Abstract
Probiotics are live bacteria found in food that assist the body's defence mechanisms against pathogens by reconciling the gut microbiota. Probiotics are believed to aid with gut health, the immune system, and brain function, among other factors. They've furthermore been shown to help with constipation, high blood pressure, and skin issues. The global probiotics market has been incrementally growing in recent years, as consumers' demand for healthy diets and wellness has continued to increase. This has prompted the food industry to develop new probiotic-containing food products, as well as researchers to explore their specific characteristics and impacts on human health. Although most probiotics are fastidious microorganisms that are nutritionally demanding and sensitive to environmental conditions, they become less viable as they are processed and stored. In this review we studied the current literature on the fundamental idea of probiotic bacteria, their medical benefits, and their selection, characterization, and implementations.
Graphical Abstract
Keywords: Probiotics, Stability, Selection, Characterization, Applications
Introduction
Probiotic strains have seen increasing commercial interest in recent years. Since most of the probiotics currently available are fastidious microorganisms, nutritionally demanding and sensitive to environmental conditions, they become less viable as they are processed and stored. Elie Metchnikoff brought the idea of probiotics in the early nineties(Amara and Shibl, 2015a). Research in probiotics gradually gained pace in the past two decades and various advancements were observed with respect to the selection of probiotics, their isolation and characterisation. Probiotics fall under the category of functional food, a term that gained popularity as healthy foods in the past few years (Jackson et al., 2019a). Global markets have witnessed considerable interest in probiotics for various medical conditions, along with gaining popularity as health supplements among millions of people across the globe (Jackson et al., 2019).
Probiotics are bacteria, moulds, and yeasts with lactic acid bacteria being most common (Iqbal et al., 2021). A few examples include Lactobacillus bulgaricus (L. bulgaricus), Lactobacillus plantarum, Bifidobacterium species, and Escherichia coli. It is generally believed that these microbes contribute to the spread of diseases and, as a consequence, harm and deteriorate human health. There exist two kinds of bacteria, the good bacteria are required for the proper functioning of the body and are commonly found in the gut and other locations of the body including mouth, urinary tract, vagina etc. (Hemarajata and Versalovic, 2013). Probiotics are basically made up of these good bacteria, whose consumption can provide benefits such as supporting immune function(Nazir et al., 2018), controlling inflammation, increase digestion (Kaur et al., 2021), break down and absorption of medications(Nazir et al., 2018).
Certain medical conditions can be treated by increasing the amount of probiotics in the body which includes diarrhoea, gum diseases, irritable bowel syndrome, inflammatory bowel disease, yeast infections, lactose intolerance etc. (Foster and Zhou, 2015). However, the viability of probiotics continues to be a technological and marketing challenge for industries. Reduction of viability during processing and storage still continues to be one of the major challenges. This study focuses on the selection and characterisation of probiotics and their stability analysis to understands conditions under which the probiotic stability is affected to assist future research on solving this problem..
Literature search
Using the electronic databases Google Scholar, PubMed, and Web of Science without any limitations on language or time, a comprehensive search strategy was utilized to identify articles published by mid-2022. Research articles mentioning “probiotic” in the title and abstract were searched. To obtain more precise results, an advanced search was conducted with filters such as selection, stability, and characterization terms including “probiotics”. We screened additional reviews and systematic reviews to identify potentially related citations. Manual searching was conducted to circumvent the elimination of pertinent articles.
Probiotics and food products
The gut microbes refer to a wide catalogue of microbes and their genes inhabiting the gut. The amount of microbes present in the human body outnumber the human somatic cells (Kerry et al., 2018). From the beginning of early life, the immune system’s development and sustaining homeostasis are strongly affected by the configuration of microbial communities present in the gut (Yadav et al., 2018; Zhao, 2010). Probiotics have a lot of beneficial properties which are proven to be remediable and salubrious (Fig. 1). In addition, probiotics are coadjutants in treating metabolic disorders, which includes obesity, metabolic syndrome and type 2 diabetes. Probiotics exhibit many beneficial effects such as antimicrobial, anti-carcinogenic and anti-pathogenic properties (Shah and Swami, 2017).
Fig. 1.
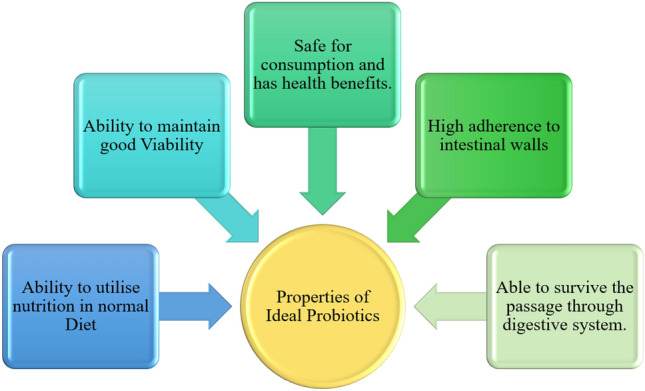
Properties of an ideal probiotics
Currently, there are a wide variety of food products containing probiotic strains (Table 1). Milk-based products account for the majority of market sales, including fermented milks, cheese, ice cream, buttermilk, milk powder, and yogurts (Damián et al., 2022). As a non-dairy food application, soy-based products, nutrition bars, cereals, and juices can be used to deliver probiotics to the consumer (Aspri et al., 2020). As part of the evaluation of the effectiveness of probiotic strains added to foods and beverages, the product’s safety and compatibility with the microorganisms, as well as its processing, packaging, and storage conditions, should be taken into account (George Kerry et al., 2018). The worldwide market for probiotic food has grown substantially every year due to the potential health advantages. However, certain food with probiotic claims is a problem because of the difficulty of surviving and maintaining the probiotic cells which are included in the food at the time of processing and storage (Rodrigues et al., 2020).
Table 1.
Various food probiotics, manufacturers and benefit on body
| Food used for delivery of probiotic | Strain | Organism present | Amount to be consumed / dosage | Benefit on body | Manufacture s and country | References |
|---|---|---|---|---|---|---|
| DanActive fermented milk |
Lactobacillus casei DN-114 001 |
L. casei Immunitas | 200 g | Fosters digestion process, improves function of intestines, helping to avoid constipation | Danone (Paris, France) | (Rizzoli and Biver, 2018) |
| Kefir | Lactobacillus, bifidobacterium | Lactobacillus | 200–300 ml | Boost immune system, improves bone health | Lifeway Foods, Kefir, USA | (Rizzoli and Biver, 2018) |
| Tempeh | Bacteria | Firmicutes | 100 g | Reduce cholesterol, improve digestive health and reduce inflammation | Taiwan Tempeh Food Business Co., Ltd. Taiwan | (Stephanie et al., 2017) |
| Kimchi | Lactobacillus | Lactobacillus | 0.5–7.5 oz (15 -210 g) | Regulate immune system, promote weight loss, fight inflammation | Madge’s food company, USA | (Park et al., 2017) |
| Miso | Saccharomyces | Saccharomyces cereviase | 6 g per day | Enhance gut bacteria. Overall physical and mental health | Hikari, Miso, Japan | (Ito, 2020) |
| Kombucha | Acetic acid bacteria | Acetobacteraceae | 100–120 ml | Strengthening immune system, helps in gout, rheumatism, liver function | Zoh Probiotics, India | (Watawana et al., 2015) |
| Pickles | L. plantarum, L. brevis, | Lactobacillus, Enterococcus | – | Fight diseases | – | (Behera et al., 2020a) |
| Enterococcus faecalis | ||||||
| Traditional buttermilk | L. acidophilus | Lactobacillus | 8 oz | Helps in treating digestive health issues as irritable bowel syndrome | – | (Rawat et al., 2018) |
| Natto | Bacillus subtilis | Bacillus | 100–200 g | Stronger bones, healthier heart and immune system | True basics, India | (Sella et al., 2021) |
| Cheese | Bifidiobacterium longum, L. acidophilus | Bifidiobacteria, lactobacillus | 42 g | Beneficial for immune system | Nestle, India | (Hammam, 2020) |
| Miso soup | T. halophilus, Saccharomyces cerevisiae | Tetragenococcus, | 200 to 240 ml | Reduce risk of inflammatory bowel diseases | Hikari, Miso, Japan | (Allwood et al., 2021) |
| Saccharomyces | ||||||
| Sourdough bread | Lactic acid bacteria and yeast | Saccharomyces cerevisiae | 56 g | Improves gut health,better digestion | Swiss bake, Switzerland | (Longoria-García et al., 2018) |
| Yoghurt | L. bulgaricus, S. thermophilus | Lactobacillus | 3 cups | Strengthen immune system | Danone, (Paris, France) | (Meybodi et al., 2020) |
| Soft cheese | L. lactis | Lactococcuss | 3 servings | Healthy weight gain, prevent osteoporosis | (El-Sayed and El-Sayed, 2021) | |
| Acidophilus milk | L. acidophilus | Lactobacillus | – | Preventing intestinal infections | (Farag et al., 2020) | |
| Sour pickles | L. plantarum | Lactobacillus | – | Helps digestion | (Behera et al., 2020b) |
Engineering probiotics with tailored functional properties and its applications
The process of encapsulating one substance into another is referred to as encapsulation (Levi et al., 2011). Encapsulation is done to enhance their viability and stability during production and storage (Rodrigues et al., 2020). As carrier material, the substance used for encapsulation must be both safe and cost-effective. Electron microscopy can be used to analyse the size and structure of the microcapsule before and after incorporating the probiotics. Sometimes along with carrier material, some cryoprotectant or bioactive compounds might increase the stability (Šipailienė and Petraitytė, 2018). The process maintains cell viability even under harsh conditions in the gastric environment and allows the cell to be released in a controlled manner. The coating material selection is very significant since it determines the effectiveness of encapsulation.
