Abstract
After a decades-long pause, psychedelics are again being intensely investigated for treating a wide range of neuropsychiatric ailments including depression, anxiety, addiction, post-traumatic stress disorder, anorexia, and chronic pain syndromes. The classic serotonergic psychedelics psilocybin and lysergic acid diethylamide and nonclassic psychedelics 3,4-methylenedioxymethamphetamine and ketamine are increasingly appreciated as neuroplastogens given their potential to fundamentally alter mood and behavior well beyond the time window of measurable exposure. Imaging studies with psychedelics are also helping advance our understanding of neural networks and connectomics. This resurgence in psychedelic science and psychedelic-assisted therapy has potential significance for the fields of neurosurgery and neuro-oncology and their diverse and challenging patients, many of whom continue to have mental health issues and poor quality of life despite receiving state-of-the-art care. In this study, we review recent and ongoing clinical trials, the set and setting model of psychedelic-assisted therapy, potential risks and adverse events, proposed mechanisms of action, and provide a perspective on how the safe and evidence-based use of psychedelics could potentially benefit many patients, including those with brain tumors, pain syndromes, ruminative disorders, stroke, SAH, TBI, and movement disorders. By leveraging psychedelics' neuroplastic potential to rehabilitate the mind and brain, novel treatments may be possible for many of these patient populations, in some instances working synergistically with current treatments and in some using subpsychedelic doses that do not require mind-altering effects for efficacy. This review aims to encourage broader multidisciplinary collaboration across the neurosciences to explore and help realize the transdiagnostic healing potential of psychedelics.
KEY WORDS: Addiction; Anxiety; Depression; Lysergic acid diethylamide; N-methyl-3,4-methylenedioxyamphetamine; Post-traumatic stress disorder; Psilocybin; Psychedelic; Serotonin
ABBREVIATIONS:
- AE
adverse event
- AUD
alcohol use disorder
- BP
blood pressure
- CIA
Central Intelligence Agency
- CSTC
cortico-striatial-thalamo-cortical
- CT
clinical trial
- DMN
default mode network
- DMT
N,N-dimethyltryptamine
- ED
ego dissolution
- HA
headache
- KAT
ketamine-assisted therapy
- LSD
lysergic acid diethylamide
- MA
meta-analyses
- MDMA
3,4-methylenedioxymethamphetamine
- ME
mystical experience
- N/V
nausea or vomiting
- OCD
obsessive-compulsive disorder
- PAT
psychedelic-assisted therapy
- PFC
prefrontal cortex
- PTSD
post-traumatic stress disorder
- SAH
subarachnoid hemorrhage
- SUD
substance use disorders
- TBI
traumatic brain injury
- TMS
transcranial magnetic stimulation.
“It does not seem to be an exaggeration to say that psychedelics, used responsibly and with proper caution, would be for psychiatry what the microscope is for biology and medicine, or the telescope is for astronomy.” Stanislav Grof1
After a decades-long moratorium, investigations of psychedelic-assisted therapy (PAT) and psychedelics as neuroplastogens are making a resurgence in behavioral health and neuroscience. Over the last 2 decades, multiple clinical trials with psilocybin, lysergic acid diethylamide (LSD), 3,4-methylenedioxymethamphetamine (MDMA), and other psychedelics have been completed or are ongoing for patients with depression, anxiety, addiction, post-traumatic stress disorder (PTSD), and terminal illness.2-6 The results from Phase 2 trials with psilocybin for depression and anxiety and a recent Phase 3 trial for MDMA for PTSD are promising in both efficacy and safety.7-11 Functional imaging and clinical investigations with psychedelics are examining neural networks, neural correlates of subjective experience, and the interplay between meditation, contemplative practice, and music.12-19 The capacity of psychedelics to potentiate neuroplasticity is being explored for stroke, TBI, neurodegenerative disorders, and chronic pain.20-26
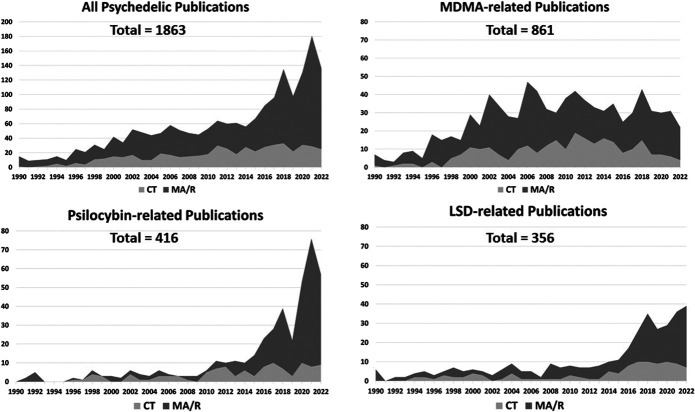
Considering the massive societal and economic burden of mental illness, the relative ineffectiveness of many current therapies and the transdiagnostic reach of psychedelics, the renewed interest in these plant and fungal medicines, and more recently discovered semisynthetic and synthetic molecules may offer one of the most impactful developments in the biomedical sciences.5,20,27,28 This academic fervor is highlighted by the dramatic increase in psychedelic peer-reviewed publications (Figure 1). While recent articles have explored the potential of psychedelics in the psychiatry, palliative care, addiction, and neuropharmacology literature, there are no articles within the neurosurgical and neuro-oncology literature, 2 disciplines largely left outside this resurgence, yet whose challenging patient populations often still need innovative approaches.3,4,29-31
FIGURE 1.
Peer-reviewed publications in psychedelic science from 1990 through June 2022: The four graphs show over the last 3 decades an initial slow then rapid rise in articles focused on clinical applications of all psychedelics, MDMA, psilocybin, and LSD. Publications are shaded to distinguish between CT results or MA and R (Methodology: PubMed was searched using the terms: psychedelic, clinical trial, LSD, 3,4-MDMA, and psilocybin for both human and animal investigations.). CT, clinical trial; LSD, lysergic acid diethylamide; MA, meta-analyses; MDMA, 3,4-methylenedioxymethamphetamine; R, reviews.
This review assesses the state of psychedelic research and offers a perspective on how PAT may help address mental health issues common in neurosurgery and neuro-oncology patients and how psychedelics' neuroplastic potential could benefit other neurosurgical conditions, while also reviewing potential adverse consequences and safety issues surrounding PAT. We focus on psilocybin, LSD, and MDMA (all currently non-US Food and Drug Administration (FDA)–approved Schedule 1 psychedelics) and discuss ketamine because it is currently the only FDA-approved drug with psychedelic properties available to mental health providers for off-label use outside clinical trials.32
NOMENCLATURE
Three terms most often used for plant, fungal, and synthetic medicines that can occasion nonordinary states of consciousness are psychedelic, meaning “mind manifesting;” entheogen, meaning “accessing the divine within;” and hallucinogen, meaning “wandering in mind.”29,30 In this study, we use “psychedelic” because it is believed to best capture the essence of what these medicines can elicit. Classic psychedelics include fungal-based psilocybin, semisynthetic LSD, plant-based N,N-dimethyltryptamine, and cactus-based mescaline; all are predominantly serotonin 2A receptor (5-HT2AR) agonists and occasion similar kinds of nonordinary experiences.33 Synthetically produced MDMA is considered an atypical psychedelic that occasions empathy and emotional openness; the terms entactogen and empathogen are used to describe its effects. MDMA triggers synaptic release of serotonin and other monoamine neurotransmitters, as well as increases in oxytocin, cortisol, and prolactin.29,34 Ketamine, a dissociative anesthetic, occasions a different nonordinary state of consciousness in subanesthetic doses from the classic psychedelics or MDMA and thus can be considered an atypical psychedelic. It is predominantly an N-methyl-D-aspartate-glutamate-receptor antagonist and currently used off-label in subpsychedelic doses to treat depression and other mood disorders and in PAT at higher subanesthetic doses.30,32,35
BRIEF HISTORICAL CONTEXT
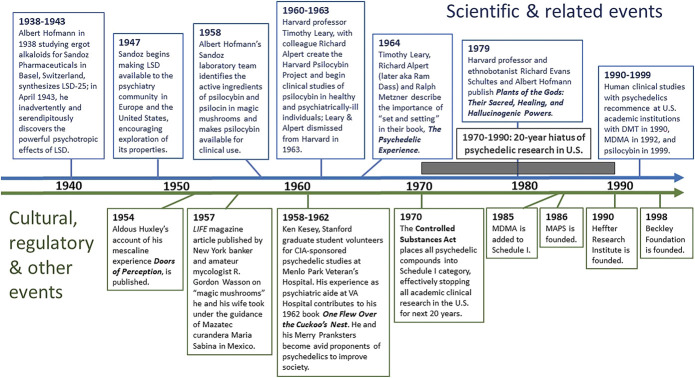
Naturally occurring psychedelics including psilocybin-containing mushrooms, mescaline-containing cacti (peyote and San Pedro), and N,N-dimethyltryptamine-containing ayahuasca brew have been used in shamanic cultures in some instances for more than 5000 years in healing and other ritual ceremonies.36,37 The modern era of psychedelics arguably began with Arthur Heffter, a German pharmacologist who in 1897 isolated mescaline as the psychotropically active alkaloid in peyote.33 Perhaps more important was the serendipitous discovery of the powerful psychotropic effects of LSD by Swiss chemist Albert Hofmann in 1943 and isolation of psilocybin as the psychotropically active alkaloid in magic mushrooms in 1958 by Hofmann et al33,38,39 (Figure 2). Chemist Alexander Shulgin and his wife Ann synthesized and tested multiple new psychedelic compounds during the 1960s and 1970s, including a revised synthesis of MDMA in 1976, and published many of their observations in academic journals.40 From the early 1950s with LSD, and late 1950s with psilocybin, PAT for depression, anxiety, and alcoholism was practiced across North America and Europe with hundreds of clinical studies involving thousands of patients.28,41 However, amid widespread and rapidly increasing use of LSD and psilocybin beyond the confines of clinical care and academic institutions, and perceived counterculture threat, the US Congress passed the Controlled Substances Act in 1970 placing all psychedelics into Schedule I, effectively stopping all clinical research for the next 2 decades.38,39,41,42 MDMA was added to Schedule I in 1985.43 Despite the Controlled Substances Act, the belief that PAT held great potential for contributing to well-being endured and work was pursued by intrepid scientists, practitioners, and advocates, eventually leading to the current resurgence in psychedelic studies.28,44
FIGURE 2.
