Abstract
Objective:
Asthma is a chronic lung disease comprising multiple endotypes, and characterized by periodic exacerbations. A diverse array of T cells have been shown to contribute to all endotypes of asthma in pathogenic and regulatory roles. Here, we review the contributions of CD4+, CD8+, and unconventional T cells in allergic and non-allergic asthma.
Data Sources:
Review of published literature pertaining to conventional and unconventional T-cell types in asthma.
Study Selections:
Recent peer-reviewed articles pertaining to T cells in asthma, with additional peer-reviewed studies for context.
Results:
Much research in asthma has focused on the roles of CD4+ Th cells. Roles for Th2 cells in promoting allergic asthma pathogenesis have been well-described, and the recent description of pathogenic Th2a cells provides additional insight into these responses. Other Th types, notably Th1 and Th17, have been linked to neutrophilic and steroid-resistant asthma phenotypes. Beyond CD4+ T cells, CD8+ Tc2 cells are also strongly associated with allergic asthma. An emerging area for study is unconventional T-cell types, including gd T, iNKT, and MAIT cells. While data in asthma remains limited for these cells, their ability to bridge innate and adaptive responses likely makes them key players in asthma. A number of asthma therapies target T-cell responses, and, although data are limited, appear to modulate T-cell populations.
Conclusion:
Given the diversity and heterogeneity of asthma and T-cell responses, there remain many rich avenues for research to better understand the pathogenesis of asthma. Despite the breadth of T cells in asthma, approved therapeutics remain limited to Th2 networks.
Keywords: Asthma, CD4+ T helper cells, CD8+ Cytotoxic T cells, γδT cells, iNKT cells, MAIT cells, Biologic therapies
Introduction
Asthma is a chronic respiratory disease characterized by periods of reversible airway hyperreactivity that affects approximately 260 million people worldwide and caused an estimated 460,000 deaths in 20191. Asthma is a heterogeneous disease, including both allergic and non-allergic endotypes2. Allergic asthma is characterized by the induction of IgE antibodies to otherwise innocuous aeroallergens, is the most prevalent form of asthma in children, and accounts for approximately 50% of asthma in adults3, whereas non-allergic asthma is typically adult-onset. While standard therapies result in disease control for the majority of asthma patients, a subset of 5–10% of patients remain refractory to treatment and frequently utilize healthcare resources4. A greater understanding of asthma pathogenesis is essential in order to effectively treat patients in whom standard care fails.
A diverse array of T-cells contribute to asthma development and exacerbation. These T-cells are influenced by a myriad of factors, including allergen and virus exposures, sex hormones, and obesity. While early life exposures and infections are important in immune development and asthma inception5–7, asthma and immune responses evolve over a lifetime8,9, adding to the complexity of T-cell mechanisms underlying asthma. In this review, we will discuss the roles of T-cells in asthma, with a major focus on the roles of CD4+ T-cells in allergic asthma and the impact of therapeutic intervention on T-cell populations.
Current perspectives on CD4+ T-cells in asthma
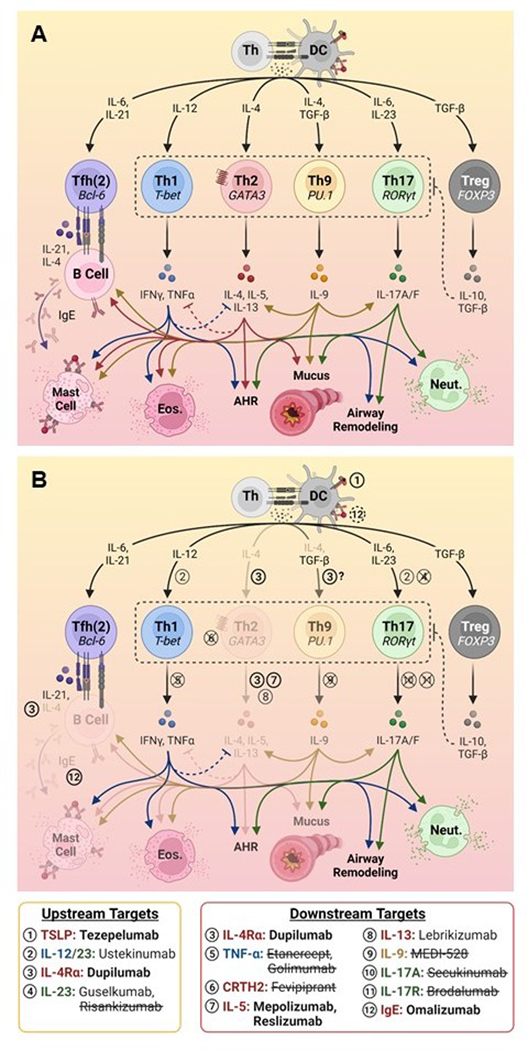
CD4+ T helper (Th) cells, can differentiate into a number of subsets with distinct functions, and are a major focus of asthma research. These functions encompass promotion of allergic inflammation (Th2, Th9), anti-viral and extracellular bacteria immune defense (Th1, Th17), immune regulation (Treg), and promotion of antibody responses (Tfh). Furthermore, Th-subset differentiation is plastic, and can be impacted by local inflammatory cues10. Here, we review the roles of diverse Th-subsets in allergic and non-allergic asthma, depicted in Figure 1.
Figure 1. Differentiation and therapeutic targeting of Th cells involved in asthma pathogenesis.
(A) Overview of the differentiation of Th subsets, production of key effector cytokines, and their downstream effects. (B) Therapeutic targeting of T-cell responses, denoted numerically. Treatments approved for use in asthma are denoted in bold font, with faded downstream immune pathways; treatments that are no longer in development and/or that lacked efficacy in clinical trials for asthma are struck through; treatments requiring further investigation are depicted in normal font. Figure created using BioRender.com.
AHR, airway hyperreactivity; DC, dendritic cell; Eos., eosinophil; Neut., neutrophil, Th, T helper
Th2 cells and pathogenic subsets:
Of the Th-subsets, Th2 cells are the most commonly ascribed to asthma pathogenesis, due to their key role in allergen responses. Th2-polarization is induced by dendritic cells (DC) that are primed by the innate cytokines IL-25, IL-33, and thymic stromal lymphopoietin (TSLP)11, leading to the upregulation of the transcription factor GATA3 in T-cells12. Upon allergen exposure, Th2 cells promote IgE production (IL-4, IL-13), mucus hypersecretion (IL-13), and eosinophil recruitment to the airways (IL-5), culminating in airway hyperreactivity (AHR)11. Th2 cells also express the chemoattractant receptors CCR4 and CRTH2, which aid in the trafficking of cells to inflamed tissue13,14. Recent work has shown that cells in different locations perform distinct functions, with circulating Th2 cells trafficking to the lung and promoting eosinophil recruitment, while tissue-resident cells promote mucus production and eosinophil activation15, and can induce AHR in the absence of peripheral Th2 cells16. Th2 responses have also been associated with BMI and AHR in mouse models of high-fat diet-induced obesity and asthma17, and sputum IL-5 is elevated in individuals with obese versus non-obese severe asthma18. This may be mediated via leptin, which can promote the proliferation, survival, and cytokine production of Th2 cells19.
Within the Th2 subset, there is a recently-described subpopulation of pathogenic cells known as Th2a. These cells are characterized by their co-expression of CRTH2, the tissue-homing integrin CD49d, and the natural killer (NK)-cell receptor CD161, and their capacity to secrete IL-4, IL-5, IL-9, and IL-1320,21. Th2a share features with pathogenic effector Th2 cells (peTh2), a subpopulation defined by the surface markers CRTH2 and CD161, and high expression of IL-5 and IL-1322. Th2a are the predominant phenotype of allergen-specific CD4+ T-cells and are present at increased frequencies in allergic individuals and reduced frequencies in desensitized individuals after immunotherapy, suggesting a key role for this population in allergic responses21,23,24. In addition to canonical and pathogenic Th2 cells, there exist “atypical” Th2 cells with mixed phenotypes. One such population is Th2/Th17 cells which co-express the transcription factors GATA3 and RORγt, and secrete IL-4 and IL-17A25, a key driver of neutrophilic inflammation. These cells are enriched in the airways of individuals with severe asthma and induce asthma exacerbations in mice25,26. Overall, Th2 cells are a key player in the pathogenesis of allergic asthma, but are not the only Th subset to play a role.
Th1 cells:
Th1 cells are key mediators of anti-viral responses. Th1-skewing is induced by IL-12, resulting in expression of the transcription factor T-bet and signature cytokines (IFN-γ, TNF-α). Much discussion of Th1 cells in allergic asthma has focused on Th1/Th2 counter-regulation27–31, and the notion that Th1 responses are deficient in allergic asthmatics, particularly in the context of respiratory viral infections32,33. By contrast, other studies have either failed to identify deficient anti-viral responses in allergic asthmatics, or observed enhanced responses34–38. It is possible that temporal differences account for these differences, with deficient early anti-viral responses giving rise to elevated viral titers and inflammation later in infection39,40, although data are conflicting34,37,41,42.
Mouse models are contradictory as to the roles of Th1 cells in asthma. Mice lacking Th1 cells spontaneously develop AHR, and Th1-cell adoptive transfer suppresses Th2 inflammation and AHR, supporting a Th2 counter-regulatory role43,44. Conversely, other mouse studies have demonstrated increased airway inflammation and cooperative recruitment of Th2 cells into the airways following Th1-cell adoptive transfer45–48, an IFN-γ requirement for the induction of AHR49, and a role for IFN-γ in RSV-induced exacerbations50. In humans, elevated IFN-γ and Th1 cells have been observed in the lungs of asthmatics51–55, including during exacerbation56,57. Th1 cells have also been implicated in severe, steroid-resistant58–62 and obesity-associated asthma63–65. There are several potential pathogenic effects of Th1-associated cytokines. IFN-γ can activate mast cells and induce AHR66,67, and TNF-α is a potent pro-inflammatory mediator with potential roles in the recruitment of immune cells and airway remodeling68–71. The complex interplay between Th1 and Th2 cells in viral and non-viral asthma exacerbations remains a key research topic.
Th17 cells:
Th17 cells are potent promoters of neutrophilic inflammation, and play a key role in host defense against extracellular bacteria. Th17 cells express the transcription factor RORγt and differentiate in response to IL-6 and IL-23, as well as neutrophil cytoplasts released by NETosis72. Th17 cells produce the signature cytokines IL-17A and IL-17F, as well as IL-21 and IL-22. IL-17 exerts pleiotropic effects in the airways, including promotion of neutrophilia, mucus production, AHR, and remodeling73,74. Numerous studies report increased Th17 cells and IL-17 in the blood and airways of asthmatic patients, which links with neutrophilia, AHR, and reduced asthma control75–77. IL-17 also exerts differential effects on allergic sensitization in mice depending on timing, with IL-17 promoting eosinophilic inflammation during asthma development, but suppressing eosinophilic inflammation in established disease78. Interestingly, rhinovirus, a major trigger of allergic asthma, has been shown to downregulate IL-17A in a mouse model of allergic asthma79. Th17 inflammation is also a feature of non-allergic asthma when compared to children with allergic asthma80. In mice, adoptive transfer of Th17 confers steroid-resistant AHR, which is reduced in mice that lack Th17 cells81,82. Interestingly, increased production of IL-17 by Th17 cells has been observed in female versus male severe asthma patients, as well as in mouse models in response to estradiol, implicating Th17 cells in sex differences in asthma83.
Th9 cells:
First described in 200884,85, Th9 cells are characterized by their production of IL-9 and expression of the lineage-defining transcription factor PU.1, which is induced by IL-4 and TGF-β84–87. In contrast with Th2a cells, Th9 cells lack expression of IL-4 and IL-1385. In mice, adoptive transfer of Th9 cells are sufficient to induce AHR to a similar extent as Th2 cells88,89. Importantly, AHR following the adoptive transfer of Th9, but not Th2 cells, is steroid-resistant in mice90. In humans, circulating Th9 cells are more frequent in allergic asthmatic versus healthy controls, and are linked to increased IgE89,91. IL-9+ T-cells are also increased in the lungs of asthmatics, with IL-9 levels linked to reduced FEV192. IL-9 promotes the survival and proliferation of cells that express IL-9R, including its major cellular target, mast cells93. Importantly, IL-9 activates Th2 and Th17 cells and promotes Th17 differentiation in mice94–96, while it inhibits IFN-γ production by CD4+ T-cells97. IL-9 has also been shown to directly promote mucin expression by airway epithelial cells98,99, IgE production by B cells in humans97,100,101, and may be a survival factor for eosinophils91, indicating a potential key role in asthma pathogenesis.
