Abstract
Introduction
Effective, brief, low-cost interventions for suicide attempt survivors are essential to saving lives and achieving the goals of the National Strategy for Suicide Prevention and Zero Suicide. This study aims to examine the effectiveness of the Attempted Suicide Short Intervention Program (ASSIP) in averting suicide reattempts in the United States healthcare system, its psychological mechanisms as predicted by the Interpersonal Theory of Suicide, and the potential implementation costs, barriers and facilitators for delivering it.
Methods and analysis
This study is a hybrid type 1 effectiveness–implementation randomised controlled trial (RCT). ASSIP is delivered at three outpatient mental healthcare clinics in New York State. Participant referral sites include three local hospitals with inpatient and comprehensive psychiatric emergency services, and outpatient mental health clinics. Participants include 400 adults who have had a recent suicide attempt. All are randomised to ‘Zero Suicide-Usual Care plus ASSIP’ or ‘Zero Suicide-Usual Care’. Randomisation is stratified by sex and whether the index attempt is a first suicide attempt or not. Participants complete assessments at baseline, 6 weeks, and 3, 6, 12 and, 18 months. The primary outcome is the time from randomisation to the first suicide reattempt. Prior to the RCT, a 23-person open trial took place, in which 13 participants received ‘Zero Suicide-Usual Care plus ASSIP’ and 14 completed the first follow-up time point.
Ethics and dissemination
This study is overseen by the University of Rochester, with single Institutional Review Board (#3353) reliance agreements from Nathan Kline Institute (#1561697) and SUNY Upstate Medical University (#1647538). It has an established Data and Safety Monitoring Board. Results will be published in peer-reviewed academic journals, presented at scientific conferences, and communicated to referral organisations. Clinics considering ASSIP may use a stakeholder report generated by this study, including incremental cost-effectiveness data from the provider point of view.
Trial registration number
Keywords: PSYCHIATRY, Suicide & self-harm, Adult psychiatry
Strengths and limitations of this study.
This study is a multisite, single-blind, hybrid type 1 effectiveness–implementation randomised controlled trial design.
The conception and design of this study was developed in consultation with community stakeholder groups and a patient advocate with lived experience of recovery from suicide attempts.
The inclusion criteria for participants with suicide attempts are broad to ensure the findings are relevant to real-world settings, as exclusions for co-occurring disorders and other health conditions tend to reduce adoption.
The Attempted Suicide Short Intervention Program is delivered in-person and via telehealth to increase participant engagement and to ensure the findings are generalisable to real-world settings that offer both treatment modalities.
The primary limitation of this study is the heterogeneity of the ‘Zero Suicide-Usual Care’ condition, and, thus, the difficulty in assessing the treatment received by participants in this condition.
Introduction
Suicide is a major public health problem,1 and the 10th leading cause of adult death in the United States (US).2 Approximately 1.3 million adults attempt suicide in the US annually,3 as do millions more worldwide. In 2015, approximately 854,000 US adults received medical attention for a suicide attempt, and 571,000 stayed overnight or longer in a hospital.4 Each attempt increases risk of future attempts and death,5 6 with an estimated 3.4%–8.6% of attempt survivors subsequently dying by suicide.7 8
Few evidence-based interventions exist for suicidal behaviour, and most take 3–12 months.9–11 The Attempted Suicide Short Intervention Program (ASSIP) is a brief manualised three-session intervention that can be used alongside usual care to reduce the risk of suicidal behaviour.12 In the first efficacy trial of ASSIP, the intervention group had 80% fewer reattempts and an average 72% fewer hospital days over 24 months compared with treatment as usual.13 14 Such a large effect on suicide attempts and hospital utilisation is unprecedented for a brief intervention. However, real-world effectiveness, cost-effectiveness, and the mechanisms of ASSIP have not yet been examined.
ASSIP’s effectiveness may result from its impact on belongingness and burdensomeness, constructs posited by the Interpersonal Theory of Suicide as central to suicide risk.15 16 ASSIP uses a novel, video-based inquiry to help the individual understand their attempt, experience connectedness and their value to others, and formulate steps for safety and recovery, leading to a collaboratively developed, individualised, written case formulation and prevention plan. By eliciting the narrative story and exploring personal vulnerabilities, ASSIP provides a poignant therapeutic experience of being understood and valued and gives insight into needs for connection, contribution, and meaning. This study tests hypotheses suggesting that the mechanisms for ASSIP’s effectiveness include reducing perceptions of burdening others and low belonging.
