Summary
Background
For ventilator-associated pneumonia (VAP), the safety of short-course versus long-course antibiotic therapy is still debated, especially regarding documented VAP due to non-fermenting Gram-negative bacilli (NF-GNB). The aim of this meta-analysis was to assess the rates of recurrence and relapse of VAP in patients receiving short-course (≤8 days) and long-course (≥10–15 days) of antibiotic therapy.
Methods
The protocol for this study was registered in the PROSPERO database (ID: CRD42022365138). We performed an electronic search of the relevant literature and limited our search to data published from 2000 until September 1, 2022. We searched for randomized controlled trials (RCTs) in the United States National Library of Medicine, Cochrane Database of Systematic Reviews (CDSR) and the Cochrane Central Register of Controlled Trials (CENTRAL), Embase, National Institutes of Health PubMed/MEDLINE, web of science and Google Scholar databases. The primary endpoint was the recurrence and relapses of VAP, secondary endpoints were 28-day mortality, mechanical ventilation duration, number of extra-pulmonary infections and length of ICU stay.
Findings
We identified five relevant studies involving 1069 patients (530 patients in the short-course group and 539 patients in the long-course group). The meta-analysis did not reveal any significant difference between short and long-course antibiotic therapy for recurrence and relapses of VAP (odd ratio “OR” = 1.48, 95% confidence intervals (CI) [0.96, 2.28], p = 0.08 and OR = 1.45, 95% CI [0.94, 2.22], p = 0.09, respectively), including those due to NF-GNB (OR = 1.90, 95% CI [0.93, 3.33], p = 0.05 and OR = 1.76, 95% CI [0.93, 3.33], p = 0.08, respectively). No difference was found for 28 days-mortality (OR = 1.24, 95% CI [0.92, 1.67], p = 0.16), mechanical ventilation duration, number of extra-pulmonary infections and length of ICU stay. However, short-course therapy significantly increased the number of antibiotic-free days.
Interpretation
Our meta-analysis showed that short-course antibiotic therapy did not result in increased number of recurence and relapses of VAP, suggesting that short-course should be preferred to reduce the exposure to antibiotics.
Funding
None.
Keywords: Ventilator-associated pneumonia, Antibiotic duration, Gram-negative bacilli, Recurrence, Systematic review, Meta-analysis
Research in context.
Evidence before this study
We sought trials in the United States National Library of Medicine, Cochrane Database of Systematic Reviews (CDSR) and the Cochrane Central Register of Controlled Trials (CENTRAL), Embase, National Institutes of Health PubMed/MEDLINE, web of science and Google Scholar databases. The MEDLINE and Embase strategies were run simultaneously as a multi-file search in Ovid, and the results were de-duplicated using the Ovid duplication tool. We only included RCTs comparing short-course antibiotic therapy (≤8 days) versus long-course antibiotic therapy (≥10–15 days) for VAP in adult patients (age ≥18 years). We did not use language restrictions. Only articles published in peer-reviewed journals were considered. Data from controlled clinical trials, non-comparative studies, review articles, editorial letters, abstract only, comments, and case series (fewer than ten cases) were excluded. RCTs were evaluated according to the Consolidated Standards of Reporting Trials (CONSORT) Statement of quality assessment. Studies with a score <14/25 were excluded. The Cochrane tool for bias assessment was used to assess the risk of bias in RCTs (RoB2).
Added value of this study
Short-course antibiotic therapy for VAP did not significantly affect the rate of recurrence relapses, and mortality compared with long-course antibiotic therapy. However, for VAP due to NF-GNB, even if a higher risk of recurrence is reported, it did not translate into clinical outcomes such as mortality and duration of ICU stay. In addition, short-course therapy had several desirable consequences namely decreased antibiotic exposure, reduced antibiotic resistance, and lower overall costs.
Implications of all the available evidence
Tailored strategies (including clinical and biological endpoints) should be tested in RCTs in order to individualize antibiotic duration treatment.
Introduction
In intensive care unit (ICU) patients, ventilator-associated pneumonia (VAP) is a frequent condition associated with poor outcome. Its incidence ranges from 2 to 16 episodes per 1000 ventilator days.1 VAP is associated with high mortality and morbidity rates, including prolonged hospitalization and increased healthcare resource utilization.2 Despite guidelines on the treatment of VAP, the optimal duration of antibiotic therapy remains debated, especially for VAP due to non-fermenting Gram negative bacteria (NF-GNB).1,3,4
In patients with VAP that are not due to NF-GNB, a previous meta-analysis found that a short-course of antibiotic therapy did not increase the risk of recurrence or mortality while it reduced the emergence of resistant bacteria.5 However, this evidence was from a sub-group analyses with important limitations, namely the fact that it focused not only on VAP but on all types of hospital-acquired pneumonia, only two randomized controlled trials (RCT) were included on patients with VAP due to NF-GNB and one of the included RCTs was an abstract. On the other hand, a recent RCT found an increased rate of recurrence when patients with VAP due to NF-GNB received a short-course of antibiotic treatment.6
Therefore, the aim of this meta-analysis was to assess the safety of short-course antibiotic therapy compared to a long-course antibiotic therapy for VAP, especially late onset VAP due to NF-GNB. The primary endpoint was a composite outcome including the recurrence and relapses of VAP and secondary endpoints were 28-day mortality, mechanical ventilation duration, number of extra-pulmonary infections, and ICU length of stay.
Methods
This systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines 20207 and AMSTAR 2 (Assessing the methodological quality of systematic reviews) Guidelines.8 The protocol is registered in the PROSPERO database of systematic ID: CRD 42022365138. No Ethical Approval or consent is required as this research project is a systematic review of previous studies.
