Abstract
INTRODUCTION
Electronic pressure-sensitive walkways are commonly available solutions to quantitatively assess gait parameters for clinical and research purposes. Many studies have evaluated their measurement properties in different conditions with variable findings. In order to be informed about the current evidence of their reliability for optimal clinical and scientific decision making, this systematic review provided a quantitative synthesis of the test-retest reliability and minimal detectable change of the captured gait parameters across different test conditions (single and cognitive dual-task conditions) and population groups.
EVIDENCE ACQUISITION
A literature search was conducted in PubMed, Embase, and Scopus until November 2021 to identify articles that examined the test-retest reliability properties of the gait parameters captured by pressure-sensitive walkways (gait speed, cadence, stride length and time, double support time, base of support) in adult healthy individuals or patients. The methodological quality was rated using the Consensus-Based Standards for the Selection of Health Measurement Instruments Checklist. Data were meta-analyzed on intraclass correlation coefficient to examine the test-retest relative reliability. Quantitative synthesis was performed for absolute reliability, examined by the weighted average of minimal detectable change values.
EVIDENCE SYNTHESIS
A total of 44 studies were included in this systematic review. The methodological quality was adequate in half of the included studies. The main finding was that pressure-sensitive walkways are reliable tools for objective assessment of spatial and temporal gait parameters both in single-and cognitive dual-task conditions. Despite few exceptions, the review identified intraclass correlation coefficient higher than 0.75 and minimal detectable change lower than 30%, demonstrating satisfactory relative and absolute reliability in all examined populations (healthy adults, elderly, patients with cognitive impairment, spinocerebellar ataxia type 14, Huntington’s disease, multiple sclerosis, Parkinson’s disease, rheumatoid arthritis, spinal cord injury, stroke or vestibular dysfunction).
CONCLUSIONS
Current evidence suggested that, despite different populations and testing protocols used in the included studies, the test-retest reliability of the examined gait parameters was acceptable under single and cognitive dual-task conditions. Further high-quality studies with powered sample sizes are needed to examine the reliability findings of the currently understudied and unexplored pathologies and test conditions.
Key words: Rehabilitation, Stroke, Gait analysis
Introduction
Gait assessment has progressively become an important aspect in the current scientific research, as well as in the rehabilitation practice.1 Its primary aim was to possibly discriminate between normal and abnormal gait, helping to reveal potential alterations induced by aging and/or pathologies, guiding interventions, and monitoring individual progress over time.1-3 Changes in gait performance, including shorter stride length, lower stride velocity, increased double support time, and larger stride width, demonstrated to correlate with the risk of falls, cognitive impairment, or even risk of early mortality.4-6 In the last years, gait assessment in dual-task conditions has attracted more and more attention in clinical practice and research. This condition refers to the ability of a subject to perform two distinct tasks simultaneously, such as walking while performing cognitive demands or carrying out a glass of water. Emerging evidence suggests that dual-task paradigms are more effective than single-task conditions in detecting gait alteration in early disease stages7 or specific pathological states and risk factors, including fall risk8 and freezing of gait in patients with Parkinson’s disease.9, 10 In addition, dual-task walking might better reflect daily-living walking11 and, thus, could be a better assessment method to predict community mobility after a hospital discharge.12
Standardized scales and tests are mainly used to assess single-task and dual-task walking in clinical practice and research.13, 14 Although easy to administer and minimally time-consuming, they might convey a lack of specificity, poor accuracy and could be biased by subjective evaluations.15, 16 Over the years, different technological solutions have been proposed to overcome these limitations, including 3-dimensional motion capture systems, pressure-sensitive systems, stride analyzers, and wearable sensors.17, 18 Among currently available technologies, electronic pressure-sensitive walkways are one of the most used solutions, thanks to their ease of use.19 Dedicated software allows to quickly collect gait spatial and temporal parameters through a semi-automated procedure from the embedded pressure sensors, arranged along the walkway, that allow the detection of footfall location and timing during walking.20
In light of their extensive use as assessment tools, it is important to achieve a better knowledge of their measurement properties. Test-retest reliability is the first fundamental measurement property to be considered since it is a crucial prerequisite of measurement validation. Test-retest reliability refers to the ability of a tool to generate repeatable and consistent values between different test occasions.21 A tool with acceptable test-retest reliability ensures that the acquired data are accurate and reflect the subject’s real performance.21 This property should be primarily investigated before examining individual changes over time21 to discern real meaningful changes from irrelevant or artefactual ones due to measurement errors.22 Test-retest reliability could be presented as relative or absolute measures. Relative reliability is frequently measured through the intraclass correlation coefficient (ICC), an index used to describe the degree to which individuals obtain similar values during repeated measurements. Instead, the minimal detectable change (MDC95) is one of the most common absolute reliability indices. MDC95 provides the minimal magnitude of change required by a specific measure to be considered real beyond the measurement error.22
To date, no systematic appraisal of studies has been conducted to evaluate the reliability and measurement errors of pressure-sensitive walkways across different test conditions. Performing a systematic review with meta-analysis on this research topic could be appropriate to provide a quantitative synthesis and a clear determination of the consistency of the computed gait parameters. Therefore, the purpose of this review was to systematically investigate the reliability and the minimal detectable change of the gait parameters captured via electronic pressure-sensitive walkways. The review is intended to compare findings across two different test conditions (i.e. single- and dual-task conditions) in healthy and pathological populations to better guide researchers and clinicians in the use and interpretation of data captured by the aforementioned systems.
Evidence acquisition
Protocol
The systematic review and meta-analysis were consistent with the Preferred Reporting Items for Systemic Reviews and Meta-analysis (PRISMA) statement23 and Consensus-based Standards for the Selection of Health Measurement Instruments (COSMIN) methodology.24 The review protocol has not been prospectively recorded in an online register.
Database and search strategy
Three online databases (PubMed, Embase, and Scopus) were systematically searched until November 2021. The search keywords “walkway,” “gait,” “reliability,” and “reproducibility” and their related MeSH and Entree search terms (“gait” and “reproducibility of results”) were appropriately combined using the Boolean operators “AND” and “OR” to identify potentially relevant articles. The key words “reliability” and “reproducibility” were chosen to find articles primarily focused on the measurement property examined in this review. The broad terms “walkway” and “gait” were used for two main reasons. They identify all the possible terms used to identify the examined systems, such as pressure-sensitive walkways or gait mats. Furthermore, they detect articles focused on reliability findings of other gait assessment tools, which could also include the reliability results of pressure-sensitive walkways, often used as gold-standard tools to validate novel tools. The keywords related to gait parameters and electronic walkways names were not included in the search strategy to keep a broad query and avoid retrieval bias.
The electronic search was finally complemented by screening the reference lists of included studies.
Supplementary Digital Material 1: Supplementary Table I presents the search strategies for each online database.
Selection criteria
The inclusion criteria for this systematic review were: 1) full-text articles addressing test-retest reliability, standard error of measurement, or minimal detectable change of gait parameters captured via pressure-sensitive walkways; 2) articles assessing healthy or clinical populations with age higher than 18 years old; and 3) assessment of at least one of the gait parameters selected as the most representative outcomes for the different gait domains:25 gait speed (cm/s) and stride length (cm) for pace domain, cadence (step/min) for rhythm domain, double support time (% Gait Cycle, GC) for phase domain and base (cm) for base of support domain.
Figure 1 depicts a schematic representation of the pressure-sensitive walkway systems for gait analysis and the gait parameters under study.
Figure 1.
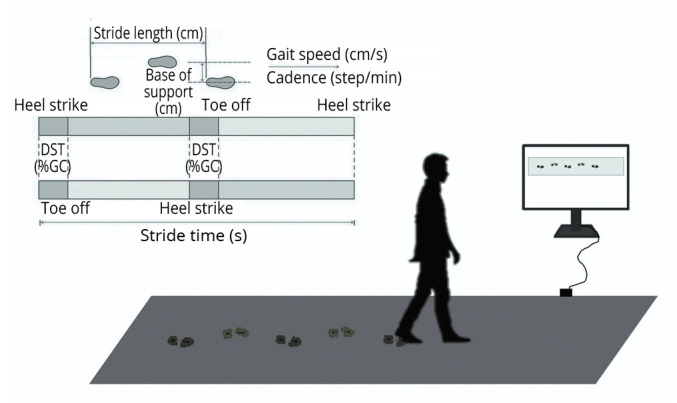
—Schematic representation of the examined gait parameters and the pressure-sensitive walkway set-up.
Articles were excluded if the time interval between repeated sessions was higher than one month to avoid potential changes in individuals’ gait patterns.
No explicit language, date, or document format restrictions were applied to reduce reporting bias.
Study selection and data extraction
The study selection was conducted by one reviewer (MP), who merged the bibliographic results and removed duplicates among databases. A second reviewer (MG) independently verified the study selection accuracy according to the criteria listed above.
