Abstract
Background and aims
HDL particles may act to buffer host cells from excessive inflammatory mediators. The aim of this study is to investigate if the lipid profile provides a prognostic biomarker for COVID-19 outcomes.
Methods
This was a prospective study of the characteristics of 125 adult COVID-19 patients with a lipid profile performed on the day of admission analyzed with regard to clinical outcomes.
Results
Seventy-seven patients (61.2%) were men, with a mean age of 66.3 (15.6) years. 54.1% had bilateral pneumonia. The all-cause mortality rate during hospitalization was 20.8%. We found a direct association between more severe disease assessed by the WHO classification, admission to the ICU and death with more pronounced lymphopenia, higher levels of CRP, ferritin (p < 0.001), D-dímer and lactate dehydrogenase (LDH) all statistically significant. Lower leves of HDL-c and LDL-c were also associated with a worse WHO classification, ICU admission, and death,. HDL-c levels were inversely correlated with inflammatory markers CRP (r = −0.333; p < 0.001), ferritin (r = −0.354; p < 0.001), D-dímer (r = −0.214; p < 0.001), LDH (r = −0.209; p < 0.001. LDL-c levels were significantly associated with CRP (r = −0.320; p < 0.001) and LDH (r = −0.269; p < 0.001). ROC curves showed that HDL [AUC = 0.737(0.586–0.887), p = 0.005] and lymphocytes [AUC = 0.672(0.497–0.847], p < 0.043] had the best prognostic accuracy to predict death. In a multivariate analysis, HDL-c (β = −0.146(0.770–0.971), p = 0.014) and urea (β = 0.029(1.003–1.057), p = 0.027) predicted mortality.
Conclusion
Hypolipidemia including HDL levels at admission identifies patients with a higher risk of death and worse clinical manifestations who may require more intensive care.
Keywords: COVID-19, HDL, LDL, Biommarker, Inflammation
Abbreviations: HDL-c, High density cholesterol; LDL-c, Low density cholesterol; COVID-19, SARS-Cov2 Disesae
Highlights
-
•
125 patients with a lipid profile at admission were included from the first wave of COVID disease.
-
•
Need of ICU admission and mortality was associated to lower levels of HDL-c and LDL-c.
-
•
HDL-c levels were inversely correlated with the inflammatory markers associated with the “cytokine storm”.
-
•
HDL-c at admission identifes patients with a higher risk of death and a worse prognostic course.
1. Introduction
A highly infectious novel coronavirus, designated as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first identified in Wuhan, China in December 2019 [1]. This virus is currently the cause of a global pandemic, producing hundreds of thousands of hospital admissions and deaths, with enormous impact on health and serious economic consequences for society. On 1st February 2020, the first case of a SARS-CoV-2 positive patient was diagnosed in Spain. Subsequently, the first cases diagnosed in the autonomous region of Catalonia were recognized on 5th March [2].
Overactivation of the immune system due to SARS-CoV-2 infection causes a surge of proinflammatory factors, often loosely called “cytokine storm,” resulting in host organ damage, such as lung injury, increased lung endothelial and epithelial permeability, impaired gas exchange and severe respiratory failure with a high mortality rate [[3], [4], [5]]. Recent studies point to a relationship between the disease severity and low lipid levels although its relationship with the hyperinflammatory status and the immune response has not been clearly investigated in patients with COVID-19 [[6], [7], [8], [9], [10], [11], [12]].
The acute-phase response (APR) is a complex reaction of the host induced by several mechanisms such infection, trauma, burns, ischemia and maligant growth. It is accompained by changes in plasma proteins including C-reactive protein (CRP), serum amyloid A (SAA) and several cytokines that induce an inflammatory state. Other consistent metabolic alterations have been classically associated with the APR consistent with hypertriglyceridemia but also significant changes in other lipid levels and lipoproteins composition and oxidation. Although the mechanism by which infection/inflammation decreases cholesterol levels has not been thoroughly studied in the APR, lipopolysacharide (LPS) and cytokines decrease total serum cholesterol levels by stimulating de novo cholesterol synthesis, decreasing lipoprotein clearance and decreasing the conversion of cholesterol into bile acids [13].
High-density lipoprotein particles (HDL) and the major protein, apolipoprotein A-1 (apoA-1), play key roles in attenuating the risk of atherosclerotic cardiovascular disease. HDL functions not only to maintain homeostasis of cholesterol metabolism but also to regulate the acute immune response after infections and other environmental stresses such as severe burns, autoimmune diseases or cancer [[14], [15], [16], [17]].
Both HDL and apoA-1 play a protective role in pneumonia in promoting lung health [[18], [19], [20]]. In addition, a relationship has been demonstrated between serum lipid levels, mortality rates, sepsis and radiological extent in community-acquired pneumonia [13,[21], [22], [23], [24], [25]].
HDL exerts antioxidative, anti-inflammatory, anti-apoptotic, and vasoprotective properties [16], and it reduces the cholesterol content of immune cells by decreasing receptor localization and signaling within lipid rafts [23,26].
HDL levels may regulate the exaggerated inflammatory response against infection in COVID-19 patients. In this study, the clinical characteristics of 125 adult COVID-19 patients with a lipid profile performed on the day of admission are presented to elucidate the association between HDL-c levels and the risk for developing severe events.
2. Methods
2.1 Study population
This prospective study was conducted on hospitalized patients with COVID-19 disease from the “first-wave” (from 15th March to the 30th of June 2020) in the Hospital Universitari de Sant Joan, in Reus, Spain. This hospital has 392 beds provided for hospitalization and social health care and is part of the Hospital Network for Public Use in Catalonia. It acts as a general hospital for a population of over 175,000 inhabitants, including primary care centers and residences for the elderly in the area.
