Abstract
Gene regulatory networks (GRNs) serve as useful abstractions to understand transcriptional dynamics in developmental systems. Computational prediction of GRNs has been successfully applied to genome-wide gene expression measurements with the advent of microarrays and RNA-sequencing. However, these inferred networks are inaccurate and mostly based on correlative rather than causative interactions. In this review, we highlight three approaches that significantly impact GRN inference: (1) moving from one genome-wide functional modality, gene expression, to multi-omics, (2) single cell sequencing, to measure cell type-specific signals and predict context-specific GRNs, and (3) neural networks as flexible models. Together, these experimental and computational developments have the potential to significantly impact the quality of inferred GRNs. Ultimately, accurately modeling the regulatory interactions between transcription factors and their target genes will be essential to understand the role of transcription factors in driving developmental gene expression programs and to derive testable hypotheses for validation.
Keywords: developmental biology, functional genomics, gene expression and regulation, gene regulatory networks
Introduction
Multicellular organisms develop from a single fertilized egg, guided by the genetic information encoded in the genome. Cell lineages diverge and form tissues and organs, based on the interplay between signaling pathways, biomechanical forces [1] and the regulation of gene expression programs [2]. While development is controlled on many levels, transcription regulation is crucial [3]. To better understand these regulatory principles in development and evolution, it is essential to construct informative models of gene regulation.
Transcription is regulated by transcription factors (TFs) within the chromatin context [4]. TFs bind the DNA either directly, mostly in a sequence-specific manner [5], or indirectly via other TFs [6]. They can recruit various other proteins, such as co-activators, RNA polymerase, chromatin remodelers and histone modifying enzymes, to remodel or stabilize the chromatin or to activate or repress transcription [7,8]. In metazoans, TFs form up to 8% of the known proteome [9,10], with DNA binding domains and affinities being highly conserved between metazoans [11–13]. They bind specific DNA motifs that are clustered in relatively short cis-regulatory elements (CREs) that can be categorized as promoters, enhancers and insulators [14]. The exact function of an element depends on the combination of bound transcription factors, which is influenced by motif specificity, distance between motifs and motif directionality [15–19]. Core regulatory modules and pathways involved in germ layer and axis formation are deeply conserved in metazoans [20].
A useful abstraction to study transcription regulation is a network of transcription factors and their target genes. This concept of a gene regulatory network (GRN) was introduced in 1969 by Roy Britten and Eric Davidson and later experimentally demonstrated in sea urchin embryos [21,22]. GRNs serve to predict the effect of transcription factor expression on gene transcription and to derive testable hypotheses for validation. More generally, they function to model cell type specification and differentiation in development as well as regulatory perturbations in disease. GRNs have been constructed, mostly based on experimental loss-of-function and gain-of-function studies, for a variety of developmental models. Examples include germ layer formation in echinoderms [23–25] and frogs [26–29], neural crest formation [30,31], the Drosophila gap gene network [32] and hematopoietic development [33–35]. However, experimental elucidation of a limited number of interactions is hard to scale. Regulatory interactions are highly context-specific [17,36] and most remain unknown [8,37].
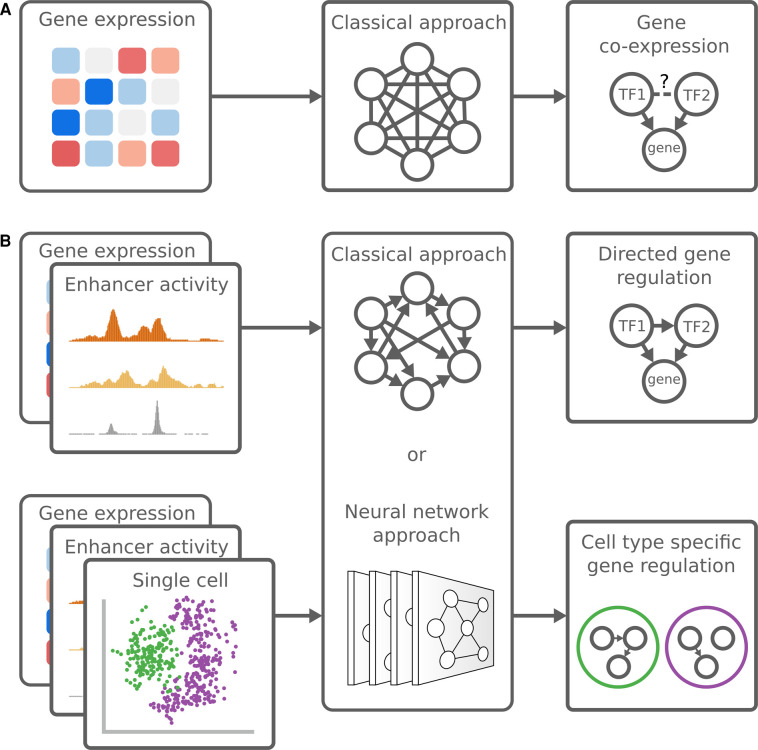
Computational inference of genome-wide GRNs was made possible with the advent of expression microarrays. Expression levels between transcription factors and their target genes tend to correlate [38] and genes with similar mRNA expression patterns are more likely to be regulated by a common transcription factor [39,40]. This led to the conception of gene co-expression networks, where functional connections between genes are inferred by expression pattern similarity. WGCNA [41] and ARACNe [42] were among the first gene co-expression-based tools and remain popular. Presently, a multitude of GRN inference methods exists. Reviews on the technical details can be found here [14,43–45]. Recent advances in experimental and computational techniques means that GRN inference has progressed beyond simple co-expression. In this review, we will highlight three approaches that have the potential to significantly impact GRN modeling: (1) moving from one modality, gene expression, to multi-omics, (2) single cell sequencing for cell type-specific signal and (3) neural networks as flexible gene regulatory models (Figure 1).
Figure 1. Schematic overview of different gene regulatory network inference approaches.
(A) Classical approaches, e.g. correlation, regression or mutual information, can be applied on gene expression data to generate undirected co-expression networks. With prior knowledge about TFs the directionality between TF and target gene can be inferred, however, the directionality between two TFs cannot be established. (B) More recent approaches combine multiple types of genome-wide functional data (multi-omics), with either a classical approach or neural networks to identify directed gene regulatory networks. Single cell sequencing allows for the identification of cell type specific regulatory networks.
Multi-omics to capture gene regulation
Gene regulation by TFs is mediated through CREs including promoters and enhancers. By incorporating TF binding at enhancers, regulatory networks can be constrained by direct, causal relationships. Ideally, binding of TFs would be determined experimentally with chromatin immunoprecipitation followed by sequencing (ChIP-seq) [46] or related techniques [47–49]. While large compendia of TF binding profiles in different cell types have been collected for humans [37], this effort remains unfeasible for less well-studied organisms, including most developmental model systems. With sufficient training data, TF binding can be computationally imputed [50–65], however, this does not necessarily generalize across species [66]. As a result, most current approaches use relatively simple models that combine experimentally measured CRE activity with TF binding motifs to computationally predict TF binding.
Putative CREs and their activity can be mapped genome-wide using chromatin accessibility assays, such as DNase I hypersensitive sites sequencing (DNAse-seq) [67] and Assay for Transposase-Accessible Chromatin using sequencing (ATAC-seq) [68]. The number of reads in an element can then be used as a measure for CRE activity in the experimental system [69]. ATAC-seq especially has been widely applied in developmental model systems, as it is experimentally relatively straightforward [31,70–78]. The chromatin environment can supply additional information on CRE location, function and activity. For instance, the transcriptional co-activator p300 is a histone acetyltransferase and can acytelate lysine 27 of histone H3 (H3K27ac). ChIP-seq using antibodies specific to p300 or H3K27ac can therefore identify active enhancers and promoters [79,80]. Other histone modifications that can be linked to CRE activity include H3K4me1 (enhancers) and H3K4me3 (promoters) [81].
CRE activity is determined by (in)direct binding of several TFs [82,83]. Therefore, characterizing TF binding at enhancers can identify their relative importance to the function of an enhancer. One approach to infer TF binding from genome-wide DNA accessibility is digital genomic footprinting [84], which has been used to directly infer GRNs [85,86]. However, sequence bias of the enzymes needs to be taken into account and TFs with more dynamic binding kinetics, such as some nuclear receptors, are not detected by footprint analysis [87–89]. Regardless, footprint analysis using cleavage bias correction can still be informative, especially in differential conditions [90,91]. A more routinely applied approach is to combine TF binding probabilities derived from TF motif scores with DNA accessibility. In some approaches, these are used as priors or constraints on network topology, where the network is inferred from gene expression measurements [92–94]. In alternative approaches, TF motif scores and accessibility are combined with RNA expression using regression models or co-variation of accessibility and expression [95–100].
