Abstract
Photodynamic therapy (PDT) is applied as a robust therapeutic option for tumor, which exhibits some advantages of unique selectivity and irreversible damage to tumor cells. Among which, photosensitizer (PS), appropriate laser irradiation and oxygen (O2) are three essential components for PDT, but the hypoxic tumor microenvironment (TME) restricts the O2 supply in tumor tissues. Even worse, tumor metastasis and drug resistance frequently happen under hypoxic condition, which further deteriorate the antitumor effect of PDT. To enhance the PDT efficiency, critical attention has been received by relieving tumor hypoxia, and innovative strategies on this topic continue to emerge. Traditionally, the O2 supplement strategy is considered as a direct and effective strategy to relieve TME, whereas it is confronted with great challenges for continuous O2 supply. Recently, O2-independent PDT provides a brand new strategy to enhance the antitumor efficiency, which can avoid the influence of TME. In addition, PDT can synergize with other antitumor strategies, such as chemotherapy, immunotherapy, photothermal therapy (PTT) and starvation therapy, to remedy the inadequate PDT effect under hypoxia conditions. In this paper, we summarized the latest progresses in the development of innovative strategies to improve PDT efficacy against hypoxic tumor, which were classified into O2-dependent PDT, O2-independent PDT and synergistic therapy. Furthermore, the advantages and deficiencies of various strategies were also discussed to envisage the prospects and challenges in future study.
Keywords: Photodynamic therapy, Hypoxia, Tumor, Nanomaterials
Graphical abstract
Latest progresses in the development of innovative strategies are summarized to improve photodynamic therapy (PDT) efficacy against hypoxic tumor, including O2-dependent PDT, O2-independent PDT and synergistic therapy.
1. Introduction
Delivery of oxygenated blood is severely hampered owing to the uncontrolled proliferation of tumor cells and unorganized growth of vasculature. Such oxygen diffusion limitation leads to the hypoxic tumor microenvironment (TME). Almost all solid tumors are featured by hypoxia, whose oxygen partial pressure is below 10 mm Hg [1,2]. Numerous studies supported that the hypoxia-induced invasive potential of tumor cells played a crucial role in metastasis [3]. Additionally, the activity of hypoxia-inducible factor (HIF-1) is increased under hypoxic condition, which contributes to drug resistance in tumor therapy [4]. The TME is slightly acidic under hypoxia. Meanwhile, glucose transporters and glycolysis-related genes are both up-regulated when mediated by HIF-1, leading to an enhanced anaerobic glycolysis in cancer cells and further aggravating the acidic environment [5]. Moreover, the tumor genetic phenotype is altered under the hypoxic condition, which affects the protein expression in tumor cells, enabling the tumors to exhibit higher hypoxia adaptability for enhanced tumor aggressiveness [6]. In short, TME is closely related to rapid malignancy progression and poor clinical prognosis, which is regarded as a significant target for clinical cancer treatment [7].
PDT has received increasing attention as an effective approach to cancer treatment for its high selectivity, absence of cumulative toxicity, lack of acquired or intrinsic resistance mechanisms, and its non-invasive nature [8]. There are two main mechanisms of PDT, i.e. type I and type II PDT, which are both composed of the PS, light with a specific wavelength, and oxygen dissolved in the cells [9]. Currently, most cases of cancer treatments are based on the type II mechanism. After PS is activated by an appropriate wavelength of light, the energy would be transferred directly from PS to the oxygen molecule, resulting in the generation of reactive oxygen species (ROS) [10]. Vascular destruction, cell apoptosis and immune response induced by ROS are proved critical in cancer treatment [11]. Previous studies have indicated that PDT is an O2 consumption process, and the rapid O2 consumption will exacerbate the tumor hypoxia to reduce the PDT efficacy. Moreover, tumor hypoxia can also promote drug resistance and lead to tumor recurrence [12]. PDT has been widely used for the treatment of superficial tumors, such as superficial basal cell carcinoma (BCC) and Bowen's disease (BD), which are less affected by the hypoxia condition. However, most of the PSs have the maximal absorptions ranging from visible to near-infrared (NIR) wavelength, resulting in a limited penetration depth (less than 1 cm) [13]. Additionally, the exciting light of PSs will be absorbed or scattered by body tissues [14], which will further hinder the application of PDT in deep tumors. In recent years, PDT has gradually been used in bladder cancer, cholangiocarcinoma and breast cancer in clinic. [15,16]. Several studies have confirmed that tumor hypoxia is happened at the early stage of tumor occurrence and development. Alleviating tumor hypoxia and reducing O2 consumption might provide promising strategies to improve the PDT efficacy. Therefore, it is necessary to give a comprehensive and in-depth summary of the whole scene of recent strategies to boost PDT efficiency on hypoxic tumor. The main contents of this review are divided into the following three parts, i.e. oxygen-dependent PDT, oxygen-independent PDT and synergistic therapy.
2. Oxygen-dependent PDT
2.1. Hyperbaric oxygen therapy
Hyperbaric O2 therapy (HBOT) is a treatment method based on the elevated O2 partial pressure. With the increased atmospheric pressure and inhaled O2 concentration, the alveolar partial pressure elevates in proportion. The amount of dissolved O2 (DO) in the plasma is subsequently amplified [17], thereby increasing O2 tissue delivery to enhance PDT. In addition, HBOT is beneficial to strengthen antimicrobial activity and attenuate HIF-mediated effects to alleviate tumor hypoxia. Notably, it can also significantly lessen the formation of oxidative stress, thus increasing the body's healing capacity. Inflammation is reduced through vasoconstriction and angiogenesis [18]. Based on the above advantages, HBOT is widely used in clinical practice, but obvious deficiencies are still observed during HBOT. For example, O2 poisoning is the most typical adverse effect. Besides, the strategy may also enhance the side effects of drugs and cause middle ear barotrauma, which needs further optimization [19].
Hyperbaric oxygen (HBO) can assist PDT to strengthen the decomposition of collagen in the tumor extracellular matrix (ECM). Based on this property, Yang et al. proposed a synergistic strategy of upconversion nano-photosensitizer (UNPSs) in combination with HBO. HBO could promote the diffusion of O2 and UNPSs into the deep tumors to enhance the PDT efficacy in vivo [20]. Surprisingly, the synergistic effect also significantly improved the therapeutic effect at a low laser power density, which also exhibited a good biological safety. In addition, the researchers also proposed a method to co-administer DOX-loaded liposomes (Doxil) with HBO [21]. Among which, HBO could not only reduce the collagen deposition at the tumor ECM to improve the tumor penetration of Doxil, but also promote the sensitivity of tumor cells to enhance the antitumor efficacy.
Moreover, HBO is able to regulate the abnormal mechanical TME and enhance oxygen diffusion in the chaotic blood vessels of solid tumors. Therefore, researchers synthesized HBO-PD-1 Ab with the programmed cell death-1 antibody (PD-1 Ab) for enhanced penetration and retention (EPR) effect of tumor tissue [22]. Notably, HBO could significantly disrupt hypoxia-mediated immunosuppression. It helped PD-1 Ab to trigger potent cytotoxic T lymphocytes and long-lasting immune memory, acting to inhibit tumor recurrence. Furthermore, it was also demonstrated that HBO therapy could directly eliminate the formation of cancer stem cell-like cells (CSCs) and cancer metastasis. HBO therapy promoted the commercialized nanomedicine delivery in stroma-rich solid tumors [19], showing a promise to cure stroma-rich solid tumors in the clinic.
In addition, Yang et al. also deeply investigated the antitumor effect of the combination of HBO, gemcitabine (GEM) with Abraxane on mice with pancreatic ductal adenocarcinoma tumors, in order to further explore the mechanisms of HBO to dysregulate tumor. Then a useful conclusion has been made that HBO therapy could selectively attenuate hypoxia and directly inhibit cancer-related fibroblasts (CAFs) and ECM [23]. Nonetheless, the detailed molecular mechanism of HBO-induced CAFs inhibition has not yet been concluded.
2.2. Vascular normalization
TME is characterized by hypoxia. Distorted blood vessels and abnormally proliferated tumor cells lead to hypoxia in the tumor, which is further aggravated by a large amount of O2 consumption during PDT [24]. Vascular endothelial growth factor (VEGF) is one of the most important O2 regulatory cytokines in tumors. As the expression of VEGF increases, tumors promote angiogenesis, inducing tumor growth and metastasis [25]. At the same time, VEGF increases the immunosuppression in TME as an immunosuppressive factor. It reduces the infiltration of immune cells, while increases the difficulty of tumor treatment [26]. In addition, VEGF promotes VE-cadherin endocytosis on cell membrane through phosphorylation, thus promoting tumor metastasis [27]. As a result, many well-designed nanoplatforms improve the hypoxia state of tumors through vascular normalization, and reverse the immunosuppressive microenvironment to promote cancer treatment. However, vascular normalization strategy is short of uniform evaluation criteria, which greatly impacts the widespread clinical application. Specific molecular mechanisms are needed to be further studied for cancer treatment [28,29].
Aiming to effectively improve PDT and enhance therapeutic immunity, Luan et al. designed a CAM NP nanoplatform, which integrated chlorine e6 (Ce6), axitinib (AXT) and dextro-1-methyl tryptophan (1MT) [4]. Among which, AXT could inhibit VEGF to improve abnormal tumor blood vessels and increase blood perfusion thereby improving local hypoxia. Meanwhile, 1MT exerted a strong antitumor effect by inhibiting the activity of indoleamine 2,3-dioxy-genase (IDO) to reduce the immunosuppression of the tumor.
In addition, Tang et al. prepared tumor-derived exosome/AEGEN mixed nanocapsules (DES), which significantly inhibited tumor growth through effective tumor penetration and PDT [30]. First, AIEs generated ROS to kill tumor cells. Tumor-derived exosomes could simultaneously enhance the tumor targeting ability of AIE PS. Besides, the PDT effect of DES could be effectively improved by dexamethasone-induced normalization of tumor vascular function and hypoxic remission. At the same time, Xu et al. prepared a novel nanoplatform SPMI/3 by modifying polydopamine (PDA) nanoparticles (NPs) with sialic acid (SA)-polyethylene glycol (PEG), and loading the PSs polysaccharide cyanine (ICG) and 3PO [27]. Among which, 3PO inhibited the production of VEGF and reduced the endocytosis of ve-cadherin. Simultaneously, SA increased the tumor targeting ability of SPMI/3. The combination of PDT and PTT under the laser irradiation could well suppress the tumor growth and metastasis, providing a new direction for TME regulation in the treatment of hypoxic tumors.
2.3. H2O2 decomposition
The H2O2 level is elevated in tumors compared with normal tissues due to cell proliferation and angiogenesis. Catalase (CAT) catalyzes the decomposition of H2O2 into oxygen and water utilizing the increased H2O2 level in tumors, which improves the PDT efficiency by generating oxygen [31]. Besides, nanoenzymes also exhibit excellent biocatalytic performances to decompose H2O2, which are composed of metal or metal oxide nanomaterials, such as Ir, Pt, MnO2, and CeO2 [32]. Based on the previous studies, MnO2 nanosystems had been confirmed to not only generate O2 by triggering tumor H2O2 decomposition, but also served as a class of markedly T1- magnetic resonance imaging (MRI) agent, which benefited PDT enhancement with high safety [33].
CAT is an effective material to improve hypoxia in tumors [34], and fluoride can promote the transmembrane ability of CAT to avoid degradation. Based on the above principles, Shen et al. designed a fluorinated chitosan drug for oral cancer to achieve catalase transmission, which assembled fluoroalkyl and Ce6 onto branched chitosan (CS) and encapsulated CAT to construct CAT-FC-Ce6 NPs [35]. The nanocomplex possessed strong transmembrane ability, which could be effectively taken up by tumor cells, and decomposed H2O2 in cells to generate O2 to improve the hypoxia TME as well as the curative effect of PDT. Both In vivo and in vitro experiments demonstrated that the nanosystem had good targeting ability and biological safety, suggesting that it was a brand new and effective nanodrug for cancer therapy. Although CAT has a wide range of sources and a high activity, its stability in physiological environment is poor as a biological enzyme. How to improve the stability of CAT through safe and effective methods will become a major problem that must be solved in the clinical transformation [36,37].
Hollow polydopamine (HPDA) NPs with unique structure and feature are not only an excellent drug carrier system, but also can control drug release by assembling various shell structures. Ma et al. designed a therapeutic nanoplatform (HPDA@MnO2@Ce6/DOX@PEG-RGD, herein called HPMRCD) by coating HPDA with MnO2 to form HPDA@MnO2, then functioning with the modified PEG to load Ce6, doxorubicin (DOX) and PDA, which exhibited great performance on PDT improvement and TME responded-tumor MRI. Notably, HPMRCD had the capacity to precisely target the tumor site because of the acidity and abundant H2O2 in TME. Ce6, DOX, as well as sufficient O2 supplement were sped up to release as the depolymerization of HPMRCD, hence possessing a potency for tumor inhibition through the synergy of chemotherapy and PDT [33]. Additionally, Tang et al. also devised and created an intelligent glutathione (GSH) depletion and NIR regulation nanoplatform (MUM NPs) employing aggregation-induced emission (AIE) PSs with ROS production capability [38], which consisted of free AIE-active radioactive PSs, MnO2, and upconversion nanoparticles (UCNPs). Notably, owing to the introduction of UCNPs, AIE PSs were able to be activated to generate OH· under NIR irritation. In addition to the O2-producing function, MnO2 also presented great potency in reducing the GSH level and further turning the generated Mn+ into OH· through a Fenton-like reaction, thereby simultaneously achieving the relief of hypoxia and ROS depletion. Furthermore, the bimodal-MRI imaging function also provided accurate guidance for deep tumor treatment, and significantly boosted the efficiency of PDT in tumor treatment.
Ceria nanoparticles (CNPs) possess powerful enzyme-like activities, which can catalyze the decomposition of tumor endogenous H2O2 and effectively improve the tumor oxidation level. Inspired by this, Zhou et al. synthesized 6-hydrate (Ce) and ethylene glycol (EG) in a nitric acid solution [39], and then modified it with bioderived ATP and PS loading to obtain the hollow ceria nanozymes (ATP-HCNPs@Ce6) with a sufficient hollow layer and uniform shape. According to the oxygen production experiments, HCNPs showed great O2 releasing ability under a weakly acidic lysosomal TME at pH 5 owing to the ATP modification, which could be exploited to achieve precise manipulation of tumor hypoxia. In addition, the inflammatory infiltration and death regions of the tumor were found larger after treatment with ATP-HCNPs@Ce6. In summary, this synthetic method not only retained the enzyme mimic activity of CNPs, but also gained great drug delivery potential to overcome hypoxia in PDT. Nevertheless, issues were put forward that CNPs were seldom clinically converted for loading therapeutic drugs, so further studies were necessary to optimize this approach.
Pt not only acts as a kind of chemotherapeutic agent, but also plays a significant role in CAT-mimicry to facilitate PDT. Fang et al. developed nano-Pt/VP@MLip, which utilized both CAT-like activity and chemotherapeutic efficacy of Pt [40]. The nano-Pt was encapsulated in the liposomes by reversed-phase evaporation technology, and the PS verteporfin (VP) was further added to obtain nano-Pt/VP@Lipo. The complex was subsequently hybridized to cell membrane (CM) of RAW264.7 macrophage (Mφ) fabricating nano-Pt/VP@MLipo, aiming to gain extra biomimetic and targeting characteristics. Nano-Pt catalyzed the decomposition of H2O2 in tumor cells to provide O2 and enhanced the chemotherapy effect of VP. At the same time, the singlet oxygen (1O2) generated from the PDT process could cause damage to the liposome membrane, which accelerated the release of ultra-small and nano-Pt to enhance chemotherapy. After nano-Pt/VP@MLipo treatment, more ROS could be detected in 4T1 cells and tumor spheres, presenting a good cytotoxic effect. More importantly, the system possessed good biocompatibility and no obvious toxicity, hence performing a multifunctional antineoplastic effect to overcome hypoxic tumors. Nevertheless, nano-Pt itself also has a dose-dependent cytotoxicity like other platinum drugs. It still needs further data support to achieve clinical transformation.
2.4. Water splitting
Water splitting material is receiving extensive attention as an environmental protection and energy-saving method in PDT applications. Water provides a limitless supply of raw materials for water splitting reactions as the most abundant molecule in living organisms, thus elevating the level of O2 in vivo therapy [41].
CaO2 had been previously applied as an O2 generator to relieve oxygen deficiency in the hypoxic tumor. Its oxygen production mechanism was as Eq. 1 [42].
| CaO2 +H2O → 0.5O2 + Ca(OH)2 | (1) |
However, due to the limitation of acidic TME, the actual O2 production of CaO2 was restricted and CaO2 practically had a tendency to produce H2O2 Eq. 2 rather than O2 [43].
| CaO2 + 2H2O → H2O2 + Ca(OH)2 | (2) |
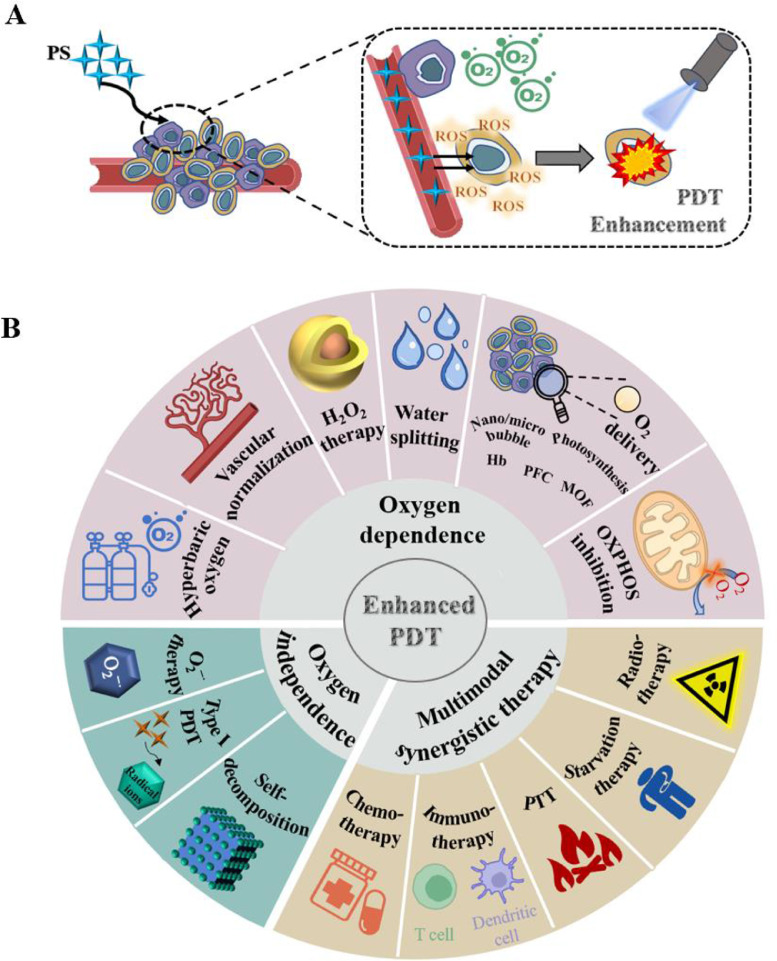
CaO2 can produce O2 and H2O2 from H2O, and H2O2 can further produce O2 under the catalysis of the catalase while CAT is unstable in the TME. However, its capacity to produce O2 is constrained due to the instability of CAT in tumors. Fig. 1 and Table 1, Table 2, Table 3.
