delivery with worthwhile patient benefits, which has enabled better organisation of the OPCU and greater accessibility for patients attending in person. It is a replicable method that is applicable in other PS.
Key messages.
What is already known on this topic
The disruption caused by the COVID-19 pandemic has accelerated the drawing up of a legal framework allowing the implementation of telepharmacy and has favoured the development of different models of telepharmacy and remote medication dispensing in hospital pharmacy services.
New technologies allow remote patient care with hospital medication.
What this study adds
Patient satisfaction with telepharmacy shows the high acceptance of this new model and the need to develop new patient care systems adapted to current circumstances and needs.
How this study might affect research, practice or policy
This study benefits health professionals because it provides a replicable method to implement telepharmacy in hospitals beyond the COVID-19 pandemic.
ejhpharm-2021-003194supp002.pdf (168KB, pdf)
Acknowledgments
We would like to thank the staff at the PS for all their work and collaboration.
Footnotes
Twitter: @JuliaBodega
Contributors: MG-C, AS-A, EV-E and PP-H conceived the study. MG-C, AS-A and EV-E analysed, interpreted the data and wrote the paper; MG-C, AS-A and EV-E included data of patients; MG-C and AS-A performed the statistical analyses; MG-C, AS-A, EV-E, AP-B, JBA and RFP reviewed the manuscript and contributed to the final draft. MG-C is responsible for the overall content as the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. Not applicable.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
Ethics approval
This study involves human participants and was approved by Comité de Ética del Hospital General Universitario de Castellón.ID: FARTEL-01 Participants gave informed consent to participate in the study before taking part.
References
- 1. Ministerio de Sanidad . Orden SND/293/2020, de 25 de marzo, POR La que Se establecen condiciones a la dispensación Y administración de medicamentos en El ámbito del Sistema Nacional de Salud, ante La situación de crisis sanitaria ocasionada POR El COVID-19. Boletín Of del Estado 2020:61561–7. [Google Scholar]
- 2. Ministerio de la Presidencia Relaciones con las Cortes y Memoria Democrática . Real Decreto 463/2020, de 14 de marzo, POR El que Se declara El estado de alarma para La gestión de la situación de crisis sanitaria ocasionada POR El COVID-19. Boletín Of del Estado 2020;67:25390–400. [Google Scholar]
- 3. Sociedad Española de Farmacia Hospitalaria . Proyecto MAPEX: MARCO estratégico en telefarmacia, 2020. [Google Scholar]
- 4. Taberner Bonastre P, Tomas Sanchez M, Gallart Lopez M. 2SPD-001 Describing a thickener home delivery protocol and the benefits of its implementation. In: Section 2: selection, procurement and distribution. British Medical Journal Publishing Group, 2019: A19.2–A19. [Google Scholar]
- 5. Cordero-Ramos J, Ú B-R, Fernandez-González-Caballos J. 2SPD-001 Implementation of home delivery and telepharmacy systems in a third level hospital. In: Section 2: selection, procurement and distribution. British Medical Journal Publishing Group, 2020: A9.2–10. [Google Scholar]
- 6. Cazorla Poderoso L, Navarro Aznarez H, Perez Moreno M. 4CPS-395 Pharmacy service’s adaptation to the COVID-19 pandemic: telepharmacy and home drug delivery. In: Section 4: clinical pharmacy services. British Medical Journal Publishing Group, 2021: A111.1–A111. [Google Scholar]
- 7. Ibarra Barrueta O, Morillo Verdugo R. Lo que debes Saber sobre La ADHERENCIA al tratamiento, 2017. [Google Scholar]
- 8. ASHP . ASHP Statement on Telepharmacy. In: Best practices. American Society of Health-System pharmacists, 2019: 22–7. [DOI] [PubMed] [Google Scholar]
- 9. Margusino-Framiñán L, Fernández-Llamazares CM, Negro-Vega E, et al. Outpatients' opinion and experience regarding Telepharmacy during the COVID-19 pandemic: the Enopex project. J Multidiscip Healthc 2021;14:3621–32. 10.2147/JMDH.S343528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Departamento de Desarrollo Económico Sostenibilidad y Medio Ambiente . Gobierno Vasco. Herramienta de Cálculo de Huella de Carbono en base a la norma UNE-ISO 16064-1 Y al estándar GHG protocol. Serv. web del GOB. Vasco, 2021. Available: https://www.euskadi.eus/informacion/acciones-voluntarias-para-la-reduccion-de-emisiones-en-empresas/web01-a2ingkli/es/
- 11. Tortajada-Goitia B, Morillo-Verdugo R, Margusino-Framiñán L, et al. Survey on the situation of telepharmacy as applied to the outpatient care in hospital pharmacy departments in Spain during the COVID-19 pandemic. Farm Hosp 2020;44:135–40. 10.7399/fh.11527 [DOI] [PubMed] [Google Scholar]
