Abstract
Objective:
To appraise the evidence that pathophysiological mechanisms and individualized treatment directed at those mechanisms provide an alternative approach to the treatment of patients with irritable bowel syndrome (IBS).
Design:
A PubMED-based literature review of mechanisms and treatment of IBS was conducted independently by the 2 authors, and any differences of perspective or interpretation of the literature were resolved following discussion.
Results:
The availability of several noninvasive clinical tests can appraise the mechanisms responsible for symptom generation in IBS, including rectal evacuation disorders, abnormal transit, visceral hypersensitivity or hypervigilance, bile acid diarrhea, sugar intolerances, barrier dysfunction, the microbiome, immune activation, and chemicals released by the latter mechanism. The basic molecular mechanisms contributing to these pathophysiologies are increasingly recognized, offering opportunities to intervene with medications directed specifically to food components, receptors, and potentially the microbiome. Although the evidence supporting interventions for each mechanism is not at the same level of proof, the current state-of-the-art provides the opportunity to advance the practice from treatment based on symptoms to individualization of treatment guided by pathophysiology and clinically identified biomarkers.
Conclusion:
These advances augur well for the implementation of evidence-based individualized treatment for patients with IBS based on actionable biomarkers or psychological disturbances.
INTRODUCTION
The objectives of this review are to review some of the pathophysiological principles involved in irritable bowel syndrome (IBS), the actionable biomarkers that can be used to identify and specifically treat mechanisms resulting in the symptoms of IBS, and treatments based on the pathophysiology or predominant symptoms.
The mechanisms underlying IBS include central nervous system hypervigilance, psychosocial factors, genetic predisposition, and mechanisms directly involving the gastrointestinal tract. Although it is commonly perceived that IBS is a disorder of gut-brain interaction, it is relevant to note that there are gut-specific mechanisms that can be corrected without use of central neuromodulators. There is a role for hypnotherapy, psychotherapy, and central neuromodulators in the appropriate patients with IBS. However, it is important to recognize opportunities for addressing the mechanisms or pathophysiology in the gut. Thus, in addition to the irritable bowel, there is accumulated evidence that the gut may also be irritated in IBS by products of digestion, neurotransmitters, prior enteritis, the microbiome, mucosal immune activation, and increased mucosal permeability.1 These factors lead to altered gut motor function, altered sensation, and rectal evacuation disorders.
UPDATE ON PATHOPHYSIOLOGY OF IBS AND ITS DIAGNOSIS
The pathophysiological features of significance in IBS are evacuation disorders, abnormal colonic transit, bile acid diarrhea, increased colonic and rectal sensation, disaccharidase deficiency, local immune reactions to food, and altered microbiota.
Through studies that were designed to demonstrate the pathophysiology, several diagnostic tests have been developed to facilitate recognition of the mechanisms leading to patient symptoms.
a. Rectal evacuation disorders:
Evacuation disorders mimic the symptoms of IBS with constipation: reduced emptying of the left colon leads to distension, bloating, abdominal pain, and constipation. Evacuation disorders may result in delayed colonic transit, particularly in the left colon.2 In clinical practice, two general types of pelvic floor dysfunction are recognized: spastic evacuation disorders in which the puborectalis is spastic (“dyssynergia”),3 or the anal sphincter does not relax (“anismus”); a second category represents a flaccid disorder, especially in descending perineum syndrome,4 which typically affects older patients, particularly women who have had three or four vaginal deliveries,4 or Ehlers-Danlos syndrome, hypermobility or vascular types, with loss of connective tissue support of the perineum.5, 6 The anchor of diagnosis of rectal evacuation disorders is anorectal manometry with balloon expulsion, and the most useful parameters are increased resting anal sphincter pressure, markedly negative rectoanal pressure differential, and prolonged balloon expulsion time4 relative to normal values based on sex and age.
b. Motor dysfunction
has been demonstrated noninvasively using radiopaque markers studies Or scintigraphy. A Swedish study7 showed that about a third of patients with diarrhea and a third of those with constipation have transit abnormalities. Transit measurement is not indicated at the first encounter and may be indicated after insufficient response with first-line therapies such as treatment with loperamide for IBS-diarrhea (IBS-D) or with fiber and osmotic laxative for IBS-constipation (IBS-C). Measurement of transit is particularly relevant in patients with IBS-C. Using scintigraphy to measure transit, there is a significant relationship between the emptying of the proximal colon at 36 hours and the 24-hour stool weight.8 In addition, the distribution of radiolabeled colonic content differed significantly in IBS-D compared to healthy controls, with more isotope appearing in the stool and rectosigmoid and less in the descending colon.8 The transit profile in the colon was abnormal at 24 or 48 hours respectively in patients with IBS-D and IBS-C, and patients with mixed or alternating IBS had a transit profile quite similar to that of patients with IBS-D at 48 hours.9 Transit measurements are established as a diagnostic biomarker with the important proviso to exclude rectal evacuation disorders in patients with evidence of slow colonic transit.2 However, transit measurements cannot differentiate IBS-D from functional diarrhea or IBS-C from functional constipation, and this is understandable given the transition of these symptom complexes.10 In patients with rapid colonic transit associated with IBS-D, the objective measurement may corroborate the patient’s report of severity of diarrhea or impact the choice of pharmacological treatment such as addition of second-line treatment such as a 5-hydroxytryptamine (5-HT3) antagonist to the first-line treatment with loperamide. In case of slow transit, objective measurement of transit may indicate the need for the addition of a secretagogue to a first-line osmotic laxative for constipation in IBS-C.
c. IBS is associated with visceral hypersensitivity or hypervigilance to visceral signaling. In a classical study by Ritchie et al.,11 patients with IBS had rectal hypersensitivity to a distended balloon and more patients had evidence of pain sensation at lower volumes of distension in IBS compared to healthy controls. Further studies at UCLA12 showed two types of increased rectal sensation: hypersensitivity or hyperalgesia. Thus, there are patients in whom distension of the balloon in the rectum leads to pain or other sensations at lower thresholds of distension, whereas those who have a normal threshold of distension experience increased discomfort or hyperalgesia, consistent with hypervigilance to or reduced downregulation of normal visceral afferent input. Importantly, the pain scores reported by patients are rather subjective and strongly influenced by the psychological burden of the patient,13 questioning to what extent this test is actually assessing visceral afferent dysfunction. Live calcium recordings from rectal biopsies however demonstrated increased excitability of submucosal neurons in response to agonists of the pro-nociceptive transient receptor potential (TRP) channels [TRP vanilloid (TRPV1, TRPV4) and TRP ankyrin 1 (TRPA1)].14,15 Although submucosal neurons are most likely not involved in pain signaling, these observations indicate that the submucosal microenvironment contains TRP channel sensitizing factors that might equally affect visceral afferents. These data favor interventions directed at peripheral mechanisms involved in aberrant pain signaling in addition to opportunities to target central mechanisms associated with visceral hypervigilance.
d. One in four patients with IBS-D actually has idiopathic bile acid diarrhea.16 Primary bile acids, chenodeoxycholic acid and cholic acid, are derived from cholesterol, undergo taurine or glycine conjugation (which increases their solubility), and pass into the small intestine after meal ingestion and gall bladder contraction to facilitate fat digestion and absorption. About 90-95% of the bile acids are reabsorbed in the terminal ileum by the active transporter, apical sodium-coupled bile acid transporter. Bile acids undergo enterohepatic cycling, and the remaining 5-10% pass into the colon where they can increase the permeability because of their detergent effects. Once in the colon, the primary bile acids are deconjugated with removal of glycine and taurine and are converted to secondary bile acids through 7α dehydroxylation or 7β epimerization by the colonic microbiota. The main secondary bile acids are lithocholic acid, deoxycholic acid, and ursodeoxycholic acid. In the colon, bile acids cause increased secretion, increased mucosal permeability, and stimulate motility (e.g., high amplitude colonic contractions).17 Bile acid diarrhea affects both adolescents and adults.17, 18
Patients with bile acid diarrhea have increased fasting serum 7 alpha-hydroxy-4 cholesten-3-one (7αC4), an indirect marker for bile acid synthesis in the liver.19 Ileal absorption of bile acids normally stimulates the enterocyte nuclear receptor, farnesoid X receptor (FXR), leading to synthesis of fibroblast growth factor 19 (FGF-19), a portal circulation hormone that reaches the hepatocyte and inhibits bile acid synthesis. Thus, there is a reciprocal relationship between FGF-19 and serum 7αC4. The rate of synthesis of bile acids (indirectly measured by serum 7αC4) is directly proportional to fecal bile acid excretion over 48 hours. There are three biochemical parameters validated for diagnosis of bile acid diarrhea20: total 48-hour fecal bile acid, increased fecal primary bile acids in the stool, and fasting serum C4 (collected before 9:00 a.m.). An additional method available in some countries is the scintigraphic test measuring 75-selenium homocholic acid taurine (75SeHCAT) retention after 7 days. Recent validation of combined fasting serum 7αC4 and primary bile acids in a single sample of stool21 or fecal bile acid concentration in a single stool sample22 provide opportunities for simplifying the diagnosis and decreasing the costs for diagnosis compared to the 75SeHCAT retention test.