The study builds effectiveness research into a federally funded Zero Suicide (ZS) grant to New York State (NYS). ZS is a systems approach to suicide prevention whereby health systems assess their practices to identify gaps in service delivery.17 In 2017, the NYS Office of Mental Health (OMH) launched a post-attempt treatment service as part of their ZS strategy that included the first public sector implementation of ASSIP in the US. NYS-OMH invested in clinical training, personnel, and cross-system leadership to support referrals to the service. With support from NYS-OMH, we assembled an investigator team to conduct effectiveness research on this promising intervention. The current study is responsive to Notice of Interest MH-17-031, which requested applications that leverage partnerships with state systems and ZS Substance Abuse and Mental Health Services Administration grants.18
We describe the protocol as a multisite, single-blind, hybrid type 1 effectiveness–implementation randomised controlled trial (RCT) of ASSIP.19 20 This study aims to (1) determine the effectiveness of ASSIP, (2) examine potential mechanisms predicted by the Interpersonal Theory of Suicide, (3) explore cost-effectiveness, and (4) evaluate implementation barriers and facilitators.
Methods and analysis
Study design
Participants (N=400) are randomised to receive ZS-Usual Care (UC) plus ASSIP (treatment) or UC only (control). UC comprises a heterogeneous variety of mental health services, many implementing ZS quality improvement protocols. No one in the study receives less than the care typically offered for suicidal behaviour. Those receiving ASSIP receive the therapy in person or via telehealth, following normal practice in participating clinical services, with delivery method based on participant preference, COVID-19 safety requirements and telehealth availability.
Assessments are conducted in person and remotely at baseline and remotely at 6 weeks and 3, 6, 12, and 18 months. The primary outcome variable is time from randomisation to first suicide reattempt. We hypothesise that those assigned to UC plus ASSIP will have lower risk of repeated attempt across 18 months compared with UC only. This study is overseen by the University of Rochester’s Institutional Review Board (IRB) and registered at ClinicalTrials.gov: NCT03894462. The community-based mental health clinics delivering ASSIP ceded IRB review to the University of Rochester, per revised Common Rule regulations. In addition, this study follows Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) reporting guidelines.21
Recruitment sites
ASSIP referrals come from Upstate University Hospital, Syracuse; St. Joseph’s Health Hospital, Syracuse; University of Rochester Medical Center, Rochester; and local outpatient mental health clinics. Most outpatient clinics in both communities have participated in the NYS-OMH ZS project. Clinical directors and unit leaders from each site provide permission to enroll on-site prior to study initiation.
Clinical sites
ASSIP is delivered at Upstate University Hospital, Syracuse; Hutchings Psychiatric Center, Syracuse; and Strong Behavioral Health, Rochester by licensed therapists trained in ASSIP through the ZS project. Therapists are not considered research staff, but provide ASSIP as an outpatient, postattempt clinical service. Telehealth delivery is provided via each site’s Health Insurance Portability and Accountability Act (HIPAA)-compliant video conference platform.
Participants
Potentially eligible individuals are identified by consulting their medical record and treatment team. They are approached by someone involved in their care and provided with information about the study. Those who consent are given a full research assessment battery and compensation for their time before being randomly assigned. Participants may grant permission to share a templated brief report from the battery with their outpatient provider.
Inclusion criteria: (1) 18+ years, (2) suicide attempt within past 60 days, defined by self-report of suicide attempt with intent to die using a validated item,22 and (3) ability and willingness to provide permission to contact at least one person if needed to promote participant safety or to reach the participant for follow-up. Exclusion criteria: (1) psychotic symptoms or other factors that preclude ability to consent or complete baseline assessment, (2) inability to communicate in English, and (3) residence outside NYS during the time period the participant is eligible to receive ASSIP.
Assessments
Table 1 shows the research assessment battery. All scales selected for this study are validated measures with established psychometric properties. To determine effectiveness, our primary measure is time to first suicide reattempt. This information is collected via clinical interview on the Columbia Suicide Severity Rating Scale23 with an added procedure for determining the date of the attempt24 or via electronic medical records if participants cannot be reached. The Interpersonal Needs Questionnaire is used to assess potential mediators: thwarted belongingness and perceived burdensomeness.25
Table 1.
