Abstract
The mitochondrial heat shock protein Hsp70 (mtHsp70) is essential for driving translocation of preproteins into the matrix. Two models, trapping and pulling by mtHsp70, are discussed, but positive evidence for either model has not been found so far. We have analyzed a mutant mtHsp70, Ssc1-2, that shows a reduced interaction with the membrane anchor Tim44, but an enhanced trapping of preproteins. Unexpectedly, at a low inner membrane potential, ssc1-2 mitochondria imported loosely folded preproteins more efficiently than wild-type mitochondria. The import of a tightly folded preprotein, however, was not increased in ssc1-2 mitochondria. Thus, enhanced trapping by mtHsp70 stimulates the import of loosely folded preproteins and reduces the dependence on the import-driving activity of the membrane potential, directly demonstrating that trapping is one of the molecular mechanisms of mtHsp70 action.
Two energy sources are required for import of precursor proteins across the mitochondrial inner membrane into the matrix (19, 28, 30, 35). The electrical potential gradient (Δψ) across the inner membrane initiates translocation of the amino-terminal signal sequences (presequences) of the preproteins across the membrane. Then a molecular chaperone (5, 7, 15), the matrix heat shock protein 70 (mtHsp70), encoded in Saccharomyces cerevisiae by the essential gene SSC1, promotes further translocation by a direct and ATP-dependent interaction with the preprotein in transit (20, 36). It cooperates with two essential partner proteins: Tim44, a peripheral subunit of the inner membrane translocase (21, 34, 38), and Mge1, a nucleotide-exchanging cofactor (39, 45, 46). Studies with temperature-sensitive yeast SSC1 mutants showed that mtHsp70 is also required for the unfolding of the polypeptide chain during the translocation process (8, 20, 44, 48).
In order to come to a molecular understanding of the mechanism of preprotein translocation, it will be of central importance to understand how the two energy sources, Δψ and ATP-mtHsp70, are converted into import-driving forces for preproteins. It is undisputed that the membrane potential (negative on the matrix side) exerts an electrophoretic effect on the positively charged presequences (11, 16, 24, 41). Additionally, Δψ supports the dimerization of Tim23 of the inner membrane translocase and thus promotes its interaction with presequences (3). In contrast, the mode of action of mtHsp70 is controversial. Three major views are currently debated. (i) The Brownian ratchet or trapping model predicts that movement of the polypeptide chain is driven solely by Brownian motion. Binding of mtHsp70 to the polypeptide chain emerging on the matrix side would render protein translocation vectorial (2, 10, 27, 38, 41, 42). In the trapping model, unfolding of the preprotein prior to import is a passive reaction caused by spontaneous molecular breathing. (ii) According to the pulling or motor model, mtHsp70 plays a more active role (13, 17, 26, 31, 45, 48). While simultaneously interacting with Tim44 and the preprotein in transit, mtHsp70 might generate an inward-directed force on the preprotein by an ATP-dependent conformational change. Thereby, translocation of the preprotein and destabilization of preprotein domains on the cytosolic side are promoted. (iii) It has also been suggested that a combination of both mechanisms is required to explain the full activity of mtHsp70 in preprotein unfolding and translocation (31, 44, 48). Pulling should favor the unfolding of folded domains, while trapping is the major mechanism to promote translocation of unfolded polypeptide chains.
Two experimental approaches have been exploited previously to define the function of mtHsp70 in protein import. On the one hand, preprotein import rates were compared to preprotein unfolding rates in solution to address the question of whether unfolding is an active or passive process (10, 18, 22, 26). However, these studies eventually came to the conclusion that their results were compatible with either model of mtHsp70 action. On the other hand, studies analyzing mutant forms of mtHsp70 showed a different behavior concerning unfolding and trapping of preproteins, indicating that a single mechanism such as trapping only was not sufficient to explain all functions of mtHsp70 in protein import (8, 20, 47, 48). Moreover, a puzzling observation was that enhanced trapping of preproteins did not increase the efficiency of import, raising doubts if trapping could actually function as an import-driving mechanism in mitochondrial preprotein translocation (44).
Thus, none of the studies conducted so far have provided positive experimental evidence for either mechanism of mtHsp70 action. For this report we performed a systematic characterization of ssc1-2 mutant mitochondria. We asked if the alteration in mtHsp70 affected the membrane potential dependence of protein import and compared the interactions of the mutant mtHsp70 with its three partners during translocation, i.e., preprotein, Tim44, and Mge1. We unexpectedly found that at a low membrane potential, ssc1-2 mitochondria were more efficient in protein import than wild-type mitochondria. The enhanced trapping of preproteins by the mutant mtHsp70 stimulated preprotein import when Δψ was limiting. Trapping-stimulated import, however, was only possible with loosely folded preproteins, not with a preprotein carrying a tightly folded domain. These results provide direct evidence that trapping of preproteins is one of the molecular mechanisms by which mtHsp70 drives protein import.
MATERIALS AND METHODS
Import of preproteins into isolated mitochondria.
Mitochondria were isolated from S. cerevisiae cells of wild-type strain PK82 (MATα his4-713 lys2 ura3-52 Δtrp1 leu2-3,112) and ssc1-2 temperature-sensitive strain PK81 (MATα his4-713 lys2 ura3-52 Δtrp1 leu2-3,112 ssc1-2 [LEU2]) (8) grown on YPG (1% yeast extract, 2% Bacto-peptone, 3% glycerol) at the permissive temperature. For the synthesis of radiolabeled preproteins, in vitro translation in rabbit reticulocyte lysate (Amersham Pharmacia Biotech) in the presence of [35S]methionine/cysteine after in vitro transcription by SP6 polymerase (Stratagene) was performed.
For the in vitro import reactions, the isolated mitochondria were diluted in import buffer (1% [wt/vol] fatty acid-free bovine serum albumin [BSA], 250 mM sucrose, 80 mM KCl, 5 mM MgCl2, 10 mM MOPS[morpholinepropanesulfonic acid]-KOH, pH 7.2) (1, 40) to a final concentration of 25 to 50 μg of mitochondrial protein/ml. To induce the mutant phenotype, the import reactions were shifted to 37°C for 15 min. After heat shock, ATP (final concentration, 2 mM) and NADH (final concentration, 2 mM) were added. Where indicated, 10 μM heme was added to the import buffer and the reticulocyte lysate. When import was performed under conditions of a decreased Δψ, oligomycin (20 μM) and carbonyl cyanide m-chlorophenylhydrazone (CCCP) at the indicated concentrations (9, 24) were added. For the generation of membrane-spanning translocation intermediates, 5 μM methotrexate was added to the import reaction where indicated. After incubation for 5 min at 25°C, the import reactions were started by the addition of reticulocyte lysate containing radiolabeled preprotein and incubated at 25°C. The import reactions were stopped by the addition of 1 μM valinomycin and cooling on ice. To remove unimported preproteins, the import reactions were treated with proteinase K (final concentration, 40 μg/ml) for 15 min on ice. After inhibition of the protease by the addition of 1 mM phenylmethylsulfonyl fluoride (PMSF), the mitochondria were reisolated, and the samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Coimmunoprecipitation experiments.