The specificity of the serological tests approximates that of the 75SeHCAT and 48-hour fecal bile acid excretion tests. In addition, the combination of fasting serum 7αC4 and primary bile acids in a single stool21 has 68% sensitivity at 80% specificity receiver operating characteristic curve – area under the curve (ROCAUC 0.86)] for diagnosing bile acid diarrhea, relative to the gold standard 48-hour fecal bile acid excretion. It is anticipated that this simple combined serum and single stool test will become available in clinical practice in the near future. One could legitimately ask: Why still include the patients who have evidence of bile salt diarrhea in IBS using Rome IV criteria, and should this group be excluded in future clinical trials of IBS-D? The current approach in IBS is to make a symptom-based diagnosis, and therefore, in the absence of simple and inexpensive screening tests, patients with bile acid diarrhea are included in IBS-D or functional diarrhea. With the introduction and widespread availability of simple combined serum and single stool test, patients with bile acid diarrhea should be excluded from IBS-D diagnosis or clinical trials, just as patients with celiac disease (with the same population prevalence of about 1%) are excluded based on screening serological testing for celiac disease.
e. Carbohydrate maldigestion or malabsorption.
The normal small intestine very avidly absorbs monosaccharides and disaccharides in the presence of normal disaccharidases; usually, these are absorbed within the first two meters of the small intestine,23, 24 and the absorption of monosaccharides is greater in the jejunum than the ileum.25 Monosaccharides and disaccharides are absorbed from the intestinal lumen at equal rates. Monosaccharides are transported by carrier-mediated mechanisms across the enterocyte brush border, and up to 50% of this transport is dependent on a sodium ion (Na+) gradient. There are 5 functional mammalian facilitated hexose carriers characterized by molecular cloning: 3 high affinity transporters of glucose (GLUT-1, GLUT-3, and GLUT-4) and one low-affinity transporter (GLUT-2), whereas GLUT-5 is primarily a fructose carrier. Because their Michaelis-Menten constant (Km) values (that is, substrate concentration at which the reaction velocity is 50% of the Vmax) are below the normal blood glucose concentration (6mmol/L), the high-affinity transporters function at rates close to maximal velocity. Transport of glucose across the apical brush border of intestinal (and kidney) epithelial cells is an active process that requires the presence of a sodium (Na+) gradient, maintained by Na+, potassium (K+), and a group of enzymes that catalyze the hydrolysis of a phosphate bond in adenosine triphosphate (ATPases).26 Any maldigested or malabsorbed carbohydrates that reach the colon are metabolized by colonic bacteria with production of gas, carbon dioxide (CO2), and water, and increased osmotic load leading to diarrhea. In fact, 25% to 75% of patients with disaccharidase deficiency meet IBS criteria.27
Lactase deficiency:
It is estimated that 65% of the human population has, to some extent, a reduced ability to digest lactose after infancy.28 The highest prevalence is in southeast Asia and South Africa, with lower prevalence in the Mediterranean littoral and far lower prevalence in more northern latitudes. It is relevant that, when lactose intake is limited to the equivalent of 240 ml of milk or less a day, symptoms are likely to be negligible and the use of lactose-digestive aids unnecessary.29
Sucrase-isomaltase deficiency:
Recent literature in adults has identified sucrase isomaltase deficiency in adults with symptoms of IBS-D.30-33 This condition is more clearly recognized in pediatric practice. Four genetic mutations in the sucrase or in the isomaltase domain account for the most common nucleotide changes in children with congenital sucrase-isomaltase deficiency.30 In adults, the same 4 mutations in the sucrase or isomaltase gene and one other mutation controlling the stalk that anchors the protein in the cell membrane have been identified.31 The latter mutation has been shown to be associated with increased stool frequency.32 Sucrase maltase deficiency is more prevalent in patients with IBS than in controls: in one study,31 2.1% in IBS versus 1.2% in controls, and in another study,32 4% in IBS versus 2.8% in controls. Studies using the UK Biobank showed that patients with an International Classification of Diseases, 10th revision (ICD-10) code diagnosis of IBS were more likely to have a significant odds ratio for sucrase-isomaltase deficiency compared to controls, in contrast to patients with self-diagnosis of IBS33 for whom the odds ratio was not significant. With more widespread recognition and availability of screening tests, sucrase-isomaltase deficiency would be separated from IBS-D.
f. Barrier dysfunction.
Several published studies have documented increased intestinal or colonic permeability in patients with IBS;34 the increased permeability predisposes to immune activation or inflammation.35 A systematic review identified that permeability was increased compared to healthy controls in IBS-D (9/13 studies) and in post-infectious-IBS (4/4 studies), but only in a minority of IBS-C (2/7 studies)36. In addition, there was a positive association between loss of barrier function and symptoms such as abdominal pain and changes in bowel function.36 The increased permeability was particularly noted in patients with bile acid diarrhea whose permeability was increased relative to IBS-D.37 Alternatively, increased permeability may be secondary to immune or mast cell activation.38 Although the systematic review36 suggested that urine collections of orally-administered probe molecules at 0-8 hours reflect proximal gastrointestinal (GI) permeability, and 0-24 hours reflect lower GI permeability, a combined study of permeability using oral probes and imaging within the GI tract of concomitantly-administered radioisotopic markers show those timings reflect both proximal and distal GI permeability since urine collections at 0-2 hours reflect small bowel, 2-8 hours reflect both small bowel and colon, and 8-24 hours reflect exclusively colonic permeability.
g. Immune activation.
Several lines of evidence document mucosal immune activation in IBS.
g.(i). Numbers and activation of immunocytes:
There is a higher number and activation of mucosal B cells and plasma cells in close proximity to mast cells, consistent with a local adaptive immune activation in IBS, with no increase in serum immunoglobulin G (IgG) in contrast to increased luminal IgG.39 In addition, recently acquired mechanistic evidence demonstrates increased release of nociceptive mediators by immune cells and the intestinal epithelium, leading to increased excitability of pro-nociceptive receptors of neurons leading to visceral hypersensitivity.38 Single-center, proof-of-concept studies have documented the clinical efficacy in relief of pain as well as downregulation of nociceptive functions with non-sedating histamine H1 receptor (H1R) antagonist in IBS.15
g. (ii). Mucosal expression of immune mechanisms
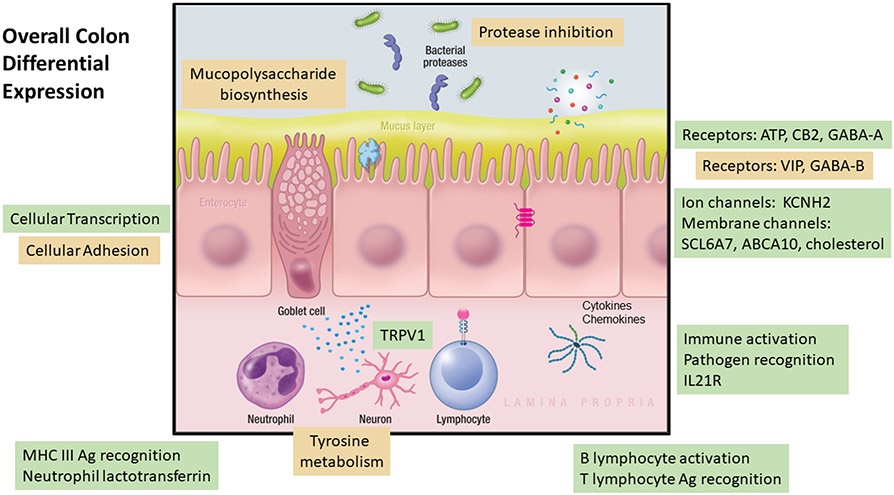
The relationships of mucosal inflammation or immune activation and symptoms or subgroups of IBS have been studied. Evidence of immune activation in the rectum and left colon was documented, though there was no relationship to symptoms or predominant bowel disturbance.40,41 In a study of colonic mucosal biopsies from patients with IBS (30 females with IBS-C, and 31 females and 13 males with IBS-D) there were differential expressions of 181 genes in ascending colon and 199 genes in rectosigmoid colon. The majority were gene upregulations in IBS-D, with functions reflecting activation of inflammation genes, transient receptor potential vanilloid 1 (TRPV1) (visceral hypersensitivity) and neurotransmitters/receptors [specifically purinergic, gamma-aminobutyric acid (GABA), and cannabinoid] (Figure 1). Although gene differential expressions in the ascending and rectosigmoid colon mucosa of the IBS-C and IBS-D were different, the diverse upregulated genes involved immune functions, receptors, transmitters, ion channels, and transporters in both IBS subgroups. Conversely, there was reduced expressions of peptidase inhibitor (PI) PI15 and PI16 genes that inhibit proteases in IBS-D, suggesting vulnerability of the mucosa to the effects of proteases (e.g. pancreatic or bacterial) in IBS-D.42 The differential immune activation in ascending colon mucosal biopsies in 11 patients with bile acid diarrhea (BAD) and 33 controls with IBS-D showed greater activation in BAD43 which is consistent with the detergency and mucosal irritation by bile acids, particularly the di-α hydroxy bile acids, chenodeoxycholic acid and deoxycholic acid.44 There were minimal differences in mucosal expression between ileal biopsies from patients with IBS-C or IBS-D and healthy controls.44 However, extensive studies conducted using jejunal mucosa obtained from patients with IBS have documented aberrant immunological responses, increased humoral immunity, disturbed molecular and functional intestinal epithelial barrier, impaired bile acid metabolism, proximity of plasma cells to nerves, mast cell activation and protease and neuropeptide signaling and dysbiosis that may be related to the origin of symptoms in IBS patients. These data also suggest the role of the small bowel in the pathophysiology of IBS, particularly IBS-D.45-48
Figure 1. Cellular mechanisms that are similarly or differentially expressed in colonic mucosa of patients with IBS-D compared with mucosa from patients with IBS-C. Mechanisms that appear in green boxes showed increased differential expression; those in orange boxes showed decreased expression; those in blue boxes showed similar expression in mucosa from IBS-D compared with mucosa from IBS-C.
