Abstract
We aimed to identify caregiver characteristics associated with the trajectory of quality of life (QoL) in Parkinson’s disease (PD). We fit a growth mixture model to longitudinal data from the Parkinson Foundation Parkinson’s Outcomes Project (POP) to identify the heterogeneity of QOL trajectories in PD. We then used multinomial logistic regression to model baseline factors that predicted class membership. Baseline growth models were fit to QOL scores measured over 4 disease duration time points. A random intercept and slope model was determined to best fit the data. Next, growth mixture models (1, 2, 3, 4, and 5-class) were fit with covariates (Hoehn & Yahr, sex, and depression) and a three-class model was found to provide the best fit. Class 1 (problematic class (10.0%)) represented individuals with poor QOL at baseline and minor improvement over time. Class 2 (moderate class (32.6%)) represented individuals with moderate QOL at baseline with slight worsening over time. Class 3 (favorable class (56.9%)) represented individuals with good QOL at baseline and slight worsening over time. Multinomial regression revealed that lower caregiver strain, better mobility, and better verbal fluency at baseline predicted membership in the favorable compared to the moderate class. Worse mobility and younger age predicted membership in the problematic compared to the moderate class. While previous studies have reported on the association between mobility and cognition, the novel finding of an association between caregiver strain and PD QOL trajectory suggests caregiver strain is important to measure and address in future research and practice.
Keywords: Parkinson’s disease, quality of life, caregiver strain, verbal fluency, mobility
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disease characterized by motor and non-motor symptoms that impair quality of life (QoL) and functioning in activities of daily living (1–3). QoL is a multi-dimensional construct that encompasses physical, mental, and social functioning (4). Generally, investigations of QoL in PD have explored the effect of specific disease symptoms on QoL (3,5). Several studies have identified motor symptoms such as postural instability (2), axial rigidity (6), and tremor (7) as contributing to lower QoL in this population. Additionally, studies have elucidated the negative impact of neuropsychiatric symptoms, including cognitive impairment (8,9) anxiety (10,11), apathy (12), and depression(3,13). Other non-motor PD symptoms that can influence QoL are fatigue, autonomic dysfunction, and sleep disturbances such as nocturnal akinesia, excessive daytime sleepiness, and rapid eye movement behavior disorder (12,14).
Previous research has also demonstrated that QoL in PD generally declines as the disease progresses (15,16). To address the significant impact that PD has on QoL, several interventions have been developed (17–19). However, not all people with PD are eligible for these interventions and QoL remains negatively affected in PD, precipitating the need to look beyond the traditional symptom-focused approach and develop more accessible interventions that can improve QoL for a greater proportion of this population.
Another potential route to improve QoL for people with PD may involve the caregiver. Caregivers provide physical and emotional care for the patient, often help manage medical regimens (20,21), and are frequently required of PD patients at some point during their disease course (22). In the general population, caregiver characteristics have been linked to both positive and negative care recipient outcomes. The presence of a caregiver is associated with a reduced risk of nursing home entry (23), fewer unmet patient needs (24), improved medication and treatment adherence (25) and better quality of patient care (26). In contrast, caregiver distress has been associated with increased risk of institutionalization (27,28). Caregiver burden and the quality of the caregiver-care recipient relationship have been found to predict QoL and psychological wellbeing of the care recipient (29,30). In the PD population, studies have observed associations between caregiver strain and care recipient QoL, with greater caregiver strain associated with worse QoL for the care recipient (31,32). However, these studies have primarily relied on cross-sectional and univariable analyses, preventing any evaluation of the directionality of the relationship between the caregiver factor and care recipient QoL.
To understand the role of the caregiver in QoL for people with PD, the current study aimed to examine factors that predict trajectories of QoL in this population, with a focus on caregiver strain as a significant driver of QoL decline. Due to the variable presentation of PD, it is reasonable to assume heterogeneity in QoL trajectories (33) and growth mixture models can be used to identify subpopulations of persons who share similar patterns of change in a variable, such as QoL, over time (34). We hypothesized that higher caregiver strain would be associated with an unfavorable QoL trajectory during mid-stage PD.
Methods
Participants
This study used data from the Parkinson Foundation Parkinson’s Outcomes Project (POP), a longitudinal, observational study of PD patients (35). The purpose of this project is to evaluate the quality of care at the Parkinson Foundation’s Centers of Excellence and to identify factors that can improve health outcomes for people with PD. Participants are evaluated annually at 21 different Centers of Excellence, in Canada, the Netherlands, Israel, and the United States. The study began in 2009 and enrollment is ongoing. Inclusion criteria for general study participation are (1) physician diagnosis of idiopathic Parkinson’s disease, and (2) at least 1 year of available follow-up data. There are currently over 13,000 PD participants enrolled in the POP study, and approximately 11,000 have a caregiver.
For this analysis, only participants with a caregiver were included. Time in this analysis was defined by disease duration or time since diagnosis. We included patients who had disease durations between 8 and 11 years in our analysis. This time frame was selected based on findings in the literature that most people with PD experience a worsening of symptoms that require greater caregiver involvement around disease durations of 10 years (36,37). The final analytic sample included 1,349 patients with idiopathic PD. Figure 1 presents the participant data flow. The study was approved by the Johns Hopkins School of Medicine Institutional Review Board.
Figure 1.
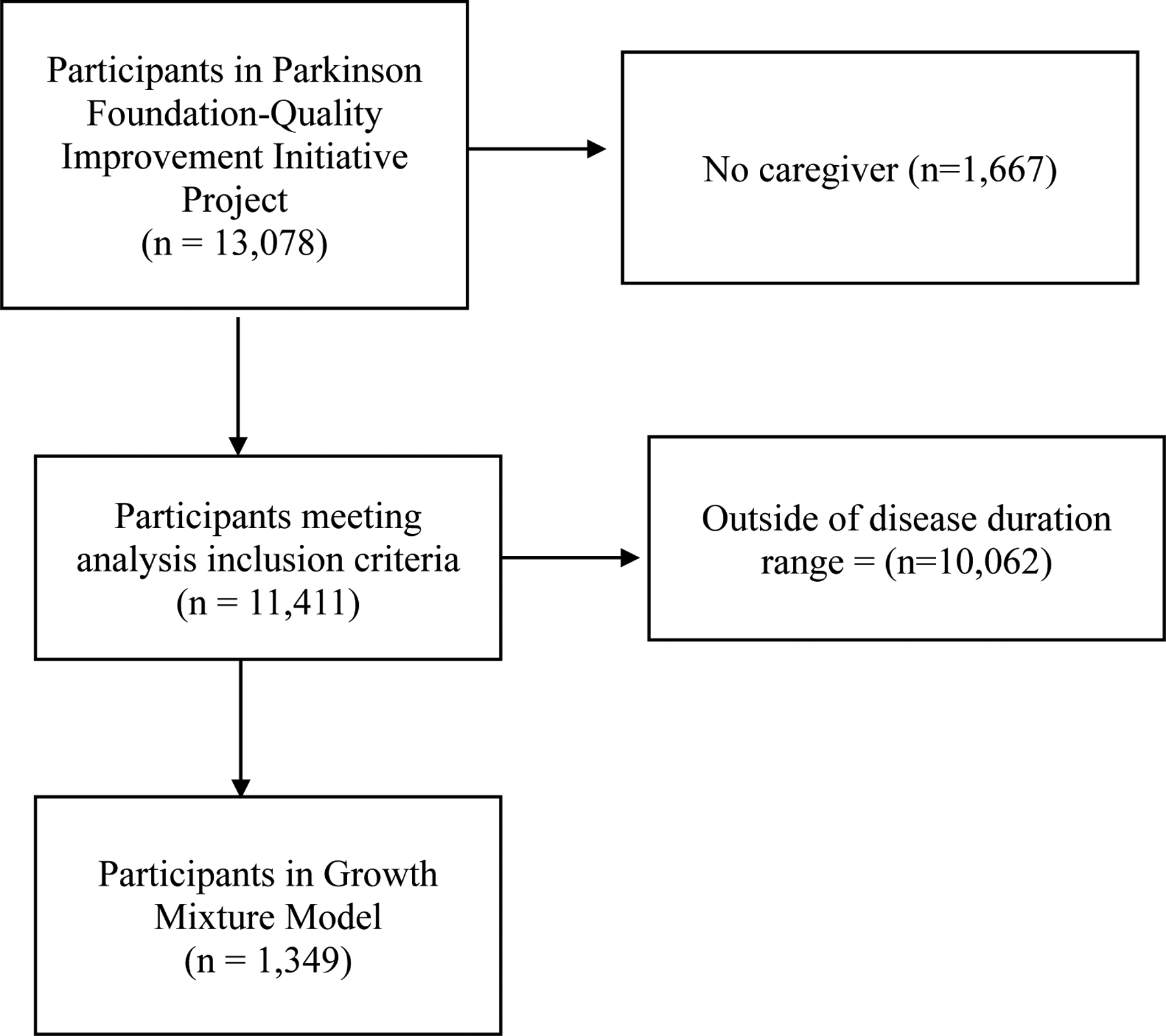
Participant Data Flow
Measures
Parkinson’s disease Questionnaire-39.
