Key Points
Question
Does automated quarterly antibiotic prescribing feedback with peer benchmarking over 2 years reduce antibiotic prescribing in the second year of the intervention among primary care physicians who are the top 75% prescribers of antibiotics (ie, with medium to high antibiotic prescription rates)?
Findings
In this randomized clinical trial of 3426 primary care physicians in Switzerland, there was a 4% relative increase in antibiotic prescribing during the second year of the intervention (2019) compared with the baseline year (2017). The median annual antibiotic prescribing rate per 100 consultations was 8.2 in the feedback and audit group and 8.4 in the control group in the second year of the intervention.
Meaning
Among primary care physicians with medium to high antibiotic prescription rates, antibiotic prescribing audit and feedback did not reduce antibiotic prescribing.
Abstract
Importance
Antibiotics are commonly prescribed in primary care, increasing the risk of antimicrobial resistance in the population.
Objective
To investigate the effect of quarterly audit and feedback on antibiotic prescribing among primary care physicians in Switzerland with medium to high antibiotic prescription rates.
Design, Setting, and Participants
This pragmatic randomized clinical trial was conducted from January 1, 2018, to December 31, 2019, among 3426 registered primary care physicians and pediatricians in single or small practices in Switzerland who were among the top 75% prescribers of antibiotics. Intention-to-treat analysis was performed using analysis of covariance models and conducted from September 1, 2021, to January 31, 2022.
Interventions
Primary care physicians were randomized in a 1:1 fashion to undergo quarterly antibiotic prescribing audit and feedback with peer benchmarking vs no intervention for 2 years, with 2017 used as the baseline year. Anonymized patient-level claims data from 3 health insurers serving roughly 50% of insurees in Switzerland were used for audit and feedback. The intervention group also received evidence-based guidelines for respiratory tract and urinary tract infection management and community antibiotic resistance information. Physicians in the intervention group were blinded regarding the nature of the trial, and physicians in the control group were not informed of the trial.
Main Outcomes and Measures
The claims data used for audit and feedback were analyzed to assess outcomes. Primary outcome was the antibiotic prescribing rate per 100 consultations during the second year of the intervention. Secondary end points included overall antibiotic use in the first year and over 2 years, use of quinolones and oral cephalosporins, all-cause hospitalizations, and antibiotic use in 3 age groups.
Results
A total of 3426 physicians were randomized to the intervention (n = 1713) and control groups (n = 1713) serving 629 825 and 622 344 patients, respectively, with a total of 4 790 525 consultations in the baseline year of 2017. In the entire cohort, a 4.2% (95% CI, 3.9%-4.6%) relative increase in the antibiotic prescribing rate was noted during the second year of the intervention compared with 2017. In the intervention group, the median annual antibiotic prescribing rate per 100 consultations was 8.2 (IQR, 6.1-11.4) in the second year of the intervention and was 8.4 (IQR, 6.0-11.8) in the control group. Relative to the overall increase, a –0.1% (95% CI, –1.2% to 1.0%) lower antibiotic prescribing rate per 100 consultations was found in the intervention group compared with the control group. No relevant reductions in specific antibiotic prescribing rates were noted between groups except for quinolones in the second year of the intervention (–0.9% [95% CI, –1.5% to –0.4%]).
Conclusions and Relevance
This randomized clinical trial found that quarterly personalized antibiotic prescribing audit and feedback with peer benchmarking did not reduce antibiotic prescribing among primary care physicians in Switzerland with medium to high antibiotic prescription rates.
Trial Registration
ClinicalTrials.gov Identifier: NCT03379194
This randomized clinical trial investigates the effect of quarterly audit and feedback on antibiotic prescribing among primary care physicians in Switzerland with medium to high antibiotic prescription rates.
Introduction
Most antibiotics in human medicine are prescribed in primary care for respiratory tract and urinary tract infections,1,2,3,4 contributing to increased population-level antibiotic resistance.5,6,7 Effective strategies to reduce antibiotic prescribing in primary care, like face-to-face education or communication training for primary care physicians, are resource intense, costly, challenging to apply on a large scale, and may not reach clinicians with high prescription rates who are not motivated to participate in such interventions.8,9 Therefore, system-wide strategies to improve antibiotic use in primary care are needed. Peer comparison audit and feedback can be an effective tool to modify physician behavior and be applied on a health system level as a low-cost intervention for reducing antibiotic prescribing in primary care.10,11,12 Only a few randomized clinical trials have evaluated audit and feedback interventions for antibiotic prescribing in primary care at the health system level. These trials have produced inconsistent results,10,12,13,14 which may be owing to the intensity and type of feedback interventions, different settings, and baseline level of prescribing.
A nationwide pilot trial in Switzerland that investigated the feasibility and effectiveness of quarterly personalized prescription feedback to primary care physicians based on aggregated practice-based claims data did not observe a reduction in antibiotic prescribing in the intervention group.11 Feedback on that trial was based on aggregated information and did not allow ascribing antibiotic prescriptions to individual patients. Thus, patient factors, such as comorbidities, that may influence prescribing behavior and adverse consequences of the intervention, could not be adequately considered.
The main objective of the present randomized clinical trial was to investigate the effect of patient-level claims data audit and feedback with peer benchmarking provided to physicians compared with no intervention on antibiotic prescribing in primary care. Adverse health outcomes (all-cause hospitalizations and infection-related hospitalizations) were a key secondary end point. An additional goal was to explore the feasibility of developing a nationwide antibiotic prescribing monitoring program using claims data.