Reproduced from reference 42, Am J Physiol Gastrointest Liver Physiol. 2022;323:G88-G101.
g.(iii). Immune activation and inflammation in diagnosis and treatment
To date, the role of mucosal immune activation in IBS has not been extensively explored in clinical diagnosis other than the appreciation of the overlap of symptoms between microscopic colitis and IBS-D49 and the recommendation to exclude microscopic colitis by measuring fecal calprotectin or lactoferrin. Other studies explored the efficacy of the anti-inflammatory 5-aminosalicylcic acid compounds in the treatment of IBS, without evidence of clinical benefit.50,51 Nevertheless, current evidence suggesting that immune activation contributes to the pathology seen in patients with IBS has been summarized elsewhere52 including the role of mast cells.
It is also conceivable that more widespread screening for bile acid diarrhea [in the overlap with IBS-D or microscopic colitis (see above)] may identify patients for more tailored treatments such as with bile acid sequestrants or, in the future, FXR agonists. Similarly, screening in urine for mast cell mediators53 such as histamine, p-hydroxybenzoic acid, and azelaic acid, or in feces might identify patients in whom mast cell activation underlies visceral hypersensitivity, providing support to treat these patients with mast cell stabilizers such as ketotifen54 or histamine H1 receptor antagonists such as ebastine.15
h. Chemicals released in association with immune activation.
Several chemical and molecular factors in the intestine are reported to be altered and to have potentially significant roles in IBS, particularly in IBS-D. These include bile acids, short-chain fatty acids, mucosal barrier proteins, mast cell products such as histamine, proteases, and tryptase, enteroendocrine cell products, and mucosal messenger ribonucleic acid (mRNAs), proteins and microRNAs.55
The biochemical mechanisms have been reviewed recently with particular emphasis on how immune mediators, particularly those released by mast cells, can directly activate or sensitize pain-transmitting nerves, leading to increased pain signalling and abdominal pain.52 The putative mechanisms include several mechanisms involved in visceral hypersensitivity such as histamine, serotonin, proteases, and nerve growth factor (NGF), all of which have been demonstrated in mucosa of patients with IBS, as reviewed by Aguilera-Lizarraga et al.53 Thus, histamine acts on histamine H1 receptors to sensitize TRPV1, TRPA1, and TRPV4 channels via H1R. Histamine and serotonin increase TRPV4 expression and translocation to the membrane in nociceptors leading to neuron hypersensitivity. Trypsin and other proteases in the mucosa lead to protease-activated receptor 2 [(PAR2); a gene-protein-coupled receptor (GPCR)] endocytosis mediating persistent afferent hyperexcitability, likely through sensitization of the same TRP channels. Increased levels of NGF, produced by mast cells, increase nerve fibre density. Along the same line, increased nerve sprouting was also observed with increased levels of brain-derived neurotrophic factor (BDNF).52
The origin of mucosal immune activation in IBS is thought to result from altered food-derived or bacterial products following dysbiosis,56 or a disrupted epithelial response. For example, the bacterial metabolite, tryptamine, which stimulates colonic mucosal secretion, was increased in patients with IBS-D and was associated with an enrichment in inflammation-related pathways;57 however, the studies were conducted in small numbers of patients with flares in IBS and require replication. Another example is the demonstration that a Klebsiella aerogenes strain, carrying a histidine decarboxylase gene variant, produces high amounts of histamine leading to mastocytosis, and to mast cell activation via histamine-H4 receptors leading to the release of histamine and proteases that induce visceral hypersensitivity.58
Alternatively, it is hypothesized that mast cell activation may be directly induced by bacterial or food-derived products, as well as by neurogenic inflammation and psychological stress. The evidence of food antigen induced local inflammation in 12 patients with IBS compared to 8 healthy controls (with allergic diathesis or mast cell problems excluded in all participants) was demonstrated in elegant studies of intramucosal injection of the antigens (soy, wheat, gluten, and milk) into rectosigmoid mucosa and observation of mucosal reactions. This study characterized a peripheral mechanism that underlies food-induced abdominal pain upon loss of local oral tolerance, mediated by food antigen specific IgE-dependent activation of mast cells in the colon,59 and resulting in sensitization of TRPV1 mediated by H1R in IBS. These findings explain the observation that treatment with ebastine, a H1R antagonist, reduced visceral hypersensitivity and abdominal pain in patients with IBS.15 Along the same line, confocal laser endomicroscopy studies had previously shown that the duodenal mucosa of patients with IBS undergoes profound structural remodeling upon exposure to food antigens.60 Prior study has also documented that gluten intolerance without celiac disease is more likely in carriers of the HLA DQ2/8 genotype.61
i. The microbiome.
The healthy intestinal microbial community can be characterized in terms of diversity, stability and resistance, and resilience.62 Intestinal dysbiosis refers to the compositional and functional alterations of the gut microbiome and may be associated with one or more of the following non-mutually exclusive characteristics: bloom of pathobionts, loss of commensals, and loss of diversity. The mechanisms that contribute to the development and maintenance of a dysbiotic state are infection and inflammation, diet and xenobiotics, genetics, familial transmission, and other causes such as circadian disruption, maternal high-fat diet, pregnancy, and physical injury.63
A systematic review and meta-analysis of the literature on microbiota in IBS concluded that there is not a true microbial signature associated with IBS, no overt or demonstrable differences between microbiome of patients with IBS-D compared to IBS-C, and the quality of evidence is not ideal.64 Further longitudinal studies of the microbiome conducted in about 30 individuals with IBS-C, IBS-D, and healthy controls also showed significant overlap, though there was evidence of differences in beta-diversity between the groups. Moreover, it is interesting to note that there appeared to be differences in the microbiota in about 6 patients with IBS-D and 6 patients with IBS-C who experienced a flare of their symptoms.57 The diagnostic and therapeutic significance of the characterization of the microbiome in IBS has still not reached clinical significance, and the role of fecal microbiota transplantation is discussed below.
ACTIONABLE BIOMARKERS
Table 1 summarizes the pathophysiological mechanisms discussed above and illustrates the application of the pathophysiology-actionable biomarker approach to managing patients with IBS.65
Table 1. Application of pathophysiology-actionable biomarker approach to managing patients with IBS.
(derived from ref. 65, Gut 2020;69:1730-1737)
| Pathophysiology | Diagnostic test | Actionable biomarker |
|---|---|---|
| Evacuation disorders | Anorectal manometry, balloon expulsion, ? defecography | Spastic pelvic floor or descending perineum |
| Transit | Radiopaque markers, scintigraphy, wireless motility capsule | Accelerated transit at 24h or delayed transit at 48h on scintigraphy |
| Sensation and central nervous system hypervigilance | Rectal sensation to balloon distension, e.g., during anorectal manometry (ARM) | Rectal sensation recorded during ARM as symptoms: first sensation, gas, urge, pain |
| Psychosocial factors | HAD, IBS-QOL, PAC-SYM, PAC-QOL | Anxiety, depression, general pain conditions |
| Bile acid diarrhea | Serum 7αC4, primary or total bile acids (single or 48h stool), 75-SeHCAT retention | Increased bile acid synthesis or excretion |
| Disaccharidase deficiency | Lactose-hydrogen breath test, duodenal biopsy measurements of disaccharidases | Lactose and sucrose intolerance with objective test results |
| Celiac disease | TTG-IgA, anti-gliadin IgA | Diagnostic tests for coeliac disease |
| Local immune reactions to foods or mucosal inflammation | Fecal calprotectin, careful dietary history, specific inquiry on fructans and galactans, consider gluten intolerance | Colonoscopy for microscopic colitis, experimental studies, screen for HLA DQ2/8 with gluten intolerance in absence of proven celiac disease |
| Microbiome | N/A | N/A |
HAD=Hospital Anxiety and Depression scale; IBS-QOL=Irritable Bowel Syndrome-Quality of Life questionnaire; PAC-SYM= Patient Assessment of Constipation-Symptoms questionnaire; PAC-QOL= Patient Assessment of Constipation-Quality of Life questionnaire; N/A=not applicable; 7αC4=7α-hydroxy-4-cholesten-3-one; 75-SeHCAT=75-selenium homocholic acid taurine; TTG = tissue transglutaminase; HLA DQ2/8= human leukocyte antigen DQ2/8
The utility of many of these tests and their sensitivity, specificity, and predictive values have been reported, albeit predominantly from the studies previously reported from Mayo Clinic where this approach has been put into practice.2, 4, 65-73 It is important to note that the most invasive biomarkers are based on physical examination, noninvasive tests other than the minimally invasive anorectal manometry and balloon expulsion tests.