We selected patients with a SARS-CoV-2 infection demonstrated by reverse transcription-polymerase chain reaction (RT-PCR) and with a lipid profile performed at the time that SARS-Cov2 infection was diagnosed.
Tests were carried out with the VIASURE SARS-CoV-2 Real Time PCR Detection Kit that detects ORF1ab and N genes (CerTest Biotec, Zaragoza, Spain). RNA was extracted in a QIAcube apparatus with RNeasy reagents (Qiagen N.V., Hilden, Germany) according to the manufacturer's instructions, and analyses were carried out in a 7500 Fast RT-PCR System (Applied Biosystems, Foster City, CA,USA). We recorded demographic data, comorbidities, cardiovascular risk factors, and treatments [13].
All participants in the study older than 18 years of age with a confirmed diagnosis of SARS-CoV-2 infection by PCR were included in the analysis. We excluded hospitalized patients with suspected SARS-CoV-2 infection but without laboratory confirmation, or patients with laboratory diagnosis of SARS-CoV-2 infection who did not require hospitalization.
The WHO classification was applied to grade the severity of illness as follows: 7: Mechanical ventilation with extracorporeal membrane oxygenation (ECMO), vasopressor support; 6: Intubation with mechanical ventilation; 5: Noninvasive ventilation (CPAP or BiPAP) or high-flow oxygen; 4:Oxygen by mask or nasal googles; 3: Hospitalized with no oxygen therapy.
Patients not hospitalized that correspond to the number 1 and 2 regarding WHO scale were not included (2: Infection with limitation of activity; 1:Infection without limitation).
All-cause mortality was analyzed as a different group not included in the initial baseline clinical severity classification of the patients at admission but considered as a clinical manifestation that ocurred during the follow-up of the study.
This study was approved by the ethical committee (Institutional Review Board) of Hospital Universitari de Sant Joan (Resolution CEIM 040/2018, amended on 16 April 2020).
2.2 Statistical analysis
Data were processed and statistically analyzed using SPSS statistical package version 25.0 (Chicago, IL, USA).
According to similar studies published about HDL and mortality in COVID (Masana etl al.) and considering the 20% of mortality in our cohort, to obtain significant differences regarding HDL cholesterol concentration the sample size necessary to achieve sufficient statistical power to achieve a significance of less than 0.05 to reject the null hypothesis has been calculated not to be less than 100 patients.
Normality distribution of continuous variables was assessed by the Kolmogorov-Smirnov test.. Results of continuous variables were expressed as medians and (standard deviation) We found that age, TG, ApoA-1 and APoB did not showed normal distribution so results are expressed with median and the interquartile rank in parenthesis. Categorical variables are expressed as percentage. Frequency comparison was performed by chi-square tests. Two-group comparison of normally distributed data was performed by the Students t-test. For multivariate comparisons, one-way analysis of variance with least square difference for posthoc comparison was applied. Bivariate correlation analyses were performed using Pearson test.
Multivariate analyses were performed to determine factors independently associated with ICU admission, death and extent of radiologic involvement. The variables included in the model were those analytical variables associated with mortality in the univariate analyses (CRP, Ferritin, D-dímer, LDH, HDL, LDL, urea, glucose and age). Results of multivariate analyses are reported as odds ratios with 95% confidence intervals and p-values. We estimated receiver-operating-characteristics (ROC) curve and Kaplan-Meier surival curve. All testing was two-tailed and p values below 0.05 were considered to be statistically significant.
3. Results
3.1. General characteristics of study population
A total number of 125 hospitalized patients with confirmed COVID-19 infection and an available lipid profile performed at the time on admission were admitted to the study. Briefly, the mean age of the patients was 66.3(15.6) years. Seventy-seven patients (61.2%) were men. The clinical general characteristics are shown in Table 1.
Table 1.
General characteristics of the population.
| General characteristics of all the patients | N = 125 |
|---|---|
| Age, years, mean(SD) | 66.3(15.6) |
| Gender (men), n(%) | 77(61.6) |
| Hypertension(%) | 65(52) |
| Dyslipidemia, n(%) | 37(29.6) |
| Diabetes mellitus, n(%) | 31(24.8) |
| Smoking, n(%) | 39.2 |
| Current, n(%) | 9(7.2) |
| Never-smoker, n(%) | 100(80) |
| Ex-smoker | 16(12.8) |
| Alcoholism, n(%) | 6(4.8) |
| Cardiovascular disease, n(%) | 62(49.6) |
| OCPD, n(%) | 17(13.6) |
| Renal chronic disease, n(%) | 20(16) |
| Chronic hepatic disease, n(%) | 2(1.6) |
| Neurologic disease, n(%) | 23(18.4) |
| HIV, n(%) | 1(0.8%) |
| Neoplasm, n(%) | 15(12) |
| Clinical presentation, n(%) | |
|
84(61.6) |
|
77(61.6) |
|
81(64.8) |
|
36(28.8) |
| Radiologic findings, n(%) | |
|
33(27) |
|
1(13.9) |
|
6(4.9) |
|
66(54.1) |
| ICU, n(%) | 31(24.8) |
| WHO classification | |
|
24(19.2) |
|
65(52) |
|
8(6.4) |
|
28(22.4) |
|
9(11.2) |
| All-cause mortality, n(%) | 26(20.8) |
OPCD: obstuctive Pulmonary Chronic Disease; HIV: Human immunodeficiency virus; ICU: intensive care unit.
The all-cause mortality rate during hospitalization was 20.8%, most patients were admitted with bilateral pneumonia (54.1%) and the predominant symptoms were fever (67.2%), cough (61.6%) and dyspnea (64.8%). The mean number of days from the beginning of the symptoms until the diagnosis was 5.9 (4.6) days. The most prevalent stage regarding the WHO classification was category 4, those patients with oxygen support by mask or high-flow oxygen therapy (52%).