Tregs:
As their name suggests, regulatory T-cells (Tregs) play a key role in tolerance and the control of inflammation in asthma. Tregs express the transcription factor FOXP3, high levels of CD25, and the anti-inflammatory cytokines IL-10 and TGF-β. In keeping with their suppressive role, reduced frequencies and functional impairment of Tregs, and Treg/effector cell imbalance have been described in asthma56,102–106, as has loss of Treg function following rhinovirus infection107. Reduced circulating Tregs have also been reported for non-allergic late-onset asthma61, and Treg-associated genes in the lung are inversely correlated with asthma severity108. In contrast, increased numbers of Tregs have also been reported in the asthmatic lung and following allergen exposure, possibly in response to increased inflammation and/or due to reduced regulatory capacity109–111.
In mice, the presence of dysfunctional Tregs in early life promote progression to an asthmatic phenotype later in life112. Furthermore, Tregs can convert to pathogenic types in response to inflammatory signals including IL-4 and viral infection113,114. Accordingly, circulating CRTH2+ Tregs that produce IL-4, IL-5, and IL-13 are increased in allergic asthmatics, link to poor asthma control, and induce bronchial epithelial barrier dysfunction in vitro115,116. Similarly, dysregulated ST2+ Tregs, which produce IL-5 and IL-13, have been reported in the airways of mice117,118. IFN-γ+ and IL-17+ Tregs are also associated with reduced lung function in allergic asthmatics119. Androgen receptor signaling stabilizes Treg function and inhibits ST2+ Treg generation, potentially linking Treg dysfunction to increased asthma prevalence in female adults120. Treg dysfunction is also thought to play a role in obesity-associated asthma121,122, possibly attributable to inhibition by leptin123.
Tfh cells:
T follicular helper cells (Tfh) play a key role in the establishment of allergic sensitization through the promotion of IgE production124. Named for their capacity to enter B-cell follicles in secondary lymphoid organs, Tfh are essential in the regulation of germinal center reactions, and express the follicle-homing chemokine receptor CXCR5, the transcription factor Bcl-6, and IL-21. In keeping with a role in promoting IgE production, increased numbers of circulating Tfh have been observed in asthmatics, have been found to correlate with elevated IgE, and have been shown to increase IgE production in in vitro co-culture assays125,126. Tfh are further subcategorized according to expression of cytokines and chemokine receptors broadly analogous to corresponding Th types, and link to the production of Ig subclasses127. Accordingly, circulating Tfh2-polarized cells have been linked to elevated IgE and FeNO in allergic asthmatic subjects128, whereas regulatory B cells and Tfr are decreased in asthma129,130. Recently, IL-13+ IL-4+ IL-5+ IL-21− Tfh, termed Tfh13, were linked to high-affinity IgE production in house dust mite (HDM)-sensitized mice131. Furthermore, IL-4+ Tfh are required for the development of Th2 effectors and can differentiate into lung-homing Th2 effectors upon secondary HDM challenge in mice, implicating Tfh in the development of Th2 responses132.
CD8+ T-cells in asthma
The role of CD8+ T-cells in asthma has been less well-studied than CD4+ T-cells. CD8+ T cytotoxic (Tc) cells play key roles in host defense against intracellular pathogens and cancers, and are named for their capacity to directly kill infected cells. Similar to CD4+ cells, CD8+ cells differentiate into diverse subsets, including Tc1, characterized by the production of TNF-α and IFN-γ. In certain mouse and rat models of asthma, CD8+ T-cells fulfill a protective role through the production of IFN-γ and counter-regulation of Th2 responses133–138. CD8+ T-cells in adult male mice produce greater quantities of IFN-γ that suppress IL-4 production by CD4+ cells139, consistent with reduced asthma prevalence in adult males. In contrast, mice lacking CD8+ T-cells do not develop airway inflammation and hyperreactivity in response to HDM sensitization and challenge140. Type 2 cytokine-producing Tc2 cells have also been described in allergic asthma (reviewed in 141), are linked to increased AHR, and provide a steroid-resistant source of type 2 cytokines in both human and mouse models of allergic asthma142,143. Tc2 differentiation and activation occurs in response to type 2 cytokines, mast cell-derived lipid mediators (LTE4 and PGD2), allergen exposure, and hypoxia144–147. In some cases, CD8+ cells are more strongly linked to asthma severity than their CD4+ counterparts148, making them an important topic for research.
Defining roles for unconventional T-cells in asthma
Unconventional T cells, including γδT-cells, invariant NKT (iNKT) cells, and mucosal-associated invariant T (MAIT) cells, are an emerging field of study. In contrast with MHC I- and II-restricted conventional T-cells, unconventional T-cells do not recognize peptides presented by MHC molecules. Instead, these cells recognize a more limited set of antigens presented by MHC-I-like molecules, with a more limited receptor repertoire. These cells share features of innate immunity, responding rapidly after antigen encounter149. Their features are extensively described in 150–154, and summarized in Table 1. Further research is needed to define the role of unconventional T-cell types in asthma.
Table 1.
Comparison of human conventional and unconventional T cells.
| Conventional | Unconventional | ||||
|---|---|---|---|---|---|
| CD4+ (Th) cell | CD8+ (Tc) cell | γδT cell | iNKT cell | MAIT cell | |
| TCR chain repertoire | αβ (Diverse) | γδ (Limited) | Invariant αβ (Limited) | ||
| Antigen | Peptides | Peptides | Phosphoantigens | Glycolipids | Microbial metabolites |
| Restriction | MHC II | MHC I | Butyrophylin-dependent | CD1 | MR1 |
| CD4+ | + | − | + (rare) | +* | + (rare) |
| CD8+ | − | + | + (minor) | +* | + (major) |
| DN | − | − | + (major) | +* | + (minor) |
| CD161+ | +/++ (Th17) | + | +++ | +++ | ++++ |
| Initial response | 7–10 days | Hours-days | |||
γδ T-cells:
While conventional T-cells express TCRs composed of α and β chains, a minority of T-cells instead express γ and δ chains. These γδT-cells respond to phosphorylated antigens and lipoproteins in an MHC-independent manner, and have a number of potential functions, including cytotoxic killing, cytokine production, and antigen presentation150,154. Mice that lack γδT-cells demonstrate increased AHR upon allergen challenge, suggesting a regulatory role155–157. Accordingly, reduced numbers of γδT cells have been reported in the blood of asthmatic patients158,159, although others have found no differences in asthmatic versus healthy individuals160. In mice and humans, airway γδT-cells are increased in response to respiratory viral infection in asthma, and inhibition of γδT-cell responses in mice results in increased AHR and eosinophilic inflammation161. In contrast, other mouse studies report an essential role for γδT cells in the induction of IgE, production of Th2 cytokines, and eosinophil recruitment162,163. In humans, γδT-cells that produce IL-5, IL-13, and reduced IFN-γ have been observed in the asthmatic lung after allergen challenge164. In mice, γ-chain usage appears to be linked to suppressive versus pro-inflammatory roles, with Vγ4+ cells suppressing inflammation, and Vγ1+ cells promoting inflammation165,166.
Invariant Natural Killer T-cells and Mucosal-Associated Invariant T-cells:
Invariant natural killer T (iNKT) and MAIT-cells share many similarities and a few key differences. Both express invariant chain TCRs that recognize unconventional antigens presented by MHC I-like molecules, can be further classified according to single-positive and double-negative (DN) expression of CD4 and CD8, and express the NK-cell receptor CD161167,168.
Invariant natural killer T-cells recognize glycolipid antigens presented by CD1d molecules169. The potential role of iNKTs in asthma is controversial170. In some mouse models of asthma, IL4+ and IL-13+ iNKTs are both necessary and sufficient for the induction of AHR171–173. In contrast, similar induction of allergic inflammation in the lung was observed in mice that lack CD1d, and therefore iNKTs, as compared to mice with normal iNKT populations174. In humans, there are conflicting reports about the presence of iNKTs in the asthmatic lung, with some reporting increased numbers in the lungs of severe asthmatics as compared with controls175, and others reporting no differences176,177. More recently, increased frequencies of circulating iNKTs were observed in children within 24 hours of an asthma attack178, as well as in severe treatment-refractory asthma, although this was independent of atopy179. Furthermore, IL-4 production by iNKTs was elevated in children undergoing asthma exacerbation178, and circulating IL-4+ iNKTs in adult asthmatics links to reduced FEV1180. Activation of iNKTs has also been linked to obesity and asthma in mice181.
MAIT-cells are activated via TCR recognition of microbial vitamin B metabolites presented by MR1 molecules182,183, or else in a receptor-independent manner by cytokines184. Little is known about MAIT-cells in asthma. In keeping with a potential regulatory function, MAIT-cell numbers are reduced in the blood and lungs of patients with asthma185, and MAIT-cells regulate ILC2 responses and AHR in mouse models of Alternaria challenge186. Furthermore, increased MAIT-cell numbers at 1 year of age has been linked to reduced risk of developing asthma by 7 years187, suggesting that MAIT play a protective role in asthma development. In contrast, IL-17+ MAIT-cells have been observed in asthmatic children and adults with neutrophilic asthma, and are associated with symptoms and an exacerbator phenotype188–190.
Impact of therapeutic interventions on T-cell populations
Inhaled and oral steroids, which broadly and non-specifically modulate immune responses and are generally considered immunosuppressive, are commonly prescribed for the treatment of asthma. Importantly, glucocorticoid receptors are expressed by T-cells, among other cell types. Th subsets are thought to exhibit differential sensitivity to both direct and indirect steroid effects, with steroids inhibiting Th1 and Th2, but not Th17 cytokine production191,192. However, for many individuals the use of steroids does not result in asthma control. The development of targeted biologic therapies represent a major advance in the treatment of asthma. Here, we will review the potential effects of targeted biologic therapies on T-cell responses; however, it is important to note that these cytokines and receptors are also expressed by and impact other cell types beyond the scope of this review.
Th2-targeted therapies:
Th2 inflammatory pathways constitute a major target for therapeutic intervention in asthma, and data are limited about impacts on T-cell responses. Therapies that directly target Th2-skewing and activation are expected to enact a pronounced effect on Th2 cell populations. The recently approved monoclonal therapy tezepelumab targets TSLP, which plays a key role in Th2 cell differentiation193–196. Although tezepelumab decreased serum IL-5 and IL-13 in adult asthmatics, no effect was observed on airway submucosal CD3+ or CD4+ T-cells197. Research in mice suggests that TSLP blockade provides the greatest benefit during the initiation of allergic disease, versus established disease198. Anti-IL-4Ra (dupilumab), which targets both IL-4 and IL-13 signaling, is approved for use in eosinophilic asthma. Dupilumab disrupts Th2-skewing by IL-4, and blocks effects of Th2 cell-derived IL-4 and IL-13. While studies of effects on T-cells in asthma in humans are lacking, studies in atopic dermatitis found decreases in Th2 and increases in Th17 cells in the circulation199–201; however, no long-term changes in Th-skewing were detectable after 1 year199.