Enhancers regulate transcription via context-dependent enhancer-promoter interactions [101], usually within a transcriptionally active domain [102]. Combined with TF binding data, these interactions allow for the inference of directed GRNs. Enhancer-promoter interactions can be identified experimentally with Chromatin Conformation Capture techniques [103–105], although this is still uncommon in non-model systems. Inferring interaction between enhancers to target genes is an active field of research. The most commonly used heuristic is to link enhancers to the nearest gene. However, this heuristic is often still incorrect [106,107]. Accuracy can be improved by combining enhancer to gene distance with TF-target gene co-expression [108]. Finally, Activity-by-Contact based models significantly outperform the nearest gene heuristic by using enhancer to gene distance and enhancer activity [109].
By combining gene expression data with at least one source of enhancer data (e.g. accessibility or interaction data), directed regulatory networks may be inferred with significantly higher accuracy compared with traditional co-expression approaches [98,110,111]. Not only does the combined approach filter out spurious interactions and add causality, but it also reduces the biases introduced by singular approaches. Therefore, we believe that the use of multiple omics will become dominant in all modalities of GRN inference approaches.
Single cell sequencing for cell type specific regulation
Developmental transcription regulation has mainly been studied by either in situ hybridization [112], which maps the spatial distribution of gene expression of a small set of genes, or bulk gene expression studies [113]. The latter measures the whole transcriptome as a compound signal of all the different cells present in the sample. Single cell sequencing is a fast developing technique to measure the gene expression of individual cells separately, with newer techniques even capable of tagging cells to their spatial coordinates [114,115]. These techniques increase the number of measurements from a handful to several (tens to hundreds of) thousands. This substantial increase in data allows for interesting new ways of GRN inference, but poses new challenges as well.
The output of a single cell experiment generally consists of count tables containing several thousands of cells with low coverage, e.g. only a few thousand of measured transcripts per cell. The low coverage makes the detection of relations between lowly expressed genes difficult. Although it is possible to artificially increase the sequencing depth by simulation (imputation), this does not seem to improve GRN inference [116,117]. Furthermore, it is important to note that cells are repeated measures [118], meaning that the cells come from the same environmental and genetic background, which breaks most statistical assumptions. Computationally clustering related cells, called pseudobulk or meta-cells [119], and using their combined signal solves the issues of low coverage and repeated measures, and still yields cell-type specific signals.
Since fundamentally there are small differences between bulk and pseudobulk data, it is not uncommon to apply bulk GRN inference approaches, such as gene co-expression, ARACNE [42] and GENIE3 [120], to pseudobulk data without much adjustment.
The large number of cells, however, allows for specialized single cell GRN approaches. These include mutual information in combination with partial information decomposition [121], gene coexpression [122], self organizing maps [123], or a combination of single cell RNA-seq and single cell ATAC-seq coexpression and/or bayesian ridge regression [124–126]. Other approaches first order cells by their inferred temporal ordering and then infer the gene-gene relations on this pseudotime, with the assumption that these orderings, also called trajectories, represent cell lineages [127]. Pseudotime can be estimated by simply following the first principal component, or finding the minimal spanning tree between clusters [128], where more advanced methods smoothen the tree [129,130]. A downside of these techniques is that they can not infer the directionality of the relationships. To computationally obtain this directionality, the ratio between spliced and unspliced transcripts per gene can be used as a proxy for whether or not a gene is actively transcribed. By applying this logic across all genes and all cells, one can infer a vector field of velocities of cells which then can be used to get a temporal cell ordering with a start and end [131,132]. These orderings then allow for inferring ordinary differential equations [133,134], Granger causality [135–137], boolean networks [138] or autoregressive models [139]. Most of these methods assume Gaussian noise for gene expression, even though transcription occurs in bursts [140,141], a phenomenon that can only be captured on a single cell level. These dynamics can be modeled as a Markov process including transcriptional bursting and degradation [142]. Theoretically these mechanistic models could be great tools for hypothesis generation, but more work is needed to prove their practical usefulness. Even though the aforementioned GRN inference methods were developed for single cell data specifically, many fail to show consistent improvement over methods that were developed for bulk data, and are seemingly barely any better than purely random models [117,143,144]. Moreover, the added complexity and number of cells leads to computational scaling issues, with some methods taking several days to weeks to finish [117].
Single cell sequencing has the advantage that it disentangles the composite signal present in all biological tissues. The increased number of measurements allows for more complex GRN definitions and inference. Finally, it allows for the inference of fine grained temporal orderings necessary for GRN inference. Even though single cell GRN inference methods have not yet brought the improvements over bulk methods we hoped for, we still expect single cell GRN inference to become the new standard of the field.
Neural networks as flexible gene regulatory models
Computational inference of a GRN depends on a lot of implicit assumptions. For example, a common assumption is that the relationship between genes is additive, which means that the effect on a gene equals the sum of the effects of two regulators separately, but in reality, gene-gene relationships are more complex and for example can include multiplicative effects [145]. A type of model that requires little explicit specification about the possible relationships in the data, but automatically learns these relationships, is an Artificial Neural Network (ANN). ANNs have been successfully applied in a variety of settings, with famously complex problems such as protein folding [146], image recognition [147], and the board game Go [148]. The successes of ANNs in these unrelated fields shows great promise for application in the field of gene regulatory inference.
Just like GRNs, ANNs consist of nodes and edges. Each edge multiplies the signal from the previous node to the next, and by applying a function to the sum of all the incoming edges the value in the next node is calculated. By adding multiple layers of nodes in between the in- and output nodes (this is where the term deep neural network comes from), a network is formed that is capable of learning more and more complex interactions. Learning happens by giving the model examples of input data and expected output, and based on this information the model iteratively updates (learns) its edge weights. After training, hypotheses can easily be tested by systematically querying the model for the predicted effect of certain changes. See [149] for an excellent review on the topic applied to genomics.
ANNs in genomics were first applied to predict the output of a genomic assay, for instance histone modifications in a certain cell type, by using only the DNA sequence as input. Early models showed that convolutional neural networks are capable of predicting functional effects of noncoding variants from short (10–1000 bp) genomic sequences alone [150,151]. These types of models can be used to discover composite motifs and periodic binding [15]. Additionally, these models are capable of learning complex and distal biological relations, as increasing the input sequence to 131 kb still improves accuracy [152].
Whereas ANNs in genomics have mainly been popularized on sequence data, adoption for GRN inference has been relatively slow. Different approaches consist of self-organizing maps [123], variational autoencoders [153], extreme learning machines [154], or graph convolutional neural networks [155,156]. Even though these networks differ in architectural designs, they all report higher levels of accuracy over non-ANN approaches. However, without independent benchmark studies it is hard to verify these results.
The main strength of ANNs is that they can approximate any continuous relationship in the data [157,158], with the downside that large amounts of training data are required. This makes the combination of single cell sequencing and ANNs promising, as current single cell GRN inference approaches have scaling issues [117] and ANNs train relatively fast with the use of GPUs (specialized graphics cards). Fundamentally, understanding how ANNs work is, however, much harder than understanding the classical models typically used for GRN inference. This causes ANNs to be met with skepticism and the persistent misconception that ANNs only function as a black box for predictions and its logic can not be interpreted [159]. We expect ANNs to become commonplace in the field of GRN inference due to their successes in other fields, ease of implementation with high-level programming libraries [160,161], and availability of sufficient training data due to single cell sequencing.
Discussion
Traditional GRNs, mostly based on gene co-expression, have so far served as a useful abstraction to understand regulatory dynamics in developmental systems. However, the way GRNs are currently derived suffers from two fundamental problems. First, the classic GRN that describes TF to target gene relations remains a simplified model and, by design, cannot properly reflect the full complexity of gene regulation. In addition, they are mostly based on mRNA expression as a measure of protein expression, even though this relation is not always linear [162]. In addition, any other types of regulation between transcript and protein product, such as mRNA degradation and post-translational modification, are usually ignored. Second, experiments generally have more features (i.e. genes measured) than samples which is also known as ‘the curse of dimensionality'. In this underdetermined system, many different models can potentially fit to the data, and it is both practically and theoretically impossible to identify the correct model with certainty [163]. It then should not come as a surprise that benchmarks consistently demonstrate that the quality of the inferred GRNs is low [143,144,164–168]. Based on these observations it is clear that our current approach to infer GRN is not sustainable and design changes are needed. Ultimately, we expect the field to move towards GRNs inferred from neural networks trained on single cell multi-omics data.
