Abstract
Cardiac rehabilitation (CR) is a guideline-recommended, multidisciplinary program of exercise training, risk factor management, and psychosocial counseling for people with cardiovascular disease (CVD) that is beneficial but underused and with substantial disparities in referral, access, and participation. The emergence of new virtual and remote delivery models has the potential to improve access to and participation in CR and ultimately improve outcomes for people with CVD. Although data suggest that new delivery models for CR have safety and efficacy similar to traditional in-person CR, questions remain regarding which participants are most likely to benefit from these models, how and where such programs should be delivered, and their effect on outcomes in diverse populations. In this review, we describe important gaps in evidence, identify relevant research questions, and propose strategies for addressing them. We highlight 4 research priorities: (1) including diverse populations in all CR research; (2) leveraging implementation methodologies to enhance equitable delivery of CR; (3) clarifying which populations are most likely to benefit from virtual and remote CR; and (4) comparing traditional in-person CR with virtual and remote CR in diverse populations using multicenter studies of important clinical, psychosocial, and cost-effectiveness outcomes that are relevant to patients, caregivers, providers, health systems, and payors. By framing these important questions, we hope to advance toward a goal of delivering high-quality CR to as many people as possible to improve outcomes in those with CVD.
Keywords: cardiac rehabilitation, coronary disease, heart failure, telemedicine
Cardiac rehabilitation (CR) is a guideline-recommended, multidisciplinary program of exercise training, risk factor management, and psychosocial counseling for people with cardiovascular disease (CVD).1–9 Outpatient programs of up to 36 supervised, in-person sessions over 12 weeks traditionally have been delivered directly by teams at CR centers. CR reduces rates of hospitalization and mortality and improves quality of life.10,11 However, only an estimated 1 in 4 eligible patients enrolls in CR,12,13 and there are persistent disparities in participation based on sex, race and ethnicity, socioeconomic status, and geographic location.14–16 Fewer patients complete the full CR program.
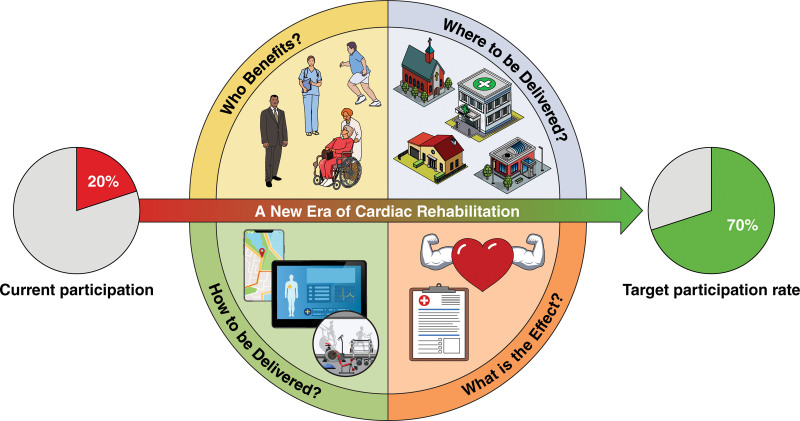
Studies suggest that new CR delivery models, such as virtual or remote CR, with prescribed exercise conducted outside of the CR center, have similar efficacy and safety to in-person CR in low- and moderate-risk patients.17,18 Virtual and remote CR programs have been implemented in the Kaiser and Veterans Affairs health systems for many years.17,19 During the coronavirus disease 2019 (COVID-19) public health emergency, many programs implemented virtual and remote CR.20 Still, questions remain regarding whether implementation of virtual and remote programs will improve access and equity, who may benefit from evolving delivery models, how and where these programs should be delivered, and what the effect on outcomes in diverse populations will be (Figure 1).
Figure 1.
Remaining questions about virtual and remote cardiac rehabilitation.
The objectives of this review are to describe gaps in knowledge of these evolving CR delivery models, identify effective research questions, and propose strategies to address these questions. We aim for this review to serve as a catalyst for future research to help achieve the goal of equitably improving health in patients with CVD from diverse racial and ethnic groups.
Health Disparities and Health Equity
Despite calls to improve referral, enrollment, and participation in CR,21 not all people who are eligible have the opportunity to benefit because disparities in program delivery persist.12 Women,12 people from historically underrepresented racial or ethnic groups (such as Blacks, Asians, and Hispanics),12,22 individuals with multiple CVD risk factors,12 people with lower incomes,23 candidates who are uninsured or underinsured,22,24 those who live in CR deserts,12,16 people who smoke,25,26 and persons who are eligible for CR because of a nonsurgical diagnosis continue to have disproportionately lower CR participation compared with their counterparts.12,26,27 Many of these groups also have greater CVD morbidity and mortality and stand to benefit from CR. For example, Black patients have greater CVD mortality than their non-Hispanic White counterparts,28 and this mortality gap may be partially attributed to disparities in CR referral.29
Inequities in CR uptake are largely attributed to geographic inaccessibility to CR, physician bias in CR referral decisions and care processes, lack of adequate or tailored education and care coordination, and factors that make CR attendance disproportionately challenging for certain patients. These barriers include the time and cost of transportation to and from a facility-based CR program, cost-prohibitive copayments or coinsurance that accrue for each CR session attended, and competing responsibilities (eg, work, child care, and elder care). Such barriers make it difficult for people to attend traditional in-person CR. Lack of racial and ethnic concordance with CR program staff and participants may compound these barriers in some instances.16,30,31 Addressing these barriers requires a greater understanding of factors that perpetuate disparities, strategies that may be used to increase CR participation among historically underserved groups, and opportunities to align incentives to implement effective strategies.
Numerous studies documenting disparities in CR uptake call for additional research on approaches to increase participation among groups with disproportionately low CR participation rates.12,32,33 A systematic review of approaches to increase CR uptake indicated that only 6 trials (23.1%) applied strategies to increase use of CR in previously underrepresented populations (ie, women and older adults).34 TAKEheart, a national initiative led by the Agency for Healthcare Research and Quality, is leading affinity groups to identify new strategies. This 3-year initiative helps hospitals implement automatic referrals with care coordination to optimize enrollment of all eligible patients into CR. The accelerated implementation of virtual CR with synchronous audiovisual communication that occurred during the COVID-19 public health emergency raised questions about the potential role this new approach may have on disparities in CR uptake. Research is needed to answer these questions.
The existence of CR deserts in both rural and urban settings also creates disparate CR participation by geographic location.16 The use of virtual and remote CR has promise in extending the reach of CR programs to accommodate patients with geographic barriers or competing priorities. The success of these models in regions lacking physical access to CR may depend upon the identification of best practices in communicating the value and usefulness of virtual and remote CR to certain populations.35 Insurance coverage for virtual and remote models will be needed to facilitate the reach of these models. In addition, the success of these models in communities will depend on reliable internet access. Whether new delivery models will ultimately improve access in CR deserts remains to be determined.
Apart from geographic location, there is strong evidence that disparate referral explains much of the disproportionately low CR participation rate among women, members of racial and ethnic minority groups, older adults, and those with multiple cardiovascular risk factors.36,37 The leading strategy to correct for inequitable CR referral patterns is to circumvent the implicit and explicit bias in CR referral33 by implementing automatic, or “opt out,” CR referral processes.14,26,38 A recent quality improvement project making CR referral the default option for eligible patients led to a significant 47% increase in CR referrals.39 However, implementing an automatic referral order is only the first step in initiating a successful referral. The likelihood of participation is significantly greater when a clinician referral for CR is made with a strong recommendation by the physician and in the context of care coordination.26,34 Although increasingly more studies support the use of strategies to combat implicit clinician attitudes and referral patterns,40 there are no data on the effect that these approaches have on advancing equity in CR referral patterns, attendance, and completion. Overall, there is a need to ascertain the degree to which subjective clinician decision-making contributes to inequities in CR participation and potential strategies to address this conundrum.