In addition to dupilumab, numerous therapies target T-cells downstream of Th-skewing. The CRTH2 antagonist fevipiprant, which blocks activation and chemotaxis of CRTH2+ cells, has also been explored as a type 2-targeted therapy. In vitro inhibition studies showed effects on human CD4+ and CD8+ T-cell populations, including inhibition of Th2 and Tc2 cytokine production, cell survival, migration, and tissue-remodeling networks in Tc2 cells202,203. Fevipiprant was pulled from development due to insufficient efficacy during phase 3 clinical trials204,205. Multiple therapies that target IL-5 are approved for use in allergic asthma. While mepolizumab and reslizumab block soluble IL-5, benralizumab also promotes antibody-directed cell-mediated cytotoxic killing of IL-5Ra+ cells, including eosinophils and basophils206. Increases in Tregs have been reported following mepolizumab treatment in patients with severe eosinophilic asthma207. Interestingly, reduced frequencies of CD8+ and NKT-like cells were observed following treatment with mepolizumab, but not benralizumab208. Anti-IL-13 (lebrikizumab) is also under investigation for use in asthma, but T-cell data are lacking. While anti-IgE (omalizumab) is not expected to directly affect T-cells, reductions in IgE-facilitated antigen presentation and/or alterations in the inflammatory milieu might alter T-cell responses. Some studies have found no difference in T-cell numbers or function in the blood of asthmatics treated with omalizumab37,209, whereas decreased numbers of lung CD4+, CD8+, and IL-4+ cells, and circulating IL-13+ T-cells have been observed following treatment210,211. Further, studies found decreased circulating Tfh, Tfh2, and type 2 cytokine production, and/or increased Treg and Tfr cells following omalizumab, indicating a shift from a Th2 to a regulatory immune state130,212–214. While studies of the immune impacts of long-term treatment with omalizumab are limited, CD4+ T-cell activation in the circulation has been observed in asthmatics receiving treatment for at least 3 years215.
Th17-targeted therapies:
Th17 cells pose attractive therapeutic targets in allergic asthma, particularly in individuals with neutrophilic inflammation. IL-17A disruption by blocking antibodies and IL-17R knockout reduces AHR and neutrophil recruitment into the airways in mice, and abolishes airway smooth muscle contraction in tracheal rings stimulated in vitro73,216,217, although impacts did not extend to Th2 cells216. Similarly, administration of anti-IL-23 in an OVA mouse model of asthma results in reduction of AHR and lung-infiltrating Th17 and Tc17 cells218, supporting the strategy of targeting Th17 inflammation in asthma.
While biologics targeting Th17 pathways are approved for a number of diseases including severe plaque psoriasis and psoriatic arthritis, none are currently approved for use in asthma. Phase 2 trials for anti-IL-17A and anti-IL-17R in asthmatics (secukinumab and brodalumab, respectively) were terminated due to a lack of improvement in asthma control (ClinicalTrials.gov Identifiers: NCT01478360 and NCT01902290), and results are not yet available from a phase 2 trial of anti-IL-17A/IL-17AF (NCT03299686). Anti-IL-23 therapies that target Th17 development are also available (anti-IL-12/23, ustekinumab; anti-IL23, guselkumab & risankizumab). A recent phase 2a study of risankizumab for severe asthma resulted in worse patient outcomes, with a reduced time to first asthma worsening, and increased exacerbation rates in those treated with the drug219. Interestingly, risankizumab was found to downregulate a number of T-cell-associated genes in the sputum (CD3E, CD8A), including genes encoding RORγt (RORC), T-bet (TBX21), genes involved in T-cell anti-viral responses and cytotoxic killing (IFNG, GZMB), indicating impacts on Th1, Th17, and CD8+ T cells219. This additional impact on airway immunity might contribute to poor outcomes. It is also likely that Th17-focused drugs would have greater efficacy if targeted to patients with Th17-high, neutrophilic asthma.
Other Th-targeted therapies:
The use of therapies targeting Th1, Th9, and Tfh pathways have also been proposed. Antagonism of the Th1 and pro-inflammatory cytokine TNF-α by etanercept in severe asthmatics resulted in improvement of lung function and restoration of steroid sensitivity, further supporting a role for Th1 responses in steroid-resistant asthma220,221. However, etanercept and another anti-TNF-α therapy, golimumab, did not significantly improve lung function or exacerbation rates and, importantly, the golimumab study was terminated early due to increased risk for infection and malignancies in the treatment group222,223. The Th17-targeting therapy ustekinumab is known to also inhibit Th1-skewing224; however, data are lacking in asthma. Although IL-9 blockade has shown promise in mouse models of asthma88, the anti-IL-9 monoclonal antibody MEDI-528 failed to significantly improve asthma control or lung function in a DBPC trial225. Dupilumab could also affect Th9-skewing, but data are currently lacking. In mice, administration of anti-ICOS-L, which disrupts ICOS/ICOS-L interactions necessary for Tfh phenotype maintenance226, depleted lung Tfh, and resulted in reduced IgE production, AHR, and IL-13227. While no anti-ICOS-L therapies are currently under investigation for asthma, ICOS-L antagonists are in development for cancer immunotherapy228. Thus, there remains a need for therapies that target non-Th2 pathways.
Conclusion
T cells perform diverse roles in the immunopathogenesis of asthma, from conventional CD4+ and CD8+ T-cells, to innate-like unconventional T-cell types. Great strides have been made in understanding mechanisms of Th2 responses in allergic asthma, including the identification of pathogenic Th2 subsets. Beyond Th2 cells, there is an increasing appreciation of the roles of diverse Th-cells in asthma, including Th17-associated neutrophilic asthma and Th1-associated severe steroid-resistant asthma. CD8+ T-cells and unconventional T-cell types, including γδT, iNKT, and MAIT cells, have also been linked to asthma outcomes in both pathogenic and protective capacities. While much remains unclear about unconventional T-cell responses, these cells are capable of acting in a manner reminiscent of Th2 and Th17 cells, in addition to providing regulatory functions.
While there are indications that asthma treatment with biologics such as anti-IgE can shift T-cell populations away from Th2 types, more research is needed into the effects of Th2-targeted biologics on T-cells in asthmatics, including the long-term effects of treatment, and persistence beyond the withdrawal of treatment. Although a number of Th2-targeted therapeutics are currently approved for use in asthma, there remains a need for additional treatments targeting other, non-allergic aspects of the asthmatic immune response. It is important to note that, while Th2-targeted drugs are employed in patients with Th2 biomarkers including elevated IgE and eosinophil counts, similar screening strategies are lacking for non-Th2 therapies. Given the heterogeneity of asthma and the difficulty in identifying effective asthma therapeutics across multiple inflammatory pathways, the importance of understanding patients’ underlying immune profiles for the appropriate selection of therapeutics is clear.
Key Messages.
Th2 cells, including the recently described pathogenic subsets of Th2a and peTh2 cells, play a key role in the establishment and exacerbation of allergic asthma.
Non-Th2 cell subsets provide complex pathogenic (Th1, Th9, Th17, Tfh, Treg) and protective (Th1, Treg) roles in allergic asthma, as well as neutrophilic, obesity-associated, and steroid-resistant asthma.
Unconventional T cells (γδ T, iNKT, MAIT) may play key roles in both asthma protection and pathogenesis, and have been shown to produce classic Th2 and Th17 cytokines.
Approved biologic therapies that target Th2 pathways both upstream (tezepelumab, dupilumab) and downstream (omalizumab, meoplizumab, benralizumab, dupilumab) of Th2-skewing have been shown to decrease Th2 responses in asthmatic patients.
There is a need for asthma therapies that target non-Th2 pathways, although these treatments may only be efficacious in specific disease endotypes.
Funding:
Salary support provided by NIH/NIAID U01 AI125056 (LM) and T32 AI007496 (NB)
Abbreviations:
- AHR
airway hyperreactivity
- DC
dendritic cell
- DN
double negative
- HDM
house dust mite
- iNKT
invariant natural killer T
- MAIT
mucosal-associated invariant T
- NK
natural killer
- peTH2
pathogenic effector Th2
- Tc
T cytotoxic
- TCR
T cell receptor
- Tfh
T follicular helper
- Th
T helper
- Treg
T regulatory
- TSLP
thymic stromal lymphopoietin
Footnotes
Conflicts of Interest: None
Trial Registration: Not Applicable
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skloot GS. Asthma phenotypes and endotypes: A personalized approach to treatment. Curr Opin Pulm Med. 2016;22:3–9. doi: 10.1097/MCP.0000000000000225 [DOI] [PubMed] [Google Scholar]
- 3.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2014;16(1):45–56. doi: 10.1038/ni.3049 [DOI] [PubMed] [Google Scholar]
- 4.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 5.Paul AGA, Muehling LM, Eccles JD, Woodfolk JA. T cells in severe childhood asthma. Clin Exp Allergy. 2019;49(5):564–581. doi: 10.1111/cea.13374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raedler D, Schaub B. Immune mechanisms and development of childhood asthma. Lancet Respir Med. 2014;2(8):647–656. doi: 10.1016/S2213-2600(14)70129-8 [DOI] [PubMed] [Google Scholar]
- 7.Prescott SL. Early origins of allergic disease: A review of processes and influences during early immune development. Curr Opin Allergy Clin Immunol. 2003;3(2):125–132. doi: 10.1097/00130832-200304000-00006 [DOI] [PubMed] [Google Scholar]
- 8.Gelfand EW. Pediatric asthma: A different disease. Proc Am Thorac Soc. 2009;6(3):278–282. doi: 10.1513/pats.200808-090RM [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc R Soc B Biol Sci. 2015;282(1821). doi: 10.1098/rspb.2014.3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy KM, Stockinger B. Effector T cell plasticity: Flexibility in the face of changing circumstances. Nat Immunol. 2010;11(8):674–680. doi: 10.1038/ni.1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akdis CA, Arkwright PD, Brüggen MC, Busse W, Gadina M, Guttman- Yassky E, et al. Type 2 immunity in the skin and lungs. Allergy. 2020;75(7):1582–1605. doi: 10.1111/all.14318 [DOI] [PubMed] [Google Scholar]
- 12.Zheng WP, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for TH2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. [DOI] [PubMed] [Google Scholar]
- 13.Mikhak Z, Fukui M, Farsidjani A, Medoff BD, Tager AM. Contribution of CCR4 and CCR8 to antigen-specific TH2 cell trafficking in allergic pulmonary inflammation. J Allergy Clin Immunol. 123(1):67–73.e3. doi: 10.1016/j.jaci.2008.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagata K, Tanaka K, Ogawa K, Kemmotsu K, Imai T, Yoshie O, et al. Selective expression of a novel surface molecule by human Th2 cells in vivo. J Immunol. 1999;162(3):1278–1286. [PubMed] [Google Scholar]
