Abstract
Effective treatments for smoking cessation exist but are underused. Proactive chronic care approaches may enhance the reach of cessation treatment and reduce the prevalence of smoking in healthcare systems. This pragmatic study evaluated a population-based Comprehensive Tobacco Intervention Program (CTIP) implemented in all (6) adult primary care clinics in a Madison, Wisconsin, USA healthcare cooperative, assessing treatment reach, reach equity, and effectiveness in promoting smoking cessation. CTIP launched in 3 waves of 2 clinics each in a multiple baseline design. Electronic health record (EHR) tools facilitated clinician-delivered pharmacotherapy and counseling; guiding tobacco care managers in phone outreach to all patients who smoke; and prompting multimethod bulk outreach to all patients on a smoking registry using an opt-out approach. EHR data were analyzed to assess CTIP reach and effectiveness among 6894 adult patients between January 2018 and February 2020. Cessation treatment reach increased significantly after CTIP launch in 5 of 6 clinics and was significantly higher when clinics were active vs. inactive in CTIP [Odds Ratio (OR) range=2.0–3.0]. Rates of converting from current to former smoking status were also higher in active vs. inactive clinics (OR range=2.2–10.5). Telephone treatment reach was particularly high in historically underserved groups, including African-American, Hispanic, and Medicaid-eligible patients. Implementation of a comprehensive, opt-out, chronic-care program aimed at all patients who smoke was associated with increases in the rates of pharmacotherapy and counseling delivery and quitting smoking. Proactive outreach may help reduce disparities in treatment access.
Keywords: smoking cessation, electronic health record, opt-out, proactive outreach, system change, primary care
Introduction1
Primary care offers a favorable context for intervening with patients who smoke [1–4]. However, it has proven difficult to engineer effective smoking interventions well-suited for broad dissemination in real-world settings. Approaches that rely upon direct intervention by primary care clinicians have yielded disappointing results [5–8] and highlight challenges to delivering comprehensive treatment (i.e., both counseling and pharmacotherapy) in primary care, including clinician time pressure and lack of awareness of cessation resources [9–10]. These barriers have led to ask-advise-refer/connect approaches that shift the task of providing cessation treatment to external services, such as tobacco quitlines [11]. Unfortunately, results show little use of such referrals by clinic staff [12–14] and low rates (<30%) of enrollment in quitline services among referred patients [15–17].
Electronic health record (EHR) tools can prompt primary care teams to assess smoking status and refer patients to treatment resources. This approach has enhanced identification of smoking and treatment [18–21]. Additionally, closed-loop EHR-enabled referral (‘eReferral’ [12]), methods that inform clinicians of referral outcomes increase quitline referral engagement [22]. A recent cluster-randomized controlled trial of quitline eReferral showed that patient acceptance of quitline calls increased about 4-fold versus fax referral [13]. Electronic referral strategies appear to be especially effective in engaging traditionally underserved populations in smoking treatment, such as African-American patients and those eligible for Medicaid [23]. However, engagement rates remain fairly low: only about 4–5% of patients who smoke receive quitline treatment, even when 18–20% accept eReferral [13,20].
The current study evaluated the reach and effectiveness of a comprehensive tobacco intervention program (CTIP) designed to address gaps in treatment access in primary care. CTIP incorporates features of prior EHR-based systems (e.g., EHR-prompted smoking status assessment, advice, treatment offer, medication orders, and closed-loop eReferral to cessation resources [12–13]), and added features designed to address limitations of past strategies. These included 1) an opt-out approach [24] to increase the rate of smoking cessation treatment acceptance; 2) multiple smoking cessation treatment options to increase treatment acceptance; 3) a smoking registry to guide multimedia outreach to increase smoking treatment reach; 4) yearly or more frequent targeted phone outreach to all patients who smoke, conducted by healthcare system tobacco cessation outreach specialists (TCOS); 5) post target quit day (TQD) outreach to re-engage those who relapsed; 6) TCOS coordination of smoking care with primary care clinicians to increase clinician engagement in smoking interventions; and 7) phone-based TCOS-delivered smoking intervention to reduce attrition caused by patients’ failing to accept out-of-state tobacco quitline calls. In essence, CTIP integrated diverse, complementary strategies to address gaps in smoking treatment in primary care.
The current research used the Reach, Effectiveness, Adoption, Implementation, Maintenance framework [25] to evaluate CTIP reach and effectiveness. This research focused on three key dimensions of CTIP population impact: 1) reach: the proportion of patients in a patient population who smoked and who received cessation treatment; 2) representativeness of reach across patient subpopulations based on sex, race, ethnicity, insurance, and age; and 3) effectiveness as indicated by conversion from current to former smoking status across the entire healthcare system, as documented in the EHR. The current study used a pragmatic multiple baseline design [26–27] to evaluate CTIP effects by comparing active clinics with clinics that had not yet implemented CTIP (providing control for secular trends), and by examining within-clinic increases in treatment reach and effectiveness following CTIP implementation.
Methods
Design
CTIP was launched in 3 waves across 3 successive quarters, as per a concurrent multiple baseline design (see Fig. 1) [26–27]. This pragmatic Type-II hybrid effectiveness-implementation [28] evaluation of CTIP balanced efforts to control for confounding effects of time with constraints related to the number and variability of clinics. The design assessed the extent to which CTIP-associated changes in treatment reach and effectiveness replicated across the 6 clinics in the health system. Assessment of CTIP reach, representativeness of reach, and effectiveness (change in smoking status), used EHR data collected during clinical service delivery. Power and sample size justification analyses were not conducted, as the goal of CTIP was to offer treatment to every patient who smoked and to have an effect at the population level. Based on historical data, we expected at least 4000 patients to be eligible for CTIP.
Fig. 1. Schedule for the introduction of the CTIP in the multiple baseline design.
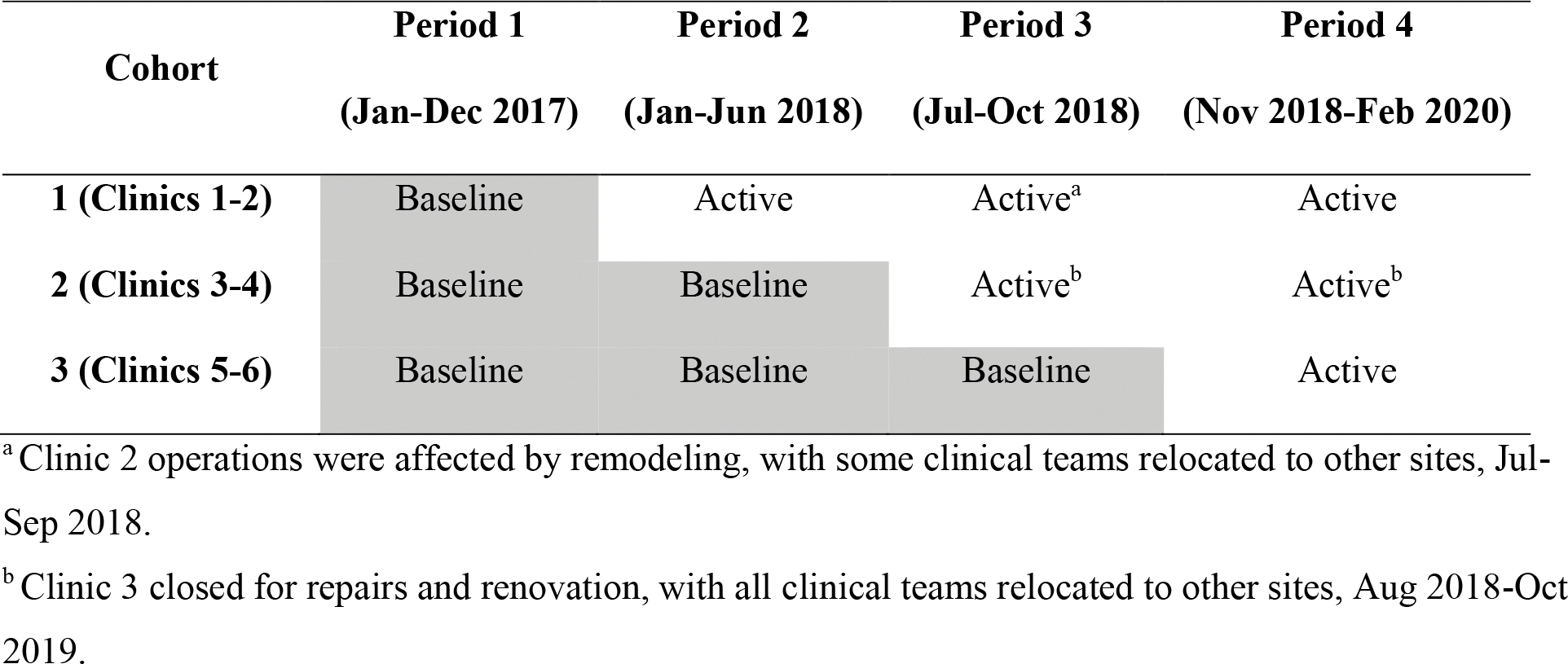
Each cohort comprised two different primary care clinics.