Having said that, it is not enough to just naively apply single cell multi-omics ANNs. By adding more modalities, and making GRNs more complex, networks become even more underdetermined. This is why most multi-omics approaches use the new modalities to prune the possible TF-target gene relations, which actually reduces the degrees of freedom [98,122,125,126]. Moreover, one can use time-series data to further prune TF-target gene interactions [169], although time-series multi-omics GRN inference tools are still relatively uncommon [170–173]. In addition, computational methods such as regularization [174] and dropout [175] constrain the problem in such a way that you end up with the simplest fit out of likely possible fits. In addition, recent developments have made it possible to measure multiple modalities in the same cell, such as combined ATAC-seq and RNA-seq [176–178], which offers new, exciting opportunities for combining single cell sequencing with multi-omics data. ANNs, finally, have been made relatively easy to implement, can learn any type of interaction, and make no assumptions about the data (such as normality), which makes them extremely powerful GRN tools. However, it is not yet clear what the optimal architecture is for these networks, and interpreting the learned network from the ANN remains difficult.
GRN inference has become a data science, and it is time that we start treating it as such. Integrating multiple omics, several thousands of cells, and training complex machine learning models requires specialized knowledge. Common mistakes, such as treating cells from the same sample as independent [118], double dipping [179], and data leakage [180], can be avoided by proper data science training, but are unfortunately still common. Comparing the quality of GRN inference methods requires standardized benchmarks with multiple datasets, preferably a mix of experimental data and simulated data [181–183]. Simulated data has the advantage that the ground truth is known which makes benchmarking straightforward, but has the clear disadvantage that the quality of simulated data depends on its assumptions and may actually not be representative of real biological data. The DREAM challenges [164,184] and BEELINE platform [144] are great examples, with predefined datasets and quality metrics. Only by measuring network accuracy in equal settings will it be possible to properly compare methods. It is however important to note that the goal of GRN inference is to gain mechanistic insights, as opposed to getting an optimal benchmark score, which makes fair comparison between approaches hard.
All together, we expect the field of transcription regulation in development to move towards increasingly multimodal GRN inference techniques to identify causal relations between genes. Single cell sequencing adds a cell type-specific precision which bulk sequencing can not provide. Finally, we expect the adoption of artificial neural networks as the field matures in technology and formal training, as these methods are inherently more powerful as previously used techniques.
Perspectives
Gene regulatory networks have served as powerful models to understand gene regulatory programs in development and disease. Amongst others, these networks have been applied to model developmental patterning, to identify relevant transcription factors for cell fate transitions and to characterize deregulated transcriptional programs in disease.
We believe three relatively recent developments will impact the computational inference of GRNs. The combination of multiple data modalities, such as RNA expression and DNA accessibility, help to constrain GRN topology and to predict directed networks. Single cell sequencing will become the de facto standard, as it allows for cell type-specific models and is able to provide the high number of measurements that are needed. Finally, artificial neural networks have the capability to create flexible and powerful models of gene regulation, which will benefit efficient and accurate GRN inference.
The developments outlined above have the potential to significantly improve GRN inference. To fully exploit these approaches we have to implement common data science practices, and develop community-driven benchmarks to consistently measure the performance of different techniques.
Abbreviations
- ANN
artificial neural network
- ATAC-seq
assay for Transposase-Accessible Chromatin using sequencing
- CREs
cis-regulatory elements
- DNAse-seq
DNase I hypersensitive sites sequencing
- GRN
gene regulatory network
- TFs
transcription factors
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
Netherlands Organization for Scientific Research [NWO grant 016.Vidi.189.081 to S.J.v.H.].
Author Contributions
Maarten van der Sande: writing — original draft and writing — review and editing. Siebren Frölich: writing — original draft and writing — review and editing. Simon J. van Heeringen: writing — review and editing, funding acquisition, and supervision.
References
- 1.Mammoto, A., Mammoto, T. and Ingber, D.E. (2012) Mechanosensitive mechanisms in transcriptional regulation. J. Cell Sci. 125, 3061–3073 10.1242/jcs.093005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cameron, R.A., Hough-Evans, B.R., Britten, R.J. and Davidson, E.H. (1987) Lineage and fate of each blastomere of the eight-cell sea urchin embryo. Genes Dev. 1, 75–85 10.1101/gad.1.1.75 [DOI] [PubMed] [Google Scholar]
- 3.Cooper, G.M. (2000) Regulation of Transcription in Eukaryotes. In The Cell: A Molecular Approach, 2nd edn, Sinauer Associates, Sunderland, MA: https://www.ncbi.nlm.nih.gov/books/NBK9904/ [Google Scholar]
- 4.Li, Y.J., Fu, X.H., Liu, D.P. and Liang, C.C. (2004) Opening the chromatin for transcription. Int. J. Biochem. Cell Biol. 36, 1411–1423 10.1016/j.biocel.2003.11.003 [DOI] [PubMed] [Google Scholar]
- 5.McMahon, A.P., Novak, T.J., Britten, R.J. and Davidson, E.H. (1984) Inducible expression of a cloned heat shock fusion gene in sea urchin embryos. Proc. Natl Acad. Sci. U.S.A. 81, 7490–7494 10.1073/pnas.81.23.7490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordân, R., Hartemink, A.J. and Bulyk, M.L. (2009) Distinguishing direct versus indirect transcription factor–DNA interactions. Genome Res. 19, 2090–2100 10.1101/gr.094144.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, H. and Pugh, B.F. (2021) What do transcription factors interact with? J. Mol. Biol. 433, 166883 10.1016/j.jmb.2021.166883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaquerizas, J.M., Kummerfeld, S.K., Teichmann, S.A. and Luscombe, N.M. (2009) A census of human transcription factors: function, expression and evolution. Nat. Rev. Genet. 10, 252–263 10.1038/nrg2538 [DOI] [PubMed] [Google Scholar]
- 9.Lambert, S.A., Jolma, A., Campitelli, L.F., Das, P.K., Yin, Y., Albu, M.et al. (2018) The human transcription factors. Cell 172, 650–665 10.1016/j.cell.2018.01.029 [DOI] [PubMed] [Google Scholar]
- 10.Sebé-Pedrós, A., Chomsky, E., Pang, K., Lara-Astiaso, D., Gaiti, F., Mukamel, Z.et al. (2018) Early metazoan cell type diversity and the evolution of multicellular gene regulation. Nat. Ecol. Evol. 2, 1176–1188 10.1038/s41559-018-0575-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nitta, K.R., Jolma, A., Yin, Y., Morgunova, E., Kivioja, T., Akhtar, J.et al. (2015) Conservation of transcription factor binding specificities across 600 million years of bilateria evolution. eLife 4, e04837 10.7554/eLife.04837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt, D., Wilson, M.D., Ballester, B., Schwalie, P.C., Brown, G.D., Marshall, A.et al. (2010) Five-vertebrate ChIP-seq reveals the evolutionary dynamics of transcription factor binding. Science 328, 1036–1040 10.1126/science.1186176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villar, D., Flicek, P. and Odom, D.T. (2014) Evolution of transcription factor binding in metazoans: mechanisms and functional implications. Nat. Rev. Genet. 15, 221–233 10.1038/nrg3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine, M. and Davidson, E.H. (2005) Gene regulatory networks for development. Proc. Natl Acad. Sci. U.S.A. 102, 4936–4942 10.1073/pnas.0408031102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avsec, Ž, Weilert, M., Shrikumar, A., Krueger, S., Alexandari, A., Dalal, K.et al. (2021) Base-resolution models of transcription-factor binding reveal soft motif syntax. Nat. Genet. 53, 354–366 10.1038/s41588-021-00782-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown, C.D., Johnson, D.S. and Sidow, A. (2007) Functional architecture and evolution of transcriptional elements that drive gene coexpression. Science 317, 1557–1560 10.1126/science.1145893 [DOI] [PubMed] [Google Scholar]