Proteins interacting with Tim44 were isolated by immunoprecipitations using antibodies against Tim44 (48). Mitochondria were lysed in lysis buffer A (0.3% Triton X-100, 30 mM Tris-HCl, 5% glycerol, 1 mM PMSF, pH 7.4) containing the indicated concentrations of KCl. Additionally, the lysis buffer contained either 5 mM ATP and 7 mM MgCl2 or 5 mM EDTA. Mitochondrial lysates were incubated with affinity-purified antibodies covalently coupled to protein A-Sepharose and washed with lysis buffer. Bound proteins were eluted by incubation with 100 mM glycine, pH 2.5, and analyzed by SDS-PAGE and Western blotting. After import of radiolabeled preproteins, mitochondria were lysed in lysis buffer B (100 mM KCl, 10 mM Tris-HCl, 5 mM EDTA, 0.1% Triton, pH 7.5). ATP and MgCl2 were added in the absence of EDTA and the salt concentration was changed where indicated. The newly imported proteins bound to mtHsp70 were isolated by immunoprecipitation (20) and detected by SDS-PAGE and autoradiography.
Assessment of mitochondrial membrane potential.
To assess the membrane potential Δψ of isolated yeast mitochondria, the fluorescence quenching of 3,3′-dipropylthiadicarbocyanine iodide (DiSC3; Molecular Probes, Inc.) was measured as described (9). The measurements were performed using a Perkin-Elmer LS 50B luminescence spectrometer at 25°C, excitation at 622 nm, emission at 670 nm, and slit size of 5 nm. The measurements were carried out using a buffer containing 600 mM sorbitol, 1% (wt/vol) BSA, 10 mM MgCl2, 0.5 mM EDTA, 20 mM potassium phosphate, pH 7.4. While constantly recording the fluorescence, the following reagents were added successively to 3 ml of buffer: 3 μl of DiSC3 (in ethanol; 2 μM final concentration), 20 μl of mitochondria (in SEM buffer [250 mM sucrose, 1 mM EDTA, 10 mM MOPS-KOH, pH 7.2]; 33 μg of mitochondrial protein/ml final concentration), and finally 3 μl of valinomycin (in ethanol; 1 μM final concentration) to dissipate Δψ. For the assessment of a decreased membrane potential, 20 μM oligomycin and different concentrations of CCCP were added prior to the addition of mitochondria. Δψ can be relatively assessed by the difference in fluorescence before and after the addition of valinomycin.
RESULTS AND DISCUSSION
In the presence of uncoupler, protein import into ssc1-2 mitochondria is more efficient than into wild-type mitochondria.
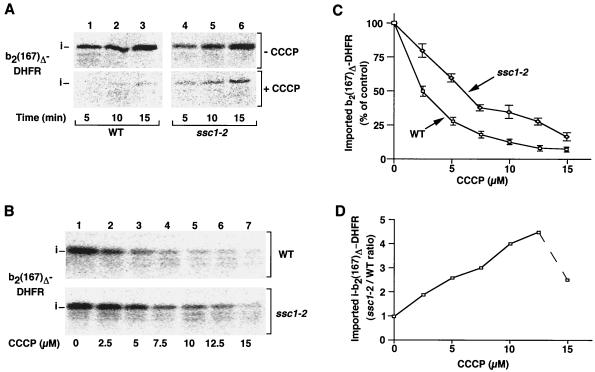
We asked if alterations of mtHsp70 activity affected the requirement for the second energy source of import, the inner membrane potential. To discriminate between the individual contributions of the two import-driving forces, Δψ and mtHsp70, we performed import experiments into wild-type and ssc1-2 mutant mitochondria under conditions of decreased membrane potential. ssc1-2 mutant yeast cells and the corresponding wild-type cells were grown at permissive conditions, and the mitochondria were isolated and incubated at the nonpermissive temperature of 37°C to induce the mutant phenotype. In this way indirect effects of the ssc1-2 mutation on biogenesis of mitochondria and cells were minimized. We used the model preprotein b2(167)Δ-DHFR, a fusion protein between an amino-terminal portion of cytochrome b2 and the entire mouse dihydrofolate reductase (DHFR) molecule (47), which depends strongly on Δψ for import (11). In b2(167)Δ-DHFR, a 19-residue segment of the intermembrane space-sorting sequence of cytochrome b2 has been deleted (Δ47–65), and thus the fusion protein is transported into the mitochondrial matrix and processed to the intermediate-sized form by the matrix-processing peptidase (47). The preprotein was synthesized in reticulocyte lysate in the presence of [35S]methionine/cysteine and efficiently imported into isolated energized wild-type and ssc1-2 mitochondria (44, 47, 48), as shown in the upper panel of Fig. 1A by processing to the intermediate form and protection against added proteinase K. In parallel, we decreased the membrane potential by incubating the isolated mitochondria with the protonophore CCCP, leading to a partial uncoupling of mitochondria. The ATP levels were kept high by addition of ATP (to promote full activity of the Hsp70 system), while oligomycin was included to inhibit the F0F1 ATPase and thereby prevent generation of a Δψ by a reverse action of the ATPase (4, 9, 11, 24). While the import of b2(167)Δ-DHFR into wild-type mitochondria was almost blocked by the CCCP treatment (Fig. 1A, lower panel, lanes 1 to 3), we surprisingly found that the mutant mitochondria still imported significant amounts of the preprotein (Fig. 1A, lower panel, lanes 4 to 6). However, this effect of the ssc1-2 mutation could not fully substitute for the import-driving activity of Δψ since a complete dissipation of Δψ before the import reaction by the potassium ionophore valinomycin (in the presence of potassium in the medium) entirely blocked protein import in the presence and absence of CCCP (32, 44, 48) (data not shown).
FIG. 1.