- 15.Rahimi RA, Nepal K, Cetinbas M, Sadreyev RI, Luster AD. Distinct functions of tissue-resident and circulating memory Th2 cells in allergic airway disease. J Exp Med. 2020;217(9):e20190865. doi: 10.1084/jem.20190865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner DL, Goldklang M, Cvetkovski F, Paik D, Trischler J, Barahona J, et al. Biased Generation and In Situ Activation of Lung Tissue-Resident Memory CD4 T Cells in the Pathogenesis of Allergic Asthma. J Immunol. 2018;200:1561–1569. doi: 10.4049/jimmunol.1700257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinkerton JW, Kim RY, Brown AC, Rae BE, Donovan C, Mayall JR, et al. Relationship between type 2 cytokine and inflammasome responses in obesity-associated asthma. J Allergy Clin Immunol. Published online 2021:1–11. doi: 10.1016/j.jaci.2021.10.003 [DOI] [PubMed] [Google Scholar]
- 18.Desai D, Newby C, Symon FA, Haldar P, Shah S, Gupta S, et al. Elevated sputum interleukin-5 and submucosal eosinophilia in obese individuals with Severe Asthma. Am J Respir Crit Care Med. 2013;188(6):657–663. doi: 10.1164/rccm.201208-1470OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng H, Zhang X, Castillo EF, Luo Y, Liu M, Yang XO. Leptin enhances TH2 and ILC2 responses in allergic airway disease. J Biol Chem. 2016;291(42):22043–22052. doi: 10.1074/jbc.M116.743187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pacheco KA, Tarkowski M, Klemm J, Rosenwasser LJ. CD49d expression and function on allergen-stimulated T cells from blood and airway. Am J Respir Cell Mol Biol. 1998;18(2):286–293. doi: 10.1165/ajrcmb.18.2.2687 [DOI] [PubMed] [Google Scholar]
- 21.Wambre E, Bajzik V, DeLong JH, O’Brien K, Nguyen QA, Speake C, et al. A phenotypically and functionally distinct human TH2 cell subpopulation is associated with allergic disorders. Sci Transl Med. 2017;9:eaam9171. doi: 10.1126/scitranslmed.aam9171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitson-Salazar A, Yin Y, Wansley DL, Young M, Bolan H, Arceo S, et al. Hematopoietic prostaglandin D synthase defines a proeosinophilic pathogenic effector human TH2 cell subpopulation with enhanced function. J Allergy Clin Immunol. 2016;137(3):907–918. doi: 10.1016/j.jaci.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 23.Bajzik V, DeBerg HA, Garabatos N, Rust BJ, Obrien KK, Nguyen Q, et al. Oral desensitization therapy for peanut allergy induces dynamic changes in peanut- specific immune responses. Allergy. 2022;In Press. doi: 10.1111/all.15276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monian B, Tu AA, Ruiter B, Morgan DM, Petrossian PM, Smith NP, et al. Peanut Oral Immunotherapy Suppresses Clonally Distinct Subsets of T Helper Cells. JCI. 2022;132(2):e150634. doi: 10.2139/ssrn.3737147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang YH, Voo KS, Liu B, yu Chen C, Uygungil B, Spoede W, et al. A novel subset of CD4+ Th2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207(11):2479–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014;134(5):1175–1186. doi: 10.1016/j.jaci.2014.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lynch JP, Werder RB, Simpson J, Loh Z, Zhang V, Haque A, et al. Aeroallergen-induced IL-33 predisposes to respiratory virus-induced asthma by dampening anti-viral immunity. J. Allergy Clin Immunol. 2016;138(5):1326–1337. doi: 10.1016/J.JACI.2016.02.039 [DOI] [PubMed] [Google Scholar]
- 28.Duerr CU, McCarthy CDA, Mindt BC, Rubio M, Meli AP, Pothlichet J, et al. Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat Immunol. 2015;17(1):65–75. doi: 10.1038/ni.3308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Contoli M, Ito K, Padovani A, Poletti D, Marku B, Edwards MR, et al. TH2 cytokines impair innate immune responses to rhinovirus in respiratory epithelial cells. Allergy. 2015;70:910–920. doi: 10.1111/all.12627 [DOI] [PubMed] [Google Scholar]
- 30.Pritchard AL, Carroll ML, Burel JG, White OJ, Phipps S, Upham JW. Innate IFNs and plasmacytoid dendritic cells constrain TH2 cytokine responses to rhinovirus: A regulatory mechanism with relevance to asthma. J Immunol. 2012;188(12):5898–5905. doi: 10.4049/jimmunol.1103507 [DOI] [PubMed] [Google Scholar]
- 31.Pritchard AL, White OJ, Burel JG, Upham JW. Innate interferons inhibit allergen and microbial specific TH2 responses. Immunol Cell Biol. 2012;90:974–977. [DOI] [PubMed] [Google Scholar]
- 32.Papadopoulos NG, Stanciu LA, Papi A, Holgate ST, Johnston SL. A defective type 1 response to rhinovirus in atopic asthma. Thorax. 2002;57(4):328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and TH1/2 cytokine and IL-10 production. Proc Natl Acad Sci USA. 2008;105(36):13562–13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel DA, You Y, Huang G, Byers DE, Kim HJ, Agapov E, et al. Interferon response and respiratory virus control are preserved in bronchial epithelial cells in asthma. J Allergy Clin Immunol. 2014;134(6):1402–1412. doi: 10.1016/j.jaci.2014.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeMore JP, Weisshaar EH, Vrtis RF, Swenson CA, Evans MD, Morin A, et al. Similar colds in subjects with allergic asthma and nonatopic subjects after inoculation with rhinovirus-16. J Allergy Clin Immunol. 2009;124(2):245–252. doi: 10.1016/j.jaci.2009.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleming HE, Little FF, Schnurr D, Avila PC, Wong H, Liu J, et al. Rhinovirus-16 colds in healthy and in asthmatic subjects: Similar changes in upper and lower airways. Am J Respir Crit Care Med. 1999;160:100–108. doi: 10.1164/ajrccm.160.1.9808074 [DOI] [PubMed] [Google Scholar]
- 37.Muehling LM, Heymann PW, Wright PW, Eccles JD, Agrawal R, Carper HT, et al. Human Th1 and Th2 cells targeting rhinovirus and allergen coordinately promote allergic asthma. J Allergy Clin Immunol. 2020;146(3):555–570. doi: 10.1016/j.jaci.2020.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altman MC, Gill MA, Whalen E, Babineau DC, Shao B, Liu AH, et al. Transcriptome networks identify mechanisms of viral and nonviral asthma exacerbations in children. Nat Immunol. 2019;20:637–651. doi: 10.1038/s41590-019-0347-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makrinioti H, Bush A, Gern J, Johnston SL, Papadopoulos N, Feleszko W, et al. The Role of Interferons in Driving Susceptibility to Asthma Following Bronchiolitis: Controversies and Research Gaps. Front Immunol. 2021;12(December):1–8. doi: 10.3389/fimmu.2021.761660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veerati PC, Troy NM, Reid AT, Li NF, Nichol KS, Kaur P, et al. Airway Epithelial Cell Immunity Is Delayed During Rhinovirus Infection in Asthma and COPD. Front Immunol. 2020;11(May):1–14. doi: 10.3389/fimmu.2020.00974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng X, Lawrence MG, Payne SC, Mattos J, Etter E, Negri JA, et al. Lower viral loads in subjects with rhinovirus-challenged allergy despite reduced innate immunity. Ann Allergy, Asthma Immunol. 2022;128(4):414–422.e2. doi: 10.1016/j.anai.2022.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kennedy JL, Shaker M, McMeen V, Gern J, Carper H, Murphy D, et al. Comparison of viral load in individuals with and without asthma during infections with rhinovirus. Am J Respir Crit Care Med. 2014;189(5):532–539. Accessed March 26, 2014. http://www.ncbi.nlm.nih.gov/pubmed/24471509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finotto S, Neurath MF, Glickman JN, Qin S, Lehr HA, Green FHY, et al. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science (80- ). 2010;295:336–339. [DOI] [PubMed] [Google Scholar]
- 44.Cohn L, Homer RJ, Niu N, Bottomly K. T helper 1 cells and interferon γ regulate allergic airway inflammation and mucus production. J Exp Med. 1999;190(9):1309–1318. doi: 10.1084/jem.190.9.1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hansen G, Berry G, DeKruyff RH, Umetsu DT. Allergen-specific TH1 cells fail to counterbalance TH2 cell-induced airway hyperreactivity but cause severe airway inflammation. J Clin Invest. 1999;103:175–183. doi: 10.1172/JCI5155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Randolph D, Carruthers CJ, Szabo SJ, Murphy KM, Chaplin DD. Modulation of airway inflammation by passive transfer of allergen-specific TH1 and TH2 cells in a mouse model of asthma. J Immunol. 1999;162:2375–2383. [PubMed] [Google Scholar]
- 47.Randolph DA, Stephens R, Carruthers CJL, Chaplin DD. Cooperation between TH1 and TH2 cells in a murine model of eosinophilic airway inflammation. J Clin Invest. 1999;104:1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stephens R, Randolph DA, Huang G, Holtzman MJ, Chaplin DD. Antigen-nonspecific recruitment of TH2 cells to the lung as a mechanism for viral infection-induced allergic asthma. J Immunol. 2002;169(10):5458–5467. doi: 10.4049/jimmunol.169.10.5458 [DOI] [PubMed] [Google Scholar]
- 49.Hessel E, Van Oosterhout A, Van Ark I, Van Esch B, Hofman G, Van Loveren H, et al. Development of airway hyperresponsiveness is dependent on interferon-gamma and independent of eosinophil infiltration. Am J Respir Cell Mol Biol. 1997;16(14):325–334. doi: 10.1165/ajrcmb.16.3.9070618 [DOI] [PubMed] [Google Scholar]
- 50.Nguyen TH, Maltby S, Tay HL, Eyers F, Foster PS, Yang M. Identification of IFN-γ and IL-27 as Critical Regulators of Respiratory Syncytial Virus–Induced Exacerbation of Allergic Airways Disease in a Mouse Model. J Immunol. 2018;200(1):237–247. doi: 10.4049/jimmunol.1601950 [DOI] [PubMed] [Google Scholar]
- 51.Krug N, Madden J, Redington AE, Lackie P, Djukanovic R, Schauer U, et al. T-cell cytokine profile evaluated at the single cell level in BAL and blood in allergic asthma. Am J Respir Cell Mol Biol. 1996;14:319–326. [DOI] [PubMed] [Google Scholar]
- 52.Cembrzyñska-Nowak M, Szklarz E, Inglot AD, Teodorczyk-Injeyan JA. Elevated release of tumor necrosis factor-α and interferon-γ by bronchoalveolar leukocytes from patients with bronchial asthma. Am Rev Respir Dis. 1993;147:291–295. [DOI] [PubMed] [Google Scholar]
- 53.Pene J, Chevalier S, Preisser L, Venereau E, Guilleux MH, Ghannam S, et al. Chronically inflamed human tissues are infiltrated by highly differentiated TH17 lymphocytes. J Immunol. 2008;180:7423–7430. doi: 10.4049/jimmunol.180.11.7423 [DOI] [PubMed] [Google Scholar]
- 54.Shannon J, Ernst P, Yamauchi Y, Olivenstein R, Lemiere C, Foley S, et al. Differences in airway cytokine profile in severe asthma compared to moderate asthma. Chest. 2008;133:420–426. doi: 10.1378/chest.07-1881 [DOI] [PubMed] [Google Scholar]
- 55.Wisniewski JA, Muehling LM, Eccles JD, Capaldo BJ, Agrawal R, Shirley DA, et al. TH1 signatures are present in the lower airways of children with severe asthma, regardless of allergic status. J Allergy Clin Immunol. 2018;141(6):2048–2060. doi: 10.1016/j.jaci.2017.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mamessier E, Nieves A, Lorec AMM, Dupuy P, Pinot D, Pinet C, et al. T-cell activation during exacerbations: A longitudinal study in refractory asthma. Allergy Eur J Allergy Clin Immunol. 2008;63(9):1202–1210. doi: 10.1111/j.1398-9995.2008.01687.x [DOI] [PubMed] [Google Scholar]
