Abstract
Numerous signaling molecules associate with lipid rafts, either constitutively or after engagement of surface receptors. One such molecule, phospholipase Cγ-1 (PLCγ1), translocates from the cytosol to lipid rafts during T-cell receptor (TCR) signaling. To investigate the role played by lipid rafts in the activation of this molecule in T cells, an influenza virus hemagglutinin A (HA)-tagged PLCγ1 was ectopically expressed in Jurkat T cells and targeted to these microdomains by the addition of a dual-acylation signal. Raft-targeted PLCγ1 was constitutively tyrosine phosphorylated and induced constitutive NF-AT-dependent transcription and interleukin-2 secretion in Jurkat cells. Tyrosine phosphorylation of raft-targeted PLCγ1 did not require Zap-70 or the interaction with the adapters Lat and Slp-76, molecules that are necessary for TCR signaling. In contrast, the Src family kinase Lck was required. Coexpression in HEK 293T cells of PLCγ1-HA with Lck or the Tec family kinase Rlk resulted in preferential phosphorylation of raft-targeted PLCγ1 over wild-type PLCγ1. These data show that localization of PLCγ1 in lipid rafts is sufficient for its activation and demonstrate a role for lipid rafts as microdomains that dynamically segregate and integrate PLCγ1 with other signaling components.
The plasma membrane lipid bilayer contains membrane domains with distinct composition (32). These membrane lipid rafts, alternatively known as detergent-insoluble membranes, detergent-resistant membranes, detergent-insoluble glycolipid-rich membranes, triton-insoluble floating fraction, or glycosphingolipid-enriched membranes, were initially defined by their insolubility in cold, nonionic detergents, a characteristic that, together with their comparatively high buoyancy, facilitates their isolation on sucrose gradients (2). Lipid rafts are enriched in sphingolipids stabilized by intercalating cholesterol (2, 11) and form liquid-ordered assemblies separate from the phospholipid bilayer. These special physicochemical characteristics are associated with distinct functions, including a role in signal transduction (32).
Numerous observations support a function for lipid rafts in T-cell receptor (TCR) signaling. Several molecules implicated in TCR signal transduction associate constitutively with lipid rafts. They include two Src kinase family members, Lck and Fyn, implicated in TCR signal initiation (31). The T-cell-specific adapter Lat is also constitutively associated with lipid rafts, where it functions as an activation scaffold after tyrosine phosphorylation (8, 19, 46, 47). Furthermore, TCR signaling requires proper compartmentalization of Lck to the lipid rafts (12) and a Lat mutant which failed to associate with lipid rafts was not phosphorylated and resulted in defective TCR signaling (48).
In addition to molecules constitutively present in the lipid rafts, TCR engagement induces raft recruitment of proximal and distal elements of the TCR activation sequence, including the TCR-associated ζ chains, and several adapter and effector molecules (22, 42, 48). Moreover, disruption of raft organization by cholesterol sequestration impairs T-cell activation (42).
A central issue in the role played by membrane rafts in signal transduction is whether recompartmentalization of effector molecules to the lipid rafts controls their activation status and induces downstream signaling events. In TCR signal transduction, a fraction of phospholipase Cγ-1 (PLCγ1), a cytosolic protein, is inducibly recompartmentalized to the lipid rafts (42, 48). PLCγ1-mediated hydrolysis of phosphatidylinositol (4,5)-bisphosphate to inositol (1,4,5)-trisphosphate and diacylglycerol controls Ca2+ mobilization and protein kinase C activation, respectively (25), critical steps that regulate interleukin 2 (IL-2) transcription (4). We therefore questioned whether PLCγ1 activation is controlled by recompartmentalization to the lipid rafts.
The TCR-induced signaling cascade ensues with Lck and Fyn activation, leading to the phosphorylation of conserved immunereceptor tyrosine-based activation motifs (ITAMs) on the TCR-associated CD3 and ζ chains (13). Phosphorylated ITAMs recruit the T-cell-specific kinase, Zap-70, which is subsequently activated via Lck phosphorylation (13). Activated Zap-70 phosphorylates Lat (40, 47) and a second T-cell-specific adapter, Slp-76 (43). PLCγ1 then engages tyrosine-phosphorylated Lat, thereby localizing PLCγ1 to the lipid rafts (40, 47). PLCγ1 activation is regulated by tyrosine phosphorylation (39) via a mechanism that, in addition to Zap-70 and Lat (40, 47), requires Slp-76 (43) and members of the Tec kinase family, Itk and Rlk (27). Interestingly, tyrosine-phosphorylated PLCγ1 is enriched in lipid rafts (42, 48).
To investigate if the activation status of PLCγ1 is controlled by recompartmentalization to the lipid rafts, we engineered a form of the enzyme that was constitutively present in the lipid rafts. Expression of a raft-targeted form of the enzyme in Jurkat T leukemia cells resulted in the constitutive tyrosine phosphorylation and activation of PLCγ1 with ensuing Ca2+-dependent transcriptional activation. Phosphorylation of raft-targeted PLCγ1 required Lck but dispensed with Zap-70, Lat, and Slp-76. Thus, relocalization to membrane rafts is sufficient for PLCγ1 phosphorylation and bypasses TCR engagement and the activation of early steps in the TCR-signaling sequence.
MATERIALS AND METHODS
Plasmids, cell lines, and reagents.
The HA-tagged bovine PLCγ1 in the expression vector pCIneo (Promega, Madison, Wis.) has been previously described (33). The amino-terminal palmitoylation signal sequence, (M)GCVQCKDKEA, and the control sequence, (M)ASVQCKDKEA, were introduced by PCR using appropriate oligonucleotides. The amplified fragments were shuttled via XbaI and BamHI sites into a pBluescript II SK(−) vector (Stratagene, La Jolla, Calif.) and excised via XbaI and Eco72I and cloned into PLCγ1-HA in pCIneo. pSX-LckY505F, encoding an activated form of Lck, was a gift of R. Perlmutter (Merck Co. Inc, Rahway, N.J.). pSX-Zap-70Y492F, encoding a partially active form of Zap-70, was described previously (15, 37). pR1k-GFP, encoding an R1k fusion protein with green fluorescent protein in the pCI vector, was a gift from P. Schwartzberg (National Institute for Human Genome Research, Bethesda, Md.). The pSRa-Flag-PTEN (WT-PTEN) and pSRa-Flag-PTEN C124S (PTEN C/S) constructs, encoding the wild-type protein and a catalytically inactive mutant, respectively, were described previously (29). All Jurkat lines were maintained in RPMI 1640 (Life Technologies, Gaithersburg, Md.) containing 10% fetal bovine serum. The Zap-70-negative cell line P116 and a stable Zap-70-reconstituted line were from R. T. Abraham (Duke University, Durham, N.C.). The Slp-76-deficient Jurkat clone J14, the stable Slp-76-reconstituted clone J14-76-11, and the anti-clonotypic TCR antibody C305, were gifts from A. Weiss (University of California, San Francisco, Calif.). Human embryonic kidney HEK 293T cells were maintained in Dulbecco's modified Eagle's medium (Life Technologies) containing 10% fetal bovine serum. The anti-influenza virus hemagglutinin (HA) antibody 12CA5 was a gift from A. Weissman (National Cancer Institute, Bethesda, Md.). The anti-HA antibody 3F10 was from Boehringer Mannheim (Indianapolis, Ind.). The antiphosphotyrosine antibody 4G10 and the anti-LAT rabbit polyclonal antiserum were from Upstate Biotechnology (Lake Placid, N.Y.). The rabbit antiphospholipase Cγ1 [pY783] phospho-specific antibody was from Biosource (Camarillo, Calif.). The antibody to phospho-Akt (Ser 473) was from Cell Signaling Technology (Beverly, Mass.), and the anti-Flag antibody (M2) was from Sigma (St. Louis, Mo.).
Transfection assays.
Jurkat E6.1 cells, Jurkat TAg cells, and all Jurkat derivatives were grown to log phase and transfected by using a square-wave electroporator. HEK 293T cells were grown to subconfluence, serum starved overnight, and transfected with the indicated constructs (0.3 μg of each constructs when not otherwise indicated) by using LipofectAMINE (Life Technologies).
Cell activation, immunoprecipitation, and immunoblotting.