The outcome of interest for this investigation was QoL measured using the Parkinson’s Disease Questionnaire-39 (PDQ-39) (38). This questionnaire assesses mobility, activities of daily living, emotional well-being, stigma, social support, cognition, communication, and bodily discomfort, and is recommended by the Movement Disorders Society. Scores range from 0 to 156, with higher scores indicating worse QoL.
Modified Caregiver Strain Index.
The Modified Caregiver Strain Index (MCSI) was used to screen for caregiver strain and includes questions that evaluate the following domains: financial, physical, psychological, social, and personal strain (39). Each question was scored from 0 (“never”) to 4 (“a great deal”) for a total score range of 0–72. Higher scores indicate worse caregiver strain. While no validated measure of caregiver strain in PD exists, the MSCI is frequently used to evaluate caregiver strain in older adults and has been used for Parkinson’s disease caregivers (40,41).
Disease severity.
Disease stage for each patient was evaluated using the Hoehn and Yahr Scale (42).
Cognitive functioning.
Two cognitive tests were administered and included in this analysis. Verbal fluency was measured by asking participants to name as many animals as they could in one minute (43). The final score was the total number of unique animals named. Verbal fluency reliably identifies problems with attention and executive dysfunction in people with PD (44,45). In addition, a 5-word recall test was administered to evaluate memory (46).
Physical functioning.
The Timed Up and Go test (TUG) was administered to all participants during clinical visits. This test evaluates physical performance by examining the participant’s ability to rise from a seated chair position, walk 3 meters, turn, walk back, and sit down. This procedure is timed with longer times indicating worse physical performance. The TUG test is strongly associated with other physical functioning factors, such as functional mobility, gait speed, and falls in older adults (47).
Analytic Plan
The analysis was based on a sample of 1,349 patients who had data collected at baseline (defined as disease duration=8 years). Descriptive statistics were conducted using Stata Version 16 (48). Growth mixture models were fit in MPlus Version 8.3 (49). Growth mixture modeling (GMM) was used to identify subpopulations in the larger sample, describing change over time within each sub-population or class, and evaluating differences in longitudinal change among these classes (50). These sub-populations were identified using latent trajectory classes (51). Trajectories were determined based on PDQ-39 scores at following disease durations: 8 years (T0), 9 years (T1), 10 years (T2), and 11 years (T3). The intercept parameter (estimated baseline value of QoL score) and the probability of class membership were adjusted for disease severity (H&Y stage), sex, and depression diagnosis (Figure 2). We hypothesized that individuals would cluster into several empirically derived and distinct QoL trajectories. In addition, we hypothesized that the baseline presence of high caregiver strain would be associated with inclusion into the class with the fastest decline in QoL while the absence of caregiver strain at baseline would predict inclusion into the class with the slowest decline in QoL.
Figure 2.
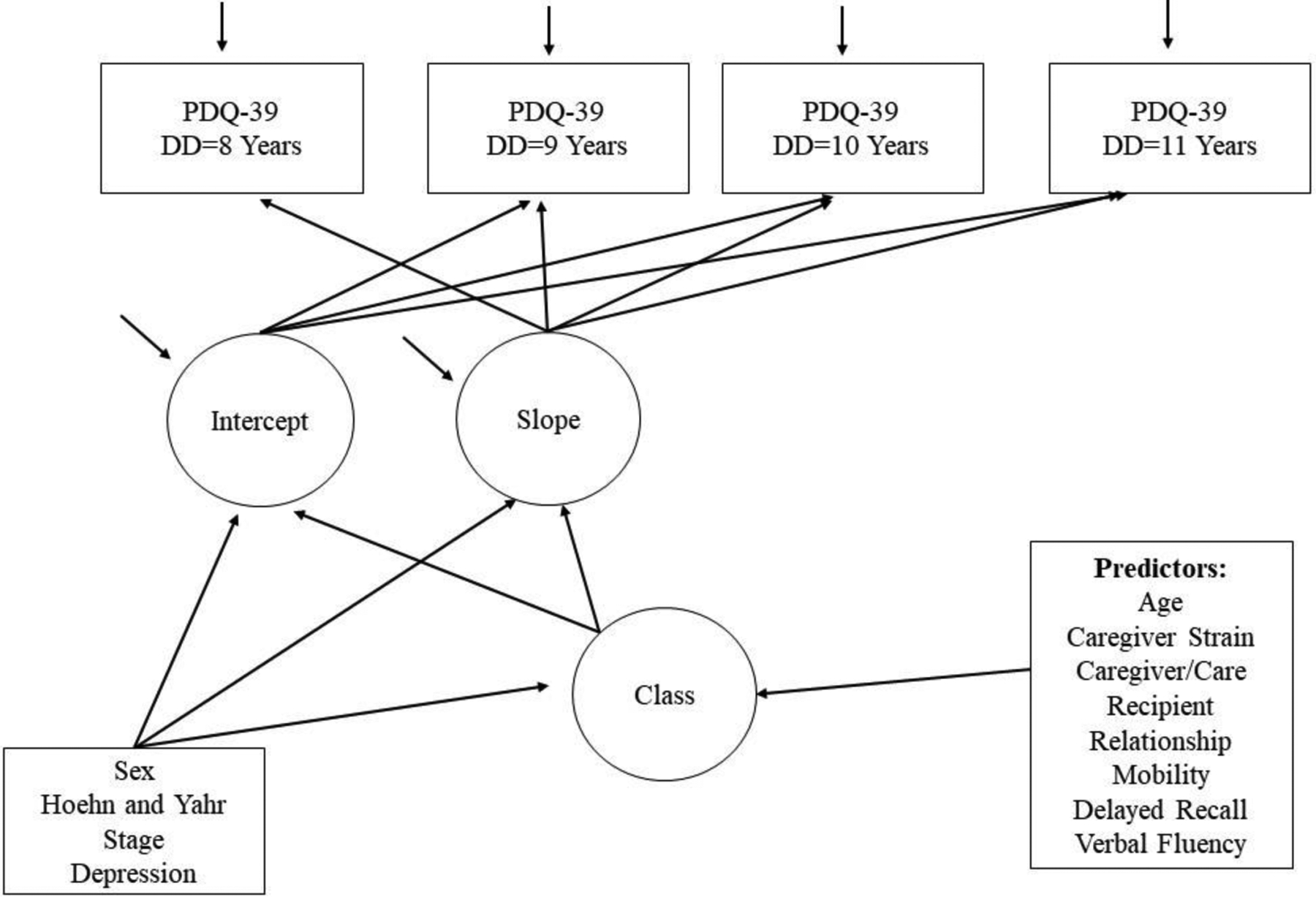
Hypothesized Growth Mixture Model Diagram for PDQ-39 over four time points in patients with Parkinson’s disease
Square boxes = observed variables, Circles = latent variables
First, a single class model was specified to examine the overall baseline growth function for QoL over time. The baseline growth models were fit to PDQ-39 scores measured over 4 time points to determine if an intercept only, slope, quadratic, or a latent basis function best fit the data. Covariates (Hoehn & Yahr stage, sex, and depression) were also added to the model.
