Abstract
Background:
Nitrate and trihalomethanes (THMs) in drinking water are widespread and are potential human carcinogens.
Objective:
We evaluated the association between drinking-water exposure to nitrate and THMs and prostate cancer.
Methods:
During the period 2008–2013, 697 hospital-based incident prostate cancer cases (97 aggressive tumors) and 927 population-based controls were recruited in Spain, providing information on residential histories and type of water consumed. Average nitrate and THMs levels in drinking water were linked with lifetime water consumption to calculate waterborne ingestion. Odds ratios (OR) and 95% confidence intervals (CI) were estimated using mixed models with recruitment area as random effect. Effect modification by tumor grade (Gleason score), age, education, lifestyle, and dietary factors was explored.
Results:
Mean () adult lifetime waterborne ingested nitrate (milligrams per day), brominated (Br)-THMs (micrograms per day), and chloroform (micrograms per day) were 11.5 (), 20.7 (), and 15.1 () in controls. Waterborne ingested nitrate vs. was associated with an OR of 1.74 (95% CI: 1.19, 2.54) overall, and 2.78 (95% CI: 1.23, 6.27) for tumors with Gleason scores . Associations were higher in the youngest and those with lower intakes of fiber, fruit/vegetables, and vitamin C. Waterborne ingested THMs were not associated with prostate cancer. Residential tap water levels of Br-THMs and chloroform showed, respectively, inverse and positive associations with prostate cancer.
Conclusions:
Findings suggest long-term waterborne ingested nitrate could be a risk factor of prostate cancer, particularly for aggressive tumors. High intakes of fiber, fruit/vegetables and vitamin C may lower this risk. Association with residential levels but not ingested chloroform/Br-THM may suggest inhalation and dermal routes could be relevant for prostate cancer. https://doi.org/10.1289/EHP11391
Introduction
Prostate cancer has become widespread worldwide,1,2 with 1,414,259 estimated new cases in 2020 (7.3% of all cancer sites),2 and the prostrate is the leading incident cancer site in Spanish men (22% of all cancers sites).3 However, the etiology of prostate cancer remains largely unknown, and it is one of the few types of cancer for which the International Agency for Research on Cancer (IARC) has not identified a clear carcinogenic agent.4 Currently recognized risk factors are nonmodifiable, including age, ethnicity, and family history of cancer (including genetic heritage).1,5,6 Aggressive and fatal prostate cancers have been suggested to have different underlying causes in comparison with slow-growing tumors with an indolent course.7–9 Other suggested risk factors, particularly for advanced-stage and aggressive prostate cancer, are lifestyle/behaviors such as smoking, unhealthy diet, overweight status, and lack of exercise,9–11 as well as exposure to endocrine-disrupting chemicals12,13 such as Agent Orange (i.e., dioxins)8 and pesticides.14 An association between nitrite and nitrate from food additives and prostate cancer has also been recently reported.15 Cancer mortality maps reporting spatial and temporal distribution within countries and globally suggest that environmental exposures may contribute to prostate cancer development and could partly explain increasing incidence rates.16–18
Nitrate occurrence in the water cycle is rising worldwide because of growing use of nitrogen fertilizers and intensive farming.19 Human exposure to nitrate mainly occurs through ingestion of food and drinking water.20 Ingested nitrate is reduced to nitrite, which can react with amines and amides under acidic conditions in the stomach to form N-nitroso compounds. Ingested nitrate or nitrite under conditions that result in endogenous nitrosation is probably carcinogenic to humans.21,22 There are limited epidemiological studies seeking to disclose the relationship between nitrate exposure from drinking water and cancer, and, to date, consistent evidence has only been established with colorectal cancer.19 Disinfectants added to raw water to inactivate microbial pathogens result in the formation of several disinfection by-products (DBPs). DBPs constitute a complex mixture of chemicals formed as by-products of the reactions of the disinfectants applied to drinking-water.23 Chlorine is the most widespread disinfectant used worldwide, and trihalomethanes (THMs) and haloacetic acids are the DBPs formed at the highest concentrations after chlorination. When water containing ammonia is chlorinated, chloramines are formed, which, in turn, lead to the formation of nitrogenated by-products such as the carcinogenic N-nitrosamines.24 Several DBPs are genotoxic in vitro and carcinogenic in animal experiments,25,26 and the IARC has classified some DBPs as possible human carcinogens.27–29
This study was designed to evaluate the association between prostate cancer and long-term exposure to nitrate and THMs in drinking water. Because risk factors for advanced-stage and aggressive prostate cancer may differ from slow-growing tumors7,18 we also evaluated the associations by Gleason score ( vs. as aggressive prostate cancer). We further investigated the effect modification by age, dietary factors, education, and adherence to a healthy lifestyle.
Methods
Study Design and Population
The MCC-Spain study (http://www.mccspain.org) is a multicase–control study conducted in different provinces in Spain between 2008 and 2013. MCC-Spain included breast, colorectal, prostate, and gastroesophageal cancer, as well as chronic lymphocytic leukemia cases, along with a common pool of population-based controls.30 Cases were histologically confirmed incident prostate cancer patients [International Classification of Diseases, Tenth Revision (ICD)-10 C61 and D07.5], identified through active searches that included periodic visits to hospital departments, and were interviewed closely after diagnosis (median of 58 d). Controls were selected from the general population, identified from the lists of randomly selected family practitioners in primary health centers, and were frequency matched to cases by age for each region (12 recruitment areas).30 Inclusion criteria required participants to be 20–85 y old, to have the ability to understand and answer the questionnaire, and to have lived for at least 6 months in the study area. The study protocol was approved by the ethics committee at all collaborating institutions, and each participant signed an informed consent form prior to enrollment. The overall response rate (subjects interviewed divided by subjects interviewed plus refusals) was 72% for prostate cancer cases and 53% for controls, leading to 996 prostate cancer cases and 1,281 controls recruited in the areas included in the present analysis (Asturias, Barcelona, Cantabria, Madrid, and Valencia).
Data Collection
Those who agreed to participate answered a structured, computerized questionnaire administered by trained personnel in a face-to-face interview to gather information on anthropometrics (self-reported), sociodemographics, lifestyle factors, and personal and family medical history. Participants provided full address, year started and stopped living in all the residences where they lived for at least 12 months since age 18 y until the time of the interview, and the type of water consumed in each residence (municipal, bottled, well, other). Amount (glasses per day) of water ingested on average lifetime at home, work, and other places was ascertained. A final section evaluating the reliability of the interview was completed by the interviewer. Dietary habits the year before the interview were collected through a self-administered semiquantitative food frequency questionnaire, including a total of 140 food items, previously validated in Spain.31 Questionnaires used are available online (http://mccspain.org).
The Gleason score was collected from the pathological records. Two prostate cancer grading categories were constructed: low- to medium-grade prostate cancer (Gleason score ) and high-grade/aggressive prostate cancer (Gleason score ).32,33
Nitrate and THM Levels in Municipal Drinking Water
We designed a structured questionnaire aimed at water utilities, local authorities, and/or health authorities to collect drinking water source (surface or ground water proportion) and treatment in the study areas back to 1940. In addition, available data from routine monitoring in the drinking water treatment plants and the distribution network were collected for nitrate and THMs (chloroform, bromodichloromethane, dibromochloromethane, and bromoform). We targeted data collection among study municipalities that contributed up to 80% of person-years.
For the years 2004–2010, centralized routine monitoring data was provided by the SINAC (Spanish National Information System on Water for Consumption), that includes information at the water-zone level introduced by water supply operators from public or private companies or municipalities, as well as from public or private laboratories. The water zone, which mostly corresponds to municipality, was defined as a geographical area supplied by water with a homogeneous source and treatment and whose quality in the water distributed in the networks can be considered homogeneous. We linked each postal code from the residence to the corresponding water zone.
The distribution of the sampling points and the sampling frequency varied greatly, depending on the population served, extension of the water zone, and the year, and could be more than once a day (e.g., Madrid), up to once every 3 months, or once a year in less-populated areas. Measurements below the analytical limit of quantification (QL) were substituted with half the QL (QL/2).34 If the QL was missing, we imputed half of the most frequently reported.
Nitrate and THM Levels in Nonmunicipal Drinking Water
We measured nitrate in the 9 most-consumed bottled water brands in Spain using ultraviolet spectrophotometry, with detection/quantification limit. Nitrate concentrations were in the range of .35 THMs were previously measured in 15 popular bottled water brands in Spain through purge-and-trap and gas chromatography. Mean concentrations for chloroform, bromodichloromethane, dibromochloromethane, and bromoform were ,36 and limits of detection were, respectively, 0.015, 0.004, 0.005, and We used THM data from 56 measurements in different Spanish areas that were supplied by chlorinated ground water. Average concentrations were 0.3, 0.3, 0.8, and for chloroform, bromodichloromethane, dibromochloromethane, and bromoform, respectively. Nitrate data in private wells were not available.
Estimation of Long-Term Levels in Municipal Drinking Water
We calculated the annual average levels of nitrate and THMs at the water zone level. Years without measurements were assigned the average of all available measurements in the water zone if the water source and treatment did not change over the years. In the case of changes in the water source and/or treatment, procedures to back-extrapolate were applied.
For THMs, because their concentrations in surface water are generally higher than in ground sources,37 we used surface water percentage as a weight to back extrapolate individual THM concentrations when water source changed through linear interpolation, assuming that concentrations increased proportionately to the percentage of surface water. Likewise, water zones with changes in treatment over the years and THMs measurements were used to estimate the change percentage of THMs concentrations after introducing such treatments. These percentages were applied as a weight to back-extrapolate THM concentrations in areas with changes in these specific treatments when measurements were unavailable. Before chlorination started, THMs concentrations were assumed to be zero. Total THMs (TTHM) levels were calculated by adding up chloroform, bromodichloromethane, dibromochloromethane, and bromoform concentrations.
For years without nitrate measurements in water zones where water source changed over the years, the groundwater percentage was used as a weight to back-extrapolate concentrations using linear interpolation, assuming that nitrate levels were higher with increasing groundwater proportion.19 In municipalities without any nitrate measurement (covering of the total person-years), we imputed the levels of neighboring municipalities supplied with similar ground water proportion plus or minus 10%.
