Abstract
Purpose:
Niemann-Pick Disease, type C1 (NPC1) is a neurodegenerative, lysosomal disorder caused by pathogenic variants in NPC1. Disease progression is monitored using the NPC Neurological Severity Scale (NPC-NSS), but there are currently no established validated or qualified biomarkers. Neurofilament light chain (NfL) is being investigated as a biomarker in multiple neurodegenerative diseases.
Methods:
Cross-sectional and longitudinal cerebral spinal fluid (CSF) samples were obtained from 116 individuals with NPC1. NfL levels were measured using a solid-phase sandwich ELISA and compared to age-appropriate non-NPC1 comparison samples.
Results:
Median levels of NfL were elevated at baseline (1152 [680–1840] pg/ml) in NPC1 compared to controls (167 [82–372] pg/ml; p<0.001). Elevated NfL levels were associated with more severe disease as assessed by both the 17-domain and 5-domain NPC-NSS. Associations were also observed with ambulation, fine motor, speech, and swallowing scores. Although treatment with the investigational drug 2-hydroxypropyl-β-cyclodextrin (Adrabetadex) did not decrease CSF NfL levels, miglustat therapy over time was associated with a decrease (OR=0.77, 95% CI 0.62–0.96).
Conclusions:
CSF NfL levels are increased in individuals with NPC1, are associated with clinical disease severity and are decreased with miglustat therapy. These data suggest that NfL is a biomarker that may have utility in future therapeutic trials.
Introduction
Niemann-Pick Disease, type C (NPC) is a lysosomal disorder caused by biallelic pathogenic variants in either NPC1 or NPC2.1 NPC1 is a large transmembrane protein located on the lysosomal membrane and NPC2 is a small soluble protein within the lysosome. Impaired function of either of these proteins results in impaired intracellular cholesterol trafficking and the accumulation of unesterified cholesterol in late endosomes and lysosomes. 2 NPC is estimated to affect approximately 1/120,000 – 150,000 births worldwide.1
NPC can present at any age with a wide range of clinical presentations. Systemic symptoms can be apparent at birth and include neonatal cholestasis, jaundice, or hepatosplenomegaly. Neonatal liver disease can be severe but is often transitory. The onset of neurological symptoms can be insidious and present in childhood as learning delays or clumsiness with progression to cerebellar ataxia, dysarthria, and dysphagia.1 Individuals with neurological symptom onset in adulthood tend to present with cognitive and psychiatric symptoms.3 Nearly all NPC patients will develop the characteristic vertical supranuclear gaze palsy (VSGP).
Severity and progression of NPC1 can be quantitatively characterized using the NPC Neurological Severity Scale (NSS).4 The NPC-NSS has been shown to have high interrater reliability4 and ascertain clinically significant aspects of the disease.5 The NPC-NSS quantifies neurological disease progression by scaling severity of disease in nine major (ambulation, cognition, fine motor, speech, swallowing, eye movement, seizures, hearing, memory) and eight minor (auditory brainstem response, behavior, gelastic cataplexy, hyperreflexia, incontinence, narcolepsy, psychiatric, respiratory status) domains. Higher NSS scores indicate increased disease burden and can be used to track NPC1 disease progression.4 For use in therapeutic trials, the NPC-NSS has been condensed to a 5-domain (ambulation, cognition, fine motor, speech, and swallowing) scale which represents core domains most important to families and clinicians.6 Rate of progression can be accounted for by age-adjusting the NPC-NSS and is represented by the Age Adjusted Severity Index (ASIS) score.6
There is currently no FDA-approved therapy for NPC. Miglustat is a small iminosugar that inhibits the production of sphingolipids. It is FDA-approved to treat type I Gaucher disease.7 Although miglustat has been approved for use to treat individuals with NPC by the EMA and other regulatory agencies since 2009, it is not labeled for treatment of NPC in the United States and is used off-label. 2-hydroxypropyl-β-cyclodextrin (HPβCD, VTS-270/adrabetadex) administered intrathecally has been under investigation for treatment of neurological symptoms of NPC for the past eight years.8 Intravenous adrabetadex may also be effective in treating neonatal liver disease.9 Identification of biomarkers which reflect NPC-associated progression of central nervous system (CNS) damage would be beneficial in monitoring both disease progression and therapeutic responses.
Neurofilaments are neuronal cytoskeletal proteins classified as light, medium, or heavy chains based on molecular mass. Neurofilament light chain (NfL) is an intermediate-sized fiber (~10nm) that is primarily found in neuronal axons and has a low rate of turnover.10 They are thought to have a role in axonal stability, radial growth, and interact with multiple other proteins and organelles.11 NfL and other neurofilaments are released into the CSF during neuroaxonal damage and therefore can be used as a marker of neurodegeneration. NfL is detectable in serum12, although at approximately 100-fold lower concentration. Neurofilaments have been studied as markers of neuronal injury in multiple neurological disorders such as epilepsy, multiple sclerosis, dementia, amyotrophic lateral sclerosis, Huntington disease, stroke, and traumatic brain injury.13–18 These studies have shown that the level of NfL detected in serum and CSF increases with disease severity and in some cases also showed improvement in NfL levels with treatments.19–23
In this study we hypothesized that CSF NfL levels would be elevated in individuals with NPC1 and would be associated with clinical aspects of the disease. To test this hypothesis, we analyzed cross-sectional and longitudinal CSF samples collected from participants in a natural history/observational trial and those receiving HPβCD in a therapeutic trial or via expanded access. The data in this paper show that CSF NfL levels are elevated in individuals with NPC1, are positively associated with clinically relevant disease signs/symptoms and are reduced in individuals treated with miglustat.
Methods and Materials
Study Participant Recruitment and Sample Collection
This study was performed both at the NIH Clinical Center in Bethesda, Maryland, and at Rush University Medical Center (RUMC) in Chicago, Illinois. Both the NICHD and RUMC Institutional Review Boards approved the study elements relevant to their site. A total of 116 NPC1 participants were evaluated between 2006 and 2019. The population was derived from an observational/natural history study at the NIH Clinical Center in Bethesda, Maryland (NCT00001367), from a phase I/II intrathecal trial of adrabetadex (NCT01747135), from a phase II/III intrathecal trial of adrabetadex (NCT02534844) and from individuals receiving intrathecal adrabetadex through an expanded access program (IND 119856). Written informed consent was obtained from guardians or participants. Assent was obtained when applicable. During each visit, participants were clinically examined and disease severity was assessed using the NPC-NSS4 along with the American Speech-Language-Hearing Association’s National Outcomes Measurement System Dysphagia subdomain, (ASHA-NOMS, at NIH only) and the Penetration-Aspiration Scale (PAS), derived from videofluoroscopic evaluations of swallowing function.24,25 Annual severity increment score (ASIS) was calculated by dividing the total severity score by the age of the participant to measure rate of disease progression in each participant.6 CSF was obtained during each visit. If adrabetadex was administered, it was done after CSF collection. CSF was also obtained from non-NPC comparison groups. (n=30). Collection of CSF from unaffected children is precluded by ethical and statutory considerations. Anonymized, residual, unused pediatric CSF samples (pediatric laboratory controls) were obtained from patients being evaluated for non-NPC-indications (n=9). Samples with elevated blood counts or positive cultures were excluded. Age, sex, and clinical information was available. We did not have control over how these pediatric laboratory control samples were handled prior to obtaining them. Thus, we also used samples collected from individuals with either Creatine Transporter Deficiency (CTD; NCT02931682) (n=9) or Smith-Lemli-Opitz Syndrome (SLOS; NCT00001721) (n=12). These samples were collected, processed and stored in a manner identical to the NPC1 CSF. Although CTD and SLOS are neurodevelopmental disorders, they are distinctly different from NPC1 and do not involve neurodegeneration. Pairwise comparison of the three comparison cohorts did not reveal any significant differences in NfL levels.