After the bibliographic search phase, retrieved articles were filtered based on title and abstract. All titles and abstracts were independently screened by two blinded reviewers (MP, MG) and full-text of the potentially relevant articles were analyzed in-depth to examine their eligibility. If an eligible article assessed different population samples, each sample was considered as a separate study as required by COSMIN methodology.24
For each included study, the two reviewers (MP, MG) independently collected data using a standardized data extraction form, to retrieve information regarding participants’ characteristics, measurement procedures, gait parameters, reliability estimates. Disagreements between the two reviewers (MP, MG) were discussed until consensus was reached.
Quality assessment
The methodological quality was examined using the reliability quality assessment of the COSMIN risk of bias checklist24, 26 by two independent reviewers with methodological and clinical expertise (MP, MG). Any disagreements in ratings were resolved through discussion. The COSMIN checklist was chosen because it is a well-recognized and reliable assessment method to evaluate methodological quality of studies, which was developed through a multidisciplinary and international Delphi study.27 The COSMIN checklist was specifically developed for patient-reported outcome measures, but it could be applied in systematic reviews aimed at assessing psychometric properties of other outcome measures.27 The COSMIN risk of bias assessment for reliability studies is composed of five different items (i.e. stable condition of participants, appropriate time interval between testing sessions, similar measurement conditions between testing sessions, appropriate statistical methods, and other important flaws) scored using a 4-point Likert type scale to grade the quality of the studies as very good, adequate, doubtful or inadequate.24, 26 The overall study quality score was given by the lowest rating of any item, according to the “worst score counts” principle.24, 26
Data analysis
The analyses were distinctly performed for trials carried out in: 1) single-task condition while walking at a comfortable speed; and 2) cognitive dual-task condition while walking at a comfortable speed.
The primary outcome of the study was the gait speed and its reliability. Secondary outcome measures were the reliability of stride length, cadence, stride time, double support time, and base of support. For each examined outcome measure, test-retest correlation values (i.e. intraclass correlation values) reported in the original articles were collected and meta-analyzed to derive an overall estimate of the test-retest relative reliability (hereafter ICC). The 95% confidence intervals (CIs) were generated using a random-effects model with weighting of individual point estimates based on study samples. ICC findings were conventionally interpreted using the following metrics: poor (ICC<0.500), moderate (ICC: 0.500-0.749), good (ICC: 0.750-0.899) and excellent (ICC≥0.900) test-retest relative reliability.28 Thus, a value of 0.75 is commonly used as the cut-off point to discriminate acceptable values from suboptimal ones.28
Values of minimum detectable change at 95% confidence level (MDC95) were quantitatively summarized. MDC95 was used to provide a threshold amount of change in scores required to be 95% confident that a true change beyond that of measurement error had occurred.28 MDC95 values were directly collected from the results of included study findings or calculated from the ICC values and the pooled standard deviation of the test- and retest values (SDtest-retest) (Eq. 1):22
| (1) |
Pooled estimates of MDC95 values (hereafter MDC95 for simplicity) were computed by weighted mean and standard deviation, with weighting of individual point estimates based on study samples.
Finally, the percentage of the MDC95 compared to the mean test-retest value (Meantest-retest) of the outcome (hereafter MDC95%) was computed to present a relative amount of random measurement errors (Eq. 2):
| (2) |
For interpretation, minimal detectable changes (MDC95-MDC95%) should be as small as possible to increase the absolute reliability of the measure and lower measurement error. Some studies suggested that an MDC95% is considered acceptable when lower than 30%.29-31
A subgroup analysis based on participants’ health status was ultimately performed to determine if the different health condition of the participants is a confounding factor of the reliability results.
Statistical analysis
All the analyses were performed using R software (version 4.0.0; R Foundation, Vienna, Austria) with the Metafor function32 and MATLAB software (R2020b, MathWorks Inc., Natick, MA, USA).
Evidence synthesis
Search and selection
Figure 2 illustrates the PRISMA flow diagram depicting the review selection process and the number of articles yielded at each stage of the literature search. Overall, the search strategies identified a total of 8270 articles. Following the removal of duplicates, screening of title/abstracts, and full-text screening, a total of 44 studies from 32 independent articles were eligible for the quantitative analysis and were included in the current systematic review.
Figure 2.
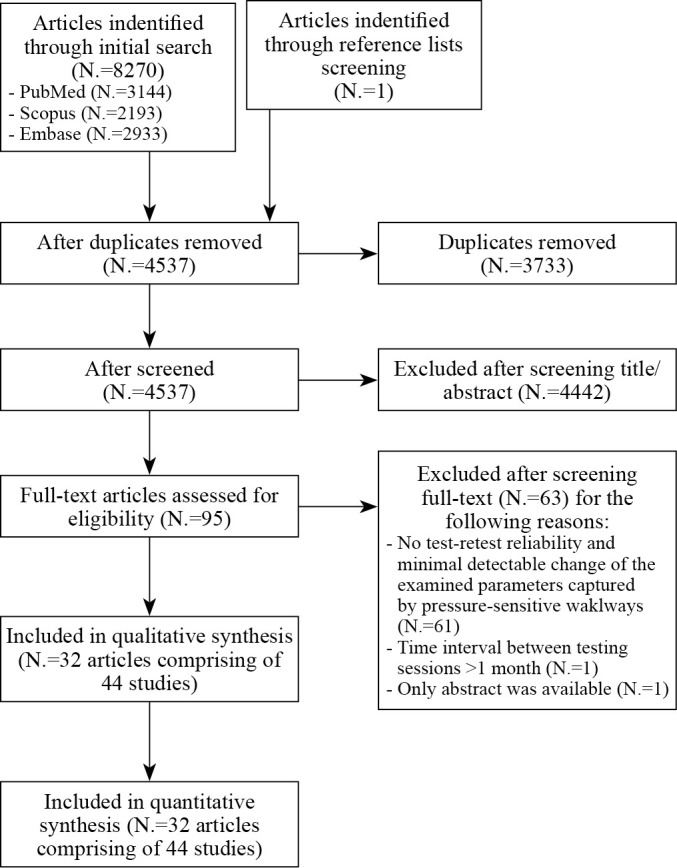
—PRISMA flow diagram of selection process in the systematic review.
Methodological quality
The COSMIN ratings for each study are summarized in Supplementary Digital Material 2: Supplementary Table II and revealed that half of the included studies had a satisfactory methodological quality. There was a total of 10 studies with very good methodological quality and 12 studies with adequate quality. Twenty-two studies were instead judged doubtful or inadequate, mainly due to an inappropriate time interval between test and retest sessions. Methodological quality evaluations are reported in the supplementary materials (Supplementary Digital Material 3: Supplementary Table III).
Characteristics of the included studies
General characteristics of the included studies are reported in Supplementary Table II: studies are grouped by health status. Then, the studies of each health status are reported in chronologic order. The samples included participants with or without pathology. Most studies included healthy adults (AH) (11 studies;31, 33-42 198 subjects) and elderly (EY) (9 studies;40, 41, 43-48 882 subjects). The most common pathology was stroke (SK) (10 studies;49-54 248 subjects). Few studies included individuals with other pathologies, such as: cognitive impairment (CI) (4 studies;46, 55-57 131 subjects), multiple sclerosis (MS) (3 studies;31, 33, 39 101 subjects), spinal cord injury (SCI) (2 studies;58, 59 39 subjects), rheumatoid arthritis (RA) (1 study;60 50 subjects), vestibular dysfunction (VD) (1 study;61 35 subjects), Parkinson’s disease (PD) (1 study;62 32 subjects), Huntington’s disease (HD) (1 study;63 12 subjects), spinocerebellar ataxia type 14 (CA) (1 study;38 8 subjects). The sample size (N) widely varied from 8 to 558 participants across the studies, with only 14 studies including N.≥30 participants. The mean age of the participants spanned from 20 to 84 years.
Characteristics of the included pressure-sensitive walkways
Four different pressure-sensitive walkways were examined in the included studies. The most common pressure-sensitive walkway employed was the GAITRite® (CIR Systems Inc., Havertown, PA, USA), which was used in 39 studies, followed by GaitMat II (EQ Inc., Chalfont, PA, USA) in 2 studies, Zeno™ Walkway (Protokinetics LLC, Haverton, PA, USA) in 2 studies and USEW62 in one study. The GAITRite® (CIR Systems Inc.), GaitMat II (EQ Inc.), and Zeno™ Walkway (Protokinetics LLC) have a rectangular shape and they examine straight-line walking. The GaitMat II (EQ Inc.) is 4 meters long, whereas the GAITRite® (CIR Systems Inc.) and Zeno™ Walkway (Protokinetics LLC) are available in different lengths. The included studies using GAITRite® (CIR Systems Inc.) adopted different mat lengths, varying from 3.5 up to 9 meters. The two studies using the Zeno™ Walkway (Protokinetics LLC) adopted the 4.3-m long system. The USEW is composed of twelve pressure modules, arranged to form a U-shaped walkway that allows straight-line walking and turning evaluations.
