Abstract
The bioeconomy drives the development of life science and biotechnology as a blueprint for the future development of human society, and offers a cross‐cutting perspective on the societal transformation towards long‐term sustainability and the transition away from the non‐renewable economy. Moreover, the sustainable bioeconomy strategies are consistent with the United Nation's (UN) Sustainable Development Goals (SDG) and are becoming the centre of the achievement for SDG. The Chinese ‘14th Five‐Year Plan for Bioeconomy Development’ (2021–2025), including the development goals of China's bioeconomy containing biomedicine, agriculture, bio‐manufacturing and bio‐security as a strategic priority, is discussed. The plan offers three pathways to improve bioeconomy, including technological innovation, industrialisation and policy supports. Finally, it concludes China's first bioeconomy development plan as a success, suggesting the key role of industrial biotechnology in bioeconomy.
Keywords: 14th Five‐Year Plan for Bioeconomy Development, bioeconomy, biotechnology, China, next‐generation industrial biotechnology
This paper discusses the Chinese ‘14th Five‐Year Plan for Bioeconomy Development’ (2021–2025), including the development goals of China's bioeconomy containing biomedicine, agriculture, bio‐manufacturing and bio‐security as a strategic priority. The plan offers three pathways to improve bioeconomy including technological innovation, industrialisation and policy supports. China's first bioeconomy development plan as a success, suggesting the key role of industrial biotechnology in bioeconomy.
1. INTRODUCTION
How to reduce the consumption of non‐renewable resources while sustainably improving the living quality of human with economic development has become the top priority of the 21st century in all societies. Encouragingly, over 50 nations, including the European Union (EU), the United States (US), China, India, South Africa, etc., have reached a consensus to launch sustainable strategies based on bioeconomy driven by innovative life sciences and biotechnology‐related research and development (R&D) [1]. According to the estimates of current studies, around US$1–2 trillion of annual global investments for agriculture, green chemicals, biofuels, bioenergy, and biotechnological services are required in the next three decades, equal to 1.3%–2.6% of global gross domestic product (GDP) [2]. The sustainable bioeconomy strategies are consistent with the United Nations' (UN) Sustainable Development Goals (SDG) and are becoming the centre of the SDG achievement [3]. While the bioeconomy has opened new opportunities for innovation, job creation, and economic growth, it has brought challenges.
The concept of bioeconomy was first proposed as ‘bio‐based economy’ in the policy of ‘Developing and Promoting Biobased Products and Bioenergy’ issued by the United States [4]. Subsequently, bioeconomy becomes popular after the Organization for Economic Co‐operation and Development launched its 2004 report on Biotechnology for Sustainable Growth and Development [5], defining the bioeconomy as ‘an economy that uses renewable biological resources, efficient biological processes and ecological industrial clusters to produce sustainable bio‐based products, thus creating jobs and incomes’ [5]. Subsequently, the EU issued a report defining bioeconomy as a knowledge‐based bioeconomy, which transforms life science knowledge into new, sustainable, ecologically efficient and competitive products that can allow future societies to no longer rely solely on fossil fuels for energy and industrial feedstocks [6]. Moreover, the US government conisders bioeconomy in a report entitled ‘the National Bioeconomy Blueprint’ as an economic form based on the application of bioscience research and innovation to create economic activities and public welfare benefits [7]. Recently, the Chinese government announced a policy entitled ‘14th Five‐Year Plan for Bioeconomy Development’ and defined the bioeconomy as ‘based on the protection, development and utilization of bioresources, to drive the development of life science and biotechnology for promoting a blueprint for the sustainable development of human society’ [8].
As a country with rich bioresources and large biological product consumption market, China has a strong domestic market with a full industrial system. Therefore, its bioeconomy development plan has attracted worldwide attention due to its large market. Statistically, the biotechnology industry has contributed RMB2 trillion to China's bioeconomy by 2011 [9], and it was reported that China's bioeconomy maintained an annual 20% growth from 2013 to 2015 [9]. Meanwhile, the Chinese government has been investing US$3.8 billion over the period of 2008–2020 in biotechnology R&D [10]. The bioeconomy has become one of the pillar industries of China over the decade.
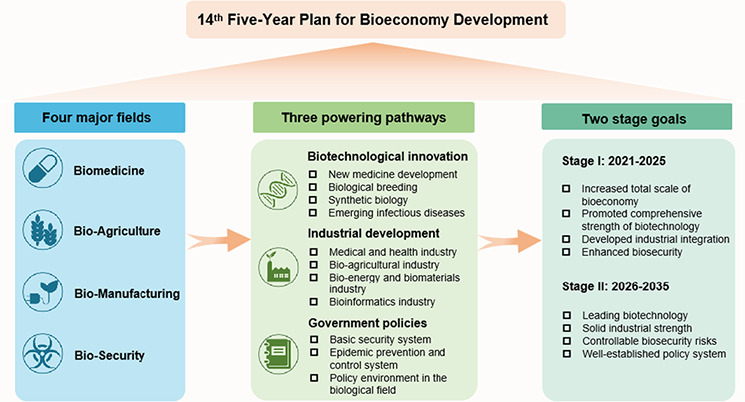
This paper focuses on the Chinese ‘14th Five‐Year Plan for Bioeconomy Development’ (2021–2025), outlining the development goals of China's bioeconomy in four major fields, including biomedicine, bio‐agriculture, bio‐manufacturing and bio‐security as a strategic priority (Figure 1). Three pathways are offered to improve the development of bioeconomy, including innovation, industrial development and government policy supporting. Finally, it concludes that the China's first bioeconomy development plan was a success and proposes industrial biotechnology as a key for bioeconomy development.
FIGURE 1.