Beyond CR referral, limited data are available regarding how to ideally tailor education, counseling, and care coordination to optimize the likelihood of enrollment, no matter the mode of CR delivery. Best practices to ensure patient activation include gathering information about the patient, providing culturally and linguistically appropriate patient education and counseling, building rapport with the patient, and facilitating the patient’s next steps.41 Limited data exist on how these critical steps can be implemented to bolster CR participation among underrepresented groups. For example, women may be more likely to enroll in CR if they are informed about the complementary psychosocial services and peer support provided.42 Community health workers or CR ambassadors may be vital resources in identifying and addressing participation barriers for patients who are members of minority groups.43 Patients with limited English proficiency likely require the use of interpreters or bilingual staff to facilitate CR education and counseling. Motivational interviewing may be especially beneficial in recruiting patients with time constraints, conflicting priorities, or lack of social support. Studies to assess the usefulness of these types of approaches are essential to ensure patients at highest risk of nonparticipation receive the support needed to enroll in and adhere to CR, enabling them to benefit from this established intervention.
Advancing equity in CR also requires examination of strategies to overcome disincentives to initiate and maintain participation. Frequently cited patient-level barriers to participation include inadequate health insurance, copayments of up to $250 per session, and transportation and parking costs.44 Individuals who face transportation challenges, family or work obligations, depression, anxiety, or low social support are less likely to use CR.45 A recent study demonstrated that more cost sharing was associated with less CR participation, reinforcing the need for insurers to explore alternative policies to potentially incentivize uptake.46 The possible role that hybrid CR delivery models and emerging value-based payment models may have on these patient-level barriers to CR participation remains unclear.
Health literacy is critical to successful CVD management. Individuals with limited health literacy may experience barriers in referral to, engagement with, and participation in CR and thus fail to realize the associated physiological and psychosocial benefits.47 With the advent of virtual and remote CR, concerns remain about the potential for exacerbating the digital divide.20 Early studies using telehealth during the COVID-19 pandemic suggest that individuals from racial or ethnic minority groups and older adults are less likely to access these services.48 Digital literacy may be a potential barrier to uptake of new delivery models, but valid assessment of digital literacy remains a challenge because there are no widely accepted, standardized methods of measurement.19,49
There is a need to explore the ethics of referring patients to CR when there are insurmountable barriers to participation. Identifying and addressing the barriers to enrollment and participation and outlining the role programs play to minimize or eliminate those barriers are crucial components to consider. What resources may be provided when traditional, virtual, or remote CR is not feasible? These are critical questions that remain unanswered.
Patient Selection
The concept of CR delivery outside the traditional CR center is not new; safe and effective telephone-based home rehabilitation programs have been in place since the 1980s. However, over the past decade, the increasing availability of portable electronic devices coupled with development of mobile health applications has greatly expanded capabilities for virtual and remote CR. For example, most CR mobile applications are now capable of accurately quantifying related measures (eg, step count, walking speed, and heart rate) that were traditionally labor-intensive to assess.50 In addition, CR software can employ best practices for user engagement to facilitate long-term adherence and self-management.
Despite these technological innovations, it remains unclear which patients are optimal candidates for virtual and remote CR. Several trials have focused on relatively young, homogenous populations. In a frequently cited Australian study, 120 patients were randomized to remote smartphone-based home CR versus standard facility-based CR after an acute myocardial infarction; mean age was 56 years, and 87% were men.51 Although these patients were well-served by virtual and remote CR, other populations (older adults, women, and people from historically underrepresented racial and ethnic minority groups) typically have numerous barriers to attending in-person CR32 and therefore the greatest potential to benefit from novel delivery approaches. For example, many older adults face unique barriers to participating in in-person CR, including transportation issues (lack of a vehicle or vision or hearing impairment that precludes driving), cognitive impairment, and physical limitations. This population may simultaneously face barriers to engaging with novel platforms; for example, in older adults, the use of portable electronic devices may be limited by impairments in fine motor skills or sensory capabilities.50 Furthermore, as traditional in-person encounters are replaced by virtual or remote encounters, feelings of social isolation could be exacerbated, and fewer opportunities for connection to peers and trusted health care providers are made available. For optimal adherence, the patient’s preference in mode of delivery should be assessed and honored when selecting a participation model.
In addition to selecting relevant demographic groups for virtual and remote CR, acuity of illness is another relevant consideration. Although exercise-related adverse events during CR are rare,52–54 they can occur. Understanding the safety of virtual and remote CR in higher-risk populations requires more data.17 In-person programs may be more appropriate for certain high-risk patients (eg, left ventricular assist device recipients, survivors of exercise-related sudden cardiac death, or patients with complex adult congenital heart disease).
CR is indicated after acute myocardial infarction, percutaneous coronary intervention, coronary artery bypass surgery, heart valve repair or replacement, or heart transplant, or in patients with chronic stable angina or systolic heart failure.1–8 Supervised exercise therapy is recommended in the American Heart Association/American College of Cardiology guidelines for management of lower extremity peripheral vascular disease.55 Whether CR may benefit patients with other indications, such as heart failure with preserved or intermediate ejection fraction, atrial fibrillation, or cancer, requires further study. The fundamental questions regarding new delivery models are largely similar for these emerging indications. When studying these potential new indications, there is also the opportunity to evaluate virtual and remote CR delivery models and whether these may be appropriate for such populations.
The COVID-19 pandemic accelerated the trend toward novel care delivery models within CR. Nevertheless, future research to determine which patients are best served by virtual and remote CR will help determine the appropriate course of therapy.
Delivery Models and Technology
Delivery
Newer delivery models for CR represent alternatives to the wholly “brick and mortar” CR facility.21 The core components of CR can be delivered synchronously (eg, patients exercising during CR session with staff) or asynchronously (eg, patients exercising on their own, not with staff during CR sessions), in-person or virtually (eg, with use of 2-way audiovisual communication) or remotely (eg, with use of remote transmission of data or telephone-only), or using a hybrid approach.20 Virtual and remote CR delivery can involve technologies including telephone, internet, video, mobile applications, and wearables. Delivery can be either one-on-one or group. It is unknown which of these models is optimal for which patients, either individually or in combination.
Many examples of new delivery models are emerging. A recent study found similar improvements in exercise capacity in patients participating in in-person, virtual (one-on-one synchronous), and hybrid CR.56 An ongoing randomized trial will compare outcomes between in-person CR and hybrid CR with virtual group exercise sessions (ATTEND [The Improving Attendance to Cardiac Rehabilitation Trial]; URL: https://www.clinicaltrials.gov; Unique identifier: NCT03646760). The Veterans Affairs health system has many sites that offer remote or virtual CR with weekly one-on-one telephone or video visits between CR providers and patients.57 Kaiser Southern California has implemented an asynchronous CR program using a smartwatch and mobile application to record exercise sessions with weekly telephone visits with a CR provider.58 A recent study in a diverse population also found that adding a mobile technology application to an in-person CR program was associated with increased attendance and adherence.59
CR has historically been delivered by hospital outpatient providers or physician offices. Entrepreneurs have recently launched virtual CR programs in which the physician refers the patient to the virtual CR program and the patient participates in virtual CR visits with CR staff employed by the third-party program. There have been no published data on the efficacy or safety of these third-party programs.
CR is an individualized, patient-centered program. The future of CR will likely evolve toward an individualized, patient-centered approach with regard to delivery mode. Patients have historically only had the option of in-person CR. Future programs will likely include options for participation with in-person visits, virtual one-on-one visits, virtual group visits, and asynchronous exercise with or without remote monitoring. Some patients may participate exclusively through one delivery mode, and others will participate through a hybrid of 2 or more delivery modes.
Format
When considering innovative approaches to CR, it is apparent that significant knowledge gaps exist about the optimal delivery format. In-person CR historically focused on exercise-based rehabilitative services, including 3 sessions per week for 12 weeks. CR now embraces a more comprehensive approach to CVD risk reduction with the inclusion of CR core components such as nutritional counseling, weight management, risk factor modification, medication adherence, behavioral therapy, peer support, tobacco cessation, and psychological well-being.9 Innovative modes of delivery (synchronous or asynchronous remote) should provide higher flexibility in the delivery format of these core components of CR.