Transfected Jurkat cells were cultured at 0.5 × 106 /ml for 24 h and treated with 0.1 mg of DNase per ml, and nonviable cells were removed by Ficoll gradient centrifugation immediately prior to experimental use. For stimulation, 107 Jurkat cells were treated with medium or C305 antibody for 2 min at 37°C. The cells were lysed in a buffer composed of 60 mM Tris-HCl (pH 7.8), 150 mM NaCl, 5 mM EDTA, 10% glycerol, and 60 mM n-octyl-β-d-glucopyranoside (Sigma) as the detergent, together with protease and phosphatase inhibitors. HEK 293T cells were harvested 48 h after transfection in lysis buffer, and the protein concentration was determined. A 3-mg portion of protein per sample was immunoprecipitated. Cleared lysates were precipitated with specific antibodies prebound to protein A or protein A/G beads. Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblot analysis with 125I-protein A or chemiluminescence for detection. Radioimmunoblots were scanned on a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.) and analyzed with no manipulation except for adjustment of the exposure range.
Metabolic labeling of Jurkat cells.
Transiently transfected Jurkat cells (8.5 × 106) were transferred to 1 ml of RPMI containing 5 mM sodium pyruvate, 5% saline-dialyzed fetal calf serum, and 0.25 mCi of [3H]myristate or [3H]palmitate (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.). After 3 h at 37°C, the cells were placed in lysis buffer. Anti-HA precipitates were resolved by SDS-PAGE (10% polyacrylamide) (InVitrogen, Carlsbad, Calif) and then treated with En3Hance (Dupont NEN, Boston, Mass.) for 1 h followed by 5% polyethylene glycol 8000 for 30 min. Dried gels were exposed to film for 4 weeks.
Subcellular fractionation and isolation of lipid rafts.
Cell fractionation was performed as previously described (5). To isolate lipid rafts, transiently transfected cells (22 × 106) were suspended in lysis buffer without detergent or glycerol and sonicated five times with 5-s pulses. Samples were adjusted to a total volume of 1 ml in lysis buffer containing 1% Brij 58 (Pierce, Rockford, Ill.) and maintained on ice for 1 h. The lysates were then mixed with 1 ml of 85% sucrose in 60 mM Tris HCl (pH 7.8) containing 150 mM NaCl and 5 mM EDTA and transferred to polyallomer centrifuge tubes (Beckman, Palo Alto, Calif.). A 2-ml volume of 30% sucrose was layered on top, followed by 1 ml of 5% sucrose. Samples were centrifuged in a Beckman SW55Ti rotor at 200,000 × g for 16 to 18 hr at 4°C, and 400-μl fractions were recovered from the top and immunoprecipitated and/or subjected to SDS-PAGE and immunoblot analysis.
Immunofluorescence confocal microscopy.
Cells were stained with tetramethylchodamine-5-isothiocyanate (TRITC) (rod)-conjugated cholera toxin B subunit (CT-B; List, Campbell, Calif.), aggregated with an anti-CT-B antibody fixed in 2.0% formaldehyde in phosphate-buffered saline (PBS), and permeabilized by immersion in PBS–0.1% Triton X-100. The cells were stained with anti-HA in PBS containing 4 mg of normal goat serum per ml and 4 mg of human immunoglobulins per ml, washed, reacted with fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Molecular Probes), washed, and mounted in glycerol. Internal controls, performed by omitting the primary antibody, show no detectable FITC staining (data not shown). Confocal analysis was carried out with a TCS 4D confocal setup (Leica) mounted on a Leitz DMRB microscope, equipped with a 100×/1.3 NA oil immersion objective. High-resolution fluorescence images were obtained by exciting FITC and rhodamine at 488 and 552 nm, respectively, with an argon ion laser. Images were acquired with an averaging function line-by-line, top down, with a scanning mode format of 512 × 512 pixels. Single optical sections of FITC signal, merged with the corresponding rhodamine images, were elaborated by a two-dimensional image-processing system.
NF-AT luciferase assay.
Jurkat cells were transfected with 3 or 5 μg of WT-PLCγ1-HA or Palm-PLCγ1-HA along with 10 μg of an NF-AT–Luc plasmid containing a luciferase reporter under the control of the IL-2 minimal promoter and three optimized NF-AT sites (a gift of G. Crabtree, Stanford University, Stanford, Calif.) and 10 μg of pADβ (Clontech, Palo Alto, Calif.), a control vector for transfection efficiency in which the expression of β-galactosidase is regulated by the adenovirus major late promoter. At 3 h after transfection, the cells were incubated with medium alone, 10 nM phorbol myristate acetate (PMA), or 10 nM PMA plus 1 μM ionomycin for additional 6 h. The cells were disrupted in lysis buffer (Promega) and assayed using luciferin (Promega) and for β-galactosidase activity using Galacton Plus and Emerald Enhance (Tropix, Bedford, Mass.).
IL-2 enzyme-linked immunosorbent assay.
Jurkat cells were transfected with 20 μg of WT-PLCγ1-HA or Palm-PLCγ1-HA along with 20 μg of WT-PTEN or PTEN C/S. At 3 h after transfection, duplicate samples were incubated at 106 cells/ml with medium alone, 10 nM PMA, or 10 nM PMA plus 1 μM ionomycin for an additional 24 h. Supernatants were harvested and assayed for IL-2 by using a QuantiGlo IL-2 chemiluminescence immunoassay kit (R&D System, Minneapolis, Minn.) as specified by the manufacturer.
RESULTS
Acylation constitutively targets PLCγ1 to lipid rafts.
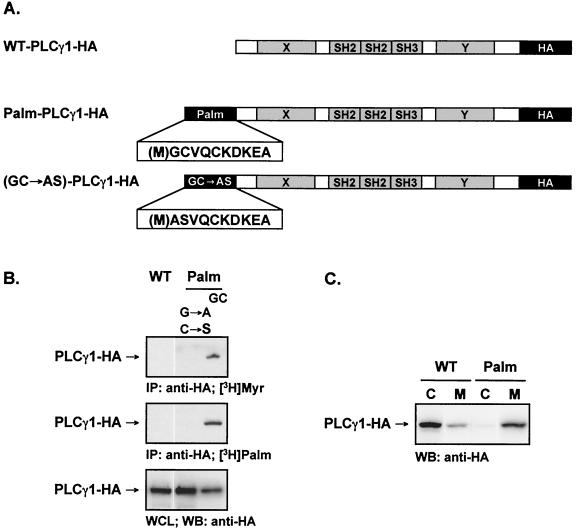
Numerous proteins are posttranslationally modified by the covalent addition of lipid moieties. Acylation by the covalent addition of myristate to an N-terminal glycine via an amide linkage and/or that of palmitic acid to cysteine residues confers specific targeting properties to the cytoplasmic leaflet of membrane microdomains (32, 36). The acylation sequence of the Src family member Fyn, a dually acylated protein, is sufficient for targeting Fyn to lipid rafts (41). To force the compartmentalization of PLCγ1 to the lipid rafts, we engineered a dually acylated form of an influenza virus HA-tagged PLCγ1 with an N-terminal myristoylation and palmitoylation motif from the human Fyn sequence (Palm-PLCγ1-HA) (Fig. 1A). In addition, an unmodified PLCγ1-HA (wild type, WT-PLCγ1-HA) and a control protein in which the glycine and cysteine acyl acceptors of the acylation motif were mutated (GC→AS) were constructed as controls.
FIG. 1.
Characterization and subcellular fractionation of wild-type and acylated PLCγ1 in Jurkat cells. (A) Schematics of the constructs encoding WT-PLCγ1-HA and Palm-PLCγ1-HA. (B) Metabolic labeling of WT-PLCγ1-HA and Palm-PLCγ1-HA. Jurkat TAg cells were transiently transfected with the indicated constructs, labeled with [3H]myristate (top panel) or [3H]palmitate (middle panel), and lysed. Anti-HA precipitates were resolved by SDS-PAGE. A parallel gel loaded with whole-cell lysates (WCL) was immunoblotted (WB) with anti-HA antibody to control for PLCγ1-HA expression (bottom panel). (C) Subcellular fractionation of WT-PLCγ1-HA and Palm-PLCγ1-HA. Jurkat TAg cells were transiently transfected with the indicted constructs and fractionated into cytosol (C) and membrane (M) fractions. A 25-μg amount of protein was resolved by SDS-PAGE and immunoblotted with anti-HA.
The acylation of the engineered forms of PLCγ1 was confirmed by metabolic labeling of proteins transiently expressed in Jurkat cells. Both [H3]myristate and [H3]palmitate were incorporated by the dually acylated Palm-PLCγ1-HA, whereas neither WT-PLCγ1-HA nor the control protein GC→AS-PLCγ1-HA incorporated detectable levels of radiolabeled lipids (Fig. 1B). The subcellular localization of transiently transfected WT-PLCγ1-HA and Palm-PLCγ1-HA was obtained by separation into soluble (cytoplasmic) and particulate (membrane-rich) fractions (Fig. 1C). WT-PLCγ1-HA was detected almost exclusively in the cytosolic fraction, while Palm-PLCγ1-HA was found mostly in the membrane fraction.