The most prevalent co-morbidity was cardiovascular disease in 49.6% of the patients. With respect to the prevalence of the cardiovascular risk factors, 52% of the patients had hypertension, 29.6% dyslipidemia and 24.8% diabetes.
A total number of 32 patients were admitted to the ICU (25.6%). Of these patients, 28 received mechanical ventilation with thraqueal intubation and none of them were referred to ECMO.
3.2. Differences regarding clinical, analytical variables and lipid profile regarding the WHO classification
Results are shown in Table 2. We did not find differences regarding age, gender or time from initial symptoms between groups. Fever, cough and dyspnea were more prevalent in those patients that required more aggressive respiratory support.
Table 2.
General characteristics of the patients regarding WHO clinical severity classification.
| Variables | WHO: 3 N = 24 |
WHO: 4 N = 65 |
WHO: 5 N = 10 |
WHO: 6 N = 17 |
WHO: 7 N = 9 |
P |
|---|---|---|---|---|---|---|
| Gender, Male, % | 50 | 58.5 | 87.5 | 58.8 | 90.9 | 0.458 |
| Age, years | 74(58) | 66(73) | 77(64) | 63(20) | 67(73) | 0.379 |
| Time initial symptoms since diagnosis, days | 3.6 | 5.2 | 3.3 | 7 | 5.9 | 0.587 |
| Hypertension, % | 50 | 52.3 | 62.5 | 52.9 | 45.5 | 0.974 |
| Diabetes mellitus, % | 29.2 | 23.1 | 25 | 23.5 | 27.3 | 0.970 |
| Dyslipidemia, % | 37.5 | 23.1 | 37.3 | 35.3 | 36.4 | 0.547 |
| Cardiovascular Disease, % | 45.8 | 49.2 | 62.5 | 52.9 | 45.5 | 0.401 |
| OPCD, % | 8.3 | 20 | 0 | 11.8 | 0 | 0.195 |
| Chronic neurologic disease,%, | 45.8 | 16.9 | 12.5 | 0 | 0 | 0.001∗ |
| Hepatic disease, % | 0 | 1.5 | 0 | 0 | 0 | 0.221 |
| Renal chronic Disease, % | 12.5 | 18.5 | 25 | 11.8 | 0 | 0.290 |
| HIV, % | 0 | 0 | 10 | 5.9 | 0 | 0.954 |
| Neoplasm, % | 12.3 | 9.2 | 25 | 11.8 | 9.1 | 0.361 |
| Statins (pre-admission), % | 16.7 | 27.7 | 25 | 35.3 | 36.4 | 0.143 |
| ACE(pre-admission), % | 16.7 | 23.1 | 12.5 | 35.3 | 36.4 | 0.304 |
| Corticoids (pre-admission), % | 4.2 | 7.7 | 12.5 | 11.8 | 0 | 0.705 |
| Symptoms | ||||||
|
33.3 | 72.3 | 62.5 | 82.4 | 90.9 | 0.001∗ |
|
41.7 | 64.6 | 50 | 94.1 | 45.5 | 0.001∗ |
|
29.2 | 66.2 | 75 | 100 | 72.7 | 0.001∗ |
|
33.3 | 23.1 | 0 | 29.4 | 36.4 | 0.537 |
| Bilateral pneumonia by CXR, % | 0 | 53.8 | 70 | 93.3 | 100 | 0.001∗ |
| Exitus,% | 0 | 21.5 | 60 | 20 | 27.3 | 0.003∗ |
| ICU, % | 0 | 1.5 | 40 | 100 | 100 | 0.000∗ |
| COVID-19 treatments | ||||||
|
15.5 | 87.3 | 75 | 88.2 | 100 | 0.401 |
|
79.2 | 89.1 | 75 | 70.6 | 90.0 | 0.105 |
|
20.8 | 41.3 | 50 | 70.6 | 90.9 | 0.002∗ |
|
8.3 | 17.9 | 37.5 | 29.4 | 36.4 | 0.705 |
|
0 | 1.6 | 12.5 | 0 | 0 | 0.266 |
|
0 | 0 | 25 | 47.1 | 54.5 | 0.000∗ |
| Biochemical | ||||||
|
135.3(16.6) | 110.8(40.9) | 161.0(45.4) | 157.2(61.7) | 173.6(7.6) | 0.003∗ |
|
33.2(12.8) | 40.8(31.4) | 70.6(37.1) | 55.3(42.7) | 51.09(20.2) | 0.025∗ |
| Inflammatory markers | ||||||
|
1524,2(689.9) | 1120.0(753.6) | 803.7(554.9) | 961.7(392.7) | 654.5(292.8) | 0.002∗ |
|
5.11(6.9) | 8.3(8.7) | 17.2(14.2) | 17.1(9.1) | 23.3(10.8) | 0.000∗ |
|
64.5(34.5) | 76.9(36.0) | 76.0(28.3) | 80.7(29.9) | 76.5(30.9) | 0.594 |
|
430.5(444.9) | 896.2(644.9) | 1406.4(750.4) | 1046.8(640.6) | 1677.7(427.6) | 0.000∗ |
|
1318.7(1399.2 | 1265.5(1319.2) | 5151.7(5453.3) | 2850.0(2365.) | 6231.0(1443.) | 0.011∗ |
|
228.9(84.6) | 301.5(121.05) | 452.3(147.5) | 329.3(103.5) | 430.4(203.9) | 0.000∗ |
| Lipid profile | ||||||
|
155.67(35.2) | 135.3(31.8) | 143.6(50.6) | 116.6(28.4) | 119.6(27.7) | 0.002∗ |
|
36.7(9.5) | 33.3(11.9) | 30.6(10.6) | 29.6(8.9) | 22.1(8.4) | 0.005∗ |
|
89.8(28.5) | 71.4(24.9) | 76.6(37.8) | 98.2(22.3) | 55.1(21.2) | 0.000∗ |
|
136(177) | 147(431) | 195(240) | 167(335) | 189(275) | 0.404 |
|
91.5(34) | 84(90) | 88(54) | 115.5(76) | 91.5(35) | 0.227 |
|
109(78) | 97(108) | 119(99) | 143(64) | 95.5(9) | 0.114 |
OPCD: Obstructive pulmonary chornic disease; HIV: Human immunodeficiency virus; ACE: Angiotensine conversor enzyme, CRP: C reactive protein, ESR: erythrocyte sedimentation rate, LDH: lactate dehydrogenase, TC: total cholesterol, HDL-c: high density lipoprotein, LDL-c: low density lipoprotein, TG: triglyceride, ApoA1: apolipoprotein-A1; ApoB: apolipoprotein-B.