Individual Exposures in the Study Population
Average nitrate and THMs concentrations in residential tap water.
We used municipality and year to link municipal levels in drinking water with residential histories of study participants from age 18 y to 2 y before the interview. We estimated the average concentration of nitrate (milligrams per liter) and THMs (micrograms per liter) for this period, henceforth referred to as “lifetime” or “long-term exposure.” Generally, because participants lived in three residences on average during the exposure window period, they were assigned to the water zone where they had lived the longest as of the date of the interview ( y).
Average ingested nitrate and THMs.
To calculate waterborne ingested nitrate (milligrams per day) and THMs (micrograms per day), we assigned levels in drinking water by year according to the water type consumed at home, including municipal (tap), bottled, and private well/other water. Nitrate and THMs levels in municipal water were assigned for tap-water consumption. Nitrate levels in the sampled bottled waters (range )35 were averaged using the sales frequency of each brand as a weight, leading to of , which was assigned to study participants consuming bottled water. Because nitrate levels in well water were not available, waterborne ingested nitrate was considered missing for years when well-water consumption was reported (). A zero THM level was assigned to bottled-water consumers, according to a previous study.36 THM values assigned for well-water consumers were 0.3, 0.3, 0.8, and for chloroform, bromodichloromethane, dibromochloromethane, and bromoform, respectively. The annual nitrate and THMs estimates were averaged from age 18 y to 2 y before the interview and multiplied by the average daily water intake at the residence. Total amount of ingested water was ascertained as the number of water glasses per day consumed on average by the participant at home (liters per day, assuming ). Water intakes that equaled zero and those above the 99th percentile (), considered implausible, were treated as missing values in the analyses.
Covariables
Age (years), education (less than primary school, primary school, secondary school, university), self-reported weight and height 1 y before the interview to compute body mass index (BMI; kilograms per square meter), family history of prostate cancer (yes, no), smoking (never, former, current), and physical activity were considered. Smokers were defined as those smoking at least one cigarette per day for months. Former smokers were defined as those who quit smoking y before the interview. Physical activity was ascertained through open questions on any type of physical activity practiced in life, years, and frequency (hours/week), to calculate metabolic equivalents (METs) from age 16 y to 2 y before the interview. We estimated a cancer prevention score based on The World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) cancer prevention recommendations38 based on six items: BMI, physical activity, consumption of foods and drinks that promote weight gain, plant foods, animal foods, and alcohol. Briefly, the method of estimating the score according to the standardized scoring system for 2018 WCRF/AICR cancer prevention recommendations39 is based on the following criteria: 1 point was assigned when the recommendation was met, 0.5 point when it was partially met, and 0 points when it was not met. The score of each recommendation was added to obtain the total score, which ranged from a minimum value of 0 to a maximum value of 6 points, with higher values indicating high compliance with the cancer prevention recommendations.40 We collected information on self-reported family history of prostate cancer (i.e., malignant tumors in first-degree relatives). Based on food composition tables, frequencies in servings per day of red and processed meat were converted to grams per day and total dietary fiber intake and vitamin intake.
Statistical Analyses
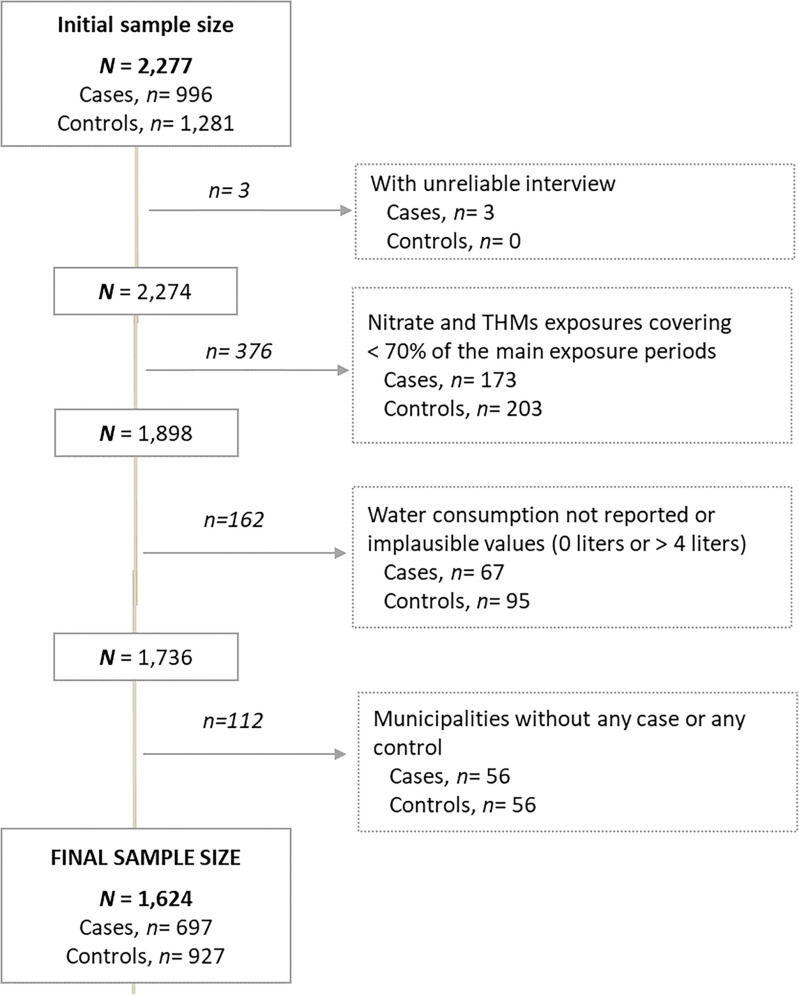
The initial sample of prostate cancer cases and controls was 2,277 (996 cases, 1,281 controls). Number of controls was greater than the number of cases because the controls were matched to different cancer sites. We excluded subjects with interviews qualified as unreliable by the trained interviewers ( cases); those with nitrate or THMs estimates covering fewer than 70% of the years between age 18 y to 2 y before the interview () and those reporting no water consumption or implausible values were also excluded (). Finally, to have a similar geographical distribution of cases and controls, only municipalities with at least one case and one control were included ( excluded). The final sample included 1,624 subjects, 697 cases (590 low- to medium-grade, tumors, 97 high-grade tumors, and 10 without this information) and 927 controls with ages between 38 and 85 y. (Figure 1). Characteristics of cases and controls excluded from the study () are presented in Supplemental Table 1.
Figure 1.
Flowchart showing exclusions of study participants from the Multicase–Control Study in Spain (MCC-Spain). The main exposure periods were from 18 y of age to 2 y before the interview. The interviewers rated the quality of the interview, and those unreliable or inconsistent were excluded. A total of 653 participants were excluded from the study.
Table 1.
Characteristics and drinking-water contaminant exposures of the study population from the Multicase–Control Study in Spain (MCC-Spain): 697 cases, 927 controls ().
| Controls (%) or mean () | Cases (%) or mean () | |||
|---|---|---|---|---|
| Total | Low- to medium-grade tumors (Gleason score ) | High-grade tumors (Gleason score ) | ||
| Number of participants | 927 | 697 | 590 | 97 |
| Characteristics | ||||
| Age (y) | 66.6 (8.3) | 66.0 (7.3) | 65.6 (7.2) | 68.7 (7.7) |
| Educational level (%) | ||||
| Less than primary | 157 (16.9) | 156 (22.4) | 128 (21.7) | 26 (26.8) |
| Primary school | 300 (32.4) | 276 (39.6) | 234 (39.7) | 39 (40.2) |
| Secondary school | 262 (28.3) | 157 (22.5) | 139 (22.0) | 23 (23.7) |
| University | 208 (22.4) | 108 (15.5) | 98 (16.6) | 9 (9.3) |
| Family history of prostate cancer (first degree) (%) | 108 (11.7) | 144 (20.7) | 122 (20.7) | 20 (20.6) |
| Smoking status (%) | ||||
| Never | 237 (25.6) | 218 (31.3) | 180 (30.5) | 34 (35. 1) |
| Former | 480 (51.8) | 349 (50.1) | 296 (50.2) | 47 (48.5) |
| Current smoker | 210 (22.7) | 130 (18.7) | 114 (19.3) | 16 (16.5) |
| WCRF/AICR cancer prevention score | 3.4 (1.0) | 3.3 (0.9) | 3.3 (0.9) | 3.4 (1.0) |
| Intake of red and processed meat (g/d) | 73.3 (38.0) | 77 (40.6) | 77.1 (40.9) | 75.4 (40.2) |
| Intake of total fiber (g/d) | 11.3 (3.8) | 11.1 (3.6) | 11.1 (3.6) | 11.3 (3.7) |
| Intake of fruit and vegetables (g/d) | 486 (277) | 501 (245) | 499 (239) | 524 (275) |
| Intake of vitamin C (mg/d) | 148 (91) | 150 (80) | 150 (78) | 156 (94) |
| Intake of vitamin E (mg/d) | 10.4 (5.45) | 10.6 (5.2) | 10.6 (5.1) | 11.1 (5.9) |
| Recruitment area (%) | ||||
| Asturias | 47 (5.1) | 8 (1.2) | 8 (1.36) | 0 (0) |
| Barcelona | 421 (45.4) | 301 (43.2) | 252 (42.7) | 49 (50.5) |
| Cantabria | 120 (12.9) | 101 (14.5) | 84 (14.2) | 14 (14.4) |
| Madrid | 276 (29.8) | 239 (34.3) | 206 (34.9) | 27 (27.8) |
| Valencia | 63 (6.8) | 48 (6.9) | 40 (6.8) | 7 (7.2) |
| Drinking-water contaminant exposures | ||||
| Average concentrations in residential tap water | ||||
| Nitrate (mg/L) | 7.2 (4.0) | 7.1 (4.2) | 7.1 (4.14) | 7.8 (4.1) |
| Brominated trihalomethanes () | 34.7 (33.0) | 28.3 (27.1) | 28.1 (27.2) | 30.6 (26.7) |
| Chloroform () | 20.7 (8.0) | 21.4 (8.4) | 21.6 (8.4) | 20.4 (8.1) |
| Average waterborne ingestion | ||||
| Nitrate (mg/d) | 11.5 (9.0) | 12.8 (10.8) | 12.8 (11.1) | 13.2 (9.2) |
| Brominated trihalomethanes () | 20.7 (32.4) | 19.2 (29.2) | 19.2 (28.1) | 20.6 (36.1) |
| Chloroform () | 15.1 (14.7) | 15.4 (14.0) | 15.8 (14.2) | 12.9 (13.4) |
Note: Continuous variables are presented as means and standard deviation and categorical variables as percentage (%) and number of subjects (n). A total of 171 subjects had missing data for WCRF/AICR cancer prevention score and dietary variables (intake of red and processed meat, total fiber, fruit and vegetables, vitamin C, and vitamin E). The mismatch between total cases and the sum of early-stage (low- to medium-grade tumors) and aggressive (high-grade tumors) prostate cancer is because there are 10 subjects without information on the grade of the tumor. Brominated trihalomethanes include bromodichloromethane, dibromochloromethane, and bromoform. WCRF/AICR cancer prevention score is based on the WCRF/AICR cancer prevention recommendations. SD, standard deviation; WCRF/AICR, World Cancer Research Fund/American Institute for Cancer Research.
