Abstract
In this study, we aimed to evaluate the association between general and central obesity, and their changes with risk of knee osteoarthritis (OA) using retrospective cohort data collected from the Korean National Health Insurance Service. We studied 1,139,463 people aged 50 and over who received a health examination in 2009. To evaluate the association between general and/or central obesity and knee OA risk, a Cox proportional hazard models were used. Additionally, we investigate knee OA risk according to the change in obesity status over 2 years for subjects who had undergone health examinations for 2 consecutive years. General obesity without central obesity (HR 1.281, 95% CI 1.270–1.292) and central obesity without general obesity (HR 1.167, 95% CI 1.150–1.184) were associated with increased knee OA risk than the comparison group. Individuals with both general with central obesity had the highest risk (HR 1.418, 95% CI 1.406–1.429). This association was more pronounced in women and younger age group. Remarkably, the remission of general or central obesity over two years was associated with decreased knee OA risk (HR 0.884; 95% CI 0.867–0.902; HR 0.900; 95% CI 0.884–0.916, respectively). The present study found that both general and central obesity were associated with increased risk of knee OA and the risk was highest when the two types of obesity were accompanied. Changes in obesity status have been confirmed to alter the risk of knee OA.
Subject terms: Diseases, Health care, Risk factors
Introduction
Knee osteoarthritis (OA) is a common progressive multifactorial disorder1. It causes chronic pain, functional impairment, decreased quality of life, and economic burden2. The reported prevalence of OA in adults varies from 1.6 to 27.1%, depending on the definition of OA, study population and country. Recent systematic review and meta-analysis, including 88 studies, reported the global pooled prevalence of knee osteoarthritis over the age of 40 as 22.9% (95% CI 19.8–26.1%)3. It also shows sex and ethnic differences. Generally, women are affected more than men4. Additionally, although Asians are relatively thin, the knee OA prevalence in Asia is higher than that in Europe and North America3.
Knee OA accounts for nearly 80% of the international burden of OA5. In Korea, knee OA ranks fifth in the number of outpatients over the age of 60 (third among chronic diseases), and the total treatment cost of outpatient and inpatient care reached almost 1.14 billion dollars in 20206. Obesity is an important risk factor in the development and progression of knee OA7. As the prevalence of obesity is steadily increasing worldwide8,9, the problem of increasing numbers of patients with knee OA is emerging.
The association between body mass index (BMI), a commonly used indicator of general obesity, and knee OA is also well known4. However, BMI makes it difficult to discriminate between body fat and lean mass or reveal central obesity10,11. Recently, waist circumference (WC) was reported to be a more appropriate indicator of central obesity-associated health risk than other obesity markers11,12. Asians are more likely to be centrally obese than Europeans with the same BMI13,14, and the difference in central fat mass can be significant even at the same BMI15,16. Previous studies have reported the relationship between knee OA and obesity based on only the BMI category4,17–19. Previous studies have evaluated the individual and combined effects of general and central obesity on knee OA. However, no nationwide studies have been conducted on the effect of obesity status and change on the risk of knee OA48,49. Understanding the association of obesity and changes in obesity status with risk of knee OA is important for developing efficient public health strategies.
The aim of this study was to evaluate the association between general and/or central obesity and the risk of knee OA in a nationwide population-based cohort study using the Korean National Health Insurance Service database. We further assessed how two-year changes in obesity status affected the risk of knee OA.
Materials and methods
Data and study population
The present study was conducted with retrospective cohort data obtained from the Korean National Health Insurance Service (KNHIS). The KNHIS is a government-run single insurance company with approximately 97% of the people subscribed. The cohort sample extracted through the KNHIS database represents the actual Korean population20,21. The KNHIS database includes enrollee anthropometric characteristics, medical institution use, disease codes identified by physicians, and medication prescription claims data22–24. The national medical screening program recommends that all enrollees over the age of 40 years undergo a general health examination at least once every two years20,25,26. Routine health examination obtains demographic data thorough standardized measurements and information on drinking, smoking, physical activity, and medical history through self-report surveys.
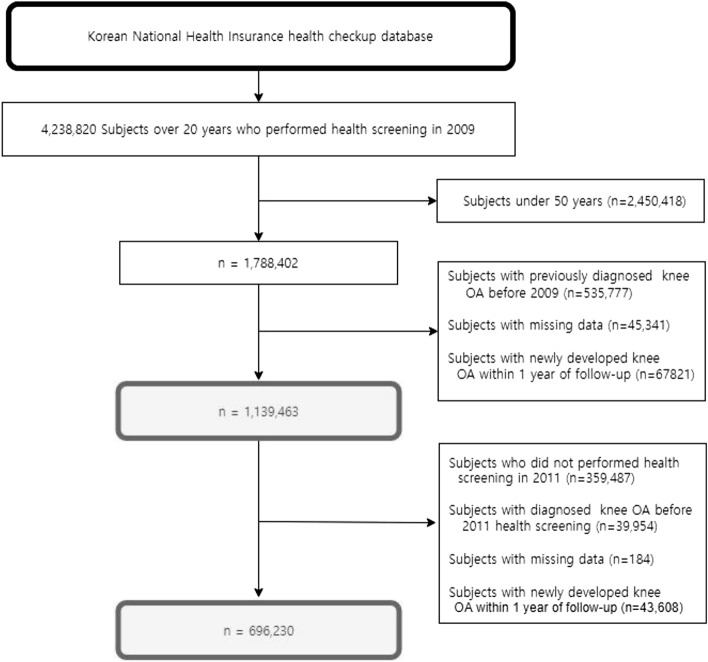
This nationwide retrospective cohort database includes a random sample of 1,788,402 people aged over 50 years at the index year of 2009. To minimize reversed causality and confounders, subjects diagnosed with knee OA prior to the index year and those with knee OA within one year were excluded. Subjects with missing values were also excluded. Finally, a retrospective analysis was conducted on 1,139,463 subjects (Fig. 1). The study cohort was followed up from the index date to the date of knee OA diagnosis or until 31 December 2018. The mean follow-up period was 6.53 ± 2.71 years.
Figure 1.
Flow chart of the cohort selection.
General health behaviors and comorbidities
Information on lifestyle-related factors was collected using the questionnaires. The categories of smoking status were as follows: never, former and current smoker. Drinking status was categorized as none, moderate (1–< 30 g/day), and heavy (≥ 30 g/day). Regular exercise was defined as when (1) moderate physical activity for > 30 min, ≥ 5 times/week or (2) vigorous physical activity for > 20 min, ≥ 3 times/week27. Income status was classified into quartiles based on the annual insurance premium to the KNHIS.
