Abstract
Memory T (Tm) cells protect against Ags that they have previously contacted with a fast and robust response. Therefore, developing long-lived Tm cells is a prime goal for many vaccines and therapies to treat human diseases. The remarkable characteristics of Tm cells have led scientists and clinicians to devise methods to make Tm cells more useful. Recently, Tm cells have been highlighted for their role in coronavirus disease 2019 vaccines during the ongoing global pandemic. The importance of Tm cells in cancer has been emerging. However, the precise characteristics and functions of Tm cells in these diseases are not completely understood. In this review, we summarize the known characteristics of Tm cells and their implications in the development of vaccines and immunotherapies for human diseases. In addition, we propose to exploit the beneficial characteristics of Tm cells to develop strategies for effective vaccines and overcome the obstacles of immunotherapy.
Keywords: CD8-positive T lymphocytes, Immunological memory, Immunotherapy, Vaccines, T cell exhaustion, Cell based therapy, Cell engineering
INTRODUCTION
One remarkable feature of immunity is that immune cells can remember the Ags they experienced before (1,2,3). Immunological memory can be manifested in various forms, including long-lived plasma cells, Tm cells, and memory B cells. Among these cells, Tm cells, which develop from effector T cells after Ag clearance, are well-documented for their robust response to previously experienced (2). Tm cells are also long-lived without further antigenic stimulation and maintain homeostasis via steady self-renewal in a cytokine-dependent manner (4,5). Owing to these characteristics, Tm cells have been considered effective in fighting secondary infections or tumors (6,7).
In recent years, Tm cells have attracted considerable attention in vaccine development and immunotherapy to treat tumors. Since numerous infectious diseases require new or improved vaccines, the paradigm has shifted to employing the robust T cell response to fight these diseases effectively (8,9). In addition, the burgeoning field of cancer immunotherapy has benefited from the technology that can generate tumor-infiltrating lymphocytes (TILs) (10,11,12).
This review provides an overview of the basic features of Tm cells and the emerging research trends in Tm cells related to vaccines and immunotherapy. First, we aimed to revisit the roles of Tm cells and summarize the reports on their fundamental properties. Second, based on our knowledge of Tm cells, we propose strategies to utilize Tm cells or modified Tm cells to mount effective defense mechanisms mediated by vaccines or immunotherapy.
CARDINAL FEATURES OF Tm CELLS
Differentiation of memory CD8+ T cells
When foreign agents enter the body, Ag-presenting cells (APCs) ingest the Ags and present the peptides of these Ags to CD8+ T cells through MHC class I molecules (13). Triggered by TCR signaling, naïve T (Tn) cells start to expand clonally and differentiate into effector CD8+ T (Teff) cells, also called CTLs, to perform critical effector functions such as inducing target cell death using cytotoxic molecules (14). Teff cells can also be distinguished from Tn cells based on their phenotypes, such as the differential expression of surface markers, including CD44, IL-7 receptor alpha-chain (IL-7Rα, CD127), L-selectin (CD62L), CC-chemokine receptor 7 (CCR7), KLRG1, CD27, and other markers. These molecules modulate Teff cell’s localization, effector functions, and potential to become Tm cells. A majority of the activated Teff cells are short-lived following Ag clearance and die via apoptosis. However, a small Teff cell population survives to differentiate into Tm cells that persist in the host. This phenomenon is illustrated by the cell fate decision model during acute viral infections (15). According to this model, Teff cells can be distinguished by two major surface markers: KLRG1 and CD127 (IL-7Rα). Short-lived effector T cells (SLECs) display a KLRG1hiCD127lo phenotype and perform cytotoxic effector functions to eliminate invading Ags. On the other hand, memory precursor effector T cells (MPECs) are KLRG1loCD127hi and develop into long-lived Tm cells.
Altogether, the fates of Teff cells have already been determined during T cell activation by Ags, and MPECs distinguished by KLRG1 and CD127 markers are precursors of Tm cells. Thus, effectively producing a larger number of MPECs has been a popular strategy that can utilize engineered Tm cells for immunotherapy with enhanced efficacy.
Subsets of memory CD8+ T cells
When Tm cells develop from MPECs, they differentiate into three major subsets, which can be distinguished based on their phenotypes and distinct roles. First, circulating Tm cells are either central memory T (Tcm; CD62LhiCCR7hi) cells that can circulate through secondary lymphoid organs (16) or effector memory T (Tem; CD62LloCCR7lo) cells that circulate among peripheral tissues (16,17). As these subsets differ in their migratory capacities, they survey different organs to detect secondary infections. In addition, these 2 subsets of Tm cells express distinct transcription factors that guide the development of Tem and Tcm cells. Transcription factors such as TCF1, BCL6, EOMES, and ID3 help Tcm cells retain their stemness and comparatively low cytotoxic activity. In contrast, transcription factors in Tem cells, including T-BET, BLIMP-1, and ID2, lead to the rapid acquisition of strong effector functions in target sites (18,19). These surface molecules and transcription factors imply that these two circulating T cells specialize in different roles despite having migratory features (3).
In contrast to circulating Tm cells, the third subset, tissue-resident memory T (Trm) cells, is located in non-lymphoid tissues and does not enter the blood. Trm cells are the first line of defense against reinfections owing to their rapid cytotoxicity at the target sites (20,21). The transcription factors HOBIT, EOMES, and BLIMP-1, are shown to regulate the differentiation of Trm cells and were identified based on the expression of CD103 and CD69 on the surfaces of these cells, which promote tissue retention (22).
In addition to traditional Tm subsets, two Tm cell subsets expressing CD45RA were found in humans. Although CD45RO which is the shorter isoform of CD45 on Ag encountered Tm cells and CD45RA, the long isoform of CD45, is expressed on Tn cells in humans, newly discovered Tm cells express CD45RA after TCR-dependent activation. One of these subsets consists of stem cell-like memory T (CD45RA+CD62LhiCCR7hi) cells which have more naïve-like phenotype such as enhanced homeostatic self-renewal, proliferative capacity, and multipotent differentiation potentials compared to Tcm and Tem cells (23,24). The other CD45RA re-expressing Tm cells discovered in circulation of humans are called as CD45RA expressed terminally differentiated effector memory T cells and they are CD45RA+CD62LloCCR7lo (3,17,25). These subsets are considered terminally differentiated with low proliferative capacities, but they are highly cytotoxic by enhanced production of cytotoxic molecules such as granzyme and perforin (25,26). These cells may be differentiated from Teff and Tem cells by undergoing repetitive proliferation (27) but detailed mechanisms of this differentiation pathways are yet to be determined.
Other subsets including CX3CR1int peripheral memory T cells (28), CD27loCD43lo Tm cells (29), and IFN-γhi Tem cells (T death intermediate memory) (30) have also been found and their roles were suggested, but these populations have been relatively less examined.