Data collection measures
| Domain | Type | Measure/description | Purpose | Schedule |
| Demographics | 12 items | Sex, gender identity, sexuality, ethnicity, race, education level, marital status, employment status, living situation, veteran status, federal assistance use and income | Descriptive | Baseline |
| Suicide attempt | Interview | Columbia Suicide Severity Rating Scale 36 |
Primary outcome | Baseline, follow-ups |
| Suicidal ideation | 4 items | Depressive Symptom Inventory: suicidality subscale37 | Covariate | Baseline, follow-ups |
| Global health | 10 items | Patient-Reported Outcomes Measurement Information System (PROMIS)—Global Health38 | Descriptive | Baseline, follow-ups |
| Mental health treatment | 5 items | National Survey on Drug Use and Health field Interview39 | Characterise care | Baseline, follow-ups |
| Substance use | 12 items | Alcohol and Substance Use Form40 and Alcohol, Smoking and Substance Involvement Screening41 | Descriptive | Baseline, follow-ups |
| Thwarted belonging and burdensomeness | 9 items | Interpersonal Needs Questionnaire25 | Mediator | Baseline, follow-ups |
| Depression | 8 items | PROMIS-Computer Adapted Tests for Depression42 | Covariate | Baseline, follow-ups |
| Social isolation | 11 items | Berkman-Syme Social Network Index43 | Covariate | Baseline, follow-ups |
| Meaning making of stress | 6 items | Integration of Stressful Life Experiences Scale—Short Scale44 | Moderator | Baseline, follow-ups |
| Self-compassion | 10 items | Self-Compassion Scale: self-kindness and judgement subscales45 | Moderator | Baseline, follow-ups |
| Self-entrapment | 4 items | Short Defeat and Entrapment Scale: entrapment subscale46 | Mediator | Baseline, follow-ups |
| Sleep | 12 items | Insomnia Severity Index47 and Behavioral Risk Factor Surveillance System48 | Covariate | Baseline, follow-ups |
| COVID-19 | 6 items | Stressors in relation to the global pandemic | Covariate | Baseline, follow-ups |
| ASSIP certification and equipment cost | Cost | NYS-OMH expenditures for providing ASSIP certification training | Cost-effectiveness | Preimplementation |
| Time preparing for and delivering ASSIP | Cost | Time spent per week on training, delivering, prepping, and post-ASSIP tasks | Cost-effectiveness | Preimplementation, baseline and follow-ups |
| Ongoing ASSIP case supervision | Cost | Time spent by supervisors providing ASSIP-specific supervision | Cost-effectiveness | Follow-ups |
ASSIP, Attempted Suicide Short Intervention Program; NYS, New York State; OMH, Office of Mental Health.
Incremental costs are captured through NYS-OMH accounting of their ZS budget expenditures on training and supervision for ASSIP therapists at clinic launch and on-boarding of new therapists, equipment costs, and dedicated administrative and supervisory full time equivalent beyond normal outpatient operations. Therapists and supervisors log weekly hours for study-related activities. Supervisors report time spent facilitating ASSIP referrals, providing ASSIP-specific case supervision and marketing the clinical service in the community.
An exploratory step uses electronic medical records from local hospitals to measure 18 months of psychiatric hospital and emergency department use following the index suicide attempt. Record requests include type of visit, length of stay, International Classification of Diseases - Tenth Revision (ICD-10) diagnostic and procedure codes, medications, diagnosis-related group, and physician discharge summary. We code any visit with deliberate self-harm as ‘suicide related’ and examine these in our analyses.
We conduct a qualitative evaluation of how ASSIP can be adapted for optimal real-world uptake, and of implementation barriers and facilitators. The evaluation focuses on the clinics where ASSIP is being tested and three additional clinics not implementing ASSIP (identified with help from NYS-OMH). Key informant interviews are conducted with clinic staff and licensed therapists trained in ASSIP. Semistructured interview guides are used to elicit information specific to provider and staff type (i.e., administrators, ASSIP therapists) as well as common core questions about what helped and hindered implementation, and recommendations for future implementation.26 Interviews are audio recorded and transcribed. Data are content analysed with line-by-line analysis leading to the development and refinement of themes.
Randomisation
Randomisation is stratified by sex (male/female)4 and prior suicide attempt (yes/no).27 28 The randomisation plan includes blocking to ensure that an equal number of participants are assigned to each treatment group in a particular stratum after a certain number of participants have been enrolled in that stratum (block size). Study staff follow a scripted procedure for instructing participants about next steps in the study and in their care.
Blinding
The participants, therapists, fidelity raters, and study staff who conduct the baseline assessment are not blind to treatment allocation. To prevent bias during follow-up assessments, study staff do not conduct baseline assessment and follow-up assessments with the same participant. Additionally, participants are repeatedly instructed to avoid discussing their random assignment with study staff during follow-up.