Protein import into ssc1-2 mitochondria shows a lower sensitivity to a reduction of the inner membrane potential. (A) Isolated ssc1-2 and wild-type (WT) mitochondria were subjected to a heat shock at 37°C and subsequently incubated with reticulocyte lysate containing 35S-labeled b2(167)Δ-DHFR in the absence or presence of 15 μM CCCP at 25°C. The import reactions were stopped after the indicated times and treated with proteinase K to remove nonimported preproteins. After reisolation of the mitochondria, the import reactions were analyzed by SDS-PAGE and digital autoradiography. (B and C) b2(167)Δ-DHFR was imported into isolated mitochondria in the presence of the indicated concentrations of CCCP at 25°C for 5 min. The amount of imported protein was quantified by digital autoradiography. The amount of protein imported in the absence of CCCP was set to 100% (control). Bars indicate the standard errors of the means (from six independent experiments). (D) Calculation of the ratio of protein import into ssc1-2 versus wild-type mitochondria as quantified in panel C.
For a more detailed comparison of the import into wild-type and ssc1-2 mitochondria under a decreased membrane potential, we performed import in the presence of increasing concentrations of CCCP to gradually lower the Δψ (Fig. 1B) (9, 11, 24). The addition of CCCP led to a rapid decrease in import of b2(167)Δ-DHFR into wild-type mitochondria (Fig. 1B, upper panel; Fig. 1C). In contrast, the effect of CCCP on the import of b2(167)Δ-DHFR into ssc1-2 mitochondria was significantly milder (Fig. 1B, lower panel). Quantification demonstrated that the preprotein import into ssc1-2 mitochondria exhibited a remarkably higher resistance to the uncoupler than that into wild-type mitochondria over a broad range of titrations (Fig. 1C). When we calculated the ratio of import into ssc1-2 versus wild-type mitochondria for each concentration of CCCP used, it became apparent that the relative import into ssc1-2 mitochondria compared to wild-type mitochondria increased strongly with a decreasing membrane potential (Fig. 1D). These results indicate that the lower the membrane potential becomes, the better is the relative import stimulation by Ssc1-2 until very high concentrations of CCCP reduce the Δψ so strongly that it is below a threshold value required for the initiation of preprotein translocation (24, 32).
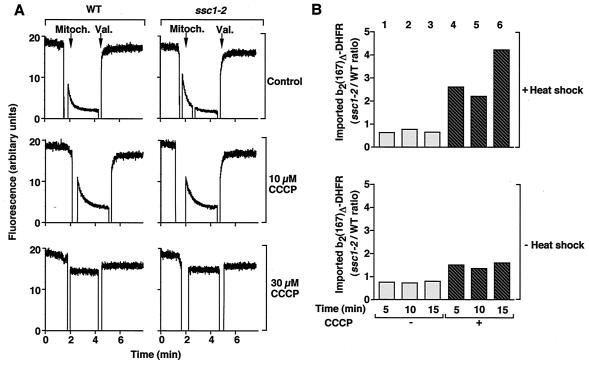
The possibility that the maintenance of a membrane potential in ssc1-2 mitochondria shows a lower sensitivity to CCCP than in wild-type mitochondria was of concern. We therefore assessed the membrane potential of ssc1-2 and wild-type mitochondria with the potential-sensitive dye DiSC3 (9, 11). The membrane potential generated by the isolated mitochondria is indicated by the difference in fluorescence before and after the addition of valinomycin (Fig. 2A). ssc1-2 and wild-type mitochondria not only exhibited a similar fluorescence quenching in the absence of uncoupler (Fig. 2A, upper panels) but also underwent a comparable decrease in fluorescence quenching after the addition of CCCP (Fig. 2A, middle and lower panels). This demonstrates that ssc1-2 mitochondria also maintain a membrane potential similar to that of wild-type mitochondria in the presence of CCCP.
FIG. 2.
Enhancement of protein import in ssc1-2 mitochondria depends on the induction of the mtHsp70 mutant phenotype. (A) Comparison of the sensitivity to CCCP between wild-type and ssc1-2 mitochondria (Mitoch.). The membrane potential of isolated mitochondria from wild-type (WT) and ssc1-2 mitochondria (preincubated at 37°C) was assessed at 25°C in the absence (control) or presence of different concentrations of CCCP using the potential-dependent fluorescent dye DiSC3. The difference in fluorescence before and after the addition of valinomycin (Val.) represents an assessment of the magnitude of Δψ. To inhibit the F0F1-ATPase, oligomycin (20 μM) was included in the buffer. (B) Import stimulation depends on the induction of the mutant phenotype. Isolated wild-type and ssc1-2 mitochondria were heat-shocked for 15 min at 37°C prior to the import reaction or left at 25°C as indicated. b2(167)Δ-DHFR was imported into the mitochondria in the absence or presence of 15 μM CCCP. The import reactions were subsequently treated as described in the legend to Fig. 1. The ratio of protein import into ssc1-2 and wild-type mitochondria was determined.
Does the import stimulation in ssc1-2 mitochondria depend specifically on the in vitro induction of the mutant phenotype by incubation of the isolated mitochondria at 37°C? We compared the import of b2(167)Δ-DHFR into ssc1-2 and wild-type mitochondria that were either heat-shocked prior to import at 37°C (Fig. 2B, upper panel) or kept at 25°C (Fig. 2B, lower panel). The ratio of preprotein import into ssc1-2 mitochondria and wild-type mitochondria is shown. In the absence of CCCP, the import ratio was close to 1 with or without heat shock (Fig. 2B, upper and lower panels, columns 1 to 3). In the presence of CCCP, however, a clear difference was apparent. Only when the mitochondria were heat-shocked prior to import was import into ssc1-2 mitochondria strongly enhanced compared to wild-type mitochondria (Fig. 2B, upper panel, columns 4 to 6), while without a heat shock, the import into ssc1-2 mitochondria was only slightly enhanced (Fig. 2B, lower panel, columns 4 to 6). The in vitro induction of the phenotype thus correlates well with the strong temperature sensitivity of the ssc1-2 phenotype in vivo (8, 20, 44). We conclude that the induction of the ssc1-2 mutant phenotype is required for the higher efficiency of protein import into ssc1-2 mitochondria compared to wild-type mitochondria at a decreased Δψ.
Differential interaction of Ssc1-2 with Tim44, Mge1, and substrate protein.