Timeline of pivotal events and people in psychedelic history 1938 to 1999: Beginning with Albert Hofmann's synthesis of LSD-25 in 1938 and ending in the 1990s with the recommencement of psychedelic clinical studies in the United States and Europe, landmark scientific, cultural, and regulatory events and key individuals are noted. For more in-depth historical accounting of these and other pivotal events and people, readers are referred to these references:36,38-44. CIA, Central Intelligence Agency; DMT, N,N-dimethyltryptamine; LSD, lysergic acid diethylamide; MAPS, Multidisciplinary Association for Psychedelic Studies; MDMA, 3,4-methylenedioxymethamphetamine. © Pacific Neuroscience Institute Foundation, 2022. Used with permission.
CLINICAL TRIALS WITH PSILOCYBIN, LSD, AND MDMA
A current search of the National Institutes of Health-maintained database (clinicaltrials.gov) yields 100 clinical trials for psilocybin (21 completed), 21 for LSD (12 completed), and 67 for MDMA (40 completed). Table 1 summarizes actions and targets of these Schedule 1 psychedelics and ketamine.
TABLE 1.
Overview of Neurohormonal Targets, Acute Actions, and Potential Adverse Events of Psilocybin, LSD, and MDMA in PAT Clinical Trials and Ketamine in KAT Clinical Practice
| Drug and DEA schedule | Therapeutic dose range and route | Main neurotransmitter effects | Main hormonal effects | Hemodynamic effects and other systemic AEs or side effects | Duration of effect; number of doses over time | Major clinical indications | Other potential indications | Main cognitive and perceptual effects | Main emotional effects: positive and negative | Mystical experience and ego dissolution | ME and ED predictive of efficacy |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Psilocybin Schedule 1 In Phase 2 trials |
20-30 mg oral | Serotonin (5HT-2A), Dopamine (partial) |
Mild or moderate rise in cortisol, prolactin at higher doses | Mild increase in BP and pulse; pupil dilation, loss of appetite. In minority: N/V, headache, tremulousness, and/or fatigue in some | 4-6 h; 1-2 sessions separated by 4-6 wk | Depression, anxiety, distress of terminal illness and cancer, AUD | Grief, nicotine and opioid addiction, OCD, anorexia | Moderate to strong visual hallucinations, audio-visual synesthesias, alterations of space and time | Positive: Sense of well-being, empathy, openness, and trust; negative: anxiety, fear, and/or paranoia, suicidal ideation in minority | ME and ED in large minority or majority at higher doses | Yes |
|
LSD Schedule 1 In Phase 2 trials |
100-250 µgm oral | Serotonin (5HT-2A) | Mild or moderate rise in cortisol, prolactin, oxytocin at higher doses | Mild increase in BP and pulse; pupil dilation, loss of appetite; in minority: N/V, headache and/or fatigue | 8-12 h; 1-2 sessions separated by 4-6 wks | Anxiety, depression, cluster headache | AUD, nicotine and opioid addiction | Moderate to strong visual hallucinations, audio-visual synesthesias, alterations of space and time | Positive: Sense of well-being, empathy, openness, and trust; negative: anxiety, fear, and/or paranoia in minority | ME and ED in large minority or majority at higher doses | Yes |
|
MDMA Schedule 1 In Phase 3 trials |
80-180 mg oral | Serotonin (5HT-2A) Dopamine Norepinephrine |
Significant rise in oxytocin; modest increase in cortisol, prolactin | Modest increase in BP and pulse; transient and mild/moderate muscle tightness, loss of appetite, nausea, hyperhidrosis, and/or feeling cold | 4-6 h; 3 sessions separated by 4 wks | PTSD | Anxiety, depression, AUD, anorexia, and other eating disorders | Mild visual and auditory perceptual changes; no hallucinations | Positive: Reduced fear and anxiety, sense of well-being, increased trust and empathy; negative: anxiety and/or distrust, hostility, and/or suicidal ideation in minority | ME and ED in minority | No |
|
Ketamine Schedule 3 Off-label use |
0.5–1.5 mg kg IV or IM | NMDA-glutamate receptor antagonist | Mild increase in cortisol and prolactin | Mild increase in BP; N/V in minority | 1-2 h; 4-8 sessions over 4-6 wks | Depression, anxiety, PTSD | OCD, SUD | Vivid imagery, derealization, disembodiment, impaired control and vigilance | Positive: Sense of unity, openness; negative: anxiety, agitation in minority | Variably at higher doses | Mixed results |
AE, adverse event; AUD, alcohol use disorder; BP, blood pressure; ED, ego dissolution; KAT, ketamine-assisted psychotherapy; ME, mystical experience; NMDA, N-methyl-D-aspartate; N/V, nausea or vomiting; PTSD, post-traumatic stress disorder; SUD, substance use disorder (relevant references7-11,29,32,34,35,38,47,49,66).
Psilocybin
Studies of psilocybin-assisted therapy have focused on depression and anxiety, existential distress, and substance use disorders (SUD). Recent trials are targeting demoralization, grief, anorexia, obsessive-compulsive disorder (OCD), headache, and fibromyalgia. The 2 largest randomized double-blind Phase 2 trials for patients with cancer with anxiety and/or depression were conducted at Johns Hopkins (N = 51 subjects) and New York University (N = 29 subjects); 6 months after a single psilocybin session, preceded and followed by supportive psychotherapy, 60% to 80% of subjects had durable reductions in anxiety and/or depression.9,11 Notably, in these 2 studies, at 6 months after psilocybin-assisted therapy, 67% to 70% of subject rated their psilocybin experience as one of the “top 5 most meaningful of life” and 52% to 70% as one of the “top 5 most spiritually significant of life.”9,11 Similar promising results were shown by the same groups in pilot studies for nicotine addiction and alcohol use disorder (AUD); a recent larger placebo-controlled trial (N = 95) for AUD showed a significant decrease in heavy drinking days over 32 weeks of follow-up.45-47 The FDA designated psilocybin a “breakthrough therapy” for treatment-resistant depression in 2018 and for major depressive disorder in 2019. Currently, 2 randomized placebo-controlled Phase 2 trials for depression have completed enrollment for treatment-resistant depression and major depressive disorder, and Phase 3 trials are soon getting underway for both indications.
LSD
Studies in healthy subjects have focused on neuroplasticity and comparison with other psychedelics, while treatment studies are assessing utility for anxiety, depression, and cluster headache.14,17,38,48,49 The fewer LSD trials likely reflects residual stigma of LSD as a counterculture drug and duration of an LSD journey averaging 8-12 hours (compared with 4-6 hours for psilocybin), making the treatment model more demanding on staffing and resources. Nonetheless, given similarities between psilocybin and LSD, future LSD trials for mood disorders and addiction are likely.49
MDMA
Studies have focused on PTSD (6 completed Phase 2 trials, 1 completed Phase 3 trial), demonstrating strong efficacy and safety across all types of severe PTSD.2,10 In the recent Phase 3 trial, after 3 MDMA psychotherapy sessions (18 weeks postbaseline) combined with psychotherapy, 28/42 (67%) of participants in the MDMA group no longer met criteria for PTSD, compared with 12/37 (32%) of those receiving placebo.10 Should the second Phase 3 trial yield similar results, FDA approval of MDMA-assisted therapy for PTSD is likely. Other therapeutic targets being studied with MDMA include anxiety, eating disorders, and AUD.
