Summary
Background
This study aims to evaluate whether changes in therapeutic strategies have improved survival of patients diagnosed with hormone receptor positive (HR+), HER2 negative (HER2−) advanced breast cancer (ABC) in real-world.
Methods
All 1950 patients systemically treated for HR+/HER2− ABC and diagnosed between 2008 and 2019 in eight hospitals were retrieved from the SONABRE Registry (NCT-03577197). Patients were categorized per three-year cohorts based on year of ABC diagnosis. Tests for trend were used to examine differences in baseline characteristics, Kaplan–Meier methods and Cox proportional hazards for survival analyses, and competing-risk methods for 3-year use of systemic therapy.
Findings
Over time, patients were older (≥70 years, 37%, n = 169/456 in 2008–2010, 47%, n = 233/493 in 2017–2019, p = 0.004) and more often had multiple metastatic sites at ABC diagnosis (48%, n = 220/456 in 2008–2010, 56%, n = 275/493 in 2017–2019, p = 0.002). Among patients with metachronous metastases the prior exposure to (neo-) adjuvant therapies increased over time (chemotherapy, 38%, n = 138/362 in 2008–2010, 48%, n = 181/376 in 2017–2019, p = <0.001; endocrine therapy, 64%, n = 231/362 in 2008–2010, 72%, n = 271/376 in 2017–2019, p = <0.001). Overall survival significantly improved from median 31.1 months (95% CI:28.2–34.3) for patients diagnosed in 2008–2010 to 38.4 months (95% CI:34.0–41.1) in 2017–2019 (adjusted hazard ratio = 0.76, 95% CI:0.64–0.90; p = 0.001). Three-year use of CDK4/6 inhibitors increased from 0% for patients diagnosed in 2008–2010 to 54% for diagnosis in 2017–2019. Conversely, three-year use of chemotherapy was 50% versus 36%, respectively.
Interpretation
Over time, patients diagnosed with HR+/HER2− ABC presented with less favourable patient characteristics. Nevertheless, we observed that overall survival of ABC increased between 2008 and 2019, with increased use of endocrine/targeted therapies.
Funding
The SONABRE Registry is supported by the Netherlands Organization for Health Research and Development (ZonMw: 80-82500-98-8003); Novartis BV; Roche; Pfizer; and Eli Lilly & Co. Funding sources had no role in the writing of the manuscript.
Keywords: Real-world, Metastatic breast cancer, Survival, Registry, CDK4/6 inhibitors
Research in context.
Evidence before this study
The treatment landscape of HR+/HER2− advanced breast cancer is changing. New available therapies include amongst others mTOR inhibitors and CDK4/6 inhibitors. Phase 3 clinical trials on CDK4/6 inhibitors have all proved progression-free survival benefit. The overall survival data show a positive trend but with mixed results in terms of statistical significance. There is no current data on the overall survival of the total HR+/HER2− population since the implementation of CDK4/6 inhibitors. Until 2016 overall survival results in population-based studies showed a constant survival rate.
Added value of this study
This is the first population-based cohort study that reveals an improvement in overall survival for the HR+/HER2− ABC population in recent years. Interestingly, this study also reveals that patients and tumour characteristics at time of advanced breast cancer diagnosis have become more unfavourable.
Implications of all the available evidence
Changed treatment patterns now do result in an overall survival improvement in real-life, underlining the effectiveness of new targeted therapies in HR+/HER2− breast cancer treatment worldwide, while delaying the use of chemotherapy.
Introduction
Advanced – metastatic – breast cancer (ABC) is generally a disease with no curative treatment options. Palliative antitumor therapies are prescribed to lengthen overall survival and improve quality of life. Improvement of overall survival in the real-world setting, due to the introduction of new systemic therapies, was previously only reported in patients with human epidermal growth factor receptor 2 (HER2) positive disease.1
Currently CDK4/6 inhibitors added to endocrine therapy has become the new preferred type of palliative therapy in first-line for HR+/HER2− ABC.2, 3, 4, 5, 6 The MONALEESA-2 trial on ribociclib and letrozole in first-line has reported a median overall survival time of over 5 years in the experimental treatment arm, an improvement of more than 12 months as compared with the placebo arm.7 The MONARCH 3 study has shown similar survival gains with abemaciclib in a second interim analysis, from a median survival of 54.5 months in the placebo group to a median 67.1 months in the treatment group.8 It remains unclear why the PALOMA-2 study, using palbociclib as CDK4/6 inhibitor, did not show an improvement in overall survival, whereas the gain in progression-free survival was seen at a comparable hazard ratio.9 Apart from the survival outcome, prior real-world studies have shown that use of CDK4/6 inhibitors delays the use of chemotherapy.10,11 Delay of chemotherapy is generally positive for patients’ quality of life, as chemotherapy usually is more toxic for patients than endocrine and targeted therapy. Accordingly, in daily practice the treatment patterns for patients diagnosed with HR+/HER2− ABC are changing.
Patients with metachronous distant metastases may already have received (neo-)adjuvant anthracyclines with cyclofosfamide (AC), taxanes, and/or aromatase inhibitor therapy at primary breast cancer diagnosis. Obviously, the intensified early breast cancer treatment has led to a significant reduction in risk of recurrence and a better relative survival for patients diagnosed with early breast cancer.12, 13, 14 But, for those who yet face a distant recurrence during follow-up, these shifts in the early breast cancer treatments may have changed the ABC patient baseline characteristics and may have worsened prognosis at time of ABC diagnosis. Conversely, the increased use of CDK4/6 inhibitors in daily practice is expected to lead to an improved overall survival for patients diagnosed with ABC since 2017. In this real-world study, we therefore aimed to assess trends in overall survival, patient characteristics, and systemic therapies of patients diagnosed with HR+/HER2− ABC between 2008 and 2019 in the Netherlands.