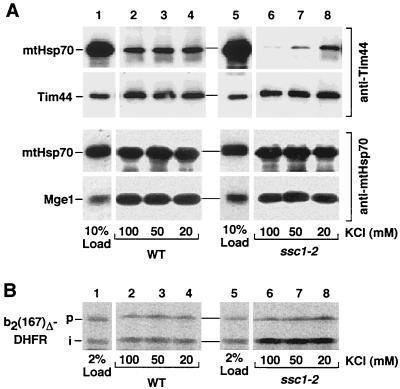
How can a mutation that leads to a temperature-sensitive lethal phenotype (8, 20) cause more efficient protein import than the wild-type situation? Since the import motor complex includes mtHsp70, preprotein, Tim44, and Mge1, we asked if and how the ssc1-2 mutation affected the interactions in the motor complex. The interaction of Ssc1-2 with one or two partners has been investigated in individual studies (8, 20, 23, 38, 44, 45, 46, 47, 48), but no systematic analysis of the interaction properties of Ssc1-2 with all three partners has been performed. Isolated ssc1-2 and wild-type mitochondria were incubated at 37°C and lysed by nonionic detergent. Tim44-mtHsp70 complexes were coprecipitated by affinity-purified Tim44 antibodies. Under standard conditions (≈100 mM salt), Ssc1-2 was not found in immunoprecipitates with Tim44 to a significant extent (Fig. 3A, upper panel, lane 6) in contrast to wild-type mtHsp70 (Fig. 3A, upper panel, lane 2) (38, 44, 45, 48). The defect in interaction of Ssc1-2 with Tim44 has been shown to be dependent on the induction of the phenotype by heat shock (38). The coprecipitation of Mge1 with mtHsp70 was similar for both wild-type mitochondria and ssc1-2 mitochondria (Fig. 3A, lower panel, lanes 2 and 6) (46).
FIG. 3.
Mutant mtHsp70 Ssc1-2 shows a differential interaction with the partner proteins Tim44, Mge1, and substrate proteins. (A) Interaction with Tim44 and Mge1. After incubation at 37°C, isolated wild-type (WT) and ssc1-2 mitochondria were lysed by Triton X-100 at the indicated concentrations of KCl and subjected to coimmunoprecipitation with antibodies directed against Tim44 (upper panel) or mtHsp70 (lower panel) as described in Materials and Methods. Upon SDS-PAGE, precipitated proteins were detected by Western blot analysis using antibodies against mtHsp70, Tim44, and Mge1, and 10% of the amount of lysed mitochondria is shown as a control. (B) Interaction with preprotein. The interaction of mtHsp70 with the preprotein b2(167)Δ-DHFR after import into wild-type and ssc1-2 mitochondria was assayed by coimmunoprecipitation with anti-mtHsp70 and digital autoradiography as described in Materials and Methods, and 2% of the total amount of the imported preprotein is shown as a control. p and i, precursor and intermediate forms of b2(167)Δ-DHFR, respectively.
To analyze the interaction of Ssc1-2 with substrate proteins, we performed coimmunoprecipitation experiments with b2(167)Δ-DHFR. Radiolabeled b2(167)Δ-DHFR was imported into wild-type and ssc1-2 mitochondria with similar efficiency. From 2 to 5% of the imported and processed protein was precipitated together with mtHsp70 in wild-type mitochondria (Fig. 3B, lanes 1 to 4), representing a typical yield for coprecipitation of imported proteins with mtHsp70 (20, 42, 44, 48). The yield of coprecipitation of the intermediate form [i-b2(167)Δ-DHFR] with Ssc1-2 was increased to 10 to 15% (Fig. 3B, lanes 5 to 8) (23, 44). Thus, of the three partners of mtHsp70, only the interaction with preproteins is enhanced by the ssc1-2 mutation, suggesting that this characteristic of Ssc1-2 is the likely explanation for the improved import of preproteins at low Δψ, i.e., that the increased trapping of preproteins by Ssc1-2 stimulates protein import.
However, the observed lack of interaction of Ssc1-2 with Tim44 raises a conceptual problem. It was demonstrated that Tim44 is required for the efficient transfer of mtHsp70 to translocating preproteins (12). The current trapping model, also termed the hand-over-hand model, includes the interaction of mtHsp70 with Tim44 so that the chaperone is positioned directly at the exit of the inner membrane import channel. This will allow an efficient trapping of preprotein segments immediately after their passage through the channel (2, 10, 27, 39, 41). To explain the results presented here in view of the hand-over-hand model, one would have to assume that the interaction between Ssc1-2 and Tim44 is not completely blocked but only labilized. Ssc1-2 may still interact with Tim44 but not with full stability, and therefore may be released by the treatment of mitochondria with detergent and salt.
To address this possibility, we tested milder conditions for the lysis of mitochondria and eventually observed that at low salt, Ssc1-2 was indeed found in a complex with Tim44 (Fig. 3A, upper panel, lane 8). The low salt condition did not unspecifically increase binding of proteins to mtHsp70, since the binding of Mge1 and b2(167)Δ-DHFR to both wild-type and mutant mtHsp70 was unchanged (Fig. 3A, lower panel, and 3B, lanes 3, 4, 7, and 8), as was the case with the interaction of Tim44 with wild-type mtHsp70 (Fig. 3A, upper panel, lanes 3 and 4). Moreover, the addition of Mg-ATP induced the release of all three partners, Tim44, Mge1, and substrate, from wild-type mtHsp70 as well as Ssc1-2 independently of the salt concentration (not shown), confirming the specificity of interaction. We conclude that Ssc1-2 is able to interact with all three partner proteins of the translocation process, but with a different efficiency than wild-type mtHsp70: a higher yield for substrate, an unchanged behavior for Mge1, and a lower stability and yield for Tim44. Since Ssc1-2 indeed can interact with Tim44, our findings are compatible with the current trapping (hand-over-hand) model.
Enhanced trapping stimulates import of b2(167)Δ-DHFR into ssc1-2 mitochondria at low Δψ.
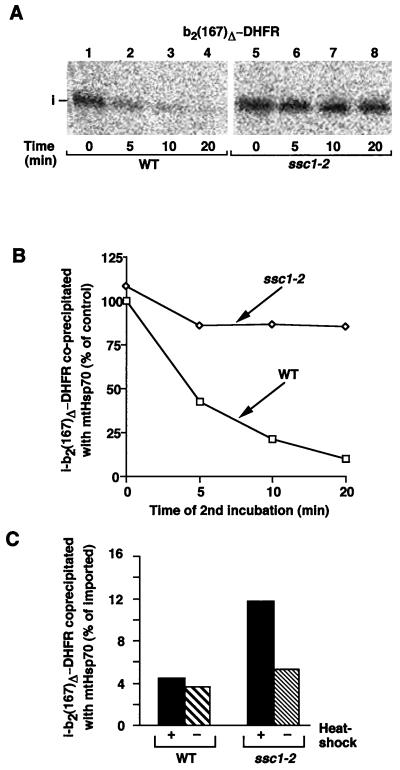
We asked if trapping of b2(167)Δ-DHFR by Ssc1-2 was correlated with the stimulation of protein import. To monitor the time course of interaction of mtHsp70 with the preprotein, we used a two-step approach. b2(167)Δ-DHFR was imported into energized ssc1-2 and wild-type mitochondria (after heat shock) for a short time, and then the membrane potential was completely dissipated by addition of valinomycin to prevent import of further preproteins. In the following chase incubation, the time dependence of interaction of b2(167)Δ-DHFR with mtHsp70 molecules was determined by lysis of mitochondrial aliquots with nonionic detergent and coprecipitation with anti-mtHsp70 (Fig. 4A). In wild-type mitochondria, the association of b2(167)Δ-DHFR with mtHsp70 molecules decreased rapidly (Fig. 4A, lanes 1 to 4; Fig. 4B), whereas in ssc1-2 mitochondria, b2(167)Δ-DHFR interacted efficiently with mutant mtHsp70 molecules for a longer time (Fig. 4A, lanes 5 to 8; Fig. 4B). This finding demonstrates a strongly prolonged interaction between b2(167)Δ-DHFR and Ssc1-2 molecules. In the typical time spans of import experiments (5 to 15 min), the net association of b2(167)Δ-DHFR with Ssc1-2 molecules is thus enhanced about three- to fivefold compared to wild-type mtHsp70.
