Abstract
Blood-based assays using various technologies and biomarkers are in commercial development for the purpose of detecting multiple cancer types concurrently at an early stage of disease. These multicancer early detection (MCED) assays have the potential to improve the detection of cancers, particularly those for which no current screening modality exists. However, the unknown clinical benefits and harms of using MCED assays for cancer screening necessitate the development and implementation of a randomized controlled trial (RCT) to ascertain their clinical effectiveness. This was the consensus of experts at a National Cancer Institute–hosted workshop to discuss initial design concepts for such a trial. Using these assays to screen simultaneously for multiple cancers poses novel uncertainties for patient care compared with conventional screening tests for single cancers, such as establishing the diagnostic workup to confirm the presence of cancer at any organ site; clarifying appropriate follow-up for a positive assay for which there is no definitive diagnosis; identifying potential harms such as overdiagnosis of indolent disease; determining clinically effective and efficient strategies for disseminating MCED screening in real-world practice; and understanding the ethical implications, such as potentially alleviating or exacerbating existing health disparities. These assays present new and complex challenges for designing an RCT. Issues that emerged from the meeting centered around the need for a flexibly designed, clinical utility RCT to rigorously capture the evidence required to fully understand the net benefit of this promising technology. Specific topic areas were endpoints, screening protocols, recruitment, diagnostic pathway, pilot phase, data elements, specimen collection, and ethical considerations.
Blood-based assays using various technologies are in commercial development for the purpose of detecting multiple cancer types concurrently at early stage of disease. These assays are denoted multicancer early detection or MCED assays. The current standard of care for early detection of cancer, however, is to screen asymptomatic, ostensibly healthy individuals at risk for a single cancer type using organ-specific tests. A positive screen triggers an organ-specific diagnostic process that typically involves imaging and a tissue biopsy for histopathologic evaluation.
MCED assays differ from organ-specific cancer screening in several ways: 1) types of cancers potentially detected; 2) types of analytes measured, such as circulating tumor DNA mutation, DNA methylation or fragmentation patterns, or circulating protein biomarkers; and 3) artificial intelligence or machine learning modeling methods to determine what combination and level of those analytes constitute a positive test result (1-10). Many assays, in addition to identifying an overall likelihood of cancer, generate a tissue-of-origin (TOO) prediction regarding the most probable organ site(s) of cancer. To date, published studies of MCED assays have generally focused on validating diagnostic performance in persons already diagnosed with cancer (1,2,6,7,10,11). These case-control studies differ in methods, design, and identification of cases and controls. Some prospective studies in asymptomatic participants (7,10) have been conducted but were not designed to assess any clinical utility endpoints, such as cancer-related mortality. A randomized trial with clinical utility endpoints of a single MCED test recently completed enrollment in the United Kingdom; however, results are not expected for several years (12).
Using these assays to screen simultaneously for multiple cancers poses novel uncertainties for patient care compared with conventional screening tests for single cancers. They include 1) establishing the diagnostic workup necessary to confirm the presence of cancer at any organ site; 2) clarifying appropriate follow-up for positive assays for which there is no definitive diagnosis; 3) identifying potential harms, such as anxiety or depression from false-positive screens and overdiagnosis of indolent disease; 4) determining clinically effective and efficient strategies for disseminating MCED screening in real-world practice; and 5) understanding ethical implications, such as alleviation or exacerbation of current health disparities.
Because of the unique features of MCED assays in the cancer screening space, in October 2021, the National Cancer Institute convened a 2-day workshop focused on the question of how to evaluate the performance of MCED tests.
Goals of the workshop
Participants in the workshop included academic cancer biomarker investigators, primary care physicians with cancer screening expertise, clinical trials experts, and others. The goals of the workshop were to assess
the need for conducting a randomized controlled trial (RCT) to evaluate the performance and utility of MCED screening tests;
various design issues of a putative RCT of MCED screening tests; and
the need for supporting studies for a RCT, including a pilot trial and observational studies.
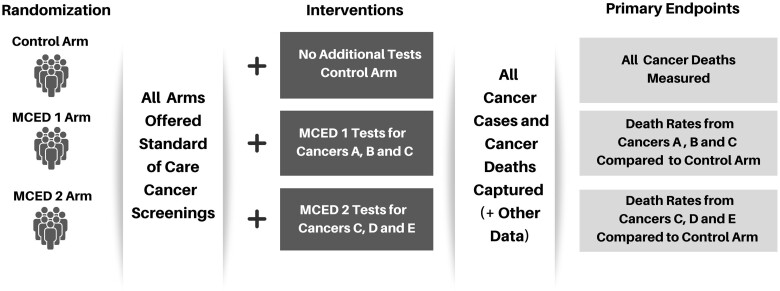
For goal #2, to facilitate discussion, a prototype trial incorporating a platform type design (13), where several intervention arms are compared with a single control arm, was presented by the National Cancer Institute. As shown in Figure 1, each intervention arm offers screening with 1 MCED assay along with standard-of-care screening vs a control arm of standard-of-care screening alone.