Altogether, Tm cells are classified into different subsets depending on their migratory capacities, cytotoxicity, and lifespan; thus, regulating the Tm cell subset population is important for developing vaccines and T cell-based immunotherapy.
Homeostasis of memory CD8+ T cells
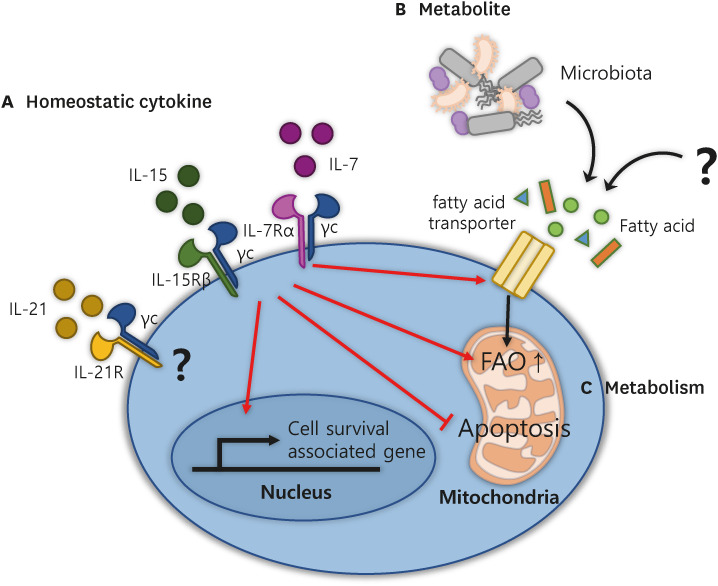
Tm cells can sustain their state, called a quiescent stage, so homeostatic signals are required to maintain the signature of Tm cells for longer periods. The best-known drivers for this phenomenon are homeostatic cytokines, such as IL-7 and IL-15 (Fig. 1A) (31,32,33,34). IL-7 is produced locally within the T cell zone of secondary lymphoid organs (16) by fibroblastic reticular cells wrapped around the conduits. These cells also secrete CCL19 and CCL21 (ligands for CCR7), thereby attracting CCR7+ Tm cells and possibly providing survival signals to these recruited cells (35). T cell responses to IL-7 occur through the modulation of the IL-7Rα (CD127) chain receptor, which dimerizes with the γc (common gamma, CD132) chain and initiates the activation of JAK1 and JAK3, which are associated with the IL-7Rα and γc chains, respectively (36). Thus, IL-7 binding recruits and activates STAT5A and STAT5B to induce heterodimerization, resulting in the translocation of STAT5 into the nucleus to transcribe cell survival-associated genes.
Figure 1. The components for enhancing CD8+ Tm cell homeostasis and functions. Simplified representation of the three requirements of CD8+ Tm cells for development and homeostasis: (A) homeostatic cytokines such as IL-7, IL-15, and IL-21, (B) metabolites, and (C) metabolic pathways. (A) Cytokine stimulation maintains homeostatic proliferation by increasing the expression of survival-associated genes and inhibiting mitochondrial-mediated apoptosis. In addition, these cytokines enhance the surface expression of fatty acid transporters to enhance the uptake of FAs into cells and convert metabolism from glycolysis to FAO, a hallmark of Tm cells. (B) Metabolites: FAs produced by microbiota or other fuel for FAO in mitochondria for Tm cells. (C) The metabolism of Tm cells relies mainly on FAO, which is induced by (A) homeostatic cytokines and (B) metabolites.
IL-15 is expressed by diverse cell types, including non-lymphoid cells, such as stromal cells, myeloid cells, and dendritic cells (37). This cytokine boosts the basal Tm cell homeostatic proliferation rate depending on signals from cells that come in contact with IL-15 expressed on APCs, such as dendritic cells (DCs) (38). Tm cells express high levels of IL-15 receptors, composed of IL-15Rβ (CD122) and γc chains that activate JAK1 and JAK3, respectively.
Upon stimulation with IL-7 and IL-15, Tm cells promote cell survival by preventing the mitochondrial pathway of apoptosis (Fig. 1A). In this respect, the pro-survival proteins BCL-2 and MCL-1 play a dominant role. BCL-2 and MCL-1 function by directly blocking the activity of the key apoptotic regulators Bax and Bak, which are derived from activating Bim and Bid, respectively (39). Thus, Tm cells terminally inhibit apoptosis by releasing cytochrome C and other molecules from the mitochondria to initiate caspase activation (40).
IL-21 is another member of the γc chain cytokine family that has been shown to have favorable effects on Tm differentiation and maintenance (41). IL-21 cooperates with IL-10 to promote the maturation of Tm cells, especially Trm cells, which is mediated by the transcription factor STAT3. On the other hand, IL-21 also stimulates the activation and clonal expansion of Ag-specific CD8+ Tm cells (42). For these reasons, the function of IL-21 in Tm cell differentiation remains controversial as to whether it induces the quiescent state of Tm cells or whether it influences the active form of Tm cells.
Taken together, the homeostatic cytokines IL-7 and IL-15 maintain the survival of Tm cells through the JAK and STAT signaling pathways and by preventing the mitochondrial pathway of apoptosis. These homeostatic cytokines can boost treatment using Tm cells, which induce active and passive immune responses.
Memory CD8+ T cell metabolism
The metabolic pathway in T cells is regulated by the stages of T cell activation and differentiation, such as quiescent Tn and Tm cells, activated Teff cells, and exhausted T (Tex) cells (43). Both Tn and Tm cells are in a quiescent stage during the steady state, but only Tm cells rely on catabolic metabolism for low-energy consumption, which mainly uses long-chain or short-chain fatty acids (LCFAs or SCFAs, respectively) for long-term survival until they respond upon re-exposure to foreign Ags in the body (Fig. 1B) (44). Since these findings have attracted attention, several reports have indicated that changes in cellular lipid metabolism have critical effects on Tm cell proliferation and fate decisions (45). For instance, glucose, glutamine, LCFAs, and SCFAs can be acquired by Tm cells to fuel oxidative phosphorylation (OXPHOS). For these reasons, the intrinsic pathways of glycolysis or fatty acid oxidation (FAO) have been considered potential targets for the modulation of Tm cell development. However, heterogeneous Tm populations differ in their preferential usage of different metabolites and, subsequently, T cell metabolic pathways; therefore, carefully design to target one of these pathways are needed.