- 57.Ghebre MA, Pang PH, Desai D, Hargadon B, Newby C, Woods J, et al. Severe exacerbations in moderate-to-severe asthmatics are associated with increased pro-inflammatory and type 1 mediators in sputum and serum. BMC Pulm Med. 2019;19:144. doi: 10.1186/s12890-019-0906-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gauthier M, Chakraborty K, Oriss TB, Raundhal M, Das S, Chen J, et al. Severe asthma in humans and mouse model suggests a CXCL10 signature underlies corticosteroid-resistant Th1 bias. JCI insight. 2017;2(13):e94580. doi: 10.1172/jci.insight.94580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oriss TB, Raundhal M, Morse C, Huff RE, Das S, Hannum R, et al. IRF5 distinguishes severe asthma in humans and drives Th1 phenotype and airway hyperreactivity in mice. JCI Insight. 2017;2(10):e91019. doi: 10.1172/jci.insight.91019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raundhal M, Morse C, Khare A, Oriss TB, Milosevic J, Trudeau J, et al. High IFN-γ and low SLPI mark severe asthma in mice and humans. J Clin Invest. 2015;125(8):3037–3050. doi: 10.1172/JCI80911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernandes JS, Araujo MI, de Almeida TVVS, Andrade LS, Lopes DM, de Mello LM, et al. Impaired immunoregulatory network of the CD4 T lymphocytes in refractory asthma. Clin Exp Allergy. 2019;49(5):644–654. doi: 10.1111/cea.13351 [DOI] [PubMed] [Google Scholar]
- 62.Camiolo MJ, Zhou X, Oriss TB, Yan Q, Gorry M, Horne W, et al. High-dimensional profiling clusters asthma severity by lymphoid and non-lymphoid status. Cell Rep. 2021;35:108974. doi: 10.1016/j.celrep.2021.108974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rastogi D, Canfield SM, Andrade A, Isasi CR, Hall CB, Rubinstein A, et al. Obesity-associated asthma in children a distinct entity. Chest. 2012;141(4):895–905. doi: 10.1378/chest.11-0930 [DOI] [PubMed] [Google Scholar]
- 64.Rastogi D, Nico J, Johnston AD, Tobias TAM, Jorge Y, Macian F, et al. CDC42-related genes are upregulated in helper T cells from obese asthmatic children. J Allergy Clin Immunol. 2018;141(2):539–548.e7. doi: 10.1016/j.jaci.2017.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alhamdan F, Marsh LM, Pedersen F, Alhamwe BA, Thölken C, Pfefferle PI, et al. Differential Regulation of Interferon Signaling Pathways in CD4+ T Cells of the Low Type-2 Obesity-Associated Asthma Phenotype. Int J Mol Sci. 2021;22(18):10144. doi: 10.3390/ijms221810144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu M, Eckart MR, Morgan AA, Mukai K, Butte AJ, Tsai M, et al. Identification of an IFN-gamma/mast cell axis in a mouse model of chronic asthma. J Clin Invest. 2011;121(8):3133–3143. doi: 10.1172/JCI43598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin C, Uhlig S, Ullrich V. Cytokine-induced bronchoconstriction in precision-cut lung slices is dependent upon cyclooxygenase-2 and thromboxane receptor activation. Am J Respir Cell Mol Biol. 2001;24(2):139–145. doi: 10.1165/ajrcmb.24.2.3545 [DOI] [PubMed] [Google Scholar]
- 68.Paulsson Y, Austgulen R, Hofsli E, Heldin CH, Westermark B, Nissen-Meyer J. Tumor Necrosis Factor-Induced Expression of Platelet-Derived Growth Factor A-Chain Messenger RNA in Fibroblasts. Exp Cell Res. 1989;180:490–496. [DOI] [PubMed] [Google Scholar]
- 69.Palombella VJ, Mendelsohn J, Vilček J. Mitogenic action of tumor necrosis factor in human fibroblasts: Interaction with epidermal growth factor and platelet- derived growth factor. J Cell Physiol. 1988;135(1):23–31. doi: 10.1002/jcp.1041350104 [DOI] [PubMed] [Google Scholar]
- 70.Vieira SM, Lemos HP, Grespan R, Napimoga MH, Dal-Secco D, Freitas A, et al. A crucial role for TNF-α in mediating neutrophil influx induced by endogenously generated or exogenous chemokines, KC/CXCL1 and LIX/CXCL5. Br J Pharmacol. 2009;158(3):779–789. doi: 10.1111/j.1476-5381.2009.00367.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamamoto H, Sedgwick JB, Busse WW. Differential regulation of eosinophil adhesion and transmigration by pulmonary microvascular endothelial cells. J Immunol. 1998;161:971–977. [PubMed] [Google Scholar]
- 72.Krishnamoorthy N, Douda DN, Brüggemann TR, Ricklefs I, Duvall MG, Abdulnour REE, et al. Neutrophil cytoplasts induce TH17 differentiation and skew inflammation toward neutrophilia in severe asthma. Sci Immunol. 2018;3(26):1–14. doi: 10.1126/sciimmunol.aao4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, et al. IL-17A produced by αβ T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med. 2012;18(4):547–554. doi: 10.1038/nm.2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Pagé N, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108(3):430–438. doi: 10.1067/mai.2001.117929 [DOI] [PubMed] [Google Scholar]
- 75.Kerzel S, Dehne J, Rogosch T, Schaub B, Maier RF, Zemlin M. TH17 cell frequency in peripheral blood from children with allergic asthma correlates with the level of asthma control. J Pediatr. 2012;161(6):1172–1174. doi: 10.1016/j.jpeds.2012.07.051 [DOI] [PubMed] [Google Scholar]
- 76.Wei Q, Liao J, Jiang M, Liu J, Liang X, Nong G. Relationship between Th17-mediated immunity and airway inflammation in childhood neutrophilic asthma. Allergy, Asthma Clin Immunol. 2021;17:4. doi: 10.1186/s13223-020-00504-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barczyk A, Pierzcha W, Sozañska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med. 2003;97(6):726–733. doi: 10.1053/rmed.2003.1507 [DOI] [PubMed] [Google Scholar]
- 78.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, et al. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203(12):2715–2725. doi: 10.1084/jem.20061401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Graser A, Ekici AB, Sopel N, Melichar VO, Zimmermann T, Papadopoulos NG, et al. Rhinovirus inhibits IL-17A and the downstream immune responses in allergic asthma. Mucosal Immunol. 2016;9(5):1183–1192. doi: 10.1038/mi.2015.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raedler D, Ballenberger N, Klucker E, Böck A, Otto R, Prazeres Da Costa O, et al. Identification of novel immune phenotypes for allergic and nonallergic childhood asthma. J. Allergy Clin Immunol. 2015;135(1):81–91. doi: 10.1016/j.jaci.2014.07.046 [DOI] [PubMed] [Google Scholar]
- 81.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, et al. Antigen-Specific T Cell Sensitization Is Impaired in IL-17-Deficient Mice, Causing Suppression of Allergic Cellular and Humoral Responses. Immunity. 2002;17(3):375–387. doi: 10.1016/S1074-7613(02)00391-6 [DOI] [PubMed] [Google Scholar]
- 82.McKinley L, Alcorn JF, Peterson A, DuPont RB, Kapadia S, Logar A, et al. TH17 Cells Mediate Steroid-Resistant Airway Inflammation and Airway Hyperresponsiveness in Mice. J Immunol. 2008;181(6):4089–4097. doi: 10.4049/jimmunol.181.6.4089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Newcomb DC, Cephus JY, Boswell MG, Fahrenholz JM, Langley EW, Feldman AS, et al. Estrogen and progesterone decrease let-7f microRNA expression and increase IL-23/IL-23 receptor signaling and IL-17A production in patients with severe asthma. J Allergy Clin Immunol. 2015;136(4):1025–1034.e11. doi: 10.1016/j.jaci.2015.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, et al. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3− effector T cells. Nat Immunol. 2008;9(12):1347–1355. doi: 10.1038/ni.1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, et al. Transforming growth factor-β “reprograms” the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9(12):1341–1346. doi: 10.1038/ni.1659 [DOI] [PubMed] [Google Scholar]
- 86.Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010;11(6):527–534. doi: 10.1038/ni.1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schmitt E, Germann T, Goedert S, Hoehn P, Huels C, Koelsch S, et al. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J Immunol. 1994;153(9):3989–3996. [PubMed] [Google Scholar]
- 88.Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010;33:192–202. doi: 10.1016/j.immuni.2010.07.014 [DOI] [PubMed] [Google Scholar]
- 89.Jones CP, Gregory LG, Causton B, Campbell GA, Lloyd CM. Activin A and TGF-β promote TH9 cell-mediated pulmonary allergic pathology. J Allergy Clin Immunol. 2012;129(4):1000–1010.e3. doi: 10.1016/j.jaci.2011.12.965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saeki M, Kaminuma O, Nishimura T, Kitamura N, Mori A, Hiroi T. Th9 cells induce steroid-resistant bronchial hyperresponsiveness in mice. Allergol Int. 2017;66:S35–S40. doi: 10.1016/j.alit.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 91.Hoppenot D, Malakauskas K, Lavinskienė S, Bajoriūnienė I, Kalinauskaitė V, Sakalauskas R. Peripheral blood Th9 cells and eosinophil apoptosis in asthma patients. Medicina (Kaunas). 2015;51(1):10–17. doi: 10.1016/j.medici.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 92.Shimbara A, Christodoulopoulos P, Soussi-Gounni A, Olivenstein R, Nakamura Y, Levitt RC, et al. IL-9 and its receptor in allergic and nonallergic lung disease: Increased expression in asthma. J Allergy Clin Immunol. 1999;104:108–115. doi: 10.1016/S0091-6749(00)90185-4 [DOI] [PubMed] [Google Scholar]
- 93.Matsuzawa S, Sakashita K, Kinoshita T, Ito S, Yamashita T, Koike K. IL-9 Enhances the Growth of Human Mast Cell Progenitors Under Stimulation with Stem Cell Factor. J Immunol. 2003;170(7):3461–3467. doi: 10.4049/jimmunol.170.7.3461 [DOI] [PubMed] [Google Scholar]
- 94.Nowak EC, Weaver CT, Turner H, Begum-Haque S, Becher B, Schreiner B, et al. IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med. 2009;206(8):1653–1660. doi: 10.1084/jem.20090246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009;106(31):12885–12890. doi: 10.1073/pnas.0812530106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Heusch M, Steenwinckel V, Cochez PM, Louahed J, Warnier G, Lemaire MM, et al. IL-9 exerts biological function on antigen-experienced murine T cells and exacerbates colitis induced by adoptive transfer. Eur J Immunol. 2020;50(7):1034–1043. doi: 10.1002/eji.201948430 [DOI] [PubMed] [Google Scholar]
- 97.Jia L, Wang Y, Li J, Li S, Zhang Y, Shen J, et al. Detection of IL-9 producing T cells in the PBMCs of allergic asthmatic patients. BMC Immunol. 2017;18:38. doi: 10.1186/s12865-017-0220-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Longphre M, Li D, Gallup M, Drori E, Ordoñez CL, Redman T, et al. Allergen-induced IL-9 directly stimulates mucin transcription in respiratory epithelial cells. J Clin Invest. 1999;104(10):1375–1382. doi: 10.1172/JCI6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Louahed J, Toda M, Jen J, Hamid Q, Renauld JC, Levitt RC, et al. Interleukin-9 upregulates mucus expression in the airways. Am J Respir Cell Mol Biol. 2000;22(6):649–656. doi: 10.1165/ajrcmb.22.6.3927 [DOI] [PubMed] [Google Scholar]
- 100.Dugas B, Renauld JC, Pène J, Bonnefoy JY, Peti- Frère C, Braquet P, et al. Interleukin- 9 potentiates the interleukin- 4- induced immunoglobulin (IgG, IgM and IgE) production by normal human B lymphocytes. Eur J Immunol. 1993;23(7):1687–1692. doi: 10.1002/eji.1830230743 [DOI] [PubMed] [Google Scholar]
- 101.Fawaz LM, Sharif-Askari E, Hajoui O, Soussi-Gounni A, Hamid Q, Mazer BD. Expression of IL-9 receptor α chain on human germinal center B cells modulates IgE secretion. J Allergy Clin Immunol. 2007;120(5):1208–1215. doi: 10.1016/j.jaci.2007.08.022 [DOI] [PubMed] [Google Scholar]
- 102.Hartl D, Koller B, Mehlhorn AT, Reinhardt D, Nicolai T, Schendel DJ, et al. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol. 2007;119(5):1258–1266. doi: 10.1016/j.jaci.2007.02.023 [DOI] [PubMed] [Google Scholar]
