Abstract
Hepatocellular carcinoma (HCC) mortality remains high mainly due to late diagnosis as a consequence of failed early detection. Professional societies recommend semi-annual HCC screening in at-risk chronic liver disease patients to increase the likelihood of curative treatment receipt and improve survival. However, recent dynamic shift of HCC etiologies from viral to metabolic liver diseases has significantly increased the potential target population for the screening, whereas annual incidence rate has become substantially lower. Thus, with the contemporary HCC etiologies, the traditional screening approach might not be practical and cost-effective. HCC screening consists of (i) definition of rational at-risk population, and subsequent (ii) repeated application of early detection tests to the population at regular intervals. The suboptimal performance of the currently available HCC screening tests highlights an urgent need for new modalities and strategies to improve early HCC detection. In this review, we overview recent developments of clinical, molecular, and imaging-based tools to address the current challenge, and discuss conceptual framework and approaches of their clinical translation and implementation. These encouraging progresses are expected to transform the current “one-size-fits-all” HCC screening into individualized precision approaches to early HCC detection and ultimately improve the poor HCC prognosis in the foreseeable future.
Keywords: Hepatocellular carcinoma, early detection, risk stratification, biomarkers, personalized screening
Graphical Abstract
INTRODUCTION
Primary liver cancer is the fourth leading cause of cancer-related death worldwide, with an estimated 0.8 million deaths in 2020.[1] More than 80% of primary liver cancers are hepatocellular carcinoma (HCC) that develop in patients with chronic infection of hepatitis B virus (HBV), hepatitis C virus (HCV), excess alcohol intake, and metabolic disorders, including non-alcoholic fatty liver disease (NAFLD)/metabolic dysregulation-associated fatty liver disease (MAFLD).[2, 3] In the U.S., the overall HCC incidence rate has been increasing in more than half of the states.[4] Despite the improvement in early HCC detection and the advance in treatment over the past decades, 5-year overall survival rate of HCC is still dismal at around 20%.[5] Given the survival benefit of diagnosing HCC at early stages amenable to potentially curative treatment, current clinical practice guidelines recommend regular HCC screening in at-risk chronic liver disease patients.[6–8] However, the recommended screening is utilized only in less than 25% of HCC patients in the U.S. due to various logistical barriers, thus effectiveness of the screening is significantly impaired.[9–11] Furthermore, application of the screening has been more challenging along with the drastic changes in the HCC etiology landscape over the past decade, namely sharp decline of active HCV infection with the widespread use of new-generation anti-HCV drugs and global epidemic of obesity and metabolic disorders.[12, 13] In addition, sensitivity of the current HCC screening test is suboptimal, and it leads to failures in early HCC diagnosis.[14, 15] Thus, new tools and strategies are urgently needed to enable more effective HCC screening with improved utilization and early HCC detection to substantially improve poor HCC mortality.
To address this urgent and growing unmet medical need, new biomarkers will have a significant role by redefining the high-risk target population for HCC screening and by enabling more sensitive and accurate detection of early-stage HCC. Cancer biomarker development is a challenging process that involves costly and lengthy test development and validation.[16] To streamline and facilitate clinical translation of experimental cancer biomarkers, several national and international efforts have been made to develop resources for high-quality validation of promising biomarker candidates under federally-funded consortia such as the U.S. National Cancer Institute (NCI) Early Detection Research Network (EDRN).[17] In parallel, development of highly sensitive omics profiling technologies has enabled the interrogation of various cancer-associated molecular information in body fluid samples such as blood and urine, so-called “liquid biopsy”, as potential HCC screening biomarkers.[18] In this review, we outline the limitations of the current HCC screening strategy, discuss the conceptual framework of precision medicine approaches to overcome the challenges, and overview new developments on the horizon to refine HCC risk stratification and early detection with a special focus on new biomarkers that will likely impact HCC screening program and eventually reduce HCC mortality.
LIMITATIONS AND UNMET NEEDS IN HCC SCREENING
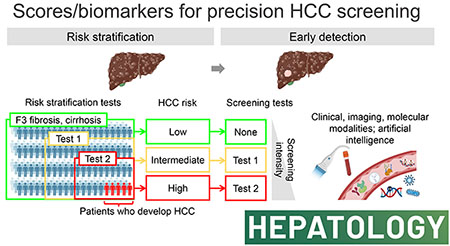
Professional societies recommend semi-annual HCC screening with abdominal ultrasound and alpha-fetoprotein (AFP) to improve early detection, curative treatment receipt, and survival in patients at risk of HCC development.[8] HCC screening consists of the following two components: (i) definition of target population, and (ii) repeated application of HCC detection tests at regular intervals (Figure 1A). A positive detection test triggers the procedure of HCC diagnosis with either contrast-enhanced dynamic computed tomography (CT)/magnetic resonance imaging (MRI) or histological assessment.[6] Efficacy of each component is limited by suboptimal performance of currently available modalities as detailed in the following sections. The complexity of the screening algorithm further compromises its effectiveness due to various logistical issues in its clinical implementation at patients, providers, and systems levels in the real-world setting.[19] Model-based simulation has been utilized to estimate efficacy and effectiveness of the HCC screening protocol based on cost-effectiveness, and revealed critical factors such as HCC incidence rates in the target population and performance of HCC detection tests.[13]
Figure 1.
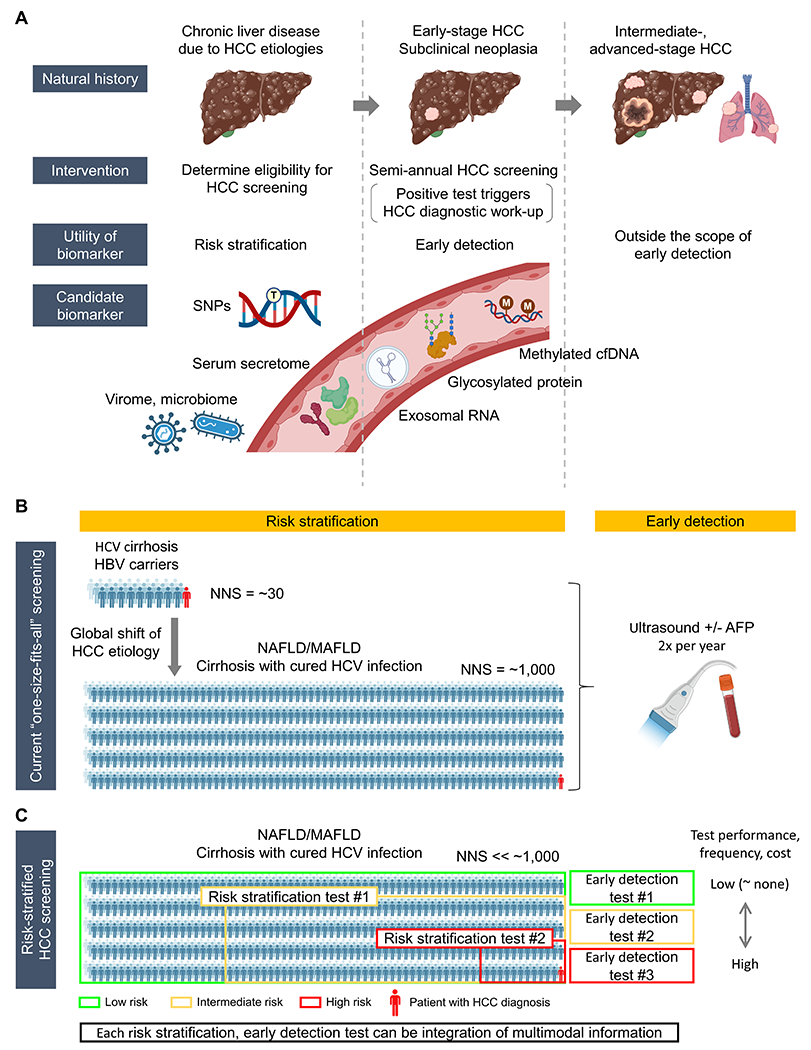
Conceptual framework and clinical implementation strategies of biomarker-guided precision HCC screening. (A) HCC risk stratification and early detection along the natural history of HCC development and progression. Risk stratification is the first step to identify specific patient population with elevated HCC risk (left). Subsequently, to the high-risk population, repeated HCC detection tests are applied at regular interval for diagnosis of early-stage HCC (middle). Intermediate- to advanced-stage HCC is theoretically outside the concept of HCC screening for early detection (right). New early detection biomarkers should achieve higher sensitivity compared to the current modalities, while maintaining a high specificity, ideally in less-invasively accessible biospecimens. Anticipated high sensitivity of the early detection biomarkers may lead to detection of subclinical neoplasia which is not recognizable with the current diagnostic tools such as contrast-enhanced dynamic MRI (i.e., false negative biomarker test based on MRI as goldstandard). Specific recall policies need to be developed according to confirmed association of the detection with subsequent HCC diagnosis. (B) Global shift of HCC etiology from viral to metabolic liver diseases over the past decade and accompanying drastic increase of the number needed to screen (NNS) for the current “one-size-fits-all” HCC screening. (C) Risk stratification by stepwise application of integrative HCC risk biomarkers to identify high-risk patients to focus the effort and resource of HCC screening. Tailored HCC detection tests are regularly applied according to predicted HCC risk by altering intensity of screening. Both HCC risk stratification biomarkers and early detection biomarkers can be integration of multimodal information, e.g., clinical, molecular and/or imaging features.
Increasingly elusive target population for HCC screening
Target population for the screening has been defined based on model-based cost-effectiveness, balancing number needed to screen (NNS) to detect one HCC case, associated net medical care costs, and net patient survival according to specific clinical context. For example, the screening was deemed cost-effective in cirrhosis patients with annual HCC incidence rate of 1.5% or greater.[6] This assumption was relevant when active HCV infection was the dominant cirrhosis etiology, where annual HCC incidence was as high as 8%.[12] However, the assumption no longer holds with the dynamic change in the landscape of liver disease etiology over the past decade, namely the sharp switching from active to cured HCV infection with the widespread use of new generation anti-virals and increase of metabolic liver diseases, particularly NAFLD.[20, 21] In these emerging at-risk populations, annual HCC incidence rate barely reaches the traditional threshold of 1.5% to justify HCC screening as a cost-effective intervention. After pharmacological cure of chronic HCV infection, i.e., sustained virologic response (SVR), annual HCC incidence rate is reduced to 0.5% to 2.1% in patients with advanced fibrosis or cirrhosis.[22] A recent simulation analysis suggested that the semi-annual screening is still cost-effective in SVR patients with advanced fibrosis or cirrhosis until age 60 to 70, but with a substantially loosened cut-off of incremental cost-effectiveness ratio (ICER) < $150,000 that is three-times higher than the traditionally used cut-off of < $50,000, which may not be globally acceptable.[23] In histologically confirmed NAFLD cirrhosis patients, annual HCC incidence rate is only 0.1% to 0.6%.[24, 25] Of note, unlike viral hepatitis- and alcohol-related liver diseases, HCC can develop even before establishing cirrhosis in > 30% of NAFLD-related HCC patients.[26] It highlights necessity of expanding the target population for HCC screening by including patients with F3 fibrosis, although this is practically infeasible given that the guideline-recommended “one-size-fits-all” HCC screening is applied only in less than a quarter of the patients.[10] Furthermore, the NNS will become unrealistically large if we adopt the recently proposed redefinition of metabolic liver disease, namely MAFLD, which is estimated to affect half of overweight/obese adults globally (Figure 1B).[27]
In addition, given that vast majority of the patients undergoing the screening will not develop HCC during their lifetime, unnecessary harms due to over-screening patients with indolent disease will become unignorable with the large NNS.[28] Thus, HCC risk stratification is urgently and increasingly needed to redefine the target population to enable cost-effective and practically feasible HCC screening, especially with the dynamically changing landscape of liver disease etiology.
Suboptimal performance of HCC detection tests
For HCC detection at early stage amenable to potentially curative treatments, sensitivity should be sufficiently high, while maintaining specificity to minimize false positives. Ultrasound is currently the standard screening test used in clinical practice, although its sensitivity is only around 50%.[15] Even combined with AFP, sensitivity is still around 70% to detect early-stage HCC.[15] In addition, this performance may be overestimation due to inclusion of phase 2 biomarker studies in the meta-analysis. Performance of ultrasound will be further impaired due to the increase of obese NAFLD patients.[29] Other clinically available markers, AFP-L3% and des-gamma-carboxy prothrombin (DCP), show similarly suboptimal performance.
Frequency of HCC screening in the era of precision medicine
Currently, the HCC screening test is performed at 6-month interval based on clinically observed superior efficacy in comparison to longer interval and non-inferiority to shorter interval with theoretical justification according to the tumor volume doubling time.[30–32] However, this guideline-recommended “one-size-fits-all” strategy disregards considerable inter-tumor/patient heterogeneity in the doubling time and frequency of multicentric carcinogenesis; the 6-month interval may not be optimal for each individual patient.[32] Indeed, a Markov model-based simulation analysis suggested that shorter interval for high-risk patients and longer interval for low-risk patients could enable more cost-effective HCC screening compared to the uniform 6-month interval for all when overall annual HCC incidence rate is > 3%.[33] This suggests that the screening interval can be tailored according to predicted individual HCC risk.
CONCEPTUAL FRAMEWORK OF PRECISION HCC SCREENING
General principles in precision HCC screening
To address the limitations in the current HCC screening and improve its effectiveness, performance of the risk stratification and early detection tests should be improved, and the tests should be rationally embedded and sequenced in an HCC screening algorithm. To improve performance of each test, integration of multimodal information (e.g., clinical, molecular, and/or imaging variables) has been often employed for both risk stratification and early detection. In addition, for risk stratification, sequential application of multiple tests has been proposed for stepwise enrichment of high-risk population to improve efficacy and feasibility of subsequent regular application of early detection tests.[34] Early detection tests should be applied according to predicted HCC risk to avoid under-screening of high-risk patients (which can lead to failed early detection) and over-screening of low-risk patients (which can lead to unnecessary harms due to the screening tests[28]). Clinical implementation of new tests in the HCC screening protocol should be guided based on trade-offs between multiple factors, including logistical feasibility and costs of the tests, accessibility to the biospecimens and other information used in the testing algorithm, among many others, to maximize its effectiveness with improved “precision” in risk stratification and early detection.
Integrative HCC screening scores/biomarkers to improve precision
Integration of multimodal information has been attempted to improve test efficacy. It has been empirically known that AFP elevation is associated with long-term HCC risk, besides its use as an HCC detection marker, reflecting chronic liver injury and regeneration underlying carcinogenic hepatic tissue milieu.[13, 35] A blood-based Prognostic Liver Secretome signature (PLSec) was integrated with AFP to achieve robust long-term HCC risk stratification in cirrhotics.[36] Integration of etiology-specific “plug-in” biomarker with etiology-agnostic backbone biomarker is a strategy for refining HCC risk stratification according to liver disease etiology as shown in a recent proof-of-concept study.[37] Non-invasive scores (NISs) or non-invasive tests (NITs) also represent the integrative approach, combining a handful number of clinical variables (e.g., patient age, sex) and biochemical tests (e.g., AFP, hepatic transaminases). Many of these clinical variable-based NISs/NITs were originally developed for other purposes such as detection of advanced liver fibrosis and subsequently associated with adverse outcomes, including HCC development, in systematic retrospective assessment, although associated outcomes vary across studies.[38] Integration of imaging modalities (e.g., acoustic elastography, magnetic resonance elastography [MRE]) and NISs/NITs (e.g., Fibrosis-4 [FIB-4] index) have been developed for non-invasive detection of advanced fibrosis, and were subsequently associated with adverse outcomes, including HCC development.[39, 40] Germline DNA variants such as single nucleotide polymorphisms (SNPs) have been heavily studied as potential HCC risk stratification biomarkers on easily accessible biospecimens such as buccal swab. More recently, their combinations have been evaluated as polygenic risk scores (PRSs), mostly tailored for metabolic liver diseases.[41] While the genetic scores show promising HCC risk association, a recent nationwide population-level biobank study suggested that additional prognostic information gained by PRSs on top of NISs/NITs may be limited unless the target population is carefully chosen.[42] The Liver Cancer Risk test algorithm (LCR1-LCR2) is an integration of clinical demographics and several biochemical test, which has been validated for high negative predictive value (NPV) > 99% in patients with viral hepatitis.[43] Integration of multimodal information has also been explored for early HCC detection tests such as the GALAD score, combining patient age and sex with AFP, AFP-L3%, and DCP.
Sequential application of HCC screening scores/biomarkers to improve effectiveness
Sequential application of HCC risk assessments for stepwise enrichment of high-risk population will be a rational strategy given the explosive growth of potential at-risk population with the NAFLD/MAFLD epidemic, which has been transforming HCC screening like finding a needle in a large haystack. Indeed, stepwise enrichment of NAFLD patients who need medical attention/intervention has been actively explored,[34] and HCC risk stratification could be added as a subsequent step.[44] Desired characteristics of HCC risk stratification biomarkers would depend on target population for the tests. For instance, cheap assay costs and robust performance in less-invasively accessible specimens would be valued over high accuracy for the first step of HCC risk stratification applied to a large population (e.g., adult NAFLD patients). If the first risk assessment is performed in general population, the tests may be tailored to also cover other cancer types and chronic diseases. Subsequent step(s) of risk stratification can be performed in the narrowed target population with more expensive tests with higher accuracy to identify a substantially small subset of patients as a high-risk group for certain interventions (e.g., HCC screening, chemoprevention) with enhanced efficacy of the interventions. In a nationwide population-based study involving 266,687 individuals, a stepwise risk enrichment with first NIS/NIT followed by PRS successfully enriched individuals at risk of severe liver diseases.[45]
Model-based assessment of precision HCC screening strategies
Given that the entire HCC screening protocol is complex with many modifiable parameters, it is challenging to evaluate net benefit of new risk-stratified HCC screening algorithms in a prospective controlled clinical trial. Instead, Markov model-based simulation analysis has been widely used to estimate net survival benefit and cost-effectiveness of experimental HCC screening strategies, in which plausible ranges of model parameters such as screening utilization rate can be assessed as sensitivity analysis.[46] The first cost-effectiveness analysis of risk-stratified HCC screening strategies, comparing 2 non-risk-stratified and 14 risk-stratified strategies, showed that risk-stratified screening utilizing new tests are substantially more cost-effective than the current non-stratified screening.[33] Various key parameters such as imaging modalities, screening interval and duration, and harms from HCC screening can be incorporated in the modeling.[23, 33, 46–52] Model-based simulation also provides insight into benchmarks to meet for experimental biomarkers in development. For example, a hypothetical risk stratification biomarker enables cost-effective HCC screening for majority of top-performing risk-stratified algorithms when it achieves risk stratification at hazard ratio > 2 in cirrhosis patients dominantly affected with chronic HCV infection.
Clinical implementation of precision HCC screening
The risk-stratified approach is essentially tailoring of screening intensity, regarding test modality and frequency, according to predicted risk level; more intensive/frequent screening is offered to high-risk patients, whereas less intensive/frequent or no screening is offered to low-risk patients. Practical feasibility and acceptance from the professional societies and practitioners will be the key in clinical implementation of risk-stratified HCC screening protocol. A questionnaire-based study showed that physicians are receptive to tailoring HCC screening modality for each patient when individual HCC risk can be quantitatively estimated.[53] Alteration of screening frequency, including dropping from the screening, will need attention on specific test performance metric, e.g., high NPV to justify exclusion from the screening, balanced with physician’s and patient’s perspective and preference. Ethical issues and potential psychological harms such as anxiety will need to be properly considered to justify exclusion of low-risk individuals from the screening. Patients with advanced fibrosis or cirrhosis may need monitoring/care for liver failure and portal hypertension regardless of HCC risk, and it may be logistically sensible to concurrently assess presence of nodular lesions with low-cost modalities such ultrasound and/or AFP during the clinic visits. Nevertheless, the guideline-recommended semi-annual screening is currently utilized in a small subset (< 25%) of the target population due to the limited medical resources,[10] and risk stratification would help identify high-risk patients to be prioritized for the screening. Biomarker-based HCC risk level may change over time in response to influential events (e.g., antiviral therapies, body weight loss, aging) depending on the type of biological information the biomarker captures. Repeated assessment may be needed for such biomarkers, considering possibility of altering subsequent HCC screening strategy. Indeed, naturally occurring modulation of HCC risk level measured by a hepatic transcriptome signature over a median interval of 2.3 years was associated with future HCC development in a cohort of NAFLD cirrhosis patients.[37]
TECHNICAL ASPECTS OF HCC BIOMARKER DEVELOPMENT
Phases of cancer screening biomarker development
To streamline and facilitate development of cancer screening biomarkers, a five-phase conceptual framework was proposed in conjunction with the NCI EDRN (Figure 2A).[54] Phase 1 studies are preclinical exploration of candidate biomarkers in biospecimens not necessarily collected with intention of biomarker research. Phase 2 studies aim at clinical assay development, encompassing clinical assay implementation, optimization, and preliminary estimation of performance typically in cross-sectional series of HCC patients and matched controls. Analytical algorithm should be established as detailed in the next section. Clinical confounding variables such as patient sex, age, liver disease etiology and severity, particularly fibrosis stage, should be properly controlled to avoid over- or under-estimation of the test performance in anticipated target patient population. Phase 3 studies are retrospective analysis of biospecimens with longitudinal follow-up information; samples are collected before HCC development or formal HCC diagnosis and patients who develop HCC during subsequent follow-up are compared to control patients matched for confounding variables who are HCC-free over certain follow-up time. Phase 3 studies will provide more accurate estimate of biomarker performance in the screening setting. Comparison to standard care is also within the scope of phase 3 study. As generic resources for phase 3 biomarker studies, prospectively developed patient cohorts accompanied with biorepository have been developed to enable high-quality biomarker evaluation by utilizing the prospective specimen collection, retrospective blinded evaluation (PRoBE) or “prospective-retrospective” design.[54, 55] Samples collected at the time of cancer diagnosis would allow conduct of phase 2 studies. The EDRN Hepatocellular carcinoma Early Detection Strategy (HEDS) study[56] and Texas HCC Consortium (THCCC)[57] are examples of nationwide and statewide multicenter cohorts, respectively, for phase 3 HCC biomarker validation. Phase 4 studies are prospective evaluation of candidate biomarkers in the screening setting to determine performance of the biomarkers, i.e., cancer detection rate and false referral rate based on standard-care diagnostic test’s result, in the target patient population. A positive test triggers the standard-care diagnostic procedure to determine an HCC diagnosis, following practice guidelines. Phase 5 studies evaluate whether HCC screening interventions that incorporate new biomarkers reduce HCC burden and mortality in the target population. This phase will prospectively determine clinical impact of new cancer screening biomarkers measured by reduction in cancer mortality and net medical care costs.[58] These phases provide roadmap for rigorous evaluation and development of cancer screening biomarkers. However, this is a costly and lengthy process that limits cancer screening biomarker development. To overcome the challenge and accelerate clinical translation of promising candidate biomarkers, innovative approaches such as adaptive trial design are urgently needed.
Figure 2.
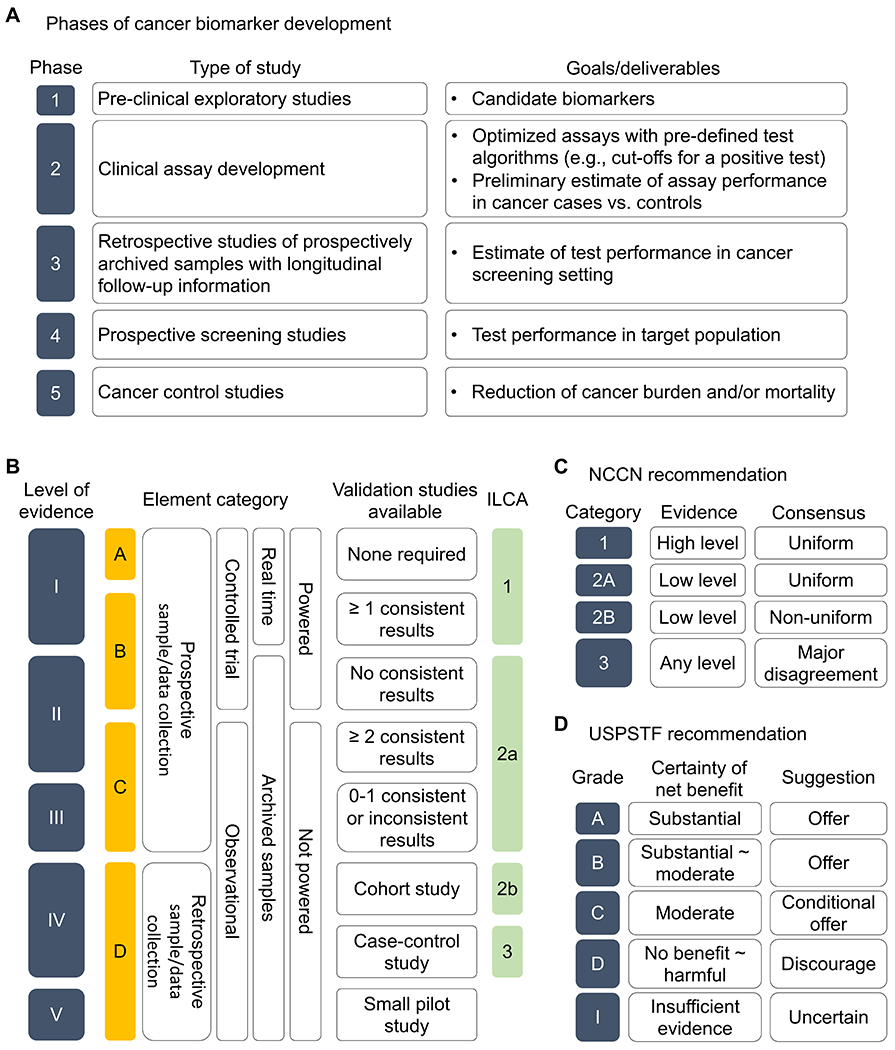
(A) Phases of cancer screening biomarker development.[54] (B) Levels of evidence (LOE) for cancer screening biomarkers, defined based on the element category and status of validation studies are determined according to the study design elements.[55, 238] Correspondence to the LOE defined in the International Liver Cancer Association (ILCA) white paper[239] is shown. (C) Categories of recommendation for clinical implementation by the National Comprehensive Cancer Network (NCCN) according to the levels of scientific evidence and consensus among the NCCN expert panel. (D) Grades of recommendation for clinical implementation by the U.S. Preventive Services Task Force (USPSTF) according to certainty of net benefit for preventive intervention.
Analytical validity and clinical utility of cancer screening biomarker
Analytical validity of new cancer biomarkers should be established in clinically applicable assays. For each molecular probe in the assays, reproducibility of its measurement should be confirmed, and magnitude of variation should be determined across day-to-day and inter-operator/laboratory variations measured by correlation coefficient, coefficient of variation, and/or other relevant statistics in technical and/or biological replicates. Reference standards will ensure proper adjustment of the measurements for experimental batch difference as needed. Cut-off values and/or analytical algorithms to call positivity of the tests should be pre-determined in derivation/training dataset(s), which should be applied in independent validation dataset(s) without any modification based on information from the validation set(s) to avoid information leak. For biomarkers that provide quantitative estimates (e.g., predicted probability of HCC incidence), proper calibration should be performed to ensure agreement between predicted and observed measures.
Clinical utility is critical in determining which candidate biomarkers warrant further clinical development and translation to ensure that the biomarkers provide clinically actionable information. Clinically meaningful effect size (e.g., magnitude of HCC risk association measured by hazard ratio, performance of early HCC detection measured by area under receiver operating characteristic [AUROC] curve) should be defined a priori, and sample size to detect the effect size should be defined for independent validation of a candidate biomarker. Comparison to or integration with existing clinical scores and/or biomarkers should be performed to determine whether additional information gained by the new biomarker justifies costs and efforts of its clinical development. Performance metrics for risk stratification biomarker include Harrel’s C-index (a.k.a. concordance index), time-dependent AUROC curve, explained variation (R2), Brier score, Royston’s D index, Akaike information criterion (AIC), and Bayesian information criterion (BIC) to assess discrimination and/or goodness of fit. Performance metrics for early detection biomarker include contingency table statistics such as sensitivity, specificity, positive/negative-predictive values, AUROC curve. Reporting guidelines help ensure proper assessment for diagnostic/prognostic biomarkers (e.g., STARD, REMARK, TRIPOD) available via the enhancing the quality and transparency of health research (equator) network (www.equator-network.org/reporting-guidelines).