Spearman correlations between tap water residential concentrations and waterborne ingested nitrate, Br-THMs (sum of bromodichloromethane, dibromochloromethane, and bromoform), and chloroform were examined. Drinking-water exposures were categorized into tertiles defined according to the distribution among controls. The main models estimated the association between prostate cancer and lifetime waterborne ingested nitrate, TTHMs, chloroform and Br-THMs, expressed in tertiles and continuous (per 5-unit increment). We estimated odds ratios (OR) and 95% confidence intervals (CI) of prostate cancer using mixed models with recruitment area (Asturias, Barcelona, Cantabria, Madrid, Valencia) as random effect. To test for linear trends (p-trend) across increasing categories of exposure, the median concentration within each category was treated as a continuous variable in the model.
To test whether the associations varied across tumor grade and aggressiveness, we used multinomial logistic regression models with Gleason scores and for the two categories, with control group as the reference (base outcome), and OR [also referred to as relative risk ratios (RRRs)] and their 95% CI were estimated. Heterogeneity of effects for the two grades of tumor severity was tested using the Wald statistic. Smoothed spline with three degrees of freedom from general additive models (GAM) were used to visually display the exposure–response relationships on continuous variables. We further explored the associations using concentrations in residential tap water as exposure, because it might be a better indicator of an exposure though multiple routes (not just ingestion), which is especially relevant for THMs.
All models were adjusted for recruitment area, age, and education. Further adjustment included first-degree family history of prostate cancer, smoking status, and WCRF/AICR cancer prevention score. An additional model was reported with mutual adjustments between nitrate, chloroform, and Br-THMs levels. Multicollinearity was explored using the variance inflation factor (VIF). A mean VIF of 1.43 was obtained, and all variable categories had a VIF . We used stochastic regression (which adds a random error term that appropriately reproduces the correlation between X and Y) to impute 171 missing values in the WCRF/AICR cancer prevention score. There were no missing data for the other covariates of adjustment.
Additionally, subgroup analyses were performed for waterborne nitrate ingested, stratifying the sample (above or below the median among controls) by the following suspected effect modifiers: age ( y vs. y), education (primary school vs. ), WCRF/AICR score (3.5 vs. ), intakes of red and processed meat ( vs. ), total fiber ( vs. ), total fruit and vegetables ( vs. ), vitamin C ( vs. ), and vitamin E ( vs. ). Interaction -value was obtained using the likelihood ratio test of the models with and without the multiplicative interaction term.
All -values presented are two-tailed; was considered statistically significant. Analyses were performed using STATA (version 16.0; Stata Corp.).
Results
Approximately 330 water zones with data on nitrate and THM levels were involved during the study exposure window (Supplemental Figure 1; Excel Table S1). Mean (plus or minus SD) values for average lifetime waterborne ingested nitrate (milligrams per day), Br-THMs (micrograms per day) and chloroform (micrograms per day) were 11.5 (9.0), 20.7 (32.4), and 15.1 (14.7), respectively, in controls; and 12.8 (10.8), 19.2 (29.2), and 15.4 (14.0) in cases. The average age was 66.6 (8.3) y old for controls and 66.0 (7.3) for cases. On average, cases had lower education, had twice as frequent family history of prostate cancer (first degree), and had consumed slightly more red and processed meat in comparison with controls. The recruitment area contributing with the largest population was Barcelona (44% of all subjects) followed by Madrid (30%), whereas Asturias was the province with the greatest difference in percentage of cases (1.6%) in comparison with controls (5.5%) (Table 1).
The proportion (in person-years) of municipal, bottled- and well-water consumption was approximately 78%, 20%, and 2%, respectively, during the exposure window. The average water intake was for cases and for controls. Spearman correlations between tap-water residential concentrations and waterborne ingested contaminants were moderate, rho 0.67 for nitrate and for Br-THM and chloroform (Supplemental Figure 2).
Waterborne Ingested Nitrate
Considering the mutually adjusted models, lifetime average waterborne ingested nitrate was positively associated with prostate cancer when comparing the highest with the lowest exposure category (, 95% CI: 1.19, 2.54; -trend 0.002). For each increase of waterborne ingested nitrate, the overall OR of prostate cancer increased by 22% (; 95% CI: 1.12, 1.33) (Table 2). When examining prostate cancer by tumor severity, comparing extreme categories, the OR for low- to medium-grade prostate cancer (Gleason score ) was 1.59, 95% CI: 1.05, 2.39; -trend 0.014, and for high-grade (Gleason score , i.e., aggressive prostate cancer) was 2.78, 95% CI: 1.23, 6.27; -trend 0.002 (Wald test ) (Table 3).
Table 2.
Association between prostate cancer and lifetime average waterborne ingested nitrate and trihalomethanes (THMs). Multicase–Control Study in Spain (MCC-Spain): 697 cases, 927 controls ().
| Exposure | Controls | Cases | OR (95% CI) Multivariable adjusteda | OR (95% CI) Multivariable adjustedb | OR (95% CI) Multivariable adjustedc |
|---|---|---|---|---|---|
| Nitrate (mg/d) | |||||
| Tertile 1 () | 309 | 221 | 1.00 | 1.00 | 1.00 |
| Tertile 2 (5.5–13.8) | 309 | 209 | 1.11 (0.82, 1.49) | 1.09 (0.81, 1.48) | 1.16 (0.85, 1.57) |
| Tertile 3 () | 309 | 267 | 1.58 (1.12, 2.23) | 1.54 (1.08, 2.19) | 1.74 (1.19, 2.54) |
| -Trend | 927 | 697 | 0.004 | 0.007 | 0.002 |
| Per | 927 | 697 | 1.13 (1.06, 1.21) | 1.13 (1.05, 1.21) | 1.22 (1.12, 1.33) |
| TTHMs () | |||||
| Tertile 1 () | 309 | 243 | 1.00 | 1.00 | 1.00 |
| Tertile 2 (13.7–37.5) | 309 | 221 | 0.88 (0.68, 1.13) | 0.87 (0.67, 1.13) | 0.87 (0.67, 1.13) |
| Tertile 3 () | 309 | 233 | 0.95 (0.73, 1.23) | 0.91 (0.70, 1.18) | 0.90 (0.69, 1.17) |
| -Trend | 927 | 697 | 0.783 | 0.535 | 0.508 |
| Per | 927 | 697 | 1.00 (0.98, 1.01) | 0.99 (0.98, 1.01) | 0.99 (0.98, 1.01) |
| Brominated THMs () | |||||
| Tertile 1 () | 311 | 254 | 1.00 | 1.00 | 1.00 |
| Tertile 2 (3.2–16.8) | 307 | 225 | 0.91 (0.71, 1.16) | 0.89 (0.69, 1.14) | 0.82 (0.61, 1.08) |
| Tertile 3 () | 309 | 218 | 0.90 (0.67, 1.20) | 0.86 (0.64, 1.16) | 0.65 (0.42, 1.00) |
| -Trend | 927 | 697 | 0.575 | 0.447 | 0.109 |
| Per | 927 | 697 | 0.99 (0.98, 1.01) | 0.99 (0.97, 1.01) | 0.99 (0.97, 1.01) |
| Chloroform () | |||||
| Tertile 1 () | 309 | 212 | 1.00 | 1.00 | 1.00 |
| Tertile 2 (5.4–19.1) | 309 | 255 | 1.17 (0.90, 1.51) | 1.16 (0.89, 1.51) | 1.35 (0.98, 1.87) |
| Tertile 3 () | 309 | 230 | 1.01 (0.76, 1.33) | 0.98 (0.74, 1.31) | 1.19 (0.80, 1.77) |
| -Trend | 927 | 697 | 0.795 | 0.671 | 0.925 |
| Per | 927 | 697 | 0.99 (0.95, 1.03) | 0.99 (0.95, 1.02) | 0.98 (0.93, 1.02) |
Note: ORs and 95% CI were calculated using mixed models with recruitment area (Asturias, Barcelona, Cantabria, Madrid, Valencia) as random effect. Brominated THMs includes bromodichloromethane, dibromochloromethane, and bromoform. TTHMs includes chloroform, bromodichloromethane, dibromochloromethane, and bromoform. CI, confidence interval; OR, odds ratio; THMs, trihalomethanes; TTHMs, total trihalomethanes; WCRF/AICR, World Cancer Research Fund/American Institute for Cancer Research.
Model adjusted for age (years) and educational level (less than primary, primary school, secondary school, university).
Model further adjusted for first-degree family history of prostate cancer (yes, no), smoking status (never, former, current smoker), and the WCRF/AICR cancer prevention score.
Model 2 mutually adjusted for the other corresponding components, i.e., total THMs (nitrate model), nitrate (THMs model), chloroform and nitrate (brominated THMs model), brominated THMs and nitrate (chloroform model).
Table 3.