Fig. 1.
Schematic illustration of photodynamic therapy (PDT) on tumor. (A) Mechanism of PDT enhancement without hypoxia at tumor site. (B) Overview of strategies to enhance PDT on hypoxic tumor.
Table 1.
Summary of the advantages and disadvantages of oxygen-dependent phototherapy.
| Strategies | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|
| Hyperbaric oxygen therapy |
|
|
[17,19] | |
| Vascular normalization |
|
|
[28,29] | |
| H2O2 decomposition | Catalase |
|
|
[36,37] |
| Nanoenzymes |
|
|
[149,150] | |
| Water splitting | CaO2 |
|
|
[45,46] |
| C3N4 |
|
|
[50,47] | |
| TiO2 |
|
|
[47,53] | |
| Hb-based oxygen carriers |
|
|
[151,152] |
|
| PFCs-based oxygen carriers |
|
|
[153,154] | |
| Microbubble/ nanobubble-based oxygen carriers |
|
|
[74,155] | |
| MOFs-based oxygen carriers |
|
|
[156,157] | |
| Biomimetic photosynthesis |
|
|
[46,87] | |
| OXPHOS inhibition |
|
|
[90] |
Table 2.
Summary of the advantages and disadvantages of oxygen-independent phototherapy.
| Strategies | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Superoxide anions therapy |
|
|
[95,158] |
| Type I PDT |
|
|
[95,99] |
| Self-decomposition |
|
|
[159] |
Table 3.
Summary of the advantages and disadvantages of photodynamic-derived multimodal synergistic therapy.
| Strategies | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Synergistic PDT/chemotherapy |
|
|
[160,161] |
| Synergistic PDT/immunotherapy |
|
|
[162,163] |
| Synergistic PDT/ PTT |
|
|
[161,164] |
| Synergistic PDT/ starvation therapy |
|
|
[142,143] |
| Synergistic PDT/ radiotherapy | Better penetration depths into tumor
|
|
[165,166] |
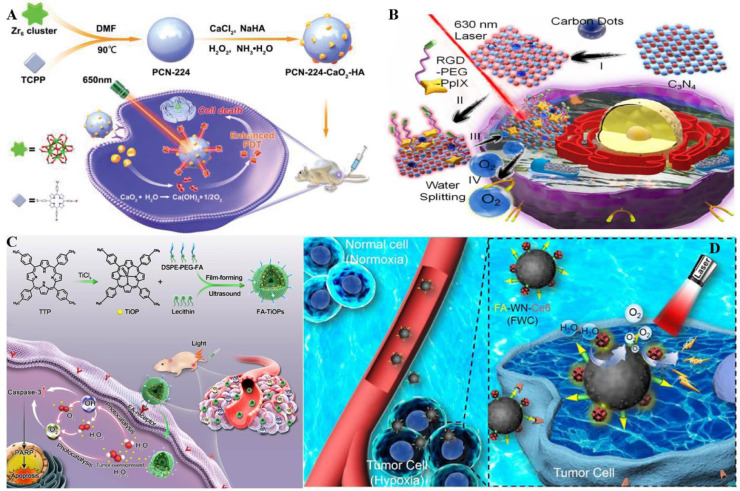
A CaO2-based nanostructure for enhanced self-administered oxygen had been reported by Zhang et al. aiming to achieve tumor hypoxia relief (Fig. 2A) [44]. PCN-224-CaO2-HA was obtained by combining porphyrin-based metal-organic cytoskeleton (PCN-224) with hyaluronate (HA)-modified CaO2, which generated sufficient O2 to address the PDT limitation via the reaction between CaO2 and H2O. Furthermore, except for the protection of CaO2, HA could also target the CD44 receptors, hence achieving precise treatment on 4T1 and MCF-7 cells. Although this pathway has a relatively slow rate of O2 release, its good biocompatibility promises clinical translation [45,46].
Fig. 2.
Representative method of O2 production by light-driven water splitting. (A) The PCN-224-CaO2—HA NPs were tail vein injected, HA maintained the stability and targeted tumor cells, while CaO2 reacted with H2O to generate O2 for tumor hypoxia relief. Reproduced from [44] with permission from Royal Society of Chemistry. (B) PCCN accumulated at tumor sites tumor cells and C3N4 participated in water splitting to produce enough O2 to promote PDT. And carbon point modification could enhance red light absorption. Reproduced from [49] with permission from American Chemical Society. (C) Under light treatment, FA targeted and accumulated in tumor cells, while TiOP splitting H2O to generate abundant O2 and H2O2, relieving tumor hypoxia and further generating ROS to induce tumor cell apoptosis. Reproduced from [52] with permission from American Chemical Society. (D) FWC NPs possessed great EPR effect. Water splitting was catalyzed by WN to elevate the ROS level, thereby optimizing PDT efficacy. Reproduced from [54] with permission from WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
C3N4 can be activated by blue light (∼420 nm) and then transfers energy to water, triggering the water splitting reaction [47]. C3N4 has the advantage of both small and adjustable band gap among various types of water splitting materials. Furthermore, it had been widely accepted to be a biocompatible material due to its metal-free element [48]. Zhang et al. had the idea of exploiting the vast quantity of H2O components in the human body and employing C3N4 as a water-splitting material to generate O2 to ameliorate tumor hypoxia (Fig. 2B) [49]. To expand the infrared light absorption range of C3N4, Zhang et al. added carbon dots to C3N4 nanocomposite (CCN) to improve the water splitting efficiency. After that, a porphyrin-containing photosensitizer (PpIx) and Arg-Gly-Asp (RGD) were combined with the CCN to create a multifunctional nanocomposite (PCCN). Results showed that under the irritation of the 630 nm laser, both the DO concentration and the generated ROS level were increased instantaneously in anoxic PBS with dispersed PCCN. Moreover, the group treated with PCCN was monitored to have less liver and lung metastasis compared with the other groups based on the animal experiment, indicating that this nanocomposite tended to display excellent antitumor efficiency for wide applicability with biocompatibility. The results are very promising. But given the high requirements for light irradiation power and the low split efficiency of C3N4, there is still much work to be done in this field [47,50].
Ultraviolet (UV) light can be absorbed by TiO2 nanomaterials to convert O2 and H2O into 1O2 and OH·, thus harming tumor tissues. Nevertheless, the activation of TiO2 is limited due to the relatively low penetration of UV light. UCNPs can effectively tackle this problem by converting NIR to UV light. In actual operation, TiO2 is activated under the 980 nm NIR laser in tumor tissues and highly cytotoxic ROS is generated to inhibit the tumor growth. Its mechanism was as Eq. 3 [51].
| (3) |
To overcome the hypoxic TME, a new type of nanostructure (FA−TiOPs) based on TiO−porphyrin was engineered by Cai et al. (Fig. 2C) [52]. Aiming at improving the biocompatibility and solubility capacity of the system, liposome-loaded TiO−porphyrin (TiOP) was subsequently modified with folic acid (FA). Furthermore, the drug had the potential for accumulating in the tumor through FA-mediated targeting in vivo. Additionally, TiOP could decompose the water into OH·, O2−· and H2O2 under photocatalysis, which could be converted to 1O2 via further excitation of TiOP. The generated OH· and 1O2 in turn induced tumor cell apoptosis by regulating predictive markers (Caspase-3 and PARP protein) to achieve tumor therapy. It must be noted that UV excitation does not provide an optimal penetration depth when comparing to NIR light. It causes tissue overheating and toxicity, hindering the preclinical research and clinical application of TiO2 [47,53].
Recently, a special focus on tungsten nitride (WN) was made to resolve hypoxia. For instance, Zhang et al. explored a WN-based O2 self-sufficient nanoplatform (FWC NPs) by coupling chimeric peptides Ce6- poly (ethylene glycol)-cysteine (Ce6-PEG-Cys) and folic acid-poly (ethylene glycol)-thiol (FA-PEG-SH) on the surface of WNNPs to relieve the hypoxia state at tumor sites (Fig. 2D) [54], which would seriously comprise the PDT efficiency. Moreover, FA with recognition feature endowed the nanomedicine to selectively aggregate in tumors. On the one hand, Ce6 produced ROS to eliminate the tumor upon being excited. On the other hand, WN supplied O2 through water cleavage to compromise the O2 consumption during the PDT process, which proved to be an appealing approach for PDT-induced tumor inhibition with low systemic toxicity.
2.5. Oxygen delivery pathway
To boost PDT efficiency, certain oxygen donors are utilized to deliver oxygen to tumor areas, such as hemoglobin (Hb), perfluorocarbon (PFC), or photosynthetic microorganism.
2.5.1. Hemoglobin-based oxygen carriers
Hb can bind four oxygen molecules in a reversible manner to transport O2 into tissue in the form of HbO2 [55]. As an endogenous protein in erythrocytes, its unique biosafe feature makes it considered as an excellent oxygen carrier for hypoxic tumors. Nonetheless, cell-free Hb is limited in biomedical applications owing to its poor stability, short circulation time and nephrotoxicity [56]. Notably, the above issues can be addressed by utilizing Hb-based carriers with chemically modified or microencapsulated, enabling its widespread use in O2-enhanced PDT.
When man-made materials enter the body, their properties suffer since their non-homology stimulated the immune response [57]. Concurrently, an aggressive mmRBC (AmmRBC) was developed by Zhang et al. to address the toxicity of hemoglobin in circulation due to its oxidability [58]. Inspired by the oxidation resistance of polydopamine (PDA), the complex formed by PDA, PDA-adsorbed PS, and Hb were encapsulated into the RBC membrane (RBCM) comprised biovesicles, which were developed through biomembrane recombination technology. The CAT and superoxide dismutase (SOD)-like functions of PDA helped AmmBRC display excellent tumor accumulation ability according to the obtained results. And the fantastic in situ oxygen production capacity of AmmBRC indicated that it could be served as an effective oxygen carrier to boost PDT efficiency.
Hb-linked conjugated polymer nanoparticles (CPNs) possess amplified light harvesting ability and fantastic photostability [59]. Drawing support from these properties, Wang et al. engineered a novel nanoplaform based on CPNs (Hb-NPs@liposome) for enhanced PDT [60]. Hb-NPs were firstly synthesized by covalently conjugating with Hb and NPs, and were subsequently encapsulated inside the fusogenic liposomes, which prevented oxidation during circulation. Chemiluminescence emitted by luminol could be absorbed by Hb-NPs under the extraneous addition of H2O2 and luminol, thereby generating cytotoxic ROS via chemiluminescence resonance energy transfer (CRET). Hb-NPs@liposome exhibited great self-luminescent and supply oxygen capacity, indicating a novel pathway for efficiency PDT.
Human serum albumin (HSA) in the human body is widely applied for its various advantages, including low immunogenicity and stimuli-responsive [61]. HSA was employed to hybrid with Hb, synthesizing a hybrid protein oxygen carrier (HPOC) by Cai et al., which exhibited great performance on O2 supplement and tumor targetin [62]. Additionally, DOX and Ce6 were loaded in HPOC to fabricate DC-HPOCs. The cellular uptake of DOX and Ce6 was improved due to the active targeting function of DC-HPOCs, and the expression levels of HIF-1α, MDR1, and P-gp were subsequently down-regulated, thus restraining DOX efflux. Consequently, this nanoplaform based on HPOC served as a promising avenue to achieve enhanced PDT and chemotherapy simultaneously. Nonetheless, the tumor-targeting function of DC-HPOCs was needed to monitor in the practical application, owing to the saturation of HSA-binding proteins.
2.5.2. Perfluorocarbon-based oxygen carriers
Numerous studies had revealed that PFCs show great oxygen solubility and high safety in humans, enabling oxygen transport by being encapsulated in various nanocarrier species to improve the oxygen levels in tumors [63]. Compared with Hb, the weak van der Waals interactions between PFC chains contribute to a higher gas solubility in the fluorous phase, helping alleviate the hypoxia situation in vivo more extensively [64]. It is noted that PFCs present excellent biocompatibility, helping alleviate the oxygen deficit situation [65]. However, the hypoxic TME can only be partially treated owing to the low loading capability of PFCs, thus showing limited potency to alleviate PDT [66].
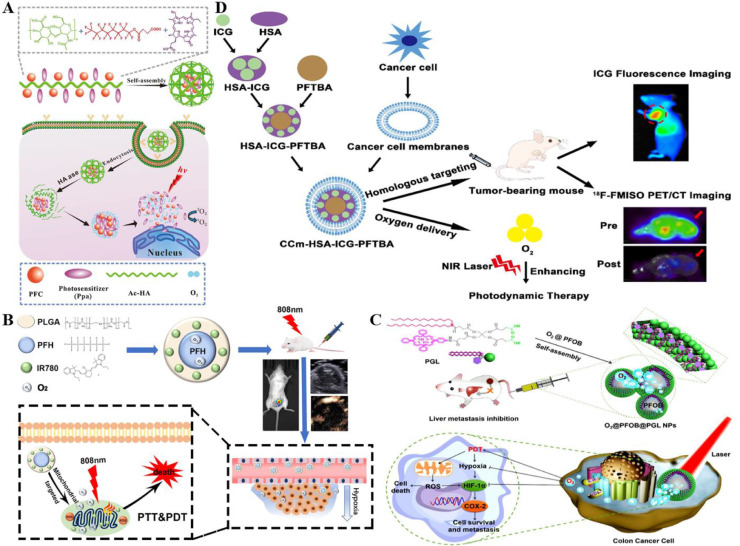
PFC was exploited to covalently immobilize with acetylated-hyaluronic acid (Ac-HA) to form Ac-HA-PFC owing to the tumor-targeting function and good biocompatibility of hyaluronic acid (HA). Then Ac-HA-PFC was conjugated with Pyropheophorbide a (Ppa), thereby forming the conjugates (Ac-HA-PFC-Ppa) by Zhou et al. (Fig. 3A) [67]. This O2-supplying system based on fluorinated hyaluronic PS showed great potency in dissolving PS and 1O2 generating capacity, thus providing a practical and convenient strategy for PDT enhancement in clinical application.
Fig. 3.
Representative nanostructures based on PFCs pathway for O2 carrying. (A) PFCs and Ppa were anchored to the polymer chain of HA to prepare Ac-HA-PFC-Ppa. Amphiphilic conjugates could self-assemble into micelles to perform intracellular tracking of cancer cells and elicit PDT efficacy. Reproduced from [67] with permission from Elsevier Ltd.. (B) PFC NPs for imaging-guided tumor PDT/PTT and therapeutic effect of intracellular tracking on cancer cells. Reproduced from [68] with permission from Chen et al.. (C) By ultrasonic dispersion of PFOB liquid into PGL NPs, an O2 self-supplemented PDT nanosystem of O2@PFOB@PGL NPs was fabricated to effectively treat liver metastasis of colon cancer. Reproduced from [63] with permission from American Chemical Society. (D) Bionic nanoparticles CCm-HSA-ICG-PFTBA delivered O2 and showed great potency on O2 self-supplement and PDT improvement. Reproduced from [70] with permission from The Author(s).
PFH and IR 780 were encapsulated inside a PLAG shell to construct a novel imaging-guided O2 delivering system by Niu et al. utilizing the ultrasound (US) contrast enhancement performance of liquid PFC and imaging characteristic of IR 780 (Fig. 3B) [68]. The system possessed great efficiency on ROS production benefiting from the exceptionally great O2 loading capacity of PFH and the preferentially mitochondrial-accumulating function of IR 780, thereby significantly enhancing PDT efficacy. Furthermore, IR 780 facilitated the PTT efficiency by serving as a class of remarkable fluorescent agents, thus achieving the combination of PDT and PTT in tumor treatment.
In addition, Dai et al. engineered a novel nanoplatform based on perfluorooctylbromide (PFOB) liquid (O2@PFOB@PGL NPs) for oxygen self-supplement (Fig. 3C) [63]. PFOB liquid was ultrasonically dispersed into porphyrin grafted lipid (PGL) NPs, resulting in a high 1O2 generation level and decreased porphyrins fluorescence loss owing to the ordered arrangement of the complexes. Moreover, O2@PFOB@PGL NPs possessed great potency on precise PDT to achieve O2 replenishment via the guidance of fluorescence/CT imaging. Besides, both HIF-1α and COX-2 expressions were found down-regulated in the experimental results, implying that O2@PFOB@PGL NPs served as a prominent system in cancer metastasis.
As Cancer cell membranes (CCm) display fantastic performance on tumor targeting and immune evasion [69], CCm were utilized as coats of the nanomedicine by Lan et al. (Fig. 3D) [70]. Perfluorotributylamine (PFTBA) was combined with ICG as a kind of PFC derivative with high O2 solubility and was subsequently encapsulated inside HSA to form a biomimetic O2 self-supplemented system (CCm–HAS-ICG-PFTBA). The hypoxia condition was found significantly alleviated in vivo based on the F-FMISO PET/CT imaging results, thereby exhibiting great potency on oxygen self-supplemented and PDT enhancement.
2.5.3. Microbubble/ nanobubble-based oxygen carriers
The core of nanobubbles or microbubbles is composed of medical gas, and the exterior is prepared by various shells, such as phospholipids, polymers, protein and surfactants. Its gas-bearing characteristics make it possess a photoacoustic image feature. It ameliorates tumor hypoxia mainly in two ways: one was intravenous injection of MNBs, which burst the bubble by using high-intensity ultrasound to release its internal gas [71]. The second method is to utilize the principle of gas diffusion along the concentration gradient to release the gas independently through the environmental gas concentration difference [72].
Zhang et al. designed a pH-responsive nanobubble shelled by acetylated dextran (AC-DEX) to deliver oxygen to tumor tissue more efficiently [73]. To be specific, a DCM solution containing AC-DEX and decane was emulsified in a lipid solution consisting of DPPC, DPPG, and DSPE-PEG to produce a nanoemulsion. The O2 nanobubbles were synthesized after a series of phase separation, freeze-drying, and O2 delivery. Acid-catalyzed hydrolysis of AC DEX to water-soluble dextran in an acidic environment led to the release of O2 from nanobubbles, which addressed the limitation of hypoxic tumor PDT. The intratumoral oxygen level provided by AC-DEX oxygen nanobubbles was found to increase approximately six-fold compared with the oxygen level initially based on the in vivo experiments.