FIG. 4.
Increased import of b2(167)Δ-DHFR into ssc1-2 mitochondria correlates with enhanced trapping by mtHsp70. (A and B) Imported b2(167)Δ-DHFR shows increased binding to the mutant mtHsp70 Ssc1-2. Radiolabeled b2(167)Δ-DHFR was imported into isolated mitochondria (after incubation at 37°C). The import was stopped after 5 min by addition of 1 μM valinomycin, and the mitochondria were further incubated at 25°C for the indicated times. The mitochondria were reisolated and lysed, and imported proteins bound to mtHsp70 were analyzed by coimmunoprecipitation, SDS-PAGE, and digital autoradiography. The amount of protein coprecipitated from wild-type (WT) mitochondria lysed directly after the addition of valinomycin (0 min) was set to 100% (control). i, matrix-targeted intermediate form. (C) The increased interaction of Ssc1-2 with imported preprotein is dependent on the induction of the temperature-sensitive phenotype. b2(167)Δ-DHFR was imported for 5 min into ssc1-2 and wild-type mitochondria that had been shifted to 37°C or kept at the permissive temperature prior to import. After the import reaction, the mitochondria were lysed and subjected to coimmunoprecipitation as described for panel A. The total amount of imported protein was set at 100%.
To control whether the enhanced binding of preprotein by Ssc1-2 was specifically related to the temperature-sensitive phenotype, b2(167)Δ-DHFR was imported into mitochondria isolated from wild-type or ssc1-2 mitochondria. Prior to import, the mitochondria were either shifted to 37°C or kept at the permissive temperature. After import, the mitochondria were lysed and subjected to immunoprecipitation with mtHsp70 antibodies. Quantification of the coprecipitated amounts confirmed that the enhanced association of preproteins with Ssc1-2 was indeed dependent on the induction of the phenotype by heat shock (Fig. 4C).
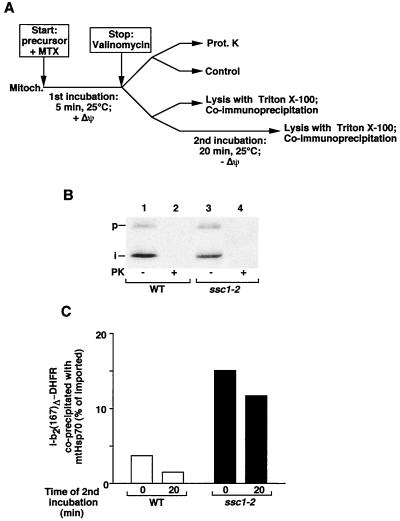
Does the enhanced association of preproteins with Ssc1-2 occur during membrane translocation, or is it only indirectly caused by a delay in folding of the fully imported protein (20, 44)? We used the possibility to arrest b2(167)Δ-DHFR during import as membrane-spanning intermediate by stabilizing the folding state of the DHFR moiety by addition of the specific ligand methotrexate (MTX) (42, 44). b2(167)Δ-DHFR was accumulated in wild-type or ssc1-2 mitochondria in the presence of MTX and analyzed for interaction with mtHsp70 (Fig. 5A). Complete arrest of the preprotein intermediate was confirmed by the full accessibility of the intermediate form to proteinase K added to the mitochondria (Fig. 5B, lanes 2 and 4). Mitochondria that were not protease treated were lysed and subjected to immunoprecipitation by anti-mtHsp70 antibodies either directly after stop of the import reaction or after an additional incubation at 25°C. Comparison of the precipitated amounts of preproteins reveals an enhanced and prolonged binding by Ssc1-2 to the translocation intermediate (Fig. 5C). Since the MTX-arrested preproteins are true translocation intermediates, the increased interaction with Ssc1-2 is directly relevant for the translocation reaction. Hence, it can be excluded that the increased interaction of preproteins with Ssc1-2 seen here is caused by a retarded folding process in the matrix.
FIG. 5.
Preproteins in transit show an enhanced interaction with Ssc1-2. (A) Experimental approach. (B and C) b2(167)Δ-DHFR was imported for 5 min at 25°C into ssc1-2 and wild-type (WT) mitochondria (Mitoch.) in the presence of 5 μM MTX. Import was stopped by the addition of 1 μM valinomycin. One portion of the import reaction was treated with proteinase K (Prot. K in A, PK in B), and one was left untreated to assay the formation of membrane-spanning intermediates (B). The other portions of the import reaction were subjected to lysis and coimmunoprecipitation by antibodies directed against mtHsp70 either directly or after an additional incubation at 25°C (C). All samples were analyzed by SDS-PAGE and digital autoradiography. The total amount of accumulated protein was set at 100%.
Why does this enhanced trapping of b2(167)Δ-DHFR not stimulate the import into fully energized ssc1-2 mitochondria? When preproteins are incubated with mitochondria at a high membrane potential, the amino-terminal presequence is efficiently inserted into the inner membrane. A high membrane potential keeps the preprotein segment in the mitochondria, while at a low membrane potential the rate of backward diffusion might be increased. When the membrane potential is lowered, an enhanced trapping of mtHsp70 can partly compensate for the destabilization of the preprotein in transit, eventually resulting in an increase in the relative import efficiency. However, also under conditions of a full membrane potential, the interaction between mtHsp70 and the preprotein in transit is absolutely essential for the completion of translocation. With an mtHsp70 mutant, Ssc1-3, which is inactivated by an alteration in the ATPase domain and is not able to bind to preproteins in transit, full translocation is blocked completely after the amino-terminal presequence has reached the matrix (8). With fully energized mitochondria, an additional trapping effect is not apparent due to the high import rate. When saturating amounts of preproteins are added to mitochondria, mtHsp70 molecules must be recycled by release from already imported proteins in order to bind to newly importing preproteins. The delayed release of proteins from Ssc1-2 might thereby impair the import reaction. Indeed, when preproteins in saturating amounts were added to fully energized ssc1-2 mitochondria, the translocation across the membranes was delayed (22).