Next, a growth mixture model with K latent classes (K = 2, 3, 4, and 5-class) was fit. Several model fit statistics contributed to decisions about which model best fit the data, including Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), the Lo-Mendell-Rubin Likelihood Ratio test (LMR LRT), and entropy (51). From the final GMM model, we determined (1) the proportion of the sample that belongs in each profile (class prevalence) and (2) the intercept and slope for each trajectory conditioned upon membership in each class. Multiple sets of starting values were used to avoid local solutions in the estimation procedure. Finally, we examined class size and avoided models where any one class contained less than 5% of the overall sample.
Following the model selection, we determined predictors of class membership using multinomial regression. As class membership is a latent variable, assigning individual classes as if they were observed and regressing class membership on predictors would introduce bias into the analysis. Therefore, we used a corrected 3-step multinomial logistic regression to predict class membership using baseline caregiver variables (type of caregiver-care recipient relationship and caregiver strain) and other clinical variables that have been previously considered to influence QoL and that were available in this data set (52–54).
Results
Sample Characteristics
The mean age of the sample of 1,349 patients at baseline was 68.3 years (95% CI: 67.9 to 68.9) with 482 females (34.3%). The mean age of PD onset was 55.4 years (95% CI: 54.8 to 55.9). Disease severity as measured by the Hoehn and Yahr scale included, 4.3% HYI, 57.3% HYII, 30.6% HYIII, 6.8% HYIV, and 0.7% HYV. Table 1 presents the descriptive statistics of the sample by disease duration.
Table 1.
Clinical characteristics of the study sample
| Disease Duration | ||||
|---|---|---|---|---|
| 8 years | 9 years | 10 years | 11 years | |
| Age (years) | 68.3 (9.1) | 68.7 (8.9) | 68.6 (8.9) | 69.3 (8.6) |
| Female (%) | 33.5 | 33.1 | 32.3 | 33.5 |
| Hoehn and Yahr Stage (%) | ||||
| HY I | 4.3 | 3.3 | 3.2 | 2.1 |
| HY II | 57.3 | 57.1 | 50.9 | 50.1 |
| HY III | 30.6 | 32.0 | 36.1 | 35.5 |
| HY IV | 6.8 | 6.5 | 8.5 | 10.6 |
| HY V | 0.7 | 1.1 | 1.3 | 1.3 |
| Verbal Fluency | 18.6 (6.6) | 18.4 (6.5) | 18.5 (6.8) | 17.7 (6.6) |
| Delayed word recall | 3.4 (1.4) | 3.4 (1.5) | 3.4 (1.4) | 3.4 (1.5) |
| Timed Up and Go | 13.3 (7.1) | 13.4 (6.9) | 13.5 (7.2) | 14.2 (7.5) |
| PDQ-39 Total Score | 40.8 (26.3) | 41.7 (25.8) | 43.3 (26.4) | 46.9 (27.0) |
| Depression Diagnosis (%) | 2.4 | 6.2 | 9.2 | 12.4 |
Baseline Growth Model
The estimated mean change in the PDQ-39 total score for the entire sample was 2.88 (SD: 1.65). The individual trajectories for each participant were heterogeneous (SD range 25.8–27.0 across the 4 years of follow-up), with individual trajectories varying from the estimated average trajectory (Figure 3). Therefore, a latent growth curve model was appropriate to model the heterogeneity in the data.
Figure 3.
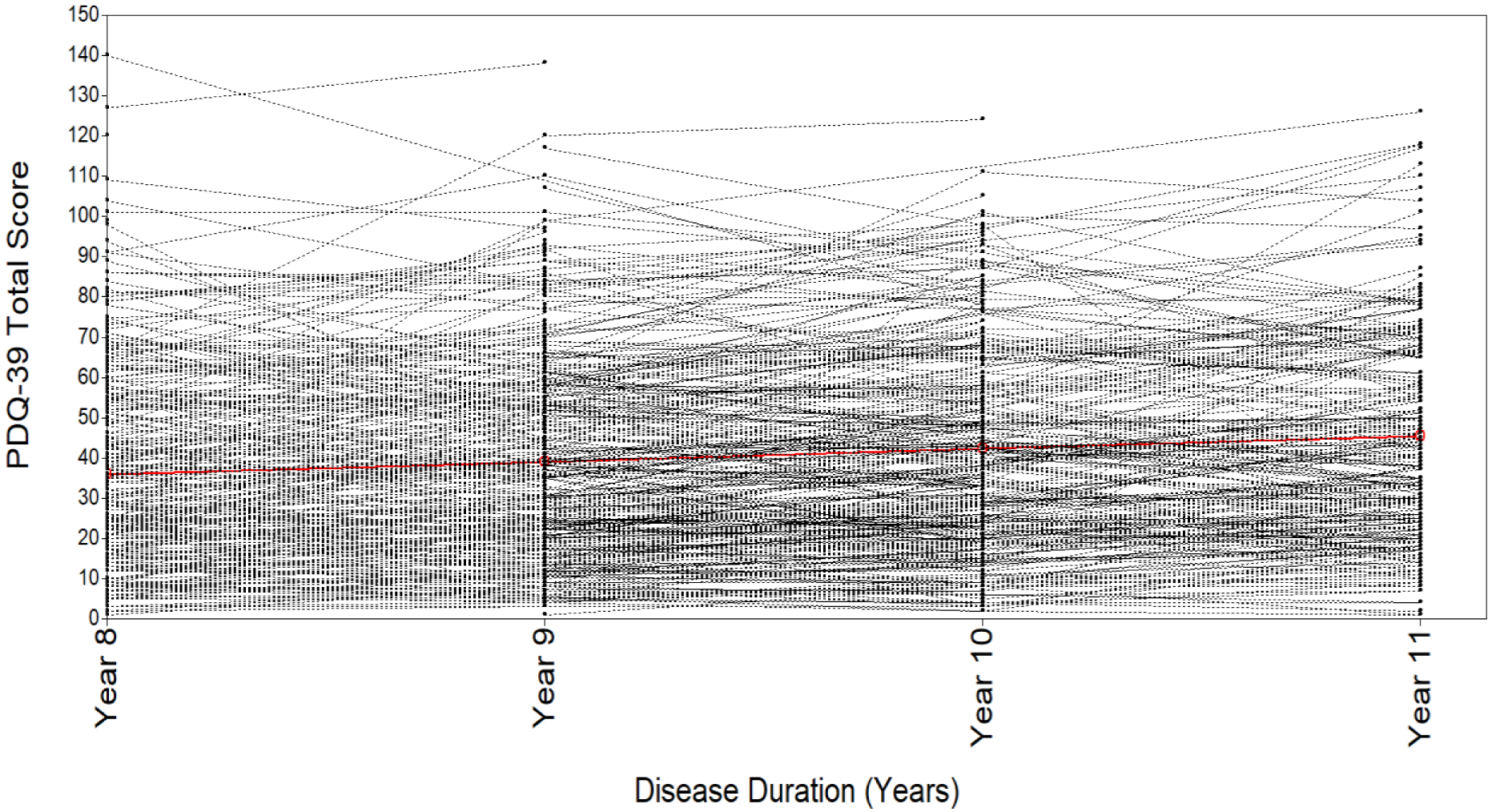
Spaghetti Plot of Individual QoL Trajectories
Fit statistics for all tested models are presented in Table 2. The fit statistics for the intercept only model represented poor fit. The TLI, CFI, RMSEA, and chi-square test of model fit represented a good fit for the slope growth model, quadratic growth model, and latent basis growth model. However, the AIC and sample-size adjusted BIC were lowest for the slope growth model, indicating a better fit. Therefore, an intercept and slope model was ultimately selected.