Figure 1.
Platform study design schema. MCED = multicancer early detection
In this article, we summarize the workshop discussions on the various issues. Where there was a clear consensus among workshop attendees, we so state. In the case of varying opinions, we present the authors’ current viewpoint, informed by arguments presented on all sides of the issue.
The need for an MCED randomized trial
There was agreement among workshop participants that rigorous evaluation of MCED assays intended for cancer screening is greatly needed, especially because at least one MCED assay is already in clinical use. Although it is hard to predict how quickly MCED assays will disseminate in the population, or the extent to which control arm participants in the proposed RCT would seek screening with MCED assays outside of the trial, blood-based screening tests could have rapid uptake. Comparisons were made to how the blood-based prostate-specific antigen test was widely adopted for screening without proper evaluation of potential benefits vs harms. Establishing clinical validity (ie, sensitivity and specificity) of MCED tests was deemed insufficient; a determination of clinical utility based on a RCT with appropriate primary endpoints would be required. A general design as illustrated in Figure 1 was endorsed by workshop participants.
Various clinical and operational aspects of the design of an MCED RCT differ from those of a single cancer screening trial. To refine these prior to the start of a main trial, workshop participants discussed the need for a pilot study. Further justification for a pilot is bolstered by previous experience in the Prostate, Lung, Colorectal, and Ovarian (PLCO) screening trial (14,15), National Lung Screening Trial (NLST) (16,17), and UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) (15,19).
To be compatible with the planned multiarm platform design of the main trial (Figure 1), the pilot study should have a similar design. Intervention arm participants would be offered screening with MCED tests over multiple rounds, with all participants offered standard-of-care screening. Blood samples would be collected in all study arms. If large enough, the pilot could provide updated estimates of cancers identified in each arm to help refine the study design assumptions for the definitive trial.
The best time to evaluate MCED assays in RCTs was generally agreed to occur before regulatory approval by the Food and Drug Administration and before health insurance reimbursement becomes broadly available. That these technologies continue to be developed and refined was cited as a reason to make the trial structure flexible. Accordingly, assays could be assessed continually over time by implementing an adaptive, platform trial design that includes early stopping rules and a transparent process for incorporating new arms for additional promising MCEDs as they emerge.
There was also discussion on the need for supporting observational studies, prior to and/or simultaneous with the pilot RCT, to independently validate MCED diagnostic performance and help assess which MCED tests merit inclusion in the pilot and/or main-phase RCT. These studies include using existing cohorts, such as National Institutes of Health’s All of Us cohort (20), to assess prospective MCED performance in asymptomatic persons.
Primary and secondary endpoints for an MCED trial
The objective of cancer screening is to improve overall clinical outcomes for persons screened through early detection and treatment of identified disease. Measures of population mortality, in particular, death rates from the cancer(s) being screened for among the entire trial arm population, provide the most rigorous, unequivocal endpoint for measuring improved outcomes (21). Population mortality measures are not affected by lead time and length biases that compromise other potential endpoints, such as survival or proportional stage shift (22,23). Moreover, potential harms are assessed to determine whether the mortality reduction outweighs the harms from screening.
Most major cancer screening trials evaluated screening for single cancers with mortality from that cancer as the primary endpoint. This includes trials for low-dose computed tomography lung cancer screening, mammography for breast cancer screening, endoscopy and stool-testing for colorectal cancer screening, and prostate-specific antigen for prostate cancer screening (24-29).
In considering a primary endpoint of cancer mortality for an MCED RCT, a central challenge entails deciding which cancer organ sites to include. One option is mortality from all cancer sites. However, these assays are not usually designed to detect all possible cancers, and different assays may detect different subsets (a “basket”) of cancer types. Therefore, another option for the primary endpoint for a particular assay arm is a composite endpoint of deaths from each of the target cancers in the prespecified basket for that assay. As shown in Figure 1, if MCED 1 detects cancers A, B, and C, the primary outcome compares death rates from those 3 cancers in arm 1 with death rates from those same cancers in the control arm.
Key secondary endpoints are all-cause mortality, all-cancer mortality (if not the primary endpoint), incidence of advanced stage disease, and harms such as false-positives, invasive procedures, serious adverse events, patient psychological distress and experiences with care, and overdiagnosis. It is important that harms and safety be assessed as stringently as benefits.