Electronic searches
We performed an electronic search of the relevant literature and limited our search to data published from 2000 until September 1, 2022. To increase the consistency of our results and in order to reduce heterogeneity, we selected this limited study period. We did not use language restrictions. We sought trials in the United States National Library of Medicine, Cochrane Database of Systematic Reviews (CDSR) and the Cochrane Central Register of Controlled Trials (CENTRAL), Embase, National Institutes of Health PubMed/MEDLINE, web of science and Google Scholar databases. The MEDLINE and Embase strategies were run simultaneously as a multi-file search in Ovid, and the results were de-duplicated using the Ovid duplication tool. We used the following keywords: “antibiotic”, “ventilator-associated pneumonia”, “VAP”, “intensive care unit”, “treatment”, “therapy”, and “randomized controlled trial”. We checked the reference list of included trials manually to identify additional studies. Additionally, we searched several clinical trial registries (ClinicalTrial.gov), Current Controlled Trials, Australian New Zealand Clinical Trials Registry (www.actr.org.au), Prospero registration and University Hospital Medical Information Network Clinical Trials Registry (www.umin.ac.jp/ctr) to identify ongoing trials.
Study selection
Two authors (M.A.D. and M.A.C.) performed independent and blinded record screening. Data extraction was performed by the same two authors and results were cross-checked to ensure consensus. Disagreements were resolved by discussion after consulting a third review team member (BD). We only included RCTs comparing short-course antibiotic therapy (≤8 days) versus long-course antibiotic therapy (≥10–15 days) for VAP in adult patients (age ≥18 years). We did not use language restrictions. Only articles published in peer-reviewed journals were considered. Data from controlled clinical trials, non-comparative studies, review articles, editorial letters, abstract only, comments, and case series (fewer than ten cases) were excluded.
Assessment of the studies' quality and risk of bias
Two authors (M.A.D. and M.A.C.) evaluated the methodology of the studies that responded to the inclusion criteria; in case of discordance or absence of consensus on some records, the senior author (BD) was consulted. RCTs were evaluated according to the Consolidated Standards of Reporting Trials (CONSORT) Statement of quality assessment.9 Studies with a score <14/25 were excluded. The Cochrane tool for bias assessment was used to assess the risk of bias in RCTs (RoB2).10 We evaluated the bias in five distinct domains (A. randomization process, B. deviations from intended interventions, C. the bias in the measurement of outcome, D. bias to missing outcome data, E. bias in selecting the reported results). Within each domain, one or more signalling questions lead to judgments of “low risk of bias,” “some concerns,” or “high risk of bias”.
Data extraction and outcomes
Data, including the first authors name, year of publication, country, Sequential Organ Failure Assessment (SOFA) score, Simplified Acute Physiology score (SAPS II) score, age, VAP definition, population, antibiotic regimen (type, duration), VAP type (early and late onset), follow-up and CONSORT score, were extracted from the studies.
We conducted our search based on the PICO approach. Population was adult patients with VAP. Intervention and comparator groups were short versus long-duration antibiotic therapy for VAP. The primary outcome was a composite endpoint combining VAP recurrence and relapses during the ICU stay. Recurrence was defined as clinical suspicion of VAP after at least 48 h without effective treatment and confirmed with positive microbiological culture. Relapses were defined as microbiologically documented VAP due to the same pathogen. Secondary outcomes were 28-day mortality (If no such data were reported, then available mortality data of at least 28 and up to 90 days were used), length of ICU stay, invasive mechanical ventilation duration, antibiotic-free days during ICU stay, number of extra-pulmonary infections and acquisition of multidrug-resistant (MDR) pathogens.
Subgroup analyses were conducted in patients with late-onset VAP and those with VAP due to NF-GNB.
Corresponding authors of the five selected studies were contacted in order to perform a meta-analysis on individual data. Of those, one refused to provide the data, one accepted, one no had longer the data, and two did not reply.
Missing data
In case of unclear bias domains or missing primary outcomes information, authors were contacted by e-mail. If data were not numerically reported we extracted them from figures. If we did not have clear information, studies were excluded.
Summary of findings
Two authors (M.A.D. and M.A.C.) independently assessed the certainty of the evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE).11 We considered the study limitations constancy of effect, imprecision, indirectness, and publication bias. We assessed the certainty of evidence as high, moderate, low, or very low. If appropriate, we considered the following criteria for upgrading the evidence: large effect, dose–response gradient, and plausible confounding effect. We used the methods and recommendations described in sections 8.5 and 8.7 and chapters 11 and 12 of the Cochrane Handbook for Systematic Reviews of Interventions. We used GRADEpro GDT software to prepare the summary of findings tables. The reasons for downgrading or upgrading the included studies are described in the footnotes with comments.
Statistics
Handling continuous data
Continuous data were analyzed using Review Manager 5.3.5 statistical package from Cochrane collaboration for meta-analysis.12 When mean and standard deviation (SD) were not reported, they were estimated from the provided interquartile range (IR) and median based on the formula described by Hozo et al.13 If the sample size was >25 patients, then the mean was equal to the median. In addition, SD was calculated as IR/4 for a sample size <70 patients and IR/7 for a sample size >70 patients.
Assessment of heterogeneity
We used the Cochrane Chi2 test (Q-test), the I2 statistic, and the variance TAU2 to estimate the degree of heterogeneity.14 Funnel plots identified studies resulting in heterogeneity. A subgroup analysis was performed when all the included studies reported the outcome.
Evaluation of size effect
We used the RevMan 5.4 statistical package from the Cochrane collaboration for meta-analysis.12 We selected the mean difference (MD) as an effective measure for continuous data. Odds ratios (OR) with 95% confidence intervals (95% CI) were calculated for dichotomous variables. The random-effects model was used, and the significance threshold was fixed at 0.05.
Role of the funding source
There was no funding source for this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit to publication.