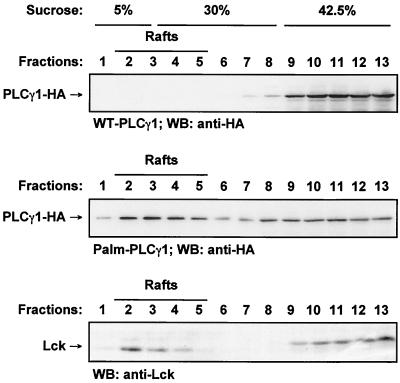
To further ascertain the membrane microdomain localization of the dually acylated form of PLCγ1, lipid rafts were isolated by ultracentrifugation over a discontinuous sucrose gradient from lysates of Jurkat T cells expressing WT-PLCγ1-HA or Palm-PLCγ1-HA. Among a total of 13 fractions collected, fractions at the interface between 5 and 30% sucrose contained the low-density membrane fraction, as shown by the presence of Lck, a protein which is in large part constitutively associated with this microdomain fraction (Fig. 2, bottom panel). Immunoblotting with anti-HA showed a large portion of Palm-PLCγ1-HA constitutively present within the raft fraction from unstimulated cells (Fig. 2, middle panel). No WT-PLCγ1-HA (or the GC→AS control protein [data not shown]) was detected in this fraction (Fig. 2, top panel).
FIG. 2.
Lipid raft compartmentalization of wild-type and acylated PLCγ1 in Jurkat cells. Lysates from transiently transfected Jurkat TAg cells were separated on discontinuous sucrose gradients. Fractions were analyzed by SDS-PAGE and immunoblotted (WB) with anti-HA (top and middle panels) or for endogenous Lck (Santa Cruz) (bottom panel).
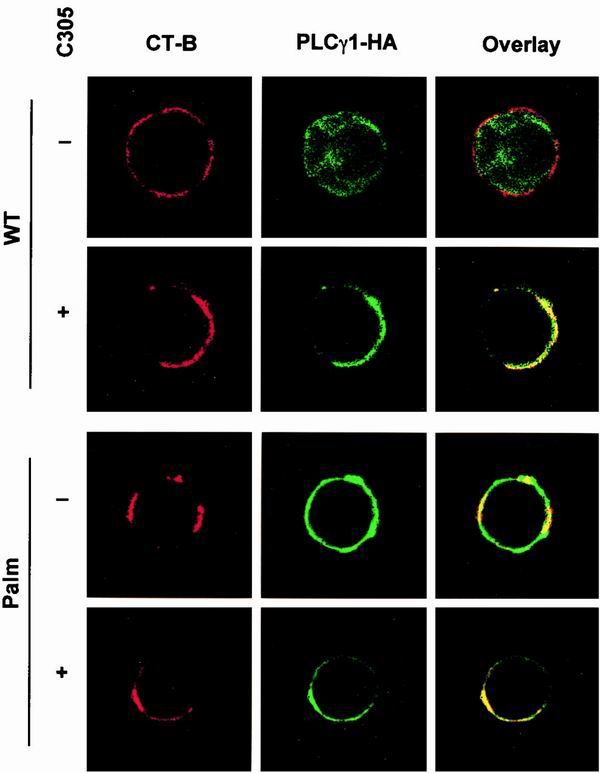
The subcellular distribution conferred by acylation was further visualized in Jurkat cells by confocal microscopy with TRITC-labeled CT-B, which binds with strong affinity to GM1, as a raft marker (24). WT-PLCγ1-HA was present as cytoplasmic fluorescence in unstimulated cells, while Palm-PLCγ1-HA was constitutively localized at the cell membrane (Fig. 3). Overlay of anti-HA and TRITC staining in resting cells showed constitutive colocalization of Palm-PLCγ1-HA with CT-B, which was insensitive to TCR stimulation. WT-PLCγ1-HA was inducibly colocalized with CT-B on TCR stimulation. These data demonstrate that Palm-PLCγ1-HA is constitutively present in lipid rafts of resting Jurkat cells.
FIG. 3.
Confocal analysis of the subcellular distribution of wild-type and acylated PLCγ1 in Jurkat cells. Transiently transfected Jurkat TAg cells were activated with an anti-TCR (C305) or left unstimulated as indicated. Cells were stained with CT-B and aggregated with anti-CT-B antibody to visualize lipid rafts (left panels, red). Transfected PLCγ1 was stained with anti-HA (middle panels, green). Areas of colocalization are shown in yellow in overlay images (right panels).
Raft-targeted PLCγ1 is constitutively tyrosine phosphorylated and induces Ca2+-dependent transcriptional activation.
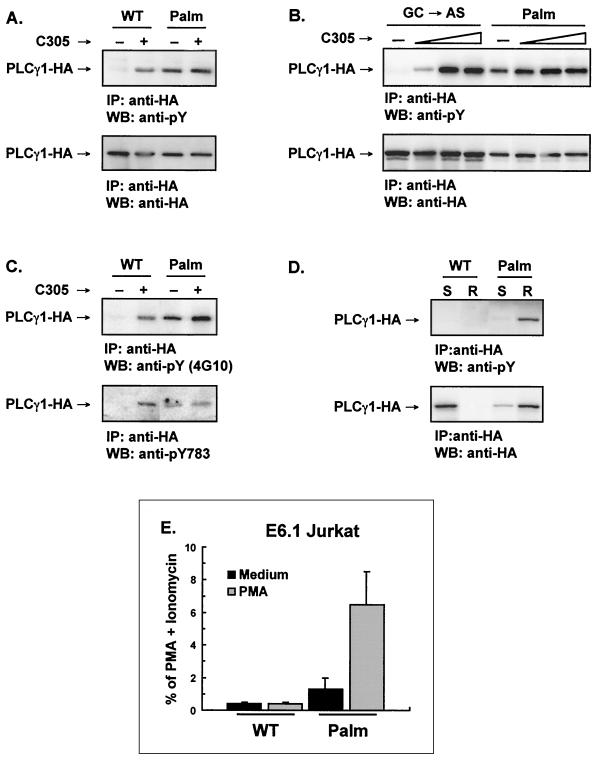
The ability to constitutively localize Palm-PLCγ1-HA to the rafts allowed us to test whether raft recruitment is sufficient for PLCγ1 activation and the transmission of signals downstream of PLCγ1. Since phosphorylation of tyrosine residues controls PLCγ1 enzymatic activation and tyrosine-phosphorylated PLCγ1 is abundant in lipid rafts (42, 48), we first questioned whether forcing PLCγ1 to the rafts affected its phosphorylation status. To test this possibility, WT-PLCγ1-HA and Palm-PLCγ-HA were transfected in Jurkat T cells and immunoprecipitated from lysates with anti-HA and probed by immunoblotting with antiphosphotyrosine antibody. While WT-PLCγ1-HA was tyrosine phosphorylated only on TCR triggering, Palm-PLCγ1-HA was constitutively phosphorylated to an extent similar to that observed for TCR-stimulated WT-PLCγ1-HA (Fig. 4A). This constitutive phosphorylation could be augmented by TCR engagement only marginally (Fig. 4B). Tyr783, a residue critical for PLCγ1 activation (14), was among the tyrosine residues constitutively phosphorylated in Palm-PLCγ1-HA (Fig. 4C). The relationship between subcellular localization and the phosphorylation status of wild-type and acylated PLCγ1 in resting cells is shown in Fig. 4D. Fractions from sucrose gradient floatation corresponding to detergent-soluble and detergent-resistant portions were pooled and probed by immunoblotting with anti-HA and antiphosphotyrosine. WT-PLCγ1-HA was exclusively present in the soluble fraction and was not phosphorylated, while Palm-PLCγ1-HA was mostly compartmentalized to the lipid rafts and constitutively phosphorylated. The small amount of Palm-PLCγ1-HA detected in the soluble fraction is most probably due to contamination during sucrose gradient flotation of the raft fractions.
FIG. 4.