Although cancer mortality remains the most rigorous primary endpoint, some workshop participants expressed interest in using the rate of advanced (late-stage) cancer as the primary endpoint, which could provide earlier trial conclusions. One example of using late-stage incidence as the primary endpoint is the current tomosynthesis mammographic imaging screening trial (TMIST) comparing digital mammography with tomosynthesis (30). Use of this endpoint was justified based on mammography screening RCTs, where there was strong correlation between reduction in advanced (ie, stages II-V) breast cancer and reduction in breast cancer mortality (28).
Workshop discussants who were against using late-stage incidence as a primary outcome measure argued that a late-stage incidence reduction might not translate into a mortality benefit for all cancers in a particular assay basket. They cited the UKCTOCS trial, which demonstrated a significant reduction in stage IV ovarian cancer incidence in the multimodal screening vs control arm but ultimately did not result in an ovarian cancer–related mortality benefit (24).
Although the incremental benefit of earlier vs later treatment of a cancer is a prominent factor in determining the benefit of a reduction in late-stage incidence, for some cancers even early stage disease has poor outcomes. For example, early stage pancreatic cancer has worse 5-year survival than late-stage prostate cancer (31,32). So, a given reduction in late-stage incidence may translate into different mortality reductions (including possibly no mortality reduction) for different cancer types. Unlike established cancer screening tests, there have been no RCTs of MCED assays used for cancer screening with mortality and advanced stage data to use for validation of surrogates.
Another issue involves the definition of advanced stage disease. Some workshop participants advocated for the use of stages III-V across cancer types; others suggested using organ-specific definitions. Nevertheless, after the completion of a MCED trial with a cancer mortality endpoint, the incidence of advanced stage disease should be investigated as a potential surrogate for mortality to determine if this endpoint can be appropriately defined and validated.
Assessing all the arguments presented, the authors believe that cancer mortality for a basket of cancers predefined for each MCED arm, as displayed in Figure 1, is the preferred primary endpoint for a trial.
Feasible screening protocols for an MCED trial
A trial design with annual MCED screening for 3-5 years, and total follow-up of 7 years for the primary endpoint, was proposed. Modeling analyses suggest that for a design with a cancer mortality endpoint, a trial with 5 annual screens and 7 years of total follow-up offers a substantial increase in power over a design with only 3 annual screens and 5 years of total follow-up. An advantage of annual screening is simplicity. Additionally, the basket of cancer types included in the primary endpoint will likely cover a broad array of cancer types, including many, such as pancreatic and ovarian, with aggressive trajectories. Therefore, a relatively short screening interval, such as annual, could maximize potential benefit without undue participant burden. The idea of testing at other frequencies, based on individual risk, was discussed as a possibility, although this would increase the complexity of the trial.
An argument was offered that screening frequency should be driven more by the underlying natural history and treatment of the cancers than by the assays. Yet little is known about the natural history of most types of cancer. In screening trials, cancer detection rates are generally greater on the first screen compared with later screens. Whether that pattern continues in an MCED trial will depend on the cancer types that a particular assay targets. This may be relevant for determining the number of screening rounds in a trial.
Workshop discussants agreed with the need to make standard-of-care screening available to all study participants if they chose to accept it. However, there was disagreement about the extent to which the trial should encourage such screening. On one hand, treating standard-of-care screening as usual care, without undue encouragement by the trial, would be a better way of assessing real-world harms, such as attrition of standard-of-care screening in the intervention arms. On the other hand, it might be more ethically justifiable to actively encourage people in all arms to receive standard-of-care screening. However, if the trial significantly encourages it, there will be a higher rate of standard-of-care screening in all arms, and the results may not be as generalizable to real-world practice experiences of the broader population. Overall, the authors’ preferred approach is that all trial participants should be encouraged to receive standard-of-care screening.
Target populations for an MCED trial
In considering the target population for an MCED trial, 2 related issues were discussed: 1) what the eligible population should be and 2) how that population should be recruited. The eligible population should be representative of the eventual intended use population and have a high-enough event rate of the primary outcome (eg, cancer mortality) to enable a feasible sample size of the trial. These characteristics can be at odds, as representativeness may be sacrificed to increase the primary outcome event rate. This was seen in the NLST with respect to the smoking history eligibility requirements (33). Typically, higher event rates lead to a smaller required trial sample size and/or a shorter study duration.