Methods
Study Design
This pragmatic randomized clinical trial was conducted from January 1, 2018, to December 31, 2019, among primary care physicians in Switzerland with medium to high antibiotic prescription rates and was based on routinely collected individual claims data of 3 large health insurers in Switzerland (Sanitas, CSS, and Helsana) providing coverage for approximately 50% of Swiss residents of all ages. We used pseudonymized physician and anonymized patient identifiers that were created by data managers of the insurance companies to ensure confidentiality. Claims data were formatted by data managers of the health insurers according to a standard protocol that allowed for data import and the identification of the relevant physician population, the generation of the antibiotic prescribing feedback, and the generation of the full claims data set for the final analysis (eFigure 1 in Supplement 1). The study protocol was approved by all ethics committees in Switzerland (Leitethikkommission Nordwest- und Zentralschweiz, Project-ID 2017-00888) and need for consent of participating physicians and their patients was waived based on article 34b of the Swiss Federal Act on Research involving Human Beings, which defines the rules for the exceptional use of health data without formal consent of participants. Details of the trial protocol have been previously published15 (trial protocol in Supplement 2). All analyses followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline using intention-to-treat principles based on the final data set, which became available in fall 2020.16,17,18 We also conducted a per-protocol analysis and 3 post hoc sensitivity analyses by excluding physicians who were identified as outliers for antibiotic-related end points at baseline, first year of the intervention, and second year of the intervention, by excluding practices of 1 to 2 or more than 3 physicians working under the same license number. The trial is registered at ClinicalTrials.gov (NCT03379194).
Participants
We included board-certified primary care physicians and pediatricians with an individual practicing license number (Zentralregisternummer) identified under a unique address who were among the top 75% prescribers of antibiotics (ie, with medium to high prescription rates) with at least 100 patient contacts per year and identified 3426 from 4888 physicians assessed for eligibility in the claims data set of 2016. License numbers of large group practices and hospital-based ambulatory facilities were excluded, but we included practices where more than 1 physician was working under 1 license number (eTable 10 in Supplement 1). Because of the delayed administrative processing of claims data, the 2016 data set was taken for identification of physicians and sample size calculation.
Randomization and Masking
Eligible physicians were randomized to the intervention and control groups in a 1:1 ratio using a computer-generated algorithm in R, version 4.0.2 (R Group for Statistical Computing).19 Physicians in the intervention groups were formally blinded regarding the fact of being included in an intervention trial, and physicians in the control group were not informed that their antibiotic prescription was monitored for the duration of the trial. As all trial-relevant data were collected by automated processes for claims data by health insurances, the outcome assessment may also be considered formally blinded.
Procedures
Continuously updated quarterly antibiotic prescription feedback contained the personal overall prescription rates and antibiotic type per 100 consultations and year, as well as the personal prescription rates for the 3 months of the same period of the preceding year, with each category compared with the prescription rates of peer physicians. In addition, each mailing contained a message for action (eFigure 2 in Supplement 1).15 The first mailing was sent on December 22, 2017, and was followed by 7 additional quarterly mailings, with the last mailing sent in late September 2019.
Because there is a 6-month delay in health insurance billing records, processing the yearly prescription feedback was based on the data from 9 months before the respective mailing. The first feedback mailing was based on antibiotic prescription rates between April 2016 and March 2017 (eFigure 3 in Supplement 1). Quarterly prescription feedback was prepared by 1 trial statistician (G.M.) with no further involvement in the analysis, and printed, packaged, and mailed by staff not otherwise involved in the trial.15
With the first postal mailing, all primary care physicians in the intervention group received an accompanying letter explaining the intervention, a response card for physicians wishing to opt out, and evidence-based guidelines in German and French on antibiotic prescribing for respiratory tract and urinary tract infections.11,15,20 With the second feedback mailing, information on antibiotic resistance and its regional distribution from the Swiss Centre for Antibiotic Resistance21 was provided. By the end of the study, 65 practices had closed and 53 had withdrawn consent to participate further in the study (eFigure 4 in Supplement 1). All information material and guidelines were also made available to physicians in the intervention group on a password-protected trial website.
Outcomes
The primary outcome was the overall antibiotic prescription rate per 100 consultations in the second year of intervention (long-term intervention effect). The secondary outcomes were (1) overall antibiotic use per 100 patient consultations in the first year (short-term intervention effect) (2) and over 2 years while considering 2 repeated measurements, over the first and the second year of intervention; (3) use of broad-spectrum antibiotics (quinolones and oral cephalosporins) per 100 patient consultations; (4) all-cause hospitalizations and infection-related hospitalizations15; (5) antibiotic use in 3 specific patient age groups (≤5 years, 6-65 years, and >65 years). The last 3 secondary outcomes were to be evaluated separately over the second and first years of the intervention. The detail of the calculation of prescription rates is provided in the eAppendix in Supplement 1.
Statistical Analysis
Statistical analysis was conducted from September 1, 2021, to January 31, 2022. Details of the sample size calculation are provided elsewhere.15 We calculated monthly and annual medians for the number of antibiotic prescriptions per 100 consultations, with associated IQRs, per physician license number (the unit of analysis) for the baseline year (2017) and the intervention years (2018-2019). The primary and secondary outcomes were modeled using analysis of covariance,22 with the intervention as a factor of interest, baseline antibiotic prescription rates in 2017 and comorbidities (immunosuppression-like conditions, metabolic disorders, cardiovascular disease, neurologic disorders, respiratory disease, and other conditions) as covariates, and an interaction term for the intervention with time. We report the coefficient estimates in percentage change in prescriptions per 100 consultations with the respective 95% CIs.
