Abstract
Purpose
The natural history of microinvasive (T1mi) breast cancer is uncertain. The objective was to evaluate long-term local and distant recurrence rates following breast conserving surgery (BCS) in a prospective cohort of patients with T1mi compared to T1a-2 disease who received whole breast irradiation (WBI) in the context of a randomized trial of hypofractionation.
Methods
1234 patients with T1-2 N0 breast cancer were randomized to receive adjuvant WBI of 42.5Gy in 16 daily fractions, or 50Gy in 25 daily fractions after BCS. An analysis of patients with T1mi tumors compared with T1a-2 disease was performed. Kaplan-Meier estimates of local recurrence (LR), distant recurrence, and overall survival (OS) were compared using the log-rank test.
Results
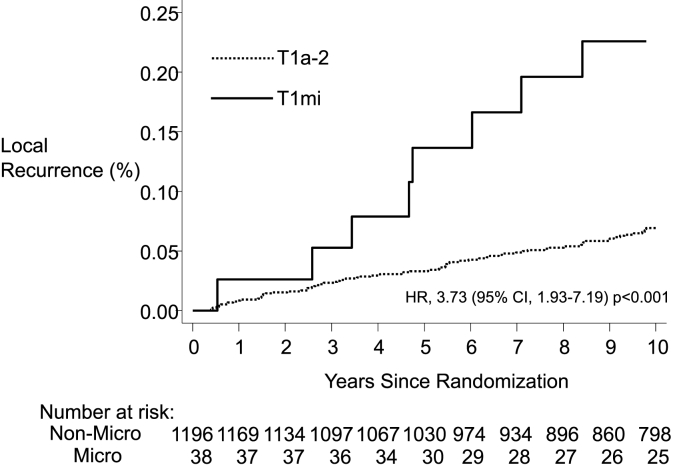
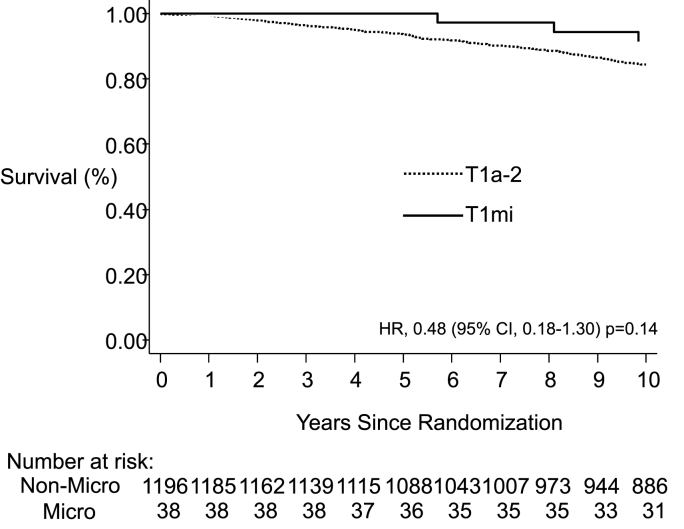
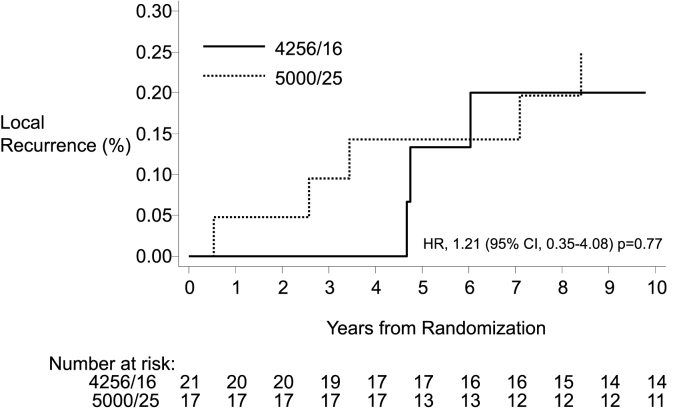
Median follow-up was 12 years. T1mi was found in 3% (n = 38) of patients. The 10-year LR rate was 22.6% in T1mi vs. 6.9% in T1a-2 breast cancer [hazard ratio (HR) = 3.73; 95% confidence interval (CI): 1.93, 7.19; p < 0.001]. The 10-year risk of distant recurrence was 5.1% for T1mi, and 12.1% for T1a-2 disease (HR = 0.56; 95% CI: 0.19, 1.84; p = 0.36). Ten-year OS was 91.5% in T1mi and 84.4% in T1a-2 disease, (HR = 0.48; 95% CI: 0.18, 1.30; p = 0.14). Rates of LR did not differ whether treated by hypofractionation or conventional fractionation (HR = 1.21; 95% CI: 0.35, 4.18; p = 0.77).