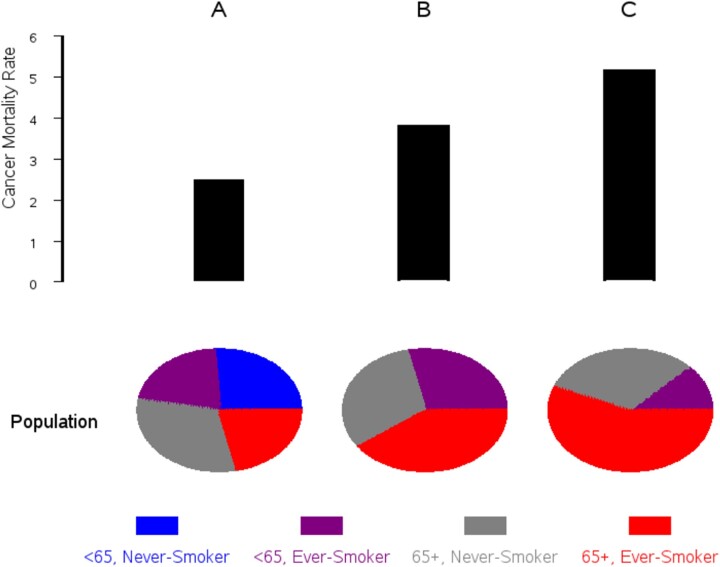
Cancer mortality increases substantially with age; therefore, age is the primary eligibility factor. Possible minimum ages are 45 or 50 years, and maximum ages are 70 or 75 years. Because MCED assays target a broad range of cancers, with potentially different risk factors, it is not as straightforward to select for high risk as it would be for a trial of a single cancer screening modality. A simple risk model was developed for this workshop using data from PLCO. From 1993 to 2001, PLCO randomly assigned almost 155 000 men and women aged 55-74 years to an intervention or control arm; intervention arm participants received periodic screening for lung, colorectal, and either prostate or ovarian cancer (14). Body mass index, family history of cancer, and several smoking history variables assessed in a baseline questionnaire from participants in both arms were used to predict 7-year all-cancer mortality (Figure 2). Based on this model, restricting eligible participants to the top 50% or 25% of risk appreciably increases the event rate of all-cancer mortality and thus lowers required sample size. However, recruiting based on higher risk skews the trial population toward current smokers and older individuals and could adversely affect representativeness.
Figure 2.
Enriching the target population for high risk. Using Prostate, Lung, Colorectal, Ovarian Cancer Screening Trial data, panels A, B, and C represent the population aged 55-75 years, the entire population (A), the top 50% risk (B), and the top 25% risk (C). Risk is for all-cancer mortality over 7 years and is based on model incorporating smoking history, body mass index, family history, and demographics.
In a general population study, individuals at very high cancer risk (eg, carriers of highly penetrant mutations that cause hereditary breast and ovarian cancer syndrome and hereditary nonpolyposis colorectal cancer) would not be excluded. However, these patients often undergo more intensive cancer screening regimens. A separate study, enrolling these individuals and using those MCED assays that target their high-risk cancers, might be more appropriate.
The authors believe that a general population approach should be used for the pilot phase, with an age range of roughly 50-75 years. The pilot results can help assess the feasibility of recruiting high-risk groups and refine population-risk profiles, which can help inform the main trial on this issue.
There was consensus that adequate representation of populations traditionally underrepresented in research is critical, including African American, Latino, and Native American individuals; rural populations; and the under- and uninsured. Adequate implies at least proportional representation in terms of the eligible age group but could also mean proportional to the cancer burden in the age-eligible population.
As a general strategy, large health-care systems have recently used patient portals, text messaging, and social media to recruit research participants, whereas prior trials have used mass mailings. A trial communications center that can directly interact with potential participants and respond to questions or concerns would be useful.
For traditionally underrepresented groups, a digital divide exists in the United States, particularly among the socially disadvantaged (34). Therefore, relying only on social media and other digital channels may not be effective in ensuring representativeness. Community outreach and engagement-based strategies that partner with trusted local organizations and develop culturally appropriate messaging strategies may be an effective way to recruit people (35). One method to help ensure adequate enrollment of underrepresented groups, which was used in some COVID-19 trials, was to close enrollment to non-Hispanic White individuals after they reached a given target and only recruit thereafter from other populations (including African American, Latino, and Native American individuals). For underinsured and uninsured individuals, it is necessary to implement specific outreach that identifies a means for coverage of downstream costs related to managing positive screening tests and treating diagnosed cancers.