Due to the recent development in research, probiotics have also found applications in treating ulcers (Khoder et al., 2016), inflammatory bowel diseases (IBD), and tissue repair (Abraham and Quigley, 2017) and modulation of stem cells (Al-Yassir et al., 2021). Among others, when the stomach is subjected to damage, its surface may erode, which leads to cancer and ulcer. The protective effects of healthy multibacterial strains modulate the dividing stem cells and secretory cell lineage of the stomach. Cells of the stomach showed regeneration with increased stem cell proliferation and increased production of mucus which essentially protects the gut (Haghshenas et al., 2017). Probiotics have also shown the potential to treat IBD (Abraham and Quigley, 2017). Following the consumption of probiotics, cells that had previously displayed symptoms of IBD showed anti-inflammatory and pro-regenerative effects to revive the tissues and cells of the affected areas(Lee et al., 2018).
Probiotics enhance the healing of wounds present in the GI tract, and stimulate fibroblast proliferation migration. Probiotics also enhance the fortification of the epithelial barrier (Lee et al., 2018). Studies have shown that bacterial strains of probiotic bacteria react with compounds such as antioxidants, antimicrobial agents, and flavours, resulting in an effect that is a combination of the compounds as they interact (Markowiak and Śliżewska, 2017). A cumulative effect is generally observed by this, and composition variations may also influence flavour, microbial composition, and quality. Probiotics also improved disease resistance and broiler chicken performance. (Terpou et al., 2019a).
Furthermore, the encapsulation of the bacterial strains ensures the strains are delivered and protected under conditions like increased temperature, variation in pH, etc. essentially protecting the strains from degradation by gut physiological conditions (Markowiak and Śliżewska, 2017). Encapsulation increases the life of probiotic strain and enhances its activity. There are various methods by which bacterial strains (probiotics) can be encapsulated, which can be broadly categorised into two types: liquid delivery system or encapsulation by liquid system and solid delivery system or encapsulation by the solid system (Fenster et al., 2019).
Criteria of selecting probiotics and their action mechanism
Probiotics are often described as “a mono- or mixed culture of live microorganisms which, when applied to man or animals, beneficially affect the host by improving the properties of indigenous microflora” (Iqbal et al., 2021). The term “Probiotics” has its word root in Greek which means “prolife” etymologically. With time various definitions evolved such as, “growth promoting factors produced by microorganisms”, “Microbial cells which transit the GI tract and which, in doing so, benefit the health of consumer”(Liu et al., 2018a). To be considered as a probiotic, the bacteria is supposed to have certain characters (Fig. 1). Lactobacillus and Bifidiobacteria are the commonly used strain considered as probiotics. Many other strains are also used as probiotics. The important criteria to consider in a strain as probiotics includes (1) the ability to survive in a digestive tract environment which require acid and bile tolerance, (2) an inhabitant of a normal intestinal tract, (3) expected to be safe for host consumption, (4) should be able to adhere to the intestines and colonise there, (5) should be able to produce antimicrobial compounds like bacterions to fight off pathogens, (6) has to be non-pathogenic to host.
Currently, a wide array of commercial probiotics products are available and provide desired medical benefits when ingested in appropriate dosages (Vijayalakshmi et al., 2020). Even though a lot of strains from genera such as Propionibacterium, Enterococcus, and Escherichia are available (Plaza-Diaz et al., 2019), the criteria for selecting probiotics depend on factors that should enhance the efficiency and stability of probiotics. They include easy to store without loss of viability; easy to grow in cheap and straight forward fermentation medium to maximum concentration; should be able to withstand physical handling without losing viability, withstand processing, should process tolerance to digestive juices as it passes through the gastrointestinal tract and adhesion onto a specific body site (Yadav and Shukla, 2017). Probiotics possess different mechanisms of action which includes short-chain fatty acid production, immunomodulation, and stimulation of mucosal barrier function and lowering of gut pH. In several studies conducted, it was found that probiotics have an influence on acquired and innate immunity responses through induction of phagocytosis IgA secretion and modifying T-cell responses (Yeşilyurt et al., 2021). Most of the health benefits regarding the use of probiotics are related with gastrointestinal tract. Probiotic mechanisms in gastrointestinal tract can be classified into mucosal, luminal and submucosal (Lee et al., 2018).
Probiotics in food and human health
Food products containing probiotic strains are available in a wide variety. The most commonly used products in markets include diary based products like cheese, fermented milks, ice cream, buttermilk, yoghurts and milk powder (Sharifi-Rad et al., 2020). The other category of available products is non-dairy products like cereals, nutrition bars, juices, and so on (Table 1). A number of studies conducted on health benefits of probiotics have shown numerous results, which includes promoting intestinal health, reduction of serum cholesterol, improved immune responses, prevention of cancer, treatment of acute diarrhoeal diseases etc. (Martín and Langella, 2019).
Isolation of probiotics
Lactic acid bacteria (LAB) are generally considered as safe (GRAS) microorganisms. They are found in water, soil manure and in habitats with nutrient sources. LAB identification steps from natural resources are sampling and isolation, 16S rDNA fragment amplification and sequencing, PCR fingerprinting and data analysis, low pH and high bile salt tolerance assessment, antimicrobial activity. (Haghshenas et al., 2017). The first step in the isolation of probiotic LAB from uncommon sources or non-intestinal sources is to cultivate them on a high nutritional medium that is different or modified from the conventional de Man, Rgosa, and Sharp (MRS) medium. A medium consisting mainly of glucose, yeast extract, and peptone (GYP) was used to cultivate LAB isolated from paddy rice silage, crop and silage fermentation (Kerry et al., 2018). A GYP plus BM medium was successfully used to cultivate LAB isolated from soil (rhizospheres of fruit trees and soil around animal farms). To cultivate malolactic-producing LAB such as Oenococcus oeni from red wine-making in Japan, BM medium containing tomato juices, peptone, liver extracts, and glucose is used. A modified MRS medium is also used to screen different parts of the GI tract of animals in LAB. For LAB isolates from animals' GI tracts to grow properly, pH conditions and nutrients may be required as well as some substrates. A modified MRS medium containing 0.3–1% (w/v) CaCO3 was used to isolate LAB from the GI tracts of animals. Various animals, including chickens (Bayane et al., 2010), cattle (Puphan et al., 2015), and dogs (Nazir et al., 2018), have been successfully isolated using this medium. In addition to raising LAB from fermented foods made from fish (Pla-chom) and beef (Mum), the medium has also been used to cultivate LAB from a variety of raw materials. Fermented foods, such as pickles, were fermented in acidic conditions using a medium containing mainly glucose, yeast extract, peptone, and 0.5% (w/v) CaCO3.
The fermentation is carried out carefully, and after fermentation, the cells are separated from the spent medium through centrifugation resulting in a concentration of cells (Shokryazdan et al., 2017). Depending on the application of the final product, stabilizer solutions are added (cryoprotectants) to probiotic mixture before freezing. After mixing the probiotic concentrate with various freezing processes can be done for cryopreservation. These frozen cans are shipped to various companies for incorporating probiotics into food and beverages. After lyophilisation, it is milled into powder form with defined practical size and density (Loh et al., 2015). The milled materials are later blended with bulking agent, functional ingredients depending upon the needs of customer. This blend is then used to make finished products (Fenster et al., 2019).
Bioengineering of probiotics: technologies currently used to enhance cell viability
Bioengineered probiotics with numerous immunogenic or antagonistic properties could be useful for improving human health. In contrast to conventional drug administration methods, these bacteria are tailored to deliver drugs, therapeutic proteins, or gene therapy vectors precisely and with a higher degree of site specificity (Kumar et al., 2015).
Many factors are found to affect the viability of cells present in probiotics. Chemical factors such as oxygen levels, redox potential along with additives, antimicrobial compounds affect viability during storage, whereas biological factors are strain type, natural microflora product, enzymes produced, post acidification and various pathogenic or spoilage microorganism occurrences (Silva et al., 2016). Similarly, physical factors include drying conditions and temperature will affect the probiotic viability (Fenster et al., 2019). Strategies to enhance the cell viability include selecting suitable strain, which serves a crucial role in improving viability. In order to prompt cell inactivation and to intensify cell stability, physical stress is applied (temperature stress, osmotic stress, oxygen stress). The selection of proper food packaging systems can influence viability including packaging methods like oxygen scavengers, vacuum packaging can significantly improve probiotics viability (Terpou et al., 2019b).
Encapsulation technologies are expected to enhance stability, ensure better handling and storage of probiotic cultures (Ren et al., 2019). As most probiotics are of intestinal origin, they are unsuitable for growth on diary- based media and mostly get inactivated on exposure to high heat, acid during processing (Corcoran et al., 2005). Technological challenges associated with maintaining a high number of probiotic organisms in food, the capability of the culture to retain viability in the food matrix environment, maintenance of its characteristics during consumption are also of concern. These technological approaches used for the preparation of cultures may affect their viability and functionality. Sometimes, cell injury can also happen during the application of these technologies.
Probiotic encapsulation technology has rapidly emerged in the past decade. With the help of this technology, many microorganisms have been immobilised with semipermeable materials to facilitate their delivery (Hassan et al., 2019). Despite the benefit of increased viability and shelf life, it faces many challenges, including developing microencapsulation equipment, selection of non-toxic materials for encapsulation, development of beads or capsules from polymers, the determination of appropriate mechanism during probiotic release. One of the most important challenge is of the cost, encapsulated end products can be very costly. This is because their development demands both time and financial resources. The use of natural polymers will increase the cost further, such as milk proteins seems more costly than carbohydrates. These techniques also require certain raw materials, which includes oil and emulsifiers in order to stabilize the capsule (Aragón-Rojas et al., 2019).
Functionalities of probiotics
Probiotics are live microorganisms which upon ingestion in sufficient concentrations can exert health benefits on the host. The data obtained from the literature of probiotics in the treatment of various diseases are organized as a summary in Tables 2 and 3. The Food and Agriculture Organization of United Nations and the World Health Organization developed this definition, which has become the term of reference for science and regulation ever since (The Food and Agriculture Organization, 2021).