Results
Study identification and characteristics
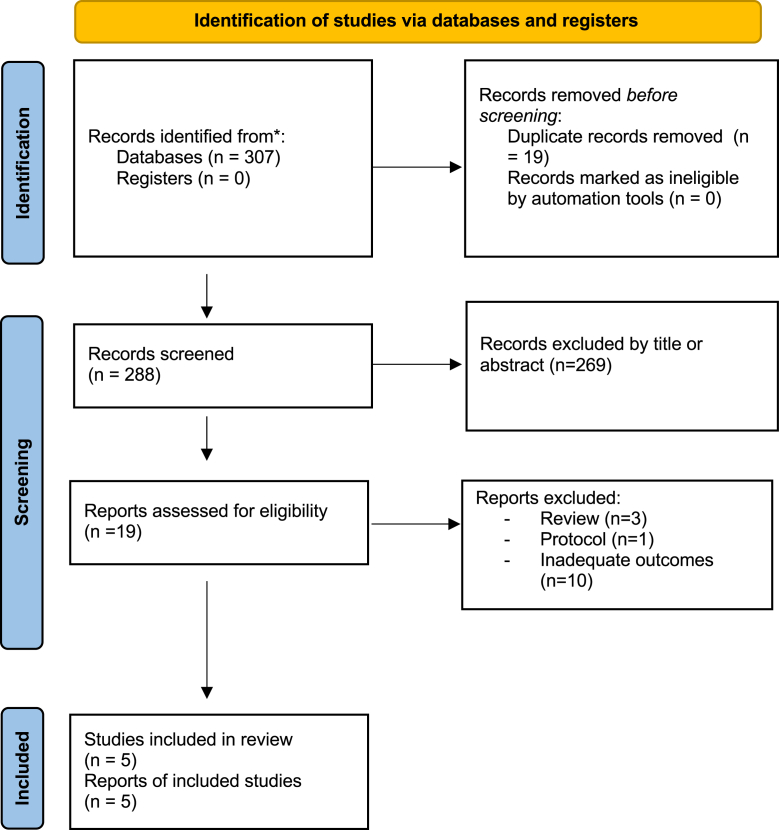
Through our literature search, we identified 307 articles (Fig. 1). After excluding duplicates (n = 19) and screening based on titles and abstracts (n = 269), 19 relevant articles were selected for full-text screening. Fourteen articles were excluded for the following reasons: ten studies used a de-escalation protocol or biomarker to guide the duration of therapy,15, 16, 17, 18, 19, 20, 21, 22, 23, 24 three studies were systematic reviews or meta-analyses,2,5,25 and one study was published as protocol.26 Finally, we retained five RCTs, including 1069 patients (530 in the short-course and 539 the long-course group6,27, 28, 29, 30) published from November 2003 to May 2022. Regarding the type of VAP, one trial included patients with early-onset VAP,30 three included patients with late-onset VAP,6,27,28 and the remaining study included patients with mixed type of VAP with less than 20% of patients having early-onset VAP.29 The most common classes of antibiotic used in four RCTs were broad-spectrum beta-lactams combined with aminoglycosides or fluoroquinolone. However, the study by Kollef et al.28 used carbapenem in 100% of cases, with doripenem in the short-course group and imipenem-cilastatin in the long-course group. For microbiology data, two studies included only VAP due to Gram-negative bacteria,6,28 and Fekih et al. included more than 80% of VAP due to NF-GNB.25 Regarding antibiotic therapy duration, three trials6,27,30 had a duration of 8 days for the short-course and 15 days for the long-course, while the remaining trials had a duration of 8 versus 10 days and 7 versus 10 days, respectively.28,29
Fig. 1.
PRISMA 2020 flow-diagram of the retained studies.
Primary outcome: Recurrence and relapses of pneumonia
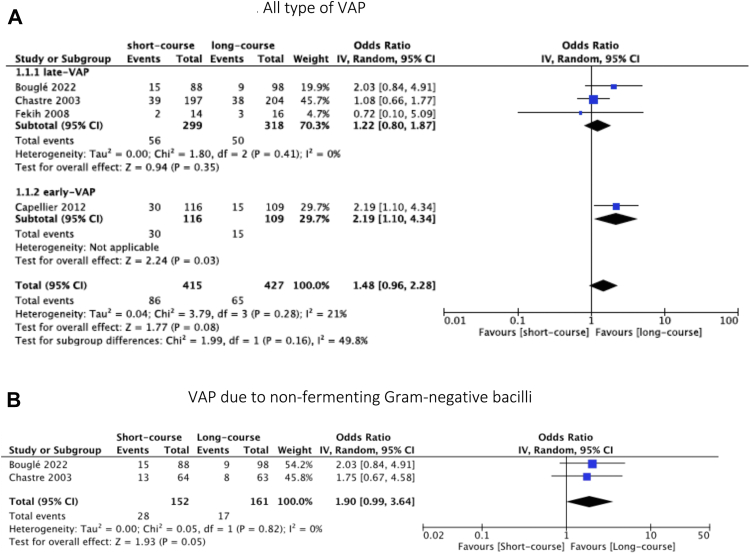
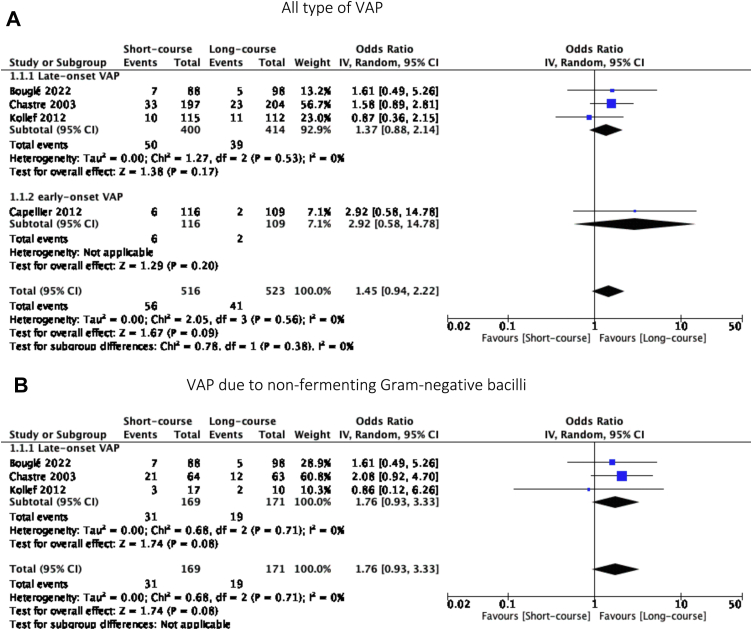
Recurrence and relapses of VAP were reported in four studies.6,27,29,30 There were no significant difference between short-course and long-course antibiotic therapy (OR = 1.48, 95% CI [0.96, 2.28], p = 0.08 and OR = 1.45, 95% CI [0.94, 2.22], p = 0.09, I2 statistic = 21% and 0%, respectively).