Diagnostic follow-up after positive MCED tests
Once a positive MCED result is found, the next step is determining whether cancer exists and, if so, where. Most assays indicate a potential TOO, providing a direction for starting the diagnostic process. Others not providing a TOO instead may trigger whole-body imaging scans as the first step in the diagnostic pathway. Although assays may indicate likely TOO(s), companies generally do not specify the diagnostic process; thus, it has the potential to be highly variable. Additionally, little is known about determining cancer risk for people with positive assay results but no definitive finding of cancer.
The appropriate number and type of diagnostic tests to evaluate positive assay results may vary according to the predicted TOO and patient characteristics (eg, co-morbidities and patient preference). Variation in the diagnostic approach also may result from clinicians’ practice preferences and the availability of follow-up care. A predefined, standardized approach to diagnostic testing for a variety of organ sites (diagnostic pathway) may need to be worked out for each assay evaluated in the trial. For example, a trial-specific diagnostic process was implemented for the UKCTOCS trial (18).
Even with standardized diagnostic pathways, sufficient flexibility is needed to accommodate clinical judgement for individual patient scenarios. For most large cancer screening trials, diagnostic pathways were not incorporated into the trials themselves. Yet, because MCED assays for screening are new, clinicians may welcome the guidance of trial-specific diagnostic pathways, particularly when the initial workup does not reveal a cancer diagnosis. Although standard approaches exist for incidental findings in some imaging modalities, the potential for harms in the diagnosis process exists, including complications from diagnostic testing, lack of follow-up, patient distress around out-of-pocket costs and other matters, and lack of a definitive diagnosis.
One drawback to implementing a predefined, trial-specific diagnostic pathway in MCED trials is that the interpretation of study findings may be restricted to the assay plus the diagnostic pathway. However, even without trial-defined pathways, the interpretation of findings still is dependent on the diagnostic process used in the trial.
The authors believe that having a predefined, trial-specific diagnostic pathway is the optimal approach and ensures the appropriate care for the study participants. The pathway algorithm should be evaluated in the pilot phase and modified as necessary.
Collecting data for the trial
Collection of high-quality data is crucial to the success of an MCED trial. However, there may be trade-offs between costs, time lags, quality, and trial data completeness. Although clinically actionable results will be supplied to the trial by the companies, it is desirable to obtain as much information on assay results as possible, including raw test data, which may be helpful in ancillary trial research.
For collection of the primary outcome of mortality from all or a subset of cancers, linkage with the National Death Index is simple and inexpensive and provides timely and highly accurate results. Cancer incidence and stage, for all or most cancer sites, are important secondary outcomes. Other cancer screening trials (eg, PLCO and NLST) initially employed a large staff to obtain and abstract relevant medical records, a labor-intensive and costly process when ascertaining all-cancer incidence. These trials later switched to passive follow-up via state cancer registry linkages. In PLCO, the passive linkage approach gave similar ascertainment rates as active follow-up and had high levels of agreement with active follow-up on cancer characteristics (36). With the development of the Virtual Pooled Registry (37), such efforts will be greatly simplified by having a single, common application process that covers most states rather than having separate applications for each, as was the case with PLCO and NLST. A drawback of state registry linkages is the lag time of up to 2 years for available data. Another disadvantage is the availability of stage information, especially tumor-nodes-metastasis stage, which is often missing in registries. Other possible approaches involve linking with electronic medical records (EMR) from large health-care systems.
To mitigate potential harms and costs of diagnostic follow-up and to understand the extent of patients’ “diagnostic odysseys,” it is critical to obtain detailed data on diagnostic follow-up, whether performed under trial auspices or in the community. Data on community-performed diagnostic tests are typically harder to obtain. Vital information on the outcomes of procedures, including adverse events, are more difficult to obtain than the fact of the procedures themselves. For example, knowing whether there were positive tests that were never pursued further is of interest. Complications of diagnostic procedures should be captured to fully assess harms. EMRs may be useful in collecting these data, especially from patients enrolled in large health-care systems. It is also important to capture patient-reported outcomes related to diagnostic follow-up of positive screens (eg, anxiety, patient experience with care, lost time from work). Patient-reported outcomes should also be collected in a subset of those without positive screens.
The authors believe that a combination of approaches, including direct collection of data by trial staff, linkage with EMRs, and linkage with the National Death Index and state cancer registries will be needed. How these different approaches will work for different types of data should be assessed in the pilot trial.
Collecting specimens for testing and future use
It is unclear how best to develop and implement a standard operating procedure for specimen collection if each MCED assay has different collection and processing requirements. Yet, common collection processes are necessary to ensure uniformity in target analyte preservation if MCED assays are evaluated against a common control arm. If each assay has sufficiently different requirements for the blood collection, specimen processing prior to shipping, conditions for transportation, and storage, sites may have to coordinate different types of collection kits for the platform trial.