Import of a tightly folded preprotein is not enhanced in ssc1-2 mitochondria.
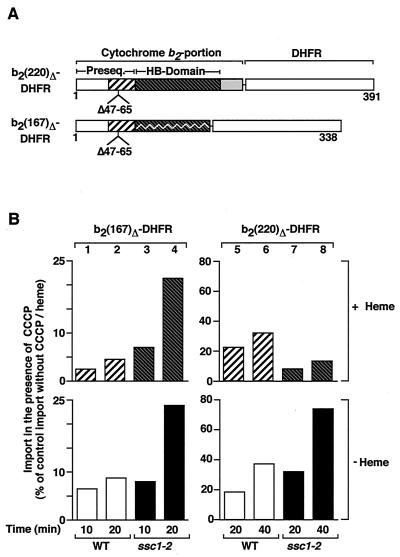
The model that mtHsp70 plays a dual role in protein import, i.e., trapping of loosely folded preproteins and pulling of folded domains to support their unfolding (44), has been based on the observation that fully energized ssc1-2 mitochondria are able to import loosely folded proteins but are impaired in the import of preproteins with tightly folded domains (44, 47, 48). The results presented here show, however, that the strong import-stimulating activity of an enhanced trapping is usually masked at high import rates due to the fully established membrane potential, raising the crucial question of whether a hypothetical unfolding activity of trapping has also been masked at a high Δψ. To address this problem, we used an additional preprotein, b2(220)Δ-DHFR, whose only difference from b2(167)Δ-DHFR is the presence of an intact heme-binding domain in the middle part of the protein (Fig. 6A). In the presence of heme, this noncovalent heme-binding domain is tightly folded, and import of the preprotein is impaired in energized ssc1-2 mitochondria compared to energized wild-type mitochondria (14, 47, 48). In b2(167)Δ-DHFR, the heme-binding domain is truncated (Fig. 6A), which leads to a loss of stable folding (48).
FIG. 6.
Import of a tightly folded preprotein is not enhanced in ssc1-2 mitochondria. (A) Fusion proteins used in this experiment. b2(220)Δ-DHFR consists of residues 1 to 220 of the wild-type cytochrome b2 precursor fused to the entire mouse DHFR with a deletion of residues 47 to 65 in the presequence. The mature part (residues 81 to 220) contains a complete heme-binding (HB) domain (residues 81 to 181). b2(167)Δ-DHFR consists of residues 1 to 167 of cytochrome b2 (excluding residues 47 to 65) and DHFR. The truncation of the heme-binding domain leads to a less stable folding of this domain. (B) b2(167)Δ-DHFR and b2(220)Δ-DHFR were imported at 25°C into wild-type (WT) and ssc1-2 mitochondria under nonpermissive conditions in the presence or absence of 5 μM CCCP with or without the addition of 10 μM heme. The import reactions were subsequently treated as described in the legend to Fig. 1. The amount of protein imported into wild-type mitochondria in the absence of CCCP and heme after the maximal import time [20 min for b2(167)Δ-DHFR and 40 min for b2(220)Δ-DHFR] was set at 100% (control).
We imported b2(167)Δ-DHFR and b2(220)Δ-DHFR in the presence of both heme and CCCP. b2(167)Δ-DHFR was imported more efficiently into ssc1-2 than wild-type mitochondria (Fig. 6B, upper panel, columns 3 and 4 versus 1 and 2), demonstrating that the addition of heme did not affect the import stimulation by enhanced trapping of this preprotein. However, the import of b2(220)Δ-DHFR was not enhanced in ssc1-2 mitochondria (Fig. 6B, upper panel, columns 7 and 8 versus 5 and 6). To test if the import of b2(220)Δ-DHFR can be stimulated by enhanced trapping at all, we performed the same experiment in the absence of heme but presence of CCCP (Fig. 6B, lower panels). Now the import of b2(220)Δ-DHFR was clearly stronger into ssc1-2 mitochondria than wild-type mitochondria (Fig. 6B, lower panel, columns 7 and 8 versus 5 and 6). Therefore, the stabilization of the folded state of the heme-binding domain of b2(220)Δ-DHFR by heme is selectively responsible for the lack of import stimulation by enhanced trapping. We conclude that enhanced trapping of preproteins by mtHsp70 can stimulate the import of loosely folded preproteins but not of preproteins with a tightly folded domain, demonstrating that the trapping model alone is not sufficient to describe the full function of mtHsp70.
Conclusions.
We report the first direct evidence that enhanced trapping of preproteins by mtHsp70 leads to their enhanced import, proving that trapping is one of the molecular mechanisms of mtHsp70 in promoting preprotein translocation into mitochondria. Previous studies with ssc1-2 mitochondria did not unravel an increased protein import efficiency compared to wild-type mitochondria because those studies were performed with fully energized mitochondria maintaining a high inner membrane potential. Therefore, the wild-type mitochondria was already importing preproteins at the maximal rate and thus an enhanced trapping in ssc1-2 mitochondria was not able to further increase the import rate. Our results imply a close functional relation between the two import-driving forces, Δψ and mtHsp70, since the import stimulation by enhanced trapping becomes stronger with a lower Δψ. However, enhanced trapping cannot suppress a complete dissipation of Δψ, in agreement with the observations that Δψ is essential for the initiation of translocation across the inner membrane (24, 32, 37). It has been discussed that Δψ promotes reversible translocation of the presequence, while mtHsp70 stabilizes the presequence in the matrix and drives translocation of the mature portion of preproteins (6, 41, 42). We show that upon Δψ-dependent initiation of translocation, both Δψ and mtHsp70 exert import-driving activities that are so closely related that they can at least partially substitute for each other. However, only import of loosely folded preproteins, not of a tightly folded preprotein, is stimulated by enhanced trapping, suggesting that trapping is not the only mechanism of mtHsp70 action.
Posttranslational protein import into the endoplasmic reticulum (ER) requires the lumenal Hsp70 BiP, yet is independent of a membrane potential (19, 33, 35, 43, 49). Matlack et al. (25) showed that protein translocation in a reconstituted ER system could be driven by binding to lumenal antibodies, suggesting that BiP also functioned by a trapping mechanism, although the import efficiency of the reconstituted antibody-trapping system was lower than that of BiP. These results with the reconstituted ER system are now complemented by the mitochondrial system, in which enhanced trapping by mtHsp70 indeed can increase the import efficiency in the complete organellar context. It has not yet been studied which forces drive a tightly folded preprotein into the ER (29). In view of the findings with mitochondrial import, it is tempting to speculate that with both mitochondria and ER, trapping of preproteins is not the only mechanism of Hsp70 systems to drive preprotein translocation.