Table 2.
Baseline Growth Model Fit Statistics
| TLI>0.95 | CFI>0.95 | RMSEA<0.06 | Chi-square test of model fit (p-value) | AIC | Sample-Size Adjusted BIC | |
|---|---|---|---|---|---|---|
| Intercept only | 0.929 | 0.906 | 0.119 | 0.0000 | 28921.647 | 28934.125 |
| Intercept and slope | 1.000 | 1.000 | 0.000 | 0.8670 | 28761.030 | 28779.747 |
| Intercept, slope, quadratic | 1.000 | 1.000 | 0.000 | 0.4470 | 28767.740 | 28794.775 |
| Latent basis | 1.000 | 1.000 | 0.000 | 0.8326 | 28764.032 | 28786.908 |
Growth Mixture Model
The fit statistics for the 1-class, 2-class, 3-class, 4-class, and 5-class models are reported in Table 3. The LMR-LRT value, one of the most reliable test statistics, was considered first to indicate model fit. This p-value was significant when comparing the 2-class model to the 1-class model, and the 3-class model to the 2-class model; however, it was no longer significant for other comparisons (i.e., 4-class model to the 3-class model or the 5-class model to the 4-class model). The 3-class model had the lowest BIC statistic, indicating good fit. Another consideration to ensure the clinical utility of the model was class prevalence. In the 3-class model, all classes contained at least 10% of the population. Due to the LMR-LRT and BIC values and the class prevalence, the 3-class model was selected.
Table 3.
Growth Mixture Model Fit Statistics *
| 1 Class | 2 Classes | 3 Classes | 4 Classes | 5 Classes | |
|---|---|---|---|---|---|
| No. of parameters | 12 | 18 | 24 | 30 | 36 |
| Log Likelihood | −13567.559 | −13486.941 | −13445.196 | −13427.934 | −13413.590 |
| AIC | 27159.119 | 27009.882 | 26938.391 | 26915.867 | 26899.180 |
| BIC | 27221.604 | 27103.610 | 27063.362 | 27072.081 | 27086.637 |
| N-adjusted BIC | 27183.485 | 27046.432 | 26987.124 | 26976.783 | 26972.280 |
| Lo-Mendell-Rubin probability | N/A | 0.0000 | 0.0033 | 0.3478 | 0.6465 |
| Entropy | N/A | 0.772 | 0.769 | 0.741 | 0.765 |
| Smallest class (%) | N/A | 18.3 | 10.0 | 7.1 | 1.0 |
Adjusted for Hoehn and Yahr stage, sex, and depression diagnosis.
Class Characteristics
The parameter estimates and class prevalence for the growth curves for the three latent classes are reported in Table 4. The first latent class, which we labeled as the problematic class (10.0%), included participants with poor QoL at baseline and slight improvement over time. The second latent class, which we labeled as the moderate class (32.6%), included participants with moderate QoL at baseline and slight worsening in QoL over time. The third class, which we labeled as the favorable class (57.4%) included participants with good QoL at baseline and only slight worsening of QoL over time. Figure 4 displays the trajectories of each class.
Table 4.
Parameter estimates for growth functions for the three-class general growth mixture model for PDQ-39 (Hoehn and Yahr stage, sex, and depression diagnosis adjusted)
| Class 1 | Class 2 | Class 3 | |
|---|---|---|---|
| Name | “Problematic” | “Moderate” | “Favorable” |
| Prevalence | 10.0% | 32.6% | 57.4% |
| Mean PDQ-39 at baseline | 74.3 | 37.95 | 8.95 |
| Rate of change in PDQ-39 | −2.75 | 3.61 | 3.79 |
Figure 4.
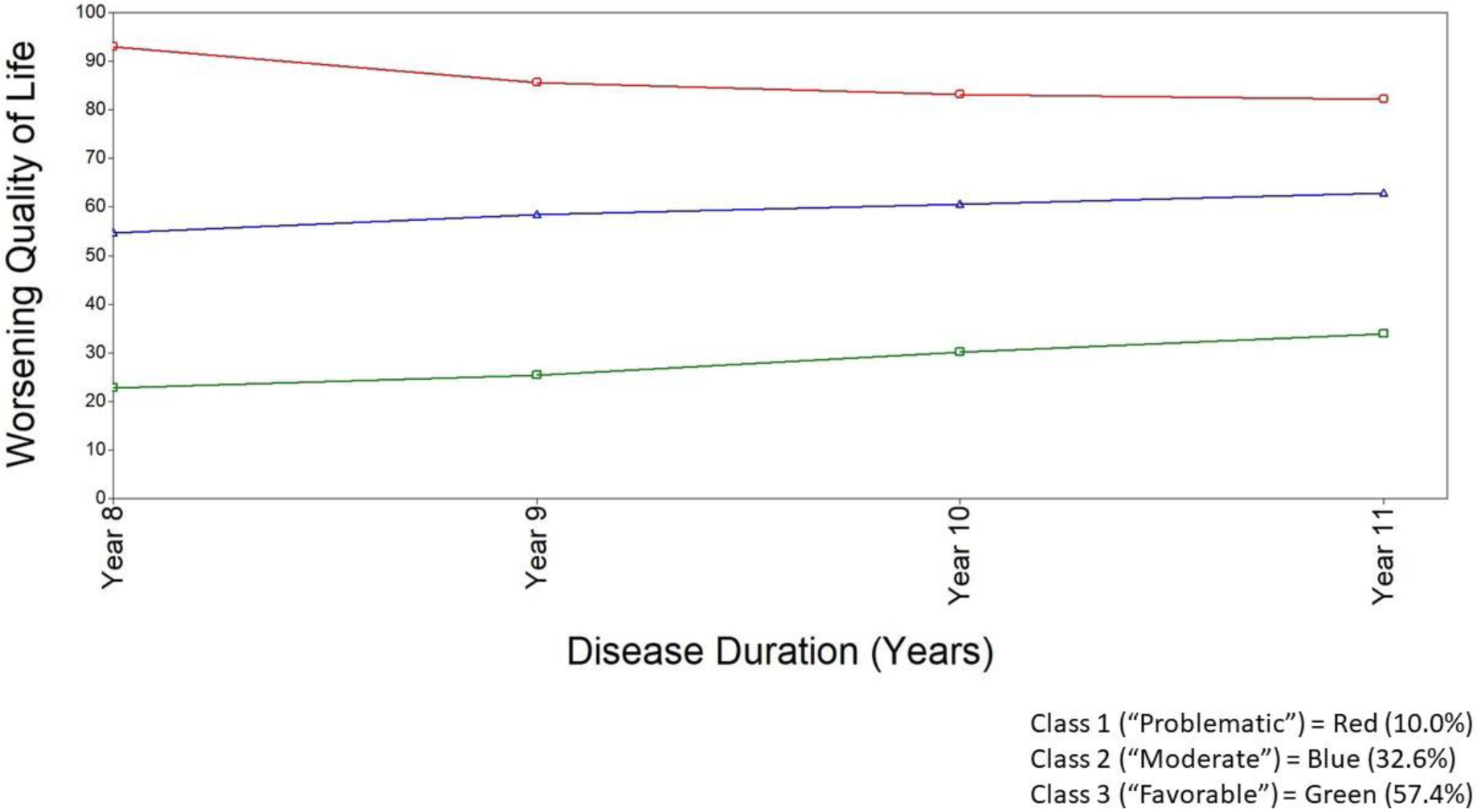
Model estimated growth trajectories for PDQ-39 over four time points for the three-class growth mixture model (controlling for HY, sex, and depression)
Predictors of Class Membership
Potential predictors of QoL trajectory listed in Table 1 were examined using multinomial logistic regression, with predicted class membership in our final 3-class model as the dependent variable. The results of this regression analysis are summarized in Table 5. This analysis was based on 549 participants and the reference class was the moderate class. The factors associated with membership in the problematic class compared to the moderate class were TUG time and age. For each additional second of TUG time, the odds of being in the problematic class compared to the moderate class increased by 6% (OR: 1.064, 95% CI: 1.007 to 1.125). For each additional year of age, the odds of being in the problematic class compared to the moderate class decreased by 6% (OR: 0.936, 95% CI: 0.877 to 0.999). The factors associated with membership in the favorable class compared to the moderate class were lower caregiver strain (OR: 0.883, 95% CI: 0.810 to 0.933), better verbal fluency (OR: 1.056, 95% CI: 1.000 to 1.115), and shorter TUG time (OR: 0.868, 95% CI: 0.810 to 0.930). For each additional point on the MSCI (i.e. increased caregiver burden), the odds of being in the favorable class compared to the moderate class decreased by 12%. For each additional word on the verbal fluency test the odds of being in the favorable class compared to the moderate class increased by 6%. For each additional second of TUG time, the odds of being in the favorable class compared to the moderate class decreased by 13%.
Table 5.
Predictors of class membership (Reference: Moderate class)
| Problematic Class versus Moderate Class | OR (95% CI) |
|---|---|
| Age | 0.936 (0.877 to 0.999)* |
| Verbal Fluency | 0.879 (0.770 to 1.004) |
| Delayed Recall | 0.793 (0.570 to 1.103) |
| Timed Up and Go test1 | 1.064 (1.007 to 1.125)* |
| Caregiver Relationship | 1.505 (0.537 to 4.219) |
| Modified Caregiver Strain Index | 1.054 (0.999 to 1.111) |
| Favorable Class versus Moderate Class | |
| Age | 1.013 (0.977 to 1.050) |
| Verbal Fluency2 | 1.056 (1.000 to 1.115) |