Graphic scheme of China 14th Five‐Year Plan for Bioeconomy Development (2021–2025).
2. DEVELOPMENT GOALS OF CHINESE BIOECONOMY
To advance the Chinese bioeconomy during the 14th Five‐Year Plan period (2021–2025), the National Development and Reform Commission of China interprets the key areas in the plan: strengthening the innovation foundation of bioeconomy, cultivating the bioeconomy industry, especially the biomanufacturing industry, and achieving the high‐quality development of bioeconomy. According to the plan, China will take steps to accelerate the developments of healthcare, agriculture, bioenergy, bio‐environmental protection and bioinformatics. The two‐stage goals can be summarised as follows:
Stage I: (2021–2025)
By 2025, Chinese bioeconomy should be increased in its scale with enhanced comprehensive strength in science and technology combined with industrial integration and enhanced bio‐security. The bioeconomy will protect and use biological resources, deeply integrating medicine, healthcare, agriculture, forestry, energy, environmental protection, materials, and other sectors so as to allow bioeconomy to become a key driving force for high‐quality developments.
Stage II: (2026–2035)
By 2035, China is set to be at the forefront in the world in terms of the comprehensive strength of its bioeconomy. In details, China will basically establish a new stage of development featuring advanced technology, strong bioindustries, extensive integration and applications, strong resource support, controllable bio‐risks, and complete institutional R&D systems.
3. FOUR MAJOR FIELDS POWERING THE BIOECONOMY
3.1. Biomedicine
To meet the new challenges of switching ‘treatment‐centered medicine’ to ‘health‐centered medicine’, the ‘14th Five‐Year Plan for Bioeconomy Development’ has set a high expectation for biomedicine (Figure 2), especially the uses of principles of biology, biochemistry and genetic engineering to develop new drugs and medical equipments.
FIGURE 2.
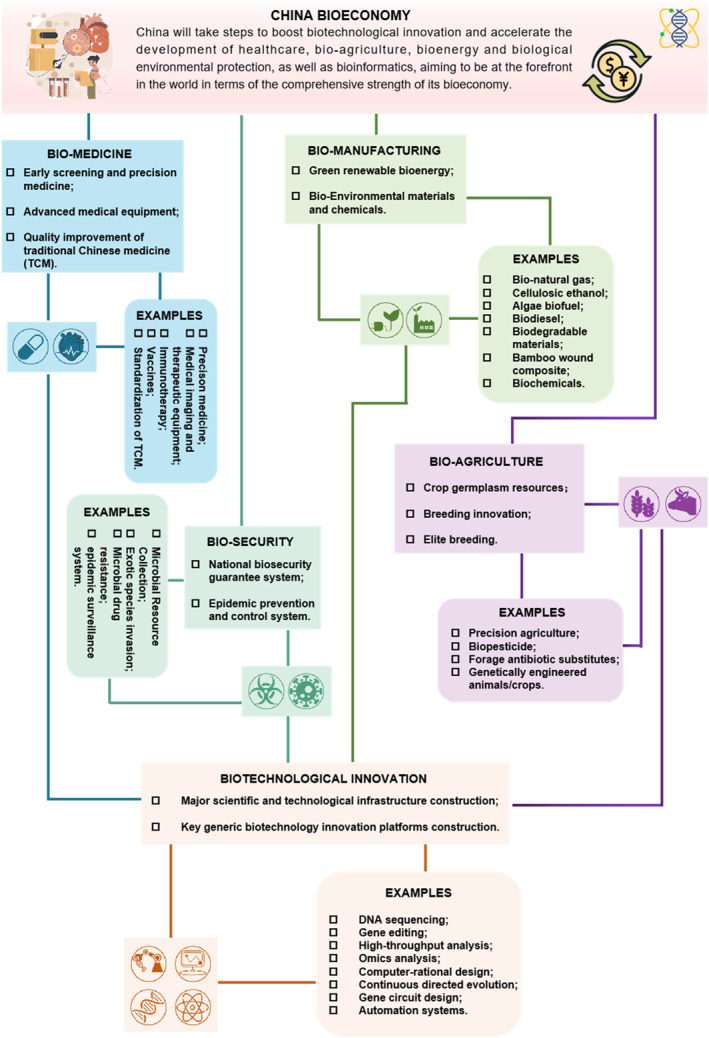
The roadmap of China bioeconomy: examples falling under the bioeconomy definition from the ‘14th Five‐Year Plan for Bioeconomy Development’ Report.
It reports that the Chinese biomedical market, as one of the most promising ones in the global pharmaceutical areas, is expected to exceed RMB 800 billion in 2025 with an annual growth rate of more than 20% [11]. In 2020, the sales of the biological drug market accounted for approximately 18.6% of the entire medical market [11]. According to the ‘2021 Drug Evaluation Report’ released by National Medical Product Administration in June 2022, 47 innovative drugs were reviewed and approved in 2021, setting a new high record [12]. In July 2022, a total of 57,398 new biomedical companies were established. The top 10 provinces, including Guangdong, Shandong, Henan, Jiangsu, Beijing, Zhejiang, Anhui, Shanghai, Hubei, and Jiangxi, account for 62.94% of the country's new biomedical enterprises [13].
Because of the COVID‐19 pandemic, vaccine research and production in China have significantly improved in recent years. The National Institutes for Food and Drug Administration reported the production a total of 810 million doses of vaccines in 2020, a year‐on‐year increase of 23% [14]. Main biomedical companies in China, including Beijing Institute of Biological Products, Sinovac Life Sciences, CanSino Biologics, and Wuhan Institute of Biological Products, can now produce over 5.5 billion doses of COVID‐19 vaccines each year [14].