This study was approved by the Health Sciences Institutional Review Board of the University of Wisconsin, USA. Because EHR data collected during routine clinical care were analyzed anonymously, informed consent was not collected from patients. The study was not preregistered as it was a program evaluation rather than a randomized controlled trial.
Healthcare system and patient population
The non-profit, member-owned healthcare system comprises 6 primary care clinics. It provides primary and specialty care to over 60,000 members living in urban, suburban, and rural communities in and around Madison, Wisconsin. The target population for CTIP was adult primary care patients (≥18 years) satisfying criteria for current cigarette smoking via an EHR-based smoking registry. Those who used other forms of tobacco or nicotine exclusively were not targeted for CTIP.
Procedures
Prior to launch, EHR tools described in supplementary materials were built in collaboration with Epic Systems Corp. (Verona, WI) and customized to the healthcare system to enable CTIP. These tools are now part of the foundation program in Epic [29] that houses the records of more than 250 million Americans [30]. A new TCOS position was developed in the healthcare system’s Population Health Department. Two full-time TCOSs were hired and trained (a first in Period 1, a second in Period 2) by the healthcare system regarding system procedures, policies, workflows, and EHR tools. TCOSs completed accredited training in tobacco treatment. A third, part-time TCOS assisted with some outreach calls. At each clinic launch, research staff provided on-site training for clinic staff regarding the CTIP EHR tools and the role of the TCOS.
Intervention Overview
CTIP entailed 3 types of proactive offers of smoking cessation treatment, all implemented using an opt-out approach in which all patients meeting eligibility criteria for a smoking registry in the past 1–3 years (see supplementary materials for detailed registry criteria) were offered treatment unless they opted-out of CTIP. The 3 types of treatment outreach were prompted and facilitated by the EHR (see supplementary materials) and included: 1) clinician-delivered cessation treatment during telehealth or face-to-face encounters; 2) proactive TCOS phone outreach, and 3) quarterly bulk outreach via mail or EHR patient portal. Outreach efforts would occur no more often than once every 90 days; this limit was initially set to 30 days but later increased to 90 days based on stakeholder input.
All outreach efforts promoted both medication and counseling, and all patients who agreed to set a TQD were provided TCOS support by phone 2–5 days before, 3–7 days after, and 4–6 weeks after their TQD. Outreach efforts were designed to reach every patient who smoked. For those who had telehealth or face-to-face encounters with clinicians, clinicians were prompted to offer treatment at the encounter and TCOS called those who did not set a TQD with the clinician 1–2 weeks later to again offer treatment. In addition, TCOS called all patients who smoked who were not seen in their clinic in the past year to offer cessation treatment. Interpreters were available at clinic visits and TCOS calls for non-English speakers. (See the supplementary material for intervention and EHR tool information).
Measures
Data on outreach efforts, contact rates, treatment activities/reach, smoking status, patient demographics (sex, race, ethnicity, and age at the most recent visit), insurance type, clinic visits, and assigned primary care clinic were collected from the EHR rather than from ad hoc research assessments. Insurance type varied across time within patients, so each insurance type (commercial, Medicare, Medicaid, other or unknown) was coded as binary (1=ever had this insurance, 0=never had this insurance). To prevent patient identification by researchers, patient visits were aggregated to the patient-month level by the healthcare system, with counts of CTIP treatment eligibility, offers, and delivery aggregated each month for each patient. Data from a clinic’s launch month were not analyzed because encounter timing relative to CTIP launch could not be determined within that month.
Treatment reach was computed as the number of patients receiving a treatment component (as indicated by billing, medication, or CTIP order-set data) divided by the total number of patients eligible for that component (e.g., recent visit at which the EHR alert fired). Separate reach outcomes were computed for smoking cessation pharmacotherapy, cessation counseling, any cessation treatment (medication or counseling), and TCOS services.
Effectiveness was measured by computing the rate at which patients on the smoking registry in each study period converted to “former smoking” status, as recorded in the EHR. Self-reported abstinence was also collected by the TCOS at calls 3–7 days and 4–6 weeks post-TQD. Multiple members of a patient’s care team could update patient smoking status, including TCOS, who routinely updated status for patients who reported at least 1 week of abstinence at a follow-up call 4–6 weeks post-TQD or at outreach calls.
Data analysis
Summary statistics (counts and percentages for categorical variables, means and standard deviations for continuous variables) were computed to characterize patients who would meet smoking registry criteria pre- and/or post-CTIP-implementation, by clinic. Differences between the demographic and insurance composition of patient panels for each clinic were tested with chi-square tests.
Reach was computed at the patient (rather than encounter) level. Many patients were seen in multiple clinics, so patients were not nested within a single clinic (e.g., 20.9% of patients attended visits at multiple clinics and 12.0% of patients switched their assigned clinic during CTIP). Given this sharing of patients across clinics, a multilevel analytical approach was not used, and chi-square tests, odds ratios (OR), and 95% confidence intervals (CI) were used to assess pre-post-CTIP changes within clinics and to test differences during each study period between clinics active vs. inactive in CTIP [26]. To assess representativeness of reach, chi-square and t-tests were used to compare CTIP treatment reach across patient subpopulations defined by sex, race, ethnicity, insurance type, or age.
To assess CTIP effectiveness, chi-square tests, OR, and 95% CI were used to compare smoking quit rates between the CTIP-inactive and -active clinics within periods 2 and 3, holding time constant. Rates of converting to former smoking were also compared across period 1 (pre-implementation) and 4 (post-implementation) at the system level.
Results
Descriptive statistics
Characteristics of smoking registry patients
Characteristics of smoking registry patients are summarized by clinic in Table 1. Clinic patient panels varied significantly in terms of race, ethnicity, and insurance type. Overall, most patients were coded in the EHR as White, non-Hispanic, and commercially insured at some point. Panel characteristics were largely stable across time, but missing data rates fell from pre- to post-implementation for race, ethnicity, and insurance.
Table 1.