Conclusions
The risk of LR was considerably higher in patients with T1mi compared to T1a-2 tumors, but OS remained very good. Future research should evaluate the utility of wider local excision and boost radiation to optimize local control for microinvasive breast cancer.
Keywords: Microinvasive breast cancer, Breast conserving surgery, Hypofractionation, Local recurrence
Highlights
-
•
Patients with microinvasive breast cancer have a high risk of local recurrence.
-
•
Both the rate of invasive and non-invasive local recurrences are increased.
-
•
The risk of distant recurrence and death is not increased.
-
•
Methods to reduce the risk of local recurrence should be considered.
1. Introduction
Microinvasive breast cancer (T1mi) is defined as malignant cells invading beyond the cellular basement membrane into adjacent breast tissue with no invasive focus greater than 1 mm in the greatest dimension [1]. It is usually associated with ductal carcinoma in situ (DCIS), but not always. The incidence of microinvasive carcinoma is low, reported to be between <1% and 2.4% [2]. Management practices and guideline development is complicated by the rarity of this diagnosis and the use of varying criteria to define this entity within the literature [3]. These lesions have historically been analyzed together with a spectrum of breast lesions, including lobular and ductal carcinoma in situ, and lobular or ductal carcinoma < 5 mm, termed “minimally invasive carcinoma.” [4] Previous studies of microinvasive disease have been limited by small sample sizes and often retrospective designs [[5], [6], [7], [8], [9], [10]].
The natural history of this pathologic entity still remains uncertain and it is not clear if microinvasive breast cancer behaves more like DCIS or invasive carcinoma. Small retrospective series suggest that the natural history of microinvasive breast cancer is similar to that of DCIS, with a similar risk of occult nodal involvement and local recurrence [6,9,11]. In the literature, the risk of occult nodal involvement is rare with patients having an overall good prognosis [7,10,[12], [13], [14]].
The majority of women diagnosed with T1mi breast cancer undergo breast conserving surgery (BCS). Adjuvant whole breast irradiation (WBI) is usually given to reduce the risk of local recurrence based on randomized trials of its efficacy in invasive breast cancer and DCIS [15,16]. Hypofractionation rather than conventional fractionation may be used based on results of randomized trials in invasive disease [[17], [18], [19]]. Patients with microinvasive breast cancer were included in the randomized trials of hypofractionation for invasive breast cancer, but their outcomes have not been separately reported.
The purpose of this study was to evaluate the risk of local and distant recurrence in patients with microinvasive disease compared to other T1-2 N0 tumors in the context of a randomized trial of hypofractionated WBI compared to conventional fractionation following BCS. An additional objective was to assess the efficacy of hypofractionated WBI in patients with microinvasive breast cancer.
2. Patients and Methods
2.1. Study patients
Patients with invasive carcinoma of the breast ≤5 cm who were pathologically node negative treated with BCS and level I and II axillary dissection were eligible for enrollment in the Canadian Hypofractionation Trial. Patients with a positive surgical margin, defined as invasive carcinoma or DCIS on the specimen inked margin, were excluded. Comprehensive eligibility and exclusion criteria have been previously described [20]. Patients with stage T1miN0 disease were defined as invasive breast cancer ≤1 mm in greatest dimension as per the AJCC 8th edition staging manual [1]. The study protocol was approved by the ethics review board at each participating centre and informed consent was obtained from all patients.