Effects for the overall time of the intervention (24 months) were assessed with a linear mixed model, including the intervention, time (baseline and 24 months), and an interaction term for the intervention with time. From the model including the same covariates as the primary analysis and randomized physicians as random effects, we derived mean percentage changes from baseline for the intervention and control groups. Because of the skewed nature of antibiotic prescription rates, we log-transformed rates and back-transformed model estimates with log-log model formulations.
The number of hospitalizations (overall and infection related) were modeled using Poisson regressions, including the intervention as a factor of interest and all covariates from the primary analysis. Finally, we conducted stratified analyses by age groups as for the primary analysis over the respective periods (eAppendix in Supplement 1). To report some of the baseline characteristics (Table), we used patient-level data, but for modeling the data were aggregated on the physician license number. Statistical analyses were performed using R, version 4.0.2 (R Group for Statistical Computing).19
Table. Baseline Characteristics in Baseline Year 2017.
| Characteristic | Participants, No. (%) | ||
|---|---|---|---|
| Total (N = 1 252 169 [100]) | Intervention (n = 629 825 [50.3]) | Control (n = 622 344 [49.7]) | |
| No. of patients, median (IQR) | 337 (236-480) | 338 (233-482.2) | 336 (238-475) |
| Patients’ age, y | |||
| ≤5 | 187 557 (15.0) | 95 468 (7.6) | 92 089 (7.4) |
| 6-64 | 711 234 (56.8) | 358 622 (28.6) | 352 612 (28.2) |
| ≥65 | 353 378 (28.2) | 175 735 (14.0) | 177 643 (14.2) |
| Female patients | 686 633 (54.8) | 344 098 (27.5) | 3425 35 (55.0) |
| Comorbiditiesa | |||
| Immunosuppression-like conditionsb | 1 400 627 (100) | 699 822 (55.9) | 700 805 (56.0) |
| Metabolic disorders | 1 270 058 (100) | 635 431 (50.0) | 634 627 (50.0) |
| Cardiovascular disease and neurologic disorders | 2 523 931 (100) | 1 260 454 (49.9) | 1 263 477 (50.1) |
| Respiratory disease | 834 878 (100) | 417 552 (50.0) | 417 326 (33.3) |
| Otherc | 2 836 897 (100) | 1 419 263 (50.0) | 1 417 634 (50.0) |
| Consultations | |||
| No. (%) | 4 790 525 (100) | 2 402 119 (50.1) | 2 388 406 (49.9) |
| Mean (SD) | 1447.7 (805.9) | 1454.1 (817.1) | 1441.4 (794.7) |
| Median (IQR) | 1283 (869-1861) | 1301 (872.5-1859.3) | 1271 (865-1862) |
Multiple comorbidities per patient are possible.
Classification according to pharmacy cost groups.
Psychiatric disorders, gastric acid–related disorders, and iron deficiency anemia.
Results
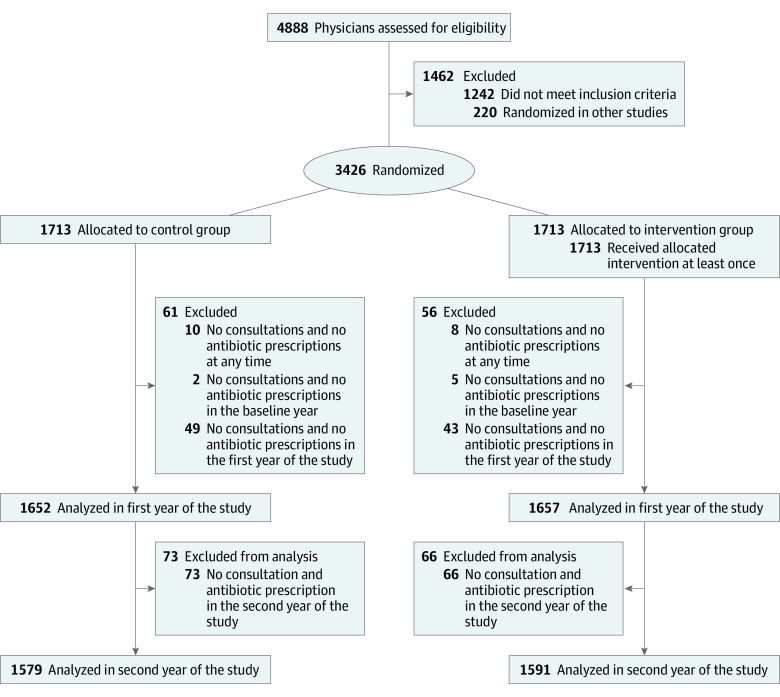
Of 4888 physicians assessed for eligibility, 3426 were randomized to the intervention (n = 1713) and control groups (n = 1713), with 1591 and 1579 physicians, respectively, being available for analysis for the second year of the intervention (Figure 1). At baseline in 2017, physicians in the intervention group served 629 825 patients and physicians in the control group 622 344 patients and prescribed 212 933 antibiotics in the intervention group and 211 825 antibiotics in the control group, for a total of 2 402 119 consultations in the intervention group and 2 388 406 consultations in the control group. A total of 53 physicians opted out of the intervention (eFigure 4 in Supplement 1). Patients’ characteristics were well balanced between study groups (Table).
Figure 1. Flow Diagram of Disposition of Primary Care Physicians and Pediatricians (Intention-to-Treat Analysis).
Median annual antibiotic prescription rates per 100 consultations in the year preceding the trial were 8.4 (IQR, 6.3-11.5) in the intervention group and 8.4 (IQR, 6.4-11.6) in the control group. In the intervention group, prescription rates per 100 consultations were 8.3 (IQR, 6.2-11.7) in the first year and 8.2 (IQR, 6.1-11.4) in the second year; in the control group, prescription rates per 100 consultations were 8.5 (IQR, 6.3-12.0) in the first year and 8.4 (IQR, 6.0-11.8) in the second year. Monthly median prescription rates are provided in Figure 2.