- 17.Farley, E.K., Olson, K.M., Zhang, W., Rokhsar, D.S. and Levine, M.S. (2016) Syntax compensates for poor binding sites to encode tissue specificity of developmental enhancers. Proc. Natl Acad. Sci. U.S.A. 113, 6508–6513 10.1073/pnas.1605085113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong, E.S., Zheng, D., Tan, S.Z., Bower, N.I., Garside, V., Vanwalleghem, G.et al. (2020) Deep conservation of the enhancer regulatory code in animals. Science 370, eaax8137 10.1126/science.aax8137 [DOI] [PubMed] [Google Scholar]
- 19.Zeitlinger, J. (2020) Seven myths of how transcription factors read the cis-regulatory code. Curr. Opin. Syst. Biol. 23, 22–31 10.1016/j.coisb.2020.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martindale, M.Q. (2005) The evolution of metazoan axial properties. Nat. Rev. Genet. 6, 917–927 10.1038/nrg1725 [DOI] [PubMed] [Google Scholar]
- 21.Britten, R.J. and Davidson, E.H. (1969) Gene regulation for higher cells: a theory. Science 165, 349–357 10.1126/science.165.3891.349 [DOI] [PubMed] [Google Scholar]
- 22.Davidson, E.H., Rast, J.P., Oliveri, P., Ransick, A., Calestani, C., Yuh, C.H.et al. (2002) A provisional regulatory gene network for specification of endomesoderm in the sea urchin embryo. Dev. Biol. 246, 162–190 10.1006/dbio.2002.0635 [DOI] [PubMed] [Google Scholar]
- 23.Cary, G.A., McCauley, B.S., Zueva, O., Pattinato, J., Longabaugh, W. and Hinman, V.F. (2020) Systematic comparison of sea urchin and sea star developmental gene regulatory networks explains how novelty is incorporated in early development. Nat. Commun. 11, 6235 10.1038/s41467-020-20023-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peter, I.S. and Davidson, E.H. (2011) A gene regulatory network controlling the embryonic specification of endoderm. Nature. 474, 635–639 10.1038/nature10100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saudemont, A., Haillot, E., Mekpoh, F., Bessodes, N., Quirin, M., Lapraz, F.et al. (2010) Ancestral regulatory circuits governing ectoderm patterning downstream of nodal and BMP2/4 revealed by gene regulatory network analysis in an echinoderm. PLoS Genet. 6, e1001259 10.1371/journal.pgen.1001259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charney, R.M., Paraiso, K.D., Blitz, I.L. and Cho, K.W.Y. (2017) A gene regulatory program controlling early Xenopus mesendoderm formation: Network conservation and motifs. Semin. Cell Dev. Biol. 66, 12–24 10.1016/j.semcdb.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koide, T., Hayata, T. and Cho, K.W.Y. (2005) Xenopus as a model system to study transcriptional regulatory networks. Proc. Natl Acad. Sci. U.S.A. 102, 4943–4948 10.1073/pnas.0408125102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rankin, S.A., Kormish, J., Kofron, M., Jegga, A. and Zorn, A.M. (2011) A gene regulatory network controlling hhex transcription in the anterior endoderm of the organizer. Dev. Biol. 351, 297–310 10.1016/j.ydbio.2010.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinner, D., Kirilenko, P., Rankin, S., Wei, E., Howard, L., Kofron, M.et al. (2006) Global analysis of the transcriptional network controlling Xenopus endoderm formation. Development 133, 1955–1966 10.1242/dev.02358 [DOI] [PubMed] [Google Scholar]
- 30.Lukoseviciute, M., Gavriouchkina, D., Williams, R.M., Hochgreb-Hagele, T., Senanayake, U., Chong-Morrison, V.et al. (2018) From pioneer to repressor: bimodal foxd3 activity dynamically remodels neural crest regulatory landscape in vivo. Dev. Cell 47, 608–628.e6 10.1016/j.devcel.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams, R.M., Candido-Ferreira, I., Repapi, E., Gavriouchkina, D., Senanayake, U., Ling, I.T.C.et al. (2019) Reconstruction of the global neural crest gene regulatory network in vivo. Dev. Cell 51, 255–276.e7 10.1016/j.devcel.2019.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaeger, J. (2011) The gap gene network. Cell. Mol. Life Sci. 68, 243–274 10.1007/s00018-010-0536-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kueh, H.Y. and Rothenberg, E.V. (2012) Regulatory gene network circuits underlying T cell development from multipotent progenitors. Wiley Interdiscip. Rev. Syst. Biol. Med. 4, 79–102 10.1002/wsbm.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pimanda, J.E. and Göttgens, B. (2010) Gene regulatory networks governing haematopoietic stem cell development and identity. Int. J. Dev. Biol. 54, 1201–1211 10.1387/ijdb.093038jp [DOI] [PubMed] [Google Scholar]
- 35.Singh, H., Khan, A.A. and Dinner, A.R. (2014) Gene regulatory networks in the immune system. Trends Immunol. 35, 211–218 10.1016/j.it.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 36.Ryan, G.E. and Farley, E.K. (2020) Functional genomic approaches to elucidate the role of enhancers during development. WIREs Syst. Biol. Med. 12, e1467 10.1002/wsbm.1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunham, I., Kundaje, A., Aldred, S.F., Collins, P.J., Davis, C.A., Doyle, F.et al. (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 10.1038/nature11247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ideker, T., Thorsson, V., Ranish, J.A., Christmas, R., Buhler, J., Eng, J.K.et al. (2001) Integrated genomic and proteomic analyses of a systematically perturbed metabolic network. Science 292, 929–934 10.1126/science.292.5518.929 [DOI] [PubMed] [Google Scholar]
- 39.Allocco, D.J., Kohane, I.S. and Butte, A.J. (2004) Quantifying the relationship between co-expression, co-regulation and gene function. BMC Bioinformatics 5, 18 10.1186/1471-2105-5-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eisen, M.B., Spellman, P.T., Brown, P.O. and Botstein, D. (1998) Cluster analysis and display of genome-wide expression patterns. Proc. Natl Acad. Sci. U.S.A. 95, 14863–8 10.1073/pnas.95.25.14863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang, B. and Horvath, S. (2005) A general framework for weighted gene co-expression network analysis. Stat. Appl. Genet. Mol. Biol. 4, Article 17 10.2202/1544-6115.1128 [DOI] [PubMed] [Google Scholar]
- 42.Margolin, A.A., Nemenman, I., Basso, K., Wiggins, C., Stolovitzky, G., Favera, R.D.et al. (2006) ARACNE: an algorithm for the reconstruction of gene regulatory networks in a mammalian cellular context. BMC Bioinformatics 7, S7 10.1186/1471-2105-7-S1-S7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chasman, D. and Roy, S. (2017) Inference of cell type specific regulatory networks on mammalian lineages. Curr. Opin. Syst. Biol. 2, 130–139 10.1016/j.coisb.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delgado, F.M. and Gómez-Vela, F. (2019) Computational methods for gene regulatory networks reconstruction and analysis: a review. Artif. Intell. Med. 95, 133–145 10.1016/j.artmed.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 45.Mercatelli, D., Scalambra, L., Triboli, L., Ray, F. and Giorgi, F.M. (2020) Gene regulatory network inference resources: a practical overview. Biochim. Biophys. Acta Gene Regul. Mech. 1863, 194430 10.1016/j.bbagrm.2019.194430 [DOI] [PubMed] [Google Scholar]
- 46.Robertson, G., Hirst, M., Bainbridge, M., Bilenky, M., Zhao, Y., Zeng, T.et al. (2007) Genome-wide profiles of STAT1 DNA association using chromatin immunoprecipitation and massively parallel sequencing. Nat. Methods 4, 651–657 10.1038/nmeth1068 [DOI] [PubMed] [Google Scholar]
- 47.He, Q., Johnston, J. and Zeitlinger, J. (2015) ChIP-nexus enables improved detection of in vivo transcription factor binding footprints. Nat. Biotechnol. 33, 395–401 10.1038/nbt.3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rhee, H.S. and Pugh, B.F. (2011) Comprehensive genome-wide protein-DNA interactions detected at single-nucleotide resolution. Cell 147, 1408–1419 10.1016/j.cell.2011.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaya-Okur, H.S., Wu, S.J., Codomo, C.A., Pledger, E.S., Bryson, T.D., Henikoff, J.G.et al. (2019) CUT&tag for efficient epigenomic profiling of small samples and single cells. Nat. Commun. 10, 1930 10.1038/s41467-019-09982-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keilwagen, J., Posch, S. and Grau, J. (2019) Accurate prediction of cell type-specific transcription factor binding. Genome Biol. 20, 9 10.1186/s13059-018-1614-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li, H., Quang, D. and Guan, Y. (2019) Anchor: trans-cell type prediction of transcription factor binding sites. Genome Res. 29, 281–292 10.1101/gr.237156.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quang, D. and Xie, X. (2019) Factornet: A deep learning framework for predicting cell type specific transcription factor binding from nucleotide-resolution sequential data. Methods 166, 40–47 10.1016/j.ymeth.2019.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen, C., Hou, J., Shi, X., Yang, H., Birchler, J.A. and Cheng, J. (2021) DeepGRN: prediction of transcription factor binding site across cell-types using attention-based deep neural networks. BMC Bioinformatics 22, 38 10.1186/s12859-020-03952-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mariani, L., Weinand, K., Gisselbrecht, S.S. and Bulyk, M.L. (2020) MEDEA: analysis of transcription factor binding motifs in accessible chromatin. Genome Res. 30, 736–748 10.1101/gr.260877.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li, H. and Guan, Y. (2021) Fast decoding cell type–specific transcription factor binding landscape at single-nucleotide resolution. Genome Res. 31, 721–731 10.1101/gr.269613.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bruse, N. and van Heeringen, S.J. (2018) Gimmemotifs: an analysis framework for transcription factor motif analysis. bioRxiv 10.1101/474403 [DOI] [Google Scholar]