Patient-level counts (and percentages) of demographic groups and mean age and number of visits (and standard deviation) for all patients who smoke seen in clinic, by implementation phase (pre vs. post), overall (collapsed across clinics) and by clinic.
| N | Overalla | Clinic 1 | Clinic 2 | Clinic 3 | Clinic 4 | Clinic 5 | Clinic 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Prea | Posta | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| 7077 | 6672 | 312 | 401 | 1420 | 1697 | 1118 | 1023 | 1369 | 1408 | 713 | 508 | 2409 | 2001 | |
|
| ||||||||||||||
| Sex b | ||||||||||||||
| Women | 3187 (45.0) | 3075 (46.1) | 156 (50.0) | 197 (49.1) | 687 (48.4) | 828 (48.8) | 507 (45.3) | 487 (47.6) | 591 (43.2) | 632 (43.2) | 345 (48.4) | 262 (51.6) | 1045 (43.4) | 896 (44.8) |
| Men | 3885 (54.9) | 3583 (53.7) | 156 (50.0) | 204 (50.9) | 733 (51.6) | 865 (51.0) | 611 (54.7) | 535 (52.3) | 776 (56.7) | 773 (56.7) | 368 (51.6) | 246 (48.4) | 1361 (56.5) | 1098 (54.9) |
| Unknown | 5 (0.1) | 14* (0.2) | 0 | 0 | 0 | 4 (0.2) | 0 | 1 (0.1) | 2 (0.1) | 3 (0.2) | 0 | 0 | 3 (0.1) | 7 (0.3) |
|
| ||||||||||||||
| Race c d | ||||||||||||||
| African American | 746‡ (10.5) | 718‡ (10.8) | 4 (1.3) | 7 (1.7) | 97 (6.8) | 125 (7.4) | 76 (6.8) | 72 (7.0) | 209 (15.3) | 208 (14.8) | 55 (7.7) | 44 (8.7) | 349 (14.5) | 306 (15.3) |
| Other minority group | 308† (4.4) | 277‡ (4.2) | 5 (1.6) | 6 (1.5) | 40 (2.8) | 47 (2.8) | 52 (4.7) | 44 (4.3) | 70 (5.1) | 73 (5.2) | 30 (4.2) | 18 (3.5) | 123 (5.1) | 99 (4.9) |
| White | 5059‡ (71.5) | 4875*‡ (73.1) | 264 (84.6) | 357 (89.0) | 1051 (74.0) | 1282 (75.5) | 928 (83.0) | 839 (82.0) | 920 (67.2) | 969 (68.8) | 446 (62.6) | 356** (70.1) | 1626 (67.5) | 1340 (67.0) |
| Unknown | 964‡ (13.6) | 802*‡ (12.0) | 39 (12.5) | 31* (7.7) | 232 (14.3) | 243 (14.3) | 62 (5.5) | 68 (6.6) | 170 (12.4) | 158 (11.2) | 182 (25.5) | 90** (17.7) | 311 (12.9) | 256 (12.8) |
|
| ||||||||||||||
| Ethnicity d | ||||||||||||||
| Hispanic | 268† (3.8) | 252‡ (3.8) | 6 (1.9) | 8 (2.0) | 41 (2.9) | 59 (3.5) | 31 (2.8) | 21 (2.1) | 54 (3.9) | 71 (5.0) | 26 (3.6) | 17 (3.3) | 118 (4.9) | 91 (4.5) |
| Not Hispanic | 5789‡ (81.8) | 5555*° (83.3) | 247 (79.2) | 332 (82.8) | 1102 (77.6) | 1360 (80.1) | 1029 (92.0) | 934 (91.3) | 1137 (83.1) | 1177 (83.6) | 501 (70.3) | 395** (77.8) | 1987 (82.5) | 1657 (82.8) |
| Unknown | 1020‡ (14.4) | 865*‡ (13.0) | 59 (18.9) | 61 (15.2) | 277 (19.5) | 278* (16.4) | 58 (5.2) | 68 (6.6) | 178 (13.0) | 160 (11.4) | 186 (26.1) | 96 (18.9) | 304 (12.6) | 253 (12.6) |
|
| ||||||||||||||
| Insurance d, e | ||||||||||||||
| Commercial | 5711‡ (80.7) | 5508**° (82.6) | 264 (84.6) | 354 (88.3) | 1198 (84.4) | 1452 (85.6) | 924 (82.6) | 858 (83.9) | 1087 (79.4) | 1145 (81.3) | 645 (90.5) | 455 (89.6) | 1803 (74.8) | 1544 (77.2) |
| Medicaid | 1547‡ (21.9) | 1461‡ (21.9) | 41 (13.1) | 45 (11.2) | 250 (17.6) | 300 (17.7) | 197 (17.6) | 197 (19.3) | 327 (23.9) | 315 (22.4) | 102 (14.3) | 75 (14.8) | 714 (29.6) | 623 (31.1) |
| Medicare | 657‡ (9.3) | 587† (8.8) | 36 (11.5) | 42 (10.5) | 116 (8.2) | 124 (7.3) | 129 (11.5) | 114 (11.1) | 122 (8.9) | 111 (7.9) | 36 (5.0) | 36 (7.1) | 240 (10.0) | 193 (9.6) |
| None or unknown | 2726‡ (38.5) | 2030***†(30.4) | 97 (31.1) | 116 (28.9) | 589 (41.5) | 560*** (33.0) | 396 (35.4) | 273*** (26.7) | 585 (42.7) | 456*** (32.4) | 173 (24.7) | 123 (24.2) | 989 (41.1) | 633*** (31.6) |
|
| ||||||||||||||
| Pregnant | 43 (0.6) | 55 (0.8) | 0 | 0 | 3 (0.2) | 14* (0.8) | 5 (0.4) | 4 (0.4) | 14 (1.0) | 15 (1.1) | 1 (0.1) | 2 (0.4) | 22 (0.9) | 24 (1.2) |
|
| ||||||||||||||
| Agef M (SD) | 42.0 (14.0) | 42.9 (14.2) | 45.0 (14.2) | 46.6 (14.2) | 43.3 (13.6) | 43.4 (13.7) | 43.5 (14.5) | 44.3 (14.5) | 42.0 (13.9) | 42.1 (14.0) | 37.3 (13.2) | 40.7 (14.7) | 41.3 (13.9) | 42.2 (14.2) |
|
| ||||||||||||||
| Clinic visits M (SD) | 2.6 (3.3) | 2.7 (3.4) | 2.1 (2.9) | 3.3 (4.0) | 1.9 (2.2) | 2.2 (2.7) | 2.3 (3.0) | 0.9 (1.4) | 2.2 (2.6) | 2.5 (2.9) | 1 (1.7) | 1 (1.4) | 2.9 (3.8) | 2.3 (3.1) |
The overall total is less than the total summed across clinics because some patients were assigned to multiple clinics in a given phase.
Transgendered individuals were coded in their self-identified gender category rather than sex at birth. Those with non-binary identities or missing sex were coded as missing.
Patients identified as American Indian/Native Alaskan, Asian, or Hawaiian/Pacific Islander were coded as members of Other Minority groups to prevent inadvertent identification of patients in these low-frequency categories.
Clinics varied significantly in terms of distribution of patients in one category vs. all others for race, ethnicity, and insurance. The P value of Chi-Square tests with 5 degrees of freedom are indicated with the following symbols:
P<.05
P<.01
P<.001
Age was coded in 5-year bins up to age 75 to avoid inadvertent identification of patients; continuous age was computed by taking the midpoint of 5-year bins, or 78 if over 75.
Insurance often changed over time for patients, so each type of insurance variable was coded as ever present or absent in the pre- or post-implementation period for each patient. For this reason, the sum of people with each insurance type exceeds the total sample size.
P<.05
P<.01
P<.001 for Chi-square tests of within-clinic pre- to post-implementation differences for each category vs. all others.
Patient flow
Fig. 2 provides an expanded Consolidated Standards of Reporting Trials (CONSORT) diagram that depicts patient volumes and treatment reach by study period. Adult patient panel size and smoking prevalence varied across study periods in this open cohort. Smoking prevalence in each period was lower than in the surrounding county (10–15% [31]).