Comorbidities were defined per a previously validated method28–31. Hypertension, type 2 diabetes mellitus (T2DM) and dyslipidemia were defined using a diagnostic combination identified through the ICD-10 code and claims data of related drugs or the measured value of the health examination. Other comorbidities considered in this study included heart failure (HF), chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), end-stage renal disease (ESRD), stroke, liver cirrhosis (LC), dementia, and cancer. Each definition is presented in Table S1. Blood collection was performed after fasting for at least 8 h from midnight to check the concentrations of glucose and creatine and determine the lipid profile.
Definitions of general and central obesity
General obesity was defined as BMI ≥ 25 kg/m2, and BMI was calculated as the weight in kilograms divided by the square of the height in meters11. Following World Health Organization (WHO) recommendations for Asians, individuals were categorized as (1) underweight (BMI < 18.5 kg/m2), (2) normal (≥ 18.5 to < 23 kg/m2, reference group), (3) overweight (≥ 23 to < 25 kg/m2), (4) class 1 obese (≥ 25 to 30 kg/m2), and (5) class 2 obese (≥ 30 kg/m2)32.
WC was measured in the centerline between the rib cage and the iliac crest when the participant exhaled while standing by trained inspectors33. Regarding central obesity, subjects were grouped into 6 levels at 5 cm WC intervals as follows: (1) < 80 cm in men and < 75 cm in women, (2) 80–< 85 cm in men and 75–< 80 cm in women, (3) 85–< 90 cm in men and 80–< 85 cm in women (reference group), (4) 90–< 95 cm in men and 85–< 90 cm in women, (5) 95–< 100 cm in men and 90–< 95 cm in women, and (6) ≥ 100 cm in men and ≥ 95 cm in women. According to the definition of the Korean Society for the Study of obesity, central obesity was defined as a WC ≥ 90 cm in men and ≥ 85 cm in women33.
The change in obesity was evaluated as changes in obesity status between two years of the individuals who underwent routine health examination in both 2009 and 2011. Subjects were classified based on the presence of general or central obesity in the preceding and subsequent health examinations. This evaluation was conducted on 696,230 subjects by applying identical exclusion criteria for the study cohort (Fig. 1).
Definition of knee OA
The primary endpoint was the newly developed knee OA. Based on a previous validation study, knee OA was defined by knee OA diagnostic code (M17) or any OA diagnostic code (M15, polyarthrosis or M19, other arthrosis) in combination with a procedure for a knee X-ray in the same claim34.
Ethical considerations
The entire process of this study complied with the ethical norms of the Declaration of Helsinki. This study was approved by both the KNHIS and the IRB of the Catholic University of Korea (IRB No. VC22ZISI0014), and informed consent was exempted IRB of the Catholic University of Korea due to the anonymity of the data and the retrospective nature of the study.
Statistical analysis
Continuous variables are presented as the means and standard deviations and were compared using the t test or ANOVA. Categorical variables are expressed as numbers and percentages and were compared by means of the chi-square test. Incidence rates were calculated by dividing the number of incident cases by the total number of person-years of follow-up and expressed as per 1,000 person-years. To evaluate the risk of knee OA associated with BMI and WC, we assessed the hazard ratios (HRs) and 95% confidence intervals (CIs) using Cox’s proportional hazards models. The variables used for adjustment from Model 1 to Model 4 are as follows: (model 1) adjusted for age and sex, (modle2) adjusted for model 1 plus income, smoking, drinking, exercise, (model 3) adjusted for model 2 plus hypertension, T2DM, dyslipidemia, (model 4) adjusted for model 3 plus ESRD, COPD, stroke, LC, HF, dementia, CKD and cancer. To assess the increasing category of obesity associated with risk of knee OA, P for linear trend was applied with linear regression, using the ordinal number assigned to each category of obesity. Stratified analyses according to sex and age were performed on the combination of general and central obesity and interaction tests were performed using a likelihood ratio test.
A P values provided are two-sided, with the level of significance at 0.05. All statistical analysis procedures were performed with SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
The descriptive baseline characteristics of study populations are summarized in Table 1. Overall, 33.5% of subjects had general obesity (BMI ≥ 25 kg/m2, Table S2), and 46.9% had central obesity (WC ≥ 90 cm in males, ≥ 85 cm in females, Table S3). Among the study population, 403,050 (35.4%) developed knee OA during the follow-up period. The baseline mean BMI and WC of the knee OA group were 24.2 ± 2.9 kg/m2 and 82.1 ± 8.3 cm, respectively, and those of the non-OA group were 23.7 ± 2.9 kg/m2 and 81.8 ± 8.3 cm, respectively (both P < 0.0001). The knee OA group was more likely to be older and comprised more females, less smokers, less alcohol consumption, more hypertension, and more dyslipidemia than the non-OA group.
Table 1.
Baseline characteristics of study participants according to knee OA development.