Intervention
Participants assigned to UC plus ASSIP receive ASSIP therapy and are then referred back to their existing provider or to a new mental health provider if they do not have one. ASSIP is a manualised,12 three-session intervention using video-based inquiry with established interpersonal strategies to (a) create a therapeutic experience of being understood, valued, and connected, (b) stimulate insight into the unmet needs and thwarted goals that led to the attempt, and (c) create plans and promote hope for meeting these needs in the future. In session 1, the therapist empathically guides the participant in telling the story of their attempt, which is video recorded. In session 2, the therapist and participant sit side by side to view selections from the video, working together to understand the feelings and events that preceded the attempt. Special attention is given to uncovering the point(s) at which the ‘suicidal mind’ took over (i.e., when they acted on false or distorted beliefs about their connections and value to others). The participant is assigned a homework task that invites them to learn more about these distortions and how to avoid them. In session 3, the therapist shares a written first-person narrative ‘formulation’ of the participant’s story. Together they adjust this formulation, then develop a plan for addressing short-term safety and the longer-term drivers of suicide ideation and behaviour. For 2 years following treatment, the participant receives a Caring Contact29–31 letter from the therapist every 3–6 months. In response to the COVID-19 pandemic, therapists and participants wear personal protective equipment during in-person sessions and ASSIP is delivered via telehealth when needed.
Control
Participants assigned to UC continue with their existing provider or are referred to a new provider if they do not have one. UC includes a variety of mental health services in the regions where the study is conducted, most of which are implementing ZS quality improvement protocols. Per ZS, those who do not engage in care typically experience enhanced transition and follow-up contact post discharge. Describing UC is important in this real-world study because participants in both study conditions are free to pursue a variety of treatment options or none at all.
Fidelity
Fidelity is assessed through independent review of a randomly selected sample (20%) of cases by an investigator who is a certified ASSIP therapist and trainer. Ratings are based on a recent ASSIP therapist fidelity measure and coding manual.32 Informed by prior work in therapist fidelity measurement,33 both ‘adherence’ (compliance with technical procedures) and ‘competence’ (therapeutic skill) are assessed. Fidelity scores for each are derived by dividing the obtained score by the maximum possible score to generate a percentage. Scores are generated for: narrative interview, video playback, homework review, case formulation and prevention plan, and caring letters.12 The scores are provided to study therapists and integrated into ongoing supervision.
Patient and public involvement
The conception and design of this study was developed in consultation with community stakeholder groups. The study team also includes a patient advocate and advisor with lived experience of recovery from suicide attempts. Once the study is complete, a stakeholder summary report will be written and disseminated to organisations and localities considering implementing ASSIP as part of their ZS strategy.
Safety procedures
ASSIP is provided by licensed mental health professionals following routine clinic protocols for in-person, over the phone, or video conference crises. Therapists are accustomed to treating high-risk individuals. Study staff followed an IRB-approved risk management protocol and received supervision from investigators on all clinical interviews.
Given the nature of this study, suicide attempts and psychiatric hospitalisations are expected to occur and to be non-study related in most cases. As such, these serious adverse events (SAEs) are reported in summary reports to the Data and Safety Monitoring Board (DSMB), with annual reports to the National Institute of Mental Health (NIMH) and IRB. Suicide deaths are reported to the DSMB within 48 hours of discovery, with investigators’ judgments as to whether a relationship to the study can be ruled out. The DSMB members either concur or request additional investigation as an SAE. Suicide deaths are reported to the IRB and NIMH within 5 business days of discovery with the investigators’ and DSMB’s determination regarding whether a relationship to the study can/cannot be ruled out and any recommendations made by the DSMB.
After reviewing the circumstances and consulting with coinvestigators, a designated unblinded coinvestigator can reclassify an adverse event as an SAE. SAEs deemed unexpected and study related are reported to the IRB and NIMH within 5–7 working days of discovery. If considered related to the study, unanticipated adverse events involving risks to participants or others are reported by the principal investigator and/or DSMB to the IRB, and the IRB promptly informs the NIMH. The DSMB, IRB, and NIMH can recommend corrective actions to be taken by the investigators.
Sample size considerations
The primary outcome variable is time from randomisation to first suicide reattempt. Under the assumption of proportional hazards, the sample size for a time-to-event outcome depends on the anticipated incidence of the event in the control group, the treatment effect to be detected (or the anticipated incidence in the ASSIP group), assumed rates of participant withdrawal, and the specified significance level and power. Assuming a sample size of 400, table 2 identifies treatment effects that can be detected with 80%–90% power using a log-rank test and a two-tailed 5% significance level for varying 18-month incidence of suicide reattempt. The detectable treatment effects on suicide attempts thus span those observed in the two most relevant trials: Brown and colleagues'9 study of cognitive–behavioural therapy for suicide prevention (42% vs 24%) and Gysin-Maillart and colleagues'13 study of ASSIP (27% vs 8%).