Fig. 2. Expanded CONSORT diagram.
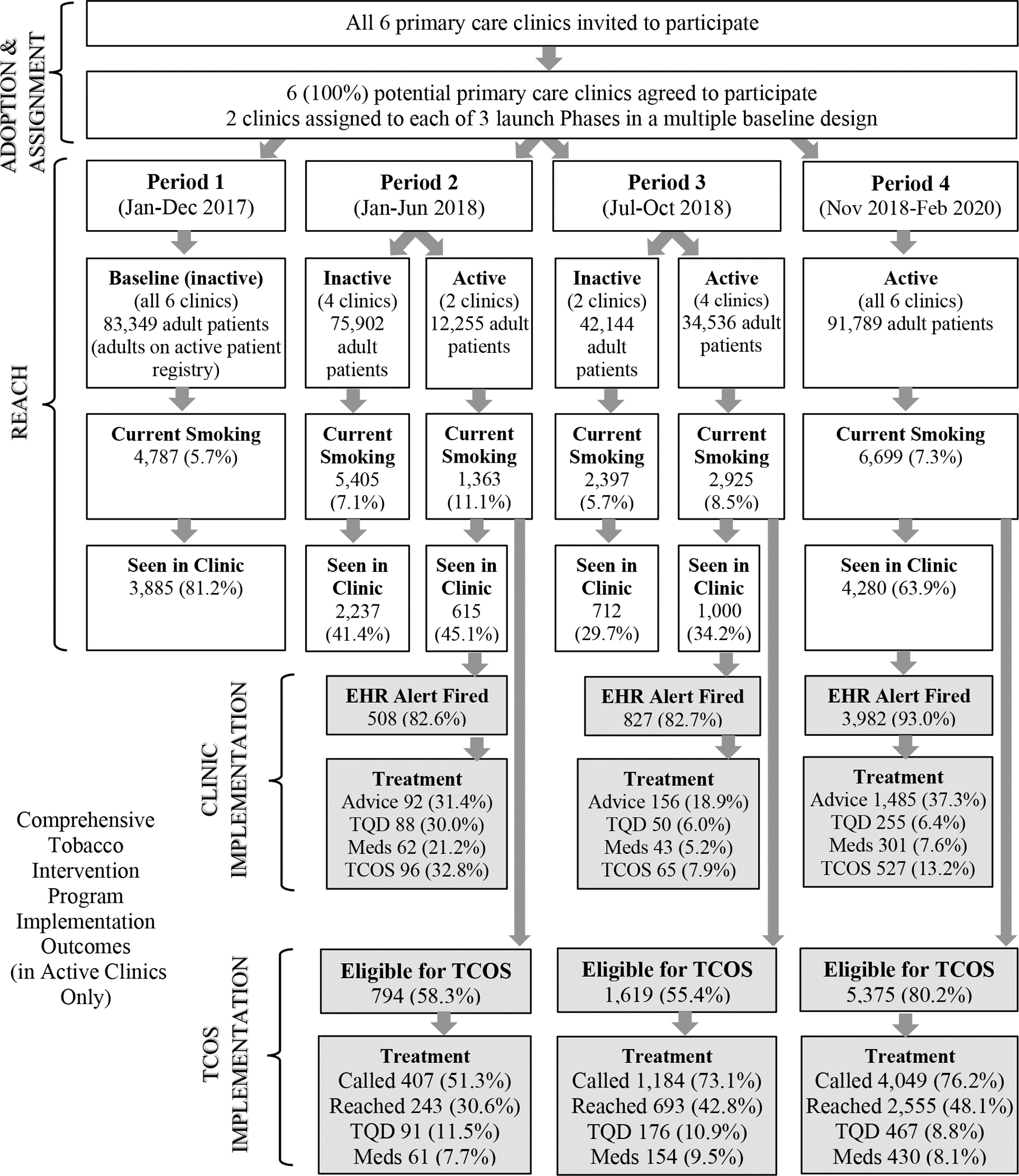
Number (and percentage from the previous step in the flow diagram) of patients retained in each step are displayed by study period and implementation status (active vs. inactive). Gray shaded boxes show rates of CTIP treatment activities post-implementation (periods 2–4) including: clinician advice to quit smoking (Advice), setting a target quit date (TQD), ordering smoking cessation medication (Meds), clinician referral to the Tobacco Cessation Outreach Specialist (TCOS), proactive calls from the TCOS (Called), and phone contact with the TCOS (Reached).
Clinician intervention reach by period
Fig. 2 shows rates of treatment reach, in clinic and via the TCOS, by study period, collapsed across active clinics. The EHR alert for clinicians fired for the vast majority (82.6% to 93.0%) of registry patients seen in active clinics (Fig. 2); this is below 100% because patients could join the smoking registry after encounters. Overall, 18.9%–37.3% of patients for whom the EHR alert fired received advice to quit during a clinic visit and 5.2%–32.8% received treatment (quit date setting, medication) from clinicians.
TCOS intervention reach by period
Fig. 2 shows that, collapsed across active clinics, 55.4%–80.2% of patients on the smoking registry were due for outreach from the TCOS at least once during each active period. Of these, 30.6%–48.1% were contacted, 8.8%–11.5% set a target quit date (TQD), and 8.1%–9.5% were ordered new or additional stop-smoking medications. Only 0.7% of patients on the smoking registry opted out of CTIP outreach permanently.
Change in reach by clinic and period
Table 2 displays clinic-specific rates of receiving medication and/or counseling from the clinician and/or TCOS, at the patient level (collapsed across visits), for each study period. Treatment reach rates varied significantly across clinics in Period 1 (pre-CTIP). Within clinics, significant differences in pre- vs. post-implementation reach rates were observed in 5 of the 6 clinics, with variable effect sizes across clinics (e.g., for any cessation treatment: Clinic 1 OR=1.98, 95% CI=1.41–2.77; Clinic 2 OR=0.95, 95% CI=0.79–1.13; Clinic 3 OR=1.25, 95% CI=1.01–1.54; Clinic 4 OR=2.57, 95% CI=2.19–3.02; Clinic 5 OR=5.12, 95% CI=3.75–7.00; Clinic 6 OR=3.71, 95% CI=3.23–4.26). Only Clinic 2 did not show a significant increase in rates of treatment (P=.53). Clinic 2 was undergoing construction for most of Period 3, and this caused disruptions in care (e.g., some clinicians shifted practice locations). The same pattern of results held for medication, cessation counseling, or any treatment (medication and/or counseling).
Table 2.