Focusing on late-onset VAP, the primary outcome was similar in the two groups (Figs. 2A and 3A).
Fig. 2.
Forest plot of recurrence.
Fig. 3.
Forest plot of relapses.
For VAP due to NF-GNB, three RCTs reported data on the recurrence and relapses of VAP.6,27,28 We did not find a significant difference in the recurrence and relapses of VAP between the two groups (OR = 1.90, 95% CI [0.93, 3.33], p = 0.05 and OR = 1.76, 95% CI [0.93, 3.33], p = 0.08, I2 = 0 respectively) (Figs. 2B and 3B).
Secondary outcomes
28-Days mortality
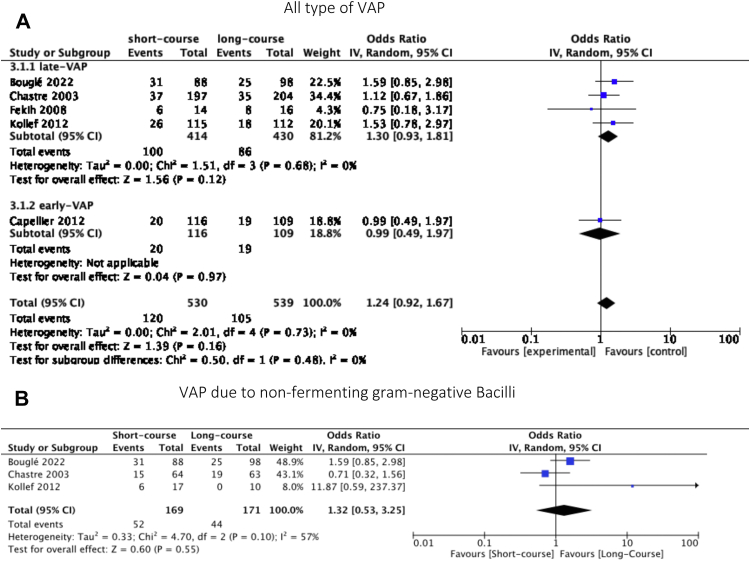
Data on mortality were reported in all selected studies. No significant difference were found between the two groups (OR = 1.24, 95% CI [0.92, 1.67], p = 0.16; I2 statistic = 0, p = 0.73). In the subgroup of late-onset VAP,6,27, 28, 29 there were no significant difference between the two groups (OR = 1.30 95% CI [0.93, 1.81], p = 0.12) (Fig. 4). For VAP due to NF-GNB, we did not find a significant difference between short-course and long-course groups either (OD = 1.32, 95% CI [0.53, 3.25], p = 0.55; I2 statistic = 57, p = 0.10).
Fig. 4.
Forest plot of mortality.
Length of intensive care unit stay
Three studies reported data on the ICU length of stay.6,29,30 Pooled results showed no significant difference between short-course and long-course antibiotic therapy (MD = −0.05, 95% CI [−0.96, 0.85], p = 0.91; I2 statistic = 0, p = 0.55). Similar findings were reported in a subgroup analysis (For late-onset VAP, MD = −0.28, 95% CI [−1.53, 0.97], p = 0.66). For VAP due to NF-GBN, only one trial reported data on this outcome showing no difference between the groups (MD = 0, 95% CI [−7, 6]).6
Mechanical invasive ventilation duration
Data on duration of mechanical ventilation were provided in three6,29,30 and no significant difference was found between the two groups (MD = −1.05, 95% CI [−3.47, 1.37], p = 0.39, I2 = 86%).
Antibiotic-free days during ICU stay
Data on antibiotic-free days were reported by three studies.6,27,29 The number of antibiotic-free days was significantly higher in the short-course antibiotic therapy group than in the long-course antibiotic therapy group (MD = 3.99, 95% CI [2.46, 5.52], p < 0.01; I2 statistic = 85%, p = 0.002). Similar results were observed in the subgroup of patients treated for VAP due to NF-GBN (MD = 4.96, 95% CI [4.34, 5.58], p < 0.01; I2 statistic = 0%, p = 0.68).
Number of extra-pulmonary infections
Data on the incidence of extra-pulmonary infections were reported in four studies.6,27,29,30 The incidence of extra-pulmonary infections did not differ significantly between the short-course therapy group and the long-course therapy group (OR = 1.32, 95% CI [0.77, 2.26], p = 0.32; I2 = 0%).
Acquisition of multidrug-resistant pathogens
Two trials assessed data on acquiring MDR pathogens following antibiotic therapy for VAP6,27 and found a similar proportion of MDR pathogens acquisition during ICU stay (OR = 0.7, 95% CI [0.43, 1.13], p = 0.14; I2 = 0%)
Sensitivity and subgroup analysis
An exploratory sensitivity analysis was conducted excluding one RCT with a high risk of bias.30 In addition, a sensitivity analysis was performed by removing each study from the meta-analysis to verify the robustness of the obtained conclusions. Pooled results showed that, regarding mechanical invasive ventilation duration, heterogeneity was not reduced (Supplemental Fig. S1).