TREATMENT STRATEGY
First Steps
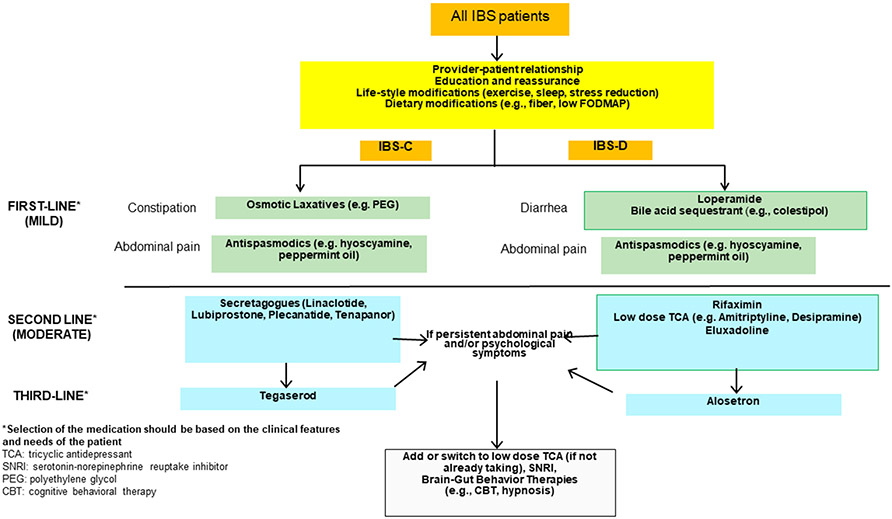
Recommendations from several gastroenterology societies (European, American, Canadian, Japanese, British societies)74-80 provided general principles regarding education, doctor-patient relationship, diverse diet options, and first line symptomatic treatments including osmotic laxatives for constipation, loperamide for diarrhea, simple psychotherapy, and first line anti-spasmodics. Some guidelines go on to prioritize the sequence of pharmacological agents and brain-gut behavior therapy that are recommended for moderate and severe IBS.78,79 Based on the rich evidence of mechanisms and biomarkers identified in IBS and documented in this review, using the algorithmic approach to treatment based on symptoms and response to the three tiers of treatment (Figure 2), one might miss opportunities for optimizing management of IBS.
Figure 2. Clinical decision support tool developed by the AGA Guideline Committee for diarrhea or constipation in IBS.
Reproduced from references 78 and 79. Note that tegaserod has since been withdrawn and is unavailable for prescription.
Dietary Approaches
Dietary approaches include increase in soluble fiber, low FODMAP diet, and gluten-free diet. Soluble fiber or psyllium is more efficacious than bran if patients have abdominal pain and discomfort with IBS.81 There are several relatively small low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet trials and several systematic reviews and meta-analyses in the literature. In addition to questions that have been posed regarding blinding and trial design, the efficacy over placebo or other diets [e.g., National Institute of Clinical Excellence (NICE) and British Dietetic Association recommended diets] is marginal or quite comparable.82,83
It is nevertheless intriguing that basic science studies show that low FODMAP diet may reduce the transfer of endotoxin (lipopolysaccharide) across the mucosa in an animal model, and this is associated with less contraction of the abdominal musculature measured by electromyography (EMG) in response to exposure of the rectal mucosa of the animal to the supernatant of stool of patients with IBS, which presumably has the high level of fecal endotoxin.84,85 A recent study indicated that FODMAPs favor the production of fecal histamine by Klebsiella aerogenes in a subgroup of IBS patients, leading to mast cell accumulation and visceral hypersensitivity in mice.58 These patients had high urinary histamine, indicating that urinary histamine might be a biomarker to identify patients that may benefit from low FODMAP diet or treatment with H1R antagonists.58 Moreover, a re-analysis of data from a trial of low FODMAP diet in IBS,53 revealed a moderate correlation (r=0.44, P=0.009) between visceral pain severity and the concentration of urinary histamine.58 It has recently been proposed that not all FODMAPs are created equal and that fructans are significant detrimental molecules.86,87 This observation was consistent with physiology of saccharide absorption in the human small intestine (as discussed above).
The American College of Gastroenterology guideline supports a limited trial of a low FODMAP diet to improve global symptoms while acknowledging that this is a conditional recommendation based on very low-quality evidence and a high risk of bias.74 A recent American Gastroenterological Association clinical practice update guideline88 recommended following a three-phase sequence: 1) restriction (lasting no more than 4-6 weeks), 2) reintroduction of FODMAP foods, and 3) personalization based on results from reintroduction. However, managing the reintroduction and personalization of the diet has not been adequately studied and there are several potential deficiencies including complexity, cost, greatest effectiveness when administered by a specialized GI dietician, and possible negative or unknown impacts on quality of life, the gut microbiota, potential for development of avoidant/restrictive food intake disorder (ARFID), cibophobia, and nutritional deficiencies. Thus, in addition to strategies for lactose intolerance, a case can be made for individualizing food restriction such as avoiding fructans, galactans, and sugar alcohols that are poorly metabolized in the human small intestine, reach the colon, and are fermented by bacteria to increase osmolality and gas production.
The evidence of efficacy of gluten withdrawal in patients with IBS is unproven based on two randomized, controlled trials involving 111 participants who responded to a gluten-free diet and then randomized to continue the diet or diet "spiked" with gluten (RR=0.42; 95% CI 0.11 to 1.55).89 However, a prospective study of 50 patients with IBS documented that the presence of antigliadin IgG was associated with overall reductions in symptoms (adjusted odds ratio compared with patients without this antibody, 128.9; 95% CI, 1.16-1427.8; P=0.04).These data suggest that antigliadin IgG can be used as a biomarker to identify patients with IBS who might have reductions in symptoms, particularly diarrhea, on a gluten-free diet.90 However, it has been demonstrated that the presence of IgG antibodies is also a sign of exposure to a food antigen.91
A novel approach to correcting sucrase-isomaltase deficiency has been reported, and it is analogous to supplementation of lactase enzyme in patients with hypolactasia. This approach utilizes a commercially available enzyme called sacrosidase, which was shown to reduce symptoms and breath hydrogen in a sucrose challenge test in a 23-year-old patient with postprandial diarrhea since infancy associated with bloating, abdominal pain, and nausea.92
Pharmacological Agents
Table 2 summarizes the evidence of the efficacy of treatments with diverse pharmacological approaches in IBS based on summary analyses such as systematic reviews and meta-analyses.93-95
Table 2. Summary of current medications approved (in at least some countries) for treatment of IBS symptoms.
| Class of Rx | Examples | Mechanisms of action | Efficacy on SRMA [OR or RR (95%CI)]/ RCTs/ NNT |
|---|---|---|---|
| PAIN | |||
| Anti-spasmodics | Hyoscine, otilonium, pinaverium, cimetropium | Inhibition of muscarinic Ach receptors, or block Ca++ channels, GI smooth muscle | May be effective; OR 0.68 (0.57 to 0,71) Overall NNT 5; NNT for: hyoscine 3.5, otilonium 4.5, cimetropium 3, pinaverium 3 |
| Peppermint Oil | block L-type Ca++ channels on muscle, activate TRPM8 receptors on nociceptive afferents | Effective: OR: 0.43 (0.32 to 0.59); Global: RR 2.23 (1.78 to 2.81) Overall NNT 2.5; RCT of sustained release formulation: ↓ pain, bloat, urgency but not total IBS scores | |
| Anti depressants | slow (TCA) or fast (SSRI, SNRI) transit | Psychological, antinociceptive effects | Effective OR: 0.67 (0.58 to 0.77) for global; OR 0.62 (0.43 to 0.88) for abdo. pain; NNT 4 |
| DIARRHEA | |||
| Opioid agents | Loperamide | μ-opioid agonist inhibits secretion, transit | Unknown for IBS; effective for diarrhea |
| Eluxadoline | κ-, and μ-opioid receptor agonist and δ-opioid receptor antagonist | Effective for FDA composite endpoint; 100mg: OR 0.87 (0.83 to 0.91); 75mg: 0.89 (0.84 to 0.94); RCTs: Effective for diarrhea and composite diarrhea + pain; not for pain alone | |
| 5-HT3 receptor antagonists | Ondansetron Alosetron Ramosetron | Retard colonic transit and reduce visceral pain | Effective: Global RR 1.60 (1.49 to 1.72); Pain RR 1.30 (1.22 to 1.39); FDA composite: OR 0.69 (0.60 to 0.80) RCTs: Class effective for all symptoms: diarrhea; composite diarrhea + pain; and pain alone. Ondansetron efficacy for diarrhea, urgency, bloating, not for pain |
| Bile acid sequestrants | Cholestyramine Colestipol Colesevelam | Bind intraluminal bile acids | Unknown; Effective in open label studies; ineffective in one, single center RCT |
| Antibiotic | Rifaximin | Non-absorbable antibiotic | Effective: In 2012 SRMA: Global: OR 1.57 (1.22 to 2.01); Bloating: OR 1.55 (1.23 to 1.96); In 2020 SRMA: FDA composite: OR: 0.92 (0.86 to 0.98) Global OR: 0.91 (0.77, 1.07) |
| CONSTIPATION | |||
| Osmotic | PEG3350 | Osmotic secretion | Effective: improves SBMs, CSBMs, consistency straining but not pain, bloating or incomplete evacuation |
| Secretory | Lubiprostone | Chloride channel (C1C2) activation and CFTR stimulate Cl” secretion | Effective: Lubiprostone 8μg RR: 0.85 (0.78 to 0.96) for FDA endpoint |
| Linaclotide | Guanylate cyclase C activator, stimulate Cl− and water secretion via CFTR; visceral analgesia | Effective: Adequate relief IBS: RR 1.95 (1.3 to 2.9); Abdo pain: RR 1.58 (1.02 to 2.46) RR 0.81 (0·76 to 0·86) for 290μg for FDA endpoint | |
| Plecanatide | Effective: Using FDA- endpoint 6mg: RR 0.87 (0·81 to 0·94); 3mg RR 0.88 (0·82 to 0·94) | ||
| Anti-absorptive | Tenapanor | NHE3 inhibitor stimulates Na+, water secretion | Effective at 50mg b.i.d. dose; RR 0.85 (0.79 to 0.82) for FDA endpoint; NNTs for CSBM and combined CSBM + >30% pain reduction: 7-9; NNT for abdo. pain reduction >30% alone: 11 |
| 5-HT4 receptor agonists | Tegaserod | Stimulate colonic motility and transit | Effective: tegaserod 6mg b.i.d. showed RR, 0.85 (0.80—0.91) for FDA endpoint |
In clinical practice, pharmacological agents are often prescribed in patients with IBS. For relief of pain associated with IBS, there are limited data on efficacy of antispasmodics, with the greatest efficacy reported for agents acting on calcium channels (most unavailable in many countries such as USA) or peppermint oil. A single-center study documented improvement of pain in IBS patients treated with the H1 receptor blocker, ebastine.15 Although widely used and recommended, the evidence in support of central neuromodulators (mostly antidepressants) is relatively weak as it is based on only three high quality trials, possible publication bias, and overestimated efficacy by inclusion of smaller trials with unprecedented response rates (e.g. 10% responders in placebo arm).96,97 Analysis of publications with network meta-analysis reported that, among medications for the relief of pain in IBS, the order for relative efficacy was tricyclic agents, followed by antispasmodics and peppermint oil, with non-significant benefit for selective serotonin reuptake inhibitors (SSRIs) and ispaghula husks.96
The first-line approach of treatment of constipation (PEG 3350) has not been formally evaluated in IBS-C. On the other hand, there are extensive studies of diverse chloride secretagogues (lubiprostone, linaclotide, and plecanatide), and the sodium hydrogen exchanger inhibitor 3 (NHE3), tenapanor. A systematic review and meta-analysis documented the efficacy of all these medications in achieving the USA Food and Drug Administration (FDA) recommended composite endpoint for IBS-C, that is the relief of constipation and pain components.94 Although the 5-HT4 receptor agonist, tegaserod, was approved for patients with IBS-C under the age of 65 years without cardiovascular disease, it was recently withdrawn from some markets (e.g., USA) for commercial reasons.