Figure 2. Median Antibiotic Prescription Rates per Month.
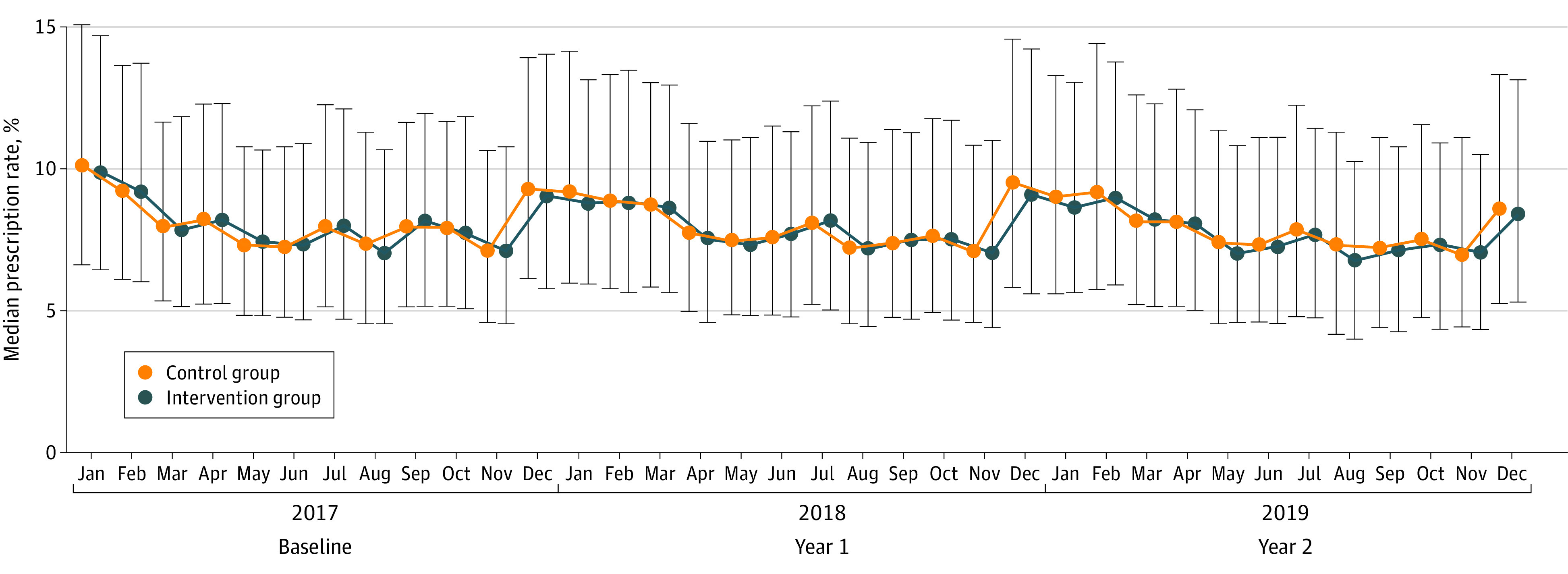
Error bars indicate IQRs.
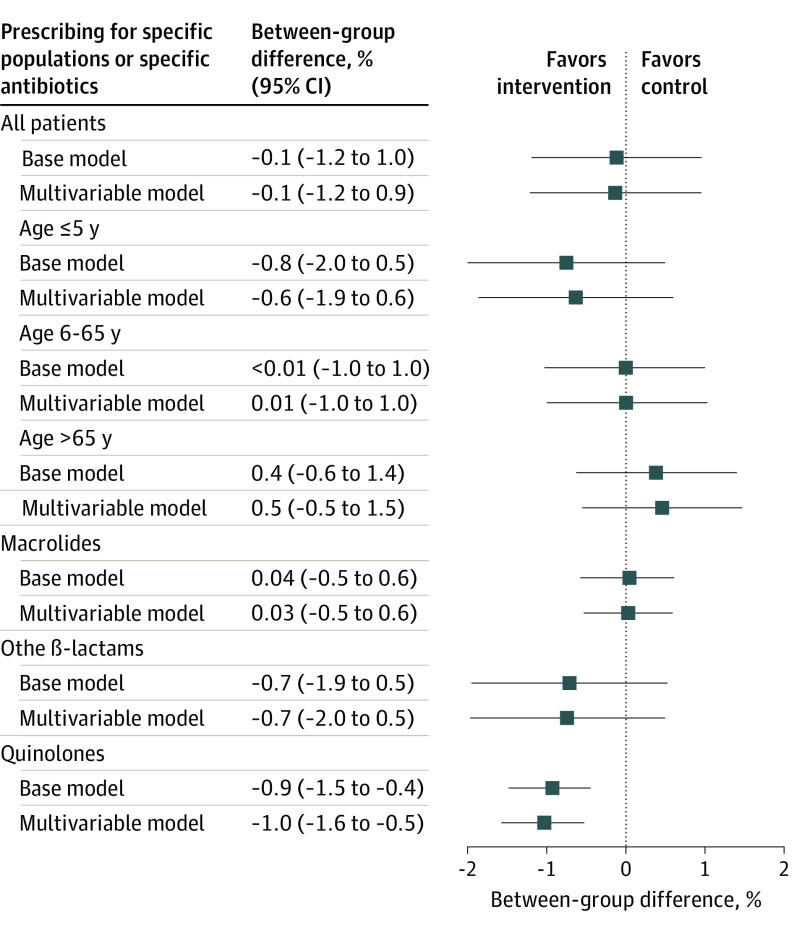
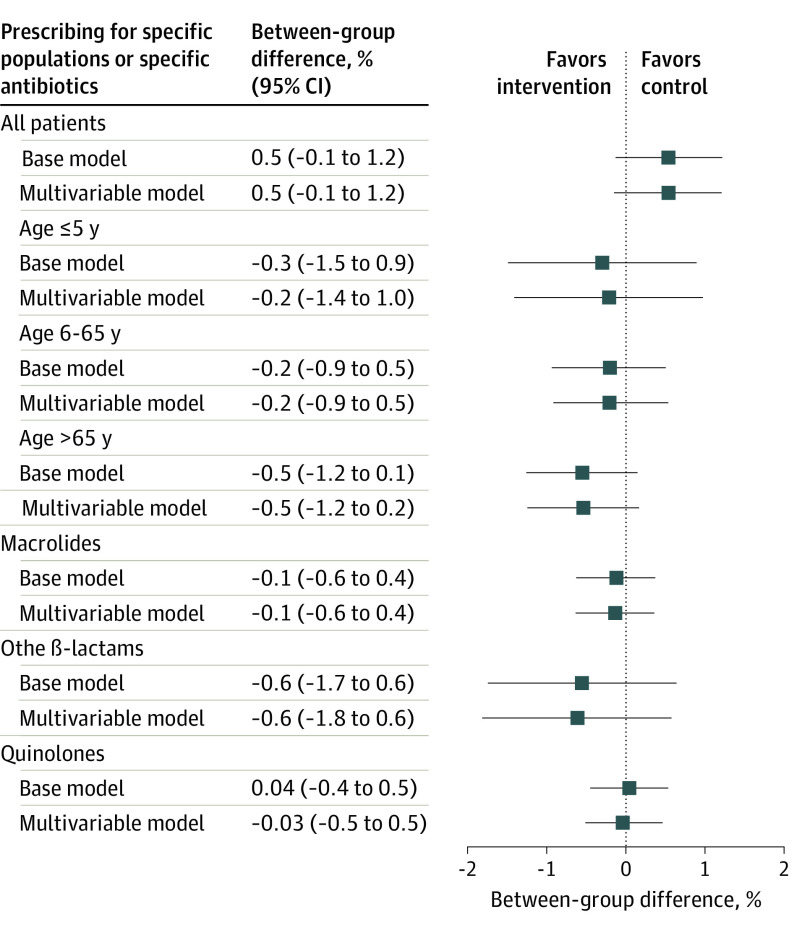
In comparison with the year 2017 prior to the intervention, there was a 4.2% (95% CI, 3.9%-4.6%) increase in the antibiotic prescription rate during the intervention phase for the entire cohort (eTable 1 in Supplement 1). Relative to these increased prescription rates in 2017, there was a small statistically nonsignificant –0.1% (95% CI, –1.2% to 51.0%) reduction in antibiotic prescriptions per 100 consultations in the feedback group compared with the control group in the second year of the intervention (primary end point) (Figure 3). During the first year and the entire trial period, antibiotic prescription rates in the intervention group additionally increased during the first year by 0.5% (95% CI, –0.1% to 1.2%) and during the entire trial period by 0.5% (95% CI, –0.2% to 1.3%) when compared with the control group (Figure 4). Findings were similar when restricting the analysis to practices with fewer than 3 physicians working under 1 license number (eTable 8 in Supplement 1).
Figure 3. Change in Prescription Rates per 100 Consultations by Physicians in the Intervention vs the Control Group in the Second Year of the Intervention (Intention-to-Treat Analysis) Relative to the Baseline Year 2017.
The base model is treatment variable adjusted to baseline prescription rate and the multivariable model is adjusted to the predefined comorbidities.
Figure 4. Change in Prescription Rates per 100 Consultations by Physicians in the Intervention vs the Control Group in the First Year of the Intervention (Intention-to-Treat Analysis) Relative to the Baseline Year 2017.
The base model is treatment variable adjusted to baseline prescription rate and the multivariable model is adjusted to the predefined comorbidities.