SET AND SETTING MODEL OF PSYCHEDELIC-ASSISTED THERAPY
The “set and setting” PAT model, established in the 1960s with its roots in shamanic cultures, is currently part of all clinical trial models and is designed to achieve a transformative yet safe patient experience (Figure 3).50,51 Set refers to the mindset of the patient as informed by the current state of mind and one's life history. Setting refers to the environment in which the medicine session or “journey” occurs including the physical space, the music, and the guides.50,52,53 Patient preparation with psychological counseling occurs over several sessions with trained guides (typically a male and female dyad).54 A sense of trust, safety, and expectations is established, and the subject reflects on their life history and intentions while developing an attitude of fortitude and curiosity about the coming journey. A mantra often encouraged is “trust, let go, be open.” The journey which lasts several hours takes place in a comfortable room with appropriate art and lighting, the patient reclining on a bed or sofa with eyeshades; music is of a new age or classical genre, evocative and nonlyrical that builds to support the internal journey and help achieve a peak experience.50,52-54 Confronting challenging material may lead to profound and beneficial psychological change. In many journeys, particularly with psilocybin and LSD, the patient will have what is termed a mystical experience.49,53,55
FIGURE 3.
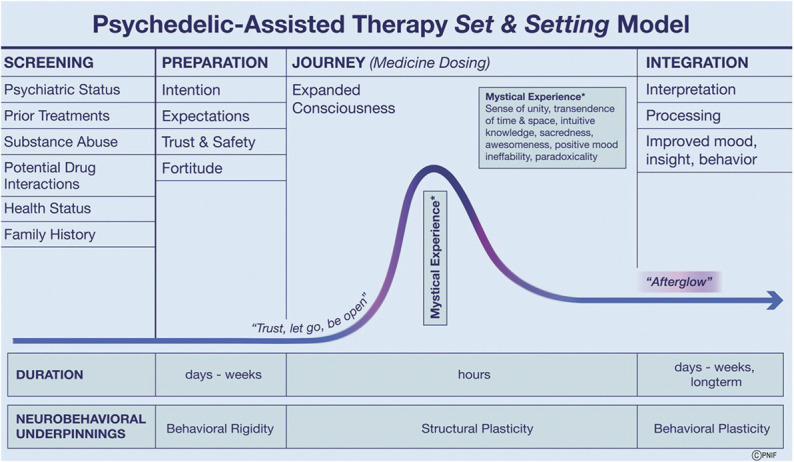
Psychedelic-assisted therapy is akin to a surgical procedure, the success and safety of which depends on experienced guides, patient screening and preparation, an optimized “set and setting,” safety protocols, and rigorous postjourney psychological counseling (integration).50,54 Depicted are the phases in current clinical trials of patient screening, preparation, journey, and integration. After detailed patient screening for appropriateness of participation and exclusion criteria, preparation with psychological counseling occurs over several sessions in the days or weeks before the medicine session. The first integration session occurs the day after the journey, in the so-called “afterglow” period followed by subsequent sessions over weeks in which the patient strives to integrate the experience into their life and favorably alter their behavior and outlook.53 For example, a dying individual may find meaning and resolution leading to long-term reduction in demoralization, hopelessness, and death anxiety, resulting in improved quality of life and of death.11,68 The neurobehavioral and structural underpinnings of the transformative process that may occur with PAT are postulated to result from the neuroplastogenic properties of psychedelic medicines.20,30,61,70 PAT, psychedelic-assisted therapy. Adapted from Majic et al.53 © Pacific Neuroscience Institute Foundation, 2022. Used with permission.
SAFETY, POTENTIAL ADVERSE EVENTS, AND ETHICAL ISSUES
The requirements to conduct clinical trials with non-FDA–approved investigational Schedule 1 psychedelics such as psilocybin, LSD and MDMA are rigorous. An MD must be on site and readily available, as well as a clinical psychologist both trained in PAT. The other guides can be other health care providers (eg, registered nurses, physician assistants, nurse practitioners, marriage and family therapists) who have undergone PAT training such as provided by California Institute of Integral Studies or Multidisciplinary Association for Psychedelic Studies. Given the powerful nature of the psychedelic experience and the vulnerable psychological state patients enter during and in the initial days postjourney, having well-trained guides especially in understanding and managing the spectrum of emotional and perceptual responses to psychedelics is essential.54 With experienced and skilled guides and a well-prepared patient, PAT is typically deeply meaningful, albeit at times challenging. The risk of a “bad trip” is low, and hallucinogen-persisting perceptual disorder is rare.50,53 Serotonin toxicity has not been reported in clinical trials to date, although most trials have excluded coadministration of psychedelics with serotonergic medications such as antidepressants.56 As recently emphasized, as such trials and treatments move forward, psychedelic practitioners must adhere to the highest ethical standards focused on patient safety and well-being and embrace a rigorous system of peer review and supervision.50,54,57
From a physiological perspective, the classic psychedelics and MDMA are notable for their high safety profile and low addictive potential.50,58 As detailed in Table 1, psilocybin and LSD cause mild-to-moderate elevations in blood pressure, pulse, and respirations, while MDMA can cause modest increases in blood pressure; all 3 are at the low end of the Harms Scale compared with alcohol, heroin, and cocaine.9,10,49,58
In modern clinical trials of psilocybin, LSD, and MDMA using the “set and setting” paradigm, there have been few serious adverse events, largely because of safety guidelines, and careful patient screening, excluding those with a personal or family history of mental illness with psychotic potential such as schizophrenia and bipolar disorder.7,9-11,49,50 In 7 of the most recent randomized placebo-controlled trials for high-dose psilocybin, LSD, or MDMA (total N = 249 patients), there were no serious medical or psychiatric adverse events reported.7-11,47,49
MECHANISMS OF ACTION AND DURABILITY OF PSYCHEDELICS
One of the fascinating questions surrounding psychedelics is how they work and what brain changes are induced long-term.59-61 How can 1, 2, or 3 psychedelic journeys, accompanied by psychotherapy, lead to months or even years of durable behavioral change? Based on multiple neuroimaging studies, the most widely accepted mechanism of action is that by stimulating 5-HT2A receptors, the classic psychedelics (psilocybin and LSD) loosen processes that normally act to constrain neural systems involved in cognition, perception, emotion, and sense of self.5,12,16,17,59,60,62 Three leading models have emerged as to how the classic psychedelics alter neural circuitry leading to this neuroplastic state: the cortico-striatial-thalamo-cortical model,12,16,61,63,64 the “relaxed beliefs under psychedelics” model,5,60,65 and the cortico-claustro-cortical model.12,59 To varying degrees, all 3 models involve the medial prefrontal cortex, posterior parietal and sensory cortices, and other subcortical structures; they all implicate increased excitability of pyramidal neurons in the prefrontal cortex (PFC), leading to dampening of cortical rhythmicity and disruption of large-scale networks such as the default mode network and reduced segregation between networks (Figure 4A).12,16,59-61
FIGURE 4.
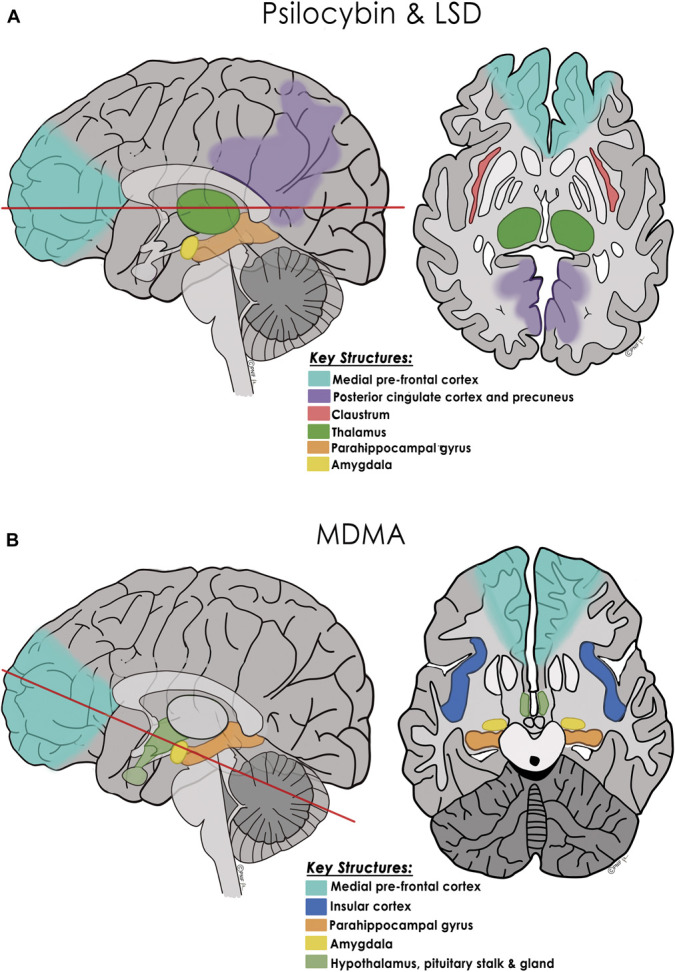
Proposed key neural structures and mechanism of action of the classic psychedelics and MDMA. A, Psilocybin and LSD: Based on functional neuroimaging studies, sagittal and axial drawings show major brain regions altered by psilocybin and LSD (predominantly 5-HT2A receptor agonists): pyramidal neurons in the medial PFC, the DMN (including the posterior cingulate cortex and precuneus), and other networks such as the salience network, the thalamus, claustrum, parahippocampal gyri, and amygdala (red line in sagittal image denotes axial cut level).12,16,59-61,64 Three functional models have been proposed for how the serotonergic psychedelics alter these cortical and subcortical structures and their regional connectivity. While they differ as to the predominance of “top-down” or “bottom-up” loosening, all 3 models propose that the normally highly filtered flow of sensory information and access to autobiographical information is dampened or partially disrupted in the acute psychedelic state. This more connected state is proposed to lead to the commonly experienced auditory-visual synesthesias, visual hallucinations, alterations of space and time, alterations of one's sense of self, and in many patients, a mystical experience.59,60,63 The CSTC model proposes that activation of medial prefrontal cortex 5-HT2AR–containing neurons projecting to the striatum disrupts thalamocortical gating leading to reduced thalamic filtering of sensory information and increased signaling to the cerebral cortex, triggering synesthesias, cognitive, and emotional changes, and in some patients, ego dissolution.16,61,63,64 The “relaxed beliefs under psychedelics” model proposes that psilocybin and LSD increase information flow from the hippocampus and parahippocampal gyrus to higher cortical areas, including the DMN, which relax high-level prior beliefs of one's sense of self, ego, and social identity.60 This temporary disruption of the normal “top-down” constraint imposed over neural networks and sensory processing, along with favorable changes in amygdala responsiveness, is believed to halt or dampen ruminative thoughts and potentiate a more functionally connected and flexible brain.5,65 The cortico-claustro-cortical model, focuses on the claustrum, situated between the insula and the putamen with a high density of 5-HT2A receptors and considered critical in maintaining cortical networks including the DMN and frontoparietal network.59 Based on fMRI data, psilocybin acutely decreases claustrum connectivity to auditory and other cortical areas with resultant alterations in perception and attention; this decreased cognitive control correlates with measures of a “mystical experience.”12,59 B, MDMA: Based on neuroimaging studies, sagittal and axial drawings show major brain regions and neurohormonal circuits altered by MDMA including connections between the medial PFC, the amygdala, parahippocampal gyri, and insula, as well as the hypothalamic-pituitary axis.34 (Red line in sagittal image denotes axial cut level.) While MDMA does not seem to cause as much increased connectivity between neural networks as do psilocybin and LSD, MDMA-induced release of oxytocin (facilitated by serotonin efflux) affects connectivity between the medial PFC and amygdala, dampening amygdalar activation which may contribute to prosocial effects and memory reconsolidation. MDMA-induced cortisol release may also affect the amygdala and hippocampus, allowing extinction learning by facilitating emotional engagement despite revisiting fear and anxiety related to a traumatic event.34 CSTC, cortico-striatial-thalamo-cortical; DMN, default mode network; LSD, lysergic acid diethylamide; MDMA, 3,4-methylenedioxymethamphetamine. © Pacific Neuroscience Institute Foundation, 2022. Used with permission.
