Abstract
This study examines the Food and Drug Administration’s accelerated approval pathway and whether preapproval initiation was associated with faster conversion to traditional approval or withdrawal for drugs with nononcology indications.
The accelerated approval pathway allows the US Food and Drug Administration (FDA) to approve drugs that demonstrate an effect on a surrogate end point that is reasonably likely to predict clinical benefit. Following accelerated approval, manufacturers are required to verify clinical benefit in confirmatory trials. Delays in confirmatory trial completion have led to proposals for reforming the accelerated approval pathway.1 One proposal would require a confirmatory trial initiation prior to accelerated approval.2 An FDA analysis found an association between preapproval initiation and faster (from the date of accelerated approval) completion of confirmatory trials for oncology indications that ultimately received traditional approval or were withdrawn.3 We extended the FDA analysis by also including nononcology indications and evaluating whether preapproval initiation was associated with timelier conversion to traditional approval or withdrawal.
Methods
We identified all accelerated approval indications between September 1, 2002, and December 31, 2018, and their regulatory outcomes (ie, conversion to traditional approval or withdrawal) by December 31, 2021. For each indication, we extracted confirmatory trial requirements and target completion dates from approval letters at Drugs@FDA. We linked trial requirements to corresponding information at ClinicalTrials.gov.4 We extracted the date on which the first participant was enrolled in the trial and noted whether this occurred before or after accelerated approval (eAppendix in Supplement 1). The Harvard Pilgrim Health Care Institutional Review Board determined the study exempt from review.
We used t tests to compare proportions of indications that were converted to traditional approval or withdrawn for drug indications with and without preapproval trial initiation and which had 3- or 5-year follow-up since accelerated approval or target completion dates before July 2021. These measures are in line with prior work on confirmatory trials making explicit the duration of patient exposure to drugs with uncertain benefits,4 and they assess compliance with timelines agreed on by sponsors and the FDA. A 2-sided P value of .05 was the threshold for statistical significance. We also used Kaplan-Meier methods, censoring studies that had not been completed by December 31, 2021, to evaluate the association between pre–accelerated approval trial initiation and regulatory outcomes. All analyses were performed using Stata version 17 (StataCorp).
Results
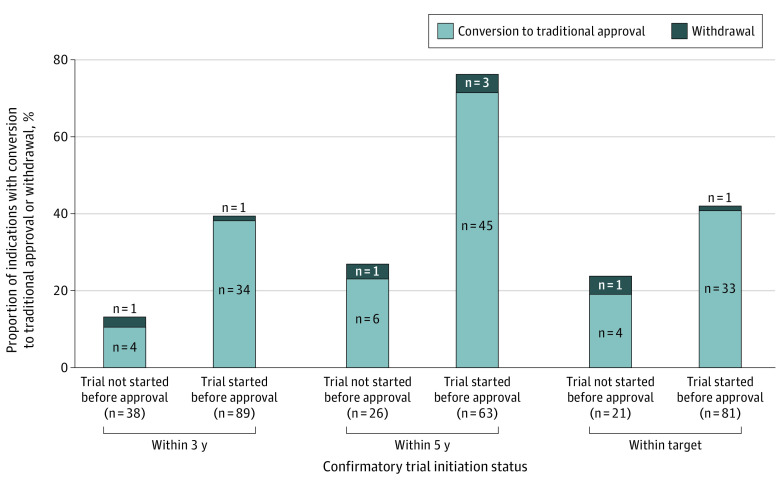
Among 127 accelerated approval indications with confirmatory trial requirements, 89 (70%) had confirmatory trials started before approval. Accelerated approval indications with confirmatory trials started before approval had higher proportions of conversion to traditional approval or withdrawal by 3 years (39.3% [35/89] vs 13.2% [5/38]; P = .003) and 5 years (76.2% [48/63] vs 26.9% [7/26]; P < .001) than indications that did not have pre–accelerated approval trial initiation. Regulatory outcomes within target timelines were not statistically significantly different between indications with and without preapproval confirmatory trial initiation (42.0% [34/81] vs 23.8% [5/21]; P = .13) (Figure 1).
Figure 1. Proportions of Indications With Conversion to Traditional Approval or Withdrawal by Preapproval Initiation Status of Confirmatory Trials.
The numbers underneath the bars indicate the numbers of indications assessed.