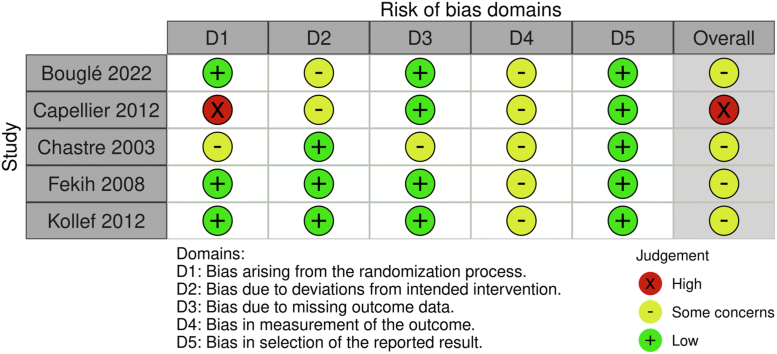
Quality assessment of the included studies
The CONSORT and RoB2 for the included clinical trials are reported in Table 1 and Fig. 5, respectively. The summary of evidence findings is reported in Table 2.
Table 1.
Demographic data of the retained studies.
| Author (year) | Country | SOFA | SAPS II | Age | VAP definition | Population (short versus long) | ATB regimen (type, duration) | VAP type | Microbiology | Follow-up | CONSORT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-Bouglé et al. (2022) | France | 7.45 ± 3.9 | 45.05 ± 17.15 | 59.4 ± 17.35 |
Clinical suspicion (≥two criteria including fever >38.5 °C, leukocytosis >109/L or leukopenia <4.108/L, purulent tracheobronchial secretions, and a new or persistent infiltrate on chest radiography) Confirmation by positive culture of a respiratory sample |
186 patients (88 versus 98) | β-Lactam with aminoglycosides or fluoroquinolone (8 days versus 15 days) | Late onset VAP | Pseudomonas aeruginosa | 90 days | 22/25 |
| 2-Capellier et al. (2012) | France | – | 39.45 ± 12.95 | 48.9 ± 19.55 | At least 2 or 3 of the following clinical criteria (temperature > 38.3 °C, leukocyte count > 10,000/mm3, excessive purulent or mucopurulent bronchial secretion) and the radiological criterion Confirmation by bronchoalveolar lavage culture |
225 patients (116 versus 109) | beta-lactams for 8 or 15 days combined with an aminoglycoside for the first 5 days | Early onset VAP | Gram + cocci (55.8%) Gram- Bacilli (40.6%) Others (3.6%) |
90 days | 21/25 |
| 3-Fekih et al. (2008) | Tunisia | – | 42.75 ± 3.9 | 52.5 ± 3.9 |
Clinical suspicion (≥two criteria including fever >38.5 °C, leukocytosis >109/L or leukopenia <4.108/L, purulent tracheobronchial secretions, and a new or persistent infiltrate on chest radiography) Confirmation by positive culture of a respiratory sample |
30 patients (14 versus 16) | beta-lactams for 8 or 10 days combined with an aminoglycoside for the first 5 days | Late onset VAP (83.4%) Early onset VAP (16.6%) |
Gram- Bacilli (89.7%) Others (10.3%) |
28 days | 17/25 |
| 4-Kollef et al. (2012) | USA | 5.8 ± 2.55 | – | 56.1 ± 17.53 | Confirmation by bronchoalveolar lavage culture | 227 patients (115 versus 112) | 7-day doripenem versus 10-day imipenem-cilastatin Adjunctive therapy was allowed at the discretion of the treating physician |
Late onset VAP | Gram- bacilli | 28 days | 19/25 |
| 5-Chastre et al. (2003) | France | 7.35 ± 4 | 45 ± 15 | 60.5 ± 17 |
Clinical suspicion (≥ two criteria including fever>38.5 °C, leukocytosis >109/L or leukopenia <4.108/L, purulent tracheobronchial secretions, and a new or persistent infiltrate on chest radiography) Confirmation by positive culture of a respiratory sample |
401 patients (197 versus 204) | Broad-spectrum beta-lactam (8 days versus 15 days) combined with at least an aminoglycoside or a fluoroquinolone | Late onset VAP | Gram- bacilli (56.6%) Gram- cocci (43.4%) |
90 days | 21/25 |
ATB: antibiotic; CONSORT: Consolidated Standards of Reporting Trials; SAPS II: Simplified Acute Physiology score; SOFA: Sequential Organ Failure Assessment score; VAP: ventilator-associated pneumonia.
Fig. 5.
Risk of bias.
Table 2.
Summary of evidence findings.