While the neural underpinnings remain to be clarified, the psychedelic-induced state seems to allow one to recollect and process deep autobiographical information, achieve new understandings of one's self and relationships with others, and gain greater appreciation of one's place within the cosmos.5,61,62 This state is closely linked to the concept of “mystical experience” which in multiple studies with classic psychedelics predicts therapeutic efficacy.9,11,45,47,55 “Ego dissolution” is another term used to describe a loss of the usual sense of self as separate entity.13 The quality and degree of psychedelic-induced ego dissolution and mystical experience are typically measured in clinical trials with validated psychometric instruments such as the 5-Dimensional Altered States of Consciousness Questionnaire and the Mystical Experience Questionnaire.9,11,45,47,49,55,61
With MDMA-assisted therapy, a mystical experience and ego dissolution are less often achieved and may not correlate with efficacy.66 MDMA triggers synaptic release of serotonin, norepinephrine, and dopamine and elevations of oxytocin, cortisol, and prolactin, acting predominantly in pathways involved in emotional and memory processing including the PFC, amygdala, hippocampus, and insular cortex34,66 (Figure 4B). Increased connectivity between the amygdala and hippocampus, reduced insula activation, and reduced medial PFC connectivity to the amygdala augmented by increased oxytocin, and cortisol levels are hypothesized to facilitate one's ability to reevaluate traumatic events without fear and anxiety, allowing a favorable reorganization of such memories.4,34
Ketamine has been shown in a nonpsychedelic model of subanesthetic IV dosing to reliably produce rapid but short-lived relief of depression and suicidal ideation.32 Since the early 1970s, ketamine has also been known to occasion a potentially therapeutic nonordinary state of consciousness. More recently, an effective ketamine-assisted therapy (KAT) model for depression has been developed using intramuscular, sublingual, or intranasal dosing.35 KAT seems to result in a dose-dependent “time-out” from usual mind negativity, often a dissociative state in which one feels a separation from their body, intense visions, and even what has been called a near-death experience.35 Neuroimaging studies suggest this experience results from ill-defined interactions on the PFC, anterior and posterior cingulate cortex, and subcortical structures including the putamen, thalamus, amygdala, and hippocampus.32,67 Importantly, ketamine has abuse and addiction potential.32,35
The durability of PAT with sustained resolution of symptoms seen in recent clinical trials of psilocybin and MDMA and depicted in Figure 3 9-11,68,69 is likely related to multiple factors that may include psychedelic-induced synaptogenesis and neurogenesis, upregulation of plasticity-promoting genes in the PFC and hippocampus, and of brain-derived neurotrophic factor, mammalian target of rapamycin, and other neurotrophins.20,30,34,70 Classic psychedelics and ketamine trigger neuritogenesis and synaptogenesis in in vitro and in vivo models.20,26,71 It has been proposed that ketamine, using N-methyl-D-aspartate-glutamate receptor antagonism, and the classic psychedelics, using 5-HT2AR agonism, cause similar downstream changes: a glutamate surge, activating PFC pyramidal neurons to release brain-derived neurotrophic factor and mammalian target of rapamycin, which in turn upregulate neuroplasticity genes promoting synaptogenesis and “adaptive rewiring” leading to lasting behavioral and mood improvements.70
Thus, PAT with only 1 or few medicine sessions offers a paradigm shift for treating mental health issues, sharply diverging from the daily pharmacopeia approach commonly practiced today. Instead of blunting or suppressing negative thoughts and emotions, these medicines seem to facilitate an expansive psychospiritual approach, allowing deep personal exploration, promoting structural neuroplasticity, and connectivity that may underlie their durability of effect.
POTENTIAL APPLICATIONS OF PSYCHEDELICS IN NEUROSURGERY AND NEURO-ONCOLOGY
Several patient groups may benefit by leveraging (1) the psychedelic properties to heal the mind using PAT and/or (2) the neuroplastic properties to heal the brain (Figure 5). These “mind-altering” and “brain-altering” approaches may overlap and, in some disorders, could potentially be combined with existing treatments such as neuromodulation techniques including deep brain stimulation (DBS), transcranial magnetic stimulation (TMS), and stem cell therapies.72-75
FIGURE 5.
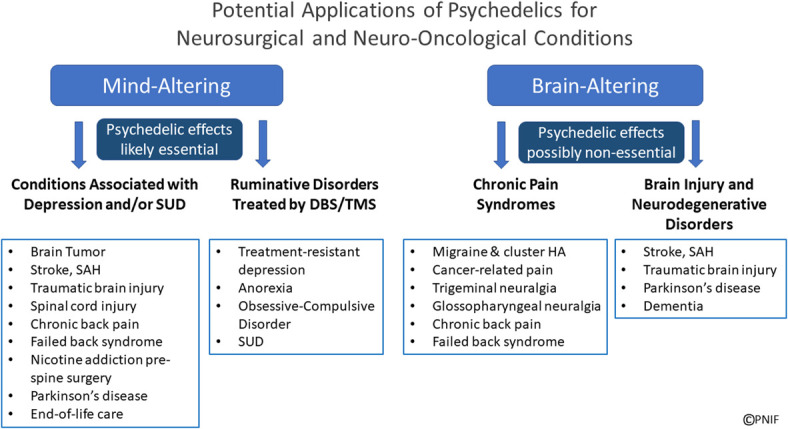
Neurosurgery and neuro-oncology patient groups potentially affected by psychedelic-based treatments: Depicted are patient populations who may benefit from psychedelic-assisted therapy (“mind-altering” treatments) for depression, anxiety, and/or addiction (SUD), as well as those in clinical trials with DBS or TMS for ruminative disorders. Patients who might benefit from the neuroplastic potential of psychedelics (possibly without psychedelic effects at lower doses) are depicted as “brain-altering,” including patients with chronic pain syndromes and those with brain injury (stoke, SAH, TBI) and neurodegenerative disorders. The potential overlap and synergy of these “mind-altering” and “brain-altering” psychedelic-based approaches warrant further investigation. DBS, deep brain stimulation; HA, headache; SAH, subarachnoid hemorrhage; SUD, substance use disorders; TBI, traumatic brain injury; TMS, transcranial magnetic stimulation. © Pacific Neuroscience Institute Foundation, 2022. Used with permission.
MOOD DISORDERS AND ADDICTION
As shown in Table 2, potential targets for PAT are the depression, anxiety, and SUD that are common, under-reported, and often undertreated in patients with brain tumors, stroke, SAH, TBI, spinal cord injury, chronic spinal disability, other pain syndromes, and Parkinson disease.3,4,76-104 For example, psilocybin-assisted therapy may help some of these patients gain greater acceptance of their disability, reduce apathy, and promote living more fully.82,83
TABLE 2.