| Knee osteoarthritis | |||
|---|---|---|---|
| No (n = 736,413) | Yes (n = 403,050) | P-value | |
| Age | |||
| Mean | 58.5 ± 7.7 | 59.9 ± 7.6 | < 0.0001 |
| Categories, n (%) | |||
| 50–59 | 460,080 (62.5) | 212,070 (52.6) | < 0.0001 |
| 60–69 | 194,718 (26.4) | 137,412 (34.1) | |
| 70–79 | 70,034 (9.5) | 48,661 (12.1) | |
| 80– | 11,581 (1.6) | 4907 (1.2) | |
| Male, n (%) | 483,669 (65.7) | 179,481 (44.5) | < 0.0001 |
| Low income < 25%, n (%) | 166,502 (22.6) | 95,279 (23.6) | < 0.0001 |
| Type 2 DM, n (%) | 109,443 (14.9) | 55,838 (13.9) | < 0.0001 |
| Hypertension, n (%) | 302,508 (41.1) | 179,064 (44.4) | < 0.0001 |
| Dyslipidemia, n (%) | 177,167 (24.1) | 112,025 (27.8) | < 0.0001 |
| Smoking, n (%) | |||
| Non | 401,912 (54.6) | 278,925 (69.2) | < 0.0001 |
| Ex | 154,072 (20.9) | 60,821 (15.1) | |
| Current | 180,429 (24.5) | 63,304 (15.7) | |
| Alcohol, n (%) | |||
| Non | 410,314 (55.7) | 264,540 (65.6) | < 0.0001 |
| Mild | 265,261 (36.0) | 112,450 (27.9) | |
| Heavy | 60,838 (8.3) | 26,060 (6.5) | |
| Regular exercise, n (%) | 165,294 (22.5) | 88,377 (21.9) | < 0.0001 |
| Cancer, n (%) | 15,865 (2.2) | 8846 (2.2) | 0.1569 |
| ESRD, n (%) | 789 (0.1) | 318 (0.1) | < 0.0001 |
| COPD, n (%) | 46,233 (6.3) | 33,449 (8.3) | < 0.0001 |
| Stroke, n (%) | 18,511 (2.5) | 11,520 (2.9) | < 0.0001 |
| LC, n (%) | 3826 (0.5) | 1511 (0.4) | < 0.0001 |
| HF, n (%) | 5620 (0.8) | 3827 (1.0) | < 0.0001 |
| Dementia, n (%) | 5406 (0.7) | 2848 (0.7) | 0.0981 |
| CKD, n (%) | 63,877 (8.7) | 37,821 (9.4) | < 0.0001 |
| Height, cm | 162.7 ± 8.2 | 159.8 ± 8.4 | < 0.0001 |
| Weight, kg | 62.9 ± 10.2 | 62.0 ± 10.0 | < 0.0001 |
| BMI, kg/m2 | 23.7 ± 2.9 | 24.2 ± 2.9 | < 0.0001 |
| WC, cm | 81.8 ± 8.3 | 82.1 ± 8.3 | < 0.0001 |
| Glucose, mg/dL | 102.8 ± 28.8 | 101.3 ± 25.9 | < 0.0001 |
| SBP, mmHg | 126.1 ± 15.8 | 126.0 ± 15.7 | 0.043 |
| DBP, mmHg | 78.1 ± 10.3 | 77.7 ± 10.1 | < 0.0001 |
| Total cholesterol, mg/dL | 199.6 ± 37.8 | 201.4 ± 38.1 | < 0.0001 |
| HDL-C, mg/dL | 54.8 ± 28.7 | 55.9 ± 31.5 | < 0.0001 |
| LDL-C, mg/dL | 117.7 ± 39 | 119.3 ± 39.3 | < 0.0001 |
| Triglyceridea, mg/dL | 121.8 (121.64–121.95) | 119.87 (119.67–120.07) | < 0.0001 |
| e-GFR, mL/min/1.73 m2 | 83.2 ± 35.2 | 83.2 ± 32.6 | 0.9422 |
Continuous variables were presented using mean and standard deviation. Categorical variables are expressed in numbers and percentages.
DM diabetes mellitus, ESRD end-stage renal disease, COPD chronic obstructive pulmonary disease, LC liver cirrhosis, HF hear failure, CKD chronic kidney disease, BMI body mass index, WC waist circumference, SBP systolic blood pressure, DBP diastolic blood pressure, HDL high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, e-GFR estimated glomerular filtration rate.
aGeometric mean values for triglyceride.
Risk of knee OA according to general or central obesity
We assessed the risk of knee OA according to BMI and WC categories (Table 2). After adjustments for confounders, the risk of knee OA increased significantly with increasing BMI and WC, and a dose-dependent association was identified (both P for trend < 0.0001). The HRs of knee OA for general obesity and central obesity compared to comparison group were 1.337 (95% CI 1.328–1.345) and 1.289 (95% CI 1.280–1.298), respectively. Of note, compared to that of the comparison group, the risk of knee OA was 73.4% higher for groups with BMIs ≥ 30.0 kg/m2 and 30.1% higher for groups with WCs ≥ 100 cm in men and ≥ 95 cm in women, respectively.
Table 2.
Incidence rate and hazard ratio for the risk of knee OA according to BMI and WC.
| IR (per 1000) | HR (95% CI) | ||||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | ||
| BMI, kg/m2 | |||||
| –< 18.5 | 38.85 | 0.749 (0.731, 0.767) | 0.752 (0.734, 0.770) | 0.753 (0.736, 0.772) | 0.749 (0.732, 0.768) |
| 18.5–< 23 | 47.59 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| 23–< 25 | 53.88 | 1.204 (1.194, 1.213) | 1.202 (1.192, 1.212) | 1.200 (1.190, 1.209) | 1.201 (1.191, 1.211) |
| 25–< 30 | 61.57 | 1.424 (1.413, 1.435) | 1.419 (1.409, 1.430) | 1.415 (1.404, 1.426) | 1.417 (1.406, 1.428) |
| 30– | 81.16 | 1.747 (1.716, 1.778) | 1.740 (1.709, 1.771) | 1.734 (1.703, 1.766) | 1.734 (1.703, 1.766) |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | |
| BMI, kg/m2 | |||||
| –< 25 | 49.89 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| 25– | 62.91 | 1.350 (1.342, 1.359) | 1.345 (1.337, 1.354) | 1.335 (1.327, 1.344) | 1.337 (1.328, 1.345) |
| P value | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | |
| WC, cm (male/female) | |||||
| − < 80/− < 75 | 46.36 | 0.765 (0.758, 0.771) | 0.767 (0.760, 0.774) | 0.770 (0.763, 0.776) | 0.769 (0.762, 0.776) |
| − < 85/− < 80 | 50.89 | 0.902 (0.894, 0.910) | 0.902 (0.894, 0.910) | 0.903 (0.896, 0.911) | 0.904 (0.896, 0.912) |
| − < 90/− < 85 | 56.36 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| − < 95/− < 90 | 61.57 | 1.103 (1.092, 1.114) | 1.102 (1.091, 1.113) | 1.101 (1.090, 1.112) | 1.101 (1.090, 1.112) |
| − < 100/− < 95 | 69.18 | 1.193 (1.177, 1.208) | 1.191 (1.175, 1.206) | 1.190 (1.174, 1.205) | 1.189 (1.173, 1.205) |
| 100–/95– | 79.75 | 1.304 (1.282, 1.327) | 1.303 (1.280, 1.326) | 1.302 (1.280, 1.325) | 1.301 (1.278, 1.324) |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | |
| WC, cm (male/female) | |||||
| − < 90/− < 85 | 50.93 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| 90–/85– | 65.53 | 1.306 (1.297, 1.315) | 1.302 (1.292, 1.311) | 1.289 (1.280, 1.299) | 1.289 (1.280, 1.298) |
| P value | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | |
Model 1 was adjusted for age, sex. Model 2 was adjusted for age, sex, income, smoking, alcohol intake and regular exercises. Model 3 was adjusted for age, sex, income, smoking, alcohol intake, regular exercises, diabetes, hypertension and dyslipidemia. Model 4 was adjusted for age, sex, income, smoking, alcohol intake, regular exercises, diabetes, hypertension, dyslipidemia, cancer, end-stage renal disease, chronic obstructive pulmonary disease, stroke, liver cirrhosis, hear failure, dementia and chronic kidney disease.