Table 2.
Probiotics in the treatment of various cancers and gastro-intestinal diseases
| Name of gastrointestinal disease | Symptoms of the disease | Strain of probiotic recommended | Amount of Probiotic administered | Disease Cured in | References |
|---|---|---|---|---|---|
| Acute infectious diarrhea | Changes in bowel movement, increase in the water content, and frequency of stools | Saccharomyces boulardii | 250 and 750 mg | ~ 24 h-73 h | (Dinleyici et al., 2015) |
| Ulcerative Colitis | Diffuse mucosal inflammation, bloody diarrhoea, and abdominal pain | E. coli and Lactobacillus GG | 100 mg to 200 mg per day | 6 months | (Kaur et al., 2020) |
| Antibiotic-Associated Diarrhea | Severity to result in colitis, electrolyte disturbance, and bowel perforation | L. acidophilus, L. casei | 5 × 109 viable bacteria | 4 weeks | (Goldenberg et al., 2017b) |
| Crohn’s Disease | Fever, fatigue, Blood in your stool, and mouth sores | Bifidobacterium longum | 2 × 1011 freeze-dried viable | 6 months | (O’Callaghan and van Sinderen, 2016b) |
| Traveller’s Diarrhea | An urgent need to defecate, Nausea, Vomiting | Lacticaseibacillus rhamnosus | 2 × 109 CFU | 1 week | (Giddings et al., 2016) |
| C. difficile diarrhea | Waterydiarrhea, Rapid heart rate, nausea Lossof appetite, Weight loss | S. boulardii | 1000 mg per day | 4 weeks | (Moré and Swidsinski, 2015) |
| Irritable Bowel Syndrome | Discomfort or pain associated with an alteration in bowel habits constipation | Propionibacterium freudenreichiis sp shermanii | 1 × 107 CFU | 23 weeks | (Didari, 2015) |
| C. albicans-associated gut discomfort | Abdominal or pelvic pain, Blood in your urine | L. rhamnosus; B. animalis subsp. lactis | 1 × 109 CFU | 14 weeks | (Severance et al., 2017) |
| Colorectal cancer | persistent change in bowel habits, Rectal bleeding | Lactobacillus and Bifidobacterial | 30 × 107 CFU | 6 months | (Zaharuddin et al., 2019) |
| Head and neck Squamous Cell Carcinoma | A persistent sore throat, and pain or difficulty swallowing | Bifidobacterium breve lw01 | 1 × 109 CFU | 3 weeks | (Johnson et al., 2020) |
| Severe acute pancreatitis | Fever, Higher heart rate, Swollen and tender belly | L. plantarum | 3 × 107 cfu | 2 weeks | (Oláh and Jr, 2014) |
Table 3.
Probiotic organisms and their applications
| Genus | Species | Applications | Reference |
|---|---|---|---|
| Lactobacillus | L. rhamnosus | Reduction of viral-associated pulmonary damage; prevention and reduction of severity of atopic dermatitis in children; reduction of risk for developing allergic disease; reduction of risk for rhinovirus infections in preterm infants; protection of human colonic muscle from lipopolysaccharide-induced damage | (Ammoscato et al., 2013; Zelaya et al., 2014) |
| L. acidophilus | Treatment of travellers’ diarrhoea; reduction of hospital stay of children with acute diarrhoea; antifungal activity; prevention or treatment of bacterial vaginosis; treatment of C. difficile-associated diarrhoea; reduction of irritable bowel syndrome symptoms | (McFarland, 2007; Phavichitr et al., 2019) | |
| L. plantarum | Prevention of endotoxin production; antifungal activity; reduction of irritable bowel syndrome symptoms | (Cortés-Zavaleta et al., 2014; Lee et al., 2014; Wright et al., 2015) | |
| L. casei | Treatment of functional constipation in adults; immunomodulatory mechanisms; improvement of rheumatoid arthritis status; protection against Salmonella infection; prevention of Salmonella-induced synovitis; treatment of intravaginal staphylococcosis | ||
| L. delbrueckii subsp. bulgaricus | Antibiotic resistance of yogurt starter culture; enhancement of systemic immunity in elderly; antibacterial action against E. coli; modulation of brain activity | (Moro-García et al., 2013; Tillisch et al., 2013) | |
| L. brevis | Protective role in bile salt tolerance; reduction in plague acidogenicity | (Suzuki et al., 2013) | |
| L. fermentum | Prevention or treatment of bacterial vaginosis; blockage of adherence of pathogenic microorganisms on vaginal epithelium; antistaphylococcal action; potential for reduction of insulin resistance and hypercholesterolemia | (Tomaro-Duchesneau et al., 2014) | |
| L. reuteri | Reduction of low-density lipoprotein cholesterol; reduction of onset of gastrointestinal disorders in infants; reduction of frequency of proven sepsis, feeding intolerance and duration of hospital stay in preterm infants | (Caramia et al., 2013; DiRienzo, 2014) | |
| Bifidobacterium | B. infantis | Reduction of irritable bowel syndrome symptoms; reduction of necrotizing enterocolitis in preterm infants | (Li et al., 2013) |
| B. animalis subsp. lactis | Treatment of functional constipation in adults; reduction of total cholesterol; reduction of risk of upper respiratory illness | (Pinto et al., 2014) | |
| B. breve | Prevention and treatment of necrotizing enterocolitis in newborns; reduction of necrotizing enterocolitis with Bifidobacteria cocktail; reduction of cholesterol | (Bordoni et al., 2013; Janvier et al., 2014) | |
| Saccharomyces | S. boulardi | Treatment of travellers’ diarrhoea; treatment and reduction of diarrhoea duration regardless of cause; treatment of irritable bowel syndrome; treatment of moderate ulcerative colitis; treatment and reduction of recurrent pseudomembrane colitis infection caused by C. difficile; treatment of acute gastroenteritis in children | (Fernandez et al., 2014) |
| Lactococcus | L. lactis subsp. lactis | Treatment of antibiotic-associated diarrhoea; antimicrobial and probiotic properties | (Lee et al., 2013) |
| Enterococcus | E. durans | Antibiotic and antioxidant activity; adherence to colonic tissue and anti-inflammatory activity | (Pieniz et al., 2013) |
| E. faecium | Treatment of antibiotic-associated diarrhoea; efficient animal probiotic | (Cao et al., 2013) |
Probiotics in gastrointestinal ailments
Probiotics have advantageous benefits in treating gastrointestinal ailments like necrotizing enterocolitis, traveller’s diarrhea, antibiotic associated diarrhea, irritable bowel syndrome (IBS), recurrent Clostridium difficile, inflammatory bowel disease (IBD), Crohn’s disease, Helicobacter pylori, ulcerative colitis, etc. (Liu et al., 2018b). The gastrointestinal Tract (GI) sustains a diverse range of microbial population that actively or passively regulates the metabolism and immunological system of the host. The significance of intestinal microbiota, one of the priorities of probiotic therapies, on the physiology of immune cells can be emphasized to cure inflammatory conditions and gastric ailments (Sales-Campos et al., 2019).
Organisms that normally populate the gut may undergo dysbiosis due to antibiotic treatment (Kim et al., 2017). When the healthy balance in the gut is disrupted, harmful, threatening organisms, especially Clostridium difficile is known to colonize the gut. A study assessed the effectiveness of probiotics containing S. boulardii or L. acidophilus with L casei at a dosage of 10–50 billion CFU/day amongst participants taking antibiotics for preventing Clostridium difficile associated diarrhea (Johnson et al., 2012). They concluded that there was a 60% decrease in the risk of contracting Clostridium difficile associated diarrhea when probiotics are consumed with antibiotics (Goldenberg et al., 2017a).
There are copious studies that successfully enabled the effectiveness of probiotic strains on diverse gastrointestinal ailments. The consumption of multistrain probiotic consolidating Streptococci, lactic acid bacteria, and Bifidobacteria in notable amounts (6 g/day) can help maintain remission and prevent acute pouchitis a study illustrated (Amara and Shibl, 2015a). It also demonstrated that the number of Lactobacilli and Bifidobacteria in the patients with pouchitis was elevated and also remained significantly increased later of the administration. Moreover, there was a substantial decrease/improvement in symptoms like involuntary defecation, abdominal cramps, faecal consistency, mucus, and urge to evacuate stools during the intervention period (LeBlanc et al., 2021). As we are well versed in the fact that our behaviour and mood is affected by the gut microbiota; patients associated with Irritable bowel syndrome most commonly exhibit the symptoms of depression and anxiety, as compared to the healthy population. The vagus nerve which is the 10th cerebral nerve plays a very significant role in the communication cascade of microbiota-brain axis (Breit et al., 2018). Administration of Lactobacillus rhamnosus JB-1 resulted in the benefit of amelioration of anxiety and depression- like disorders (Horvat et al., 2021).
Antipathogenic and antiviral activity
Probiotics is known to exhibit antimicrobial effect against numerous microorganisms, including pathogens and virus etc. (Denkova and Kostov, 2017). There are several mechanisms proposed on the action of probiotics manifesting the antipathogenic properties. When ingested orally, probiotics attach to the intestinal mucosa running through the stomach, hindering the pathogenic bacteria from adhering to the epithelial tissue (Islam, 2016). Marhamatizadeh et al., (2013) concluded that the growth of Salmonella enteritidis, Staphylococcus aureus or Escherichia coli was restricted by the intake of probiotics, whereas it had no considerable effect on the growth of certain selected LAB such as Lactobacillus rhamnosus, L. plantarum, Leuconostoc mesenteroides therefore inferring its anti-microbial properties positively. Probiotics also provided a barrier which was showcased by a reduced reactivity of the host epithelium to opportunistic pathogenic allergies (Clavel et al., 2017). Lactobacilli attach to the receptors on the surface epithelial cells and exhibit competitive inhibition with enteric pathogens. The study also suggested that the vitality and virulence properties of the E. coli 0157:H7 and other diarrhea-inducing E. coli could be reduced by lactic acid producing bacteria (Saxena et al., 2015).