Prevalence Rates of Depression, Anxiety, and SUD in Select Neurosurgical Diagnoses
| Diagnosis | Depression (range) | Anxiety (range) | SUDa (range) |
|---|---|---|---|
| Brain tumor | 3%-41% | 5%-48% | NA |
| Stroke | 20%-60% | 8%-29% | 8%-56% |
| Aneurysmal subarachnoid hemorrhage | 0%-62% | 40%-59% | 19%-33% |
| Traumatic brain injury | 17%-61% | 18%-60% | 5%-28% |
| Spinal cord injury | 19%-26% | 15%-32% | 17%-68% |
| Chronic lower back pain/failed back syndrome | 20%-64% | 15%-28% | 12%-36% |
| Trigeminal neuralgia/other pain syndromes | 15%-65% | 19%-50% | 8%-12% |
| Parkinson disease | 2%-57% | 25%-52% | NA |
Regarding patients with brain tumor, despite having high rates of anxiety and depression, all PAT clinical trials have excluded such patients given concern that psychedelics may decrease seizure threshold.78,103,104 However, there are no reports of seizures in recent clinical trials.7-9,11 Notably, tricyclic antidepressants, selective serotonin reuptake inhibitors, and monoamine oxidase inhibitors all lower seizure threshold and have other side effects including cognitive impairment, sleep disturbances, and sexual dysfunction.105,106 Interestingly, fMRI data show psilocybin induces decoupling of the medial temporal lobe to higher cortical areas and reduces interhemispheric communication, which may lower seizure risk.13 Given the safety and efficacy thus far for psilocybin-assisted therapy, it is possible that many patients with brain tumor with depression, existential distress, fear of dying, and/or suicidal ideation could benefit.9,11,68,78,107 Particularly in patients with malignant gliomas or metastatic brain tumors with a curtailed life expectancy, the relative immediacy of psilocybin on mood may be the greatest argument for its use. However, neural network integrity or lack thereof related to brain tumor location, surgical approach, and adjuvant therapies, may in part determine PAT efficacy and safety.108,109
Use of PAT for neurosurgical patients with SUD should also be considered.46,79,110,111 For example, in spine surgery candidates, given that smoking is associated with lower fusion rates, and psilocybin-assisted therapy has shown promise in early trials for nicotine and opioid addiction, PAT could be considered preoperatively to improve fusion rates and to reduce opioid dependence postsurgery which may occur in up to 1/3 of such patients.6,45,100,112,113
RUMINATIVE DISORDERS, SUD, AND PTSD BEING TARGETED WITH NEUROMODULATION
DBS and TMS have both shown variable efficacy or been considered for treating OCD, anorexia, SUD, depression, and PTSD.72-74,114-119 DBS which stereotactically targets focal nuclei and TMS which focally targets broader brain regions contrasts with the global approach of ingesting a 5-HT2A-R agonist to more broadly alter neural networks. As psilocybin-assisted therapy has shown early efficacy for depression and addiction and is in Phase 2 trials for OCD and anorexia, the relative roles of PAT vs DBS and/or TMS warrant investigation. Such studies might show one approach outperforms another for certain subgroups in efficacy, safety, and cost, or PAT combined with neuromodulation may prove to be synergistic.5,7,9,11,45,46,74
CHRONIC PAIN
LSD was shown to be effective for patients with severe cancer-related pain in the 1960s. Recently, both psilocybin and LSD have shown efficacy for chronic pain, cluster headache, and phantom-limb pain in pilot trials, and a new trial is opening for chronic low back pain.21,24,120-122 Classic psychedelics may not only favorably alter the perception and emotional overlay of pain but may potentiate downstream control through 5-HT2A receptors involved in antinociceptive actions of the ventromedial medulla, thereby augmenting descending spinal cord inhibitory pathways.120 Investigation of psychedelics for managing chronic spinal pain, failed back syndrome, cancer-related pain, trigeminal neuralgia, migraine, and cluster headache seems warranted.21,81,120 A potential benefit of psychedelic-based therapies for chronic pain may be to help resolve coexisting opioid and/or alcohol addiction.100,102,110,123
BRAIN INJURY, STROKE, AND NEURODEGENERATIVE DISEASE
As psychedelics can potentiate neuroplasticity, their possible role in treating TBI, stroke, aneurysmal SAH, dementia, and Parkinson disease warrants exploration.22,30,34,70,124,125 One approach may be to combine psychedelic therapies with TMS or stem cell therapy, both of which have shown limited success in clinical studies for these disorders.75,126,127
PSYCHEDELICS AND CONNECTOMICS
Another potential interface of psychedelics with neurosurgery and neuro-oncology is in gaining a clearer understanding of neural networks related to cognition and emotion in both healthy and neuropathological conditions. The Human Connectome Project has helped spawn a highly detailed yet evolving perspective on functional connectivity networks, which are foundational in models of depression, anxiety, cognitive deficits, and mechanisms of action for psychedelics.12,59,60,109,128-130 Neurosurgery has been at the forefront of brain mapping and increasingly using more sophisticated Human Connectome Project–generated maps in preoperative planning and postoperative assessments for patients with intra-axial brain tumors.108,109,131 The potential synergy of psychedelics as neuroplastogens, together with, neuromodulation techniques such as TMS and DBS, monitored by brain connectomics data, provides an opportunity to reimagine multimodal care for patients with brain tumors, brain injury, and neurodegenerative disorders.132
MULTIDISCIPLINARY PSYCHEDELIC RESEARCH
Leading psychedelic research centers have formed over the last 2 decades at Johns Hopkins University, New York University, Imperial College London, and the University of Zurich with many others recently created. In the past several years, for-profit startups have helped fuel psychedelic research. Until recently, there has been limited interest and support of psychedelic research at National Institutes of Health, but this is changing as evidenced by the 2022 National Institute on Drug Abuse and National Institute of Mental Health-sponsored “Workshop on Psychedelics as Therapeutics: Gaps, Challenges and Opportunities.”133 Going forward, broader multidisciplinary collaboration will help explore novel applications of psychedelic-based therapies. For example, our Pacific Neuroscience Institute Treatment & Research In Psychedelics program formed in 2019 is led by an addiction medicine specialist (K.H.) in collaboration with a psycho-oncologist (S.G.), neuro-oncologist (A.S.), and neurosurgeon (D.F.K.) and staffed by psychedelic guides (K.S.). We offer KAT and have completed enrollment for 2 psilocybin-assisted therapy clinical trials in collaboration with Usona Institute, including a pilot trial for AUD and a Phase 2 multicenter trial for major depression.
CONCLUSION
The fields of neurosurgery and neuro-oncology have contributed immensely to the betterment of patients with a wide spectrum of challenging neuropathology. The nascent field of psychedelic science offers potential new avenues for advancing and reshaping our approach to behavioral health care and to understanding and harnessing neuroplasticity. This review attempts to demonstrate convergent opportunities for psychedelic-based therapies within neurosurgery and neuro-oncology. While additional and larger clinical trials are clearly needed to better determine the potential risks and benefits of PAT across different patient populations, we encourage the neurosurgical and neuro-oncology communities to engage in this rapidly evolving field, thinking broadly and creatively as to how the safe and evidence-based use of psychedelics may affect patient care and research. Through such collaborative efforts in which psychedelics are viewed as both investigative and therapeutic molecules, we may enhance our patients' quality of life while gaining a better understanding of neural networks, neuroplasticity, the underpinnings of mental anguish, and the nature of mind itself.
Acknowledgments
We acknowledge Professor David E. Presti for his helpful critique and detailed suggestions on the manuscript. We also acknowledge collaborations from Carmen Kelly for creating Figure 3 and from Josh Emerson for creating Figure 4A and 4B.
Contributor Information
Keith Heinzerling, Email: keith.heinzerling@providence.org.
Akanksha Sharma, Email: akanksha.sharma@providence.org.
Shanthi Gowrinathan, Email: shanthi.gowrinathan@providence.org.
Karina Sergi, Email: karina.sergi@providence.org.
Regin Jay Mallari, Email: reginjay.mallari@providence.org.
Funding
This work has been in part supported by a gift from the Annenberg Foundation and support from Pacific Neuroscience Institute Foundation and Saint John's Health Center Foundation. Dr Kelly, Dr Heinzerling, and Dr Sergi receive clinical trial support from Usona Institute.