Neurofilament Light Chain Assay
NfL levels in CSF were measured using a solid-phase sandwich ELISA kit (UmanDiagnostics, Umea, Sweden; Catalog number: 10–7002 RUO) per manufacturer’s instructions. Briefly, frozen CSF samples were thawed on ice, spun at 300g for 5 min, supernatants were collected and diluted 1:2 with sample diluent provided in the kit, aliquoted randomly on 96-well plates, and analyzed in singlets. All measurements were performed on coded samples and the diagnostic code was broken only after NfL levels were measured and quality controlled. Lower limit of detection (LLOD) of the assay is 33 pg/ml; the mid-point value of 16.5 pg/ml between the 0 and LLOD was used for samples with NfL concentrations below LLOD. All CSF samples were analyzed in 2 batches (plates). A control CSF sample was analyzed in each batch; coefficient of variation (CV) for control sample across the batches was 4.7%, validating assay precision and reproducibility.
Statistical Analysis
Data were described using frequencies and percentages and mean ± standard deviation or median (inter-quartile range), as appropriate. All distributions were assessed for normality, and data were analyzed accordingly. Comparisons between groups (NPC and non-NPC controls) were carried out using t-tests or Wilcoxon rank-sum tests, and Fisher’s exact tests if nominal. One way analysis of variance (ANOVA) was used for comparing NfL among age categories in NPC1 and non-NPC1 controls. Spearman correlation coefficients with Fisher’s z transformation was used to assess specific correlations at baseline and last visit, and results are reported as rs and 95% Confidence Intervals (CI). These data were also log-transformed to aid their visualization in scatterplots. Longitudinal analyses were carried out using generalized linear models (proc genmod in SAS) with procedures for ordinal multinomial outcomes (eg, speech, memory, ASHA-NOMS) and continuous measurement outcomes (eg, NSS, 5-domain scale). Outcome probabilities for severity of disease (disease progression) were modeled, and accounted for repeated measures and within subject correlations. All models included NfL (per pg/L). Initial NfL data was reported in pg/ml; however, for meaningful interpretations and plotting the forest plots, the models used larger units of pg/L. The covariates in the longitudinal models included duration of follow-up (years), age (years), duration of neurological symptoms (years), seizures (none or mild vs severe), miglustat therapy (yes vs no), and HPβCD therapy (yes vs no). For seizures none/mild was defined as a seizure domain NSS score of 0,1 or 2 and severe was defined as a score of 3 or 5. Sensitivity testing showed that inclusion of sex in the models does not have any impact on the outcome conclusions. This is consistent with minimal gender differences reported for NfL 26–28. Sensitivity testing also showed no differences by recruitment site. Final reduced models varied by outcome and included covariates that remained associated with each outcome after iterative analytic steps. Sensitivity analyses were carried out for 1) the mix of non-NPC controls and 2) exclusion of 41 participants with only one visit from correlations on last visit data. These results supported combining non-NPC controls and including participants with no follow-up in baseline and last visit cross-sectional analyses (details are not shown). Odds Ratios (OR) and 95% CIs describe the magnitude and direction of the effects, and p-values are reported where appropriate. Data were analyzed using SAS v9.4 (SAS Institute, Inc, Cary, NC).
Results
Characteristics of Study Participants
Baseline characteristics of the NPC1 study participants and non-NPC1 controls are reported in Table 1. Demographics did not differ between recruitment sites (Supplemental Table 1). Non-NPC1 pediatric control CSF samples were from pediatric patients who had an indication other than NPC for lumbar puncture occurring in the emergency room. Median age of NPC1 study participants was 11.9 (4.4–19.7) years compared to median age of non-NPC1 controls (10.6 [5.4–14.0] years; p=0.35). NPC1 participants had approximately equal distributions of each sex; however, non-NPC1 controls were predominantly male (76.7% in non-NPC1 vs 48.3% in NPC1; p=0.007). Sex distribution skewing toward males was due to inclusion of CTD controls, though it has been found that there is minimal to no association between NfL levels and sex 26–28. Seventy-five (64.7%) NPC1 study participants had at least one follow-up visit, with 3.3 ± 3.1 visits on average (median 2.0 [1.0–4.0]) throughout their study participation; they were followed for up to 13.5 years (median 1.7 [0.0–4.0]).
Table 1:
Demographic and clinical characteristics of Niemann-Pick Disease, Type C1 (NPC1) study participants and non-NPC1 pediatric controls.
| Characteristics | NPC1 Cohort (n=116) | Non-NPC1 Controls (n=30) |
|---|---|---|
|
| ||
| Age at baseline - years | ||
| Mean ± SD | 14.8 ± 13.8 | 10.3 ± 5.5 |
| Median (IQR) | 11.9 (4.4 – 19.7) | 10.6 (5.4 – 14.0) |
| Range | 0.3 – 68.2 | 2.6 – 21.0 |
| P-value (NPC vs non-NPC) | 0.35 | |
|
| ||
| Sex - number (%) | ||
| Male | 56 (48.3) | 23 (76.7) |
| Female | 60 (51.7) | 7 (23.3) |
| P-value (NPC vs non-NPC) | 0.007 | |
|
| ||
| NPC NSS at baseline (4) | ||
| Mean ± SD | 15.1 ± 10.4 | N/A |
| Median (IQR) | 14.0 (6.0 – 21.0) | |
| Range | 0 – 42 | |
|
| ||
| Treatment status at baseline - number (%) | ||
| Miglustat | 53 (45.7) | N/A |
| HPβCD | 3 (2.6) | |
HPβCD = 2-hydroxypropyl-β-cyclodextrin; IQR = inter-quartile (25th-75th percentile) range; NPC = Niemann-Pick Disease, Type C1; NSS = NPC Neurological Severity Scale; SD = standard deviation
Elevation of NfL Levels in NPC1
NfL levels in CSF were substantially elevated in participants with NPC1 compared to non-NPC1 controls. Mean NfL level in NPC1 was (1478 ± 1548 pg/mL; median 1152 [680–1840]), while in controls it was (374 ± 633; median 167 [82–372]; p<0.001) (Figure 1). NPC1 NfL CSF levels were then partitioned by age groups as described by Vanier.1 Mean CSF NfL levels were elevated in all age categories relative to non-NPC1 controls, but decreased slightly across the four age categories (Figure 1). Although the number of CSF samples from NPC1 individuals with no neurological symptoms was low (n=6), the baseline mean CSF NfL level (1004 ± 500 pg/ml) was not significantly different (p=0.55, Mann Whitney test) than mean baseline level for 110 individuals manifesting neurological signs/symptoms (1504 ± 1583 pg/ml).
Figure 1. Distribution of cerebrospinal fluid NfL levels in individuals with Niemann-Pick disease, type C1 (NPC1).