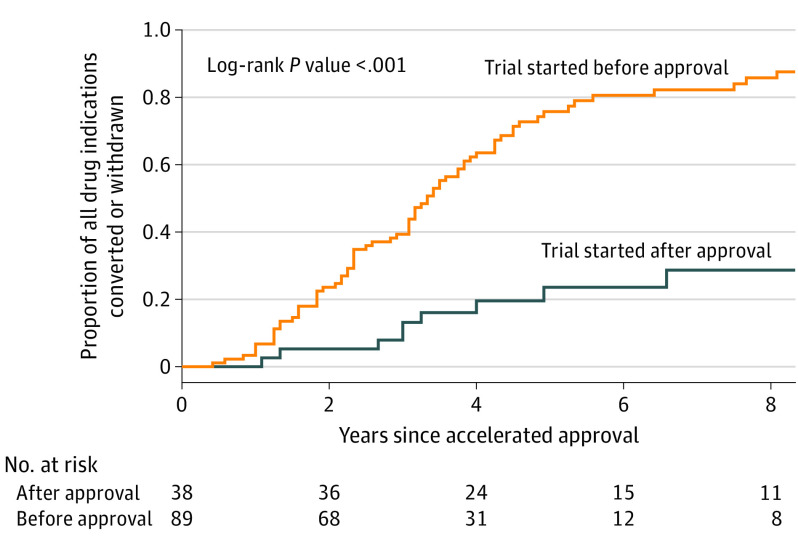
In the Kaplan-Meier analysis, indications with preapproval trial initiation had faster conversion to traditional approval or withdrawal than those without (P < .001) (Figure 2). Among indications with confirmatory trials started after approval, trial initiation took a median of 18.5 months (IQR, 13-30 months).
Figure 2. Time to Conversion to Traditional Approval or Withdrawal by Preapproval Confirmatory Trial Initiation Status.
Discussion
Indications with accelerated approval between 2002 and 2018 that had confirmatory trials started before approval had faster conversion to traditional approval or withdrawal than those that did not, thereby reducing the duration of patient exposure to therapies with uncertain efficacy. However, there was no statistically significant difference in rates of regulatory outcomes within target timelines by preapproval trial initiation status. Target timelines may have taken into consideration other factors that affect confirmatory trial completion and allowed for delayed starts. That there was a substantial lag between approval and trial initiation for indications without pre–accelerated approval trial initiation suggests a need for policies mandating timely confirmatory trials. Such policies need to reduce total time to verify clinical benefit without delaying accelerated approval.
This analysis has limitations. First, some confirmatory trials may have been missed due to limitations in publicly available information.5 Second, reasons for starting confirmatory trials before or after accelerated approval are not known. Third, conversion to traditional approval was used as a proxy for verification of benefit but may not have been based on evidence of clinical benefit.6
Overall, these results suggest that reforming the accelerated approval pathway by requiring preapproval initiation of confirmatory trials may result in timelier regulatory action on drugs initially approved on the basis of limited evidence.
Section Editors: Jody W. Zylke, MD, Deputy Editor; Kristin Walter, MD, Senior Editor.
eAppendix
Data Sharing Statement
References
- 1.US Department of Health and Human Services Office of Inspector General . Delays in confirmatory trials for drug applications granted FDA’s accelerated approval raise concerns. September 2022. Accessed October 11, 2022. https://oig.hhs.gov/oei/reports/OEI-01-21-00401.pdf
- 2.Brennan Z. FDA commissioner on accelerated approval reforms: “Need to address as soon as possible.” Endpoints News. October 19, 2022. Accessed October 19, 2022. https://endpts.com/fda-commissioner-on-accelerated-approval-reforms-need-to-address-as-soon-as-possible/
- 3.Fashoyin-Aje LA, Mehta GU, Beaver JA, Pazdur R. The on- and off-ramps of oncology accelerated approval. N Engl J Med. 2022;387(16):1439-1442. doi: 10.1056/NEJMp2208954 [DOI] [PubMed] [Google Scholar]
- 4.Naci H, Smalley KR, Kesselheim AS. Characteristics of preapproval and postapproval studies for drugs granted accelerated approval by the US Food and Drug Administration. JAMA. 2017;318(7):626-636. doi: 10.1001/jama.2017.9415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herder M. Pharmaceutical drugs of uncertain value, lifecycle regulation at the US Food and Drug Administration, and institutional incumbency. Milbank Q. 2019;97(3):820-857. doi: 10.1111/1468-0009.12413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gyawali B, Hey SP, Kesselheim AS. Assessment of the clinical benefit of cancer drugs receiving accelerated approval. JAMA Intern Med. 2019;179(7):906-913. doi: 10.1001/jamainternmed.2019.0462 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix
Data Sharing Statement