Currently available MCED assays use blood plasma, whose collection entails minimally invasive phlebotomy at an ethically acceptable maximum volume of 60 mL per draw per study participant, with an approximate plasma yield of 40 mL The standard operating procedure–required infrastructure and laboratory training and expertise for specimen processing and quality control raise questions about the ability of community primary care sites to participate in the study, which may have important implications on trial population diversity. Another ethical issue is the acceptable maximum amount of collected specimens for future research on MCED test development.
Even though all reported MCED assays use blood, in the future they could use other biospecimens (eg, urine). A platform RCT should therefore have a flexible and adaptive design to allow inclusion of new intervention arms by accommodating specimen requirements of new assays as they become available.
Ethical considerations in designing an MCED trial
No medical intervention is completely free of potential harms. To date, limited information exists on the potential benefits and harms to persons who have cancer screening with MCED assays. Potential harms to participants include psychological morbidity (eg, anxiety) because of false-positive tests, findings of indolent “incidentalomas,” or untreatable disease conditions; unnecessary diagnostic procedures and complications; and deferral of standard-of-care screening tests due to false reassurance from negative MCED test results. Other downstream harms include morbidity resulting from overdiagnosis and treatment of indolent, screening-detected cancers.
When institutional review boards approve federally funded research, they are charged with determining that risks to participants are minimized and reasonable in relation to anticipated benefits (38). Early detection of cancer, even via noninvasive means, is not a benefit per se. Meaningful benefit requires an evaluation of the clinical utility of the screening, which is best assessed in a RCT.
Ethical issues also include societal concerns, particularly related to health disparities. Historically, marginalized racial and ethnic minority and geographically isolated communities have experienced economic, educational, and geographic disadvantages that limit health-care access and may require additional safeguards to protect their rights and welfare under the Federal Policy for the Protection of Human Subjects (38). It is critical for the trial population to be nationally representative with respect to race and ethnicity, geography, insurance status, and other factors (eg, education, income) so that trial results will be widely applicable. Downstream costs to trial participants, including diagnostic follow-up tests, must be covered for the uninsured and underinsured to ensure their participation. New technologies such as MCEDs may increase current disparities if equal access to testing and follow-up care is not ensured. On the other hand, efficacious MCEDs could decrease current disparities if they are made accessible in medically underserved communities. Thus, information from RCTs is necessary to understand the impact that the assays will have on existing disparities.
Another potential harm is the erosion of public trust in cancer research if the trial is not conducted equitably and transparently. Skepticism of medical science and expert recommendations is widespread in the United States and raises the need for caution before new interventions are implemented (39). Care must be taken not only to determine whether MCED screening is effective and safe but to help the general public understand the supporting scientific evidence.
A final ethical issue, mentioned previously, concerns the extent to which standard-of-care screening should be promoted across trial arms. On one hand, the ethical principle of justice supports efforts to maximize access to and utilization of standard-of-care screening and follow-up care among all individuals in all study arms (particularly those from underserved and underrepresented populations) to promote health equity. On the other hand, such efforts may reduce the generalizability of study findings if they enhance rates of screening and follow-up care compared with real-world clinical settings (which reflect limitations in access to care). The design of an MCED trial will require an acceptable resolution of this tension between the ethical principle of justice and the scientific goal of external validity; some sacrifice of generalizability may be justified if it improves health-care access and health equity and yet still enables evaluation of the comparative effectiveness of MCED and conventional screening strategies.
Discussion
MCED assays have the potential to improve the detection of cancers, particularly those for which no current screening modality exists. However, the overall benefits and harms of screening with these assays need to be better understood. The consensus of the workshop was that a flexibly designed, clinical utility RCT is needed to rigorously capture the evidence required to fully understand the net benefit of this promising technology. The authors recommend an RCT that recruits participants aged 50-75 years from a representative population with a primary endpoint of cancer mortality from a predefined basket of cancers specific to the MCED assays. The RCT should include a predefined trial-specific diagnostic pathway for a positive MCED test result.
Contributor Information
Lori M Minasian, Division of Cancer Prevention, National Cancer Institute, Bethesda, MD, USA.
Paul Pinsky, Division of Cancer Prevention, National Cancer Institute, Bethesda, MD, USA.
Hormuzd A Katki, Division of Cancer Epidemiology and Genetics, Biostatistics Branch, National Cancer Institute, Bethesda, MD, USA.
Tony Dickherber, Center for Scientific Strategic Initiatives, National Cancer Institute, Bethesda, MD, USA.
Paul K J Han, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD, USA.
Lyndsay Harris, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Bethesda, MD, USA.