Disclosures
Dr Kelly has stock in MindMed, Numinus, and Noetic Fund. Dr Heinzerling is a consultant for MindMed. Dr Sergi is a consultant for Field Trip Health and has stock in Compass Pathways, Field Trip Health, and MindMed. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1.Grof S. LSD Psychotherapy, 2nd ed. Hunter House; 1994. [Google Scholar]
- 2.Mithoefer MC, Feduccia AA, Jerome L, et al. MDMA-assisted psychotherapy for treatment of PTSD: study design and rationale for phase 3 trials based on pooled analysis of six phase 2 randomized controlled trials. Psychopharmacology (Berl). 2019;236(9):2735-2745. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Andersen KAA, Carhart-Harris R, Nutt DJ, Erritzoe D. Therapeutic effects of classic serotonergic psychedelics: a systematic review of modern-era clinical studies. Acta Psychiatr Scand. 2021;143(2):101-118. [DOI] [PubMed] [Google Scholar]
- 4.Mithoefer MC, Grob CS, Brewerton TD. Novel psychopharmacological therapies for psychiatric disorders: psilocybin and MDMA. Lancet Psychiatry. 2016;3(5):481-488. [DOI] [PubMed] [Google Scholar]
- 5.Nutt D, Erritzoe D, Carhart-Harris R. Psychedelic psychiatry's brave new world. Cell. 2020;181(1):24-28. [DOI] [PubMed] [Google Scholar]
- 6.Bogenschutz MP, Johnson MW. Classic hallucinogens in the treatment of addictions. Prog Neuro-Psychopharmacol Biol Psychiatry. 2016;64:250-258. [DOI] [PubMed] [Google Scholar]
- 7.Carhart-Harris R, Giribaldi B, Watts R, et al. Trial of psilocybin versus escitalopram for depression. N Engl J Med. 2021;384(15):1402-1411. [DOI] [PubMed] [Google Scholar]
- 8.Davis AK, Barrett FS, May DG, et al. Effects of psilocybin-assisted therapy on major depressive disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78(5):481-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol (Oxf, Engl). 2016;30(12):1181-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell JM, Bogenschutz M, Lilienstein A, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27(6):1025-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross S, Bossis A, Guss J, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol (Oxf, Engl). 2016;30(12):1165-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett FS, Krimmel SR, Griffiths RR, Seminowicz DA, Mathur BN. Psilocybin acutely alters the functional connectivity of the claustrum with brain networks that support perception, memory, and attention. Neuroimage. 2020;218:116980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lebedev AV, Lövdén M, Rosenthal G, Feilding A, Nutt DJ, Carhart-Harris RL. Finding the self by losing the self: neural correlates of ego-dissolution under psilocybin. Hum Brain Mapp. 2015;36(8):3137-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaelen M, Roseman L, Kahan J, et al. LSD modulates music-induced imagery via changes in parahippocampal connectivity. Eur Neuropsychopharmacol. 2016;26(7):1099-1109. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths RR, Johnson MW, Richards WA, et al. Psilocybin-occasioned mystical-type experience in combination with meditation and other spiritual practices produces enduring positive changes in psychological functioning and in trait measures of prosocial attitudes and behaviors. J Psychopharmacol (Oxf, Engl). 2018;32(1):49-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preller KH, Duerler P, Burt JB, et al. Psilocybin induces time-dependent changes in global functional connectivity. Biol Psychiatry. 2020;88(2):197-207. [DOI] [PubMed] [Google Scholar]
- 17.Carhart-Harris RL, Muthukumaraswamy S, Roseman L, et al. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci U S A. 2016;113(17):4853-4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petri G, Expert P, Turkheimer F, et al. Homological scaffolds of brain functional networks. J R Soc Interf. 2014;11(101):20140873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smigielski L, Scheidegger M, Kometer M, Vollenweider FX. Psilocybin-assisted mindfulness training modulates self-consciousness and brain default mode network connectivity with lasting effects. Neuroimage. 2019;196:207-215. [DOI] [PubMed] [Google Scholar]
- 20.de Vos CMH, Mason NL, Kuypers KPC. Psychedelics and neuroplasticity: a systematic review unraveling the biological underpinnings of psychedelics. Front Psychiatry. 2021;12:724606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castellanos JP, Woolley C, Bruno KA, Zeidan F, Halberstadt A, Furnish T. Chronic pain and psychedelics: a review and proposed mechanism of action. Reg Anesth Pain Med. 2020;45(7):486-494. [DOI] [PubMed] [Google Scholar]
- 22.Saeger HN, Olson DE. Psychedelic-inspired approaches for treating neurodegenerative disorders. J Neurochem. 2022;162(1):109-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vollenweider FX, Kometer M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci. 2010;11(9):642-651. [DOI] [PubMed] [Google Scholar]
- 24.Elman I, Pustilnik A, Borsook D. Beating pain with psychedelics: matter over mind? Neurosci Biobehav Rev. 2022;134:104482. [DOI] [PubMed] [Google Scholar]
- 25.Lukasiewicz K, Baker JJ, Zuo Y, Lu J. Serotonergic psychedelics in neural plasticity. Front Mol Neurosci. 2021;14:748359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raval NR, Johansen A, Donovan LL, et al. A Single dose of psilocybin increases synaptic density and decreases 5-HT(2A) receptor density in the pig brain. Int J Mol Sci. 2021;22(2):835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly JR, Gillan CM, Prenderville J, et al. Psychedelic therapy's transdiagnostic effects: a research domain criteria (RDoC) perspective. Front Psychiatry. 2021;12:800072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doblin RE, Christiansen M, Jerome L, Burge B. The past and future of psychedelic science: an introduction to this issue. J Psychoactive Drugs. 2019;51(2):93-97. [DOI] [PubMed] [Google Scholar]
- 29.Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410. [DOI] [PubMed] [Google Scholar]
- 30.Inserra A, De Gregorio D, Gobbi G. Psychedelics in psychiatry: neuroplastic, immunomodulatory, and neurotransmitter mechanisms. Pharmacol Rev. 2021;73(1):202-277. [DOI] [PubMed] [Google Scholar]
- 31.Byock I. Taking psychedelics seriously. J Palliat Med. 2018;21(4):417-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bravo G, Grant R, Bennett R. Ketamine. In: Grob CS, Grigsby J, eds. Handbook of medical hallucinogens. Guilford Press; 2021:327-344. [Google Scholar]
- 33.Nichols DE, Nichols CD. The pharmacology of psychedelics. In: Grob CS, Grigsby J, eds. Handbook of Medical Hallucinogens. Guilford Press; 2021:3-28. [Google Scholar]
- 34.Feduccia AA, Mithoefer MC. MDMA-assisted psychotherapy for PTSD: are memory reconsolidation and fear extinction underlying mechanisms? Prog Neuro-psychopharmacol Biol Psychiatry. 2018;84(Pt A):221-228. [DOI] [PubMed] [Google Scholar]
- 35.Dore J, Turnipseed B, Dwyer S, et al. Ketamine assisted psychotherapy (KAP): patient demographics, clinical data and outcomes in three large practices administering ketamine with psychotherapy. J Psychoactive Drugs. 2019;51(2):189-198. [DOI] [PubMed] [Google Scholar]
- 36.Winkelman MJ. Anthroplogy, shamanism, and hallucinogens. In: Grob CS, Grigsby J, eds. Handbook of Medical Hallucinogens. Guilford Press; 2021:46-67. [Google Scholar]
- 37.Rodríguez Arce JM, Winkelman MJ. Psychedelics, sociality, and human evolution. Front Psychol. 2021;12:729425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panik K, Presti D. LSD. In: Grob CS, Grigsby J, eds. Handbook of Medical Hallucinogens. Guilford Press; 2021:159-180. [Google Scholar]
- 39.Hofmann A. LSD My Problem Child: Reflections on Sacred Drugs, Mysticism and Science. MAPS; 1979. [Google Scholar]
- 40.Benzenhöfer U, Passie T. Rediscovering MDMA (ecstasy): the role of the American chemist Alexander T. Shulgin. Addiction (Abingdon, Engl). 2010;105(8):1355-1361. [DOI] [PubMed] [Google Scholar]
- 41.Aday JS, Bloesch EK, Davoli CC. Beyond LSD: a broader psychedelic Zeitgeist during the early to mid-20(th) century. J Psychoactive Drugs. 2019;51(3):210-217. [DOI] [PubMed] [Google Scholar]
- 42.Stevens J. Storming Heaven: LSD and the American Dream. Grove Press; 1987. [Google Scholar]
- 43.Holland J, ed. Ecstasy: The Complete Guide: A Comprehensive Look at the Risks and Benefits of MDMA. Park Street Press; 2001. [Google Scholar]
- 44.Carhart-Harris RL, Goodwin GM. The therapeutic potential of psychedelic drugs: past, present, and future. Neuropsychopharmacology. 2017;42(11):2105-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse. 2017;43(1):55-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PC, Strassman RJ. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol (Oxf, Engl). 2015;29(3):289-299. [DOI] [PubMed] [Google Scholar]