| Recurrence compared to placebo for [VAP] | |||||
|---|---|---|---|---|---|
| Setting: Intervention: Recurrence; Comparison: placebo | |||||
| Outcomes | No of participants (studies) Follow-up |
Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects |
|
| Risk with placebo | Risk difference with Recurrence | ||||
| Recurrence | 842 (4 RCTs) | ⨁⨁⨁◯ Moderate |
OR 1.48 (0.96–2.28) | 152 per 1000 | 58 more per 1000 (5 fewer to 138 more) |
| Recurrence - late-VAP | 617 (3 RCTs) | ⨁⨁⨁◯ Moderate |
OR 1.22 (0.80–1.87) | 157 per 1000 | 28 more per 1000 (27 fewer to 101 more) |
| Recurrence - early-VAP | 225 (1 RCT) | ⨁⨁◯◯ Low |
OR 2.19 (1.10–4.34) | 138 per 1000 | 121 more per 1000 (12 more to 272 more) |
| Recurrence-GNB | 313 (2 RCTs) | ⨁⨁◯◯ Low |
OR 1.90 (0.99–3.64) | 106 per 1000 | 78 more per 1000 (1 fewer to 195 more) |
| Relapses compared to placebo for [VAP] | |||||
|---|---|---|---|---|---|
| Setting: Intervention: Relapses; Comparison: placebo | |||||
| Outcomes | No of participants (studies) Follow-up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects |
|
| Risk with placebo | Risk difference with Relapses | ||||
| Relapses | 1039 (4 RCTs) | ⨁⨁⨁◯ Moderate |
OR 1.45 (0.94–2.22) | 78 per 1000 | 31 more per 1000 (4 fewer to 80 more) |
| Relapses - Late-VAP | 814 (3 RCTs) | ⨁⨁⨁◯ Moderate |
OR 1.37 (0.88–2.14) | 94 per 1000 | 31 more per 1000 (10 fewer to 88 more) |
| Relapses - early-VAP | 225 (1 RCT) | ⨁⨁◯◯ Low |
OR 2.92 (0.58–14.78) | 18 per 1000 | 33 more per 1000 (8 fewer to 198 more) |
| Relapses-GNB | 340 (3 RCTs) | ⨁⨁⨁◯ Moderate |
OR 1.76 (0.93–3.33) | 111 per 1000 | 69 more per 1000 (7 fewer to 183 more) |
| Mortality compared to placebo for [VAP] | |||||
|---|---|---|---|---|---|
| Setting: Intervention: Mortality; Comparison:placebo | |||||
| Outcomes | No of participants (studies) Follow-up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects |
|
| Risk with placebo | Risk difference with Mortality | ||||
| Mortality | 1069 (5 RCTs) | ⨁⨁⨁⨁ High |
OR 1.24 (0.92–1.67) | 195 per 1000 | 36 more per 1000 (13 fewer to 93 more) |
| Mortality - late-VAP | 844 (4 RCTs) | ⨁⨁⨁⨁ High |
OR 1.30 (0.93–1.81) | 200 per 1000 | 45 more per 1000 (11 fewer to 112 more) |
| Mortality - early-VAP | 225 (1 RCT) | ⨁⨁◯◯ Low |
OR 0.99 (0.49–1.97) | 174 per 1000 | 1 fewer per 1000 (81 fewer to 119 more) |
| Mortality-GNB | 340 (3 RCTs) | ⨁⨁⨁◯ Moderate |
OR 1.32 (0.53–3.25) | 257 per 1000 | 57 more per 1000 (102 fewer to 272 more) |
∗The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: confidence interval; OR: odds ratio.
GRADE Working Group grades of evidence.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Discussion
This systematic review and meta-analysis did not find a significant difference between short-course (≤8 days) and long-course (≥10–15 days) antibiotic therapy on the recurrence and relapses of VAP, even for the VAP due to NF-GNB and in the late-onset VAP subgroup. Furthermore, secondary analyses did not reveal any difference on mortality, ICU stay duration, mechanical ventilation duration, number of extra-pulmonary infections and MDR emergence according to the duration of antibiotic therapy. However, short-course antibiotic therapy significantly increased the number of antibiotic-free days.
The reduced antibiotic exposure was not associated with a rise in recurrence or relapses of VAP, and this was confirmed even for VAP due to NF-GNB. Chastre et al.,27 one of the principal studies assessing VAP due to NF-GNB (37% of the included patients in our study), defined recurrent pneumonia based on only microbiological criteria. This may have led to an over-estimation of this outcome, especially for Pseudomonas aeruginosa, which was confirmed in subsequent studies.31,32 In fact, a positive microbiological sample may reflect persisting colonization rather than infection.2,3 Moreover, in the short-course group, patients were followed for more time off antibiotics which may led to an over-estimation of recurrence rates.33 In a recent RCT including only patients with VAP due to NF-GNB, the increased rate of relapse did not transfer into clinical outcomes, including the ICU length of stay and mortality rates.6 Although, due to the low enrollement, the trial was underpowered and also failed to show the gain associated with the usee of short-course therapy for VAP caused by NF-GBN.34 In a retrospective study on VAP due to NF-GNB, Hedrick et al.35 did not find an association between recurrence of VAP and duration of antibiotic therapy. However, their results should be interpreted with caution due to several limitations, namely the retrospective nature of the study, the majority of patients being surgical patients and a large difference between patients in terms of antibiotic type and duration.
The majority of the published trials mentioned above argue against the use of short-course antibiotic therapy for VAP due to NF-GBN. However, due to the high risk of relapses, biomarkers such as procalcitonin (PCT) could be used to monitor the efficacy of treatment and hereby the duration needed duration in addition to predicting recurrences and relapses.36,37 Indeed, Wongsurakiat et al.38 proposed a protocol based on clinical pulmonary infection score (CPIS) and spot serum PCT in order to guide discontinuation of antibiotic therapy in case of VAP due to NF-GBN. Furthermore, in the PRORATA trial that evaluated a strategy of antibiotic sparing guided by PCT dosage in ICU patients, Bouadma et al.24 observed in the subgroup of patients with VAP an increase of more than 3 days free of antibiotics at day 28 in patients in the PCT guided strategy (3.1 [0.7–5.6]). But, one of the limits of using PCT is the falsy low or high PCT levels which may lead to innapropriate discontinuation or continuation of antibiotic therapy.3 However, tailored strategies (including clinical and biological endpoints) should be tested in RCTs in order to individualize antibiotic duration treatement.
As mentioned, this review did not demonstrate an increase mortality nor an association with prolonged ICU or hospital stay or mechanical ventilation duration. These findings are similar to previous meta-analyses.5,25 However, these previous studies pooled data from patients with both early- and late-onset pneumonia, making their results difficult to interpret. Indeed, it is well known that late-onset VAP are caused by more resistant pathogens and associated with worse outcomes.39,40 Therefore, we conducted subgroup analyses to assess the effects of late-onset VAP and no differences were reported between both regimens.