- 57.Schreiber, J., Bilmes, J. and Noble, W.S. (2020) Completing the ENCODE3 compendium yields accurate imputations across a variety of assays and human biosamples. Genome Biol. 21, 82 10.1186/s13059-020-01978-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng, J., Xu, M., Liu, Y. and Huang, W. (2022) AttBind: Prediction of Transcription Factor Binding Sites Across Cell-types Based on Attention Mechanism. In 2022 7th International Conference on Computer and Communication Systems (ICCCS), pp. 135–139 [Google Scholar]
- 59.Behjati Ardakani, F., Schmidt, F. and Schulz, M.H. (2019) Predicting transcription factor binding using ensemble random forest models. F1000Res. 7, 1603 10.12688/f1000research.16200.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trotter, M.V., Nguyen, C.Q., Young, S., Woodruff, R.T. and Branson, K.M. (2021) Epigenomic language models powered by cerebras. arXiv 10.48550/arXiv.2112.07571 [DOI] [Google Scholar]
- 61.Yi, R., Cho, K. and Bonneau, R. (2022) NetTIME: a multitask and base-pair resolution framework for improved transcription factor binding site prediction. Bioinformatics 38, 4762–4770 10.1093/bioinformatics/btac569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pap, G., Zoltán, G., Ádám, K., Tóth, L. and Hegedus, Z. (2021) Transcription factor binding site detection using convolutional neural networks with a functional group-based data representation. J. Phys. Conf. Ser. 1824, 012001 10.1088/1742-6596/1824/1/012001 [DOI] [Google Scholar]
- 63.Karimzadeh, M. and Hoffman, M.M. (2022) Virtual ChIP-seq: predicting transcription factor binding by learning from the transcriptome. Genome Biol. 23, 126 10.1186/s13059-022-02690-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koo, P.K. and Ploenzke, M. (2020) Deep learning for inferring transcription factor binding sites. Curr. Opin. Syst. Biol. 19, 16–23 10.1016/j.coisb.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kundaje, A., Boley, N., Kuffner, R., Heiser, L., Costello, J., Stolovitzky, G.et al. (2017) ENCODE-DREAM in vivo transcription factor binding site prediction challenge. Synapse 10.7303/syn6131484 [DOI] [Google Scholar]
- 66.Cochran, K., Srivastava, D., Shrikumar, A., Balsubramani, A., Hardison, R.C., Kundaje, A.et al. (2022) Domain-adaptive neural networks improve cross-species prediction of transcription factor binding. Genome Res. 32, 512–523 10.1101/gr.275394.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boyle, A.P., Davis, S., Shulha, H.P., Meltzer, P., Margulies, E.H., Weng, Z.et al. (2008) High-resolution mapping and characterization of open chromatin across the genome. Cell 132, 311–322 10.1016/j.cell.2007.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buenrostro, J.D., Giresi, P.G., Zaba, L.C., Chang, H.Y. and Greenleaf, W.J. (2013) Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 10.1038/nmeth.2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buenrostro, J., Wu, B., Chang, H. and Greenleaf, W. (2015) ATAC-seq: a method for assaying chromatin accessibility genome-wide. Curr. Protoc. Mol. Biol. 109, 21.29.1–21.29.9 10.1002/0471142727.mb2129s109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sebé-Pedrós, A., Saudemont, B., Chomsky, E., Plessier, F., Mailhé, M.P., Renno, J.et al. (2018) Cnidarian cell type diversity and regulation revealed by whole-organism single-cell RNA-seq. Cell 173, 1520–1534.e20 10.1016/j.cell.2018.05.019 [DOI] [PubMed] [Google Scholar]
- 71.Marlétaz, F., Firbas, P.N., Maeso, I., Tena, J.J., Bogdanovic, O., Perry, M.et al. (2018) Amphioxus functional genomics and the origins of vertebrate gene regulation. Nature 564, 64–70 10.1038/s41586-018-0734-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Uesaka, M., Kuratani, S., Takeda, H. and Irie, N. (2019) Recapitulation-like developmental transitions of chromatin accessibility in vertebrates. Zool. Lett. 5, 33 10.1186/s40851-019-0148-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pálfy, M., Schulze, G., Valen, E. and Vastenhouw, N.L. (2020) Chromatin accessibility established by Pou5f3, Sox19b and Nanog primes genes for activity during zebrafish genome activation. PLoS Genet. 16, e1008546 10.1371/journal.pgen.1008546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shashikant, T., Khor, J.M. and Ettensohn, C.A. (2018) Global analysis of primary mesenchyme cell cis-regulatory modules by chromatin accessibility profiling. BMC Genomics 19, 206 10.1186/s12864-018-4542-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Madgwick, A., Magri, M.S., Dantec, C., Gailly, D., Fiuza, U.M., Guignard, L.et al. (2019) Evolution of embryonic cis-regulatory landscapes between divergent phallusia and ciona ascidians. Dev. Biol. 448, 71–87 10.1016/j.ydbio.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 76.Esmaeili, M., Blythe, S.A., Tobias, J.W., Zhang, K., Yang, J. and Klein, P.S. (2020) Chromatin accessibility and histone acetylation in the regulation of competence in early development. Dev. Biol. 462, 20–35 10.1016/j.ydbio.2020.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bright, A.R., van Genesen, S., Li, Q., Grasso, A., Frölich, S., van der Sande, M.et al. (2021) Combinatorial transcription factor activities on open chromatin induce embryonic heterogeneity in vertebrates. EMBO J. 40, e104913 10.15252/embj.2020104913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang, H., Luan, Y., Liu, T., Lee, H.J., Fang, L., Wang, Y.et al. (2020) A map of cis-regulatory elements and 3D genome structures in zebrafish. Nature 588, 337–343 10.1038/s41586-020-2962-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Visel, A., Blow, M.J., Li, Z., Zhang, T., Akiyama, J.A., Holt, A.et al. (2009) ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature 457, 854–858 10.1038/nature07730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Creyghton, M.P., Cheng, A.W., Welstead, G.G., Kooistra, T., Carey, B.W., Steine, E.J.et al. (2010) Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl Acad. Sci. U.S.A. 107, 21931–6 10.1073/pnas.1016071107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heintzman, N.D., Stuart, R.K., Hon, G., Fu, Y., Ching, C.W., Hawkins, R.D.et al. (2007) Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat. Genet. 39, 311–318 10.1038/ng1966 [DOI] [PubMed] [Google Scholar]
- 82.Simeone, A., Pannese, M., Acampora, D., D'Esposito, M. and Boncinelli, E. (1988) At least three human homeoboxes on chromosome 12 belong to the same transcription unit. Nucleic Acids Res. 16, 5379–5390 10.1093/nar/16.12.5379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mitchell, P.J. and Tjian, R. (1989) Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science 245, 371–378 10.1126/science.2667136 [DOI] [PubMed] [Google Scholar]
- 84.Hesselberth, J.R., Chen, X., Zhang, Z., Sabo, P.J., Sandstrom, R., Reynolds, A.P.et al. (2009) Global mapping of protein-DNA interactions in vivo by digital genomic footprinting. Nat. Methods 6, 283–289 10.1038/nmeth.1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Neph, S., Vierstra, J., Stergachis, A.B., Reynolds, A.P., Haugen, E., Vernot, B.et al. (2012) An expansive human regulatory lexicon encoded in transcription factor footprints. Nature 489, 83–90 10.1038/nature11212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neph, S., Stergachis, A.B., Reynolds, A., Sandstrom, R., Borenstein, E. and Stamatoyannopoulos, J.A. (2012) Circuitry and dynamics of human transcription factor regulatory networks. Cell 150, 1274–1286 10.1016/j.cell.2012.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.He, H.H., Meyer, C.A., Hu, S.S., Chen, M.W., Zang, C., Liu, Y.et al. (2014) Refined DNase-seq protocol and data analysis reveals intrinsic bias in transcription factor footprint identification. Nat. Methods 11, 73–78 10.1038/nmeth.2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sung, M.H., Baek, S. and Hager, G.L. (2016) Genome-wide footprinting: ready for prime time? Nat. Methods 13, 222–228 10.1038/nmeth.3766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sung, M.H., Guertin, M.J., Baek, S. and Hager, G.L. (2014) DNase footprint signatures are dictated by factor dynamics and DNA sequence. Mol. Cell 56, 275–285 10.1016/j.molcel.2014.