- 47.Bogenschutz MP, Ross S, Bhatt S, et al. Percentage of heavy drinking days following psilocybin-assisted psychotherapy vs placebo in the treatment of adult patients with alcohol use disorder: a randomized clinical trial. JAMA Psychiatry. 2022;79(10):953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dolder PC, Schmid Y, Müller F, Borgwardt S, Liechti ME. LSD acutely impairs fear recognition and enhances emotional empathy and sociality. Neuropsychopharmacology. 2016;41(11):2638-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holze F, Ley L, Müller F, et al. Direct comparison of the acute effects of lysergic acid diethylamide and psilocybin in a double-blind placebo-controlled study in healthy subjects. Neuropsychopharmacology. 2022;47(6):1180-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson M, Richards W, Griffiths R. Human hallucinogen research: guidelines for safety. J Psychopharmacol (Oxf, Engl). 2008;22(6):603-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winkelman MJ. The evolved psychology of psychedelic set and setting: inferences regarding the roles of shamanism and entheogenic ecopsychology. Front Pharmacol. 2021;12:619890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barrett FS, Robbins H, Smooke D, Brown JL, Griffiths RR. Qualitative and quantitative features of music reported to support peak mystical experiences during psychedelic therapy sessions. Front Psychol. 2017;8:1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Majić T, Schmidt TT, Gallinat J. Peak experiences and the afterglow phenomenon: when and how do therapeutic effects of hallucinogens depend on psychedelic experiences? J Psychopharmacol (Oxf, Engl). 2015;29(3):241-253. [DOI] [PubMed] [Google Scholar]
- 54.Phelps J, Henry J. Foundations for training psychedelic therapists. Curr Top Behav Neurosci. 2022;56:93-109. [DOI] [PubMed] [Google Scholar]
- 55.Barrett FS, Johnson MW, Griffiths RR. Validation of the revised Mystical Experience Questionnaire in experimental sessions with psilocybin. J Psychopharmacol (Oxf, Engl). 2015;29(11):1182-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malcolm B, Thomas K. Serotonin toxicity of serotonergic psychedelics. Psychopharmacology (Berl). 2022; 239(6):1881-1891. [DOI] [PubMed] [Google Scholar]
- 57.Anderson BT, Danforth AL, Grob CS. Psychedelic medicine: safety and ethical concerns. Lancet Psychiatry. 2020;7(10):829-830. [DOI] [PubMed] [Google Scholar]
- 58.Nutt DJ, King LA, Phillips LD. Drug harms in the UK: a multicriteria decision analysis. Lancet. 2010;376(9752):1558-1565. [DOI] [PubMed] [Google Scholar]
- 59.Doss MK, Madden MB, Gaddis A, et al. Models of psychedelic drug action: modulation of cortical-subcortical circuits. Brain. 2021;145(2):441-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carhart-Harris RL, Friston KJ. REBUS and the anarchic brain: toward a unified model of the brain action of psychedelics. Pharmacol Rev. 2019;71(3):316-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vollenweider FX, Preller KH. Psychedelic drugs: neurobiology and potential for treatment of psychiatric disorders. Nat Rev Neurosci. 2020;21(11):611-624. [DOI] [PubMed] [Google Scholar]
- 62.Tagliazucchi E, Roseman L, Kaelen M, et al. Increased global functional connectivity correlates with LSD-induced ego dissolution. Curr Biol. 2016;26(8):1043-1050. [DOI] [PubMed] [Google Scholar]
- 63.Preller KH, Razi A, Zeidman P, Stämpfli P, Friston KJ, Vollenweider FX. Effective connectivity changes in LSD-induced altered states of consciousness in humans. Proc Natl Acad Sci U S A. 2019;116(7):2743-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Preller KH, Burt JB, Ji JL, et al. Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. Elife. 2018;7:e35082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roseman L, Demetriou L, Wall MB, Nutt DJ, Carhart-Harris RL. Increased amygdala responses to emotional faces after psilocybin for treatment-resistant depression. Neuropharmacology. 2018;142:263-269. [DOI] [PubMed] [Google Scholar]
- 66.Holze F, Vizeli P, Müller F, et al. Distinct acute effects of LSD, MDMA, and D-amphetamine in healthy subjects. Neuropsychopharmacology. 2020;45(3):462-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McMillan R, Muthukumaraswamy SD. The neurophysiology of ketamine: an integrative review. Rev Neurosci. 2020;31(5):457-503. [DOI] [PubMed] [Google Scholar]
- 68.Ross S, Agin-Liebes G, Lo S, et al. Acute and sustained reductions in loss of meaning and suicidal ideation following psilocybin-assisted psychotherapy for psychiatric and existential distress in life-threatening cancer. ACS Pharmacol Transl Sci. 2021;4(2):553-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gukasyan N, Davis AK, Barrett FS, et al. Efficacy and safety of psilocybin-assisted treatment for major depressive disorder: prospective 12-month follow-up. J Psychopharmacol (Oxf, Engl). 2022;36(2):151-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aleksandrova LR, Phillips AG. Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol Sci. 2021;42(11):929-942. [DOI] [PubMed] [Google Scholar]
- 71.Ly C, Greb AC, Cameron LP, et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 2018;23(11):3170-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Antonelli M, Fattore L, Sestito L, Di Giuda D, Diana M, Addolorato G. Transcranial magnetic stimulation: a review about its efficacy in the treatment of alcohol, tobacco and cocaine addiction. Addict Behav. 2021;114:106760. [DOI] [PubMed] [Google Scholar]
- 73.Sonmez AI, Camsari DD, Nandakumar AL, et al. Accelerated TMS for depression: a systematic review and meta-analysis. Psychiatry Res. 2019;273:770-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee DJ, Lozano CS, Dallapiazza RF, Lozano AM. Current and future directions of deep brain stimulation for neurological and psychiatric disorders. J Neurosurg. 2019;131(2):333-342. [DOI] [PubMed] [Google Scholar]
- 75.Willing AE, Das M, Howell M, Mohapatra SS, Mohapatra S. Potential of mesenchymal stem cells alone, or in combination, to treat traumatic brain injury. CNS Neurosci Ther. 2020;26(6):616-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fakhoury M, Shakkour Z, Kobeissy F, Lawand N. Depression following traumatic brain injury: a comprehensive overview. Rev Neurosci. 2021;32(3):289-303. [DOI] [PubMed] [Google Scholar]
- 77.Medeiros GC, Roy D, Kontos N, Beach SR. Post-stroke depression: a 2020 updated review. Gen Hosp Psychiatry. 2020;66:70-80. [DOI] [PubMed] [Google Scholar]
- 78.Loughan AR, Lanoye A, Aslanzadeh FJ, et al. Fear of cancer recurrence and death anxiety: unaddressed concerns for adult neuro-oncology patients. J Clin Psychol Med Settings. 2021;28(1):16-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mahta A, Anderson MN, Azher AI, et al. Short- and long-term opioid use in survivors of subarachnoid hemorrhage. Clin Neurol Neurosurg. 2021;207:106770. [DOI] [PubMed] [Google Scholar]
- 80.Chang B, Zhu W, Li S. Effects of depression and anxiety on microvascular decompression outcome for trigeminal neuralgia patients. World Neurosurg. 2019;128:e556-e561. [DOI] [PubMed] [Google Scholar]
- 81.Melek LN, Devine M, Renton T. The psychosocial impact of orofacial pain in trigeminal neuralgia patients: a systematic review. Int J Oral Maxillofac Surg. 2018;47(7):869-878. [DOI] [PubMed] [Google Scholar]
- 82.Tang WK, Wang L, Tsoi KKF, Yasuno F, Kim JS. Apathy after subarachnoid haemorrhage: a systematic review. J Psychosomatic Res. 2022;155:110742. [DOI] [PubMed] [Google Scholar]
- 83.Arnould A, Rochat L, Azouvi P, Van der Linden M. A multidimensional approach to apathy after traumatic brain injury. Neuropsychol Rev. 2013;23(3):210-233. [DOI] [PubMed] [Google Scholar]
- 84.Williams R, Murray A. Prevalence of depression after spinal cord injury: a meta-analysis. Arch Phys Med Rehabil. 2015;96(1):133-140. [DOI] [PubMed] [Google Scholar]
- 85.Orhurhu V, Urits I, Olusunmade M, et al. Trends of co-morbid depression in hospitalized patients with failed back surgery syndrome: an analysis of the nationwide inpatient sample. Pain Ther. 2018;7(2):217-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wellisch DK, Kaleita TA, Freeman D, Cloughesy T, Goldman J. Predicting major depression in brain tumor patients. Psychooncology. 2002;11(3):230-238. [DOI] [PubMed] [Google Scholar]
- 87.Schrag A, Taddei RN. Depression and anxiety in Parkinson's disease. Int Rev Neurobiol. 2017;133:623-655. [DOI] [PubMed] [Google Scholar]
- 88.Pranckeviciene A, Bunevicius A. Depression screening in patients with brain tumors: a review. CNS Oncol. 2015;4(2):71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Appleby BS, Appleby KK, Rabins PV. Predictors of depression and anxiety in patients with intracranial neoplasms. J Neuropsychiatry Clin Neurosci. 2008;20(4):447-449. [DOI] [PubMed] [Google Scholar]
- 90.Burton CAC, Murray J, Holmes J, Astin F, Greenwood D, Knapp P. Frequency of anxiety after stroke: a systematic review and meta-analysis of observational studies. Int J Stroke. 2013;8(7):545-559. [DOI] [PubMed] [Google Scholar]