Table 2.
Power analysis for aim 1
| Power | Control group incidence | ASSIP group incidence | HR |
| 80% | 20.0% | 9.3% | 0.44 |
| 25.0% | 13.2% | 0.49 | |
| 30.0% | 17.2% | 0.53 | |
| 35.0% | 21.5% | 0.56 | |
| 40.0% | 25.9% | 0.59 | |
| 90% | 20.0% | 7.9% | 0.37 |
| 25.0% | 11.5% | 0.42 | |
| 30.0% | 15.4% | 0.47 | |
| 35.0% | 19.5% | 0.50 | |
| 40.0% | 23.8% | 0.53 |
Treatment effects detectable with 80%–90% power assuming different 18-month suicide attempt incidence rates in control group (N=400 participants).
Primary statistical model
Statistical analysis of the primary outcome variable will involve fitting a Cox proportional hazards regression model with treatment group as the independent variable and prior suicide attempt and sex as covariates. This model will determine a 95% CI for the adjusted HR for the treatment group comparison; a likelihood ratio test will be performed for the significance of the HR. A Kaplan-Meier curve will describe cumulative probability of suicide reattempt over time in each treatment group. For participants who do not have a suicide reattempt, event times will be censored at the last participant contact.
Mediation analyses
The hypothesised mediators (perceptions of low belonging and burdening others) will be measured throughout follow-up (6 weeks, 3, 6, 12, and 18 months). The number of observed mediators per participant depends on time to first suicide reattempt. We will leverage a survival mediational g-formula function to estimate direct and indirect effects,34 35 specifying parametric regression models for the time-varying mediators and estimating model parameters using maximum likelihood. The Cox proportional hazards regression model for the primary outcome will include treatment group, prior suicide attempt, referral source, and sex as baseline covariates, and the mediators as time-varying covariates. Parameter estimates will be added into the survival mediational g-formula, providing estimates of the target parameters.
Cost-effectiveness analysis
Our cost-effectiveness analysis takes the perspective of a behavioural health organisation currently adopting the ZS model and considering how to invest resources toward evidence-based treatment. We aim to ascertain the cost per suicide attempt averted, defined as the difference in number of suicide attempts in the control group and treatment groups during the 18 months post randomisation.
Intervention costs include (1) NYS expenditures on therapist training and certification, (2) cost of time spent by therapists training in, preparing to deliver, and delivering ASSIP and (3) cost of time spent on ASSIP-specific case supervision. To calculate the value of personnel time, total time spent will be multiplied by position-specific salaries from the US Bureau of Labor Statistics, inflated to reflect employee benefits. We will apply Center for Medicare & Medicaid Services hospital accounting data to derive emergency department and inpatient hospital costs. We will then ascertain whether the costs of providing ASSIP plus estimated costs associated with the change in subsequent emergency department and hospital inpatient use will be less than the costs associated with subsequent emergency department and hospital inpatient use for UC during the 18-month follow-up.
To assess cost-effectiveness, we will combine the cost data with number of suicide attempts averted. If ASSIP is less costly and more effective, then ASSIP will be considered dominant and more cost-effective. If ASSIP does not result in the same or better outcomes at a lower cost, then the trade-off between cost and effectiveness will be calculated via an incremental cost-effectiveness ratio.
Ethics and dissemination
To maximise efficiency and consistency across participating institutions, we used a single IRB (University of Rochester) to streamline review and approval. Whenever possible, we used the Streamlined, Multisite, Accelerated Resources for Trials IRB Reliance platform (SMART IRB), a national Reliance Agreement platform, to document IRB approval among the participating sites (Nathan Kline Institute and SUNY Upstate Medical University). All investigators and research staff attended a course in Human Subjects Protection and Good Clinical Practice.
Results will be published in peer-reviewed academic journals, presented at scientific conferences and communicated to referral organisations. Clinics considering implementing ASSIP as part of their ZS strategy will receive a stakeholder report including incremental cost-effectiveness data.