ACKNOWLEDGMENTS
We thank E. Craig for the ssc1-2 mutant, B. Guiard for the b2-DHFR constructs, P. Rehling for critically reading the manuscript, and N. Zufall for expert technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft, the Sonderforschungsbereich 388, and the Fonds der Chemischen Industrie/BMBF.
REFERENCES
- 1.Alconada A, Gärtner F, Hönlinger A, Kübrich M, Pfanner N. Mitochondrial receptor complex from Neurospora crassa and Saccharomyces cerevisiae. Methods Enzymol. 1995;260:263–286. doi: 10.1016/0076-6879(95)60144-9. [DOI] [PubMed] [Google Scholar]
- 2.Bauer M F, Hofmann S, Neupert W, Brunner M. Protein translocation into mitochondria: the role of TIM complexes. Trends Cell Biol. 2000;10:25–31. doi: 10.1016/s0962-8924(99)01684-0. [DOI] [PubMed] [Google Scholar]
- 3.Bauer M F, Sirrenberg C, Neupert W, Brunner M. Role of Tim23 as voltage sensor and presequence receptor in protein import into mitochondria. Cell. 1996;87:33–41. doi: 10.1016/s0092-8674(00)81320-3. [DOI] [PubMed] [Google Scholar]
- 4.Bömer U, Maarse A, Martin F, Geissler A, Merlin A, Schonfisch B, Meijer M, Pfanner N, Rassow J. Separation of structural and dynamic functions of the mitochondrial translocase: Tim44 is crucial for the inner membrane import sites in translocation of tightly folded domains, but not of loosely folded preproteins. EMBO J. 1998;17:4226–4237. doi: 10.1093/emboj/17.15.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukau B, Horwich A. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 6.Cyr D M, Stuart R, Neupert W. A matrix ATP requirement for presequence translocation across the inner membrane of mitochondria. J Biol Chem. 1993;268:23751–23754. [PubMed] [Google Scholar]
- 7.Ellis R J, van der Vies S. Molecular chaperones. Annu Rev Biochem. 1991;60:321–347. doi: 10.1146/annurev.bi.60.070191.001541. [DOI] [PubMed] [Google Scholar]
- 8.Gambill B D, Voos W, Kang P, Miao B, Langer T, Craig E, Pfanner N. A dual role for mitochondrial heat shock protein 70 in membrane translocation of preproteins. J Cell Biol. 1993;123:109–117. doi: 10.1083/jcb.123.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gärtner F, Voos W, Craig E, Cumsky M, Pfanner N. Mitochondrial import of subunit Va of cytochrome c oxidase characterized with yeast mutants. Independence from receptors, but requirement for matrix hsp70 translocase function. J Biol Chem. 1995;270:3788–3795. doi: 10.1074/jbc.270.8.3788. [DOI] [PubMed] [Google Scholar]
- 10.Gaume B, Klaus C, Ungermann C, Guiard B, Brunner M. Unfolding of preproteins upon import into mitochondria. EMBO J. 1998;17:6497–6507. doi: 10.1093/emboj/17.22.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geissler A, Krimmer T, Bömer U, Guiard B, Rassow J, Pfanner N. Membrane potential-driven protein import into mitochondria: the sorting sequence of cytochrome b2 modulates the Δψ-dependence of translocation of the matrix-targeting sequence. Mol Biol Cell. 2000;11:3977–3991. doi: 10.1091/mbc.11.11.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geissler A, Krimmer T, Schönfisch B, Meijer M, Rassow J. Biogenesis of the yeast frataxin homolog Yfh1p: Tim44-dependent transfer to mtHsp70 facilitates folding of newly imported proteins in mitochondria. Eur J Biochem. 2000;267:3167–3180. doi: 10.1046/j.1432-1327.2000.01334.x. [DOI] [PubMed] [Google Scholar]
- 13.Glick B S. Can Hsp70 proteins act as force-generating motors? Cell. 1995;80:11–14. doi: 10.1016/0092-8674(95)90444-1. [DOI] [PubMed] [Google Scholar]
- 14.Glick B S, Wachter C, Reid G, Schatz G. Import of cytochrome b2 to the mitochondrial intermembrane space: the tightly folded heme-binding domain makes import dependent upon matrix ATP. Protein Sci. 1993;2:1901–1917. doi: 10.1002/pro.5560021112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartl F U. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 16.Haucke V, Schatz G. Reconstitution of the protein insertion machinery of the mitochondrial inner membrane. EMBO J. 1997;16:4560–4567. doi: 10.1093/emboj/16.15.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horst M, Oppliger W, Feifel B, Schatz G, Glick B S. The mitochondrial protein import motor: dissociation of mitochondrial hsp70 from its membrane anchor requires ATP binding rather than ATP hydrolysis. Protein Sci. 1996;5:759–767. doi: 10.1002/pro.5560050421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang S, Ratliff K, Schwartz M, Spenner J, Matouschek A. Mitochondria unfold precursor proteins by unraveling them from their N-termini. Nat Struct Biol. 1999;6:1132–1138. doi: 10.1038/70073. [DOI] [PubMed] [Google Scholar]
- 19.Jensen R E, Johnson A. Protein translocation: is Hsp70 pulling my chain? Curr Biol. 1999;9:R779–R782. doi: 10.1016/S0960-9822(00)80012-3. [DOI] [PubMed] [Google Scholar]
- 20.Kang P J, Ostermann J, Shilling J, Neupert W, Craig E, Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990;348:137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- 21.Kronidou N G, Oppliger W, Bolliger L, Hannavy K, Glick B, Schatz G, Horst M. Dynamic interaction between Isp45 and mitochondrial hsp70 in the protein import system of the yeast mitochondrial inner membrane. Proc Natl Acad Sci USA. 1994;91:12818–12822. doi: 10.1073/pnas.91.26.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim J H, Martin F, Guiard B, Pfanner N, Voos W. The mitochondrial Hsp70-dependent import system actively unfolds preproteins and shortens the lag phase of translocation. EMBO J. 2001;20:941–950. doi: 10.1093/emboj/20.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q, Krzewska J, Liberek K, Craig E. Mitochondrial Hsp70 Ssc1: role in protein folding. J Biol Chem. 2001;276:6112–6118. doi: 10.1074/jbc.M009519200. [DOI] [PubMed] [Google Scholar]
- 24.Martin J, Mahlke K, Pfanner N. Role of an energized inner membrane in mitochondrial protein import: Δψ drives the movement of presequences. J Biol Chem. 1991;266:18051–18057. [PubMed] [Google Scholar]