2.2. Radiation and systemic treatment
Prior to randomization patients were considered for systemic therapy according to individual institutional guidelines. Patients were randomly assigned to receive radiation therapy to the whole breast of hypofractionation of 42.5Gy in 16 fractions over 22 days, or conventional fractionation: 50Gy in 25 fractions over 35 days. External beam radiation was delivered with a tangential parallel opposed pair beam arrangement. Wedge composition was used to ensure uniform dose distribution at the central plane. Boost radiation to the tumor bed and regional nodal irradiation were not permitted.
2.3. Patient follow-up and outcomes
After completion of radiation, patients were assessed on a 6-monthly basis for 5 years, and then yearly. Bilateral mammograms were obtained at 6 months after WBI and then annually. Local recurrences were defined as any invasive or in-situ recurrence in the ipsilateral/treated breast and were histologically confirmed. Distant recurrence included any recurrence in the ipsilateral regional nodes, bone, liver, lung or central nervous system, and were confirmed by imaging or histologically if possible.
2.4. Statistical analysis
Patients with microinvasive breast cancer were compared to patients with T1a-2 tumors (>1–50 mm). Fisher's exact test was used to determine differences in baseline characteristics for the patient groups. Cumulative incidence of local recurrence was defined as the time from randomization until local recurrence as a first event. Patients were censored at distant recurrence, last follow-up, or death, whichever occurred first. Cumulative incidence of distant recurrence was defined as time from randomization until any distant recurrence. Patients were censored at last follow-up, or death. Overall survival (OS) was defined as death from any cause. Patients were censored at last follow-up.
The outcomes of local recurrence, distant recurrence and OS were estimated using the Kaplan-Meier method and patient groups were compared using the log-rank test. Cox proportional hazards model was used to estimate the relationship between local recurrence and microinvasive disease (yes, no) age (50 years, ≥50 years), tumor size (<2 cm, ≥2 cm), grade (low, intermediate, and high), estrogen receptor status (negative, positive and equivocal), systemic therapy (none, tamoxifen or chemotherapy) and radiotherapy fractionation (hypofractionation, conventional fractionation).
3. Results
Between April 1993 and September 1996, 1234 patients with T1-2 N0 invasive breast cancer were randomized to hypofractionated or conventional fractionated WBI. Microinvasive carcinoma occurred in 38 patients (3%). Median follow-up was 12 years for the entire cohort. Tamoxifen was used in 18% of patients with T1mi disease, compared to 41% of patients with T1a-2 tumors. No patient with microinvasive disease received cytotoxic chemotherapy. Baseline characteristics, other than the use of systemic therapy, did not differ between the microinvasive breast cancer subgroup and the remaining study patients (Table 1).
Table 1.
Patient and treatment characteristics.
| n (%) | T1mi n = 38 (%) | T1a-2 n = 1196 (%) | p value |
|---|---|---|---|
| Age | |||
| <50 | 6 (16) | 299 (25) | 0.25 |
| ≥50 | 32 (84) | 897 (75) | |
| Tumor Grade | |||
| I | 14 (37) | 441 (37) | 0.85 |
| II | 16 (42) | 504 (42) | |
| III | 7 (18) | 231 (19) | |
| Unknown | 1 (3) | 20 (2) | |
| Estrogen receptor | |||
| Positive | 22 (58) | 863 (72) | 0.07 |
| Negative | 13 (34) | 255 (21) | |
| Equivocal | 1 (3) | 57 (5) | |
| Missing | 2 (5) | 21 (2) | |
| Systemic Therapy | |||
| None | 31 (82) | 572 (48) | <0.001 |
| Tamoxifen | 7 (18) | 493 (41) | |
| Chemotherapy | 0 (0) | 131 (11) | |
| Radiation regimen | |||
| Conventional | 21 (55) | 591 (49) | 0.51 |
| Hypofractionated | 17 (45) | 605 (51) | |
Eight local recurrences occurred after BCS and WBI for T1mi breast cancer. Of these recurrences, 5 (62.5%) were invasive carcinoma and 3 (37.5%) were DCIS. In the remaining patients with T1a-2 disease (n = 1196) there were 73 local recurrences, of which 64 (87.7%) were invasive and 9 (12.3%) were DCIS. The 10-year local recurrence rate (invasive and non-invasive recurrences) was 22.6% in the T1mi patients, and 6.9% in the T1a-2 cohort [hazard ratio (HR) 3.73; 95% confidence interval (CI): 1.93–7.19; p < 0.001] (Fig. 1). The rate of invasive local recurrences at 10 years was 14.3% in the T1mi patients, and 6.1% in the T1a-2 cohort (HR = 2.94; 95% CI: 1.37, 6.25; p = 0.004). The rate of non-invasive local recurrence at 10 years was also increased for T1mi patients compared to the T1a-2 cohort (8.3% vs. 0.8%, HR = 9.1; 95% CI: 2.5, 33.1; p < 0.001).