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li, Z., Schulz, M.H., Look, T., Begemann, M., Zenke, M. and Costa, I.G. (2019) Identification of transcription factor binding sites using ATAC-seq. Genome Biol. 20, 45 10.1186/s13059-019-1642-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bentsen, M., Goymann, P., Schultheis, H., Klee, K., Petrova, A., Wiegandt, R.et al. (2020) ATAC-seq footprinting unravels kinetics of transcription factor binding during zygotic genome activation. Nat. Commun. 11, 4267 10.1038/s41467-020-18035-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miraldi, E.R., Pokrovskii, M., Watters, A., Castro, D.M., De Veaux, N., Hall, J.A.et al. (2019) Leveraging chromatin accessibility for transcriptional regulatory network inference in T Helper 17 cells. Genome Res. 29, 449–463 10.1101/gr.238253.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Siahpirani, A.F. and Roy, S. (2017) A prior-based integrative framework for functional transcriptional regulatory network inference. Nucleic Acids Res. 45, e21 10.1093/nar/gkw963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sonawane, A.R., DeMeo, D.L., Quackenbush, J. and Glass, K. (2021) Constructing gene regulatory networks using epigenetic data. NPJ Syst. Biol. Appl. 7, 45 10.1038/s41540-021-00208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Madsen, J.G.S., Rauch, A., Van Hauwaert, E.L., Schmidt, S.F., Winnefeld, M. and Mandrup, S. (2018) Integrated analysis of motif activity and gene expression changes of transcription factors. Genome Res. 28, 243–255 10.1101/gr.227231.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kamal, A., Arnold, C., Claringbould, A., Moussa, R., Servaas, N., Kholmatov, M.et al. (2022) GRaNIE and GRaNPA: Inference and evaluation of enhancer-mediated gene regulatory networks applied to study macrophages. bioRxiv 10.1101/2021.12.18.473290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schmidt, F., Kern, F., Ebert, P., Baumgarten, N. and Schulz, M.H. (2019) TEPIC 2—an extended framework for transcription factor binding prediction and integrative epigenomic analysis. Bioinformatics 35, 1608–1609 10.1093/bioinformatics/bty856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu, Q., Georgiou, G., Frölich, S., van der Sande, M., Veenstra, G.J.C., Zhou, H.et al. (2021) ANANSE: an enhancer network-based computational approach for predicting key transcription factors in cell fate determination. bioRxiv 10.1101/2020.06.05.135798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ghaffari, S., Hanson, C., Schmidt, R.E., Bouchonville, K.J., Offer, S.M. and Sinha, S. (2021) An integrated multi-omics approach to identify regulatory mechanisms in cancer metastatic processes. Genome Biol. 22, 19 10.1186/s13059-020-02213-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vijayabaskar, M.S., Goode, D.K., Obier, N., Lichtinger, M., Emmett, A.M.L., Abidin, F.N.Z.et al. (2019) Identification of gene specific cis-regulatory elements during differentiation of mouse embryonic stem cells: an integrative approach using high-throughput datasets. PLoS Comput. Biol. 15, e1007337 10.1371/journal.pcbi.1007337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Levine, M. (2010) Transcriptional enhancers in animal development and evolution. Curr. Biol. 20, R754–R763 10.1016/j.cub.2010.06.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nora, E.P., Lajoie, B.R., Schulz, E.G.. Giorgetti, L., Okamoto, I., Servant, N.et al. (2012) Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 485, 381–385 10.1038/nature11049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dekker, J., Rippe, K., Dekker, M. and Kleckner, N. (2002) Capturing chromosome conformation. Science 295, 1306–1311 10.1126/science.1067799 [DOI] [PubMed] [Google Scholar]
- 104.Lieberman-Aiden, E., van Berkum, N.L., Williams, L., Imakaev, M., Ragoczy, T., Telling, A.et al. (2009) Comprehensive mapping of long range interactions reveals folding principles of the human genome. Science 326, 289–293 10.1126/science.1181369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mifsud, B., Tavares-Cadete, F., Young, A.N., Sugar, R., Schoenfelder, S., Ferreira, L.et al. (2015) Mapping long-range promoter contacts in human cells with high-resolution capture Hi-C. Nat. Genet. 47, 598–606 10.1038/ng.3286 [DOI] [PubMed] [Google Scholar]
- 106.Sanyal, A., Lajoie, B.R., Jain, G. and Dekker, J. (2012) The long-range interaction landscape of gene promoters. Nature 489, 109–113 10.1038/nature11279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li, G., Ruan, X., Auerbach, R.K., Sandhu, K.S., Zheng, M., Wang, P.et al. (2012) Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation. Cell 148, 84–98 10.1016/j.cell.2011.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marbach, D., Lamparter, D., Quon, G., Kellis, M., Kutalik, Z. and Bergmann, S. (2016) Tissue-specific regulatory circuits reveal variable modular perturbations across complex diseases. Nat. Methods 13, 366–370 10.1038/nmeth.3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fulco, C.P., Nasser, J., Jones, T.R., Munson, G., Bergman, D.T., Subramanian, V.et al. (2019) Activity-by-Contact model of enhancer-promoter regulation from thousands of CRISPR perturbations. Nat. Genet. 51, 1664–1669 10.1038/s41588-019-0538-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mercatelli, D. and Lopez-Garcia, G. (2020) Giorgi FM. corto: a lightweight R package for gene network inference and master regulator analysis. Bioinformatics 36, 3916–3917 10.1093/bioinformatics/btaa223 [DOI] [PubMed] [Google Scholar]
- 111.Glass, K., Huttenhower, C., Quackenbush, J. and Yuan, G.C. (2013) Passing messages between biological networks to refine predicted interactions. PLoS ONE 8, e64832 10.1371/journal.pone.0064832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jensen, E. (2014) Technical review: in situ hybridization. Anat. Rec. 297, 1349–1353 10.1002/ar.22944 [DOI] [PubMed] [Google Scholar]
- 113.Wang, Z., Gerstein, M. and Snyder, M. (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 10, 57–63 10.1038/nrg2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Longo, S.K., Guo, M.G., Ji, A.L. and Khavari, P.A. (2021) Integrating single-cell and spatial transcriptomics to elucidate intercellular tissue dynamics. Nat. Rev. Genet. 22, 627–644 10.1038/s41576-021-00370-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Borm, L.E., Mossi Albiach, A., Mannens, C.C.A., Janusauskas, J., Özgün, C., Fernández-García, D.et al. (2022) Scalable in situ single-cell profiling by electrophoretic capture of mRNA using EEL FISH. Nat. Biotechnol., 1–10 10.1038/s41587-022-01455-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ly, L.H. and Vingron, M. (2022) Effect of imputation on gene network reconstruction from single-cell RNA-seq data. Patterns 3, 100414 10.1016/j.patter.2021.100414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stone, M., Li, J., McCalla, S.G., Siahpirani, A.F., Periyasamy, V., Shin, J.et al. (2022) Identifying strengths and weaknesses of methods for computational network inference from single cell RNA-seq data. bioRxiv 10.1101/2021.06.01.446671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zimmerman, K.D., Espeland, M.A. and Langefeld, C.D. (2021) A practical solution to pseudoreplication bias in single-cell studies. Nat. Commun. 12, 738 10.1038/s41467-021-21038-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Baran, Y., Bercovich, A., Sebe-Pedros, A., Lubling, Y., Giladi, A., Chomsky, E.et al. (2019) Metacell: analysis of single-cell RNA-seq data using K-nn graph partitions. Genome Biol. 20, 206 10.1186/s13059-019-1812-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huynh-Thu, V.A., Irrthum, A., Wehenkel, L. and Geurts, P. (2010) Inferring regulatory networks from expression data using tree-Based methods. PLoS ONE 5, e12776 10.1371/journal.pone.0012776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chan, T.E., Stumpf, M.P.H. and Babtie, A.C. (2017) Gene regulatory network inference from single-cell data using multivariate information measures. Cell Syst. 5, 251–267.e3 10.1016/j.cels.2017.