Overall patient-level counts (and %) of smoking cessation treatment delivered by clinic staff or TCOS to patients assigned to or seen in each clinic, by study period. Pre-launch rates are unbolded; post-launch rates are bolded.
|
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Received any cessation treatment (medication and/or counseling) | Prescribed cessation medication | Received cessation counseling from clinician and/or TCOS | ||||||||||
| Period 1 | Period 2 | Period 3 | Period 4 | Period 1 | Period 2 | Period 3 | Period 4 | Period 1 | Period 2 | Period 3 | Period 4 | |
| Clinic | Jan-Dec 2017 | Jan-Jun 2018 | Jul-Oct 2018 | Nov 2018-Feb 2020 | Jan-Dec 2017 | Jan-Jun 2018 | Jul-Oct 2018 | Nov 2018-Feb 2020 | Jan-Dec 2017 | Jan-Jun 2018 | Jul-Oct 2018 | Nov 2018-Feb 2020 |
|
|
||||||||||||
| 1 | 50aa (15.6%) | 85 (30.8%) | 40 (18.0%) | 90 (29.3%) | 48aa (15.0%) | 77 (27.9%) | 37 (16.7%) | 81 (26.4%) | 8 aa (2.5%) | 53 (19.2%) | 23 (10.4%) | 40 (13.0%) |
| 2 | 203 (15.6%) | 177 (15.6%) | 93 (8.9%) | 263 (18.7%) | 153 (11.8%) | 138 (12.1%) | 83 (8.0%) | 224 (15.9%) | 89 (6.8%) | 105 (9.2%) | 42 (4.0%) | 108 (7.7%) |
| 3 | 127a (13.6%) | 60 (7.4%) | 76 (10.1%) | 142 (15.4%) | 108a (11.5%) | 50 (6.1%) | 69 (9.1%) | 128 (13.9%) | 47 a (5.0%) | 18 (2.2%) | 32 (4.2%) | 27 (2.9%) |
| 4 | 190aa (16.1%) | 76 (7.8%) | 162 (18.6%) | 397 (32.1%) | 130aa (11%) | 63 (6.4%) | 127 (14.6%) | 339 (27.4%) | 111 aa (9.4%) | 20 (2.0%) | 110 (12.6%) | 216 (17.5%) |
| 5 | 27aa (4.5%) | 24 (6.4%) | 23 (7.2%) | 120 (23.6%) | 24aa (4.0%) | 23 (6.1%) | 16 (5.0%) | 92 (18.1%) | 9 aa (1.5%) | 7 (1.9%) | 11 (3.4%) | 93 (18.3%) |
| 6 | 218aa (10.9%) | 121 (7.2%) | 110 (7.3%) | 521 (26.1%) | 168aa (8.4%) | 103 (6.2%) | 102 (6.7%) | 469 (23.5%) | 106 aa (5.3%) | 40 (2.4%) | 25 (1.7%) | 266 (13.3%) |
|
|
||||||||||||
| X2 | 58.5b | 140.6c | 36.6c | 85.4b | 41.9b | 105.9c | 17.1c | 76.6b | 55.5b | 188.4c | 62.1c | 138.5b |
| n | 6334 | 5251 | 4721 | 6375 | 6334 | 5251 | 4721 | 6375 | 6334 | 5251 | 4721 | 6375 |
| OR | -- | 2.88c | 1.88c | 2.14d | -- | 2.71c | 1.78c | 2.75d | -- | 5.56c | 3.85c | 2.14d |
| 95%CI | -- | 2.41–3.45 | 1.53–2.32 | 1.95–2.35 | -- | 2.23–3.29 | 1.43–2.22 | 2.49–3.04 | -- | 4.24–7.30 | 2.69–5.51 | 1.89–2.45 |
| P | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
|
|
||||||||||||
2×2 Chi-square test of within-clinic differences in treatment rates across pre- vs. post-implementation phases, collapsed across periods within phases, p<.05
P<.001
Chi-square test indicates significant variability in treatment rates across clinics in period 1 or period 4
2×2 Chi-square test and odds ratio (OR) and 95% confidence interval (95% CI) of differences between clinics that are actively implementing CTIP vs. in baseline in periods 2 or 3.
OR of treatment in period 4 vs. period 1, collapsed across clinics.
Comparisons of clinics actively implementing the program vs. those still in baseline in the same period were significant in both Periods 2 and 3 (Table 2). In Period 4, when CTIP was active in all clinics, clinics continued to vary significantly in reach of any treatment, medication ordering, and cessation counseling with OR 2.14–2.75, relative to Period 1 treatment rates.
Reach of TCOS phone outreach by call type
Table 3 displays the proportion of patients eligible for each type of TCOS phone outreach post-implementation across all clinics who: were reached by a TCOS; set a new TQD; confirmed working toward an existing TQD (if applicable); or were ordered new or additional medication. There was no proactive patient outreach available pre-implementation for comparison.
Table 3.
Patient-level reach (n and % of those eligible for outreach in each row) of TCOS services post-implementation, by outreach type.
| Outreach Type (n eligible) | Reached by Phone | Created Quit Plan | Confirmed Quit Pland | Reported Abstinencee | Ordered Medication |
|---|---|---|---|---|---|
|
| |||||
| Annuala (not seen in clinic in >365 days, n=2014) | 1093 (54.3%) | 181 (9.0%) | -- | -- | 182 (9.0%) |
| Recent visit (after recent clinic visit, with no TQDb, n=3964) | 2006 (50.6%) | 417 (10.5%) | -- | -- | 360 (9.1%) |
| Pre-quit (2–5 days before a TQD, n=886) | 429 (48.4%) | 116 (13.1%) | 252 (28.4%) | -- | 46 (5.2%) |
| Post-quit (3–7 days after a TQD, n=948) | 434 (45.8%) | 107 (11.3%) | 237 (25.0%) | 319 (33.6%) | 34 (3.6%) |
| Follow-up (4–6 weeks after a TQD, n=869) | 396 (45.6%) | 87 (10.0%) | -- | 175 (20.1%) | 46 (5.3%) |
| Patient-initiatedc (if patient called TCOS, N=6894) | 454 (6.6%) | 164 (2.4%) | -- | -- | 197 (2.9%) |
To be included in this category, patients had to appear on the list of patients who have not had a visit in at least a year for at least 2 consecutive months, as many patients would appear on the list for only a few days, too short a time window for outreach to occur.
TQD=target quit date, or planned day to quit smoking.
The denominator used to compute rates of patient-initiated contact was the number of patients on the smoking registry in an active clinic in periods 2–4.
Patient confirmed that they were still trying to adhere to the quit plan and the target quit smoking date.
Counts and percentages are the number of patients due for each post-TQD outreach call who reported they were no longer smoking.
Overall, at least 50% of those eligible for TCOS-initiated Recent Visit outreach or Annual outreach and at least 45% of those due for a call around the TQD were reached by phone (Table 3). The patient-level reach rates in Table 3 are slightly higher than the contact rates in Fig. 2, due to cumulative effects that accrue when collapsing across period. Calls tied to a TQD had a narrower window than the Recent Visit and Annual calls, which may have contributed to their lower contact rates. Of note, 41.5% of patients with a future TQD reported actively working toward quitting by confirming their planned quit date or setting a new one; 70.0% with a TQD either reported abstinence or working toward an existing or new quit date in a call 3–7 days post-TQD; and 30.1% of those due for follow-up 4–6 weeks after a TQD either reported abstinence or set a new quit date.
Nearly 7% of patients initiated contact with the TCOS (Table 3). Of these, 36.1% created set a TQD and 43.4% were ordered cessation medication. Rates of TCOS referral of patients to the Wisconsin Tobacco Quit Line and/or SmokefreeTXT were low (<1.5%) across all TCOS calls.
Representativeness of reach
Rates of receiving any cessation treatment (medication and/or counseling) and cessation-specific services pre- and post-CTIP were compared across patient subpopulations defined by sex, race, ethnicity, insurance type, or age (Table 4). Women received treatment at higher rates than did men both before and during CTIP. Pre-CTIP, White patients were treated at higher rates than were members of minoritized groups. During CTIP, African-American patients received TCOS services at higher rates than did members of other racial groups and no significant racial disparities in access to any treatment were observed. Pre-CTIP, Hispanic patients were treated at significantly lower rates than were other patients. This gap was no longer significant during CTIP, and Hispanic patients accepted TCOS services at particularly high rates. Patients who were ever uninsured were treated at the lowest rates before and during CTIP. Before CTIP, patients who ever had Medicare were most likely to be treated; during CTIP treatment rates were similar among all insured patients. Patients who ever had Medicaid were especially likely to receive TCOS services post-CTIP. Significant age differences were observed, such that patients who received any treatment, including TCOS services (or pre-CTIP equivalent), were significantly older than were those who did not receive treatment, both before and during CTIP.