| Delayed Recall | 0.936 (0.694 to 1.262) |
| Timed Up and Go test | 0.868 (0.810 to 0.930)* |
| Caregiver Relationship | 1.384 (0.234 to 8.191) |
| Modified Caregiver train Index3 | 0.883 (0.836 to 0.933)* |
Higher scores othe Timed Up and Go test indicate longer time (seconds) to complete the task, meaning worse motor function.
Higher scores on verbal fluency indicate better verbal fluency (more words).
Higher scores on the Modified Caregiver Strain Index indicate worse caregiver strain.
p<0.05
Discussion
Our study is the first to examine the relationship between caregiver strain and QoL trajectories in people with PD. We identified 3 groups of people with PD defined by their QoL change over years 8 to 11 of disease duration, when up to 46% of people with PD develop dementia, imbalance is more prevalent, and many become dependent on caregivers for some tasks (55). Lower caregiver strain, when adjusting for motor and cognitive performance of patients independently predicted membership in a class with a favorable trajectory. This finding contributes to existing studies that have demontrated the association between caregiver strain and PD patient QoL (56,57); however, these previous studies have been cross-sectional and the findings could be explained with reverse causality. Our longitudinal analysis adjusted for several influential variables that likely confound the relationship between caregiver strain and QoL implicating caregiver strain as a causal contributor to QoL trajectory. This finding also provides provides evidence that a caregiver intervention may improve PD care recipeint QoL. Caregiver interventions have improved outcomes for both caregivers and care recipients in other disease populations (e.g., cancer, dementia, stroke, and cardiovascular disease), but have not thoroughly been studied in PD. As caregiver strain was found to be related to patients’ QoL, improvement in QoL for PD patients may hinge on addressing caregiver strain.
Additional predictors of class membership were TUG performance, age, and verbal fluency. Participants with worse performance on the TUG and younger age were more likely to be included in the problematic class than the moderate class. In contrast, participants with better performance on the TUG and better verbal fluency were more likely to be included in the favorable class than the moderate class. The impact of motor functioning on QoL in PD has previously been described (3,58–60); however, our study was the first to investigate the role of motor impairment in trajectories of QoL in PD (60). Similarly, several studies have elucidated the negative impact of specific domains of cognitive impairment on QoL in PD, such as attention (61), memory (62,63), self-rated cognitive function (64), and verbal fluency (63). The current study supplies additional evidence of the impact of verbal fluency and mobility on QoL trajectories in people with PD, suggesting that mobility and verbal fluency should be prioritized in this population through referrals to physical therapy and speech therapy. Furthermore, as TUG performance was associated with membership in both the favorable and problematic class, TUG scores may be useful for differentiating QoL trajectories in the general PD population. In order to reduce risk of membership in the problematic class, individuals with slow TUG scores may require additional services, such as physical therapy or referrals to exercise programs.
In addition to identifying predictors of QoL trajectories in this population, our study contributes to the existing evidence on how QoL changes in people with PD. The observed rate of change in QoL over time in this sample has been reported in other longitudinal investigations of QoL in PD (60,65); however, this analysis was one of the first to employ a growth mixture model (66) and was the first anlaysis to include caregiver factors as predictors of QoL change. Horvath et al. (2017) determined clinically meaningful change in PDQ-39 to be around −4.72 for worsening and 4.22 for improvement (67). The changes in QoL over the 4 observed years in the current study were: class 1 (−11), class 2 (14.4), and class 3 (15.2), surpassing the clinically meaningful change. Notably, patients in the moderate class and the favorable class, with a better QoL at baseline, suffer the largest degree of QoL worsening during years 8–11 of the disease, a time when patients are likely to transition in their level of caregiver needs and independence to some degree (36,68). As such, it might be these patients who can be targeted with preventative interventions versus those in the problematic class, who require mitigating interventions.
Our study had several strengths. First, it employed a longitudinal anlaysis to understand QoL in this population. QoL generally declines over time in people with PD and therefore studying QoL longitudinally is critical (15,16). Furthermore, our study employed growth mixture models to model heterogeneity of QoL in PD. This approach can improve the ability to detect important associations that can be missed by conventional models of longitudinal analyses that assume a mean trajectory for the entire sample (69). Lastly, our study was the first to examine the influence of caregiver strain on PD QoL change, providing new evidence of the role of the careigver in predicting the change in QoL of people with PD over time.