BMI body mass index, WC waist circumference, HR hazard ratio, CI confidence interval, OA osteoarthritis, IR incidence rate.
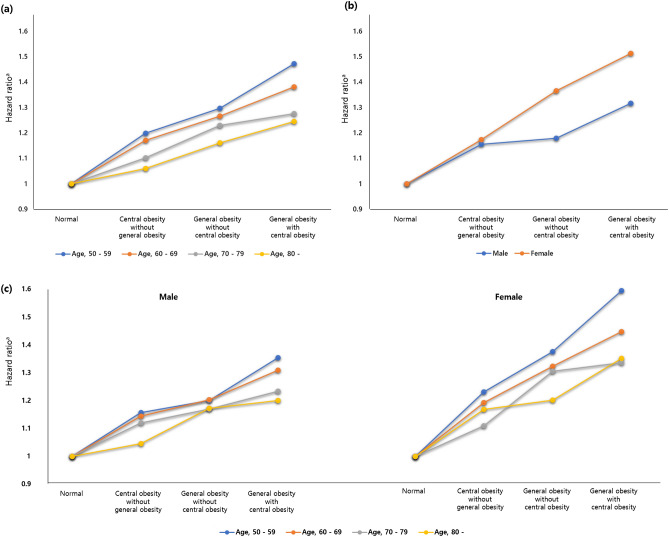
In the analysis of knee OA risk according to general and/or central obesity, the highest risk was identified in those who had both general with central obesity (HR 1.418; 95% CI 1.406–1.429) (Table 3). Even having only central obesity without general obesity increased the risk (HR 1.167; 95% CI 1.150–1.184) (Table 3). In the subgroup analysis, females (HR 1.513; 95% CI 1.496–1.529) were more associated with knee OA risk than males (HR 1.317; 95% CI 1.302–1.332) when accompanied by general and central obesity (Table 4, Fig. 2). It has been found that younger age groups have a higher HR of knee OA associated with general and/or central obesity (Table 4, Fig. 2).
Table 3.
Incidence rate and hazard ratio for the risk of knee OA according to general obesity and/or central obesity composition.
| General obesity | Central obesity | IR (per 1000) | HR (95% CI) | |||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||
| No | No | 49.06 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Yes | 61.49 | 1.173 (1.156, 1.190) | 1.171 (1.154, 1.188) | 1.168 (1.152, 1.185) | 1.167 (1.150, 1.184) | |
| Yes | No | 58.69 | 1.290 (1.279, 1.301) | 1.286 (1.275, 1.297) | 1.279 (1.268, 1.290) | 1.281 (1.270, 1.292) |
| Yes | 66.58 | 1.432 (1.421, 1.443) | 1.426 (1.415, 1.437) | 1.417 (1.406, 1.429) | 1.418 (1.406, 1.429) | |
Model 1 was adjusted for age, sex. Model 2 was adjusted for age, sex, income, smoking, alcohol intake and regular exercises. Model 3 was adjusted for age, sex, income, smoking, alcohol intake, regular exercises, diabetes, hypertension and dyslipidemia. Model 4 was adjusted for age, sex, income, smoking, alcohol intake, regular exercises, diabetes, hypertension, dyslipidemia, cancer, end-stage renal disease, chronic obstructive pulmonary disease, stroke, liver cirrhosis, hear failure, dementia and chronic kidney disease.
HR hazard ratio, CI confidence interval, OA osteoarthritis, IR incidence rate.
Table 4.
Incidence rate and hazard ratio for the risk of knee OA of the general obesity and/or central obesity composition according to age and sex.
| General obesity | Central obesity | IR (per 1000) | HR (95% CI) | |||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||
| Age categories | ||||||
| 50–59 | P for interaction < 0.0001 | |||||
| No | No | 41.70 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Yes | 47.74 | 1.204 (1.176, 1.232) | 1.202 (1.174, 1.230) | 1.200 (1.172, 1.228) | 1.198 (1.170, 1.227) | |
| Yes | No | 50.79 | 1.304 (1.290, 1.319) | 1.300 (1.286, 1.315) | 1.295 (1.280, 1.310) | 1.296 (1.281, 1.311) |
| Yes | 55.56 | 1.484 (1.468, 1.501) | 1.477 (1.461, 1.494) | 1.470 (1.454, 1.487) | 1.470 (1.453, 1.486) | |
| 60–69 | ||||||
| No | No | 59.69 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Yes | 71.34 | 1.175 (1.149, 1.202) | 1.173 (1.147, 1.200) | 1.172 (1.146, 1.199) | 1.170 (1.143, 1.196) | |
| Yes | No | 75.20 | 1.271 (1.252, 1.291) | 1.268 (1.249, 1.288) | 1.263 (1.244, 1.282) | 1.265 (1.246, 1.285) |
| Yes | 81.24 | 1.389 (1.371, 1.407) | 1.384 (1.366, 1.402) | 1.379 (1.361, 1.397) | 1.379 (1.361, 1.398) | |
| 70–79 | ||||||
| No | No | 66.63 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Yes | 77.83 | 1.105 (1.070, 1.141) | 1.103 (1.069, 1.139) | 1.103 (1.068, 1.139) | 1.102 (1.067, 1.137) | |
| Yes | No | 83.07 | 1.230 (1.194, 1.267) | 1.227 (1.191, 1.265) | 1.223 (1.187, 1.260) | 1.229 (1.193, 1.267) |
| Yes | 87.72 | 1.278 (1.250, 1.307) | 1.275 (1.247, 1.304) | 1.272 (1.244, 1.301) | 1.275 (1.247, 1.304) | |
| 80– | ||||||
| No | No | 58.94 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Yes | 67.25 | 1.061 (0.972, 1.159) | 1.060 (0.971, 1.157) | 1.060 (0.971, 1.158) | 1.061 (0.972, 1.159) | |
| Yes | No | 70.02 | 1.169 (1.038, 1.317) | 1.165 (1.035, 1.313) | 1.161 (1.031, 1.308) | 1.161 (1.031, 1.308) |
| Yes | 76.06 | 1.253 (1.16, 1.355) | 1.25 (1.157, 1.352) | 1.248 (1.154, 1.349) | 1.244 (1.151, 1.345) | |
| Sex category | ||||||
| Male | P for interaction < 0.0001 | |||||
| No | No | 36.08 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Yes | 44.74 | 1.168 (1.144, 1.193) | 1.163 (1.138, 1.187) | 1.160 (1.136, 1.185) | 1.156 (1.132, 1.181) | |
| Yes | No | 40.40 | 1.188 (1.172, 1.204) | 1.180 (1.165, 1.196) | 1.174 (1.159, 1.190) | 1.179 (1.164, 1.195) |
| Yes | 47.30 | 1.335 (1.320, 1.351) | 1.323 (1.308, 1.338) | 1.315 (1.300, 1.330) | 1.317 (1.302, 1.332) | |
| Female | ||||||
| No | No | 67.38 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Yes | 90.01 | 1.175 (1.153, 1.198) | 1.177 (1.154, 1.200) | 1.174 (1.151, 1.197) | 1.174 (1.151, 1.197) | |