Anti-cholesterol, anti-obesity and anti-diabetic effect of probiotics
Obesity has become an alarming disease as it leads to several chronic ailments such as cardiovascular diseases, diabetes, insulin resistance of hepatic and skeletal muscle, and few forms of cancer (Fruh, 2017). Medication through drugs gives undesirable side effects and there is a high risk of post-surgery infections which may lead to dysbiosis of gut microbiota. Comparative surveys of gut bacteria of obese patients showed fewer Bacteroidetes and more firmicutes (Puphan et al., 2015). Hence, dietary inclusions of probiotics may help upregulate and maintain the ratio of Bacteroidetes and firmicutes, therefore, improving the state of obese patients (Stojanov et al., 2020).
Bifidobacterium is a common bacteria found in the human intestine. It can aid Bacteroides in the degradation of polysaccharides and limit the absorption of exogenous cholesterol from the small intestine (O’Callaghan and van Sinderen, 2016a). According to a study, administering strain B. L66-5 resulted in BW reduction, and a reduction in hepatic adiposity, all of which aided in the management of obesity (Shen et al., 2013).
Cholesterol accumulation is a life-threatening condition in which the oxidation of cholesterol causes arterial plaque formation, which contributes to cardiovascular disease (Soliman, 2018). Probiotic products' efficiency in the enhancement of health and disease reduction is due to their bioactive components. Probiotics have been suggested as a beneficial dietary strategy for lowering total cholesterol, particularly in people with borderline blood cholesterol. Probiotic strains such as L5022, LA2404, LA2410, and BB5286 have witnessed the ability to remove cholesterol (Miremadi et al., 2014).
Antimutagenic and anticarcinogenic properties of probiotics
Cancer is defined as a spontaneous and unregulated division/proliferation of cells that results in the destruction of body tissue. Cancer is one of the most common malignant diseases, with significant fatality rates all around the world (Sawant and Shegokar, 2014). While chemotherapy destroys cancer cells over time, it also harms healthy cells and results in drug resistance (Sawant and Shegokar, 2014). Probiotics act on cancerous and mutagenic cells by exhibiting few mechanisms which include degradation, binding and inhibition of mutagens; probiotics also aid in prevention of procarcinogens and also alter deleterious and highly reactive carcinogens (Kumar et al., 2010). They assist in lowering the pH of the gut with the help of short chain fatty acids made during the catabolism of undigestible carbohydrates. They modulate and strengthen host’s innate immunity by secreting anti-inflammatory molecules.
Live entire probiotic strains of Lactobacillus fermentum RM28 and Enterococcus faecium RM11 in fermented milks induced anti-proliferation in colon cancer cells (Amara and Shibl, 2015b). A study found that administration of L. acidophilus to tumour-bearing mice transformed their cytokine production into a Th1 protective pattern, which is good for anti-tumour immunity (Maroof et al., 2012a). As a result, decreased tumour growth rate and increased lymphocyte proliferation are also beneficial (Maroof et al., 2012b). Due to the key properties connected to the presence of arginine deaminase activity, Lactococcus lactis provides value to a probiotic product. This has metabolic features that can inhibit opportunistic infections by depriving them of arginine, a critical source of nitrogen, carbon, and energy for bacteria (Verdenelli et al., 2014).
Probiotics in urogenital infections
Probiotics containing Lactobacillus bacteria of human origin for ameliorating urogenital infections is proved to be very effective. Problems due to antimicrobial treatments such as yeast and bacterial resistance, recurrent infections have become prominent (Maroof et al., 2012a). Canadian urologist Andrew Bruce first conceived that Lactobacilli content of vaginal microbiota acts as a barrier and prevents urogenital infections. The Lactobacillus adheres to the vaginal epithelium and enhances anti-pathogenicity controlling vaginal microflora including Candida albicans (Verdenelli et al., 2014).
In a study, the bacteriocin produced by all Lactobacilli isolates against Candida strains was analysed using the method agar antagonism, however no inhibitory action was found, whereas the radial method yielded considerable antipathogenic results (Maroof et al., 2012a). L. plantarum 319 was shown to have potent inhibitory properties. This existence of normal intestinal L. plantarum, in the vaginal habitat has been linked to a lower incidence of bacterial vaginosis in the past (Martín et al., 2012).
Role of symbiotic probiotics
The human gut commensal microbiota thrives by maintaining a symbiotic relationship with the host, hence, forming a complex population of microorganisms. It plays a very prominent role in maintaining a balanced homeostasis and also aids in the formation of adaptive immune system. The human digestive system can be very challenging for the bacteria, especially, if it is prescribed to be taken orally and is poorly formulated. There is a possibility that the probiotic bacteria will not reach the intended location say, small intestine in the viable state. In such circumstances, the human gut microbiota will not be altered by probiotics. Hence, probiotic multi strain bacteria, known as symbionts confer additional and effective health benefits. The symbiotic preparation, symbiotic drink, administered in multi organ failure (MOF) patients, for 7 days resulted in positive alterations to improve the early lactate levels and late fibrinogen/D-dimer levels also, mucosa colonization by Candida (Martín-Consuegra et al., 2014).
Moens et al., (2019) concluded that the probiotic supplement (Symprove™), containing Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus rhamnosus and Enterococcus faecium has displayed to ameliorate clinical symptoms, severity scores in IBS and to reduce abdominal pain and remarkably weaken manifestation of irregularity in bowel movements and menorrhoea symptoms in diverticular disease. Symprove proved to exhibit high tolerance against gastric acids in in-vivo studies anti-pathogenicity against Clostridium difficile (Moens et al., 2019).
Fortification of probiotics in food products
Food fortification, in general, is defined as adding one or more components of food as to enhance its nutritional concentrations for beneficial outcomes (Chadare et al., 2019). Food fortification is an essential technique for the enhancement of nutrition in quality and quantity of food. It is very cost-efficient and, considering the crucially of food safety and quality, it aims at promoting public health and safety. Consumption of fortified foods with probiotics is progressively increasing presently due to nutrition deficiencies in certain societies and at certain ages as evaluated from the literature on various probiotics (Table 1). Food fortification normally prioritizes natural resources such as fruit, cereals, milk, vegetables, grains, and so on, in the best way to improve the nutritional value with minimal side effects (Gahruie et al., 2015). The natural health-promoting factors provide benefits to the customers, such as maintaining normal blood pressure, and sustaining balanced cardiovascular, immune and nervous systems (Chadare et al., 2019).
Weaning foods
Weaning foods are the first solid foods consumed by an infant. Weaning foods provide the baby with nutritional benefits to nurture the baby’s growth (Bassey et al., 2013). A shift from whole breast milk feeding to weaning foods might alter the infant’s digestion and cause minor digestive issues like diarrhea. Hence, probiotic infant formula might help maintain homeostasis. Bifidobacterium lactis (BB-12) and Lactobacillus reuteri are normally comprised in the probiotic infant’s formula. Porridge contains lactic acid bacteria that synthesize feruloyl esterase, was isolated and characterised for their probiotic potential. The strain Lactobacillus fermentum produces galactosidase and glutamate decarboxylase enzymes, as well as its potential to lower cholesterol in vitro, serves as a marker for use in the implementation of functional foods (Kumar et al., 2021).
Confectionary
Chocolate is, in essence, prepared in a butter matrix composed of cocoa mass and sugar suspended in it. Chocolates have been considered to be fortified with probiotic strains since long time due to its popularity among kids and also among the elderly. This is considered nutritionally and economically advantageous. Probiotic chocolates have a significant effect on the nervous system and relieve stress (Faccinetto-Beltrán et al., 2021). Probiotic strains are fortified along with other nourishing foods like dry fruits to make nutrition bars in the market. They provide as immediate energy and nurture the individual, providing homeostasis (Barat and Ozcan, 2018). Fermented ice cream formulation has the notable capacity to be used as a functional product. The use of soy milk or coconut milk gives an advantage as the growth of bifidum bacteria is enriched and also the final product can be consumed by lactose-intolerant individuals acting as a nutritious alternative (Palka and Newerli-Guz., 2018).
Health mix
Kefir grains and kefir cultures are used to make fermented milk called kefir. Kefir helps the body with healing and stabilizing functions as it contains essential vitamins, minerals, and amino acids (Egea et al., 2022), which also comprises easily digestible complete proteins. Kefir provides a wide range of benefits if consumed. It has been claimed to be effective against a variety of diseases. Various intestinal disorders, difficulty in bowel moments etc, can be treated by regular consumption of kefir (Leite et al., 2013). Fruit juices are popular for their rich nutritional values, fruit juices are fortified with probiotic enzymes to cater numerous diseases and also maintain a healthy system. Probiotic strain influences the viability, i.e., a few strains of L. plantarum, L. acidophilus and L. casei can thrive in fruit matrices as they are capable of sustaining acidic environs (Egea et al., 2022). Microencapsulation may create a more suitable anaerobic habitat for vulnerable probiotic bacteria, as well as act as an enhanced physical barrier from the fruit juice's severe acidic conditions, hence, being more effective.
Bakery products
One of the prominent item, when it comes to bakery products is bread. Bread is prepared in different forms and shapes to facilitate eating and preparation of desired food items. A study was conducted aimed at obtaining symbiotic bread. Encapsulation of L. casei 431 and Lactobacillus acidophilus LA-5 combined with calcium alginate starch with Hi-maize resistance and chitosan coating was achieved by emulsion process (Maroof et al., 2012a). The bread loaves were baked by encapsulating probiotics and inoculating them into the bread dough. After baking, the viability of encapsulated probiotics was analysed; also, the sensory assessment was performed. The standard requirement for probiotic products was met by the symbiotic bread (Ardabili et al., 2016).