Increasing ageing population in China will drive the increasing demand for cancer treatment drugs, as the population aged 65 and above in China has reached 200.56 million, an increase of 3.34 million over 2020, accounting for 14.2% of the total population in 2021 [15].
Chimeric antigen receptor T cell (CAR‐T) immunotherapy is a new type of cell therapy that has developed rapidly in recent years and has achieved successes in the treatment of various haematological tumours. Different from traditional cancer treatment drugs, CAR‐T cell therapy mainly uses T cells to activate the body's natural host defence mechanism, thus specifically recognising and killing tumour cells [16]. According to the Frost & Sullivan report, the market size of Chinese cellular immunotherapy products is expected to increase from RMB 1.3 billion to 10.2 billion from 2021 to 2023 with an annual growth rate of 181.5%. In 2021, the first and second CAR‐T drugs produced by Fosun Kite and JW Therapeutics were approved by National Medical Products Administration for clinical usages.
New biomedicines are important to implement the Healthy China strategy, is of great significance for addressing the challenges of population ageing and protecting people's lives and health.
3.2. Bio‐agriculture
In response to the new trends of ‘solving subsistence’ to ‘nutrition diversification’, the development of agriculture to modernising agricultures and the meeting of people's new expectations for higher levels of food consumptions are the priority (Figure 2). The ‘14th Five‐Year Plan’ for the development of bioeconomy puts forward the goal of focussing on the modernising bio‐agriculture, which is the agricultural industrial system based on biological principles relying on various biological processes to maintain soil fertility for crop nutrition, and establishment of an effective biological system to prevent weeds and pests. At present, bio‐agriculture mainly includes five main areas, including genetically engineering crops, animal vaccine, bio‐feeds, non‐chemical pest control and bio‐pesticides [17]. Among them, genetically engineering crops have become one of the fastest growing, most widely used and most promising area. Genetically modified (GM) breeding approaches use one or more foreign genes integrated into the genome of plant species via modern molecular biology techniques to improve plant characteristics [17]. Transgenic plant breeding is the fastest growing area of agricultural biotechnology applications during the period of late 20th and early 21st centuries. Since the first GM crop became available in 1996, the cumulative areas cultivated with GM‐bred crops worldwide reached 2.15 billion hectares by 2017, including 1.04 billion hectares of GM soybeans, 640 million hectares of GM maize, 340 million hectares of GM cotton, and 130 million hectares of GM oilseed rape [18].
These GM crops have provided foods, feeds, fibres, and fuels to 7.6 billion people worldwide with benefits of substantial agricultures, environments, economy, health, and social stability to farmers [18]. Transgenic animal breeding technology has also shown promises in improving livestock production traits, disease resistance, and the production of unconventional livestock products. In 1997, the birth of Dolly, the cloned sheep, was of great significance for the application of cloning technology to reproduce livestock species [19]. Transgenic cows are dairy breeds in which scientists have GM cow embryos using transgenic technology to achieve the desired effects. The existing reported transgenic dairy breeds include transgenic cows that produce breast milk‐like product with antibacterial and antiviral effects, cows with protection against bovine tuberculosis, ‘low lactose cows’, and cloned cows with trans‐human defensin genes [20]. In 2009, recombinant human antithrombin III (ATryn) was approved to enter the U.S. market, and transgenic animal pharmaceuticals are gradually becoming commercialised. Currently, more than 40 valuable pharmaceutical proteins, such as human serum albumin, long‐acting tissue fibrinogen activation (TFA), and human haemoglobin, can be produced from the mammary glands of transgenic animals. It brings revolutionary changes to human medicine and health, biomaterials, and other biofields [21]. Transgenic animal breeding technology will also become one of the revolutionary ways to breed new animal species [20].
The animal vaccine industry has been strongly supported by the national industrial policy and is a key direction encouraged by the State Administration. Animal vaccine developments include research, application, quality inspection, and the latest progress of vaccines for Class A diseases, vaccines for multiple animal co‐morbidities, vaccines for cattle diseases, vaccines for sheep diseases, vaccines for horse diseases, vaccines for swine diseases, vaccines for poultry diseases, and vaccines for other animal diseases et al. [21]. Biofeeds refer to the feed products developed through bio‐engineering technologies, such as fermentation processes, enzyme engineering, protein engineering and genetic engineering, using feed ingredients and additives allowed by the relevant national regulations [22]. L‐threonine and L‐lysine can effectively promote the growth of livestock and poultry, improve meat quality, and increase nutritional values [23]. Therefore, the addition of feed‐grade amino acids is of greater significance to the development of pastoral farming and the improvement of people's living standards [24].
In addition, biopesticide refers to the use of living organisms including fungi, bacteria, insect viruses, GM organisms, natural predators, etc., or their metabolites of pheromones, growth factors, sodium naphthalene acetate, etc. against agricultural pests [25]. China has more than 260 biopesticide manufacturers, accounting for about 10% of the country's pesticide manufacturers, with an annual output of nearly 130,000 tons of biopesticide formulations, the annual output values approximately RMB 3 billion, accounting for about 9% of the total output values of pesticides [26]. Presently, the types of biological pesticides in China have been commonly used including microbial pesticides, agricultural antibiotics, plant‐derived pesticides, biochemical pesticides and natural enemy insect pesticides, plant growth regulator‐type pesticides and other six major types, and several other biological pesticides [25]. Therefore, the development of bio‐agriculture helps grow the bioeconomy especially the agricultural ones.