Methods
Patient selection
Data for this study were obtained from the Southeast Netherlands advanced breast cancer (SONABRE) registry. The SONABRE Registry (NCT-03577197) is an observational cohort study, including all patients (≥18 years) with an ABC diagnosis in the Southeast of the Netherlands. The registry includes patient characteristics, tumour specifications and treatment information for the primary tumour and metastatic disease, extracted from medical files by specially trained registrars. When information on vital status was unknown, it was checked in the Dutch Municipal Administrative Database. The registry was approved by the Medical Research Ethics Committee of Maastricht University Medical Centre (15-4-239). For the current study, patients were selected when diagnosed with HR+/HER2− ABC from 2008 until 2019 and systemically treated in one of eight hospitals where registration was complete, including one academic comprehensive cancer centre, four teaching and three non-teaching hospitals. Last follow-up was collected in 2021 and data lock was on October 15, 2021.
Definitions
The HR+/HER2− subtype was defined as the presence of oestrogen receptor and/or progesterone receptor of ≥10% and a negative in situ hybridization for HER2 or a IHC score of 0 or +1. Biopsy of a metastatic site was used, if unavailable the latest biopsy results were used (recurrence or primary tumour). Patients for whom HER2− status was unknown were included when they never had received HER2− targeted therapy. Metastatic-free interval (MFI) was defined as the time between date of diagnosis of the primary tumour and date of diagnosis of the first distant metastasis. Year of diagnosis was clustered per 3-year period: 2008–2010, 2011–2013, 2014–2016 and 2017–2019. We chose to divide the cohort into 3-year periods to identify the role of new implemented therapies in ABC in specific years.
Endpoints
The primary endpoint was median overall survival of patients per 3-year period of diagnosis. Overall survival was defined as the time between date of ABC diagnosis and date of death. Secondary endpoints included patient and tumour characteristics at time of ABC diagnosis and the use of systemic therapies within three years since ABC diagnosis. The 3-year use of systemic therapy represents the proportion of patients who started the therapy of interest within 3 years following ABC diagnosis, and included chemotherapy, endocrine monotherapy, CDK4/6 inhibitors with endocrine therapy, and mTOR inhibitors with endocrine therapy.
Statistical analyses
Differences in patient and tumour characteristics were analysed using the Mantel–Haenszel test for trend. Median overall survival was estimated by Kaplan Meier methodology. All patients still alive were censored at the date of last follow-up. Kaplan–Meier curves were curtailed when only 10% of patients were in follow-up.15 We adjusted overall survival for potential confounders in a Cox proportional hazard analysis with the pre-specified factors: age at diagnosis, metastatic-free interval, number of metastatic sites, and metastatic localization. This analysis was performed in the total study population (model 1), for patients with metachronous metastases (model 2) and for patients with metachronous metastases including a correction for prior exposure to (neo-) adjuvant systemic therapies (model 3). All potential predictors with a p-value of <0.2 in the univariable analysis were included in the main multivariable model. All reported p-values are two-sided and considered statistically significant at ≤0.05. Finally, three-year use of therapies was calculated using competing-risk methodology, considering start of the therapy of interest as ‘event’ and death without use of the therapy of interest as ‘competing event’, and censoring patients in follow-up at the date of last update. The 3-year period is specifically chosen as it matches the expected median overall survival based on the data of patients in 2007–2009 in a prior study of the SONABRE (i.e. 24.8 months16), as we considered that therapies used in the period before the median survival time point could influence the median survival results. The 3-year period also matches median follow-up time for patients diagnosed in 2017–2019 (i.e. 34.4 months).
Results
A total of 1950 patients were systemically treated for HR+/HER2− ABC in 2008–2019 and included in this study. Overall, median age at ABC diagnosis was 66 years (Table 1). The WHO performance status was 0–1 for 83% of patients. The majority of patients (72%) was diagnosed with carcinoma of no special type (NST). Metastatic-free interval was categorized as <3 months (23%), 3–60 months (26%), 60–120 months (23%) and >120 months (28%). Overall, 54% of patients had visceral metastases and 3% central nervous system metastases.
Table 1.
Patient characteristics at time of advanced breast cancer diagnosis for the total study population, and for patients diagnosed per period 2008–2010, 2011–2013, 2014–2016 and 2017–2019.