Association between prostate cancer and lifetime average waterborne ingested nitrate by tumor grade (according to Gleason score). Multicase–Control Study in Spain (MCC-Spain): 687 cases, 927 controls ().
| Exposure | Controls | Cases | OR (95% CI) Multivariable adjusteda | OR (95% CI) Multivariable adjustedb | OR (95% CI) Multivariable adjustedc |
|---|---|---|---|---|---|
| Nitrate waterborne ingestion (mg/d) | |||||
| Low- to medium-grade Gleason score | |||||
| Tertile 1 () | 309 | 190 | 1.00 | 1.00 | 1.00 |
| Tertile 2 (5.5–13.8) | 309 | 179 | 1.10 (0.80, 1.52) | 1.09 (0.79, 1.50) | 1.13 (0.81, 1.56) |
| Tertile 3 () | 309 | 221 | 1.52 (1.04, 2.21) | 1.47 (1.01, 2.15) | 1.59 (1.05, 2.39) |
| -Trend | 927 | 590 | 0.015 | 0.024 | 0.014 |
| Per | 927 | 590 | 1.14 (1.06, 1.23) | 1.14 (1.06, 1.22) | 1.23 (1.13, 1.35) |
| High-grade Gleason score | |||||
| Tertile 1 () | 309 | 27 | 1.00 | 1.00 | 1.00 |
| Tertile 2 (5.5–13.8) | 309 | 25 | 0.92 (0.46, 1.83) | 0.90 (0.45, 1.81) | 1.12 (0.55, 2.29) |
| Tertile 3 () | 309 | 45 | 1.80 (0.84, 3.85) | 1.77 (0.82, 3.82) | 2.78 (1.23, 6.27) |
| -Trend | 927 | 97 | 0.035 | 0.039 | 0.002 |
| Per | 927 | 97 | 1.07 (0.94, 1.23) | 1.07 (0.93, 1.23) | 1.18 (1.01, 1.39) |
Note: The number of these analyses is 1,614 (10 cases had no information on Gleason score). Multinomial logistic regression models with the two categories of tumor grade Gleason score and , with control group as the reference (base outcome). ORs and 95% CI were calculated using multinomial (polytomous) logistic regression. Wald test -values for heterogeneity of effects were 0.1894. CI, confidence interval; OR, odds ratio; THMs, trihalomethanes; WCRF/AICR, World Cancer Research Fund/American Institute for Cancer Research.
Model adjusted for recruitment area (Asturias, Barcelona, Cantabria, Madrid, Valencia), age (years) and educational level (less than primary, primary school, secondary school, university).
Model further adjusted for first-degree family history of prostate cancer (yes, no), smoking status (never, former, current smoker) and the WCRF/AICR cancer prevention score.
Model 2 mutually adjusted for total THMs.
We also explored whether other factors (Supplemental Table 2) modified the association between waterborne ingested nitrate and prostate cancer. In the analyses stratified by several factors (Table 4), those with greatest impact on the estimates were age and dietary fiber intake, followed by intakes of fruit and vegetables and vitamin C, although interaction -values were not statistically significant. Comparing highest vs. lowest categories, waterborne ingested nitrate was significantly associated with a higher odds of prostate cancer in the youngest (1.75, 95% CI: 1.07, 2.89; -trend 0.007) but not in the oldest [1.23, 95% CI: 0.75, 2.01; -trend 0.505 (p for interaction 0.095)]. Likewise, the OR of prostate cancer for those with the lowest intake of total fiber () was 2.34 (95% CI: 1.39, 3.94; -trend 0.001), whereas for those with the highest intake (), the OR was 0.95 (95% CI: 0.52, 1.73; -trend 0.709). Interaction -value was 0.102. Comparable results were observed when comparing prostate cancer likelihood in those with higher and lower intakes of fruit and vegetables and vitamin C. Finally, waterborne ingested nitrate was associated with prostate cancer only in those participants with highest education attained, without significant interaction (Table 4).
Table 4.
Subgroup analysis. Association between waterborne ingested nitrate and prostate cancer by age, education, WCRF score adherence, and intakes of red and processed meat, fiber, total fruit and vegetables, vitamin C and vitamin E (above and below the median among controls). Multicase–Control Study in Spain (MCC-Spain): 629 cases, 824 controls ().
| By age | y () | p Interaction | y () | ||
|---|---|---|---|---|---|
| Ingested nitrate (mg/d) | Controls/Cases | OR | 0.095 | Controls/Cases | OR |
| Tertile 1 () | 156/124 | 1.00 | — | 153/97 | 1.00 |
| Tertile 2 (5.5–13.8) | 165/98 | 1.02 (0.67, 1.54) | — | 144/111 | 118 (0.76, 1.84) |
| Tertile 3 () | 154/151 | 1.75 (1.07, 2.89) | — | 155/116 | 1.23 (0.75, 2.01) |
| p-Trend | 475/373 | 0.007 | — | 452/324 | 0.505 |
| Per | 475/373 | 1.15 (1.04, 1.28) | — | 452/324 | 1.08 (0.98, 1.19) |
| By education | Primary school or less () | — | Secondary school or higher () | ||
| Ingested nitrate (mg/d) | Controls/Cases | OR | 0.495 | Controls/Cases | OR |
| Tertile 1 () | 140/124 | 1.00 | — | 169/97 | 1.00 |
| Tertile 2 (5.5–13.8) | 146/119 | 0.87 (0.53, 1.44) | — | 163/90 | 1.40 (0.90, 2.16) |
| Tertile 3 () | 171/189 | 1.17 (0.54, 2.54) | — | 138/78 | 1.89 (1.09, 3.29) |
| p-Trend | 457/432 | 0.215 | — | 470/265 | 0.032 |
| Per | 457/432 | 1.07 (0.98, 1.16) | — | 470/265 | 1.22 (1.08, 1.38) |
| By WCRF/AICR score | Score () | p Interaction | Score () | ||
| Ingested nitrate (mg/day) | Controls/Cases | OR | 0.844 | Controls/Cases | OR |
| Tertile 1 () | 156/113 | 1.00 | — | 121/79 | 1.00 |
| Tertile 2 (5.5–13.8) | 160/115 | 1.26 (0.82, 1.92) | — | 113/71 | 0.96 (0.60, 1.54) |
| Tertile 3 () | 165/145 | 1.74 (1.07, 2.82) | — | 109/106 | 1.43 (0.84, 2.44) |
| p-Trend | 481/373 | 0.021 | — | 343/256 | 0.110 |
| Per | 481/373 | 1.12 (1.02, 1.23) | — | 343/256 | 1.13 (1.01, 1.26) |
| By intake of red and processed meat | Intake () | p Interaction | Intake () | ||
| Ingested nitrate (mg/d) | Controls/Cases | OR | 0.705 | Controls/Cases | OR |
| Tertile 1 () | 150/78 | 1.00 | — | 127/114 | 1.00 |
| Tertile 2 (5.5–13.8) | 140/86 | 1.14 (0.73, 1.78) | — | 133/100 | 1.05 (0.67, 1.64) |
| Tertile 3 () | 122/100 | 1.66 (0.99, 1.78) | — | 152/151 | 1.39 (0.84, 2.29) |
| p-Trend | 412/264 | 0.043 | — | 412/365 | 0.128 |
| Per | 412/264 | 1.12 (1.01, 1.24) | — | 412/365 | 1.11 (1.01, 1.23) |
| By intake of total fiber | Intake () | p Interaction | Intake () | ||
| Ingested nitrate (mg/d) | Controls/Cases | OR | 0.102 | Controls/Cases | OR |
| Tertile 1 () | 145/97 | 1.00 | — | 132/95 | 1.00 |
| Tertile 2 (5.5–13.8) | 136/113 | 1.48 (0.94, 2.32) | — | 137/73 | 0.75 (0.45, 1.23) |
| Tertile 3 () | 131/142 | 2.34 (1.39, 3.94) | — | 143/109 | 0.95 (0.52, 1.73) |
| p-Trend | 412/352 | 0.001 | — | 412/277 | 0.709 |
| Per | 412/352 | 1.17 (1.05, 1.30) | — | 412/277 | 1.06 (0.97, 1.17) |
| By intake of fruit and vegetables | Intake () | p Interaction | Intake () | ||
| Ingested nitrate (mg/d) | Controls/Cases | OR | 0.783 | Controls/Cases | OR |
| Tertile 1 () | 135/84 | 1.00 | — | 142/108 | 1.00 |
| Tertile 2 (5.5–13.8) | 149/98 | 1.26 (0.81, 1.97) | — | 124/88 | 0.88 (0.60, 1.28) |
| Tertile 3 () | 128/116 | 2.07 (1.24, 3.45) | — | 146/135 | 1.01 (0.71, 1.45) |
| p-Trend | 412/298 | 0.003 | — | 412/331 | 0.799 |
| Per | 412/298 | 1.20 (1.08, 1.34) | — | 412/331 | 1.02 (0.95, 1.10) |
| By intake of vitamin C | Intake () | p Interaction | Intake () | ||
| Ingested nitrate (mg/d) | Controls/Cases | OR | 0.987 | Controls/Cases | OR |
| Tertile 1 () | 131/82 | 1.00 | — | 146/110 | 1.00 |
| Tertile 2 (5.5–13.8) | 148/95 | 1.29 (0.81, 2.07) | — | 125/91 | 0.99 (0.65, 1.50) |
| Tertile 3 () | 133/111 | 1.78 (1.04, 3.02) | — | 141/140 | 1.31 (0.81, 2.13) |
| p-Trend | 412/288 | 0.034 | — | 412/341 | 0.198 |
| Per | 412/288 | 1.17 (1.05, 1.31) | — | 412/341 | 1.07 (0.97, 1.17) |
| By intake of vitamin E | Intake () | p Interaction | Intake () | ||
| Ingested nitrate (mg/d) | Controls/Cases | OR | 0.764 | Controls/Cases | OR |
| Tertile 1 () | 140/95 | 1.00 | — | 137/97 | 1.00 |
| Tertile 2 (5.5–13.8) | 147/87 | 1.05 (0.68, 1.62) | — | 126/99 | 1.20 (0.77, 1.88) |
| Tertile 3 () | 125/101 | 1.59 (0.95, 2.65) | — | 149/150 | 1.54 (0.93, 2.54) |
| p-Trend | 412/283 | 0.047 | — | 412/346 | 0.084 |
| Per | 412/283 | 1.13 (1.02, 1.26) | — | 412/346 | 1.10 (0.99, 1.21) |
Note: These analyses are performed excluding 171 subjects with missing data in the dietary variables. WCRF/AICR cancer prevention score is based on the WCRF/AICR cancer prevention recommendations. OR and 95% CI were calculated using mixed models with recruitment area (Asturias, Barcelona, Cantabria, Madrid, Valencia) as random effect. Model adjusted for age (years), educational level (less than primary, primary school, secondary school, university), first-degree family history of prostate cancer (yes, no), smoking status (never, former, current smoker) and the WCRF/AICR cancer prevention score. —, no data; CI, confidence interval; OR, odds ratio; WCRF/AICR, World Cancer Research Fund/American Institute for Cancer Research.