Christos Patriotis, Division of Cancer Prevention, National Cancer Institute, Bethesda, MD, USA.
Sudhir Srivastava, Division of Cancer Prevention, National Cancer Institute, Bethesda, MD, USA.
Carol J Weil, Division of Cancer Prevention, National Cancer Institute, Bethesda, MD, USA.
Philip C Prorok, Division of Cancer Prevention, National Cancer Institute, Bethesda, MD, USA.
Philip E Castle, Division of Cancer Prevention, National Cancer Institute, Bethesda, MD, USA.
Funding
Not applicable.
Notes
Role of the funder: Not applicable
Author disclosures: There are no disclosures to report by any authors related to any information in this manuscript. PEC, a JNCI Associate Editor and coauthor on this manuscript, was not involved in the editorial review or decision to publish this commentary.
Author contributions: Writing—original draft and writing—review and editing: LM, PP, HK, TD, PH, LH, CP, SS, CW, PP, PC.
Acknowledgements: The authors thank the entire MCED Trial Team: Philip E. Castle, PhD, MPH; Kathleen Carroll, PhD, MBA; Tony Dickherber, PhD; Lyndsay Harris, MD; Paul Han, MD; Hormuzd A. Katki, PhD; Elyse LeeVan, MD; Jack Lee, PhD; Gwen Moulton, BA; Lori M. Minasian, MD; Christos Patriotis, PhD; Paul Pinsky, PhD; Michael Pollack, PhD; Philip Prorok, PhD; Kara Smigel, MS; Sudhir Srivastava, PhD, MPH; Sarah Temkin, MD; and Carol Weil, JD. The authors would also like to acknowledge and thank our colleagues at the U.S. Food and Drug Administration, Dan Edelman, PhD; Bowen Cui, PhD; Kristofor Langlais, PhD; and Wendy Rubinstein, MD, PhD, for their engagement at the MCED workshop and MCED activities.
Disclaimer: The views expressed in the manuscript are those of the authors, not of the National Cancer Institute.
Data availability
There are no data associated with this commentary.
References
- 1. Nuzzo PV, Berchuck JE, Korthauer K, et al. Detection of renal cell carcinoma using plasma and urine cell-free DNA methylomes. Nat Med. 2020;26(7):1041-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hinestrosa JP, Kurzrock R, Lewis JM, et al. Early-stage multi-cancer detection using an extracellular vesicle protein-based blood test. Commun Med. (Lond). 2022;2(29). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Song CX, Yin S, Ma L, et al. 5-hydroxymethylcytosine signatures in cell-free DNA provide information about tumor types and stages. Cell Res. 2017;27(10):1231-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liang N, Li B, Jia Z, et al. Ultrasensitive detection of circulating tumour DNA via deep methylation sequencing aided by machine learning. Nat Biomed Eng. 2021;5(6):586-599. [DOI] [PubMed] [Google Scholar]
- 5. Abraham J, Heimberger AB, Marshall J, et al. Machine learning analysis using 77,044 genomic and transcriptomic profiles to accurately predict tumor type. Transl Oncol. 2021;14(3):101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cristiano S, Leal A, Phallen J, et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature. 2019;570(7761):385-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu MC, Oxnard GR, Klein EA, et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol. 2020;31(6):745-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feng M, Ye X, Chen B, et al. Detection of circulating genetically abnormal cells using 4-color fluorescence in situ hybridization for the early detection of lung cancer. J Cancer Res Clin Oncol. 2021;147(8):2397-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang HY, Chen CH, Shi S, et al. Improving multi-tumor biomarker health check-up tests with machine learning algorithms. Cancers (Basel. 2020;12(6):1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lennon AM, Buchanan AH, Kinde I, et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science. 2020;369(6499). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen JD, Li L, Wang Y, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. 2018;359(6378):926-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neal RD, Johnson P, Clarke CA, et al. Cell-free DNA-based multi-cancer early detection test in an asymptomatic screening population (NHS-Galleri): design of a pragmatic, prospective randomised controlled trial. Cancers (Basel). 2022;14(19):4818. doi: 10.3390/cancers14194818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park JJH, Detry MA, Murthy S, et al. How to use and interpret the results of a platform trial: users’ guide to the medical literature. JAMA. 2022;327(1):67-74. [DOI] [PubMed] [Google Scholar]
- 14. Prorok PC, Andriole GL, Bresalier RS, et al. ; for the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial Project Team. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial. Control Clin Trials. 2000;21(suppl 6):273s-309s. [DOI] [PubMed] [Google Scholar]
- 15. Gohagan JK, Prorok PC, Greenwald P, et al. The PLCO cancer screening trial: background, goals, organization, operations, results. Rev Recent Clin Trials. 2015;10(3):173-180. [DOI] [PubMed] [Google Scholar]
- 16. Aberle DR, Berg CD, Black WC, et al. ; for the National Lung Screening Trial Research Team. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gohagan J, Marcus P, Fagerstrom R, et al. ; for the Writing Committee, Lung Screening Study Research Group. Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT scan vs chest radiograph: the lung screening study of the National Cancer Institute. Chest. 2004;126(1):114-121. [DOI] [PubMed] [Google Scholar]
- 18. Menon U, Gentry-Maharaj A, Hallett R, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol. 2009;10(4):327-340. [DOI] [PubMed] [Google Scholar]
- 19. Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387(10022):945-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.All of Us Research Program. National Institutes of Health. https://allofus.nih.gov/. Accessed August 24, 2022.