However, nanobubbles suffer from the disadvantage of poor stability, which leads to a shortened half-life and limited application. In response to this problem, Sun et al. developed biogenic gas vesicles (GVs), a gas-filled compartment whose surfaces were decorated with a layer of liposomes [74]. The hypoxic state of cells could be reversed based on the in vitro results, and lipid-GVs (O2) exhibited a long circulation time according to the in vivo experiments. More importantly, this novel strategy possessed potency in long-term storage and no obvious toxicity, suggesting it was an efficient O2 delivery pathway to overcome the bottleneck of PDT in the hypoxic tumor.
2.5.4. Metal−organic frameworks (MOFs)-based oxygen carriers
Nanoscale metal-organic frameworks (nMOFs) are porous materials with a high surface area and excellent flexibility [75]. Growing studies had reported that nMOFs such as ZIF series and MIL series, had been widely used in drug delivery, luminescence and multiphase catalysis [76]. The encapsulated PS in the micropores of MOF nanomaterials minimized the aggregation of small molecule PSs due to the ordered crystal structure of MOFs, facilitating the generation of reactive 1O2. Besides, the porous structure of nMOFs can promote the diffusion of ROS, which effectively alleviates the hypoxia of tumor tissues and enhances the PDT efficiency [77]. In addition, nanoenzymes with high stability and catalase-like activity can be decorated on the photosensitivities integrated MOF, which cause severe damage to hypoxic tumors through a higher level of 1O2 [78].
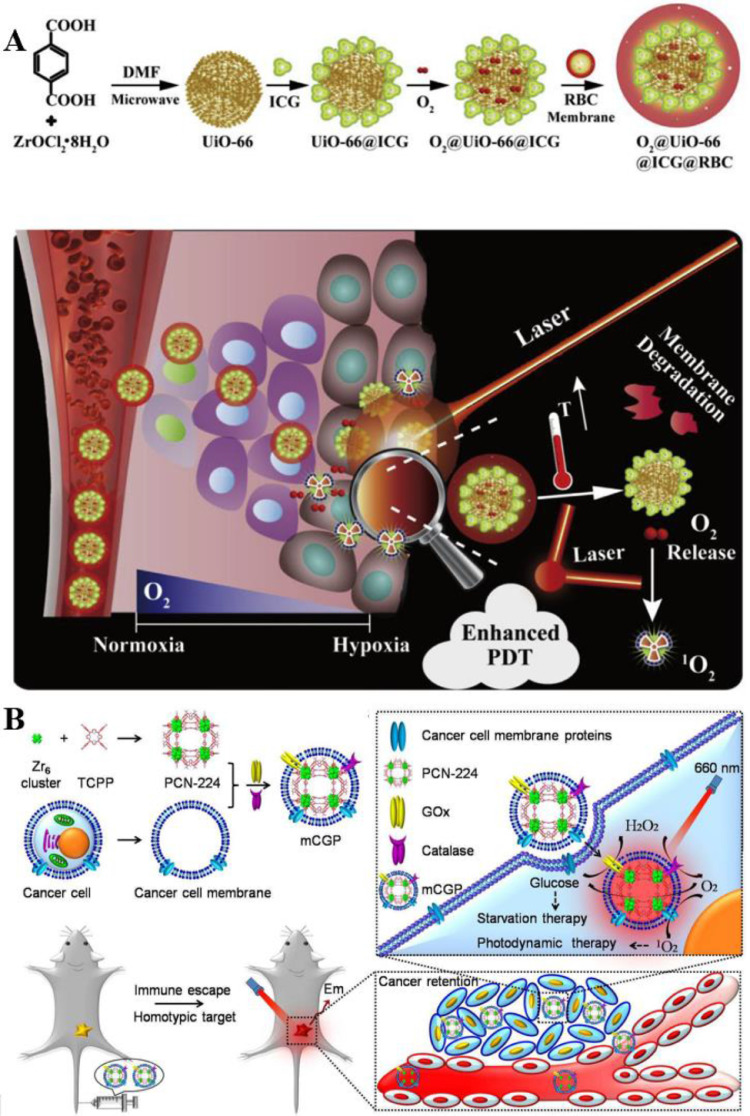
MOFs are regarded as prominent nanomaterials due to their significant gas storage capacity in the porous structure [79]. For example, Li et al. developed a MOFs-based nanoplatform for O2-evolutionary PDT (O2@UiO-66@ICG@RBC) (Fig. 4A) [80]. UiO-66 was coordinated with ICG conjugate utilizing its good biocompatibility and excellent loading capacity as an O2 carrier. And the complex was then encapsulated in the RBC membrane, which avoided being eliminated by the adaptive immune system. 1O2 was generated by ICG to degrade the erythrocyte membrane under laser irradiation at 808 nm. More importantly, the photothermal property of ICG enabled O2 release promotion from UiO-66, significantly improving PDT effectiveness on hypoxic tumors and offering a brand biomimetic strategy for O2-evolving PDT.
Fig. 4.
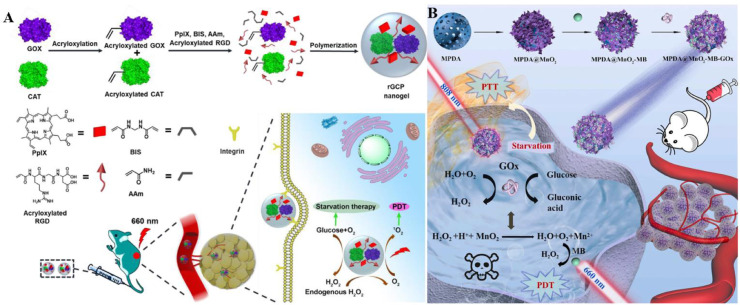
Representative nanostructured delivery system based on MOFs. (A) The RBC membranes coating protected biomimetic O2@UiO-66@ICG@RBC from attacking by the immune systems, enhancing the EPR effect. Under laser irradiation, the RBC membrane was degraded to release ICG and UiO-66, and ICG promoted O2 release from UiO-66 thus enhancing PDT. Reproduced from [80] with permission from Elsevier Ltd.. (B) MEM@Catalast@gox@PCN-224 escaped from the immune recognition and targeted tumor cells through CCm. CAT catalyzed endogenous H2O2 to generate O2, while GOx decomposed glucose in tumor tissues, facilitating the synergistic therapeutic effects of starvation therapy and PDT. Reproduced from [81] with permission from American Chemical Society.
More nutrition and energy are needed by abnormally proliferated cancer cells via the up-regulation of the aerobic glycolysis according to the Warburg effect. Therefore, effectively cutting off the nutritional supply and metabolic pathways of tumor cells are regarded as a promising approach to treating cancer [75]. Zhang et al. prepared a cascade bioreactor for cancer-targeted starvation and PDT treatment (mCGP, MEM@Catalast@gox@PCN-224). The Zr-MOF of PCN-224 was utilized as a nanoPSs to exert PDT efficacy (Fig. 4B) [81]. Meanwhile, glucose oxidase (GOx) and CAT were encapsulated inside the porphyrin-based Zr-MOF of PCN-224, and the complex was then coated with the cancer cell membrane to form the bioreactor. Consequently, mCGP exhibited fantastically performance both on O2 production and O2 consumption benefiting from the H2O2 decomposition catalyzing capacity as well as glucose metabolism blocking ability based on the cascade reactions.
2.5.5. Biomimetic photosynthesis
Since PDT is highly dependent on O2 and most of the malignant tumors are often in the hypoxic condition, the PDT application is seriously limited in hypoxic tumors. In this regard, photosynthetic microorganisms (chlorella, cyanobacteria, etc.) are introduced to produce O2 through photosynthesis under light conditions, thus reversing the hypoxia state in hypoxic tumor cells [82]. In addition, the process of bio-oxygen production by photosynthetic microorganisms under light conditions are also characterized by controllability and continuity. As a natural PS, chlorophyll released from algae can further enhance PDT by producing ROS that killed cancer cells [83]. For instance, as a kind of algae, cyanobacteria improves the hypoxic TME by utilizing water as an electron donor and releasing O2 via absorbing sunlight for photosynthesis [84].
Li et al. designed a novel bioreactor for cyanobacteria termed as Cyan@BPNSs inspired by the ideal oxygen-supplying function of cyanobacteria [85], which were modified with inorganic two-dimensional black phosphorus nanosheets (BPNSs). Substantial O2 was generated in situ continuously owing to the photosynthesis of cyanobacteria, and O2 was further turned into cytotoxicity 1O2 by the activation of BPNSs. The enhanced effect of this innovative bioreactor on PDT was demonstrated through in vivo and in vitro experiments on 4T1 tumor cells and corresponding xenograft models. This hybridization of microorganisms with inorganic nano PS expanded the scope of microbial nanomedicines and provided a promising avenue for PDT of hypoxic tumors.
Similarly, the mechanism of microbial photosynthesis to produce oxygen for tumor hypoxia combat was employed by Wu et al. [86]. But the difference was that they used PFC to collect oxygen, which further increased the oxygen concentration in the hypoxic TME. It was worth mentioning that Chlorella was an immune stimulant for PDT-induced antitumor immunity enhancement owing to the high level of 70 β-glucan and ARS-2 in it, which exerted a highly antineoplastic effect via activating NF-κB pathway and TLR2. A 90% inhibitory efficiency on tumors was observed based on the in vitro experimental result, indicating that it was an appealing approach through the combination of PDT and immunity activation for patient at the advanced stages.
However, there are some challenges in the currently developed O2 delivery pathways. For instance, it is difficult to get satisfactory treatment effect when Hb-based oxygen carriers possess short blood circulation. Meanwhile, the PDT efficiency would be hindered owing to the premature release of PFCs-based oxygen carriers in vivo. While MOFs-based oxygen carriers tend to cause damage to normal tissues due to long circulation half-lives. Furthermore, microbubble/nanobubble-based oxygen carriers are short of safety, which will cause biotoxicity after repeated administration. Biomimetic photosynthesis is safe but unstable, which has great defects in deep-seated tumor treatment [46,87].
2.6. Oxidative phosphorylation (OXPHOS) inhibition
The energy needed for tumor cell growth can be continuously supplied through aerobic respiration (Mito-AR) or glycolysis. Among them, Mito-AR produces adenosine triphosphate (ATP) through OXPHOS and electron transport chains while consuming oxygen at the same time [88]. Therefore, hyperactive Mito-AR in tumors exacerbates the O2 consumption, which hinderes the efficacy of PDT. Inhibition of OXPHOS can reduce oxygen consumption, and effectively increase the partial pressure of O2 in tumor cells, hence improving the hypoxic environment and further enhancing the effect of PDT. PSs such as porphyrins and Ce6, significantly block the respiratory chain and inhibit the OXPHOS of cells by targeting cytochrome c oxidase and F0F1ATP synthase in the mitochondrial inner membrane, thereby inhibiting the proliferation of tumor cells and enhancing their sensitivity to antitumor drugs [84].
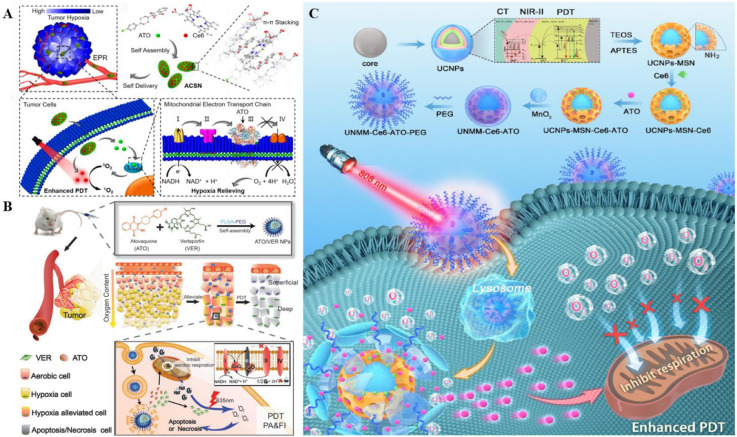
In recent years, carrier-free drug systems have received immense attention for safety reasons [89]. For instance, a carrier-free nanoplatform (ACSN) was designed and engineered by Li et al. (Fig. 5A) [90]. Self-assembling OXPHOS inhibitor ATO with Ce6 via π -π stacking and hydrophobic interaction, ACSN was constructed to display excellent PDT enhanced efficacy in hypoxic TME.ACSN was firstly accumulated in the tumor tissue taking advantage of the performed EPR effect and subsequently infiltrated into the tumor cells to target mitochondria. The physiological function of electron transfer chain (ETC) was seriously interfered with the mitochondrial complex III inhibition in OXPHOS, thus causing diminished O2 consumption and hypoxia relief for PDT improvement. Apart from the great O2 replenished advantage, this carrier-free ACSN was proved to possess potency on high drug loading as well as low systemic toxicity, offering a prominent strategy for cancer treatment.
Fig. 5.
Representative nanocomplexes based on mitochondrial oxidative phosphorylation inhibitor ATO. (A) ATO and Ce6 self-assembled ACSN by beta-action, which was self-delivered to the tumor site. ATO inhibited mitochondrial respiration by inhibiting mitochondrial enzyme III, thus reducing O2 consumption and enhancing the PDT effect. Reproduced from [90] with permission from American Chemical Society. (B) ATO and VER were encapsulated in PLGA-PEG vectors by self-assembly, which released the ATO and VER through the lysosomes after being engulfed by the cells. The ATO hindered mitochondrial respiration, thereby enhancing the oxygen content and the ability of the VER to produce ROS. Reproduced from [91] with permission from WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim. (C) UNMM-Ce6-ATO-PEG blocked OXPHOS and utilized MnO2 to increase O2 level, boosting PDT efficacy with MRI and CT assistance capacity. Reproduced from [92] with permission from Elsevier Ltd.
Fan et al. established a dual-drug nano-system based on ATO (Fig. 5B) in order to penetrate deep into the solid tumor and further relieve the hypoxic TME [91]. The nanomedicine with the size of less than 50 nm was fabricated by encapsulating ATOs-VER self-assembled complex inside the poly (lactide-co-glycolide)-block-poly (ethylene glycol) methyl ether (PLGA-PEG). As a homolog of coenzyme Q in mitochondria, ATO was proved to not only act on complex III to inhibit mitochondrial respiration but also impair cellar repair ability by affecting dihydroorotate dehydrogenase (DHODH) and pyrimidine function, hence leading to PDT enhancement. Additionally, it was noted that VER possessed tumor targeting capacity and fluorescence imaging property in the human body, providing an efficient eradication manner in tumor therapy.
Some scholars have recently proposed an integration strategy of simultaneously reducing O2 consumption and increasing O2 supply in the tumor site. For example, Yuan et al. developed a novel multilayer nanosystem (UNMM-Ce6-ATO-PEG), which consisted of manganese dioxide (MnO2) in the outermost layer, Ce6 and ATO-loaded mesoporous silicon nanoshell (MSN) in the intermediate layer, and the UCNPs in the inner core portion (Fig. 5C) [92]. The O2 level was verified to be elevated via O2 supplement from MnO2 degradation and O2 economizing from the OXPHOS blocking ability of ATO, thereby boosting PDT efficiency in hypoxic tumors. Furthermore, UCNPs-MSN-MnO2 nanocomposites (UNMM) were able to serve as a class of contrast agent to assist magnetic resonance imaging (MRI) and computed tomography (CT) owing to the NIR light conversion capacity of UCNPs.
Nevertheless, cancer cells will enhance aerobic glycolysis to compensate for the energy production disorder and affect the effect of antitumor drugs, which is a process known as the Warburg effect. Therefore, cutting off the energy supply pathways of OXPHOS and glycolysis at the same time is the most direct strategy for cancer treatment [93]. Of note, OXPHOS inhibitors exhibit short blood retention time and poor bioavailability. Thus, the hypoxia alleviation effect should be optimized by adjusting the drug dosage and matching the irradiation time [90,94]. Moreover, more sophisticated mechanisms of the clinical available drugs are waited to be uncovered to remodel the tumor hypoxia microenvironment, which may also promote the clinical translation for hypoxic tumor treatment.
3. Oxygen-independent PDT
3.1. Superoxide anions therapy
PDT-induced ROS achieves an excellent killing effect on tumor cells, including 1O2, hydroxyl radical (OH·), hydrogen peroxide (H2O2) and superoxide anions (O2−·). Increasing evidences suggest that O2−· therapy can generate molecular oxygen in electron-transfer photoreactions via the one-electron reduction form, which is expected to be an effective scheme to overcome hypoxic TME and enhance PDT efficacy [95]. Since type I PDT is independent of the presence of oxygen, O2−· can solve the hypoxic problem by avoiding direct and rapid oxygen depletion in type II PDT. Recent studies have shown that O2−· bound to proteins, DNA or liposomes, would lead to disordered cellular metabolism and the intracellular components damage. Besides, partial O2−· is transformed into high toxic OH· through SOD-mediated cascade reactions to enhance the therapeutic effect of PDT [96]. The generated O2−· can further participate in superoxide dismutase-triggered catalytic cascades to reduce the PDT demand of O2 in an oxygen recyclable manner. Furthermore, O2−· can participate in the Harper-Weiss reaction or the Fenton reaction to realize the recycling production and utilization of molecular oxygen, thus compensating for oxygen depletion during treatment [97]. Therefore, O2−· therapy is considered as a great way to improve hypoxic-tumor PDT.
In addition to hypoxia in TME, tumor metastasis occurring after PDT treatment is also a major factor affecting the efficacy of PDT in clinical application [98]. Boron difluoride dipyrromethene (BODIPY) was employed to combine with the vascular disrupting agent (VDA) by Dong et al. to fabricate BDPVDA utilizing the type I PDT triggered characteristics. Then BDPVDA was subsequently encapsulated inside electron-rich amphiphilic polymers (MPEG-PPDA) [99], thereby obtaining an innovative nanostructure (PBV NPs) for dual-pattern cancer therapy, i.e. O2−· photogenerator-based PDT and vascular destroying avenue. Herein, abundant O2−· was generated via core-shell electron transfer upon light irritation, which effectively led to tumor cell apoptosis. Moreover, VDA was released from the PBV NPs owing to the TME acidity and the ester bond destruction by lysosomes, which achieved great metastasis suppression efficiency.
Peng et al. devised and synthesized a promising system (SORgenTAM) based on the antiestrogenic drug tamoxifen (TAM) for both O2−· generation and mitochondrial respiration inhibition, aiming to further tackle the hypoxia issue in PDT application [100]. Cellular O2 consumption rate (OCR) was monitored to be significantly slowed down by affecting the complex I in the mitochondrial ETC, thus alleviating the hypoxic status-induced PDT limitation [101]. Besides, it was worth mentioning that substantial cytotoxic O2−· was generated via O2-independent type I PDT, which avoided O2 consumption in PDT treatment. Additionally, part of the produced O2−· was converted into OH· through a biological cascade, which enhanced photodamage to tumor cells and achieve O2 recycling. More importantly, SORgenTAM achieved both tumor boundary recognition and accurate tissue diagnosis in mouse models without obvious side effects during the whole therapeutic course, providing an unprecedented effective PDT-mediated anticancer approach. Even so, the development of superoxide anion based PDT is restricted by the scarce PSs. Moreover, unwanted damage on normal tissue is frequently happened during PDT. The development of a safe and effective strategy for precise PDT is urgently needed.