Issues in clinical deployment and implementation of cancer screening biomarkers
Analytically and clinically validated biomarkers would undergo the process of clinical deployment and implementation, including commercial product development, regulatory approval, coding for health insurance coverage, and incorporation in clinical practice guidelines, which can hugely vary across geographic regions and countries. In the U.S., while it keeps evolving, there are two major paths under oversight by the FDA: (i) in vitro diagnostic devices (IVDs) as commercial medical devices with 510(k) clearance, and (ii) laboratory developed tests (LDTs) as home-grown tests performed at each diagnostic lab.[59] FDA guidance documents are available for several relevant types of biomarkers and topics such as circulating tumor DNA (ctDNA)-based tests and LDTs (www.fda.gov/regulatory-information). Clinical biomarker tests must be conducted in diagnostic laboratories certified for Clinical Laboratory Improvement Amendments (CLIA) and in accordance with state-specific regulations. Coverage by health insurance is critical for physicians to order the tests. Other local/regional agencies such as European Medicines Agency (EMA) employ similar but their own procedure.[60] Coding for the tests, e.g., current procedural terminology (CPT) codes, is needed for insurance coverage as billable medical procedures. Centers for Medicare & Medicaid Services (CMS) regularly updates the billing and coding policies according to specific indications (www.cms.gov/medicare-coverage-database).
For decision of payers and policy makers, incorporation of the tests into clinical practice guidelines/guidance is important, which should be based on the level of available evidence (Figure 2B). Public organizations such as the Biomarkers Compendium of National Comprehensive Cancer Network (NCCN) (www.nccn.org) and the U.S. Preventive Services Task Force (USPSTF) (www.uspreventiveservicestaskforce.org) also provide regularly updated guidelines and recommendations for cancer biomarkers and screening algorithms graded by quality of available evidence (Figure 2C, D).[61] Post-marketing clinical utility validation, including the phase 5 biomarker validation study, will further support the use of biomarker tests and may result in indication for additional diseases and/or clinical scenarios. With the sharply expanding clinical and commercial interests especially in circulating cancer biomarkers so-called “liquid biopsy”, several federally-funded and private consortia have been established to facilitate clinical translation of this type of biomarkers, including Blood Profiling Atlas in Cancer (BloodPAC) and NCI Division of Cancer Prevention’s Liquid Biopsy Consortium.[62] Further, engagement of practitioners who order the tests and medical staffs via education, training, and/or incentive will be important to ensure proper adherence to the new biomarker-based care.
Emerging technologies/methodologies with potential utility in HCC screening
The requirement of clinic visits at 6-month interval is a significant logistical hurdle in the current ultrasound-based HCC screening protocol.[11, 63] Body fluid (e.g., plasma, urine)-based tests are expected to be available in clinic in near future and alleviate the burden as overviewed in subsequent sections. A functional in vivo genetic screening suggested that there may be a new class of HCC risk-associated DNA variants, somatic DNA mutations in PKD1, KMT2D, and ARID1A genes in cirrhotic liver that confers protective effect against carcinogenesis.[64] Point-of-care (POC) biochemical tests and imaging devices have been actively explored as potential options to substantially improve receipt of the regular screening examination particularly in developing regions with limited access to medical care.[65–68] These new technologies could be combined with software as a medical device (SaMD), incorporating artificial intelligence (AI) and machine learning/deep learning (ML/DL) for widespread application.[69] Several promising examples are overviewed in the following sections.
HCC RISK STRATIFICATION SCORES AND BIOMARKERS
Numerous HCC risk-associated clinical and molecular scores and biomarkers have been reported to date. None of them has been adopted into clinical practice yet, but some scores/biomarkers have shown promising performance in more advanced stages of clinical validation as summarized below (Table 1, Supplementary table 1).
Table 1.
HCC risk stratification scores and biomarkers (with independent validation).
| Biomarker type | Score/biomarker | Biomarker development phase | Level of evidence (Simon et al./ILCA) | Variables | Study design | Enrollment | Endpoint (HCC) | Major etiology | Region/country | No. subjects | Race/ethnicity | Cirrhosis | Independent validation | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical NIS/NIT | aMAP risk score | 3 | II/2a | Age, sex, albumin-bilirubin, platelets | Cohort | Prospective-retrospective | Development (3/5y) | HBV, HBV on NA, HCV, HCV post-SVR, alcohol | International; UK; Egypt; Japan; China; Egypt; Australia, UK | 3,688 + 13,686; 2,139 + 606; 2.085; 1,113; 1,042; 3,075; 269 | Asian, Caucasian, Black | 11% + 27%; 100% + 100%; 100% (F3-4); 100%; 66%; 100% (F3-4); 100% | In independent studies | [73, 74, 240–243] |
| ADRESS-HCC | 3 | II/2a | Age, sex, diabetes, race, etiology, Child-Pugh score | Cohort | Prospective-retrospective | Development (1y) | HCV, alcohol, NASH, HBV, other | U.S., China | 17,124 + 17,808 + 1,050 | Caucasian, Hispatnic/Latino, Black, Asian | 100% + 100% + 100% | Within the study | [244] | |
| LCR1-LCR2 | 3 | II/2a | Age, sex, apolipoprotein A1, haptoglobin, GGT, alpha2-macroglobulin | Cohort | Prospective-retrospective | Development | HCV, HBV | France; Europe, Asia, Africa; Europe, Asia, Africa | 4,944 + 4,948; 4,903; 3,520 | Caucasian, Asian, Black | 15% + 14%; 22%; 9% | In independent studies | [43, 245, 246] | |
| CU-HCC | 3 | II/2a | Age, albumin, bilirubin, HBV-DNA, cirrhosis | Cohort | Prospective-retrospective | Development (5y) | HBV | Hong Kong; Korea; Korea; Canada.; Hong Kong; Korea; U.S. | 1,005 + 424; 1,308; 1,330; 2,105; 1,531; 1,092; 3,101 | Asian, Caucasian, Black | 38% + 16%; 18%; 25%; 22%; 18%; 37%; 32% | In independent studies | [247–253] | |
| REACH-B | 3 | II/2a | Age, sex, ALT, HBeAg, HBV-DNA | Cohort | Prospective-retrospective | Development (3/5/10y) | HBV | Taiwan; Korea; Korea; Canada; Hong Kong; Korea | 3,584 + 1,505; 1,308; 1,241; 2,105; 1,531; 1,092 | Asian, Caucasian | 0% + 18%, 18%, 25%, 22%, 37% | In independent studies | [248–252, 254] | |
| GES score | 3 | II/2a | Age, sex, fibrosis stage, albumin, AFP | Cohort | Prospective-retrospective | Development (1/2/3y) | HCV post-SVR with DAA | Egypt; Egypt; International | 2,372 + 687 + 1,341; 3,075; 12,038 | n.a. | 100% + 100% + 100% (all F3-4); 100% (F3-4); 44% | In independent studies | [242, 255–257] | |
| REACH-B2 | 3 | III/2a | Age, sex, ALT, family history of HCC, HBeAg, HBV-DNA, HBsAg, Genotype | Cohort | Prospective-retrospective | Development (5/10/15y) | HBV | Taiwan | 3,340 (2 : 1 for training and validation) | Asian | 0% | Within the study | [258] | |
| UM regression model | 3 | III/2a | Machine-learning (23 clinical variables) | Cohort | Prospective-retrospective | Development (3/5y) | HCV, cryptogenic, alcohol, other | U.S. | 442 + 1,050 | Caucasian, Black, Hispanic | 100% + 41% | Within the study | [259] | |
| Hung et al | 3 | III/2a | Age, sex, ALT, previous liver disease, history of HCC, smoking, HBV/HCV infection | Cohort | Prospective-retrospective | Development (3/5/10y) | HBV, HCV | Taiwan | 8,252 + 4,125 | n.a. | n.a. | Within the study | [260] | |
| LSM-HCC | 3 | III/2a | Age, LSM, albumin, HBV-DNA | Cohort | Prospective-retrospective | Development (3/5y) | HBV | Hong Kong; Korea; Korea | 1,035 + 520; 1,308; 1,241 | Asian | 32% + 31%; 18%; 24% | In independent studies | [248, 261, 262] | |
| NGM1/2-HCC | 3 | III/2a | Age, sex, family history of HCC, alcohol, ALT, HBeAg | Cohort | Prospective-retrospective | Development (5/10y) | HBV | Taiwan; Canada | 2,435 + 1,218; 2,105 | Asian, Caucasian | n.a.; 25% | In independent study | [250, 263] | |
| RWS-HCC | 3 | III/2a | Age, sex, cirrhosis, AFP | Cohort | Prospective-retrospective | Development | HBV | Singapore; U.S. | 538 + 3,353; 3,101 | Asian, Caucasian, Black | 15% + n.a.; 32% | In independent study | [253, 264] | |
| GAG-HCC | 3 | III/2a | Age, sex, HBV-DNA, core promoter mutations, cirrhosis | Cohort | Prospective-retrospective | Development (5/10y) | HBV, HBV on NA | Taiwan; Korea; Korea; Taiwan; Hong Kong; Korea; Japan; Korea; Canada | 820; 1,330; 3,001; 1,325; 1,531; 1,308; 225; 1,092; 2,105 | Asian, Caucasian | 15%; 46%; 19%; 36%; 22%; 18%; 26%; 37%; 25% | In independent studies | [248–252, 265–268] | |
| REVEAL-HCV | 3 | III/2a | Age, ALT, AST/ALT ratio, HCV-RNA, cirrhosis, HCV genotype | Cohort | Prospective-retrospective | Development (5/10/15y) | HCV | Taiwan | 1,095 + 572 | n.a. | 1% + 7% | Within the study | [269] | |
| Ganne-Carri et al | 3 | III/2a | Age, alcohol, platelets, GGT, SVR | Cohort | Prospective-retrospective | Development (1/3y) | HCV, HCV post-SVR | France; Switzerland, Belgium | 720 + 360; 192 | Caucasian | 100% + 100%; 100% | In independent study | [270, 271] | |
| Semmler et al. | 3 | III/2a | Age, albumin, LSM, AFP, alcohol consumption | Cohort | Prospective-retrospective | Development (4y) | HCV post-SVR with DAA | Austria, Spain | 475 + 1,500 | Caucasian | 100% +100% (F3-4/HVPG≥6 mmHg/LSM ≥10 kPa) | Within the study | [77] | |
| Pons et al. | 3 | III/2a | albumin, LSM | Cohort | Prospective-retrospective | Development (1y) | HCV post-SVR with DAA | Spain | 290 + 282 | Caucasian | 100% + 100% (LSM ≥10 kPa) | Within the study | [272] | |
| FIB-4 | 2 | IV/2b | FIB-4 (AST, ALT, platelets, age) | Cohort | Retrospective | Development | HBV, HCV, alcohol, NAFLD | Korea; Italy; Korea; Germany; Japan | 986; 4,492; 6,661; 29,999; 3,823 | Asian, Caucasian | 9%; n.a.; n.a.; n.a.; n.a. | In independent studies | [273–277] | |
| THRI | 2 | IV/2b | Age, sex, etiology, platelets | Cohort | Retrospective | Development (5/10y) | HCV, HBV, steatohepatitis, PBC, AIH | Canada, Netherlands; China; Turkey; Sweden | 2,079 + 1,144; 2,836; 1,287; 2,491 | Asian, Caucasian | 100% + 100%; 100%; 100%; 100% | In independent studies | [72, 278–280] | |
| Hughes et al | 2 | IV/2b | AFP | Cohort | Retrospective | Development | HCV, HBV | Japan, Scotland | 3,450 + 4,754 | Asian, Caucasian | n.a. | Within the study | [35] | |
| AGED | 2 | IV/2b | Age, sex, HBeAg, HBV-DNA | Cohort | Retrospective | Development | HBV | China | 628 + 1,663 | Asian | 0% + 0% | Within the study | [281] | |
| D2AS risk score | 2 | IV/2b | Age, sex, HBV-DNA | Cohort | Retrospective | Development (3/5y) | HBV | Korea | 971 + 507 | Asian | 0% + 0% | Within the study | [282] | |
| PAGE-B | 2 | IV/2b | Age, sex, platelets | Cohort | Retrospective | Development (5y) | HBV, HBV under NA | Europe; Korea; Hong Kong; Turkey; U.S. | 1,325 + 490; 1,330; 32,150; 647; 3,101 | Caucasian, Asian, Black | 20% + 48%; 46%; 14%; 9%; 32% | In independent studies | [249, 253, 283–285] | |
| Modified PAGE-B | 2 | IV/2b | Age, sex, platelets, albumin | Cohort | Retrospective | Development (5y) | HBV on NA | Korea; Korea; Turkey; U.S. | 2,001 + 1,000; 3,171; 647; 3,101 | Asian, Caucasian, Black | 19% + 20%; 33%; 9%; 32% | In independent studies | [253, 266, 285, 286] | |
| CAGE-B | 2 | IV/2b | Age, cirrhosis | Cohort | Retrospective | Development | HBV on NA | Europe; Korea; Korea; Korea | 1,427; 1,763; 1,557; 734 | Caucasian, Asian | 26%; 37%; 28%; 47% | In independent studies | [287–290] | |
| SAGE-B | 2 | IV/2b | Age, LSM | Cohort | Retrospective | Development | HBV on NA | Europe; Korea; Korea; Korea | 1,427; 1,763; 1,557; 734 | Caucasian, Asian | 26%; 37%; 28%; 47% | In independent studies | [287–290] | |
| Modified REACH-B | 2 | IV/2b | Age, LSM, sex, ALT, HBeAg | Cohort | Retrospective | Development | HBV on NA | Korea; Korea | 192; 1,308 | Asian | 40%; 18% | In independent study | [248, 291] | |
| HCC-RESCUE | 2 | IV/2b | Age, sex, cirrhosis | Cohort | Retrospective | Development | HBV on NA | Korea; Korea; Turkey; U.S. | 990 + 1,071; 3,171; 647; 3,101 | Asian, Caucasian, Black | 61% + 65%; 33%; 9%; 32% | In independent studies | [253, 285, 286, 292] | |
| CAMPAS model score | 2 | IV/2b | Age, sex, cirrhosis, platelets, albumin, LSM | Cohort | Retrospective | Development (7y) | HBV on NA | Korea | 1,511 + 252 | Asian | 40% + n.a. | Within the study | [293] | |
| GBM-based model | 2 | IV/2b | Age, sex, cirrhosis, platelets, ETV or TDF, ALT, HBV-DNA, albumin, bilirubin, HBeAg | Cohort | Retrospective | Development | HBV on NA | Korea, Greece, Italy, German | 6,051 + 5,817 + 1,640 | Asian, Caucasian | 50% + 35% + 27% | Within the study | [294] | |
| ALT flare | 2 | IV/2b | ALT | Cohort | Retrospective | Development | HBV on NA | China, U.S. | 8,152 + 4,893 | Asian, Caucasian | 18% + 17% | Within the study | [294] | |
| REAL-B | 2 | IV/2b | Age, sex, alcohol, diabetes, cirrhosis, platelets, AFP | Cohort | Retrospective | Development (3/5/10y) | HBV on NA | U.S., Asia-Pacific; U.S. | 5,365 + 2,683; 3,101 | Asian, Caucasian, Black | 20% + 22%; 32% | In independent study | [253, 295] | |
| AASL-HCC score | 2 | IV/2b | Age, sex, albumin, cirrhosis | Cohort | Retrospective | Development (3/5y) | HBV on NA | Korea; U.S. | 944 + 298; 3,101 | Asian, Caucasian, Black | 39% + 39%; 32% | In independent study | [253, 296] | |
| CAMD score | 2 | IV/2b | Age, sex, cirrhosis, diabetes | Cohort | Retrospective | Development (1/2/3y) | HBV on NA | Taiwan, Hong Kong; Korea; U.S. | 23,851 + 19,321; 3,277; 3,101 | Asian, Caucasian, Black | 26% + 7%; 32%; 32% | In independent studies | [253, 297, 298] | |
| APA-B | 2 | IV/2b | Age, platelets, AFP | Cohort | Retrospective | Development | HBV on NA | Taiwan; U.S. | 883 + 442; 3,101 | Asian, Caucasian, Black | 36% + 37%; 32% | In independent study | [253, 267] | |
| HCC-SVR score | 2 | IV/2b | Sex, FIB-4, AFP | Cohort | Retrospective | Development | HCV post-SVR | Korea; Egypt | 669 + 524; 3,075 | Asian | 17% + 21%; 66% | In independent study | [242] | |
| ADRES score | 2 | IV/2b | Sex, SVR24, FIB-4, AFP | Cohort | Retrospective | Development (1/2y) | HCV post-SVR with DAA | Japan; Egypt | 484 + 585; 3,075 | Asian | n.a.; 66% | In independent study | [242, 299] | |
| HEPATHER HCC score | 2 | IV/2b | Age, sex, HCV Genotype, hypercholesterolemia, albumin, bilirubin, esophageal varices, FIB-4 | Cohort | Retrospective | Development | HCV post-SVR with DAA | France, Egypt | 3,531 + 3,075 | n.a. | 69% + 100% (all F3-4) | Within the study | [300] | |
| Watanabe et al. | 2 | IV/2b | Sex, FIB-4, albumin | Cohort | Retrospective | Development (1/2y) | HCV post-SVR with DAA | Japan; Egypt | 1,174; 3,075 | Asian | n..a.; 100% (F3-4) | In independent study | [242, 301] | |
| Alonso López et al. | 2 | IV/2b | 2 models: albumin, LSM, 1y-ΔLSM; albumin, FIB-4, 1y-FIB-4, 1y-GGT | Cohort | Retrospective | Development | HCV post-SVR with DAA | Spain; Egypt | 993; 3,075 | Caucasian | 100% (F3-4/LSM >9.5 kPa) + 100% (F3-4) | In independent study | [242, 302] | |
| Tani et al. | 2 | IV/2b | Age, AFP | Cohort | Retrospective | Development | HCV post-SVR with DAA | Japan; Egypt | 1,088; 3,075 | Asian | 18%; 100% (F3-4) | In independent study | [242, 303] | |
| Abe et al. | 2 | IV/2b | ALBI score, platelets, diabetes | Cohort | Retrospective | Development (1/2/3/4y) | HCV post-SVR with DAA | Japan; Egypt | 188; 3,075 | Asian | 100%; 100% (F3-4) | In independent study | [242, 304] | |
| Hu et al. | 2 | IV/2b | Age, bilirubin, AFP, SVR, cirrhosis | Cohort | Retrospective | Development | HCV, HCV post-SVR | Taiwan; Egypt | 665 + 78; 3,075 | Asian | 28% + 29%; 100% (F3-4) | In independent study | [242, 305] | |
| Sinn et al | 2 | IV/2b | Age, sex, smoking, diabetes, total cholesterol, ALT | Cohort | Retrospective | Development (10y) | non-HCV, non-HBV, non-alcohol | Korea | 467,206 + 91,357 | Asian | n.a., general population | Within the study | [306] | |
| SNP | Genetic risk score | 3 | III/2a | SNPs of PNPLA3, TM6SF2, HSD17B13 | Cohort | Prospective-retrospective | Developement | General population | Denmark, UK | 110,761 + 334,691 | Caucasian | 0.4% + 0.1% | Within the study | [88] |
| Genetic and Metabolic Staging (GEMS) scoring | 3 | III/2a | SNPs of PNPLA3, TM6SF2, HSD17B13, age, diabetes, platelets, HDL, albumin | Cohort | Prospective-retrospective | Liver related event (HCC + liver decompensation) | NAFLD | Germany, UK | 546 + 303,075 | Caucasian | 100% + n.a. | Within the study | [307] | |
| EGF | 2 | n.a. | EGF 61AG (rs4444903, A>G) | Meta-analysis of 16 case-control studies | Retrospective | Presence | HBV, HCV | France, Italy, China, Egypt, Japan, U.S. | 2,475 : 5,381 | Asian, European, Black | n.a. | In independent studies | [81] | |
| IFNL3 | 2 | n.a. | IFNL3 (rs12979860: C>T, rs8099917: T>G) | Meta-analysis of 24 case-control studies | Retrospective | Presence | HBV, HCV, HCV post-SVR | China, Japan | 4,212 : 5,489 | Asian, European | n.a. | In independent studies | [82] | |
| MICA | 2 | n.a. | MICA (rs2596542, C>T) | Meta-analysis of 11 case-control studies | Retrospective | Presence | HCV | Japan, China, Switzerland, Italy, Egypt, Taiwan, Vietnam | 4,678 : 16,867 | Asian, European | n.a. | In independent studies | [308] | |
| KIF1B or 1p36.22 | 2 | n.a. | KIF1B or 1p36.22 (rs17401966, A>G) | Meta-analysis of 19 case-control studies | Retrospective | Presence | HBV | China, Japan, Thailand | 8,741 : 10,812 | Asian | n.a. | In independent studies | [309] | |
| STAT4 | 2 | n.a. | STAT4 (rs7574865, G>T) | Meta-analysis of 7 case-control studies | Retrospective | Presence | HBV | China, Vietnam, Korea, Thailand | 2,028 : 9,388 | Asian | n.a. | In independent studies | [310] | |
| PNPLA3 | 2 | n.a. | PNPLA3 (rs738409: C>G) | Meta-analysis of 6 case-control studies | Retrospective | Presence | NAFLD, alcohol, HCV | Europe, Japan | 544 : 1,543 | European | n.a. | In independent studies | [311] | |
| TM6SF2 | 2 | n.a. | TM6SF2 (rs58542926: C>T) | Meta-analysis of 5 case-control studies | Retrospective | Presence | NAFLD, alcohol | Europe, Thailand | 2,594 : 4,279 | European | n.a. | In independent studies | [85] | |
| HSD17B13 | 2 | IV/3 | HSD17B13 (rs72613567: TA) | Case-control | Retrospective | Presence | NAFLD, alcohol | Europe | 1,109 : 2,206 | European | 49% : 79% | Within the study | [312] | |
| WNT3A-WNT9A | 2 | IV/3 | WNT3A-WNT9A (rs708113: T>A) | Case-control | Retrospective | Presence | Alcohol | Europe | 775 : 1,332 + 874 : 1,059 | European | 80% : 94% + 83% : 96% (all F3-4) | Within the study | [87] | |
| Polygenic risk scores (PRS-HFC, PRS-5) | 2 | IV/3 | SNPs of PNPLA3, TM6SF2, MBOAT7, GCKR, HSD17B13 + hepatic fat | Case-control | Retrospective | Presence | NAFLD | Italy, UK, Germany | 226 : 2,340 + 84 : 343 + 202 : 363,846 | Caucasian | n.a. : 13% + n.a. : 21% + n.a. : 0.4% | Within the study | [41] | |
| Tissue transcriptome | Prognostic liver signature (PLS) | 3 | II/2a | 186 mRNAs | Cohort | Prospective-retrospective | Development, recurrence | HCV, HBV, alcohol, NAFLD | Italy; U.S.; Japan | 216; 145; 263 | Caucasian, Asian | 100%; 100%; n.a. | In independent studies | [90–92] |
| PLS-NAFLD | 3 | II/2a | 133 mRNAs | Cohort | Prospective-retrospective | Development, recurrence | NAFLD | Japan | 48 + 106 + 59 | Asian | 90% + 25% + 41% (all F3-4) | Within the study | [37] | |
| Circulating proteins/nucleic acids | Prognostic Liver Secretome signature (PLSec)-AFP | 3 | II/2a | 8 proteins + AFP | Cohort | Prospective-retrospective | Developement | HCV, HCV post-SVR, non-viral | U.S., Japan | 331 + 164 + 146 | Caucasian, Asian | 100% + 74% + 80% | Within the study | [36] |
| PLSec-NAFLD | 3 | II/2a | 4 proteins | Cohort | Prospective-retrospective | Developement | NAFLD | U.S. | 59 | Caucasian | 100% | Within the study | [37] | |
| miRNA | 3 | III/2a | 5 miRNAs | Cohort | Prospective-retrospective | Developement | HBV, HCV | Taiwan | 220 + 110 | Asian | 100% + 100% | Within the study | [313] | |
| Imaging-based | MEFIB | 2 | n.a. | MRE, FIB-4 | Cohort | Meta-analysis of 4 cohort studies | Developement | NAFLD | U.S., Japan, Turkey | 2,018 | Caucasian, Asian, Hispanic | n.a. | In independent studies | [40] |
| Pathogen-based | Serum virome | 3 | III/2a | Viral exposure signature | Case-control + Cohort | Retrospective + Prospective-retrospective | Development | HCV | U.S. | 150 : 337 + 173 | Caucasian, Black | n.a. + 25% | Within the study | [137] |
| Gut micriobiome | 1 | n.a. | Stool microbiome signature | Case-control | Prospective-retrospective | Presence | HBV | China | 75 : 40 + 30 : 56 | Asian | n.a. | Within the study | [132] | |
| Serum microbiome | 1 | n.a. | 5-microbiome signature | Case-control | Retrospective | Presence | HBV | Korea | 79 : 83 + 79 + 83 | Asian | n.a. : 100% + n.a. : 100% | Within the study | [314] |
Prospective-retrospective enrollment indicates Prospective sample collection–Retrospective-Blinded Evaluation (PRoBE) design.
No. subjects for training and validation sets are separately shown with “+” in between. No. subjects of different studies are separately shown with “;” in between. No. subjects for case-control studies are shown as HCC case : control.
HCC, hepatocellular carcinoma; ILCA, International Liver Cancer Association; NIS, Non-invasive score; NIT, non-invasive tests; HBV, hepatitis B virus; HCV, hepatitis C virus; NASH, non-alcoholic steatohepatitis; NAFLD, non-alcoholic fatty liver disease; PBC, primary biliary cirrhosis; AIH, autoimmune hepatitis; NA, nucleoside analogue; SVR, sustained virologic response; ADRESS, Age, Diabetes, Race, Etiology of cirrhosis, Sex, and Severity of liver dysfunction; REACH-B, risk estimation for hepatocellular carcinoma in chronic hepatitis B; UM, University of Michigan; LSM, liver stiffness measurement; NGM, nomogram; GAG-HCC, Guide with Age, Gender, HBV DNA, Core promoter mutations and Cirrhosis-HCC; RWS-HCC, Real-world risk score for HCC; REVEAL-HCV, Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer in HCV; FIB-4, fibrosis-4; THRI, Toronto HCC risk index; AGED, Age, Gender, HBeAg and HBV DNA; D2AS, HBV DNA, age, and sex; PAGE-B, platelets, age, and gender; CAGE-B, cirrhosis and age; SAGE-B, stiffness and age; HCC-RESCUE, HCC-Risk Estimating Score in CHB patients Under Entecavir; CAMPAS, Cirrhosis, Age, Male, Platelet, Albumin, liver Stiffness; GBM, gradient-boosting machine; ALT, alanine transaminase; REAL-B, Real-world Effectiveness from the Asia Pacific Rim Liver Consortium for HBV; AASL-HCC, Age, albumin, sex, liver cirrhosis-HCC; CAMD, cirrhosis, age, male sex, and diabetes mellitus; APA-B, age, platelet count, and AFP; ADRES, After DAAs Recommendation for Surveillance; PRS, polygenic risk scores; PRS-HFC, PRS of hepatic fat content; PLS, Prognostic Liver Signature; PLSec, Prognostic Liver Secretome signature; MEFIB index, an idex calculated from magnetic resonance elastography and FIB-4; MRE, magnetic resonance elastography.