Raft-targeted PLCγ1 is constitutively tyrosine phosphorylated and induces NF-AT activation in Jurkat cells. (A) Transfected Jurkat E6.1 cells were activated with an anti-TCR antibody (C305) as indicated. Lysates were immunoprecipitated (IP) with anti-HA, resolved by SDS-PAGE, immunoblotted (WB) with an antiphosphotyrosine 4G10 (anti-pY, upper panel), stripped, and reprobed with anti-HA (lower panel). (B) Jurkat E6.1 cells were transiently transfected with the control GC→AS PLCγ1-HA or Palm-PLCγ1-HA and stimulated with increasing amounts of anti-TCR antibody (C305). Lysates were immunoprecipitated with anti-HA, resolved by SDS-PAGE, immunoblotted with 4G10 (anti-pY, upper panel), stripped, and reprobed with anti-HA (lower panel). (C) Lysates from transfected E6.1 Jurkat cells were immunoprecipitated with anti-HA, resolved by SDS-PAGE, and immunoblotted with 4G10 (anti-pY, upper panel) or a phospho-specific anti-PLCγ1[pY783] (lower panel). (D) The detergent-soluble (S) and raft (R) fractions were separated by discontinuous sucrose gradient, pooled, immunoprecipitated with anti-HA, resolved by SDS-PAGE, immunoblotted with 4G10 (anti-pY, upper panel), stripped, and reprobed with anti-HA (lower panel). (E) Jurkat cells were transiently transfected with the indicated constructs along with an NF-AT luciferase reporter and an adenovirus major late promoter β-galactosidase-encoding vector to control for transfection efficiency. Transfected cells were treated with medium alone, a phorbol ester (PMA), or PMA plus ionomycin. Data were normalized for β-galactosidase activity and expressed as the percentage of maximum activation with PMA plus ionomycin. The data shown are the means and standard errors of the mean of four separate experiments.
The activation of events downstream of PLCγ1 in Jurkat cells expressing Palm-PLCγ1-HA was investigated by measuring the induction of gene transcription by the nuclear factor of activated T cells (NF-AT), an IL-2 transcriptional activator (4). NF-AT is translocated to the nucleus via a Ca2+-sensitive, calcineurin-dependent dephosphorylation mechanism, where, in concert with AP-1, it binds to the triplicate NF-AT binding sites of the reporter. Expression of Palm-PLCγ1-HA resulted in a low-level constitutive NF-AT transcriptional activation, which was further increased by the concomitant treatment with a phorbol ester (Fig. 4E). Phorbol esters activate Ras and induce optimal levels of the AP-1 component of NF-AT-dependent transcription (4). NF-AT induction was negligible in cells expressing WT-PLCγ1-HA irrespective of phorbol ester addition, indicating that the Ca2+-dependent component of NF-AT activation was absent in these cells. These data indicate that raft-targeted PLCγ1 is constitutively active and can induce the Ca2+-sensitive component of NF-AT-dependent transcription.
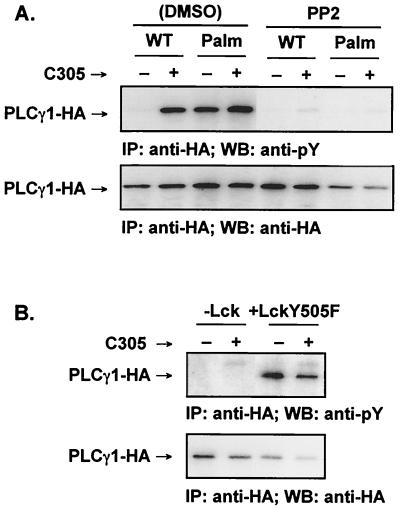
Tyrosine-phosphorylation of raft-targeted PLCγ1 requires Lck.
Since Lck is necessary for Ca2+ mobilization (34), we investigated the role of Src kinases in the phosphorylation of raft-targeted PLCγ1. Treatment with the Src kinase inhibitor 4 - amino - 5 - (4 - chlorophenyl) - 7 - (t - butyl)pyrazolo - [3,4 - d]pyrimidine (PP2) (9) abolished Palm-PLCγ1-HA tyrosine phosphorylation in both unstimulated and TCR-stimulated Jurkat cells (Fig. 5A). Furthermore, transient expression of Palm-PLCγ1-HA in JCaM1.6, a Jurkat line deficient in functional Lck (34), did not result in constitutive tyrosine phosphorylation, and phosphorylation was not detectable following TCR engagement (Fig. 5B). Coexpression of Lck in JCaM1.6 cells led to the reconstitution of Palm-PLCγ1-HA phosphorylation. Therefore, Lck is required for the phosphorylation of raft-targeted PLCγ1.
FIG. 5.
Tyrosine phosphorylation of raft-targeted PLCγ1 in Jurkat cells requires Lck. (A) Transiently transfected Jurkat E6.1 cells were cultured in medium containing vehicle alone (0.2% dimethyl sulfoxide [DMSO]) or 20 μM PP2. The cells were activated with anti-TCR antibody (C305) as indicated. Lysates were immunoprecipitated (IP) with anti-HA, resolved by SDS-PAGE, immunoblotted (WB) with 4G10 (anti-pY, upper panel), stripped and reprobed with anti-HA (lower panel). (B) The Lck-defective Jurkat cell line JCam1.6 was transfected with Palm-PLCγ1-HA and cotransfected with an empty vector or pSX-LckY505F. Lysates were immunoprecipitated with anti-HA, resolved by SDS-PAGE, immunoblotted with 4G10 (anti-pY, upper panel), stripped, and reprobed with anti-HA (lower panel).
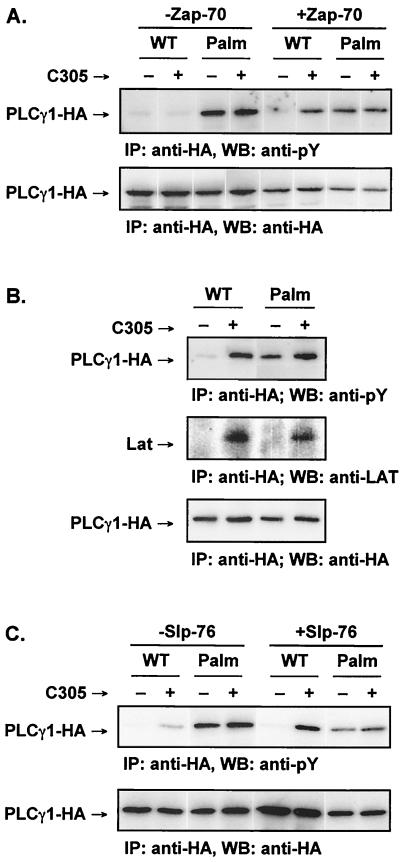
Tyrosine-phosphorylation of raft-targeted PLCγ1 does not require Zap-70 or interaction with the adapters Lat and Slp-76.
Since Lck phosphorylates and activates Zap-70 (3, 13), whose expression is required for PLCγ1 activation (40), we questioned whether Zap-70 played a role in the phosphorylation of raft-targeted PLCγ1. WT-PLCγ1-HA or Palm-PLCγ1-HA was expressed in the Zap-70-deficient Jurkat cell somatic mutant P116 (40). P116 cells display no detectable tyrosine phosphorylation of WT-PLCγ1-HA in either unstimulated or stimulated cells, while the phosphorylation defect was fully reversed in P116 cells stably transfected with Zap-70 (Fig. 6A). Palm-PLCγ1-HA, however, was constitutively tyrosine phosphorylated in the Zap-70-deficient P116 cells, demonstrating that phosphorylation of raft-targeted PLCγ1 is independent of Zap-70.
FIG. 6.
Tyrosine phosphorylation of raft-targeted PLCγ1 in Jurkat cells is independent of Zap-70, Lat, and Slp-76. (A) The Zap-70-negative Jurkat line P116 and a stable Zap-70-reconstituted line were transiently transfected and activated as indicated. Lysates were immunoprecipitated (IP) with anti-HA, resolved by SDS-PAGE, immunoblotted (WB) with 4G10 (anti-pY, upper panel), stripped, and reprobed with anti-HA (lower panel). (B) Jurkat E6.1 cells were transfected and activated as indicated. Lysates were immunoprecipitated with anti-HA, resolved by SDS-PAGE, and separately immunoblotted with 4G10 (anti-pY) (top panel), with anti-Lat (middle panel), or with anti-HA (bottom panel). Calf intestinal phosphatase was used to remove phosphate groups from the anti-HA immunoprecipitates probed with anti-Lat to enhance blotting. (C) The Slp-76-deficient Jurkat clone J14 and a stable Slp-76-reconstituted clone (J14-76-11) were transiently transfected and activated as indicated. Lysates were immunoprecipitated with anti-HA, resolved by SDS-PAGE, immunoblotted with 4G10 (anti-pY) (upper panel), stripped, and reprobed with anti-HA (lower panel).