3.2. Type I PDT
The selective accumulation of PS in tumors induce the creation of cytotoxic chemicals and the devastation of tumor tissues upon being exposed to visible light of a particular wavelength and the presence of molecular oxygen in cells [102]. PS can briefly change from the ground state to the singlet state (1PS*) after absorbing photons from light, which lasts only for a limited period (calculated in nanoseconds). Then 1PS* converts to an excited triplet state (3PS*) with a long lifetime and reacts with the surrounding molecular molecules to transfer energy to molecular O2 in two ways, namely type I PDT and type II PDT. Molecular O2 receives energy directly from the PSs in the excited triplet state during type II reaction, resulting in the formation of highly active 1O2 [9]. Therefore, type II PDT leads to a progressive decline of oxygen in tumors and hypoxia weakens the photodynamic efficacy of PS, thereby preventing PDT from exerting its therapeutic potential [9]. However, 3PS* reacts with organic molecules directly and forms radical substances through the transfer of hydrogen or electrons during the type I reaction, thus avoiding oxygen restriction. ROS including O2−·, hydroperoxide radical (HOO·), H2O2, and OH· are reaction products of free radical substances and cellular oxygen, which cause tumor cell apoptosis and trigger immune responses to further attack tumor cells [103].
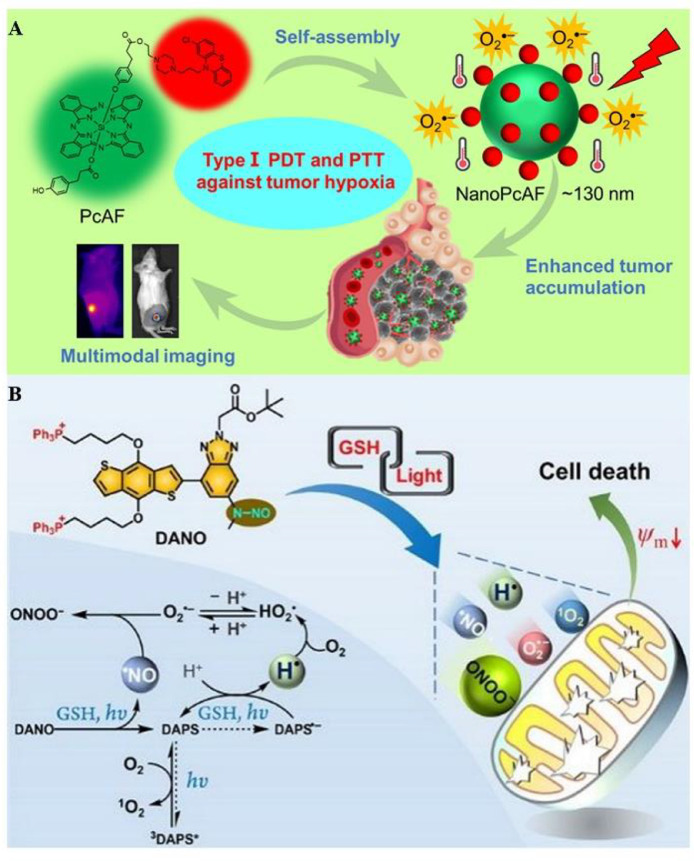
Increasing numbers of type I PSs have been developed since the type I PDT was first proposed in 1991 [104]. For instance, Li et al. designed and synthesized a multifunctional nanostructure (NanoPcAF) for overcoming hypoxic tumors on the basis of the modified phthalocyanine (Fig. 6A) [105], which was fabricated by the self-assembly of PcAF in aqueous solutions. PcAF contributed to transferring type II PDT to type I PDT as a class of versatile silicon (IV) phthalocyanine derivatives, thereby offering an O2-independent pathway to clinical cancer therapy. Furthermore, PcAF also exhibited fancy performance on photothermal conversion efficiency, facilitating hypoxic tumor destruction efficacy via both PDT and PTT. It was worth noting that a substantially high level of NanoPcAF accumulation was monitored in the tumor site with no significant toxicity after systemic administration according to the experimental results.
Fig. 6.
Typical oxygen-independent type I PDT strategies. (A) NanoPcAF was obtained in aqueous solutions through a spontaneous assembly process, realizing the transition from type II PDT to type I PDT, with excellent photothermal conversion efficiency for enhancing PDT. Reproduced from [105] with permission from American Chemical Society. (B) DANO targeted mitochondria and achieved anti-hypoxia PDT through dual type (both type I and type II reactions) and tandem cascade reactions. Reproduced from [106] with permission from American Chemical Society.
Cascade Reactions achieve the synergistic effect of multiple reactive species and significantly boost the antitumor effect of PDT. Recently, Wang et al. reported a molecular system (DANO) developed by coupling a NO· photogenerator to a π-conjugated donor−acceptor (D−A) structure (Fig. 6B) [106]. DANO targeted mitochondria with the coactivation of GSH and light, achieving excellent performance on the tumor treatment through dual type PDT (type I and II PDT). Of special note, the peroxynitrite (ONOO−), O2−·, and hydroperoxyl radical (HO2·) yield were verified to facilitate PDT efficacy via tandem cascade reactions. Furthermore, DANO exhibited excellent potency on low-light dose activated-PDT due to its fluorescence characteristic and the broad two-photon absorption cross section under irritation. This novel system was expected to present low nonspecific phototoxicity owing to the high expression of GSH in tumor sites.
Unlike type II process, ROS are generated by the direct transfer of electrons or hydrogen atoms when PS interacts with the substrate in type I process. Substantial oxygen consumption is not involved in the process so the type I PDT breaks through the hypoxic limitation during type II process and proceeds even more efficiently under hypoxic conditions. Whereas, the mechanism of transformation from type II to type I pathway is still immature and further investigation is needed [107].
3.3. Self-decomposition
PDT is popular for several advantages, such as minimal invasiveness high and spatiotemporal controllability, etc. But oxygen dependence limits its application owing to the relatively low oxygen concentration in the tumor site. Surprisingly, self-decomposition compounds possess the ability to release oxygen in hypoxic tumors, which can alleviate the hypoxic microenvironment caused by PDT. Nevertheless, high biosafety and precise O2 release are essential requirements for them [108]. At present, the main used self-decomposition compounds are calcium peroxide (CaO2) and platinum (IV) -azide complex, etc.
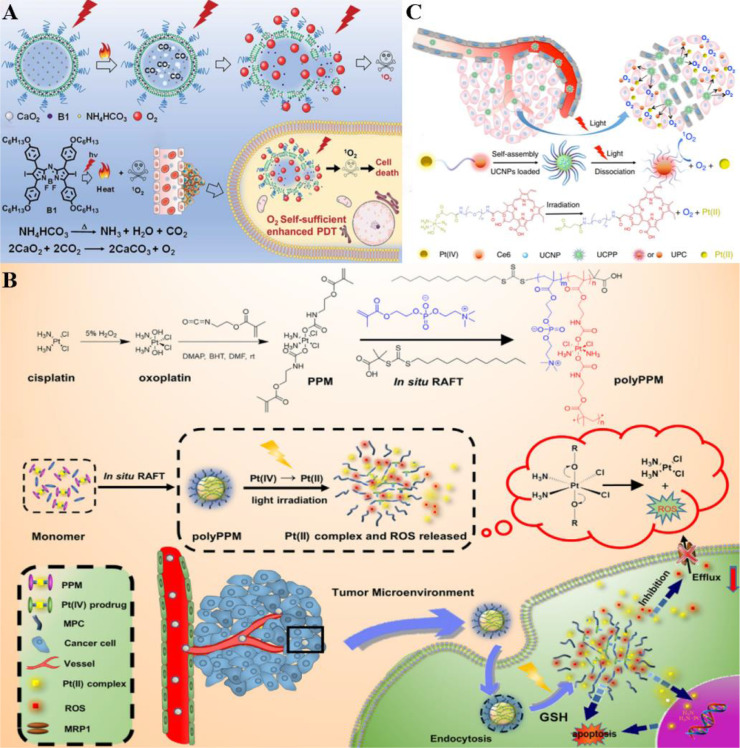
Halogenated aza-BODIPY (B1) was exploited to assemble with CaO2 NPs and NH4HCO3 by Zhao et al. considering that it was a class of ideal PS and organic photothermal agent (Fig. 7A) [109]. Then the complex was loaded into polyethylene glycol (PEG) shelled liposome to synthesize CaO2/B1/NH4HCO3 lipo, which performed EPR effect in tumor tissue and good biocompatibility. Of special note, the decomposition of NH4HCO3 and CO2 bubbles generation was induced by the generated heat from B1 upon light irritation, thereby subsequently triggering the reaction between CO2 and CaO2 to achieve O2 supplement for PDT enhancement without accompanying harmful by-products.
Fig. 7.
Representative oxygen generators via self-decomposition for achieving oxygen self-sufficiency. (A) CaO2/B1/NH4HCO3 lipo absorbed NIR to decompose NH4HCO3 to produce CO2 under irradiation, which further induced O2 release from CaO2 NPs and enhanced 1O2 generation. Reproduced from [109] with permission from Royal Society of Chemistry. (B) PolyPPM absorbed long-wavelength light and converted it into short-wavelength light, which promoted the rapid decomposition of Pt (IV) to release O2 and Pt (II). O2 could further generate ROS, while Pt (II) could be used for chemotherapy to realize synergistic PDT chemotherapy, and significantly enhanced the anti-cancer efficacy. Reproduced from [110] with permission from The Author(s). (C) UCPP produced ROS in an O2-independent manner through light response, and generated Pt (II) for chemotherapy, showing excellent chemo-photodynamic therapy efficacy. Reproduced from [111] with permission from Royal Society of Chemistry.
Zhang et al. designed and synthesized an appealing platinum(IV) complex-based system (polyPPM) for synergistic PDT and chemotherapy improvement with the Pt(II) species generation capacity upon light activated, (Fig. 7B) [110]. Notably, platinum (IV) diazide complex not only exhibited fantastic potency on Pt (II) species production as a type of effective chemotherapeutics but was also proved to display excellent performance on self-generated ROS without endogenous O2 consumption. As 2-methacryloyloxyethyl phosphorylcholine (MPC) monomer possessed the ability to increase drug solubility and blood circulation time, it was employed to copolymerize with the platinum(IV) complex-based prodrug monomer (PPM) to obtain the nanoscale hydrogel-like polyPPM, which enhanced drug cellular accumulation via increasing uptake and drug resistance suppressing. Whereas, the efficiency was restricted by shallow penetration owing to the relatively short wavelength of light irritation. An optimized manner was called for to tackle this issue and achieve satisfying results.
The obstacle related to light wavelength was resolved in the study of Yan et al. [111]. Similarly, the complex cis, trans, cis-[Pt(N3)2(OH)2(NH3)2] (Pt(IV)) was utilized as a prominent anticancer drug to assembled with Ce6 and PEG drawing support from its O2 self-producing feature. The complex was subsequently combined with UCNPs to obtain UCNPs-embedded NPs (UCPP) (Fig. 7C) [112]. UCPP decomposition was triggered to generate O2 upon a 980 nm laser-excited, hence overcoming tumor hypoxia for O2-independent PDT enhancement with the combination of chemotherapy effect.
4. Photodynamic-derived multimodal synergistic therapy
PDT produces cytotoxic reactive oxygen substances by combining nontoxic PS, light and oxygen to kill cancer cells through apoptosis and necrosis [113]. A series of events can lead to direct tumor cell death, microvascular injury and induction of local inflammatory responses in the presence of O2. However, PDT is an oxygen-consuming process and its photochemical reaction may only occur in the initial stage. The treatment efficiency of PDT is greatly reduced to a certain extent with the O2 reduction [114]. To address the above problems, the synergy of PDT with other treatments, such as chemotherapy, immunotherapy, and PTT have been put forward and receive wide attention for multiple advantages in overcoming tumor hypoxia and improving therapeutic effectiveness.
4.1. Synergistic photodynamic and chemotherapy
In recent years, chemical-photodynamic collaborative cancer therapy has been extensively studied as a prominent strategy for cancer therapy. Researchers have proposed that a hypoxic microenvironment can be created to activate the hypoxia-responsive anticancer drugs owing to the low oxygen level in tumor tissue and PDT-induced oxygen consumption. In general, chemotherapy prodrug with low-toxicity is converted to a free radical through one-electron reduction. When O2 is present, the unpaired electron can be transformed from the free radical to O2 molecule, reproducing prodrug in a reverse reaction [115]. On the contrary, the free radical continuously undergoes further reduction and ultimate toxic species are synthesized. Five classes of bioreductive compounds have been developed to serve as hypoxia-activated prodrugs, i.e. nitro(hetero)cyclic compounds, aromatic N-oxides, aliphatic N-oxides, quinones, and metal complexes. Typical examples of these prodrugs and their mechanisms of action are illustrated below (Table 4) [116,117]. However, this combination therapy will cause cell damage in normal tissues in some ways. In this regard, the combination of chemotherapy and PDT using polymer nanocarriers has been proposed by researchers to boost the efficiency of anticancer therapy. The synergistic therapy can not only further reduce the side effects of chemotherapy drugs but also improve the biocompatibility of PSs [118,119].
Table 4.
Representative samples of hypoxia responsive prodrug.
| Type | Agent | Reduction | Mechanism of action |
|---|---|---|---|
| Nitro(hetero)cyclic compounds | Evofosfmide (TH-302) | 1e− | DNA alkylation |
| PR-104 | 1e−/2e− | DNA interstrand cross-links | |
| Etanidazole | 1e− | DNA alkylation | |
| Misonidazole | 1e− | DNA alkylation | |
| Quinones | Apaziquone (EO9) | 1e−/2e− | DNA alkylation |
| quinone mitomycin C (MMC) | 1e−/2e− | DNA interstrand cross-links | |
| Porfiromycin | 1e− | DNA interstrand cross-links | |
| Indolequinone | 1e− | DNA alkylation | |
| Aromatic N-oxides | Tirapazamine (TPZ) | 1e− | Topoisomerase ll poisoning and DNA doublestrand breaks |
| SN30000 | 1e− | Complex DNA damage | |
| QdNOs | 2e− | Reduce hypoxic gene expression | |
| Aliphatic N-oxides | Banoxantrone (AQ4N) | 2e− | DNA binding and Topoisomerase II inhibition |
| OCT 1002 | 2e− | DNA binding and Topoisomerase II inhibition | |
| Transition metals complexes | Pt(IV) | 2e− | DNA binding |
| Au(I) | 2e− | ROS generation | |
| Cu(II) | 2e− | ROS generation |
Additionally, chemotherapeutic agents such as taxanes, gemcitabine, and cisplatin are used to modify the disordered structure of tumor microvasculature, which is promising to achieve elevated blood perfusion and oxygen level in tumors. Studies have also pointed out that chemotherapeutic drugs can also increase oxygen levels in addition to destroying tumor tissue cells, resulting in improving the effectiveness of PDT [120]. Since this synergistic strategy possesses a relatively weak ability to deliver drugs accurately to tumor cells, it is of significance to integrate various delivery modes targeting a single internal or external signal aiming at lessening side effects in the course of treatment [119].
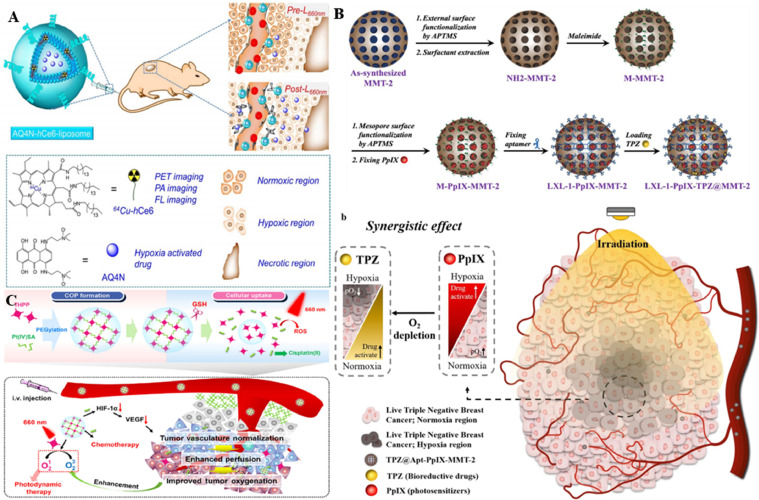
Hypoxic-activated precursors alone generally provide unsatisfactory antineoplastic effects because they are ineffective against tumor cells that are near the vasculature with an adequate oxygen supply. Liu et al. designed a multifunctional therapeutic system to tackle this problem (Fig. 8A) [120], implying that on the one hand, the hypoxic environment could hamper PDT efficiency. While on the other hand, it could enhance the antineoplastic efficacy of chemotherapy via the activation of hypoxia-selective prodrugs. A liposome-based nanostructure (AQ4N-hCe6-liposome) was formed by simultaneously encapsulating the hydrophilic AQ4N molecule as a hypoxia-activated prodrug and the hCe6 as a photosensitizer into PEGylated liposomes, which achieved PDT-induced hypoxia-activated therapy. The Ce6-mediated PDT process exacerbated the local hypoxic TME upon 660 nm laser irradiation and resulted in the conversion of the hypoxia-activated prodrug AQ4N to AQ4, thus achieving high cytotoxicity. Furthermore, mice receiving intravenous AQ4N-hCe6-liposomes were monitored to show more potent antitumor effects than the control group owing to the cascade of hypoxia-activated chemotherapy and PDT. Additionally, AQ4N-hCe6-liposomes also served as a kind of multifunctional probe for multimodal imaging with excellent EPR effects, indicating that it was a great potential synergistic cancer treatment strategy in clinical practice for in vivo tracking. Nonetheless, the strategy still had a large room to improve because the combination of hypoxia-responsive anticancer drugs with PDT had the likelihood to cause cell damage in normal tissue during the treatment.
Fig. 8.