Although this study had several strengths, there are some limitations that are important to acknowledge. Only a few caregiver variables (i.e., MSCI and caregiver-care recipient relationship) were available in the current dataset. Other caregiver factors are associated with QoL of the care recipient, such as caregiver mental health (70,71), QoL for the caregiver (72–74), and relationship quality with the care recipient (75,76). Future research should examine the influence of additional caregiver factors on QoL trajectories for people with PD. For the PD variables, we only included verbal fluency, TUG time, disease severity, depression, and sex in our analysis in order to evaluate if caregiver strain was an independent predictor of membership in a class determined by changes in QoL. While other variables are included in the POP dataset and could influence QoL, our variable selection was informed by the literature and therefore we feel our analysis adequately addresses our research question. One variable that has previously been shown to influence QoL is motor functioning and the severity of neuropsychiatric symptoms (12,58–60,77–79). While we included a measure of mobility and depression, other measures (i.e., UPDRS, NMS) are missing from the dataset or were not consistenly captured, preventing their inclusion. These factors are important to consider in future work.
Lastly, our study was not population based because all the data were collected from Centers of Excellence. Not all individuals with PD can access Centers of Excellence for movement disorders and therefore participants in the current study may experience a different disease course than a community-based sample. Additionally, we examined individual-level variables and did not include a multilevel modeling approach to investigate the influence of study site on QoL. However, this is the first investigation of caregiver factors that are associated with QoL trajectories and it is important to use a large and well-characterized Parkinson’s disease sample, making the POP cohort the most appropriate. Future research examining a community-based sample and analyses employing a multi-level modeling approach is needed.
Conclusion
Our findings highlight the influence of caregiver strain, motor function, and cognitive function on QoL trajectories for people with PD. The findings can inform novel interventions targeting caregiver strain to improve the QoL for the caregiver and care recipient. Our research emphasizes the importance of considering the caregiver and their health during clinical visits for the care recipient as this can indicate future outcomes for the person with PD as well. We recommend that a caregiver strain measure be administered to caregivers when possible, during clinic visits to identify caregivers who may require referrals to health services. Future research is needed to identify additional caregiver factors that could contribute to QoL. A longtiudinal study conducted in a community-based sample with extensive caregiver factors (e.g., caregiver QOL, mental health, caregiver/care recipient relationship quality) and care recipient factors (e.g. NMS severity and MDS-UPDRS scores), would address some of the limitations of the current analysis.
We identified three quality of life (QOL) trajectories of people with PD for longer than 8 years.
Lower caregiver strain independently predicted membership in a class with a favorable QOL trajectory.
Better performance on a verbal fluency task also predicted membership in a class with a favorable QOL trajectory.
Differing performance on a mobility task was associated with membership in a favorable and problematic class.
Acknowledgements:
Kate Perepezko and the study described in this manuscript was funded by the NIH/NIA as part of a F31 award (1F31AG066316). Jared T Hinkle Receives tuition and stipend support through the Medical Scientist Training Program at the Johns Hopkins School of Medicine (NIH/NIGMS 5 T32 GM007309). The data used for this analysis was provided by the Parkinson Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure/Conflict of Interest Statement: The other authors report no conflicts of interest, financial disclosures, or funding pertinent to this manuscript. Greg Pontone has consulted for Acadia Pharmaceuticals Inc.
References
- (1).Jankovic J Parkinson’s disease: clinical features and diagnosis. Journal of Neurology, Neurosurgery & Psychiatry 2008;79(4):368–376. [DOI] [PubMed] [Google Scholar]
- (2).Schrag A, Jahanshahi M, Quinn N. How does Parkinson’s disease affect quality of life? A comparison with quality of life in the general population. Movement Disorders 2000;15(6):1112–1118. [DOI] [PubMed] [Google Scholar]
- (3).Kuhlman GD, Flanigan JL, Sperling SA, Barrett MJ. Predictors of health-related quality of life in Parkinson’s disease. Parkinsonism & related disorders 2019. Aug;65:86–90. [DOI] [PubMed] [Google Scholar]
- (4).O Ja, BW, Leonardi M, Opara J. Quality of life in Parkinson`s Disease. Journal of Medicine and Life ;5:375. [PMC free article] [PubMed] [Google Scholar]
- (5).Soh S, Morris ME, McGinley JL. Determinants of health-related quality of life in Parkinson’s disease: A systematic review. Parkinsonism related disorders 2010;17(1):1–9. [DOI] [PubMed] [Google Scholar]
- (6).Cano-de-la-Cuerda Roberto, Vela-Desojo Lydia, Miangolarra-Page Juan Carlos, Macías-Macías Yolanda, Muñoz-Hellín Elena. Axial rigidity and quality of life in patients with Parkinson’s disease: a preliminary study. Qual Life Res 2011. Aug 01,;20(6):817–823. [DOI] [PubMed] [Google Scholar]
- (7).Louis ED, Machado DG. Tremor-related quality of life: A comparison of essential tremor vs. Parkinson’s disease patients. Parkinsonism & Related Disorders 2015. -July;21(7):729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Karlsen KH, Larsen JP, Tandberg E, Mæland JG. Influence of clinical and demographic variables on quality of life in patients with Parkinson’s disease. Journal of neurology, neurosurgery and psychiatry 1999. Apr;66(4):431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson’s disease? Journal of neurology, neurosurgery and psychiatry 2000. Sep;69(3):308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Quelhas R, Costa M. Anxiety, Depression, and Quality of Life in Parkinson’s Disease. The journal of neuropsychiatry and clinical neurosciences 2009. Oct;21(4):413–419. [DOI] [PubMed] [Google Scholar]
- (11).Hanna KK, Cronin-Golomb A. Impact of Anxiety on Quality of Life in Parkinson’s Disease. Parkinson’s disease 2012. Dec 01,;2012:640707–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Movement disorders 2009;24(11):1641–1649. [DOI] [PubMed] [Google Scholar]
- (13).Scherbaum R, Hartelt E, Kinkel M, Gold R, Muhlack S, Tönges L. Parkinson’s Disease Multimodal Complex Treatment improves motor symptoms, depression and quality of life. J Neurol 2020;267(4):954–965. [DOI] [PubMed] [Google Scholar]
- (14).Zhang Y, Chen W, Lu L, de Wang C, Zhang X. Multiple comorbid sleep disorders adversely affect quality of life in Parkinson’s disease patients. NPJ Parkinson’s disease 2020;6(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Reuther M, Spottke EA, Klotsche J, Riedel O, Peter H, Berger K, et al. Assessing health-related quality of life in patients with Parkinson’s disease in a prospective longitudinal study. Parkinsonism Relat Disord 2007;13(2):108–114. [DOI] [PubMed] [Google Scholar]