HCC risk scores based on clinical variables
Many clinical HCC risk scores have been proposed in various regional populations, representing diverse HCC etiology and race/ethnicity, based on etiology-agnostic clinical variables such as age, sex, hepatic transaminases, and platelet count with or without etiology-specific variables such as status of viral hepatitis, alcohol abuse, and metabolic disorders. These scores are readily available and could be useful as the initial step of risk enrichment followed by application of more accurate molecular risk biomarkers tailored for specific clinical context. Some of the scores were developed in a cohort of patients with various HCC etiologies within a specific region, which may compromise general applicability of the scores to other regions with distinct etiology. Some scores were developed in more homogeneous population such as patients with HBV infection, in which head-to-head comparison between the scores clarified superior performance of several scores such as REAL-B and PAGE-B.[70, 71] Toronto HCC risk index[72] and aMAP risk score are examples of externally validated etiology-agnostic risk scores.[73] In a systematic comparison between six clinical HCC risk scores in HCV-cured cirrhosis patients in the U.K., aMAP score outperformed other scores.[74] This study also found that age plays a substantial role in the risk prediction, and their performance was suboptimal in the older patient subgroup. In viral hepatitis patients, quickly evolving anti-viral therapies will be critical confounding factors in the risk score performance. New-generation anti-HBV drugs under development may have a significant impact in predicting HBV-related HCC risk, while viral control/cure may not eliminate the risk as observed in HCV-cured cirrhosis patients who are at risk for nearly a decade.[75] Serum AFP is currently used as an HCC detection tumor marker, while it is frequently selected as a variable in HCC risk scores. It is empirically known that mild AFP elevation is often observed when hepatic injury and regeneration occur following a transient flare of hepatic inflammation due to active HCV infection even in the absence of HCC. Indeed, AFP elevation can be observed more than a decade before HCC diagnosis.[36, 76] Interestingly, baseline AFP levels decrease along with a resolution of hepatic inflammation after achieving HCV cure, namely SVR, and AFP elevation post-SVR is more specifically associated with HCC risk.[77]
Combinations of clinical variables have been explored to develop NIS/NITs mostly to detect liver disease severity such as fibrosis stage in viral hepatitis and NAFLD.[34] Not surprisingly, some of the NISs/NITs such as the FIB-4 were associated with future HCC risk in retrospective assessment (Table 1). In regional and national NAFLD cohorts, aspartate aminotransferase to platelet ratio index (APRI) and FIB-4 showed the highest association with cirrhosis-related morbidity, including HCC development, among 20 NISs/NITs.[42] Together with the scores specifically developed for HCC risk, the NISs/NITs may enable convenient risk enrichment in large patient population for further biomarker-based risk stratification and/or indication for chemopreventive interventions.
While most of the clinical risk scores were derived from conventional regression modeling, AI/ML/DL-based approaches have also been emerging. In 48,151 patients with HCV cirrhosis, recurrent neural network models outperformed logistic regression-based model in predicting 3-year HCC risk.[78] These promising results demonstrate utility of the new approaches, whereas there are several caveats such as overfitting to specific datasets/cohorts and the black-box nature of the DL/AI models that precludes adjustment guided by human interpretation. To avoid the issues and ensure transparency in model building, reproducible performance, and general applicability of DL/AI-based diagnostic/prognostic models, methodological and reporting guidelines have been developed.[79]
Germline DNA variants
As indicators of genetic susceptibility to HCC, SNPs have been extensively studied in the settings of genome-wide association study (GWAS) or hypothesis-driven single-gene analysis. The major logistical advantages of SNPs include easy access via readily available biospecimens such as buccal swab and the discrete measurement of genotypes less affected by assay conditions.[80] Prevalence of risk alleles/genotypes often varies across patient populations, and therefore may be associated with racial/ethnic and/or other disparities. Vast majority of the SNPs were evaluated in comparison between HCC cases and matched controls, and thus phase 3 validation (i.e., analysis of samples obtained before HCC development) is needed. SNPs in EGF, IFNL3, and MICA genes were associated with viral HCC risk, whereas SNPs in PNPLA3, TM6SF2, and HSD17B13 genes were associated mainly with metabolic etiology-related HCC.[81–86] A SNP in WNT3A-WNT9A was recently identified for its association with alcohol-related HCC.[87] Despite the logistical advantages, magnitude of HCC risk association for these individual SNPs is generally modest with odds ratio of 1.5 or less. To overcome the limited risk association of single SNP and improve risk enrichment, combinations of multiple SNPs have been explored as polygenic risk scores in HCV-SVR and NAFLD patients.[83, 88] However, a recent national biorepository-based study reported that additional prognostic information gained by such multi-SNP scores beyond readily available NISs/NITs is likely minimal.[41] This may not necessarily indicate that the SNP-based risk assessment is useless given that information about several confounding factors was not available in the population-based study, but suggest that specific clinical contexts/scenarios should be carefully considered when applying the SNP-based scores to maximize their utility.
Tissue-based molecular HCC risk biomarkers
Tissue transcriptome has been extensively studied as a direct source to interrogate molecular aberrations that drive HCC development.[80] Prognostic Liver Signature (PLS) is an example of hepatic transcriptome signature predictive of long-term HCC risk in all major viral and metabolic HCC etiologies.[89–93] Of note, PLS can be induced by HBV, HCV, ethanol, or free fatty acids in a cell culture model called cell culture-derived PLS (cPLS) for high-throughput drug screening and functional study.[94, 95] Such transcriptomic signatures can capture various types of molecular dysregulations involved in the mechanisms of hepatocarcinogenesis, including hepatic injury and regeneration,[96] HCC-promoting status of hepatic stellate cells,[97–99] and presence of pathogenic histological structures such as ectopic lymphoid structure as a niche supporting malignant transformation.[100]
Tissue-based histopathological HCC risk scores/biomarkers
Histological fibrosis stage is associated with magnitude of future HCC risk, although sampling bias in liver biopsy and low inter-observer agreement impair its reproducibility.[13] Collagen proportionate area based on immunostaining of fibrous tissue enables more robust and quantitative measurement of fibrosis severity and reliable HCC risk estimation.[101] Second harmonic generation/two-photon excitation fluorescence microscopy combined with artificial intelligence enables more precise quantification and characterization of collagen in liver tissue to monitor subtle change in fibrosis,[102] which may refine HCC risk prediction. Infiltrating HCC risk-driving immune cell types, e.g., CXCR6+ PD-1+ CD8 T cells and IDO1+ conventional dendritic cells, can be conveniently estimated based on tissue transcriptome in NAFLD-affected livers.[37]
Body fluid-based HCC risk biomarkers
Body fluid such as blood, urine, ascites, and bile can serve as windows to detect hepatic or systemic molecular dysregulations associated with HCC risk less invasively compared to liver tissue biopsy. Serum cytokines such as interleukin (IL)-6, IL-17, and IL-27 and serum proteins such as laminin γ2 monomer and insulin-like growth factor (IGF)-I were reported as correlates of HCC risk.[103–108] A serum surrogate of tissue-based PLS, Prognostic Liver Secretome signature (PLSec), was developed as a “liquid liver biopsy”, and its combination with AFP (PLSec-AFP) was validated as an etiology-agnostic HCC risk biomarker in cirrhosis from mixed etiologies and HCV-SVR.[36, 109] PLSec-AFP also predicted development of hepatic decompensation in cirrhosis patients.[110] NAFLD-specific “plug-in” module, PLSec-NAFLD, refined HCC risk prediction with the etiology-agnostic PLSec-AFP as a proof of concept of integrative test to optimize prognostic performance according to specific clinical context.[37] Tissue transcriptome signatures can be converted by a generic computational pipeline, TexSEC (www.texsec-app.org), to facilitate development of non-invasive biomarkers reflecting hepatic tissue-based molecular information.[36, 111] Chemical modifications of serum proteins such as glycomics-based GlycoCirrhoTest represent another type of proteome-based HCC risk biomarker.[112] Metabolomic and lipidomic profiling by mass spectrometry (MS) and/or nuclear magnetic resonance spectroscopy in body fluid samples can also be non-invasive HCC risk biomarkers.[113] Liquid-chromatography-MS analysis identified serum metabolites associated with HCC risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort and a Korean prospective cohort.[114, 115]. Plasma phenylalanine and glutamine levels were associated with HCC incidence in Asian patients mainly affected with viral hepatitis.[116] Phenylalanyl-tryptophan and glycocholate were also identified as a serum metabolite biomarker in combination with AFP to detect pre-clinical HCC.[117]
Imaging-based HCC risk scores/biomarkers
The Liver Imaging Reporting and Data System (LI-RADS) category 3 and 4 (LR-3, LR-4) indicate suspicious hepatic nodules with no definite features of HCC, which are observed in one-fourth of the patients enrolled in HCC screening program.[118] Presence of these intermediate lesions is associated with elevated risk of HCC development not necessarily from the index lesions; 32% and 21% of HCC diagnoses following detection of LR-3 and LR-4 lesions were made elsewhere in the liver, respectively.[119, 120] These data suggest that the presence of LR-3/LR-4 legions may have utility for HCC risk stratification. An MRI radiomic feature-based model was developed to predict 3-year HCC risk in HBV cirrhosis patients (AUROC 0.64 in external validation).[121] This study supports radiomics as a promising tool for HCC risk stratification, although its reproducibility across different MRI systems is low.[122] Deep learning model of radiomic elastography features was used to determine liver fibrosis stage in chronic hepatitis B patients.[123] Hepatic venous pressure gradient (HVPG) is an interventional radiology-based measure of liver disease severity, which was correlated with HCC risk.[124] To circumvent the transcatheter-based procedure to measure HVPG, CT-based radiomics model, auto-machine-learning HVPG, was developed to non-invasively detect HVPG ≥ 20 mmHg (AUROC, 0.81 in internal test set).[125] Integrative scores combining imaging modalities and clinical variables/scores have also been actively explored mainly as tools to measure disease severity in NAFLD, and then assessed for risk of developing lethal complications, including HCC. FibroScan-AST (FAST) score was initially developed to detect significant disease activity and fibrosis in NAFLD patients,[126] The score was later shown to be associated with HCC risk in HCV-cured patients, but not in NAFLD patients.[127, 128] Similarly, MRE-FIB-4 (MEFIB) index was developed to estimate fibrosis severity in NAFLD patients, and later was found to be associated with adverse outcomes, including HCC development.[40]
Pathogen-related HCC risk biomarkers
Microbiome in the digestive tract and changes in its composition, namely dysbiosis, are associated with exacerbating or protective effects on liver disease severity and HCC risk via cellular signaling such as toll-like receptor pathway, metabolites, bile acids, fatty acids, lipopolysaccharide, and other biomolecules.[129–131] Several intestinal bacteria such as Enterococcus, Limnobacter, and Phyllobacterium, oral Cyanobacteria, and duodenal Alloprevotella were associated with elevated HCC risk, whereas probiotic bacteria may attenuate HCC risk.[132–136] These reported HCC risk associations are likely influenced by variations between patient populations defined by dietary habits, host genetics/race, and geographic environmental factors, which need to be addressed before their application as HCC risk biomarkers. History of viral exposure measured by a viral exposure signature was associated with future HCC development.[137, 138]. Genomic integrations of HBV and adeno-associated virus 2 were associated with HCC risk even after seroclearance of hepatitis B surface antigen.[139] These pathogen-related features may serve as a new class of HCC risk biomarkers upon successful high-quality validation.
Environmental exposure-related HCC risk biomarkers
Food contamination with carcinogens such as aflatoxin B1 and aristolochic acid is known to increase HCC risk, not exclusively in developing countries.[2, 140] Several genetic aberrations have been reported as characteristic molecular features of dietary carcinogen exposure such as C>A transversions, hotspot somatic mutations in TP53, ADGRB1, and NEIL1 genes, high-level mutation-associated neoantigens, and infiltrating lymphocytes, and PD-L1 over-expression.[141–143] Prevalence of the aflatoxin exposure-related features in HCC patients was 9.8% in China, whereas the prevalence in patients from other regions was 0.4%-3.5%. A mutational signature of aristolochic acid exposure was observed in nearly 80% of Taiwanese HCC patients.[144] Prevalence of the mutational signature of aristolochic acid exposure in HCC patients ranged from 2.7% to 47% in Asia and from 1.7% to 4.8% in North America and Europe. These features may serve as HCC risk biomarkers according to their regional prevalence and magnitude of risk association that influence cost-effectiveness of HCC screening with the assays. The hotspot TP53 R249S was frequently observed in Hispanic HCC patients in South Texas, but its detection in cfDNA was not useful as HCC risk biomarker.[145]
Therapeutically modifiable HCC risk biomarkers
The HCC risk scores and/or biomarkers may identify at-risk liver disease patients who should be considered for preventive interventions because of elevated HCC risk (prognostic enrichment) and/or anticipated benefit of such intervention (predictive enrichment)[146] (Figure 3A). Many HCC risk scores based on readily available clinical variables (e.g., sex, age) and SNPs will allow convenient and low-cost enrichment of target population for HCC chemopreventive therapies. However, these features are not therapeutically modifiable, and therefore cannot be used to monitor therapeutic response. In contrast, other types of HCC risk biomarkers measuring abundance of functional biomolecules such as transcripts and proteins may enable real-time monitoring of dynamic change in HCC risk status in response to medical interventions. Such biomarkers may allow monitoring of biological response to chemopreventive therapies to gauge therapeutic modulation of HCC risk level in hepatic tissue milieu and/or systemic condition, which is distinct from measuring effect on direct molecular target of the therapy (Figure 3B). If the biomarker measurement is quantitatively correlated with future HCC incidence, the modulation may serve as surrogate biological endpoints in HCC chemoprevention clinical trials to infer anticipated reduction of future HCC incidence (Figure 3C). This is distinct from a surrogate biological endpoint that measures effect of tested agent on direct molecular targets (i.e., on-target effect). Such functional HCC risk biomarkers may resolve the long-standing logistical hurdle for chemoprevention clinical trials that typically require a large sample size and lengthy follow-up time exceeding the timeframe of typical clinical trials and studies.[13] In a previous HCC chemoprevention trial with S-adenosylmethionine (SAMe) in HCV cirrhosis patients, modulation of AFP was assessed as surrogate endpoint of HCC risk.[147] This trial failed to show decrease of AFP levels, and the concept of surriogate biomarkers for HCC risk is yet to be demonstrated.
Figure 3.
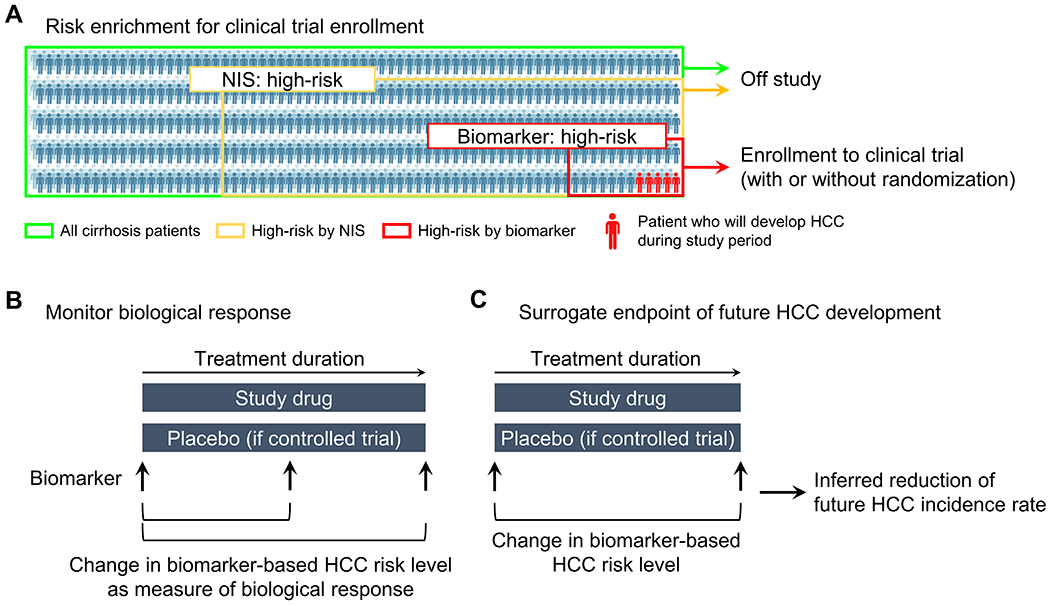
Potential use of HCC risk biomarkers in chemoprevention clinical trials. (A) Risk enrichment to select participants to be enrolled in chemoprevention clinical trials. Stepwise approach can be employed to identify super high-risk subgroup to increase HCC incidence rate for detection of chemopreventive effect in shorter time period with smaller sample size compared to conventional all-comer enrollment.[92] (B) Use of therapeutically modifiable HCC risk biomarker to monitor effect of experimental intervention on quantitative molecular HCC risk level. (C) Use of therapeutic modulation of HCC risk biomarker as a surrogate endpoint to estimate reduction of future HCC incidence.
Therapeutic modulation of hepatic transcriptome signatures were associated with magnitude of future HCC risk and prognosis in chronic liver disease patients treated with anti-HCV, bariatric surgery, and lipophilic statin.[37, 92, 93, 148] Of note, such transcriptome signatures can be modeled in cell culture model for in vitro high-throughput screening and functional assessment of experimental chemopreventive agents.[94, 95] Similarly, abundance of proteins in blood circulation was associated with reduction of HCC risk level after successful HCV cure by direct-acting antivirals that reflect reduced HCC incidence in subsequent clinical follow-up.[36] These promising observations have led to ongoing and planned HCC chemoprevention clinical trials of various agents using HCC risk biomarkers as surrogate endpoints for HCC incidence (NCT02273362, NCT05028829).
HCC EARLY DETECTION SCORES AND BIOMARKERS
Performance of the current standard-care HCC early detection tests, ultrasound and AFP, is suboptimal and needs improvement. To address the unmet need, new approaches have been explored by developing new biomarkers and imaging techniques integrated with existing tests (Table 2, Supplementary table 2), many of which are under active clinical testing (Table 3).
Table 2.
HCC early detection scores and biomarkers.
| Biomarker type | Score/biomarker (cutoff) | Biomarker development phase | Level of evidence (Simon et al./ILCA) | Variables | Study design | Enrollment | Major etiology | Region/country | No. subjects | Race/ethnicity | Cirrhosis (HCC : control) | Definition of early-stage HCC | Sensitivity | Specificity | AUROC | Other endpoints | Independent validation | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical tumor markers | AFP | 2-4 | n.a. | AFP | Meta-analysis of 30 cohort & case-control studies | Retrospective, prospective | HBV, HCV, alcohol, NAFLD | Korea, U.S., Taiwan, Japan, Italy, Egypt, Canada, Indonesia, France, Australia, Belgium, Spain | n.a. | n.a. | n.a. | BCLC 0/A or within Milan | 49% | 88% | n.a. | n.a. | In independent studies | [15] |
| AFP | 4 | n.a. | AFP | Meta-analysis of 11 cohort studies | Prospective | HCV, HBV, alcohol, NAFLD | U.S., Japan, Egypt, Italy, Korea, France | n.a. | n.a. | n.a. | BCLC 0/A or within Milan | 55% | 90% | n.a. | n.a. | In independent studies | [15] | |
| AFP | 3 | n.a. | AFP | Meta-analysis of 18 cohort studies | Prospective-retrospective | HBV, HCV, alcohol, NAFLD | Korea, Taiwan, U.S., Italy, Japan, Canada, Indonesia, Australia, Belgium, Spain | n.a. | n.a. | n.a. | BCLC 0/A or within Milan | 38% | 90% | n.a. | n.a. | In independent studies | [15] | |
| AFP (20 ng/mL) | 2 | n.a. | AFP | Meta-analysis of 6 case-control studies | Retrospective | HBV, HCV | China, Japan, U.S. | 1,722 | n.a. | n.a. | Resectable | 65% | 80% | n.a. | n.a. | In independent studies | [315] | |
| AFP (25/15 ng/mL) | 3/4 | IV/2b | AFP | Cohort | Prospective | HBV | U.S. (Alaska) | 32 HCC patients from 1,487 AFP-screened patients : 12 historcal HCC patients with no screening | n.a. | n.a. | Single, <6 cm | n.a. | n.a. | n.a. | 5y survival = 42% : 0%; 10y survival = 30% : 0% | In independent studies | [316] | |
| AFP (20 ng/mL) | 3 | III/2a | AFP | Cohort | Prospective-retrospective | HCV, alcohol, NASH | U.S.; U.S. (VA system) | 355 + 484 | Caucasian, Black, Latino; Caucasian | 100% + 100% | BCLC 0/A; single, ≤5 cm | 58%; 19% | 92%; 96% | n.a.; 0.71 | n.a. | In independent studies | [154, 156] | |
| AFP-L3% (n.a.) | 2/3 | n.a. | AFP-L3% | Meta-analysis of 6 cohort & case-control studies | Retrospective, prospective | HBV, HCV, alcohol | China, U.S., Germany, Japan, Korea | 497 (497) : 1950 | n.a. | n.a. | BCLC 0/A, AJCC I | 34% | 92% | 0.76 | n.a. | In independent studies | [153] | |
| AFP-L3% (10%) | 3 | III/2a | AFP-L3% | Cohort | Prospective-retrospective | HCV, alcohol, NASH | U.S.; U.S. (VA system) | 355 + 484 | Caucasian, Black, Latino; Caucasian | 100% + 100% | BCLC 0/A; single, ≤5 cm | 74%; 27% | 83%; 95% | n.a.; 0.64 | n.a. | In independent studies | [154, 156] | |
| DCP (n.a.) | 2/3 | n.a. | DCP | Meta-analysis of 11 cohort & case-control studies | Retrospective, prospective | HCV, HBV | U.S., Japan, China, Germany, France | 1,316 (1,316) : 1,892 | n.a. | n.a. | Single, <3cm | 64% | 87% | 0.86 | n.a. | In independent studies | [155] | |
| DCP (7.5 ng/mL) | 3 | III/2a | DCP | Cohort | Prospective-retrospective | HCV, alcohol, NASH | U.S.; U.S. (VA system) | 355 + 484 | Caucasian, Black, Latino; Caucasian | 100% + 100% | BCLC 0/A; single, ≤5 cm | 26%; 12% | 92%; 99% | n.a.; 0.72 | n.a. | In independent studies | [154, 156] | |
| Clinical scores | GALAD score (−0.63) | 2 | n.a. | Gender, age, AFP, AFP-L3%, DCP | Meta-analysis of 7 case-control studies | Retrospective | HBV, HCV, alcohol, NASH | U.S., Europe, Asia | 1,183 (1,183) : 2,838 | Caucasian, Asian, Hispanic, Black | n.a. | BCLC 0-A, AJCC I/II, within Milan | 69% | 91% | 0.83 | n.a. | In independent studies | - |
| GALAD score (−0.63) | 3 | n.a. | Gender, age, AFP, AFP-L3%, DCP | Meta-analysis of 2 cohort studies | Prospective-retrospective | HCV, alcohol, NASH | U.S.; U.S. (VA system) | 849 | Caucasian, Black, Latino; Caucasian | 100% | BCLC 0/A; single, ≤5 cm | 58% | 83% | 0.73 | n.a. | In independent studies | - | |
| HES algorithm | 3 | III/2a | AFP, change in AFP over the last year, age, platelets, ALT, and interaction terms | Cohort | Prospective-retrospective | HCV, alcohol, NASH | U.S.; U.S. (VA system) | 355 + 484 | Caucasian, Black, Latino; Caucasian | 100% + 100% | BCLC 0/A; single, ≤5 cm | 42%; 27% | 91%; 95% | n.a.; 0.76 | n.a. | In independent studies | [154, 156] | |
| Doylestown algorithm | 2 | IV/3 | Age, gender, logAFP, alkaline phosphatase, ALT | Case-control | Retrospective | HBV, HCV, others | U.S. | 165 (101) : 195 + 432 (225) : 438 + 113 (113) : 586 + 425 (140) : 804 | n.a. | 100% : 100% + 100% : 100% + 100% : 100% + 100% : 100% | BCLC 0/A | 43% (validation 1) ; 58% (validation 2) ; 35% (validation 3) | 95% (validation 1) ; 90% (validation 2) ; 95% (validation 3) | 0.81 (validation 1) ; 0.89 (validation 2) ; 0.77 (validation 3) | n.a. | In independent studies | [174] | |
| ASAP model | 2 | IV/3 | Gender, age, AFP, DCP | Case-control | Retrospective | HBV | China | 908 (318) : 603 + 286 (n.a.) : 211 | Asian | n.a. : 52% + n.a. : 46% | BCLC 0/A | 74% | 90% | n.a. | n.a. | Within the study | [317] | |
| AFP, DCP, D-dimer | 2 | IV/3 | AFP, DCP, D-dimer | Case-control | Retrospective | HBV | China | 59 (59) : 143 | Asian | n.a. : 100% | Single, ≤5 cm | 93% | 84% | 0.96 | n.a. | No | [318] | |