Representative nanomedicines for photodynamic synergistic chemotherapy. (A) The AQ4N-hCe6-liposomes gradually accumulated at the tumor site, inducing severe tumor hypoxia and showing hypoxia-dependent cytotoxicity to kill cancer cells. Reproduced from [120] with permission from American Chemical Society. (B) DNA aptamer LXL-1 targeted tumor cells to deliver the NPs to the tumor site after intravenous administration of TPZ@LXL-1-PpIX-MMT-2. PDT was initiated by the PpIX, aggravating tumor hypoxia and enhancing the antitumor activity of TPZ to achieve the synergistic effect of PDT and chemotherapy. Reproduced from [121] with permission from The Author(s). (C) The THPP-Pt-PEG COP showed efficient tumor-site accumulation via the EPR effect. cis-Pt (IV) SA dissociated the NPs to rapidly release the drug. And THPP induced PDT process to produce ROS under light irradiation, realizing the combination of PDT-chemotherapy. Reproduced from [122] with permission from Tsinghua University Press and Springer-Verlag GmbH Germany.
Hypoxic regions are frequently found in triple-negative breast cancer (TNBC) compared with other types of breast cancer, hence making it more difficult to treat. To effectively treat TNBC, Ho et al. devised a new strategy to combine PDT with bioredox therapy, exploiting adverse hypoxia conditions to increase the effects of bioreductive precursors drugs (Fig. 8B) [121]. As protoporphyrin IX (PpIX) possessed the O2 consumption and ROS generation feature, PpIX and the bioreactive precursor tirapazamine (TPZ) were utilized to integrate with hollow mesoporous silica NPs (HMSN MMT-2) carrier. The complex was then modified with the DNA aptamer LXL-1 to design an innovative drug delivery system (TPZ@LXL-1-PpIX-MMT-2) with the remarkable function of selectively targeting MDA-MB-231 in human breast cancer cells. Of special note, the low microenvironmental oxygen levels contributed to the activation of the TPZ and the generation of toxic free radicals, which not only eradicated the hypoxic tumor cells, but also promoted the therapeutic effect of PDT. The study confirmed that TPZ@LXL-1-PpIX-MMT-2 had the ability to target tumor cells both in vitro and in vivo, facilitating the selective accumulation at tumor sites and displaying a satisfying tumor-killing effect within both normoxic and hypoxic areas. This nanotherapy not only enhanced the retention of chemotherapeutic agents in tumors, but also reduced drug accumulation in other non-target organs, further suggesting that it was a promising therapeutic strategy for TNBC.
Improving the PDT efficacy by elevating O2 through chemotherapy was also considered as a promising approach except for enhancing the chemotherapy efficacy through PDT-induced oxygen deprivation. Chemical therapeutic drugs are exploited to eliminate tumor tissue and simultaneously regulate the chaotic structure of tumor microvasculature. Consequently, efficient perfusion of blood at tumor sites is elevated and PDT efficacy is improved due to the less O2 consumption of the dying tumor tissue.
Covalent-organic polymers (COPs) were fabricated by cross-linking the chemotherapy-promoting cis-Pt(IV)SA with the PS meso-tetra(p-hydroxyphenyl) porphine (THPP). COPs were further combined with PEG to obtain THPP-Pt-PEG COPs, which could exist as a stable NP in an aqueous solution (Fig. 8C) [122]. Since THPP-Pt-PEG COPs had a longer blood circulation time, the nanomedicine showed effective tumor accumulation after intravenous injection to mice. Alternatively, THPP-Pt-PEG COPs were essentially a class of biodegradable polymer that could be effectively cleared by kidneys to minimize its long-term toxicity owing to its decomposable characteristic. Whereas, issues had been put forward that chemotherapeutic drugs were not precise enough to be delivered to tumor cells by merely synergistic photodynamic and chemotherapy. As a consequence, it was of great necessity to apply external or internal stimuli to an intelligent delivery system, which could further minimize the side effects during the treatment.
4.2. Synergistic photodynamic and immunotherapy
Recently, immunotherapy has emerged as a powerful clinical manner for treating cancer owing to its capability of activating the immune system to eliminate tumors. PDT can simultaneously elicit immunogenic cell death (ICD) and induce cytotoxic lymphocyte (CTL) mediated antitumor immunities apart from direct cytotoxic effects on cancer cells [123]. These immunologic effects propose an appealing clinical treatment strategy to combine immunotherapy and PDT to bring synergistic effects on the hypoxic tumor. Unfortunately, hypoxia leads to immunosuppression via multiple mechanisms as with PDT inhibition [124].
Multiple mechanisms are involved in the development of tumor hypoxia. Among which, the severely aberrant tumor blood artery is just one of many factors. Blood flow resistance is caused by the disorganized and dysfunctional tumor vasculature, which limits perfusion and drug delivery. Furthermore, blood vessels can form further from the cells, affecting the oxygen supply of the cells in the periphery [125]. In addition, the abnormal blood vessels will trigger an immunosuppressive microenvironment thus hindering cancer immunotherapy. It is also worth mentioning that the vascular shutdown induced by PDT would further aggravate hypoxia and in turn limits immunotherapy and PDT efficiency. Therefore, strategies that normalization of the aberrant vasculature may improve hypoxia and facilitate antitumor therapy. Immune checkpoint blockade (ICB) is the most thoroughly studied type of immunotherapy to date. Antibodies are used to disrupt harmful immune regulatory pathways and exert their anticancer impact by targeting immune suppressive signals and resuscitating the host immune system [126]. Recent studies have suggested that ICB could alter the TME by restoring normalcy to the aberrant tumor vasculature. There is a strong evidence that targeting tumor vasculature with a low-dose antiangiogenic VEGFR2 antibody could induce tumor vascular normalization and reprogram the TME, which can alleviate hypoxia. ICB can also induce tumor-vascular normalization and decrease hypoxia through the engagement of TH1 cells [127]. These studies points to the combination of PDT with immunotherapy as a workable new cancer treatment approach.
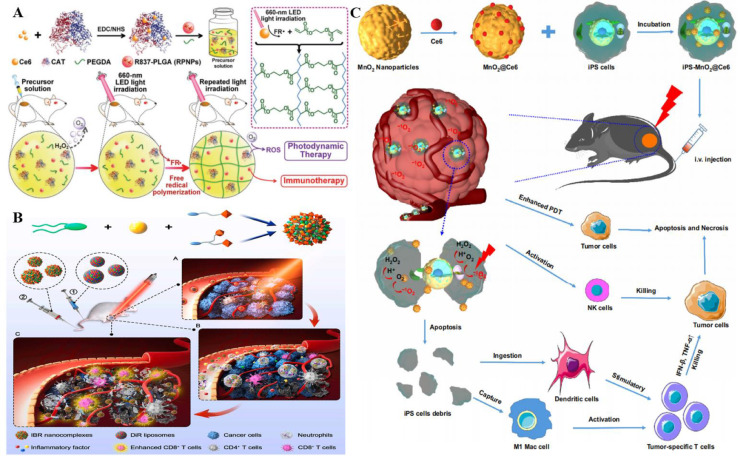
PDT can simultaneously elicit ICD and induce CTL-mediated antitumor immunities besides direct cytotoxic effects on cancer cells [123]. These immunologic effects propose an attractive clinical treatment option to combine the immunotherapy and PDT for synergistic effects on hypoxic tumors. Liu et al. designed and developed an in situ gelation system by modifying CAT with Ce6, and then mixed it with a biodegradable polymer (poly (ethylene glycol) double acrylate (PEGDA) as well as the immune adjuvant R837 (Fig. 9A) [128], thereby fabricating R837-loaded PLGA NPs (RPNPs). The polymerization of PEGDA was able to form in situ hydrogels after the intratumor injection of precursor solution, thus retaining efficient activity in tumor sites for a long period. This tumor-resident in-situ hydrogel alleviated the hypoxic environment of tumors by decomposing H2O2 to O2 via Ce6-CAT. And this continuous tumor hypoxia relief ability was confirmed by in vitro experiments with quantitative measurements using PAI. Notably, R837-loaded PLGA NPs under repeated laser stimulation could lead to multiple rounds of PDT with continuous cytotoxic ROS generation and significantly enhance the immune response via the PDT-mediated ICD. More importantly, multi-round PDT combined with α-CTLA4 checkpoint blockade significantly suppressed the activation of regulatory T cells (Treg), causing long-term immune memory to protect from tumor rechallenge.
Fig. 9.
Representative nanocomposites for photodynamic synergistic immunotherapy. (A) RPNPs alleviated the hypoxic TME and triggered multiple rounds of PDT under repeated laser stimulation, simultaneously enhancing the immune response via the PDT-mediated ICD. Reproduced from [128] with permission from WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim. (B) SA-2@NCs generated ROS to induce acute inflammation and rapidly activated neutrophils to infiltrate tumors. The activated neutrophils subsequently internalized SA-coated IBR and carried it to the tumor site. Reproduced from [129] with permission from Elsevier Ltd.. (C) iPS-MnO2@Ce6 performed great tumor homing ability and accumulated at tumor sites, mediating PDT and enhancing antitumor immunity. Reproduced from [130] with permission from Elsevier Ltd..
Neutrophils play an increasingly important role in cancer treatment as the major immune cell in human body. Deng et al. developed a strategy to target the activation of the peripheral blood neutrophils (PBN) for realizing the accumulation of the antitumor agent ibrutinib (IBR) at the tumor sites, which explored the potential of neutrophils for PDT enhancement (Fig. 9B) [129]. First, liposomes loaded with PS DIR (DiR-lipos) were injected into the tail vein of tumor-bearing mice. Then DiR-lipos were irradiated with NIR light to generate ROS by performing PDT/PTT treatment to induce acute inflammation, so that the PBNs were rapidly activated and infiltrated at the tumor site. Afterward, the nanocomplex (SA-2@NCs) that targeted activated PBN was constructed by encapsulating IBR with targeted sialic acid (SA) derivative. The accumulation of the IBR in the tumors was achieved upon internalization by the activated neutrophils. The results indicated that DiR-mediated PDT/PTT therapy combined with SA-2@NCs-would mediate antitumor immunity therapy to promote T cell infiltration and inhibit tumor growth as well as metastasis, thus exerting potent effects in cancer treatment.
As induced pluripotent stem cells (iPSs) share nearly identical antigens with tumor cells and overcame ethical constraints, they possess great potential in tumor immunotherapy. Cui et al. engineered an iPS platform for the precise delivery of nanoprobes by attaching Ce6 to MnO2 NPs (MnO2@Ce6) due to the tumor homing ability of iPS cells (Fig. 9C) [130]. MnO2@Ce6 was subsequently loaded into mitomycin-treated iPS to synthesize iPS-MnO2@Ce6, which actively targeted the tumor in vivo. A large amount of O2 was produced by consuming H+ in the acidic TME to alleviate the tumor hypoxia since MnO2 was highly reactive to H2O2. The treated mouse models exhibited significant tumor growth inhibition and lower mortality compared with the control group. It was worth noting that iPS not only promoted the aggregation of the NPs at the tumor site, but could also be cleaved by the ROS generated in the PDT process. The synergy between PDT and immunotherapy was achieved by releasing the tumor antigens to produce an effective antitumor immune response.
4.3. Synergistic photodynamic and photothermal therapy
The PS is ingested by tumor tissue in PDT. The PS is activated to deliver energy to intratumoral O2 under the irradiation of specific wavelength light, generating highly active 1O2 and producing the cytotoxic effect. Therefore, the treatment effect highly depends on the intratumoral O2 concentration and PS concentration. While the fundamental principle of photothermal treatment is that light energy is transformed into heat to destroy tumor cells under the exposure of a certain wavelength of light when tumor tissues absorb photothermal conversion materials. The application of novel materials show that PTT has an enhanced effect on PDT in tumors, thereby eliciting obvious synergistic effects. The photothermal effect can enhance the transport of PSs inside tumor cells and increase the distribution of PSs in tumors. Besides, the photothermal effect also enhances the blood flow of local tumors, which increases the oxygen concentration in tumor tissue. The simultaneous elevation of PS and O2 concentration greatly improves the therapeutic efficiency of PDT. Concurrently, PDT also has a synergistic effect on PTT. The 1O2 generated in the PTT process can destroy heat shock protein C, thereby weakening the protective effect of protein C against tumors in the PTT process [131]. Nevertheless, there is still a certain shortage in the synergistic effect between PDT and PTT. The irradiation spectral areas of PDT and PTT are not the same, therefore it is often necessary for practical operations to adjust different spectra for irradiation and prolong the treatment time. Single laser-activated therapies that are synergistic for PDT and PTT have been developed so far in response to this problem, but are still under continuous investigation. In addition, the toxicity to the body from the reagents used under the synergistic therapy is also a problem that needed to be improved [132].
Transferrin-receptor (TfR) is proved to overexpress in multiple cancers [133]. Wang et al. prepared Tf-IR780 NPs with transferrin-loaded IR-780 [134], which solved the problem of PTT/PDT synergistic therapy requiring two different wavelengths. Tf-IR780 NPs realized the targeted imaging and cancer treatment under single NIR (808 nm) irradiation benefiting from the tumor targeting function of transferrin. Tf-IR780 NPs compensated for the disadvantages of IR780 such as poor solubility and targeting deficiency, implying that it was a promising manner for image-guided cancer phototherapy. Tf-IR780 NPs had good tumor suppressive ability and a high cellular uptake rate compared with the PBS-injected group according to the mouse experiments.
From another perspective, Ren et al. explored a new material (BSA/SAs-NMOFs) by modifying iron-porphyrin MOFs with bovine serum albumin (BSA) and sulfonamides (SAs), which could realize guided synergistic PTT/PDT under imaging [135]. BSA/SAs-NMOFs possessed the ability to display T1-T2 dual mode MRI as iron ion-based nMOFs were potential MRI contrast candidates with magnetic capacity and nontoxic property, which offered reliable and more precise for cancer treatment. Additionally, BSA/SAs-NMOFs exhibited excellent simultaneously PDT and PTT effectiveness accompanied by multiple advantages such as good biocompatibility and long circulation time in the blood benefiting from the targeting ability of Carbonic anhydrases (CA) IX at tumor sites and the satisfying half time of BSA. It was worth mentioning that BSA/SAs-NMOFs performed well even under hypoxic conditions via remarkable ROS generation with a great photothermal conversion efficiency of 40.53%.
Different from the above two perspectives, professor Lu et al. sought solutions for better PTT/PDT enhancement from the field of mitochondria. Lu et al. developed a mitochondria-targeted nanoplatfrom (PTPF-MitP) [136], which possessed an excellent performance in activating the apoptosis pathway in cancer treatment under hypoxic TME through the release of apoptotic factor cytochrome C. Moreover, PTPF-MitP also had the ability to trigger H2O2 decomposition serving as a nanoenzyme, thus further tackling the issue of hypoxia-induced PDT limitation. Cell viability was observed to significantly decrease in groups treated with PTPF-MitP compared with the PTPF group, implying the high cytotoxicity of MitP served as a mitochondrial targeting unit. In addition, the tumor temperature was monitored to rise to 45 °C in mice after being injected with PTPF-MitP, which offered a suitable condition for PTT to exert its better effect. It was surprising to find that the tumor volume was significantly controlled in PTPF-MitP when compared with the control group, indicating the remarkable cancer therapeutical efficacy of the combination of PDT and PTT under the hypoxic condition.
4.4. Synergistic photodynamic and starvation therapy
It is well known that vigorous nutrition consumption is a major property of malignancies that needed strong nutrient intake. Starvation therapy is anticipated to become an important strategy for cancer treatment via cutting off nutrient supply [137]. There are two major ways to cut off the nutrient supply of cancer cells: blocking their access to blood by intervening in tumor angiogenesis and inhibiting the metabolism of cancer cells to induce starvation status . In recent years, the combination therapy based on starvation therapy has been continuously proposed for maximizing the therapeutic efficiency [138]. Among which, PDT synergistic starvation therapy has drawn rising interest.
As both starvation therapy and PDT are affected by a tumor hypoxia environment [139], it is important to develop a nanoplatform that provided sufficient O2 to enhance the efficacy of PDT synergistic starvation therapy. He et al. co-polymerized porphyrin and Arg-Gly-Asp (RGD) on the surface of GOx and CAT to design an enzyme nanogel (rGCP nanogel) (Fig. 10A) [139], realizing the sufficient supply of O2 and H2O2 via endogenous circulation and greatly improving the PDT efficiency. RGCP nanogels targeted tumor cells in the action of Arg-Gly-Asp (RGD) after intravenous injection. GOx deprived the O2 and glucose in cancer cells to produce H2O2, thereby hindering the energy supply of tumors. Meanwhile, CAT decomposed H2O2 to provide sufficient O2 due to the sufficient supplementation of H2O2, which not only ameliorated hypoxia and boosted PDT efficacy, but also promoted the glucose catabolism of GOx to enhance the effect of starvation therapy. It was worth noting that the rapid catalysis of cytotoxic H2O2 by CAT would further prevent the damage to normal cells in addition to the targeting effect of RGD, thus achieving low-toxic synergistic cancer therapy.
Fig. 10.
Typical nanostructures for photodynamic synergistic starvation therapy. (A) Co-polymerization of the porphyrin and RGD to the GOX and CAT surfaces finally yielded the rGCP nanogels, which produced H2O2 at the tumor site. CAT broke down H2O2 to produce O2, ultimately enhancing 1O2 production and realizing PDT and starvation synergistic therapy. Reproduced from [139] with permission from Acta Materialia Inc. Published by Elsevier Ltd. (B) MB and GOx were encapsulated to the MDPA for the MPDA@MnO2−MB-GOx synthesis. MnO2 catalyzed O2 production of H2O2 to alleviate tumor hypoxia. While GOx catalyzed glucose generation of H2O2 to further enhance O2 production, eventually achieving enhancement of PTT, PDT, and starvation therapies with O2 self-supplement. Reproduced from [141] with permission from Elsevier B.V..
Mesoporous polydopamine (MPDA) NP is not only an ideal drug carrier, but also an ideal photothermal agent due to its unique structure and feature [140]. Therefore, Shi et al. designed a multifunctional nanoamplifier (MPDA@MnO2-MB-GOx) (Fig. 10B) [141]. First, MPDA@MnO2 was synthesized using MPDA coated with MnO2 nanosheets. Then, MPDA@MnO2-MB was synthesized by electrostatic adsorption and methylene blue (MB) loading. Finally, glucose oxidase (GOx) was encapsulated to obtain MPDA@MnO2-MB-GOx, which was utilized to synergistically enhance photothermal, photodynamic, and starvation therapy in the hypoxic tumor. The system exhibited great potential for starvation therapy through tumor growth inhibition benefiting from the glucose metabolism blocking feature of GOx. Notably, MnO2 nanosheets in MPDA@MnO2-MB-GOx nanomaterials could not only react with the endogenous substrate H2O2 in the TME to produce an oxygen-enhanced treatment of PDT, but also cooperated with the GOx catalytic reaction, which further improved the therapeutic effect of PDT and the catalytic effect of GOx. Recent studies have indicated that starvation therapy is characterized with high safety and good tumor inhibition effect. Synergistic PDT/starvation therapy is expected to be applied in clinic. However, it is important to note that cancer cells may generate adaptations in a low-energy environment caused by prolonged starvation, leading to the poor efficacy of long-term treatment. Consequently, it is of significance to explore how to optimize the therapeutic effect in combination with PDT and starvation therapy [142,143].