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aibar, S., González-Blas, C.B., Moerman, T., Huynh-Thu, V.A., Imrichova, H., Hulselmans, G.et al. (2017) SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 10.1038/nmeth.4463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jansen, C., Ramirez, R.N., El-Ali, N.C., Gomez-Cabrero, D., Tegner, J., Merkenschlager, M.et al. (2019) Building gene regulatory networks from scATAC-seq and scRNA-seq using linked self organizing maps. PLoS Comput. Biol. 15, e1006555 10.1371/journal.pcbi.1006555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.González-Blas, C.B., Winter, S.D., Hulselmans, G., Hecker, N., Matetovici, I., Christiaens, V.et al. (2022) SCENIC+: single-cell multiomic inference of enhancers and gene regulatory networks. bioRxiv 10.1101/2022.08.19.504505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jiang, J., Lyu, P., Li, J., Huang, S., Blackshaw, S., Qian, J.et al. (2022) IReNA: integrated regulatory network analysis of single-cell transcriptomes and chromatin accessibility profiles. bioRxiv 10.1101/2021.11.22.469628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kamimoto, K., Hoffmann, C.M. and Morris, S.A. (2020) Celloracle: dissecting cell identity via network inference and in silico gene perturbation. bioRxiv 10.1101/2020.02.17.947416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Packer, J.S., Zhu, Q., Huynh, C., Sivaramakrishnan, P., Preston, E., Dueck, H.et al. (2019) A lineage-resolved molecular atlas of C. elegans embryogenesis at single cell resolution. Science 365, eaax1971 10.1126/science.aax1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wolf, F.A., Hamey, F.K., Plass, M., Solana, J., Dahlin, J.S., Göttgens, B.et al. (2019) PAGA: graph abstraction reconciles clustering with trajectory inference through a topology preserving map of single cells. Genome Biol. 20, 59 10.1186/s13059-019-1663-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qiu, X., Mao, Q., Tang, Y., Wang, L., Chawla, R., Pliner, H.et al. (2017) Reversed graph embedding resolves complex single-cell developmental trajectories. Nat. Methods 14, 979–982 10.1038/nmeth.4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Street, K., Risso, D., Fletcher, R.B., Das, D., Ngai, J., Yosef, N.et al. (2018) Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics 19, 477 10.1186/s12864-018-4772-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bergen, V., Lange, M., Peidli, S., Wolf, F.A. and Theis, F.J. (2020) Generalizing RNA velocity to transient cell states through dynamical modeling. Nat. Biotechnol. 38, 1408–1414 10.1038/s41587-020-0591-3 [DOI] [PubMed] [Google Scholar]
- 132.La Manno, G., Soldatov, R., Zeisel, A., Braun, E., Hochgerner, H., Petukhov, V.et al. (2018) RNA velocity of single cells. Nature 560, 494–498 10.1038/s41586-018-0414-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aubin-Frankowski, P.C. and Vert, J.P. (2020) Gene regulation inference from single-cell RNA-seq data with linear differential equations and velocity inference. Bioinformatics 36, 4774–4780 10.1093/bioinformatics/btaa576 [DOI] [PubMed] [Google Scholar]
- 134.Matsumoto, H., Kiryu, H., Furusawa, C., Ko, M.S.H., Ko, S.B.H., Gouda, N.et al. (2017) SCODE: an efficient regulatory network inference algorithm from single-cell RNA-Seq during differentiation. Bioinformatics 33, 2314–2321 10.1093/bioinformatics/btx194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Deshpande, A., Chu, L.F., Stewart, R. and Gitter, A. (2022) Network inference with granger causality ensembles on single-cell transcriptomic data. Cell Rep. 38, 110333 10.1016/j.celrep.2022.110333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Papili Gao, N., Ud-Dean, S.M.M., Gandrillon, O. and Gunawan, R. (2018) SINCERITIES: inferring gene regulatory networks from time-stamped single cell transcriptional expression profiles. Bioinformatics 34, 258–266 10.1093/bioinformatics/btx575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Qiu, X., Rahimzamani, A., Wang, L., Ren, B., Mao, Q., Durham, T.et al. (2020) Inferring causal gene regulatory networks from coupled single-cell expression dynamics using scribe. Cell Syst. 10, 265–274.e11 10.1016/j.cels.2020.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Woodhouse, S., Piterman, N., Wintersteiger, C.M., Göttgens, B. and Fisher, J. (2018) SCNS: a graphical tool for reconstructing executable regulatory networks from single-cell genomic data. BMC Syst. Biol. 12, 59 10.1186/s12918-018-0581-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sanchez-Castillo, M., Blanco, D., Tienda-Luna, I.M., Carrion, M.C. and Huang, Y. (2018) A Bayesian framework for the inference of gene regulatory networks from time and pseudo-time series data. Bioinformatics 34, 964–970 10.1093/bioinformatics/btx605 [DOI] [PubMed] [Google Scholar]
- 140.Chubb, J.R., Trcek, T., Shenoy, S.M. and Singer, R.H. (2006) Transcriptional pulsing of a developmental gene. Curr. Biol. 16, 1018–1025 10.1016/j.cub.2006.03.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Larsson, A.J.M., Johnsson, P., Hagemann-Jensen, M., Hartmanis, L., Faridani, O.R., Reinius, B.et al. (2019) Genomic encoding of transcriptional burst kinetics. Nature 565, 251–254 10.1038/s41586-018-0836-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ventre, E., Herbach, U., Espinasse, T., Benoit, G. and Gandrillon, O. (2022) One model fits all: combining inference and simulation of gene regulatory networks. bioRxiv 10.1101/2022.06.19.496754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen, S. and Mar, J.C. (2018) Evaluating methods of inferring gene regulatory networks highlights their lack of performance for single cell gene expression data. BMC Bioinformatics 19, 232 10.1186/s12859-018-2217-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pratapa, A., Jalihal, A.P., Law, J.N., Bharadwaj, A. and Murali, T.M. (2020) Benchmarking algorithms for gene regulatory network inference from single-cell transcriptomic data. Nat. Methods 17, 147–154 10.1038/s41592-019-0690-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim, D., Risca, V., Reynolds, D., Chappell, J., Rubin, A., Jung, N.et al. (2021) The dynamic, combinatorial cis-regulatory lexicon of epidermal differentiation. Nat. Genet. 53, 1564–1576 10.1038/s41588-021-00947-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jumper, J., Evans, R., Pritzel, A., Green, T., Figurnov, M., Ronneberger, O.et al. (2021) Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 10.1038/s41586-021-03819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Krizhevsky, A., Sutskever, I. and Hinton, G.E. (2012) ImageNet Classification with Deep Convolutional Neural Networks. In Advances in Neural Information Processing Systems (Pereira, F., Burges, C.J.C., Bottou, L. and Weinberger, K.Q., eds), Curran Associates, Inc., Red Hook/New York/United States: https://proceedings.neurips.cc/paper/2012/file/c399862d3b9d6b76c8436e924a68c45b-Paper.pdf [Google Scholar]
- 148.Silver, D., Huang, A., Maddison, C.J., Guez, A., Sifre, L., van den Driessche, G.et al. (2016) Mastering the game of Go with deep neural networks and tree search. Nature 529, 484–489 10.1038/nature16961 [DOI] [PubMed] [Google Scholar]
- 149.Eraslan, G., Avsec, Ž, Gagneur, J. and Theis, F.J. (2019) Deep learning: new computational modelling techniques for genomics. Nat. Rev. Genet. 20, 389–403 10.1038/s41576-019-0122-6 [DOI] [PubMed] [Google Scholar]
- 150.Alipanahi, B., Delong, A., Weirauch, M.T. and Frey, B.J. (2015) Predicting the sequence specificities of DNA- and RNA-binding proteins by deep learning. Nat. Biotechnol. 33, 831–838 10.1038/nbt.3300 [DOI] [PubMed] [Google Scholar]
- 151.Zhou, J. and Troyanskaya, O.G. (2015) Predicting effects of noncoding variants with deep learning–based sequence model. Nat. Methods 12, 931–934 10.1038/nmeth.3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kelley, D.R., Reshef, Y.A., Bileschi, M., Belanger, D., McLean, C.Y. and Snoek, J. (2018) Sequential regulatory activity prediction across chromosomes with convolutional neural networks. Genome Res. 28, 739–750 10.1101/gr.227819.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shu, H., Zhou, J., Lian, Q., Li, H., Zhao, D., Zeng, J.et al. (2021) Modeling gene regulatory networks using neural network architectures. Nat. Comput. Sci. 1, 491–501 10.1038/s43588-021-00099-8 [DOI] [PubMed] [Google Scholar]
- 154.Rubiolo, M., Milone, D.H. and Stegmayer, G. (2018) Extreme learning machines for reverse engineering of gene regulatory networks from expression time series. Bioinformatics 34, 1253–1260 10.1093/bioinformatics/btx730 [DOI] [PubMed] [Google Scholar]
- 155.Dutil, F., Cohen, J.P., Weiss, M., Derevyanko, G. and Bengio, Y. (2018) Towards gene expression convolutions using gene interaction graphs. ArXiv 10.48550/arXiv.1806.06975 [DOI] [Google Scholar]
- 156.Wang, J., Ma, A., Ma, Q., Xu, D. and Joshi, T. (2020) Inductive inference of gene regulatory network using supervised and semi-supervised graph neural networks. Comput. Struct. Biotechnol. J. 18, 3335–3343 10.1016/j.csbj.2020.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cybenko, G. (1989) Approximation by superpositions of a sigmoidal function. Math. Control. Signals Syst. 2, 303–314 10.1007/BF02551274 [DOI] [Google Scholar]
- 158.Hornik, K., Stinchcombe, M. and White, H. (1989) Multilayer feedforward networks are universal approximators. Neural Netw. 2, 359–366 10.1016/0893-6080(89)90020-8 [DOI] [Google Scholar]
- 159.Zhang, Y., Tiňo, P., Leonardis, A. and Tang, K. (2021) A survey on neural network interpretability. IEEE Trans. Emerg. Top. Comput. Intell. 5, 726–742 10.1109/TETCI.2021.3100641 [DOI] [Google Scholar]
- 160. Francois C. Keras. 2015.