- 103.Provoost S, Maes T, Van Durme YM, Gevaert P, Bachert C, Schmidt-Weber CB, et al. Decreased FOXP3 protein expression in patients with asthma. Allergy Eur J Allergy Clin Immunol. 2009;64(10):1539–1546. doi: 10.1111/j.1398-9995.2009.02056.x [DOI] [PubMed] [Google Scholar]
- 104.Lin YL, Shieh CC, Wang JY. The functional insufficiency of human CD4+CD25high T-regulatory cells in allergic asthma is subjected to TNF-α modulation. Allergy Eur J Allergy Clin Immunol. 2008;63:67–74. doi: 10.1111/j.1398-9995.2007.01526.x [DOI] [PubMed] [Google Scholar]
- 105.Huang F, Yin JN, Wang HB, Liu SY, Li YN. Association of imbalance of effector T cells and regulatory cells with the severity of asthma and allergic rhinitis in children. Allergy Asthma Proc. 2017;38(6):e70–e77. doi: 10.2500/aap.2017.38.4076 [DOI] [PubMed] [Google Scholar]
- 106.Kinoshita T, Baatjes A, Smith SG, Dua B, Watson R, Kawayama T, et al. Natural regulatory T cells in isolated early responders compared with dual responders with allergic asthma. J Allergy Clin Immunol. Published online October 15, 2013:1–8. doi: 10.1016/j.jaci.2013.08.025 [DOI] [PubMed] [Google Scholar]
- 107.Jansen K, Wirz OF, van de Veen W, Tan G, Mirer D, Sokolowska M, et al. Loss of regulatory capacity in Treg cells following rhinovirus infection. J Allergy Clin Immunol. Published online 2021. doi: 10.1016/j.jaci.2021.05.045 [DOI] [PubMed] [Google Scholar]
- 108.Weathington N, O’Brien ME, Radder JE, Whisenant TC, Bleecker ER, Busse WW, et al. BAL cell gene expression in severe asthma reveals mechanisms of severe disease and influences of medications. Am J Respir Crit Care Med. 2019;200(7):837–856. doi: 10.1164/rccm.201811-2221OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smyth LJC, Eustace A, Kolsum U, Blaikely J, Singh D. Increased airway T regulatory cells in asthmatic subjects. Chest. 2010;138(4):905–912. doi: 10.1378/chest.09-3079 [DOI] [PubMed] [Google Scholar]
- 110.Thunberg S, Gafvelin G, Nord M, Grönneberg R, Grunewald J, Eklund A, et al. Allergen provocation increases TH2-cytokines and FOXP3 expression in the asthmatic lung. Allergy Eur J Allergy Clin Immunol. 2010;65(3):311–318. doi: 10.1111/j.1398-9995.2009.02218.x [DOI] [PubMed] [Google Scholar]
- 111.Lee JH, Yu HH, Wang LC, Yang YH, Lin YT, Chiang BL. The levels of CD4+CD25+ regulatory T cells in paediatric patients with allergic rhinitis and bronchial asthma. Clin Exp Immunol. 2007;148:53–63. doi: 10.1111/j.1365-2249.2007.03329.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lynch JP, Werder RB, Curren BF, Sikder MAA, Ullah A, Sebina I, et al. Long-lived regulatory T cells generated during severe bronchiolitis in infancy influence later progression to asthma. Mucosal Immunol. 2020;13(4):652–664. doi: 10.1038/s41385-020-0268-8 [DOI] [PubMed] [Google Scholar]
- 113.Krishnamoorthy N, Khare A, Oriss TB, Raundhal M, Morse C, Yarlagadda M, et al. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat Med. 2012;18(10):1525–1530. doi: 10.1038/nm.2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jansen K, Wirz OF, van de Veen W, Tan G, Mirer D, Sokolowska M, et al. Loss of regulatory capacity in Treg cells following rhinovirus infection. J Allergy Clin Immunol. 2021;148(4):1016–1029.e16. doi: 10.1016/j.jaci.2021.05.045 [DOI] [PubMed] [Google Scholar]
- 115.Chantveerawong T, Sangkangjanavanich S, Chiewchalermsri C, Pradubpongsa P, Mitthamsiri W, Jindarat S, et al. Increased circulating CRTH2+Tregs are associated with asthma control and exacerbation. Allergy Eur J Allergy Clin Immunol. 2022;77(2):681–685. doi: 10.1111/all.15145 [DOI] [PubMed] [Google Scholar]
- 116.Jansen K, Satitsuksanoa P, Wirz OF, Schneider SR, van de Veen W, Tan G, et al. T regulatory cells from atopic asthmatic individuals show a Th2- like phenotype. Allergy. 2022;77(4):1320–1324. doi: 10.1111/all.15193 [DOI] [PubMed] [Google Scholar]
- 117.Chen CC, Kobayashi T, Iijima K, Hsu FC, Kita H. IL-33 dysregulates regulatory T cells and impairs established immunologic tolerance in the lungs. J Allergy Clin Immunol. 2017;140(5):1351–1363.e7. doi: 10.1016/j.jaci.2017.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Koh B, Ulrich BJ, Nelson AS, Panangipalli G, Kharwadkar R, Wu W, et al. Bcl6 and Blimp1 reciprocally regulate ST2+ Treg–cell development in the context of allergic airway inflammation. J Allergy Clin Immunol. 2020;146(5):1121–1136.e9. doi: 10.1016/j.jaci.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xin L, Gao J, Ge X, Tian C, Ma W, Tian Z, et al. Increased pro-inflammatory cytokine-secreting regulatory T cells are correlated with the plasticity of T helper cell differentiation and reflect disease status in asthma. Respir Med. 2018;143:129–138. doi: 10.1016/j.rmed.2018.09.007 [DOI] [PubMed] [Google Scholar]
- 120.Gandhi VD, Cephus JY, Norlander AE, Chowdhury NU, Zhang J, Ceneviva ZJ, et al. Androgen receptor signaling promotes Treg suppressive function during allergic airway inflammation. J Clin Invest. 2022;132(4). doi: 10.1172/JCI153397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Donma M, Karasu E, Ozdilek B, Turgut B, Topcu B, Nalbantoglu B, et al. CD4+, CD25+, FOXP3+ T Regulatory Cell Levels in Obese, Asthmatic, Asthmatic Obese, and Healthy Children. Inflammation. 2015;38(4):1473–1478. doi: 10.1007/s10753-015-0122-4 [DOI] [PubMed] [Google Scholar]
- 122.Guo Y, Shi J, Wang Q, Hong L, Chen M, Liu S, et al. Metformin alleviates allergic airway inflammation and increases Treg cells in obese asthma. J Cell Mol Med. 2021;25(4):2279–2284. doi: 10.1111/jcmm.16269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dias ASO, Santos ICL, Delphim L, Fernandes G, Endlich LR, Cafasso MOSD, et al. Serum leptin levels correlate negatively with the capacity of vitamin D to modulate the in vitro cytokines production by CD4+ T cells in asthmatic patients. Clin Immunol. 2019;205:93–105. doi: 10.1016/j.clim.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 124.Kobayashi T, Iijima K, Dent AL, Kita H. Follicular helper T cells mediate IgE antibody response to airborne allergens. J Allergy Clin Immunol. 2017;139(1):300–313.e7. doi: 10.1016/j.jaci.2016.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gong F, Su Q, Jiang D, Chen J, Pan Y, Huang X. High frequency of circulating follicular helper T cells in patients with bronchial asthma. Clin Lab. 2014;60(6):963–968. doi: 10.7754/Clin.Lab.2013.130427 [DOI] [PubMed] [Google Scholar]
- 126.Gong F, Zhu HY, Zhu J, Dong QJ, Huang X, Jiang DJ. Circulating CXCR5+CD4+ T cells participate in the IgE accumulation in allergic asthma. Immunol Lett. 2018;197:9–14. doi: 10.1016/j.imlet.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 127.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gong F, Qian C, Zhu H, Zhu J, Pan Y, Dong Q, et al. Circulating follicular T-helper cell subset distribution in patients with asthma. Allergy Asthma Proc. 2016;37(6):154–161. doi: 10.2500/aap.2016.37.3982 [DOI] [PubMed] [Google Scholar]
- 129.Kamekura R, Shigehara K, Miyajima S, Jitsukawa S, Kawata K, Yamashita K, et al. Alteration of circulating type 2 follicular helper T cells and regulatory B cells underlies the comorbid association of allergic rhinitis with bronchial asthma. Clin Immunol. 2015;158(2):204–211. doi: 10.1016/j.clim.2015.02.016 [DOI] [PubMed] [Google Scholar]
- 130.Bergantini L, d’Alessandro M, Cameli P, Pianigiani T, Fanetti M, Sestini P, et al. Follicular T Helper and Breg Cell Balance in Severe Allergic Asthma Before and After Omalizumab Therapy. Mol Diagnosis Ther. 2021;25(5):593–605. doi: 10.1007/s40291-021-00545-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gowthaman U, Chen JS, Zhang B, Flynn WF, Lu Y, Song W, et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science (80- ). 2019;365:eaaw6433. doi: 10.1126/science.aaw6433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ballesteros-Tato A, Randall TD, Lund FE, Spolski R, Leonard WJ, León B. T Follicular Helper Cell Plasticity Shapes Pathogenic T Helper 2 Cell-Mediated Immunity to Inhaled House Dust Mite. Immunity. 2016;44(2):259–273. doi: 10.1016/j.immuni.2015.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Takeda K, Dow SW, Miyahara N, Kodama T, Koya T, Taube C, et al. Vaccine-Induced CD8 + T Cell-Dependent Suppression of Airway Hyperresponsiveness and Inflammation. J. Immunol. 2009;183:181–190. doi: 10.4049/jimmunol.0803967 [DOI] [PubMed] [Google Scholar]
- 134.Suzuki M, Maghni K, Molet S, Shimbara A, Hamid QA, Martin JG. IFN-γ secretion by CD8+T cells inhibits allergen-induced airway eosinophilia but not late airway responses. J Allergy Clin Immunol. 2002;109(5):803–809. doi: 10.1067/mai.2002.123233 [DOI] [PubMed] [Google Scholar]
- 135.Stock P, Kallinich T, Akbari O, Quarcoo D, Gerhold K, Wahn U, et al. CD8+ T cells regulate immune responses in a murine model of allergen-induced sensitization and airway inflammation. Eur J Immunol. 2004;34(7):1817–1827. doi: 10.1002/eji.200324623 [DOI] [PubMed] [Google Scholar]
- 136.McMenamin BC, Holt PG. The natural immune response to inhaled soluble protein antigens involves major histocompatibility complex (MHC) class I-restricted CD8+ T cell-mediated but MHC class II-restricted CD4+ T cell-dependent immune deviation resulting in selective suppression o. J Exp Med. 1993;178:889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Renz H, Lack G, Saloga J, Schwinzer R, Bradley K, Loader J, et al. Inhibition of IgE production and normalization of airways responsiveness by sensitized CD8 T cells in a mouse model of allergen-induced sensitization. J Immunol. 1994;152:351–360. [PubMed] [Google Scholar]
- 138.Thomas MJ, Noble A, Sawicka E, Askenase PW, Kemeny DM. CD8 T Cells Inhibit IgE Via Dendritic Cell IL-12 Induction That Promotes Th1 T Cell Counter-Regulation. J Immunol. 2002;168:216–223. doi: 10.4049/jimmunol.168.1.216 [DOI] [PubMed] [Google Scholar]
- 139.Ito C, Okuyama-Dobashi K, Miyasaka T, Masuda C, Sato M, Kawano T, et al. CD8+ T Cells Mediate Female-Dominant IL-4 Production and Airway Inflammation in Allergic Asthma. Shiku H, ed. PLoS One. 2015;10(10):e0140808. doi: 10.1371/journal.pone.0140808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Raemdonck K, Baker K, Dale N, Dubuis E, Shala F, Belvisi MG, et al. CD4+ and CD8+ T cells play a central role in a HDM driven model of allergic asthma. Respir Res. 2016;17:45. doi: 10.1186/s12931-016-0359-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hinks TSC, Hoyle RD, Gelfand EW. CD8 + Tc2 cells: underappreciated contributors to severe asthma. Eur Respir Rev. 2019;28:190092. doi: 10.1183/16000617.0092-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang L, Netto KG, Zhou L, Liu X, Wang M, Zhang G, et al. Single-cell transcriptomic analysis reveals the immune landscape of lung in steroid-resistant asthma exacerbation. Proc Natl Acad Sci. 2021;118(2):e2005590118. doi: 10.1073/pnas.2005590118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kelly C, Stenton SC, Ward C, Bird G, Hendrick DJ, Walters EH. Lymphocyte subsets in bronchoalveolar lavage fluid obtained from stable asthmatics, and their correlations with bronchial responsiveness. Clin Exp Allergy. 1989;19:169–175. doi: 10.1111/j.1365-2222.1989.tb02360.x [DOI] [PubMed] [Google Scholar]