4.5. Synergistic photodynamic and radiotherapy
Radiotherapy is a common clinical therapeutic approach for solid tumors, which employ rays and proton beams to kill tumor cells and reduce the risk of tumor recurrence [144]. However, the effect of radiotherapy is limited due to the radio-resistance of some tumors. The X-ray dose needs serious control according to the radiation dose tolerance in normal tissues. Surprisingly, the X-ray dose could be effectively reduced through the synergy of PDT and radiotherapy, thus diminishing the side effects of radiation therapy. Meanwhile, the defect of low radiation wavelength penetration in PDT can be improved by X-ray with high penetration ability, showing great potency to deep tumors [145,146]. There are two main mechanisms in the PDT synergistic radiotherapy. To be specific, PDT can be activated through radiotherapy pathway. In addition, therapeutic efficacy can reach a higher level via the mutual promotion between radiotherapy and PDT.
Won et al. prepared PEG-BR/CWO NPs with PEGylated bilirubin packaging CaWO4. CaWO4 emitted UV-A and visible light when X-ray irradiation stimulated the radioluminescent CaWO4 inside the particles [147]. Its rearrangement eventually caused PEG-BR cleavage, and the formed BR produced ROS under UV-A and visible light to kill tumor cells. This combined effect of PDT and chemotherapy took advantage of the penetration of X-ray to deep tissues. Furthermore, PDT could be also activated indirectly upon the irradiation of X-ray. Therefore, it overcame the disadvantage of low penetration in PDT and realized the treatment of deep tissues, which exhibited better therapeutic effect against head and neck cancer compared with radiotherapy alone.
Moreover, Ju et al. synthesized the Gd2O3@BSA-BSA-Ce6 (BGBC) variable-size nanoprobes using the crosslinking method [148]. Such probes could be substantially enriched at the tumor site based on the EPR effect of solid tumors. After this, Ce6 catalyzed the production of ROS under irradiation (660 nm), splitting the probes into small particles of Gd2O3@BSA and BSA-Ce6. The small Gd2O3@BSA and BSA-Ce6 particles had the ability to penetrate into the deep tumor. Under the strong penetrating X-ray irradiation, Gd2O3@BSA directly destroyed the DNA or catalytically generated ROS to indirectly destroy the cells, eventually leading to the death of the tumor cells. In this process, the ROS generated during PDT promoted the entry of radiotherapy drugs into the deep tumor tissues, enabling the tissue-penetrating X-ray to achieve enhanced treatment.
5. Conclusion and perspective
Hypoxic TME is well known as one of the main limiting factors of PDT. And PDT is an oxygen-consuming process, which may exacerbate tumor hypoxia and lead to drug resistance. Therefore, it is highly warranted to develop strategies for tumor hypoxia alleviation or break the limitation of hypoxia to promote PDT efficiency. In recent years, an increasing number of studies have found that nanomedicine delivery systems can regulate hypoxic TME via different routes to enhance PDT. In this review, effective strategies as well as their advantages and disadvantages for improving PDT were summarized systematically.
In this review, the oxygen-dependent PDT strategies were divided into three categories: (1) Oxygen generator: catalase-mediated PDT realized the PDT amplification via H2O2 decomposition with wide biological sources and high activity; nanoenzymes-mediated PDT was easily stored; water splitting route was simple to prepare with low price and an unlimited supply of raw material. (2) Oxygen carriers: Hb-based oxygen carriers possessed normal metabolic avenue of Hb; PFCs-based oxygen carriers allowed stable O2 loading without being influenced by temperature and pH with reliable biosafety; microbubble/nanobubble-based oxygen carriers improved the deep tumor penetration through mechanical cavitation; MOFs-based oxygen carriers had high specific surface area and porous network for ROS diffusion. Ultimately, the biomimetic photosynthesis pathway had the ability to continuously generate O2 with high biosafety, which could significantly enhance the treatment of PDT. (3) Oxygen consumption reduction: OXPHOS inhibition strategy economized O2 via ETC blocking. Furthermore, oxygen-independent PDT was also one of the major avenues to solving the tumor hypoxia dilemma. For instance, superoxide anions therapy achieved cyclic O2 utilization via cascade reaction, causing irreversible damage to tumor cells. Type I PDT could trigger PDT by the fluorescence or NIR absorption without O2 participation, and efficiently generated ROS. Self-decomposition strategies could achieve precise O2 release at hypoxic sites, thus relieving the restriction from the hypoxic TME. Photodynamic-derived multimodal synergistic therapies were also summarized to enhance PDT including synergistic PDT with chemotherapy, immunotherapy, PTT and starvation therapy.
Nevertheless, currently developed nanodelivery platforms still have some drawbacks, such as poor biocompatibility, inherent toxicity, low efficiency, unstable activity, and complex preparation process, etc. For example, the pathway based on CAT and MOFs was limited in clinical transformation due to its instability. Furthermore, repeated administration of nano-bubble based oxygen carrier would cause toxic reaction. In addition, synergistic immunotherapy had potential immune activation response, leading to hemolysis. Additionally, challenges remained including the inhibition effect of hypoxia alleviation to tumor metastasis, as well as the simultaneous oxygen supply and PS activation to achieve amplified PDT efficiency. Overcoming the shortcomings and improving the techniques should be the focus of the next research step. In addition, although numerous innovative nanoparticle delivery systems had been proposed and developed, and validated in animal models in regulating tumor hypoxia to improve PDT, most of these protocols had not been approved for clinical application yet. Existing studies support that some pathways to alleviate tumor hypoxia have the promise of clinical transformation. For example, HBO therapy has received FDA approval as an effective oxygenation method and has been routinely used for decades in clinics. Although most current strategies to alleviate tumor hypoxia through HBO therapy are still in the laboratory stage, they can quickly translate into clinical trials targeting patients with hypoxic tumors. Moreover, O2 leakage and the inability to deliver to the deep tumor region are also significant problems in O2 delivery. To this end, light-triggered cluster polymer vesicles were proposed for light-induced hypoxic tumor ablation. The vesicles were able to penetrate into the deep tumor and provide O2 supply under light irradiation to enhance the PDT efficacy on hypoxic tumor. Combination therapy strategies exhibit great potential for clinical translation. Especially, clinical available drugs, which can alleviate tumor hypoxia, will be appealing to synergize with PDT and to treat hypoxic tumors. However, the synergistic mechanism and the drug pairs for chemotherapy alleviated tumor hypoxia are waited to be uncovered. Moreover, small molecular drugs are fast to be excreted, and the hypoxia alleviation effect should be improved by adjusting the irradiation time or drug dosage. Therefore, the clinical PDT treatment of cancer still remains an ongoing challenge. The next step should focus on the leap from preclinical animal models to clinical trials and O2 supply quantification, realizing the clinical transformation of PDT drugs. Besides, although PDT in combination with other treatment modalities have made good progress in clinical trials, further exploration is still needed to optimize the controllability and stability of continuous oxygen supply, thus significantly improving the problems of tumor metastasis and invasion to some extent. Only when these deficiencies are solved one by one, the therapeutic effect of PDT in hypoxic tumors will be significantly enhanced and more widely used in other clinical aspects.
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Shao C., Yang F., Miao S., Liu W., Wang C., Shu Y., et al. Role of hypoxia-induced exosomes in tumor biology. Mol Cancer. 2018;17(1):120. doi: 10.1186/s12943-018-0869-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jing X., Yang F., Shao C., Wei K., Xie M., Shen H., et al. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. 2019;18(1):157. doi: 10.1186/s12943-019-1089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saxena K., Jolly M.K., Balamurugan K. Hypoxia, partial EMT and collective migration: emerging culprits in metastasis. Transl Oncol. 2020;13(11) doi: 10.1016/j.tranon.2020.100845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tirpe A.A., Gulei D., Ciortea S.M., Crivii C. Berindan-Neagoe I. Hypoxia: overview on hypoxia-mediated mechanisms with a focus on the role of HIF genes. Int J Mol Sci. 2019;20(24):6140. doi: 10.3390/ijms20246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang G., Li J., Wang X., Ma Y., Yin X., Wang F., et al. The reverse Warburg effect and 18F-FDG uptake in non-small cell lung cancer A549 in mice: a pilot study. J Nucl Med. 2015;56(4):607–612. doi: 10.2967/jnumed.114.148254. [DOI] [PubMed] [Google Scholar]
- 6.Erin N., Grahovac J., Brozovic A., Efferth T. Tumor microenvironment and epithelial mesenchymal transition as targets to overcome tumor multidrug resistance. Drug Resist Updat. 2020;53 doi: 10.1016/j.drup.2020.100715. [DOI] [PubMed] [Google Scholar]
- 7.Khalaf K., Hana D., Chou J.T., Singh C., Mackiewicz A., Kaczmarek M. Aspects of the tumor microenvironment involved in immune resistance and drug resistance. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.656364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Y., Ye J., Li Z., Chen H., Gao Y. Recent progress in sono-photodynamic cancer therapy: from developed new sensitizers to nanotechnology-based efficacy-enhancing strategies. Acta Pharm Sin B. 2021;11(8):2197–2219. doi: 10.1016/j.apsb.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y., Zhao D., Wang G., Wang Y., Cao L., Sun J., et al. Recent progress of hypoxia-modulated multifunctional nanomedicines to enhance photodynamic therapy: opportunities, challenges, and future development. Acta Pharm Sin B. 2020;10(8):1382–1396. doi: 10.1016/j.apsb.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansoori B., Mohammadi A., Amin Doustvandi M., Mohammadnejad F., Kamari F., Gjerstorff M.F., et al. Photodynamic therapy for cancer: role of natural products. Photodiagnosis photodyn Ther. 2019;26:395–404. doi: 10.1016/j.pdpdt.2019.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correia J.H., Rodrigues J.A., Pimenta S., Dong T., Yang Z. Photodynamic therapy review: principles, photosensitizers, applications, and future directions. Pharmaceutics. 2021;13(9):1332. doi: 10.3390/pharmaceutics13091332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du J., Shi T., Long S., Chen P., Peng X. Enhanced photodynamic therapy for overcoming tumor hypoxia: from microenvironment regulation to photosensitizer innovation. Coord Chem Rev. 2021;427(6) [Google Scholar]
- 13.Sengar P., Juárez P., Verdugo-Meza A., Arellano D.L., Jain A., Chauhan K., et al. Development of a functionalized UV-emitting nanocomposite for the treatment of cancer using indirect photodynamic therapy. J Nanobiotechnology. 2018;16(1):19. doi: 10.1186/s12951-018-0344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sengar P., Garcia-Tapia K., Chauhan K., Jain A., Juarez-Moreno K., Borbón-Nuñez H.A., et al. Dual-photosensitizer coupled nanoscintillator capable of producing type I and type II ROS for next generation photodynamic therapy. J Colloid Interface Sci. 2019;536:586–597. doi: 10.1016/j.jcis.2018.10.090. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee S.M., MacRobert A.J., Mosse C.A., Periera B., Bown S.G., Keshtgar M.R.S. Photodynamic therapy: inception to application in breast cancer. Breast. 2017;31:105–113. doi: 10.1016/j.breast.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 16.Moole H., Tathireddy H., Dharmapuri S., Moole V., Boddireddy R., Yedama P., et al. Success of photodynamic therapy in palliating patients with nonresectable cholangiocarcinoma: a systematic review and meta-analysis. World J Gastroenterol. 2017;23(7):1278–1288. doi: 10.3748/wjg.v23.i7.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei F., Rees T.W., Liao X., Ji L., Chao H. Oxygen self-sufficient photodynamic therapy. Coord Chem Rev. 2021;432 [Google Scholar]
- 18.Senniappan K., Jeyabalan S., Rangappa P., Kanchi M. Hyperbaric oxygen therapy: can it be a novel supportive therapy in COVID-19? Indian J Anaesth. 2020;64(10):835–841. doi: 10.4103/ija.IJA_613_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X., Ye N., Xiao C., Wang X., Li S., Deng Y., et al. Hyperbaric oxygen regulates tumor microenvironment and boosts commercialized nanomedicine delivery for potent eradication of cancer stem-like cells. Nano Today. 2021;40 [Google Scholar]
- 20.Li J., Huang J., Ao Y., Li S., Miao Y., Yu Z., et al. Synergizing upconversion nanophotosensitizers with hyperbaric oxygen to remodel the extracellular matrix for enhanced photodynamic cancer therapy. ACS Appl Mater Interfaces. 2018;10(27):22985–22996. doi: 10.1021/acsami.8b07090. [DOI] [PubMed] [Google Scholar]
- 21.Wu X., Zhu Y., Huang W., Li J., Zhang B., Li Z., et al. Hyperbaric oxygen potentiates doxil antitumor efficacy by promoting tumor penetration and sensitizing cancer cells. Adv Sci (Weinh) 2018;5(8) doi: 10.1002/advs.201700859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X., Ye N., Liu S., Guan J., Deng Q., Zhang Z., et al. Hyperbaric oxygen boosts PD-1 antibody delivery and T Cell infiltration for augmented immune responses against solid tumors. Adv Sci (Weinh) 2021;8(15) doi: 10.1002/advs.202100233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X., Ye N., Xu C., Xiao C., Zhang Z., Deng Q., et al. Hyperbaric oxygen regulates tumor mechanics and augments Abraxane and gemcitabine antitumor effects against pancreatic ductal adenocarcinoma by inhibiting cancer-associated fibroblasts. Nano Today. 2022;44 [Google Scholar]
- 24.Yang M., Li J., Gu P., Fan X. The application of nanoparticles in cancer immunotherapy: targeting tumor microenvironment. Bioact Mater. 2020;6(7):1973–1987. doi: 10.1016/j.bioactmat.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yue D., Cai X., Fan M., Zhu J., Tian J., Wu L., et al. An alternating irradiation strategy-driven combination therapy of PDT and RNAi for highly efficient inhibition of tumor growth and metastasis. Adv Healthc Mater. 2021;10(8) doi: 10.1002/adhm.202001850. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Y., Ren X., Hou Z., Wang N., Jiang Y., Luan Y. Engineering a photosensitizer nanoplatform for amplified photodynamic immunotherapy via tumor microenvironment modulation. Nanoscale Horiz. 2021;6(2):120–131. doi: 10.1039/d0nh00480d. [DOI] [PubMed] [Google Scholar]
- 27.Xu X., Chen M., Lou X., Du Y., Shu G., Qi J., et al. Sialic acid-modified mesoporous polydopamine induces tumor vessel normalization to enhance photodynamic therapy by inhibiting VE-cadherin internalization. Chem Eng J. 2021;414 [Google Scholar]
- 28.Une N., Takano-Kasuya M., Kitamura N., Ohta M., Inose T., Kato C., et al. The anti-angiogenic agent lenvatinib induces tumor vessel normalization and enhances radiosensitivity in hepatocellular tumors. Med Oncol. 2021;38(6):60. doi: 10.1007/s12032-021-01503-z. [DOI] [PubMed] [Google Scholar]
- 29.Xu Z., Guo C., Ye Q., Shi Y., Sun Y., Zhang J., et al. Endothelial deletion of SHP2 suppresses tumor angiogenesis and promotes vascular normalization. Nat Commun. 2021;12(1):6310. doi: 10.1038/s41467-021-26697-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu D., Duo Y., Suo M., Zhao Y., Xia L., Zheng Z., et al. Tumor-exocytosed exosome/aggregation-induced emission luminogen hybrid nanovesicles facilitate efficient tumor penetration and photodynamic therapy. Angew Chem Int Ed Engl. 2020;59(33):13836–13843. doi: 10.1002/anie.202003672. [DOI] [PubMed] [Google Scholar]
- 31.Wang X.H., Wei X.F., Liu J.H., He D., Sun D.D., Yang W., et al. Oxygen self-supplying enzymatic nanoplatform for precise and enhanced photodynamic therapy. Adv Therap. 2022 [Google Scholar]
- 32.Dai Y., Ding Y., Li L. Nanozymes for regulation of reactive oxygen species and disease therapy. Chin Chem Lett. 2021;32(9):2715–2728. [Google Scholar]
- 33.Wang H., Wang W., Liu L., Wang M., Li G., Li H., et al. Biodegradable hollow polydopamine@manganese dioxide as an oxygen self-supplied nanoplatform for boosting chemo-photodynamic cancer therapy. ACS Appl Mater Interfaces. 2021;13(48):57009–57022. doi: 10.1021/acsami.1c18601. [DOI] [PubMed] [Google Scholar]
- 34.Liu P., Xie X., Shi X., Peng Y., Ding J., Zhou W., et al. Oxygen-self-supplying and HIF-1α-inhibiting core–shell nanosystem for hypoxia-resistant photodynamic therapy. ACS Appl Mater Interfaces. 2019;11(51):48261–48270. doi: 10.1021/acsami.9b18112. [DOI] [PubMed] [Google Scholar]