Yogurt
Yogurt products supplement its customers with high levels of nutrition. Probiotic strains such as L. acidophilus, S. acidophiles etc. are used for fortification with yogurt (Chandan, 2017), which acts as a substitute for lactose-intolerant patients. The lactase enzyme contained in the yogurt bacteria digests the lactose in the gut lumen and is released into the small intestine where these bacteria are lysed by bile acids (Chandan, 2017). This helps balance gut homeostasis and treats diarrhea, constipation, and so on. There is a wide range of choices available in probiotic yogurt ranging from plain, flavoured, and Greek yogurts which are again available according to the composition of fats and protein, e.g., low fat, high protein etc. Greek yogurt is very famous in consumers as it offers high protein value. There are various brands selling yogurt in India namely epigamic, mother diary, milk mist etc. (Chandan, 2017).
Probiotics and food safety
Studies have been conducted on the health effects of probiotics on a wide variety of diseases. However, limited information is available about the safety and gastrointestinal tolerance of probiotic-containing products for long-term consumption and disorders caused by certain probiotics, such as Lactobacilli opportunistic pathogens. In some cases, these products may be sold without legal oversight. Populations at risk have reported cases of bacteraemia and adverse effects of probiotics (Nazir et al., 2018). Until recently, there has not been enough research on the long-term effect of prolonged probiotic use, particularly in new-borns.
A Probiotic Safety Assessment study uses a number of methods: 1. Probiotic strain intrinsic properties study. 2. Molecular mechanism of drug action of probiotic strains based on their pharmacokinetics. 3. An analysis of the interactions between probiotics and their hosts (Saarela et al., 2000). An immunocompromised host can be evaluated using animal models to determine the safety of the new probiotic. Assays of probiotic bacteria's safety have now been conducted using gnotobiotic mice with immune deficiencies (Amara and Shibl, 2015a). It is expected that the results of the animal studies will be reflected in the human studies as well.
Four broad categories can be used to categorize the need for further research on probiotics. First, a better understanding of host and microbial agents that contribute to lactobacillus infections is needed. A second requirement is to prove the efficacy of probiotics in treating or preventing diseases and infections. When probiotic efficacy is proven, it is necessary to understand the mechanisms of action of these organisms. Furthermore, based on previous criteria, it is necessary to research and develop improved probiotics, including new probiotic species being used as medical therapists or therapeutic supplements, as well as vaccines made from probiotics.
Dimensions of probiotic safety include the following characteristics (Amara and Shibl, 2015a):
Probiotic strains for human consumption should preferably come from human origin.
It is imperative that they are isolated from the healthy gastrointestinal tracts of humans.
It is important that they do not have any history of pathogenicity.
Infectious endocarditis or gastrointestinal disorders should not be linked to them.
Decongestion and hydroxylation should not destroy bile salts in the small intestine.
It is not recommended that they have genes that are transferable to antibiotic resistance. And so on.
To assess the safety of the new probiotics, it is important to identify them (Amara and Shibl, 2015a). There are two main reasons for identifying in the clinical setting: diagnostics and epidemiology. Since physiological characteristics are involved (Amara and Shibl, 2015a), molecular studies should be conducted for identification. Food supplements containing probiotics are not subject to any legal standards around the world. Based on the final consumer, the target species, the environment, and workers' safety during production or application, safety aspects are considered to be in contact with microorganisms (Amara and Shibl, 2015a).
Future perspectives of probiotics
Research on probiotics is abundant, but most of them are based on high-throughput analyses of the gut microbiota that provide snapshots of its diversity, but these studies yield little insight into how this complex system works. It is likely that future research efforts will focus on generating and combining data from multiple omics platforms. Using these tools, we will be able to characterize the microbiome from the genetic make-up and transcription products (metagenomics/metatranscriptomics), to the proteins and metabolic products (metabolomics/metabonomics). An important future challenge of microbiome research will be to link these meta-omics datasets through a mechanistic model of the microbiome to form a systems-level framework (Rao et al., 2009). These multiple technologies, however, will allow us to gain a deeper understanding of the workings of this complex ecosystem and identify the functionally important microbial components. Having a better understanding of this will allow for the creation of tailored probiotic treatments tailored to specific indications and diagnoses, which will ultimately lead to robust RCTs for clinical benefit based on individual strains.
The development of new technologies and methods for probiotics offers exciting possibilities for research and application. Enhanced tools allowing real-time studies in humans and following a microbe as it consolidates into an existing microbiota, as well as systems that can quantify levels of health, will drive this field forward (Spacova et al., 2020). In light of research, future physical exams will include information about microbes present, how they interact with the host, and the effect of environmental factors (like drugs, nutrients) (Lebeer et al., 2011).
Conclusion
Scientific studies have demonstrated how important probiotic consumption is for enhancing the quality of life, and probiotic-containing foods and supplements are becoming more and more ubiquitous. The viability of probiotics in the product up until consumption and during delivery throughout the gastrointestinal tract, sensory qualities of the product, consumers’ favourable perceptions of foods versus drugs, the economic perspective, and even the shelf life of the products are some of the many variables that affect a customer's consumption patterns. Today’s markets provide a diverse selection of foods containing probiotic strains, and it has been discovered that consuming them in the recommended dosage offers a number of health advantages. In recent years, probiotics have been bioengineered using a variety of cutting-edge fermentation technologies. The product may be exposed to a variety of conditions during production and storage that impact its stability and viability. Temperature, water activity, oxygen concentration, pH, and other microbes are a few of these variables. A financial burden on manufacturers precludes the implementation of probiotics into many product categories that may impairs efficacy.
Acknowledgements
The authors would like to thank the financial support received from the fundamental research funds for SRMIST, Chennai, India.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shreyasi Pramanik and Swethaa Venkatraman have equally contributed first authors.
Contributor Information
Shreyasi Pramanik, Email: shreyasip99@gmail.com.
Swethaa Venkatraman, Email: swethaav96@gmail.com.
Pothiyappan Karthik, Email: pkarthikbiotech@gmail.com.
Vinoth Kumar Vaidyanathan, Email: vinothkv@srmist.edu.in.
References
- Abraham BP, Quigley EMM. Probiotics in inflammatory bowel disease. Gastroenterology Clinics of North America. 2017;46:769–782. doi: 10.1016/j.gtc.2017.08.003. [DOI] [PubMed] [Google Scholar]
- Allwood JG, Wakeling LT, Bean DC. Fermentation and the microbial community of Japanese koji and miso: a review. Journal of Food Science. 2021;86:2194–2207. doi: 10.1111/1750-3841.15773. [DOI] [PubMed] [Google Scholar]
- Al-Yassir F, Khoder G, Sugathan S, Saseedharan P, al Menhali, A., Karam, S.M., Modulation of stem cell progeny by probiotics during regeneration of gastric mucosal erosions. Biology (basel) 2021;10:596. doi: 10.3390/biology10070596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara AA, Shibl A. Role of probiotics in health improvement, infection control and disease treatment and management. Saudi Pharmaceutical Journal. 2015;23:107–114. doi: 10.1016/j.jsps.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammoscato F, Scirocco A, Altomare A, Matarrese P, Petitta C, Ascione B, Caronna R, Guarino M, Marignani M, Cicala M, Chirletti P, Malorni W, Severi C. Lactobacillus rhamnosus protects human colonic muscle from pathogen lipopolysaccharide-induced damage. Neurogastroenterology & Motility. 2013;25:984–e777. doi: 10.1111/nmo.12232. [DOI] [PubMed] [Google Scholar]
- Aragón-Rojas S, Quintanilla-Carvajal MX, Hernández-Sánchez H, Hernández-Álvarez AJ, Moreno FL. Encapsulation of lactobacillus fermentum K73 by refractance window drying. Scientific Reports. 2019;9:5625. doi: 10.1038/s41598-019-42016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspri M, Papademas P, Tsaltas D. Review on non-dairy probiotics and their use in non-dairy based products. Fermentation. 2020;6:30. doi: 10.3390/fermentation6010030. [DOI] [Google Scholar]
- Barat A, Ozcan T. Growth of probiotic bacteria and characteristics of fermented milk containing fruit matrices. International Journal of Dairy Technology. 2018;71:120–129. doi: 10.1111/1471-0307.12391. [DOI] [Google Scholar]