Although this meta-analysis shows promising results, it should be interpreted carefully, given several limitations. First, only five trials were included with a small sample size (1069 patients). Secondary, only three trials assessed data on VAP due to NF-GNB (330 patients), Bouglé et al.6 trial being the major “driver” of the results. Nevertheless, this study indeed lacked power as it included only 33% of the patients initially planned. In addition, one of the major criticisms of Bouglé et al. trial is the fact that, in order to assess the recurrence, the patients in short-course group were followed for more time off antibiotics, which may explain the higher reported rates of recurrence. Besides, one limitation was the lack of standardized definitions of the assessed outcomes. The timing of mortality assessment differed in the included studies (90 days in one RCT, 28 days in three RCTs and 21 days in the remaining trial). In addition, the protocol of the short and long course antibiotic therapy changed as one trial used a short course of 7 days and a long course of 10 days. There were also differences in antibiotics used between groups, especially in the trial conducted by Kollef et al. as doripenem and imipenem were used in the short and long arms, respectively. Moreover, the heterogeneity, which was higher in three outcomes (mortality, antibiotic-free days and mechanical ventilation duration), was probably explained by the limitations cited previously. Finally, the effect estimates for the primary outcome were quite large and confidence intervals barely surpassed one thus begging the question if larger studies or adding more studies would push this signal to significance.
As a conclusion, short-course antibiotic therapy for VAP did not significantly affect the rate of recurrence relapses, and mortality compared with long-course antibiotic therapy. However, for VAP due to NF-GNB, even if a higher risk of recurrence is reported, it did not translated into clinical outcomes such as mortality and duration of ICU stay. In addition, short-course therapy had several desirable consequences namely decreased antibiotic exposure, reduced antibiotic resistance, and lower overall costs.
Contributors
M.A.D.: conceptualization, writing of the original draft and final approval of the version. E.D.: writing review and editing. M.A.C.: writing review and editing. J.B.: writing review and editing. A.B.: writing review and editing. M.L.: writing review and editing. F.D.: project administration, writing of the original draft and final approval of the version. B.D.: project administration, writing of the original draft and final approval of the version.
Declaration of interests
The authors declare that they have no conflict of interest.
Acknowledgements
We thank all the contributors for their expertise and assistance throughout all aspects of our study and for their help in writing the manuscript.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101880.
Appendix A. Supplementary data
References
- 1.Torres A., Niederman M.S., Chastre J., et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT) Eur Respir J. 2017;50(3) doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 2.Dimopoulos G., Matthaiou D.K. Duration of therapy of ventilator-associated pneumonia. Curr Opin Infect Dis. 2016;29(2):218–222. doi: 10.1097/QCO.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 3.Kalil A.C., Metersky M.L., Klompas M., et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leone M., Bouadma L., Bouhemad B., et al. Hospital-acquired pneumonia in ICU. Anaesth Crit Care Pain Med. 2018;37(1):83–98. doi: 10.1016/j.accpm.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Pugh R., Grant C., Cooke R.P., Dempsey G. Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst Rev. 2015;2015(8) doi: 10.1002/14651858.CD007577.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouglé A., Tuffet S., Federici L., et al. Comparison of 8 versus 15 days of antibiotic therapy for Pseudomonas aeruginosa ventilator-associated pneumonia in adults: a randomized, controlled, open-label trial. Intensive Care Med. 2022;48(7):841–849. doi: 10.1007/s00134-022-06690-5. [DOI] [PubMed] [Google Scholar]
- 7.Page M.J., Moher D., Bossuyt P.M., et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shea B.J., Reeves B.C., Wells G., et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358 doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antes G. The new CONSORT statement. BMJ. 2010;340 doi: 10.1136/bmj.c1432. [DOI] [PubMed] [Google Scholar]
- 10.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 11.Balshem H., Helfand M., Schünemann H.J., et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Cochrane handbook for systematic reviews of interventions [internet] https://handbook-5-1.cochrane.org/ Available from:
- 13.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stolz D., Smyrnios N., Eggimann P., et al. Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia: a randomised study. Eur Respir J. 2009;34(6):1364–1375. doi: 10.1183/09031936.00053209. [DOI] [PubMed] [Google Scholar]
- 16.Ammar M.A., Hilal A., Abdalla W. The role of lung ultrasound in procalcitonin-guided antibiotic discontinuation in ventilator-associated pneumonia. Indian J Anaesth. 2022;66(6):431–435. doi: 10.4103/ija.ija_989_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nobre V., Harbarth S., Graf J.D., Rohner P., Pugin J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177(5):498–505. doi: 10.1164/rccm.200708-1238OC. [DOI] [PubMed] [Google Scholar]
- 18.Micek S.T., Ward S., Fraser V.J., Kollef M.H. A randomized controlled trial of an antibiotic discontinuation policy for clinically suspected ventilator-associated pneumonia. Chest. 2004;125(5):1791–1799. doi: 10.1378/chest.125.5.1791. [DOI] [PubMed] [Google Scholar]