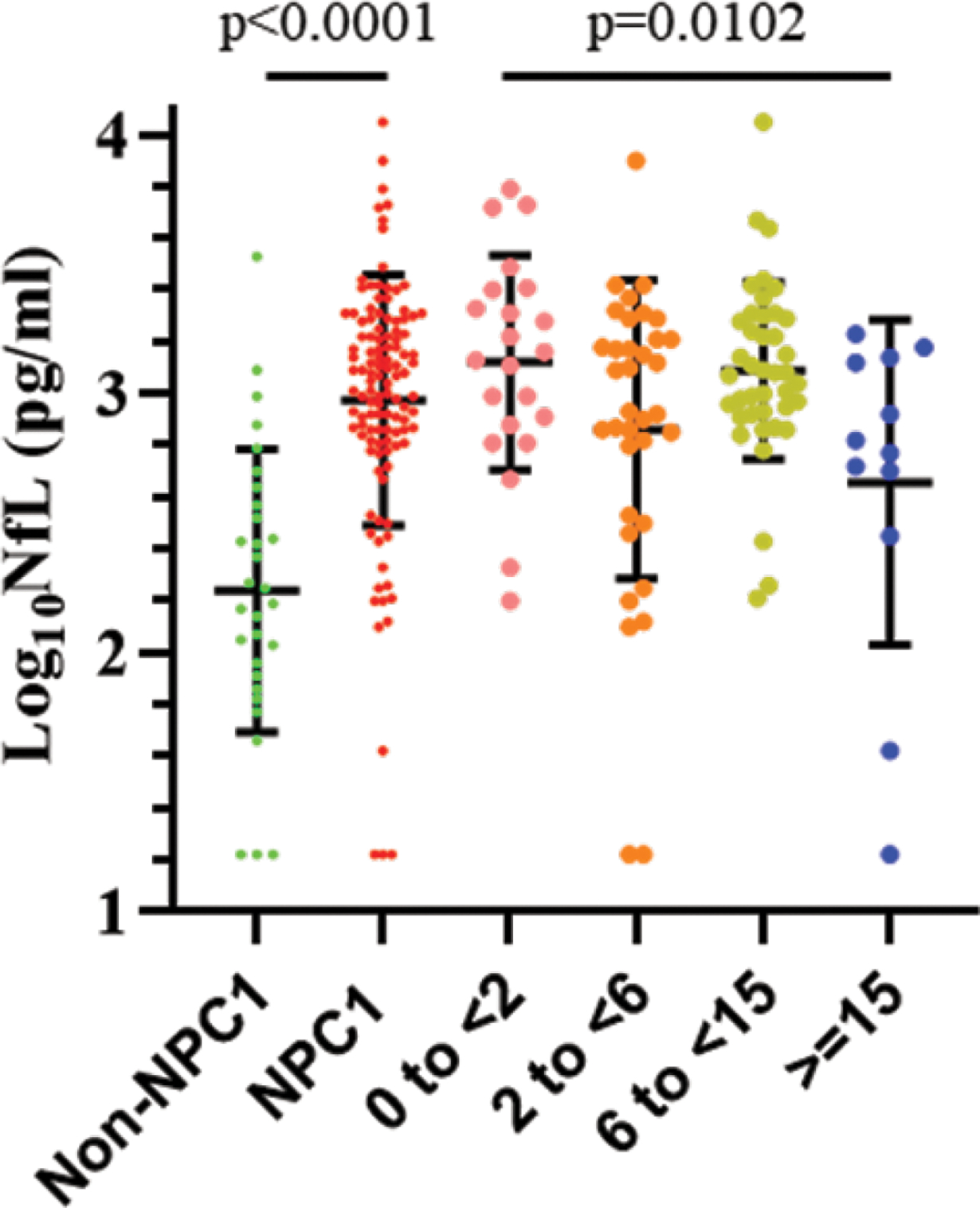
CSF NfL levels were significantly elevated (p<0.0001) in individuals NPC1 (red) relative to non-NPC1 controls (green). Age stratification, in years, based on neurological onset (early infantile (pink), late infantile (orange), juvenile (olive), adolescent/adult (blue)) demonstrated a slight but significant decrease (p=0.0102, one way ANOVA) from younger to older cohorts. Error bars indicate the mean ± SD.
Correlation of NfL Levels with Outcome Measurements
NfL levels in CSF of NPC1 study participants correlated with the severity of disease measured by the NPC-NSS (Figure 2A). At the baseline visit there was a weak, but notable correlation (rs=0.33, 95% CI 0.16–0.49; p<0.001) with 17-domain NPC-NSS and analogously weak correlation with the 5-domain NPC-NSS (rs=0.34, 95% CI 0.17–0.49; p<0.001) (Figure 2B). For all outcome measurements except 5-domain cognition and hearing, correlation between NfL level and disease severity became stronger between baseline and last visit (Table 2). Correlation figures for each outcome measurement at the baseline and last visits can be found in Supplementary Figures 1A–M.
Figure 2: Positive correlation at baseline visit of NfL levels to Niemann-Pick Disease, Type C1 (NPC1).
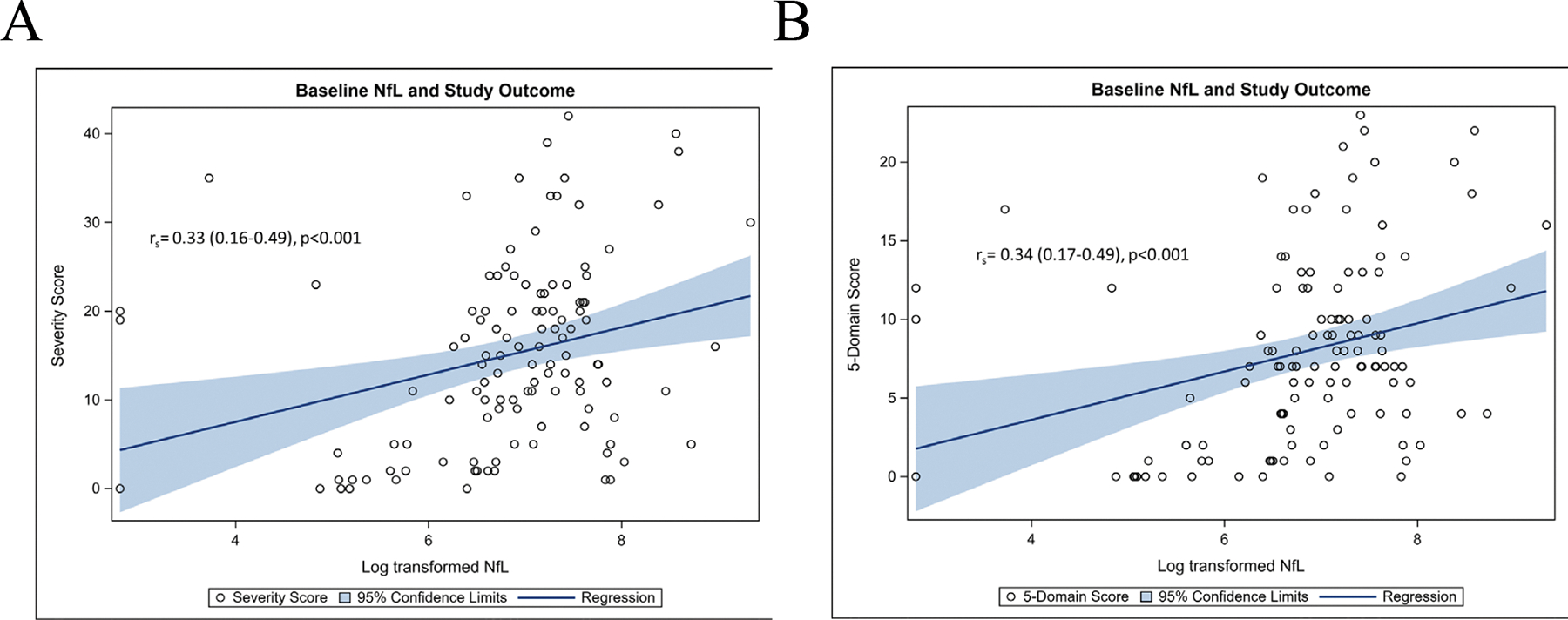
A. 17-domain Neurological Severity Score (NSS) and B. 5-domain scale. NfL was measured in units of pg/ml and presented in each panel in log-transformed scale.