Zap-70 phosphorylation of Lat is an essential step linking the TCR to PLCγ1 tyrosine phosphorylation and Ca2+ mobilization (8, 47). We next investigated whether phosphorylated raft-targeted PLCγ1 associated with Lat. Jurkat cells were transfected with WT-PLCγ1-HA or Palm-PLCγ1-HA followed by detection of Lat in anti-HA precipitates from either resting or TCR-activated cells. As expected, both WT-PLCγ1-HA and Palm-PLCγ1-HA bound Lat upon TCR triggering (Fig. 6B). No detectable Lat, however, coprecipitated with Palm-PLCγ1-HA from resting Jurkat cells, despite constitutive phosphorylation of the raft-targeted PLCγ1. Furthermore, no tyrosine-phosphorylated band in the 36 to 38-kDa range corresponding to phospho-Lat was observed in antiphosphotyrosine blots of anti-HA precipitates from resting cells (data not shown). Therefore, tyrosine phosphorylation of raft-targeted PLCγ1 ensues in the absence of detectable Lat interaction.
Another Zap-70 substrate, Slp-76, is necessary for TCR-induced PLCγ1 phosphorylation (38, 43). To explore the role played by this adapter in the constitutive tyrosine phosphorylation of raft-targeted PLCγ1, we expressed WT-PLCγ1-HA or Palm-PLCγ1-HA in the Slp-76-deficient Jurkat somatic mutant J14 (43). TCR-induced phosphorylation of WT-PLCγ1-HA was only minimal in this cell line but was fully restored in a J14 cell line stably reconstituted with Slp-76 (Fig. 6C). Palm-PLCγ1-HA, however, was constitutively phosphorylated in J14 cells, consistent with the lack of a Zap-70 requirement in the phosphorylation of raft-targeted PLCγ1. Therefore, forced PLCγ1 compartmentalization to the lipid rafts leads to its constitutive tyrosine phosphorylation without requiring Zap-70 and Zap-70-dependent events. These data further suggest that a tyrosine kinase that phosphorylates PLCγ1 is present within the raft microdomains and can interact with raft-targeted PLCγ1 independently of Lat and Slp-76.
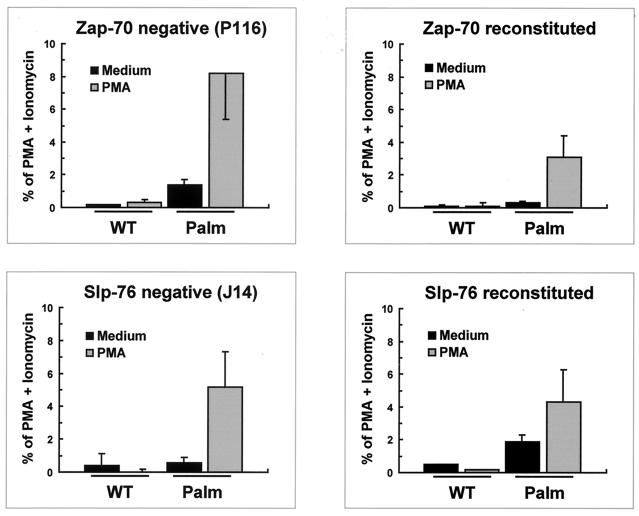
Expression of Palm-PLCγ1-HA in P116 Jurkat cells (Zap-70-deficient cells) or J14 Jurkat cells (Slp-76-deficient cells) resulted in constitutive and PMA-enhanced NF-AT activation (Fig. 7), similar to what was observed in the corresponding reconstituted cell lines or wild-type Jurkat cells (Figure 4E). Slp-76-reconstituted cells and, to a lesser extent, Zap-70-reconstituted cells consistently showed diminished Palm-PLCγ1-HA-induced NF-AT activation compared to the corresponding deficient lines. The reason for this phenomenon is unclear and might reflect clonal variation in response sensitivity. Nonetheless, these data indicate that raft-targeted PLCγ1 is constitutively active and can induce the Ca2+-sensitive component of NF-AT-dependent transcription, bypassing the requirement for Zap-70 and the adapter Slp-76.
FIG. 7.
NF-AT activation by raft-targeted PLCγ1 does not require Zap-70 or Slp-76. The Zap-70-negative Jurkat line P116, a Zap-70-reconstituted P116 cell-derived line, the Slp-76-deficient Jurkat clone J14, and a Slp-76-reconstituted J14 cell clone (J14-76-11) were transiently transfected with the indicated PLCγ1 constructs along with a NF-AT luciferase reporter and an adenovirus major late promoter β-galactosidase-encoding vector to control for transfection efficiency. Transfected cells were treated with medium alone, PMA, or PMA plus ionomycin. Data were normalized for β-galactosidase activity and expressed as percentage of maximum activation with PMA plus ionomycin. The data shown are the means and standard errors of the mean of three separate experiments.
Reconstitution of PTEN in Jurkat T cells does not affect the constitutive tyrosine phosphorylation of Palm-PLCγ1-HA or decrease NF-AT induction or IL-2 production in cells expressing raft-targeted PLCγ1.
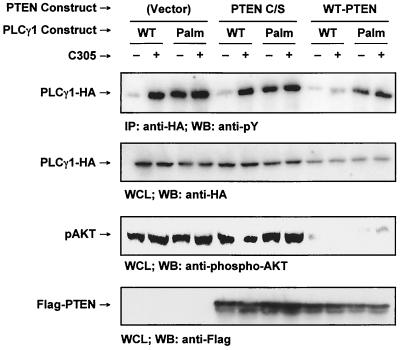
Membrane localization of certain members of the Tec kinase family is mediated by the high-affinity interaction of the pleckstrin homology domain of these kinases with specific D3 phosphoinositides, principally phosphatidylinositol (3, 4, 5)-trisphosphate (PIP3) (17, 27, 28). Localization of Tec kinases at the plasma membrane promotes their activation (16). PIP3 levels are regulated by the D3 phosphoinositide phosphatase PTEN, whose expression is deficient in Jurkat T cells (29). The resulting high basal levels of D3 phosphoinositides in these cells lead to the constitutive membrane localization of the Tec kinase Itk and contribute to the hyperresponsiveness of Jurkat cells to exogenous stimuli (29, 30). To investigate whether the lack of PTEN plays a role in the phosphorylation of raft-targeted PLCγ1 in Jurkat cells, we have transiently reconstituted these cells with WT-PTEN or an inactive form of PTEN (PTEN C/S) along with WT-PLCγ1-HA or Palm-PLCγ1-HA (Fig. 8). Nearly equivalent levels of PTEN expression were observed in cells transfected with the PTEN constructs. Furthermore, reconstitution of PTEN activity by the wild-type protein was confirmed by the reduced levels of phosphorylation of the ubiquitous serine/threonine kinase Akt (protein kinase B) on Ser473, a residue whose phosphorylation is critically dependent on the presence of D3 phosphoinositides. The levels of tyrosine phosphorylation of WT-PLCγ1-HA were greatly reduced on TCR stimulation, confirming the critical role played by D3-phosphoinositides in the activation of cytoplasmic PLCγ1, possibly by regulating its interaction with the plasma membrane. Interestingly, PTEN reconstitution did not affect the tyrosine phosphorylation of Palm-PLCγ1-HA. Therefore, PTEN expression levels do not contribute to the constitutive phosphorylation of raft-targeted PLCγ1.
FIG. 8.
PTEN expression does not affect tyrosine phosphorylation of raft-targeted PLCγ1 in Jurkat T cells. Jurkat TAg cells were transiently transfected with 20 μg of empty vector, Flag-PTEN C/S, or Flag-WT-PTEN along with 20 μg of WT-PLCγ1-HA or Palm-PLCγ1-HA. The cells were treated as indicated. Cell lysates were immunoprecipitated (IP) with anti-HA, resolved by SDS-PAGE, and immunoblotted (WB) with 4G10 (anti-pY) (top panel). Whole-cell lysates (WCL) were immunoblotted with anti-HA (second panel from the top), an anti-phospho-Akt (pAKT, Ser473) (second panel from the bottom), or anti-Flag (bottom panel).