All these systems embedded pressure sensors activated at heel strike and deactivated at toe-off, for collecting footprints while an individual walked along the mat. The spatial resolution of the matrix of pressure sensors varies across the four systems. The GAITRite® (CIR Systems Inc.) and Zeno™ Walkway (Protokinetics LLC) have a slight better spatial resolution (1.3 cm) than the GaitMat II (EQ Inc.) (1.5 cm). No information about spatial resolution was reported for USEW system.
Footprints were collected at a mean sampling frequency of 80 Hz (range: 32-180Hz) and were processed by ad-hoc application software to collect spatial and temporal gait parameters. The included pressure-sensitive walkways adopt different processing software systems, which could lead to disparities in the gait parameter calculations. For instance, GAITRite® (CIR Systems Inc.) software measures the gait speed as the ratio between the distance travelled and the ambulation time using only the first and the last foot contacts.64 In contrast, the Protokinetics Movement Analysis Software (PKMAS) of the Zeno™ Walkway (Protokinetics LLC) computes it as the ratio between the sum of the stride length and the sum of the stride time taking into account all footfalls.64 A previous study evaluated the possible differences between these two processing software systems.65 The authors compared the GAITRite® (CIR Systems Inc.) software with the Protokinetics Movement Analysis Software (PKMAS) of the Zeno™ Walkway (Protokinetics LLC), using the same footsteps data collected from older adults with the GAITRite® (CIR Systems Inc.) walkway.65 The authors showed that minimal differences occurred between the two software systems in the most of gait parameters, suggesting they may be used interchangeably.65 To our knowledge, no other studies compared differences between GaitMat II and USEW software systems with PKMAS and GAITRite® (CIR Systems Inc.) software.
Characteristics of the included walking protocols
All gait evaluations were carried out in controlled laboratory conditions. The majority of the studies asked participants to walk at their self-selected walking speed along a path, long from 5 up to 30 meters. If necessary, they were allowed to use their walking aids, but the therapist’s assistance was not permitted. Few studies asked participants to perform some practice walks before testing trials to become familiar to walk on the mat.
Different walking protocols were adopted in the included studies. A total of 21 studies used an intra-session test-retest reliability design. They usually assessed walking performance in a single session with 2 testing trials (range: 2-4 trials), with a time between testing trials varying from 2 minutes to 1 hour. The remaining 23 studies employed an inter-session test-retest reliability design. The gait assessment in two testing sessions was the most selected choice (N.=21, 91%), while the number of walking trials in each testing session widely varied from 1 to 10 across studies. Several studies did not report the duration of the resting break between walking trials. When reported, 10-minute break was usually allowed between each testing trial in a testing session. The testing sessions were generally spaced one week apart.
In addition, different test conditions were employed. A total of 41 studies assessed the test-retest reliability of gait parameters while participants were instructed to walk at their self-selected comfortable speed without performing any other task (i.e. single-task condition). Fifteen studies from 10 independent articles assessed walking in cognitive dual-tasking condition, asking participants to walk while counting backwards (-1, -3, or -7 from a fixed or random number) or reciting words that start with a predefined letter. Because of the limited number of studies (N.=2) investigating motor dual-tasking conditions (i.e. walking carrying a tray with glasses), the analysis was focused on cognitive dual-tasking conditions only.
Data pooling
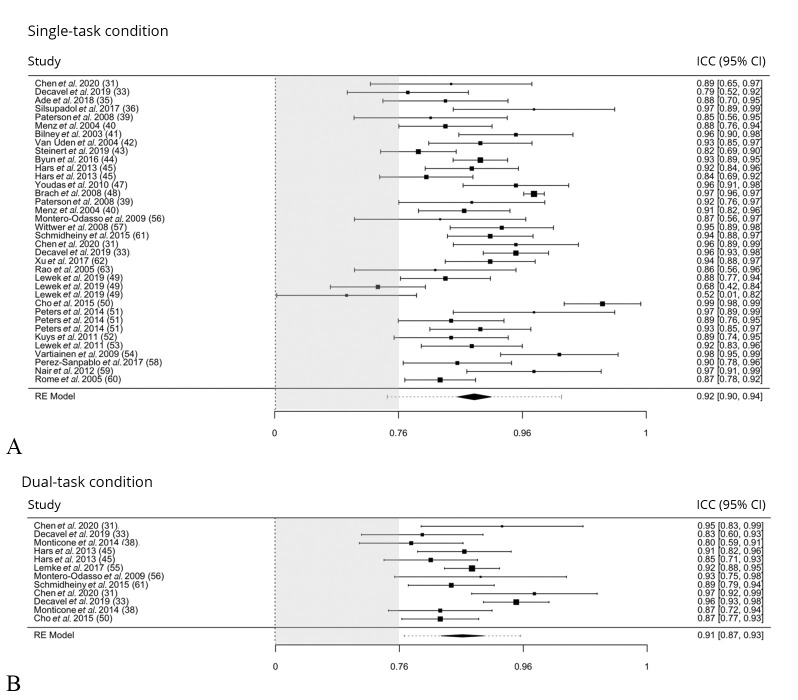
In this section, results are presented for all outcome measures, starting from the gait speed, selected as the primary outcome measure. The meta-analysis results of test-retest reliability of the gait speed are reported in the forest plots in Figure 3.31, 33, 34, 36, 37, 39-45, 47-63
Figure 3.
—Test-retest relative reliability of gait speed. A) Results in single-task condition; and B) in dual-task condition.31, 33, 34, 36, 37, 39-45, 47-63 Forest plot displaying intraclass correlation coefficients (ICC) data (and 95% confidence interval) of the gait speed, primary outcome measure of the review. Grey box indicates the area of suboptimal ICC findings.
Figure 3A31, 33, 34, 36, 37, 40-45, 47-54, 56-63 shows the results of the studies examining gait speed in single-task condition (i.e. those having a black square both in the “Gait Speed” and “Single-task” columns in Supplementary Table II), whereas Figure 3B31, 33, 39, 45, 50, 55, 56, 61 displays the results of the studies reporting gait speed in dual-task condition (i.e. those having a black square both in the “gait speed” and “dual-task” columns in Supplementary Table II).
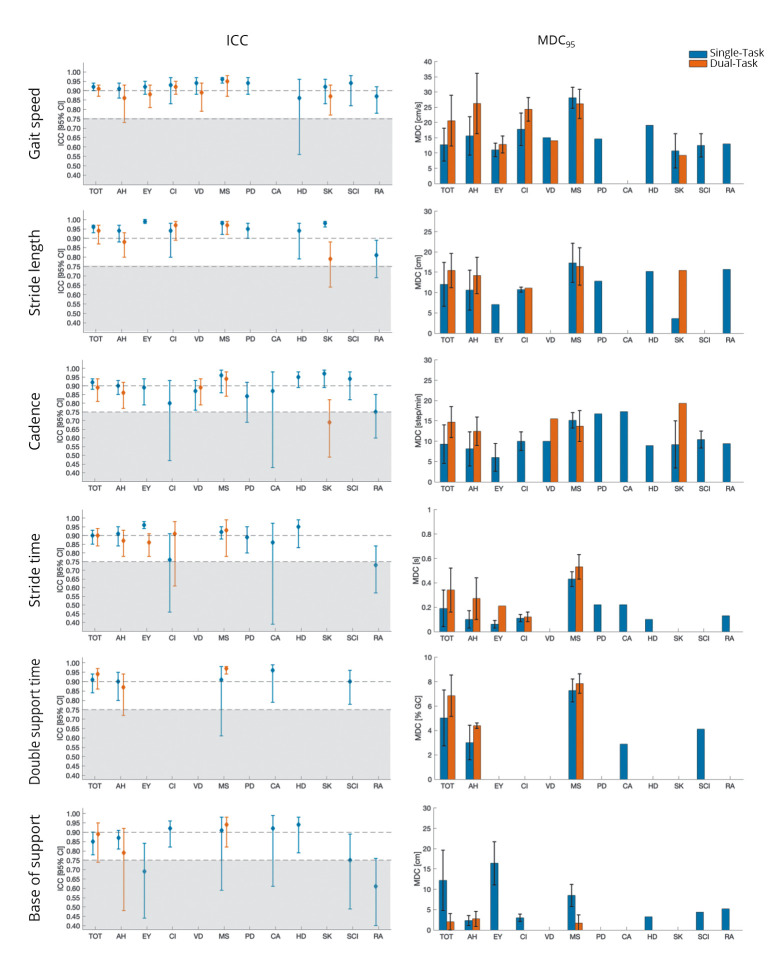
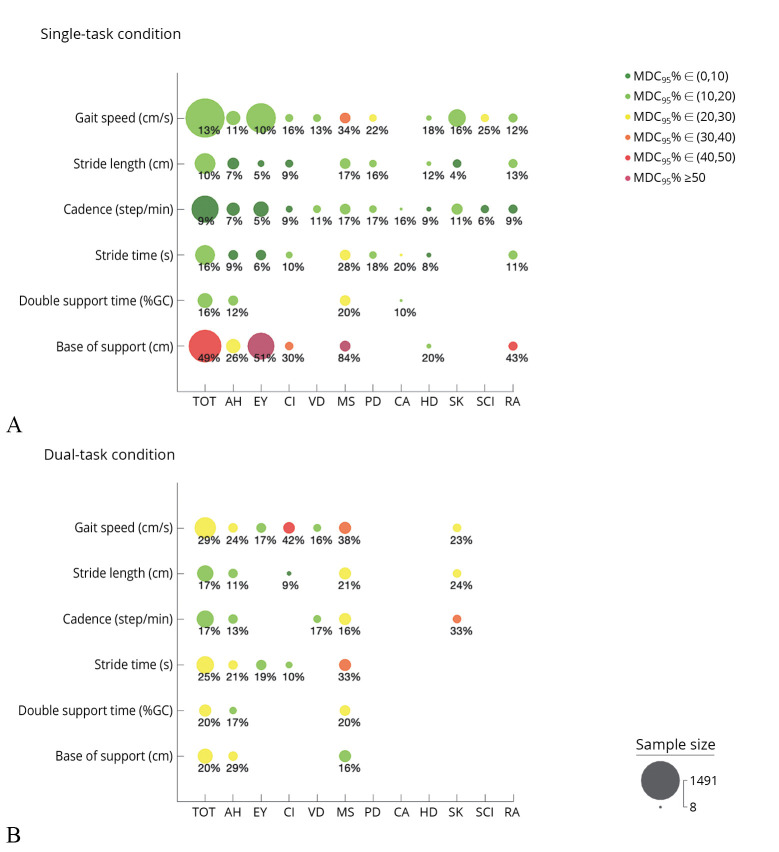
The results of the forest plots (i.e., the pooled estimates of ICC with 95% confidence intervals) and the pooled MDC95 for all outcome measures, health conditions, and task conditions are summarized in Figure 4. Finally, the results of MDC95% are summarized in Figure 5.