- Bassey FI, Mcwatters KH, Edem CA, Iwegbue CMA. Formulation and nutritional evaluation of weaning food processed from cooking banana, supplemented with cowpea and peanut. Food Science Nutritions. 2013;1:384–391. doi: 10.1002/fsn3.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayane A, Diawara B, Dubois RD, Destain J, Roblain D, Thonart P. Isolation and characterisation of new spore-forming lactic acid bacteria with prospects of use in food fermentations and probiotic preparations. African Journal of Microbiology Research. 2010;4:1016–1025. [Google Scholar]
- Behera SS, EI Sheikha AF, Hammami R, Kumar A. Traditionally fermented pickles: how the microbial diversity associated with their nutritional and health benefits? Journal of Functional Foods. 2020;70:103971. doi: 10.1016/j.jff.2020.103971. [DOI] [Google Scholar]
- Behera SS, Priyadarshini M, Kumar A. Optimization for bio-processing of elephant foot yam (Amorphophallus paeoniifolius) into lacto-pickle using taguchi statistical approach. Journal of Food Measurement and Characterization. 2020;14:1470–1480. doi: 10.1007/s11694-020-00397-1. [DOI] [Google Scholar]
- Horvat B.I, Gobin I, Kresović A, Hauser G. How can probiotic improve irritable bowel syndrome symptoms? World Journal of Gastrointestinal Surgery. 2021;13:923–940. doi: 10.4240/wjgs.v13.i9.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordoni A, Amaretti A, Leonardi A, Boschetti E, Danesi F, Matteuzzi D, Roncaglia L, Raimondi S, Rossi M. Cholesterol-lowering probiotics: in vitro selection and in vivo testing of bifidobacteria. Applied Microbiology and Biotechnology. 2013;97:8273–8281. doi: 10.1007/s00253-013-5088-2. [DOI] [PubMed] [Google Scholar]
- Breit S, Kupferberg A, Rogler G, Hasler G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front Psychiatry. 2018 doi: 10.3389/fpsyt.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao GT, Zeng XF, Chen AG, Zhou L, Zhang L, Xiao YP, Yang CM. Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poultry Science. 2013;92:2949–2955. doi: 10.3382/ps.2013-03366. [DOI] [PubMed] [Google Scholar]
- Caramia G, Ciccarelli S, Stolfi I. Management strategies in the treatment of neonatal and pediatric gastroenteritis. Infection and Drug Resistance. 2013 doi: 10.2147/IDR.S12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadare FJ, Idohou R, Nago E, Affonfere M, Agossadou J, Fassinou TK, Kénou C, Honfo S, Azokpota P, Linnemann AR, Hounhouigan DJ. Conventional and food-to-food fortification: an appraisal of past practices and lessons learned. Food Science & Nutrition. 2019;7:2781–2795. doi: 10.1002/fsn3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandan RC. An overview of yogurt production and composition, yogurt in health and disease prevention. Elsevier, pp. 31–47 (2017) 10.1016/B978-0-12-805134-4.00002-X
- Clavel T, Gomes-Neto JC, Lagkouvardos I, Ramer-Tait AE. Deciphering interactions between the gut microbiota and the immune system via microbial cultivation and minimal microbiomes. Immunological Reviews. 2017;279:8–22. doi: 10.1111/imr.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran BM, Stanton C, Fitzgerald GF, Ross RP. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Applied and Environmental Microbiology. 2005;71:3060–3067. doi: 10.1128/AEM.71.6.3060-3067.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés-Zavaleta O, López-Malo A, Hernández-Mendoza A, García HS. Antifungal activity of lactobacilli and its relationship with 3-phenyllactic acid production. International Journal of Food Microbiology. 2014;173:30–35. doi: 10.1016/j.ijfoodmicro.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Damián MR, Cortes-Perez NG, Quintana ET, Ortiz-Moreno A, Garfias Noguez C, Cruceño-Casarrubias CE, Sánchez Pardo ME, Bermúdez-Humarán LG. Functional foods, nutraceuticals and probiotics: a focus on human health. Microorganisms. 2022;10:1065. doi: 10.3390/microorganisms10051065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkova Z, Kostov G. Antimicrobial activity of probiotic microorganisms: mechanisms of interaction and methods of examination. (2017)
- Didari T. Effectiveness of probiotics in irritable bowel syndrome: updated systematic review with meta-analysis. World Journal of Gastroenterology. 2015;21:3072. doi: 10.3748/wjg.v21.i10.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinleyici EC, Dalgic N, Guven S, Metin O, Yasa O, Kurugol Z, Turel O, Tanir G, Yazar AS, Arica V, Sancar M, Karbuz A, Eren M, Ozen M, Kara A, Vandenplas Y. Lactobacillus reuteri DSM 17938 shortens acute infectious diarrhea in a pediatric outpatient setting. Journal De Pediatria (rio j) 2015;91:392–396. doi: 10.1016/j.jped.2014.10.009. [DOI] [PubMed] [Google Scholar]
- DiRienzo DB. Effect of probiotics on biomarkers of cardiovascular disease: implications for heart-healthy diets. Nutrition Reviews. 2014;72:18–29. doi: 10.1111/nure.12084. [DOI] [PubMed] [Google Scholar]
- El-Sayed HS, El-Sayed SM. A modern trend to preserve white soft cheese using nano-emulsified solutions containing cumin essential oil. Environmental Nanotechnology Monitoring & Management. 2021;16:100499. doi: 10.1016/j.enmm.2021.100499. [DOI] [Google Scholar]
- Egea MB, dos Santos DC, de Oliveira Filho JG, da Ores J, Takeuchi KP, Lemes AC. A review of nondairy kefir products: their characteristics and potential human health benefits. Critical Reviews in Food Science and Nutrition. 2022;62:1536–1552. doi: 10.1080/10408398.2020.1844140. [DOI] [PubMed] [Google Scholar]
- Faccinetto-Beltrán P, Gómez-Fernández AR, Orozco-Sánchez NE, Pérez-Carrillo E, Marín-Obispo LM, Hernández-Brenes C, Santacruz A, Jacobo-Velázquez DA. Physicochemical properties and sensory acceptability of a next-generation functional chocolate added with omega-3 polyunsaturated fatty acids and probiotics. Foods. 2021;10:333. doi: 10.3390/foods10020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag MA, El Hawary EA, Elmassry MM. Rediscovering acidophilus milk, its quality characteristics, manufacturing methods, flavor chemistry and nutritional value. Critical Reviews in Food Science and Nutrition. 1–20 (2020) 10.1080/10408398.2019.1675584 [DOI] [PubMed]
- Fenster K, Freeburg B, Hollard C, Wong C, Rønhave Laursen R, Ouwehand A. The production and delivery of probiotics: a review of a practical approach. Microorganisms. 2019;7:83. doi: 10.3390/microorganisms7030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez B, Hammami R, Savard P, Jean J, Fliss I. Pediococcus acidilactici UL5 and Lactococcus lactis ATCC 11454 are able to survive and express their bacteriocin genes under simulated gastrointestinal conditions. Journal of Applied Microbiology. 2014;116:677–688. doi: 10.1111/jam.12391. [DOI] [PubMed] [Google Scholar]
- Foster JA, Zhou L. Psychobiotics and the gut-brain axis: in the pursuit of happiness. Neuropsychiatric Disease and Treatment. 2015 doi: 10.2147/NDT.S61997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruh SM. Obesity. Journal of the American Association of Nurse Practitioners. 2017;29:S3–S14. doi: 10.1002/2327-6924.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George Kerry R, Patra JK, Gouda S, Park Y, Shin H-S, Das G. Benefaction of probiotics for human health: a review. Journal of Food and Drug Analysis. 2018;26:927–939. doi: 10.1016/j.jfda.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings SL, Stevens AM, Leung DT. Traveler’s diarrhea. Medical Clinics of North America. 2016;100:317–330. doi: 10.1016/j.mcna.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg JZ, Yap C, Lytvyn L, Lo CK-F, Beardsley J, Mertz D, Johnston BC. Probiotics for the prevention of clostridium difficile-associated diarrhea in adults and children. Cochrane Database of Systematic Reviews. 2017 doi: 10.1002/14651858.CD006095.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghshenas B, Nami Y, Almasi A, Abdullah N, Radiah D, Rosli R, Barzegari A, Khosroushahi AY. Isolation and characterization of probiotics from dairies. Iranian Journal of Microbiology. 2017;9(4):234–243. [PMC free article] [PubMed] [Google Scholar]
- Gahruie HH, Eskandari MH, Mesbahi G, Hanifpour MA. Scientific and technical aspects of yogurt fortification: a review. Food Science and Human Wellness. 2015;4:1–8. doi: 10.1016/j.fshw.2015.03.002. [DOI] [Google Scholar]
- Hassan ME, Yang Q, Xiao Z, Liu L, Wang N, Cui X, Yang L. Impact of immobilization technology in industrial and pharmaceutical applications. 3 Biotech. 9: 440 (2019) 10.1007/s13205-019-1969-0 [DOI] [PMC free article] [PubMed]
- Hammam AR, Elfaruk MS, Ahmed ME, Sunkesula V. Characteristics and technological aspects of the Egyptian cheeses. International Journal of Current Microbiology and Applied Sciences. 2020;9(6):3338–3354. doi: 10.20546/ijcmas.2020.906.397. [DOI] [Google Scholar]
- Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therapeutic Advances in Gastroenterology. 2013;6:39–51. doi: 10.1177/1756283X12459294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K. Review of the health benefits of habitual consumption of miso soup: focus on the effects on sympathetic nerve activity, blood pressure, and heart rate. Environmental Health and Preventive Medicine. 2020;25:45. doi: 10.1186/s12199-020-00883-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal Z, Ahmed S, Tabassum N, Bhattacharya R, Bose D. Role of probiotics in prevention and treatment of enteric infections: a comprehensive review. 3 Biotech. 11: 242 (2021) 10.1007/s13205-021-02796-7 [DOI] [PMC free article] [PubMed]
- Islam SU. Clinical uses of probiotics. Medicine. 2016;95:e2658. doi: 10.1097/MD.0000000000002658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SA, Schoeni JL, Vegge C, Pane M, Stahl B, Bradley M, Goldman VS, Burguière P, Atwater JB, Sanders ME. Improving end-user trust in the quality of commercial probiotic products. Frontier Microbiology. 2019 doi: 10.3389/fmicb.2019.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvier A, Malo J, Barrington KJ. Cohort study of probiotics in a North American neonatal intensive care unit. The Journal of Pediatrics. 2014;164:980–985. doi: 10.1016/j.jpeds.2013.11.025. [DOI] [PubMed] [Google Scholar]