We did not find differences between the cardiovascular risk factors or cardiovascular diseases. There were no differences in the proportion of patients treated with statins or antihypertensive treatment regarding the WHO classification.
Of patients with mild COVID-19 that did not required oxygen supplementation (WHO:3 n = 24) 45.8% presented with chronic neurologic diseases (dementia or convalescent from ictus). This finding was related to a nosocomial outbreak in the sociosanitary area of the hospital that was occupied by patients under rehabilitation and convalescent from neurologic diseases. Most of these patients were asymptomatic for COVID-19 disease and none of them presented bilateral infiltrates in the CXR (19.2%).
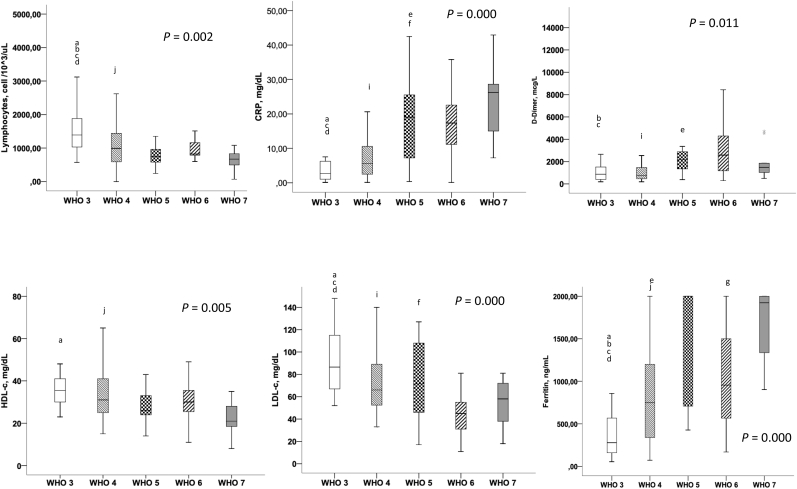
In accordance with previous published results in COVID-19, we found a direct association between more severe disease and the presence of more pronounced lymphopenia (p < 0.001) and higher levels of the known inflammatory markers such as CRP (p < 0.001), ferritin (p < 0.001), D-dímer (p < 0.001) and lactate dehydrogenase (LDH) (p < 0.001).
In the same way as the inflammatory markers, we confirmed that lower levels of HDL-c and lower levels of LDL-c were associated with a more severe presentation of the disease.
3.3. Characteristics of the COVID-19 patients that were admitted to the ICU
Results are shown in Table 3. Of the 32 patients admitted to the ICU department, 75% were men (p = 0.053) and with a mean of 7.3 days from the initial symptoms (p < 0.001). We did not find differences regarding age, the presence of cardiovascular risk factors nor other co-morbidities, except for the lack of patients with neurologic diseases in the ICU (p < 0.001).
Table 3.
Main clinical and biochemical variables, inflammatory markers and lipid profile regarding mortality and ICU admission.
| EXITUS |
P | ICU |
P | |||
|---|---|---|---|---|---|---|
| NO N = 99 |
YES N = 26 |
NO N = 94 |
YES N = 31 |
|||
| Clinical variables | ||||||
|
63(167) | 74 (51) | 0.003 | 70(73) | 61(51) | NS |
|
57.6 | 76.9 | 0.055 | 57 | 75 | 0.053 |
|
5.9 | 2.7 | 0.009 | 4.4 | 7.3 | 0.001 |
|
45.5 | 76.9 | 0.004 | 52.7 | 50 | NS |
|
20.2 | 42.3 | NS | 25.8 | 21.9 | NS |
|
30.3 | 26.9 | NS | 29 | 31.9 | NS |
|
43.4 | 73.1 | 0.026 | 49.5 | 50 | NS |
|
12.1 | 19.2 | NS | 15.1 | 9.4 | NS |
|
17.2 | 23.1 | NS | 24.7 | 0 | 0.002 |
|
0.26 | 3.8 | NS | 1.1 | 3.1 | NS |
|
13.1 | 26.9 | NS | 19.4 | 6.3 | 0.057 |
|
0 | 1 | NS | 1 | 0 | NS |
|
7.1 | 30.8 | 0.004 | 12.9 | 9.4 | NS |
|
21.2 | 34.6 | NS | 25.8 | 18.8 | NS |
|
22.2 | 30.8 | NS | 20.4 | 34.4 | NS |
|
7.1 | 7.7 | NS | 7.5 | 6.3 | NS |
| Biochemical variables | ||||||
|
123.1(61.9) | 158.5(66.6) | 0.012 | 121.7(60.8) | 158.3(67.7) | 0.005 |
|
37.5(22.1) | 69.7(45.7) | 0.000 | 41.7(31.6) | 51.3(29.7) | NS |
|
1.24(3.8) | 1.26(0.7) | NS | 1.34(3.9) | 0.98(0.55) | NS |
| Inflammatory markers | ||||||
|
1182.3(715.3) | 858.1(565.4) | 0.034 | 1196.2(763.9) | 878.4(367.9) | 0.026 |
|
9.1(9.7) | 17.31(11.2) | 0.000 | 7.65(8.3) | 19.9(10.9) | 0.000 |
|
72.4(33.7) | 86.5(37.4) | NS | 72.4(34.9) | 81.8(31.0) | NS |
|