Table 4.
Representativeness of patient-level reach of smoking cessation treatment pre- (N=7077) vs. post-implementation (N=6936) across all clinics.
| Pre-implementation (N=7077) | Post-implementation (N=6936) | Pre-implementation (N=7077) | Post-implementation (N=6936) | |
|---|---|---|---|---|
|
| ||||
| Received any cessation treatment | Received any cessation treatment | Received cessation specialist counselinga | Received TCOS treatment | |
|
| ||||
| Sex | ||||
| Men | 762 (18.0%)** | 946 (25.3%)** | 125 (3.0%) | 453 (12.1%)*** |
| Women | 710 (20.4%)** | 914 (28.7%)** | 80 (2.3%) | 496 (15.6%)*** |
| Other/unknown | 3 (60.0%)* | 5 (33.3%) | 0 (0.0%) | 3 (20.0%) |
|
| ||||
| Race | ||||
| White | 1086 (19.7%)* | 1393 (27.5%) | 163 (3.0%)** | 668 (13.2%)*** |
| African American | 140 (18.0%) | 208 (28.2%) | 13 (1.7%) | 152 (20.6%)*** |
| Other minorityb | 48 (15.4%) | 57 (20.5%)* | 13 (4.2%) | 34 (12.2%) |
| Unknown | 201 (17.5%) | 207 (24.4%) | 16 (1.4%)* | 98 (11.5%) |
|
| ||||
| Ethnicity | ||||
| Hispanic | 31 (10.9%)*** | 59 (22.8%) | 4 (1.4%) | 37 (14.3%)* |
| Not Hispanic | 1207 (19.7%) | 1571 (27.3%) | 174 (2.8%) | 796 (13.8%) |
| Unknown | 237 (19.4%) | 235 (25.6%) | 27 (2.2%) | 119 (13.0%) |
|
| ||||
| Insurance c | ||||
| Commercial | 1219 (19.5%) | 1549 (27.0%) | 168 (2.7%) | 758 (13.2%)* |
| Medicare | 175 (24.8%)*** | 174 (28.9%) | 26 (3.7%) | 100 (16.6%) |
| Medicaid | 328 (19.8%) | 424 (28.3%) | 45 (2.7%) | 269 (18.0%)*** |
| Uninsured | 499 (16.7%)*** | 481 (22.8%)*** | 68 (2.3%) | 237 (11.2%)*** |
|
| ||||
| Age | 45.2 (12.7)† | 46.0 (12.9)† | 44.7 (13.6)† | 46.3 (12.8)† |
P<.001
P<.01
P<.05 in a Chi-square test comparing the group in this row against all other rows for this variable (or, for insurance, against those who never had this type of insurance) within pre- or post-implementation phase
P<.001 in a t-test of differences in mean age for patients who did vs. did not receive treatment, with those receiving treatment being significantly older than those not receiving treatment (pre-CTIP mean age was 41.0, SD=14.0; post-CTIP mean age was 43.1 SD=14.1)
Prior to CTIP implementation, a health educator specializing in smoking cessation treatment could receive referrals for smoking cessation counseling and some behavioral health clinicians offered smoking cessation counseling. These were the pre-CTIP services most analogous to TCOS services and were therefore used as a comparator in this analysis.
Other minority includes Asian, American Indian/Alaska Native, and Hawaiian/Other Pacific Island.
Because insurance can and often did change over the post-implementation period for patients, each type of insurance variable was coded as binary, with 1 indicating that a patient ever had that insurance type post-implementation, and 0 indicating the patient never had that insurance type. For this reason, the sum of people with each insurance type exceeds the total sample size.
Effectiveness
The rates at which patients on the smoking registry switched from active smoking to former smoking in the EHR are displayed in Fig. 3. Raw rates of change increased from 0.4% in inactive clinics to 6.9% in active clinics in Period 2, from 0.7% in inactive clinics to 6.6% in active clinics in Period 3, and from 2.1% at baseline (Period 1) to 10.5% in Period 4. To account for improved ascertainment of smoking status through TCOS, we adjusted conversion rates to remove from the numerator all patients who reported they no longer smoked at an Annual or Recent Visit TCOS call, but never received CTIP treatment. The denominator remained all patients on the smoking registry (or equivalent) in the period of interest. The numerator adjustment is shown by gray bars in Figure 3, and represents 35.1%, 40.4%, 58.1% of the changes to former smoking in active clinics in Periods 2, 3, and 4, respectively. After removing these never-treated former smoking patients, adjusted conversion rates remained significantly higher in active clinics than their comparators: Period 2, 4.5% active vs. 0.4% inactive (Chi square (N=6 768)=142.65, OR=10.50, 95% CI=6.52–16.91); Period 3, 3.9% active vs. 0.7% inactive (Chi square (N=5 322)=58.46, OR=6.09, 95% CI=3.60–10.30); Period 4, 4.4% vs. Period 1, 2.1%, Chi square (N=11 486)=44.57, OR=2.15, 95% CI=1.71–2.71).
Fig. 3.
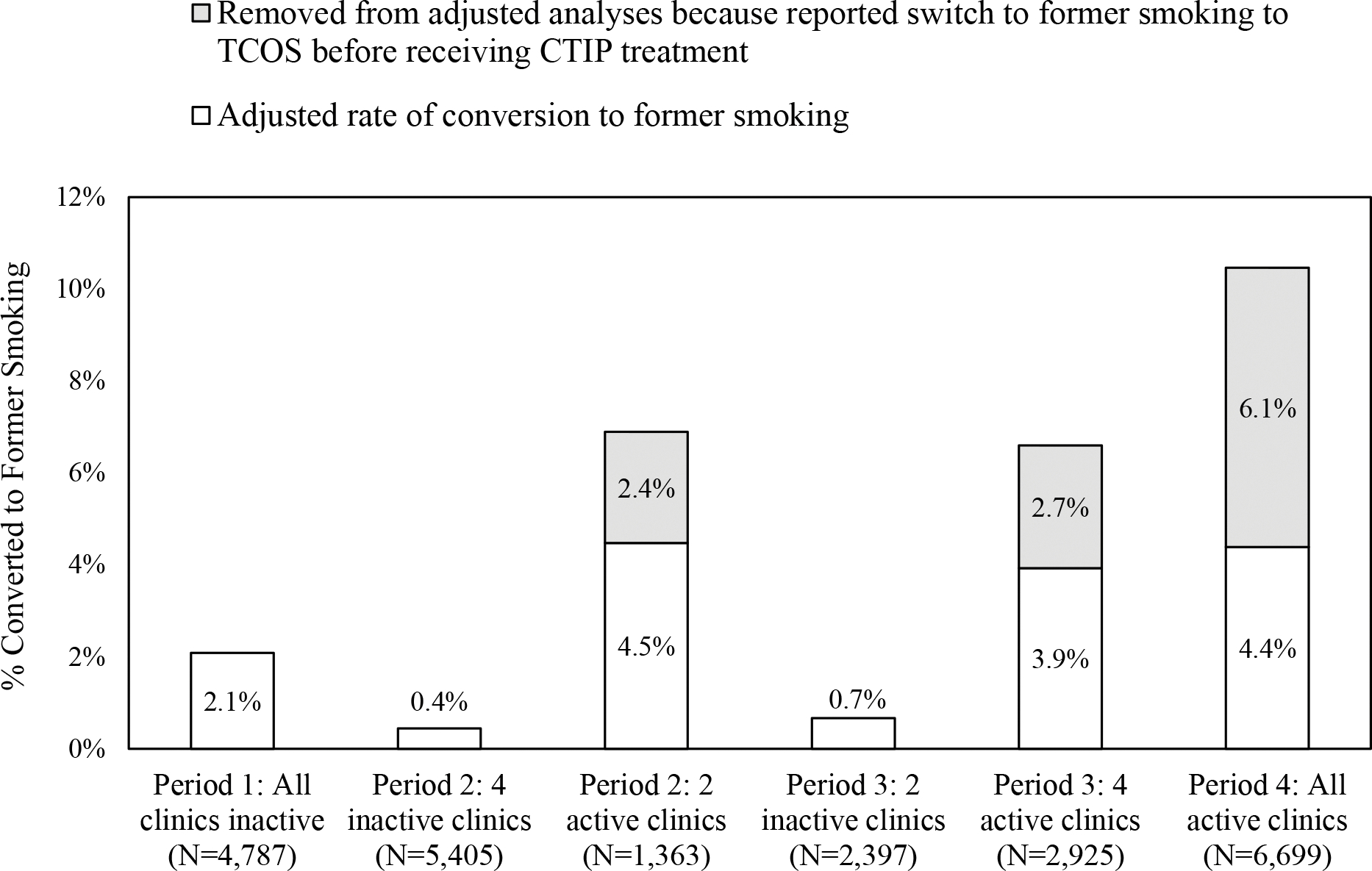
Raw and adjusted rates of converting from current to former smoking in the electronic health record by period and CTIP activity status
Discussion
The introduction of a comprehensive, health system-wide primary care tobacco intervention program, CTIP, coincided with significant increases in rates of delivery of smoking treatment, including pharmacotherapy and counseling, and increased smoking cessation rates. A distinguishing feature of CTIP was proactive outreach by phone to both patients who recently saw a primary care provider and those who had not visited a clinic for at least a year. Both primary care clinicians and tobacco cessation specialists delivered smoking cessation advice and support in CTIP.