Prescription rates for specific antibiotics also increased during the intervention period when compared with the baseline year (eTable 2 in Supplement 1). Relative to these increased rates, small relative reductions in antibiotic prescriptions were noted in the intervention group compared with the control group during both years of the intervention (eTable 4 and eTable 6 in Supplement 1); these reductions were not statistically significant with the exception of quinolone prescriptions during the second year of the intervention (–0.9%; 95% CI, –1.5% to –0.4%) (Figure 3). No statistically significant differences in antibiotic prescription rates were noted between the feedback and control groups for all prespecified age-related subgroup analyses (eTables 2, 3, and 5 in Supplement 1). Estimates from the per-protocol analysis likewise showed no reductions in antibiotic prescriptions between both groups (eTable 9 in Supplement 1). Also, no differences in infection-related and overall hospitalization rates were found between both groups during all observation periods (eTable 7 in Supplement 1).
Discussion
In this nationwide insurance claims data–based pragmatic randomized clinical trial, primary care physicians and pediatricians in Switzerland with the highest prescription rates were randomized to receive regular prescribing feedback, the provision of community-based antibiotic resistance data, and evidence-based guidelines for respiratory tract and urinary tract infections vs not receiving any information. For the primary end point, the overall antibiotic prescription rate in the second year of the intervention, a very small—from a public health perspective, not relevant—reduction of antibiotic prescription rates was noted. All prespecified subgroup analyses showed also no difference between the 2 groups, with the exception of a −0.9% difference in quinolone prescriptions between the intervention and control groups during the second year of the intervention.