Evidence suggests that early initiation of CR after an index event or hospitalization increases enrollment.60 New delivery models should seek to initiate CR early after an index event and may help to expand program capacity and reduce wait time.
For newer, more innovative CR delivery models, such as virtual and remote approaches, the duration associated with optimal outcomes is uncertain, although most studies have assessed the effect of ≥12 weeks of therapy.17 With an updated understanding of overall CVD health and CR services to maximize secondary prevention, the duration of CR could be longer than 12 weeks. For example, to achieve greater improvements in cardiorespiratory fitness, the duration of exercise-based CR may be closer to 6 months.61 Furthermore, with the advent of low-cost remote monitoring and readily accessible technologies capable of connecting patients with caregivers outside the conventional in-person setting, prolonged surveillance and additional follow-up is now possible. When considering other objectives of CR, such as nutritional modification, weight reduction, and CVD risk factor control, the optimal number and frequency of CR sessions is uncertain—both for in-person and virtual and remote approaches—but studies consistently show a positive correlation between the amount of exercise and clinical benefits.62 In addition, the optimal modes and intensities of interventions are not determined. For example, although medically supervised high-intensity interval training appears to be relatively safe and effective in select patients, including those with stable heart failure, and across settings,63 the importance of and appropriate balance between high-intensity interval training and moderate-intensity continuous training are unclear relative to long-term outcomes in individuals with CVD. Furthermore, the preferred strategies to optimize adherence to lifestyle changes and cardioprotective medications have not been specifically studied in the CR setting.
Technology
The role of technology in supporting delivery of CR is increasingly being recognized. In 2013, a mobile technology framework for CR was introduced.64 Subsequent innovation and the shift toward virtual care during the COVID-19 pandemic have accelerated technology adoption in CR. Delivery of CR services at a patient’s home using technology is not a replacement for in-person CR, but rather an opportunity to reach more patients or bolster the effects of in-person CR. It offers an alternative to overcome barriers to in-person CR, such as time and travel. Hybrid CR, which combines in-person and remote components, requires further investigation as a promising opportunity for extending reach, overcoming barriers, and improving outcomes. When using technology, it is important that a virtual or remote approach continues to embrace the established standards and best practices of in-person CR.20
The use of technology in delivering CR has not been standardized. A plethora of terms are used to describe these delivery platforms: telemedicine, telehealth, eHealth, mobile health, digital health, remote care, and virtual care. A recent systematic review on technology in CR uses the terminology “digital health intervention,” referring to use of technologies beyond the telephone, including the internet, wearable devices, and mobile applications.65 This terminology is consistent with the verbiage of the World Health Organization proposal for shared language to describe the uses of digital technology for health.66
The most commonly reported modalities for digital health interventions in CR, as summarized in a recent systematic review, included smartphones or mobile devices (n=20/31 studies [65%]), web-based portals (n=18/31 studies [58%]), and email/short message service communication (n=11/31 studies [35%]).65 Other technology modalities for CR and CVD care more broadly may include video communication platforms, pedometers and accelerometers, tele-ECG, chest and wrist heart rate sensors, blood pressure monitors, and artificial intelligence.67 Multiple proprietary commercial mobile applications have emerged to support the expansion of virtual and remote CR services. Future research should identify effective combinations of digital health tools for achieving CR goals; this will likely vary by patient population.
Although in an early stage of development, use of technology in CR delivery can enable a program to offer a flexible and patient-centered hybrid program with both in-person and virtual/remote options.68 In the virtual model, synchronous audiovisual technology is used to supervise patient exercise and to provide education in real-time. Synchronous interventions could also be used to improve access to CR services, such as consultation with a registered dietitian for weight management or with a behavioral therapist for smoking cessation. An asynchronous remote model may also be offered using wearable and smartphone-based technology to monitor exercise sessions and deliver patient education.20
How Does Technology Change CR?
Implementing the components of CR using technology replaces some in-person communications, representing an example of human factor engineering. Early research has focused on the feasibility of various technologies by participants eligible for CR and the acceptability of specific relevant solutions.69 A more foundational question is as follows: What, if anything, changes about the delivery of CR when it is mediated through technology? How might technology alter the core components comprising the intervention? To provide an example of how technology may alter the core components, we discuss the core component of patient education.
Patient education is both a core component of CR and infused throughout the intervention.9 Effective patient education is a foundational competency for professionals delivering the nutritional counseling and psychosocial management, as well as the exercise training and physical activity counseling components of CR to help patients acquire relevant knowledge and skills. Patient education has several goals, including the acquisition of knowledge (eg, what foods to eat and how long to exercise), skill acquisition (eg, how to read nutrition labels, how to use the exercise equipment correctly, and how to exercise at the correct intensity without equipment), persuasion (eg, adopting correct beliefs and helpful intentions regarding the role of physical activity in secondary prevention), and the application of knowledge (eg, adopting a physically active lifestyle in the patient’s specific circumstances).
The goals of patient education are achieved through different methods. For example, teaching declarative knowledge (eg, facts), or knowledge acquisition, is accomplished most efficiently by repeated recall of the material.70 In contrast, teaching procedural knowledge (ie, skill acquisition) is accomplished most effectively by showing patients how to perform the skill, followed by deliberate practice with feedback.71 How will patient education be delivered, considering that different educational goals align with different methods? Whether synchronous or asynchronous, and whether automated (eg, videos or readings) or individually administered, patient education will be ineffective if it does not use appropriate methods. For example, the efficiencies conferred by online videos and readings compared with group patient education discussions or brief encounters with staff “during exercise” may result in an erosion of knowledge acquisition in remotely delivered CR.
With respect to the goal of teaching patients to apply their knowledge to real-world situations, innovative delivery methods may have the inherent advantage of greater ecological validity than traditional center-based CR. For example, during nutritional counseling, participants could show the dietician their kitchen and the contents of their refrigerator and pantry. Registered dieticians remotely observing the patient’s living environment may be able to make observations and suggestions, which may be more readily adopted given the power of learning in the context in which the knowledge will be applied.
Additional research is needed to understand how the mode of delivery could change each of the core components. There is often an assumption that supervised exercise is the primary component of CR. This is not necessarily true. Delivery of comprehensive CR is superior to providing only selected core components.72 A systematic review and network meta-analysis evaluating the unique and joint contributions of CR supported delivery of all of the core components and concluded that exercise training, psychosocial management, and risk factor modification each contributed directly to the effectiveness of CR.73 However, psychosocial management was also the component least likely to be delivered, partly because of limited human resources (eg, licensed mental health providers). The scientific statement on home-based CR reported that in the studies reviewed, psychosocial management was “not always described clearly” when offered.17 We anticipate that innovative delivery models may also neglect the delivery of certain core components, particularly psychosocial management.
Barriers to Implementing Delivery Models That Use Technology
Potential barriers to CR may include a lack of strong leadership commitment, staff education and training, incorporation of the remote delivery technology to the preexisting information technology of the institution, suboptimal perceived return on investment, and the creation of a new clinical workflow as well as buy-in from the patient. Each barrier can potentially take months to overcome, which makes it almost impossible for the implementation to succeed without a facility champion who is engaged and committed to the task. For example, it may take 3 to 6 months to finalize contracts with a remote delivery system vendor and incorporate it into the existing infrastructure.
Financial barriers related to insurance reimbursement and patient copays or coinsurance also remain. Potential facilitators of the implementation of virtual and remote delivery models would include the continued extension of reimbursement for CR using virtual and remote delivery beyond the pandemic. For Medicare and Medicaid beneficiaries, this would require the continuation of the revised definition of “direct supervision” to include the use of audio/visual communication and the Centers for Medicare & Medicaid Services to interpret existing legislation to include virtual or remote delivery or the passage of legislation by Congress to explicitly allow new delivery models. For private payors, it would be possible now to allow virtual and remote delivery, as has been done by several payors in Michigan.74 Accountable care organizations may also support expanding programs with new delivery models to improve access, as CR reduces hospitalizations and may result in net cost savings for the population. Hospitals and health systems must also consider the costs of the technology platforms and the challenge of creating a business model for new delivery models when data on costs and outcomes are limited.