- 144.Hilvering B, Hinks TSC, Stöger L, Marchi E, Salimi M, Shrimanker R, et al. Synergistic activation of pro-inflammatory type-2 CD8+ T lymphocytes by lipid mediators in severe eosinophilic asthma. Mucosal Immunol. 2018;11(5):1408–1419. doi: 10.1038/s41385-018-0049-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ning F, Takeda K, Schedel M, Domenico J, Joetham A, Gelfand EW. Hypoxia enhances CD8+ TC2 cell–dependent airway hyperresponsiveness and inflammation through hypoxia-inducible factor 1α. J Allergy Clin Immunol. 2019;143(6):2026–2037.e7. doi: 10.1016/j.jaci.2018.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Miyahara N, Takeda K, Kodama T, Joetham A, Taube C, Park JW, et al. Contribution of Antigen-Primed CD8 + T Cells to the Development of Airway Hyperresponsiveness and Inflammation Is Associated with IL-13. J Immunol. 2004;172(4):2549–2558. doi: 10.4049/jimmunol.172.4.2549 [DOI] [PubMed] [Google Scholar]
- 147.Croft BM, Carter L, Swain SL, Dutton RW. Generation of Polarized Antigen-specific CD8 Effector Populations: Reciprocal Action of Interleukin (IL)-4 and IL-12 in Promoting Type 2 versus Type 1 Cytokine Profiles. J Exp Med. 1994;180:1715–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Den Otter I, Willems LNA, Van Schadewijk A, Van Wijngaarden S, Janssen K, De Jeu RC, et al. Lung function decline in asthma patients with elevated bronchial CD8, CD4 and CD3 cells. Eur Respir J. 2016;48(2):393–402. doi: 10.1183/13993003.01525-2015 [DOI] [PubMed] [Google Scholar]
- 149.Kabelitz D γδ T-cells: Cross-talk between innate and adaptive immunity. Cell Mol Life Sci. 2011;68(14):2331–2333. doi: 10.1007/s00018-011-0696-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Godfrey DI, Uldrich AP, Mccluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat Immunol. 2015;16(11):1114–1124. doi: 10.1038/ni.3298.1114 [DOI] [PubMed] [Google Scholar]
- 151.Kalyan S, Kabelitz D. Defining the nature of human γδ T cells: A biographical sketch of the highly empathetic. Cell Mol Immunol. 2013;10:21–29. doi: 10.1038/cmi.2012.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Roberts S, Girardi M. Conventional and Unconventional T Cells. In: Gaspari AA, Tyring SK, eds. Clinical and Basic Immunodermatology. Springer, London; 2008:85–104. [Google Scholar]
- 153.Gao Y, Williams AP. Role of innate T cells in anti-bacterial immunity. Front Immunol. 2015;6:302. doi: 10.3389/fimmu.2015.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ribot JC, Lopes N, Silva-Santos B. γδ T cells in tissue physiology and surveillance. Nat Rev Immunol. 2021;21(4):221–232. doi: 10.1038/s41577-020-00452-4 [DOI] [PubMed] [Google Scholar]
- 155.Lahn M, Kanehio A, Takeda K, Joetham A, Schwarze J, Köhler G, et al. Negative regulation of airway responsiveness that is dependent on γδ T cells and independent of αβ T cells. Nat Med. 1999;5(10):1150–1156. doi: 10.1038/13476 [DOI] [PubMed] [Google Scholar]
- 156.Cui ZH. Reversal of Allergic Airway Hyperreactivity after Long-term Allergen Challenge Depends on gd T Cells. Am J Respir Crit Care Med. 2003;168(11):1324–1332. doi: 10.1164/rccm.200305-634oc.r1 [DOI] [PubMed] [Google Scholar]
- 157.Murdoch JR, Gregory LG, Lloyd CM. γδT cells regulate chronic airway inflammation and development of airway remodelling. Clin Exp Allergy. 2014;44:1386–1398. doi: 10.1111/cea.12395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schauer U, Dippel E, Gieler U, Brauer J, Jung T, Heymanns J, et al. T cell receptor γδ bearing cells are decreased in the peripheral blood of patients with atopic diseases. Clin Exp Immunol. 2008;86(3):440–443. doi: 10.1111/j.1365-2249.1991.tb02950.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mota-Pinto A, Todo A, Alves V, Santos A, Santos M. Regulatory T cells in elderly patients with asthma. J Investig Allergol Clin Immunol. 2011;21(3):199–206. [PubMed] [Google Scholar]
- 160.Urboniene D, Babusyte A, Lötvall J, Sakalauskas R, Sitkauskiene B. Distribution of γδ and other T-lymphocyte subsets in patients with chronic obstructive pulmonary disease and asthma. Respir Med. 2013;107(3):413–423. doi: 10.1016/j.rmed.2012.11.012 [DOI] [PubMed] [Google Scholar]
- 161.Glanville N, Message SD, Walton RP, Pearson RM, Parker HL, Laza-Stanca V, et al. γδT cells suppress inflammation and disease during rhinovirus-induced asthma exacerbations. Mucosal Immunol. 2013;6(6):1091–1100. doi: 10.1038/mi.2013.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zuany-Amorim C, Ruffié C, Hailé S, Vargaftig BB, Pereira P, Pretolani M. Requirements for Γδ T cells in allergic airway inflammation. Science (80- ). 1998;280(5367):1265–1267. doi: 10.1126/science.280.5367.1265 [DOI] [PubMed] [Google Scholar]
- 163.Hamzaoui A, Kahan A, Ayed K, Hamzaoui K. T cells expressing the γδ receptor are essential for TH2-mediated inflammation in patients with acute exacerbation of asthma. Mediat Inflamm. 2002;11:113–119. doi: 10.1080/09629350220131971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Krug N, Erpenbeck VJ, Balke K, Petschallies J, Tschernig T, Hohlfeld JM, et al. Cytokine profile of bronchoalveolar lavage-derived CD4+, CD8+, and γδ T cells in people with asthma after segmental allergen challenge. Am J Respir Cell Mol Biol. 2001;25:125–131. [DOI] [PubMed] [Google Scholar]
- 165.Lahn M, Kanehiro A, Takeda K, Terry J, Hahn YS, Aydintug MK, et al. MHC class I-dependent Vγ4+ pulmonary T cells regulate αβ T cell-independent airway responsiveness. Proc Natl Acad Sci U S A. 2002;99(13):8850–8855. doi: 10.1073/pnas.132519299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hahn YS, Taube C, Jin N, Sharp L, Wands JM, Aydintug MK, et al. Different Potentials of γδ T Cell Subsets in Regulating Airway Responsiveness: Vγ1+ Cells, but Not Vγ4+ Cells, Promote Airway Hyperreactivity, Th2 Cytokines, and Airway Inflammation. J Immunol. 2004;172(5):2894–2902. doi: 10.4049/jimmunol.172.5.2894 [DOI] [PubMed] [Google Scholar]
- 167.Dusseaux M, Martin E, Serriari N, Péguillet I, Premel V, Louis D, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161 hi IL-17-secreting T cells. Blood. 2011;117(4):1250–1259. doi: 10.1182/blood-2010-08-303339 [DOI] [PubMed] [Google Scholar]
- 168.Fergusson JR, Smith KE, Fleming VM, Rajoriya N, Newell EW, Simmons R, et al. CD161 defines a transcriptional and functional phenotype across distinct human T cell lineages. Cell Rep. 2014;9(3):1075–1088. doi: 10.1016/j.celrep.2014.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Godfrey DI, Uldrich AP, Mccluskey J, Rossjohn J, Moody DB. The burgeoning family of non- - - conventional T cells. Nat Publ Gr. 2015;16(11):1114–1123. doi: 10.1038/ni.3298 [DOI] [PubMed] [Google Scholar]
- 170.Thomas SY, Chyung YH, Luster AD. Natural killer T cells are not the predominant T cell in asthma and likely modulate, not cause, asthma. J Allergy Clin Immunol. 2010;125(5):980–984. doi: 10.1016/j.jaci.2010.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9(5):582–588. doi: 10.1038/nm851 [DOI] [PubMed] [Google Scholar]
- 172.Lisbonne M, Diem S, de Castro Keller A, Lefort J, Araujo LM, Hachem P, et al. Cutting Edge: Invariant Vα14 NKT Cells Are Required for Allergen-Induced Airway Inflammation and Hyperreactivity in an Experimental Asthma Model. J Immunol. 2003;171(4):1637–1641. doi: 10.4049/jimmunol.171.4.1637 [DOI] [PubMed] [Google Scholar]
- 173.Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, et al. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc Natl Acad Sci U S A. 2006;103(8):2782–2787. doi: 10.1073/pnas.0510282103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Das J, Eynott P, Jupp R, Bothwell A, Van Kaer L, Shi Y, et al. Natural killer T cells and CD8+ T cells are dispensable for T cell-dependent allergic airway inflammation. Nat Med. 2006;12(12):1345–1346. doi: 10.1038/nm1206-1345 [DOI] [PubMed] [Google Scholar]
- 175.Matangkasombut P, Marigowda G, Ervine A, Idris L, Pichavant M, Kim HY, et al. Natural killer T cells in the lungs of patients with asthma. J Allergy Clin Immunol. 2009;123(5):1181–1186. doi: 10.1016/j.jaci.2009.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mutalithas K, Croudace J, Guillen C, Siddiqui S, Thickett D, Wardlaw A, et al. Bronchoalveolar lavage invariant natural killer T cells are not increased in asthma. J Allergy Clin Immunol. 2007;119(5):1274–1276. doi: 10.1016/j.jaci.2007.01.038 [DOI] [PubMed] [Google Scholar]
- 177.Vijayanand P, Seumois G, Pickard C, Powell Robert M., Angco G, Sammut D, et al. Invariant natural killer T cells in asthma and chronic obstructive pulmonary disease. N Engl J Med. 2007;356:1410–1422. doi: 10.1111/j.1440-1843.2011.02104.x [DOI] [PubMed] [Google Scholar]
- 178.Carpio-Pedroza JC, Vaughan G, Del Rio-Navarro BE, Del Rio-Chivardi JM, Vergara-Castaneda A, Jimenez-Zamudio LA, et al. Participation of CD161+ and invariant natural killer T cells in pediatric asthma exacerbations. Allergy Asthma Proc. 2013;34(1):84–92. doi: 10.2500/aap.2013.34.3619 [DOI] [PubMed] [Google Scholar]
- 179.Antunes L, Duarte de Souza AP, de Araújo PD, Pinto LA, Jones MH, Stein RT, et al. iNKT cells are increased in children with severe therapy-resistant asthma. Allergol Immunopathol (Madr). 2018;46(2):175–180. doi: 10.1016/j.aller.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 180.Shim JU, Koh Y Il. Increased Th2-like invariant natural killer T cells in peripheral blood from patients with asthma. Allergy, Asthma Immunol Res. 2014;6(5):444–448. doi: 10.4168/aair.2014.6.5.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chen YP, Zhang JH, Li CQ, Sun QX, Jiang XH. Obesity enhances Th2 inflammatory response via natural killer T cells in a murine model of allergic asthma. Int J Clin Exp Med. 2015;8(9):15403–15412. [PMC free article] [PubMed] [Google Scholar]
- 182.Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717–723. doi: 10.1038/nature11605 [DOI] [PubMed] [Google Scholar]
- 183.Le Bourhis L, Martin E, Péguillet I, Guihot A, Froux N, Coré M, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11(8):701–708. doi: 10.1038/ni.1890 [DOI] [PubMed] [Google Scholar]
- 184.Van Wilgenburg B, Scherwitzl I, Hutchinson EC, Leng T, Kurioka A, Kulicke C, et al. MAIT cells are activated during human viral infections. Nat Commun. 2016;7. doi: 10.1038/ncomms11653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hinks TSC, Zhou X, Staples KJ, Dimitrov BD, Manta A, Petrossian T, et al. Innate and adaptive T cells in asthmatic patients: Relationship to severity and disease mechanisms. J Allergy Clin Immunol. 2015;136(2):323–333. doi: 10.1016/j.jaci.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ye L, Pan J, Pasha MA, Shen X, D’Souza SS, Fung ITH, et al. Mucosal-associated invariant T cells restrict allergic airway inflammation. J Allergy Clin Immunol. 2020;145(5):1469–1473.e4. doi: 10.1016/j.jaci.2019.12.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chandra S, Wingender G, Greenbaum JA, Khurana A, Gholami AM, Ganesan AP, et al. Development of Asthma in Inner-City Children: Possible Roles of MAIT Cells and Variation in the Home Environment. J Immunol. 2018;200(6):1995–2003. doi: 10.4049/jimmunol.1701525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lezmi G, Abou Taam R, Dietrich C, Chatenoud L, de Blic J, Leite-de-Moraes M. Circulating IL-17-producing mucosal-associated invariant T cells (MAIT) are associated with symptoms in children with asthma. Clin Immunol. 2018;188:7–11. doi: 10.1016/j.clim.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 189.Lezmi G, Abou-Taam R, Garcelon N, Dietrich C, Machavoine F, Delacourt C, et al. Evidence for a MAIT-17–high phenotype in children with severe asthma. J Allergy Clin Immunol. 2019;144(6):1714–1716.e6. doi: 10.1016/j.jaci.2019.08.003 [DOI] [PubMed] [Google Scholar]