Table 2:
Correlations between NfL and outcome measurements at baseline and last visit in patients with Niemann-Pick Disease, Type C1 (NPC1).
| Outcome Measurement | Correlation at Baseline rs (95% CI), p-value (n=116) |
Correlation at Final Visit rs (95% CI), p-value (n=116)a |
|---|---|---|
|
| ||
| NPC NSS | 0.33 (0.16–0.49), p<0.001 | 0.40 (0.24–0.54), p<0.001) |
|
| ||
| ASIS | 0.27 (0.10–0.43), p=0.003 | 0.36 (0.19–0.51), p<0.001 |
|
| ||
| Five-Domain scale | 0.34 (0.17–0.49), p<0.001 | 0.43 (0.27–0.57), p<0.001 |
| Ambulation | 0.27 (0.10–0.43), p=0.003 | 0.50 (0.35–0.63), p<0.001 |
| Fine Motor | 0.32 (0.14–0.47), p<0.001 | 0.40 (0.23–0.54), p<0.001 |
| Speech | 0.26 (0.08–0.42), p=0.005 | 0.34 (0.17–0.49), p<0.001 |
| Swallowing | 0.31 (0.13–0.46), p<0.001 | 0.37 (0.20–0.52), p<0.001 |
| Cognition | 0.36 (0.19–0.51), p<0.001 | 0.30 (0.13–0.46), p<0.001 |
|
| ||
| Eye Movement | 0.24 (0.06–0.41), p=0.008 | 0.31 (0.13–0.46), p<0.001 |
|
| ||
| Hearing | 0.14 (−0.04–0.32), p=0.13 (n=114) |
0.11 (−0.07–0.29), p=0.24 (n=114) |
|
| ||
| Memory | 0.33 (0.16–0.49), p<0.001 | 0.35 (0.18–0.50), p<0.001 |
|
| ||
| ASHA-NOMS | 0.15 (−0.05–0.34), p=0.15 (n=94) |
0.25 (0.04–0.44), p=0.021 (n=84) |
|
| ||
| PAS | 0.12 (−0.08–0.32), p=0.25 (n=94) |
0.19 (−0.02–0.39), p=0.071 (n=84) |
n=41 patients had only one visit and their baseline and last visits overlapped; sensitivity analyses excluding them from cross-sectional analyses of last visit data did not reveal clinically meaningful changes in conclusions.
ASHA-NOMS = American Speech-Language-Hearing Association’s National Outcomes Measurement System; ASIS = Annual Severity Increment Score (calculated by dividing NSS by age); CI = confidence interval; NfL = neurofilament light chain, measured in pg/mL; NPC NSS = NPC Neurological Severity Scale; PAS = Penetration-Aspiration Scale; rs = Spearman’s Correlation Coefficient with Fisher’s z Transformation
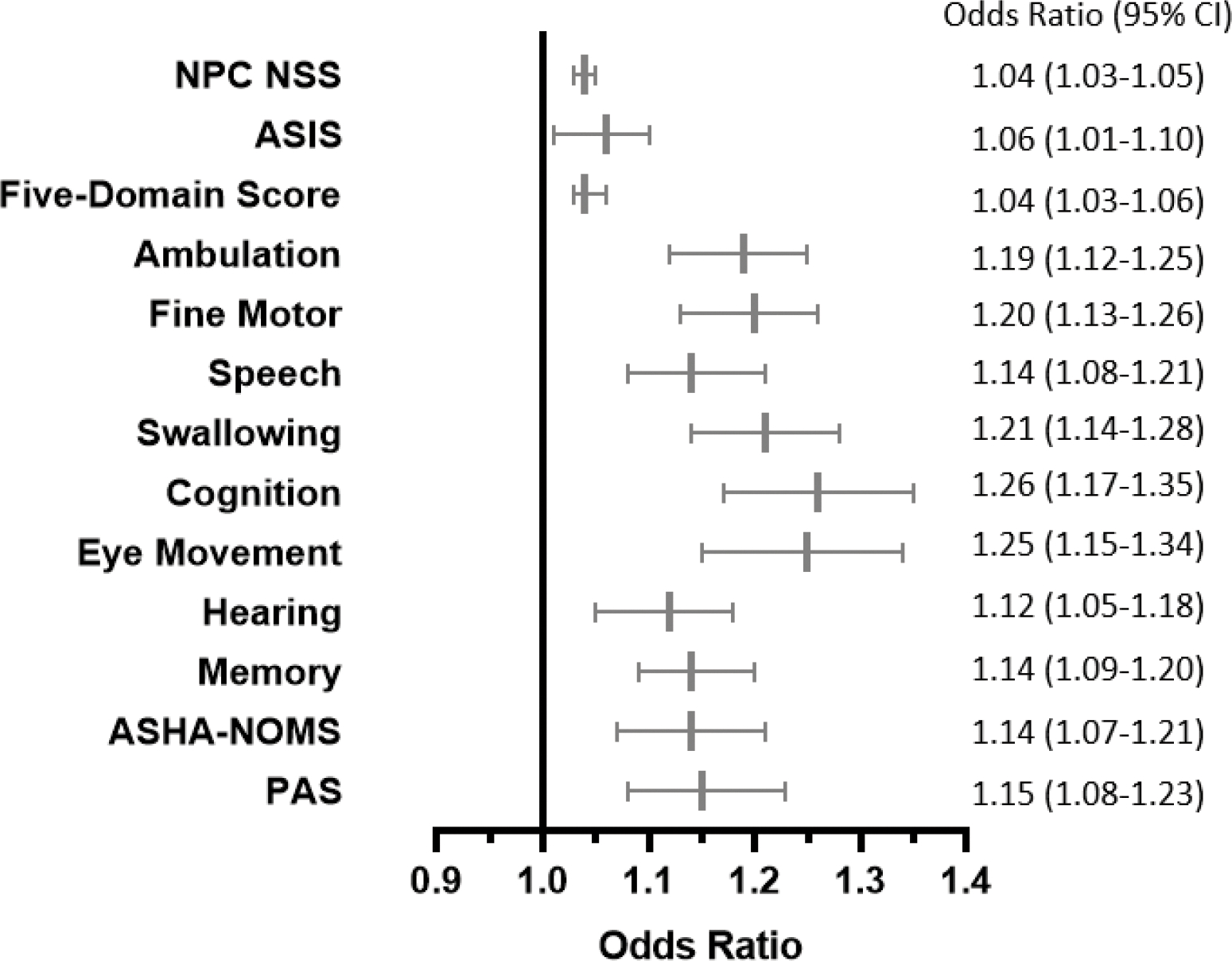
Using longitudinal data, odds ratios for risk of more severe disease were calculated for each outcome measurement. Odds ratios >1 indicate increased severity of disease associated with increased CSF NfL levels over time (Figure 3). Elevated CSF NfL levels were associated with increased disease severity as assessed by the 17-domain NPC-NSS (OR=1.03, 95% CI 1.002–1.05), Annual Severity Increment Score (ASIS, OR=1.08, 95% CI 1.02–1.15), 5-domain NPC-NSS (OR=1.03, 95% CI 1.002–1.05) and four of five individual NPC-NSS domains (ambulation [OR=1.30, 95% CI 1.05–1.60], fine motor [OR=1.27, 95% CI 1.07–1.52], speech [OR=1.25, 95% CI 1.05–1.48], and swallowing [OR=1.28, 95% CI 1.07–1.54]). CSF NfL levels were not associated with disease progression as assessed by the cognition domain. Other components of the 17-domain NPC NSS that showed an association with CSF NfL levels were eye movement (OR=1.25, 95% CI 1.04–1.50), hearing (OR=1.18, 95% CI 1.03–1.36) and memory (OR=1.17, 95% CI 1.009–1.35). Independent assessments of swallowing, ASHA-NOMS (OR=1.05, 95% CI 0.91–1.22) and PAS (OR=1.09, 95% CI 0.94–1.27), were not associated with CSF NfL levels (Figure 3). These data are consistent with the conclusion that elevated NfL levels are associated with NPC1 disease progression.