Fig. 1.
Kaplan-Meier curves for Local Recurrence (invasive and in-situ) for patients with T1mi and T1a-2 tumors.
A sensitivity analysis was undertaken to assess the rate of local recurrence in patients with T1mi breast cancer compared to those with T1a (>1–5 mm) tumors (n = 46). The 10-year local recurrence rate was 22.6% for microinvasive disease compared to only 2% in patients with T1a disease (p = 0.001). Tamoxifen was rarely used in patients with T1a tumors (n = 4) and no patient received chemotherapy.
In the patients with microinvasive breast cancer, there were no regional recurrences at 10 years and only 2 distant recurrences. Rates of distant recurrence at 10 years were 5.4% in the T1mi group and 12.1% in the T1a-2 group (HR = 0.59; 95% CI: 0.19, 1.84; p-value = 0.36). OS was 91.5% in the T1mi patient cohort, and 84.4% in the T1a-2 patients (HR = 0.48; 95% CI: 0.18, 1.30; p = 0.14) (Fig. 2).
Fig. 2.
Kaplan-Meier curves for overall survival for patients with T1mi and T1a-2 tumors.
Cox-regression multivariate analysis was undertaken to assess variables associated with local tumor recurrence. T1mi status (HR = 2.86; 95% CI: 1.30, 6.29; p = 0.009) and tumor size >2 cm (HR = 1.80; 95% CI: 1.11, 2.91; p = 0.02) were independently prognostic for local recurrence (Table 2).
Table 2.
Multivariate analysis for local recurrence.
| HR (95% CI) | p-value | |
|---|---|---|
| Treatment: Hypofractionated vs Conventional Fractionation | 1.01 (0.67–1.52) | 0.95 |
| Microinvasive: yes vs no | 2.86 (1.30–6.29) | 0.009 |
| Age: <50 vs ≥ 50 | 1.18 (0.75–1.87) | 0.48 |
| Grade (ref = low) | ||
| High | 1.58 (0.82–3.04) | 0.39 |
| Intermediate | 1.22 (0.74–2.04) | |
| Size: >2 cm vs ≤ 2 cm | 1.80 (1.11–2.91) | 0.02 |
| ER status (ref = negative) | ||
| positive | 0.58 (0.34–0.97) | 0.11 |
| equivocal | 0.78 (0.32–1.86) | |
| Systemic Therapy: yes vs no | 0.80 (0.51–1.25) | 0.32 |
Within the subgroup of patients with microinvasive breast cancer, local recurrence rates did not differ by radiation treatment regimen (hypofractionation vs. conventional fractionation, HR = 1.21; 95% CI: 0.35, 4.18; p = 0.77) (Fig. 3).
Fig. 3.
Local recurrence rates for patients with microinvasive breast cancer treated with hypofractionated (4256cGy/16) or conventional (5000cGy/25) fractionation.
4. Discussion
In this study we report the analysis of patients with T1mi N0 breast cancer compared to patients with T1a-2 N0 treated by BCS. Patients received WBI either with hypofractionation or conventional fractionation as part of a randomized trial. The risk of local recurrence was significantly higher with microinvasive disease compared to T1a-2 tumors. This was due both to an increase in invasive and non-invasive local recurrences. The increase in local recurrence was unlikely due to decreased use of systemic therapy as the risk remained increased when T1mi was compared to T1a tumors where systemic therapy was also uncommonly used. Despite the increased risk in local recurrence, the risk of distant recurrence and death was less in patients with microinvasive disease compared to T1a-2 tumors although no significant differences were observed.