Numerous logistical, ethical, and clinical issues occur with telehealth, such as lack of privacy, internet access, and technical difficulties. Until recently, these and other aspects of telehealth were not typically addressed in training programs for CR staff.75 Although staff and patient training will be necessary, the specific telehealth competencies required for the effective delivery of CR have not yet been defined.
Outcomes
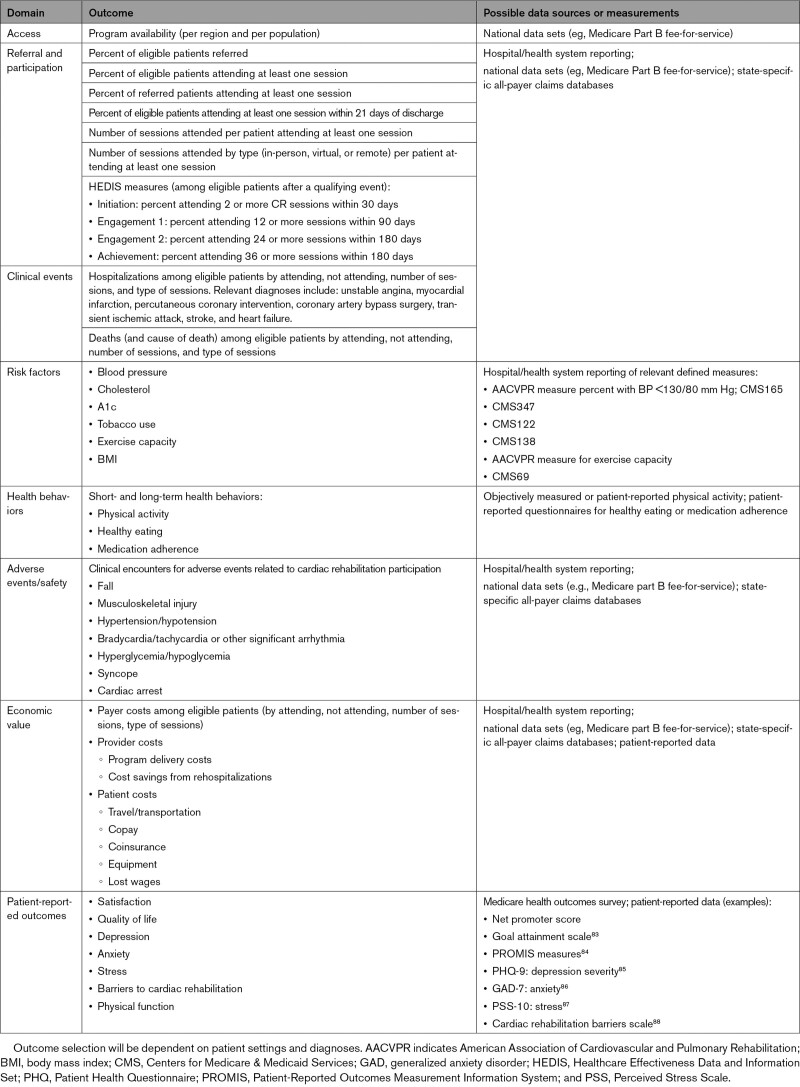
In-person CR is supported by decades of robust evidence from randomized trials, meta-analyses, and observational studies across all indications for CR that consistently show improved outcomes.10,11,76–82 In order for new delivery models to become widely adopted, the evidence base will need to demonstrate to patients, caregivers, clinicians, hospitals, and payors that new models have acceptable health, safety, and cost-effectiveness outcomes. The body of evidence generated by the research community must address a range of important results, including access, referral, and participation; clinical events (eg, hospitalization and mortality); CVD risk factors (eg, high blood pressure, high blood cholesterol level, diabetes, tobacco use, exercise capacity, and weight); adverse events/safety; economic value; and patient-reported outcomes (eg, satisfaction, quality of life, and mental health; Table 1).
Table 1.
Suggested Outcomes to Include in Studies of Cardiac Rehabilitation
Given the marked and persistent underuse of CR,12 new models will be judged by whether they provide greater availability of programs, especially in CR deserts, and improvements in referral, enrollment, timeliness of enrollment, adherence, and completion in a more diverse population. Existing professional society performance measures8 and Healthcare Effectiveness Data and Information Set measures89 offer relevant metrics for research studies and public reporting.
Improvement in use cannot come at the expense of intervention quality. The core components of CR should continue to apply to new delivery models.20 These models should adopt similar standards for developing exercise prescriptions, promoting improvement in cardiorespiratory fitness, and supporting lifestyle behavior change, which are known to enhance health outcomes.1
Improving CR use benefits patients and payors alike.90 Data on cost-effectiveness of contemporary CR models are limited. However, historical data and recent reports outside of the United States suggest that compared with in-person CR, new delivery models may result in similar overall costs.17,91 Economic studies are needed to quantify the contemporary, long-term cost-effectiveness of CR from the payor perspective. Costs to health systems with regard to delivery of CR and savings related to rehospitalization must also be studied. Also relevant are patient and caregiver costs related to CR, including travel/transportation, copays, coinsurance, equipment, and lost wages. There is substantial interest in the role of eliminating cost-sharing or providing financial incentives for improving participation in CR.92
In addition to these outcomes, greater attention is needed in defining, measuring, and improving patient-centered outcomes in CR—particularly patient satisfaction. Little is known about how to measure or improve patient satisfaction in CR or whether changes in programming can affect this perception.93 In an era of increased focus on patient experience, this represents an important gap in both measurement and intervention.
Future Directions
CR has an important and positive effect for eligible patients with CVD. Because of disparities in access, referral, and participation, the benefits of CR remain largely unrealized by large numbers of eligible people. New innovations in delivery are needed to meaningfully improve access, participation, and outcomes. However, there are important questions remaining about how to make CR participation more accessible, affordable, and equitable so that every eligible person can receive the proven benefits.