Figure 3: Higher NfL levels over time are associated with increased risk of more severe disease as measured using a variety of outcome measurement tools in longitudinal analysis.
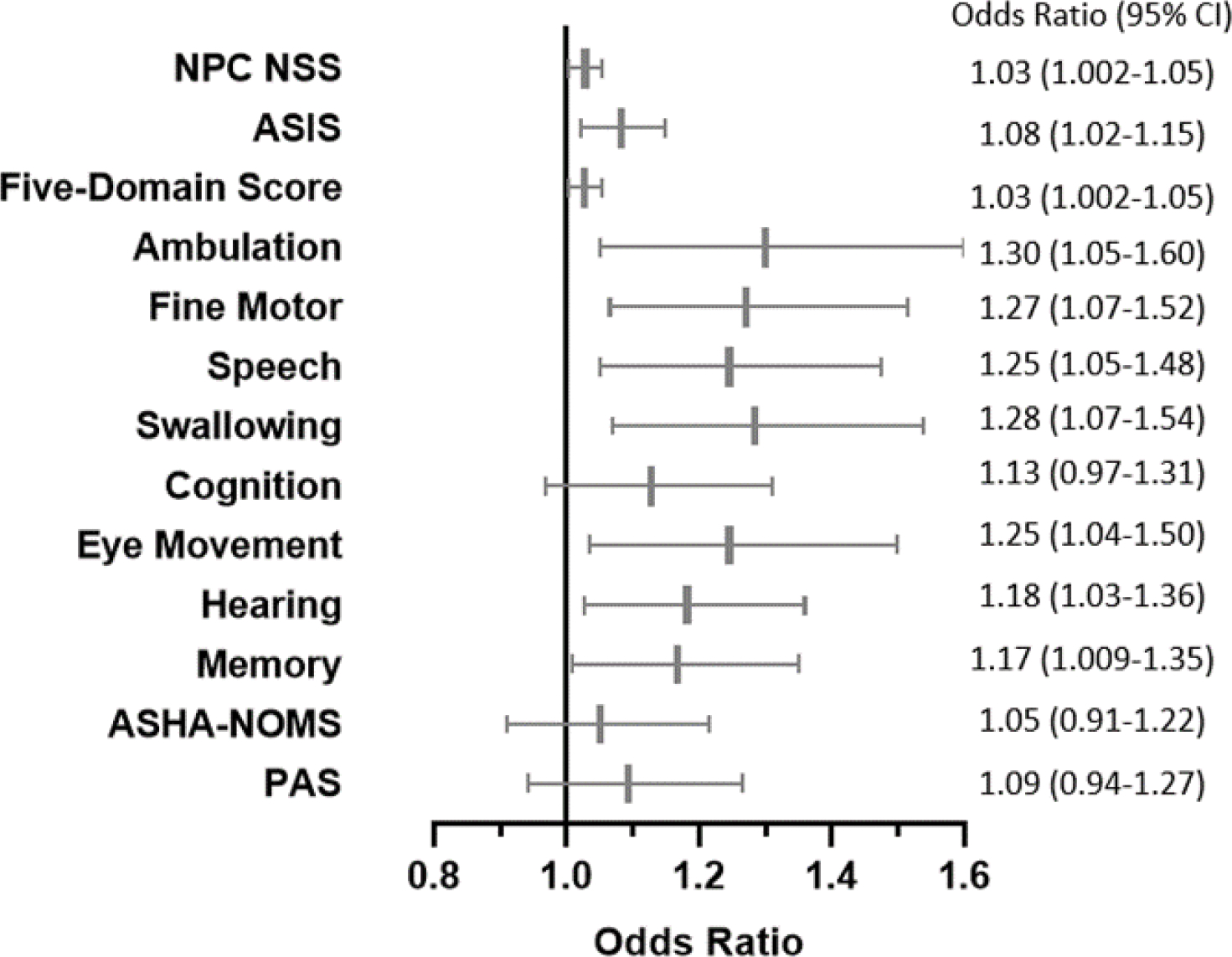
Odds Ratio (OR) and 95% Confidence Intervals (CI) are shown in a forest plot and table. For meaningful clinical interpretation, the ORs are per pg/L units of elevated NfL, adjusted for covariates in final reduced models.
Seizures and treatment of seizures can impact assessment of neurological function. Thus, seizure status was analyzed as a covariate in the longitudinal models testing the association between NfL and each disease severity measurement. Participants who had never had seizures, had had a single seizure, or had only had very rare seizures were grouped together compared to participants who had seizures that were either controlled well with medication or not well-controlled (a rating of 0, 1, or 2 on the severity scale versus a rating of 3 or 5). The absence of seizures requiring antiepileptic therapy was independently protective of disease severity by all measurements except hearing, ASHA-NOMS, and PAS (Figure 4). These data are consistent with the conclusion that seizures are a prognostic indicator of poorer clinical outcome.
Figure 4: The independent associations between seizure severity score and various outcomes of NPC disease severity from longitudinal analysis models.