Waterborne Ingested THMs
The relationship between waterborne ingested Br-THMs and prostate cancer showed an inverse pattern (Table 2; Figure 2). However, neither waterborne ingested TTHM (OR 0.90, 95% CI: 0.69, 1.17; -trend 0.508), Br-THMs (OR 0.65, 95% CI: 0.42, 1.00; -trend 0.109), nor chloroform (OR 1.19, 95% CI: 0.80, 1.77; -trend 0.925) were significantly associated with prostate cancer (Table 2).
Figure 2.
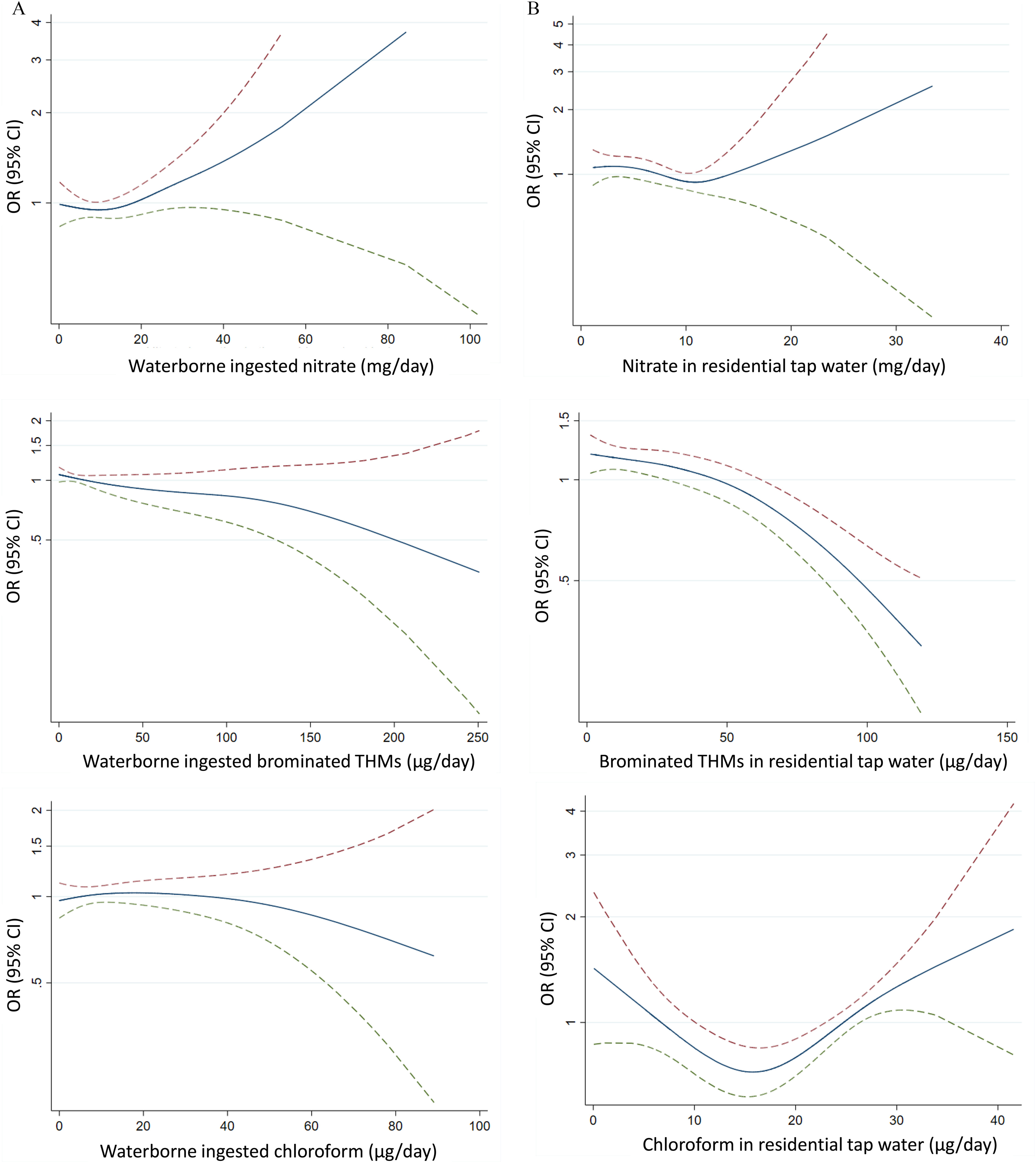
Exposure–response relationship between prostate cancer and waterborne ingested nitrate, brominated THMs, and chloroform (expressed as ORs). Multicase–Control Study in Spain (MCC-Spain): 697 cases, 927 controls (). Smoothed spline with three degrees of freedom from general additive models adjusted for recruitment area (Asturias, Barcelona, Cantabria, Madrid, Valencia), age (years), educational level (less than primary, primary school, secondary school, university), first-degree family history of prostate cancer (yes, no), smoking status (never, former, current smoker) and the WCRF/AICR cancer prevention score. The dashed lines represent the 95% CIs. Upper CI red and lower green color. Note: CI, confidence interval; OR, odds ratio; THMs, trihalomethanes; WCRF/AICR, World Cancer Research Fund/American Institute for Cancer Research.
Residential Nitrate and THM Concentrations
Residential nitrate levels were associated with prostate cancer; OR for increase of residential nitrate level was 1.59 (95% CI: 1.13, 2.25) (Table 5). Br-THMs were inversely associated with prostate cancer both comparing extreme tertiles and the continuous exposure, OR 0.92 (95% CI: 0.89, 0.95) for increase (Table 5). Although the relationship between residential chloroform levels and prostate cancer was not linear (Figure 2), those with the highest residential chloroform exposure were more likely to develop prostate cancer than those with lowest residential exposure (OR 2.61, 95% CI: 1.55, 4.39; -trend ) (Table 5).
Table 5.
Association between prostate cancer and lifetime average nitrate and trihalomethanes concentrations in residential tap water. Multicase–Control Study in Spain (MCC-Spain): 697 cases, 927 controls ().
| Exposure | Controls | Cases | OR (95% CI) Multivariable adjusteda | OR (95% CI) Multivariable adjustedb | OR (95% CI) Multivariable adjustedc |
|---|---|---|---|---|---|
| Nitrate (mg/L) | |||||
| Tertile 1 () | 309 | 237 | 1.00 | 1.00 | 1.00 |
| Tertile 2 (2.8–10.0) | 309 | 242 | 1.32 (0.73, 2.38) | 1.33 (0.73, 2.44) | 1.37 (0.75, 2.51) |
| Tertile 3 () | 309 | 218 | 1.30 (0.69, 2.43) | 1.33 (0.70, 2.52) | 1.52 (0.78, 2.97) |
| -trend | 927 | 697 | 0.473 | 0.441 | 0.214 |
| Per | 927 | 697 | 1.18 (0.88, 1.59) | 1.18 (0.87, 1.60) | 1.59 (1.13, 2.25) |
| TTHMs () | |||||
| Tertile 1 () | 309 | 231 | 1.00 | 1.00 | 1.00 |
| Tertile 2 (32.5–64.4) | 309 | 278 | 1.14 (0.81, 1.61) | 1.11 (0.79, 1.58) | 1.06 (0.75, 1.51) |
| Tertile 3 () | 309 | 188 | 0.88 (0.57, 1.36) | 0.86 (0.55, 1.33) | 0.78 (0.49, 1.24) |
| -Trend | 927 | 697 | 0.322 | 0.274 | 0.172 |
| Per | 927 | 697 | 0.93 (0.90, 0.96) | 0.92 (0.89, 0.95) | 0.92 (0.89, 0.95) |
| Brominated THMs () | |||||
| Tertile 1 () | 309 | 259 | 1.00 | 1.00 | 1.00 |
| Tertile 2 (8.9–44.6) | 309 | 261 | 0.86 (0.53, 1.40) | 0.87 (0.53, 1.42) | 0.68 (0.39, 1.20) |
| Tertile 3 () | 309 | 177 | 0.57 (0.32, 0.99) | 0.57 (0.32, 1.01) | 0.48 (0.25, 0.93) |
| -Trend | 927 | 697 | 0.007 | 0.007 | 0.017 |
| Per | 927 | 697 | 0.93 (0.90, 0.96) | 0.93 (0.90, 0.96) | 0.92 (0.89, 0.95) |
| Chloroform () | |||||
| Tertile 1 () | 309 | 183 | 1.00 | 1.00 | 1.00 |
| Tertile 2 (18.4–25.5) | 311 | 228 | 2.02 (1.36, 3.02) | 2.19 (1.46, 3.29) | 2.18 (1.43, 3.34) |
| Tertile 3 () | 307 | 286 | 2.37 (1.52, 3.71) | 2.55 (1.61, 4.02) | 2.61 (1.55, 4.39) |
| -Trend | 927 | 697 | |||
| Per | 927 | 697 | 1.20 (1.02, 1.42) | 1.21 (1.02, 1.43) | 1.07 (0.94, 1.23) |
Note: OR and 95% CI were calculated using mixed models with recruitment area (Asturias, Barcelona, Cantabria, Madrid, Valencia) as random effect. Brominated THMs includes bromodichloromethane, dibromochloromethane, and bromoform. TTHMs includes chloroform, bromodichloromethane, dibromochloromethane, and bromoform. CI, confidence interval; OR, odds ratio; THMs, trihalomethanes; TTHMs, total trihalomethanes; WCRF/AICR, World Cancer Research Fund/American Institute for Cancer Research.
Model adjusted for age (years) and educational level (less than primary, primary school, secondary school, university).
Model further adjusted for first degree family history of prostate cancer (yes, no), smoking status (never, former, current smoker) and the WCRF/AICR cancer prevention score.
Model 2 mutually adjusted for the other corresponding components, i.e., total THMs (nitrate model), nitrate (THMs model), chloroform and nitrate (brominated THMs model), brominated THMs and nitrate (chloroform model).