| Characteristics | Total (n = 1950) | 2008–2010 (n = 456) | 2011–2013 (n = 521) | 2014–2016 (n = 480) | 2017–2019 (n = 493) | p-value for Trend |
|---|---|---|---|---|---|---|
| Sex | 0.36 | |||||
| Female | 1932 (99) | 450 (99) | 516 (99) | 477 (99) | 489 (99) | |
| Age (in years) | 0.004 | |||||
| Median (range) | 66 (25–98) | 65 (33–95) | 67 (33–94) | 67 (32–95) | 69 (25–98) | |
| <70 years | 1158 (59) | 287 (63) | 314 (60) | 297 (62) | 260 (53) | |
| ≥70 years | 792 (41) | 169 (37) | 207 (40) | 183 (38) | 233 (47) | |
| WHO status | 0.57 | |||||
| 0–1 | 1204 (83) | 153 (86) | 324 (81) | 342 (83) | 385 (82) | |
| 2–4 | 251 (17) | 25 (14) | 74 (19) | 69 (17) | 83 (18) | |
| Unknown | 495 | 278 | 123 | 69 | 25 | |
| Comorbidities | 0.001 | |||||
| Any | 1137 (58) | 237 (52) | 305 (59) | 282 (59) | 313 (64) | |
| Histology | 0.43 | |||||
| Ductal (NST) | 1412 (72) | 328 (72) | 375 (72) | 343 (72) | 366 (74) | |
| Lobular | 412 (21) | 94 (21) | 118 (23) | 110 (23) | 90 (18) | |
| Other/unknown | 126 (7) | 34 (7) | 28 (5) | 27 (6) | 37 (8) | |
| MFI | 0.71 | |||||
| <3 months | 447 (23) | 94 (21) | 122 (23) | 114 (24) | 117 (24) | |
| 3–60 months | 510 (26) | 147 (32) | 117 (23) | 120 (25) | 126 (26) | |
| 60–120 months | 443 (23) | 101 (22) | 122 (23) | 119 (25) | 101 (21) | |
| >120 months | 550 (28) | 114 (25) | 160 (31) | 127 (27) | 149 (30) | |
| Number of metastatic sites | 0.002 | |||||
| Single | 907 (47) | 236 (52) | 257 (49) | 196 (41) | 218 (44) | |
| Multiple | 1043 (54) | 220 (48) | 264 (51) | 284 (59) | 275 (56) | |
| Sites of metastases | ||||||
| Bones | 1477 (76) | 345 (76) | 408 (78) | 351 (73) | 373 (76) | 0.53 |
| Bone-only | 639 (33) | 165 (36) | 190 (37) | 135 (28) | 149 (30) | 0.006 |
| Soft tissuea | 663 (34) | 125 (27) | 158 (30) | 196 (41) | 184 (37) | <0.001 |
| Visceralb | 1057 (54) | 242 (53) | 271 (52) | 269 (56) | 275 (56) | 0.23 |
| CNSc | 67 (3) | 18 (4) | 16 (3) | 19 (4) | 14 (3) | 0.52 |
| (Neo-)adjuvant chemotherapyd | <0.001 | |||||
| Taxane or AC | 276 (18) | 83 (23) | 82 (21) | 74 (20) | 37 (10) | |
| Taxane and AC | 334 (22) | 33 (9) | 72 (18) | 106 (29) | 123 (33) | |
| Other adjuvant chemotherapy | 84 (6) | 22 (6) | 29 (7) | 12 (3) | 21 (6) | |
| No (neo-)adjuvant chemotherapy | 809 (54) | 224 (62) | 216 (54) | 174 (48) | 195 (52) | |
| (Neo-)adjuvant ETd | <0.001 | |||||
| AI with/without tamoxifen | 638 (42) | 115 (32) | 152 (38) | 198 (54) | 173 (46) | |
| Tamoxifen only, or other | 397 (26) | 116 (32) | 103 (26) | 80 (22) | 98 (26) | |
| No (neo-)adjuvant ET | 468 (31) | 131 (36) | 144 (36) | 88 (24) | 105 (28) |
Data are given as number (%) unless otherwise indicated.
ABC, advanced breast cancer; AC, anthracyclines and cyclophosphamide; AI, aromatase inhibitors; CNS, central nervous system; ET, endocrine therapy; MFI, metastatic-free interval; WHO, World Health Organization.
Soft tissue, lymph nodes, skin and eye.
Liver, lung, pleura, peritoneum, gastrointestinal tract, other.
Brain and leptomeningeal.
Among patients with recurrent metastases (excluding patients with de novo ABC).
The proportion of patients aged above 70 years significantly increased from 41% in 2008–2010 to 47% in 2017–2019 (p = 0.004). In line, the proportion of patients with comorbidities at time of ABC diagnosis increased from 52% in cohort 2008–2010 to 64% in cohort 2017–2019 (p = 0.001). In more recent years, more patients had multiple metastatic sites at time of ABC diagnosis, increasing from 48% in 2008–2010 to 56% in 2017–2019 (p = 0.002). Metastatic-free interval distribution remained constant over time (p = 0.71). Among patients with metachronous metastases (i.e. MFI >3 months) the use of (neo-) adjuvant chemotherapy significantly increased from 38% in 2008–2010 to 48% in 2017–2019 (p = <0.001) (baseline characteristics for this group are specified in Supplementary Table S1). Patients diagnosed with ABC in more recent years had more frequently received prior taxane and AC as (neo-)adjuvant chemotherapy, 9% in 2008–2010 versus 33% in 2017–2019 (p = <0.001). In addition, more patients had received prior adjuvant endocrine therapy, 64% in 2008–2010 versus 72% in 2017–2019 (p = <0.001). The use of prior adjuvant aromatase inhibitors increased from 32% in 2008–2010 to 46% in 2017–2019 (p = <0.001).