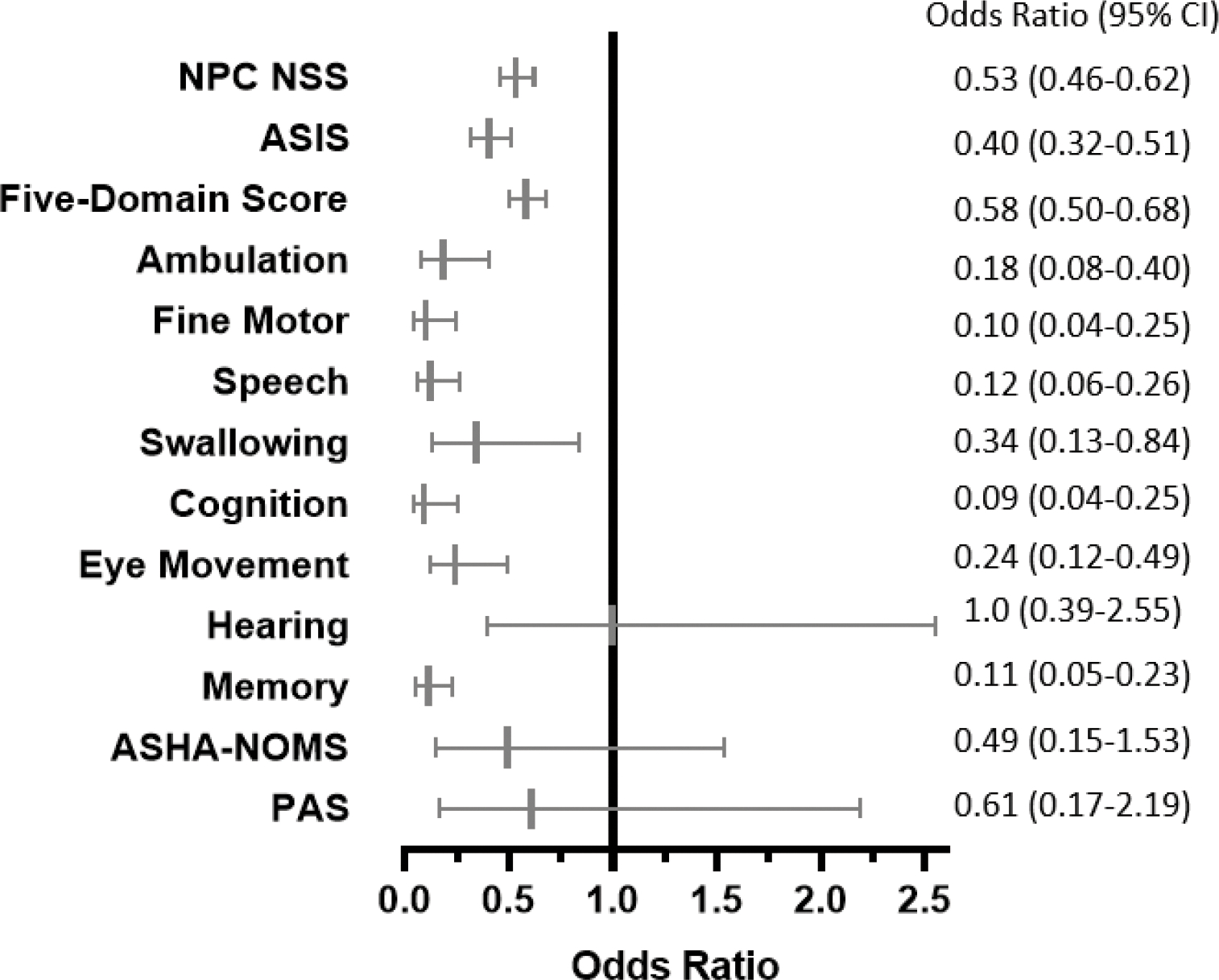
Absent/mild seizures compared to severe are shown as a forest plot and table. Results are from final reduced models of NfL and outcome measurements; where an association was not observed between the covariate and outcome, results from the full model are shown.
The independent effects of a longer duration of neurological symptoms were also observed for each outcome measurement. Worsening neurological symptoms can be linked to progressive neurodegeneration, which can be reflected by higher NfL levels.20 Though NfL levels can fluctuate over time, when observed longitudinally, having a longer duration of neurological symptoms was independently associated with higher NfL levels. This was assessed using NfL levels as an independent variable and duration of neurological symptoms as the outcome (Figure 5). These data are consistent with the conclusion that having neurological symptoms for a longer time is associated with more severe disease as measured by NfL levels.
Figure 5: The independent associations between duration of neurological symptoms and various outcomes of NPC disease severity from longitudinal analysis models.
Duration of neurological symptoms in years are shown as a forest plot and table. Results are from final reduced models of NfL and outcome measurements; where an association was not observed between the covariate and outcome, results from the full model are shown.
Effects of Treatment on NfL Levels in NPC1
Many individuals with NPC1 were receiving intrathecal HPβCD via trials investigating safety and efficacy or expanded access. At baseline, 3 of 116 (2.6%) participants were on HPβCD. By the last visit, 49 (42.2%) were on HPβCD. Over time, we did not observe a protective effect of HPβCD on NfL levels (OR=1.25, 95% CI 0.99–1.58) (Supplementary Figure 2A). In fact, when adjusting the longitudinal data for the protective effect of miglustat, HPβCD was associated with increased NfL levels (OR=1.35, 95% CI 1.09–1.67). HPβCD use as a covariate in longitudinal models was not associated with any domains except an apparent protective effect for eye movement (OR=0.40, 95% CI 0.23–0.69), but increased risk of disease severity by ASHA-NOMS (OR=2.66, 95% CI 1.06–6.64) and PAS scales (OR=2.49, 95% CI 1.01–6.13) (Supplementary Figure 2B).
At the baseline visit, out of 116 participants, 53 (45.7%) were on miglustat. Off-label use of miglustat is frequently precluded by cost and insurance denial. By the last visit, 66 (56.9%) participants were on miglustat therapy. Over time, miglustat use was found to be associated with lower NfL levels in CSF (OR=0.77, 95% CI 0.62–0.96) (Figure 6A). In the longitudinal models testing NfL levels on outcome measurements, miglustat use as a covariate conferred an independent protective effect on disease severity in multiple domains including the 17-domain NPC NSS (OR=0.75, 95% CI 0.65–0.86), ASIS (OR=0.75, 95% CI 0.61–0.92), 5-domain NPC NSS (OR=0.68, 95% CI 0.57–0.79), and four of the five individual domains (ambulation [OR=0.21 95% CI 0.10–0.44;], fine motor [OR=0.23, 95% CI 0.11–0.48], swallowing [OR=0.29, 95% CI 0.14–0.62], and cognition [OR=0.46, 95% CI 0.23–0.93]). An association was also found in the memory domain (OR=0.31, 95% CI 0.17–0.57) as well as with ASHA-NOMS (OR=0.24, 95% CI 0.10–0.57) and PAS (OR=0.35, 95% CI 0.14–0.87). Miglustat use did not show a longitudinal effect with the speech (OR=0.60, 95% CI 0.31–1.17), eye movement (OR=0.57, 95% CI 0.28–1.15), or hearing domains (OR=1.97, 95% CI 0.93–4.19) (Figure 6B).
Figure 6: Covariate effects of treatments on various NPC outcome measurements.
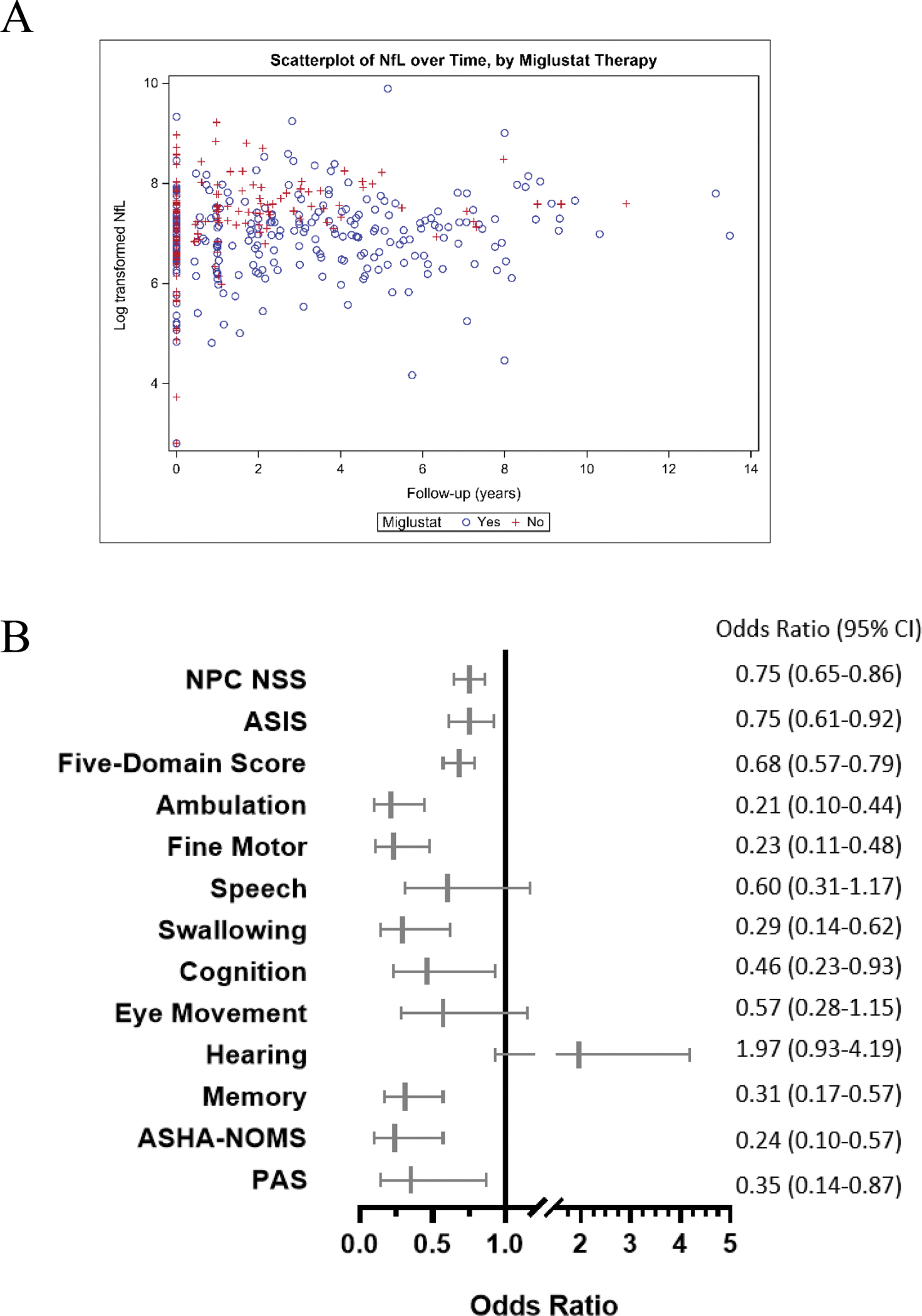
Results are from final reduced models of NfL and outcome measurements in longitudinal analysis; where an association was not observed between the covariate and outcome, results from the full model are shown. A. Scatterplot of NfL levels over time grouped by miglustat therapy. B. Miglustat therapy as a forest plot and table.