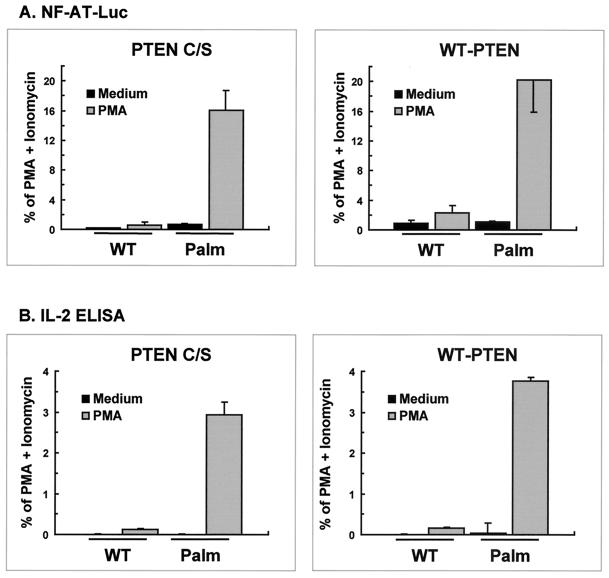
To ascertain whether PTEN reconstitution affected the transcriptional activation induced by raft-targeted PLCγ1, Jurkat cells were transfected with WT-PLCγ1-HA or Palm-PLCγ1-HA along with PTEN C/S or WT-PTEN and were assayed for NF-AT induction or IL-2 production. Consistent with the lack of effect on PLCγ1 tyrosine phosphorylation, PMA-induced NF-AT transcriptional activation in cells expressing Palm-PLCγ1-HA was unaffected by reconstitution with PTEN (Fig. 9A). Furthermore, cells expressing raft-targeted PLCγ1 secreted substantial levels of IL-2 when treated with PMA, in contrast to undetectable levels from cells expressing WT PLCγ1 (Fig. 9B). IL-2 production in cells expressing Palm-PLCγ1-HA was also unaffected by PTEN reconstitution. In all experiments, parallel samples were processed for PLCγ1-HA expression and phospho-Akt levels by Western blot analysis that confirmed that WT-PTEN reconstitution effectively reduced or abolished the constitutive levels of phosphorylation of Akt in Jurkat cells (data not shown). These data indicate that raft-targeted PLCγ1 is constitutively active and can induce the Ca2+-sensitive component of NF-AT-dependent transcription leading to IL-2 secretion.
FIG. 9.
PTEN reconstitution does not affect NF-AT activation or IL-2 secretion in Jurkat T cells expressing raft-targeted PLCγ1. (A). Jurkat TAg cells were transiently transfected with 20 μg of Flag-PTEN C/S or Flag-WT-PTEN along with 5 μg of WT-PLCγ1-HA or Palm-PLCγ1-HA, a NF-AT luciferase reporter, and an adenovirus major late promoter β-galactosidase-encoding vector as described in Materials and Methods. Transfected cells were treated with medium alone, PMA, or PMA plus ionomycin. Parallel samples were processed for PLCγ1-HA expression by immunoblot analysis. Data were normalized for PLCγ1-HA expression levels and presented as percentage of maximum activation with PMA plus ionomycin. The data shown are the means and standard error of the mean of four separate experiments. (B) Jurkat TAg cells were transiently transfected with Flag-PTEN C/S or Flag-WT-PTEN along with WT-PLCγ1-HA or Palm-PLCγ1-HA. Transfected cells were treated with medium alone, PMA, or PMA plus ionomycin. Parallel samples were processed for PLCγ1-HA expression by immunoblot analysis. Data were normalized for PLCγ1-HA expression and are presented as a percentage of maximum activation with PMA plus ionomycin. IL-2 secretion by cells treated with PMA plus ionomycin ranged from 2,400 to 3,900 U per 5 × 104 cells. ELISA, enzyme-linked immunosorbent assay.
Raft-targeted PLCγ1 is preferentially phosphorylated by Rlk or Lck in HEK 293T cells.
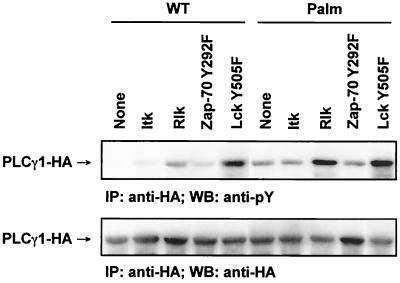
In addition to Lck and Zap-70, the Tec family kinases Itk and Rlk are involved in PLCγ1 activation. T lymphocytes from Itk−/−/Rlk−/− mice display a decrease in early PLCγ1 phosphorylation together with impaired Ca2+ mobilization, indicating that Tec kinases regulate TCR-induced PLCγ1 activation (26). To investigate the ability of these kinases to phosphorylate wild-type or raft-targeted PLCγ1, we transfected HEK 293T cells with WT-PLCγ1-HA or Palm-PLCγ1-HA along with Itk, Rlk, or active forms of Lck or Zap-70. HEK 293T cells do not express any of these proteins, allowing reconstitution of their activity in the absence of interference by endogenous kinases.
Ectopic expression of the different kinases in HEK 293T cells was confirmed by immunoblotting (data not shown). Phosphorylation of WT-PLCγ1-HA or Palm-PLCγ1-HA was minimal to negligible when coexpressed in HEK 293T cells with Itk or a partially active form of Zap-70 (Zap-70 Y492F) (Fig. 10). Coexpression of PLCγ1 with Rlk or an active form of Lck (Lck Y505F) resulted in increased tyrosine phosphorylation of PLCγ1. Similar results were obtained with wild-type Lck (data not shown). Notably, Palm-PLCγ1-HA was preferentially phosphorylated compared to WT-PLCγ1-HA when cotransfected with Rlk or Lck Y505F, although cells expressing Lck Y505F displayed a less pronounced preferential effect between raft-targeted and wild-type PLCγ1 than did those expressing Rlk. Palm-PLCγ1-HA associated with lipid rafts in HEK 293T cells (data not shown), suggesting that raft compartmentalization favored PLCγ1 interaction with Lck or Rlk.
FIG. 10.
Preferential phosphorylation of raft-targeted PLCγ1 by Rlk and Lck in HEK 293T cells. HEK 293T cells were transiently transfected with 1 μg of PLCγ1-HA and 0.3 μg of the indicated kinase plasmids. Lysates were immunoprecipitated (IP) with anti-HA, resolved by SDS-PAGE, immunoblotted (WB) with 4G10 (anti-pY) (upper panel), stripped, and reprobed with anti-HA (lower panel).
DISCUSSION
Our data show that compartmentalization of PLCγ1 to the lipid rafts is sufficient to trigger constitutive tyrosine phosphorylation and activation of this molecule. Phosphorylation of raft-targeted PLCγ1 required Lck but was independent of Zap-70, Slp-76, or the interaction with Lat.
Effectors and regulatory molecules appear to be segregated in resting cells based in part on their “solubility” in lipid rafts. Receptor-induced recompartmentalization to these membrane microdomains may promote their interaction by increasing their local concentration or via the formation of localized scaffolds with molecules constitutively present in this compartment. In the experiments reported here, we focused on TCR-induced translocation of PLCγ1 to the lipid rafts as a regulatory mechanism controlling its phosphorylation and activation.
Under physiologic conditions of TCR signaling, tyrosine-phosphorylated Lat serves as a raft-associated scaffold that recruits PLCγ1 via direct binding to its amino-terminal SH2 domain (33, 40, 47). We therefore anticipated that a raft-targeted PLCγ1 could bypass the need for Lat interaction. Accordingly, neither Lat nor Zap-70, the kinase responsible for Lat phosphorylation (47), was required to phosphorylate raft-targeted PLCγ1. Phosphorylated Lat recruits Slp-76 to the lipid rafts via the intercession of another adapter, Gads (21, 49). It has been proposed that Slp-76 nucleates a molecular complex with PLCγ1 and Itk (35). While an association between Slp-76 and Itk has been observed in vivo (35), the interaction between PLCγ1 and phosphorylated Slp-76 has been inferred from SH2 fusion protein studies (10) and appears to be redundant, given the direct interaction between Lat and PLCγ1. Recently, however, a raft-targeted Slp-76 has been shown to substitute for Lat in PLCγ1 activation (1), suggesting that a direct Slp-76/PLCγ1 interaction can take place. Cells deficient in Slp-76 recruit PLCγ1 to Lat, but no PLCγ1 tyrosine phosphorylation and activation is observed in these cells. This contrasts with our observation of no requirement for Slp-76 for tyrosine phosphorylation and activation of raft-targeted PLCγ1. Lat interaction, however, may not directly correlate with raft compartmentalization, since not all Lat is compartmentalized to the lipid rafts. Indeed, even phosphorylated Lat can be found outside of the raft compartment (48). A plausible explanation in light of our data is that Lat functions by initially recruiting PLCγ1 with Slp-76 stabilizes the compartmentalization of PLCγ1 to the lipid rafts. This is consistent with other data showing that inactivation of the Gads binding sites in Lat (and thereby the Slp-76 recruitment sites) blocks stable association of PLCγ1 with Lat and PLCγ1 activation (49). Furthermore, Gads-deficient thymocytes exhibit significantly reduced PLCγ1 phosphorylation and Gads-deficient T cells fail to flux Ca2+ on CD3 activation (45). Experiments are in progress to determine if Slp-76 and Lat cooperate in TCR-induced PLCγ1 compartmentalization to the lipid rafts.