Several smaller trials that were conducted in selected general practices used antibiotic prescription feedback in combination with personalized expert feedback,23 academic detailing,9 a practice accreditation program,24 or in combination with decision support systems12,25,26 and found a relative reduction in antibiotic prescriptions of approximately 5%. Some of these trials recruited motivated primary care physicians already engaged in education programs to reduce antibiotic prescriptions or offered financial incentives for trial participation.25 Only a few trials used routine prescription feedback for clinicians in primary care with high antibiotic prescription rates on the health system level addressing the entire primary care physician community. In a trial in the UK, a 3.3% reduction in antibiotic prescriptions over 6 months was found when a single letter from England’s chief medical officer with information on high prescription rates was sent to the top 20% of antibiotic prescribers.10 In a Canadian trial, the same intervention approach was used for the 25% top prescribers in Ontario and a nonstatistically significant 3.4% reduction over 12 months was found.14 In a trial from New Zealand, a single letter providing peer antibiotic comparison found reduced antibiotic use only among clinicians with high prescription rates but not those with low prescription rates.27 Another trial from Australia provided antibiotic prescription feedback twice over 6 months in unselected rural practices and found no difference in antibiotic prescribing.13
The negative findings from this trial are in contrast with results from the UK and Canadian trials, which both included system-wide practices. First, antibiotic prescription rates in the UK trial were about 30% higher than in the present trial.10,14 The Canadian trial was based on drug sales data with no denominator information. The present trial included—for reasons of sample size—about 80% of eligible practices and represents a more heterogeneous group of practices in terms of antibiotic prescribing patterns. Switzerland has one of the lowest antibiotic consumption rates among European countries.28,29,30 Thus, it seems difficult to further reduce antibiotic prescribing with our chosen approach in a setting with already low prescription rates, although we know that prescriptions of antibiotics in primary care for upper respiratory tract and urinary tract infections are still too high31 and the spread of multidrug resistance remains a problem in particular for gram-negative bacteria expressing extended-spectrum β-lactamases.21,32
Limitations and Strengths
Our trial has several limitations. Due to the long processing time of claims data by health insurers, prescription feedback was sent to physicians with a delay of 6 months, making it likely less relevant or more difficult to interpret in the actual clinical situation.33 Swiss claims data do not contain any diagnostic information from primary care; therefore, it was not possible to provide feedback on the appropriateness of antibiotic prescriptions. Legal issues in regard to the privacy of health data are major obstacles to overcome this data deficit in Switzerland. Social scientists emphasize the social normative aspects when aiming at behavior change in antibiotic stewardship interventions and the necessity to link peer comparisons appropriately with the top percentage of prescribers.33 We chose a mean prescription benchmark rather than practices with the top percentage of prescribers as a reference to acknowledge prescribing variations due to differences in the case mix and the lack of diagnostic data for the reason for antibiotic prescriptions. Other investigators have advocated the use of the top percentage of prescribers as a reference standard.33 Finally, our trial does not cover the entire Swiss population.
A relative increase in antibiotic prescriptions of 4% was noted in both years of the intervention compared with the baseline in 2017. The Swiss Ministry of Health also reported higher rates of influenza-like illnesses and invasive Haemophilus influenzae infections during the intervention period of 2018-2019 when compared with 2017.34 This finding illustrates the importance of evaluating feedback interventions for antibiotic prescribing over sufficiently long periods to accurately account for seasonality effects.
The strengths of this trial are the formal blinding of physicians and data analysis, the conduct of intention-to-treat and per-protocol analyses, the completeness and external validity of our data, and the use of nonaggregated patient-level claims data for certain end points. In our statistical approach—contrary to previous trials10,14—we integrated baseline prescription data, abstained from excluding practices with extreme prescription data, and included covariates to address the patient case mix. Because of the large sample, all our estimates include small 95% CIs. Finally, we were able to explore the theoretical harm from the intervention by the analysis of hospitalization data.
Conclusions
In this randomized clinical trial, quarterly detailed claims data–based prescription feedback to primary care physicians did not reduce antibiotic prescriptions during a 2-year intervention in the Swiss primary health care setting with already low antibiotic use when compared with other European countries. Whether health system–wide antibiotic stewardship programs with more individually tailored information on the appropriateness of antibiotic prescriptions, eventually combined with individual physician-targeted incentives, might achieve further reductions in antibiotic use should be evaluated in future trials. Such trials, however, will need much more detailed, routinely collected diagnostic and laboratory patient data.
eAppendix.