Discussion
Though there are clinical measurements available to monitor the severity and progression of NPC1, an objective and sensitive biomarker that links neurological progression to neuroaxonal damage would strengthen evidence of potential therapeutic effect of applied treatments by providing cellular specificity. Three additional studies have reported increased serum NfL levels in NPC1; however, none of these smaller studies have provided an in-depth look at associations with clinical outcome measures and response to therapeutic interventions.29–31 In this study we have demonstrated that CSF NfL is elevated in NPC1 patients compared to age appropriate non-NPC1 controls. In addition, longitudinally, higher levels are shown to be associated with more severe disease as measured across a variety of clinically relevant outcome measurements, including the NPC Neurological Severity Score4, age-adjusted total severity score (ASIS), the condensed 5-domain scale32, and four (Ambulation, Fine Motor, Speech, and Swallow) of the five elements of the 5-domain score. Only the cognition domain was not associated. This may be because deterioration of cognitive skills is often insidious, and changes only very gradually as measured by the scale. Of note, the increased risk of more severe disease was lower in the 5-domain scale than each independent domain measured separately. This is due to heterogeneity among participants in symptom presentation, causing changes in individual domains to become more significant independently but is blunted when combining them into the 5-domain score. Correlation of higher NfL levels with more severe disease was seen to increase over time across all measurements, which is likely due to participants having more severe disease at their final visit compared to their baseline visit.
Though more severe disease as reported by participants and their parents was found to be associated with higher NfL levels, quantitative measurements using ASHA-NOMS and PAS did not. This may be because these measurements represent a single point in time and are performed in a very controlled setting, while guardians and participants are likely able to describe more subtle worsening of symptoms over time. Additionally, there may be a time lag between the reported swallow domain and the formal swallowing assessments. Furthermore, the lack of association may be due to the smaller sample size of participants who were evaluated using ASHA-NOMS and PAS.
Status epilepticus and prolonged seizures have been independently associated with increased CSF NfL levels based on samples collected during hospitalization.33,34 Though we did not record the timing between the last known seizure and CSF sampling, our samples were generally collected when patients were at their baseline health status. A more severe history of seizures was independently associated with increased risk of elevated NfL levels across each outcome measurement except hearing, ASHA-NOMS, and PAS. Worsening of neurological symptoms is due to progressive neurodegeneration and as expected, a longer duration of neurological symptoms was also independently associated with increased NfL levels.
This study does have a number of potential limitations. Obtaining CSF from healthy children is precluded by ethical and statutory considerations. Using healthy adult CSF samples as controls would be confounded by the strong positive correlation of CSF NfL levels with increasing age 26–28. Thus, we elected to use CSF from three separate comparison cohorts. These included residual laboratory samples and samples from two different disorders, CTD and SLOS. The CTD and SLOS samples where obtained, processed and stored in a manner identical to the NPC1 samples. This allowed us to control for potential issues related to handling of the laboratory control samples. CTD and SLOS are neurodevelopmental disorders which are distinctly different in etiology and pathology from a neurodegenerative disorder such as NPC1. Pairwise comparison of these three cohorts did not reveal any substantial differences in NfL levels, thus our conclusion that NfL levels are elevated in individuals with NPC1 is more likely than a conclusion that CSF NfL levels are decreased in three independent comparison cohorts. While this is a large study for an ultrarare disease such as NPC1, it is still a relatively small study in comparison to studies evaluating the utility of biomarkers in more common disorders. That limits the number of correlations with other phenotypes and biomarkers that can be investigated. Because collection of blood is easier than collection of CSF, future work will evaluate the clinical utility of measuring serum NfL in NPC1.
We had anticipated, based on previous data showing decrease in CSF calbindin D levels after treatment with intrathecal HPβCD in our phase 1/2 study 8 that we might also see a decrease in CSF NfL levels in individuals treated with intrathecal HPβCD. We did not observe this. Both calbindin D and NfL are markers of neuronal damage, but CSF calbindin D is likely originating from cerebellar Purkinje neurons whereas NfL is a more general marker of neuronal damage. It is plausible that cerebellum is exposed to higher concentrations of HPβCD administered by lumbar infusion than other areas of the brain. It is also plausible that penetration of HPβCD into deeper brain structures is limited when delivered by lumbar infusion. This contrasts with miglustat where delivery to neurons is via the brain capillary network, a more efficient mechanism of drug delivery that is unfortunately precluded by the very limited blood-brain-barrier penetration of HPβCD.
Many of the NPC1 study participants were already on miglustat when they presented for their baseline visit. The use of miglustat was associated with both lower NfL levels and less severe disease based on outcome measurements, strongly suggesting that miglustat inhibits neuroaxonal damage associated with NPC1. Given that miglustat has been shown to delay onset of swallowing dysfunction35 and prolongs survival36 for individuals with NPC1, NfL has the potential to be a surrogate biomarker for NPC disease progression.
Supplementary Material
Acknowledgements:
This work was supported by the intramural research programs of the Eunice Kennedy Shriver National Institutes of Child Health and Human Development (ZIA HD008989). The NIH Natural History trial has been supported by the Ara Parseghian Medical Research Foundation. We would like to express our appreciation to the participants in our clinical trials.
Footnotes
Conflicts of Interest Statement: The authors report no conflicts of interest.
Ethics Declaration: The Eunice Kennedy Shriver National Institute of Child Health and Human Development approved this study. Recent ongoing ethical review has been provided by the National Institutes of Health Institutional Review Board. All institutions involved in human participant research received local IRB approval. Written informed consent was obtained from guardians or participants. Assent was obtained when applicable. Data was deidentified by coding. This study adhered to the principles set out in the Declaration of Helsinki.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability:
Coded data is available to IRB approved investigators consistent with NIH intramural research policies.