Tcm and Trm cells use different substrates, although all Tm cells rely primarily on FAO for their energy demands. For example, Tcm cells engage in a futile cycle with the uptake of glucose and glutamine to generate fatty acids (FAs) for FAO fuel under ex vivo conditions. However, this subset of Tm cells uses lower amounts of FAs compared to Teff or Trm cells and even persists in a lipid-depleted medium (46). Tem cells are metabolically active, employing diverse substrates, including glucose and FAs, to fuel glycolysis and OXPHOS, respectively. However, they rely less on OXPHOS than Tcm or Trm cells do. During their development into Trm cells, these Trm cell precursors upregulate the expression of lipid chaperones, including fatty acid-binding proteins 4/5 and a lipid-scavenger cell-surface receptor, CD36 (47). These lipid chaperones facilitate the acquisition of more FAs directly from the microenvironment, providing sufficient exogenous FAs to fuel mitochondrial respiration compared to Tcm cells.
Cytokines also play a central role in Tm metabolism and homeostasis. IL-7 promotes the expression of the glycerol channel aquaporin 9, which mediates glycerol import into Tm cells (Fig. 1B) (48). Imported glycerol fuels the synthesis and storage of FAs and triglycerides within Tm cells for survival. Another homeostatic cytokine, IL-15, also contributes to Tm cell metabolism. By employing in vitro differentiation systems in which IL-2 or IL-15 induces CD44hiCD62Llo Teff cells or CD44hiCD62Lhi Tcm cells, respectively, CD44hiCD62Lhi Tcm cells not only have impaired glucose uptake to generate FAs, but they also contain elongated mitochondria (49). Because mitochondrial elongation accelerates the metabolic shift from glycolysis to OXPHOS (50), IL-15 provides a favorable metabolic switch for Tm cells. In contrast, IL-2-induced Teff cells displayed fragmented mitochondria. Taken together, these studies signify that metabolites such as FAs are available for Tm cells and that FAO-related pathways in Tm cells play vital roles in the homeostasis and functional activity of these cells.
Correlation between the microbiota and memory CD8+ T cells
The microbiota is composed of various living organisms, such as bacteria and viruses, which influence Tm cell homeostasis and survival mediated by microbial metabolic products such as SCFAs (51). SCFAs induce the switching of Tm cell metabolism toward OXPHOS and FAO after binding to the SCFA receptors GPR41 and GPR43 and induce the transition from activated T cells into long-lived Tm cells (52). This finding was confirmed by showing that Teff cells in germ-free mice failed to transition into long-lived Tm cells, suggesting that SCFA-producing microbiota instructed transition into Tm cells and provided fundamental metabolites for Tm cell homeostasis; however, it was difficult to identify specific strains of microbiota to produce these SCFAs (52). Another study highlighted the role of the microbiota in the development of Tm cells based on their finding that SCFAs also promote IL-10 production mediated by CD4+ regulatory T cells, which contribute differentiation into CD8+ Tm cells during acute viral infection (53). Taken together, these microbiota-derived components can influence Tm cell differentiation and survival.
MEMORY CD8+ T CELLS AS KEY DRIVERS FOR IMMUNOTHERAPY AND VACCINE
Over the past few decades, T cells have been studied as a useful tool for treating various disorders, especially infectious diseases, and cancers. In this section, we discuss recent research on T cell-mediated therapies and describe the potential of Tm or memory-like T cells as therapeutics for these diseases.
Memory CD8+ T cells correlate with vaccine efficacy
One important rationale for using Tm cells as a therapeutic is their fundamental feature that they react faster and more strongly than Tn cells do. Recently, people who have a weakened immune system, especially those aged 65 years or older, have been found to be more likely to experience severe symptoms of coronavirus disease 2019 (COVID-19) for a longer period compared to younger people (54). This phenomenon is explained by the reduced immune response in the elderly, known as immunosenescence. Due to immunosenescence, elderly people experience difficulties in producing effector and memory cells after vaccination; thus, they are vulnerable to disorders caused by infections and tumors. One explanation for the development of immunosenescence can be traced to the study of hematopoietic stem cells (HSCs), which can differentiate into progenitor cells of myeloid and lymphoid lineages in primary lymphoid organs (55). As all lymphocytes are derived from HSCs, they can lose their ability to differentiate into lymphoid lineages and generate functional lymphocytes such as Teff and Tm cells as they age. Another suggested explanation is the abnormality in functional lymphocyte differentiation. Increased inflammatory cytokine microenvironments in the elderly induces the expansion of CD28loCD8+ T cells, a hallmark of senescent T cells (56). Therefore, the Ag-induced proliferation of senescent T cells is profoundly impaired; however, their proliferative response to homeostatic cytokines is normal (57). Furthermore, CD8+ T cell clonal expansion was observed after influenza vaccination in older adults who were limited to vaccine-specific Ab production (58,59). For these reasons, the vaccines that trigger active adaptive immune responses can elicit different effects depending on the individual’s immune system. Therefore, we suggest the effective generation of vaccine-induced memory cells, especially Tm cells is important to minimize the variations of immune response to infections.
Memory CD8+ T cells correlate with cancer immunotherapies
The numbers of infiltrating Tm cells into tumor cells, especially Trm cells, in various clinical tumor samples, such as melanoma, non-small cell lung cancer, breast cancer, cervical cancer, and ovarian cancer, were positively correlated with improved outcomes (60). In addition, Trm cells express high levels of T cell intracellular Ag 1 and perforin, indicating increased cytotoxic activity compared to non-resident CD103lo T cells from the same patient. It was also shown that the numbers of circulating T cells, such as Tcm and Teff cells, were positively correlated with immune responses and positive therapeutic progress to immune checkpoint inhibitors (ICIs) in some patients with cancer (61,62). Although circulating T cells still need to infiltrate tumor sites to fight against tumors, the presence of Tm cells in the blood determines whether patients will have favorable outcomes or responses to ICIs.
Memory CD8+ T cell dysfunction
Although T cells possess great potential to fight against various diseases, including infectious diseases and cancers, CD8+ T cells occasionally have difficulties in curing these diseases. Although many diverse reasons have been presented to explain this, we want to focus on exhausted T cells. Exhaustion is a dysfunctional T cell state caused by persistent Ag stimulation, including chronic viral infection and tumor cells (63,64).
Only a few studies have investigated the exhaustion of Tm cells. West et al. (65) revealed that CD8+ Tm cells rapidly disappeared during high viral loads or persistent Ag stimulation. As Tm cells become exhausted, they have a decreased ability to proliferate and produce effector cytokines (65). One major hypothesis for Tm cell exhaustion is that Tm cells are intensively regulated to prevent excessive immune responses, even though the same strength or duration of Ag signals could induce the normal activation of Tn cells. Because Tm cells have more and larger TCR oligomers at their surface, Tm cells are ready to respond to Ag re-exposure; increased TCR expression levels are directly responsible for the enhanced sensitivity of Tm cells compared to Tn cells (66). Similarly, another study showed that TCR strength altered the functional activity of tumor specific CD8+ T cells. As TCR signal strength increased, the anti-tumor effector functions of Tm cells were reduced, and the transcriptional programming of exhaustion increased (67). Thus, fine-tuning the optimal TCR signal strength for Tm cells is proposed to be vital for blocking T cell exhaustion.