| Glycome + tumor marker + clinical variables | Doylestown Plus Algorithm | 3 | III/2a | Age, logAFP, PEG-precipitated IgG, fucosylated kininogen | Cohort | Prospective-retrospective | HCV, alcohol, NASH | U.S. | 29 (17) : 58 (matched) | Caucasian | 100% : 100% | BCLC 0/A | 80% | 90% | n.a. | n.a. | F/u study of Wang et al. | [176] |
| Doylestown Plus Algorithm | 2 | IV/3 | Age, gender, logAFP, alkaline phosphatase, ALT, fucosylated kininogen | Case-control | Retrospective | HCV, HBV, others | U.S. | 115 (69) : 93 | n.a. | 100% : 100% | Within Milan | 86% | 95% | 0.97 | n.a. | F/u study of Wang et al. | [175] | |
| N-glycopeptide N241_A4G4F2S4, AFP, Age | 1/2 | IV/3 | N-glycopeptide N241_A4G4F2S4, AFP, Age | Case-control | Retrospective | NASH | China | 32 (32) : 46 | Asian | n.a. : 100% | AJCC I/II | 72% | 90% | 0.9 | n.a. | Internal (cross-validation) | [319] | |
| Plasma cfDNA + tumor marker + clinical variables | HCCscreen | 3 | III/2a | Mutations in TP53, CTNNB1, AXIN1, TERT promoter, HBV integration breakpoint, AFP, DCP | Cohort | Prospective-retrospective | HBV | China | 331 | Asian | 0% : 11% | BCLC 0/A | 100% | 94% | n.a. | PPV = 17% | Within the study | [188] |
| HelioLiver test | 2 | IV/3 | 28 methylation markers, age, sex, AFP, AFP-L3%, DCP | Case-control | Retrospective | HBV, others | China | 46 : 236 + 122 (37) : 125 | Asian | n.a. + 37% : 37% | AJCC I/II | 76% | 91% | 0.92 | n.a. | Within the study | [320] | |
| Multitarget HCC blood test (mt-HBT) | 2 | IV/3 | 3 cfDNA methylation markers (HOXA1, TSPYL5, B3GALT6), sex, AFP | Case-control | Retrospective | HCV, alcohol, NASH, HBV | U.S., France, Germany, Italy, Spain, Taiwan, Thailand | 136 (81) : 404 + 156 (78) : 245 | Caucasian, Black, Asian | 96% : 93% + 97% : 92% | BCLC 0/A | 82% | 87% | 0.92 | n.a. | F/u study of Chalasani et al. | [167] | |
| Multitarget HCC panel | 2 | IV/3 | 4 cfDNA methylation markers (HOXA1, EMX1, TSPYL5, B3GALT6), AFP, AFP-L3% | Case-control | Retrospective | HCV, NAFLD, alcohol, HBV | U.S., France, Germany, Italy, Spain, Taiwan, Thailand | 135 (76) : 302 | Caucasian, Black, Asian | 90% : 87% | BCLC 0/A | 71% | 90% | 0.88 | n.a. | F/u study of Kiesel et al. | [180] | |
| CtDNA mutations, AFP, DCP | 2 | IV/3 | CtDNA mutations, AFP, DCP | Case-control | Retrospective | HBV | Korea | 102 (43) : 41 | Asian | 59% : 22% | BCLC A | n.a. | n.a. | 0.87 | n.a. | No | [321] | |
| Plasma cfDNA/ctDNA | Methylated SEPT9 | 2 | n.a. | Methylated SEPT9 | Meta-analysis of 6 case-control studies | Retrospective | NAFLD, HBV, HCV, alcohol, others | China, Japan, U.S., France, Germany, UK | 500 : 949 | n.a. | n.a. | n.a. | 80% (any stage) | 90% (any stage) | 0.92 (any stage) | n.a. | In independent studies | [186] |
| 32 5hmC markers | 1/2 | IV/3 | 32 5hmC markers | Case-control | Retrospective | HBV | China | 335 (335) : 263 + 220 (220) : 129 + 24 (24) : 180 | Asian | 70% : 28% + n.a. : 26% + n.a. : 0% | BCLC 0/A | 83% (validation 1) ; n.a. (validation 2) | 67% (validation 1) ; n.a. (validation 2) | 0.85 (validation 1) ; 0.92 (validation 2) | n.a. | Within the study | [187] | |
| CfDNA fragmentomics-based machine learning model | 1/2 | IV/3 | cfDNA fragmentomics | Case-control | Retrospective | HBV | China | 192 (134) : 170 + 189 (140) : 165 | Asian | 46% : 57% + 29% : 29% | BCLC 0/A | 90% (BCLC 0), 97% (BCLC A); | n.a. | n.a. | n.a. | Within the study | [190] | |
| HIFI score | 1/2 | IV/3 | 5hmC, motif, fragmentation, nucleosome footprint | Case-control | Retrospective | HBV | China | 225 (108) : 607 + 95 (35) : 100 + 131 (58) : 1800 | Asian | 61% : 57% + 64% : 100% + 70% : 100% | n.a. | 96%; 95% (any stage) | 95%; 98% (any stage) | 1.00; 1.00 (any stage) | n.a. | Within the study | [191] | |
| 6+1 cfDNA methylation markers | 1/2 | IV/3 | HOXA1, EMX1, AK055957, ECE1, PFKP, CLEC11A, B3GALT6 | Case-control | Retrospective | HCV, alcohol, NAFLD | U.S. | 95 (46) : 51 | n.a. | 98% : 100% | n.a. | 95% (any stage) | 86% (any stage) | 0.93 (any stage) | n.a. | In independent studies | [179] | |
| 7 cfDNA methylation markers | 1/2 | IV/3 | ASCL2, LDHB, LGALS3, LOXL3, PLXND1, OSR1, RASSF2 | Case-control | Retrospective | NAFLD, alcohol, HCV, others | Germany (training), U.S. (validation) | 46 : 41 + 60 (49) : 103 | n.a. | 100% : 100% + 100% : 100% | n.a. | 57% (any stage) | 97% (any stage) | 0.85 (any stage) | n.a. | Within the study | [183] | |
| ctDNA somatic copy number aberration-based machine learning model | 1/2 | IV/3 | Somatic copy number aberration | Case-control | Retrospective | HBV | China | 108 (73) : 101 + 38 (38) : 38 + 51 (51) : 48 | Asian | 77% : 42% + 68% : 58% + 63% : 46% | BCLC 0/A | 56% (validation 1) ; 53% (validation 2) | 90% (validation 1) ; 96% (validation 2) | 0.92 (validation 1) ; 0.81 (validation 2) | n.a. | Within the study | [322] | |
| 10 ctDNA methylation markers | 1/2 | IV/3 | BMPR1A, PSD, ARHGAP25, KLF3, PLAC8, ATXN1, Chr 6 : 170, Chr 6 : 3, ATAD2, Chr 8 : 20 | Case-control | Retrospective | HBV, HCV, NAFLD | China | 715 : 560 + 383 : 275 | Asian | n.a. : 0% | n.a. | 83% (any stage) | 91% (any stage) | 0.94 (any stage) | n.a. | Within the study | [323] | |
| CTC | 4 mRNA markers | 2 | IV/3 | EpCAM, CD90, CD133, CK19 | Case-control | Retrospective | HBV | China | 200 (131) : 101 + 195 (94) : 200 | Asian | 78% : n.a. + 80% : n.a. | BCLC 0/A | 85% | 93% | 0.93 | n.a. | Within the study | [196] |
| 9 mRNA markers | 1/2 | IV/3 | AFP, ALB, APOH, FABP1, FGB, FGG, AHSG, RBP4,TF | Case-control | Retrospective | HBV, HCV, alcohol | U.S. | 16 (9) : 57 | n.a. | n.a. : 25% | n.a. | n.a. | n.a. | 0.88 (any stage) | n.a. | Internal (cross-validation) | [195] | |
| EpCAMmRNA+ CTCs | 2 | IV/3 | EpCAMmRNA+ CTCs | Case-control | Retrospective | HBV | China | 157 (119) : 120 | Asian | 82% : n.a. | n.a. | 43% (any stage) | 97% (any stage) | 0.70 (any stage) | n.a. | No | [194] | |
| Circulating ncRNA | 8 miRNA markers | 1/2 | IV/3 | miR-320b, miR-663a, miR-4448, miR-4651, miR-4749-5p, miR-6724-5p, miR-6877-5p, miR-6885-5p | Case-control | Retrospective | HCV, HBV, others | Japan | 172 (108) : 64 + 173 (123) : 75 | Asian | n.a. : 69% + n.a. : 65% | AJCC I/II | 98% | n.a. | 0.99 (any stage) | n.a. | Within the study | [198] |
| miR-10a, miR-125b | 1/2 | IV/3 | miR-10a, miR-125b | Case-control | Retrospective | HBV | China | 65 : 75 | Asian | n.a. | n.a. | 99% (any stage) | 99% (any stage) | 0.99 (any stage) | n.a. | No | [199] | |
| miR-16 | 2 | IV/3 | miR-16 | Case-control | Retrospective | n.a. | China | 100 (100) : 20 | Asian | n.a. : 100% | BCLC 0/A | 87% | 90% | 0.94 | n.a. | No | [324] | |
| lncRNA-AF085935 | 2 | IV/3 | lncRNA-AF085935 | Case-control | Retrospective | HBV; HBV | China; Egypt | 137 : 104 + 70 : 70 | Asian; n.a. | n.a. | n.a.; n.a. | n.a.; 56% (any stage) | n.a.; 96% (any stage) | 0.86 (any stage); 0.81 (any stage) | n.a. | In independent studies | [200, 201] | |
| lncRNA-uc003wbd | 2 | IV/3 | lncRNA-uc003wbd | Case-control | Retrospective | HBV; HBV | China; Egypt | 137 : 104 + 70 : 70 | Asian; n.a. | n.a. | n.a.; n.a. | n.a.; 87% (any stage) | n.a.; 96% (any stage) | 0.70 (any stage); 0.96 (any stage) | n.a. | In independent studies | [200, 201] | |
| 2 lncRNA markers | 1/2 | IV/3 | MIR4435-2HG, lnc-POLD3-2 in PBMCs | Case-control | Retrospective | HBV | Thailand | 100 (35) : 200 | Asian | 80% : 6% | BCLC 0/A | 85% | n.a. | n.a. | n.a. | No | [325] | |
| CircPanel | 1/2 | IV/3 | hsa_circ_0000976, hsa_circ_0007750, hsa_circ_0139897 | Case-control | Retrospective | HBV | China | 158 (54) : 102 + 152 (59) : 104 + 290 (88) : 160 | Asian | 70% : 49% + n.a. : 48% + n.a. : 50% | Single, ≤ 3 cm | 83% (validation 1) ; 86% (validation 2) | 84% (validation 1) ; 86% (validation 2) | 0.85 (validation 1) ; 0.85 (validation 2) | n.a. | Within the study | [202] | |
| hTERT mRNA | 2 | IV/3 | hTERT mRNA | Case-control | Retrospective | HBV, HCV | Vietnam | 170 (92) : 170 | Asian | 100% : 100% | BCLC 0/A | 88% | 96% | 0.94 | n.a. | No | [326] | |
| EV-related biomarker | 3-small RNA cluster signature | 1/2 | IV/3 | smRC_119591, smRC_135709, smRC_48615 | Case-control | Retrospective | n.a. | U.S. | 105 (105) : 85 | n.a. | 67% : 72% | BCLC 0/A | 86% | 91% | 0.87 | n.a. | Internal (cross-validation) | [206] |
| 3 lncRNA markers and AFP | 1/2 | IV/3 | ENSG00000248932.1, ENST00000440688.1, ENST00000457302.2, AFP | Case-control | Retrospective | HBV, HCV | China | 20 + 180 : 200 | Asian | n.a. | n.a. | n.a. | n.a. | 0.87 (any stage) | n.a. | Within the study | [207] | |
| LINC00853 | 1/2 | IV/3 | LINC00853 | Case-control | Retrospective | HBV | Korea | 90 (46) : 63 | Asian | n.a. : 56% | mUICC I/II | 91% | 85% | 0.95 | n.a. | No | [205] | |
| Lnc85 | 1/2 | IV/3 | Lnc85 | Case-control | Retrospective | n.a. | China | 112 : 43 | Asian | n.a. : 100% | n.a. | 80% (any stage) | 74% (any stage) | 0.89 (any stage) | n.a. | No | [327] | |
| 4 miRNA markers | 1/2 | IV/3 | miR-10b-5p, miR-221-3p, miR-223-3p, miR-21-5p | Case-control | Retrospective | HCV, HBV | India | 38 (20) : 60 | Asian | 71% : 42% | n.a. | 58% (any stage) | 95% (any stage) | 0.80 (any stage) | n.a. | No | [328] | |
| miR-10b-5p | 1/2 | IV/3 | miR-10b-5p | Case-control | Retrospective | HBV | Korea | 90 (46) : 60 | Asian | n.a. : 55% | mUICC I/II | 94% | 78% | 0.95 | n.a. | No | [204] | |
| lncRNA, miRNA markers and AFP | 1/2 | IV/3 | ENSG00000258332.1, LINC00635, AFP | Case-control | Retrospective | HBV | China | 60 (16) : 96 + 55 : 60 | Asian | 70% : 0% + n.a. : 0% | n.a. | 85% (any stage) | 85% (any stage) | 0.89 (any stage) | n.a. | Within the study | [329] | |
| 2 miRNA markers and AFP | 1/2 | IV/3 | miR-122, miR-148a, AFP | Case-control | Retrospective | HBV | China | 50 (37) : 40 | Asian | n.a. : 100% | Within Milan | 87% | 90% | 0.95 | n.a. | No | [330] | |
| 10 mRNA markers | 2 | IV/3 | AFP, GPC3, ALB, APOH, FABP1, FGB, FGG, AHSG, RBP4,TF | Case-control | Retrospective | HCV, alcohol, NAFLD | U.S. | 36 (36) : 26 | Caucasian, Asian, Hispanic, Black | 100% : 100% | BCLC 0/A | 94% | 89% | 0.93 | n.a. | No | [208] | |
| HCC EV ECG score | 2 | IV/3 | EpCAM+ CD63+ EV, CD147+ CD63+ EV, GPC3+ CD63+ EV | Case-control | Retrospective | HCV, alcohol, NAFLD, HBV | U.S. | 45 (45) : 61 + 35 (35) : 37 | Caucasian, Hispanic, Asian | 82% : 100% + 86% : 100% | BCLC 0/A | 91% | 81% | 0.93 | n.a. | Within the study | [210] | |
| EV number | 2 | IV/3 | Amount of AnnexinV+ EpCAM+ ASGPR1+ EV | Case-control | Retrospective | n.a. | Germany | 86 : 49 | n.a. | n.a. : 100% | n.a. | 81% (any stage) | 47% (any stage) | 0.73 (any stage) | n.a. | No | [331] | |
| EV number | 2 | IV/3 | Amount of total EVs | Case-control | Retrospective | HBV, alcohol | China | 48 (48) : 40 | Asian | n.a. : 100% | AJCC I/II | 63% | 89% | 0.83 (AJCC I), 0.94 (AJCC II) | n.a. | No | [332] | |
| Serum protein | Golgi protein 73 | 2 | n.a. | Golgi protein 73 | Meta-analysis of 3 case-control studies | Retrospective | HCV, HBV, alcohol, others | U.S., China | 354 (354) : 581 | n.a. | n.a. | AJCC I/II | 79% | 62% | n.a. | n.a. | In independent studies | [219] |
| Osteopontin | 2 | n.a. | Osteopontin | Meta-analysis of 4 case-control studies | Retrospective | HBV | China, Thailand, Australia | 511 (511) : 523 | n.a. | n.a. | BCLC 0/A | 49% | 72% | n.a. | n.a. | In independent studies | [214] | |
| Midkine | 2 | n.a. | Midkine | Meta-analysis of 4 case-control studies | Retrospective | HBV, HCV | China, Australia, Egypt | n.a. | n.a. | n.a. | BCLC 0/A | 84% | 82% | 0.87 | n.a. | In independent studies | [220] | |
| Midkine | 3 | III/2a | Midkine | Cohort | Prospective-retrospective | HCV, HBV | Australia | 28 : 84 (matched) | n.a. | n.a. | n.a. | 67% (any stage) | n.a. | n.a. | n.a. | No | [333] | |
| AKR1B10 | 2 | IV/3 | AKR1B10 | Case-control | Retrospective | HBV | China | 209 (79) : 50 + 204 (75) : 60 | Asian | 48% : 80% + 50% : 63% | BCLC 0/A | 61% | 86% | 0.76 | n.a. | Within the study | [217] | |
| A panel of 7 autoantibodies | 1/2 | IV/3 | CIAPIN1, EGFR, MAS1, SLC44A3, ASAH1, UBL7, ZNF428 | Case-control | Retrospective | HBV, alcohol | China | 282 (60) : 130 + 279 (59) : 119 | Asian | n.a. : 100% + n.a. : 100% | BCLC 0/A | 70% | 91% | 0.88 | n.a. | Within the study | [221] | |
| MAP panel | 1/2 | IV/3 | 17 proteins, AFP, DCP | Case-control | Retrospective | HBV, HCV | Korea | 199 (199) : 199 + 85 (85) : 85 + 109 (109) : 50 | Asian | 62% : 79% + 66% : 75% + 83% : 100% | Within Milan | 81% (validation 1) ; 90% (validation 2) | 82% (validation 1) ; 98% (validation 2) | 0.91 (validation 1) ; 0.97 (validation 2) | n.a. | Within the study | [334] | |
| Metabolite Classifier | 1/2 | IV/3 | Benzoic acid, Creatine, Citrulline | Case-control | Retrospective | T2DM | China | 58 (n.a.) : 96 | Asian | n.a. | AJCC I/II | 92% | 82% | 0.94 | n.a. | No | [335] | |
| Urine-based biomarker | A 2-stage model : AFP then a ctDNA panel of mutation and 2 methylation markers | 1/2 | IV/3 | AFP, TP53 mutation and 2 methylation markers (mRASSF1A, mGSTP1) | Case-control | Retrospective | HBV, HCV, others | U.S., Taiwan | 186 (86) : 423 | n.a. | n.a. : 34% | BCLC 0/A | 92% (BCLC 0), 77% (BCLC A) | 90% (BCLC 0), 90% (BCLC A) | n.a. | n.a. | Internal (cross-validation) | [222] |
| miR-93-5p | 2 | IV/3 | miR-93-5p | Case-control | Retrospective | HBV | China | 130 (64) : 65 | Asian | 100% : 0% | AJCC I/II | 88% | 95% | 0.90 | n.a. | No | [223] | |
| Surface-enhanced Raman spectroscopy with SVM algorithm | 1/2 | IV/3 | Surface-enhanced Raman spectroscopy | Case-control | Retrospective | n.a. | China | 55 : 49 | Asian | n.a. : 100% | n.a. | 80% (any stage) | 76% (any stage) | n.a. | n.a. | Internal (cross-validation) | [225] | |
| Imaging | Ultrasound | 2-4 | n.a. | Ultrasound | Meta-analysis of 34 cohort & case-control studies | Retrospective, prospective | HCV, HBV, alcohol, NAFLD | U.S., Korea, Taiwan, Thailand, France, Japan, Italy, Egypt, Canada, Argentina, India, Pakistan, Switzerland, Belgium, Australia, Spain | 13,544 | n.a. | n.a. | BCLC 0/A or within Milan | 52% | 88% | n.a. | n.a. | In independent studies | [15] |
| Ultrasound | 4 | n.a. | Ultrasound | Meta-analysis of 17 cohort studies | Prospective | HCV, HBV, alcohol, NAFLD | U.S., Korea, Thailand, France, Japan, Italy, Egypt, Canada, India, Pakistan | n.a. | n.a. | n.a. | BCLC 0/A or within Milan | 53% | 90% | n.a. | n.a. | In independent studies | [15] | |
| Ultrasound | 3 | n.a. | Ultrasound | Meta-analysis of 15 cohort studies | Prospective-retrospective | HCV, HBV, alcohol, NAFLD | U.S., Korea, Taiwan, Thailand, Japan, Italy, Argentina, Switzerland, Belgium, Australia, Spain | n.a. | n.a. | n.a. | BCLC 0/A or within Milan | 46% | 90% | n.a. | n.a. | In independent studies | [15] | |
| MRI | 2, 4 | n.a. | ECA-enhanced MRI, HBA-enhanced MRI, non-contrast AMRI | Meta-analysis of 5 cohort & case-control studies | Retrospective, prospective | HBV, HCV, alcohol | Korea, Turkey, U.S., Australia | 107 (107) : 1,237 | n.a. | n.a. | BCLC 0/A | 83% | 95% | n.a. | n.a. | In independent studies | [336] | |
| MRI | 4 | III/2a | Gadoxetic acid-enhanced MRI | Cohort | Prospective | HBV | Korea | 407 | Asian | 100% | BCLC 0, A | 85% (BCLC 0), 86% (BCLC A) | 97% | 0.90 (BCLC 0) | n.a. | No | [226] | |
| AMRI | 2-4 | n.a. | Non-contrast, HBA-enhanced, and dynamic ECA-enhanced AMRI | Meta-analysis of 6 cohort & case-control studies | Retrospective, prospective | HBV, HCV, alcohol | Korea, Australia | n.a. | n.a. | n.a. | BCLC 0 | 69% | n.a. | n.a. | n.a. | In independent studies | [227] | |
| AMRI | 4 | III/2a | Non-contrast AMRI | Cohort | Prospective | HBV, HCV, alcohol | Australia | 192 | n.a. | n.a. | BCLC 0/A | 83% | 98% | n.a. | n.a. | In independent studies | [230] | |
| AMRI | 4 | III/2a | Non-contrast AMRI | Cohort | Prospective | HCV | Egypt | 41 | n.a. | 100% | n.a. | 100% (any stage) | 100% (any stage) | n.a. | n.a. | In independent studies | [337] | |
| AMRI | 3 | III/2a | Non-contrast AMRI | Cohort | Prospective-retrospective | HBV | Korea | 382 with high-risk | Asian | 100% | n.a. | 79% (any stage) | 98% (any stage) | n.a. | n.a. | In independent studies | [231] | |
| Triple-phase CT | 5 | II/2a | Iodine-enhanced CT | RCT | Prospective | HCV | U.S. (VA system) | CT : US = 80 : 83 | White, Black | 100% | n.a. | 67% (any stage) | 94% (any stage) | n.a. | n.a. | No | [232] | |
| Dual-phase low-dose CT | 4 | III/2a | Iodine-enhanced CT | Cohort | Prospective | HBV | Korea | 137 with high-risk | Asian | 92% | BCLC 0, A | 82% (BCLC 0), 86% (BCLC A) | 96% | n.a. | n.a. | No | [233] | |
| CEUS | 4 | III/2a | Sonazoid-enhanced ultrasound | Cohort | Prospective intra-individual comparison design | HBV | Korea | 524 | Asian | 100% | BCLC 0/A | n.a. | n.a. | n.a. | Detection rate = 1.1%; false referral rate = 1.1% | No | [235] | |
| CEUS | 5 | II/2a | Sonazoid-enhanced ultrasound | RCT | Prospective | HCV, HBV | Japan | CEUS : US = 309 : 313 | Asian | 100% | n.a. | 100% (any stage) | 96% (any stage) | n.a. | n.a. | No | [236] | |
| Imaging + tumor marker | Ultrasound + AFP | 2-4 | n.a. | Ultrasound, AFP | Meta-analysis of 14 cohort & case-control studies | Retrospective, prospective | HBV, HCV, alcohol, NAFLD | Taiwan, Thailand, Egypt, U.S., Canada, Korea, Australia, Belgium, Spain | 7,140 | n.a. | n.a. | BCLC 0/A or within Milan | 74% | 84% | n.a. | n.a. | In independent studies | [15] |
| Ultrasound + AFP (20 ng/mL) | 5 | II/2a | Ultrasound, AFP | Cohort | Prospective | HBV | China (Shanghai) | Screening : control = 9,373 : 9,443 | Asian | n.a. | Single, <5 cm | n.a. | n.a. | n.a. | Screening arm vs. control arm : Standardized incidence (per 100,000) = 279 : 267; Early-stage HCC = 39 (45%) : 0 (0%); 5-year survival = 46% : 0% | No | [150] | |
| GALADUS score | 2 | IV/3 | GALAD score, ultrasound | Case-control | Retrospective | HCV, NAFLD, alcohol, HBV | U.S. | 111 (60) : 180 | Caucasian, Asian | 98% : 86% | BCLC 0/A | 88% | 94% | 0.97 | n.a. | No | [165] |
Prospective-retrospective enrollment indicates Prospective sample collection–Retrospective-Blinded Evaluation (PRoBE) design.
No. subjects for case-control studies are shown as HCC case (early-stage HCC) : control. No. subjects for training and validation sets are separately shown with “+” in between. No. subjects of different studies are separately shown with “;” in between.
Performance metrics for early-stage HCC within 6 months of diagnosis are presented in cohort studies unless indicated otherwise.
HCC, hepatocellular carcinoma; ILCA, International Liver Cancer Association; AFP, alpha-fetoprotein; AUROC, area under receiver operating characteristic curve; HBV, hepatitis B virus; HCV, hepatitis C virus; NASH, non-alcoholic steatohepatitis; NAFLD, non-alcoholic fatty liver disease; BCLC, Barcelona clinic liver cancer; AJCC, American Joint Committee on Cancer; DCP, des-gamma-carboxy prothrombin; GALAD, Gender, Age, AFP-L3%, AFP, and DCP; HES, Hepatocellular Carcinoma Early Detection Screening; PEG, polyethylene glycol; cfDNA, cell-free DNA; ctDNA, circulating tumor DNA; CTC, circulating tumor cell; ncRNA, noncoding RNA; EV, extracellular vesicle; AKR1B10, aldo-keto reductase family 1 member 10; SVM, support vector machine; MRI, magnetic resonance imaging; ECA, extracellular contrast agent; HBA, hepatobiliary agent; AMRI, abbreviated MRI; CT, computed tomography; US, ultrasound; CEUS, contrast-enhanced ultrasound.
Table 3.
Ongoing clinical trials evaluating risk stratification and early detection biomarkers for HCC.
| Biomarker type | Type of test/biomarker | Trial name | Test/biomarker | Target population | Biomarker development phase | Planned no. subjects | Country | Anticipated completion year | NCT No. |
|---|---|---|---|---|---|---|---|---|---|
| Risk stratification | Imaging, clinical test/feature | STARHE | Deep learning of clinical, biological, elastography/ultrasound features | Advanced fibrosis/cirrhosis on HCC screening | 1-3 | 400 | France | 2023 | NCT04802954 |
| SNP | n.a. | MMP1-1607 1G/2G (rs1799750) | Egyptian HCV cirrhosis | 1/2 | 200 | Egypt | 2022 | NCT03722628 | |
| Risk stratification/early detection | Circulating biomarker, microbiome, imaging | ELEGANCE | miRNA panel (early detection), microbiome/MRI/urine, plasma metabolome (risk prediction) | Chronic liver disease from HBV, HCV, or NAFLD | 1-3 | 2,000 | Singapore | 2025 | NCT04965259 |
| Early detection | Circulating biomarker | n.a. | Serum visfatin, vaspin | HCV-related HCC, cirrhosis, healthy controls | 1/2 | 100 | Egypt | 2022 | NCT04763707 |
| Circulating biomarker, clinical test/feature | n.a. | Glycotest HCC panel | HCC, cirrhosis | 2 | 766 | U.S. | 2022 | NCT03878550 | |
| Circulating biomarker | n.a. | lncRNAs-WRAP53, UCA1 | HCC, cirrhosis, and healty controls | 1/2 | 80 | Egypt | 2022 | NCT05088811 | |
| Circulating biomarker, clinical test/feature | ALTUS | mt-HBT, Oncoguard Liver (Multi-target HCC blood test) | Cirrhosis, HBV carriers | 4 | 3,000 | U.S. | 2025 | NCT05064553 | |
| Circulating biomarker, clinical test/feature | LIVER-1 | HelioLiver Test | HCC, controls undergoing routine imaging surveillance for HCC | 4 | 1,200 | U.S. | 2024 | NCT05199259 | |
| Circulating biomarker, clinical test/feature | HEPATIC | HelioLiver Test | HCC, cirrhosis, and HD controls | 2 | 1,000 | China | 2022 | NCT05053412 | |
| Circulating biomarker | SEPT9-CROSS | Epi proColon 2.0 CE (Plasma mSEPT9) | HCC cases and cirrhosis controls | 2 | 530 | France | 2023 | NCT03311152 | |
| Circulating biomarker | n.a. | ctDNA methylation and fragmentation markers, miRNA7, CTC | Esophageal cancer, gastric cancer, colorectal cancer, HCC, healthy controls, pre-cancer | 1-3 | 2,430 | China | 2022 | NCT05431621 | |
| Imaging | FASTRAK | non-contrast AMRI | Compensated cirrhosis | 4 | 944 | France | 2027 | NCT05095714 | |
| Imaging | n.a. | Low-contrast dose CT and deep learning-based reconstruction | Patients undergoing CT for HCC diagnosis or surveillance | 1-3 | 90 | Korea | 2022 | NCT04027556 | |
| Imaging | n.a. | Gadolinium-enhanced AMRI | Cirrhosis | 4 | 150 | U.S. | 2023 | NCT04288323 | |
| Imaging | n.a. | non-contrast AMRI, MRI | Cirrhosis AND reduced visualisation on ultrasound | 4 | 476 | Australia, New Zealand | 2027 | NCT04455932 | |
| Imaging | n.a. | CEUS and MRI | Cirrhosis, chronic liver disease from HBV, atypical hyperplasia nodules | 4 | 100 | China | 2023 | NCT05286099 | |
| Imaging | n.a. | Short MRI surveillance (SMS) protocol | High-risk cirrhosis and/or chronic liver disease | 4 | 470 | Netherlands | 2026 | NCT05429190 | |
| Clinical test/score, imaging | n.a. | HBsAg, AFP, ultrasound | Populations in Zhongshan City | 5 | 20,000 | China | 2023 | NCT02501980 | |
| Circulating biomarker, clinical test/feature, imaging | FAST-MRI Study | GALAD score, ctDNA, nc-AMRI | Cirrhosis | 4 | 820 | U.S. | 2025 | NCT04539717 | |
| Clinical test/score, imaging | STOP-HCC | GALAD score, ultrasound | Compensated cirrhosis | 4 | 1,600 | Saudi Arabia, Vietnam | 2032 | NCT05342350 | |
| Clinical test/score, imaging | n.a. | AFP, AFP-L3%, DCP, ultrasound, CT | Cirrhosis | 4 | 1,418 | Korea | 2026 | NCT04414956 | |
| Circulating biomarker, clinical test/feature, imaging | n.a. | Genetron HCC Methylation PCR Kit, AFP, ultrasound, MRI | HCC, cirrhosis, chronic liver disease; cirrhosis, chronic liver disease under surveillance | 1-4 | 4,816 | China | 2022 | NCT05343832 | |
| Circulating biomarker, imaging | n.a. | EV-RNA, imaging | HCC, biliary tract cancer, cirrhosis, chronic liver disease | 1/2 | 1,810 | U.S. | 2023 | NCT02908048 | |
| Circulating biomarker | n.a. | Chiroptical, Raman, infrared spectroscopy | HCC, cirrhosis, healty controls | 1/2 | 250 | Czechia | 2022 | NCT04221347 | |
| Monitor change in HCC risk level | Tissue transcriptome, immunostaining biomarker | n.a. | PLS, phospho-EGFR staining | Compensated cirrhosis | 1/2 | 25 | U.S. | 2022 | NCT02273362 |
| Circulating biomarker | n.a. | PLSec | Compensated cirrhosis | 2 | 60 | U.S. | 2026 | NCT05028829 |
ClinicalTrials.gov accessed in July 2022.