eFigure 1. Flow Diagram of the Data Processing Steps
eFigure 2. English Translation of the Feedback Intervention
eFigure 3. Statistical Analysis Flow
eFigure 4. Flow Diagram of Physician Disposition for Per-Protocol Analysis
eTable 1. Summary of Yearly Prescription Rates per 100 Consultations
eTable 2. Change of Antibiotic Prescription Rates per 100 Consultations in the Second Year of Intervention for all Patients and Different Antibiotic Groups
eTable 3. Change of Antibiotic Prescription Rates per 100 Consultations in the Second Year of Intervention for Different Age Groups
eTable 4. Change of Antibiotic Prescription Rates per 100 Consultations in the First Year of Intervention for all Patients and Different Antibiotic Groups
eTable 5. Change of Antibiotic Prescription Rates per 100 Consultations in the First Year of Intervention for Different Age Groups
eTable 6. Change of Antibiotic Prescription Rates per 100 Consultations for 24 Months of Intervention
eTable 7. All-Cause and Infection-Related Hospitalisations by Consultations
eTable 8. Change of Antibiotic Prescription Rates per 100 Consultations for Different Outcomes Including Only Practice License Numbers With up to 3 General Practitioners (Sensitivity Analysis)
eTable 9. Change of Antibiotic Prescription Rates per 100 Consultations for Per Protocol Analysis
eTable 10. Number of General Practitioners Under One Practice License Number for the Included General Practitioners in the Study for the Intention to Treat Analysis
Trial Protocol
Data Sharing Statement
References
- 1.Akkerman AE, Kuyvenhoven MM, Verheij TJ, van Dijk L. Antibiotics in Dutch general practice: nationwide electronic GP database and national reimbursement rates. Pharmacoepidemiol Drug Saf. 2008;17(4):378-383. doi: 10.1002/pds.1501 [DOI] [PubMed] [Google Scholar]
- 2.Hawker JI, Smith S, Smith GE, et al. Trends in antibiotic prescribing in primary care for clinical syndromes subject to national recommendations to reduce antibiotic resistance, UK 1995-2011: analysis of a large database of primary care consultations. J Antimicrob Chemother. 2014;69(12):3423-3430. doi: 10.1093/jac/dku291 [DOI] [PubMed] [Google Scholar]
- 3.Petersen I, Hayward AC; SACAR Surveillance Subgroup . Antibacterial prescribing in primary care. J Antimicrob Chemother. 2007;60(suppl 1):i43-i47. doi: 10.1093/jac/dkm156 [DOI] [PubMed] [Google Scholar]
- 4.Smucny J, Fahey T, Becker L, Glazier R. Antibiotics for acute bronchitis. Cochrane Database Syst Rev. 2004;(4):CD000245. [DOI] [PubMed] [Google Scholar]
- 5.Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096. doi: 10.1136/bmj.c2096 [DOI] [PubMed] [Google Scholar]
- 6.Goossens H, Ferech M, Vander Stichele R, Elseviers M; ESAC Project Group . Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365(9459):579-587. doi: 10.1016/S0140-6736(05)17907-0 [DOI] [PubMed] [Google Scholar]
- 7.Hillier S, Roberts Z, Dunstan F, Butler C, Howard A, Palmer S. Prior antibiotics and risk of antibiotic-resistant community-acquired urinary tract infection: a case-control study. J Antimicrob Chemother. 2007;60(1):92-99. doi: 10.1093/jac/dkm141 [DOI] [PubMed] [Google Scholar]
- 8.Butler CC, Simpson SA, Dunstan F, et al. Effectiveness of multifaceted educational programme to reduce antibiotic dispensing in primary care: practice based randomised controlled trial. BMJ. 2012;344:d8173. doi: 10.1136/bmj.d8173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gjelstad S, Høye S, Straand J, Brekke M, Dalen I, Lindbæk M. Improving antibiotic prescribing in acute respiratory tract infections: cluster randomised trial from Norwegian general practice (prescription peer academic detailing (Rx-PAD) study). BMJ. 2013;347:f4403. doi: 10.1136/bmj.f4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallsworth M, Chadborn T, Sallis A, et al. Provision of social norm feedback to high prescribers of antibiotics in general practice: a pragmatic national randomised controlled trial. Lancet. 2016;387(10029):1743-1752. doi: 10.1016/S0140-6736(16)00215-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemkens LG, Saccilotto R, Reyes SL, et al. Personalized prescription feedback using routinely collected data to reduce antibtiotic use in primary care: a randomized clinical trial. JAMA Intern Med. 2017;177(2):176-183. doi: 10.1001/jamainternmed.2016.8040 [DOI] [PubMed] [Google Scholar]
- 12.Gulliford MC, Prevost AT, Charlton J, et al. Effectiveness and safety of electronically delivered prescribing feedback and decision support on antibiotic use for respiratory illness in primary care: REDUCE cluster randomised trial. BMJ. 2019;364:l236. doi: 10.1136/bmj.l236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connell DL, Henry D, Tomlins R. Randomised controlled trial of effect of feedback on general practitioners’ prescribing in Australia. BMJ. 1999;318(7182):507-511. doi: 10.1136/bmj.318.7182.507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz KL, Ivers N, Langford BJ, et al. Effect of antibiotic-prescribing feedback to high-volume primary care physicians on number of antibiotic prescriptions: a randomized clinical trial. JAMA Intern Med. 2021;181(9):1165-1173. doi: 10.1001/jamainternmed.2021.2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glinz D, Mc Cord KA, Moffa G, et al. Antibiotic prescription monitoring and feedback in primary care in Switzerland: Design and rationale of a nationwide pragmatic randomized controlled trial. Contemp Clin Trials Commun. 2021;21:100712. doi: 10.1016/j.conctc.2021.100712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulz KF, Altman DG, Moher D; CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726-732. doi: 10.7326/0003-4819-152-11-201006010-00232 [DOI] [PubMed] [Google Scholar]
- 17.Juszczak E, Altman DG, Hopewell S, Schulz K. Reporting of multi-arm parallel-group randomized trials: extension of the CONSORT 2010 statement. JAMA. 2019;321(16):1610-1620. doi: 10.1001/jama.2019.3087 [DOI] [PubMed] [Google Scholar]