For IBS-D, loperamide is usually the first-line therapy, although it has not been tested in large studies in IBS. Eluxadoline has effects on multiple opioid mechanisms, and its greatest benefit is in relief of diarrhea with limited efficacy on pain. It must be used with great caution as it can cause sphincter of Oddi spasm and pancreatitis and is contraindicated in patients with cholecystectomy. As a class, 5-HT3 receptor antagonists are very efficacious for treatment of IBS-D, and network meta-analyses place them at the highest level for efficacy in the relief of abdominal pain and stool consistency, as well as global IBS symptoms, compared to rifaximin and eluxadoline.98 It is worth noting that rifaximin’s efficacy in patients with IBS-D, as demonstrated in single and repeat treatment trials was greater for global symptoms, bloating, and the composite FDA endpoint, but not for diarrhea or stool consistency.99,100 These observations are not surprising given the fact that rifaximin actually accelerates colonic transit101 and that it has only modest and transient effects on gut microbial taxa.102
Only open-label studies are available to support bile acid sequestrants efficacy in bile acid diarrhea.
Fecal Microbial Transplantation
Based on systematic reviews and meta-analyses, there is equivocal data regarding the efficacy of fecal microbiota transplantation (FMT) for IBS. A recent report of three-year outcomes of treatment of IBS with FMT provided by a single super donor103 or from treatment of IBS in primary care centers in Belgium104 support the use of FMT for IBS. However, pitfalls identified in study design and questions regarding clinical relevance of 50-point response on the 500-point IBS-Symptom Severity Scale (IBS-SSS)105,106 suggest that evidence of significant clinical efficacy or effectiveness is still required. Therefore, further research is needed to identify the beneficial microbiota and the mechanism involved to ideally transfer a selection of well-characterized “therapeutic” microbiota and to avoid the risk of introducing potential pathogens.
TREATMENT: FROM CHOICE BASED ON SYMPTOMS TO STRATEGY BASED ON PATHOPHYSIOLOGICAL MECHANISMS
Figure 2 documents the clinical decision support tool developed by the AGA guideline committee for diarrhea or constipation in IBS.78,79 It essentially provides four tiers of management: first, general measures including diet; second, first line treatments based on mild symptom severity and bowel dysfunction; third, second line approaches for moderate severity symptoms based on bowel dysfunction; and fourth, based on third line treatments or centrally directed pharmacological or behavioral treatments.
There are several controversial choices in the proposed tiers. Should bile acid sequestrants be applied as first line empiric treatment in the absence of a diagnosis, given the availability of serum or single fecal sample diagnostic tests? Where they are available, should rifaximin and eluxadoline be applied ahead of alosetron, given the level of evidence of efficacy of alosetron and the cumulated evidence of safety of that medication, the limited efficacy of rifaximin (which accelerated colonic transit) for diarrhea, and the relative risks associated with eluxadoline and contraindication in patients with prior cholecystectomy?
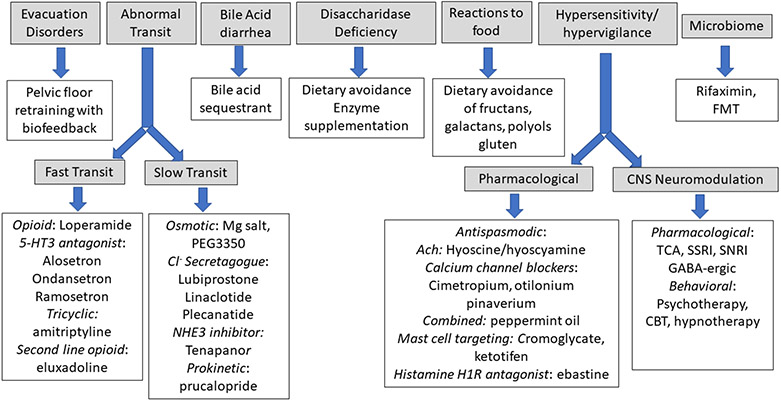
All these insights and the opportunity to individualize treatment based on identified pathophysiological mechanisms as shown in Tables 1 and 2 led to the recommendations in Figure 3 where therapeutic choices are guided by pathophysiology and biomarkers.
Figure 3. Therapeutic choices guided by pathophysiology and biomarkers.
CONCLUSION
The widespread availability of noninvasive clinical tests that can appraise the mechanisms responsible for symptom generation in IBS provides the opportunity to advance the practice from treatment based on symptoms to individualization of treatment guided by pathophysiology and clinically identified biomarkers.
SUMMARY BOX.
What is already known on this topic
The current guidelines suggest algorithms regarding the sequence of choice of medications based on predominant symptoms particularly bowel dysfunction in patients with IBS.
What this study adds
This review documents the evidence that pathophysiological mechanisms and individualized treatment directed at those mechanisms provide an alternative approach to the management of patients with irritable bowel syndrome.
How this study might affect research, practice or policy
This review focuses the attention of researchers to the translational and basic molecular mechanisms that deserve further studies to enhance the diagnosis and management of IBS, and it informs policy makers and those involved in developing guidelines for clinical practice regarding the importance of “splitting” IBS, thereby increasing the opportunities to provide specific targeted treatment.
Acknowledgements:
The authors thank Mrs. Cindy Stanislav for excellent secretarial assistance.