Trial status
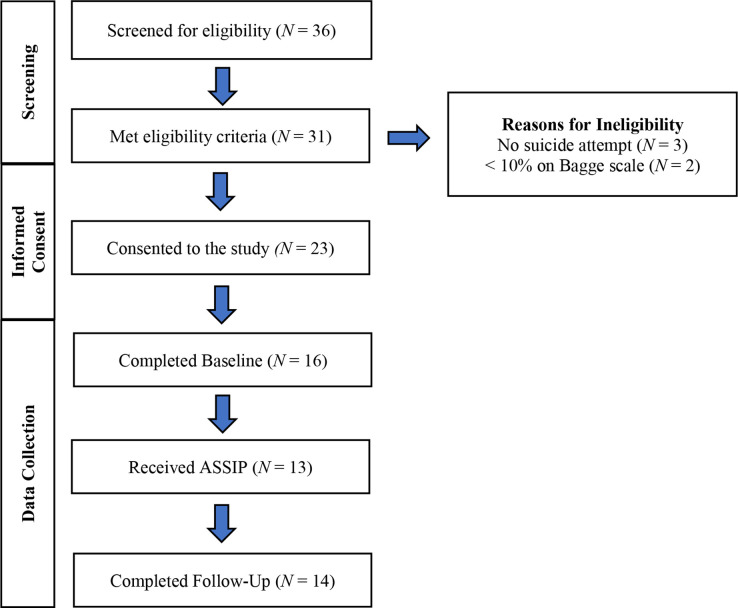
We conducted an open trial (1 October to 28 March 2021) before the RCT to ensure well-functioning recruitment procedures and data systems. Open-trial participants entered the UC plus ASSIP condition and were followed for 6 weeks. Figure 1 outlines our process for screening referrals using the procedures above. We met our enrolment target with 23 participants across all sites. Our sample’s mean age was 38 (SD=17); 70% were female and 30% male; 74% identified as Caucasian, 4% African American, 4% Asian, 13% more than one race, and 4% declined to state. Overall, 16 participants completed the baseline visit and 13 attended at least one ASSIP session. Recruitment for the RCT began on 29 March 2021. An interim analysis for futility is planned once 50% of the expected suicide reattempt events have occurred.
Figure 1.
Open-trial recruitment and enrolment flow diagram.
Effective, brief, low-cost interventions for individuals who attempt suicide are needed to save lives. This study tests the effectiveness of a highly promising new treatment and examines how it works. If the hypotheses are supported, our study will provide evidence of a brief, practical and novel therapy that reduces suicide reattempts in real-world healthcare settings. It will also provide insight into how the intervention works and pave the way for research examining strategies for spreading implementation to other sites. Cost-effectiveness data will provide states and health systems with information to direct ZS investments and will provide a basis for future research on cost-effectiveness from a societal perspective.
Supplementary Material
Acknowledgments
The authors wish to acknowledge Edmund Pizzarello for his assistance with open-trial data collection and Paul Scade for his assistance with preparing this paper. The views expressed in this paper are those of the authors and do not necessarily reflect the position or policy of the United States Department of VA, Veterans Health Administration (VHA) or the United States Government.
Footnotes
Twitter: @SJLandes
Contributors: Concept and design: AP, KC, KVO, NJ, SJL, GC, MM, AE, SR, JC, KM and DG. Acquisition, analysis or interpretation of data: All authors. Drafting of the manuscript: AP, KC, KVO and CK. Critical revision of the manuscript for important intellectual content: All authors. Statistical advice: NJ, GC, SJL, AE and MM. Administrative, technical or material support: CK, SR, JC, KM and DG. Final Approval: All authors.
Funding: This study was supported by the National Institute of Mental Health (NIMH) grant number (R01MH119264). NIMH had no role in the design of the study and will have no role in the implementation of the study, analysis of the data or writing of the manuscript.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods and analysis section for further details.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.US Surgeon, National Action Alliance for Suicide Prevention (US) . 2012 national strategy for suicide prevention: goals and objectives for action. 2012. [PubMed]
- 1.Centers for Disease Control-National Center for Injury Prevention and Control . Web-based injury statistics query and reporting system (WISQARS); 2021. Available: https://www.cdc.gov/injury/wisqars/ [Accessed Dec 2021].