On the other hand, it was also suggested that Tm cells develop from a different lineage (64) so it remains controversial whether Tm cells are exhausted; hence, it is critical to understand how Tm cells react to chronic Ag stimulation. In particular, tumor microenvironments containing immunosuppressive receptors and cytokines such as IL-10 and TGF-β secreted by myeloid-derived suppressor cells, Tregs, and other cells alter recall responses (68,69).
APPLICATION OF MEMORY CD8+ T CELLS AS IMMUNOTHERAPY AND VACCINE
In the wake of the COVID-19 pandemic, numerous studies have been conducted on immune responses against this virus in humans. Of particular interest is vaccine development using Tm cells.
Vaccine development goals
Until recently, the focus of vaccines was to elicit B cells to produce large amounts of neutralizing Abs; however, efforts to overcome the limitations of Abs and treat more diseases accompanied by clinical demands in the era of new infectious diseases with pandemic potential focused on studying Tm cells and novel Tm cell-based strategies.
In recent years, mRNA vaccines, a novel vaccine platform, have been established against COVID-19 (70). Here, mRNAs encoding viral proteins are delivered within cells via endocytosis and translated into target proteins by ribosomes in host cells. Because of this process, mRNA vaccines have more advantages in activating T cells than other vaccines do. First, mRNA can be recognized as a pathogen-associated molecular pattern, serving as an adjuvant that elicits costimulatory signals (71). In addition, translated proteins derived from mRNA vaccines are degraded by the 26S proteasome within the cytoplasm and presented on MHC class I molecules to activate CD8+ T cells. For this reason, the roles of T cells against COVID-19 infections have emerged, particularly against viruses that mutate their genes to escape neutralizing Abs (72,73). Many ongoing studies have focused on evaluating vaccine-induced T cell responses to variants such as Omicron (B.1.1.529), which carries over 30 mutations in its spike proteins (74,75). Researchers have revealed that the Omicron variant cannot completely escape from both CD8+ Teff and Tm cell responses because the dominant virus epitopes recognized by CD8+ T cells are conserved in the original wild-type virus and that T cells are cross-reactive to variants (74). In addition, the effective recognition of the Omicron variant by polyfunctional T cells can produce multiple cytokines, such as IFN-γ, IL-2, and TNF-α, in vaccinated individuals after 6 to 7 months. Furthermore, vaccine-induced T cells recognize little difference between Omicron and the original strain of COVID-19 (75). Hence, mRNA vaccines are likely to produce effective long-lived Tm cells for a long time, regardless of viral mutations.
In addition to mRNA vaccines, traditional vaccines have also been shown to have enhanced efficacy by administering homeostatic cytokines for Tm cells. In a mouse model wherein administered with recombinant human (rh) IL-7 and rhIL-15, Melchionda et al. (76) reported that the number of Ag-specific Teff cells was not only significantly increased, but the survival of resting T cells was prolonged until day 120. The impact of increasing vaccine efficacy via cytokines has also been confirmed on other vaccine platforms, similar to those applied to humans (77).
Adaptive cell therapy (ACT) with memory CD8+ T cells
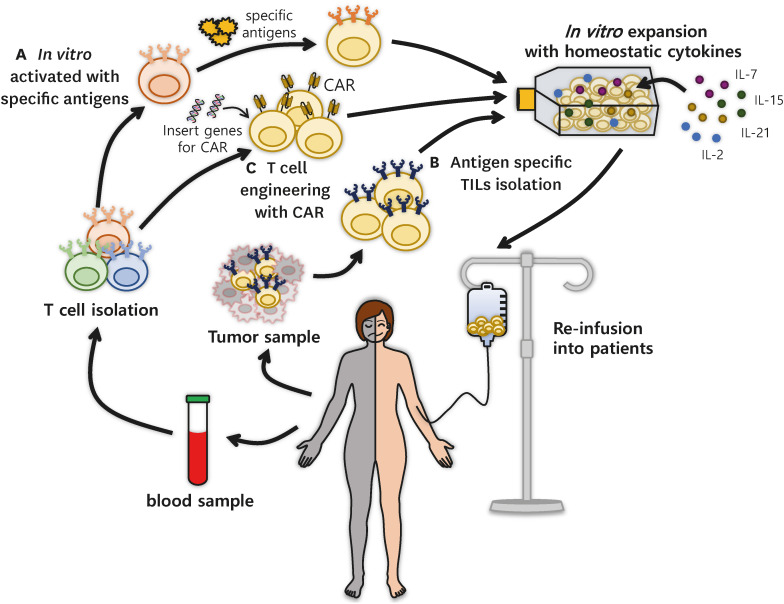
To employ effective T cells in immunotherapy, direct infusion of Tm cells that recognize cancer neoantigens through ACT is a promising strategy (Fig. 2) (78). Traditional ACTs utilize circulating or tissue-resident immune cells harvested from patients, expand them exponentially in in vitro culture systems, and re-infuse these cells into patients to mediate the clearance of infections or tumor destruction. Previously, genetically unmodified immune cells had been applied for therapy, such as circulating T cells, TILs, γδ T cells, NK cells, lymphokine-activated killer cells, and cytokine-induced killer cells (79). Because ACT employs self-derived T cells, also called autologous T cells, the biggest advantage of using patient-driven immune cells is that the transferred T cells are seldom rejected.
Figure 2. The scheme of ACT using CD8+ Tm cells. ACT is a type of treatment in which T cells isolated from patients are modified and expanded in the laboratory, so that T cells can recognize and kill cells expressing Ags after re-infusion into patients. These cells are prepared using three major protocols: (A) in vitro-activated T cells, (B) T cells isolated from tissues, and (C) T cells engineered by genetic modification. (A) Tn cells derived from a patient’s blood can be activated in vitro with the desired Ags for clonal expansion. (B) T cells harvested from tissues, especially from tumors, promptly expand in vitro with homeostatic cytokines including IL-7, IL-15, IL-21, and IL-2 and refuse to eliminate tumor cells. (C) After collection from a patient’s blood, CD8+ T cells can be genetically engineered to become CAR-T cells that have Ag receptors that recognize cancer. These CAR-T cells can also be expanded in vitro in the laboratory and then reinfused into patients for use in immunotherapy.