Funding support:
Michael Camilleri: grant R01-DK115950 from National Institutes of Health Guy Boeckxstaens: Leuven University internal funding grant C1 (C14/18/086)
Footnotes
Disclosures:
Michael Camilleri: Consulting regarding irritable bowel syndrome for Ironwood; Protagonist Therapeutics; Zealand Biopharma; Aditum Bio; Invea Therapeutics; and InveniAI Guy Boeckxstaens: No conflicts of interest to report
REFERENCES
- 1.Camilleri M Peripheral mechanisms in irritable bowel syndrome. N Engl J Med 2012;367:1626–1635 [DOI] [PubMed] [Google Scholar]
- 2.Nullens S, Nelsen T, Camilleri M, Burton D, Eckert D, Iturrino J, Vazquez-Roque M, Zinsmeister AR. Regional colon transit in patients with dyssynergic defecation or slow transit in patients with constipation. Gut 2012;61:1132–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang XJ, Chedid V, Vijayvargiya P, Camilleri M. Clinical features and associations of descending perineum syndrome in 300 adults with constipation in gastroenterology referral practice. Dig Dis Sci 2020;65:3688–3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chedid V, Vijayvargiya P, Halawi H, Park S-Y, Camilleri M. Audit of the diagnosis of rectal evacuation disorders in chronic constipation. Neurogastroenterol Motil 2019;31:e13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson AD, Mouchli MA, Valentin N, Deyle D, Pichurin P, Acosta A, Camilleri M. Ehlers Danlos syndrome and gastrointestinal manifestations: a 20-year experience at Mayo Clinic. Neurogastroenterol Motil 2015;27:1657–1666 [DOI] [PubMed] [Google Scholar]
- 6.Wang XJ, Babameto M, Babovic-Vuksanovic D, Bowen JM, Camilleri M. Audit of gastrointestinal manifestations in patients with Loeys-Dietz syndrome and vascular Ehlers-Danlos syndrome. Dig Dis Sci 2021;66:1142–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadik R, Stotzer P-O, Simrén M, Abrahamsson H. Gastrointestinal transit abnormalities are frequently detected in patients with unexplained GI symptoms at a tertiary centre. Neurogastroenterol Motil 2008;20:197–205 [DOI] [PubMed] [Google Scholar]
- 8.Vassallo M, Camilleri M, Phillips SF, Brown ML, Chapman NJ, Thomforde GM. Transit through the proximal colon influences stool weight in the irritable bowel syndrome. Gastroenterology 1992;102:102–108 [DOI] [PubMed] [Google Scholar]
- 9.Camilleri M, McKinzie S, Busciglio I, Low PA, Sweetser S, Burton D, Baxter K, Ryks M, Zinsmeister AR. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2008;6:772–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halder SL, Locke GR 3rd, Schleck CD, Zinsmeister AR, Melton LJ 3rd, Talley NJ. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology 2007;133:799–807 [DOI] [PubMed] [Google Scholar]
- 11.Ritchie J Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut 1973;14:125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology 1995;109:40–52 [DOI] [PubMed] [Google Scholar]
- 13.Dorn SD, Palsson OS, Thiwan SIM, Kanazawa M, Clark WC, van Tilburg MAL, Drossman DA, Scarlett Y, Levy RL, Ringel Y, Crowell MD, Olden KW, Whitehead WE. Increased colonic pain sensitivity in irritable bowel syndrome is the result of an increased tendency to report pain rather than increased neurosensory sensitivity. Gut 2007;56:1202–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balemans D, Aguilera-Lizarraga J, Florens MV, Jain P, Denadai-Souza A, Viola MF, Alpizar YA, Van Der Merwe S, Vanden Berghe P, Talavera K, Vanner S, Wouters MM, Boeckxstaens GE. Histamine-mediated potentiation of transient receptor potential (TRP) ankyrin 1 and TRP vanilloid 4 signaling in submucosal neurons in patients with irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2019;316:G338–G349 [DOI] [PubMed] [Google Scholar]
- 15.Wouters MM, Balemans D, Van Wanrooy S, Dooley J, Cibert-Goton V, Alpizar YA, et al. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology 2016;150:875–887.e9 [DOI] [PubMed] [Google Scholar]
- 16.Valentin N, Camilleri M, Altayar O, Vijayvargiya P, Acosta A, Nelson AD, Murad MH. Biomarkers for bile acid diarrhea in functional bowel disorder with diarrhea: a systematic review and meta-analysis. Gut 2016;65:1951–1959 [DOI] [PubMed] [Google Scholar]
- 17.Camilleri M, Nurko S. Bile acid diarrhea in adults and adolescents. Neurogastroenterol Motil 2022;34:e14287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beinvogl BC, Manini ML, Camilleri M, Donato LJ, Harmsen WS, Absah I, Burch E, Schechter NL, Nurko S. Markers of bile acid metabolism in pediatric diarrhea predominant irritable bowel syndrome and healthy controls. J Pediatr Gastroenterol Nutr 2021;72:859–865 [DOI] [PubMed] [Google Scholar]
- 19.Wong BS, Camilleri M, Carlson P, McKinzie S, Busciglio I, Bondar O, Dyer RB, Lamsam J, Zinsmeister AR. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol 2012;10:1009–1015.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vijayvargiya P, Camilleri M. Current practice in the diagnosis of bile acid diarrhea. Gastroenterology 2019;156:1233–1238 [DOI] [PubMed] [Google Scholar]
- 21.Vijayvargiya P, Camilleri M, Taylor A, Busciglio I, Loftus EV Jr, Donato L. Combined fasting serum C4 and primary bile acids from a single stool sample to diagnose bile acid diarrhea. Gastroenterology 2020;159:1952–1954.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar A, Al-Hassi HO, Jain M, Phipps O, Ford C, Gama R, Steed H, Butterworth J, McLaughlin J, Galbraith N, Brookes MJ, Hughes LE. A single faecal bile acid stool test demonstrates potential efficacy in replacing SeHCAT testing for bile acid diarrhoea in selected patients. Sci Rep 2022;12:8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahlqvist A, Borgstrom B. Digestion and absorption of disaccharides in man. Biochem J 1961;81:411–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borgstrom B, Dahlqvist A, Lundh G, Sjovall J. Studies of intestinal digestion and absorption in the human. J Clin Invest 1957;36:1521–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silk DB, Webb JP, Lane AE, Clark ML, Dawson AM. Functional differentiation of human jejunum and ileum: a comparison of the handling of glucose, peptides, and amino acids. Gut 1974;15:444–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camilleri M Integrated upper gastrointestinal response to food intake. Gastroenterology 2006;131:640–658 [DOI] [PubMed] [Google Scholar]
- 27.Misselwitz B, Butter M, Verbeke K, Fox MR. Update on lactose malabsorption and intolerance: pathogenesis, diagnosis and clinical management. Gut 2019;68:2080–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lactose intolerance. Medline Plus. National Library of Medicine: Bethesda, MD: https://ghr.nlm.nih.gov/condition/lactose-intolerance [Google Scholar]
- 29.Suarez FL, Savaiano DA, Levitt MD. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose intolerance. N Engl J Med 1995;333:1–4 [DOI] [PubMed] [Google Scholar]
- 30.Uhrich S, Wu Z, Huang J-Y, Scott CR. Four mutations in the SI gene are responsible for the majority of clinical symptoms of CSID. J Pediatr Gastroenterol Nutr 2012;55(Suppl.2):S34–S35 [DOI] [PubMed] [Google Scholar]
- 31.Henström M, Diekmann L, Bonfiglio F, Hadizadeh F, Kuech E-M, von Köckritz-Blickwede M, et al. Functional variants in the sucrase-isomaltase gene associate with increased risk of irritable bowel syndrome. Gut 2018;67:263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Etxebarria K, Zheng T, Bonfiglio F, Bujanda L, Dlugosz A, Lindberg G, et al. Increased prevalence of rare sucrase-isomaltase pathogeneic variants in irritable bowel syndrome patients. Clin Gastroenterol Hepatol 2018;16:1673–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng T, Camargo-Tavares L, Bonfiglio F, Marques F, Naim HY, D’Amato M. Rare hypomorphic sucrase isomaltase variants in relation to irritable bowel syndrome risk in UK biobank. Gastroenterology 2021;161:1712–1714 [DOI] [PubMed] [Google Scholar]
- 34.Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil 2012;24:503–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2012;303:G775–G785 [DOI] [PubMed] [Google Scholar]
- 36.Hanning N, Edwinson AL, Ceuleers H, Peters SA, De Man JG, Hassett LC, De Winter BY, Grover M. Intestinal barrier dysfunction in irritable bowel syndrome: a systematic review. Therap Adv Gastroenterol 2021;14:1756284821993586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magnus Y, BouSaba J, Sannaa W, McKinzie S, Busciglio I, Camilleri M. Bile acid diarrhea is associated with increased intestinal permeability compared with irritable bowel syndrome-diarrhea. Gastroenterology 2022;162:1343–1345.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanuytsel T, Bercik P, Boeckxstaens G. Understanding neuro-immune interactions in disorders of gut-brain interaction: from functional to immune-mediated disorders. Gut (2022, in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vicario M, González-Castro AM, Martínez C, Lobo B, Pigrau M, Guilarte M, et al. Increased humoral immunity in the jejunum of diarrhoea- predominant irritable bowel syndrome associated with clinical manifestations. Gut 2015;64:1379–1388 [DOI] [PubMed] [Google Scholar]
- 40.Bennet SMP, Sundin J, Magnusson MK, Strid H, Tap J, Derrien M, Le Nevé B, Doré J, Törnblom H, Simrén M, Öhman L. Altered intestinal antibacterial gene expression response profile in irritable bowel syndrome is linked to bacterial composition and immune activation. Neurogastroenterol Motil 2018;30:e13468. [DOI] [PubMed] [Google Scholar]