- (16).Den Oudsten BL, Van Heck GL, De Vries J. Quality of life and related concepts in Parkinson’s disease: a systematic review. Movement disorders: official journal of the Movement Disorder Society 2007;22(11):1528–1537. [DOI] [PubMed] [Google Scholar]
- (17).Chen K, Tan Y, Lu Y, Wu J, Liu X, Zhao Y. Effect of Exercise on Quality of Life in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Parkinson’s disease 2020. Jul 09,;2020:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Martinez-Martin P, Deuschl G. Effect of medical and surgical interventions on health-related quality of life in Parkinson’s disease. Movement disorders 2007. Apr 30,;22(6):757–765. [DOI] [PubMed] [Google Scholar]
- (19).Jin X, Wang L, Liu S, Zhu L, Loprinzi PD, Fan X. The Impact of Mind-Body Exercises on Motor Function, Depressive Symptoms, and Quality of Life in Parkinson’s Disease: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health 2019. Dec 18,;17(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Van Houtven CH, Voils CI, Weinberger M. An organizing framework for informal caregiver interventions: detailing caregiving activities and caregiver and care recipient outcomes to optimize evaluation efforts. BMC geriatrics 2011;11(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hand A, Oates LL, Gray WK, Walker RW. The role and profile of the informal carer in meeting the needs of people with advancing Parkinson’s disease. Aging & mental health 2019;23(3):337–344. [DOI] [PubMed] [Google Scholar]
- (22).Wong SL, Gilmour H, Ramage-Morin P. Parkinson’s disease: Prevalence, diagnosis, and impact. 2014. November 19,. [PubMed]
- (23).Miller EA, Weissert WG. Predicting elderly people’s risk for nursing home placement, hospitalization, functional impairment, and mortality: a synthesis. Medical care research and review 2000;57(3):259–297. [DOI] [PubMed] [Google Scholar]
- (24).Freedman VA, Spillman BC. Disability and care needs among older Americans. Milbank Q 2014;92(3):509–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).DiMatteo MR. Social support and patient adherence to medical treatment: a meta-analysis. Health psychology 2004;23(2):207. [DOI] [PubMed] [Google Scholar]
- (26).Zulman DM, Nazi KM, Turvey CL, Wagner TH, Woods SS, An LC. Patient interest in sharing personal health record information: a web-based survey. Ann Intern Med 2011;155(12):805–810. [DOI] [PubMed] [Google Scholar]
- (27).Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of Nursing Home Placement in Parkinson’s Disease: A Population-Based, Prospective Study. Journal of the American Geriatrics Society (JAGS) 2000. Aug;48(8):938–942. [DOI] [PubMed] [Google Scholar]
- (28).Dufournet M, Dauphinot V, Moutet C, Verdurand M, Delphin-Combe F, Krolak-Salmon P, et al. Impact of cognitive, functional, behavioral disorders, and caregiver burden on the risk of nursing home placement. Journal of the American Medical Directors Association 2019;20(10):1254–1262. [DOI] [PubMed] [Google Scholar]
- (29).Burgener S, Twigg P. Relationships Among Caregiver Factors and Quality of Life in Care Recipients with Irreversible Dementia. 2002. February;16(2). [DOI] [PubMed] [Google Scholar]
- (30).Brodaty H, Berman K. Interventions for family caregivers of people with dementia. 2008.
- (31).da Silva EG, Cuziol K, Viana MA, Barasnevicius EMA. Quality of life in patients with Parkinson’s Disease and their caregivers’ stress levels. Revista Neurociências 2008;16(2):113–117. [Google Scholar]
- (32).Peters M, Fitzpatrick R, Doll H, Playford D, Jenkinson C. Does self-reported well-being of patients with Parkinson’s disease influence caregiver strain and quality of life? Parkinsonism Relat Disord 2011;17(5):348–352. [DOI] [PubMed] [Google Scholar]
- (33).Maetzler W, Liepelt I, Berg D. Progression of Parkinson’s disease in the clinical phase: potential markers. The Lancet Neurology 2009;8(12):1158–1171. [DOI] [PubMed] [Google Scholar]
- (34).Muthén B Latent variable analysis. The Sage handbook of quantitative methodology for the social sciences 2004;345(368):106–109. [Google Scholar]
- (35).Okun MS, Siderowf A, Nutt JG, O’Conner GT, Bloem BR, Olmstead EM, et al. Piloting the NPF data-driven quality improvement initiative. Parkinsonism Relat Disord 2010;16(8):517–521. [DOI] [PubMed] [Google Scholar]
- (36).Lokk J Caregiver strain in Parkinson’s disease and the impact of disease duration. European Journal of Physical and Rehabilitation Medicine 2007. October;44(1). [PubMed] [Google Scholar]
- (37).Auyeung M, Tsoi TH, Mok V, Cheung CM, Lee CN, Li R, et al. Ten year survival and outcomes in a prospective cohort of new onset Chinese Parkinson’s disease patients. Journal of Neurology, Neurosurgery & Psychiatry 2012;83(6):607–611. [DOI] [PubMed] [Google Scholar]
- (38).Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age Ageing 1997;26(5):353–357. [DOI] [PubMed] [Google Scholar]
- (39).Oguh O, Kwasny M, Carter J, Stell B, Simuni T. Caregiver strain in Parkinson’s disease: national Parkinson Foundation Quality Initiative study. Parkinsonism Relat Disord 2013;19(11):975–979. [DOI] [PubMed] [Google Scholar]
- (40).Kelly DH, McGinley JL, Huxham FE, Menz HB, Watts JJ, Iansek R, et al. Health-related quality of life and strain in caregivers of Australians with Parkinson’s disease: An observational study. BMC neurology 2012. Jul 17;12(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Klein PJ, Rivers L. Taiji for individuals with Parkinson disease and their support partners: program evaluation. Journal of Neurologic Physical Therapy 2006;30(1):22–27. [DOI] [PubMed] [Google Scholar]
- (42).Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations the Movement Disorder Society Task Force on rating scales for Parkinson’s disease. Movement disorders 2004;19(9):1020–1028. [DOI] [PubMed] [Google Scholar]
- (43).Chou KL, Amick MM, Brandt J, Camicioli R, Frei K, Gitelman D, et al. A recommended scale for cognitive screening in clinical trials of Parkinson’s disease. Movement disorders 2010. Nov 15,;25(15):2501–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Archives of clinical neuropsychology 1999;14(2):167–177. [PubMed] [Google Scholar]
- (45).Cholerton BA, Zabetian CP, Wan JY, Montine TJ, Quinn JF, Mata IF, et al. Evaluation of mild cognitive impairment subtypes in Parkinson’s disease. Movement disorders 2014. May;29(6):756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. Journal of the American Geriatrics Society (JAGS) 2005. Apr;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- (47).Viccaro LJ, Perera S, Studenski SA. Is Timed Up and Go Better Than Gait Speed in Predicting Health, Function, and Falls in Older Adults? Journal of the American Geriatrics Society (JAGS) 2011. May;59(5):887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).StataCorp L. Stata. 2020;16.1. [Google Scholar]
- (49).Muthén LK, Muthén BO. Mplus user’s guide (Seventh). Los Angeles, CA: Muthén & Muthén; 2012. [Google Scholar]
- (50).Jung T, Wickrama KAS. An Introduction to Latent Class Growth Analysis and Growth Mixture Modeling. Social and personality psychology compass 2008. Jan;2(1):302–317. [Google Scholar]