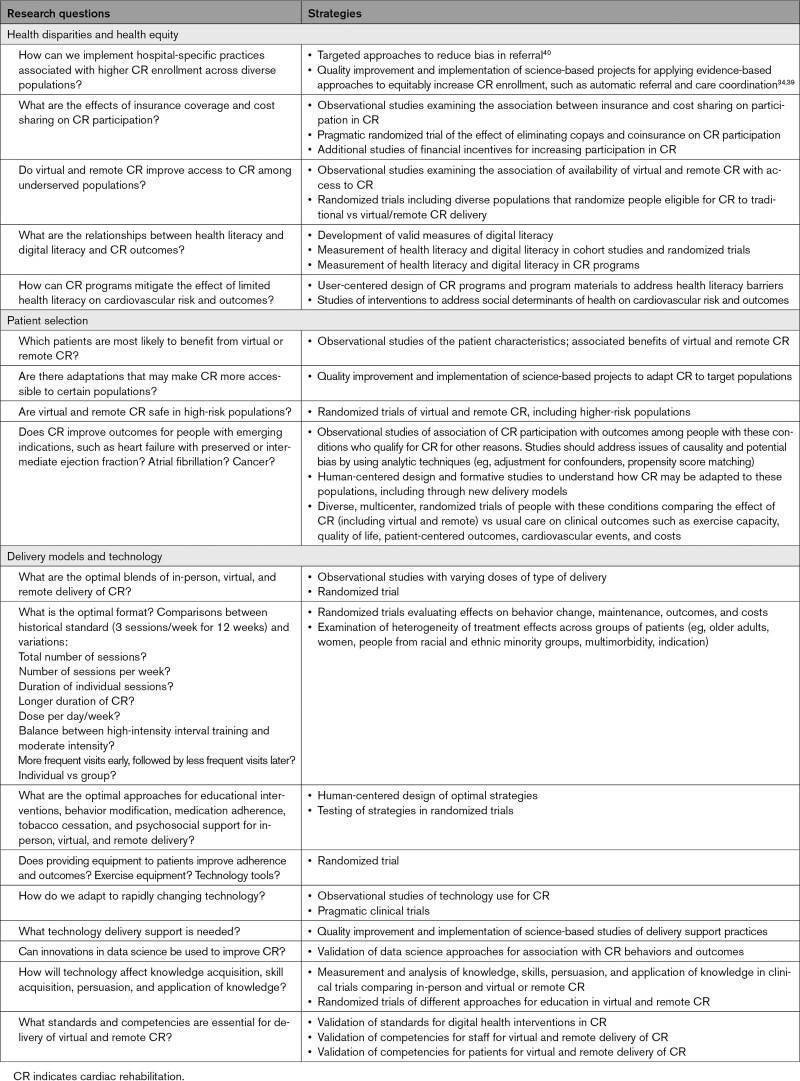
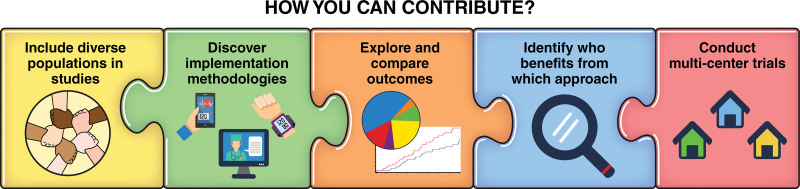
On the basis of these knowledge gaps, we identified important research questions and strategies for addressing them (Table 2). Although this does not encompass the entire range of possible inquiries, the list includes many important research questions that will help to advance knowledge related to new delivery models for CR. We encourage the scientific community to undertake these areas of research and collaborate with researchers and those newly emerging as innovators in this field (Figure 2).
Table 2.
Research Questions and Strategies for Advancing the Science of Cardiac Rehabilitation Delivery
Figure 2.
How the research community can contribute to better understanding new approaches to delivery of cardiac rehabilitation.
We highlight the following research priorities:
(1) Including diverse populations in all CR research;
(2) Leveraging implementation methodologies to enhance equitable and optimal delivery of CR;
(3) Clarifying the populations most likely to benefit from facility-based CR, virtual or remote CR, or a combination of these options (hybrid CR); and
(4) Comparing traditional in-person CR with virtual and remote CR in diverse populations using multicenter studies to identify clinical, psychosocial, and cost-effectiveness outcomes that are relevant to patients, caregivers, providers, health systems, and payors.
CR is an underused but highly beneficial intervention for people with CVD. Although important quality gaps in areas such as coronary revascularization, valvular disease interventions, and use of cardioprotective medications have narrowed, we must continue to work toward closing the gap in providing CR.
Article Information
Acknowledgments
The authors thank their patient partners, Greg Merritt and Patricia McNair; the Centers for Disease Control and Prevention Million Hearts staff and leadership, including Hilary Wall and Laurence Sperling; Susan Svencer, from the National Association of Chronic Disease Directors; and Tadashi Funahashi, Kwan Kim, Richie Grantham, and Alyssa Milan from Kaiser Permanente Southern California, and Patrick Lane of Sceyence Studios, for their assistance in developing the figures.
Sources of Funding
None.
Disclosures
Dr Beatty was formerly employed by (2018–2019) and held stock in (2019–2021) Apple, Inc, and was formerly on the board of the American Association of Cardiovascular and Pulmonary Rehabilitation (2020–2021), and has received research support from the National Heart, Lung, and Blood Institute, and the National Institute of Minority Health unrelated to this article topic.
Nonstandard Abbreviations and Acronyms
- CR
- cardiac rehabilitation
- CVD
- cardiovascular disease
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
Circulation is available at www.ahajournals.org/journal/circ
For Sources of Funding and Disclosures, see page 263.
Contributor Information
Alexis L. Beatty, Email: beattya@uw.edu.
Theresa M. Beckie, Email: tbeckie@usf.edu.
John Dodson, Email: John.Dodson@nyumc.org.
Carly M. Goldstein, Email: Carly_goldstein@brown.edu.
Joel W. Hughes, Email: jhughes1@kent.edu.
William E. Kraus, Email: william.kraus@duke.edu.
Seth S. Martin, Email: smart100@jhmi.edu.
Thomas P. Olson, Email: olson.thomas2@mayo.edu.
Quinn R. Pack, Email: Quinn.PackMD@baystatehealth.org.
Haley Stolp, Email: vul4@cdc.gov.
Randal J. Thomas, Email: thomas.randal@mayo.edu.
Wen-Chih Wu, Email: wen-chih_wu@brown.edu.
References
- 1.American Association of Cardiovascular & Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation Programs, 6th ed. Champaign, IL: Human Kinetics; 2021. [Google Scholar]
- 2.O’Gara PT, Kushner FG, Ascheim DD, Casey JDE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6 [DOI] [PubMed] [Google Scholar]
- 3.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. ; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. Circulation. 2014;130:e344–e426. doi: 10.1161/CIR.0000000000000134 [DOI] [PubMed] [Google Scholar]
- 4.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. Circulation. 2011;124:e574–e651. doi: 10.1161/CIR.0b013e31823ba622 [DOI] [PubMed] [Google Scholar]
- 5.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, Cigarroa JE, DiSesa VJ, Hiratzka LF, Hutter A, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery. Circulation. 2011;124:e652–e735. doi: 10.1161/CIR.0b013e31823c074e [DOI] [PubMed] [Google Scholar]
- 6.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, et al. ; American College of Cardiology Foundation/American Heart Association Task Force. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2012;126:e354–e471. doi: 10.1161/CIR.0b013e318277d6a0 [DOI] [PubMed] [Google Scholar]
- 7.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017;70:776–803. doi: 10.1161/CIR.0000000000000509 [DOI] [PubMed] [Google Scholar]
- 8.Thomas RJ, Balady G, Banka G, Beckie TM, Chiu J, Gokak S, Ho PM, Keteyian SJ, King M, Lui K, et al. 2018 ACC/AHA clinical performance and quality measures for cardiac rehabilitation: a report of the American College of Cardiology/American Heart Association task force on performance measures. Circ Cardiovasc Qual Outcomes. 2018;11:e000037. doi: 10.1161/HCQ.0000000000000037 [DOI] [PubMed] [Google Scholar]
- 9.Balady GJ, Williams MA, Ades PA, Bittner V, Comoss P, Foody JM, Franklin BA, Sanderson BK, Southard D; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update. Circulation. 2007;115:2675–2682. doi: 10.1161/CIRCULATIONAHA.106.180945 [DOI] [PubMed] [Google Scholar]
- 10.Anderson L, Thompson DR, Oldridge N, Zwisler A-D, Rees K, Martin N, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2016;2016:CD001800. doi: 10.1002/14651858.CD001800.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJ, Dalal H, Rees K, Singh SJ, Taylor RS. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev. 2019;1:CD003331. doi: 10.1002/14651858.CD003331.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ritchey MD, Maresh S, McNeely J, Shaffer T, Jackson SL, Keteyian SJ, Brawner CA, Whooley MA, Chang T, Stolp H, et al. Tracking cardiac rehabilitation participation and completion among Medicare beneficiaries to inform the efforts of a national initiative. Circ Cardiovasc Qual Outcomes. 2020;13:e005902. doi: 10.1161/CIRCOUTCOMES.119.005902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park LG, Schopfer DW, Zhang N, Shen H, Whooley MA. Participation in cardiac rehabilitation among patients with heart failure. J Card Fail. 2017;23:427–431. doi: 10.1016/j.cardfail.2017.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellanos LR, Viramontes O, Bains NK, Zepeda IA. Disparities in cardiac rehabilitation among individuals from racial and ethnic groups and rural communities: a systematic review. J Racial Ethn Health Disparities. 2019;6:1–11. doi: 10.1007/s40615-018-0478-x [DOI] [PubMed] [Google Scholar]