3.3. Bio‐manufacturing
In line with the new trend of ‘the pursuit of production capacity and efficiency’ to ‘adhere to the ecological priority’, the alternative development on green and low‐carbon biomasses is required to meet the new sustainable mode of production (Figure 2). Biomass energy is an important renewable energy source with basic characteristics of green, low‐carbon, and recycling demands. As an important way to build a low‐carbon ecological system in rural areas, the biomass power industry is of great significance in promoting the transformation of agricultural green development, increasing employment of rural labours, recycling bioresources, solving rural environmental pollution, and exploring potential negative greenhouse gas emission technologies [27]. Under the goal of ‘double carbon reduction strategy’, a high proportion of renewable energy has become one of the basic directions of energy transformation. As the most important renewable energy source, biomass is commonly used in many areas, such as agriculture fertilisers, feeds or power generation for heat and natural gas supplies, as well as synthetic biomaterials for constructions.
Biomass resources come from a wide range of sources, including agricultural wastes, such as wood and forest wastes, urban organic wastes, algae biomass and energy crops, as well as corn stalk, etc. [27] with the annual output of approximately 3.5 billion tons. Among them, 694 million tons of straws, 1.868 billion tons of livestock and poultry manures, and 350 million tons of forestry residues can be collected. By the end of 2020, China has invested in building 30 million kilowatts of installed capacity of biomass power generation, providing more than 110 billion kilowatt hours of clean electricity annually. The clean heating area of biomass exceeds 300 million m2. More than 7700 large‐scale biogas projects have been completed, with an annual gas production capacity of 1.37 billion cubic metres, supplying more than 478,000 households with natural gas [28]. Based on the Renewable Energy Law, China has established a system to support the development of biomass energy industry [29]. Various types of biomass energy industry development policies have been introduced, allowing the industry to achieve rapid developments. Among them, biomass power, together with hydropower, wind power, and solar power are listed as the four major renewable power generation industries in China [30]. In addition to power generation, productions of heats, liquid fuels, and solid pellet fuels have also been explored. The utilisation of biomass energy is an important element to promote the revolution of energy production and consumption and an important task to improve environmental quality and circular economy [27].
3.4. Bio‐security
Considering the new trend of changing from ‘passive defence’ to ‘active protection’, it is necessary to strengthen the construction of the national biosafety for risk prevention and governance system, meeting people's new expectations for better bio‐security (Figure 2). Bio‐safety refers to a country's effective prevention and response to the threat of dangerous biological factors and related factors, healthy development of biotechnology, people, and ecosystems being in a state relatively free from dangers and threats and the biological factors that have the ability to maintain national security and sustainable developments [31]. At present, bio‐security includes seven specific aspects: prevention and control of major new infectious diseases, animal and plant epidemics; research, development, and application of biotechnology; bio‐security management of pathogenic microorganism laboratories; the safety management of human genetic resources and biological resources; prevention of alien species invasion and protection of biodiversity; overcoming microbial drug resistance; preventing threats of biological terrorists and biological weapons. Bio‐security is of great importance both for individuals or countries, especially under the background of the occurrence of biological threat in the ‘global village’ with the rapid development of biotechnology [32]. Data from the 2019 China Ecological Environment Status Bulletin shows over 660 alien invasive species in China, such as Mikania micrantha, Alternanthera philoxeroides, Solidago canadensis, American white moth, etc [33]. Water hyacinth is a serious alien invasive species in Dianchi Lake, Kunming. Data shows that the direct economic loss caused by water hyacinth in China is nearly 10 billion RMB every year, and it costs 500 million to 1 billion yuan to treat the invasive species [33].
Bio‐security is not only an important part of national security, but also related to the common security of people all over the world. For example, the outbreak of the Ebola epidemic caused an impact on socioeconomics loss of $53 billion across West Africa from 2013 to 2016, plummeting Sierra Leone's GDP in 2015 by 20% and that of Liberia by 8% between 2013 and 2014 [34]. The Omicron variant of COVID‐19 has caused economic damages to the world due to the mandatory national lockdown and border closures [35]. According to the 2022 Global Risk Report, ‘infectious diseases’ rank the sixth among the top 10 global risks in the next decade and are also considered as one of the most important global short‐term risks [36]. To maintain bio‐safety, it is required to adhere to the principles of people‐oriented, risk prevention, classified management, and coordination with the guidance of the overall national security concept [31]. As the world's second largest economy and a responsible country, China is willing to contribute its strength and wisdom to establish the human security community on the basis of pursuing collaboration and common interests. Every coin has two sides, with the development of biotechnologies, bioeconomy has developed rapidly. However, the associated problems have gradually surfaced, especially a large number of direct or indirect operations involving genetic materials, such as DNA and RNA, which have caused serious public concerns [37]. Bioeconomy must balance biosafety and development.
4. THREE PATHWAYS FOR IMPROVING THE DEVELOPMENT OF BIOECONOMY
4.1. Biotechnological innovation
Adhering to the purpose of ‘innovating biotechnology and serving the people’ with cutting‐edge science and technology, China has gradually established a complete set of platforms based on molecular biology, with many Research and Development Centres for life science, which helps strengthen innovation and promote applications in life science and medical healths (Figure 2). At present, high‐throughput DNA and protein sequencing and gene editing have well developed in China [38]. Among DNA sequencing technologies, high‐throughput sequencing technology (NGS) has become the mainstream sequencing method in the current commercial market because of its high throughput, fast detection speed, convenience, and sensitivity that can sequence hundreds of thousands to millions of nucleic acid molecules at a short period of time [39]. It digests the genome into short fragments with more than 100 bases, sequencing the short fragments, then splicing the sequences, and finally obtaining sequence information. However, the accuracy of this technology is low, and the sequencing results should be corrected to improve the accuracy of sequencing [39]. The next‐generation sequencing for diagnosis and treatment technology allows precise high‐throughput sequencing be applied to precision medicine [40].