HCC, hepatocellular carcinoma; SNP, single nucleotide polymorphism; miRNA, micro RNA; MRI, magnetic resonance imaging; HCV, hepatitis C virus; HBV, hepatitis B virus; NAFLD, non-alcoholic fatty liver disease; ctDNA, circulating tumor DNA; CTC, circulating tumor cell; AMRI, abbreviated MRI; CT, computed tomography; CEUS, contrast-enhanced ultrasound; AFP, alpha-fetoprotein; GALAD, Gender, Age, AFP-L3%, AFP, and DCP; nc-AMRI, non-contrast AMRI; DCP, des-gamma-carboxy prothrombin; EV, extracellular vesicle; PLS, Prognostic Liver Signature; PLSec, Prognostic Liver Secretome Signature.
Clinical HCC tumor markers
AFP is the most commonly used HCC tumor marker currently incorporated in practice guideline-recommended HCC screening protocol.[7] In a recent meta-analysis of phase 2-4 biomarker studies, sensitivity of AFP for early-stage HCC is only 49% with specificity of 88%.[15] AFP can elevate due to non-malignant hepatic inflammation due to chronic hepatitis that limits specificity.[149] In the setting of HCC screening, addition of AFP improved sensitivity of ultrasound for detection of early-stage HCC from 53% to 74%.[15] AFP is the only HCC tumor marker assessed for its survival impact (i.e., phase 5 study) as a part of the recommended HCC screening protocol together with ultrasound.[8] It is ethically infeasible to conduct randomized controlled trial (RCT) comparing HCC screening vs. no screening, but one RCT conducted in China showed a 37% reduction in HCC mortality.[150] AFP-L3% is lens culinaris agglutinin-reactive fraction of AFP, which showed high specificity of 84%-98%, while sensitivity is limited to 13%-49%.[151–154] DCP, also known as protein induced by vitamin k absence or antagonist-II (PIVKA-II), showed similarly suboptimal sensitivity of 64% and specificity of 87% in a meta-analysis of mostly phase 2 studies.[155] In phase 3 studies for early-stage HCC detection, its sensitivity dropped to 12-26%.[154, 156] Given the complementary positivity of these tumor markers, their combination has been explored to improve their performance.[154, 157–159] In phase 3 studies testing their combinations, sensitivity ranged from 31% to 77% and specificity between 66% and 91% for early-stage HCC detection.[154, 159]
HCC risk scores based on tumor markers and clinical variables
Gender, Age, AFP-L3%, AFP, and DCP (GALAD) score was developed by using patient gender, age, AFP, AFP-L3%, and DCP to predict presence of HCC in 833 patients with chronic liver disease in the U.K.[160] Since the initial report, GALAD score has been extensively validated in global viral and metabolic liver disease patients from Germany, Hong Kong, Japan, China, and the U.S.,[161–168] which allowed us to perform meta-analysis by the phase of biomarker development. In meta-analysis of seven phase 2 biomarker studies, sensitivity, specificity, and AUROC for detection early-stage HCC were 69%, 91%, and 0.83, respectively, at the original cutoff of −0.63 (Figure 4, Table 2). In meta-analysis of two phase 3 studies, sensitivity, specificity, and AUROC for detection early-stage HCC were 58%, 83%, and 0.73, respectively, reiterating general limitation of phase 2 studies that can overestimate test performance. Subgroup analysis suggested that the score’s performance measured by AUROC is comparable across the HCC etiologies and geographic regions. Despite the superiority to the individual tumor markers, high false-positive rate (14% to 22%) raises concerns of potential harm and cost.[154, 156]. More recent studies have attempted to further improve performance of the score. Longitudinal measurement of GALAD achieved higher sensitivity (69%) compared to cross-sectional single-timepoint measurement (54%).[156] Integration of ultrasound (GALADUS) yielded sensitivity, specificity, and AUROC of 88%, 94%, and 0.97, respectively, for detection of early-stage HCC (Barcelona Clinic Liver Cancer [BCLC] stage 0/A).[165]
Figure 4.
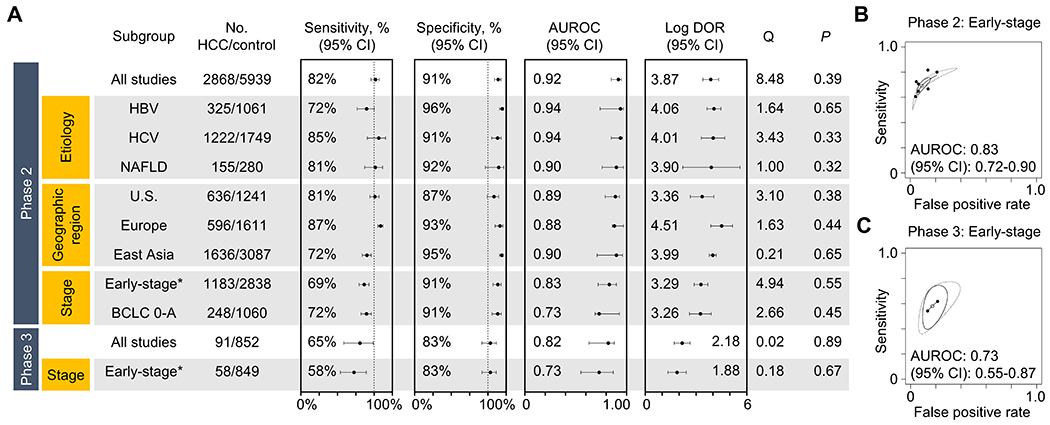
Performance of the GALAD score according to clinical subgroups and the phase of cancer screening biomarker study (meta-analysis). (A) Sensitivity, specificity, AUROC, and log diagnostic odds ratio (DOR) by clinical subgroups defined by HCC etiology, geographic region, and HCC stage. (B) Summary ROC curves for early-stage HCC in phase 2 (upper panel) and 3 (lower panel) studies are separately presented. DerSimonian and Laird random-effect method was used for the meta-analysis, and heterogeneity was assessed by Cochrane’s Q statistic. See Table 2 and Supplementary table 3 for details of the individual studies used for the meta-analysis.
Hepatocellular Carcinoma Early Detection Screening (HES) algorithm is another integrative composed of AFP, rate of AFP change within the last year, age, alanine aminotransferase (ALT), platelets, etiology, and interaction terms (AFP and ALT, and AFP and platelets) for HCC diagnosis in 6 months.[169, 170] The HES algorithm has serially validated in multiple phase 2 studies.[169–173] One of the largest studies in 709 patients reported sensitivity of 51% and specificity of 90% for early-stage HCC.[173] Phase 3 studies reported sensitivity ranging from 39% to 42% at fixed specificity of 90%.[154, 156] Its superiority to the GALAD score and individual tumor markers is yet to be conclusively determined.[154, 156]
Doylestown algorithm, comprised of age, gender, log AFP, alkaline phosphatase, and ALT, was developed for HCC detection and validated in serial phase 2 studies.[174, 175] With the addition of polyethylene glycol-precipitated IgG and fucosylated kininogen, a newer version, Doylestown Plus algorithm, was tested in a phase 3 study of 29 HCC patients and 58 matched cirrhosis controls and showed sensitivity of 80% at specificity of 90% and AUROC of 0.92 for early-stage (BCLC stage 0/A) HCC.[175, 176] Larger phase 2 study is ongoing to further validate the algorithm (NCT03878550).
Plasma cell-free DNA (cfDNA) and circulating tumor DNA (ctDNA)
cfDNA/ctDNA are fragmented DNA in circulation that are likely released from and/or associated with HCC cells and therefore may serve as a sensitive measure to detect presence of malignant cell in and/or outside the liver.[18, 177] cfDNA/ctDNA may reflect various types of biological information from the tumor, and may serve as sensitive tools to non-invasively detect early-stage HCC. Methylated cfDNA/ctDNA is cancer-specific circulating DNA fragments, and represents one of the most advanced types of HCC early detection biomarker toward clinical translation.
A 28-gene (covering 77 CpG sites) methylated cfDNA panel combined with AFP, AFP-L3%, DCP, age, and sex (HelioLiver Test) showed superior sensitivity (76%) for early-stage HCC (AJCC stage I/II) detection compared to AFP alone (57% at cutoff of 20 ng/mL) and the GALAD score (65% at cutoff of −0.63) in a phase 2 study.[178] AUROC for early-stage HCC detection for the HelioLiver Test, AFP, and the GALAD score were 0.92, 0.81, and 0.84, respectively. Another methylated cfDNA markers in three genes (HOXA1, TSPYL5, and B3GALT6) combined with AFP and sex (multi-target HCC blood test [mt-HBT] algorithm) showed sensitivity of 82% for early-stage HCC (BCLC stage 0/A) detection, which was higher than AFP (40%) and GALAD score (71%) in a phase 2 study. [167, 179, 180] AUROC for early-stage HCC detection for the mt-HBT algorithm, AFP, and the GALAD score were 0.92, 0.84, and 0.89 for GALAD, respectively. SEPT9 is involved in the process of liver carcinogenesis, and its methylation level in cfDNA showed a pooled sensitivity of 80% and specificity of 90% for all-stage HCC detection in a meta-analysis of six case-control studies conducted in Europe, Asia, and the U.S.[181–186] A 32-gene 5-hydroxymethylcytosine (5hmC) markers in cfDNA selected from genome-wide profiling showed AUROCs of 0.85 and 0.92 in detecting early-stage (BCLC stage 0/A) HCC in a phase 2 study Chinese patients with HBV infection or cirrhosis.[187]
HCC-specific somatic DNA mutations in TP53, CTNNB1, AXIN1, and TERT promoter, HBV integration breakpoint, combined with serum AFP and DCP (HCCscreen) distinguished 65 HCC patients from 70 HBV-infected patients with AUROC, sensitivity, and specificity of 0.93, 85%, and 93%, respectively, in a phase 2 study.[188] HCCscreen was positive in four patients 6-8 months prior to early-stage HCC diagnosis among 331 HBV-infected patients under HCC screening. Despite this encouraging result, the sample size was small and positive predictive value only 17%, which may cause unnecessary harms from HCC screening.[189] A new approach utilized HCC-specific length of cfDNA fragments from shallow-read whole-genome sequencing data as fragmentomics profile to detect early-stage HCC and intrahepatic cholangiocarcinoma in a phase 1/2 study.[190] Integration of four genomic features (i.e., 5hmC, motif, fragmentation, and nucleosome footprint [HIFI]) yielded AUROC of 0.996 for all-stage HCC detection.[191]
Circulating tumor cell (CTC)
CTC has shown promising capability in prognostication of HCC patients.[192] For HCC screening, sensitivity of CTC count for detecting HCC is low at 60% despite the high specificity of 95% across different CTC platforms.[193] To address the suboptimal sensitivity and overcome technical limitations in CTC enumeration, RNA-based CTC detection methods were proposed and evaluated in phase 2 studies.[194–196] Leveraging a negative enrichment platform with quantitative real-time PCR, an mRNA panel, including EPCAM, THY1 (encoding CD90), PROM1 (encoding CD133), and KRT19, for identifying a CTC subpopulation with stem-like cell features in a large multicenter cohort comprising 1,006 patients.[196] The CTC detection panel distinguished early-stage (BCLC stage 0/A) HCC from cirrhosis and HBV-infected patients with AUROC of 0.93 in a phase 2 study.[196] Of note, the AUROC remained high (0.92) in AFP-negative subgroup. Phase 3 study for CTC-based test is still lacking.
Circulating noncoding RNA (ncRNA), extracellular vesicle (EV)
Non-coding RNA such as microRNA (miRNA), long non-coding RNA (lncRNA), and circular RNA (circRNA) are regulatory RNA species involved in a wide variety of biological processes in HCC.[197] A comprehensive miRNA profiling in serum samples from 345 HCC, 139 chronic hepatitis or cirrhosis, and 1,033 non-cancer controls derived an 8-miRNA panel, which demonstrated sensitivity of 98% for detecting early-stage HCC, outperforming sensitivity of AFP (59%) and DCP (40%), in this phase 1/2 study.[198] In patients with chronic hepatitis B, a combination of miR-10a and miR-125b,[199] and lncRNA-AF085935[200, 201] were suggested as potential HCC detection biomarkers, which showed AUROC of 0.99 and 0.81-0.86, respectively, for detecting all-stage HCC. A large multi-center Chinese study proposed a 3-circRNA panel (CircPanel) for screening HBV-related HCC.[202] CircPanel showed superior performance in detecting small HCC (single and ≤ 3 cm) in three independent cohorts of HBV-related cirrhosis and chronic hepatitis patients (AUROC, 0.81 to 0.87) compared to AFP (0.65 to 0.73), which was maintained in AFP-negative cases.
Extracellular vesicles (EVs), lipid bilayer-enclosed particles released from tumor and normal cells, can serve as cargos for various biomolecules, including mRNA, ncRNA, proteins, and lipids.[203] LINC00853 and miR-10b-5p were upregulated in HCC tissues and EVs with AUROC of 0.96 and 0.94 for single small (< 2 cm) HCC, respectively[204, 205] EV-derived LINC00853 was detectable in 97% of AFP-negative HCC patients. Whole RNA sequencing of EVs identified three small RNA clusters (smRC) specific to HCC, which showed sensitivity, specificity, and AUROC of 86%. 91%, and 0.87, respectively in 105 early-stage (BCLC stage 0/A) HCC and 85 chronic liver disease patients.[206] Microarray-based screening identified three lncRNAs, and their combination with AFP yielded AUROC of 0.87, although half the HCC cases were advanced metastatic disease.[207] An HCC-specific 10-EV-mRNA panel was identified by using microfluidics combination with reverse-transcription droplet digital PCR yielded sensitivity, specificity, and AUROC of 94%, 89%, and 0.93, respectively in 36 early-stage (BCLC stage 0/A) HCC patients and 26 cirrhosis controls.[208] EV-lipidome biomarkers were identified by using ultra-high-resolution mass spectrometry to distinguish HCC and cirrhotic patients.[209] A recent phase 2 study showed that HCC EV ECG score based on EpCAM+ CD63+, CD147+ CD63+, and GPC3+ CD63+ HCC EVs, yielded AUROCs of 0.95 and 0.93 for early-stage HCC (BCLC stage 0/A) detection in the training and validation cohorts, respectively.[210] EV-based biomolecules may have a potential role in HCC early detection, although its clinical assessment is still in early phase. These promising results warrant subsequent larger phase 2 studies.
Serum protein biomarkers
Several serum protein biomarkers, e.g., Golgi protein 73, osteopontin, glypican-3, midkine, and aldo-keto reductase family 1 member 10, have been evaluated as HCC early detection biomarkers in phase 2 studies and their meta-analysis.[211–220] Their performance is generally limited at least as single biomarkers, and the previous studies have failed to demonstrate superiority and/or additive benefit to the current standard-care tumor marker, AFP. HCC-associated autoantibodies represent an alternative serum protein-based approach to identify early-stage HCC. In a phase 1/2 comprehensive seromic survey, 7-autoantibody panel was developed and validated in a large multi-center cohort, showing sensitivity, specificity, and AUROC of 70%, 91%, and 0.88, respectively, for detection of early-stage (BCLC stage 0/A) HCC.[221]
Urine-based HCC early detection biomarkers
Urine is another type of biospecimen even more accessible, i.e., less invasively obtainable, than blood. In a phase 1/2 multi-center study, a urine ctDNA panel of TP53 mutation and two methylation markers, mRASSF1A and mGSTP1 was tested in 279 chronic hepatitis B, 144 cirrhosis, and 186 HCC patients.[222] This ctDNA panel alone did not outperform AFP, showing AUROC of 0.74 and 0.85, respectively. However, when two-step strategy was applied, i.e., AFP was first applied and then the ctDNA panel was used in patients with AFP < 20 ng/mL, AUROC was improved to 0.91. Sensitivities of detecting BCLC stage 0 and A HCC tumors with this two-step strategy were 92% and 77%, respectively, at fixed specificity of 90%. Elevated levels of urine miR-93-5p showed AUROC of 0.90 in detecting early-stage HBV-related HCC, although it is likely over-estimated performance given that the controls were heathy subjects.[223] Surface-enhanced Raman spectroscopy (SERS) is a highly sensitive technique to detect low-abundant biomolecules.[224] SERS combined with support vector machine algorithm applied to urine samples yielded sensitivity of 80% and specificity of 76% for detection of all-stage HCC in 55 HCC and 49 cirrhosis controls.[225] Given the logistical advantage in sample accessibility, urine will remain a promising source of molecular information for HCC early detection.
Imaging-based HCC early detection tests
MRI with multi-phase gadoxetic acid enhancement is a standard-care test for HCC diagnostic (not early detection).[6] This full MRI study shows obviously superior sensitivity (85%) compared to ultrasound (27%) for detection of early-stage HCC, but is too costly and logistically demanding as a test repeatedly applied at regular interval (i.e., 6 months) for HCC screening.[226] To leverage the performance of MRI with limited costs and procedural requirements, abbreviated MRI (AMRI) has been actively explored to develop protocol tailored as an HCC screening test.[227, 228] AMRI protocols can be classified into three types: non-contrast-enhanced, hepatobiliary contrast-enhanced, and dynamic extracellular contrast-enhanced AMRI.[229] The overall patient-level sensitivity and specificity of AMRI for HCC detection were 86% and 94%, respectively, in a meta-analysis regardless of the AMRI type, presence of cirrhosis, and HCC etiology.[227] Of note, sensitivity for BCLC stage 0 HCC significantly dropped to 69%.[227] In a prospective study directly comparing non-contrast-enhanced AMRI and ultrasound for HCC detection in 192 patients with chronic liver disease, sensitivity of AMRI for detecting six HCC patients was inferior to ultrasound (83% vs. 100%), although the sample size is too small to make a conclusive statement.[230] Another prospective study of 382 cirrhosis patients reported that non-contrast-enhanced AMRI had better patient-level sensitivity and specificity for HCC detection compared to ultrasound (sensitivities of 79% vs. 28% and specificities of 98% vs. 94% for AMRI vs. ultrasound, respectively).[231] These inconsistent findings may be attributable to variations in liver disease severity and/or etiology as well as other clinical confounders that affect baseline HCC risk. In addition, these studies were conducted in patients mostly affected with HBV and HCV infection, and the performance in patients with NAFLD and alcohol-associated liver disease is yet to be determined in ongoing studies.
CT with multi-phase dynamic iodinated contrast enhancement is another standard-care HCC diagnostic test.[6] Given the radiation exposure, CT is not generally considered as an HCC screening test that is applied every 6 months. In a single-center RCT in 163 compensated cirrhosis patients, annual triple-phase CT and biannual ultrasound showed similar sensitivities of 67% and 71%, specificities of 94% and 98%, and early-stage HCC detection rates of 63% vs. 56%, respectively.[232] To mitigate potential harms from radiation exposure, a prospective study compared biannual dual-phase low-dose CT (LDCT) and ultrasound in 137 chronic liver disease patients.[233] In this relatively small study, the dual-phase LDCT had better sensitivity in detecting all-stage HCC and BCLC-stage 0 HCC than ultrasound (83% and 29% for all-stage HCC; 82% and 18% for BCLC stage 0 HCC, respectively), suggesting potential utility of LDCT for HCC screening.
Contrast-enhanced ultrasound (CEUS) using microbubble-based agents enables assessment of vascularity for focal liver lesions for improved early-stage HCC detection.[234] A prospective intra-individual comparison was conducted to evaluate added value of CEUS to conventional B-mode ultrasound for HCC detection in 524 patients with predominantly HBV-related cirrhosis.[235] There was no significant improvement in detecting any stage or early-stage HCC with CEUS, whereas the false referral rate for definite diagnosis was significantly lower in the CEUS group. On the other hand, a multi-center RCT enrolling 622 HCV- or HBV-infected cirrhosis patients found that CEUS-based screening had a higher sensitivity for HCC detection than conventional B-mode ultrasound (100% and 65%, respectively).[236] In addition, observed HCC size detected by CEUS was significantly smaller compared to conventional ultrasound in all patients and HCV-infected subgroup (all patients: 13.0 mm vs. 16.7 mm; HCV subgroup: 12.7 mm vs. 17.6 mm). Further studies will be needed to determine utility/role of CEUS in HCC in early HCC detection.
FUTURE DIRECTIONS AND CONCLUSIONS
The evolving landscape of HCC etiology, particularly the global rise of NAFLD/MAFLD, continues to hamper the development of effective HCC screening strategy. HCC risk post HCV cure remains high for nearly a decade when cirrhosis is present, and therefore requires HCC screening.[75] Alcohol-associated liver disease stays as a major HCC etiology with notable inter-individual heterogeneity.[237] With the etiological landscape, precision and practical feasibility will need to be carefully balanced to ensure clinically acceptable costs and complexity for actual clinical translation and implementation of the risk-stratified HCC screening strategy. Prospective biorepositories and clinical databases representing global liver disease patient population will enhance and facilitate evaluation of clinical utility for promising biomarkers across geographic regions under the PRoBE principle. Innovative clinical trial design will also expedite the sequence of validations and help timely translation of the biomarkers. Predefined framework will be needed to measure net benefit of the screening intervention in controlling HCC burden and mortality at population level. Future research will also explore use of the biomarkers and assay technologies beyond the scope of HCC screening, including assessment of therapeutic effect, monitoring of residual disease after treatment, and prediction of recurrence or progression following surgical or medical therapies. Collectively, these developments are expected to lead to a transformative improvement of HCC mortality over the next decade.
Supplementary Material
Financial support:
This work was supported by Uehara Memorial Foundation to N.F.; American College of Gastroenterology (Junior Faculty Development Award), United States Department of Defense (Peer Reviewed Cancer Research Program Career Development Award, CA191051) to J.Y.; U.S. NIH (DK099558, CA233794, CA222900, CA230694, CA255621), European Commission (ERC-2014-AdG-671231, ERC-AdG-2020-101021417), Cancer Prevention and Research Institute of Texas (RR180016, RP200554) to Y.H. The funders had no role in the collection of data; the design and conduct of the study; management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
Abbreviations:
- HCC
hepatocellular carcinoma
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- NAFLD
non-alcoholic fatty liver disease
- MAFLD
metabolic dysregulation-associated fatty liver disease
- AFP
alpha-fetoprotein
- CT
computed tomography
- MRI
magnetic resonance imaging
- SVR
sustained virologic response
- DCP
des-gamma-carboxy prothrombin
- PLSec
Prognostic Liver Secretome Signature
- NIS
Non-invasive score
- NIT
non-invasive test
- FIB-4
fibrosis-4
- SNP
single nucleotide polymorphism
- AUROC
area under receiver operating characteristic
- PLS
Prognostic Liver Signature
- HVPG
hepatic venous pressure gradient
- GALAD
Gender, Age, AFP-L3%, AFP, and DCP
- BCLC
Barcelona Clinic Liver Cancer
- cfDNA
cell-free DNA
- ctDNA
circulating tumor DNA
- CTC
circulating tumor cell
- ncRNA
noncoding RNA
- AMRI
abbreviated MRI
- CEUS
contrast-enhanced ultrasound
Footnotes
Conflict of interest:
J.Y. serves as consultant for Exact Sciences, Exelixis, and Eisai. Y.H. serves as an advisory board member for Helio Genomics and Espervita Therapeutics, and share holder for Alentis Therapeutics and Espervita Therapeutics.