Figure 4.
—Summary of the relative and absolute reliability findings. For each examined outcome measure, the test-retest relative reliability (pooled ICC [95% confidence interval, CI]) findings is presented on the left column and the weighted average of MDC95±standard deviation is shown on the right column. For each graph, the results of relative and absolute reliability in single-task and dual task conditions for the total population sample (TOT) and the subgroups are shown. Grey box indicates suboptimal ICC findings. AH: Healthy adult; EY: elderly; CI: cognitive impairment; VD: vestibular dysfunction; MS: multiple sclerosis; PD: Parkinson’s disease; CA: spinocerebellar ataxia type 14; HD: Huntington’s disease; SK: stroke; SCI: spinal cord injury; RA: rheumatoid arthritis.
Figure 5.
—Summary of MDC95% findings. A) Results of MDC95% of the gait parameters in single-task; and B) results in dual-task condition. The analysis was performed for the total population sample (TOT) and the subgroups. Dot size is proportional to the number of participants included in the analyses. MDC95% less than 30% is considered acceptable (light gray-gray dots; green-yellow in the online version), otherwise MDC95% is judged suboptimal (off-white–dark gray dots; orange-red in the online version). AH: Healthy adult; EY: elderly; CI: cognitive impairment; VD: vestibular dysfunction; MS: multiple sclerosis; PD: Parkinson’s disease; CA: spinocerebellar ataxia type 14; HD: Huntington’s disease; SK: stroke; SCI: spinal cord injury; RA: rheumatoid arthritis.
Gait speed (cm/s)
The test-retest reliability of gait speed in single-task conditions was estimated from 36 studies recruiting a total of 1491 subjects. Overall, the meta-analyzed data showed that the pooled estimate of test-retest relative reliability was excellent (ICC: 0.92 [95% CI: 0.90, 0.94]) (Figure 2). The subgroup analysis demonstrated that the reliability was good to excellent across all the different health conditions, as shown in Figure 4.
MDC95 was 12.7±5.4 cm/s (MDC95%=13%) for the entire sample of participants and varied from 10.7 to 28.1 cm/s across the different health status. MDC95% was generally deemed acceptable (11-25%) in all the examined populations, except for multiple sclerosis group (MDC95%=34%).
Regarding dual-task conditions, twelve studies including a total of 387 subjects overall found excellent test-retest relative reliability (ICC: 0.91 [95% CI: 0.87, 0.93]). The reliability findings reached good to excellent levels for all the examined health conditions (adult healthy, elderly, cognitive impairment, vestibular disease, multiple sclerosis, and stroke), as demonstrated by subgroup analysis in Figure 4. MDC95 was 20.6±8.3 cm/s for the total population sample, and it ranged from 9.2 to 26.1 cm/s across the different examined subgroups. MDC95% values were acceptable for all the health conditions (16-29%), except for patients with multiple sclerosis and cognitive impairment (38% and 42%, respectively).
Stride length (cm)
A total of 14 studies (368 subjects) reported ICC data to assess the test-retest relative reliability of stride length in single-task conditions. The meta-analysis findings suggested excellent reliability of stride length for the entire population sample (ICC: 0.96 [95% CI: 0.93, 0.97]). The subgroup analysis also verified that stride length had excellent reliability for all examined health conditions, except for rheumatoid arthritis (ICC: 0.81 [95% CI: 0.69, 0.89]). The MDC95 was 12.0±5.4 cm for the total population sample and varied from 3.6 to 17.3 cm in the different subgroups. All the MDC95 were below 17% of the mean value of stride length (MDC95%=4-17%), demonstrating always acceptable small measurement errors.
In dual-task conditions, stride length largely showed excellent relative reliability (ICC: 0.94 [95% CI: 0.87, 0.97]) in 8 studies recruiting a total of 211 subjects. In particular, the reliability was excellent for patients with multiple sclerosis or cognitive impairment (ICC: 0.97 in both groups) and good for healthy adults (ICC: 0.88 [95% CI: 0.80, 0.94]) and patients with stroke (ICC: 0.79 [95% CI: 0.64,0.88]). MDC95 was 15.4±4.2 cm and ranged from 11.1 cm to 16.4 cm across the included populations. The MDC95% of stride length under dual-task conditions was acceptable (9-24%) for all examined subgroups.
Cadence (step/min)
Excellent test-retest relative reliability (ICC: 0.92 [95% CI: 0.88, 0.94]) was found for cadence during walking in single-task condition in 24 studies (667 subjects). Good to excellent results were found in all examined population groups, with a minimum intraclass correlation coefficient of 0.75 (95% CI: 0.60, 0.85) in individuals with rheumatoid arthritis to a maximum intraclass correlation of 0.97 (95% CI: 0.90, 0.99) in stroke patients. The total population sample showed an MDC95 of 9.3±4.7 steps/min, corresponding to an MDC95% of 9%. The subgroup analyses showed MDC95 ranging from 6.0 to 17.3 step/min and all acceptable MDC95% values (range: 5-17%).
In dual-task conditions, cadence overall yielded good to excellent test-retest relative reliability (ICC: 0.89 [95% CI: 0.81, 0.94]), as reported in 8 studies (235 subjects). Similar results were found for healthy subjects, patients with vestibular dysfunction, and multiple sclerosis (ICC range: 0.86-0.94). Instead, cadence revealed a moderate reliability for stroke patients in dual-task condition (ICC: 0.69 [95% CI: 0.49, 0.82]). The MDC95 was 14.7±3.8 steps/min for the total population sample, the minimum MDC95 was registered for healthy adults (MDC95: 12.4±3.5 steps/min), and the maximum one for stroke patients (MDC95: 19.3 steps/min). MDC95% was deemed suboptimal for stroke patients (33%) and acceptable (13-17%) for all other examined populations (healthy adults, vestibular disease, multiple sclerosis).
Stride time (s)
Data from 13 studies suggested that the test-retest reliability of stride time was excellent with an ICC of 0.90 (95% CI: 0.85, 0.93). Excellent values were observed in healthy adults, elderly, multiple sclerosis, and Huntington’s disease (ICC: 0.91-0.96). Instead, stride time reliability was good for patients with Parkinson’s disease, spinocerebellar ataxia, and cognitive impairment (ICC: 0.76-0.89). Moderate reliability was found in rheumatoid arthritis (ICC: 0.73 [95% CI 0.57, 0.84]). MDC95 was 0.19±0.15 s, with an MDC95% of 16% in the total population sample. In the subgroups, the MDC95 varied from 0.06 to 0.43 s and its percentage was always below 28% of the mean value of stride time, indicating acceptable measurement errors (MDC95%: 6-28%).
The ICC data reported in 9 studies (251 subjects) for test-retest reliability of stride time during dual-task condition overall showed excellent levels (ICC: 0.90 [95% CI: 0.84, 0.94]). The subgroup analysis found good to excellent reliability for the healthy adults, elderly, cognitive impairment, or multiple sclerosis subgroups (ICC: 0.86-0.93). Regarding MDC95, the value for the total population sample was 0.34±0.18 s, corresponding to a 25% for the MDC95%. The MDC95% was acceptable for all tested populations (10-21%), except for multiple sclerosis (33%).