- 15.Beatty AL, Truong M, Schopfer DW, Shen H, Bachmann JM, Whooley MA. Geographic variation in cardiac rehabilitation participation in Medicare and Veterans Affairs populations: opportunity for improvement. Circulation. 2018;137:1899–1908. doi: 10.1161/CIRCULATIONAHA.117.029471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wall HK, Stolp H, Wright JS, Ritchey MD, Thomas RJ, Ades PA, Sperling LS. The Million Hearts Initiative: catalyzing utilization of cardiac rehabilitation and accelerating implementation of new care models. J Cardiopulm Rehabil Prev. 2020;40:290–293. doi: 10.1097/HCR.0000000000000547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas RJ, Beatty AL, Beckie TM, Brewer LC, Brown TM, Forman DE, Franklin BA, Keteyian SJ, Kitzman DW, Regensteiner JG, et al. Home-based cardiac rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation. 2019;140:e69–e89. doi: 10.1161/CIR.0000000000000663 [DOI] [PubMed] [Google Scholar]
- 18.Anderson L, Sharp GA, Norton RJ, Dalal H, Dean SG, Jolly K, Cowie A, Zawada A, Taylor RS. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev. 2017;6:CD007130. doi: 10.1002/14651858.CD007130.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakefield BJ, Drwal K, Paez M, Grover S, Franciscus C, Reisinger HS, Kaboli PJ, Accaoui RE. Creating and disseminating a home-based cardiac rehabilitation program: experience from the Veterans Health Administration. BMC Cardiovasc Disord. 2019;19:242. doi: 10.1186/s12872-019-1224-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beatty AL, Brown TM, Corbett M, Diersing D, Keteyian SJ, Mola A, Stolp H, Wall HK, Sperling LS. Million Hearts Cardiac Rehabilitation Think Tank: accelerating new care models. Circ Cardiovasc Qual Outcomes. 2021;14:e008215. doi: 10.1161/CIRCOUTCOMES.121.008215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balady GJ, Ades PA, Bittner VA, Franklin BA, Gordon NF, Thomas RJ, Tomaselli GF, Yancy CW; American Heart Association Science Advisory and Coordinating Committee. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond. Circulation. 2011;124:2951–2960. doi: 10.1161/CIR.0b013e31823b21e2 [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Sobolev M, Piña IL, Prince DZ, Taub CC. Predictors of cardiac rehabilitation initiation and adherence in a multiracial urban population. J Cardiopulm Rehabil Prev. 2017;37:30–38. doi: 10.1097/HCR.0000000000000226 [DOI] [PubMed] [Google Scholar]
- 23.Gaalema DE, Higgins ST, Shepard DS, Suaya JA, Savage PD, Ades PA. State-by-state variations in cardiac rehabilitation participation are associated with educational attainment, income, and program availability. J Cardiopulm Rehabil Prev. 2014;34:248–254. doi: 10.1097/HCR.0000000000000059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson MP, Yaser JM, Hou H, Syrjamaki JD, DeLucia A, Likosky DS, Keteyian SJ, Prager RL, Gurm HS, Sukul D. Determinants of hospital variation in cardiac rehabilitation enrollment during coronary artery disease episodes of care. Circ Cardiovasc Qual Outcomes. 2021;14:e007144. doi: 10.1161/CIRCOUTCOMES.120.007144 [DOI] [PubMed] [Google Scholar]
- 25.Gaalema DE, Cutler AY, Higgins ST, Ades PA. Smoking and cardiac rehabilitation participation: associations with referral, attendance and adherence. Prev Med. 2015;80:67–74. doi: 10.1016/j.ypmed.2015.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khadanga S, Savage PD, Gaalema DE, Ades PA. Predictors of cardiac rehabilitation participation: opportunities to increase enrollment. J Cardiopulm Rehabil Prev. 2021;41:322–327. doi: 10.1097/HCR.0000000000000573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beatty AL, Bradley SM, Maynard C, McCabe JM. Referral to cardiac rehabilitation after percutaneous coronary intervention, coronary artery bypass surgery, and valve surgery: data from the clinical outcomes assessment program. Circ Cardiovasc Qual Outcomes. 2017;10:e003364. doi: 10.1161/CIRCOUTCOMES.116.003364 [DOI] [PubMed] [Google Scholar]
- 28.Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA, Willis M, et al. Cardiovascular Health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136:e393–e423. doi: 10.1161/CIR.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 29.Li S, Fonarow GC, Mukamal K, Xu H, Matsouaka RA, Devore AD, Bhatt DL. Sex and racial disparities in cardiac rehabilitation referral at hospital discharge and gaps in long-term mortality. J Am Heart Assoc. 2018;7:e008088. doi: 10.1161/JAHA.117.008088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moghei M, Turk-Adawi K, Isaranuwatchai W, Sarrafzadegan N, Oh P, Chessex C, Grace SL. Cardiac rehabilitation costs. Int J Cardiol. 2017;244:322–328. doi: 10.1016/j.ijcard.2017.06.030 [DOI] [PubMed] [Google Scholar]
- 31.Resurrección DM, Motrico E, Rigabert A, Rubio-Valera M, Conejo-Cerón S, Pastor L, Moreno-Peral P. Barriers for nonparticipation and dropout of women in cardiac rehabilitation programs: a systematic review. J Womens Health. 2017;26:849–859. doi: 10.1089/jwh.2016.6249 [DOI] [PubMed] [Google Scholar]
- 32.Menezes AR, Lavie CJ, DeSchutter A, Milani RV. Gender, race and cardiac rehabilitation in the United States: is there a difference in care? Am J Med Sci. 2014;348:146–152. doi: 10.1097/MAJ.0000000000000306 [DOI] [PubMed] [Google Scholar]
- 33.Mead H, Ramos C, Grantham SC. Drivers of racial and ethnic disparities in cardiac rehabilitation use: patient and provider perspectives. Med Care Res Rev MCRR. 2016;73:251–282. doi: 10.1177/1077558715606261 [DOI] [PubMed] [Google Scholar]
- 34.Pio CS de A, Chaves GS, Davies P, Taylor RS, Grace SL. Interventions to promote patient utilisation of cardiac rehabilitation. Cochrane Database Syst Rev. 2019;2:CD007131. doi: 10.1002/14651858.CD007131.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schopfer DW, Nicosia FM, Ottoboni L, Whooley MA. Patient perspectives on declining to participate in home-based cardiac rehabilitation: a mixed-methods study. J Cardiopulm Rehabil Prev. 2020;40:335–340. doi: 10.1097/HCR.0000000000000493 [DOI] [PubMed] [Google Scholar]
- 36.Gregory PC, LaVeist TA, Simpson C. Racial disparities in access to cardiac rehabilitation. Am J Phys Med Rehabil. 2006;85:705–710. doi: 10.1097/01.phm.0000233181.34999.3d [DOI] [PubMed] [Google Scholar]
- 37.Brown TM, Hernandez AF, Bittner V, Cannon CP, Ellrodt G, Liang L, Peterson ED, Piña IL, Safford MM, Fonarow GC, et al. ; American Heart Association Get With The Guidelines Investigators. Predictors of cardiac rehabilitation referral in coronary artery disease patients: findings from the American Heart Association’s Get With The Guidelines Program. J Am Coll Cardiol. 2009;54:515–521. doi: 10.1016/j.jacc.2009.02.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grace SL, Russell KL, Reid RD, Oh P, Anand S, Rush J, Williamson K, Gupta M, Alter DA, Stewart DE, et al. ; Cardiac Rehabilitation Care Continuity Through Automatic Referral Evaluation (CRCARE) Investigators. Effect of cardiac rehabilitation referral strategies on utilization rates: a prospective, controlled study. Arch Intern Med. 2011;171:235–241. doi: 10.1001/archinternmed.2010.501 [DOI] [PubMed] [Google Scholar]
- 39.Adusumalli S, Jolly E, Chokshi NP, Gitelman Y, Rareshide CAL, Kolansky DM, Patel MS. Referral rates for cardiac rehabilitation among eligible inpatients after implementation of a default opt-out decision pathway in the electronic medical record. JAMA Netw Open. 2021;4:e2033472. doi: 10.1001/jamanetworkopen.2020.33472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper LA. Why Are Health Disparities Everyone’s Problem? Baltimore: Johns Hopkins University Press; 2021. [Google Scholar]