REFERENCE
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019;16:589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee YT, Singal AG, Luu M, Nissen NN, Gores GJ, Yang JD. Increasing Incidence of Intrahepatic Cholangiocarcinoma Relative to Hepatocellular Carcinoma in the United States. Gastro Hep Advances 2022;1:121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee YT, Wang JJ, Luu M, Tseng HR, Rich NE, Lu SC, Nissen NN, et al. State-Level HCC Incidence and Association With Obesity and Physical Activity in the United States. Hepatology 2021;74:1384–1394. [DOI] [PubMed] [Google Scholar]
- 5.Lee YT, Wang JJ, Luu M, Noureddin M, Kosari K, Agopian VG, Rich NE, et al. The Mortality and Overall Survival Trends of Primary Liver Cancer in the United States. J Natl Cancer Inst 2021;113:1531–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 7.Wen N, Cai Y, Li F, Ye H, Tang W, Song P, Cheng N. The clinical management of hepatocellular carcinoma worldwide: A concise review and comparison of current guidelines: 2022 update. Biosci Trends 2022;16:20–30. [DOI] [PubMed] [Google Scholar]
- 8.Singal AG, Zhang E, Narasimman M, Rich NE, Waljee AK, Hoshida Y, Yang JD, et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: A meta-analysis. J Hepatol 2022;77:128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeo YH, Hwang J, Jeong D, Dang N, Kam LY, Henry L, Park H, et al. Surveillance of patients with cirrhosis remains suboptimal in the United States. J Hepatol 2021;75:856–864. [DOI] [PubMed] [Google Scholar]
- 10.Wolf E, Rich NE, Marrero JA, Parikh ND, Singal AG. Use of Hepatocellular Carcinoma Surveillance in Patients With Cirrhosis: A Systematic Review and Meta-Analysis. Hepatology 2021;73:713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singal AG, Tiro JA, Murphy CC, Blackwell JM, Kramer JR, Khan A, Liu Y, et al. Patient-Reported Barriers Are Associated With Receipt of Hepatocellular Carcinoma Surveillance in a Multicenter Cohort of Patients With Cirrhosis. Clin Gastroenterol Hepatol 2021;19:987–995 e981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumert TF, Juhling F, Ono A, Hoshida Y. Hepatitis C-related hepatocellular carcinoma in the era of new generation antivirals. BMC Med 2017;15:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol 2018;68:526–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, Waljee AK, et al. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018;154:1706–1718 e1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singal AG, Haaland B, Parikh ND, Ozbay AB, Kirshner C, Chakankar S, Porter K, et al. Comparison of a multitarget blood test to ultrasound and alpha-fetoprotein for hepatocellular carcinoma surveillance: Results of a network meta-analysis. Hepatol Commun 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goossens N, Nakagawa S, Sun X, Hoshida Y. Cancer biomarker discovery and validation. Transl Cancer Res 2015;4:256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Z, Pepe MS. Adding Rigor to Biomarker Evaluations-EDRN Experience. Cancer Epidemiol Biomarkers Prev 2020;29:2575–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyu X, Tsui YM, Ho DW, Ng IO. Liquid Biopsy Using Cell-Free or Circulating Tumor DNA in the Management of Hepatocellular Carcinoma. Cell Mol Gastroenterol Hepatol 2022;13:1611–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singal AG, Parikh ND, Rich NE, John BV, Pillai A: Hepatocellular Carcinoma Surveillance and Staging. In: Hoshida Y, ed. Hepatocellular Carcinoma: Translational Precision Medicine Approaches. Cham (CH): Humana Press; Copyright 2019, Springer Nature Switzerland AG., 2019; 27–51. [PubMed] [Google Scholar]
- 20.Younossi ZM, Stepanova M, Ong J, Trimble G, AlQahtani S, Younossi I, Ahmed A, et al. Nonalcoholic Steatohepatitis Is the Most Rapidly Increasing Indication for Liver Transplantation in the United States. Clin Gastroenterol Hepatol 2021;19:580–589 e585. [DOI] [PubMed] [Google Scholar]
- 21.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2021;18:223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lockart I, Yeo MGH, Hajarizadeh B, Dore GJ, Danta M. HCC incidence after hepatitis C cure among patients with advanced fibrosis or cirrhosis: A meta-analysis. Hepatology 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mueller PP, Chen Q, Ayer T, Nemutlu GS, Hajjar A, Bethea ED, Peters MLB, et al. Duration and cost-effectiveness of hepatocellular carcinoma surveillance in hepatitis C patients after viral eradication. J Hepatol 2022;77:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon TG, Roelstraete B, Sharma R, Khalili H, Hagstrom H, Ludvigsson JF. Cancer Risk in Patients With Biopsy-Confirmed Nonalcoholic Fatty Liver Disease: A Population-Based Cohort Study. Hepatology 2021;74:2410–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, Loomba R, et al. Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N Engl J Med 2021;385:1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, May SB, et al. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol 2016;14:124–131 e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Ayada I, Zhang X, Wang L, Li Y, Wen T, Ma Z, et al. Estimating Global Prevalence of Metabolic Dysfunction-Associated Fatty Liver Disease in Overweight or Obese Adults. Clin Gastroenterol Hepatol 2022;20:e573–e582. [DOI] [PubMed] [Google Scholar]
- 28.Atiq O, Tiro J, Yopp AC, Muffler A, Marrero JA, Parikh ND, Murphy C, et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology 2017;65:1196–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chong N, Schoenberger H, Yekkaluri S, Fetzer DT, Rich NE, Yokoo T, Gopal P, et al. Association between ultrasound quality and test performance for HCC surveillance in patients with cirrhosis: a retrospective cohort study. Aliment Pharmacol Ther 2022;55:683–690. [DOI] [PubMed] [Google Scholar]
- 30.Santi V, Trevisani F, Gramenzi A, Grignaschi A, Mirici-Cappa F, Del Poggio P, Di Nolfo MA, et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. Journal of hepatology 2010;53:291–297. [DOI] [PubMed] [Google Scholar]
- 31.Trinchet JC, Chaffaut C, Bourcier V, Degos F, Henrion J, Fontaine H, Roulot D, et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology 2011;54:1987–1997. [DOI] [PubMed] [Google Scholar]
- 32.Nathani P, Gopal P, Rich N, Yopp A, Yokoo T, John B, Marrero J, et al. Hepatocellular carcinoma tumour volume doubling time: a systematic review and meta-analysis. Gut 2021;70:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goossens N, Singal AG, King LY, Andersson KL, Fuchs BC, Besa C, Taouli B, et al. Cost-Effectiveness of Risk Score-Stratified Hepatocellular Carcinoma Screening in Patients with Cirrhosis. Clin Transl Gastroenterol 2017;8:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anstee QM, Castera L, Loomba R. Impact of non-invasive biomarkers on hepatology practice: Past, present and future. J Hepatol 2022;76:1362–1378. [DOI] [PubMed] [Google Scholar]
- 35.Hughes DM, Berhane S, Emily de Groot CA, Toyoda H, Tada T, Kumada T, Satomura S, et al. Serum Levels of α-Fetoprotein Increased More Than 10 Years Before Detection of Hepatocellular Carcinoma. Clin Gastroenterol Hepatol 2021;19:162–170.e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujiwara N, Kobayashi M, Fobar AJ, Hoshida A, Marquez CA, Koneru B, Panda G, et al. A blood-based prognostic liver secretome signature and long-term hepatocellular carcinoma risk in advanced liver fibrosis. Med (N Y) 2021;2:836–850 e810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujiwara N, Kubota N, Crouchet E, Koneru B, Marquez CA, Jajoriya AK, Panda G, et al. Molecular signatures of long-term hepatocellular carcinoma risk in nonalcoholic fatty liver disease. Sci Transl Med 2022;14:eabo4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Younes R, Caviglia GP, Govaere O, Rosso C, Armandi A, Sanavia T, Pennisi G, et al. Long-term outcomes and predictive ability of non-invasive scoring systems in patients with non-alcoholic fatty liver disease. J Hepatol 2021;75:786–794. [DOI] [PubMed] [Google Scholar]
- 39.Woreta TA, Van Natta ML, Lazo M, Krishnan A, Neuschwander-Tetri BA, Loomba R, Mae Diehl A, et al. Validation of the accuracy of the FAST score for detecting patients with at-risk nonalcoholic steatohepatitis (NASH) in a North American cohort and comparison to other non-invasive algorithms. PLoS One 2022;17:e0266859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ajmera V, Kim BK, Yang K, Majzoub AM, Nayfeh T, Tamaki N, Izumi N, et al. Liver Stiffness on Magnetic Resonance Elastography and the MEFIB Index and Liver-Related Outcomes in Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Individual Participants. Gastroenterology 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bianco C, Jamialahmadi O, Pelusi S, Baselli G, Dongiovanni P, Zanoni I, Santoro L, et al. Non-invasive stratification of hepatocellular carcinoma risk in non-alcoholic fatty liver using polygenic risk scores. J Hepatol 2021;74:775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Innes H, Morling JR, Buch S, Hamill V, Stickel F, Guha IN. Performance of routine risk scores for predicting cirrhosis-related morbidity in the community. J Hepatol 2022. [DOI] [PubMed] [Google Scholar]
- 43.Poynard T, Lacombe JM, Deckmyn O, Peta V, Akhavan S, de Ledinghen V, Zoulim F, et al. External validation of LCR1-LCR2, a multivariable HCC risk calculator, in patients with chronic HCV. JHEP Rep 2021;3:100298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gellert-Kristensen H, Richardson TG, Davey Smith G, Nordestgaard BG, Tybjaerg-Hansen A, Stender S. Combined Effect of PNPLA3, TM6SF2, and HSD17B13 Variants on Risk of Cirrhosis and Hepatocellular Carcinoma in the General Population. Hepatology 2020. [DOI] [PubMed] [Google Scholar]
- 45.De Vincentis A, Tavaglione F, Jamialahmadi O, Picardi A, Antonelli Incalzi R, Valenti L, Romeo S, et al. A Polygenic Risk Score to Refine Risk Stratification and Prediction for Severe Liver Disease by Clinical Fibrosis Scores. Clin Gastroenterol Hepatol 2022;20:658–673. [DOI] [PubMed] [Google Scholar]
- 46.Goossens N, Bian CB, Hoshida Y. Tailored algorithms for hepatocellular carcinoma surveillance: Is one-size-fits-all strategy outdated? Curr Hepatol Rep 2017;16:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kutaiba N, Ardalan Z. Risk factors and screening intervals are crucial for evaluating the cost effectiveness of abbreviated MRI in HCC screening. J Hepatol 2021;75:1258–1259. [DOI] [PubMed] [Google Scholar]
- 48.Parikh ND, Singal AG, Hutton DW, Tapper EB. Cost-Effectiveness of Hepatocellular Carcinoma Surveillance: An Assessment of Benefits and Harms. Am J Gastroenterol 2020;115:1642–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lima PH, Fan B, Berube J, Cerny M, Olivie D, Giard JM, Beauchemin C, et al. Cost-Utility Analysis of Imaging for Surveillance and Diagnosis of Hepatocellular Carcinoma. AJR Am J Roentgenol 2019;213:17–25. [DOI] [PubMed] [Google Scholar]
- 50.Kim HL, An J, Park JA, Park SH, Lim YS, Lee EK. Magnetic Resonance Imaging Is Cost-Effective for Hepatocellular Carcinoma Surveillance in High-Risk Patients With Cirrhosis. Hepatology 2019;69:1599–1613. [DOI] [PubMed] [Google Scholar]
- 51.Nahon P, Najean M, Layese R, Zarca K, Segar LB, Cagnot C, Ganne-Carrie N, et al. Early hepatocellular carcinoma detection using magnetic resonance imaging is cost-effective in high-risk patients with cirrhosis. JHEP Rep 2022;4:100390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka H, Iijima H, Nouso K, Aoki N, Iwai T, Takashima T, Sakai Y, et al. Cost-effectiveness analysis on the surveillance for hepatocellular carcinoma in liver cirrhosis patients using contrast-enhanced ultrasonography. Hepatol Res 2012;42:376–384. [DOI] [PubMed] [Google Scholar]
- 53.Kim NJ, Rozenberg-Ben-Dror K, Jacob DA, Rich NE, Singal AG, Aby ES, Yang JD, et al. Provider Attitudes Toward Risk-Based Hepatocellular Carcinoma Surveillance in Patients With Cirrhosis in the United States. Clin Gastroenterol Hepatol 2022;20:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, Winget M, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst 2001;93:1054–1061. [DOI] [PubMed] [Google Scholar]
- 55.Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst 2009;101:1446–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borges KA, Dai J, Parikh ND, Schwartz M, Nguyen MH, Roberts LR, Befeler AS, et al. Rationale and design of the Hepatocellular carcinoma Early Detection Strategy study: A multi-center longitudinal initiative of the National Cancer Institute’s Early Detection Research Network. Contemp Clin Trials 2019;76:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanwal F, Khaderi S, Singal AG, Marrero JA, Loo N, Asrani SK, Amos CI, et al. Risk factors for HCC in contemporary cohorts of patients with cirrhosis. Hepatology 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Menon U, Gentry-Maharaj A, Burnell M, Singh N, Ryan A, Karpinskyj C, Carlino G, et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet 2021;397:2182–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Graden KC, Bennett SA, Delaney SR, Gill HE, Willrich MAV. A High-Level Overview of the Regulations Surrounding a Clinical Laboratory and Upcoming Regulatory Challenges for Laboratory Developed Tests. Lab Med 2021;52:315–328. [DOI] [PubMed] [Google Scholar]
- 60.Ritzhaupt A, Hayes I, Ehmann F. Implementing the EU in vitro diagnostic regulation - a European regulatory perspective on companion diagnostics. Expert Rev Mol Diagn 2020;20:565–567. [DOI] [PubMed] [Google Scholar]
- 61.Birkeland ML, McClure JS. Optimizing the Clinical Utility of Biomarkers in Oncology: The NCCN Biomarkers Compendium. Arch Pathol Lab Med 2015;139:608–611. [DOI] [PubMed] [Google Scholar]
- 62.Grossman RL, Dry JR, Hanlon SE, Johann DJ, Kolatkar A, Lee JSH, Meyer C, et al. BloodPAC Data Commons for Liquid Biopsy Data. JCO Clin Cancer Inform 2021;5:479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singal AG, Tiro JA, Murphy CC, Blackwell JM, Kramer JR, Khan A, Liu Y, et al. Patient-Reported Barriers Are Associated With Receipt of Hepatocellular Carcinoma Surveillance in a Multicenter Cohort of Patients With Cirrhosis. Clin Gastroenterol Hepatol 2021;19:987–995.e981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu M, Lu T, Jia Y, Luo X, Gopal P, Li L, Odewole M, et al. Somatic Mutations Increase Hepatic Clonal Fitness and Regeneration in Chronic Liver Disease. Cell 2019;177:608–621 e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng N, Fu J, Chen D, Chen S, Wang H. An antibody-free liver cancer screening approach based on nanoplasmonics biosensing chips via spectrum-based deep learning. NanoImpact 2021;21:100296. [DOI] [PubMed] [Google Scholar]
- 66.Li G, Li S, Wang Z, Xue Y, Dong C, Zeng J, Huang Y, et al. Label-free electrochemical aptasensor for detection of alpha-fetoprotein based on AFP-aptamer and thionin/reduced graphene oxide/gold nanoparticles. Anal Biochem 2018;547:37–44. [DOI] [PubMed] [Google Scholar]
- 67.Yao C, Ng E, Wang SX. An automated and mobile magnetoresistive biosensor system for early hepatocellular carcinoma diagnosis. Biosens Bioelectron 2022;202:113982. [DOI] [PubMed] [Google Scholar]
- 68.Barahman M, Grunvald E, Prado PJ, Bussandri A, Henderson WC, Wolfson T, Fowler KJ, et al. Point-of-care magnetic resonance technology to measure liver fat: Phantom and first-in-human pilot study. Magn Reson Med 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gerke S, Babic B, Evgeniou T, Cohen IG. The need for a system view to regulate artificial intelligence/machine learning-based software as medical device. NPJ Digit Med 2020;3:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu S, Zeng N, Sun F, Zhou J, Wu X, Sun Y, Wang B, et al. Hepatocellular Carcinoma Prediction Models in Chronic Hepatitis B: A Systematic Review of 14 Models and External Validation. Clin Gastroenterol Hepatol 2021;19:2499–2513. [DOI] [PubMed] [Google Scholar]
- 71.Kim HS, Yu X, Kramer J, Thrift AP, Richardson P, Hsu YC, Flores A, et al. Comparative performance of risk prediction models for hepatitis B-related hepatocellular carcinoma in the United States. J Hepatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharma SA, Kowgier M, Hansen BE, Brouwer WP, Maan R, Wong D, Shah H, et al. Toronto HCC risk index: A validated scoring system to predict 10-year risk of HCC in patients with cirrhosis. J Hepatol 2017. [DOI] [PubMed] [Google Scholar]
- 73.Fan R, Papatheodoridis G, Sun J, Innes H, Toyoda H, Xie Q, Mo S, et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J Hepatol 2020;73:1368–1378. [DOI] [PubMed] [Google Scholar]
- 74.Innes H, Jepsen P, McDonald S, Dillon J, Hamill V, Yeung A, Benselin J, et al. Performance of models to predict hepatocellular carcinoma risk among UK patients with cirrhosis and cured HCV infection. JHEP Rep 2021;3:100384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim NJ, Vutien P, Cleveland E, Cravero A, Ioannou GN. Fibrosis Stage-specific Incidence of Hepatocellular Cancer After Hepatitis C Cure With Direct-acting Antivirals: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hughes DM, Berhane S, Emily de Groot CA, Toyoda H, Tada T, Kumada T, Satomura S, et al. Serum Levels of alpha-Fetoprotein Increased More Than 10 Years Before Detection of Hepatocellular Carcinoma. Clin Gastroenterol Hepatol 2021;19:162–170 e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Semmler G, Meyer EL, Kozbial K, Schwabl P, Hametner-Schreil S, Zanetto A, Bauer D, et al. HCC risk stratification after cure of hepatitis C in patients with compensated advanced chronic liver disease. J Hepatol 2022;76:812–821. [DOI] [PubMed] [Google Scholar]
- 78.Ioannou GN, Tang W, Beste LA, Tincopa MA, Su GL, Van T, Tapper EB, et al. Assessment of a Deep Learning Model to Predict Hepatocellular Carcinoma in Patients With Hepatitis C Cirrhosis. JAMA Netw Open 2020;3:e2015626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Hond AAH, Leeuwenberg AM, Hooft L, Kant IMJ, Nijman SWJ, van Os HJA, Aardoom JJ, et al. Guidelines and quality criteria for artificial intelligence-based prediction models in healthcare: a scoping review. NPJ Digit Med 2022;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kubota N, Fujiwara N, Hoshida Y. Clinical and Molecular Prediction of Hepatocellular Carcinoma Risk. J Clin Med 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang G, Yu K, Shao L, Yu X, Hu C, Qian P, Xie H, et al. Association between epidermal growth factor gene +61A/G polymorphism and the risk of hepatocellular carcinoma: a meta-analysis based on 16 studies. BMC Cancer 2015;15:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qin S, Wang J, Zhou C, Xu Y, Zhang Y, Wang X, Wang S. The influence of interleukin 28B polymorphisms on the risk of hepatocellular carcinoma among patients with HBV or HCV infection: An updated meta-analysis. Medicine (Baltimore) 2019;98:e17275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Degasperi E, Galmozzi E, Pelusi S, D’Ambrosio R, Soffredini R, Borghi M, Perbellini R, et al. Hepatic Fat-Genetic Risk Score Predicts Hepatocellular Carcinoma in Patients With Cirrhotic HCV Treated With DAAs. Hepatology 2020;72:1912–1923. [DOI] [PubMed] [Google Scholar]
- 84.Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjaerg-Hansen A, Vogt TF, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2014;46:352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tang S, Zhang J, Mei TT, Guo HQ, Wei XH, Zhang WY, Liu YL, et al. Association of TM6SF2 rs58542926 T/C gene polymorphism with hepatocellular carcinoma: a meta-analysis. BMC Cancer 2019;19:1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bianco C, Casirati E, Malvestiti F, Valenti L. Genetic predisposition similarities between NASH and ASH: Identification of new therapeutic targets. JHEP Rep 2021;3:100284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Trépo E, Caruso S, Yang J, Imbeaud S, Couchy G, Bayard Q, Letouzé E, et al. Common genetic variation in alcohol-related hepatocellular carcinoma: a case-control genome-wide association study. Lancet Oncol 2021. [DOI] [PubMed] [Google Scholar]
- 88.Gellert-Kristensen H, Richardson TG, Davey Smith G, Nordestgaard BG, Tybjaerg-Hansen A, Stender S. Combined Effect of PNPLA3, TM6SF2, and HSD17B13 Variants on Risk of Cirrhosis and Hepatocellular Carcinoma in the General Population. Hepatology 2020;72:845–856. [DOI] [PubMed] [Google Scholar]
- 89.Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, Gupta S, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med 2008;359:1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoshida Y, Villanueva A, Sangiovanni A, Sole M, Hur C, Andersson KL, Chung RT, et al. Prognostic gene expression signature for patients with hepatitis C-related early-stage cirrhosis. Gastroenterology 2013;144:1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.King LY, Canasto-Chibuque C, Johnson KB, Yip S, Chen X, Kojima K, Deshmukh M, et al. A genomic and clinical prognostic index for hepatitis C-related early-stage cirrhosis that predicts clinical deterioration. Gut 2015;64:1296–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nakagawa S, Wei L, Song WM, Higashi T, Ghoshal S, Kim RS, Bian CB, et al. Molecular Liver Cancer Prevention in Cirrhosis by Organ Transcriptome Analysis and Lysophosphatidic Acid Pathway Inhibition. Cancer Cell 2016;30:879–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ono A, Goossens N, Finn RS, Schmidt WN, Thung SN, Im GY, Hoshida Y, et al. Persisting risk of hepatocellular carcinoma after hepatitis C virus cure monitored by a liver transcriptome signature. Hepatology 2017;66:1344–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Crouchet E, Bandiera S, Fujiwara N, Li S, El Saghire H, Fernandez-Vaquero M, Riedl T, et al. A human liver cell-based system modeling a clinical prognostic liver signature for therapeutic discovery. Nat Commun 2021;12:5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim MH, Kim MY, Salloum S, Qian T, Wong LP, Xu M, Lee Y, et al. Atorvastatin favorably modulates a clinical hepatocellular carcinoma risk gene signature. Hepatol Commun 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim JH, Sohn BH, Lee HS, Kim SB, Yoo JE, Park YY, Jeong W, et al. Genomic predictors for recurrence patterns of hepatocellular carcinoma: model derivation and validation. PLoS Med 2014;11:e1001770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ji J, Eggert T, Budhu A, Forgues M, Takai A, Dang H, Ye Q, et al. Hepatic stellate cell and monocyte interaction contributes to poor prognosis in hepatocellular carcinoma. Hepatology 2015;62:481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang DY, Goossens N, Guo J, Tsai MC, Chou HI, Altunkaynak C, Sangiovanni A, et al. A hepatic stellate cell gene expression signature associated with outcomes in hepatitis C cirrhosis and hepatocellular carcinoma after curative resection. Gut 2016;65:1754–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev 2017;121:27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Finkin S, Yuan D, Stein I, Taniguchi K, Weber A, Unger K, Browning JL, et al. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat Immunol 2015;16:1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang Y, de Boer WB, Adams LA, MacQuillan G, Bulsara MK, Jeffrey GP. Image analysis of liver biopsy samples measures fibrosis and predicts clinical outcome. J Hepatol 2014;61:22–27. [DOI] [PubMed] [Google Scholar]
- 102.Naoumov NV, Brees D, Loeffler J, Chng E, Ren Y, Lopez P, Tai D, et al. Digital pathology with artificial intelligence analyses provides greater insights into treatment-induced fibrosis regression in NASH. J Hepatol 2022. [DOI] [PubMed] [Google Scholar]
- 103.Mazziotti G, Sorvillo F, Morisco F, Carbone A, Rotondi M, Stornaiuolo G, Precone DF, et al. Serum insulin-like growth factor I evaluation as a useful tool for predicting the risk of developing hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis: a prospective study. Cancer 2002;95:2539–2545. [DOI] [PubMed] [Google Scholar]
- 104.Nakagawa H, Maeda S, Yoshida H, Tateishi R, Masuzaki R, Ohki T, Hayakawa Y, et al. Serum IL-6 levels and the risk for hepatocarcinogenesis in chronic hepatitis C patients: an analysis based on gender differences. Int J Cancer 2009;125:2264–2269. [DOI] [PubMed] [Google Scholar]
- 105.Kim JH, Kang SH, Lee M, Youn GS, Kim TS, Jun BG, Kim MY, et al. Serum Myostatin Predicts the Risk of Hepatocellular Carcinoma in Patients with Alcoholic Cirrhosis: A Multicenter Study. Cancers (Basel) 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yuan JM, Wang Y, Wang R, Luu HN, Adams-Haduch J, Koh WP, Gao YT, et al. Serum IL27 in Relation to Risk of Hepatocellular Carcinoma in Two Nested Case-Control Studies. Cancer Epidemiol Biomarkers Prev 2021;30:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yamashita T, Koshikawa N, Shimakami T, Terashima T, Nakagawa M, Nio K, Horii R, et al. Serum Laminin γ2 Monomer as a Diagnostic and Predictive Biomarker for Hepatocellular Carcinoma. Hepatology 2021;74:760–775. [DOI] [PubMed] [Google Scholar]
- 108.Liang KH, Lai MW, Lin YH, Chu YD, Lin CL, Lin WR, Huang YH, et al. Plasma interleukin-17 and alpha-fetoprotein combination effectively predicts imminent hepatocellular carcinoma occurrence in liver cirrhotic patients. BMC Gastroenterol 2021;21:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fujiwara N, Kobayashi M, Fobar AJ, Hoshida A, Marquez CA, Koneru B, Panda G, et al. A blood-based prognostic liver secretome signature and long-term hepatocellular carcinoma risk in advanced liver fibrosis. Med (N Y) 2021;2:836–850.e810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fujiwara N, Fobar AJ, Raman I, Li QZ, Marrero JA, Parikh ND, Singal AG, et al. A Blood-Based Prognostic Liver Secretome Signature Predicts Long-term Risk of Hepatic Decompensation in Cirrhosis. Clin Gastroenterol Hepatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fujiwara N, Trépo E, Raman I, Li QZ, Degré D, Gustot T, Moreno C, et al. Plasma-Signature-Model for End-Stage Liver Disease Score to Predict Survival in Severe Alcoholic Hepatitis. Clin Gastroenterol Hepatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Verhelst X, Vanderschaeghe D, Castéra L, Raes T, Geerts A, Francoz C, Colman R, et al. A Glycomics-Based Test Predicts the Development of Hepatocellular Carcinoma in Cirrhosis. Clin Cancer Res 2017;23:2750–2758. [DOI] [PubMed] [Google Scholar]
- 113.Beyoğlu D, Idle JR. Metabolomic and Lipidomic Biomarkers for Premalignant Liver Disease Diagnosis and Therapy. Metabolites 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stepien M, Keski-Rahkonen P, Kiss A, Robinot N, Duarte-Salles T, Murphy N, Perlemuter G, et al. Metabolic perturbations prior to hepatocellular carcinoma diagnosis: Findings from a prospective observational cohort study. Int J Cancer 2021;148:609–625. [DOI] [PubMed] [Google Scholar]
- 115.Jee SH, Kim M, Kim M, Yoo HJ, Kim H, Jung KJ, Hong S, et al. Metabolomics Profiles of Hepatocellular Carcinoma in a Korean Prospective Cohort: The Korean Cancer Prevention Study-II. Cancer Prev Res (Phila) 2018;11:303–312. [DOI] [PubMed] [Google Scholar]
- 116.Liang KH, Cheng ML, Lo CJ, Lin YH, Lai MW, Lin WR, Yeh CT. Plasma phenylalanine and glutamine concentrations correlate with subsequent hepatocellular carcinoma occurrence in liver cirrhosis patients: an exploratory study. Sci Rep 2020;10:10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Luo P, Yin P, Hua R, Tan Y, Li Z, Qiu G, Yin Z, et al. A Large-scale, multicenter serum metabolite biomarker identification study for the early detection of hepatocellular carcinoma. Hepatology 2018;67:662–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Konerman MA, Verma A, Zhao B, Singal AG, Lok AS, Parikh ND. Frequency and Outcomes of Abnormal Imaging in Patients With Cirrhosis Enrolled in a Hepatocellular Carcinoma Surveillance Program. Liver Transpl 2019;25:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Arvind A, Joshi S, Zaki T, Burkholder D, Parikh ND, Singal AG, North American Liver Cancer C. Risk of Hepatocellular Carcinoma in Patients With Indeterminate (LI-RADS 3) Liver Observations. Clin Gastroenterol Hepatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Onyirioha K, Joshi S, Burkholder D, Yekkaluri S, Parikh ND, Singal AG, North American Liver Cancer C. Clinical Outcomes of Patients with Suspicious (LI-RADS 4) Liver Observations. Clin Gastroenterol Hepatol 2022. [DOI] [PubMed] [Google Scholar]