References
- 1.Vanier MT Niemann-Pick disease type C. Orphanet J Rare Dis 5, 16 (2010). 10.1186/1750-1172-5-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wheeler S & Sillence DJ Niemann-Pick type C disease: cellular pathology and pharmacotherapy. J Neurochem 153, 674–692 (2020). 10.1111/jnc.14895 [DOI] [PubMed] [Google Scholar]
- 3.Nadjar Y et al. Adult Niemann-Pick disease type C in France: clinical phenotypes and long-term miglustat treatment effect. Orphanet J Rare Dis 13, 175 (2018). 10.1186/s13023-018-0913-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yanjanin NM et al. Linear clinical progression, independent of age of onset, in Niemann-Pick disease, type C. Am J Med Genet B Neuropsychiatr Genet 153B, 132–140 (2010). 10.1002/ajmg.b.30969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans W et al. International consensus on clinical severity scale use in evaluating Niemann-Pick disease Type C in paediatric and adult patients: results from a Delphi Study. Orphanet J Rare Dis 16, 482 (2021). 10.1186/s13023-021-02115-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortina-Borja M et al. Annual severity increment score as a tool for stratifying patients with Niemann-Pick disease type C and for recruitment to clinical trials. Orphanet J Rare Dis 13, 143 (2018). 10.1186/s13023-018-0880-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyseng-Williamson KA Miglustat: a review of its use in Niemann-Pick disease type C. Drugs 74, 61–74 (2014). 10.1007/s40265-013-0164-6 [DOI] [PubMed] [Google Scholar]
- 8.Ory DS et al. Intrathecal 2-hydroxypropyl-beta-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: a non-randomised, open-label, phase 1–2 trial. Lancet 390, 1758–1768 (2017). 10.1016/S0140-6736(17)31465-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynolds M et al. A phase 1/2 open label nonrandomized clinical trial of intravenous 2-hydroxypropyl-beta-cyclodextrin for acute liver disease in infants with Niemann-Pick C1. Mol Genet Metab Rep 28, 100772 (2021). 10.1016/j.ymgmr.2021.100772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalil M et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 14, 577–589 (2018). 10.1038/s41582-018-0058-z [DOI] [PubMed] [Google Scholar]
- 11.Yuan A, Rao MV, Veeranna & Nixon RA Neurofilaments and Neurofilament Proteins in Health and Disease. Cold Spring Harb Perspect Biol 9 (2017). 10.1101/cshperspect.a018309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaiottino J et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS One 8, e75091 (2013). 10.1371/journal.pone.0075091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lycke JN, Karlsson JE, Andersen O & Rosengren LE Neurofilament protein in cerebrospinal fluid: a potential marker of activity in multiple sclerosis. J Neurol Neurosurg Psychiatry 64, 402–404 (1998). 10.1136/jnnp.64.3.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosengren LE, Karlsson JE, Sjogren M, Blennow K & Wallin A Neurofilament protein levels in CSF are increased in dementia. Neurology 52, 1090–1093 (1999). 10.1212/wnl.52.5.1090 [DOI] [PubMed] [Google Scholar]
- 15.Brettschneider J, Petzold A, Sussmuth SD, Ludolph AC & Tumani H Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurology 66, 852–856 (2006). 10.1212/01.wnl.0000203120.85850.54 [DOI] [PubMed] [Google Scholar]
- 16.Rosengren LE, Karlsson JE, Karlsson JO, Persson LI & Wikkelso C Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J Neurochem 67, 2013–2018 (1996). 10.1046/j.1471-4159.1996.67052013.x [DOI] [PubMed] [Google Scholar]
- 17.Byrne LM et al. Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington’s disease: a retrospective cohort analysis. Lancet Neurol 16, 601–609 (2017). 10.1016/S1474-4422(17)30124-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shahim P, Zetterberg H, Tegner Y & Blennow K Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology 88, 1788–1794 (2017). 10.1212/WNL.0000000000003912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunnarsson M et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann Neurol 69, 83–89 (2011). 10.1002/ana.22247 [DOI] [PubMed] [Google Scholar]
- 20.Bacioglu M et al. Neurofilament Light Chain in Blood and CSF as Marker of Disease Progression in Mouse Models and in Neurodegenerative Diseases. Neuron 91, 494–496 (2016). 10.1016/j.neuron.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 21.Zanier ER et al. Neurofilament light chain levels in ventricular cerebrospinal fluid after acute aneurysmal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 82, 157–159 (2011). 10.1136/jnnp.2009.177667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nylen K et al. CSF -neurofilament correlates with outcome after aneurysmal subarachnoid hemorrhage. Neurosci Lett 404, 132–136 (2006). 10.1016/j.neulet.2006.05.029 [DOI] [PubMed] [Google Scholar]
- 23.Shahim P et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci Rep 6, 36791 (2016). 10.1038/srep36791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL & Wood JL A penetration-aspiration scale. Dysphagia 11, 93–98 (1996). 10.1007/BF00417897 [DOI] [PubMed] [Google Scholar]
- 25.Adults in Healthcare: Inpatient Rehab: National Data Report 2012–2016. (American Speech-Language-Hearing Association, 2019). [Google Scholar]
- 26.Fitzgerald KC et al. Contributors to Serum NfL Levels in People without Neurologic Disease. Ann Neurol (2022). 10.1002/ana.26446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khalil M et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun 11, 812 (2020). 10.1038/s41467-020-14612-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simren J et al. Establishment of reference values for plasma neurofilament light based on healthy individuals aged 5–90 years. Brain Commun 4, fcac174 (2022). 10.1093/braincomms/fcac174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dardis A et al. Plasma Neurofilament Light (NfL) in Patients Affected by Niemann-Pick Type C Disease (NPCD). J Clin Med 10 (2021). 10.3390/jcm10204796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eratne D et al. Cerebrospinal fluid neurofilament light chain is elevated in Niemann-Pick type C compared to psychiatric disorders and healthy controls and may be a marker of treatment response. Aust N Z J Psychiatry 54, 648–649 (2020). 10.1177/0004867419893431 [DOI] [PubMed] [Google Scholar]
- 31.Bountouvi E et al. Longitudinal Data in Patients with Niemann-Pick Type C Disease Under Combined High Intrathecal and Low Intravenous Dose of 2-hydroxylpropyl-beta-cyclodextrin. Innov Clin Neurosci 18, 11–16 (2021). [PMC free article] [PubMed] [Google Scholar]
- 32.Patterson MC et al. Validation of the 5-domain Niemann-Pick type C Clinical Severity Scale. Orphanet J Rare Dis 16, 79 (2021). 10.1186/s13023-021-01719-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsushige T et al. Serum neurofilament concentrations in children with prolonged febrile seizures. J Neurol Sci 321, 39–42 (2012). 10.1016/j.jns.2012.07.043 [DOI] [PubMed] [Google Scholar]
- 34.Giovannini G et al. Serum neurofilament light as biomarker of seizure-related neuronal injury in status epilepticus. Epilepsia 63, e23–e29 (2022). 10.1111/epi.17132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon BI et al. Association of Miglustat With Swallowing Outcomes in Niemann-Pick Disease, Type C1. JAMA Neurol 77, 1564–1568 (2020). 10.1001/jamaneurol.2020.3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patterson MC et al. Long-term survival outcomes of patients with Niemann-Pick disease type C receiving miglustat treatment: A large retrospective observational study. J Inherit Metab Dis 43, 1060–1069 (2020). 10.1002/jimd.12245 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Coded data is available to IRB approved investigators consistent with NIH intramural research policies.