833.2(652.1) | 1307.2(687.9) | 0.007 | 802.8(644.9) | 1307.2(663.5) | 0.000 |
|
2036.1(5114.9) | 2742.4(2984.5) | NS | 1479.9(1989.9) | 4051.0(8459.5) | 0.011 |
|
289.9(133.2) | 394.8(120.4) | 0.001 | 289.1(126.01) | 371.6(148.6) | 0.005 |
| Lipid profile | ||||||
|
142.7(34.3) | 117.2(24.5) | 0.014 | 143.9(34.1) | 118.4(30.3) | 0.001 |
|
33.4(11.7) | 27.4(7.7) | 0.014 | 34.25(11.2) | 26.6(94) | 0.0001 |
|
74.3(29.2) | 58.0(22.7) | 0.009 | 76.9(27.0) | 53.13(26.01) | 0000 |
|
149.4(420) | 153(194) | NS | 145(431) | 179(335) | NS |
|
92.8(95) | 89 (65) | 0.010 | 86(90) | 93.5(92) | NS |
|
106(108) | 93.8(99) | NS | 98(108) | 119(72) | NS |
OPCD: Obstructive pulmonary chornic disease; HIV: Human immunodeficiency virus; ACE: Angiotensine conversor enzyme, CRP: C reactive protein, ESR: erythrocyte sedimentation rate, LDH: lactate dehydrogenase, TC: total cholesterol, HDL: high density lipoprotein, LDL: low density lipoprotein, TG: triglyceride, ApoA1: apolipoprotein-A1, ApoB: apolipoprotein-B.
As expected, we found higher levels of the inflammatory markers: CRP:19.9(10.9)g/dl as compared to 7.6(8.3)g/dl; p < 0.001; ferritin: 1307.2(663.5)μg/dl v 802.8(644.9)μg/dl; p < 0.001; D-dímer: 4051.0(8459.5)ng/mL as compared to 1479.9(1989.9) ng/mL; p < 0.001; and LDH: 371.6(148.6) as compared to 289.1(126.0); p < 0.001).
Patients admitted to the ICU had lower levels of HDL-c at 26.56(9.4)mg/dl v. 34.5(11.2) mg/dl p < 0.001; and also LDL-c at 53.1(26.0)mg/dl v. 76.9(27.0)mg/dl; p < 0.001.
3.4. Characteristics of the COVID-19 patient deaths
In our cohort, a total of 26 patients died, with a mean age of 74.2(14.6) v. survivors at 64.2(5.3); p = 0.003. A higher proportion of patients were men (76.9%) although the difference did not reach statistical significance (p = 0.055). Regarding the co-morbidities, patients that died presented higher prevalence of hypertension (76.9% respect 45.5%; p = 0.004); diabetes (42.3% respect 20.2%; p = 0.022) cardiovascular diseases (73.1% respect 43.4%; p = 0.026) and neoplasms (30.8% respect 7.1%; p = 0.004). Table 3.
The same inflammatory markers related to the ICU admission except levels of D-dímer were higher in patients that died: CRP: 17.3(11.2)g/dl v. 9.1(9.7)g/dl, p < 0.001; ferritin:1307.2(687.9) v. 833.2(652.1), p = 0.007; LDH: 394.8(120.4) v. 289.9(133.2), p < 0.001; and lymphopenia: 858.1(566.4) v. 1182.3(715.3), p = 0.034.
With respect to the lipid parameters, levels of lipoproteins were significantly decreased. HDL-c levels: 27.4(7.7)mg/dl v. 33.4(11.7)mg/dl. p = 0.014; ApoA-1: 74.1(19.1)mg/dl v. 91.1(19.7)mg/dl,; p = 0.010); and LDL-c: 58.0(22.7)mg/dl v. 74.3(29.2)mg/dl,; p = 0.009). Table 3.
3.5. Association between the lipid profile and the inflammatory markers
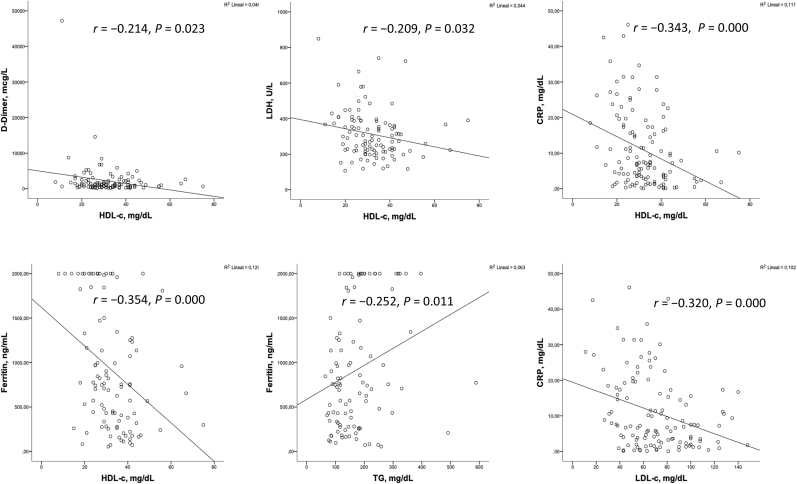
We found that the levels of HDL-c were inversely correlated with all the inflammatory markers associated with the worse outcomes, the need for admission to the ICU and the worse WHO classification. This variables are CRP (r = – 0.333; p < 0.001), ferritin (r = – 0.354; p < 0.001), D-dímer(r = – 0.214; p < 0.001), LDH (r = – 0.209; p < 0.001and also glucose (r = – 0.192; p < 0.035). Fig. 1.