- Johnson S, Maziade PJ, McFarland LV, Trick W, Donskey C, Currie B, Low DE, Goldstein EJC. Is primary prevention of clostridium difficile infection possible with specific probiotics? International Journal of Infectious Diseases. 2012;16:e786–e792. doi: 10.1016/j.ijid.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nature Reviews Disease Primers. 2020;6:92. doi: 10.1038/s41572-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur L, Gordon M, Baines PA, Iheozor-Ejiofor Z, Sinopoulou V, Akobeng AK. Probiotics for induction of remission in ulcerative colitis. Cochrane Database of Systematic Reviews. 2020 doi: 10.1002/14651858.CD005573.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur AP, Bhardwaj S, Dhanjal DS, Nepovimova E, Cruz-Martins N, Kuča K, Chopra C, Singh R, Kumar H, Șen F, Kumar V, Verma R, Kumar D. Plant prebiotics and their role in the amelioration of diseases. Biomolecules. 2021;11:440. doi: 10.3390/biom11030440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoder G, Al-Menhali AA, Al-Yassir F, Karam SM. Potential role of probiotics in the management of gastric ulcer. Experimental and Therapeutic Medicine. 2016;12:3–17. doi: 10.3892/etm.2016.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Covington A, Pamer EG. The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens. Immunological Reviews. 2017;279:90–105. doi: 10.1111/imr.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Kumar A, Nagpal R, Mohania D, Behare P, Verma V, Kumar P, Poddar D, Aggarwal PK, Henry CJK, Jain S, Yadav H. Cancer-preventing attributes of probiotics: an update. International Journal of Food Sciences and Nutrition. 2010;61:473–496. doi: 10.3109/09637480903455971. [DOI] [PubMed] [Google Scholar]
- Lebeer S, Claes IJJ, Verhoeven TLA, Vanderleyden J, de Keersmaecker SCJ. Exopolysaccharides of lactobacillus rhamnosus GG form a protective shield against innate immune factors in the intestine. Microbiology Biotechnology. 2011;4:368–374. doi: 10.1111/j.1751-7915.2010.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc JF, Segal JP, de Campos Braz LM, Hart AL. The microbiome as a therapy in pouchitis and ulcerative colitis. Nutrients. 2021;13:1780. doi: 10.3390/nu13061780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Chung M-J, Seo J-G. In vitro evaluation of antimicrobial activity of lactic acid bacteria against clostridium difficile. Toxicological Research. 2013;29:99–106. doi: 10.5487/TR.2013.29.2.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Bose S, Seo J-G, Chung W-S, Lim C-Y, Kim H. The effects of co-administration of probiotics with herbal medicine on obesity, metabolic endotoxemia and dysbiosis: a randomized double-blind controlled clinical trial. Clinical Nutrition. 2014;33:973–981. doi: 10.1016/j.clnu.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Lee E-S, Song E-J, Nam Y-D, Lee S-Y. Probiotics in human health and disease: from nutribiotics to pharmabiotics. Journal of Microbiology. 2018;56:773–782. doi: 10.1007/s12275-018-8293-y. [DOI] [PubMed] [Google Scholar]
- Levi S, Rac V, Manojlovi V, Raki V, Bugarski B, Flock T, Krzyczmonik KE, Nedovi V. Limonene encapsulation in alginate/poly (vinyl alcohol) Procedia Food Science. 2011;1:1816–1820. doi: 10.1016/j.profoo.2011.09.266. [DOI] [Google Scholar]
- Li D, Rosito G, Slagle T. Probiotics for the prevention of necrotizing enterocolitis in neonates: an 8-year retrospective cohort study. Journal of Clinical Pharmacy and Therapeutics. 2013;38:445–449. doi: 10.1111/jcpt.12084. [DOI] [PubMed] [Google Scholar]
- Liu Y, Alookaran J, Rhoads J. Probiotics in autoimmune and inflammatory disorders. Nutrients. 2018;10:1537. doi: 10.3390/nu10101537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Tran DQ, Rhoads JM. Probiotics in disease prevention and treatment. The Journal of Clinical Pharmacology. 2018;58:S164–S179. doi: 10.1002/jcph.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh ZH, Samanta AK, Sia Heng PW. Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian Journal of Pharmaceutical. 2015;10:255–274. doi: 10.1016/j.ajps.2014.12.006. [DOI] [Google Scholar]
- de Leite AM, Miguel MAL, Peixoto RS, Rosado AS, Silva JT, Paschoalin VMF. Microbiological, technological and therapeutic properties of kefir: a natural probiotic beverage. Brazilian Journal of Microbiology. 2013;44:341–349. doi: 10.1590/S1517-83822013000200001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longoria-García S, Cruz-Hernández MA, Flores-Verástegui MI, Contreras-Esquivel JC, Montañez-Sáenz JC, Belmares-Cerda RE. Potential functional bakery products as delivery systems for prebiotics and probiotics health enhancers. Journal of Food Science and Technology. 55: 833–854 (2018) 10.1007/s13197-017-2987-8 [DOI] [PMC free article] [PubMed]
- de Toro López, Martín-Consuegra I, Sanchez-Casado M, Pérez-Pedrero Sánchez-Belmonte MJ, López-Reina Torrijos P, Sánchez-Rodriguez P, Raigal-Caño A, Heredero-Galvez E, Zubigaray SB, Arrese-Cosculluela MA. Influencia de los simbióticos en la disfunción multiorgánica: ensayo aleatorizado. Medicina Clinica (barc) 2014;143:143–149. doi: 10.1016/j.medcli.2013.09.046. [DOI] [PubMed] [Google Scholar]
- Marhamatizadeh MH, Ehsandoost E, Gholami P. The influence of green tea (Camellia sinensis L.) extract on characteristic of probiotic bacteria in milk and yoghurt during fermentation and refrigerated storage. International Journal of Farming and Allied Sciences. 2013;2:599–606. [Google Scholar]
- Markowiak P, Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9:1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroof H, Hassan ZM, Mobarez AM, Mohamadabadi MA. Lactobacillus acidophilus could modulate the immune response against breast cancer in murine model. The Journal of Clinical Pharmacology. 2012;32:1353–1359. doi: 10.1007/s10875-012-9708-x. [DOI] [PubMed] [Google Scholar]
- Martín R, Langella P. Emerging health concepts in the probiotics field: streamlining the definitions. Frontier Microbiology. 2019 doi: 10.3389/fmicb.2019.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín R, Sánchez B, Suárez JE, Urdaci MC. Characterization of the adherence properties of human Lactobacilli strains to be used as vaginal probiotics. FEMS Microbiology Letters. 2012;328:166–173. doi: 10.1111/j.1574-6968.2011.02495.x. [DOI] [PubMed] [Google Scholar]
- McFarland L, v., Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Medicine and Infectious Disease. 2007;5:97–105. doi: 10.1016/j.tmaid.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Meybodi NM, Mortazavian AM, Arab M, Nematollahi A. Probiotic viability in yoghurt:a review of influential. International Dairy Journal. 2020;55:104973. [Google Scholar]
- Miremadi F, Ayyash M, Sherkat F, Stojanovska L. Cholesterol reduction mechanisms and fatty acid composition of cellular membranes of probiotic lactobacilli and bifidobacteria. Journal of Functional Foods. 2014;9:295–305. doi: 10.1016/j.jff.2014.05.002. [DOI] [Google Scholar]
- Moens F, van den Abbeele P, Basit AW, Dodoo C, Chatterjee R, Smith B, Gaisford S. A four-strain probiotic exerts positive immunomodulatory effects by enhancing colonic butyrate production in vitro. International Journal of Pharmaceutics. 2019;555:1–10. doi: 10.1016/j.ijpharm.2018.11.020. [DOI] [PubMed] [Google Scholar]
- Moré MI, Swidsinski A. Saccharomyces boulardii CNCM I-745 supports regeneration of the intestinal microbiota after diarrheic dysbiosis – a review. Clinical and Experimental Gastroenterology. 2015 doi: 10.2147/CEG.S85574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro-García MA, Alonso-Arias R, Baltadjieva M, Fernández Benítez C, Fernández Barrial MA, Díaz Ruisánchez E, Alonso Santos R, Álvarez Sánchez M, Saavedra Miján J, López-Larrea C. Oral supplementation with Lactobacillus delbrueckii subsp. bulgaricus 8481 enhances systemic immunity in elderly subjects. Age (omaha) 2013;35:1311–1326. doi: 10.1007/s11357-012-9434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazir Y, Hussain SA, Abdul Hamid A, Song Y. Probiotics and their potential preventive and therapeutic role for cancer, high serum cholesterol, and allergic and HIV diseases. BioMed Research International. 2018;2018:1–17. doi: 10.1155/2018/3428437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan A, van Sinderen D. Bifidobacteria and their role as members of the human gut microbiota. Frontier in Microbiology. 2016 doi: 10.3389/fmicb.2016.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oláh A, Jr, L.R., Enteral nutrition in acute pancreatitis: a review of the current evidence. World Journal of Gastroenterology. 2014;20:16123. doi: 10.3748/wjg.v20.i43.16123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palani Kumar MK, Halami PM, Serva Peddha M. Effect of lactobacillus fermentum MCC2760-based probiotic curd on hypercholesterolemic C57BL6 Mice. ACS Omega. 2021;6:7701–7710. doi: 10.1021/acsomega.1c00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palka A, Newerli-Guz J. Ice cream as innovative luxury products ice cream quality and consumption estimation view project artisan ice cream with a pro-health potential View project. Journal of Microbiology. 2018;55:304–310. [Google Scholar]
- Park JS, Joe I, Rhee PD, Jeong CS, Jeong G. lactic acid bacterium isolated from kimchi ameliorates intestinal inflammation in DSS-induced colitis. Journal of Microbiology. 2017;55:304–310. doi: 10.1007/s12275-017-6447-y. [DOI] [PubMed] [Google Scholar]
- Phavichitr N, Puwdee P. Tantibhaedhyangkul Rnd. cost-benefit of the probiotic treatment of childhood diarrhea cost-benefit analysis of the probiotic treatment of children hospitalized for acute diarrhea in Bangkok, Thailand. The Southeast Asian Journal of Tropical Medicine and Public Health. 2013;4(6):1065–1071. [PubMed] [Google Scholar]
- The Southeast Asian Journal of Tropical Medicine and Public Health. 4(6): 1065-71 (2013) [PubMed]