Overall survival
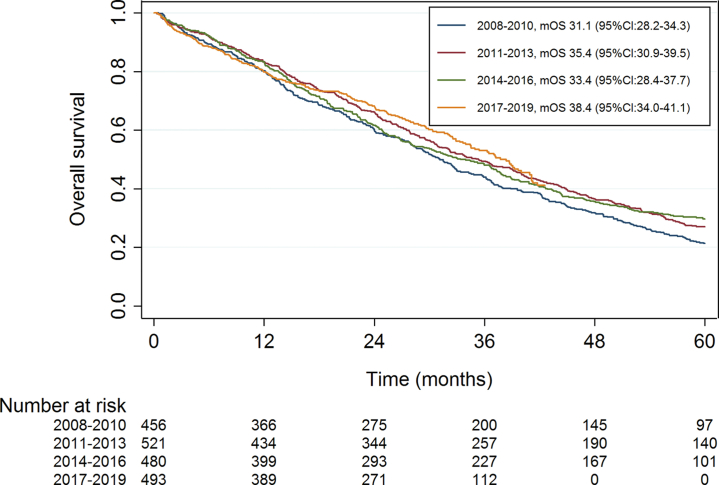
Median follow-up time of the total study population was 81.6 months, for the patients diagnosed in 2017–2019 median follow-up time was 34.4 months. Median overall survival gradually improved from 31.1 months (95% CI:28.2–34.3) for patients diagnosed with ABC in 2008–2010 to 38.4 months (95% CI:34.0–41.1) for patients diagnosed in 2017–2019 (Fig. 1). After adjustment for the pre-specified prognostic factors, the improvement in overall survival showed to be statistically significant for the patients diagnosed in the most recent cohorts with an adjusted hazard ratio of 0.85 (95% CI:0.73–0.99, p = 0.03) for those diagnosed with ABC in 2014–2016 and of 0.76 (95% CI:0.64–0.90, p = 0.001) for 2017–2019, compared with patients diagnosed in 2008–2010 (Table 2).
Fig. 1.
Overall survival in systemically treated patients diagnosed with HR+/HER2− ABC per period of diagnosis. mOS, median overall survival; CI, confidence interval.
Table 2.
The adjusted hazard ratios of overall survival per 3-year period of diagnosis for HR + /HER2− ABC patients, corrected for patient- and tumour characteristics in three models using multivariable proportional hazard analysis.
| N (%) | All patients (N = 1950, 1469 events) |
N (%) | Patients with metachronous metastases (N = 1503, 1151 events) |
|||||
|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
||||||
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |||
| Period of diagnosis | ||||||||
| 2008–2010 | 456 (23) | Ref | 362 (24) | Ref. | Ref. | |||
| 2011–2013 | 521 (27) | 0.88 (0.76–1.02) | 0.09 | 399 (27) | 0.88 (0.74–1.03) | 0.11 | 0.79 (0.67–0.94) | 0.007 |
| 2014–2016 | 480 (25) | 0.85 (0.73–0.99) | 0.03 | 366 (24) | 0.87 (0.74–1.03) | 0.12 | 0.71 (0.59–0.85) | <0.001 |
| 2017–2019 | 493 (25) | 0.76 (0.64–0.90) | 0.001 | 376 (25) | 0.75 (0.62–0.90) | 0.003 | 0.63 (0.51–0.77) | <0.001 |
| Age at diagnosis | ||||||||
| Per year | 1.02 (1.01–1.02) | <0.001 | 1.01 (1.01–1.02) | <0.001 | 1.02 (1.01–1.03) | <0.001 | ||
| Metastatic-free interval | ||||||||
| <3 months | 447 (23) | Ref. | ||||||
| 3–60 months | 510 (26) | 1.89 (1.62–2.20) | <0.001 | 510 (34) | Ref. | Ref. | ||
| 60–120 months | 443 (23) | 1.05 (0.89–1.23) | 0.58 | 443 (29) | 0.56 (0.48–0.65) | <0.001 | 0.55 (0.47–0.64) | <0.001 |
| >120 months | 550 (28) | 0.71 (0.60–0.83) | <0.001 | 550 (37) | 0.38 (0.33–0.44) | <0.001 | 0.43 (0.36–0.50) | <0.001 |
| Metastatic localization | ||||||||
| Bone-only | 639 (33) | Ref | 466 (31) | Ref. | Ref. | |||
| Soft tissue, without visceral or CNS | 230 (12) | 0.71 (0.55–0.90) | 0.005 | 169 (11) | 0.71 (0.54–0.94) | 0.02 | 0.70 (0.53–0.93) | 0.01 |
| Visceral, without CNS | 1014 (52) | 1.21 (1.00–1.47) | 0.05 | 811 (54) | 1.30 (1.05–1.61) | 0.02 | 1.29 (1.04–1.60) | 0.02 |
| CNS | 67 (3) | 1.88 (1.37–2.59) | <0.001 | 57 (4) | 1.77 (1.24–2.51) | 0.001 | 1.65 (1.16–2.35) | 0.005 |
| Number of metastatic sites | ||||||||
| Single | 907 (47) | Ref | 680 (45) | Ref. | Ref. | |||
| Multiple | 1043 (53) | 1.48 (1.25–1.77) | <0.001 | 823 (55) | 1.40 (1.15–1.70) | 0.001 | 1.43 (1.17–1.73) | <0.001 |
| (Neo-)Adjuvant chemotherapy | ||||||||
| No chemotherapy | – | – | 809 (54) | – | – | Ref. | ||
| AC or taxane | – | – | 276 (18) | – | – | 1.20 (0.98–1.46) | 0.08 | |
| AC and taxane | – | – | 334 (22) | – | – | 1.33 (1.08–1.62) | 0.007 | |
| Other chemotherapy | – | – | 84 (6) | – | – | 0.89 (0.65–1.22) | 0.48 | |
| (Neo-)Adjuvant ET | ||||||||
| No adjuvant ET | – | – | 468 (31) | – | – | Ref. | ||
| Tamoxifen or other | – | – | 397 (26) | – | – | 1.56 (1.30–1.88) | <0.001 | |
| AI with/without tamoxifen | – | – | 638 (42) | – | – | 1.88 (1.58–2.24) | <0.001 | |
ABC, advanced breast cancer; AC, anthracyclines and cyclophosphamide; AI, aromatase inhibitors; CNS, central nervous system; ET, endocrine therapy.