Discussion
This is, to our knowledge, the first study to explore the relationship between exposure to nitrate and THMs through drinking water and prostate cancer at the individual level. In this case–control study, long-term waterborne ingested nitrate was associated with an increased OR of prostate cancer, especially with aggressive tumors. Residential chloroform levels showed a nonlinear positive association, whereas brominated THM were negatively associated with prostate cancer.
We examined two distinct exposure estimates, i.e., residential levels at the tap and waterborne ingested exposure. Residential levels provide a rough estimate of exposure through multiple routes (ingestion, inhalation, dermal), which is particularly relevant for volatile and skin permeable THMs.41–43 Given that nitrate is only ingested, the waterborne ingested estimates provide a more relevant exposure in comparison with residential nitrate levels. This finding is consistent with the differences we observed between exposure metrics for nitrate and THMs, i.e., higher associations for the ingested vs. residential nitrate exposure estimates and for residential vs. ingested THMs exposure estimates.
Prostate cancer is increasingly studied as two distinct phenotypes with suggested different etiologies: one slow-growing, indolent form and an aggressive form that can be fatal. Although age is more related to early-stage and indolent prostate tumors, lifestyle factors such as obesity, cigarette smoking, Western diets,44 or exposures such as pesticides14 have been linked to advanced-stage and more aggressive prostate cancer.9 Although our study had limited power to conduct stratified analyses for the most aggressive tumors, we observed a higher effect size for aggressive in comparison with early-stage prostate cancer (based on Gleason score vs. ). This observation might suggest that nitrate could have a greater influence on prostate cancer progression than initiation. Future work on the eventual mechanisms of nitrate on prostate carcinogenesis considering the role of grade and stage are warranted.
Our findings suggested that men y old might be more susceptible to the carcinogenic effect of drinking-water nitrate on the prostate. In Spain, prostate cancer incidence at age y is increasing at a higher rate than in older men45 and follows different spatial patterns,18 supporting the hypothesis that environmental factors may be involved. Prostate tumors in younger men differ from tumors diagnosed at an older age in terms of biological features and clinical entity.46,47 On the other hand, an effect modification has been identified by education level, suggesting that unaccounted factors related to socioeconomic status may play a role in the association between nitrate and prostate cancer. This effect modification is probably independent from that of the exposure to water contaminants, because previous studies have not been able to identify a clear link between socioeconomic status and exposure to drinking water contaminants.48,49 In light of this evidence, whether subgroups within the population may respond differently to the toxicity of drinking-water nitrate needs to be further examined.50
We also performed subgroup analysis by dietary factors involved in endogenous nitrosation.50 Men with high intake of total fruit and vegetables or vitamin C did not show an association between waterborne ingested nitrate and prostate cancer, whereas in those with low intakes the associations were strong. Antioxidants, vitamins, and polyphenols present in fruits and vegetables are inhibitors of endogenous nitrosation,51–54 and epidemiological evidence suggests the role of vitamins on prostate cancer prevention.55–57 Vitamin C (ascorbate) has shown significant antitumor activity, and high-dose vitamin C has been investigated as a treatment for cancer patients.58,59 Similarly, the association between waterborne ingested nitrate and prostate cancer was only found among people who consumed low amounts of fiber. These findings are consistent with the recognized benefit of dietary fiber to the gut microbiome, with protective capacity against food-derived toxicants, including N-nitrosamine.60 Furthermore, men with aggressive prostate cancer share a specific gut microbial profile, and recent studies have associated gut microbiome–related metabolites such as choline, betaine, and PAGln61 with prostate cancer, particularly its lethal form.62 Overall, these findings may suggest microbiome disruption as a possible biological mechanism of nitrate.
We did not find significant associations between waterborne ingested THMs and prostate cancer. However, residential levels of brominated THMs showed an inverse association with prostate cancer, whereas chloroform showed a nonlinear positive association. Current evidence is limited and methodology of previous studies is not comparable with our study. Previous cohort studies have evaluated prostate cancer in relation to residential-based exposure estimates, not specifically THMs, and showed null associations.63,64 The higher associations we observed for residential levels in comparison with ingestion suggest the relevance of inhalation and dermal exposure routes. These observations are supported by experimental evidence showing a higher internal dose of THMs and longer duration in the bloodstream when exposure is through activities involving inhalation and dermal absorption in comparison with ingestion.41,65 Different effects between chloroform and brominated THMs are expected, given the different genotoxicities.66 However, the inverse association with brominated THMs was unexpected.
Although THMs have been used in epidemiological studies as surrogates of total DBPs in drinking water, they are not the most toxic,67 and correlations among specific DBPs are variable and strongly depend on raw water quality and the type of treatment (including the disinfection processes).68 During the recruitment for the present study, DBPs other than THMs were analyzed in drinking water of a representative sample across study areas.68 The median (range) concentration of 3-Chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone (MX), which is a major mutagenic constituent of DBP,69 was 16.7 (0.8–54.1) ng/L. Chloroform concentrations were positively correlated to MX, whereas Br-THMs concentrations were negatively correlated to MX.68 This correlation might explain the inverse association we observed between residential Br-THMs and prostate cancer and the positive association with chloroform.
Exposure measurement error is the main concern in this study, particularly because the exposure difference between cases and controls is small. The limited historical measurements (particularly before 1980) and the assumptions used to model historical concentrations could reduce the accuracy of exposure estimates. To minimize exposure measurement error, we included only subjects with known exposures for at least 70% of the exposure window. Inability to account for exposures outside home and use of domestic filtration systems may have introduced nondifferential misclassification in the waterborne ingested estimates. However, the reported amount of water consumed at work (: ) and other places () was smaller than that consumed at home (), and minor bias was expected. As for the use of domestic filters, a reduction of THMs levels has been reported.70 Although there were no statistics on the use of domestic filters in Spain for the study exposure window, expert knowledge suggests that the use of domestic water filters during the exposure window was most likely infrequent. Overall, the expected effect on the associations from exposure measurement error is attenuation toward the null,71 as has been shown for other residence-based exposures.72 This could partly explain the lack of association between waterborne ingested chloroform and prostate cancer, whereas residential levels were associated.
Frequency of routine monitoring by water zone is determined by the population served; thus the number of measurements and accuracy of exposure estimates are expected to be higher in large municipalities (or cities), which in turn concentrate most of the study participants. Although the number of measurements below the QL was small (e.g., for nitrate), the imputation of values below the QL may have introduced nondifferential error at the low-exposure range that may have attenuated associations for the continuous variable. However, this approach likely did not affect the results based on exposure categories, because values remain in the referent category. The use of the average instead of geometric mean to calculate long-term levels was due to the constraints of the data provided by water operators, which mostly reported averages and did not provide the raw database. We speculate that this use of the average has probably reduced the accuracy of the exposure estimates, which in turn has probably led to attenuated associations, although bias away from the null cannot be excluded.
Personal information was collected retrospectively after diagnosis, and differential recall between cases and controls may not be totally ruled out. However, the questionnaire was administered by trained personnel in a face-to-face interview, and there is no obvious link between the water questions and prostate cancer that could motivate different responses between cases and controls. Other questions more prone to differential recall bias (e.g., occupational history) are not included in the present analysis. Moreover, the interviewers rated the quality of the interview, and unreliable or inconsistent interviews were excluded from our analyses. Thus, minor differential recall bias is expected to affect the results on waterborne ingested nitrate/THMs. Selection bias arising from control sampling might be of concern. Response rates were moderate, especially among controls, and is partly explained by the population-based source as opposed to hospital-based cases. Controls had a slightly higher educational level in comparison with cases, and all logistic regression models were adjusted for education. In addition, we conducted stratified analysis to identify eventual effect modification on the associations, suggesting that unaccounted factors related to socioeconomic status may be relevant in the association with nitrate. Finally, the probability of participation can be assumed to be independent from the exposure, and nondifferential bias, if any, is expected. Residual confounding by unmeasured factors including environmental exposures with geographical distribution, such as air pollution, green spaces, neighborhoods, or other drinking-water contaminants, cannot be ruled out. It was not possible to perform analyses by recruitment area due to the limited within area variability, and we conducted mixed models with area as random effect. This approach indirectly adjusted for environmental factors geographically distributed, and expected effect on results is minimal because correlation of these factors with our main exposures is unlikely.
Strengths of this study are the relatively large sample size, the long-term exposure approach (from 18 y of age to 2 y before the study interview), and detailed individual information on a range of covariables. These elements facilitated the assessment of several potential confounders and effect modifiers and assessment of coexposure to two main water contaminants.
Conclusions
Findings suggest long-term waterborne ingested nitrate could be a risk factor of prostate cancer, particularly for aggressive tumors and in men y old. A high dietary intake of fiber, fruits and vegetables, or vitamin C may reduce this negative effect of drinking-water nitrate. Association with residential levels but not ingested chloroform/Br-THM may suggest inhalation and dermal routes could be relevant for prostate cancer. Further studies are warranted to draw firm conclusions.
Supplementary Material
Acknowledgments
The authors would like to acknowledge all the research assistants and interviewers in the study centers, the staff of all participating hospitals, and, most of all, the study participants.
The study was partially funded by the “Accion Transversal del Cancer,” approved on the Spanish Ministry Council on 11 October 2007, by the Instituto de Salud Carlos III-FEDER (PI08/1770, PI08/0533, PI08/1359, PS09/00773, PS09/01286, PS09/01903, PS09/02078, PS09/01662, PI11/01889, PI11/00226, PI12/01270, PI12/00715, PI14/0613, PI15/00914, PI17CIII/00034), by the Fundación Marqués de Valdecilla (API 10/09), by the Conselleria de Sanitat of the Generalitat Valenciana (AP_061/10), by the European Commission grants FOOD-CT-2006-036224-HIWATE, by the Spanish Association Against Cancer (AECC) Scientific Foundation, by the Catalan Government-Agency for Management of University and Research Grants (AGAUR) grants 2017SGR723 and 2014SGR850, by the Fundación Caja de Ahorros de Asturias, and by the University of Oviedo. ISGlobal acknowledges support from the Spanish Ministry of Science and Innovation through the “Centro de Excelencia Severo Ochoa 2019–2023” Program (CEX2018-000806-S) and support from the Generalitat de Catalunya through the CERCA Program.