- 18.Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG; CONSORT Group . Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308(24):2594-2604. doi: 10.1001/jama.2012.87802 [DOI] [PubMed] [Google Scholar]
- 19.R Core Team. R: a language and environment for statistical computing. 2017. R Foundation for Statistical Computing. Accessed May 10, 2022. https://www.R-project.org/
- 20.Hemkens LG, Saccilotto R, Reyes SL, et al. Personalized prescription feedback to reduce antibiotic overuse in primary care: rationale and design of a nationwide pragmatic randomized trial. BMC Infect Dis. 2016;16:421. doi: 10.1186/s12879-016-1739-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Institut für Infektionskrankheiten . Swiss Centre for Antibiotic Resistance. Accessed May 10, 2022. http://www.anresis.ch
- 22.Wang B, Ogburn EL, Rosenblum M. Analysis of covariance in randomized trials: more precision and valid confidence intervals, without model assumptions. Biometrics. 2019;75(4):1391-1400. doi: 10.1111/biom.13062 [DOI] [PubMed] [Google Scholar]
- 23.Eilermann K, Halstenberg K, Kuntz L, Martakis K, Roth B, Wiesen D. The effect of expert feedback on antibiotic prescribing in pediatrics: experimental evidence. Med Decis Making. 2019;39(7):781-795. doi: 10.1177/0272989X19866699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Velden AW, Kuyvenhoven MM, Verheij TJM. Improving antibiotic prescribing quality by an intervention embedded in the primary care practice accreditation: the ARTI4 randomized trial. J Antimicrob Chemother. 2016;71(1):257-263. doi: 10.1093/jac/dkv328 [DOI] [PubMed] [Google Scholar]
- 25.Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562-570. doi: 10.1001/jama.2016.0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linder JA, Meeker D, Fox CR, et al. Effects of behavioral interventions on inappropriate antibiotic prescribing in primary care 12 months after stopping interventions. JAMA. 2017;318(14):1391-1392. doi: 10.1001/jama.2017.11152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chappell N, Gerard C, Gyani A, et al. Using a randomised controlled trial to test the effectiveness of social norms feedback to reduce antibiotic prescribing without increasing inequities. N Z Med J. 2021;134(1544):13-34. [PubMed] [Google Scholar]
- 28.Adriaenssens N, Coenen S, Versporten A, et al. ; ESAC Project Group . European Surveillance of Antimicrobial Consumption (ESAC): outpatient quinolone use in Europe (1997-2009). J Antimicrob Chemother. 2011;66(suppl 6):vi47-vi56. doi: 10.1093/jac/dkr457 [DOI] [PubMed] [Google Scholar]
- 29.European Centre for Disease Prevention and Control . Summary of the latest data on antibiotic consumption in the European Union. ESAC-Net surveillance data. November 2016. Accessed May 10, 2022. http://ecdc.europa.eu/en/eaad/antibiotics-get-informed/antibiotics-resistance-consumption/Pages/data-reports.aspx
- 30.Federal Office of Public Health; Federal Food Safety and Veterinary Office . Swiss antibiotic resistance report 2016. FOPH publication number: 2016-OEG-30. Accessed May 10, 2022. https://www.bag.admin.ch/bag/en/home/das-bag/publikationen/broschueren/publikationen-uebertragbare-krankheiten/publikation-swiss-antibiotic-resistance-report-2016.html
- 31.Glinz D, Leon Reyes S, Saccilotto R, et al. Quality of antibiotic prescribing of Swiss primary care physicians with high prescription rates: a nationwide survey. J Antimicrob Chemother. 2017;72(11):3205-3212. doi: 10.1093/jac/dkx278 [DOI] [PubMed] [Google Scholar]
- 32.Murray CJL, Ikuta KS, Sharara F, et al. ; Antimicrobial Resistance Collaborators . Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629-655. doi: 10.1016/S0140-6736(21)02724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox CR, Doctor JN, Goldstein NJ, Meeker D, Persell SD, Linder JA. Details matter: predicting when nudging clinicians will succeed or fail. BMJ. 2020;370:m3256. doi: 10.1136/bmj.m3256 [DOI] [PubMed] [Google Scholar]
- 34.Eidgenössisches Departement des Innern Bundesamt für Gesundheitswesen . Informationsmagazin für medizinische Fachpersonen und Medienschaffende: BAG-Bulletin. February 10, 2020. Accessed May 10, 2022. https://www.bag.admin.ch/dam/bag/de/dokumente/cc/Kampagnen/Bulletin/2020/bu-7-20.pdf.download.pdf/BU_7_20_DE.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix.
eFigure 1. Flow Diagram of the Data Processing Steps
eFigure 2. English Translation of the Feedback Intervention
eFigure 3. Statistical Analysis Flow
eFigure 4. Flow Diagram of Physician Disposition for Per-Protocol Analysis
eTable 1. Summary of Yearly Prescription Rates per 100 Consultations
eTable 2. Change of Antibiotic Prescription Rates per 100 Consultations in the Second Year of Intervention for all Patients and Different Antibiotic Groups
eTable 3. Change of Antibiotic Prescription Rates per 100 Consultations in the Second Year of Intervention for Different Age Groups
eTable 4. Change of Antibiotic Prescription Rates per 100 Consultations in the First Year of Intervention for all Patients and Different Antibiotic Groups
eTable 5. Change of Antibiotic Prescription Rates per 100 Consultations in the First Year of Intervention for Different Age Groups
eTable 6. Change of Antibiotic Prescription Rates per 100 Consultations for 24 Months of Intervention
eTable 7. All-Cause and Infection-Related Hospitalisations by Consultations
eTable 8. Change of Antibiotic Prescription Rates per 100 Consultations for Different Outcomes Including Only Practice License Numbers With up to 3 General Practitioners (Sensitivity Analysis)
eTable 9. Change of Antibiotic Prescription Rates per 100 Consultations for Per Protocol Analysis
eTable 10. Number of General Practitioners Under One Practice License Number for the Included General Practitioners in the Study for the Intention to Treat Analysis
Trial Protocol
Data Sharing Statement