Double support time (% gait cycle)
Double support time was evaluated under single-task conditions by 8 studies including a total of 172 subjects. The pooled estimate of test-retest reliability in this condition suggested an excellent value of reliability between test and retest measures of double support time. ICC value was 0.91 (95% CI: 0.84, 0.94) for the total population sample and varied from 0.90 to 0.96 in the examined subgroups (healthy adults, multiple sclerosis, Parkinson’s disease, cerebellar ataxia, and spinal cord injury). MDC95 was 5.03±2.29% of the gait cycle for the total population sample (MDC95%: 17%). Regarding subgroup analysis, MDC95 values ranged from 2.87 to 7.27%. These values supported acceptable values of MDC95% (10-20%).
The test-retest relative reliability of double support time under dual-task conditions was examined by 4 studies. These studies included healthy subjects (N.=31 from 2 studies) and patients with multiple sclerosis (N.=76 from 2 studies). Overall, the reliability was excellent, demonstrated by an ICC of 0.94 [95% CI 0.86, 0.97] for the total population sample. Looking at subgroup analysis findings, test-retest reliability was good for healthy adults (ICC: 0.87 [95% CI 0.72, 0.94]) and excellent for patients with multiple sclerosis (ICC: 0.97 [95% CI: 0.95, 0.98]). In this testing condition, MDC95 was 6.84±1.7% of the gait cycle for the total population sample and varied from 4.39±0.22% of the gait cycle in the healthy subjects to 7.85±0.80% of the gait cycle in patients with multiple sclerosis. Computing MDC95%, acceptable levels of absolute reliability were reached (17-20%).
Base of support (cm)
The reliability of the base of support was assessed by 17 studies recruiting a total of 919 subjects. The results for the total population sample demonstrated good test-retest relative reliability (ICC: 0.85 [95% CI: 0.78, 0.90]). Subgroup meta-analysis found good to excellent reliability for the following groups: healthy adults, patients with cognitive impairment, multiple sclerosis, cerebellar ataxia, Huntington’s disease, and spinal cord injury (ICC range: 0.75-0.94). Moderate reliability was found for elderly and rheumatoid arthritis subgroups (ICC: 0.61-0.69).
The measurement error was suboptimal in the total population sample (MDC95%: 49%), as well as in the following subgroups: elderly subjects, patients with cognitive impairment, multiple sclerosis, and rheumatoid arthritis (MDC95%: 30-84%). Acceptable measurement error findings were achieved on healthy adults and patients with Huntington’s disease (MDC95%: 20-26%).
Six studies established the test-retest reliability of base of support under dual-task conditions. They included a total of 157 participants (56 healthy subjects and 101 patients with multiple sclerosis). Pooled estimate found good test-retest reliability (ICC: 0.89 [95% CI 0.74, 0.95], MDC95: 2.0±2.0 cm) for the entire population sample. Subgroup analysis instead revealed good reliability for healthy subjects (ICC: 0.79 [95%CI: 0.48, 0.92], MDC95: 2.7±1.9 cm) and excellent reliability (ICC: 0.94 [95% CI: 0.82, 0.98], MDC95: 1.7±2.0 cm) for patients with multiple sclerosis. Acceptable findings of MDC95% were found for the total population sample (20%) and the examined subgroups: patients with multiple sclerosis (16%) and healthy subjects (29%).
Discussion
The result of the current review revealed that pressure-sensitive walkways are highly reliable tools for the objective assessment of gait spatial and temporal parameters under single and dual-task conditions. The review included studies enrolling several populations with diverse gait patterns. Almost half of these studies (45%) was focused on healthy adults31, 33-42 and elderlies typically experiencing a lower comfortable gait speed, a wastage of muscular effort for postural maintenance, and an increased fear of falling.40, 41, 43-48 In addition, several studies included neurological conditions. The principal examined neurological patients were stroke patients characterized by gait asymmetry and steppage gait49-54 and patients with multiple sclerosis who exhibit a slower preferred gait speed, lower stride length and an increase in gait asymmetry indicating potential issues with balance control.31, 33, 39 Some studies included patients with cognitive impairment, characterized by slow gait, increase falls risk, impaired spatial orientation and decreased dual-tasking ability.46, 55-57 Instead, a limited number of studies (16%) were focused on other pathologies, including spinocerebellar ataxia type 14,38 Huntington’s disease,63 spinal cord injury,58, 59 Parkinson’s disease,62 vestibular disorder38 and rheumatoid arthritis.60 Despite the diversity of included populations and gait patterns, the current evidence suggested that the reliability of the examined gait parameters was acceptable under both single and dual-task conditions. Clinicians and researchers could use these systems to measure the effects of an intervention or changes due to disease progression without the substantial influence of labile measurements.
Inter-studies comparisons revealed that different study designs and testing protocols have been applied in the included studies. Regarding testing protocol differences, the mat length and the walking distance that participants were asked to perform were the main factors that frequently varied across the studies. Most of the studies limited the walking distance to the mat length or to a maximum of 10 meters, whereas there were some studies in which the pressure-sensitive walkway was embedded in a 20-m or 30-m walking track39, 44 or that used for the 6-minute walking test.58 Furthermore, the length of the pressure sensitive-walkways varied from 3.5 to 9 meters. This factor could influence the number of strides recorded on the mat.
The number of trial repetitions within each testing session and the different number of testing sessions were other relevant factors that changed across the studies. Finally, differences in the applied type of cognitive dual-tasking were observed, as using a serial subtraction of 1, 3, or 7 as well as reciting words with a predefined letter. All these elements revealed the lack of a common and standardized approach to examine the reliability properties of an instrument in terms of dual-task condition, walking distance, trial frequency and time interval between testing sessions. Future research is recommended to define and promote the use of a standardized protocol with optimal procedures and determine how these confounding factors impact the different subgroups of individuals. Nevertheless, all these inter-studies differences have the potential to inspect how the systems react to different test conditions, populations and protocols and increase the generalizability of the reliability findings. Therefore, in the present review, reliability data from the examined gait parameters were collected and summarized using meta-analysis and other quantitative techniques in order to look broadly at patterns and generalize findings.
Regarding relative test-retest reliability results, current evidence suggested that the test-retest relative reliability of the examined gait parameters was acceptable under single and dual-task conditions (ICC>0.75), with only a few exceptions (4 analyses out of 83: stride time and base of support in the single-task condition in patients with rheumatoid arthritis, base of support in the single-task condition in elderly and cadence in the dual-task condition in stroke patients). In deep, the pressure-sensitive walkways appear to provide similar results across different repetitions when subjects walked at their self-paced walking speed in single-task condition. Whereas, when they were asked to perform more demanding tasks as cognitive dual-tasks, the pooled reliability estimates were still from good to excellent, but they showed a slight decrease compared to the single-task values. These findings are in line with previous studies focused on cognitive dual-task condition and are possibly attributed to an attention overloading during the concomitant tasks66, 67 or to learning effects between repeated trials.31, 33, 39 With the increasing use of dual-task outcome measures in clinical trials, it is important to balance and minimize learning effects due to test familiarization to ensure that observed effects can be attributed to the intervention itself. Some techniques could be applied to decrease the likelihood of a learning effect due to practice, such as starting from a randomized number or initial letter on each dual-task test.39 However, the number of studies focusing on dual-task condition is limited with respect to single-task condition and new research on this topic is advocated.
It is important to mention that in this review the reliability point estimates were used as cut-off points to discriminate between acceptable and suboptimal reliability values. For some subgroup analyses (Figure 4), the 95% CI boundaries of ICC estimate are wide, and the lower bounds of the confidence intervals are often below the acceptability threshold (ICC>0.75). This fact is potentially generated by some limitations of the included studies. Firstly, as shown in Supplementary Table II, most of the included studies presented a fairly small sample size (<30 participants); secondarily, some gait parameters, test conditions and pathologies are currently understudied. These elements reduced the overall sample size of the meta-analyses and increased uncertainties around the point of estimate. Using the lower boundaries of 95% CIs as a cut-off point to discriminate between acceptable (95% CI lower boundaries of ICC>0.75) and suboptimal reliability, the number of suboptimal reliability findings was 27% of the total number of analyses. Future research is invited to adopt a sufficiently powered sample size and to report the consequent sample size calculation, as recommended by the guidelines for reporting reliability and agreement studies.28
Although ICC is a commonly reported measure to assess reliability, it merely informs about the magnitude of measurement error relative to the between-subject variability for the tested sample tested.28 In situations with a large range of scores, it could happen that ICC is excellent despite large between-subjects differences in the scores among test-retest trials.28 To overcome this issue, the complementary use of an absolute reliability measure as MDC95 is fundamental to provide a useful consistency indicator that is not affected by heterogeneous samples.28 MDC95 allows assessors to interpret if an observed change score is above that expected due to random measurement error and therefore if it represents a real change in gait performance. This review provided the guidance on MDC95 across different test conditions and populations, that should be used when assessing individual changes in gait performance produced by an intervention or by a disease progression, when calculating the sample sizes for a clinical trial or when applying methods to adjust for measurement error in epidemiological research.