- 121.Wei Y, Gong J, He X, Liu B, Liu T, Yang S, Zhou Z, et al. An MRI-Based Radiomic Model for Individualized Prediction of Hepatocellular Carcinoma in Patients With Hepatitis B Virus-Related Cirrhosis. Front Oncol 2022;12:800787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carbonell G, Kennedy P, Bane O, Kirmani A, El Homsi M, Stocker D, Said D, et al. Precision of MRI radiomics features in the liver and hepatocellular carcinoma. Eur Radiol 2022;32:2030–2040. [DOI] [PubMed] [Google Scholar]
- 123.Wang K, Lu X, Zhou H, Gao Y, Zheng J, Tong M, Wu C, et al. Deep learning Radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: a prospective multicentre study. Gut 2019;68:729–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Suk KT, Kim EJ, Kim DJ, Kim HS, Bang CS, Park TY, Baik GH, et al. Prognostic Significance of Hemodynamic and Clinical Stages in the Prediction of Hepatocellular Carcinoma. J Clin Gastroenterol 2017;51:285–293. [DOI] [PubMed] [Google Scholar]
- 125.Yu Q, Huang Y, Li X, Pavlides M, Liu D, Luo H, Ding H, et al. An imaging-based artificial intelligence model for non-invasive grading of hepatic venous pressure gradient in cirrhotic portal hypertension. Cell Rep Med 2022;3:100563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan WK, Yilmaz Y, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol 2020;5:362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ogawa E, Takayama K, Hiramine S, Hayashi T, Toyoda K. Association between steatohepatitis biomarkers and hepatocellular carcinoma after hepatitis C elimination. Aliment Pharmacol Ther 2020;52:866–876. [DOI] [PubMed] [Google Scholar]
- 128.Miura K, Maeda H, Morimoto N, Watanabe S, Tsukui M, Takaoka Y, Nomoto H, et al. Utility of FibroScan-based scoring systems to narrow the risk group of nonalcoholic fatty liver disease with comorbidities. World J Gastrointest Pathophysiol 2022;13:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schwabe RF, Greten TF. Gut microbiome in HCC - Mechanisms, diagnosis and therapy. J Hepatol 2020;72:230–238. [DOI] [PubMed] [Google Scholar]
- 130.Vallianou N, Christodoulatos GS, Karampela I, Tsilingiris D, Magkos F, Stratigou T, Kounatidis D, et al. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Non-Alcoholic Fatty Liver Disease: Current Evidence and Perspectives. Biomolecules 2021;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu J, Zhan Q, Fan Y, Lo EKK, Zhang F, Yu Y, El-Nezami H, et al. Clinical Aspects of Gut Microbiota in Hepatocellular Carcinoma Management. Pathogens 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ren Z, Li A, Jiang J, Zhou L, Yu Z, Lu H, Xie H, et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut 2019;68:1014–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zheng R, Wang G, Pang Z, Ran N, Gu Y, Guan X, Yuan Y, et al. Liver cirrhosis contributes to the disorder of gut microbiota in patients with hepatocellular carcinoma. Cancer Med 2020;9:4232–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hernandez BY, Zhu X, Risch HA, Lu L, Ma X, Irwin ML, Lim JK, et al. Oral Cyanobacteria and Hepatocellular Carcinoma. Cancer Epidemiol Biomarkers Prev 2022;31:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dong TS, Jacobs JP, Agopian V, Pisegna JR, Ayoub W, Durazo F, Enayati P, et al. Duodenal Microbiome and Serum Metabolites Predict Hepatocellular Carcinoma in a Multicenter Cohort of Patients with Cirrhosis. Dig Dis Sci 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thilakarathna W, Rupasinghe HPV, Ridgway ND. Mechanisms by Which Probiotic Bacteria Attenuate the Risk of Hepatocellular Carcinoma. Int J Mol Sci 2021;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu J, Tang W, Budhu A, Forgues M, Hernandez MO, Candia J, Kim Y, et al. A Viral Exposure Signature Defines Early Onset of Hepatocellular Carcinoma. Cell 2020;182:317–328.e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fujiwara N, Hoshida Y. Viral Exposure Signature Associated with Liver Cancer Risk. Trends Mol Med 2020;26:711–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tatsuno K, Midorikawa Y, Takayama T, Yamamoto S, Nagae G, Moriyama M, Nakagawa H, et al. Impact of AAV2 and Hepatitis B Virus Integration Into Genome on Development of Hepatocellular Carcinoma in Patients with Prior Hepatitis B Virus Infection. Clin Cancer Res 2019;25:6217–6227. [DOI] [PubMed] [Google Scholar]
- 140.Pollock BH, Elmore S, Romoser A, Tang L, Kang MS, Xue K, Rodriguez M, et al. Intervention trial with calcium montmorillonite clay in a south Texas population exposed to aflatoxin. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2016;33:1346–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alvarez CS, Ortiz J, Bendfeldt-Avila G, Xie Y, Wang M, Wu D, Higson H, et al. Analysis of TP53 aflatoxin signature mutation in hepatocellular carcinomas from Guatemala: A cross-sectional study (2016–2017). Health Sci Rep 2020;3:e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang W, He H, Zang M, Wu Q, Zhao H, Lu LL, Ma P, et al. Genetic Features of Aflatoxin-Associated Hepatocellular Carcinoma. Gastroenterology 2017;153:249–262 e242. [DOI] [PubMed] [Google Scholar]
- 143.Minko IG, Vartanian VL, Tozaki NN, Linde OK, Jaruga P, Coskun SH, Coskun E, et al. Characterization of rare NEIL1 variants found in East Asian populations. DNA Repair (Amst) 2019;79:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ng AWT, Poon SL, Huang MN, Lim JQ, Boot A, Yu W, Suzuki Y, et al. Aristolochic acids and their derivatives are widely implicated in liver cancers in Taiwan and throughout Asia. Sci Transl Med 2017;9. [DOI] [PubMed] [Google Scholar]
- 145.Jiao J, Niu W, Wang Y, Baggerly KA, Ye Y, Wu X, Davenport D, et al. Prevalence of Aflatoxin-associated TP53R249S mutation in Hepatocellular Carcinoma in Hispanics in South Texas. Cancer Prev Res (Phila) 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fahed AC, Philippakis AA, Khera AV. The potential of polygenic scores to improve cost and efficiency of clinical trials. Nat Commun 2022;13:2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Morgan TR, Osann K, Bottiglieri T, Pimstone N, Hoefs JC, Hu KQ, Hassanein T, et al. A Phase II Randomized, Controlled Trial of S-Adenosylmethionine in Reducing Serum alpha-Fetoprotein in Patients with Hepatitis C Cirrhosis and Elevated AFP. Cancer Prev Res (Phila) 2015;8:864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Goossens N, Hoshida Y, Song WM, Jung M, Morel P, Nakagawa S, Zhang B, et al. Nonalcoholic Steatohepatitis Is Associated With Increased Mortality in Obese Patients Undergoing Bariatric Surgery. Clin Gastroenterol Hepatol 2016;14:1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Galle PR, Foerster F, Kudo M, Chan SL, Llovet JM, Qin S, Schelman WR, et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int 2019;39:2214–2229. [DOI] [PubMed] [Google Scholar]
- 150.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Taketa K, Endo Y, Sekiya C, Tanikawa K, Koji T, Taga H, Satomura S, et al. A collaborative study for the evaluation of lectin-reactive alpha-fetoproteins in early detection of hepatocellular carcinoma. Cancer Res 1993;53:5419–5423. [PubMed] [Google Scholar]
- 152.Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, Reddy KR, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology 2009;137:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhou JM, Wang T, Zhang KH. AFP-L3 for the diagnosis of early hepatocellular carcinoma: A meta-analysis. Medicine (Baltimore) 2021;100:e27673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tayob N, Kanwal F, Alsarraj A, Hernaez R, El-Serag HB. The Performance of AFP, AFP-3, DCP as Biomarkers for Detection of Hepatocellular Carcinoma (HCC): A Phase 3 Biomarker Study in the United States. Clin Gastroenterol Hepatol 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xing H, Zheng YJ, Han J, Zhang H, Li ZL, Lau WY, Shen F, et al. Protein induced by vitamin K absence or antagonist-II versus alpha-fetoprotein in the diagnosis of hepatocellular carcinoma: A systematic review with meta-analysis. Hepatobiliary Pancreat Dis Int 2018;17:487–495. [DOI] [PubMed] [Google Scholar]
- 156.Singal AG, Tayob N, Mehta A, Marrero JA, El-Serag H, Jin Q, Saenz de Viteri C, et al. GALAD demonstrates high sensitivity for HCC surveillance in a cohort of patients with cirrhosis. Hepatology 2022;75:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fang YS, Wu Q, Zhao HC, Zhou Y, Ye L, Liu SS, Li XX, et al. Do combined assays of serum AFP, AFP-L3, DCP, GP73, and DKK-1 efficiently improve the clinical values of biomarkers in decision-making for hepatocellular carcinoma? A meta-analysis. Expert Rev Gastroenterol Hepatol 2021;15:1065–1076. [DOI] [PubMed] [Google Scholar]
- 158.Wang X, Zhang Y, Yang N, He H, Tao X, Kou C, Jiang J. Evaluation of the Combined Application of AFP, AFP-L3%, and DCP for Hepatocellular Carcinoma Diagnosis: A Meta-analysis. Biomed Res Int 2020;2020:5087643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Choi J, Kim GA, Han S, Lee W, Chun S, Lim YS. Longitudinal Assessment of Three Serum Biomarkers to Detect Very Early-Stage Hepatocellular Carcinoma. Hepatology 2019;69:1983–1994. [DOI] [PubMed] [Google Scholar]
- 160.Johnson PJ, Pirrie SJ, Cox TF, Berhane S, Teng M, Palmer D, Morse J, et al. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev 2014;23:144–153. [DOI] [PubMed] [Google Scholar]
- 161.Berhane S, Toyoda H, Tada T, Kumada T, Kagebayashi C, Satomura S, Schweitzer N, et al. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clin Gastroenterol Hepatol 2016;14:875–886 e876. [DOI] [PubMed] [Google Scholar]
- 162.Liu M, Wu R, Liu X, Xu H, Chi X, Wang X, Zhan M, et al. Validation of the GALAD Model and Establishment of GAAP Model for Diagnosis of Hepatocellular Carcinoma in Chinese Patients. J Hepatocell Carcinoma 2020;7:219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lambrecht J, Porsch-Ozcurumez M, Best J, Jost-Brinkmann F, Roderburg C, Demir M, Tacke F, et al. The APAC Score: A Novel and Highly Performant Serological Tool for Early Diagnosis of Hepatocellular Carcinoma in Patients with Liver Cirrhosis. J Clin Med 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Best J, Bilgi H, Heider D, Schotten C, Manka P, Bedreli S, Gorray M, et al. The GALAD scoring algorithm based on AFP, AFP-L3, and DCP significantly improves detection of BCLC early stage hepatocellular carcinoma. Z Gastroenterol 2016;54:1296–1305. [DOI] [PubMed] [Google Scholar]
- 165.Yang JD, Addissie BD, Mara KC, Harmsen WS, Dai J, Zhang N, Wongjarupong N, et al. GALAD Score for Hepatocellular Carcinoma Detection in Comparison with Liver Ultrasound and Proposal of GALADUS Score. Cancer Epidemiol Biomarkers Prev 2019;28:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schotten C, Ostertag B, Sowa JP, Manka P, Bechmann LP, Hilgard G, Marquardt C, et al. GALAD Score Detects Early-Stage Hepatocellular Carcinoma in a European Cohort of Chronic Hepatitis B and C Patients. Pharmaceuticals (Basel) 2021;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chalasani NP, Porter K, Bhattacharya A, Book AJ, Neis BM, Xiong KM, Ramasubramanian TS, et al. Validation of a Novel Multitarget Blood Test Shows High Sensitivity to Detect Early Stage Hepatocellular Carcinoma. Clin Gastroenterol Hepatol 2022;20:173–182 e177. [DOI] [PubMed] [Google Scholar]
- 168.Best J, Bechmann LP, Sowa JP, Sydor S, Dechene A, Pflanz K, Bedreli S, et al. GALAD Score Detects Early Hepatocellular Carcinoma in an International Cohort of Patients With Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol 2020;18:728–735 e724. [DOI] [PubMed] [Google Scholar]
- 169.El-Serag HB, Kanwal F, Davila JA, Kramer J, Richardson P. A new laboratory-based algorithm to predict development of hepatocellular carcinoma in patients with hepatitis C and cirrhosis. Gastroenterology 2014;146:1249–1255 e1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.White DL, Richardson P, Tayoub N, Davila JA, Kanwal F, El-Serag HB. The Updated Model: An Adjusted Serum Alpha-Fetoprotein-Based Algorithm for Hepatocellular Carcinoma Detection With Hepatitis C Virus-Related Cirrhosis. Gastroenterology 2015;149:1986–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tayob N, Richardson P, White DL, Yu X, Davila JA, Kanwal F, Feng Z, et al. Evaluating screening approaches for hepatocellular carcinoma in a cohort of HCV related cirrhosis patients from the Veteran’s Affairs Health Care System. BMC Med Res Methodol 2018;18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tayob N, Christie I, Richardson P, Feng Z, White DL, Davila J, Corley DA, et al. Validation of the Hepatocellular Carcinoma Early Detection Screening (HES) Algorithm in a Cohort of Veterans With Cirrhosis. Clin Gastroenterol Hepatol 2019;17:1886–1893 e1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tayob N, Corley DA, Christie I, Almers L, Rahal AK, Richardson P, White DL, et al. Validation of the Updated Hepatocellular Carcinoma Early Detection Screening Algorithm in a Community-Based Cohort of Patients With Cirrhosis of Multiple Etiologies. Clin Gastroenterol Hepatol 2021;19:1443–1450 e1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang M, Devarajan K, Singal AG, Marrero JA, Dai J, Feng Z, Rinaudo JA, et al. The Doylestown Algorithm: A Test to Improve the Performance of AFP in the Detection of Hepatocellular Carcinoma. Cancer Prev Res (Phila) 2016;9:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang M, Sanda M, Comunale MA, Herrera H, Swindell C, Kono Y, Singal AG, et al. Changes in the Glycosylation of Kininogen and the Development of a Kininogen-Based Algorithm for the Early Detection of HCC. Cancer Epidemiol Biomarkers Prev 2017;26:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Singal AG, Tayob N, Mehta A, Marrero JA, Jin Q, Lau J, Parikh ND. Doylestown Plus and GALAD Demonstrate High Sensitivity for HCC Detection in Patients With Cirrhosis. Clin Gastroenterol Hepatol 2022;20:953–955 e952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tran NH, Kisiel J, Roberts LR. Using cell-free DNA for HCC surveillance and prognosis. JHEP Rep 2021;3:100304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lin N, Lin Y, Xu J, Liu D, Li D, Meng H, Gallant MA, et al. A multi-analyte cell-free DNA-based blood test for early detection of hepatocellular carcinoma. Hepatol Commun 2022;6:1753–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kisiel JB, Dukek BA, R VSRK, Ghoz HM, Yab TC, Berger CK, Taylor WR, et al. Hepatocellular Carcinoma Detection by Plasma Methylated DNA: Discovery, Phase I Pilot, and Phase II Clinical Validation. Hepatology 2019;69:1180–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chalasani NP, Ramasubramanian TS, Bhattacharya A, Olson MC, Edwards VD, Roberts LR, Kisiel JB, et al. A Novel Blood-Based Panel of Methylated DNA and Protein Markers for Detection of Early-Stage Hepatocellular Carcinoma. Clin Gastroenterol Hepatol 2021;19:2597–2605 e2594. [DOI] [PubMed] [Google Scholar]
- 181.Oussalah A, Rischer S, Bensenane M, Conroy G, Filhine-Tresarrieu P, Debard R, Forest-Tramoy D, et al. Plasma mSEPT9: A Novel Circulating Cell-free DNA-Based Epigenetic Biomarker to Diagnose Hepatocellular Carcinoma. EBioMedicine 2018;30:138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kotoh Y, Suehiro Y, Saeki I, Hoshida T, Maeda M, Iwamoto T, Matsumoto T, et al. Novel Liquid Biopsy Test Based on a Sensitive Methylated SEPT9 Assay for Diagnosing Hepatocellular Carcinoma. Hepatol Commun 2020;4:461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lewin J, Kottwitz D, Aoyama J, deVos T, Garces J, Hasinger O, Kasielke S, et al. Plasma cell free DNA methylation markers for hepatocellular carcinoma surveillance in patients with cirrhosis: a case control study. BMC Gastroenterol 2021;21:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bannaga AS, Alvarez R, Zhou L, Petchey M, Noufaily A, Hitchins MP, Arasaradnam RP. Role of methylated septin 9 as an adjunct diagnostic and prognostic biomarker in hepatocellular carcinoma. HPB (Oxford) 2021;23:1595–1606. [DOI] [PubMed] [Google Scholar]
- 185.He N, Feng G, Zhang C, Wu F, Zhang T, Yang Y. Plasma levels of methylated septin 9 are capable of detecting hepatocellular carcinoma and hepatic cirrhosis. Mol Med Rep 2020;22:2705–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chandrapalan S, Bannaga A, Weidner A, Hitchins MP, Arasaradnam RP. A systematic review and meta-analysis: the diagnostic accuracy of methylated SEPTIN9 for the detection of hepatocellular carcinoma and the clinical evaluation of its use in combination with other surveillance modalities. Scand J Gastroenterol 2022;57:473–480. [DOI] [PubMed] [Google Scholar]
- 187.Cai J, Chen L, Zhang Z, Zhang X, Lu X, Liu W, Shi G, et al. Genome-wide mapping of 5-hydroxymethylcytosines in circulating cell-free DNA as a non-invasive approach for early detection of hepatocellular carcinoma. Gut 2019;68:2195–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Qu C, Wang Y, Wang P, Chen K, Wang M, Zeng H, Lu J, et al. Detection of early-stage hepatocellular carcinoma in asymptomatic HBsAg-seropositive individuals by liquid biopsy. Proc Natl Acad Sci U S A 2019;116:6308–6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rich NE, Singal AG. Overdiagnosis of hepatocellular carcinoma: Prevented by guidelines? Hepatology 2022;75:740–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang X, Wang Z, Tang W, Wang X, Liu R, Bao H, Chen X, et al. Ultrasensitive and affordable assay for early detection of primary liver cancer using plasma cell-free DNA fragmentomics. Hepatology 2021. [DOI] [PubMed] [Google Scholar]
- 191.Chen L, Abou-Alfa GK, Zheng B, Liu JF, Bai J, Du LT, Qian YS, et al. Genome-scale profiling of circulating cell-free DNA signatures for early detection of hepatocellular carcinoma in cirrhotic patients. Cell Res 2021;31:589–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ahn JC, Teng PC, Chen PJ, Posadas E, Tseng HR, Lu SC, Yang JD. Detection of Circulating Tumor Cells and Their Implications as a Biomarker for Diagnosis, Prognostication, and Therapeutic Monitoring in Hepatocellular Carcinoma. Hepatology 2021;73:422–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cui K, Ou Y, Shen Y, Li S, Sun Z. Clinical value of circulating tumor cells for the diagnosis and prognosis of hepatocellular carcinoma (HCC): A systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Guo W, Yang XR, Sun YF, Shen MN, Ma XL, Wu J, Zhang CY, et al. Clinical significance of EpCAM mRNA-positive circulating tumor cells in hepatocellular carcinoma by an optimized negative enrichment and qRT-PCR-based platform. Clin Cancer Res 2014;20:4794–4805. [DOI] [PubMed] [Google Scholar]
- 195.Kalinich M, Bhan I, Kwan TT, Miyamoto DT, Javaid S, LiCausi JA, Milner JD, et al. An RNA-based signature enables high specificity detection of circulating tumor cells in hepatocellular carcinoma. Proc Natl Acad Sci U S A 2017;114:1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Guo W, Sun YF, Shen MN, Ma XL, Wu J, Zhang CY, Zhou Y, et al. Circulating Tumor Cells with Stem-Like Phenotypes for Diagnosis, Prognosis, and Therapeutic Response Evaluation in Hepatocellular Carcinoma. Clin Cancer Res 2018;24:2203–2213. [DOI] [PubMed] [Google Scholar]
- 197.Pea A, Jamieson NB, Braconi C. Biology and Clinical Application of Regulatory RNAs in Hepatocellular Carcinoma. Hepatology 2021;73 Suppl 1:38–48. [DOI] [PubMed] [Google Scholar]
- 198.Yamamoto Y, Kondo S, Matsuzaki J, Esaki M, Okusaka T, Shimada K, Murakami Y, et al. Highly Sensitive Circulating MicroRNA Panel for Accurate Detection of Hepatocellular Carcinoma in Patients With Liver Disease. Hepatol Commun 2020;4:284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Li LM, Hu ZB, Zhou ZX, Chen X, Liu FY, Zhang JF, Shen HB, et al. Serum microRNA profiles serve as novel biomarkers for HBV infection and diagnosis of HBV-positive hepatocarcinoma. Cancer Res 2010;70:9798–9807. [DOI] [PubMed] [Google Scholar]
- 200.Lu J, Xie F, Geng L, Shen W, Sui C, Yang J. Investigation of serum lncRNA-uc003wbd and lncRNA-AF085935 expression profile in patients with hepatocellular carcinoma and HBV. Tumour Biol 2015;36:3231–3236. [DOI] [PubMed] [Google Scholar]
- 201.Motawi TMK, El-Maraghy SA, Sabry D, Mehana NA. The expression of long non coding RNA genes is associated with expression with polymorphisms of HULC rs7763881 and MALAT1 rs619586 in hepatocellular carcinoma and HBV Egyptian patients. J Cell Biochem 2019;120:14645–14656. [DOI] [PubMed] [Google Scholar]
- 202.Yu J, Ding WB, Wang MC, Guo XG, Xu J, Xu QG, Yang Y, et al. Plasma circular RNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma: A large-scale, multicenter study. Int J Cancer 2020;146:1754–1763. [DOI] [PubMed] [Google Scholar]
- 203.Lee YT, Tran BV, Wang JJ, Liang IY, You S, Zhu Y, Agopian VG, et al. The Role of Extracellular Vesicles in Disease Progression and Detection of Hepatocellular Carcinoma. Cancers (Basel) 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cho HJ, Eun JW, Baek GO, Seo CW, Ahn HR, Kim SS, Cho SW, et al. Serum Exosomal MicroRNA, miR-10b-5p, as a Potential Diagnostic Biomarker for Early-Stage Hepatocellular Carcinoma. J Clin Med 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kim SS, Baek GO, Ahn HR, Sung S, Seo CW, Cho HJ, Nam SW, et al. Serum small extracellular vesicle-derived LINC00853 as a novel diagnostic marker for early hepatocellular carcinoma. Mol Oncol 2020;14:2646–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.von Felden J, Garcia-Lezana T, Dogra N, Gonzalez-Kozlova E, Ahsen ME, Craig A, Gifford S, et al. Unannotated small RNA clusters associated with circulating extracellular vesicles detect early stage liver cancer. Gut 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lu Y, Duan Y, Xu Q, Zhang L, Chen W, Qu Z, Wu B, et al. Circulating exosome-derived bona fide long non-coding RNAs predicting the occurrence and metastasis of hepatocellular carcinoma. J Cell Mol Med 2020;24:1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sun N, Lee YT, Zhang RY, Kao R, Teng PC, Yang Y, Yang P, et al. Purification of HCC-specific extracellular vesicles on nanosubstrates for early HCC detection by digital scoring. Nat Commun 2020;11:4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sanchez JI, Jiao J, Kwan SY, Veillon L, Warmoes MO, Tan L, Odewole M, et al. Lipidomic Profiles of Plasma Exosomes Identify Candidate Biomarkers for Early Detection of Hepatocellular Carcinoma in Patients with Cirrhosis. Cancer Prev Res (Phila) 2021;14:955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sun N, Zhang C, Lee YT, Tran BV, Wang J, Kim H, Lee J, et al. HCC EV ECG Score: An Extracellular Vesicle-based Protein Assay for Detection of Early-Stage Hepatocellular Carcinoma. Hepatology 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Xia Y, Zhang Y, Shen M, Xu H, Li Z, He N. Golgi protein 73 and its diagnostic value in liver diseases. Cell Prolif 2019;52:e12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wen Y, Jeong S, Xia Q, Kong X. Role of Osteopontin in Liver Diseases. Int J Biol Sci 2016;12:1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhang Y, Gao J, Bao Y, Liu Y, Tong Y, Jin S, Zhao Q. Diagnostic accuracy and prognostic significance of osteopontin in liver cirrhosis and hepatocellular carcinoma: a Meta-analysis. Biomarkers 2022;27:13–21. [DOI] [PubMed] [Google Scholar]
- 214.Sun T, Tang Y, Sun D, Bu Q, Li P. Osteopontin versus alpha-fetoprotein as a diagnostic marker for hepatocellular carcinoma: a meta-analysis. Onco Targets Ther 2018;11:8925–8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Guo M, Zhang H, Zheng J, Liu Y. Glypican-3: A New Target for Diagnosis and Treatment of Hepatocellular Carcinoma. J Cancer 2020;11:2008–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gowhari Shabgah A, Ezzatifar F, Aravindhan S, Olegovna Zekiy A, Ahmadi M, Gheibihayat SM, Gholizadeh Navashenaq J. Shedding more light on the role of Midkine in hepatocellular carcinoma: New perspectives on diagnosis and therapy. IUBMB Life 2021;73:659–669. [DOI] [PubMed] [Google Scholar]
- 217.Ye X, Li C, Zu X, Lin M, Liu Q, Liu J, Xu G, et al. A Large-Scale Multicenter Study Validates Aldo-Keto Reductase Family 1 Member B10 as a Prevalent Serum Marker for Detection of Hepatocellular Carcinoma. Hepatology 2019;69:2489–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.DiStefano JK, Davis B. Diagnostic and Prognostic Potential of AKR1B10 in Human Hepatocellular Carcinoma. Cancers (Basel) 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhou Y, Yin X, Ying J, Zhang B. Golgi protein 73 versus alpha-fetoprotein as a biomarker for hepatocellular carcinoma: a diagnostic meta-analysis. BMC Cancer 2012;12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lu Q, Li J, Cao H, Lv C, Wang X, Cao S. Comparison of diagnostic accuracy of Midkine and AFP for detecting hepatocellular carcinoma: a systematic review and meta-analysis. Biosci Rep 2020;40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhang S, Liu Y, Chen J, Shu H, Shen S, Li Y, Lu X, et al. Autoantibody signature in hepatocellular carcinoma using seromics. J Hematol Oncol 2020;13:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kim AK, Hamilton JP, Lin SY, Chang TT, Hann HW, Hu CT, Lou Y, et al. Urine DNA biomarkers for hepatocellular carcinoma screening. Br J Cancer 2022;126:1432–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhou G, Zeng Y, Luo Y, Guo S, Bao L, Zhang Q. Urine miR-93-5p is a promising biomarker for early detection of HBV-related hepatocellular carcinoma. Eur J Surg Oncol 2022;48:95–102. [DOI] [PubMed] [Google Scholar]
- 224.Han XX, Rodriguez RS, Haynes CL, Ozaki Y, Zhao B. Surface-enhanced Raman spectroscopy. Nature Reviews Methods Primers 2022;1. [Google Scholar]
- 225.Dawuti W, Zheng X, Liu H, Zhao H, Dou J, Sun L, Chu J, et al. Urine surface-enhanced Raman spectroscopy combined with SVM algorithm for rapid diagnosis of liver cirrhosis and hepatocellular carcinoma. Photodiagnosis Photodyn Ther 2022;38:102811. [DOI] [PubMed] [Google Scholar]
- 226.Kim SY, An J, Lim YS, Han S, Lee JY, Byun JH, Won HJ, et al. MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA Oncol 2017;3:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gupta P, Soundararajan R, Patel A, Kumar MP, Sharma V, Kalra N. Abbreviated MRI for hepatocellular carcinoma screening: A systematic review and meta-analysis. J Hepatol 2021;75:108–119. [DOI] [PubMed] [Google Scholar]
- 228.Kim DH, Choi SH, Shim JH, Kim SY, Lee SS, Byun JH, Choi JI. Meta-Analysis of the Accuracy of Abbreviated Magnetic Resonance Imaging for Hepatocellular Carcinoma Surveillance: Non-Contrast versus Hepatobiliary Phase-Abbreviated Magnetic Resonance Imaging. Cancers (Basel) 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.An JY, Pena MA, Cunha GM, Booker MT, Taouli B, Yokoo T, Sirlin CB, et al. Abbreviated MRI for Hepatocellular Carcinoma Screening and Surveillance. Radiographics 2020;40:1916–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sutherland T, Watts J, Ryan M, Galvin A, Temple F, Vuong J, Little AF. Diffusion-weighted MRI for hepatocellular carcinoma screening in chronic liver disease: Direct comparison with ultrasound screening. J Med Imaging Radiat Oncol 2017;61:34–39. [DOI] [PubMed] [Google Scholar]
- 231.Park HJ, Jang HY, Kim SY, Lee SJ, Won HJ, Byun JH, Choi SH, et al. Non-enhanced magnetic resonance imaging as a surveillance tool for hepatocellular carcinoma: Comparison with ultrasound. J Hepatol 2020;72:718–724. [DOI] [PubMed] [Google Scholar]