Our findings are similar to other series demonstrating a relatively high rate of local recurrence with a favorable survival outcome for patients with microinvasive breast cancer treated with BCS and adjuvant WBI [9,21]. This high rate of local recurrence with a similar proportion of invasive and non-invasive recurrences is similar to the pattern of local recurrence following BCS for DCIS [15,22]. Rakovitch et al. evaluated a population cohort of patients with DCIS or microinvasive breast cancer and reported 15-year local recurrence free survival of 80.3% for patients with pure DCIS treated with BCS and WBI (without boost radiotherapy) or 81.8% for patients with 1 focus of microinvasion treated in the same way [22]. In this series ≥2 foci of microinvasion was significantly associated with higher rates of local recurrence (15-year local recurrence free survival of 70.3%) [22].
Solin et al. compared the outcomes of 39 women with microinvasive breast cancer treated with BCS followed by WBI with boost radiation to two control groups of women with invasive carcinoma or pure DCIS. The 5-year rate of local failure was significantly higher with microinvasive breast cancer [19% compared to 6% (invasive) and 2% (pure DCIS), p = 0.006] [23]. In this study the 5 year recurrence rates were similar to the 10 year rates observed in our trial despite the use of boost radiation. In contrast to our study, microinvasion was defined as a maximal extent of invasion ≤ 2 mm or invasive carcinoma comprising <10% of the tumor thus comprising a potentially higher risk group. Moreover, margin status was unknown in almost half the entire patient cohort as routine inking of surgical specimens was not performed and no endocrine therapy was used. In their study, local failure rate was only 5% in patients with negative margins (≥2 mm), compared to 35% in patients with unknown margin status, demonstrating the impact of margin status on local control. Although these differences resulted in a higher 5-year recurrence risk in the study by Solin et al. the similar trend of a higher rate of local recurrence when comparing patients with microinvasive tumors to those with T1a-2 disease is consistent.
Despite the high incidence of local recurrence, other studies have also found that the risk of distant recurrence or death is less for patients with microinvasive compared to invasive disease. In a series of 426 patients with microinvasive disease, nodal involvement was detected in only 7.7% of patients and overall survival was 98% [9]. In an analysis of the SEER database by Wang et al. of 8863 patients with T1mi, only 7.6% had nodal disease and the 10-year cancer mortality was 4% [21]. More recent data suggests that patients with microinvasive disease have a slightly higher risk of distant metastases and breast cancer mortality than DCIS, but a lower risk than invasive disease. Investigators from the UK Sloan project reported on 521 cases of microinvasive disease and compared this group to 10,764 cases of DCIS with follow-up of approximately 9 years [24]. They identified that the risk of distant metastases and breast cancer mortality was higher with microinvasion compared to DCIS (1.2% vs. 0.3%, p = 0.01 and 2.1% vs. 0.8%, p = 0.005, respectively). Sopik et al. using the SEER database reported on the largest series to date of 13,489 women with microinvasive disease [25]. Details on local recurrence and distant metastasis were not available, but the 20-year breast cancer mortality rates were higher for patients with microinvasive disease compared to DCIS (6.9% vs. 3.8%, p < 0.001). The risk of breast cancer mortality in this series for women with microinvasive disease was similar to those with invasive cancer 0.2–1 cm in size (6.8%) and less than that for women with invasive cancers 1.1–2.0 cm (12.1%).