- 3.Han B, Kott PS, Hughes A, et al. Estimating the rates of deaths by suicide among adults who attempt suicide in the United States. J Psychiatr Res 2016;77:125–33. 10.1016/j.jpsychires.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 4.Piscopo K, Lipari R, Cooney J, et al. Suicidal thoughts and behavior among adults: results from the 2015 national survey on drug use and health. NSDUH Data Rev; 2016. Available: https://www.samhsa.gov/data/
- 5.Olfson M, Wall M, Wang S, et al. Suicide following deliberate self-harm. Am J Psychiatry 2017. 10.1176/appi.ajp.2017.16111288 [DOI] [PubMed] [Google Scholar]
- 6.Bergen H, Hawton K, Waters K, et al. Premature death after self-harm: a multicentre cohort study. Lancet 2012;380:1568–74. 10.1016/S0140-6736(12)61141-6 [DOI] [PubMed] [Google Scholar]
- 7.Bostwick JM, Pankratz VS. Affective disorders and suicide risk: a reexamination. Am J Psychiatry 2000;157:1925–32. 10.1176/appi.ajp.157.12.1925 [DOI] [PubMed] [Google Scholar]
- 8.Bostwick JM, Pabbati C, Geske JR, et al. Suicide attempt as a risk factor for completed suicide: even more lethal than we knew. Am J Psychiatry 2016;173:1094–100. 10.1176/appi.ajp.2016.15070854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown GK, Ten Have T, Henriques GR, et al. Cognitive therapy for the prevention of suicide attempts. JAMA 2005;294:563. 10.1001/jama.294.5.563 [DOI] [PubMed] [Google Scholar]
- 10.Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. Guilford Press, 1993. [Google Scholar]
- 11.Jobes DA. The collaborative assessment and management of suicidality (CAMS): an evolving evidence-based clinical approach to suicidal risk. Suicide Life Threat Behav 2012;42:640–53. 10.1111/j.1943-278X.2012.00119.x [DOI] [PubMed] [Google Scholar]
- 12.Michel K, Gysin-Maillart A. ASSIP – attempted suicide short intervention program: A manual for clinicians. Hogrefe Publishing, 2015. [Google Scholar]
- 13.Gysin-Maillart A, Schwab S, Soravia L, et al. A novel brief therapy for patients who attempt suicide: a 24-months follow-up randomized controlled study of the attempted suicide short intervention program (ASSIP). PLoS Med 2016;13:e1001968. 10.1371/journal.pmed.1001968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naudet F, Sakarovitch C, Janiaud P, et al. Data sharing and reanalysis of randomized controlled trials in leading biomedical journals with a full data sharing policy: survey of studies published in the BMJ and PLOS medicine BMJ 2018;360:k400. 10.1136/bmj.k400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Orden KA, Witte TK, Cukrowicz KC, et al. The interpersonal theory of suicide. Psychological Review 2010;117:575–600. 10.1037/a0018697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu C, Buchman-Schmitt JM, Stanley IH, et al. The interpersonal theory of suicide: a systematic review and meta-analysis of a decade of cross-national research. Psychol Bull 2017;143:1313–45. 10.1037/bul0000123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brodsky BS, Spruch-Feiner A, Stanley B. The zero suicide model: applying evidence-based suicide prevention practices to clinical care. Front Psychiatry 2018;9:33. 10.3389/fpsyt.2018.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NIMH . Notice of interest to highlight high-priority time-sensitive research opportunities toward zero suicide healthcare systems. Available: https://grants.nih.gov/grants/guide/notice-files/NOT-MH-17-031.html [Accessed 24 May 2018].
- 19.Curran GM, Bauer M, Mittman B, et al. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012;50:217–26. 10.1097/MLR.0b013e3182408812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landes SJ, McBain SA, Curran GM. An introduction to effectiveness-implementation hybrid designs. Psychiatry Res 2019;280:112513. 10.1016/j.psychres.2019.112513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. Spirit 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bagge CL, Glenn CR, Lee H-J. Quantifying the impact of recent negative life events on suicide attempts. J Abnorm Psychol 2013;122:359–68. 10.1037/a0030371 [DOI] [PubMed] [Google Scholar]
- 23.Posner K, Brown GK, Stanley B, et al. The columbia-suicide severity rating scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 2011;168:1266–77. 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conner KR, Kearns JC, Esposito EC, et al. Pilot RCT of the attempted suicide short intervention program (ASSIP) adapted for rapid delivery during hospitalization to adult suicide attempt patients with substance use problems. Gen Hosp Psychiatry 2021;72:66–72. 10.1016/j.genhosppsych.2021.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Orden KA, Cukrowicz KC, Witte TK, et al. Thwarted belongingness and perceived burdensomeness: construct validity and psychometric properties of the interpersonal needs questionnaire. Psychol Assess 2012;24:197–215. 10.1037/a0025358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landes SJ, Jegley SM, Kirchner JE, et al. Adapting caring contacts for veterans in a department of veterans affairs emergency department: results from a type 2 hybrid effectiveness-implementation pilot study. Front Psychiatry 2021;12:746805. 10.3389/fpsyt.2021.746805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forman EM, Berk MS, Henriques GR, et al. History of multiple suicide attempts as a behavioral marker of severe psychopathology. Am J Psychiatry 2004;161:437–43. 10.1176/appi.ajp.161.3.437 [DOI] [PubMed] [Google Scholar]