Zhang et al. (49) have also observed TCR-induced transient Ca2+ mobilization in the absence of PLCγ1 binding to Lat, possibly by a PLCγ1-independent mechanism. PLCγ1 binding to Tyr132 of Lat, however, was still required for PLCγ1 phosphorylation, prolonged Ca2+ flux, and NF-AT activation (49). By stably targeting PLCγ1 to the rafts, we are more likely to recapitulate those events that lead to the long-term activation of PLCγ1. Whether prolonged PLCγ1 activation is due to phosphorylation of specific sites (possibly by specific kinases) or correlates with its residence time in the raft compartment remains to be established. In either case, our data suggest that a stable association of PLCγ1 with the lipid rafts results in its persistent phosphorylation and activation, with ensuing Ca2+ flux, NF-AT activation, and IL-2 secretion, by promoting approximation of PLCγ1 with critical kinase(s) or by affecting its dwelling time.
Phosphorylation of PLCγ1 in TCR signal transduction involves multiple kinases. While several pieces of evidence indicate that Lck, Zap-70, and the Tec kinases are all required, the role of individual players is less well defined. In particular, whether each kinase directly phosphorylates PLCγ1 has not been established. We observed no PLCγ1 phosphorylation when Palm-PLCγ1-HA was coexpressed with Zap-70 Y492F in HEK 293T cells. Together with the lack of a Zap-70 requirement in the phosphorylation of raft-targeted PLCγ1 in Jurkat cells, these data bring into question a direct intervention of Zap-70 in PLCγ1 phosphorylation. These data are consistent with the lack of TCR-induced PLCγ1 phosphorylation in cells defective for Slp-76, despite normal levels of Zap-70 activation, as indicated by the phosphorylation of endogenous Lat (43). A role for Slp-76 in facilitating Zap-70 interaction with PLCγ1 cannot be ruled out, albeit no stable interaction of this adapter with Zap-70 has been reported.
Lck and Rlk play a role in the phosphorylation of raft-associated PLCγ1, as shown by the Lck requirement for the phosphorylation of Palm-PLCγ1-HA in Jurkat cells and the preferential phosphorylation of raft-targeted PLCγ1 by either kinase in reconstituted HEK 293T cells. Both kinases are acylated and associate with lipid rafts or membranous vesicles (6, 27). We speculate that raft compartmentalization promotes the proximity of PLCγ1 to these kinases. Lck could function by both trans-activation of Rlk (27) and direct phosphorylation of raft-associated PLCγ1. This latter possibility is consistent with the phosphorylation of PLCγ1 in reconstituted HEK 293T cells and the observation that viral Src is capable of phosphorylating PLCγ1 (23). These data support the notion that Lck plays important signaling roles beyond the initial ITAM phosphorylation and the activation of Zap-70 (7, 44).
In contrast to Rlk, Itk failed to phosphorylate either wild-type or raft target PLCγ1 in reconstituted HEK 293T cells. While Rlk is constitutively acylated, Itk is recruited to the lipid rafts via PIP3-dependent anchoring (27). The absence of significant phosphorylation of Palm-PLCγ1-HA in HEK 293T cells coexpressing Itk may therefore be due to the absence of PIP3. In Jurkat cells, however, a PTEN defect maintains high basal levels of PIP3, favoring the constitutive enrichment of Itk, which is normally present in the cytosol, in the lipid rafts (29). Reintroduction of PTEN phosphatase into Jurkat cells restores the normal Itk distribution pattern but does not affect the constitutive tyrosine phosphorylation of raft-targeted PLCγ1. Furthermore, PTEN reconstitution does not affect PMA-enhanced NF-AT induction or IL-2 production in cells expressing raft-targeted PLCγ1. These data suggest that Itk, even when constitutively associated with the plasma membrane, plays only a minor role in the phosphorylation and activation of raft-targeted PLCγ1 in Jurkat cells, although they do not exclude a role for Itk in the phosphorylation of PLCγ1 in response to TCR activation. Furthermore, these data, taken together with the observation that the decrease in PLCγ1 phosphorylation seen in Itk-deficient lymphocytes is only partial (18, 20, 26), support the notion that kinases other than Itk contributes to PLCγ1 phosphorylation in T lymphocytes. Our study supports a model whereby translocation to the lipid rafts is rate limiting for PLCγ1 activation by promoting its interaction with regulatory kinases present in these microdomains. Lck and Rlk are two potential candidates that may preferentially phosphorylate raft-compartmentalized PLCγ1, either directly by playing a hierarchical role or indirectly by activating other downstream kinases. Thus, lipid rafts play a central role in TCR signal transduction by segregating signaling molecules in resting cells and by providing a compartment for their association and activation upon receptor engagement.
ACKNOWLEDGMENTS
We thank E. W. Shores and S. Kozlowski for discussion, comments, and review of the manuscript.
This research was supported in part by the appointment of R.R. and C.N. to the Research Participation Program at the Center for Biologies Evaluation and Research administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration.
REFERENCES
- 1.Boerth N J, Sadler J J, Bauer D E, Clements J L, Gheith S M, Koretzky G A. Recruitment of SLP-76 to the membrane and glycolipid-enriched membrane microdomains replaces the requirement for linker for activation of T cells in T cell receptor signaling. J Exp Med. 2000;192:1047–1058. doi: 10.1084/jem.192.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown D A, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 3.Chan A C, Dalton M, Johnson R, Kong G H, Wang T, Thoma R, Kurosaki T. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 1995;14:2499–2508. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crabtree G R, Clipstone N A. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu Rev Biochem. 1994;63:1045–1083. doi: 10.1146/annurev.bi.63.070194.005145. [DOI] [PubMed] [Google Scholar]
- 5.DeBell K E, Stoica B A, Veri M C, Di Baldassarre A, Miscia S, Graham L J, Rellahan B L, Ishiai M, Kurosaki T, Bonvini E. Functional independence and interdependence of the src homology domains of phospholipase C-gamma1 in B-cell receptor signal transduction. Mol Cell Biol. 1999;19:7388–7398. doi: 10.1128/mcb.19.11.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debnath J, Chamorro M, Czar M J, Schaeffer E M, Lenardo M J, Varmus H E, Schwartzberg P L. rlk/TXK encodes two forms of a novel cysteine string tyrosine kinase activated by Src family kinases. Mol Cell Biol. 1999;19:1498–1507. doi: 10.1128/mcb.19.2.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denny M F, Kaufman H C, Chan A C, Straus D B. The lck SH3 domain is required for activation of the mitogen-activated protein kinase pathway but not the initiation of T-cell antigen receptor signaling. J Biol Chem. 1999;274:5146–5152. doi: 10.1074/jbc.274.8.5146. [DOI] [PubMed] [Google Scholar]
- 8.Finco T S, Kadlecek T, Zhang W, Samelson L E, Weiss A. LAT is required for TCR-mediated activation of PLCgammal and the Ras pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 9.Hanke J H, Gardner J P, Dow R L, Changelian P S, Brissette W H, Weringer E J, Pollok B A, Connelly P A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 10.Jackman J K, Motto D G, Sun Q, Tanemoto M, Turck C W, Peltz G A, Koretzky G A, Findell P R. Molecular cloning of SLP-76, a 76-kDa tyrosine phosphoprotein associated with Grb2 in T cells. J Biol Chem. 1995;270:7029–7032. doi: 10.1074/jbc.270.13.7029. [DOI] [PubMed] [Google Scholar]
- 11.Janes P W, Ley S C, Magee A I, Kabouridis P S. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin Immunol. 2000;12:23–34. doi: 10.1006/smim.2000.0204. [DOI] [PubMed] [Google Scholar]
- 12.Kabouridis P S, Magee A I, Ley S C. S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. EMBO J. 1997;16:4983–4998. doi: 10.1093/emboj/16.16.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kane L P, Lin J, Weiss A. Signal transduction by the TCR for antigen. Curr Opin Immunol. 2000;12:242–249. doi: 10.1016/s0952-7915(00)00083-2. [DOI] [PubMed] [Google Scholar]