In our trial patients were randomized to hypofractionation (42.5Gy/16 fractions) or conventional fractionation (50Gy/25 fractions). The small number of T1mi cases limited our ability to adequately compare hypofractionation with conventional fractionation for T1 microinvasive disease. However, similar to the results of the overall trial there was no evidence that hypofractionation resulted in a higher risk of local recurrence in patients with microinvasive disease. These data are consistent with recent trials of hypofractionation for DCIS. In the Danish Breast Cancer Group (DBCG) HYPO trial, 246 patients with DCIS alone were randomized to hypofractionation (40Gy/15 fractions) compared to conventional fractionation (50Gy/25 fractions) as part of a larger randomized trial in invasive disease [26]. No differences were observed in local recurrence at a median follow-up of 7.3 years. Similarly in BIG 3–07/TROG 07.01, 503 patients with DCIS were randomized to hypofractionation (42.56Gy/16 fractions) versus conventional fractionation (50Gy/25 fractions) [27]. Again, no difference was detected in local recurrence at a median follow-up of 6.6 years. The results from our study together with results of trials in patients with DCIS and invasive disease, support the use of hypofractionation for microinvasive breast cancer.
In our hypofractionation trial, additional boost radiation to the tumor bed was not used. At the time of study initiation, boost radiation had not been demonstrated in randomized trials to be effective and investigators wished to avoid confounding of boost radiation on outcomes in the trial. Since then, boost radiation has been demonstrated to be effective in reducing the risk of local recurrence in both invasive disease and non-invasive disease. The large EORTC boost trial of 5318 patients with invasive disease demonstrated a relative reduction of local recurrence of 40% at 10 years [28], and the recent BIG 3–07/TROG 07.01 trial in 1608 patients with non-low risk DCIS demonstrated a reduction in local recurrence of a similar magnitude [27]. Microinvasive disease is not normally considered a standard indicator for boost radiation, but given our findings of a higher risk of local recurrence, and results from these trials it seems reasonable to consider boost radiation for patients with microinvasive disease especially with high risk features typically seen for DCIS such as age <50, size >2.5 cm and the presence of high grade or comedo necrosis. The high risk of local recurrence observed in our trial also suggests that other strategies to reduce local recurrence such as wider surgical margins (≥2 mm) currently recommended for DCIS [29] or the use of adjuvant endocrine therapy could also be considered to reduce risk of recurrence [30,31].
One of the strengths of our study is that a uniform inception cohort was identified. All patients were treated uniformly with BCS and axillary dissection and determined to be node negative with negative surgical margins consistent with the Society of Surgical Oncology and the American Society of Clinical Oncology practice guidelines for invasive disease [32]. All patient were then treated with WBI using hypofractionation or conventional fractionation as per the trial and then followed prospectively for 12 years. Patients were followed carefully for local and distant recurrence, which has not been possible in epidemiological databases such as SEER. We were also able to look at the impact of different radiation fractionations without the confounding of boost radiation.
An important limitation of our study is that despite its prospective nature, there were only 38 cases of microinvasive disease. Despite the small numbers, the risk of local recurrence was higher for microinvasive disease compared to T1a-2 disease. The lower bound of the CI for the HR for the direct comparison would suggest that the risk of local recurrence could be 1.9 times that for the higher staged disease. It should be noted that this study was performed in the mid-1990s. Since then the rate of local recurrence has decreased owing to improved mammographic techniques, better surgical approaches, and effective systemic therapy so the absolute rates of LR for T1mi and T1a-2 are now likely to be less. Other limitations of the study were that specific details regarding extent of associated DCIS, the number of foci of microinvasive disease and the presence of comedo necrosis were not available.
This study adds to our understanding about the natural history of microinvasive breast cancer. It demonstrates that despite a higher risk of local recurrence (both invasive and non-invasive) the risk of distant metastasis was relatively low and associated with a good prognosis. Hypofractionation was equally effective compared to conventional fractionation in preventing local recurrence. The high rate of local recurrence at 10-years for microinvasive disease observed in our trial suggests that measures to reduce risk such as wider margins of excision (≥2 mm), the use of additional boost radiation to the tumor bed, and adjuvant endocrine therapy as used for DCIS could be considered.
Ethical approval
The study protocol was approved by the Ethics Review Board at each participating centre.
Declaration of competing interest
Dr. Whelan has received in-kind funding from Exact Sciences for work not related to this study.
Acknowledgements
This study was supported by the National Cancer Institute of Canada (NCIC) and the Canadian Cancer Society (CCS). Dr. Whelan is supported by a Canada Research Chair in Breast Cancer Research.