| Yes | No | 91.13 | 1.376 (1.360, 1.392) | 1.374 (1.359, 1.390) | 1.367 (1.351, 1.383) | 1.366 (1.350, 1.382) |
| Yes | 108.14 | 1.523 (1.506, 1.539) | 1.523 (1.507, 1.540) | 1.514 (1.497, 1.530) | 1.513 (1.496, 1.529) | |
Model 1 was adjusted for age, sex. Model 2 was adjusted for age, sex, income, smoking, alcohol intake and regular exercises. Model 3 was adjusted for age, sex, income, smoking, alcohol intake, regular exercises, diabetes, hypertension and dyslipidemia. Model 4 was adjusted for age, sex, income, smoking, alcohol intake, regular exercises, diabetes, hypertension, dyslipidemia, cancer, end-stage renal disease, chronic obstructive pulmonary disease, stroke, liver cirrhosis, hear failure, dementia and chronic kidney disease.
HR hazard ratio, CI confidence interval, OA osteoarthritis, IR incidence rate.
Figure 2.
Adjusted knee OA risk for general and/or central obesity composition, according to (a) age, (b) sex and (c) age and sex. aAdjusted for age, sex, income, smoking, alcohol intake, regular exercises, diabetes, hypertension, dyslipidemia, cancer, end-stage renal disease, chronic obstructive pulmonary disease, stroke, liver cirrhosis, hear failure, dementia and chronic kidney disease.
Risk of knee OA according to changes in obesity status
Compared to those without general and central obesity over two years, the HRs of knee OA in those who developed obesity in the subsequent health examinations were 1.229 (95% CI 1.206–1.253) for general obesity and 1.226 (95% CI 1.207–1.245) for central obesity (Table 5). Remarkably, those whose general or central obese status was resolved still had a higher risk of knee OA (HR 1.216; 95% CI 1.193–1.239; HR 1.227; 95% CI 1.208–1.247, respectively). On the other hand, compared to those whose general or central obesity remained, the knee OA risks in those whose obesity status was resolved were significantly reduced by 11.6% for general obesity and 10.0% for central obesity (Table 6).
Table 5.
Incidence rate and hazard ratio for the risk of knee OA according to the change in obesity status.
| Change in obesity (pre/post) | IR (per 1000) | HR (95% CI) | |||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | ||
| General obesity | |||||
| No/no | 50.63 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| No/yes | 59.42 | 1.236 (1.213, 1.26) | 1.235 (1.212, 1.259) | 1.230 (1.207, 1.254) | 1.229 (1.206, 1.253) |
| Yes/no | 59.57 | 1.222 (1.199, 1.246) | 1.220 (1.197, 1.243) | 1.215 (1.192, 1.238) | 1.216 (1.193, 1.239) |
| Yes/yes | 64.46 | 1.388 (1.374, 1.401) | 1.383 (1.369, 1.396) | 1.374 (1.360, 1.388) | 1.375 (1.361, 1.389) |
| Central obesity | |||||
| No/no | 51.17 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| No/yes | 63.55 | 1.235 (1.216, 1.254) | 1.233 (1.214, 1.252) | 1.226 (1.208, 1.246) | 1.226 (1.207, 1.245) |
| Yes/no | 63.21 | 1.237 (1.217, 1.256) | 1.233 (1.214, 1.253) | 1.227 (1.208, 1.247) | 1.227 (1.208, 1.247) |
| Yes/yes | 67.76 | 1.382 (1.365, 1.398) | 1.376 (1.359, 1.392) | 1.365 (1.348, 1.382) | 1.363 (1.347, 1.380) |
Model 1 was adjusted for age, sex. Model 2 was adjusted for age, sex, income, smoking, alcohol intake and regular exercises. Model 3 was adjusted for age, sex, income, smoking, alcohol intake, regular exercises, diabetes, hypertension and dyslipidemia. Model 4 was adjusted for age, sex, income, smoking, alcohol intake, regular exercises, diabetes, hypertension, dyslipidemia, cancer, end-stage renal disease, chronic obstructive pulmonary disease, stroke, liver cirrhosis, hear failure, dementia and chronic kidney disease.
(Pre/post) means obesity status in the preceding and subsequent health examination.
HR hazard ratio, CI confidence interval, OA osteoarthritis, IR incidence rate.
Table 6.
Change in hazard ratio for the risk of knee OA due to the resolution of obesity in obese subjects.
| Change in obesity (pre/post) | HR (95% CI) | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| General obesity | ||||
| Yes/yes | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Yes/no | 0.881 (0.864, 0.898) | 0.882 (0.865, 0.899) | 0.884 (0.867, 0.902) | 0.884 (0.867, 0.902) |
| Central obesity | ||||
| Yes/yes | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Yes/no | 0.895 (0.879, 0.912) | 0.897 (0.880, 0.913) | 0.899 (0.883, 0.916) | 0.900 (0.884, 0.916) |
Model 1 was adjusted for age, sex. Model 2 was adjusted for age, sex, income, smoking, alcohol intake and regular exercises. Model 3 was adjusted for age, sex, income, smoking, alcohol intake, regular exercises, diabetes, hypertension and dyslipidemia. Model 4 was adjusted for age, sex, income, smoking, alcohol intake, regular exercises, diabetes, hypertension, dyslipidemia, cancer, end-stage renal disease, chronic obstructive pulmonary disease, stroke, liver cirrhosis, hear failure, dementia and chronic kidney disease.