In the current review, except for some isolated cases, the examined gait parameters showed small and satisfactory measurement errors, generally demonstrating an MDC95% lower than the 30% (Figure 5). This means that small real changes in the measurement could be confidently detected by these systems. However, it is important to note that the base of support generally presented good ICC values (0.65-0.94) but suboptimal MDC95 values (>30%), meaning that only quite large variations in this measurement could be securely detected as real. The base of support is defined as the distance between the heels when walking. The pressure-sensitive mats computed this parameter as the perpendicular distance from the center of heel of one footfall to the line of progression of the opposite foot.41 The observation of a larger MDC95 for base of support can be explained by several factors. Menz et al.41 hypothesized two possible explanations. The first hypothesis concerned a technical shortcoming of these systems. The spatial resolution of the pressure sensor grids, generally 1.3 cm, was too low in relation to the magnitude of the base of support parameter.41 Given the fact that the base of support values typically ranged from 2 up to 5 cm, the occasional failure to detect one or two 1.3 cm wide sensors from a footfall could result in a large between-trial differences and suboptimal measurement error. While, for other spatial parameters as the stride length, the adopted spatial resolution could be considered adequate because the distance between the two footfalls in the stride length measurements is considerably larger than the base of support measures. Lastly, the base of support is an important parameter in the maintenance of postural stability.68, 69 The observation of a larger MDC95 for the base of support might be caused by the inherent challenge associated with postural stability requirements during walking.68, 69 Impairments in postural stability could increase stride width variability and potentially impact the reliability measures.68, 69
To conclude, when interpreting MDC95 values, it is important to compare them to the minimal clinically important differences (MCIDs), defined as the smallest amounts of change in the domain that are considered relevant and important to patients and clinicians.22 Achieving a score at least equal to the MCID suggests that an individual has undergone a change in condition that reflects a clinically relevant change.22 Theoretically, the MDC95, which is a threshold of measurement error should be lower than the MCID.70 However, in the current scientific literature, the provision of clinically meaningful cutoff points for all the gait parameters captured by pressure-sensitive walkways is limited and the MDC95 and MCID comparisons are currently not feasible.
Methodological quality criteria are crucial for assessment and to identify efficient instruments for clinical practice. The COSMIN criteria facilitated the judgement of methodological quality of the included studies. Overall, the methodological quality was from good to excellent in more than half (51%) of the included studies. The most common methodological shortcomings were the definition of an appropriate time interval between test and re-test examinations and the evidence of stable individuals in the studies. Some studies indeed did not specify if the enrolled participants were stable during the test and retest examination period. Furthermore, there were some studies that adopted a very short time interval (in the range of a few minutes) between repeated sessions, potentially leading to the risk of fatigue or learning effects in the individuals. Indeed, a learning effect is a well-recognized factor that may influence the reliability of a given test: an individual may become more proficient in a given test with increased experience, as consecutively repeating a cognitive task. This factor might substantially decrease the amount of random error in the test findings and, hence, improve the reliability of the test. On the contrary, the concomitant fatigue could affect gait performances in continuous repetitive trials, causing an increase in the number of random errors and consequential decrease in test-retest reliability. At the same time, there were some studies that used a range of some weeks as time interval between testing sessions. This period could be too long, and individuals could potentially have an actual change in their gait patterns in the meantime, which theoretically biases the reliability findings. Future research should consider the shortcomings of the currently available articles to design upcoming studies with high methodological quality. In particular, the assessment of stable health conditions before testing trials and the adoption of optimal standardized protocols to perform test-retest examinations should be recommended.
Limitations of the study
This review has some strengths, but the results should also be interpreted in light of some limitations. The main strength laid in the rigorous systematic process and methodological quality assessment achieved by the use of PRISMA and COSMIN checklists, widely validated tools to report review findings and to examine the risk of bias in reliability studies. Another strength was the relatively comprehensive and exhaustive literature search, attested by the broad search string adopted and without the application of search filters that could potentially lead to retrieval bias. However, we did not consult all electronic databases, nor grey literature, a strategy that future reviews might consider. Thus, there is a slight possibility to miss some studies that could be relevant for the present systematic review. In addition, this review did not take into account studies enrolling very young populations (age <18 years old). Future works should include and review the reliability of gait parameters in this population sample, both in healthy and pathological conditions. Another limitation of the present review was that it was restricted to examine the measurement properties of test-retest reliability and MDC95. In addition, we did not include any other exploratory subgroup analysis to investigate how possible confounding factors influence reliability results for each subset of individuals, due to the limited number of available studies that limited possible comparisons. Finally, most of the included studies (88%) investigated the reliability properties of GAITRite® (CIR Systems Inc.) walkway, and limited information is available for the other pressure-sensitive walkways. Consequently, this review did not robustly detect which pressure-sensitive walkway differences could influence the reliability results for the different clinical populations. To the best of our knowledge, only one previous study directly compared the characteristics and the concurrent validity of two pressure-sensitive walkways (GAITRite® [CIR Systems Inc.] and Zeno™ Walkway [Protokinetics LLC]), showing moderate to excellent validity for gait parameters in older adults.64 In conclusion, future studies should examine how possible confounding factors could influence the reliability results of gait parameters captured by pressure-sensitive walkways, as well as investigate their intra- and inter-rater reliability, and concurrent validity with other gait analysis instruments and clinical tests in different clinical populations.
Conclusions
The review provides a relevant contribution to knowledge by comprehensively examining the reliability of gait spatial and temporal parameters captured by pressure-sensitive instruments in healthy individuals as well as several pathological conditions. The review generally showed from good to excellent test-retest reliability and satisfactory minimal detectable changes, despite a large variety among studies. High heterogeneity was indeed observed in the examined clinical conditions, experimental design protocols, and methodological quality. In addition, the results of the current review showed that the reliability of the gait parameters in many pathological conditions is either understudied or unexplored. Therefore, more high-quality studies devoted to investigating these understudied or unexplored conditions should warrant further investigations.
Supplementary Digital Material 1
Supplementary Table I
Search strategy.
Supplementary Digital Material 2
Supplementary Table II
Main characteristics and methodological quality of the included studies.
Supplementary Digital Material 3
Supplementary Table III
Included studies.
References
- 1.Baker R. Gait analysis methods in rehabilitation. J Neuroeng Rehabil 2006;3:4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16512912&dopt=Abstract 10.1186/1743-0003-3-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rispens SM, van Schooten KS, Pijnappels M, Daffertshofer A, Beek PJ, van Dieën JH. Identification of fall risk predictors in daily life measurements: gait characteristics’ reliability and association with self-reported fall history. Neurorehabil Neural Repair 2015;29:54–61. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24759809&dopt=Abstract 10.1177/1545968314532031 [DOI] [PubMed] [Google Scholar]
- 3.Pistacchi M, Gioulis M, Sanson F, De Giovannini E, Filippi G, Rossetto F, et al. Gait analysis and clinical correlations in early Parkinson’s disease. Funct Neurol 2017;32:28–34. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28380321&dopt=Abstract 10.11138/FNeur/2017.32.1.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu B, Hu X, Zhang Q, Fan Y, Li J, Zou R, et al. Usual walking speed and all-cause mortality risk in older people: A systematic review and meta-analysis. Gait Posture 2016;44:172–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27004653&dopt=Abstract 10.1016/j.gaitpost.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 5.Chamberlin ME, Fulwider BD, Sanders SL, Medeiros JM. Does fear of falling influence spatial and temporal gait parameters in elderly persons beyond changes associated with normal aging? J Gerontol A Biol Sci Med Sci 2005;60:1163–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16183957&dopt=Abstract 10.1093/gerona/60.9.1163 [DOI] [PubMed] [Google Scholar]