References
- 1.Amin M., Edge S., Greene F., Byrd D., Brookland R. eighth ed. Springer; New York: 2017. American joint committee on cancer: AJCC cancer staging manual; pp. 589–636. [Google Scholar]
- 2.Hoda S.A., Chiu A., Prasad M.L., et al. Are microinvasive and micrometastasis in breast cancer mountains or molehills? Am J Surg. 2000;180:305–308. doi: 10.1016/s0002-9610(00)00464-5. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi S., Vezzosi V. Microinvasive carcinoma of the breast. Pathol Oncol Res. 2008;14:105–111. doi: 10.1007/s12253-008-9054-8. [DOI] [PubMed] [Google Scholar]
- 4.Frazier T.G., Copeland E.M., Gallager H.S., et al. Prognosis and treatment in minimal breast cancer. Am J Surg. 1977;133:697–701. doi: 10.1016/0002-9610(77)90156-8. [DOI] [PubMed] [Google Scholar]
- 5.Vieira C.C., Mercado C.L., Cangiarella J.F., et al. Microinvasive ductal carcinoma in situ: clinical presentation, imaging features, pathologic findings, and outcome. Eur J Radiol. 2010;73:102–107. doi: 10.1016/j.ejrad.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 6.Parikh R.R., Haffty B.G., Lannin D., et al. Ductal carcinoma in situ with microinvasion: prognostic implications, long-term outcomes, and role of axillary evaluation. Int J Radiat Oncol Biol Phys. 2012;82:7–13. doi: 10.1016/j.ijrobp.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 7.Margalit D.N., Sreedhara M., Chen Y.H., et al. Microinvasive breast cancer: ER, PR, and HER-2/neu status and clinical outcomes after breast-conversing therapy or mastectomy. Ann Surg Oncol. 2013;20:811–818. doi: 10.1245/s10434-012-2640-8. [DOI] [PubMed] [Google Scholar]
- 8.Kwon J.H., Kim Y.J., Lee K.W., et al. Triple negativity and young age as prognostic factors in lymph node-negative invasive ductal carcinoma of 1 cm or less. BMC Cancer. 2010;10:557. doi: 10.1186/1471-2407-10-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsen C.B., Hirsch A., Eaton A., et al. Extent of microinvasion of ductal carcinoma in situ is not associated with sentinel lymph node metastases. Ann Surg Oncol. 2014;21:3330–3335. doi: 10.1245/s10434-014-3920-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasad M.L., Osborne M.P., Giri D.D., et al. Microinvasive carcinoma (T1mic) of the breast: clinicopathologic profile of 21 cases. Am J Surg Pathol. 2000;24:422–428. doi: 10.1097/00000478-200003000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Katz A., Gage I., Evans S., et al. Sentinel lymph node positivity of patients with ductal carcinoma in situ or microinvasive breast cancer. Am J Surg. 2006;191:761–766. doi: 10.1016/j.amjsurg.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Wong J.H., Kopald K.H., Morton D.L. The impact of microinvasion on axillary node metastases and survival in patients with intraductal breast cancer. Arch Surg. 1990;125:1298–1301. doi: 10.1001/archsurg.1990.01410220082011. discussion 1301-1302. [DOI] [PubMed] [Google Scholar]
- 13.Shatat L., Gloyeske N., Madan R., et al. Microinvasive breast carcinoma carries an excellent prognosis regardless of the tumor characteristics. Hum Pathol. 2013;44:2684–2689. doi: 10.1016/j.humpath.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Lyons J.M., 3rd, Stempel M., Van Zee K.J., et al. Axillary node staging for microinvasive breast cancer: is it justified? Ann Surg Oncol. 2012;19:3416–3421. doi: 10.1245/s10434-012-2376-5. [DOI] [PubMed] [Google Scholar]
- 15.Early Breast Cancer Trialists’ Collaborative Group (EBTCG) Correa C., McGale P., et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010:162–177. doi: 10.1093/jncimonographs/lgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Early Breast Cancer Trialists’ Collaborative Group. Darby S., McGale P., et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/s0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whelan T.J., Pignol J.P., Levine M.N., et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–520. doi: 10.1056/nejmoa0906260. [DOI] [PubMed] [Google Scholar]