- 35.Zhu T., Shi L., Ma C., Xu L., Yang J., Zhou G., et al. Fluorinated chitosan-mediated intracellular catalase delivery for enhanced photodynamic therapy of oral cancer. Biomater Sci. 2021;9(3):658–662. doi: 10.1039/d0bm01898h. [DOI] [PubMed] [Google Scholar]
- 36.Wang X.Y., Lin C., Chang W.J., Huang Y.H., Mi F.L. Thiolated hyaluronic acid and catalase-enhanced CD44-targeting and oxygen self-supplying nanoplatforms with photothermal/photodynamic effects against hypoxic breast cancer cells. Int J Biol Macromol. 2022;221:121–134. doi: 10.1016/j.ijbiomac.2022.08.164. [DOI] [PubMed] [Google Scholar]
- 37.Cheng X., He L., Xu J., Fang Q., Yang L., Xue Y., et al. Oxygen-producing catalase-based prodrug nanoparticles overcoming resistance in hypoxia-mediated chemo-photodynamic therapy. Acta Biomater. 2020;112:234–249. doi: 10.1016/j.actbio.2020.05.035. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y., Li Y., Zhang Z., Wang L., Wang D., Tang B.Z. Triple-Jump photodynamic theranostics: mnO2 combined upconversion nanoplatforms involving a type-i photosensitizer with aggregation-induced emission characteristics for potent cancer treatment. Adv Mater. 2021;33(41) doi: 10.1002/adma.202103748. [DOI] [PubMed] [Google Scholar]
- 39.Zhou L., Li W., Wen Y., Fu X., Leng F., Yang J., et al. Chem-inspired hollow ceria nanozymes with lysosome-targeting for tumor synergistic phototherapy. J Mater Chem B. 2021;9(10):2515–2523. doi: 10.1039/d0tb02837a. [DOI] [PubMed] [Google Scholar]
- 40.Liu X.L., Dong X., Yang S.C., Lai X., Liu H.J., Gao Y., et al. Biomimetic liposomal nanoplatinum for targeted cancer chemophototherapy. Adv Sci (Weinh) 2021;8(8) doi: 10.1002/advs.202003679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui X., Zhao Q., Huang Z., Xiao Y., Wan Y., Li S., et al. Water-splitting based and related therapeutic effects: evolving concepts, progress, and perspectives. Small. 2020;16(47) doi: 10.1002/smll.202004551. [DOI] [PubMed] [Google Scholar]
- 42.He C., Zhang X., Chen C., Liu X., Chen Y., Yan R., et al. A solid lipid coated calcium peroxide nanocarrier enables combined cancer chemo/chemodynamic therapy with O2/H2O2 self-sufficiency. Acta Biomater. 2021;122:354–364. doi: 10.1016/j.actbio.2020.12.036. [DOI] [PubMed] [Google Scholar]
- 43.Qian Y., Zhang J., Zhang Y., Chen J., Zhou X. Degradation of 2,4-dichlorophenol by nanoscale calcium peroxide: implication for groundwater remediation. Sep Purif Technol. 2016;166:222–229. [Google Scholar]
- 44.Sun X., Chen K., Liu Y., Zhang G., Shi M., Shi P., et al. Metal–organic framework combined with CaO2 nanoparticles for enhanced and targeted photodynamic therapy. Nanoscale Adv. 2021;3(23):6669–6677. doi: 10.1039/d1na00610j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li R.Q., Zhang C., Xie B.R., Yu W.Y., Qiu W.X., Cheng H., et al. A two-photon excited O2-evolving nanocomposite for efficient photodynamic therapy against hypoxic tumor. Biomaterials. 2019;194:84–93. doi: 10.1016/j.biomaterials.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 46.Hong L., Li J., Luo Y., Guo T., Zhang C., Ou S., et al. Recent advances in strategies for addressing hypoxia in tumor photodynamic therapy. Biomolecules. 2022;12(1):81. doi: 10.3390/biom12010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei F., Kuang S., Rees T.W., Liao X., Liu J., Luo D., et al. Ruthenium(II) complexes coordinated to graphitic carbon nitride: oxygen self-sufficient photosensitizers which produce multiple ROS for photodynamic therapy in hypoxia. Biomaterials. 2021;276 doi: 10.1016/j.biomaterials.2021.121064. [DOI] [PubMed] [Google Scholar]
- 48.Jiang Z., Le S., Xie Y., Huang Q., Wang B., Jiang T. mpg-C₃N₄/Ag₂O nanocomposites photocatalysts with enhanced visible-light photocatalytic performance. J Nanosci Nanotechnol. 2019;19(2):721–728. doi: 10.1166/jnn.2019.15732. [DOI] [PubMed] [Google Scholar]
- 49.Zheng D.W., Li B., Li C.X., Fan J.X., Lei Q., Li C., et al. Carbon-dot-decorated carbon nitride nanoparticles for enhanced photodynamic therapy against hypoxic tumor via water splitting. ACS Nano. 2016;10(9):8715–8722. doi: 10.1021/acsnano.6b04156. [DOI] [PubMed] [Google Scholar]
- 50.Bosio G.N., Mártire D.O. Therapy P. Carbon nitride nanomaterials with application in photothermal and photodynamic therapies. Photodiagnosis Photodyn Ther. 2021;37 doi: 10.1016/j.pdpdt.2021.102683. [DOI] [PubMed] [Google Scholar]
- 51.Sahu A., Kwon I., Tae G. Improving cancer therapy through the nanomaterials-assisted alleviation of hypoxia. Biomaterials. 2020;228 doi: 10.1016/j.biomaterials.2019.119578. [DOI] [PubMed] [Google Scholar]
- 52.Chen H., Ma A., Yin T., Chen Z., Liang R., Pan H., et al. In situ photocatalysis of tio–porphyrin-encapsulated nanosystem for highly efficient oxidative damage against hypoxic tumors. ACS Appl Mater Interfaces. 2020;12(11):12573–12583. doi: 10.1021/acsami.0c00921. [DOI] [PubMed] [Google Scholar]
- 53.Noman M.T., Ashraf M.A., Ali A. Synthesis and applications of nano-TiO2: a review. Environ Sci Pollut Res Int. 2019;26(4):3262–3291. doi: 10.1007/s11356-018-3884-z. [DOI] [PubMed] [Google Scholar]
- 54.Wang S.B., Zhang C., Liu X.H., Chen Z.X., Peng S.Y., Zhong Z.L., et al. A tungsten nitride-based O2 self-sufficient nanoplatform for enhanced photodynamic therapy against hypoxic tumors. Advanced Therapeutics. 2019;2(6) [Google Scholar]
- 55.Sen Gupta A. Hemoglobin-based oxygen carriers: current state-of-the-art and novel molecules. Shock. 2019;52:70–83. doi: 10.1097/SHK.0000000000001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li T., Jing X., Huang Y. Polymer/hemoglobin assemblies: biodegradable oxygen carriers for artificial red blood cells. Macromol Biosci. 2011;11(7):865–875. doi: 10.1002/mabi.201000469. [DOI] [PubMed] [Google Scholar]
- 57.Zhang P., Liu G., Chen X. Nanobiotechnology: cell membrane-based delivery systems. Nano Today. 2017;13:7–9. doi: 10.1016/j.nantod.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu W.L., Liu T., Zou M.Z., Yu W.Y., Li C.X., He Z.Y., et al. Aggressive man-made red blood cells for hypoxia-resistant photodynamic therapy. Adv Mater. 2018;30(35) doi: 10.1002/adma.201802006. [DOI] [PubMed] [Google Scholar]
- 59.Jiang L., Bai H., Liu L., Lv F., Ren X., Wang S. Luminescent, oxygen-supplying, hemoglobin-linked conjugated polymer nanoparticles for photodynamic therapy. Angew Chem Int Ed Engl. 2019;58(31):10660–10665. doi: 10.1002/anie.201905884. [DOI] [PubMed] [Google Scholar]
- 60.Wang S. Hemoglobin-linked conjugated polymer nanoparticles for self-luminescing and oxygen self-supplying phototherapy. Chem Int Ed. 2019;58(31) doi: 10.1002/anie.201905884. [DOI] [PubMed] [Google Scholar]
- 61.Lei C., Liu X.R., Chen Q.B., Li Y., Zhou J.L., Zhou L.Y., et al. Hyaluronic acid and albumin based nanoparticles for drug delivery. J Control Release. 2021;331:416–433. doi: 10.1016/j.jconrel.2021.01.033. [DOI] [PubMed] [Google Scholar]
- 62.Luo Z., Tian H., Liu L., Chen Z., Liang R., Chen Z., et al. Tumor-targeted hybrid protein oxygen carrier to simultaneously enhance hypoxia-dampened chemotherapy and photodynamic therapy at a single dose. Theranostics. 2018;8(13):3584–3596. doi: 10.7150/thno.25409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liang X., Chen M., Bhattarai P., Hameed S., Dai Z. Perfluorocarbon@Porphyrin nanoparticles for tumor hypoxia relief to enhance photodynamic therapy against liver metastasis of colon cancer. ACS Nano. 2020;14(10):13569–13583. doi: 10.1021/acsnano.0c05617. [DOI] [PubMed] [Google Scholar]
- 64.Krafft M. Alleviating tumor hypoxia with perfluorocarbon-based oxygen carriers. Curr Opin Pharmacol. 2020;53:117–125. doi: 10.1016/j.coph.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 65.Li N., Xu F., Cheng J., Zhang Y., Huang G., Zhu J., et al. Perfluorocarbon nanocapsules improve hypoxic microenvironment for the tumor ultrasound diagnosis and photodynamic therapy. J Biomed Nanotechnol. 2018;14(12):2162–2171. doi: 10.1166/jbn.2018.2656. [DOI] [PubMed] [Google Scholar]
- 66.Song G., Liang C., Yi X., Zhao Q., Cheng L., Yang K., et al. Perfluorocarbon-loaded hollow Bi2Se3 nanoparticles for timely supply of oxygen under near-infrared light to enhance the radiotherapy of cancer. Adv Mater. 2016;28(14):2716–2723. doi: 10.1002/adma.201504617. [DOI] [PubMed] [Google Scholar]
- 67.Li J., Xue Y., Tian J., Liu Z., Zhuang A., Gu P., et al. Fluorinated-functionalized hyaluronic acid nanoparticles for enhanced photodynamic therapy of ocular choroidal melanoma by ameliorating hypoxia. Carbohydr Polym. 2020;237 doi: 10.1016/j.carbpol.2020.116119. [DOI] [PubMed] [Google Scholar]
- 68.Chen S., Huang B., Pei W., Wang L., Xu Y., Niu C. Mitochondria-targeting oxygen-sufficient perfluorocarbon nanoparticles for imaging-guided tumor phototherapy. Int J Nanomedicine. 2020;15:8641–8658. doi: 10.2147/IJN.S281649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li M., Fang H., Liu Q., Gai Y., Yuan L., Wang S., et al. Red blood cell membrane-coated upconversion nanoparticles for pretargeted multimodality imaging of triple-negative breast cancer. Biomater Sci. 2020;8(7):1802–1814. doi: 10.1039/d0bm00029a. [DOI] [PubMed] [Google Scholar]
- 70.Fang H., Gai Y., Wang S., Liu Q., Zhang X., Ye M., et al. Biomimetic oxygen delivery nanoparticles for enhancing photodynamic therapy in triple-negative breast cancer. J Nanobiotechnology. 2021;19(1):81. doi: 10.1186/s12951-021-00827-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stride E., Segers T., Lajoinie G., Cherkaoui S., Bettinger T., Versluis M., et al. Microbubble agents: new directions. Ultrasound Med Biol. 2020;46(6):1326–1343. doi: 10.1016/j.ultrasmedbio.2020.01.027. [DOI] [PubMed] [Google Scholar]
- 72.Cavalli R., Soster M., Argenziano M. Nanobubbles: a promising efficient tool for therapeutic delivery. Ther Deliv. 2016;7(2):117–138. doi: 10.4155/tde.15.92. [DOI] [PubMed] [Google Scholar]
- 73.Song R., Peng S., Lin Q., Luo M., Chung H.Y., Zhang Y., et al. pH-Responsive oxygen nanobubbles for spontaneous oxygen delivery in hypoxic tumors. Langmuir. 2019;35(31):10166–10172. doi: 10.1021/acs.langmuir.8b03650. [DOI] [PubMed] [Google Scholar]
- 74.Song L., Wang G., Hou X., Kala S., Qiu Z., Wong K.F., et al. Biogenic nanobubbles for effective oxygen delivery and enhanced photodynamic therapy of cancer. Acta Biomater. 2020;108:313–325. doi: 10.1016/j.actbio.2020.03.034. [DOI] [PubMed] [Google Scholar]
- 75.He S., Wu L., Li X., Sun H., Xiong T., Liu J., et al. Metal-organic frameworks for advanced drug delivery. Acta Pharm Sin B. 2021;11(8):2362–2395. doi: 10.1016/j.apsb.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu B., Fu J., Zhou Y., Luo S., Zhao Y., Quan G., et al. Tailored core‒shell dual metal–organic frameworks as a versatile nanomotor for effective synergistic antitumor therapy. Acta Pharm Sin B. 2020;10(11):2198–2211. doi: 10.1016/j.apsb.2020.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guan Q., Li Y.A., Li W.Y., Dong Y.B. Photodynamic therapy based on nanoscale metal-organic frameworks: from material design to cancer nanotherapeutics. Chem Asian J. 2018;13(21):3122–3149. doi: 10.1002/asia.201801221. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y., Wang F., Liu C., Wang Z., Kang L., Huang Y., et al. Nanozyme decorated metal–organic frameworks for enhanced photodynamic therapy. ACS Nano. 2018;12(1):651–661. doi: 10.1021/acsnano.7b07746. [DOI] [PubMed] [Google Scholar]
- 79.Lismont M., Dreesen L., Wuttke S. Metal-organic framework nanoparticles in photodynamic therapy: current status and perspectives. Adv Funct Mater. 2017;27(14) [Google Scholar]
- 80.Gao S., Zheng P., Li Z., Feng X., Yan W., Chen S., et al. Biomimetic O2-evolving metal-organic framework nanoplatform for highly efficient photodynamic therapy against hypoxic tumor. Biomaterials. 2018;178:83–94. doi: 10.1016/j.biomaterials.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 81.Li S.Y., Cheng H., Xie B.R., Qiu W.X., Zeng J.Y., Li C.X., et al. Cancer cell membrane camouflaged cascade bioreactor for cancer targeted starvation and photodynamic therapy. ACS Nano. 2017;11(7):7006–7018. doi: 10.1021/acsnano.7b02533. [DOI] [PubMed] [Google Scholar]
- 82.Chang M., Feng W., Ding L., Zhang H., Dong C., Chen Y., et al. Persistent luminescence phosphor as in-vivo light source for tumoral cyanobacterial photosynthetic oxygenation and photodynamic therapy. Bioact Mater. 2022;10:131–144. doi: 10.1016/j.bioactmat.2021.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li W., Zhong D., Hua S., Du Z., Zhou M. Biomineralized biohybrid algae for tumor hypoxia modulation and cascade radio-photodynamic therapy. ACS Appl Mater Interfaces. 2020;12(40):44541–44553. doi: 10.1021/acsami.0c14400. [DOI] [PubMed] [Google Scholar]
- 84.Yuan P., Deng F.A., Liu Y.B., Zheng R.R., Rao X.N., Qiu X.Z., et al. Mitochondria targeted O2 economizer to alleviate tumor hypoxia for enhanced photodynamic therapy. Adv Healthc Mater. Jun 2021;10(12) doi: 10.1002/adhm.202100198. [DOI] [PubMed] [Google Scholar]
- 85.Qi F., Ji P., Chen Z., Wang L., Yao H., Huo M., et al. Photosynthetic cyanobacteria-hybridized black phosphorus nanosheets for enhanced tumor photodynamic therapy. Small. 2021;17(42) doi: 10.1002/smll.202102113. [DOI] [PubMed] [Google Scholar]
- 86.Wang H., Guo Y., Wang C., Jiang X., Liu H., Yuan A., et al. Light-controlled oxygen production and collection for sustainable photodynamic therapy in tumor hypoxia. Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2020.120621. [DOI] [PubMed] [Google Scholar]
- 87.Sun T., Zhang Y., Zhang C., Wang H., Pan H., Liu J., et al. Cyanobacteria-based bio-oxygen pump promoting hypoxia-resistant photodynamic therapy. Front Bioeng Biotechnol. 2020;8:237. doi: 10.3389/fbioe.2020.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang J.Q., Zhao L.P., Zhou X., Liu L.S., Zheng R.R., Deng F.A., et al. Carrier free O2-economizer for photodynamic therapy against hypoxic tumor by inhibiting cell respiration. Small. 2022;18(15) doi: 10.1002/smll.202107467. [DOI] [PubMed] [Google Scholar]
- 89.Zhang W., Wen Y., He D.X., Wang Y.F., Liu X.L., Li C., et al. Near-infrared AIEgens as transformers to enhance tumor treatment efficacy with controllable self-assembled redox-responsive carrier-free nanodrug. Biomaterials. 2019;193:12–21. doi: 10.1016/j.biomaterials.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 90.Zhao L.P., Zheng R.R., Chen H.Q., Liu L.S., Zhao X.Y., Liu H.H., et al. Self-delivery nanomedicine for O2-economized photodynamic tumor therapy. Nano Lett. 2020;20(3):2062–2071. doi: 10.1021/acs.nanolett.0c00047. [DOI] [PubMed] [Google Scholar]
- 91.Fan Y.T., Zhou T.J., Cui P.F., He Y.J., Chang X., Xing L., et al. Modulation of intracellular oxygen pressure by dual-drug nanoparticles to enhance photodynamic therapy. Adv Funct Mater. 2019;29(10) [Google Scholar]
- 92.Wang D., Xue B., Ohulchanskyy T.Y., Liu Y., Yakovlev A., Ziniuk R., et al. Inhibiting tumor oxygen metabolism and simultaneously generating oxygen by intelligent upconversion nanotherapeutics for enhanced photodynamic therapy. Biomaterials. 2020;251 doi: 10.1016/j.biomaterials.2020.120088. [DOI] [PubMed] [Google Scholar]
- 93.Mascaraque M., Delgado W.P., Nuevo T.C., Gracia C.T., Abarca L.E., González S., et al. Metformin as an adjuvant to photodynamic therapy in resistant basal cell carcinoma cells. Cancers (Basel) 2020;12(3):668. doi: 10.3390/cancers12030668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xiang Q., Qiao B., Luo Y., Cao J., Fan K., Hu X., et al. Increased photodynamic therapy sensitization in tumors using a nitric oxide-based nanoplatform with ATP-production blocking capability. Theranostics. 2021;11(4):1953–1969. doi: 10.7150/thno.52997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen D., Xu Q., Wang W., Shao J., Huang W., Dong X. Type I photosensitizers revitalizing photodynamic oncotherapy. Small. 2021;17(31) doi: 10.1002/smll.202006742. [DOI] [PubMed] [Google Scholar]
- 96.Jiang S., Xiao M., Sun W., Crespy D., Mailänder V., Peng X., et al. Synergistic anticancer therapy by ovalbumin encapsulation-enabled tandem reactive oxygen species generation. Angew Chem Int Ed Engl. 2020;59(45):20008–20016. doi: 10.1002/anie.202006649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang M., Maragani S., Huang L., Jeon S., Canteenwala T., Hamblin M.R., et al. Synthesis of decacationic fullerene decaiodides giving photoinduced production of superoxide radicals and effective PDT-mediation on antimicrobial photoinactivation. Eur J Med Chem. 2013;63:170–184. doi: 10.1016/j.ejmech.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guan M., Zhou Y., Liu S., Chen D., Ge J., Deng R., et al. Photo-triggered gadofullerene: enhanced cancer therapy by combining tumor vascular disruption and stimulation of anti-tumor immune responses. Biomaterials. 2019;213 doi: 10.1016/j.biomaterials.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 99.Chen D., Yu Q., Huang X., Dai H., Luo T., Shao J., et al. A highly-efficient type I photosensitizer with robust vascular-disruption activity for hypoxic-and-metastatic tumor specific photodynamic therapy. Small. 2020;16(23) doi: 10.1002/smll.202001059. [DOI] [PubMed] [Google Scholar]