Model 1: Total study population; Model 2: patients with metachronous metastases without correcting for adjuvant therapies; Model 3: patients with metachronous metastases with correction for adjuvant therapies.
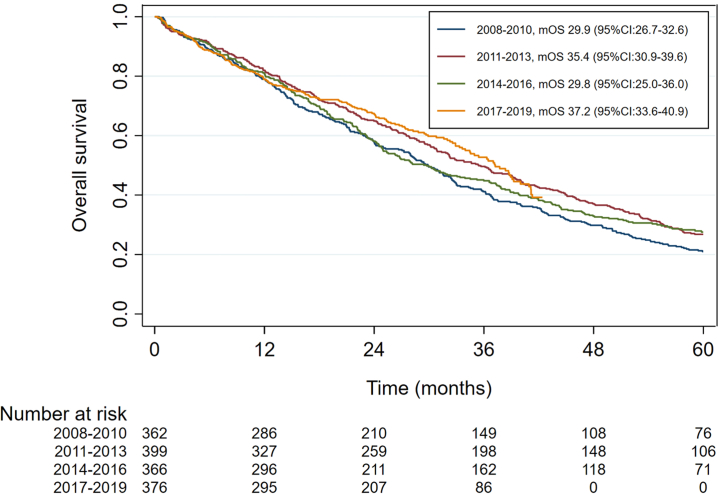
In patients with metachronous metastases, median overall survival improved from 29.9 months (95% CI:26.7–32.6) for patients diagnosed with ABC in 2008–2010 to 37.2 months (95% CI:33.6–40.9) for diagnosis in 2017–2019 (Fig. 2). The overall survival for patients diagnosed in 2017–2019 versus 2008–2010 was significantly better with an adjusted hazard ratio of 0.75 (95% CI:0.62–0.90, p = 0.003) without adjustment for prior (neo-) adjuvant systemic therapy and 0.63 (95%CI:0.51–0.77, p = <0.001) after additional correction for prior (neo-) adjuvant systemic therapy (Fig. 2, Table 2). When adjusting for prior systemic therapy for early breast cancer in patients with metachronous metastases, ABC outcome shows to improve statistically significant for patients since 2011, with an adjusted HR of 0.79 (95% CI:0.67–0.94, p = 0.007) for the 2011–2013 cohort, an adjusted HR of 0.71 (95% CI:0.59–0.85, p < 0.001) for the 2014–2016 cohort and an adjusted HR of 0.63 (95% CI:0.51–0.77, p < 0.001) for the 2017–2019 cohort, compared with cohort 2008–2010. Patients who were previously treated with (neo-) adjuvant systemic therapy had a statistically significant worse overall survival when compared with patients who did not receive (neo-) adjuvant chemotherapy or endocrine therapy. For patients who received prior (neo-) adjuvant AC and taxane chemotherapy the adjusted hazard was 1.33 (95% CI:1.08–1.62, p = 0.007) and for patients who received prior (neo-)adjuvant aromatase inhibitors (AI) with or without tamoxifen the adjusted hazard ratio was 1.88 (95% CI:1.58–2.24, p < 0.001).
Fig. 2.
Overall survival in systemically treated patients diagnosed with metachronous HR+/HER2− ABC per period of diagnosis. mOS, median overall survival; CI, confidence interval.
Systemic therapy
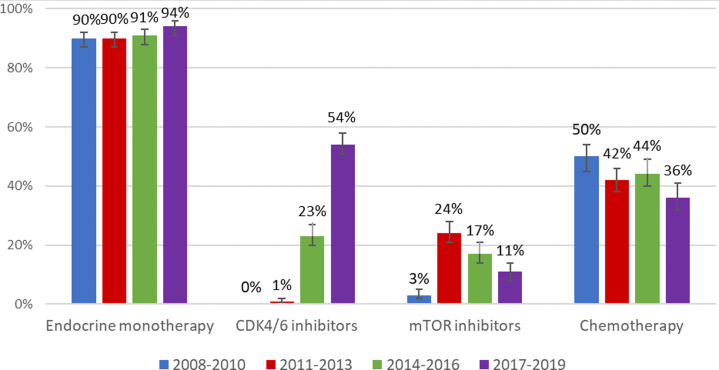
Among all systemically treated patients, use of endocrine therapy in the 3 years following ABC diagnosis was 90% for patients diagnosed with ABC in 2008–2010, 90% for cohort 2011–2013, 91% for cohort 2014–2016 and 94% for cohort 2017–2019 (Fig. 3, Supplementary Fig. S1). The use of CDK4/6 inhibitors in the first 3 years since diagnosis was 0% for patients diagnosed with ABC in 2008–2010, 1% for cohort 2011–2013, 23% for cohort 2014–2016 and 54% for cohort 2017–2019. Among the 518 lines of CDK4/6 inhibitor therapy, 431 (83%) comprised palbociclib, 86 (17%) ribociclib and 1 (<1%) abemaciclib. Three-year use of mTOR inhibitors for cohorts 2008–2010, 2011–2013, 2014–2016, and 2017–2019 were respectively 3%, 24%, 17% and 11%. Three-year use of chemotherapy decreased from 50% for patients diagnosed with ABC in 2008–2010 to 36% for those diagnosed in 2017–2019.
Fig. 3.
Cumulative use of systemic therapies during the first three years since the diagnosis of HR+/HER2− advanced breast cancer with 95% confidence intervals, per period of diagnosis. See Supplementary Fig. S1 for the graphs of the cumulative use of these systemic agents.