Fig. 2.
Bivariate correlations between lipid profile and inflammatory markers.
Fig. 1.
Differences between the WHO group [[3], [4], [5], [6], [7]] regarding the means levels of lymphocytes cell count, CRP, D-Dímer, HDL-c, LDL-c and ferritin. In this box plots there are expressed the significance between all the groups (p 0.005) and intra-groups after post-hoc analyses. a:WHO 3–4; b:WHO 3–5; c: WHO 3–6; d: WHO 3–7; e: WHO 4–5; j: WHO 4–7; g: WHO 6–7.
Lower LDL-c levels were inversely correlated with CRP (r = – 0.320; p < 0.001), and LDH (r = – 0.269; p < 0.001) but neither with ferritin nor D-dímer. Tryglycerides only were positively correlated with ferritin levels (r = 0.252; p < 0.011).
3.6. Variables that predict mortality
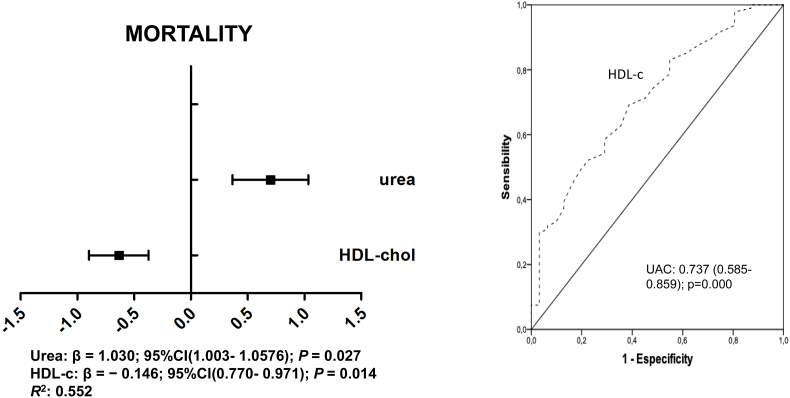
To investigate the variables that predicted the mortality rates we performed binary logistic regression analyses including in the model those analytical variables associated to mortality in the univariate analyses (CRP, Ferritin, D-dímer, TG, HDL, LDL, urea, glucose, age and statins). We found that only HDL-c (β = −0.146(0.770–0.971), P = 0.014) and urea (β = 0.027(1.003–1.057), P = 0.034) predicted mortality in our cohort of patients (Fig. 3).
Fig. 3.
Fig. 2. Receiver operating characteristics (ROC) plots of variables (A) and multivariate analyses (B) with respect mortality.
To assess the overall diagnosis accuracy we calculated the receiver operating characteristics (ROC) for each variable included in the model (Fig. 3). We found that HDL [AUC = 0.737 (0.586–0.887), p = 0.005], lymphocytes [AUC = 0.672(0.497–0.847], p < 0.043]; urea [AUC = 0.201(0.050–0.352)], p < 0.001]; ferritin [AUC = 0.295(0.146–0.445)], p < 0.016]; LDH [AUC = 0.293(0.146–0.439)], p < 0.015]; CRP [AUC = 0.224 (0.09–0.359), p < 0.001]; and D-dímer [AUC = 0.238(0.133–0.344)], p = 0.002]; neither age nor LDL-c levels were associated with the mortality rates using ROC. We did not found an association regarding the cuartiles of HDL-c levels and the survival analysis performed by Kaplan Meier.
4. Discussion
In this study we inluded a total number of 125 patients admitted with documented COVID-19 with a lipid profile performed at admission. General characteristics are similar to previous cohorts of hospitalized patients by pneumonia for SARS-Cov2 with similar mortality rates (20.8%), demographics and clinical characteristics [1]. We also observed the close association between the risk of a worse clinical outcome including mortality (20.8%) and need of ICU admission (24.8%) with the presence of cardiovascular risk factors like hypertension (52%), cardiovascular diseases(49.6%) and older age 66.3(15.6) years.
In our cohort although all patients were hospitalized, there were patients that were positive for SARS-Cov-2 but did not develop pneumonia nor respiratory symptoms (19.2%). We could include these asymptomatic patients because they were detected during a nosocomial outbreak and it allowed us to compare the differences between groups of patients at different clinical stages.
We used the WHO classification to stratify the degree of disease to analyze the risk of mortality. Another issue is that the severity of the disease is related to the degree of the respiratory dysfunction that may not be determined only by the radiologic extent. Mortality rates were similar at WHO stage 3 respect 5 and 6 although the presence of bilateral infiltrates only was in the 53.8% at stage 4. The patients that received noninvasive ventilation or high flow oxygen (WHO classification 5) are a group of patients with higher mortality rates. The characteristics reflect that this is a subgroup of patients older (although non statistically significant), higher co-morbidities like hypertension (62.5%), and cardiovascular diseases (62.5%). The more severe disease and bad outcome is also reflected by the analytical differences with significant higher levels of inflammatory markers such as ferritin (1406.4(750.4), D-dímer (5151.7(5453.3), and LDH (452.3(147.5).
We could confirm our hypothesis that low levels of HDL were associated with a more severe COVID-19 [3]. The patients with worse WHO stages [5], [6], [7] beside the lymphopenia and the inflammatory status showed a decrease in the HDL-c levels and also the LDL-c levels. If we investigate patients regarding the admission to the ICU or the risk of death we confirmed again this association between hypolipidemia, hyperinflammation and lymphopenia [6,27,28].
The inverse correlations especially between HDL-c and the inflammatory markers (ferritin, d-dimer, and LDH) support the role of this lipoprotein in the regulation of the acute phase response in severe infections such as COVID-19 [[29], [30], [31]]. Moreover Congwen et al. have recently demonstrated that S1 subunit of SARS-2S binds to the HDL scavenger receptor B type 1 to enhance viral uptake in vitro and acts as a host factor that promotes SARS-Cov-2 infection [32].