- 41.Santiago de Araújo Pio C, Beckie TM, Varnfield M, Sarrafzadegan N, Babu AS, Baidya S, Buckley J, Chen S-Y, Gagliardi A, Heine M, et al. Promoting patient utilization of outpatient cardiac rehabilitation: a joint International Council and Canadian Association of Cardiovascular Prevention and Rehabilitation position statement. Int J Cardiol. 2020;298:1–7. doi: 10.1016/j.ijcard.2019.06.064 [DOI] [PubMed] [Google Scholar]
- 42.Midence L, Arthur HM, Oh P, Stewart DE, Grace SL. Women’s health behaviours and psychosocial well-being by cardiac rehabilitation program model: a randomized controlled trial. Can J Cardiol. 2016;32:956–962. doi: 10.1016/j.cjca.2015.10.007 [DOI] [PubMed] [Google Scholar]
- 43.Carroll DL, Rankin SH, Cooper BA. The effects of a collaborative peer advisor/advanced practice nurse intervention: cardiac rehabilitation participation and rehospitalization in older adults after a cardiac event. J Cardiovasc Nurs. 2007;22:313–319. doi: 10.1097/01.JCN.0000278955.44759.73 [DOI] [PubMed] [Google Scholar]
- 44.Beckman AL, Bucholz EM, Zhang W, Xu X, Dreyer RP, Strait KM, Spertus JA, Krumholz HM, Spatz ES. Sex differences in financial barriers and the relationship to recovery after acute myocardial infarction. J Am Heart Assoc. 2016;5:e00923. doi: 10.1161/JAHA.116.003923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marzolini S, Brooks D, Oh PI. Sex differences in completion of a 12-month cardiac rehabilitation programme: an analysis of 5922 women and men. Eur J Cardiovasc Prev Rehabil. 2008;15:698–703. doi: 10.1097/HJR.0b013e32830c1ce3 [DOI] [PubMed] [Google Scholar]
- 46.Farah M, Abdallah M, Szalai H, Berry R, Lagu T, Lindenauer PK, Pack QR. Association between patient cost sharing and cardiac rehabilitation adherence. Mayo Clin Proc. 2019;94:2390–2398. doi: 10.1016/j.mayocp.2019.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magnani JW, Mujahid MS, Aronow HD, Cené CW, Dickson VV, Havranek E, Morgenstern LB, Paasche-Orlow MK, Pollak A, Willey JZ, et al. ; American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; Council on Quality of Care and Outcomes Research; and Stroke Council. Health literacy and cardiovascular disease: fundamental relevance to primary and secondary prevention: a scientific statement from the American Heart Association. Circulation. 2018;138:e48–e74. doi: 10.1161/CIR.0000000000000579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sachs JW, Graven P, Gold JA, Kassakian SZ. Disparities in telephone and video telehealth engagement during the COVID-19 pandemic. JAMIA Open. 2021;4:ooab056. doi: 10.1093/jamiaopen/ooab056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nouri SS, Avila-Garcia P, Cemballi AG, Sarkar U, Aguilera A, Lyles CR. Assessing mobile phone digital literacy and engagement in user-centered design in a diverse, safety-net population: mixed methods study. JMIR MHealth UHealth. 2019;7:e14250. doi: 10.2196/14250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bostrom J, Sweeney G, Whiteson J, Dodson JA. Mobile health and cardiac rehabilitation in older adults. Clin Cardiol. 2019;43:118–126. doi: 10.1002/clc.23306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Varnfield M, Karunanithi M, Lee C-K, Honeyman E, Arnold D, Ding H, Smith C, Walters DL. Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart. 2014;100:1770–1779. doi: 10.1136/heartjnl-2014-305783 [DOI] [PubMed] [Google Scholar]
- 52.Pack QR, Dudycha KJ, Roschen KP, Thomas RJ, Squires RW. Safety of early enrollment into outpatient cardiac rehabilitation after open heart surgery. Am J Cardiol. 2015;115:548–552. doi: 10.1016/j.amjcard.2014.11.040 [DOI] [PubMed] [Google Scholar]
- 53.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, et al. ; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pavy B, Iliou MC, Meurin P, Tabet J-Y, Corone S; Functional Evaluation and Cardiac Rehabilitation Working Group of the French Society of Cardiology. Safety of exercise training for cardiac patients: results of the French registry of complications during cardiac rehabilitation. Arch Intern Med. 2006;166:2329–2334. doi: 10.1001/archinte.166.21.2329 [DOI] [PubMed] [Google Scholar]
- 55.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FGR, Hamburg NM, Kinlay S, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2017;135:e726–e779. doi: 10.1161/CIR.0000000000000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ganeshan S, Jackson H, Grandis DJ, Janke D, Murray ML, Valle V, Beatty AL. Clinical outcomes and qualitative perceptions of in-person, hybrid, and virtual cardiac rehabilitation. J Cardiopulm Rehabil Prev. 42:338–346. doi: 10.1097/HCR.0000000000000688 [DOI] [PubMed] [Google Scholar]
- 57.Drwal KR, Wakefield BJ, Forman DE, Wu W-C, Haraldsson B, El Accaoui RN. Home-based cardiac rehabilitation: experience from the Veterans Affairs. J Cardiopulm Rehabil Prev. 2021;41:93–99. doi: 10.1097/HCR.0000000000000594 [DOI] [PubMed] [Google Scholar]
- 58.Nkonde-Price C, Reynolds K, Najem M, Yang S-J, Batiste C, Cotter T, Lahti D, Gin N, Funahashi T. Comparison of home-based vs center-based cardiac rehabilitation in hospitalization, medication adherence, and risk factor control among patients with cardiovascular disease. JAMA Netw Open. 2022;5:e2228720. doi: 10.1001/jamanetworkopen.2022.28720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imran TF, Wang N, Zombeck S, Balady GJ. Mobile technology improves adherence to cardiac rehabilitation: a propensity score–matched study. J Am Heart Assoc. 2021;10:e020482. doi: 10.1161/JAHA.120.020482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pack QR, Mansour M, Barboza JS, Hibner BA, Mahan MG, Ehrman JK, Vanzant MA, Schairer JR, Keteyian SJ. An early appointment to outpatient cardiac rehabilitation at hospital discharge improves attendance at orientation. Circulation. 2013;127:349–355. doi: 10.1161/CIRCULATIONAHA.112.121996 [DOI] [PubMed] [Google Scholar]
- 61.Hamm LF, Kavanagh T, Campbell RB, Mertens DJ, Beyene J, Kennedy J, Shephard RJ. Timeline for peak improvements during 52 weeks of outpatient cardiac rehabilitation. J Cardpulm Rehabil. 2004;24:374–380. doi: 10.1097/00008483-200411000-00002 [DOI] [PubMed] [Google Scholar]
- 62.Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation. 2010;121:63–70. doi: 10.1161/CIRCULATIONAHA.109.876383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Franklin BA, Quindry J. High level physical activity in cardiac rehabilitation: Implications for exercise training and leisure-time pursuits. Prog Cardiovasc Dis. 2022;70:22–32. doi: 10.1016/j.pcad.2021.12.005 [DOI] [PubMed] [Google Scholar]
- 64.Beatty AL, Fukuoka Y, Whooley MA. Using mobile technology for cardiac rehabilitation: a review and framework for development and evaluation. J Am Heart Assoc. 2013;2:e000568. doi: 10.1161/JAHA.113.000568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wongvibulsin S, Habeos EE, Huynh PP, Xun H, Shan R, Porosnicu Rodriguez KA, Wang J, Gandapur YK, Osuji N, Shah LM, et al. Digital health interventions for cardiac rehabilitation: systematic literature review. J Med Internet Res. 2021;23:e18773. doi: 10.2196/18773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.World Health Organization. Classification of digital health interventions v1.0. Accessed November 13, 2021. https://apps.who.int/iris/bitstream/handle/10665/260480/WHO-RHR-18.06-eng.pdf.