Discussion
This study has investigated time trends in overall survival, patient characteristics and systemic therapy for 1950 patients diagnosed with HR+/HER2− ABC in the Southeast of the Netherlands between 2008 and 2019. We observed a statistically significant and clinically relevant increasing overall survival between 2008 and 2019. This improvement was observed, even though patient characteristics at time of ABC diagnosis became more unfavourable over the years. Additionally, patients who were previously treated with (neo-) adjuvant systemic therapy had a statistically significant worse overall survival compared with patients who did not receive (neo-) adjuvant chemotherapy or endocrine therapy.
In this study, patients more recently diagnosed were older and more often had comorbidities. In the total study population 41% of patients is aged above 70 years at time of ABC diagnosis. Relevant to consider is the median age at early breast cancer diagnosis, which is about 61 years.12 We observed that 51% of patients were diagnosed with distant metastases more than five and 28% more than ten years after primary HR+/HER2− diagnosis, which also explains an elderly age at ABC diagnosis. In comparison to other cohorts, the EU-5 cohort with 82,073 patients diagnosed with HR+/HER2− ABC in 2008–2010 reported 37% of patients aged above 70 years, identical to our results (i.e. 37% in 2008–2010).17 However, the French ESME-MBC cohort includes a somewhat younger population, with a median age of 62 years in the HR+/HER2−population diagnosed in 2008–2016, as opposed to a median age of 66 years in 2008–2019 in our study.18 Differences in age distribution between HR+/HER2− ABC cohort studies might reflect differences in patient selection, by for example the registration of only patients from academic hospitals versus patients from all hospitals, or by different informed consent requirements. Remarkably, in our study we observed an increase of patients aged above 70 years from 37% in 2008–2010 to 47% in 2017–2019. Ageing of the population is a phenomenon observed throughout Europe. Between 2011 and 2021 there was an increase of the share of people aged above 65 years with 3% in the European Union and 4.2% in the Netherlands.19 Of note, the age distribution of the general population is similar in the Netherlands and Europe, with 21% of inhabitants aged above 65 years in 2021. But, in the Dutch province Limburg, where most patients included in the SONABRE Registry live, the proportion of patients aged above 65 years is slightly higher, i.e. 25%.20 We expect that the ageing of the population probably also led to the higher prevalence of comorbidities over time, although this could also have resulted from a better registration of comorbidities with the introduction of electronic health records over the study period.
This study illustrates a change in prior exposure to (neo-) adjuvant systemic therapies in patients with metachronous metastases. We observed that over time, more patients with HR+/HER2− ABC were previously exposed to a combination of AC and taxanes, and aromatase inhibitors as part of their prior (neo-) adjuvant therapy. The prior exposure to (neo-) adjuvant therapy for patients diagnosed with metachronous metastases in 2017–2019 in our study was 48% for chemotherapy and 72% for endocrine therapy. In the French ESME cohort, including patients diagnosed with ABC in 2008–2016, the prior exposure to (neo-) adjuvant chemotherapy was 68% and the prior exposure to (neo-)adjuvant endocrine therapy 83%.18 For ABC patients included in the German PREAGNANT registry and diagnosed in 2014–2017, 48% had received (neo-) adjuvant chemotherapy and 83% (neo-) adjuvant endocrine therapy.21 It should be noted, that the rate of prior systemic therapy when looking at an ABC population is not the same as the rate in early breast cancer. The differences and similarities between the cohort studies is probably the result of patient selection.
In the total study population, the median overall survival increased from 31.1 months in patients diagnosed in 2008–2010 to 38.4 months in patients diagnosed in 2017–2019. The addition of 7 months is in this context a clinically relevant improvement. The SONABRE Registry is the first population-based cohort study that reveals an improvement in overall survival for the HR+/HER2− ABC population in recent years.
The improvement in overall survival over time in our study cohort seems to be associated with changes in systemic treatment choices in the HR+/HER2− ABC setting. This is supported by the observation that the survival improvement was larger after correcting for baseline characteristics and (neo-) adjuvant therapies. The most relevant treatment changes were a lower use of chemotherapy within the first 3 years after ABC diagnosis from 50% for patients diagnosed in 2008–2010 to 36% for those diagnosed in 2017–2019, while the use of CDK4/6 inhibitors increased from 0% to 54% over the same period. Apart from the gradual implementation of CDK4/6 inhibitors, other explanations for the gradual improvement of survival could be the introduction of other more effective systemic therapies, such as everolimus, fulvestrant, capecitabine and taxane, toegether with increased focus on additional supportive care measures leading to more lines of active anti-tumour treatment.22 Based on the results from the clinical phase 3 trials, we expect that the survival of the HR+/HER2− ABC population will further increase with the ongoing implementation of CDK4/6 inhibitor therapy in the real-world setting.7, 8, 9 In a prior study of the SONABRE Registry we observed that the implementation of CDK4/6 inhibitors was somewhat lower than in other Western countries, mostly explained by the Dutch guideline recommendation that the use of CDK4/6 inhibitors in second line may be considered while awaiting the results of the SONIA study.10,23 It is of importance to notice that most patients in the SONABRE Registry were treated with palbociclib as CDK4/6 inhibitor. Although no conclusions on the effectiveness of CDK4/6 inhibitors can be made from our study, our results seem to support the hypothesis that all types of CDK4/6 inhibitors may provide an overall survival benefit, despite the ongoing discussion regarding the variable significance in the phase 3 trials. Remarkably, the three-year use of mTOR inhibitor everolimus decreased from 24% in 2011–2013 to 11% in 2017–2019. In line with guidelines, the results of the SONABRE Registry indicates that the use of everolimus is postponed to later lines of treatment since the implementation of CDK4/6 inhibitors.10 By the introduction of PI3 kinase inhibitors in more recent years, we expect that well-chosen and more effective second- and further lines of endocrine and targeted therapies may contribute to further improvements in overall survival of patients with HR+/HER2− ABC.24,25 The improved overall survival we observed in this real-world setting despite a reduced use of chemotherapy, indicates the value of endocrine and targeted therapy and fits the earlier findings of relative chemotherapy resistance in this particular breast cancer subtype.