Gene editing is a new and relatively accurate genetic engineering technology that can modify specific target genes in the genome of organisms. Gene editing tools include homologous recombination, uses of deoxyribonuclease, zinc finger nucleus, transcriptional activator‐like effector nucleus, and the CRISPR/Cas system most commonly used for precise gene editing [41]. It is based on short RNA sequences as specific recognition units and then activated to break double‐stranded DNA at the target location. CRISPR/Cas9, in particular, only needs to synthesise a new RNA to achieve gene editing [42]. Its simple design and easy procedure are the greatest advantages for regulating and observing genomic functional changes, allowing genetic research, gene therapy, and functional genetic improvements [43].
High‐throughput screening technology refers to a system based on experimental methods at the molecular and cellular levels using microplates as the carrier of experimental tools to implement the selection process with an automated detection system; it collects experimental results with sensitive and fast detection sensors to analyse and process experimental data using computers to screen thousands to millions of samples in a short period of time [44]. The advantage of high‐throughput drug screening permits the search of a wide range of natural and/or synthesis compounds for their biomedical activity as drugs, especially for screening Chinese traditional medicines that can be extracted and purified from plants [45].
4.2. Industrial development
To cultivate and expand the pillar industries of the bio‐economy, the Chinese government will promote the development of the medical and health industry, bio‐agricultural industry, bio‐energy, bio‐environmental protection industry, and bio‐informatics ones during the ‘14th Five‐Year Plan’ period. The biopharmaceutical industry accounts for more than 70% of the total bioindustry in China. It is expected that during the ‘14th Five‐Year Plan’ period, the average annual growth rate of biomedicine and related industries in China will reach approximately 10% to become the core of bioeconomic development [46].
During the ‘14th Five‐Year Plan’ period, the applied omics of agricultural biology, the new generation of biological breeding technology, the prevention and treatment technology of major animal and plant diseases, and the technology of new agricultural biological products will be further developed. In the bio‐energy and bio‐environmental protection areas, environmental protection and pollution prevention will be strengthened based on new biotechnology. More biomass energy will be used to replace traditional fossil energy [8].
As problems such as environmental pollution and global warming are becoming increasingly serious, it is of great significance to develop an environmentally friendly and sustainable bio‐manufacturing industry. For example, biodegradable plastics can be used instead of traditional chemically synthesised ones to reduce microplastic pollution [47]. Polyhydroxyalkanoates (PHA) with biodegradability, biocompatibility, their structure and property diversity will be used as bulk plastics and/or medical materials [48] as they will be produced conveniently using the next‐generation industrial biotechnology based on extremophilic microorganisms. Synthetic and systems biology, metabolic engineering, and new processing technology will be used for low‐cost production of chemicals, materials, fuels, proteins, and medicines [49].
4.3. Government policies supports
Based on the pattern of biotechnology development, the government will publish policies to deepen the reform of technological innovation, industry supervision, market applications to increase the capital investments, technological innovations, talents education, international collaborations, and acceleration of the formation of a policy environment conducive to the innovative development of the bioeconomy (Table 1). Firstly, the government should improve market accessibility and increase market demand, especially in fields related to drugs, medical devices, new food raw materials, and additives. In 2000, the ‘1035 Plan’ was implemented by the Ministry of Science and Technology, aiming in promoting the R&D of new medicinal entities. The ‘Medical Science and Technology Policy (2002010)’, ‘Bio‐Industry Development Eleventh Five‐Year Plan’, and ‘Made in China 2025’ are all geared towards supporting and expanding the capability of the biopharmaceutical landscapes [50]. With all these policies, innovative drugs are advancing by leaps and bounds, and the bioindustrial groups are gradually growing. Chinese biopharmaceutical market is growing year by year from 2010 to 2017 [51]. Secondly, the government should strengthen intellectual property protection, improve the management system of access to biological genetic resources and information sharing, and most importantly, promote the transformation of intellectual property in daily applications.
TABLE 1.
China's legislation and regulations of biotechnology: 2012–2022
| Year | Title | Fields | Main contents |
|---|---|---|---|
| 2016 | Guiding Opinions on Promoting the Healthy Development of the Pharmaceutical Industry | Biomedicine |
|
| 2016 | Pharmaceutical Industry Development Planning Guide |
|
|
| 2017 | Guidelines for Research and Evaluation of Cell Therapy Products |
|
|
| 2018 | Guidelines for the clinical application of new antitumour drugs |
|
|
| 2018 | Opinions on Reforming and Improving the Vaccine Management System |
|
|
| 2019 | Vaccine Administration Law of the People's Republic of China |
|
|
| 2019 | Drug Administration Law of the People's Republic of China |
|
|
| 2020 | Pharmacopoeia of the People's Republic of China |
|
|
| 2020 | Technical guidelines for the research and development of vaccines for the prevention of novel coronavirus |
|
|
| 2021 | The 14th Five‐Year Plan for the Development of the Biopharmaceutical Industry |
|
|
| 2022 | The 14th Five‐Year Plan for the Development of the Pharmaceutical Industry |
|
|
| 2016 | Seed Law of the People's Republic of China | Bio‐agriculture |
|
| 2019 | Issues production safety certificates to genetically modified maize and soybean |
|
|
| 2021 | Issues production safety certificates to genetically modified maize and soybean |
|
|
| 2022 | National standard for the approval of genetically modified soybean varieties |
|
|
| 2022 | National standard for the approval of genetically modified maize varieties |
|
|
| 2013 | Provisions on Application and Acceptance of New Food Raw Materials | Bio‐manufacturing |
|
| 2015 | Opinions of the State Council on Reforming the Examination and Approval System of Pharmaceutical and Medical Devices |
|
|
| 2021 | Notice of Jiangsu Provincial Government on Printing and Distributing Several Policies and Measures to Promote the High Quality Development of the Provincial Biomedical Industry |
|
|
| 2022 | Opinions of the National Energy Administration on Improving the System, Mechanism and Policy Measures for Green and Low Carbon Energy Transformation |
|
|
| 2020 | Bio‐security Law of the People's Republic of China | Bio‐security |
|
| 2021 | Notice on Printing and Issuing the Work Plan for Further Strengthening the Prevention and Control of Alien Species Invasion |
|
|
| 2022 | Issues Guide for Bio‐security Measurement of Gene Edited Crops |
|
In June 2008, the State Council issued the National Intellectual Property Strategy Outline (2021–2035). Under the guidance of this Strategy Outline, China has improved the legal system on intellectual property protection by vigorously strengthening relevant law enforcements, protecting intellectual property rights, and combating the infringement of intellectual property rights [52]. Thirdly, the government should further increase financial supports for bio‐innovations, making full use of relevant financial funds at all levels to support the bioeconomy. For example, in September 2021, Jiangsu Province published policies to promote the high‐quality development of biomedical industry, offering financial supports to researches on innovative drugs and medical devices [53]. Considering local characteristics, in December 2020, Kunming government provides financial subsidies for traditional Chinese medicine planting, processing, and many other relevant works [54].