- Pieniz S, Andreazza R, Pereira JQ, de Oliveira Camargo FA, Brandelli A. Production of selenium-enriched biomass by Enterococcus durans. Biological Trace Element Research. 2013;155:447–454. doi: 10.1007/s12011-013-9818-1. [DOI] [PubMed] [Google Scholar]
- Pinto GS, Cenci MS, Azevedo MS, Epifanio M, Jones MH. Effect of yogurt containing Bifidobacteriumanimalis subsp. lactis DN-173010 probiotic on dental plaque and saliva in orthodontic patients. Caries Research. 2014;48:63–68. doi: 10.1159/000353467. [DOI] [PubMed] [Google Scholar]
- Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Advances Nutrition. 2019;10:S49–S66. doi: 10.1093/advances/nmy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puphan K, Sornplang P, Uriyapongson S, Navanukraw C. Screening of lactic acid bacteria as potential probiotics in beef cattle. Pakistan Journal of Nutrition. 2015;14:474–479. doi: 10.3923/pjn.2015.474.479. [DOI] [Google Scholar]
- Rao AV, Bested AC, Beaulne TM, Katzman MA, Iorio C, Berardi JM, Logan AC. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathogens. 2009;1:6. doi: 10.1186/1757-4749-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat K, Kumari A, Kumar S, Kumar R, Gehlot R. Traditional fermented products of India. International Journal of Current Microbiology and Applied. 2018;7(4):1873–1883. [Google Scholar]
- Ren H, Saliu E-M, Zentek J, Goodarzi Boroojeni F, Vahjen W. Screening of host specific lactic acid bacteria active against escherichia coli from massive sample pools with a combination of in vitro and ex vivo methods. Frontier Microbiology. 2019 doi: 10.3389/fmicb.2019.02705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli RR, Biver E. Effects of fermented milk products on bone. Calcified Tissue International. (2018) 10.1007/s00223-017-0317-9 [DOI] [PubMed]
- Rodrigues FJ, Cedran MF, Bicas JL, Sato HH. Encapsulated probiotic cells: relevant techniques, natural sources as encapsulating materials and food applications – a narrative review. Food Research International. 2020;137:109682. doi: 10.1016/j.foodres.2020.109682. [DOI] [PubMed] [Google Scholar]
- Ruiz Sella SR, Bueno T, de Oliveira AA, Karp SG, Soccol CR. Bacillus subtilis natto as a potential probiotic in animal nutrition. Critical Reviews in Food Science and Nutrition. 1–18 (2021) 10.1080/07388551.2020.1858019 [DOI] [PubMed]
- Saarela M, Mogensen G, Fondén R, Mättö J, Mattila-Sandholm T. Probiotic bacteria: safety, functional and technological properties. Journal of Biotechnology. 2000;84:197–215. doi: 10.1016/S0168-1656(00)00375-8. [DOI] [PubMed] [Google Scholar]
- Sales-Campos H, Soares SC, Oliveira CJF. An introduction of the role of probiotics in human infections and autoimmune diseases. Critical Reviews in Microbiology. 2019;45:413–432. doi: 10.1080/1040841X.2019.1621261. [DOI] [PubMed] [Google Scholar]
- Sawant S, Shegokar R. Cancer research and therapy: where are we today?. International Journal of Cancer Therapy and Oncology. 2: 020408 (2014) 10.14319/ijcto.0204.8
- Saxena T, Kaushik P, Krishna Mohan M. Prevalence of E. coli O157:H7 in water sources: an overview on associated diseases, outbreaks and detection methods. Diagnostic Microbiology and Infectious Disease. 2015;82:249–264. doi: 10.1016/j.diagmicrobio.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CLG, Adamos MB, Sweeney KM, Origoni AE, Khushalani S, Dickerson FB, Yolken RH. Probiotic normalization of Candida albicans in schizophrenia: a randomized, placebo-controlled, longitudinal pilot study. Brain Behavior and Immunology. 2017;62:41–45. doi: 10.1016/j.bbi.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedain Ardabili M, Sharifan A, Ghiassi Tarzi B. An investigation of the production of synbiotic pan breads by microencapsulation. Food Technology and Biotechnology. 54 (2016) 10.17113/ftb.54.01.16.4234 [DOI] [PMC free article] [PubMed]
- Shah NJ, Swami OC. Role of probiotics in diabetes: a review of their rationale and efficacy. Diabetes. 2017;5:104–110. [Google Scholar]
- Sharifi-Rad J, Rodrigues CF, Stojanović-Radić Z, Dimitrijević M, Aleksić A, Neffe-Skocińska K, Zielińska D, Kołożyn-Krajewska D, Salehi B, Milton Prabu S, Schutz F, Docea AO, Martins N, Calina D. Probiotics: versatile bioactive components in promoting human health. Medicina (b Aires) 2020;56:433. doi: 10.3390/medicina56090433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Molecular Aspects in Medicines. 2013;34:39–58. doi: 10.1016/j.mam.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Shokryazdan P, Faseleh Jahromi M, Liang JB, Ho YW. Probiotics: from isolation to application. Journal of the American College of Nutrition. 2017;36:666–676. doi: 10.1080/07315724.2017.1337529. [DOI] [PubMed] [Google Scholar]
- Šipailienė A, Petraitytė S. Encapsulation of probiotics: proper selection of the probiotic strain and the influence of encapsulation technology and materials on the viability of encapsulated microorganisms. Probiotics Antimicrob Proteins. 2018;10:1–10. doi: 10.1007/s12602-017-9347-x. [DOI] [PubMed] [Google Scholar]
- Soliman G. Dietary cholesterol and the lack of evidence in cardiovascular disease. Nutrients. 2018;10:780. doi: 10.3390/nu10060780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spacova I, Dodiya HB, Happel A-U, Strain C, Vandenheuvel D, Wang X, Reid G. Future of probiotics and prebiotics and the implications for early career researchers. Frontier Microbiology. 2020 doi: 10.3389/fmicb.2020.01400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanov S, Berlec A, Štrukelj B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8:1715. doi: 10.3390/microorganisms8111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephanie S, Ratih NK, Soka S, Suwanto A. Effect of tempeh supplementation on the profiles of human intestinal immune system and gut microbiota. Microbiology Indonesia. 2017;11(1):2. doi: 10.5454/mi.11.1.2. [DOI] [Google Scholar]
- Suzuki S, Yakabe T, Suganuma H, Fukao M, Saito T, Yajima N. Cell-bound exopolysaccharides of lactobacillus brevis KB290: protective role and monosaccharide composition. Canadian Journal of Microbiology. 2013;59:549–555. doi: 10.1139/cjm-2013-0115. [DOI] [PubMed] [Google Scholar]
- Terpou A, Papadaki A, Lappa I, Kachrimanidou V, Bosnea L, Kopsahelis N. Probiotics in food systems: significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients. 2019;11:1591. doi: 10.3390/nu11071591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D, Legrain-Raspaud S, Trotin B, Naliboff B, Mayer EA. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–1401.e4. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaro-Duchesneau C, Saha S, Malhotra M, Jones ML, Labbé A, Rodes L, Kahouli I, Prakash S. Effect of orally administered L. fermentum NCIMB 5221 on markers of metabolic syndrome: an in vivo analysis using ZDF rats. Applied molecular biology and biotechnology. 2014;98:115–126. doi: 10.1007/s00253-013-5252-8. [DOI] [PubMed] [Google Scholar]
- Verdenelli MC, Coman MM, Cecchini C, Silvi S, Orpianesi C, Cresci A. Evaluation of antipathogenic activity and adherence properties of human lactobacillus strains for vaginal formulations. Journal of Applied Microbiology. 2014;116:1297–1307. doi: 10.1111/jam.12459. [DOI] [PubMed] [Google Scholar]
- Vieira da Silva B, Barreira JCM, Oliveira MBPP. Natural phytochemicals and probiotics as bioactive ingredients for functional foods: extraction, biochemistry and protected-delivery technologies. Trends in Food Science and Technology. 2016;50:144–158. doi: 10.1016/j.tifs.2015.12.007. [DOI] [Google Scholar]
- Vijaya Kumar B, Vijayendra SVN, Reddy OVS. Trends in dairy and non-dairy probiotic products - a review. Journal of Food Science and Technology. 2015;52:6112–6124. doi: 10.1007/s13197-015-1795-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayalakshmi S, Adeyemi DE, Choi IY, Sultan G, Madar IH, Park M-K. Comprehensive in silico analysis of lactic acid bacteria for the selection of desirable probiotics. LWT. 2020;130:109617. doi: 10.1016/j.lwt.2020.109617. [DOI] [Google Scholar]
- Watawana MI, Jayawardena N, Gunawardhana CB, Waisundara VY. Health, wellness, and safety aspects of the consumption of kombucha. Journal of Chemistry. 2015;15:134. [Google Scholar]
- Wright K, Wright H, Murray M. Probiotic treatment for the prevention of antibiotic-associated diarrhoea in geriatric patients: a multicentre randomised controlled pilot study. Australasian Journal on Ageing. 2015;34:38–42. doi: 10.1111/ajag.12116. [DOI] [PubMed] [Google Scholar]
- Yadav R, Shukla P. An overview of advanced technologies for selection of probiotics and their expediency: a review. Critical Review on Food Science and Nutrition. 2017;57:3233–3242. doi: 10.1080/10408398.2015.1108957. [DOI] [PubMed] [Google Scholar]
- Yadav R, Kumar V, Baweja M, Shukla P. Gene editing and genetic engineering approaches for advanced probiotics: a review. Critical Review on Food Science and Nutrition. 2018;58:1735–1746. doi: 10.1080/10408398.2016.1274877. [DOI] [PubMed] [Google Scholar]
- Yeşilyurt N, Yılmaz B, Ağagündüz D, Capasso R. Involvement of probiotics and postbiotics in the immune system modulation. Biologics. 2021;1:89–110. doi: 10.3390/biologics1020006. [DOI] [Google Scholar]
- Zaharuddin L, Mokhtar NM, Muhammad Nawawi KN, Raja Ali RA. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterology. 2019;19:131. doi: 10.1186/s12876-019-1047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelaya H, Tsukida K, Chiba E, Marranzino G, Alvarez S, Kitazawa H, Agüero G, Villena J. Immunobiotic lactobacilli reduce viral-associated pulmonary damage through the modulation of inflammation–coagulation interactions. International Immunopharmacology. 2014;19:161–173. doi: 10.1016/j.intimp.2013.12.020. [DOI] [PubMed] [Google Scholar]
- Zhao L. The tale of our other genome. Nature. 2010;465:879–880. doi: 10.1038/465879a. [DOI] [PubMed] [Google Scholar]