- 6.Kikkert LH, Vuillerme N, Van Campen JP, Appels BA, Hortobágyi T, Lamoth CJ. Gait characteristics and their discriminative power in geriatric patients with and without cognitive impairment. J Neuroeng Rehabil 2017;14:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bridenbaugh SA, Kressig RW. Motor cognitive dual tasking: early detection of gait impairment, fall risk and cognitive decline. Z Gerontol Geriatr 2015;48:15–21. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25633391&dopt=Abstract 10.1007/s00391-014-0845-0 [DOI] [PubMed] [Google Scholar]
- 8.Bayot M, Dujardin K, Dissaux L, Tard C, Defebvre L, Bonnet CT, et al. Can dual-task paradigms predict Falls better than single task? - A systematic literature review. Neurophysiol Clin 2020;50:401–40. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33176988&dopt=Abstract 10.1016/j.neucli.2020.10.008 [DOI] [PubMed] [Google Scholar]
- 9.Spildooren J, Vercruysse S, Desloovere K, Vandenberghe W, Kerckhofs E, Nieuwboer A. Freezing of gait in Parkinson’s disease: the impact of dual-tasking and turning. Mov Disord 2010;25:2563–70. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20632376&dopt=Abstract 10.1002/mds.23327 [DOI] [PubMed] [Google Scholar]
- 10.Pieruccini-Faria F, Jones JA, Almeida QJ. Motor planning in Parkinson’s disease patients experiencing freezing of gait: the influence of cognitive load when approaching obstacles. Brain Cogn 2014;87:76–85. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24727559&dopt=Abstract 10.1016/j.bandc.2014.03.005 [DOI] [PubMed] [Google Scholar]
- 11.Hillel I, Gazit E, Nieuwboer A, Avanzino L, Rochester L, Cereatti A, et al. Is every-day walking in older adults more analogous to dual-task walking or to usual walking? Elucidating the gaps between gait performance in the lab and during 24/7 monitoring. Eur Rev Aging Phys Act 2019;16:6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31073340&dopt=Abstract 10.1186/s11556-019-0214-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feld JA, Plummer P. Patterns of cognitive-motor dual-task interference post stroke: an observational inpatient study at hospital discharge. Eur J Phys Rehabil Med 2021;57:327–36. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32935952&dopt=Abstract 10.23736/S1973-9087.20.06273-5 [DOI] [PubMed] [Google Scholar]
- 13.Bryant MS, Workman CD, Jackson GR. Multidirectional walk test in individuals with Parkinson’s disease: a validity study. Int J Rehabil Res 2015;38:88–91. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25371075&dopt=Abstract 10.1097/MRR.0000000000000091 [DOI] [PubMed] [Google Scholar]
- 14.Ferrarello F, Bianchi VA, Baccini M, Rubbieri G, Mossello E, Cavallini MC, et al. Tools for observational gait analysis in patients with stroke: a systematic review. Phys Ther 2013;93:1673–85. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23813091&dopt=Abstract 10.2522/ptj.20120344 [DOI] [PubMed] [Google Scholar]
- 15.Schlachetzki JC, Barth J, Marxreiter F, Gossler J, Kohl Z, Reinfelder S, et al. Wearable sensors objectively measure gait parameters in Parkinson’s disease. PLoS One 2017;12:e0183989. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29020012&dopt=Abstract 10.1371/journal.pone.0183989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ambrosini E, Parati M, Peri E, De Marchis C, Nava C, Pedrocchi A, et al. Changes in leg cycling muscle synergies after training augmented by functional electrical stimulation in subacute stroke survivors: a pilot study. J Neuroeng Rehabil 2020;17:35. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32106874&dopt=Abstract 10.1186/s12984-020-00662-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muro-de-la-Herran A, García-Zapirain B, Méndez-Zorrilla A. Gait analysis methods: an overview of wearable and non-wearable systems, highlighting clinical applications. Sensors (Basel) 2014;14:3362–94. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24556672&dopt=Abstract 10.3390/s140203362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MejiaCruz Y, Franco J, Hainline G, Fritz S, Jiang Z, Caicedo JM, et al.; Mejia Cruz Y. Walking speed measurement technology: A review. Curr Geriatr Rep 2021;10:32–41. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33816062&dopt=Abstract 10.1007/s13670-020-00349-z [DOI] [PMC free article] [PubMed]
- 19.Cleland BT, Arshad H, Madhavan S. Concurrent validity of the GAITRite electronic walkway and the 10-m walk test for measurement of walking speed after stroke. Gait Posture 2019;68:458–60. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30599332&dopt=Abstract 10.1016/j.gaitpost.2018.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonough AL, Batavia M, Chen FC, Kwon S, Ziai J. The validity and reliability of the GAITRite system’s measurements: A preliminary evaluation. Arch Phys Med Rehabil 2001;82:419–25. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11245768&dopt=Abstract 10.1053/apmr.2001.19778 [DOI] [PubMed] [Google Scholar]
- 21.Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res 2005;19:231–40. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15705040&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 22.Beninato M, Portney LG. Applying concepts of responsiveness to patient management in neurologic physical therapy. J Neurol Phys Ther 2011;35:75–81. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21934362&dopt=Abstract 10.1097/NPT.0b013e318219308c [DOI] [PubMed] [Google Scholar]
- 23.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19622552&dopt=Abstract 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mokkink LB, Prinsen CA, Patrick DL, Alonso J, Bouter LM, de Vet HC, et al. COSMIN manual for systematic reviews of PROMs. COSMIN; 2018 [Internet]. Available from: https://cosmin.nl/wp-content/uploads/COSMIN-syst-review-for-PROMs-manual_version-1_feb-2018.pdf [cited 2021, Jan 4].
- 25.Hollman JH, McDade EM, Petersen RC. Normative spatiotemporal gait parameters in older adults. Gait Posture 2011;34:111–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21531139&dopt=Abstract 10.1016/j.gaitpost.2011.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mokkink LB, de Vet HC, Prinsen CA, Patrick DL, Alonso J, Bouter LM, et al. COSMIN Risk of Bias checklist for systematic reviews of Patient-Reported Outcome Measures. Qual Life Res 2018;27:1171–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29260445&dopt=Abstract 10.1007/s11136-017-1765-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res 2010;19:539–49. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20169472&dopt=Abstract 10.1007/s11136-010-9606-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 2016;15:155–63. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27330520&dopt=Abstract 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klotzbier TJ, Wollesen B, Vogel O, Rudisch J, Cordes T, Jöllenbeck T, et al. An interrater reliability study of gait analysis systems with the dual task paradigm in healthy young and older adults. Eur Rev Aging Phys Act 2021;18:17. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34344302&dopt=Abstract 10.1186/s11556-021-00271-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med 1998;26:217–38. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9820922&dopt=Abstract 10.2165/00007256-199826040-00002 [DOI] [PubMed] [Google Scholar]
- 31.Chen A, Kirkland MC, Wadden KP, Wallack EM, Ploughman M. Reliability of gait and dual-task measures in multiple sclerosis. Gait Posture 2020;78:19–25. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32179457&dopt=Abstract 10.1016/j.gaitpost.2020.03.004 [DOI] [PubMed] [Google Scholar]
- 32.Viechtbauer W. Conducting meta-analyses in R with the metafor. J Stat Softw 2010;36:1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 33.Decavel P, Moulin T, Sagawa Y, Jr. Gait tests in multiple sclerosis: reliability and cut-off values. Gait Posture 2019;67:37–42. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30269001&dopt=Abstract 10.1016/j.gaitpost.2018.09.020 [DOI] [PubMed] [Google Scholar]
- 34.van Uden CJ, Besser MP. Test-retest reliability of temporal and spatial gait characteristics measured with an instrumented walkway system (GAITRite). BMC Musculoskelet Disord 2004;5:13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15147583&dopt=Abstract 10.1186/1471-2474-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manor B, Yu W, Zhu H, Harrison R, Lo OY, Lipsitz L, et al. Smartphone app–based assessment of gait during normal and dual-task walking: demonstration of validity and reliability. JMIR Mhealth Uhealth 2018;6:e36. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29382625&dopt=Abstract 10.2196/mhealth.8815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ade V, Schalkwijk D, Psarakis M, Laporte MD, Faras TJ, Sandoval R, et al. Between session reliability of heel-to-toe progression measurements in the stance phase of gait. PLoS One 2018;13:e0200436. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30001382&dopt=Abstract 10.1371/journal.pone.0200436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silsupadol P, Teja K, Lugade V. Reliability and validity of a smartphone-based assessment of gait parameters across walking speed and smartphone locations: Body, bag, belt, hand, and pocket. Gait Posture 2017;58:516–22. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28961548&dopt=Abstract 10.1016/j.gaitpost.2017.09.030 [DOI] [PubMed] [Google Scholar]
- 38.Schmitz-Hübsch T, Brandt AU, Pfueller C, Zange L, Seidel A, Kühn AA, et al. Accuracy and repeatability of two methods of gait analysis - GaitRite™ und Mobility Lab™ - in subjects with cerebellar ataxia. Gait Posture 2016;48:194–201. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27289221&dopt=Abstract 10.1016/j.gaitpost.2016.05.014 [DOI] [PubMed] [Google Scholar]
- 39.Monticone M, Ambrosini E, Fiorentini R, Rocca B, Liquori V, Pedrocchi A, et al. Reliability of spatial-temporal gait parameters during dual-task interference in people with multiple sclerosis. A cross-sectional study. Gait Posture 2014;40:715–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25086800&dopt=Abstract 10.1016/j.gaitpost.2014.06.015 [DOI] [PubMed] [Google Scholar]