- 100.Li M., Shao Y., Kim J.H., Pu Z., Zhao X., Huang H., et al. Unimolecular photodynamic O2-economizer to overcome hypoxia resistance in phototherapeutics. J Am Chem Soc. 2020;142(11):5380–5388. doi: 10.1021/jacs.0c00734. [DOI] [PubMed] [Google Scholar]
- 101.Li M., Xiong T., Du J., Tian R., Xiao M., Guo L., et al. Superoxide radical photogenerator with amplification effect: surmounting the Achilles’ heels of photodynamic oncotherapy. J Am Chem Soc. 2019;141(6):2695–2702. doi: 10.1021/jacs.8b13141. [DOI] [PubMed] [Google Scholar]
- 102.Alzeibak R., Mishchenko T.A., Shilyagina N.Y., Balalaeva I.V., Vedunova M.V., Krysko D.V. Targeting immunogenic cancer cell death by photodynamic therapy: past, present and future. J Immunother Cancer. 2021;9(1) doi: 10.1136/jitc-2020-001926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baptista M.S., Cadet J., Di Mascio P., Ghogare A.A., Greer A., Hamblin M.R., et al. Type I and Type II photosensitized oxidation reactions: guidelines and mechanistic pathways. Photochem Photobiol. 2017;93(4):912–919. doi: 10.1111/php.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shigemitsu H., Ohkubo K., Sato K., Bunno A., Mori T., Osakada Y., et al. Fluorescein-based type I supramolecular photosensitizer via induction of charge separation by self-assembly. JACS Au. 2022;2(6):1472–1478. doi: 10.1021/jacsau.2c00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao Y.Y., Zhang L., Chen Z., Zheng B.Y., Ke M., Li X., et al. Nanostructured phthalocyanine assemblies with efficient synergistic effect of type I photoreaction and photothermal action to overcome tumor hypoxia in photodynamic therapy. J Am Chem Soc. 2021;143(34):13980–13989. doi: 10.1021/jacs.1c07479. [DOI] [PubMed] [Google Scholar]
- 106.Sun J., Cai X., Wang C., Du K., Chen W., Feng F., et al. Cascade reactions by nitric oxide and hydrogen radical for anti-hypoxia photodynamic therapy using an activatable photosensitizer. J Am Chem Soc. 2021;143(2):868–878. doi: 10.1021/jacs.0c10517. [DOI] [PubMed] [Google Scholar]
- 107.Li X., Kwon N., Guo T., Liu Z., Yoon J. Innovative strategies for hypoxic-tumor photodynamic therapy. Angew Chem Int Ed Engl. 2018;57(36):11522–11531. doi: 10.1002/anie.201805138. [DOI] [PubMed] [Google Scholar]
- 108.Zhang C., Qin W.J., Bai X.F., Zhang X. Nanomaterials to relieve tumor hypoxia for enhanced photodynamic therapy. Nano Today. 2020;35 [Google Scholar]
- 109.Yu Q., Huang T., Liu C., Zhao M., Xie M., Li G., et al. Oxygen self-sufficient NIR-activatable liposomes for tumor hypoxia regulation and photodynamic therapy. Chem Sci. 2019;10(39):9091–9098. doi: 10.1039/c9sc03161h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu S., Zhu X., Zhang C., Huang W., Zhou Y., Yan D. Oxygen and Pt (II) self-generating conjugate for synergistic photo-chemo therapy of hypoxic tumor. Nat Commun. 2018;9(1):2053. doi: 10.1038/s41467-018-04318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guo D., Xu S., Huang Y., Jiang H., Yasen W., Wang N., et al. Platinum (IV) complex-based two-in-one polyprodrug for a combinatorial chemo-photodynamic therapy. Biomaterials. 2018;177:67–77. doi: 10.1016/j.biomaterials.2018.05.052. [DOI] [PubMed] [Google Scholar]
- 112.Song H., Li W., Qi R., Yan L., Jing X., Zheng M., et al. Delivering a photosensitive transplatin prodrug to overcome cisplatin drug resistance. Chem Commun (Camb) 2015;51(57):11493–11495. doi: 10.1039/c5cc03692e. [DOI] [PubMed] [Google Scholar]
- 113.Ji B., Wei M., Yang B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics. 2022;12(1):434–458. doi: 10.7150/thno.67300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wan S.S., Zeng J.Y., Cheng H., Zhang X.Z. ROS-induced NO generation for gas therapy and sensitizing photodynamic therapy of tumor. Biomaterials. 2018;185:51–62. doi: 10.1016/j.biomaterials.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 115.Zeng Y., Ma J., Zhan Y., Xu X., Zeng Q., Liang J., et al. Hypoxia-activated prodrugs and redox-responsive nanocarriers. Int J Nanomedicine. 2018;13:6551–6574. doi: 10.2147/IJN.S173431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Anduran E., Dubois L.J., Lambin P., Winum J.-.Y. Hypoxia-activated prodrug derivatives of anti-cancer drugs: a patent review 2006 –2021. Expert Opin Ther Pat. 2022;32(1):1–12. doi: 10.1080/13543776.2021.1954617. [DOI] [PubMed] [Google Scholar]
- 117.Zhou M., Xie Y., Xu S., Xin J., Wang J., Han T., et al. Hypoxia-activated nanomedicines for effective cancer therapy. Eur J Med Chem. 2020;195 doi: 10.1016/j.ejmech.2020.112274. [DOI] [PubMed] [Google Scholar]
- 118.Chen Y., Gao Y., Li Y., Wang K., Zhu J. Synergistic chemo-photodynamic therapy mediated by light-activated ROS-degradable nanocarriers. J Mater Chem B. 2019;7(3):460–468. doi: 10.1039/c8tb03030h. [DOI] [PubMed] [Google Scholar]
- 119.He H., Zhu R., Sun W., Cai K., Chen Y., Yin L. Selective cancer treatment via photodynamic sensitization of hypoxia-responsive drug delivery. Nanoscale. 2018;10(6):2856–2865. doi: 10.1039/c7nr07677k. [DOI] [PubMed] [Google Scholar]
- 120.Feng L., Cheng L., Dong Z., Tao D., Barnhart T.E., Cai W., et al. Theranostic liposomes with hypoxia-activated prodrug to effectively destruct hypoxic tumors post-photodynamic therapy. ACS Nano. 2017;11(1):927–937. doi: 10.1021/acsnano.6b07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chou Y.T., Lin C.Y., Wen J.W., Hung L.C., Chang Y.F., Yang C.M., et al. Targeting triple-negative breast cancer with an aptame-functionalized nanoformulation: a synergistic treatment that combines photodynamic and bioreductive therapies. J Nanobiotechnology. 2021;19(1):1–15. doi: 10.1186/s12951-021-00786-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang H., Zhu W., Feng L., Chen Q., Chao Y., Dong Z., et al. Nanoscale covalent organic polymers as a biodegradable nanomedicine for chemotherapy-enhanced photodynamic therapy of cancer. Nano Res. 2018;11(6):3244–3257. [Google Scholar]
- 123.Riley R.S., June C.H., Langer R., Mitchell M. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18(3):175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chung C.H., Lu K.Y., Lee W.C., Hsu W.J., Lee W.F., Dai J.Z., et al. Fucoidan-based, tumor-activated nanoplatform for overcoming hypoxia and enhancing photodynamic therapy and antitumor immunity. Biomaterials. 2020;257 doi: 10.1016/j.biomaterials.2020.120227. [DOI] [PubMed] [Google Scholar]
- 125.Graham K., Unger E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int J Nanomed. 2018;13:6049–6058. doi: 10.2147/IJN.S140462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li W., Yang J., Luo L., Jiang M., Qin B., Yin H., et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat Commun. 2019;10(1):3349. doi: 10.1038/s41467-019-11269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Swamy K. Vascular normalization and immunotherapy: spawning a virtuous cycle. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.1002957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Meng Z., Zhou X., Xu J., Han X., Dong Z., Wang H., et al. Light-triggered in situ gelation to enable robust photodynamic-lmmunotherapy by repeated stimulations. Adv Mater. 2019;31(24) doi: 10.1002/adma.201900927. [DOI] [PubMed] [Google Scholar]
- 129.Qiu Q., Li C., Yan X., Zhang H., Luo X., Gao X., et al. Photodynamic/photothermal therapy enhances neutrophil-mediated ibrutinib tumor delivery for potent tumor immunotherapy: more than one plus one? Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2021.120652. [DOI] [PubMed] [Google Scholar]
- 130.Liu Y., Yang J., Liu B., Cao W., Zhang J., Yang Y., et al. Human iPS cells loaded with MnO2-based nanoprobes for photodynamic and simultaneous enhanced immunotherapy against cancer. Nanomicro Lett. 2020;12(1):127. doi: 10.1007/s40820-020-00452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang C., Zhu Y., Li D., Liu Y., Guan C., Man X., et al. Red phosphorus decorated TiO2 nanorod mediated photodynamic and photothermal therapy for renal cell carcinoma. Small. 2021;17(30) doi: 10.1002/smll.202101837. [DOI] [PubMed] [Google Scholar]
- 132.Younis M.R., Wang C., An R., Wang S., Younis M.A., Li Z.Q., et al. Low power single laser activated synergistic cancer phototherapy using photosensitizer functionalized dual plasmonic photothermal nanoagents. ACS Nano. 2019;13(2):2544–2557. doi: 10.1021/acsnano.8b09552. [DOI] [PubMed] [Google Scholar]
- 133.Candelaria P.V., Leoh L.S., Penichet M.L., Daniels-Wells T.R. Antibodies targeting the transferrin receptor 1 (TfR1) as direct anti-cancer agents. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.607692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang K., Zhang Y., Wang J., Yuan A., Sun M., Wu J., et al. Self-assembled IR780-loaded transferrin nanoparticles as an imaging, targeting and PDT/PTT agent for cancer therapy. Sci Rep. 2016;6(1):27421. doi: 10.1038/srep27421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhu W., Liu Y., Yang Z., Zhang L., Xiao L., Liu P., et al. Albumin/sulfonamide stabilized iron porphyrin metal organic framework nanocomposites: targeting tumor hypoxia by carbonic anhydrase IX inhibition and T1-T2 dual mode MRI guided photodynamic/photothermal therapy. J Mater Chem B. 2018;6(2):265–276. doi: 10.1039/c7tb02818k. [DOI] [PubMed] [Google Scholar]
- 136.Shang D., Yu Q., Liu W., Zhang S., Li Y., Chen J., et al. Enhanced porphyrin-based fluorescence imaging-guided photodynamic/photothermal synergistic cancer therapy by mitochondrial targeting. Sci China Mater. 2022;65(2):527–535. [Google Scholar]
- 137.Yu S., Chen Z., Zeng X., Chen X., Gu Z. Advances in nanomedicine for cancer starvation therapy. Theranostics. 2019;9(26):8026–8047. doi: 10.7150/thno.38261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dang J., Ye H., Li Y., Liang Q., Li X., Yin L.J. Multivalency-assisted membrane-penetrating siRNA delivery sensitizes photothermal ablation via inhibition of tumor glycolysis metabolism. Biomaterials. 2019;223 doi: 10.1016/j.biomaterials.2019.119463. [DOI] [PubMed] [Google Scholar]
- 139.Fan X., Luo Z., Chen Y., Yeo J.C.C., Li Z., Wu Y.L., et al. Oxygen self-supplied enzyme nanogels for tumor targeting with amplified synergistic starvation and photodynamic therapy. Acta Biomater. 2022;142:274–283. doi: 10.1016/j.actbio.2022.01.056. [DOI] [PubMed] [Google Scholar]
- 140.Yuan G., Cen J., Liao J., Huang Y., Jie L. In situ hydrogen nanogenerator for bimodal imaging guided synergistic photothermal/hydrogen therapies. Nanoscale. 2021;13(37):15576–15589. doi: 10.1039/d1nr03260g. [DOI] [PubMed] [Google Scholar]
- 141.Li S., Lin K., Hu P., Wang S., Zhao S., Gan Y., et al. A multifunctional nanoamplifier with self-enhanced acidity and hypoxia relief for combined photothermal/photodynamic/starvation therapy. Int J Pharm. 2022;611 doi: 10.1016/j.ijpharm.2021.121307. [DOI] [PubMed] [Google Scholar]
- 142.Yu Z., Zhou P., Pan W., Li N., Tang B. A biomimetic nanoreactor for synergistic chemiexcited photodynamic therapy and starvation therapy against tumor metastasis. Nat Commun. 2018;9(1):5044. doi: 10.1038/s41467-018-07197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu S., Yan T., Sun J., Li F., Xu J., Sun H., et al. Biomimetic cascade polymer nanoreactors for starvation and photodynamic cancer therapy. Molecules. 2021;26(18):5609. doi: 10.3390/molecules26185609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hughes J.R., Parsons J.L. FLASH radiotherapy: current knowledge and future insights using proton-beam therapy. Int J Mol Sci. 2020;21(18):6492. doi: 10.3390/ijms21186492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kabakov A.E., Yakimova A.O. Hypoxia-induced cancer cell responses driving radioresistance of hypoxic tumors: approaches to targeting and radiosensitizing. Cancers (Basel) 2021;13(5):1102. doi: 10.3390/cancers13051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.McBride W.H., Schaue D. Radiation-induced tissue damage and response. J Pathol. 2020;250(5):647–655. doi: 10.1002/path.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gu X., Shen C., Li H., Goldys E.M., Deng W. X-ray induced photodynamic therapy (PDT) with a mitochondria-targeted liposome delivery system. J Nanobiotechnology. 2020;18(1):87. doi: 10.1186/s12951-020-00644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hou Z., Zhou M., Ma Y., Xu X., Zhang Z., Lai S., et al. Size-changeable nanoprobes for the combined radiotherapy and photodynamic therapy of tumor. Eur J Nucl Med Mol Imaging. 2022;49(8):2655–2667. doi: 10.1007/s00259-022-05830-9. [DOI] [PubMed] [Google Scholar]
- 149.Adhikari A., Mondal S., Das M., Biswas P., Pal U., Darbar S., et al. Incorporation of a biocompatible nanozyme in cellular antioxidant enzyme cascade reverses huntington's like disorder in preclinical model. Adv Healthc Mater. 2021;10(7) doi: 10.1002/adhm.202001736. [DOI] [PubMed] [Google Scholar]
- 150.Lou X.F., Wang C., Zhang J.C., Du Y.Z., Xu X.L. A melanin-like nanoenzyme for acute lung injury therapy via suppressing oxidative and endoplasmic reticulum stress response. Pharmaceutics. 2021;13(11):1850. doi: 10.3390/pharmaceutics13111850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jia Y., Duan L., Li J. Hemoglobin-based nanoarchitectonic assemblies as oxygen carriers. Adv Mater. 2016;28(6):1312–1318. doi: 10.1002/adma.201502581. [DOI] [PubMed] [Google Scholar]
- 152.Shi X., Yang W., Ma Q., Lu Y., Xu Y., Bian K., et al. Hemoglobin-mediated biomimetic synthesis of paramagnetic O2-evolving theranostic nanoprobes for MR imaging-guided enhanced photodynamic therapy of tumor. Theranostics. 2020;10(25):11607–11621. doi: 10.7150/thno.46228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hu D.R., Zhong L., Wang M.Y., Li H.H., Qu Y., Liu Q.Y., et al. Perfluorocarbon-loaded and redox-activatable photosensitizing agent with oxygen supply for enhancement of fluorescence/photoacoustic imaging guided tumor photodynamic therapy. Adv Funct Mater. 2019;29(9) [Google Scholar]
- 154.Xing L., Gong J.H., Wang Y., Zhu Y., Huang Z.J., Zhao J., et al. Hypoxia alleviation-triggered enhanced photodynamic therapy in combination with IDO inhibitor for preferable cancer therapy. Biomaterials. 2019;(206):170–182. doi: 10.1016/j.biomaterials.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 155.Liao A. Ultrasound in biomedical engineering: ultrasound microbubble contrast agents promote transdermal permeation of drugs. Ultrasound Med Biol. 2016;24(3):86–88. [Google Scholar]
- 156.Alves S.R., Calori I.R., Tedesco A.C. Photosensitizer-based metal-organic frameworks for highly effective photodynamic therapy. Mater Sci Eng C Mater Biol Appl. 2021;131 doi: 10.1016/j.msec.2021.112514. [DOI] [PubMed] [Google Scholar]
- 157.Hidalgo T., Simón-Vázquez R., González-Fernández A., Horcajada P. Cracking the immune fingerprint of metal-organic frameworks. Chem Sci. 2021;13(4):934–944. doi: 10.1039/d1sc04112f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chelora J., Liang Y., Wei W.C., Cui X., Xiao Y., Wan Y., et al. Single molecular nanomedicine with NIR light-initiated superoxide radical, singlet oxygen and thermal generation for hypoxia-overcoming cancer therapy. Nanoscale. 2021;13(17):8012–8016. doi: 10.1039/d1nr01094h. [DOI] [PubMed] [Google Scholar]
- 159.Shih C.Y., Wang P.T., Su W.C., Teng H., Huang W.L. Nanomedicine-based strategies assisting photodynamic therapy for hypoxic tumors: state-of-the-art approaches and emerging trends. Biomedicines. 2021;9(2):137. doi: 10.3390/biomedicines9020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Deng X., Shao Z., Zhao Y. Solutions to the drawbacks of photothermal and photodynamic cancer therapy. Adv Sci (Weinh) 2021;8(3) doi: 10.1002/advs.202002504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kadkhoda J., Tarighatnia A., Barar J., Aghanejad A., Davaran S. Recent advances and trends in nanoparticles based photothermal and photodynamic therapy. Photodiagnosis Photodyn Ther. 2021 doi: 10.1016/j.pdpdt.2021.102697. [DOI] [PubMed] [Google Scholar]
- 162.Emens L.A. Immunotherapy in triple-negative breast cancer. Cancer J. 2021;27(1):59–66. doi: 10.1097/PPO.0000000000000497. [DOI] [PubMed] [Google Scholar]
- 163.Li Y., Zhang X., Liu X., Pan W., Li N., Tang B. Designing and engineering of nanocarriers for bioapplication in cancer immunotherapy. ACS Appl Bio Mater. 2020;3(12):8321–8337. doi: 10.1021/acsabm.0c01272. [DOI] [PubMed] [Google Scholar]
- 164.Wu R., Wang H., Hai L., Wang T., Hou M., He D., et al. A photosensitizer-loaded zinc oxide-polydopamine core-shell nanotherapeutic agent for photodynamic and photothermal synergistic therapy of cancer cells. Chin Chem Lett. 2020;31(1):189–192. [Google Scholar]
- 165.Liu T., Song Y., Huang Z., Pu X., Wang Y., Yin G., et al. Photothermal photodynamic therapy and enhanced radiotherapy of targeting copolymer-coated liquid metal nanoparticles on liver cancer. Colloids Surf B Biointerfaces. 2021;207 doi: 10.1016/j.colsurfb.2021.112023. [DOI] [PubMed] [Google Scholar]
- 166.Busk M., Overgaard J., Horsman M.R. Imaging of tumor hypoxia for radiotherapy:current status and future directions. Semin Nucl Med. 2020;50(6):562–583. doi: 10.1053/j.semnuclmed.2020.05.003. [DOI] [PubMed] [Google Scholar]