When CTIP was active in all 6 clinics, 24.0% of adult patients who smoked received some form of smoking treatment. This engagement rate is substantially higher than rates of quitline treatment engagement in studies of closed-loop eReferral [13, 22, 23]. For instance, a recent study [23] of quitline eReferral in 30 clinics found that only 3.6% of adults eligible for eReferral connected with the quitline.
While quitlines often reach only one-third of referred patients [15–17], TCOS reached 50% or more of those due for outreach. Moreover, roughly 1 in 6 of those reached made a quit plan and accepted smoking-cessation medication. For patients with quit plans, TCOS connected by phone with nearly half before and after a TQD, and 30.1% of patients with TQDs in the past 6 weeks reported either success in quitting or willingness to set a new quit date. This adds to evidence that comprehensive phone outreach meaningfully expands utilization of cessation treatment services [32–33].
In addition to TCOS calls, quarterly outreach by mail or EHR patient portal informed patients about cessation resources (e.g., via letters, postcards, magnets, a 2-week starter kit of nicotine patches). Fewer than 7% of patients who received such outreach initiated contact with the TCOS but this approach was relatively inexpensive, and more than one-third of those who initiated contact with a TCOS accepted pharmacotherapy.
The comprehensive program was especially effective in reaching traditionally underserved populations, particularly in terms of TCOS services. TCOS reach rates were especially high for women, African-American patients, Hispanic patients, and those with Medicaid. Similar results have been reported with other EHR-enabled smoking interventions in primary care [13, 22, 23], suggesting that such approaches reduce disparities in smoking treatment offers and access.
Rates of quitting smoking were substantially higher in clinics implementing CTIP than in those still in baseline, with increases in quit rates coinciding with CTIP implementation. In addition, quit rates more than doubled from the year before CTIP launch (2.1%) to 4.4% in the 15 months all 6 clinics were implementing CTIP; this estimated quit rate excluded patients who reported no longer smoking to TCOS, but never received CTIP treatment, to enhance the specificity of such estimates.
Despite these promising results, there is still potential for improvement. For instance, after full implementation, less than one-third of individuals who smoked received cessation counseling and/or medication. Although expecting 100% of patients who smoke to engage in cessation treatment is unrealistic, reach may be improved by motivational strategies, such as incentives for treatment engagement [34–35]. There was variability in CTIP implementation across clinics. For example, the rate of providing medication varied 6-fold across clinics (from 2.9% to 18.3%). Future research may identify causes of such variability and methods for addressing it.
Limitations
This research involved only a single healthcare system, with relatively low smoking prevalence, which may limit generalizability. The ascertainment of outcomes relied on EHR records which may have introduced error, as abstinence was not biochemically verified and smoking status assessment was not conducted at standardized, long-term follow-up time points. Some patients may have quit smoking without this being noted in the EHR, while others who dropped off the smoking registry may have had undetected relapses. Moreover, some patient-reported conversion to former smoking may reflect patient desire to stop receiving smoking-related outreach rather than true abstinence. Future studies could include independent assessments of abstinence. In addition, the current analyses do not address CTIP cost-effectiveness, replication costs, or scalability. Although CTIP was designed for dissemination, additional analyses of scalability are needed. Finally, the program involved numerous components, precluding identification of prepotent elements.
Conclusion
A comprehensive, health system-wide approach to smoking treatment in adult primary care may markedly improve smoking cessation treatment delivery and success. This program increased the reach of cessation treatment and rates of conversion to former smoking and was especially effective in engaging traditionally underserved groups in smoking treatment. In keeping with the goal articulated in the inaugural issue of Preventive Medicine 50 years ago, these results suggest ways to advance “the medical care delivery system of tomorrow” [36, p.2] through comprehensive programs that extend opt-out, remote cessation treatment offers to all individuals who smoke.
Supplementary Material
Acknowledgments
This study was funded by a grant (R35CA197573 to Michael C. Fiore) from the National Cancer Institute (www.cancer.gov). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors thank Epic Systems Corp., Verona, Wisconsin, USA, for their assistance and support in developing EHR enhancements that enabled the conduct of this research.
Footnotes
Declaration of Interests
Danielle E. McCarthy, Michael C. Fiore, and Timothy B. Baker have all received grant funding from NCI, the institute that sponsors SmokefreeTXT. Michael C. Fiore serves as a consultant to the National Cancer Institute (NCI) on tobacco cessation policy issues. Timothy B. Baker has served as a paid consultant to ICF to evaluate the portfolio of Smokefree resources via monies supplied by NCI. His consulting activities involved suggesting new research directions for digital health interventions and providing feedback to ICF and NCI on research products on such interventions. The research reported in this report was not part of his consulting work with ICF/NCI. Timothy B. Baker is Principal Investigator on a project funded by NHLBI for which Pfizer provided free active and placebo varenicline. Pfizer played no role in the design, implementation, or analysis of the project described in the present manuscript. All other authors have no conflicts to report.
The electronic health record tools described in this manuscript are marketed by Epic Systems Corporation.