- 18.START Trialists’ Group. Bentzen S.M., Agrawal R.K., et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomized trial. Lancet Oncol. 2008;9:331–341. doi: 10.1016/S1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.START Trialists’ Group. Bentzen S.M., Agrawal R.K., et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomized trial. Lancet. 2008;371:1098–1107. doi: 10.1016/S0140-6736(08)60348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whelan T., MacKenzie R., Julian J., et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst. 2002;94:1143–1150. doi: 10.1093/jnci/94.15.1143. [DOI] [PubMed] [Google Scholar]
- 21.Wang W., Zhu W., Du F., et al. The demographic features, clinicopathological characteristics and cancer-specific outcomes for patients with microinvasive breast cancer: a SEER database analysis. Sci Rep. 2017;7 doi: 10.1038/srep42045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rakovitch E., Sutradhar R., Lalani N., et al. Multiple foci of microinvasion is associated with an increased risk of invasive local recurrence in women with ductal carcinoma in situ treated with breast-conserving surgery. Breast Cancer Res Treat. 2019;178:169–176. doi: 10.1007/s10549-019-05364-z. [DOI] [PubMed] [Google Scholar]
- 23.Solin L.J., Fowble B.L., Yeh I.T., et al. Microinvasive ductal carcinoma of the breast treated with breast-conserving surgery and definitive irradiation. Int J Radiat Oncol Biol Phys. 1992;23:961–968. doi: 10.1016/0360-3016(92)90900-3. [DOI] [PubMed] [Google Scholar]
- 24.Shaaban A.M., Hilton B., Clements K., et al. The presentation, management and outcome of patients with ductal carcinoma in situ (DCIS) with microinvasion (invasion <1 mm in size) – results from the UK Sloane Project. Br J Cancer. 2022 doi: 10.1038/s41416-022-01983-4. Oct 12 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sopik V., Sun P., Narod S.A. Impact of microinvasion on breast cancer mortality in women with ductal carcinoma in situ. Breast Cancer Res Treat. 2018;167:787–795. doi: 10.1007/s10549-017-4572-2. [DOI] [PubMed] [Google Scholar]
- 26.Offersen B.V., Alsner J., Nielsen H.M., et al. Hypofractionated versus standard fractionated radiotherapy in patients with early breast cancer or ductal carcinoma in situ in a randomized phase III trial: the DBCG HYPO trial. J Clin Oncol. 2020;38:3615–3625. doi: 10.1200/jco.20.01363. [DOI] [PubMed] [Google Scholar]
- 27.Chua B.H., Link E.K., Kunkler I.H., et al. Radiation doses and fractionation schedules in non-low-risk ductal carcinoma in situ in the breast (BIG 3-07/TROG 07.01): a randomized, factorial, multicenter, open-label, phase 3 study. Lancet. 2022;400:431–440. doi: 10.1016/s0140-6736(22)01246-6. [DOI] [PubMed] [Google Scholar]
- 28.Bartelink H., Horiot J.-C., Poortmans P.M., et al. Impact of a higher radiation dose on local control and survival in breast-consering therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol. 2007;25:3259–3265. doi: 10.1200/jco.2007.11.4991. [DOI] [PubMed] [Google Scholar]
- 29.Morrow M., Van Zee K.J., Solin L.J., et al. Society of surgical Oncology-American society for radiation Oncology-American society of clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. Pract Radiat Oncol. 2016;6:287–295. doi: 10.1016/j.prro.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuzick J., Sestak I., Pinder S.E., et al. Effect of taxomifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12:21–219. doi: 10.1016/s1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher B., Dignam J., Wolmark N., et al. Tamoxifen in treatment of intraductal breast cancer: national Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/s0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 32.Moran M.S., Schnitt S.J., Giuliano A.E., et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stage I and II invasive breast cancer. J Clin Oncol. 2014;32:1507–1515. doi: 10.1200/jco.2013.53.3935. [DOI] [PubMed] [Google Scholar]