- 161.Paszke, A., Gross, S., Massa, F., Lerer, A., Bradbury, J., Chanan, G.et al. (2019) Pytorch: an imperative style, high-performance deep learning library. ArXiv 10.48550/arXiv.1912.01703 [DOI] [Google Scholar]
- 162.de Sousa Abreu, R., Penalva, L.O., Marcotte, E.M. and Vogel, C. (2009) Global signatures of protein and mRNA expression levels. Mol. Biosyst. 5, 1512–1526 10.1039/B908315D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Krishnan, A., Giuliani, A. and Tomita, M. (2007) Indeterminacy of reverse engineering of gene regulatory networks: the curse of gene elasticity. PLoS ONE 2, e562 10.1371/journal.pone.0000562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cokelaer, T., Bansal, M., Bare, C., Bilal, E., Bot, B.M., Chaibub Neto, E.et al. (2016) DREAMTools: a Python package for scoring collaborative challenges. F1000Res. 4, 1030 10.12688/f1000research.7118.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.(DREAM Challenge) C-Path Analytics - syn21760283 - Wiki [Internet]. [cited 2021 Jun 8]. Available from: https://www.synapse.org/#!Synapse:syn21760283/wiki/603540
- 166.Guo, W., Calixto, C.P.G., Tzioutziou, N., Lin, P., Waugh, R., Brown, J.W.S.et al. (2017) Evaluation and improvement of the regulatory inference for large co-expression networks with limited sample size. BMC Syst. Biol. 11, 62 10.1186/s12918-017-0440-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Marbach, D., Costello, J.C., Küffner, R., Vega, N.M., Prill, R.J., Camacho, D.M.et al. (2012) Wisdom of crowds for robust gene network inference. Nat. Methods 9, 796–804 10.1038/nmeth.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Stolovitzky, G., Monroe, D. and Califano, A. (2007) Dialogue on reverse-engineering assessment and methods. Ann. N. Y. Acad. Sci. 1115, 1–22 10.1196/annals.1407.021 [DOI] [PubMed] [Google Scholar]
- 169.Zoppoli, P., Morganella, S. and Ceccarelli, M. (2010) TimeDelay-ARACNE: Reverse engineering of gene networks from time-course data by an information theoretic approach. BMC Bioinformatics 11, 154 10.1186/1471-2105-11-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ernst, J., Vainas, O., Harbison, C.T., Simon, I. and Bar-Joseph, Z. (2007) Reconstructing dynamic regulatory maps. Mol. Syst. Biol. 3, 74 10.1038/msb4100115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schulz, M.H., Devanny, W.E., Gitter, A., Zhong, S., Ernst, J. and Bar-Joseph, Z. (2012) DREM 2.0: improved reconstruction of dynamic regulatory networks from time-series expression data. BMC Syst. Biol. 6, 104 10.1186/1752-0509-6-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ding, J., Hagood, J.S., Ambalavanan, N., Kaminski, N. and Bar-Joseph, Z. (2018) iDREM: interactive visualization of dynamic regulatory networks. PLoS Comput. Biol. 14, e1006019 10.1371/journal.pcbi.1006019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Conard, A.M., Goodman, N., Hu, Y., Perrimon, N., Singh, R., Lawrence, C.et al. (2021) TIMEOR: a web-based tool to uncover temporal regulatory mechanisms from multi-omics data. Nucleic Acids Res. 49, W641–W653 10.1093/nar/gkab384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bühlmann, P. and van de Geer, S. (2011) SpringerLink (Online service). In Statistics for High-Dimensional Data [electronic resource]: Methods, Theory and Applications. pp. 575, Berlin, Heidelberg, Springer-Verlag Berlin Heidelberg; http://archive.org/details/statisticsforhig00bhlm [Google Scholar]
- 175.Srivastava, N., Hinton, G., Krizhevsky, A., Sutskever, I. and Salakhutdinov, R. (2014) Dropout: a simple way to prevent neural networks from overfitting. J. Mach. Learn. Res. 15, 1929–1958 https://dl.acm.org/doi/10.5555/2627435.2670313 [Google Scholar]
- 176.Ma, S., Zhang, B., LaFave, L.M., Earl, A.S., Chiang, Z., Hu, Y.et al. (2020) Chromatin potential identified by shared single-Cell profiling of RNA and chromatin. Cell 183, 1103–1116.e20 10.1016/j.cell.2020.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cao, J., Cusanovich, D.A., Ramani, V., Aghamirzaie, D., Pliner, H.A., Hill, A.J.et al. (2018) Joint profiling of chromatin accessibility and gene expression in thousands of single cells. Science 361, 1380–1385 10.1126/science.aau0730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chen, S., Lake, B.B. and Zhang, K. (2019) High-throughput sequencing of the transcriptome and chromatin accessibility in the same cell. Nat. Biotechnol. 37, 1452–1457 10.1038/s41587-019-0290-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gao, L.L., Bien, J. and Witten, D. (2021) Selective inference for hierarchical clustering. arXiv 10.48550/arXiv.2012.02936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schreiber, J., Singh, R., Bilmes, J. and Noble, W.S. (2020) A pitfall for machine learning methods aiming to predict across cell types. Genome Biol. 21, 282 10.1186/s13059-020-02177-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dibaeinia, P. and Sinha, S. (2020) SERGIO: a single-cell expression simulator guided by gene regulatory networks. Cell Syst. 11, 252–271.e11 10.1016/j.cels.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li, H., Zhang, Z., Squires, M., Chen, X. and Zhang, X. (2022) Scmultisim: simulation of multi-modality single cell data guided by cell-cell interactions and gene regulatory networks. bioRxiv 11, 252–271 10.1101/2022.10.15.512320 [DOI] [Google Scholar]
- 183.Ventre, E. (2021) Reverse engineering of a mechanistic model of gene expression using metastability and temporal dynamics. In Silico Biol. 14, 89–113 10.3233/ISB-210226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.ENCODE-DREAM in vivo Transcription Factor Binding Site Prediction Challenge [Internet]. DREAM Challenges. [cited 2021 Jun 8]. Available from: https://dreamchallenges.org/encode-dream-in-vivo-transcription-factor-binding-site-prediction-challenge/