The median overall survival time of 38.4 months observed in our population is shorter than survival results reported in clinical trials and some real-world studies. Median overall survival of patients treated in the phase 3 clinical trials of CDK4/6 inhibitors is over 50 months.7, 8, 9 However, the current study is conducted in a population-based study, representing the whole population of HR+/HER2− ABC patients, which is not comparable to a clinical trial. The first-line clinical trials were performed with specific patient selections, for example only including patients with performance status 0 or 1, without progression on adjuvant endocrine therapy and without symptomatic visceral disease. The ESME cohort study reported a median overall survival of 43.3 months in patients diagnosed with HR+/HER2− ABC in 2008–2016.18 In Sweden in 2009–2016 the median overall survival of HR + ABC patients was 37.0 months.26 Apart from differences in therapy use, differences in outcomes between observational studies may be caused by differences in patient populations and selection. As previously mentioned, the ESME cohort has a younger study population, which is probably explained by the patient selection from 18 comprehensive cancer centres, whereas SONABRE includes one academic comprehensive cancer centre, four teaching and three non-teaching. Additionally, routine follow-up to detect metastases in its asymptomatic phase is not advised in the Netherlands, while in the ESME study 51% of ABC diagnoses were screen-detected. This earlier diagnosis is expected to extend the survival time by the duration of the lead time.
This real-world study is the first to show an improvement in overall survival for patients diagnosed with HR+/HER2− ABC, which is rewarding for the work in the scientific field of breast cancer treatment. The SONABRE Registry is an unselected observational cohort study in the Southeast of the Netherlands without inclusion bias. The findings are specific for the Southeast of the Netherlands, because patient characteristics and treatment choices are country- or region-specific, limiting generalizability of the study findings.27 Nevertheless, the improvement of overall survival in patients diagnosed with HR+/HER2− ABC can be expected throughout the world when improved treatment options are implemented. The analysis only included patients who were systemically treated, to prevent an underestimation of the survival in comparison to other studies. The proportion of patients not systemically treated (5% of patients with HR+/HER2− ABC in the registry) and their median overall survival (0.9–1.2 months, data not further shown) remained stable over time and we therefore not presume this could lead to a selection bias. For the definition of the HR+/HER2− we chose to include patients with an unknown HER2 status when they never had received HER2− targeted therapy, although this might have led to a misclassification in a minority of the patients. In terms of registration bias, we observed an increase in the number of soft tissue and lymph node metastases over time, but we expect this to be the consequence of registration differences. Other factors may have led to a confounding bias. For example, the overall increase of multiple metastases at ABC diagnosis could be the effect of higher quality imaging techniques and increased use of nuclear imaging, e.g. by PET-CT-scanning, over the past decade, although data to evaluate this were not available in the Registry. In the multivariate analysis of the overall survival, we have corrected for all patient and tumour characteristics that changed over time, but the results should be interpreted with caution, as residual confounding may remain. Moreover, in this analysis we focused on endocrine/targeted therapy and chemotherapy, but changes in PARP inhibitors in the (small) subset of patients with germline BRCA1/2 mutations may also have improved outcome.
The registration of outcomes in daily practice remains relevant in patients diagnosed with HR+/HER2− ABC. In the near future, the HR+/HER2− group is expected to be further subdivided into HER2 negative and HER2 low, based on new treatment opportunities. Furthermore, the introduction of new adjuvant therapies, such as adjuvant abemaciclib, might again change the characteristics of patients with ABC and possibly even lead to a downfall of survival in the ABC setting if at the same time no therapy improvements are made. Therefore, continuous monitoring of survival outcome and treatment patterns is needed.
Conclusion
This study indicates that patients, when diagnosed with HR+/HER2− ABC in more recent years more often had received prior (neo-)adjuvant therapies and tend to have less favourable patient and tumour characteristics. Nevertheless, there is an increase in the overall survival of this population. This is accompanied by an increased use of CDK4/6 inhibitors and lower use of chemotherapy in the ABC setting.
Contributors
MM, SG and VTH: Conceptualization, Methodology; FE, MD, BV, KA, MP, LW, NP and MK: Resources; MM, SG, AF and VTH: Investigation, validation; SG: Data curation, Formal analysis, Visualization; VTH: Supervision; MM: Writing original draft; MM, FE, MD, BV, KA, MP, LW, NP, JT, JH, AW, AF, MK, and VTH: Writing – review & editing.
Data sharing statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Declaration of interests
MM report grants institutional grants from Gilead. SG reports institutional grants from Novartis BV, Roche, Pfizer, Eli Lilly, Daiichi Sankyo and Gilead, and personal fees from Astra-Zeneca. VTH reports institutional grants and personal fees from Roche, Novartis, Pfizer, and Eli Lilly, personal fees from Accord Healthcare, institutional grants from AstraZeneca, Eisai, Daiichi Sankyo and Gilead. All remaining authors have declared no conflicts of interest.
Acknowledgements
We thank our SONABRE registrars of the department of Medical Oncology of Maastricht University Medical Centre (MUMC+), Maastricht, the Netherlands.