- 190.Wen X, Nian S, Wei G, Kang P, Yang Y, Li L, et al. Changes in the phenotype and function of mucosal-associated invariant T cells in neutrophilic asthma. Int Immunopharmacol. 2022;106:108606. doi: 10.1016/j.intimp.2022.108606 [DOI] [PubMed] [Google Scholar]
- 191.Georas SN. Inhaled glucocorticoids, lymphocytes, and dendritic cells in asthma and obstructive lung diseases. Proc Am Thorac Soc. 2004;1(3):215–221. doi: 10.1513/pats.200402-004MS [DOI] [PubMed] [Google Scholar]
- 192.Banuelos J, Lu NZ. A gradient of glucocorticoid sensitivity among helper T cell cytokines. Cytokine Growth Factor Rev. 2016;31(2016):27–35. doi: 10.1016/j.cytogfr.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202(9):1213–1223. doi: 10.1084/jem.20051135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang Q, Du J, Zhu J, Yang X, Zhou B. Thymic stromal lymphopoietin signaling in CD4+ T cells is required for TH2 memory. J Allergy Clin Immunol. 2015;135(3):781–791.e3. doi: 10.1016/j.jaci.2014.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lai JF, Thompson LJ, Ziegler SF. TSLP drives acute TH2-cell differentiation in lungs. J Allergy Clin Immunol. 2020;146(6):1406–1418.e7. doi: 10.1016/j.jaci.2020.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rochman Y, Dienger-Stambaugh K, Richgels PK, Lewkowich IP, Kartashov AV, Barski A, et al. TSLP signaling in CD4 + T cells programs a pathogenic T helper 2 cell state. Sci Signal. 2018;11(521). doi: 10.1126/scisignal.aam8858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Diver S, Khalfaoui L, Emson C, Wenzel SE, Menzies-Gow A, Wechsler ME, et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2021;9(11):1299–1312. doi: 10.1016/S2213-2600(21)00226-5 [DOI] [PubMed] [Google Scholar]
- 198.Jang S, Morris S, Lukacs NW. TSLP Promotes Induction of Th2 Differentiation but Is Not Necessary during Established Allergen-Induced Pulmonary Disease. PLoS One. 2013;8(2):e56433. doi: 10.1371/journal.pone.0056433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bakker DS, van der Wal MM, Heeb LEM, Giovannone B, Asamoah M, Delemarre EM, et al. Early and Long-Term Effects of Dupilumab Treatment on Circulating T-Cell Functions in Patients with Moderate-to-Severe Atopic Dermatitis. J Invest Dermatol. 2021;141(8):1943–1953.e13. doi: 10.1016/j.jid.2021.01.022 [DOI] [PubMed] [Google Scholar]
- 200.Trichot C, Faucheux L, Karpf L, Grandclaudon M, Pattarini L, Bagot M, et al. TH cell diversity and response to dupilumab in patients with atopic dermatitis. J Allergy Clin Immunol. 2021;147(2):756–759. doi: 10.1016/j.jaci.2020.05.037 [DOI] [PubMed] [Google Scholar]
- 201.Imai Y, Kusakabe M, Nagai M, Yasuda K, Yamanishi K. Dupilumab Effects on Innate Lymphoid Cell and Helper T Cell Populations in Patients with Atopic Dermatitis. JID Innov. 2021;1:100003. doi: 10.1016/j.xjidi.2021.100003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sandham DA, Barker L, Brown L, Brown Z, Budd D, Charlton SJ, et al. Discovery of Fevipiprant (NVP-QAW039), a Potent and Selective DP2 Receptor Antagonist for Treatment of Asthma. ACS Med Chem Lett. 2017;8(5):582–586. doi: 10.1021/acsmedchemlett.7b00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chen W, Luo J, Ye Y, Hoyle R, Liu W, Borst R, et al. The Roles of Type 2 Cytotoxic T Cells in Inflammation, Tissue Remodeling, and Prostaglandin (PG) D 2 Production Are Attenuated by PGD 2 Receptor 2 Antagonism. J Immunol. 2021;206(11):2714–2724. doi: 10.4049/jimmunol.2001245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Brightling CE, Gaga M, Inoue H, Li J, Maspero J, Wenzel S, et al. Effectiveness of fevipiprant in reducing exacerbations in patients with severe asthma (LUSTER-1 and LUSTER-2): two phase 3 randomised controlled trials. Lancet Respir Med. 2021;9:43–56. doi: 10.1016/S2213-2600(20)30412-4 [DOI] [PubMed] [Google Scholar]
- 205.Castro M, Kerwin E, Miller D, Pedinoff A, Sher L, Cardenas P, et al. Efficacy and safety of fevipiprant in patients with uncontrolled asthma: Two replicate, phase 3, randomised, double-blind, placebo-controlled trials (ZEAL-1 and ZEAL-2). EClinicalMedicine. 2021;35:100847. doi: 10.1016/j.eclinm.2021.100847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kolbeck R, Kozhich A, Koike M, Peng L, Andersson CK, Damschroder MM, et al. MEDI-563, a humanized anti-IL-5 receptor α mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125(6):1344–1353.e2. doi: 10.1016/j.jaci.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 207.Bergantini L, D’Alessandro M, Cameli P, Bono C, Perruzza M, Biagini M, et al. Regulatory T cell monitoring in severe eosinophilic asthma patients treated with mepolizumab. Scand J Immunol. 2021;94:e13031. doi: 10.1111/sji.13031 [DOI] [PubMed] [Google Scholar]
- 208.Bergantini L, D’Alessandro M, Cameli P, Bianchi F, Sestini P, Bargagli E, et al. Personalized Approach of Severe Eosinophilic Asthma Patients Treated with Mepolizumab and Benralizumab. Int Arch Allergy Immunol. 2020;181(10):746–753. doi: 10.1159/000508936 [DOI] [PubMed] [Google Scholar]
- 209.Gruchalla RS, Sampson HA, Liu AH, Shreffler W, Wallace PK, Togias A, et al. Effects of omalizumab on T lymphocyte function in inner-city children with asthma. Pediatr Allergy Immunol. 2016;27(3):328–331. doi: 10.1111/pai.12508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Djukanović R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med. 2004;170:583–593. doi: 10.1164/rccm.200312-1651OC [DOI] [PubMed] [Google Scholar]
- 211.Noga O, Hanf G, Brachmann I, Klucken AC, Kleine-Tebbe J, Rosseau S, et al. Effect of omalizumab treatment on peripheral eosinophil and T-lymphocyte function in patients with allergic asthma. J Allergy Clin Immunol. 2006;117(6):1493–1499. [DOI] [PubMed] [Google Scholar]
- 212.Amat F, Tallon P, Foray AP, Michaud B, Lambert N, Saint-Pierre P, et al. Control of asthma by omalizumab: the role of CD4 + Foxp3 + regulatory T cells. Clin Exp Allergy. 2016;46:1614–1616. doi: 10.1111/cea.12839 [DOI] [PubMed] [Google Scholar]
- 213.López-Abente J, Benito-Villalvilla C, Jaumont X, Pfister P, Tassinari P, Palomares O. Omalizumab restores the ability of human plasmacytoid dendritic cells to induce Foxp3 + Tregs. Eur Respir J. 2021;57:2000751. doi: 10.1183/13993003.00751-2020 [DOI] [PubMed] [Google Scholar]
- 214.Takaku Y, Soma T, Nishihara F, Nakagome K, Kobayashi T, Hagiwara K, et al. Omalizumab attenuates airway inflammation and interleukin-5 production by mononuclear cells in patients with severe allergic asthma. Int Arch Allergy Immunol. 2013;161(suppl 2):107–117. doi: 10.1159/000350852 [DOI] [PubMed] [Google Scholar]
- 215.Maggi L, Rossettini B, Montaini G, Matucci A, Vultaggio A, Mazzoni A, et al. Omalizumab dampens type 2 inflammation in a group of long-term treated asthma patients and detaches IgE from FcεRI. Eur J Immunol. 2018;48(12):2005–2014. doi: 10.1002/eji.201847668 [DOI] [PubMed] [Google Scholar]
- 216.Chesné J, Braza F, Chadeuf G, Mahay G, Cheminant MA, Loy J, et al. Prime role of IL-17A in neutrophilia and airway smooth muscle contraction in a house dust mite-induced allergic asthma model. J Allergy Clin Immunol. 2015;135(6):1643–1645.e5. doi: 10.1016/j.jaci.2014.12.1872 [DOI] [PubMed] [Google Scholar]
- 217.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes TH17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180:720–730. doi: 10.1164/rccm.200904-0573OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cheng S, Chen H, Wang A, Bunjhoo H, Cao Y, Xie J, et al. Blockade of IL-23 ameliorates allergic lung inflammation via decreasing the infiltration of Tc17 cells. Arch Med Sci. 2016;12(6):1362–1369. doi: 10.5114/aoms.2016.62923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Brightling CE, Nair P, Cousins DJ, Louis R, Singh D. Risankizumab in Severe Asthma — A Phase 2a, Placebo-Controlled Trial. N Engl J Med. 2021;385(18):1669–1679. doi: 10.1056/nejmoa2030880 [DOI] [PubMed] [Google Scholar]
- 220.Howarth PH, Babu KS, Arshad HS, Lau L, Buckley M, McConnell W, et al. Tumour necrosis factor (TNFα) as a novel therapeutic target in symptomatic corticosteroid dependent asthma. Thorax. 2005;60(12):1012–1018. doi: 10.1136/thx.2005.045260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, et al. Evidence of a Role of Tumor Necrosis Factor α in Refractory Asthma. N Engl J Med. 2006;354(7):697–708. doi: 10.1056/nejmoa050580 [DOI] [PubMed] [Google Scholar]
- 222.Wenzel SE, Barnes PJ, Bleecker ER, Bousquet J, Busse W, Dahlén SE, et al. A randomized, double-blind, placebo-controlled study of tumor necrosis factor-α blockade in severe persistent asthma. Am J Respir Crit Care Med. 2009;179(7):549–558. doi: 10.1164/rccm.200809-1512OC [DOI] [PubMed] [Google Scholar]
- 223.Holgate ST, Noonan M, Chanez P, Busse W, Dupont L, Pavord I, et al. Efficacy and safety of etanercept in moderate-to-severe asthma: A randomised, controlled trial. Eur Respir J. 2011;37(6):1352–1359. doi: 10.1183/09031936.00063510 [DOI] [PubMed] [Google Scholar]
- 224.Benson JM, Peritt D, Scallon BJ, Heavner GA, Shealy DJ, Giles-Komar JM, et al. Discovery and mechanism of ustekinumab: A human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. MAbs. 2011;3(6). doi: 10.4161/mabs.3.6.17815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Oh CK, Leigh R, McLaurin KK, Kim K, Hultquist M, Molfino NA. A randomized, controlled trial to evaluate the effect of an anti-interleukin-9 monoclonal antibody in adults with uncontrolled asthma. Respir Res. 2013;14:93. doi: 10.1186/1465-9921-14-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Weber JP, Fuhrmann F, Feist RK, Lahmann A, Al Baz MS, Gentz LJ, et al. ICOS maintains the T follicular helper cell phenotype by down-regulating krüppel-like factor 2. J Exp Med. 2015;212(2):217–233. doi: 10.1084/jem.20141432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Uwadiae FI, Pyle CJ, Walker SA, Lloyd CM, Harker JA. Targeting the ICOS/ICOS-L pathway in a mouse model of established allergic asthma disrupts T follicular helper cell responses and ameliorates disease. Allergy Eur J Allergy Clin Immunol. 2018;44:650–662. doi: 10.1111/all.13602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Solinas C, Gu-Trantien C, Willard-Gallo K. The rationale behind targeting the ICOS-ICOS ligand costimulatory pathway in cancer immunotherapy. ESMO Open. 2020;5:e000544. doi: 10.1136/esmoopen-2019-000544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.O’Reilly V, Zeng SG, Bricard G, Atzberger A, Hogan AE, Jackson J, et al. Distinct and overlapping effector functions of expanded human CD4+, CD8α+ and CD4-CD8α− invariant natural killer T cells. PLoS One. 2011;6(12):e28648. doi: 10.1371/journal.pone.0028648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Montoya CJ, Pollard D, Martinson J, Kumari K, Wasserfall C, Mulder CB, et al. Characterization of human invariant natural killer T subsets in health and disease using a novel invariant natural killer T cell-clonotypic monoclonal antibody, 6B11. Immunology. 2007;122:1–14. doi: 10.1111/j.1365-2567.2007.02647.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195(5):625–636. doi: 10.1084/jem.20011786 [DOI] [PMC free article] [PubMed] [Google Scholar]