(Pre/post) means obesity status in the preceding and subsequent health examination.
HR hazard ratio, CI confidence interval, OA osteoarthritis, IR incidence rate.
Discussion
In this nationwide population-based retrospective cohort study of 1,139,463 participants aged 50 and older, we found that (1) Higher BMI and greater WC increase the risk of knee OA in a dose-dependent manner; (2) Those who had both general and central obesity had the highest risk of knee OA and its associations were stronger in women and younger people; and (3) Changes in obesity status altered the risk of knee OA. In particular, the risk was reduced in the obesity-resolved group compared to the obesity-maintaining group.
Although obesity has been reported to be an important risk factor for knee OA, to the best of our knowledge, this is the first national cohort study to determine the risk of knee OA in Asian people with general and central obesity. General obesity is defined on the basis of BMI, a standard anthropometric parameter, and high BMI is one of the strongest risk factors for knee OA. General obesity causes greater mechanical stress due to excessive weight on the joint surface, which leads to cartilage degeneration and OA. Indeed, with a weight gain of 1 kg, there is a six-fold increase in on both sides of the knee35. A meta-analysis of 22 cohort and patient-control studies reported that the pooled odds ratio of knee OA for overweight-to-normal BMI was 2.18 (95% CI 1.86–2.55), and that for obese-to-normal BMI was 2.63 (95% CI 2.28–3.05)36. In a prospective cohort study of 105,189 patients with newly developed knee OA, obese patients had more than twice the risk of knee arthroplasty surgery than normal-weight patients37. The risk of general obesity for knee OA identified in our study is also in line with previous studies.
Traditionally, the relationship between obesity and OA has been understood in terms of mechanical load, but OA in joints that are not affected by weight, such as the hands, has also been confirmed to be related to obesity38. It is presumed to be related to cytokines secreted by adipose tissues (i.e., adipokines) that regulate bone and cartilage homeostasis39–42. Among those adipokines, leptin has been identified as a major degradation factor of cartilage and a mediator for OA. Cartilage cells have leptin receptors, and high concentrations of leptin are associated with the development and progression of OA43. The leptin concentration was found to be higher in the synovial fluid of the osteoarthritic joint than in normal tissue44. The present study supports the hypothesis about the role of regional adipose tissue in OA by confirming the independent association between central obesity and the risk of knee OA. Therefore, it is inferred that the composition of general and central obesity increases the risk of knee OA due to the synergy of mechanical stress and the degradation-mediated reaction of adipose tissue.
Our findings showed that the association between obesity and risk of knee OA was stronger in female. Although the mechanism by which sex differences affect the development of knee OA is still unclear, various factors have been suggested as possible causes. First, the normal knee joint cartilage volume of males is significantly larger than that of females45. In a study using MRI to determine changes in knee articular cartilage defects over a 2.3-year period among 211 participants without clinical OA, females had a threefold higher risk of developing knee articular cartilage defects than males, even among healthy participants45. In addition, hormonal differences between males and females are also suggested to play an important role. In particular, postmenopausal estrogen reduction in females is significantly associated with an increased risk of developing knee OA46. The presence of estrogen receptors identified in joint cartilage suggests a relationship between female hormones and joint cartilage47.
Present study confirms that younger group with obesity are more susceptible to knee OA. Several studies have reported an association between general obesity and knee osteoarthritis50,51, and the metabolic effects of central obesity are also known to be associated with osteoarthritis52. Therefore, considering the cumulative effects of both types of obesity on knee joint, obesity at an early age may be associated with a greater risk than a later age.
Identifying the risk of obesity and groups more vulnerable to its harm is useful for focusing management strategies. This study highlights the stronger association between obesity and knee OA in women and younger populations. From a public health perspective, just as screening for osteoporosis is provided to postmenopausal women in their 50 s, more attention should be sought to prevent knee OA for obese women of a similar age.
In this study, changes in obesity status altered the risk of developing OA. The fact that resolution of general and central obesity in two years reduced the risk of developing knee OA by 11.6% and 10.0%, respectively, is promising evidence for the importance of intensive interventions in obese populations. It is noteworthy that baseline normal subjects who developed new general obesity had a higher risk of knee OA than baseline general obese subjects who achieved remission of obesity for 2 years in the population aged 50 years and older (Table 5). These findings further emphasize the importance of obesity management and provide a high level of motivation for behavioral changes toward a healthy lifestyle.
The present study has several limitations. First, since the disease was defined based on the claims data in the NHIS database, the potential for bias due to misclassification cannot be overlooked, which might lead to the possibility of underestimation or overestimation. In addition, because our study was based on claims data, we could not confirm information on the grade or treatment of knee OA. Second, the change in obesity was evaluated only as a result of two years, and the cause of the change could not be identified. Third, a selection bias may exist between enrolled and missing participants for the analysis of changes in obesity status. Due to the large sample size, the difference in obesity and comorbidity between the two groups has statistically significant, but the magnitude of the difference was not large. Details are shown in Table S7. Fourth, drug use or eating habits that may affect weight were not considered. Fifth, medical history were obtained through a self-reported questionnaire. This suggests that there might be some bias that could affect the results after adjustments. Sixth, since our research was conducted with the data of the Korean NHIS, there may be limitations in generalizing our research results to other ethnic groups.
Nevertheless, our study has the strength of a nationwide population-based cohort study. The KNHIS database provides large-scale health data (big data), which is rare worldwide. In addition, this study enhances the reliability and universality of the findings by adjusting for a wide range of comorbidities. To the best of our knowledge, this is the first nationwide study to confirm that a two-year change in obesity status decreases the risk of knee OA.
Obesity and knee OA are strongly associated with personal and social burdens. Therefore, our findings support further investigation of the relationship between obesity and knee OA for the establishment of more effective OA prevention strategies.