The multivariate analyses to detect those variables that predict mortality rates showed that between all the inflammatory markers and those clinical and demographic variables associated to mortality in univariate analyses only HDL-c levels and urea predicted the risk of death in our cohort. Urea has been used in prognostic scales for pneumonia (CURB-65 and PSI-FINE). We show with these results that the determination of the lipid profile at the beginning of the infection is also an early laboratory biomarker to identify patients with a higher risk of death and a worse clinical manifestation that will need stronger ventilatory support and more resources [33].
Whether cause or a consequence of COVID-19 disease or how lipid metabolism is affected by the infection is not a question that could be answered by this cross-sectional study. Potential mechanisms related to COVID-19 dyslipidemia and few previous recently published studies also showed this association between low levels of HDL-c and the severity of COVID-19 pneumonia besides a protective factor against SARS-2 infection [[6], [9], [33], [34], [35], [36], [37]]. Previously to the SARS-Cov-2 pandemia it was pointed by several studies changes in the quantity and composition of HDL in infections and community acquired pneumonia. Furthermore, the anti-inflammatory and antioxidant properties of HDL are significantly reduced during influenza [19]. The mechanisms by which increased inflammation reduces HDL functionality are not clearly defined. In addition to its function in reverse cholesterol transport, HDL particles have several other properties that may be relevant to modulation of the immune system and the control of infectious diseases. Unfortunantly we couldn't confirm a closer relation between the number of HDL particles and the inflammatory parametres that could add information about this inflammatory regulation by HDL particles in particular since we couldn't analyze the levels of ApoA-in all the patients during the pandemia outbreak.
According to the above results HDL-c level may provide valuable and easily accessible prognostic information for clinicians when encountering patients with COVID-19 disease and facilitate more rapid intervention for those at highest risk for fatal complications related to the hyperinflammatory status.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Acknowledgement to Reus City Council (ECH 6/2020)
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.athplu.2023.01.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iftimie S., López-Azcona A.F., Vicente-Miralles M., et al. Risk factors associated with mortality in hospitalized patients with SARS-CoV-2 infection. A prospective, longitudinal, unicenter study in Reus, Spain. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0234452. Published 2020 Sep. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding Q., Lu P., Fan Y., Xia Y., Liu M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol. 2020;92(9):1549–1555. doi: 10.1002/jmv.25781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J., Jiang M., Chen X., Montaner L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. 2020;108(1):17–41. doi: 10.1002/JLB.3COVR0520-272R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponti G., Maccaferri M., Ruini C., Tomasi A., Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020;57(6):389–399. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feingold K.R. In: Endotext. South dartmouth (MA) Feingold K.R., Anawalt B., Boyce A., et al., editors. MDText.com, Inc.; November 15, 2020. Lipid and lipoprotein levels in patients with COVID-19 infections. [Google Scholar]
- 7.Tanaka S I., De Tymowski C., Assadi M., et al. Lipoprotein concentrations over time in the intensive care unit COVID-19 patients: results from the ApoCOVID study. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0239573. Published 2020 Sep. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu X., Chen D., Wu L., He G., Ye W. Declined serum high density lipoprotein cholesterol is associated with the severity of COVID-19 infection. Clin Chim Acta. 2020;510:105–110. doi: 10.1016/j.cca.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang G., Zhang Q., Zhao X., et al. Low high-density lipoprotein level is correlated with the severity of COVID-19 patients: an observational study. Lipids Health Dis. 2020;19(1):204. doi: 10.1186/s12944-020-01382-9. Published 2020 Sep. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorokin A.V., Karathanasis S.K., Yang Z.H., Freeman L., Kotani K., Remaley A.T. COVID-19-Associated dyslipidemia: implications for mechanism of impaired resolution and novel therapeutic approaches. Faseb J. 2020;34(8):9843–9853. doi: 10.1096/fj.202001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He Y., Yang F.Y., Sun E.W. Neutrophil extracellular traps in autoimmune diseases. Chin Med J. 2018;131(13):1513–1519. doi: 10.4103/0366-6999.235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khovidhunkit W., Kim M.S., Memon R.A., et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45(7):1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Gruber M., Christ-Crain M., Stolz D., et al. Prognostic impact of plasma lipids in patients with lower respiratory tract infections - an observational study. Swiss Med Wkly. 2009;139(11–12):166–172. doi: 10.4414/smw.2009.12494. [DOI] [PubMed] [Google Scholar]
- 14.Parra S., Castro A., Masana L. The pleiotropic role of HDL in autoimmune diseases. Clín Invest Arterioscler. 2015;27(2):97–106. doi: 10.1016/j.arteri.2014.09.002¡. [DOI] [PubMed] [Google Scholar]