There are 2 primary ways to prepare endogenous T cells as ACTs for clinical applications: in vitro-stimulated Teff cells derived from Tn cells with specific Ags and tissue-infiltrating Ag-reactive T cells (Fig. 2A). First, T cell differentiation is induced in vitro, in which Tn cells become Teff or Tm cells against desired Ags, such as tumor Ags, and then re-transferred into patients. An advantage of using this protocol is that the TCRs of these T cells are highly diverse, allowing tumor Ags to stimulate at least a portion of these T cell pools (80,81,82). Thus, it is likely that Tn cells can differentiate into Teff or Tm cells in vitro and respond to various agents. Additionally, Teff or Tm cell differentiation in vitro using several cytokines has been attempted to obtain specific cell subsets with the desired number of cells. ACT was applied using Tn cells to patients with cancer in 2002 (83).
Circulating CD8+ T cells have also been collected from patients with metastatic melanoma and transformed into functional effector T cells using autologous DCs with melanoma-specific peptides in vitro. These cells were then transferred to patients with subsequent IL-2 administration for further T cell expansion in vivo (83). Teff cells administered to patients were shown to infiltrate into tissues containing tumor cells with cognate Ags. Despite these theoretically anticipated advantages, the functions of T cells differentiated in culture and in patients varied, so T cell administration cannot be fully implemented in vivo. Moreover, the mechanism by which these T cells are activated in vitro are not completely understood. For example, in vitro-differentiated Tm cells stimulated with a mixture of IL-7 and IL-15 were more effective in anti-tumor immunity than Tm cells induced only with IL-2 (84). Therefore, it is essential to fully examine the factors influencing Tm cell differentiation in vitro.
Another method of ACT is to employ tissue-infiltrating cells, particularly Trm cells, which have been intensively studied for cancer therapy (Fig. 2B) (85). Although this method has limitations of Ags compared to Tn cells, it is more effective than other T cell subsets because the isolated cells are functional cells that recognize and clear specific Ags in patients. Twenty years ago, Dudley et al. (86) isolated TILs from patients with metastatic melanoma who received immunodepleting chemotherapy and expanded their cells in vitro. After transplantation of T cells into patients treated with high-dose IL-2, rapid clonal TIL expansion in vivo resulted in the impairment of tumor metastasis (86). In addition, the number of transferred T cells rapidly decreased to less than 30% after 3–4 wk, even though the Ag-specific T cells survived for over 4 months. These reports suggest that in vitro-expanded TILs have functional effects against Ags and have the potential to differentiate into Tm cells in vivo.
Until now, the ACT method using Tm cells has not been reported much compared to that using Teff cells. However, if further research provides more information regarding the differentiation of Tm cells and the modulation of Tm cell functionality in vitro and in vivo, Tm cells will probably possess more therapeutic benefits than other subsets.
ACT with memory-like chimeric antigen receptor (CAR)-T cells
The experience and knowledge gained from ACT have triggered the advent of CAR-T cell therapy (Fig. 2C). Recent advances in CAR-T therapy are currently available worldwide and extensive challenges are being addressed (79,87). CAR-T cells are modified T cells with artificial Ag receptors that are more therapeutically effective when they are less differentiated and exhausted (88,89). Recently, the use of CD8+ CAR-T cells for cancer treatment has been rapidly increasing. However, the limitations of the cytolytic reactive efficiency of CAR-T cells have not yet been closely examined. One of the hurdles to using CAR-T cells is the lack of or weak response to the killing activity of CAR-T cells in preclinical studies, particularly in patients with solid tumors. This is probably due to the limited expansion and survival of CAR-T cells in tumors, as even these T cells undergo exhaustion, suggesting that new protocols to treat these patients are clinically on demand (90,91,92).
One important attempt differentiated CAR-T cells into memory or memory-like T cells with in vitro or in vivo stimulation, and this approach has shown promising clinical outcomes (93). For example, factors such as homeostatic cytokines and intracellular metabolism for generating memory CAR-T cells have been applied in clinical trials. First, IL-7 and IL-15 were evaluated to determine whether these homeostatic cytokines increased the proliferation, survival, and cytotoxicity of CAR-T cells, as in Tm cells. CAR-T cells incubated with a mixture of IL-7 and IL-15 in vivo differentiated more into CD8+CD45RA+CCR7+ CAR-T cells with a Tcm phenotype than CAR-T cells treated with mock or IL-2 (16,94). Moreover, these CAR-T cells had increased proliferative capacity and CTL activity for anti-tumor effects and decreased Tex cell markers, such as the inhibitory receptor PD-1. These cells retained their memory phenotype even after subsequent in vivo expansion (16,95). When IL-7- or IL-7R-expressing CAR-T cells were adoptively transferred via genetic modifications, they also persisted longer with enhanced anti-tumor activity for expansion and effector functions compared to controls (96,97).
The second attempt to alter differentiation into memory-like CAR-T cells is to switch the metabolism of these CAR-T cells between glycolysis, FAO, and OXPHOS (98). For example, CAR-T cells cultured in a glutamine metabolism inhibitory condition not only retained more highly proliferative subsets, such as Tn (CCR7+CD45RA+) or Tcm (CCR7+ CD45RA−), in vitro but also had accelerated cytotoxic activities to eliminate tumor cells in vivo. These cells also undergo metabolic reprogramming of mitochondrial OXPHOS, utilizing FAs and reduced glycolysis (99).
Altogether, CAR-T cells with a Tm cell phenotype have great potential to be more effective for cancer therapy than any other subsets, but the current basic understanding of Tm cell development and function remains limited. Thus, further studies and attempts at utilizing Tm cells as therapeutics, which will bring a bright future for cancer therapies, are required.
CONCLUSION AND FUTURE PERSPECTIVES
Despite recent remarkable advances in medical science and technology, there are still inadequate vaccines or immunological therapies for pandemic infectious diseases or various cancers. In this review, we summarized the features of Tm cells and suggested the reasons why Tm cells should be used as immunotherapy. Although the most important factor in preventing infectious diseases and cancers is to increase the potency of an individual’s Tm cells. Unfortunately, many vaccines and therapies cannot take advantage of these cells due to our limited understanding of Tm cell biology. Thus, we mapped out potential protocols for differentiating and maintaining Tm cells in terms of the interaction between cytokines, microbiota, and intrinsic metabolism. Finally, to overcome the efficiency and limitations of conventional therapy, a better understanding and study of Tm cells, the most potent cells in our body, will be needed.
ACKNOWLEDGEMENTS
We thank Aryeong Choi, Heonju Song, and Hyunjin Moon for their useful and constructive comments regarding this review. This work was supported by the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (NRF-2019R1A6A1A03031807 and NRF-2021R1A2C2004279).