- 19.Labelle A.J., Schoenberg N., Skrupky L., Kollef M. D63 advances in treatment of respiratory infections [Internet] American Thoracic Society; 2012. Five versus seven day antibiotic course for the treatment of pneumonia in the intensive care unit; p. A6078.https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2012.185.1_MeetingAbstracts.A6078 (American Thoracic Society International Conference Abstracts). Available from: [Google Scholar]
- 20.Hochreiter M., Köhler T., Schweiger A.M., et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care. 2009;13(3):R83. doi: 10.1186/cc7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hellyer T.P., McAuley D.F., Walsh T.S., et al. Biomarker-guided antibiotic stewardship in suspected ventilator-associated pneumonia (VAPrapid2): a randomised controlled trial and process evaluation. Lancet Respir Med. 2020;8(2):182–191. doi: 10.1016/S2213-2600(19)30367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Z Mazlan M., A H Ismail M., Ali S., Salmuna Z.N., Wan Muhd Shukeri W.F., Omar M. Efficacy and safety of the point-of-care procalcitonin test for determining the antibiotic treatment duration in patients with ventilator-associated pneumonia in the intensive care unit: a randomised controlled trial. Anaesthesiol Intensive Ther. 2021;53(3):207–214. doi: 10.5114/ait.2021.104300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mo Y., West T.E., MacLaren G., et al. Reducing antibiotic treatment duration for ventilator-associated pneumonia (REGARD-VAP): a trial protocol for a randomised clinical trial. BMJ Open. 2021;11(5) doi: 10.1136/bmjopen-2021-050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouadma L., Luyt C.E., Tubach F., et al. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375(9713):463–474. doi: 10.1016/S0140-6736(09)61879-1. [DOI] [PubMed] [Google Scholar]
- 25.Dimopoulos G., Poulakou G., Pneumatikos I.A., Armaganidis A., Kollef M.H., Matthaiou D.K. Short- vs long-duration antibiotic regimens for ventilator-associated pneumonia. Chest. 2013;144(6):1759–1767. doi: 10.1378/chest.13-0076. [DOI] [PubMed] [Google Scholar]
- 26.Bouglé A., Foucrier A., Dupont H., et al. Impact of the duration of antibiotics on clinical events in patients with Pseudomonas aeruginosa ventilator-associated pneumonia: study protocol for a randomized controlled study. Trials. 2017;18(1):37. doi: 10.1186/s13063-017-1780-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chastre J., Wolff M., Fagon J.Y., et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588–2598. doi: 10.1001/jama.290.19.2588. [DOI] [PubMed] [Google Scholar]
- 28.Kollef M.H., Chastre J., Clavel M., et al. A randomized trial of 7-day doripenem versus 10-day imipenem-cilastatin for ventilator-associated pneumonia. Crit Care. 2012;16(6):R218. doi: 10.1186/cc11862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fekih Hassen M., Ayed S., Ben Sik Ali H., Gharbi R., Marghli S., Elatrous S. Durée de l’antibiothérapie lors du traitement des pneumopathies acquises sous ventilation mécanique: comparaison entre sept jours et dix jours. Étude pilote. Ann Fr Anesth Réanim. 2009;28(1):16–23. doi: 10.1016/j.annfar.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 30.Capellier G., Mockly H., Charpentier C., et al. Early-onset ventilator-associated pneumonia in adults randomized clinical trial: comparison of 8 versus 15 days of antibiotic treatment. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0041290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Combes A., Luyt C.E., Fagon J.Y., Wolff M., Trouillet J.L., Chastre J. Early predictors for infection recurrence and death in patients with ventilator-associated pneumonia. Crit Care Med. 2007;35(1):146–154. doi: 10.1097/01.CCM.0000249826.81273.E4. [DOI] [PubMed] [Google Scholar]
- 32.Morris A.C. Management of pneumonia in intensive care. J Emerg Crit Care Med. 2018;2:1–17. [Google Scholar]
- 33.Metersky M.L., Klompas M., Kalil A. Less is more: a 7-day course of antibiotics is the evidence-based treatment for Pseudomonas aeruginosa ventilator-associated pneumonia. Clin Infect Dis. 2022;75 doi: 10.1093/cid/ciac809. [DOI] [PubMed] [Google Scholar]
- 34.Siegrist E.A., Sassine J. Shorter might not always be better: the case for longer antibiotic therapy for Pseudomonas aeruginosa pneumonia. Intensive Care Med. 2022;48(7):963–964. doi: 10.1007/s00134-022-06754-6. [DOI] [PubMed] [Google Scholar]
- 35.Hedrick T.L., McElearney S.T., Smith R.L., Evans H.L., Pruett T.L., Sawyer R.G. Duration of antibiotic therapy for ventilator-associated pneumonia caused by non-fermentative gram-negative bacilli. Surg Infect. 2007;8(6):589–597. doi: 10.1089/sur.2006.021. [DOI] [PubMed] [Google Scholar]
- 36.Smedemark S.A., Aabenhus R., Llor C., Fournaise A., Olsen O., Jørgensen K.J. Biomarkers as point-of-care tests to guide prescription of antibiotics in people with acute respiratory infections in primary care. Cochrane Database Syst Rev. 2022;10:CD010130. doi: 10.1002/14651858.CD010130.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bréchot N., Hékimian G., Chastre J., Luyt C.E. Procalcitonin to guide antibiotic therapy in the ICU. Int J Antimicrob Agents. 2015;46:S19–S24. doi: 10.1016/j.ijantimicag.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Wongsurakiat P., Tulatamakit S. Clinical pulmonary infection score and a spot serum procalcitonin level to guide discontinuation of antibiotics in ventilator-associated pneumonia: a study in a single institution with high prevalence of nonfermentative gram-negative bacilli infection. Ther Adv Respir Dis. 2018;12 doi: 10.1177/1753466618760134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vallés J., Pobo A., García-Esquirol O., Mariscal D., Real J., Fernández R. Excess ICU mortality attributable to ventilator-associated pneumonia: the role of early vs late onset. Intensive Care Med. 2007;33(8):1363–1368. doi: 10.1007/s00134-007-0721-0. [DOI] [PubMed] [Google Scholar]
- 40.Ben Lakhal H., M'Rad A., Naas T., Brahmi N. Antimicrobial susceptibility among pathogens Isolated in early- versus late-onset ventilator-associated pneumonia. Infect Dis Rep. 2021;13(2):401–410. doi: 10.3390/idr13020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.