- (51).Muthén B, Muthén LK. Integrating person-centered and variable-centered analyses: Growth mixture modeling with latent trajectory classes. Alcoholism: Clinical and experimental research 2000;24(6):882–891. [PubMed] [Google Scholar]
- (52).Asparouhov T, Muthén B. Auxiliary variables in mixture modeling: Three-step approaches using M plus. Structural equation modeling: A multidisciplinary Journal 2014;21(3):329–341. [Google Scholar]
- (53).Bolck A, Croon M, Hagenaars J. Estimating latent structure models with categorical variables: One-step versus three-step estimators. Political analysis 2004;12(1):3–27. [Google Scholar]
- (54).Kuhlman GD, Flanigan JL, Sperling SA, Barrett MJ. Predictors of health-related quality of life in Parkinson’s disease. Parkinsonism & related disorders 2019. Aug;65:86–90. [DOI] [PubMed] [Google Scholar]
- (55).Williams-Gray CH, Mason SL, Evans JR, Foltynie T, Brayne C, Robbins TW, et al. The CamPaIGN study of Parkinson’s disease: 10-year outlook in an incident population-based cohort. Journal of neurology, neurosurgery and psychiatry 2013. Nov;84(11):1258–1264. [DOI] [PubMed] [Google Scholar]
- (56).Peters M, Fitzpatrick R, Doll H, Playford D, Jenkinson C. Does self-reported well-being of patients with Parkinson’s disease influence caregiver strain and quality of life? Parkinsonism Relat Disord 2011;17(5):348–352. [DOI] [PubMed] [Google Scholar]
- (57).da Silva EG, Cuziol K, Viana MA, Barasnevicius EMA. Quality of life in patients with Parkinson’s Disease and their caregivers’ stress levels. Revista Neurociências 2008;16(2):113–117. [Google Scholar]
- (58).Cano-de-la-Cuerda Roberto, Vela-Desojo Lydia, Miangolarra-Page Juan Carlos, Macías-Macías Yolanda, Muñoz-Hellín Elena. Axial rigidity and quality of life in patients with Parkinson’s disease: a preliminary study. Qual Life Res 2011. Aug 01,;20(6):817–823. [DOI] [PubMed] [Google Scholar]
- (59).Nocera JR, PhD, Stegemöller EL, PhD, Malaty IA, MD, Okun MS, MD, Marsiske M, PhD, Hass CJ, PhD. Using the Timed Up & Go Test in a Clinical Setting to Predict Falling in Parkinson’s Disease. Archives of physical medicine and rehabilitation 2013;94(7):1300–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Marras C, McDermott MP, Rochon PA, Tanner CM, Naglie G, Lang AE. Predictors of deterioration in health-related quality of life in Parkinson’s disease: Results from the DATATOP trial. Movement disorders 2008. Apr 15,;23(5):653–659. [DOI] [PubMed] [Google Scholar]
- (61).Lawson RA, Yarnall AJ, Duncan GW, Breen DP, Khoo TK, Williams-Gray CH, et al. Cognitive decline and quality of life in incident Parkinson’s disease: The role of attention. Parkinsonism & Related Disorders 2016;27:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Movement disorders 2009. Aug 15,;24(11):1641–1649. [DOI] [PubMed] [Google Scholar]
- (63).Dauwerse L, Hendrikx A, Schipper K, Struiksma C, Abma TA. Quality-of-life of patients with Parkinson’s disease. Brain injury 2014;28(10):1342–1352. [DOI] [PubMed] [Google Scholar]
- (64).Marras C, McDermott MP, Rochon PA, Tanner CM, Naglie G, Lang AE. Predictors of deterioration in health-related quality of life in Parkinson’s disease: Results from the DATATOP trial. Movement disorders 2008. Apr 15,;23(5):653–659. [DOI] [PubMed] [Google Scholar]
- (65).Forsaa EB, Larsen JP, Wentzel-Larsen T, Herlofson K, Alves G. Predictors and course of health-related quality of life in Parkinson’s disease. Movement disorders 2008. Jul 30,;23(10):1420–1427. [DOI] [PubMed] [Google Scholar]
- (66).Klotsche J, MSc, Reese Jens Peter, PhD, MPH, Winter Y, MD, Oertel WH, MD, Irving H, MSc, Wittchen H, PhD, et al. Trajectory Classes of Decline in Health-Related Quality of Life in Parkinson’s Disease: A Pilot Study. Value in health 2011;14(2):329–338. [DOI] [PubMed] [Google Scholar]
- (67).Horváth K, Aschermann Z, Kovács M, Makkos A, Harmat M, Janszky J, et al. Changes in quality of life in Parkinson’s disease: how large must they be to be relevant? Neuroepidemiology 2017;48(1–2):1–8. [DOI] [PubMed] [Google Scholar]
- (68).Boersma I, Jones J, Coughlan C, Carter J, Bekelman D, Miyasaki J, et al. Palliative care and Parkinson’s disease: caregiver perspectives. J Palliat Med 2017;20(9):930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Terrera GM, Brayne C, Matthews F. One size fits all? Why we need more sophisticated analytical methods in the explanation of trajectories of cognition in older age and their potential risk factors. International psychogeriatrics 2010;22(2):291–299. [DOI] [PubMed] [Google Scholar]
- (70).Bartolomei L, Pastore A, Meligrana L, Sanson E, Bonetto N, Minicuci GM, et al. Relevance of sleep quality on caregiver burden in Parkinson’s disease. Neurol Sci 2018. Feb 14,;39(5):835–839. [DOI] [PubMed] [Google Scholar]
- (71).Trang I, Katz M, Galifianakis N, Fairclough D, Sillau SH, Miyasaki J, et al. Predictors of general and health-related quality of life in Parkinson’s disease and related disorders including caregiver perspectives. Parkinsonism Relat Disord 2020;77:5–10. [DOI] [PubMed] [Google Scholar]
- (72).Martinez-Martin P, Benito-Leon J, Alonso F, Catalán Mª, Pondal M, Zamarbide I, et al. Quality of life of caregivers in Parkinson’s disease. Quality of life research 2005;14(2):463–472. [DOI] [PubMed] [Google Scholar]
- (73).Miyashita M, Narita Y, Sakamoto A, Kawada N, Akiyama M, Kayama M, et al. Health-related quality of life among community-dwelling patients with intractable neurological diseases and their caregivers in Japan. Psychiatry and clinical neurosciences 2011. Feb;65(1):30–38. [DOI] [PubMed] [Google Scholar]
- (74).Navarta-Sanchez MV, Senosiain Garcia JM, Riverol M, Ursua Sesma ME, Diaz de Cerio Ayesa S, Anaut Bravo S, et al. Factors influencing psychosocial adjustment and quality of life in Parkinson patients and informal caregivers. Qual Life Res 2016. Aug 01,; 25(8):1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Ricciardi L, Pomponi M, Demartini B, Ricciardi D, Morabito B, Bernabei R, et al. Emotional awareness, relationship quality, and satisfaction in patients with parkinson’s disease and their spousal caregivers. J Nerv Ment Dis 2015;203(8):646–649. [DOI] [PubMed] [Google Scholar]
- (76).Karlstedt M, Fereshtehnejad S, Aarsland D, Lökk J. Mediating Effect of Mutuality on Health-Related Quality of Life in Patients with Parkinson’s Disease. Parkinson’s disease 2018. Sep 16,; 2018:9548681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Schrag A, Jahanshahi M, Quinn N. How does Parkinson’s disease affect quality of life? A comparison with quality of life in the general population. Movement disorders: official journal of the Movement Disorder Society 2000;15(6):1112–1118. [DOI] [PubMed] [Google Scholar]
- (78).Morris S, Morris ME, Iansek R. Reliability of measurements obtained with the Timed “Up & Go” test in people with Parkinson disease. Phys Ther 2001;81(2):810–818. [DOI] [PubMed] [Google Scholar]
- (79).Palmerini L, Mellone S, Avanzolini G, Valzania F, Chiari L. Quantification of motor impairment in Parkinson’s disease using an instrumented timed up and go test. IEEE transactions on neural systems and rehabilitation engineering 2013;21(4):664–673. [DOI] [PubMed] [Google Scholar]