References
- 1.Rawla P. 2019. Epidemiology of prostate cancer. World J Oncol 10(2):63–89, PMID: , 10.14740/wjon1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. 2021. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249, PMID: , 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Galceran J, Ameijide A, Carulla M, Mateos A, Quirós JR, Rojas D, et al. REDECAN Working Group. 2017. Cancer incidence in Spain, 2015. Clin Transl Oncol 19(7):799–825, PMID: , 10.1007/s12094-016-1607-9. [DOI] [PubMed] [Google Scholar]
- 4.IARC (International Agency for Research on Cancer). 2018. Global Cancer Observatory: Cancer Today. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al., eds. Lyon, France: IARC. [Google Scholar]
- 5.Eeles RA, Olama AAA, Benlloch S, Saunders EJ, Leongamornlert DA, Tymrakiewicz M, et al. 2013. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat Genet 45(4):385–391, PMID: , 10.1038/ng.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan SH, Petrovics G, Srivastava S. 2018. Prostate cancer genomics: recent advances and the prevailing underrepresentation from racial and ethnic minorities. Int J Mol Sci 19(4):1255, PMID: , 10.3390/ijms19041255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. 2007. Risk factors for prostate cancer incidence and progression in the Health Professionals Follow-Up Study. Int J Cancer 121(7):1571–1578, PMID: , 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chamie K, DeVere White RW, Lee D, Ok JH, Ellison LM. 2008. Agent Orange exposure, Vietnam War veterans, and the risk of prostate cancer. Cancer 113(9):2464–2470, PMID: , 10.1002/cncr.23695. [DOI] [PubMed] [Google Scholar]
- 9.Pernar CH, Ebot EM, Wilson KM, Mucci LA. 2018. The epidemiology of prostate cancer. Cold Spring Harb Perspect Med 8(12):a030361, PMID: , 10.1101/cshperspect.a030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandaglia G, Leni R, Bray F, Fleshner N, Freedland SJ, Kibel A, et al. 2021. Epidemiology and prevention of prostate cancer. Eur Urol Oncol 4(6):877–892, PMID: , 10.1016/j.euo.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Cornago A, Key TJ, Allen NE, Fensom GK, Bradbury KE, Martin RM, et al. 2017. Prospective investigation of risk factors for prostate cancer in the UK Biobank cohort study. Br J Cancer 117(10):1562–1571, PMID: , 10.1038/bjc.2017.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. 2015. EDC-2: the Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev 36(6):E1–E150, PMID: , 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Falco M, Laforgia V. 2021. Combined effects of different endocrine-disrupting chemicals (EDCs) on prostate gland. Int J Environ Res Public Health 18(18):9772, PMID: , 10.3390/ijerph18189772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koutros S, Beane Freeman LE, Lubin JH, Heltshe SL, Andreotti G, Barry KH, et al. 2013. Risk of total and aggressive prostate cancer and pesticide use in the Agricultural Health Study. Am J Epidemiol 177(1):59–74, PMID: , 10.1093/aje/kws225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chazelas E, Pierre F, Druesne-Pecollo N, Esseddik Y, Szabo de Edelenyi F, Agaesse C, et al. 2022. Nitrites and nitrates from food additives and natural sources and cancer risk: results from the NutriNet-Santé cohort. Int J Epidemiol 51(4):1106–1119, PMID: , 10.1093/ije/dyac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quinn M, Babb P. 2002. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part I: international comparisons. BJU Int 90(2):162–173, PMID: , 10.1046/j.1464-410x.2002.2822.x. [DOI] [PubMed] [Google Scholar]
- 17.Dasgupta P, Baade PD, Aitken JF, Ralph N, Chambers SK, Dunn J. 2019. Geographical variations in prostate cancer outcomes: a systematic review of international evidence. Front Oncol 9:238, PMID: , 10.3389/fonc.2019.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez-Sanchez L, Fernández-Navarro P, López-Abente G, Nuñez O, Fernández de Larrea-Baz N, Jimenez-Moleón JJ, et al. 2019. Different spatial pattern of municipal prostate cancer mortality in younger men in Spain. PLoS One 14(1):e0210980, PMID: , 10.1371/journal.pone.0210980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward MH, Jones RR, Brender JD, de Kok TM, Weyer PJ, Nolan BT, et al. 2018. Drinking water nitrate and human health: an updated review. Int J Environ Res Public Health 15(7):1557, PMID: , 10.3390/ijerph15071557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Espejo-Herrera N, Gracia-Lavedan E, Pollan M, Aragonés N, Boldo E, Perez-Gomez B, et al. 2016. Ingested nitrate and breast cancer in the Spanish Multicase-Control Study on Cancer (MCC-Spain). Environ Health Perspect 124(7):1042–1049, PMID: , 10.1289/ehp.1510334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grosse Y, Baan R, Straif K, Secretan B, El Ghissassi F, Cogliano V, et al. 2006. Carcinogenicity of nitrate, nitrite, and cyanobacterial peptide toxins. Lancet Oncol 7(8):628–629, PMID: , 10.1016/s1470-2045(06)70789-6. [DOI] [PubMed] [Google Scholar]
- 22.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 2010. IARC monographs on the evaluation of carcinogenic risks to humans. Ingested nitrate and nitrite, and cyanobacterial peptide toxins. IARC Monogr Eval Carcinog Risks Hum 94:v–vii, 1–412, PMID: . [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson SD, Thruston Jr AD, Caughran TV, Chen PH, Collette TW, Schenck KM, et al. 2000. Identification of new drinking water disinfection by-products from ozone, chlorine dioxide, chloramine, and chlorine. Water Air Soil Pollut 123:95–102, 10.1023/A:1005265509813. [DOI] [Google Scholar]
- 24.Kristiana I, Tan J, Joll CA, Heitz A, von Gunten U, Charrois JW. 2013. Formation of N-nitrosamines from chlorination and chloramination of molecular weight fractions of natural organic matter. Water Res 47(2):535–546, PMID: , 10.1016/j.watres.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Richardson SD, Plewa MJ, Wagner ED, Schoeny R, Demarini DM. 2007. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res 636(1–3):178–242, PMID: , 10.1016/j.mrrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Wilbourn JD, Partensky C, Rice JM. 1999. Agents that induce epithelial neoplasms of the urinary bladder, renal cortex and thyroid follicular lining in experimental animals and humans: summary of data from IARC monographs volumes 1–69. IARC Sci Publ 147:191–209, PMID: . [PubMed] [Google Scholar]
- 27.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 2013. Some Chemicals Present in Industrial and Consumer Products, Food and Drinking-Water. IARC Monogr Eval Carcinog Risks Hum 101:9–549. https://publications.iarc.fr/_publications/media/download/5700/5febe9a9b9aae2224f6c0cc3db1c430149ec5593.pdf [accessed 24 February 2023]. [PMC free article] [PubMed] [Google Scholar]
- 28.Chang SL. 1982. The safety of water disinfection. Annu Rev Public Health 3:393–418, PMID: , 10.1146/annurev.pu.03.050182.002141. [DOI] [PubMed] [Google Scholar]
- 29.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1999. Some Chemicals that Cause Tumours of the Kidney or Urinary Bladder in Rodents and Some Other Substances. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 73. Lyon, France: IARC. [Google Scholar]
- 30.Castano-Vinyals G, Aragones N, Perez-Gomez B, Martén V, Llorca J, Moreno V, et al. 2015. Population-based multicase–control study in common tumors in Spain (MCC-Spain): rationale and study design. Gac Sanit 29(4):308–315, PMID: , 10.1016/j.gaceta.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Martin-Moreno JM, Boyle P, Gorgojo L, Maisonneuve P, Fernandez-Rodriguez JC, Salvini S, et al. 1993. Development and validation of a food frequency questionnaire in Spain. Int J Epidemiol 22(3):512–519, PMID: , 10.1093/ije/22.3.512. [DOI] [PubMed] [Google Scholar]
- 32.Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA, Grading Committee. 2016. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol 40(2):244–252, PMID: , 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 33.Hurwitz LM, Agalliu I, Albanes D, Barry KH, Berndt SI, Cai Q, et al. 2021. Recommended definitions of aggressive prostate cancer for etiologic epidemiologic research. J Natl Cancer Inst 113(6):727–734, PMID: , 10.1093/jnci/djaa154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Croghan C, Egeghy PP. 2003. Methods of dealing with values below the limit of detection using SAS. Southern SAS User Group 22:24. St. Petersburg, FL, September 22-24, 2003. https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NERL&dirEntryId=64046. [Google Scholar]
- 35.Espejo-Herrera N, Kogevinas M, Castaño-Vinyals G, Aragonés N, Boldo E, Ardanaz E, et al. 2013. Nitrate and trace elements in municipal and bottled water in Spain. Gac Sanit 27(2):156–160, PMID: , 10.1016/j.gaceta.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Font-Ribera L, Kogevinas M, Nieuwenhuijsen MJ, Grimalt JO, Villanueva CM. 2010. Patterns of water use and exposure to trihalomethanes among children in Spain. Environ Res 110(6):571–579, PMID: , 10.1016/j.envres.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Villanueva CM, Kogevinas M, Grimalt JO. 2003. Haloacetic acids and trihalomethanes in finished drinking waters from heterogeneous sources. Water Res 37(4):953–958, PMID: , 10.1016/s0043-1354(02)00411-6. [DOI] [PubMed] [Google Scholar]