- 91.Rabat Y, Sibon I, Berthoz S. Implication of problematic substance use in poststroke depression: an hospital-based study. Sci Rep. 2021;11(1):13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tang WK, Wang L, Kwok Chu Wong G, et al. Depression after subarachnoid hemorrhage: a systematic review. J Stroke. 2020;22(1):11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.von Vogelsang AC, Forsberg C, Svensson M, Wengström Y. Patients experience high levels of anxiety 2 years following aneurysmal subarachnoid hemorrhage. World Neurosurg. 2015;83(6):1090-1097. [DOI] [PubMed] [Google Scholar]
- 94.Azouvi P, Arnould A, Dromer E, Vallat-Azouvi C. Neuropsychology of traumatic brain injury: an expert overview. Revue Neurol. 2017;173(7-8):461-472. [DOI] [PubMed] [Google Scholar]
- 95.VanderVeen JD. TBI as a risk factor for substance use behaviors: a meta-analysis. Arch Phys Med Rehabil. 2021;102(6):1198-1209. [DOI] [PubMed] [Google Scholar]
- 96.Le J, Dorstyn D. Anxiety prevalence following spinal cord injury: a meta-analysis. Spinal Cord. 2016;54(8):570-578. [DOI] [PubMed] [Google Scholar]
- 97.Graupensperger S, Corey JJ, Turrisi RJ, Evans MB. Individuals with spinal cord injury have greater odds of substance use disorders than non-sci comparisons. Drug Alcohol Depend. 2019;205:107608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Polatin PB, Kinnedy RK, Gatchel RJ, Lillo E, Mayer TG. Psychiatric illness and chronic low-back pain. Spine. 1993;18(1):66-71. [DOI] [PubMed] [Google Scholar]
- 99.Strøm J, Bjerrum MB, Nielsen CV, et al. Anxiety and depression in spine surgery-a systematic integrative review. Spine J. 2018;18(7):1272-1285. [DOI] [PubMed] [Google Scholar]
- 100.Vraa ML, Myers CA, Young JL, Rhon DI. More than 1 in 3 patients with chronic low back pain continue to use opioids long-term after spinal fusion: a systematic review. Clin J Pain. 2022;38(3):222-230. [DOI] [PubMed] [Google Scholar]
- 101.Cheng J, Long J, Hui X, Lei D, Zhang H. Effects of microvascular decompression on depression and anxiety in trigeminal neuralgia: a prospective cohort study focused on risk factors and prognosis. Clin Neurol Neurosurg. 2017;161:59-64. [DOI] [PubMed] [Google Scholar]
- 102.Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156(4):569-576. [DOI] [PubMed] [Google Scholar]
- 103.Mainio A, Hakko H, Niemelä A, Koivukangas J, Räsänen P. Depression in relation to anxiety, obsessionality and phobia among neurosurgical patients with a primary brain tumor: a 1-year follow-up study. Clin Neurol Neurosurg. 2011;113(8):649-653. [DOI] [PubMed] [Google Scholar]
- 104.Mainio A, Tuunanen S, Hakko H, Niemelä A, Koivukangas J, Räsänen P. Decreased quality of life and depression as predictors for shorter survival among patients with low-grade gliomas: a follow-up from 1990 to 2003. Eur Arch Psychiatry Clin Neurosci. 2006;256(8):516-521. [DOI] [PubMed] [Google Scholar]
- 105.Hill T, Coupland C, Morriss R, Arthur A, Moore M, Hippisley-Cox J. Antidepressant use and risk of epilepsy and seizures in people aged 20 to 64 years: cohort study using a primary care database. BMC Psychiatry. 2015;15(1):315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu CS, Liu HY, Tsai HJ, Liu SK. Seizure Risk associated with antidepressant treatment among patients with depressive disorders: a population-based case-crossover study. J Clin Psychiatry. 2017;78(9):e1226-e1232. [DOI] [PubMed] [Google Scholar]
- 107.Vargas AS, Luís Â, Barroso M, Gallardo E, Pereira L. Psilocybin as a new approach to treat depression and anxiety in the context of life-threatening diseases-a systematic review and meta-analysis of clinical trials. Biomedicines. 2020;8(9):331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dadario NB, Brahimaj B, Yeung J, Sughrue ME. Reducing the cognitive footprint of brain tumor surgery. Front Neurol. 2021;12:711646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.De Witte NAJ, Mueller SC. White matter integrity in brain networks relevant to anxiety and depression: evidence from the human connectome project dataset. Brain Imaging Behav. 2017;11(6):1604-1615. [DOI] [PubMed] [Google Scholar]
- 110.LaRowe LR, Powers JM, Garey L, Rogers AH, Zvolensky MJ, Ditre JW. Pain-related anxiety, sex, and co-use of alcohol and prescription opioids among adults with chronic low back pain. Drug Alcohol Depend. 2020;214:108171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jacotte-Simancas A, Fucich EA, Stielper ZF, Molina PE. Traumatic brain injury and the misuse of alcohol, opioids, and cannabis. Int Rev Neurobiol. 2021;157:195-243. [DOI] [PubMed] [Google Scholar]
- 112.Carlson BB, Burton DC, Jackson RS, Robinson S. Recidivism rates after smoking cessation before spinal fusion. Orthopedics. 2016;39(2):e318-e322. [DOI] [PubMed] [Google Scholar]
- 113.Berman D, Oren JH, Bendo J, Spivak J. The effect of smoking on spinal fusion. Int J Spine Surg. 2017;11(4):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sheth SA, Bijanki KR, Metzger B, et al. Deep brain stimulation for depression informed by intracranial recordings. Biol Psychiatry. 2022;92(3):246-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Graat I, Figee M, Denys D. The application of deep brain stimulation in the treatment of psychiatric disorders. Int Rev Psychiatry. 2017;29(2):178-190. [DOI] [PubMed] [Google Scholar]
- 116.Navarro PA, Paranhos T, Lovo E, et al. Safety and feasibility of nucleus accumbens surgery for drug addiction: a systematic review. Neuromodulation. 2022;25(2):171-184. [DOI] [PubMed] [Google Scholar]
- 117.Freire RC, Cabrera-Abreu C, Milev R. Neurostimulation in anxiety disorders, post-traumatic stress disorder, and obsessive-compulsive disorder. Adv Exp Med Biol. 2020;1191:331-346. [DOI] [PubMed] [Google Scholar]
- 118.Reznikov R, Hamani C. Posttraumatic stress disorder: perspectives for the use of deep brain stimulation. Neuromodulation. 2017;20(1):7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Deogaonkar M, Ranjan M, Ranjan N, Rezai A. Deep brain stimulation for refractory depression, obsessive-compulsive disorder and addiction. Neurol India. 2020;68(8):S282-s287. [DOI] [PubMed] [Google Scholar]
- 120.Whelan A, Johnson MI. Lysergic acid diethylamide and psilocybin for the management of patients with persistent pain: a potential role? Pain Manag. 2018;8(3):217-229. [DOI] [PubMed] [Google Scholar]
- 121.Schindler EAD, Gottschalk CH, Weil MJ, Shapiro RE, Wright DA, Sewell RA. Indoleamine hallucinogens in cluster headache: results of the clusterbusters medication use survey. J Psychoactive Drugs. 2015;47(5):372-381. [DOI] [PubMed] [Google Scholar]
- 122.Ramachandran V, Chunharas C, Marcus Z, Furnish T, Lin A. Relief from intractable phantom pain by combining psilocybin and mirror visual-feedback (MVF). Neurocase. 2018;24(2):105-110. [DOI] [PubMed] [Google Scholar]
- 123.Yang AI, McShane BJ, Hitti FL, Sandhu SK, Chen HI, Lee JYK. Patterns of opioid use in patients with trigeminal neuralgia undergoing neurosurgery. J Neurosurg. 2019;131(6):1805-1811. [DOI] [PubMed] [Google Scholar]
- 124.Khan SM, Carter GT, Aggarwal SK, Holland J. Psychedelics for brain injury: a mini-review. Front Neurol. 2021;12:685085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Flanagan TW, Nichols CD. Psychedelics as anti-inflammatory agents. Int Rev Psychiatry. 2018;30(4):363-375. [DOI] [PubMed] [Google Scholar]
- 126.Chung JW, Chang WH, Bang OY, et al. Efficacy and safety of intravenous mesenchymal stem cells for ischemic stroke. Neurology. 2021;96(7):e1012-e1023. [DOI] [PubMed] [Google Scholar]
- 127.Bonaventura G, Munafò A, Bellanca CM, et al. Stem cells: innovative therapeutic options for neurodegenerative diseases? Cells. 2021;10(8):1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Glasser MF, Smith SM, Marcus DS, et al. The Human Connectome Project's neuroimaging approach. Nat Neurosci. 2016;19(9):1175-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Elam JS, Glasser MF, Harms MP, et al. The Human Connectome Project: a retrospective. Neuroimage. 2021;244:118543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sha Z, Wager TD, Mechelli A, He Y. Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biol Psychiatry. 2019;85(5):379-388. [DOI] [PubMed] [Google Scholar]
- 131.Catalino MP, Yao S, Green DL, Laws ER, Golby AJ, Tie Y. Mapping cognitive and emotional networks in neurosurgical patients using resting-state functional magnetic resonance imaging. Neurosurg Focus. 2020;48(2):E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yeung JT, Young IM, Doyen S, Teo C, Sughrue ME. Changes in the brain connectome following repetitive transcranial magnetic stimulation for stroke rehabilitation. Cureus. 2021;13(10):e19105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Health NIoM. https://www.nimh.nih.gov/news/events/2022/psychedelics-as-therapeutics-gaps-challenges-and-opportunities. 2022.