In conclusion, our nationwide retrospective cohort study confirmed that higher BMI and WC were associated with an increased risk of knee OA in a dose-dependent manner. Accompanying general obesity and central obesity increase the risk more, and these effects are stronger in women and younger age groups. Changes in obesity status have been confirmed to alter the risk of knee OA.
Supplementary Information
Acknowledgements
The authors thank the participants of the Korean National Health Insurance Service-Health Screening program.
Author contributions
D.P., K.H., and H.S.K. conceived the presented idea. D.P., S.H.K., and K.H. collected the study data and did the statistical analyses. D.P. wrote the initial draft of the paper. D.P., Y.M.P., Y.H.C., D.W.M., and S.H.K. reviewed the manuscript. K.H. and H.S.K. supervised the manuscript and they contributed equally to this manuscript. K.H. and H.S.K. share the corresponding authorship. All authors approved the paper.
Funding
The authors wish to acknowledge the financial support of Clinical Research Invigoration Project of the St. Vincent's Hospital, The Catholic University of Korea. This research was also supported by a grant of the Korea Health Industry Development Institute (KHIDI), Republic of Korea (HI19C0784).
Data availability
The data presented in this study are available in the main article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kyungdo Han and Hae-Seok Koh.
Contributor Information
Kyungdo Han, Email: hkd917@naver.com.
Hae-Seok Koh, Email: vincentos@naver.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-30727-4.
References
- 1.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet (London, England) 2019;393:1745–1759. doi: 10.1016/s0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 2.Buttgereit, F., Burmester, G. R. & Bijlsma, J. W. Non-surgical management of knee osteoarthritis: Where are we now and where do we need to go? RMD Open1, e000027. 10.1136/rmdopen-2014-000027 (2015). [DOI] [PMC free article] [PubMed]
- 3.Cui, A. et al. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClin. Med.29–30, 100587. 10.1016/j.eclinm.2020.100587 (2020). [DOI] [PMC free article] [PubMed]
- 4.Jiang L, et al. Body mass index and susceptibility to knee osteoarthritis: A systematic review and meta-analysis. Joint Bone Spine. 2012;79:291–297. doi: 10.1016/j.jbspin.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet (London, England)388, 1545–1602. 10.1016/s0140-6736(16)31678-6 (2016). [DOI] [PMC free article] [PubMed]
- 6.Statistics Korea, https://kostat.go.kr/portal/korea/kor_nw/1/6/2/index.board. Accessed 21 January 2022.
- 7.Sowers MR, Karvonen-Gutierrez CA. The evolving role of obesity in knee osteoarthritis. Curr. Opin. Rheumatol. 2010;22:533–537. doi: 10.1097/BOR.0b013e32833b4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patterson RE, Frank LL, Kristal AR, White E. A comprehensive examination of health conditions associated with obesity in older adults. Am. J. Prev. Med. 2004;27:385–390. doi: 10.1016/j.amepre.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Yoon KH, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet (London, England) 2006;368:1681–1688. doi: 10.1016/s0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 10.Seidell JC. Waist circumference and waist/hip ratio in relation to all-cause mortality, cancer and sleep apnea. Eur. J. Clin. Nutr. 2010;64:35–41. doi: 10.1038/ejcn.2009.71. [DOI] [PubMed] [Google Scholar]
- 11.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am. J. Clin. Nutr. 2004;79:379–384. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 12.Mendy, V. L. et al. The association between individual and combined components of metabolic syndrome and chronic kidney disease among African Americans: The Jackson Heart Study. PloS One9, e101610. 10.1371/journal.pone.0101610 (2014). [DOI] [PMC free article] [PubMed]
- 13.Farrell GC, Wong VW, Chitturi S. NAFLD in Asia—as common and important as in the West. Nat. Rev. Gastroenterol. Hepatol. 2013;10:307–318. doi: 10.1038/nrgastro.2013.34. [DOI] [PubMed] [Google Scholar]
- 14.Lim, U. et al. Asian women have greater abdominal and visceral adiposity than Caucasian women with similar body mass index. Nutr. Diab.1, e6. 10.1038/nutd.2011.2 (2011). [DOI] [PMC free article] [PubMed]
- 15.Organization, W. H. Obesity: Preventing and managing the global epidemic (2000). [PubMed]
- 16.Hu, L. et al. Prevalence of overweight, obesity, abdominal obesity and obesity-related risk factors in southern China. PloS one12, e0183934. 10.1371/journal.pone.0183934 (2017). [DOI] [PMC free article] [PubMed]
- 17.Zhou, Z. Y., Liu, Y. K., Chen, H. L. & Liu, F. Body mass index and knee osteoarthritis risk: A dose-response meta-analysis. Obesity (Silver Spring, Md.)22, 2180–2185. 10.1002/oby.20835 (2014). [DOI] [PubMed]
- 18.Holmberg S, Thelin A, Thelin N. Knee osteoarthritis and body mass index: A population-based case-control study. Scand. J. Rheumatol. 2005;34:59–64. doi: 10.1080/03009740510017922. [DOI] [PubMed] [Google Scholar]
- 19.Wills AK, et al. Life course body mass index and risk of knee osteoarthritis at the age of 53 years: Evidence from the 1946 British birth cohort study. Ann. Rheum. Dis. 2012;71:655–660. doi: 10.1136/ard.2011.154021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SR, Choi EK, Han KD, Cha MJ, Oh S. Trends in the incidence and prevalence of atrial fibrillation and estimated thromboembolic risk using the CHA(2)DS(2)-VASc score in the entire Korean population. Int. J. Cardiol. 2017;236:226–231. doi: 10.1016/j.ijcard.2017.02.039. [DOI] [PubMed] [Google Scholar]
- 21.Kwon H, Han KD, Park CY. Weight change is significantly associated with risk of thyroid cancer: A nationwide population-based cohort study. Sci. Rep. 2019;9:1546. doi: 10.1038/s41598-018-38203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang HK, et al. Obesity, metabolic health, and mortality in adults: A nationwide population-based study in Korea. Sci. Rep. 2016;6:30329. doi: 10.1038/srep30329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee YH, Han K, Ko SH, Ko KS, Lee KU. Data analytic process of a nationwide population-based study using national health information database established by national health insurance service. Diabetes Metab. J. 2016;40:79–82. doi: 10.4093/dmj.2016.40.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, J., Lee, J. S., Park, S. H., Shin, S. A. & Kim, K. Cohort profile: The national health insurance service-national sample cohort (NHIS-NSC), South Korea. Int. J. Epidemiol.46, e15. 10.1093/ije/dyv319 (2017). [DOI] [PubMed]