- 14.Kim H K, Kim J W, Zilberstein A, Margolis B, Kim J G, Schlessinger J, Rhee S G. PDGF stimulation of inositol phospholipid hydrolysis requires PLC-gamma 1 phosphorylation on tyrosine residues 783 and 1254. Cell. 1991;65:435–441. doi: 10.1016/0092-8674(91)90461-7. [DOI] [PubMed] [Google Scholar]
- 15.Kong G, Dalton M, Wardenburg J B, Straus D, Kurosaki T, Chan A C. Distinct tyrosine phosphorylation sites in ZAP-70 mediate activation and negative regulation of antigen receptor function. Mol Cell Biol. 1996;16:5026–5035. doi: 10.1128/mcb.16.9.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li T, Rawlings D J, Park H, Kato R M, Witte O N, Satterthwaite A B. Constitutive membrane association potentiates activation of Bruton tyrosine kinase. Oncogene. 1997;15:1375–1383. doi: 10.1038/sj.onc.1201308. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, Wahl M I, Eguinoa A, Stephens L R, Hawkins P T, Witte O N. Phosphatidylinositol 3-kinase-gamma activates Bruton's tyrosine kinase in concert with Src family kinases. Proc Natl Acad Sci USA. 1997;94:13820–13825. doi: 10.1073/pnas.94.25.13820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao X C, Littman D R. Altered T cell receptor singaling and disrupted T cell development in mice lacking Itk. Immunity. 1995;3:757–769. doi: 10.1016/1074-7613(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 19.Lin J, Weiss A, Finco T S. Localization of LAT in glycolipid-enriched microdomains is required for T cell activation. J Biol Chem. 1999;274:28861–28864. doi: 10.1074/jbc.274.41.28861. [DOI] [PubMed] [Google Scholar]
- 20.Liu K Q, Bunnell S C, Gurniak C B, Berg L J. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J Exp Med. 1998;187:1721–1727. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S K, Fang N, Koretzky G A, McGlade C J. The hematopoietic-specific adaptor protein gads functions in T-cell signaling via interactions with the SLP-76 and LAT adaptors. Curr Biol. 1999;9:67–75. doi: 10.1016/s0960-9822(99)80017-7. [DOI] [PubMed] [Google Scholar]
- 22.Montixi C, Langlet C, Bernard A M, Thimonier J, Dubois C, Wurbel M A, Chauvin J P, Pierres M, He H T. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 1998;17:5334–5348. doi: 10.1093/emboj/17.18.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakanishi O, Shibasaki F, Hidaka M, Homma Y, Takenawa T. Phospholipase C-gamma 1 associates with viral and cellular src kinases. J Biol Chem. 1993;268:10754–10759. [PubMed] [Google Scholar]
- 24.Orlandi P A, Fishman P H. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol. 1998;141:905–915. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhee S G, Bae Y S. Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem. 1997;272:15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- 26.Schaeffer E M, Debnath J, Yap G, McVicar D, Liao X C, Littman D R, Sher A, Varmus H E, Lenardo M J, Schwartzberg P L. Requirement for Tec kinases R1k and Itk in T cell receptor signaling and immunity. Science. 1999;284:638–641. doi: 10.1126/science.284.5414.638. [DOI] [PubMed] [Google Scholar]
- 27.Schaeffer E M, Schwartzberg P L. Tec family kinases in lymphocyte signaling and function. Curr Opin Immunol. 2000;12:282–288. doi: 10.1016/s0952-7915(00)00088-1. [DOI] [PubMed] [Google Scholar]
- 28.Scharenberg A M, El-Hillal O, Fruman D A, Beitz L O, Li Z, Lin S, Gout I, Cantley L C, Rawlings D J, Kinet J P. Phosphatidylinositol-3,4,5-trisphosphate (PtdIns-3,4,5-P3)/Tec kinase-dependent calcium signaling pathway: a target for SHIP-mediated inhibitory signals. EMBO J. 1998;17:1961–1972. doi: 10.1093/emboj/17.7.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shan X, Czar M J, Bunnell S C, Liu P, Liu Y, Schwartzberg P L, Wange R L. Deficiency of PTEN in Jurkat T cells causes constitutive localization of Itk to the plasma membrane and hyperresponsiveness to CD3 stimulation. Mol Cell Biol. 2000;20:6945–6957. doi: 10.1128/mcb.20.18.6945-6957.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shan X, Wange R L. Itk/Emt/Tsk activation in response to CD3 cross-linking in Jurkat T cells requires ZAP-70 and Lat and is independent of membrane recruitment. J Biol Chem. 1999;274:29323–29330. doi: 10.1074/jbc.274.41.29323. [DOI] [PubMed] [Google Scholar]
- 31.Shenoy-Scaria A M, Dietzen D J, Kwong J, Link D C, Lublin D M. Cysteine3 of Src family protein tyrosine kinase determines palmitoylation and localization in caveolae. J Cell Biol. 1994;126:353–363. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 33.Stoica B, DeBell K E, Graham L, Rellahan B L, Alava M A, Laborda J, Bonvini E. The amino-terminal Src homology 2 domain of phospholipase C gamma 1 is essential for TCR-induced tyrosine phosphorylation of phospholipase C gamma 1. J Immunol. 1998;160:1059–1066. [PubMed] [Google Scholar]
- 34.Straus D B, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 35.Su Y W, Zhang Y, Schweikert J, Koretzky G A, Reth M, Wienands J. Interaction of SLP adaptors with the SH2 domain of Tec family kinases. Eur J Immunol. 1999;29:3702–3711. doi: 10.1002/(SICI)1521-4141(199911)29:11<3702::AID-IMMU3702>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 36.Towler D A, Gordon J I, Adams S P, Glaser L. The biology and enzymology of eukaryotic protein acylation. Annu Rev Biochem. 1988;57:69–99. doi: 10.1146/annurev.bi.57.070188.000441. [DOI] [PubMed] [Google Scholar]
- 37.Wange R L, Guitian R, Isakov N, Watts J D, Aebersold R, Samelson L E. Activating and inhibitory mutations in adjacent tyrosines in the kinase domain of ZAP-70. J Biol Chem. 1995;270:18730–18733. doi: 10.1074/jbc.270.32.18730. [DOI] [PubMed] [Google Scholar]
- 38.Wardenburg J B, Fu C, Jackman J K, Flotow H, Wilkinson S E, Williams D H, Johnson R, Kong G, Chan A C, Findell P R. Phosphorylation of SLP-76 by the ZAP-70 protein-tyrosine kinase is required for T-cell receptor function. J Biol Chem. 1996;271:19641–19644. doi: 10.1074/jbc.271.33.19641. [DOI] [PubMed] [Google Scholar]
- 39.Weiss A, Koretzky G, Schatzman R C, Kadlecek T. Functional activation of the T-cell antigen receptor induces tyrosine phosphorylation of phospholipase C-gamma 1. Proc Natl Acad Sci USA. 1991;88:5484–5488. doi: 10.1073/pnas.88.13.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams B L, Schreiber K L, Zhang W, Wange R L, Samelson L E, Leibson P J, Abraham R T. Genetic evidence for differential coupling of Syk family kinases to the T-cell receptor: reconstitution studies in a ZAP-70-deficient Jurkat T-cell line. Mol Cell Biol. 1998;18:1388–1399. doi: 10.1128/mcb.18.3.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolven A, Okamura H, Rosenblatt Y, Resh M D. Palmitoylation of p59fyn is reversible and sufficient for plasma membrane association. Mol Biol Cell. 1997;8:1159–1173. doi: 10.1091/mbc.8.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 43.Yablonski D, Kuhne M R, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 44.Yamasaki S, Takamatsu M, Iwashima M. The kinase, SH3, and SH2 domains of Lck play critical roles in T-cell activation after ZAP-70 membrane localization. Mol Cell Biol. 1996;16:7151–7160. doi: 10.1128/mcb.16.12.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoder J, Pham C, Iizuka Y M, Kanagawa O, Liu S K, McGlade J, Cheng A M. Requirement for the SLP-76 adaptor GADS in T cell development. Science. 2001;291:1987–1991. doi: 10.1126/science.1057176. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W, Irvin B J, Trible R P, Abraham R T, Samelson L E. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. Int Immunol. 1999;11:943–950. doi: 10.1093/intimm/11.6.943. [DOI] [PubMed] [Google Scholar]
- 47.Zhang W, Sloan-Lancaster J, Kitchen J, Trible R P, Samelson L E. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 48.Zhang W, Trible R P, Samelson L E. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- 49.Zhang W, Trible R P, Zhu M, Liu S K, McGlade C J, Samelson L E. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell angigen receptor-mediated signaling. J Biol Chem. 2000;275:23355–23361. doi: 10.1074/jbc.M000404200. [DOI] [PubMed] [Google Scholar]