- 41.Aguilera-Lizarraga J, Florens MV, Van Brussel T, Clevers E, Van Oudenhove L, Lambrechts D, Wouters MM, Boeckxstaens GE. Expression of immune-related genes in rectum and colon descendens of irritable bowel syndrome patients is unrelated to clinical symptoms. Neurogastroenterol Motil 2019;31:e13579. [DOI] [PubMed] [Google Scholar]
- 42.Camilleri M, Magnus Y, Carlson P, Wang XJ, Chedid V, Maselli D, Taylor A, McKinzie S, Kengunte Nagaraj N, Busciglio I, Nair A. Differential mRNA expression in ileal and colonic biopsies in irritable bowel syndrome with diarrhea or constipation. Am J Physiol Gastrointest Liver Physiol. 2022;323:G88–G101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camilleri M, Carlson P, BouSaba J, McKinzie S, Vijayvargiya P, Magnus Y, Sannaa W, Wang XJ, Chedid V, Zheng T, Maselli D, Atieh J, Taylor A, Nair A, Kengunte Nagaraj N, Johnson S, Chen J, Burton D, Busciglio I. Comparison of biochemical, microbial, and mucosal mRNA expression in bile acid diarrhea and irritable bowel syndrome-diarrhea. Gut 2022. May 17:gutjnl-2022-327471. doi: 10.1136/gutjnl-2022-327471. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Camilleri M Invited Mini-Review: Bile acid detergency: permeability, inflammation and effects of sulfation. Am J Physiol-GI & Liver Physiol 2022;322:G480–G488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruno Kotska Rodiño-Janeiro Cristina Pardo-Camacho, Santos Javier, Martinez Cristina. Mucosal RNA and protein expression as the next frontier in IBS: abnormal function despite morphologically intact small intestinal mucosa. Am J Physiol Gastrointest Liver Physiol. 2019. Jun 1;316(6):G701–G719 [DOI] [PubMed] [Google Scholar]
- 46.Vicario M, González-Castro AM, Martínez C, Lobo B, Pigrau M, Guilarte M, de Torres I, Mosquera JL, Fortea M, Sevillano-Aguilera C, Salvo-Romero E, Alonso C, Rodiño-Janeiro BK, Söderholm JD, Azpiroz F, Santos J. Increased humoral immunity in the jejunum of diarrhoea-predominant irritable bowel syndrome associated with clinical manifestations. Gut. 2015. Sep;64(9):1379–88. [DOI] [PubMed] [Google Scholar]
- 47.Pardo-Camacho C, Ganda Mall JP, Martínez C, Pigrau M, Expósito E, Albert-Bayo M, Melón-Ardanaz E, Nieto A, Rodiño-Janeiro B, Fortea M, Guagnozzi D, Rodriguez-Urrutia A, Torres I, Santos-Briones I, Azpiroz F, Lobo B, Alonso-Cotoner C, Santos J, González-Castro AM, Vicario M. Mucosal Plasma Cell Activation and Proximity to Nerve Fibres Are Associated with Glycocalyx Reduction in Diarrhoea-Predominant Irritable Bowel Syndrome: Jejunal Barrier Alterations Underlying Clinical Manifestations. Cells. 2022. Jun 28;11(13):2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guilarte M, Vicario M, Martínez C, de Torres I, Lobo B, Pigrau M, González-Castro A, Rodiño-Janeiro BK, Salvo-Romero E, Fortea M, Pardo-Camacho C, Antolín M, Saperas E, Azpiroz F, Santos J, Alonso-Cotoner C. Peripheral Corticotropin-Releasing Factor Triggers Jejunal Mast Cell Activation and Abdominal Pain in Patients With Diarrhea-Predominant Irritable Bowel Syndrome. Am J Gastroenterol. 2020. Dec;115(12):2047–2059. [DOI] [PubMed] [Google Scholar]
- 49.Limsui D, Pardi DS, Camilleri M, Loftus EV Jr, Kammer PP, Tremaine WJ, Sandborn J: Symptomatic overlap between irritable bowel syndrome and microscopic colitis. Inflamm Bowel Dis 2007;13:175–181. [DOI] [PubMed] [Google Scholar]
- 50.Lam C, Tan W, Leighton M, Hastings M, Lingaya M, Falcone Y, Zhou X, Xu L, Whorwell P, Walls AF, Zaitoun A, Montgomery A, Spiller R. A mechanistic multicentre, parallel group, randomised placebo-controlled trial of mesalazine for the treatment of IBS with diarrhoea (IBS-D). Gut 2016;65:91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barbara G, Cremon C, Annese V, Basilisco G, Bazzoli F, Bellini M, Benedetti A, Benini L, Bossa F, Buldrini P, Cicala M, Cuomo R, Germanà B, Molteni P, Neri M, Rodi M, Saggioro A, Scribano ML, Vecchi M, Zoli G, Corinaldesi R, Stanghellini V. Randomised controlled trial of mesalazine in IBS. Gut 2016;65:82–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aguilera-Lizarraga J, Hussein H, Boeckxstaens GE. Immune activation in irritable bowel syndrome: what is the evidence? Nat Rev Immunol 2022. Mar 16. doi: 10.1038/s41577-022-00700-9. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 53.McIntosh K, Reed DE, Schneider T, Dang F, Keshteli AH, De Palma G, Madsen K, Bercik P, Vanner S. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut 2017;66:1241–1251 [DOI] [PubMed] [Google Scholar]
- 54.Klooker TK, Braak B, Koopman KE, Welting O, Wouters MM, van der Heide S, Schemann M, Bischoff SC, van den Wijngaard RM, Boeckxstaens GE. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut 2010;59:1213–1221 [DOI] [PubMed] [Google Scholar]
- 55.Camilleri M, Oduyebo I, Halawi H. Chemical and molecular factors in irritable bowel syndrome: current knowledge, challenges, and unanswered questions. Am J Physiol Gastrointest Liver Physiol 2016;311:G777–G784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caminero A, Meisel M, Jabri B, Verdu EF. Mechanisms by which gut microorganisms influence food sensitivities. Nat Rev Gastroenterol Hepatol 2019;16:7–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mars RAT, Yang Y, Ward T, Houtti M, Priya S, Lekatz HR, et al. Longitudinal multi-omics reveals subset-specific mechanisms underlying irritable bowel syndrome. Cell 2020; 182:1460–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Palma G, Shimbori C, Reed DE, Yu Y, Rabbia V, Lu J, Jimenez-Vargas N, Sessenwein J, Lopez-Lopez C, Pigrau M, Jaramillo-Polanco J, Zhang Y, Baerg L, Manzar A, Pujo J, Bai X, Pinto-Sanchez MI, Caminero A, Madsen K, Surette MG, Beyak M, Lomax AE, Verdu EF, Collins SM, Vanner SJ, Bercik P. Histamine production by the gut microbiota induces visceral hyperalgesia through histamine 4 receptor signaling in mice. Sci Transl Med 2022; 14:eabj1895. [DOI] [PubMed] [Google Scholar]
- 59.Aguilera-Lizarraga J, Florens MV, Viola MF, Jain P, Decraecker L, Appeltans I, et al. Local immune response to food antigens drives meal-induced abdominal pain. Nature 2021;590:151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fritscher- Ravens A, Schuppan D, Ellrichmann M, Schoch S, Röcken C, Brasch J, Bethge J, Böttner M, Klose J, Milla PJ. Confocal endomicroscopy shows food- associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2014;147:1012–1020.e3 [DOI] [PubMed] [Google Scholar]
- 61.Vazquez-Roque MI, Camilleri M, Smyrk T, Murray J, Marietta E, O’Neill J, Carlson P, Lamsam J, Janzow D, Eckert D, Burton D, Zinsmeister AR. A controlled trial of gluten-free diet in irritable bowel syndrome-diarrhea: effect on bowel frequency and intestinal functions. Gastroenterology 2013;144:903–911.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature 2012;489:220–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nature Reviews ∣ Immunology 2017;17:219–232 [DOI] [PubMed] [Google Scholar]
- 64.Pittayanon R, Lau JT, Yuan Y, Leontiadis GI, Tse F, Surette M, Moayyedi P. Gut microbiota in patients with irritable bowel syndrome – a systematic review. Gastroenterology 2019;157:97–108 [DOI] [PubMed] [Google Scholar]
- 65.Camilleri M, Chedid V. Leading Article: Actionable biomarkers: the key to resolving disorders of gastrointestinal function. Gut 2020;69:1730–1737 [DOI] [PubMed] [Google Scholar]
- 66.Brandler J, Camilleri M. Pretest and post-test probabilities of diagnoses of rectal evacuation disorders based on symptoms, rectal exam, and basic tests: a systematic review. Clin Gastroenterol Hepatol 2020;18:2479–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heinrich H, Sauter M, Fox M, Weishaupt D, Halama M, Misselwitz B, Buetikofer S, Reiner C, Fried M, Schwizer W, Fruehauf H. Assessment of obstructive defecation by high-resolution anorectal manometry compared with magnetic resonance defecography. Clin Gastroenterol Hepatol 2015;13:1310–1317, e1 [DOI] [PubMed] [Google Scholar]
- 68.Cangemi DJ, Flanagan R, Barshop K, Kuo B, Staller K. Colonic stool burden a useful surrogate for slow transit constipation as determined by a radiopaque transit study. Am J Gastroenterol 2019;114:519–523 [DOI] [PubMed] [Google Scholar]
- 69.Manabe N, Wong BS, Camilleri M, Burton D, McKinzie S, Zinsmeister AR. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil 2010;22:293–e82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sciarretta G, Furno A, Mazzoni M, et al. Post-cholecystectomy diarrhea: evidence of bile acid malabsorption assessed by SeHCAT test. Am J Gastroenterol 1992;87:1852–1854 [PubMed] [Google Scholar]
- 71.Vijayvargiya P, Camilleri M, Chedid V, Carlson P, Busciglio I, Burton D, Donato LJ. Analysis of fecal primary bile acids detects increased stool weight and colonic transit in patients with chronic functional diarrhea. Clin Gastroenterol Hepatol 2019;17:922–929.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vijayvargiya P, Camilleri M, Carlson P, Lueke A, O’Neill J, Burton D, Busciglio I, Donato L. Performance characteristics of serum C4 and FGF19 measurements to exclude the diagnosis of bile acid diarrhoea in IBS-diarrhoea and functional diarrhoea. Aliment Pharmacol Ther 2017;46:581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosado JL, Solomons NW. Sensitivity and specificity of the hydrogen breath-analysis test for detecting malabsorption of physiological doses of lactose. Clin Chem 1983;29:545–548 [PubMed] [Google Scholar]