- 25.Matlack K E, Misselwitz B, Plath K, Rapoport T A. BiP acts as a molecular ratchet during posttranslational transport of prepro-α factor across the ER membrane. Cell. 1999;97:553–564. doi: 10.1016/s0092-8674(00)80767-9. [DOI] [PubMed] [Google Scholar]
- 26.Matouschek A, Azem A, Ratliff K, Schmid K, Schatz G. Active unfolding of precursor proteins during mitochondrial protein import. EMBO J. 1997;16:6727–6736. doi: 10.1093/emboj/16.22.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moro F, Sirrenberg C, Schneider H, Neupert W, Brunner M. The TIM17 · 23 preprotein translocase of mitochondria: composition and function in protein transport into the matrix. EMBO J. 1999;18:3667–3675. doi: 10.1093/emboj/18.13.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neupert W. Protein import into mitochondria. Annu Rev Biochem. 1997;66:863–917. doi: 10.1146/annurev.biochem.66.1.863. [DOI] [PubMed] [Google Scholar]
- 29.Paunola E, Suntio T, Jamsa E, Makarow M. Folding of active β-lactamase in the yeast cytoplasm before translocation into the endoplasmic reticulum. Mol Biol Cell. 1998;9:817–827. doi: 10.1091/mbc.9.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfanner N, Craig E, Hönlinger A. Mitochondrial preprotein translocase. Annu Rev Cell Dev Biol. 1997;13:25–51. doi: 10.1146/annurev.cellbio.13.1.25. [DOI] [PubMed] [Google Scholar]
- 31.Pfanner N, Meijer M. Protein sorting: pulling in the proteins. Curr Biol. 1995;5:132–135. doi: 10.1016/s0960-9822(95)00033-9. [DOI] [PubMed] [Google Scholar]
- 32.Pfanner N, Neupert W. Transport of proteins into mitochondria: a potassium diffusion potential is able to drive the import of ADP/ATP carrier. EMBO J. 1985;4:2819–2825. doi: 10.1002/j.1460-2075.1985.tb04009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pilon M, Schekman R. Protein translocation: how Hsp70 pulls it off. Cell. 1999;97:679–682. doi: 10.1016/s0092-8674(00)80780-1. [DOI] [PubMed] [Google Scholar]
- 34.Rassow J, Maarse A, Krainer E, Kübrich M, Meijer M, Craig E, Pfanner N. Mitochondrial protein import: biochemical and genetic evidence for interaction of matrix hsp70 and the inner membrane protein MIM44. J Cell Biol. 1994;127:1547–1556. doi: 10.1083/jcb.127.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schatz G, Dobberstein B. Common principles of protein translocation across membranes. Science. 1996;271:1519–1526. doi: 10.1126/science.271.5255.1519. [DOI] [PubMed] [Google Scholar]
- 36.Scherer P E, Krieg U, Hwang S, Vestweber D, Schatz G. A precursor protein partly translocated into yeast mitochondria is bound to a 70 kd mitochondrial stress protein. EMBO J. 1990;9:4315–4322. doi: 10.1002/j.1460-2075.1990.tb07880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schleyer M, Neupert W. Transport of proteins into mitochondria: translocational intermediates spanning contact sites between outer and inner membranes. Cell. 1985;43:339–350. doi: 10.1016/0092-8674(85)90039-x. [DOI] [PubMed] [Google Scholar]
- 38.Schneider H C, Berthold J, Dietmeier K, Guiard B, Brunner M, Neupert W. Mitochondrial Hsp70/MIM44 complex facilitates protein import. Nature. 1994;371:768–774. doi: 10.1038/371768a0. [DOI] [PubMed] [Google Scholar]
- 39.Schneider H C, Westermann B, Neupert W, Brunner M. The nucleotide exchange factor MGE exerts a key function in the ATP-dependent cycle of mt-Hsp70-Tim44 interaction driving mitochondrial protein import. EMBO J. 1996;15:5796–5803. [PMC free article] [PubMed] [Google Scholar]
- 40.Söllner T, Rassow J, Pfanner N. Analysis of mitochondrial protein import using translocation intermediates and specific antibodies. Methods Cell Biol. 1991;34:345–358. doi: 10.1016/s0091-679x(08)61689-1. [DOI] [PubMed] [Google Scholar]
- 41.Ungermann C, Guiard B, Neupert W, Cyr D. The Δψ- and Hsp70/MIM44-dependent reaction cycle driving early steps of protein import into mitochondria. EMBO J. 1996;15:735–744. [PMC free article] [PubMed] [Google Scholar]
- 42.Ungermann C, Neupert W, Cyr D. The role of Hsp70 in conferring unidirectionality on protein translocation into mitochondria. Science. 1994;266:1250–1253. doi: 10.1126/science.7973708. [DOI] [PubMed] [Google Scholar]
- 43.Vogel J P, Misra L, Rose M. Loss of BiP/GRP78 function blocks translocation of secretory proteins in yeast. J Cell Biol. 1990;110:1885–1895. doi: 10.1083/jcb.110.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voisine C, Craig E, Zufall N, von Ahsen O, Pfanner N, Voos W. The protein import motor of mitochondria: unfolding and trapping of preproteins are distinct and separable functions of matrix Hsp70. Cell. 1999;97:565–574. doi: 10.1016/s0092-8674(00)80768-0. [DOI] [PubMed] [Google Scholar]
- 45.von Ahsen O, Voos W, Henninger H, Pfanner N. The mitochondrial protein import machinery: role of ATP in dissociation of the Hsp70 · Mim44 complex. J Biol Chem. 1995;270:29848–29853. doi: 10.1074/jbc.270.50.29848. [DOI] [PubMed] [Google Scholar]
- 46.Voos W, Gambill B, Laloraya S, Ang D, Craig E, Pfanner N. Mitochondrial GrpE is present in a complex with hsp70 and preproteins in transit across membranes. Mol Cell Biol. 1994;14:627–663. doi: 10.1128/mcb.14.10.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voos W, Gambill B, Guiard B, Pfanner N, Craig E. Presequence and mature part of preproteins strongly influence the dependence of mitochondrial protein import on heat shock protein 70 in the matrix. J Cell Biol. 1993;123:119–126. doi: 10.1083/jcb.123.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voos W, von Ahsen O, Müller H, Guiard B, Rassow J, Pfanner N. Differential requirement for the mitochondrial Hsp70-Tim44 complex in unfolding and translocation of preproteins. EMBO J. 1996;15:2668–2677. [PMC free article] [PubMed] [Google Scholar]
- 49.Walter P, Johnson A. Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1994;10:87–119. doi: 10.1146/annurev.cb.10.110194.000511. [DOI] [PubMed] [Google Scholar]