Lastly, as a responsible country, China has actively participated in global public health governance and will do so in the future. China will work with the international community to address the increasingly bio‐security challenges and strengthen bilateral and multilateral cooperations and exchanges in bio‐security policy formulation, risk assessment, emergency responses, information sharing, capacity building, etc.
In 1963, China sent the first medical team to Algeria, opening the door to international health assistances [55]. In 2015, China put forward the idea of the ‘Healthy Silk Road’, an important practice of building a community of human health [56]. The Chinese government will further provide financial support services, cultivate more biotechnology talents, and develop new policies in specific areas. All these done by China's government will benefit the formation of a friendly policy environment to the innovative development of bioeconomy.
5. SUMMARY AND CONCLUSIONS
Under the ‘14th Five‐Year Plan’, China will take steps to promote innovative bioeconomy, accelerating the development of healthcare, bio‐agriculture, bioenergy, environmental protection, and bioinformatics, improving the bio‐risk control, prevention, and governance system, creating a better environment for the innovative bioeconomy. This article discusses the two‐stage goals of China's bioeconomy, with four major fields powering the development of bioeconomy, including biomedicine, bio‐agriculture, bio‐manufacturing, and bio‐security as the strategic priority. Finally, three pathways for biotechnological innovation, industrial development, and government policy supports will help improve the development of bioeconomy in the following 5 years. In conclusion, the new plan will further boost innovation and foster high‐quality development to build a modern innovative ecosystem deeply integrated into the industrial and supply chains, promoting intelligent and green development of the bioeconomy industry.
AUTHOR CONTRIBUTIONS
Xu Zhang: Conceptualization; Investigation; Methodology; Resources; Software; Visualization; Writing – original draft. Cuihuan Zhao: Data curation; Investigation; Writing – original draft. Ming‐Wei Shao: Data curation; Investigation; Writing – original draft. Yi‐Ling Chen: Data curation; Investigation; Writing – original draft. Puyuan Liu: Formal analysis; Validation; Writing – review and editing. Guo‐Qiang Chen: Funding acquisition; Project administration; Supervision; Writing – review and editing.
CONFLICT OF INTEREST
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
ACKNOWLEDGEMENTS
This work was financially supported by grants from the Ministry of Science and Technology of China (Grant No. 2018YFA0900200), National Natural Science Foundation of China (Grant No. 21761132013; No. 31870859; No. 92068117), Tsinghua University‐INDITEX Sustainable Development Fund (Grant No. TISD201907), Postdoctoral Science Foundation of China (Grant No. 2022M721810), and Center of Life Sciences of Tsinghua‐Peking University. This project was also funded by the National Natural Science Foundation of China (Grant No. 31961133017, No. 31961133018, No. 31961133019). These grants are part of MIX‐UP, a joint NSFC and EU H2020 collaboration. In Europe, MIX‐UP has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 870294.