- 74.Lacy BE, Pimentel M, Brenner DM, Chey WD, Keefer LA, Long MD, Moshiree B. ACG Clinical Guideline: Management of irritable bowel syndrome. Am J Gastroenterol 2021;116:17–44 [DOI] [PubMed] [Google Scholar]
- 75.Vasant DH, Paine PA, Black CJ, Houghton LA, Everitt HA, Corsetti M, Agrawal A, Aziz I, Farmer AD, Eugenicos MP, Moss-Morris R, Yiannakou Y, Ford AC. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut 2021;70:1214–1240 [DOI] [PubMed] [Google Scholar]
- 76.Moayyedi P, Andrews CN, MacQueen G, Korownyk C, Marsiglio M, Graff L, Kvern B, Lazarescu A, Liu L, Paterson WG, Sidani S, Vanner S. Canadian Association of Gastroenterology Clinical Practice Guideline for the Management of Irritable Bowel Syndrome (IBS). J Can Assoc Gastroenterol 2019;2:6–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fukudo S, Okumura T, Inamori M, Okuyama Y, Kanazawa M, Kamiya T, Sato K, Shiotani A, Naito Y, Fujikawa Y, Hokari R, Masaoka T, Fujimoto K, Kaneko H, Torii A, Matsueda K, Miwa H, Enomoto N, Shimosegawa T, Koike K. Evidence-based clinical practice guidelines for irritable bowel syndrome 2020. J Gastroenterol 2021;56:193–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lembo A, Sultan S, Chang L, Heidelbaugh JJ, Smalley W, Verne GN. AGA Clinical Practice Guideline on the pharmacological management of irritable bowel syndrome with diarrhea. Gastroenterology 2022;163:137–151 [DOI] [PubMed] [Google Scholar]
- 79.Chang L, Sultan S, Lembo A, Verne GN, Smalley W, Heidelbaugh JJ. AGA Clinical Practice Guideline on the pharmacological management of irritable bowel syndrome with constipation. Gastroenterology 2022;163:118–136 [DOI] [PubMed] [Google Scholar]
- 80.Savarino E, Zingone F, Barberio B, Marasco G, Akyuz F, Akpinar H, Barboi O, Bodini G, Bor S, Chiarioni G, Cristian G, Corsetti M, Di Sabatino A, Dimitriu AM, Drug V, Dumitrascu DL, Ford AC, Hauser G, Nakov R, Patel N, Pohl D, Sfarti C, Serra J, Simrén M, Suciu A, Tack J, Toruner M, Walters J, Cremon C, Barbara G. Functional bowel disorders with diarrhoea: Clinical guidelines of the United European Gastroenterology and European Society for Neurogastroenterology and Motility. United European Gastroenterol J 2022;10:556–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bijkerk CJ, de Wit NJ, Muris JWM, Whorwell PJ, Knottnerus JA, Hoes AW. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial. BMJ 2009;339:b3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Black CJ, Staudacher HM, Ford AC. Efficacy of a low FODMAP diet in irritable bowel syndrome: systematic review and network meta-analysis. Gut 2022;71:1117–1126 [DOI] [PubMed] [Google Scholar]
- 83.van Lanen A-S, de Bree A, Greyling A. Efficacy of a low-FODMAP diet in adult irritable bowel syndrome: a systematic review and meta-analysis. Eur J Nutr 2021;60:3505–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou S-Y, Gillilland M 3rd, Wu X, Leelasinjaroen P, Zhang G, Zhou H, Ye B, Lu Y, Owyang C. FODMAP diet modulates visceral nociception by lipopolysaccharide-mediated intestinal inflammation and barrier dysfunction. J Clin Invest 2018;128:267–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singh P, Grabauskas G, Zhou S-Y, Gao J, Zhang Y, Owyang C. High FODMAP diet causes barrier loss via lipopolysaccharide-mediated mast cell activation. JCI Insight 2021;6:e146529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eswaran SL, Singh P, Ritkin S, Chey WD. Are all FODMAPs created equal? A blinded, randomized reintroduction trial to determine which FODMAPs drive clinical response in IBS patients. Gastroenterology 2021;160:S745 [Google Scholar]
- 87.Van den Houte K, Carbone F, Toth J, Marien Z, Schol J, Colemier E, Van den Bergh J, Vanderstappen J, Pauwels N, Matthys C, Vanuytsel T, Tack JF. Symptoms and duodenal mucosal integrity and are improved by a dietary intervention in functional dyspepsia. Gastroenterology 2021;160:S96 [Google Scholar]
- 88.Chey WD, Hashash JG, Manning L, Chang L. AGA Clinical Practice Update on the role of diet in irritable bowel syndrome: Expert Review. Gastroenterology 2022;162:1737–1745.e5. [DOI] [PubMed] [Google Scholar]
- 89.Dionne J, Ford AC, Yuan Y, Chey WD, Lacy BE, Saito YA, Quigley EMM, Moayyedi P. A systematic review and meta-analysis evaluating the efficacy of a gluten-free diet and a low FODMAPs diet in treating symptoms of irritable bowel syndrome. Am J Gastroenterol 2018;113:1290–1300 [DOI] [PubMed] [Google Scholar]
- 90.Pinto-Sanchez MI, Nardelli A, Borojevic R, De Palma G, Calo NC, McCarville J, Caminero A, Basra D, Mordhorst A, Ignatova E, Hansen S, Uhde M, Norman GL, Murray JA, Smecuol E, Armstrong D, Bai JC, Schuppan D, Collins SM, Alaedini A, Moayyedi P, Verdu EF, Bercik P. Gluten-Free Diet Reduces Symptoms, Particularly Diarrhea, in Patients With Irritable Bowel Syndrome and Antigliadin IgG. Clin Gastroenterol Hepatol. 2021. Nov; 19(11):2343–2352.e8. [DOI] [PubMed] [Google Scholar]
- 91.Ligaarden Solveig C 1, Lydersen Stian, Farup Per G. IgG and IgG4 antibodies in subjects with irritable bowel syndrome: a case control study in the general population. BMC Gastroenterol 2012; 12:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Foley A, Halmos EP, Husein DM, Fehily SR, Löscher B-S, Franke A, Naim HY, Gibson PR, D’Amato M. Adult sucrase-isomaltase deficiency masquerading as IBS. Gut 2022;71:1237–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Camilleri M Diagnosis and treatment of irritable bowel syndrome. JAMA 2021;325:865–877 [DOI] [PubMed] [Google Scholar]
- 94.Black CJ, Burr NE, Quigley EMM, Moayyedi P, Houghton LA, Ford AC. Efficacy of secretagogues in patients with irritable bowel syndrome with constipation: systematic review and network meta-analysis. Gastroenterology 2018;155:1753–1763 [DOI] [PubMed] [Google Scholar]
- 95.Black CJ, Burr NE, Ford AC. Relative efficacy of tegaserod in a systematic review and network meta-analysis of licensed therapies for irritable bowel syndrome with constipation. Clin Gastroenterol Hepatol 2020;18:1238–1239.e1 [DOI] [PubMed] [Google Scholar]
- 96.Black CJ, Yuan Y, Selinger CP, Camilleri M, Quigley EMM, Moayyedi P, Ford AC. Efficacy of soluble fibre, antispasmodic drugs, and gut-brain neuromodulators in irritable bowel syndrome: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol 2020;5:117–131 [DOI] [PubMed] [Google Scholar]
- 97.Camilleri M American College of Gastroenterology monograph on the management of irritable bowel syndrome. Expert Opin Pharmacother 2015;16:629–632 [DOI] [PubMed] [Google Scholar]
- 98.Black CJ, Burr NE, Camilleri M, Earnest DL, Quigley EMM, Moayyedi P, Houghton LA, Ford AC. Efficacy of pharmacological therapies in patients with IBS with diarrhoea or mixed stool pattern: systematic review and network meta-analysis. Gut 2020;69:74–82 [DOI] [PubMed] [Google Scholar]
- 99.Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP; TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med 2011;364:22–32 [DOI] [PubMed] [Google Scholar]
- 100.Lembo A, Pimentel M, Rao SS, Schoenfeld P, Cash B, Weinstock LB, Paterson C, Bortey E, Forbes WP. Repeat treatment with rifaximin is safe and effective in patients with diarrhea-predominant irritable bowel syndrome. Gastroenterology 2016;151:1113–1121 [DOI] [PubMed] [Google Scholar]
- 101.Acosta A, Camilleri M, Shin A, Linker Nord S, O'Neill J, Gray AV, Lueke AJ, Donato LJ, Burton DD, Szarka LA, Zinsmeister AR, Golden PL, Fodor A. Effects of rifaximin on transit, permeability, fecal microbiome, and organic acid excretion in irritable bowel syndrome. Clin Transl Gastroenterol 2016;7:e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fodor AA, Pimentel M, Chey WD, Lembo A, Golden PL, Israel RJ, Carroll IM. Rifaximin is associated with modest, transient decreases in multiple taxa in the gut microbiota of patients with diarrhoea-predominant irritable bowel syndrome. Gut Microbes 2019;10:22–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.El-Salhy M, Winkel R, Casen C, Hausken T, Gilja OH, Hatlebakk JG. Efficacy of fecal microbiota transplantation for patients with irritable bowel syndrome at 3 years after transplantation. Gastroenterology 2022. Jun 14:S0016-5085(22)00625–4. doi: 10.1053/j.gastro.2022.06.020. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 104.Carbone F, Van den Houte K, Besard L, et al. Diet or medication in primary care patients with IBS: the DOMINO study - a randomised trial supported by the Belgian Health Care Knowledge Centre (KCE Trials Programme) and the Rome Foundation Research Institute. Gut 2022. Apr 28;gutjnl-2021-325821. doi: 10.1136/gutjnl-2021-325821. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Camilleri M, Dilmaghani S. Invited Editorial: Treatment of IBS using FMT: a step forward? Gastroenterology 2022. Jul 6:S0016-5085(22)00750–8. doi: 10.1053/j.gastro.2022.06.087. Online ahead of print. [DOI] [Google Scholar]
- 106.Camilleri M Invited Letter: 50-point IBS-SSS responders but persistence of moderate severity IBS in over 40% of those on diet. Gut 2022. Jul 26:gutjnl-2022-328211. doi: 10.1136/gutjnl-2022-328211. Online ahead of print. [DOI] [PubMed] [Google Scholar]