Abbreviations
CTIP=Comprehensive Tobacco Intervention Program; EHR=Electronic health record; M=Mean; OR=Odds ratio; TCOS=Tobacco cessation outreach specialist; TQD=Target quit day; 95% CI=95% confidence interval
References
- 1.Fiore MC, Wetter DW, Bailey WC, et al. The Agency for Health Care Policy and Research Smoking Cessation Clinical Practice Guideline. JAMA. 1996;275(16):1270–1280. [PubMed] [Google Scholar]
- 2.Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, Henderson PN. Treating tobacco use and dependence: 2008 update. Rockville, MD: US Department of Health and Human Services. 2008. May 27. [Google Scholar]
- 3.Jaèn CR, Crabtree BF, Zyzanski SJ, Stange KC. Making time for tobacco cessation counseling. J Fam Pract. 1998;46:425–428. [PubMed] [Google Scholar]
- 4.McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18:156–170. [DOI] [PubMed] [Google Scholar]
- 5.Longo DR, Stone TT, Phillips RL, Everett KD, Kruse RL, Jaen CR, et al. Characteristics of smoking cessation guideline use by primary care physicians. Missouri Med. 2006;103(2):180–4. [PubMed] [Google Scholar]
- 6.DePue JD, Goldstein MG, Schilling A, Reiss P, Papandonatos G, Sciamanna C, et al. Dissemination of the AHCPR clinical practice guideline in community health centres. Tob Cont. 2002;11(4):329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papadakis S, McDonald P, Mullen KA, et al. Strategies to increase the delivery of smoking cessation treatments in primary care settings: a systematic review and meta-analysis. Prev Med. 2010;51(3–4):199–213. [DOI] [PubMed] [Google Scholar]
- 8.Piper ME, Fiore MC, Smith SS, Jorenby DE,Wilson JR, Zehner ME, et al. Use of the vital sign stamp as a systematic screening tool to promote smoking cessation. Mayo Clinic Proc. 2003;78(6):716–22. [DOI] [PubMed] [Google Scholar]
- 9.Holtrop JS, Malouin R, Weismantel D, et al. Clinician perceptions of factors influencing referrals to a smoking cessation program. BMC Fam Pract. 2008;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcy TW, Skelly J, Shiffman RN, Flynn BS. Facilitating adherence to the tobacco use treatment guideline with computer-mediated decision support systems: physician and clinic office manager perspectives. Prev Med. 2005;41(2):479–87. [DOI] [PubMed] [Google Scholar]
- 11.Bentz C, Bayley K, Bonin K, Fleming L, Hollis J, McAfee T. The feasibility of connecting physician offıces to a state-level tobacco quit line. Am J Prev Med. 2006;30(1):31–7. [DOI] [PubMed] [Google Scholar]
- 12.Adsit RT, Fox BM, Tsiolis T, Ogland C, Simerson M, Vind LM, Bell SM, Skora AD, Baker TB, Fiore MC. Using the electronic health record to connect primary care patients to evidence-based telephonic tobacco quitline services: a closed-loop demonstration project. Trans Behav Med. 2014;4:324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiore M, Adsit R, Zehner M, et al. An electronic health record-based interoperable eReferral system to enhance smoking Quitline treatment in primary care [published correction appears in J Am Med Inform Assoc. 2019;26(10):1159]. J Am Med Inform Assoc. 2019;26(8–9):778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheffer MA, Baker TB, Fraser D, Adsit R, McAfee T, Fiore MC. Fax referrals, academic detailing, and tobacco quitline use: A randomized trial. Am J Prev Med. 2012;42(1):21–8. [DOI] [PubMed] [Google Scholar]
- 15.Leuthard JL, Beebe LA, Halstead L, Olson KD, Roysdon JW. Increased evidence-based tobacco treatment through Oklahoma hospital system changes. Am J Prev Med. 2015;48(1)(suppl 1):S65–S70. [DOI] [PubMed] [Google Scholar]
- 16.Wadland WC, Holtrop JS, Weismantel D, Pathak PK, Fadel H, Powell J. Practice-based referrals to a tobacco cessation quit line: assessing the impact of comparative feedback vs general reminders. Ann Fam Med. 2007;5(2):135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willett JG, Hood NE, Burns EK, et al. Clinical faxed referrals to a tobacco quitline: reach, enrollment, and participant characteristics. Am J Prev Med. 2009;36(4):337–40. [DOI] [PubMed] [Google Scholar]
- 18.Bentz CJ, Bayley BK, Bonin KE, et al. Provider feedback to improve 5A’s tobacco cessation in primary care: A cluster randomized clinical trial. Nicotine Tob Res. 2007;9(3):341–349. [DOI] [PubMed] [Google Scholar]
- 19.Greenwood DA, Parise CA, MacAller TA, Hankins AI, Harms KR, Pratt LS, Olveda JE, Buss KA. Utilizing clinical support staff and electronic health records to increase tobacco use documentation and referrals to a state quitline. J Vasc Nurs. 2012;30(4):107–11. [DOI] [PubMed] [Google Scholar]
- 20.Linder JA, Rigotti NA, Schneider LI, Kelley JH, Brawarsky P, Haas JS. An electronic health record-based intervention to improve tobacco treatment in primary care: a cluster-randomized controlled trial. Arch Intern Med. 2009;169(8):781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindholm C, Adsit R, Bain P, Reber PM, Brein T, Redmond L, Smith SS, Fiore MC. A demonstration project for using the electronic health record to identify and treat tobacco users. WMJ. 2010. Dec;109(6):335–40. [PMC free article] [PubMed] [Google Scholar]
- 22.Flocke SA, Seeholzer E, Lewis SA, et al.12-month evaluation of an EHR-supported staff role change for provision of tobacco cessation care in 8 primary care safety-net clinics. J Gen Intern Med. 2020;35:3234–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker TB, Berg KM, Adsit RT, Skora AD, Swedlund MP, Zehner ME, McCarthy ME, Glasgow R, Fiore MC. Closed loop eReferral from primary care clinics to a state tobacco cessation quitline; an implementation and maintenance evaluation. Am J Prev Med. 2021;60(3 Suppl 2):S113–S122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richter KP, Ellerbeck ER. It’s time to change the default for tobacco treatment. Addiction. 2015;110(3):381–6. [DOI] [PubMed] [Google Scholar]
- 25.Glasgow RE, Harden SM, Gaglio B, et al. RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front Public Health. 2019;7:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazdin AE. Single-Case Research Designs: Methods for Clinical and Applied Settings. Oxford University Press; 2011, pp. 144–166. [Google Scholar]
- 27.Coon JC, Rapp JT. Application of multiple baseline designs in behavior analytic research: Evidence for the influence of new guidelines. Behavioral Interventions. 2018;33(2):160–72. [Google Scholar]
- 28.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50(3):217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epic Systems Corp. Evidence-Based Tobacco Screening and Interventions Setup and Support Guide. 2015–2017. Epic Systems Corp.: Verona, WI, USA. [Google Scholar]
- 30.Epic Systems Corporation. About Epic System [Internet]. Verona, Wisconsin (US): Epic Systems Corp; reviewed 2022 Jan 18; cited 2022 Jan 18]. Available from: https://www.epic.com/about [Google Scholar]
- 31.County Health Rankings & Roadmaps: Building a Culture of Health, County by County. 2022 Health Behavior Data, Tobacco Use, Wisconsin, Dane County. https://www.countyhealthrankings.org/app/wisconsin/2022/measure/factors/9/data [Accessed May 1, 2022].
- 32.Fu SS, van Ryn M, Sherman SE, et al. Proactive tobacco treatment and population-level cessation: a pragmatic randomized clinical trial. JAMA Intern Med. 2014;174(5):671–7. [DOI] [PubMed] [Google Scholar]
- 33.Fu SS, van Ryn M, Nelson D, et al. Proactive tobacco treatment offering free nicotine replacement therapy and telephone counselling for socioeconomically disadvantaged smokers: a randomised clinical trial. Thorax. 2016;71(5):446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baker TB, Fraser DL, Kobinsky K, Adsit R, Smith SS, Khalil L, Alaniz KM, Sullivan TE, Johnson ML, Fiore MC. A randomized controlled trial of financial incentives to low income pregnant women to engage in smoking cessation treatment: Effects on post-birth abstinence. J Consult Clin Psychol. 2018;86(5):464–473. [DOI] [PubMed] [Google Scholar]
- 35.Fraser DL, Fiore MC, Kobinsky K, Adsit R, Smith SS, Johnson ML, Baker TB. A randomized trial of incentives for smoking treatment in Medicaid members. Am J Prev Med. 2017;53(6):754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wynder EL. Preventive medicine introduction. Prev Med, 1972;.1(1):1–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