- 67.Bayoumy K, Gaber M, Elshafeey A, Mhaimeed O, Dineen EH, Marvel FA, Martin SS, Muse ED, Turakhia MP, Tarakji KG, et al. Smart wearable devices in cardiovascular care: where we are and how to move forward. Nat Rev Cardiol. 2021;18:581–599. doi: 10.1038/s41569-021-00522-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keteyian SJ, Ades PA, Beatty AL, Gavic-Ott A, Hines S, Lui K, Schopfer DW, Thomas RJ, Sperling LS. A review of the design and implementation of a hybrid cardiac rehabilitation program: an expanding opportunity for optimizing cardiovascular care. J Cardiopulm Rehabil Prev. 2022;42:1–9. doi: 10.1097/HCR.0000000000000634 [DOI] [PubMed] [Google Scholar]
- 69.Beatty AL, Magnusson SL, Fortney JC, Sayre GG, Whooley MA. VA FitHeart, a mobile app for cardiac rehabilitation: usability study. JMIR Hum Factors. 2018;5:e3. doi: 10.2196/humanfactors.8017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dunlosky J, Rawson KA, Marsh EJ, Nathan MJ, Willingham DT. Improving students’ learning with effective learning techniques: promising directions from cognitive and educational psychology. Psychol Sci Public Interest. 2013;14:4–58. doi: 10.1177/1529100612453266 [DOI] [PubMed] [Google Scholar]
- 71.Ericsson KA. Deliberate practice and acquisition of expert performance: a general overview. Acad Emerg Med. 2008;15:988–994. doi: 10.1111/j.1553-2712.2008.00227.x [DOI] [PubMed] [Google Scholar]
- 72.van Halewijn G, Deckers J, Tay HY, van Domburg R, Kotseva K, Wood D. Lessons from contemporary trials of cardiovascular prevention and rehabilitation: a systematic review and meta-analysis. Int J Cardiol. 2017;232:294–303. doi: 10.1016/j.ijcard.2016.12.125 [DOI] [PubMed] [Google Scholar]
- 73.Francis T, Kabboul N, Rac V, Mitsakakis N, Pechlivanoglou P, Bielecki J, Alter D, Krahn M. The effect of cardiac rehabilitation on health-related quality of life in patients with coronary artery disease: a meta-analysis. Can J Cardiol. 2019;35:352–364. doi: 10.1016/j.cjca.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 74.Berry R, Brawner CA, Kipa SG, Stevens C, Bloom C, Keteyian SJ. Telemedicine home-based cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2020;40:245–248. doi: 10.1097/HCR.0000000000000504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rutledge CM, O’Rourke J, Mason AM, Chike-Harris K, Behnke L, Melhado L, Downes L, Gustin T. Telehealth competencies for nursing education and practice: the four P’s of telehealth. Nurse Educ. 2021;46:300–305. doi: 10.1097/NNE.0000000000000988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Santiago de Araújo Pio C, Marzolini S, Pakosh M, Grace SL. Effect of cardiac rehabilitation dose on mortality and morbidity: a systematic review and meta-regression analysis. Mayo Clin Proc. 2017;92:1644–1659. doi: 10.1016/j.mayocp.2017.07.019 [DOI] [PubMed] [Google Scholar]
- 77.Suaya JA, Stason WB, Ades PA, Normand S-LT, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol. 2009;54:25–33. doi: 10.1016/j.jacc.2009.01.078 [DOI] [PubMed] [Google Scholar]
- 78.Beatty AL, Doll JA, Schopfer DW, Maynard C, Plomondon ME, Shen H, Whooley MA. Cardiac rehabilitation participation and mortality after percutaneous coronary intervention: insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. J Am Heart Assoc. 2018;7:e010010. doi: 10.1161/JAHA.118.010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pack QR, Goel K, Lahr BD, Greason KL, Squires RW, Lopez-Jimenez F, Zhang Z, Thomas RJ. Participation in cardiac rehabilitation and survival after coronary artery bypass graft surgery: a community-based study. Circulation. 2013;128:590–597. doi: 10.1161/CIRCULATIONAHA.112.001365 [DOI] [PubMed] [Google Scholar]
- 80.Bachmann JM, Duncan MS, Shah AS, Greevy RA, Lindenfeld J, Keteyian SJ, Thomas RJ, Whooley MA, Wang TJ, Freiberg MS. Association of cardiac rehabilitation with decreased hospitalizations and mortality after ventricular assist device implantation. JACC Heart Fail. 2018;6:130–139. doi: 10.1016/j.jchf.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bachmann JM, Shah AS, Duncan MS, Greevy RA, Graves AJ, Ni S, Ooi HH, Wang TJ, Thomas RJ, Whooley MA, et al. Cardiac rehabilitation and readmissions after heart transplantation. J Heart Lung Transplant. 2018;37:467–476. doi: 10.1016/j.healun.2017.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goel K, Pack QR, Lahr B, Greason KL, Lopez-Jimenez F, Squires RW, Zhang Z, Thomas RJ. Cardiac rehabilitation is associated with reduced long-term mortality in patients undergoing combined heart valve and CABG surgery. Eur J Prev Cardiol. 2015;22:159–168. doi: 10.1177/2047487313512219 [DOI] [PubMed] [Google Scholar]
- 83.Turner-Stokes L. Goal attainment scaling (GAS) in rehabilitation: a practical guide. Clin Rehabil. 2009;23:362–370. doi: 10.1177/0269215508101742 [DOI] [PubMed] [Google Scholar]
- 84.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, et al. ; PROMIS Cooperative Group. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 87.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 88.Shanmugasegaram S, Gagliese L, Oh P, Stewart DE, Brister SJ, Chan V, Grace SL. Psychometric validation of the Cardiac Rehabilitation Barriers Scale. Clin Rehabil. 2012;26:152–164. doi: 10.1177/0269215511410579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.National Committee for Quality Assurance. Cardiac rehabilitation: a new HEDIS measure for heart health. Accessed December 10, 2021. https://blog.ncqa.org/cardiac-rehabilitation-a-new-hedis-measure-for-heart-health.
- 90.Alter DA, Yu B, Bajaj RR, Oh PI. Relationship between cardiac rehabilitation participation and health service expenditures within a universal health care system. Mayo Clin Proc. 2017;92:500–511. doi: 10.1016/j.mayocp.2016.12.024 [DOI] [PubMed] [Google Scholar]
- 91.Brouwers RWM, van der Poort EKJ, Kemps HMC, van den Akker-van Marle ME, Kraal JJ. Cost-effectiveness of cardiac telerehabilitation with relapse prevention for the treatment of patients with coronary artery disease in the Netherlands. JAMA Netw Open. 2021;4:e2136652. doi: 10.1001/jamanetworkopen.2021.36652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gaalema DE, Savage PD, Rengo JL, Cutler AY, Higgins ST, Ades PA. Financial incentives to promote cardiac rehabilitation participation and adherence among Medicaid patients. Prev Med. 2016;92:47–50. doi: 10.1016/j.ypmed.2015.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taherzadeh G, Filippo DE, Kelly S, van Engen-Verheul M, Peek N, Oh P, Grace SL. Patient-reported outcomes in cardiac rehabilitation: what do we know about program satisfaction? A review. J Cardiopulm Rehabil Prev. 2016;36:230–239. doi: 10.1097/HCR.0000000000000142 [DOI] [PubMed] [Google Scholar]