- 40.Paterson KL, Hill KD, Lythgo ND, Maschette W. The reliability of spatiotemporal gait data for young and older women during continuous overground walking. Arch Phys Med Rehabil 2008;89:2360–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19061748&dopt=Abstract 10.1016/j.apmr.2008.06.018 [DOI] [PubMed] [Google Scholar]
- 41.Menz HB, Latt MD, Tiedemann A, Mun San Kwan M, Lord SR. Reliability of the GAITRite walkway system for the quantification of temporo-spatial parameters of gait in young and older people. Gait Posture 2004;20:20–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15196515&dopt=Abstract 10.1016/S0966-6362(03)00068-7 [DOI] [PubMed] [Google Scholar]
- 42.Bilney B, Morris M, Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture 2003;17:68–74. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12535728&dopt=Abstract 10.1016/S0966-6362(02)00053-X [DOI] [PubMed] [Google Scholar]
- 43.Steinert A, Sattler I, Otte K, Röhling H, Mansow-Model S, Müller-Werdan U. Using new camera-based technologies for gait analysis in older adults in comparison to the established GAITrite system. Sensors (Basel) 2019;20:1. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31878177&dopt=Abstract 10.3390/s20010125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Byun S, Han JW, Kim TH, Kim KW. Test-retest reliability and concurrent validity of a single tri-axial accelerometer-based gait analysis in older adults with normal cognition. PLoS One 2016;11:e0158956. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27427965&dopt=Abstract 10.1371/journal.pone.0158956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hars M, Herrmann FR, Trombetti A. Reliability and minimal detectable change of gait variables in community-dwelling and hospitalized older fallers. Gait Posture 2013;38:1010–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23790571&dopt=Abstract 10.1016/j.gaitpost.2013.05.015 [DOI] [PubMed] [Google Scholar]
- 46.Beauchet O, Freiberger E, Annweiler C, Kressig RW, Herrmann FR, Allali G. Test-retest reliability of stride time variability while dual tasking in healthy and demented adults with frontotemporal degeneration. J Neuroeng Rehabil 2011;8:37. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21745370&dopt=Abstract 10.1186/1743-0003-8-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Youdas JW, Childs KB, McNeil ML, Mueller AC, Quilter CM, Hollman JH. Responsiveness of 2 procedures for measurement of temporal and spatial gait parameters in older adults. PM R 2010;2:537–43. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20630440&dopt=Abstract 10.1016/j.pmrj.2010.02.008 [DOI] [PubMed] [Google Scholar]
- 48.Brach JS, Perera S, Studenski S, Newman AB. The reliability and validity of measures of gait variability in community-dwelling older adults. Arch Phys Med Rehabil 2008;89:2293–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19061741&dopt=Abstract 10.1016/j.apmr.2008.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewek MD, Sykes R, 3rd. Minimal Detectable Change for Gait Speed Depends on Baseline Speed in Individuals With Chronic Stroke. J Neurol Phys Ther 2019;43:122–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30702510&dopt=Abstract 10.1097/NPT.0000000000000257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho KH, Lee HJ, Lee WH. Test-retest reliability of the GAITRite walkway system for the spatio-temporal gait parameters while dual-tasking in post-stroke patients. Disabil Rehabil 2015;37:512–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24957081&dopt=Abstract 10.3109/09638288.2014.932445 [DOI] [PubMed] [Google Scholar]
- 51.Peters DM, Middleton A, Donley JW, Blanck EL, Fritz SL. Concurrent validity of walking speed values calculated via the GAITRite electronic walkway and 3 meter walk test in the chronic stroke population. Physiother Theory Pract 2014;30:183–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24164441&dopt=Abstract 10.3109/09593985.2013.845805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuys SS, Brauer SG, Ada L. Test-retest reliability of the GAITRite system in people with stroke undergoing rehabilitation. Disabil Rehabil 2011;33:1848–53. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21265611&dopt=Abstract 10.3109/09638288.2010.549895 [DOI] [PubMed] [Google Scholar]
- 53.Lewek MD, Randall EP. Reliability of spatiotemporal asymmetry during overground walking for individuals following chronic stroke. J Neurol Phys Ther 2011;35:116–21. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21934372&dopt=Abstract 10.1097/NPT.0b013e318227fe70 [DOI] [PubMed] [Google Scholar]
- 54.Vartiainen MV, Savolainen S, Alaranta HT. Reliability and agreement in gait measurements among patients with brain injury. Adv Physiother 2009;11:22–9. 10.1080/14038190701879898 [DOI] [Google Scholar]
- 55.Lemke NC, Wiloth S, Werner C, Hauer K. Validity, test-retest reliability, sensitivity to change and feasibility of motor-cognitive dual task assessments in patients with dementia. Arch Gerontol Geriatr 2017;70:169–79. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28182895&dopt=Abstract 10.1016/j.archger.2017.01.016 [DOI] [PubMed] [Google Scholar]
- 56.Montero-Odasso M, Casas A, Hansen KT, Bilski P, Gutmanis I, Wells JL, et al. Quantitative gait analysis under dual-task in older people with mild cognitive impairment: a reliability study. J Neuroeng Rehabil 2009;6:35. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19772593&dopt=Abstract 10.1186/1743-0003-6-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wittwer JE, Webster KE, Andrews PT, Menz HB. Test-retest reliability of spatial and temporal gait parameters of people with Alzheimer’s disease. Gait Posture 2008;28:392–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18321707&dopt=Abstract 10.1016/j.gaitpost.2008.01.007 [DOI] [PubMed] [Google Scholar]
- 58.Pérez-Sanpablo AI, Quinzaños-Fresnedo J, Loera-Cruz R, Quiñones-Uriostegui I, Rodriguez-Reyes G, Pérez-Zavala R. Validation of the instrumented evaluation of spatio-temporal gait parameters in patients with motor incomplete spinal cord injury. Spinal Cord 2017;55:699–704. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28244503&dopt=Abstract 10.1038/sc.2017.4 [DOI] [PubMed] [Google Scholar]
- 59.Nair PM, Hornby T G, Behrman AL. Minimal detectable change for spatial and temporal measurements of gait after incomplete spinal cord injury. Top Spinal Cord Inj Rehabil 2012;18:273–81. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23459394&dopt=Abstract 10.1310/sci1803-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rome K, Hanchard NC. Within-day reliability of temporal-spatial gait parameters associated with rheumatoid arthritic feet. Musculoskelet Care 2005;3:17–23. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17041990&dopt=Abstract 10.1002/msc.22 [DOI] [PubMed] [Google Scholar]
- 61.Schmidheiny A, Swanenburg J, Straumann D, de Bruin ED, Knols RH. Discriminant validity and test re-test reproducibility of a gait assessment in patients with vestibular dysfunction. BMC Ear Nose Throat Disord 2015;15:6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26500447&dopt=Abstract 10.1186/s12901-015-0019-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu S, Cheng N, Liu Y, Wang X, Yang X, Sun Y, et al. Reliability and validity of the assessment of Parkinson gait using the U-shape electronic walkway. Proceedings of the 2016 IEEE Advanced Information Management, Communicates, Electronic and Automation Control Conference (IMCEC); 2016 Oct 3–5; Xi’an, China. [Google Scholar]
- 63.Rao AK, Quinn L, Marder KS. Reliability of spatiotemporal gait outcome measures in Huntington’s disease. Mov Disord 2005;20:1033–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15838854&dopt=Abstract 10.1002/mds.20482 [DOI] [PubMed] [Google Scholar]
- 64.Vallabhajosula S, Humphrey SK, Cook AJ, Freund JE. Concurrent Validity of the Zeno Walkway for Measuring Spatiotemporal Gait Parameters in Older Adults. J Geriatr Phys Ther 2019;42:E42–50. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29286982&dopt=Abstract 10.1519/JPT.0000000000000168 [DOI] [PubMed] [Google Scholar]
- 65.Egerton T, Thingstad P, Helbostad JL. Comparison of programs for determining temporal-spatial gait variables from instrumented walkway data: PKmas versus GAITRite. BMC Res Notes 2014;7:542. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25134621&dopt=Abstract 10.1186/1756-0500-7-542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang L, Liao LR, Lam FM, He CQ, Pang MY. Psychometric properties of dual-task balance assessments for older adults: a systematic review. Maturitas 2015;80:359–69. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25618745&dopt=Abstract 10.1016/j.maturitas.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 67.Yang L, Lam FM, Liao LR, Huang MZ, He CQ, Pang MY. Psychometric properties of dual-task balance and walking assessments for individuals with neurological conditions: A systematic review. Gait Posture 2017;52:110–23. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27893997&dopt=Abstract 10.1016/j.gaitpost.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 68.Terrier P, Reynard F. Effect of age on the variability and stability of gait: a cross-sectional treadmill study in healthy individuals between 20 and 69 years of age. Gait Posture 2015;41:170–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25455699&dopt=Abstract 10.1016/j.gaitpost.2014.09.024 [DOI] [PubMed] [Google Scholar]
- 69.Donelan JM, Shipman DW, Kram R, Kuo AD. Mechanical and metabolic requirements for active lateral stabilization in human walking. J Biomech 2004;37:827–35. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15111070&dopt=Abstract 10.1016/j.jbiomech.2003.06.002 [DOI] [PubMed] [Google Scholar]
- 70.Beninato M, Fernandes A, Plummer LS. Minimal clinically important difference of the functional gait assessment in older adults. Phys Ther 2014;94:1594–603. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24947198&dopt=Abstract 10.2522/ptj.20130596 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table I
Search strategy.
Supplementary Table II
Main characteristics and methodological quality of the included studies.
Supplementary Table III
Included studies.