Abbreviations
- ACT
adoptive cellular therapy
- APC
Ag-presenting cell
- CAR
chimeric antigen receptor
- CCR
CC-chemokine receptor
- COVID-19
coronavirus disease 2019
- FA
fatty acid
- FAO
fatty acid oxidation
- HSC
hematopoietic stem cell
- ICI
immune checkpoint inhibitor
- LCFA
long-chain fatty acid
- MPEC
memory precursor effector T cell
- OXPHOS
oxidative phosphorylation
- rh
recombinant human
- SCFA
short-chain fatty acid
- SLEC
short-lived effector T cell
- Tcm
central memory T
- Teff
effector T
- Tem
effector memory T
- Tex
exhausted T
- TIL
tumor-infiltrating lymphocyte
- Tm
memory T
- Tn
naïve T
- Trm
tissue-resident memory T
- γc
common γ
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Choi H, Kim Y, Jung YW.
- Data curation: Choi H, Kim Y, Jung YW.
- Funding acquisition: Jung YW.
- Supervision: Choi H, Jung YW.
- Visualization: Choi H, Kim Y.
- Writing - original draft: Choi H, Kim Y, Jung YW.
- Writing - review & editing: Choi H, Jung YW.
References
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naïve and memory CD8+ T cells to antigen stimulation in vivo . Nat Immunol. 2000;1:47–53. doi: 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- 3.Martin MD, Badovinac VP. Defining memory CD8 T cell. Front Immunol. 2018;9:2692. doi: 10.3389/fimmu.2018.02692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Wu D, Yang X, Zhou S. Immunotherapeutic potential of T memory stem cells. Front Oncol. 2021;11:723888. doi: 10.3389/fonc.2021.723888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baharom F, Ramirez-Valdez RA, Khalilnezhad A, Khalilnezhad S, Dillon M, Hermans D, Fussell S, Tobin KK, Dutertre CA, Lynn GM, et al. Systemic vaccination induces CD8+ T cells and remodels the tumor microenvironment. Cell. 2022;185:4317–4332.e15. doi: 10.1016/j.cell.2022.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mateus J, Grifoni A, Tarke A, Sidney J, Ramirez SI, Dan JM, Burger ZC, Rawlings SA, Smith DM, Phillips E, et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370:89–94. doi: 10.1126/science.abd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23:186–193. doi: 10.1038/s41590-021-01122-w. [DOI] [PubMed] [Google Scholar]
- 10.Principe N, Kidman J, Goh S, Tilsed CM, Fisher SA, Fear VS, Forbes CA, Zemek RM, Chopra A, Watson M, et al. Tumor infiltrating effector memory antigen-specific CD8+ T cells predict response to immune checkpoint therapy. Front Immunol. 2020;11:584423. doi: 10.3389/fimmu.2020.584423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanc C, Hans S, Tran T, Granier C, Saldman A, Anson M, Oudard S, Tartour E. Targeting resident memory T cells for cancer immunotherapy. Front Immunol. 2018;9:1722. doi: 10.3389/fimmu.2018.01722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mami-Chouaib F, Blanc C, Corgnac S, Hans S, Malenica I, Granier C, Tihy I, Tartour E. Resident memory T cells, critical components in tumor immunology. J Immunother Cancer. 2018;6:87. doi: 10.1186/s40425-018-0399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reiser J, Banerjee A. Effector, memory, and dysfunctional CD8+ T cell fates in the antitumor immune response. J Immunol Res. 2016;2016:8941260. doi: 10.1155/2016/8941260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y, Zhang M, Ramos CA, Durett A, Liu E, Dakhova O, Liu H, Creighton CJ, Gee AP, Heslop HE, et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood. 2014;123:3750–3759. doi: 10.1182/blood-2014-01-552174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 18.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8+ T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Wherry EJ, Teichgräber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 21.Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackay LK, Minnich M, Kragten NA, Liao Y, Nota B, Seillet C, Zaid A, Man K, Preston S, Freestone D, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. 2016;352:459–463. doi: 10.1126/science.aad2035. [DOI] [PubMed] [Google Scholar]
- 23.Gattinoni L, Speiser DE, Lichterfeld M, Bonini C. T memory stem cells in health and disease. Nat Med. 2017;23:18–27. doi: 10.1038/nm.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lugli E, Galletti G, Boi SK, Youngblood BA. Stem, effector, and hybrid states of memory CD8+ T cells. Trends Immunol. 2020;41:17–28. doi: 10.1016/j.it.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verma K, Ogonek J, Varanasi PR, Luther S, Bünting I, Thomay K, Behrens YL, Mischak-Weissinger E, Hambach L. Human CD8+ CD57− TEMRA cells: too young to be called “old”. PLoS One. 2017;12:e0177405. doi: 10.1371/journal.pone.0177405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamann D, Kostense S, Wolthers KC, Otto SA, Baars PA, Miedema F, van Lier RA. Evidence that human CD8+CD45RA+CD27− cells are induced by antigen and evolve through extensive rounds of division. Int Immunol. 1999;11:1027–1033. doi: 10.1093/intimm/11.7.1027. [DOI] [PubMed] [Google Scholar]
- 28.Gerlach C, Moseman EA, Loughhead SM, Alvarez D, Zwijnenburg AJ, Waanders L, Garg R, de la Torre JC, von Andrian UH. The chemokine receptor CX3CR1 defines three antigen-experienced CD8 T cell subsets with distinct roles in immune surveillance and homeostasis. Immunity. 2016;45:1270–1284. doi: 10.1016/j.immuni.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swanson PA., 2nd . Discovery and characterization of a novel antiviral CD8 T cell response. Atlanta, GA: Emory University; 2009. [Google Scholar]
- 30.Nolz JC, Rai D, Badovinac VP, Harty JT. Division-linked generation of death-intermediates regulates the numerical stability of memory CD8 T cells. Proc Natl Acad Sci U S A. 2012;109:6199–6204. doi: 10.1073/pnas.1118868109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo . Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 32.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8+ T cells. J Exp Med. 2002;196:935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong JH, Kim SH, Kim HG, Jang JH, Son RG, Pack SP, Park YH, Kang P, Jeong KJ, Kim JS, et al. Effect of human or mouse IL-7 on the homeostasis of porcine T lymphocytes. Immune Netw. 2021;21:e24. doi: 10.4110/in.2021.21.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi H, Song H, Jung YW. The roles of CCR7 for the homing of memory CD8+ T cells into their survival niches. Immune Netw. 2020;20:e20. doi: 10.4110/in.2020.20.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carrette F, Surh CD. IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis. Semin Immunol. 2012;24:209–217. doi: 10.1016/j.smim.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vahidi Y, Faghih Z, Talei AR, Doroudchi M, Ghaderi A. Memory CD4+ T cell subsets in tumor draining lymph nodes of breast cancer patients: a focus on T stem cell memory cells. Cell Oncol (Dordr) 2018;41:1–11. doi: 10.1007/s13402-017-0352-6. [DOI] [PubMed] [Google Scholar]