- 12. Álvarez Criado J, García-Trevijano Cabetas M, Nácher J. Evaluación del servicio de entrega de medicación a domicilio desde La Farmacia Hospitalaria durante La pandemia COVID-19. Rev la OFIL 2020;30:193–9. [Google Scholar]
- 13. Zozaya N, González-Domínguez A, Calvente N, et al. Continuity of care between hospital pharmacies and community pharmacies, and costs avoided: a pilot experience in times of COVID-19 in Spain. Grhta 2021;8:8–13. 10.33393/grhta.2021.2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Margusino-Framiñán L, Illarro-Uranga A, Lorenzo-Lorenzo K, et al. Pharmaceutical care to hospital outpatients during the COVID-19 pandemic. Telepharmacy. Farm Hosp 2020;44:61–5. 10.7399/fh.11498 [DOI] [PubMed] [Google Scholar]
- 15. Peláez Bejarano A, Villar Santos P, Robustillo-Cortés MdeLA, et al. Implementation of a novel home delivery service during pandemic. Eur J Hosp Pharm 2021;28:e120–3. 10.1136/ejhpharm-2020-002500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muflih SM, Al-Azzam S, Abuhammad S, et al. Pharmacists' experience, competence and perception of telepharmacy technology in response to COVID-19. Int J Clin Pract 2021;75:e14209. 10.1111/ijcp.14209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quirós-González V, Rubio R, Pulido F. Healthcare outcomes in patients with HIV infection at a tertiary hospital during the COVID-19 pandemic. Enferm Infecc Microbiol Clin 2021;110:697–700. 10.1016/j.eimc.2021.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Megías-Vericat JE, Monte-Boquet E, Martín-Cerezuela M, et al. Pilot evaluation of home delivery programme in haemophilia. J Clin Pharm Ther 2018;43:822–8. 10.1111/jcpt.12718 [DOI] [PubMed] [Google Scholar]
- 19. Baldoni S, Amenta F, Ricci G. Telepharmacy services: present status and future perspectives: a review. Medicina 2019;55:327. 10.3390/medicina55070327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Le T, Toscani M, Colaizzi J. Telepharmacy: a new paradigm for our profession. J Pharm Pract 2020;33:176–82. 10.1177/0897190018791060 [DOI] [PubMed] [Google Scholar]
- 21. Samartín-Ucha M, Piñeiro-Corrales G, Continuity of Care Group from the EOXI Vigo . Model of teleconsultation pharmaceutical integrated in the electronic clinical history of the patient. Farm Hosp 2019;43:1–5. 10.7399/fh.10937 [DOI] [PubMed] [Google Scholar]
- 22. Van den Bosch F, Ostor AJK, Wassenberg S, et al. Impact of participation in the adalimumab (Humira) patient support program on rheumatoid arthritis treatment course: results from the passion study. Rheumatol Ther 2017;4:85–96. 10.1007/s40744-017-0061-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. León A, Cáceres C, Fernández E, et al. A new multidisciplinary home care telemedicine system to monitor stable chronic human immunodeficiency virus-infected patients: a randomized study. PLoS One 2011;6:e14515. 10.1371/journal.pone.0014515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. San José Ruiz B, Gil Lemus MA, Figuero Echeverria MP. [Pharmaceutical care and home delivery of medication to patients with chronic myeloid leukemia]. Farm Hosp 2015;39:13–22. 10.7399/fh.2015.39.1.7860 [DOI] [PubMed] [Google Scholar]
- 25. Lal R, Hillerdal GN, Shah RNH, et al. Feasibility of home delivery of pemetrexed in patients with advanced non-squamous non-small cell lung cancer. Lung Cancer 2015;89:154–60. 10.1016/j.lungcan.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 26. Lal R, Bourayou N, Hillerdal G, et al. Home administration of maintenance pemetrexed for patients with advanced non-squamous non-small cell lung cancer: rationale, practicalities and phase II feasibility study design. Health Qual Life Outcomes 2013;11:163. 10.1186/1477-7525-11-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pedersen CA, Schneider PJ, Ganio MC, et al. ASHP national survey of pharmacy practice in hospital settings: dispensing and administration—2020. Am J Heal Pharm 2021;78:1074–93. 10.1093/ajhp/zxab120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lizano-Díez I, Amaral-Rohter S, Pérez-Carbonell L. Impact of home care services on patient and economic outcomes: a targeted review. Home Health Care Manag Pract 2021;108482232110383. [Google Scholar]
- 29. Nittari G, Khuman R, Baldoni S, et al. Telemedicine practice: review of the current ethical and legal challenges. Telemed J E Health 2020;26:1427–37. 10.1089/tmj.2019.0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ejhpharm-2021-003194supp001.pdf (1.8MB, pdf)
ejhpharm-2021-003194supp002.pdf (168KB, pdf)
Data Availability Statement
No data are available. Not applicable.