Zhang, X. , et al.: The roadmap of bioeconomy in China. Eng. Biol. 6(4), 71–81 (2022). 10.1049/enb2.12026
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Svazas, M. , et al.: Sustainable supply chain of the biomass cluster as a factor for preservation and enhancement of forests. J. Int. Stud. 12(2), 309–321 (2019). 10.14254/2071-8330.2019/12-2/20 [DOI] [Google Scholar]
- 2. Kircher, M. : Bioeconomy: markets, implications, and investment opportunities. Economies 7(3), 73 (2019). 10.3390/economies7030073 [DOI] [Google Scholar]
- 3. El‐Chichakli, B. , et al.: Policy: five cornerstones of a global bioeconomy. Nature 535(7611), 221–223 (2016). 10.1038/535221a [DOI] [PubMed] [Google Scholar]
- 4. Administration of William J. Clinton. https://www.govinfo.gov/content/pkg/WCPD‐1999‐08‐16/pdf/WCPD‐1999‐08‐16‐Pg1623.pdf. (1999). Accessed Oct 2022
- 5. ‘Biotechnology for sustainable growth and development’, Organization for Economic Co‐operation and Development. https://www.oecd.org/science/emerging‐tech/33784888.pdf Accessed Oct 2022
- 6. Albrecht, J. , et al.: The knowledge based bio‐economy (KBBE) in Europe: achievements and challenges. https://www.researchgate.net/publication/315752017_The_Knowledge_Based_Bio-Economy_KBBE_in_Europe_Achievements_and_Challenges?channel=doi%26linkId=58e217db4585153bfe9a3a1b%26showFulltext=true. (2010). Accessed Oct 2022
- 7. House, T.W. : National bioeconomy blueprint. Ind. Biotechnol. 8(3), 97–102 (2012). 10.1089/ind.2012.1524 [DOI] [Google Scholar]
- 8. 14th Five‐Year Plan for Bioeconomy Development (In Chinese). https://www.ndrc.gov.cn/xxgk/zcfb/ghwb/202205/P020220510324220702505.pdf. (2022). Accessed Oct 2022
- 9. The development plan for the biological industry (In Chinese). http://www.gov.cn/zhengce/content/2013‐01/06/content_2754.htm. (2012). Accessed Oct 2022
- 10. Xiao, Z. , Kerr, W.A. : Biotechnology in China‐regulation, investment, and delayed commercialization. GM Crops Food 13(1), 86–96 (2022). 10.1080/21645698.2022.2068336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Report of market perspective and investment strategy planning on China biomedicine industry (2022–2027) (In Chinese). https://bg.qianzhan.com/report/detail/8647b9d794294177.html. (2022). Accessed Oct 2022
- 12. 2021 Drug Evaluation Report (In Chinese). https://www.nmpa.gov.cn/xxgk/fgwj/gzwj/gzwjyp/20220601110541120.html. (2022). Accessed Oct 2022
- 13. The growth trend of newly added biomedical enterprises in key areas (In Chinese). https://www.ndrc.gov.cn/fgsj/tjsj/cxhgjscyyx/202208/t20220826_1333852.html?code=%26state=123. (2022). Accessed Oct 2022
- 14. Annual report on drug supervision and administration statistics (2020) (In Chinese). https://www.nmpa.gov.cn/directory/web/nmpa/images/1624869232805095741.pdf. (2021.) Accessed Oct 2022
- 15. Wang, P.P. : Population growth and steady improvement in urbanization (In Chinese). http://www.stats.gov.cn/xxgk/jd/sjjd2020/202201/t20220118_1826609.html. (2022). Accessed Oct 2022
- 16. Majzner, R.G. , Mackall, C.L. : Clinical lessons learned from the first leg of the CAR T cell journey. Nat. Med. 25(9), 1341–1355 (2019). 10.1038/s41591-019-0564-6 [DOI] [PubMed] [Google Scholar]
- 17. Yang, H. : Bio‐agriculture: urgent need for policies to help international competition (In Chinese). China Invest. Mag. 9, 56–58 (2009) [Google Scholar]
- 18. Brief 53: global status of commercialized biotech/GM crops: 2017. https://www.isaaa.org/resources/publications/briefs/53/default.asp. (2018). Accessed Oct 2022
- 19. Wilmut, I. , et al.: Viable off spring derived from fetal and adult mammalian cells. Nature 385(6619), 810–813 (1997). 10.1038/385810a0 [DOI] [PubMed] [Google Scholar]
- 20. Park, D.S. , et al.: Current status of production of transgenic livestock by genome editing technology. J. Anim. Reprod. Biotechnol. 34(3), 148–156 (2019). 10.12750/jarb.34.3.148 [DOI] [Google Scholar]
- 21. Xie, H.H. , Wang, G.X. : Strength and weakness of animal vaccine industry in China. Asian Agric. Res. 2(4), 27–30 (2010) [Google Scholar]
- 22. Park, J.H. , Lee, S.Y. : Metabolic pathways and fermentative production of L‐aspartate family amino acids. Biotechnol. J. 5(6), 560–577 (2010). 10.1002/biot.201000032 [DOI] [PubMed] [Google Scholar]
- 23. Fazius, F. , Zaehle, C. , Brock, M. : Lysine biosynthesis in microbes: relevance as drug target and prospects for β‐lactam antibiotics production. Appl. Microbiol. Biotechnol. 97(9), 3763–3772 (2013). 10.1007/s00253-013-4805-1 [DOI] [PubMed] [Google Scholar]
- 24. Toghyani, M. , et al.: Amino acid requirements for laying hens: a comprehensive review. Poultry Sci. 100(5), 101036 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chandler, D. , et al.: The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366(1573), 1987–1998 (2011). 10.1098/rstb.2010.0390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tian, T. , et al.: Commercialization and regulatory requirements of biopesticides in China. In: Singh, H.B. , Sarma, B.K. , Keswani, C. (eds.) Agriculturally Important Microorganisms, pp. 237–254. Springer, Singapore: (2016) [Google Scholar]
- 27. He, J. , Zhu, R. , Lin, B. : Prospects, obstacles and solutions of biomass power industry in China. J. Clean. Prod. 237, 117783 (2019). 10.1016/j.jclepro.2019.117783 [DOI] [Google Scholar]
- 28. 3060 Zero‐carbon Biomass Energy Development Potential Blue Book (In Chinese). The Biomass Industry Branch of the China Industrial Development Promotion Association. http://www.cn‐bea.com/filedownload/394003, Accessed Oct 2022 [Google Scholar]
- 29. Renewable Energy law of the People's Republic of China (In Chinese). http://www.nea.gov.cn/2017-11/02/c_136722869.htm. (2017). Accessed Oct 2022