- 38.Stonier SW, Ma LJ, Castillo EF, Schluns KS. Dendritic cells drive memory CD8 T-cell homeostasis via IL-15 transpresentation. Blood. 2008;112:4546–4554. doi: 10.1182/blood-2008-05-156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dzhagalov I, Dunkle A, He YW. The anti-apoptotic Bcl-2 family member Mcl-1 promotes T lymphocyte survival at multiple stages. J Immunol. 2008;181:521–528. doi: 10.4049/jimmunol.181.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarasenko TN, Pacheco SE, Koenig MK, Gomez-Rodriguez J, Kapnick SM, Diaz F, Zerfas PM, Barca E, Sudderth J, DeBerardinis RJ, et al. Cytochrome C oxidase activity is a metabolic checkpoint that regulates cell fate decisions during T cell activation and differentiation. Cell Metab. 2017;25:1254–1268.e7. doi: 10.1016/j.cmet.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian Y, Zajac AJ. IL-21 and T cell differentiation: consider the context. Trends Immunol. 2016;37:557–568. doi: 10.1016/j.it.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moroz A, Eppolito C, Li Q, Tao J, Clegg CH, Shrikant PA. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. J Immunol. 2004;173:900–909. doi: 10.4049/jimmunol.173.2.900. [DOI] [PubMed] [Google Scholar]
- 43.Geltink RI, Kyle RL, Pearce EL. Unraveling the complex interplay between T cell metabolism and function. Annu Rev Immunol. 2018;36:461–488. doi: 10.1146/annurev-immunol-042617-053019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lochner M, Berod L, Sparwasser T. Fatty acid metabolism in the regulation of T cell function. Trends Immunol. 2015;36:81–91. doi: 10.1016/j.it.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ecker C, Guo L, Voicu S, Gil-de-Gómez L, Medvec A, Cortina L, Pajda J, Andolina M, Torres-Castillo M, Donato JL, et al. Differential reliance on lipid metabolism as a salvage pathway underlies functional differences of T cell subsets in poor nutrient environments. Cell Reports. 2018;23:741–755. doi: 10.1016/j.celrep.2018.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan Y, Tian T, Park CO, Lofftus SY, Mei S, Liu X, Luo C, O’Malley JT, Gehad A, Teague JE, et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature. 2017;543:252–256. doi: 10.1038/nature21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cui G, Staron MM, Gray SM, Ho PC, Amezquita RA, Wu J, Kaech SM. Il-7-induced glycerol transport and tag synthesis promotes memory CD8+ T cell longevity. Cell. 2015;161:750–761. doi: 10.1016/j.cell.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Huang Q, Long X, Guo X, Sun X, Jin X, Li Z, Ren T, Yuan P, Huang X, et al. Mitochondrial elongation-mediated glucose metabolism reprogramming is essential for tumour cell survival during energy stress. Oncogene. 2017;36:4901–4912. doi: 10.1038/onc.2017.98. [DOI] [PubMed] [Google Scholar]
- 51.Overacre-Delgoffe AE, Hand TW. Regulation of tissue-resident memory T cells by the Microbiota. Mucosal Immunol. 2022;15:408–417. doi: 10.1038/s41385-022-00491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bachem A, Makhlouf C, Binger KJ, de Souza DP, Tull D, Hochheiser K, Whitney PG, Fernandez-Ruiz D, Dähling S, Kastenmüller W, et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8+ T cells. Immunity. 2019;51:285–297.e5. doi: 10.1016/j.immuni.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 53.Laidlaw BJ, Cui W, Amezquita RA, Gray SM, Guan T, Lu Y, Kobayashi Y, Flavell RA, Kleinstein SH, Craft J, et al. Production of IL-10 by CD4+ regulatory T cells during the resolution of infection promotes the maturation of memory CD8+ T cells. Nat Immunol. 2015;16:871–879. doi: 10.1038/ni.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gallo A, Pero E, Pellegrino S, Macerola N, Murace CA, Ibba F, Agnitelli MC, Landi F, Montalto M. How can biology of aging explain the severity of COVID-19 in older adults. Clin Geriatr Med. 2022;38:461–472. doi: 10.1016/j.cger.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geiger H, Rudolph KL. Aging in the lympho-hematopoietic stem cell compartment. Trends Immunol. 2009;30:360–365. doi: 10.1016/j.it.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 56.Weng NP, Akbar AN, Goronzy J. CD28− T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiu WK, Fann M, Weng NP. Generation and growth of CD28nullCD8+ memory T cells mediated by IL-15 and its induced cytokines. J Immunol. 2006;177:7802–7810. doi: 10.4049/jimmunol.177.11.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saurwein-Teissl M, Lung TL, Marx F, Gschösser C, Asch E, Blasko I, Parson W, Böck G, Schönitzer D, Trannoy E, et al. Lack of antibody production following immunization in old age: association with CD8+CD28− T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 59.Sasaki S, Sullivan M, Narvaez CF, Holmes TH, Furman D, Zheng NY, Nishtala M, Wrammert J, Smith K, James JA, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest. 2011;121:3109–3119. doi: 10.1172/JCI57834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Craig DJ, Creeden JF, Einloth KR, Gillman CE, Stanbery L, Hamouda D, Edelman G, Dworkin L, Nemunaitis JJ. Resident memory T cells and their effect on cancer. Vaccines (Basel) 2020;8:562. doi: 10.3390/vaccines8040562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manjarrez-Orduño N, Menard LC, Kansal S, Fischer P, Kakrecha B, Jiang C, Cunningham M, Greenawalt D, Patel V, Yang M, et al. Circulating T cell subpopulations correlate with immune responses at the tumor site and clinical response to PD1 inhibition in non-small cell lung cancer. Front Immunol. 2018;9:1613. doi: 10.3389/fimmu.2018.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bello E, Dougan M. Elevated circulating memory T cells precede immunotherapy toxicities in melanoma. Trends Cancer. 2022;8:347–349. doi: 10.1016/j.trecan.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 63.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 64.Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, Lynn RC, Philip M, Rao A, Restifo NP, et al. Defining ‘T cell exhaustion’. Nat Rev Immunol. 2019;19:665–674. doi: 10.1038/s41577-019-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.West EE, Youngblood B, Tan WG, Jin HT, Araki K, Alexe G, Konieczny BT, Calpe S, Freeman GJ, Terhorst C, et al. Tight regulation of memory CD8+ T cells limits their effectiveness during sustained high viral load. Immunity. 2011;35:285–298. doi: 10.1016/j.immuni.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar R, Ferez M, Swamy M, Arechaga I, Rejas MT, Valpuesta JM, Schamel WW, Alarcon B, van Santen HM. Increased sensitivity of antigen-experienced T cells through the enrichment of oligomeric T cell receptor complexes. Immunity. 2011;35:375–387. doi: 10.1016/j.immuni.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 67.Shakiba M, Zumbo P, Espinosa-Carrasco G, Menocal L, Dündar F, Carson SE, Bruno EM, Sanchez-Rivera FJ, Lowe SW, Camara S, et al. TCR signal strength defines distinct mechanisms of T cell dysfunction and cancer evasion. J Exp Med. 2022;219:e20201966. doi: 10.1084/jem.20201966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jarnicki AG, Lysaght J, Todryk S, Mills KH. Suppression of antitumor immunity by IL-10 and TGF-β-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol. 2006;177:896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