- 21. Cuzick J, Cafferty FH, Edwards R, et al. Surrogate endpoints for cancer screening trials: general principles and an illustration using the UK flexible sigmoidoscopy screening trial. J Med Screen. 2007;14(4):178-185. [DOI] [PubMed] [Google Scholar]
- 22. Prorok PC, Hankey BF, Bundy BN.. Concepts and problems in the evaluation of screening programs. J Chronic Dis. 1981;34(4):159-171. [DOI] [PubMed] [Google Scholar]
- 23. Etzioni RD, Connor RJ, Porok PC, et al. Design and analysis of cancer screening trials. Stat Methods Med Res. 1995;4(1):3-17. [DOI] [PubMed] [Google Scholar]
- 24. Menon U, Gentry-Maharaj A, Burnell M, et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2021;397(10290):2182-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schoen RE, Pinsky PF, Weissfeld JL, et al. ; for the PLCO Project Team. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366(25):2345-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Church TR, Black WC, Aberle DR, et al. ; for the National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andriole GL, Crawford ED, Grubb RL 3rd, et al. ; for the PLCO Project Team. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104(2):125-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tabár L, Yen AM, Wu WY, et al. Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J. 2015;21(1):13-20. [DOI] [PubMed] [Google Scholar]
- 29. Frisell J, Lidbrink E, Hellström L, et al. Followup after 11 years–update of mortality results in the Stockholm Mammographic Screening Trial. Breast Cancer Res Treat. 1997;45(3):263-270. [DOI] [PubMed] [Google Scholar]
- 30.Digital Tomosynthesis Mammography and Digital Mammography in Screening Patients for Breast Cancer. U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT03233191?term=TMIST&cond=Breast+Cancer&draw=2&rank=2. Accessed August 24, 2022.
- 31. Cancer Stat Facts: Prostate Cancer. National Cancer Institute. https://seer.cancer.gov/statfacts/html/prost.html. Accessed August 24, 2022.
- 32. Cancer Stat Facts: Pancreatic Cancer. National Cancer Institute. https://seer.cancer.gov/statfacts/html/pancreas.html. Accessed August 24, 2022.
- 33. Pinsky PF, Church TR, Izmirlian G, et al. The National Lung Screening Trial: results stratified by demographics, smoking history, and lung cancer histology. Cancer. 2013;119(22):3976-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brewer LC, Fortuna KL, Jones C, et al. Back to the future: achieving health equity through health informatics and digital health. JMIR Mhealth Uhealth. 2020;8(1):e14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yelton B, Brandt HM, Adams SA, et al. “Talk about cancer and build healthy communities”: how visuals are starting the conversation about breast cancer within African-American communities. Int Q Community Health Educ. 2021;41(3):267-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pinsky PF, Yu K, Black A, et al. Active follow-up versus passive linkage with cancer registries for case ascertainment in a cohort. Cancer Epidemiol. 2016;45:26-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Virtual Pooled Registry, About VPR-CLS. North American Association of Cancer Registries. https://www.naaccr.org/about-vpr-cls/. Accessed April 19, 2022.
- 38.Homeland Security Department, Agriculture Department, Energy Department, National Aeronautics and Space Administration, Commerce Department, Social Security Administration, Agency for International Development, Housing and Urban Development Department, Labor Department, Defense Department, Education Department, Veterans Affairs Department, Environmental Protection Agency, Health and Human Services Department, National Science Foundation, and Transportation Department. Federal Policy for the Protection of Human Subjects. January 19, 2017. https://www.federalregister.gov/documents/2017/01/19/2017-01058/federal-policy-for-the-protection-of-human-subjects. Accessed April 19, 2022.
- 39. Cross R. Will public trust in science survive the pandemic? Chem Eng News. 2021;99:3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no data associated with this commentary.