- 15.Kaysen G.A., Ye X., Raimann J.G., et al. Lipid levels are inversely associated with infectious and all-cause mortality: international MONDO study results. J Lipid Res. 2018;59(8):1519–1528. doi: 10.1194/jlr.P084277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Lenten B.J., Hama S.Y., de Beer F.C., et al. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995;96(6):2758–2767. doi: 10.1172/JCI118345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma N.K., Tashima A.K., Brunialti M.K.C., et al. Proteomic study revealed cellular assembly and lipid metabolism dysregulation in sepsis secondary to community-acquired pneumonia. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-15755-1. Published 2017 Nov 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nandedkar S.D., Weihrauch D., Xu H., et al. D-4F, an apoA-1 mimetic, decreases airway hyperresponsiveness, inflammation, and oxidative stress in a murine model of asthma. J Lipid Res. 2011;52(3):499–508. doi: 10.1194/jlr.M012724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon E.M., Figueroa D.M., Barochia A.V., Yao X., Levine S.J. High-density lipoproteins and apolipoprotein A-I: potential new players in the prevention and treatment of lung disease. Front Pharmacol. 2016;7:323. doi: 10.3389/fphar.2016.00323. Published 2016 Sep. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodríguez M., Guardiola M., Oliva I., et al. Low-density lipoprotein net charge is a risk factor for atherosclerosis in lupus patients independent of lipid concentrations. Int J Rheum Dis. 2019;22(3):480–487. doi: 10.1111/1756-185X.13445. [DOI] [PubMed] [Google Scholar]
- 21.Kumaraswamy S.B., Linder A., Åkesson P., Dahlbäck B. Decreased plasma concentrations of apolipoprotein M in sepsis and systemic inflammatory response syndromes. Crit Care. 2012;16(2):R60. doi: 10.1186/cc11305. Published 2012 Dec 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saballs M., Parra S., Sahun P., et al. HDL-c levels predict the presence of pleural effusion and the clinical outcome of community-acquired pneumonia. SpringerPlus. 2016;5(1):1491. doi: 10.1186/s40064-016-3145-x. Published 2016 Sep. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norata G.D., Pirillo A., Ammirati E., Catapano A.L. Emerging role of high density lipoproteins as a player in the immune system. Atherosclerosis. 2012;220(1):11–21. doi: 10.1016/j.atherosclerosis.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 24.Madsen C.M., Varbo A., Tybjærg-Hansen A., Frikke-Schmidt R., Nordestgaard B.G. U-shaped relationship of HDL and risk of infectious disease: two prospective population-based cohort studies. Eur Heart J. 2018;39(14):1181–1190. doi: 10.1093/eurheartj/ehx665. [DOI] [PubMed] [Google Scholar]
- 25.Deniz O., Tozkoparan E., Yaman H., et al. Serum HDL-C levels, log (TG/HDL-C) values and serum total cholesterol/HDL-C ratios significantly correlate with radiological extent of disease in patients with community-acquired pneumonia. Clin Biochem. 2006;39(3):287–292. doi: 10.1016/j.clinbiochem.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Norata G.D., Catapano A.L. HDL and adaptive immunity: a tale of lipid rafts. Atherosclerosis. 2012;225(1):34–35. doi: 10.1016/j.atherosclerosis.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 27.Scalsky R.J., Chen Y.J., Desai K., O'Connell J.R., Perry J.A., Hong C.C. Baseline cardiometabolic profiles and SARS-CoV-2 infection in the UK biobank. Preprint. medRxiv. 2020;2020 doi: 10.1101/2020.07.25.20161091. 07.25.20161091. Published 2020 Nov 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alamdari N.M., Rahimi F.S., Afaghi S., et al. The impact of metabolic syndrome on morbidity and mortality among intensive care unit admitted COVID-19 patients. Diabetes Metabol Syndr. 2020;14(6):1979–1986. doi: 10.1016/j.dsx.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das U.N. Can bioactive lipids inactivate coronavirus (COVID-19)? Arch Med Res. 2020;51(3):282–286. doi: 10.1016/j.arcmed.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verdiá-Báguena C., Nieto-Torres J.L., Alcaraz A., et al. Coronavirus E protein forms ion channels with functionally and structurally-involved membrane lipids. Virology. 2012;432(2):485–494. doi: 10.1016/j.virol.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kočar E., Režen T., Rozman D. Cholesterol, lipoproteins, and COVID-19: basic concepts and clinical applications. Biochim Biophys Acta Mol Cell Biol Lipids. 2021;1866(2) doi: 10.1016/j.bbalip.2020.158849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei C., Wan L., Yan Q., et al. HDL-scavenger receptor B type 1 facilitates SARS-CoV-2 entry. Nat Metab. 2020;2(12):1391–1400. doi: 10.1038/s42255-020-00324-0. [DOI] [PubMed] [Google Scholar]
- 33.Masana L., Correig E., Rodríguez-Borjabad C., Anoro E., Arroyo J.A., al Jet. Group OBOTSR. EFFECT oF STATIN THERAPY oN SARS-CoV-2 INFECTION-RELATED. Eur Heart J Cardiovasc Pharmacother. 2020 doi: 10.1093/ehjcvp/pvaa128. Nov 2:pvaa128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masana L., Correig E., Ibarretxe D., Anoro E., Arroyo J.A., et al. STACOV-XULA research group Low HDL and high triglycerides predict COVID-19 severity. Sci Rep. 2021 Mar 30;11(1):7217. doi: 10.1038/s41598-021-86747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hilser J.R., Han Y., Biswas S., Gukasyan J., Cai Z., Zhu R., Tang W.H.W., Deb A., Lusis A.J., Hartiala J.A., Allayee H. Association of serum HDL-cholesterol and apolipoprotein A1 levels with risk of severe SARS-CoV-2 infection. J Lipid Res. 2021;62 doi: 10.1016/j.jlr.2021.100061. Epub 2021 Mar 2. PMID: 33667465; PMCID: PMC7923911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mostaza J.M., Salinero-Fort M.A., Cardenas-Valladolid J., Rodriguez-Artalejo F., Díaz-Almiron M., Vich-Pérez P., San Andres-Rebollo F.J., Vicente I., Lahoz C. Pre-infection HDL-cholesterol levels and mortality among elderly patients infected with SARS-CoV-2. Atherosclerosis. 2022 Jan;341:13–19. doi: 10.1016/j.atherosclerosis.2021.12.009. Epub 2021 Dec 22. PMID: 34959204; PMCID: PMC8692242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Surma S., Banach M., Lewek J. COVID-19 and lipids. The role of lipid disorders and statin use in the prognosis of patients with SARS-CoV-2 infection. Lipids Health Dis. 2021 Oct 25;20(1):141. doi: 10.1186/s12944-021-01563-0. PMID: 34689776; PMCID: PMC8542506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.