- 30. Guidelines on building a modern environmental governance system (In Chinese). http://www.gov.cn/zhengce/2020-03/03/content_5486380.htm. (2020). Accessed Oct 2022
- 31. Bio‐security law of the People's Republic of China (In Chinese). http://www.gov.cn/xinwen/2020‐10/18/content_5552108.htm. (2020). Accessed Oct 2022
- 32. Cao, C. : China's evolving biosafety/biosecurity legislations. J. Law Biosci. 8(1), lsab020 (2021). 10.1093/jlb/lsab020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bulletin on the state of China's ecological environment (2019) (In Chinese). https://www.mee.gov.cn/hjzl/sthjzk/zghjzkgb/202006/P020200602509464172096.pdf. (2020). Accessed Oct 2022
- 34. Nnaji, N.D. , et al.: The deuce‐ace of Lassa Fever, Ebola virus disease and COVID‐19 simultaneous infections and epidemics in West Africa: clinical and public health implications. Trop. Med. Health 49(1), 1–11 (2021). 10.1186/s41182-021-00390-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Im, A. , et al.: A critical analysis of the impacts of COVID‐19 on the global economy and ecosystems and opportunities for circular economy strategies. Resour. Conserv. Recycl. 164, 105169 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Climate Failure and Social crisis Top Global Risks 2022. World Economic Forum, Geneva. https://www.marshmclennan.com/content/dam/mmc-web/insights/publications/2022/global-risks-report-2022/global-risks-report-2022-press-release.pdf. (2022). Accessed Oct 2022 [Google Scholar]
- 37. Waltz, M. , et al.: The view from the benches: scientists' perspectives on the uses and governance of human gene‐editing research. CRISPR J 4, 609–615 (2021). 10.1089/crispr.2021.0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun, W. , Wang, H. : Recent advances of genome editing and related technologies in China. Gene Ther. 27(7), 312–320 (2020). 10.1038/s41434-020-0181-5 [DOI] [PubMed] [Google Scholar]
- 39. Reuter, J.A. , Spacek, D.V. , Snyder, M.P. : High‐throughput sequencing technologies. Mol. Cell 58(4), 586–597 (2015). 10.1016/j.molcel.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schmidt, B. , Hildebrandt, A. : Next‐generation sequencing: big data meets high performance computing. Drug Discov. Today 22(4), 712–717 (2017). 10.1016/j.drudis.2017.01.014 [DOI] [PubMed] [Google Scholar]
- 41. Maeder, M.L. , Gersbach, C.A. : Genome‐editing technologies for gene and cell therapy. Mol. Ther. 24(3), 430–446 (2016). 10.1038/mt.2016.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ran, F. , et al.: Genome engineering using the CRISPR‐Cas9 system. Nat. Protoc. 8(11), 2281–2308 (2013). 10.1038/nprot.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hsu, P.D. , Lander, E.S. , Zhang, F. : Development and applications of CRISPR‐Cas9 for genome engineering. Cell 157(6), 1262–1278 (2014). 10.1016/j.cell.2014.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zeng, W. , et al.: High‐throughput screening technology in industrial biotechnology. Trends Biotechnol. 38(8), 888–906 (2020). 10.1016/j.tibtech.2020.01.001 [DOI] [PubMed] [Google Scholar]
- 45. Zhu, Y. , et al.: High throughput screening for bioactive components from traditional Chinese medicine. Comb. Chem. High Throughput Screen. 13(10), 837–848 (2010). 10.2174/138620710793360257 [DOI] [PubMed] [Google Scholar]
- 46. Schmid, R.D. , Xiong, X. : Biotech in China 2021, at the beginning of the 14th five‐year period (“145”). Appl. Microbiol. Biotechnol. 105(10), 3971–3985 (2021). 10.1007/s00253-021-11317-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lambert, S. , Wagner, M. : Environmental performance of bio‐based and biodegradable plastics: the road ahead. Chem. Soc. Rev. 46(22), 6855–6871 (2017). 10.1039/c7cs00149e [DOI] [PubMed] [Google Scholar]
- 48. Tan, D. , et al.: Grand challenges for industrializing polyhydroxyalkanoates (PHAs). Trends Biotechnol. 39(9), 953–963 (2021). 10.1016/j.tibtech.2020.11.010 [DOI] [PubMed] [Google Scholar]
- 49. Zhang, X. , et al.: Synthetic biology and genome‐editing tools for improving PHA metabolic engineering. Trends Biotechnol. 38(7), 689–700 (2020). 10.1016/j.tibtech.2019.10.006 [DOI] [PubMed] [Google Scholar]
- 50. Yang, R. , et al.: Current situation and future development of the biopharmaceutical industry in China: a mixed‐method study. Front. Pharmacol. 13, 911165 (2022). 10.3389/fphar.2022.911165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang, K. , Liu, W. : The current status, trend, and development strategies of Chinese biopharmaceutical industry with a challenging perspective. Sage Open 10(1), 2158244020901529 (2020). 10.1177/2158244020901529 [DOI] [Google Scholar]
- 52. The outline of the national intellectual property strategy (In Chinese). https://www.cnipa.gov.cn/art/2020/6/5/art_407_154337.html. (2020). Accessed Oct 2022
- 53. Promoting the Jiangsu province's biomedicine industry policies and measures for high‐quality development (In Chinese). http://www.jiangsu.gov.cn/art/2021/9/26/art_46143_10027877.html?gqnahi=affiy2%26ivk_sa=1024320u. (2021). Accessed Oct 2022
- 54. Measures to promote the high‐quality development of biomedicine industry in Kunming (In Chinese). https://www.km.gov.cn/c/2020‐12‐30/3798018.shtml. (2020). Accessed Oct 2022
- 55. Tang, K. , et al.: China's silk road and global health. Lancet 390(10112), 2595–2601 (2017). 10.1016/s0140-6736(17)32898-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Three‐year implementation plan of the National Health and Family Planning Commission on promoting health exchanges and cooperation under the belt and road initiative (2015–2017) (In Chinese). http://www.nhc.gov.cn/wjw/ghjh/201510/ce634f7fed834992849e9611099bd7cc.shtml. (2015). Accessed Oct 2022
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.