- 38.Olmedo-Requena R, Lozano-Lorca M, Salcedo-Bellido I, Jiménez-Pacheco A, Vázquez-Alonso F, García-Caballos M, et al. 2020. Compliance with the 2018 World Cancer Research Fund/American Institute for Cancer Research cancer prevention recommendations and prostate cancer. Nutrients 12(3):768, PMID: , 10.3390/nu12030768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shams-White MM, Brockton NT, Mitrou P, Romaguera D, Brown S, Bender A, et al. 2019. Operationalizing the 2018 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) cancer prevention recommendations: a standardized scoring system. Nutrients 11(7):1572, PMID: , 10.3390/nu11071572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romaguera D, Gracia-Lavedan E, Molinuevo A, de Batlle J, Mendez M, Moreno V, et al. 2017. Adherence to nutrition-based cancer prevention guidelines and breast, prostate and colorectal cancer risk in the MCC-Spain case-control study. Int J Cancer 141(1):83–93, PMID: , 10.1002/ijc.30722. [DOI] [PubMed] [Google Scholar]
- 41.Ashley DL, Blount BC, Singer PC, Depaz E, Wilkes C, Gordon S, et al. 2005. Changes in blood trihalomethane concentrations resulting from differences in water quality and water use activities. Arch Environ Occup Health 60(1):7–15, PMID: , 10.3200/AEOH.60.1.7-15. [DOI] [PubMed] [Google Scholar]
- 42.Gordon SM, Brinkman MC, Ashley DL, Blount BC, Lyu C, Masters J, et al. 2006. Changes in breath trihalomethane levels resulting from household water-use activities. Environ Health Perspect 114(4):514–521, PMID: , 10.1289/ehp.8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costet N, Villanueva CM, Jaakkola JJK, Kogevinas M, Cantor KP, King WD, et al. 2011. Water disinfection by-products and bladder cancer: is there a European specificity? A pooled and meta-analysis of European case-control studies. Occup Environ Med 68(5):379–385, PMID: , 10.1136/oem.2010.062703. [DOI] [PubMed] [Google Scholar]
- 44.Chan JM, Gann PH, Giovannucci EL. 2005. Role of diet in prostate cancer development and progression. J Clin Oncol 23(32):8152–8160, PMID: , 10.1200/JCO.2005.03.1492. [DOI] [PubMed] [Google Scholar]
- 45.Zhou CK, Check DP, Lortet-Tieulent J, Laversanne M, Jemal A, Ferlay J, et al. 2016. Prostate cancer incidence in 43 populations worldwide: an analysis of time trends overall and by age group. Int J Cancer 138(6):1388–1400, PMID: , 10.1002/ijc.29894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding Y, Wu H, Warden C, Steele L, Liu X, Iterson MV, et al. 2016. Gene expression differences in prostate cancers between young and old men. PLoS Genet 12(12):e1006477, PMID: , 10.1371/journal.pgen.1006477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salinas CA, Tsodikov A, Ishak-Howard M, Cooney KA. 2014. Prostate cancer in young men: an important clinical entity. Nat Rev Urol 11(6):317–323, PMID: , 10.1038/nrurol.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castaño-Vinyals G, Cantor KP, Villanueva CM, Tardon A, Garcia-Closas R, Serra C, et al. 2011. Socioeconomic status and exposure to disinfection by-products in drinking water in Spain. Environ Health 10:18, PMID: , 10.1186/1476-069X-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vrijheid M, Martinez D, Aguilera I, Ballester F, Basterrechea M, Esplugues A, et al. 2012. Socioeconomic status and exposure to multiple environmental pollutants during pregnancy: evidence for environmental inequity? J Epidemiol Community Health 66(2):106–113, PMID: , 10.1136/jech.2010.117408. [DOI] [PubMed] [Google Scholar]
- 50.Bryan NS, Alexander DD, Coughlin JR, Milkowski AL, Boffetta P. 2012. Ingested nitrate and nitrite and stomach cancer risk: an updated review. Food Chem Toxicol 50(10):3646–3665, PMID: , 10.1016/j.fct.2012.07.062. [DOI] [PubMed] [Google Scholar]
- 51.Bartsch H, Ohshima H, Pignatelli B. 1988. Inhibitors of endogenous nitrosation. Mechanisms and implications in human cancer prevention. Mutat Res 202(2):307–324, PMID: , 10.1016/0027-5107(88)90194-7. [DOI] [PubMed] [Google Scholar]
- 52.Mergens WJ, Kamm JJ, Newmark HL, Fiddler W, Pensabene J. 1978. Alpha-tocopherol: uses in preventing nitrosamine formation. IARC Sci Publ 19:199–212, PMID: . [PubMed] [Google Scholar]
- 53.Mirvish SS. 1975. Blocking the formation of N-nitroso compounds with ascorbic acid in vitro and in vivo. Ann NY Acad Sci 258:175–180, PMID: , 10.1111/j.1749-6632.1975.tb29277.x. [DOI] [PubMed] [Google Scholar]
- 54.Mirvish SS. 1996. Inhibition by vitamins C and E of in vivo nitrosation and vitamin C occurrence in the stomach. Eur J Cancer Prev 5(suppl 1):131–136, PMID: , 10.1097/00008469-199609000-00027. [DOI] [PubMed] [Google Scholar]
- 55.Bidoli E, Talamini R, Zucchetto A, Bosetti C, Negri E, Lenardon O, et al. 2009. Dietary vitamins E and C and prostate cancer risk. Acta Oncol 48(6):890–894, PMID: , 10.1080/02841860902946546. [DOI] [PubMed] [Google Scholar]
- 56.Helzlsouer KJ, Huang HY, Alberg AJ, Hoffman S, Burke A, Norkus EP, et al. 2000. Association between alpha-tocopherol, gamma-tocopherol, selenium, and subsequent prostate cancer. J Natl Cancer Inst 92(24):2018–2023, PMID: , 10.1093/jnci/92.24.2018. [DOI] [PubMed] [Google Scholar]
- 57.McCann SE, Ambrosone CB, Moysich KB, Brasure J, Marshall JR, Freudenheim JL, et al. 2005. Intakes of selected nutrients, foods, and phytochemicals and prostate cancer risk in western New York. Nutr Cancer 53(1):33–41, PMID: , 10.1207/s15327914nc5301_4. [DOI] [PubMed] [Google Scholar]
- 58.Mastrangelo D, Pelosi E, Castelli G, Lo-Coco F, Testa U. 2018. Mechanisms of anti-cancer effects of ascorbate: cytotoxic activity and epigenetic modulation. Blood Cells Mol Dis 69:57–64, PMID: , 10.1016/j.bcmd.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 59.Vissers MCM, Das AB. 2018. Potential mechanisms of action for vitamin C in cancer: reviewing the evidence. Front Physiol 9:809, PMID: , 10.3389/fphys.2018.00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farag MA, Shakour ZTA, Elmassry MM, Donia MS. 2022. Metabolites profiling reveals gut microbiome-mediated biotransformation of green tea polyphenols in the presence of N-nitrosamine as pro-oxidant. Food Chem 371:131147, PMID: , 10.1016/j.foodchem.2021.131147. [DOI] [PubMed] [Google Scholar]
- 61.Reichard CA, Naelitz BD, Wang Z, Jia X, Li J, Stampfer MJ, et al. 2022. Gut microbiome-dependent metabolic pathways and risk of lethal prostate cancer: prospective analysis of a PLCO cancer screening trial cohort. Cancer Epidemiol Biomarkers Prev 31(1):192–199, PMID: , 10.1158/1055-9965.EPI-21-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fujita K, Matsushita M, Banno E, De Velasco MA, Hatano K, Nonomura N, et al. 2022. Gut microbiome and prostate cancer. Int J Urol 29(8):793–798, PMID: , 10.1111/iju.14894. [DOI] [PubMed] [Google Scholar]
- 63.Wilkins JR 3rd, Comstock GW. 1981. Source of drinking water at home and site-specific cancer incidence in Washington County, Maryland. Am J Epidemiol 114(2):178–190, PMID: , 10.1093/oxfordjournals.aje.a113181. [DOI] [PubMed] [Google Scholar]
- 64.Koivusalo M, Pukkala E, Vartiainen T, Jaakkola JJ, Hakulinen T. 1997. Drinking water chlorination and cancer–a historical cohort study in Finland. Cancer Causes Control 8(2):192–200, PMID: , 10.1023/a:1018420229802. [DOI] [PubMed] [Google Scholar]
- 65.Leavens TL, Blount BC, DeMarini DM, Madden MC, Valentine JL, Case MW, et al. 2007. Disposition of bromodichloromethane in humans following oral and dermal exposure. Toxicol Sci 99(2):432–445, PMID: , 10.1093/toxsci/kfm190. [DOI] [PubMed] [Google Scholar]
- 66.Landi S, Hanley NM, Warren SH, Pegram RA, DeMarini DM. 1999. Induction of genetic damage in human lymphocytes and mutations in Salmonella by trihalomethanes: role of red blood cells and GSTT1-1 polymorphism. Mutagenesis 14(5):479–482, PMID: , 10.1093/mutage/14.5.479. [DOI] [PubMed] [Google Scholar]
- 67.Plewa MJ, Muellner MG, Richardson SD, Fasano F, Buettner KM, Woo Y-T, et al. 2008. Occurrence, synthesis, and mammalian cell cytotoxicity and genotoxicity of haloacetamides: an emerging class of nitrogenous drinking water disinfection byproducts. Environ Sci Technol 42(3):955–561, PMID: , 10.1021/es071754h. [DOI] [PubMed] [Google Scholar]
- 68.Villanueva CM, Castaño-Vinyals G, Moreno V, Carrasco-Turigas G, Aragonés N, Boldo E, et al. 2012. Concentrations and correlations of disinfection by-products in municipal drinking water from an exposure assessment perspective. Environ Res 114:1–11, PMID: , 10.1016/j.envres.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 69.Komulainen H, Kosma VM, Vaittinen SL, Vartiainen T, Kaliste-Korhonen E, Lötjönen S, et al. 1997. Carcinogenicity of the drinking water mutagen 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone in the rat. J Natl Cancer Inst 89(12):848–856, PMID: , 10.1093/jnci/89.12.848. [DOI] [PubMed] [Google Scholar]
- 70.Egorov AI, Tereschenko AA, Altshul LM, Vartiainen T, Samsonov D, LaBrecque B, et al. 2003. Exposures to drinking water chlorination by-products in a Russian city. Int J Hyg Environ Health 206(6):539–551, PMID: , 10.1078/1438-4639-00244. [DOI] [PubMed] [Google Scholar]
- 71.Rothman KJ, Greenland S, Lash TL. 2008. Modern Epidemiology, Vol. 3. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins. [Google Scholar]
- 72.Wei Y, Qiu X, Yazdi MD, Shtein A, Shi L, Yang J, et al. 2022. The impact of exposure measurement error on the estimated concentration-response relationship between long-term exposure to PM2.5 and mortality. Environ Health Perspect 130(7):77006, PMID: , 10.1289/EHP10389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.