- 28.Miranda R, Scott M, Hicks R, et al. Suicide attempt characteristics, diagnoses, and future attempts: comparing multiple attempters to single attempters and ideators. J Am Acad Child Adolesc Psychiatry 2008;47:32–40. 10.1097/chi.0b013e31815a56cb [DOI] [PubMed] [Google Scholar]
- 29.Motto JA. Suicide prevention for high-risk persons who refuse treatment. Suicide Life Threat Behav 1976;6:223–30. 10.1111/j.1943-278X.1976.tb00880.x [DOI] [PubMed] [Google Scholar]
- 30.Motto JA, Bostrom AG. A randomized controlled trial of postcrisis suicide prevention. Psychiatr Serv 2001;52:828–33. 10.1176/appi.ps.52.6.828 [DOI] [PubMed] [Google Scholar]
- 31.Luxton DD, June JD, Comtois KA. Can postdischarge follow-up contacts prevent suicide and suicidal behavior? A review of the evidence. Crisis 2013;34:32–41. 10.1027/0227-5910/a000158 [DOI] [PubMed] [Google Scholar]
- 32.Gysin-Maillart A, Conner K, Pisani A, et al. ASSIP: coding manual adherence and competence scale (ACS) [Unpublished manuscript]. University Hospital of Psychiatry, University of Bern, 2021. [Google Scholar]
- 33.Shaw BF, Elkin I, Yamaguchi J, et al. Therapist competence ratings in relation to clinical outcome in cognitive therapy of depression. J Consult Clin Psychol 1999;67:837–46. 10.1037//0022-006x.67.6.837 [DOI] [PubMed] [Google Scholar]
- 34.Lin SH, Young JG, Logan R, et al. Mediation analysis for a survival outcome with time-varying exposures, mediators, and confounders. Stat Med 2017;36:4153–66. 10.1002/sim.7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng W, van der Laan M. Longitudinal mediation analysis with time-varying mediators and exposures, with application to survival outcomes. J Causal Inference 2017;5:20160006. 10.1515/jci-2016-0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Posner K, Brown GK, Stanley B, et al. The Columbia-suicide severity rating scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 2011;168:1266–77. 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Glischinski M, Teismann T, Prinz S, et al. Depressive symptom inventory suicidality subscale: optimal cut points for clinical and non-clinical samples. Clin Psychol Psychother 2016;23:543–9. 10.1002/cpp.2007 [DOI] [PubMed] [Google Scholar]
- 38.Cella D, Riley W, Stone A, et al. The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol 2010;63:1179–94. 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Center for Behavioral Health Staitistics and Quality . 2018 national survey on drug use and health (NSDUH): CAI specifications for programming (english version. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2017. [Google Scholar]
- 40.Tonigan JS, Miller WR, Brown JM. The reliability of form 90: an instrument for assessing alcohol treatment outcome. J Stud Alcohol 1997;58:358–64. 10.15288/jsa.1997.58.358 [DOI] [PubMed] [Google Scholar]
- 41.Group WAW. The alcohol, smoking and substance involvement screening test (assist): development, reliability and feasibility. Addiction 2002;97:1183–94. 10.1046/j.1360-0443.2002.00185.x [DOI] [PubMed] [Google Scholar]
- 42.Pilkonis PA, Choi SW, Reise SP, et al. Item banks for measuring emotional distress from the patient-reported outcomes measurement information system (PROMIS®): depression, anxiety, and anger. Assessment 2011;18:263–83. 10.1177/1073191111411667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of alameda County residents. Am J Epidemiol 1979;109:186–204. 10.1093/oxfordjournals.aje.a112674 [DOI] [PubMed] [Google Scholar]
- 44.Holland JM, Currier JM, Neimeyer RA. Validation of the integration of stressful life experiences scale-short form in a bereaved sample. Death Stud 2014;38:234–8. 10.1080/07481187.2013.829369 [DOI] [PubMed] [Google Scholar]
- 45.Neff KD. The development and validation of a scale to measure self-compassion. Self and Identity 2003;2:223–50. 10.1080/15298860309027 [DOI] [Google Scholar]
- 46.Griffiths AW, Wood AM, Maltby J, et al. The development of the short defeat and entrapment scale (SDES). Psychol Assess 2015;27:1182–94. 10.1037/pas0000110 [DOI] [PubMed] [Google Scholar]
- 47.Nelson DE, Holtzman D, Bolen J, et al. Reliability and validity of measures from the behavioral risk factor surveillance system (BRFSS). Soz Praventivmed 2001;46 Suppl 1:S3–42. [PubMed] [Google Scholar]
- 48.Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med 2001;2:297–307. 10.1016/s1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.