- 232.Pocha C, Dieperink E, McMaken KA, Knott A, Thuras P, Ho SB. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography -- a randomised study. Aliment Pharmacol Ther 2013;38:303–312. [DOI] [PubMed] [Google Scholar]
- 233.Yoon JH, Lee JM, Lee DH, Joo I, Jeon JH, Ahn SJ, Kim ST, et al. A Comparison of Biannual Two-Phase Low-Dose Liver CT and US for HCC Surveillance in a Group at High Risk of HCC Development. Liver Cancer 2020;9:503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Maruyama H, Shiina S. Contrast-enhanced ultrasonography: is it an ideal tool for hepatocellular carcinoma surveillance? Quant Imaging Med Surg 2019;9:1611–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Park JH, Park MS, Lee SJ, Jeong WK, Lee JY, Park MJ, Lee SS, et al. Contrast-enhanced US with Perfluorobutane for Hepatocellular Carcinoma Surveillance: A Multicenter Diagnostic Trial (SCAN). Radiology 2019;292:638–646. [DOI] [PubMed] [Google Scholar]
- 236.Kudo M, Ueshima K, Osaki Y, Hirooka M, Imai Y, Aso K, Numata K, et al. B-Mode Ultrasonography versus Contrast-Enhanced Ultrasonography for Surveillance of Hepatocellular Carcinoma: A Prospective Multicenter Randomized Controlled Trial. Liver Cancer 2019;8:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Costentin CE, Minoves M, Kotzki S, Farges O, Goutte N, Decaens T, Bailly S. Alcohol-related hepatocellular carcinoma is a heterogenous condition: Lessons from a latent class analysis. Liver Int 2022;42:1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hayes DF, Bast RC, Desch CE, Fritsche H Jr., Kemeny NE, Jessup JM, Locker GY, et al. Tumor marker utility grading system: a framework to evaluate clinical utility of tumor markers. J Natl Cancer Inst 1996;88:1456–1466. [DOI] [PubMed] [Google Scholar]
- 239.Singal AG, Hoshida Y, Pinato DJ, Marrero J, Nault JC, Paradis V, Tayob N, et al. International Liver Cancer Association (ILCA) White Paper on Biomarker Development for Hepatocellular Carcinoma. Gastroenterology 2021;160:2572–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Gui H, Huang Y, Zhao G, Chen L, Cai W, Wang H, Guo Q, et al. External Validation of aMAP Hepatocellular Carcinoma Risk Score in Patients With Chronic Hepatitis B-Related Cirrhosis Receiving ETV or TDF Therapy. Front Med (Lausanne) 2021;8:677920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Yamashita Y, Joshita S, Sugiura A, Yamazaki T, Kobayashi H, Wakabayashi SI, Yamada Y, et al. aMAP score prediction of hepatocellular carcinoma occurrence and incidence-free rate after a sustained virologic response in chronic hepatitis C. Hepatol Res 2021;51:933–942. [DOI] [PubMed] [Google Scholar]
- 242.Shiha G, Mikhail NNH, Soliman R, Hassan A, Eslam M. Predictive performance and clinical utility of HCC risk scores in chronic hepatitis C: a comparative study. Hepatol Int 2022;16:159–170. [DOI] [PubMed] [Google Scholar]
- 243.Liu K, Yip TCF, Masson S, Fateen W, Schwantes-An TH, McCaughan GW, Morgan TR, et al. Validation of the aMAP score to predict hepatocellular carcinoma development in a cohort of alcohol-related cirrhosis patients. Liver Cancer International 2022;3:99–104. [Google Scholar]
- 244.Flemming JA, Yang JD, Vittinghoff E, Kim WR, Terrault NA. Risk prediction of hepatocellular carcinoma in patients with cirrhosis: the ADRESS-HCC risk model. Cancer 2014;120:3485–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Poynard T, Peta V, Deckmyn O, Munteanu M, Moussalli J, Ngo Y, Rudler M, et al. LCR1 and LCR2, two multi-analyte blood tests to assess liver cancer risk in patients without or with cirrhosis. Aliment Pharmacol Ther 2019;49:308–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Poynard T, Lacombe JM, Deckmyn O, Peta V, Akhavan S, Zoulim F, de Ledinghen V, et al. External Validation of LCR1-LCR2, a Multivariable Hepatocellular Carcinoma Risk Calculator, in a Multiethnic Cohort of Patients With Chronic Hepatitis B. Gastro Hep Advances 2022;1:604–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wong VW, Chan SL, Mo F, Chan TC, Loong HH, Wong GL, Lui YY, et al. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clin Oncol 2010;28:1660–1665. [DOI] [PubMed] [Google Scholar]
- 248.Jung KS, Kim SU, Song K, Park JY, Kim DY, Ahn SH, Kim BK, et al. Validation of hepatitis B virus-related hepatocellular carcinoma prediction models in the era of antiviral therapy. Hepatology 2015;62:1757–1766. [DOI] [PubMed] [Google Scholar]
- 249.Lee HW, Kim SU, Park JY, Kim DY, Ahn SH, Han KH, Kim BK. External validation of the modified PAGE-B score in Asian chronic hepatitis B patients receiving antiviral therapy. Liver Int 2019;39:1624–1630. [DOI] [PubMed] [Google Scholar]
- 250.Abu-Amara M, Cerocchi O, Malhi G, Sharma S, Yim C, Shah H, Wong DK, et al. The applicability of hepatocellular carcinoma risk prediction scores in a North American patient population with chronic hepatitis B infection. Gut 2016;65:1347–1358. [DOI] [PubMed] [Google Scholar]
- 251.Wong GL, Chan HL, Chan HY, Tse PC, Tse YK, Mak CW, Lee SK, et al. Accuracy of risk scores for patients with chronic hepatitis B receiving entecavir treatment. Gastroenterology 2013;144:933–944. [DOI] [PubMed] [Google Scholar]
- 252.Kim MN, Hwang SG, Rim KS, Kim BK, Park JY, Kim DY, Ahn SH, et al. Validation of PAGE-B model in Asian chronic hepatitis B patients receiving entecavir or tenofovir. Liver Int 2017;37:1788–1795. [DOI] [PubMed] [Google Scholar]
- 253.Kim HS, Yu X, Kramer J, Thrift AP, Richardson P, Hsu YC, Flores A, et al. Comparative performance of risk prediction models for hepatitis B-related hepatocellular carcinoma in the United States. J Hepatol 2022;76:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yang HI, Yuen MF, Chan HL, Han KH, Chen PJ, Kim DY, Ahn SH, et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol 2011;12:568–574. [DOI] [PubMed] [Google Scholar]
- 255.Shiha G, Waked I, Soliman R, Elbasiony M, Gomaa A, Mikhail NNH, Eslam M. GES: A validated simple score to predict the risk of HCC in patients with HCV-GT4-associated advanced liver fibrosis after oral antivirals. Liver Int 2020;40:2828–2833. [DOI] [PubMed] [Google Scholar]
- 256.Shiha G, Soliman R, Mikhail N, Hassan A, Eslam M. Development of a simple dynamic algorithm for individualized hepatocellular carcinoma risk-based surveillance using pre- and post-treatment general evaluation score. Liver Int 2021;41:2768–2776. [DOI] [PubMed] [Google Scholar]
- 257.Shiha G, Soliman R, Mikhail NNH, Carrat F, Azzi J, Nathalie GC, Toyoda H, et al. International multicenter validation of GES score for HCC risk stratification in chronic hepatitis C patients. J Viral Hepat 2022;29:807–816. [DOI] [PubMed] [Google Scholar]
- 258.Lee MH, Yang HI, Liu J, Batrla-Utermann R, Jen CL, Iloeje UH, Lu SN, et al. Prediction models of long-term cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: risk scores integrating host and virus profiles. Hepatology 2013;58:546–554. [DOI] [PubMed] [Google Scholar]
- 259.Singal AG, Mukherjee A, Elmunzer BJ, Higgins PD, Lok AS, Zhu J, Marrero JA, et al. Machine learning algorithms outperform conventional regression models in predicting development of hepatocellular carcinoma. Am J Gastroenterol 2013;108:1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hung YC, Lin CL, Liu CJ, Hung H, Lin SM, Lee SD, Chen PJ, et al. Development of risk scoring system for stratifying population for hepatocellular carcinoma screening. Hepatology 2015;61:1934–1944. [DOI] [PubMed] [Google Scholar]
- 261.Wong GL, Chan HL, Wong CK, Leung C, Chan CY, Ho PP, Chung VC, et al. Liver stiffness-based optimization of hepatocellular carcinoma risk score in patients with chronic hepatitis B. J Hepatol 2014;60:339–345. [DOI] [PubMed] [Google Scholar]
- 262.Seo YS, Jang BK, Um SH, Hwang JS, Han KH, Kim SG, Lee KS, et al. Validation of risk prediction models for the development of HBV-related HCC: a retrospective multi-center 10-year follow-up cohort study. Oncotarget 2017;8:113213–113224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Yang HI, Sherman M, Su J, Chen PJ, Liaw YF, Iloeje UH, Chen CJ. Nomograms for risk of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. J Clin Oncol 2010;28:2437–2444. [DOI] [PubMed] [Google Scholar]
- 264.Poh Z, Shen L, Yang HI, Seto WK, Wong VW, Lin CY, Goh BB, et al. Real-world risk score for hepatocellular carcinoma (RWS-HCC): a clinically practical risk predictor for HCC in chronic hepatitis B. Gut 2016;65:887–888. [DOI] [PubMed] [Google Scholar]
- 265.Yuen MF, Tanaka Y, Fong DY, Fung J, Wong DK, Yuen JC, But DY, et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol 2009;50:80–88. [DOI] [PubMed] [Google Scholar]
- 266.Kim JH, Kim YD, Lee M, Jun BG, Kim TS, Suk KT, Kang SH, et al. Modified PAGE-B score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J Hepatol 2018;69:1066–1073. [DOI] [PubMed] [Google Scholar]
- 267.Chen CH, Lee CM, Lai HC, Hu TH, Su WP, Lu SN, Lin CH, et al. Prediction model of hepatocellular carcinoma risk in Asian patients with chronic hepatitis B treated with entecavir. Oncotarget 2017;8:92431–92441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Tawada A, Chiba T, Saito T, Ogasawara S, Suzuki E, Ooka Y, Arai M, et al. Utility of Prediction Scores for Hepatocellular Carcinoma in Patients with Chronic Hepatitis B Treated with Nucleos(t)ide Analogues. Oncology 2016;90:199–208. [DOI] [PubMed] [Google Scholar]
- 269.Lee MH, Lu SN, Yuan Y, Yang HI, Jen CL, You SL, Wang LY, et al. Development and validation of a clinical scoring system for predicting risk of HCC in asymptomatic individuals seropositive for anti-HCV antibodies. PLoS One 2014;9:e94760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Ganne-Carrie N, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, Pol S, et al. Nomogram for individualized prediction of hepatocellular carcinoma occurrence in hepatitis C virus cirrhosis (ANRS CO12 CirVir). Hepatology 2016;64:1136–1147. [DOI] [PubMed] [Google Scholar]
- 271.Marot A, Vandenbulcke H, Knebel JF, Doerig C, Moreno C, Deltenre P. External validation of the nomogram for individualized prediction of hepatocellular carcinoma occurrence in patients with hepatitis C virus-related compensated cirrhosis. Hepatology 2017;65:1419–1421. [DOI] [PubMed] [Google Scholar]
- 272.Pons M, Rodriguez-Tajes S, Esteban JI, Marino Z, Vargas V, Lens S, Buti M, et al. Non-invasive prediction of liver-related events in patients with HCV-associated compensated advanced chronic liver disease after oral antivirals. J Hepatol 2020;72:472–480. [DOI] [PubMed] [Google Scholar]
- 273.Suh B, Park S, Shin DW, Yun JM, Yang HK, Yu SJ, Shin CI, et al. High liver fibrosis index FIB-4 is highly predictive of hepatocellular carcinoma in chronic hepatitis B carriers. Hepatology 2015;61:1261–1268. [DOI] [PubMed] [Google Scholar]
- 274.Fusco M, Piselli P, Virdone S, Di Cicco P, Scognamiglio P, De Paoli P, Ciullo V, et al. Infection with hepatitis viruses, FIB-4 index and risk of hepatocellular carcinoma in southern Italy: a population-based cohort study. Infect Agent Cancer 2016;11:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Suh B, Yun JM, Park S, Shin DW, Lee TH, Yang HK, Ahn E, et al. Prediction of future hepatocellular carcinoma incidence in moderate to heavy alcohol drinkers with the FIB-4 liver fibrosis index. Cancer 2015;121:3818–3825. [DOI] [PubMed] [Google Scholar]
- 276.Loosen SH, Kostev K, Keitel V, Tacke F, Roderburg C, Luedde T. An elevated FIB-4 score predicts liver cancer development: A longitudinal analysis from 29,999 patients with NAFLD. J Hepatol 2022;76:247–248. [DOI] [PubMed] [Google Scholar]
- 277.Tamaki N, Kurosaki M, Yasui Y, Mori N, Tsuji K, Hasebe C, Joko K, et al. Change in Fibrosis 4 Index as Predictor of High Risk of Incident Hepatocellular Carcinoma After Eradication of Hepatitis C Virus. Clin Infect Dis 2021;73:e3349–e3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Zhang H, Zhu J, Xi L, Xu C, Wu A. Validation of the Toronto hepatocellular carcinoma risk index for patients with cirrhosis in China: a retrospective cohort study. World J Surg Oncol 2019;17:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Demirtas CO, Gunduz F, Kani HT, Keklikkiran C, Alahdab YO, Yilmaz Y, Duman DG, et al. External validation of the Toronto hepatocellular carcinoma risk index in Turkish cirrhotic patients. Eur J Gastroenterol Hepatol 2020;32:882–888. [DOI] [PubMed] [Google Scholar]
- 280.Astrom H, Ndegwa N, Hagstrom H. External validation of the Toronto hepatocellular carcinoma risk index in a Swedish population. JHEP Rep 2021;3:100343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Fan C, Li M, Gan Y, Chen T, Sun Y, Lu J, Wang J, et al. A simple AGED score for risk classification of primary liver cancer: development and validation with long-term prospective HBsAg-positive cohorts in Qidong, China. Gut 2019;68:948–949. [DOI] [PubMed] [Google Scholar]
- 282.Sinn DH, Lee JH, Kim K, Ahn JH, Lee JH, Kim JH, Lee DH, et al. A Novel Model for Predicting Hepatocellular Carcinoma Development in Patients with Chronic Hepatitis B and Normal Alanine Aminotransferase Levels. Gut Liver 2017;11:528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Papatheodoridis G, Dalekos G, Sypsa V, Yurdaydin C, Buti M, Goulis J, Calleja JL, et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol 2016;64:800–806. [DOI] [PubMed] [Google Scholar]
- 284.Yip TC, Wong GL, Wong VW, Tse YK, Liang LY, Hui VW, Lee HW, et al. Reassessing the accuracy of PAGE-B-related scores to predict hepatocellular carcinoma development in patients with chronic hepatitis B. J Hepatol 2020;72:847–854. [DOI] [PubMed] [Google Scholar]
- 285.Guzelbulut F, Gokcen P, Can G, Adali G, Degirmenci Salturk AG, Bahadir O, Ozdil K, et al. Validation of the HCC-RESCUE score to predict hepatocellular carcinoma risk in Caucasian chronic hepatitis B patients under entecavir or tenofovir therapy. J Viral Hepat 2021;28:826–836. [DOI] [PubMed] [Google Scholar]
- 286.Chang JW, Lee JS, Lee HW, Kim BK, Park JY, Kim DY, Ahn SH, et al. Validation of risk prediction scores for hepatocellular carcinoma in patients with chronic hepatitis B treated with entecavir or tenofovir. J Viral Hepat 2021;28:95–104. [DOI] [PubMed] [Google Scholar]
- 287.Papatheodoridis GV, Sypsa V, Dalekos GN, Yurdaydin C, Van Boemmel F, Buti M, Calleja JL, et al. Hepatocellular carcinoma prediction beyond year 5 of oral therapy in a large cohort of Caucasian patients with chronic hepatitis B. J Hepatol 2020;72:1088–1096. [DOI] [PubMed] [Google Scholar]
- 288.Ji JH, Park SY, Son WJ, Shin HJ, Lee H, Lee HW, Lee JS, et al. External validation of CAGE-B and SAGE-B scores for Asian chronic hepatitis B patients with well-controlled viremia by antivirals. J Viral Hepat 2021;28:951–958. [DOI] [PubMed] [Google Scholar]
- 289.Lim J, Chon YE, Kim MN, Lee JH, Hwang SG, Lee HC, Ha Y. Cirrhosis, Age, and Liver Stiffness-Based Models Predict Hepatocellular Carcinoma in Asian Patients with Chronic Hepatitis B. Cancers (Basel) 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Chon HY, Lee JS, Lee HW, Chun HS, Kim BK, Tak WY, Park JY, et al. Predictive Performance of CAGE-B and SAGE-B Models in Asian Treatment-Naive Patients Who Started Entecavir for Chronic Hepatitis B. Clin Gastroenterol Hepatol 2022;20:e794–e807. [DOI] [PubMed] [Google Scholar]
- 291.Lee HW, Yoo EJ, Kim BK, Kim SU, Park JY, Kim DY, Ahn SH, et al. Prediction of development of liver-related events by transient elastography in hepatitis B patients with complete virological response on antiviral therapy. Am J Gastroenterol 2014;109:1241–1249. [DOI] [PubMed] [Google Scholar]
- 292.Sohn W, Cho JY, Kim JH, Lee JI, Kim HJ, Woo MA, Jung SH, et al. Risk score model for the development of hepatocellular carcinoma in treatment-naive patients receiving oral antiviral treatment for chronic hepatitis B. Clin Mol Hepatol 2017;23:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Lee HW, Park SY, Lee M, Lee EJ, Lee J, Kim SU, Park JY, et al. An optimized hepatocellular carcinoma prediction model for chronic hepatitis B with well-controlled viremia. Liver Int 2020;40:1736–1743. [DOI] [PubMed] [Google Scholar]
- 294.Kim HY, Lampertico P, Nam JY, Lee HC, Kim SU, Sinn DH, Seo YS, et al. An artificial intelligence model to predict hepatocellular carcinoma risk in Korean and Caucasian patients with chronic hepatitis B. J Hepatol 2022;76:311–318. [DOI] [PubMed] [Google Scholar]
- 295.Yang HI, Yeh ML, Wong GL, Peng CY, Chen CH, Trinh HN, Cheung KS, et al. Real-World Effectiveness From the Asia Pacific Rim Liver Consortium for HBV Risk Score for the Prediction of Hepatocellular Carcinoma in Chronic Hepatitis B Patients Treated With Oral Antiviral Therapy. J Infect Dis 2020;221:389–399. [DOI] [PubMed] [Google Scholar]
- 296.Yu JH, Suh YJ, Jin YJ, Heo NY, Jang JW, You CR, An HY, et al. Prediction model for hepatocellular carcinoma risk in treatment-naive chronic hepatitis B patients receiving entecavir/tenofovir. Eur J Gastroenterol Hepatol 2019;31:865–872. [DOI] [PubMed] [Google Scholar]
- 297.Hsu YC, Yip TC, Ho HJ, Wong VW, Huang YT, El-Serag HB, Lee TY, et al. Development of a scoring system to predict hepatocellular carcinoma in Asians on antivirals for chronic hepatitis B. J Hepatol 2018;69:278–285. [DOI] [PubMed] [Google Scholar]
- 298.Kim SU, Seo YS, Lee HA, Kim MN, Kim EH, Kim HY, Lee YR, et al. Validation of the CAMD Score in Patients With Chronic Hepatitis B Virus Infection Receiving Antiviral Therapy. Clin Gastroenterol Hepatol 2020;18:693–699 e691. [DOI] [PubMed] [Google Scholar]
- 299.Hiraoka A, Kumada T, Ogawa C, Kariyama K, Morita M, Nouso K, Toyoda H, et al. Proposed a simple score for recommendation of scheduled ultrasonography surveillance for hepatocellular carcinoma after Direct Acting Antivirals: multicenter analysis. J Gastroenterol Hepatol 2019;34:436–441. [DOI] [PubMed] [Google Scholar]
- 300.Azzi J, Dorival C, Cagnot C, Fontaine H, Lusivika-Nzinga C, Leroy V, De Ledinghen V, et al. Prediction of hepatocellular carcinoma in Hepatitis C patients with advanced fibrosis after sustained virologic response. Clin Res Hepatol Gastroenterol 2022;46:101923. [DOI] [PubMed] [Google Scholar]
- 301.Watanabe T, Tokumoto Y, Joko K, Michitaka K, Horiike N, Tanaka Y, Tada F, et al. Predictors of hepatocellular carcinoma occurrence after direct-acting antiviral therapy in patients with hepatitis C virus infection. Hepatol Res 2019;49:136–146. [DOI] [PubMed] [Google Scholar]
- 302.Alonso Lopez S, Manzano ML, Gea F, Gutierrez ML, Ahumada AM, Devesa MJ, Olveira A, et al. A Model Based on Noninvasive Markers Predicts Very Low Hepatocellular Carcinoma Risk After Viral Response in Hepatitis C Virus-Advanced Fibrosis. Hepatology 2020;72:1924–1934. [DOI] [PubMed] [Google Scholar]
- 303.Tani J, Morishita A, Sakamoto T, Takuma K, Nakahara M, Fujita K, Oura K, et al. Simple scoring system for prediction of hepatocellular carcinoma occurrence after hepatitis C virus eradication by direct-acting antiviral treatment: All Kagawa Liver Disease Group Study. Oncol Lett 2020;19:2205–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Abe K, Wakabayashi H, Nakayama H, Suzuki T, Kuroda M, Yoshida N, Tojo J, et al. Factors associated with hepatocellular carcinoma occurrence after HCV eradication in patients without cirrhosis or with compensated cirrhosis. PLoS One 2020;15:e0243473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Hu CC, Weng CH, Hua MC, Chang PH, Lin CL, Chen YT, Chien CH, et al. New Scoring Method to Predict Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis C After Pegylated Interferon and Ribavirin Therapy. J Interferon Cytokine Res 2020;40:82–91. [DOI] [PubMed] [Google Scholar]
- 306.Sinn DH, Kang D, Cho SJ, Paik SW, Guallar E, Cho J, Gwak GY. Risk of hepatocellular carcinoma in individuals without traditional risk factors: development and validation of a novel risk score. Int J Epidemiol 2020;49:1562–1571. [DOI] [PubMed] [Google Scholar]
- 307.Pennisi G, Pipitone RM, Enea M, De Vincentis A, Battaglia S, Di Marco V, Di Martino V, et al. A Genetic and Metabolic Staging System for Predicting the Outcome of Nonalcoholic Fatty Liver Disease. Hepatol Commun 2022;6:1032–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Wang H, Cao H, Xu Z, Wang D, Zeng Y. SNP rs2596542G>A in MICA is associated with risk of hepatocellular carcinoma: a meta-analysis. Biosci Rep 2019;39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Zhang YF, Zeng XL, Lu HW, Ji H, Lu L, Liu PD, Hong RF, et al. Association between KIF1B (rs17401966) polymorphism and hepatocellular carcinoma susceptibility: a meta-analysis. Onco Targets Ther 2018;11:3225–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Shi H, He H, Ojha SC, Sun C, Fu J, Yan M, Deng C, et al. Association of STAT3 and STAT4 polymorphisms with susceptibility to chronic hepatitis B virus infection and risk of hepatocellular carcinoma: a meta-analysis. Biosci Rep 2019;39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Singal AG, Manjunath H, Yopp AC, Beg MS, Marrero JA, Gopal P, Waljee AK. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: a meta-analysis. Am J Gastroenterol 2014;109:325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Yang J, Trepo E, Nahon P, Cao Q, Moreno C, Letouze E, Imbeaud S, et al. A 17-Beta-Hydroxysteroid Dehydrogenase 13 Variant Protects From Hepatocellular Carcinoma Development in Alcoholic Liver Disease. Hepatology 2019;70:231–240. [DOI] [PubMed] [Google Scholar]
- 313.Huang YH, Liang KH, Chien RN, Hu TH, Lin KH, Hsu CW, Lin CL, et al. A Circulating MicroRNA Signature Capable of Assessing the Risk of Hepatocellular Carcinoma in Cirrhotic Patients. Sci Rep 2017;7:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Cho EJ, Leem S, Kim SA, Yang J, Lee YB, Kim SS, Cheong JY, et al. Circulating Microbiota-Based Metagenomic Signature for Detection of Hepatocellular Carcinoma. Sci Rep 2019;9:7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Colli A, Nadarevic T, Miletic D, Giljaca V, Fraquelli M, Stimac D, Casazza G. Abdominal ultrasound and alpha-foetoprotein for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease. Cochrane Database Syst Rev 2021;4:CD013346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.McMahon BJ, Bulkow L, Harpster A, Snowball M, Lanier A, Sacco F, Dunaway E, et al. Screening for hepatocellular carcinoma in Alaska natives infected with chronic hepatitis B: a 16-year population-based study. Hepatology 2000;32:842–846. [DOI] [PubMed] [Google Scholar]
- 317.Yang T, Xing H, Wang G, Wang N, Liu M, Yan C, Li H, et al. A Novel Online Calculator Based on Serum Biomarkers to Detect Hepatocellular Carcinoma among Patients with Hepatitis B. Clin Chem 2019;65:1543–1553. [DOI] [PubMed] [Google Scholar]
- 318.Peng F, Yuan H, Zhou YF, Wu SX, Long ZY, Peng YM. Diagnostic Value of Combined Detection via Protein Induced by Vitamin K Absence or Antagonist II, Alpha-Fetoprotein, and D-Dimer in Hepatitis B Virus-Related Hepatocellular Carcinoma. Int J Gen Med 2022;15:5763–5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Lin Y, Zhu J, Zhang J, Dai J, Liu S, Arroyo A, Rose M, et al. Glycopeptides with Sialyl Lewis Antigen in Serum Haptoglobin as Candidate Biomarkers for Nonalcoholic Steatohepatitis Hepatocellular Carcinoma Using a Higher-Energy Collision-Induced Dissociation Parallel Reaction Monitoring-Mass Spectrometry Method. ACS Omega 2022;7:22850–22860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Lin N, Lin Y, Xu J, Liu D, Li D, Meng H, Gallant MA, et al. A multi-analyte cell-free DNA-based blood test for early detection of hepatocellular carcinoma. Hepatol Commun 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Lee HW, Kim E, Cho KJ, Park HJ, Seo J, Lee H, Baek E, et al. Applications of molecular barcode sequencing for the detection of low-frequency variants in circulating tumour DNA from hepatocellular carcinoma. Liver Int 2022. [DOI] [PubMed] [Google Scholar]
- 322.Tao K, Bian Z, Zhang Q, Guo X, Yin C, Wang Y, Zhou K, et al. Machine learning-based genome-wide interrogation of somatic copy number aberrations in circulating tumor DNA for early detection of hepatocellular carcinoma. EBioMedicine 2020;56:102811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Xu RH, Wei W, Krawczyk M, Wang W, Luo H, Flagg K, Yi S, et al. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat Mater 2017;16:1155–1161. [DOI] [PubMed] [Google Scholar]
- 324.Fang Y, Yan D, Wang L, Zhang J, He Q. Circulating microRNAs (miR-16, miR-22, miR-122) expression and early diagnosis of hepatocellular carcinoma. J Clin Lab Anal 2022;36:e24541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Kunadirek P, Pinjaroen N, Nookaew I, Tangkijvanich P, Chuaypen N. Transcriptomic Analyses Reveal Long Non-Coding RNA in Peripheral Blood Mononuclear Cells as a Novel Biomarker for Diagnosis and Prognosis of Hepatocellular Carcinoma. Int J Mol Sci 2022;23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Nguyen HB, Le XT, Nguyen HH, Vo TT, Le MK, Nguyen NT, Do-Nguyen TM, et al. Diagnostic Value of hTERT mRNA and in Combination With AFP, AFP-L3%, Des-gamma-carboxyprothrombin for Screening of Hepatocellular Carcinoma in Liver Cirrhosis Patients HBV or HCV-Related. Cancer Inform 2022;21:11769351221100730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Huang X, Sun L, Wen S, Deng D, Wan F, He X, Tian L, et al. RNA sequencing of plasma exosomes revealed novel functional long noncoding RNAs in hepatocellular carcinoma. Cancer Sci 2020;111:3338–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Ghosh S, Bhowmik S, Majumdar S, Goswami A, Chakraborty J, Gupta S, Aggarwal S, et al. The exosome encapsulated microRNAs as circulating diagnostic marker for hepatocellular carcinoma with low alpha-fetoprotein. Int J Cancer 2020;147:2934–2947. [DOI] [PubMed] [Google Scholar]
- 329.Xu H, Chen Y, Dong X, Wang X. Serum Exosomal Long Noncoding RNAs ENSG00000258332.1 and LINC00635 for the Diagnosis and Prognosis of Hepatocellular Carcinoma. Cancer Epidemiol Biomarkers Prev 2018;27:710–716. [DOI] [PubMed] [Google Scholar]
- 330.Wang Y, Zhang C, Zhang P, Guo G, Jiang T, Zhao X, Jiang J, et al. Serum exosomal microRNAs combined with alpha-fetoprotein as diagnostic markers of hepatocellular carcinoma. Cancer Med 2018;7:1670–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Julich-Haertel H, Urban SK, Krawczyk M, Willms A, Jankowski K, Patkowski W, Kruk B, et al. Cancer-associated circulating large extracellular vesicles in cholangiocarcinoma and hepatocellular carcinoma. J Hepatol 2017;67:282–292. [DOI] [PubMed] [Google Scholar]
- 332.Wang W, Li H, Zhou Y, Jie S. Peripheral blood microvesicles are potential biomarkers for hepatocellular carcinoma. Cancer Biomark 2013;13:351–357. [DOI] [PubMed] [Google Scholar]
- 333.Vongsuvanh R, van der Poorten D, Iseli T, Strasser SI, McCaughan GW, George J. Midkine Increases Diagnostic Yield in AFP Negative and NASH-Related Hepatocellular Carcinoma. PLoS One 2016;11:e0155800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Kim JY, Kim J, Lim Y-S, Gwak G-Y, Yeo I, Kim Y, Lee J, et al. Proteome Multimarker Panel for the Early Detection of Hepatocellular Carcinoma: Multicenter Derivation, Validation, and Comparison. ACS Omega 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Cao LL, Han Y, Pei L, Yue ZH, Liu BY, Cui JW, Jia M, et al. A Serum Metabolite Classifier for the Early Detection of Type 2 Diabetes Mellitus-Positive Hepatocellular Cancer. Metabolites 2022;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Kim DH, Choi SH, Shim JH, Kim SY, Lee SS, Byun JH, Kim KW, et al. Magnetic Resonance Imaging for Surveillance of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Diagnostics (Basel) 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ahmed NNA, El Gaafary SM, Elia RZ, Abdulhafiz EM. Role of abbreviated MRI protocol for screening of HCC in HCV related cirrhotic patients prior to direct-acting antiviral treatment. Egyptian Journal of Radiology and Nuclear Medicine 2020;51. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