- 69.Sawant DV, Yano H, Chikina M, Zhang Q, Liao M, Liu C, Callahan DJ, Sun Z, Sun T, Tabib T, et al. Adaptive plasticity of IL-10+ and IL-35+ Treg cells cooperatively promotes tumor T cell exhaustion. Nat Immunol. 2019;20:724–735. doi: 10.1038/s41590-019-0346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corbett KS, Edwards DK, Leist SR, Abiona OM, Boyoglu-Barnum S, Gillespie RA, Himansu S, Schäfer A, Ziwawo CT, DiPiazza AT, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verbeke R, Lentacker I, De Smedt SC, Dewitte H. Three decades of messenger RNA vaccine development. Nano Today. 2019;28:100766 [Google Scholar]
- 72.Garcia-Beltran WF, Lam EC, Astudillo MG, Yang D, Miller TE, Feldman J, Hauser BM, Caradonna TM, Clayton KL, Nitido AD, et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184:476–488.e11. doi: 10.1016/j.cell.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cao Y, Wang J, Jian F, Xiao T, Song W, Yisimayi A, Huang W, Li Q, Wang P, An R, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602:657–663. doi: 10.1038/s41586-021-04385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu J, Chandrashekar A, Sellers D, Barrett J, Jacob-Dolan C, Lifton M, McMahan K, Sciacca M, VanWyk H, Wu C, et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron. Nature. 2022;603:493–496. doi: 10.1038/s41586-022-04465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jung MK, Jeong SD, Noh JY, Kim DU, Jung S, Song JY, Jeong HW, Park SH, Shin EC. BNT162b2-induced memory T cells respond to the Omicron variant with preserved polyfunctionality. Nat Microbiol. 2022;7:909–917. doi: 10.1038/s41564-022-01123-x. [DOI] [PubMed] [Google Scholar]
- 76.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bai C, Zhou L, Tang J, He J, Han J, Niu H, Zhu B. Fusion cytokines IL-7-linker-IL-15 promote mycobacterium tuberculosis subunit vaccine to induce central memory like T cell-mediated immunity. Vaccines (Basel) 2020;8:715. doi: 10.3390/vaccines8040715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang F, Jin H, Wang J, Sun Q, Yan C, Wei F, Ren X. Adoptive cellular therapy (ACT) for cancer treatment. Adv Exp Med Biol. 2016;909:169–239. doi: 10.1007/978-94-017-7555-7_4. [DOI] [PubMed] [Google Scholar]
- 80.Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human αβ T cell receptor diversity. Science. 1999;286:958–961. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- 81.Nikolich-Žugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4:123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 82.de Greef PC, Oakes T, Gerritsen B, Ismail M, Heather JM, Hermsen R, Chain B, de Boer RJ. The naive T-cell receptor repertoire has an extremely broad distribution of clone sizes. eLife. 2020;9:e49900. doi: 10.7554/eLife.49900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Beumer-Chuwonpad A, Taggenbrock RL, Ngo TA, van Gisbergen KP. The potential of tissue-resident memory t cells for adoptive immunotherapy against cancer. Cells. 2021;10:2234. doi: 10.3390/cells10092234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hupperetz C, Lah S, Kim H, Kim CH. Car t cell immunotherapy beyond haematological malignancy. Immune Netw. 2022;22:e6. doi: 10.4110/in.2022.22.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Curran KJ, Pegram HJ, Brentjens RJ. Chimeric antigen receptors for T cell immunotherapy: current understanding and future directions. J Gene Med. 2012;14:405–415. doi: 10.1002/jgm.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 90.Baumeister SH, Murad J, Werner L, Daley H, Trebeden-Negre H, Gicobi JK, Schmucker A, Reder J, Sentman CL, Gilham DE, et al. Phase I trial of autologous CAR T cells targeting NKG2D ligands in patients with AML/MDS and multiple myeloma. Cancer Immunol Res. 2019;7:100–112. doi: 10.1158/2326-6066.CIR-18-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gumber D, Wang LD. Improving CAR-T immunotherapy: overcoming the challenges of T cell exhaustion. EBioMedicine. 2022;77:103941. doi: 10.1016/j.ebiom.2022.103941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11:69. doi: 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Golubovskaya V, Wu L. Different subsets of T cells, memory, effector functions, and CAR-T immunotherapy. Cancers (Basel) 2016;8:36. doi: 10.3390/cancers8030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gargett T, Brown MP. Different cytokine and stimulation conditions influence the expansion and immune phenotype of third-generation chimeric antigen receptor T cells specific for tumor antigen GD2. Cytotherapy. 2015;17:487–495. doi: 10.1016/j.jcyt.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 95.Zhou J, Jin L, Wang F, Zhang Y, Liu B, Zhao T. Chimeric antigen receptor T (CAR-T) cells expanded with IL-7/IL-15 mediate superior antitumor effects. Protein Cell. 2019;10:764–769. doi: 10.1007/s13238-019-0643-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.He C, Zhou Y, Li Z, Farooq MA, Ajmal I, Zhang H, Zhang L, Tao L, Yao J, Du B, et al. Co-expression of IL-7 improves NKG2D-based CAR T cell therapy on prostate cancer by enhancing the expansion and inhibiting the apoptosis and exhaustion. Cancers (Basel) 2020;12:1969. doi: 10.3390/cancers12071969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shum T, Omer B, Tashiro H, Kruse RL, Wagner DL, Parikh K, Yi Z, Sauer T, Liu D, Parihar R, et al. Constitutive signaling from an engineered IL7 receptor promotes durable tumor elimination by tumor-redirected T cells. Cancer Discov. 2017;7:1238–1247. doi: 10.1158/2159-8290.CD-17-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rostamian H, Fallah-Mehrjardi K, Khakpoor-Koosheh M, Pawelek JM, Hadjati J, Brown CE, Mirzaei HR. A metabolic switch to memory CAR T cells: Implications for cancer treatment. Cancer Lett. 2021;500:107–118. doi: 10.1016/j.canlet.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 99.Shen L, Xiao Y, Zhang C, Li S, Teng X, Cui L, Liu T, Wu N, Lu Z. Metabolic reprogramming by ex vivo glutamine inhibition endows CAR-T cells with less-differentiated phenotype and persistent antitumor activity. Cancer Lett. 2022;538:215710. doi: 10.1016/j.canlet.2022.215710. [DOI] [PubMed] [Google Scholar]