- 25.Noh J, Han KD, Ko SH, Ko KS, Park CY. Trends in the pervasiveness of type 2 diabetes, impaired fasting glucose and co-morbidities during an 8-year-follow-up of nationwide Korean population. Sci. Rep. 2017;7:46656. doi: 10.1038/srep46656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song SO, et al. Background and data configuration process of a nationwide population-based study using the Korean national health insurance system. Diabetes Metab. J. 2014;38:395–403. doi: 10.4093/dmj.2014.38.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SR, Han KD, Choi EK, Oh S, Lip GYH. Nonalcoholic fatty liver disease and the risk of atrial fibrillation stratified by body mass index: A nationwide population-based study. Sci. Rep. 2021;11:3737. doi: 10.1038/s41598-021-83367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee KR, Hwang IC, Han KD, Jung J, Seo MH. Waist circumference and risk of breast cancer in Korean women: A nationwide cohort study. Int. J. Cancer. 2018;142:1554–1559. doi: 10.1002/ijc.31180. [DOI] [PubMed] [Google Scholar]
- 29.Kang SH, et al. Underweight is a risk factor for atrial fibrillation: A nationwide population-based study. Int. J. Cardiol. 2016;215:449–456. doi: 10.1016/j.ijcard.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 30.Choi YJ, et al. Temporal trends of the prevalence and incidence of atrial fibrillation and stroke among Asian patients with hypertrophic cardiomyopathy: A nationwide population-based study. Int. J. Cardiol. 2018;273:130–135. doi: 10.1016/j.ijcard.2018.08.038. [DOI] [PubMed] [Google Scholar]
- 31.Choi YJ, et al. Reconsidering the cut-off diastolic blood pressure for predicting cardiovascular events: A nationwide population-based study from Korea. Eur. Heart J. 2019;40:724–731. doi: 10.1093/eurheartj/ehy801. [DOI] [PubMed] [Google Scholar]
- 32.Organization, W. H. The Asia-Pacific perspective: Redefining obesity and its treatment (2000).
- 33.Lee SY, et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res. Clin. Pract. 2007;75:72–80. doi: 10.1016/j.diabres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Park HR, et al. Validation of algorithms to identify knee osteoarthritis patients in the claims database. Int. J. Rheum. Dis. 2019;22:890–896. doi: 10.1111/1756-185x.13470. [DOI] [PubMed] [Google Scholar]
- 35.Felson DT. Weight and osteoarthritis. J. Rheumatol. Suppl. 1995;43:7–9. [PubMed] [Google Scholar]
- 36.Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: A systematic review and meta-analysis. Osteoarth. Cartil. 2010;18:24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Leyland, K. M. et al. Obesity and the relative risk of knee replacement surgery in patients with knee osteoarthritis: A prospective cohort study. Arthritis Rheumatol. (Hoboken, N.J.)68, 817–825. 10.1002/art.39486 (2016). [DOI] [PubMed]
- 38.Yusuf E, et al. Association between weight or body mass index and hand osteoarthritis: A systematic review. Ann. Rheum. Dis. 2010;69:761–765. doi: 10.1136/ard.2008.106930. [DOI] [PubMed] [Google Scholar]
- 39.Coppari R, Bjørbæk C. Leptin revisited: its mechanism of action and potential for treating diabetes. Nat. Rev. Drug Discovery. 2012;11:692–708. doi: 10.1038/nrd3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gualillo O. Further evidence for leptin involvement in cartilage homeostases. Osteoarth. Cartil. 2007;15:857–860. doi: 10.1016/j.joca.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 41.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scotece M, et al. Adipokines as drug targets in joint and bone disease. Drug Discov. Today. 2014;19:241–258. doi: 10.1016/j.drudis.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Scotece M, Mobasheri A. Leptin in osteoarthritis: Focus on articular cartilage and chondrocytes. Life Sci. 2015;140:75–78. doi: 10.1016/j.lfs.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 44.Thijssen E, van Caam A, van der Kraan PM. Obesity and osteoarthritis, more than just wear and tear: pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology (Oxford) 2015;54:588–600. doi: 10.1093/rheumatology/keu464. [DOI] [PubMed] [Google Scholar]
- 45.Hanna, F. S. et al. Women have increased rates of cartilage loss and progression of cartilage defects at the knee than men: A gender study of adults without clinical knee osteoarthritis. Menopause (New York, N.Y.)16, 666–670. 10.1097/gme.0b013e318198e30e (2009). [DOI] [PubMed]
- 46.Hame SL, Alexander RA. Knee osteoarthritis in women. Curr. Rev. Musculoskelet. Med. 2013;6:182–187. doi: 10.1007/s12178-013-9164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richmond RS, Carlson CS, Register TC, Shanker G, Loeser RF. Functional estrogen receptors in adult articular cartilage: estrogen replacement therapy increases chondrocyte synthesis of proteoglycans and insulin-like growth factor binding protein 2. Arthritis Rheum. 2000;43:2081–2090. doi: 10.1002/1529-0131(200009)43:9<2081::aid-anr20>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 48.Lyu L, et al. Causal relationships of general and abdominal adiposity on osteoarthritis: A two-sample Mendelian randomization study. J. Clin. Med. 2022;12:1. doi: 10.3390/jcm12010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janssen I, Mark AE. Separate and combined influence of body mass index and waist circumference on arthritis and knee osteoarthritis. Int. J. Obes. (Lond) 2006;30:1223–1228. doi: 10.1038/sj.ijo.0803287. [DOI] [PubMed] [Google Scholar]
- 50.Gelber AC, et al. Body mass index in young men and the risk of subsequent knee and hip osteoarthritis. Am. J. Med. 1999;107:542–548. doi: 10.1016/s0002-9343(99)00292-2. [DOI] [PubMed] [Google Scholar]
- 51.Hochberg MC, et al. The association of body weight, body fatness and body fat distribution with osteoarthritis of the knee: Data from the Baltimore Longitudinal Study of Aging. J. Rheumatol. 1995;22:488–493. [PubMed] [Google Scholar]
- 52.van Saase JL, Vandenbroucke JP, van Romunde LK, Valkenburg HA. Osteoarthritis and obesity in the general population: A relationship calling for an explanation. J. Rheumatol. 1988;15:1152–1158. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the main article.