Footnotes
Translation: For the Dutch translation of the abstract see Supplementary Materials section.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2022.100573.
Appendix A. Supplementary data
References
- 1.Gobbini E., Ezzalfani M., Dieras V., et al. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur J Cancer. 2018;96:17–24. doi: 10.1016/j.ejca.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Gennari A., André F., Barrios C.H., et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–1495. doi: 10.1016/j.annonc.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Burstein H.J., Somerfield M.R., Barton D.L., et al. Endocrine treatment and targeted therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: ASCO guideline update. J Clin Oncol. 2021;39(35):Jco2101392. doi: 10.1200/JCO.21.01392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston S., O'Shaughnessy J., Martin M., et al. Abemaciclib as initial therapy for advanced breast cancer: MONARCH 3 updated results in prognostic subgroups. npj Breast Cancer. 2021;7(1):80. doi: 10.1038/s41523-021-00289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rugo H.S., Finn R.S., Dieras V., et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174(3):719–729. doi: 10.1007/s10549-018-05125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hortobagyi G.N., Stemmer S.M., Burris H.A., et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 7.Hortobagyi G.N., Stemmer S.M., Burris H.A., et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022;386(10):942–950. doi: 10.1056/NEJMoa2114663. [DOI] [PubMed] [Google Scholar]
- 8.Goetz M.P., Toi M., Huober J., et al. LBA15 MONARCH 3: interim overall survival (OS) results of abemaciclib plus a nonsteroidal aromatase inhibitor (NSAI) in patients (pts) with HR+, HER2- advanced breast cancer (ABC) Ann Oncol. 2022;33:S1384. doi: 10.1016/j.annonc.2024.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Finn R.S., Rugo H.S., Dieras V.C., et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): analyses from PALOMA-2. J Clin Oncol. 2022;40(17_suppl):LBA1003–LBA. [Google Scholar]
- 10.Meegdes M., Geurts S.M.E., Erdkamp F.L.G., et al. The implementation of CDK 4/6 inhibitors and its impact on treatment choices in HR+/HER2- advanced breast cancer patients: a study of the Dutch SONABRE Registry. Int J Cancer. 2021;150(1) doi: 10.1002/ijc.33785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneeweiss A., Ettl J., Lüftner D., et al. Initial experience with CDK4/6 inhibitor-based therapies compared to antihormone monotherapies in routine clinical use in patients with hormone receptor positive, HER2 negative breast cancer — data from the PRAEGNANT research network for the first 2 years of drug availability in Germany. Breast. 2020;54:88–95. doi: 10.1016/j.breast.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Meer D.J., Kramer I., van Maaren M.C., et al. Comprehensive trends in incidence, treatment, survival and mortality of first primary invasive breast cancer stratified by age, stage and receptor subtype in The Netherlands between 1989 and 2017. Int J Cancer. 2021;148(9):2289–2303. doi: 10.1002/ijc.33417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 14.Cardoso F., Kyriakides S., Ohno S., et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 15.Pocock S.J., Clayton T.C., Altman D.G. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet. 2002;359(9318):1686–1689. doi: 10.1016/S0140-6736(02)08594-X. [DOI] [PubMed] [Google Scholar]
- 16.Lobbezoo D.J., van Kampen R.J., Voogd A.C., et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat. 2013;141(3):507–514. doi: 10.1007/s10549-013-2711-y. [DOI] [PubMed] [Google Scholar]
- 17.DeKoven M., Bonthapally V., Jiao X., et al. Treatment pattern by hormone receptors and HER2 status in patients with metastatic breast cancer in the UK, Germany, France, Spain and Italy (EU-5): results from a physician survey. J Compar Effectiv Res. 2012;1(5):453–463. doi: 10.2217/cer.12.43. [DOI] [PubMed] [Google Scholar]
- 18.Deluche E., Antoine A., Bachelot T., et al. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008–2016. Eur J Cancer. 2020;129:60–70. doi: 10.1016/j.ejca.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Population structure and ageing Eurostat Statistics explained, 2022. Available from: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Population_structure_and_ageing.
- 20.Age groups of inhabitants per province in the Netherlands, 2022. Available from: https://allecijfers.nl/ranglijst/inwoners-naar-leeftijd-per-provincie-in-nederland/.
- 21.Hartkopf A.D., Huober J., Volz B., et al. Treatment landscape of advanced breast cancer patients with hormone receptor positive HER2 negative tumors – data from the German PRAEGNANT breast cancer registry. Breast. 2018;37:42–51. doi: 10.1016/j.breast.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Temel J.S., Greer J.A., Muzikansky A., et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 23.van Ommen-Nijhof A., Konings I.R., van Zeijl C.J.J., et al. Selecting the optimal position of CDK4/6 inhibitors in hormone receptor-positive advanced breast cancer - the SONIA study: study protocol for a randomized controlled trial. BMC Cancer. 2018;18(1):1146. doi: 10.1186/s12885-018-4978-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.André F., Ciruelos E., Rubovszky G., et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380(20):1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 25.Rugo H.S., Lerebours F., Ciruelos E., et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22(4):489–498. doi: 10.1016/S1470-2045(21)00034-6. [DOI] [PubMed] [Google Scholar]
- 26.Valachis A., Carlqvist P., Ma Y., et al. Overall survival of patients with metastatic breast cancer in Sweden: a nationwide study. Br J Cancer. 2022;127(4):720–725. doi: 10.1038/s41416-022-01845-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jansen Christel, Bas Amesz V. 2020. Improving time to patients access to innovative oncology therapies in Europe. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.