Highlights
-
•
Improvement of therapeutic ratio by novel unconventional radiotherapy approaches.
-
•
Immunomodulation using high-dose spatially fractionated radiotherapy.
-
•
Boosting radiation anti-tumor effects by adding an immune-mediated cell killing.
Keywords: Novel Unconventional Radiotherapy, Bystander effect, Abscopal effect, Immunomodulation, Spatially fractionated radiotherapy, GRID, LATTICE, PATHY, PULSAR, FLASH, Low doses, High doses
1. Introduction
Recently, remarkable technological, physical and biological developments in the field of radiation oncology have resulted in improved treatment effectiveness and reduced complication rates thanks to the high-precision in tumor identification, localization and dose delivery. These advances unfortunately do not apply to all clinical situations, not always necessarily translating into improved local control and survival. A significant percentage of cancer patients, for example, including those affected by unresectable bulky tumors still remain unsuitable for conventional radio-chemotherapy. These patients are predestined to palliative or best supportive care and from that the need to improve treatment outcomes for this patient population arises. Fortunately, multiple studies are now ongoing that employ alternative delivery methods of radiation in order to address the limitations of conventional radiotherapy, especially when complex clinical scenarios are involved. In order to present and to discuss the currently available data in regard to novel, unconventional radiotherapy techniques, the MedAustron Center for Ion Therapy hosted the new ESTRO (European Society for Therapeutic Radiation Oncology) focus group, organizing an online seminar that focused on emerging spatially fractionated radiotherapy (SFRT) approaches with a potential to increase the radiation therapeutic ratio, encompassing the three radiation oncology disciplines: clinical radiation oncology, radiobiology and radiation medical physics. Innovation in radiation oncology is facing two main line of resistance and skepticism, the first being intrinsic and driven by a high degree of conservatism in the field and the second being extrinsic and driven by lack of strong supporting industries unlike what is happening in the field of medical oncology with the input of pharmaceutical industry.
This event was held on October 6th, 2022. The seminar program was multidisciplinary by essence including clinical radiation oncology, radiobiology and radiation medical physics, and can be found at the following link: https://www.medaustron.at/wp-content/uploads/2022/08/20221006_Seminar_Unconventional-Radiotherapy-Techniques_v1.0.pdf.
The whole event has been video-registered and made free-available at the following link for all interested scientists: https://youtube.com/playlist?list = PL5IOhHd3LZtmmS4D7Zm4140ZRWnBGUMeS.
Here we discussed current status and future prospective of innovative, unconventional radiotherapy techniques.
2. Clinical use of novel unconventional radiotherapy approaches
2.1. GRID radiotherapy
Spatial fractionation was introduced early to overcome the problem of superficial energy deposition of cathode-ray based radiation treatment. SFRT challenges some of the classical dogmas in conventional radiotherapy by using a highly spatial dose modulation [1], [2]. In order to provide greater depth dose to deep-seated tumors in the pelvis, the open field was divided into smaller subsections of open and closed fields by treating through a “sieve” or a “grid” made of lead and rubber placed on the patient's skin. This allowed for much higher skin dose tolerance by sparing some areas, and also much higher dose delivery to deeper tumors. This spatially separated the delivered dose into regions of high dose or ‘peaks’ and regions of low dose or ‘valleys’ [1], [2], [3]. SFRT allows for the delivery of high radiation doses only in some areas within tumors, especially bulky tumors, avoiding the induction of detrimental toxicities to surrounding tissues. A significant increase in normal tissue dose tolerances was observed both in early clinical trials and in small animal experiments, to a significant increase in normal tissue dose tolerances [1], [2], [3]. Tumor control effectiveness is maintained or even enhanced in some configurations as compared with conventional radiotherapy [1], [2], [3]. With the invention of megavoltage energy linear accelerators a few decades ago, the concept of spatial fractionation was largely abandoned.
In the modern era, a metal block added to the linear accelerator was re-introduced in order to treat difficult bulky tumors. Spatially fractionated GRID radiation therapy debulks locally advanced tumors with an initial priming dose of 15–20 Gy in a single fraction with heterogeneous dosimetry similar to an interstitial catheter implant. Clinically, the combination of high GRID dose and the spatial delivery suggests a different radiobiology as compared to conventional 1.8–2 Gy per fraction [4].
SFRT can promote immunological upregulation to help with cell kill (cytokine as well as cell-mediated) as well as a local bystander and distant abscopal effects to help shrink very large masses [5]. For palliative treatment, a 78% overall response, 20% complete response, and 73% mass effect response rate can be seen [6]. The GRID has been used in the definitive treatment of head and neck cancers and sarcoma. For head and neck, GRID can increase the overall response rate by 10% compared to the standard treatment [7].
The original “spatial” concept of GRID now includes LATTICE and microbeam arrangements to deliver similar heterogeneous dose in newer configurations. Based on the available data, more than 400 patients have been treated with GRID radiotherapy [1], however the actual number may reach up to 1000 patients taking into account the unpublished data.
2.2. LATTICE radiotherapy
Built upon the clinical experience of GRID SFRT, and inspired by the biological insights of bystander/abscopal effects, the technique of LATTICE radiation therapy (LRT) was proposed to deliver SFRT to deep-seated tumors using 3D configurations in 2010 [8] and in the same year, first clinical application of LRT was reported [9].
Employing modern radiation delivery systems with intensity/arc-modulated focused photon beams, or charged particle beams, LRT aims to deliver an array of high dose islands (vertices) within the gross target volume (GTV), creating peak-valley-like, and highly heterogeneous dose distribution. 3D LRT can effectively minimize dose outside of the GTV, thus achieving an even higher degree of toxicity control. More than 150 patients were treated with LRT for various indications in the first 10 years since its technical inception. The early clinical experience demonstrated the feasibility and safety of LRT, and led to a series of preliminary technical guidelines [10]. Based on the published literature and private communication, the clinical implementation of LRT has increased rapidly in the last 4 years, with more than 25 RT centers worldwide currently using LRT for their patients. Technically, LRT can be readily implemented in any facility that has commissioned a SRS/SBRT program; it is similar to delivering a SRS/SBRT to multiple tumors simultaneously in a confined area.
The clinical objective of a LRT varies based on clinical indication. Most of LRT cases have been intended for dose boosting, or tumor radiation-debulking. Using LRT to mediate anti-tumor bystander/abscopal effects has been under active investigation [11] and attempted clinically [12]. However, it is to be acknowledged that the biological mechanisms of SFRT in general, beyond classically understood DNA- damage pathways involving double strand breaks (DSB), have yet to be further investigated. Concerted efforts are needed to more systemically explore the biological foundation and clinical efficacies of LATTICE SFRT.
2.3. Personalized ultrafractionated stereotactic adaptive radiation therapy (PULSAR) radiotherapy
Sophisticated new radiotherapy treatment platforms have been commissioned to carry out specialized imaging, quantify changes in the imaging, and respond with a modified (“adapted”) plan – all while the patient lies on the table for treatment. The key opportunity to exploit with this adaptive approach is to personalize the therapy for an individual patient. Such would be a dramatic change from routine practice of a “one-size-fits-all” class solution for treating patients with radiotherapy.
Conventional radiotherapy giving daily doses of 2–3 Gy exploits differences in repair between targeted tumor and normal tissue. Anatomically, though, changes induced by this therapy are most often subtle, reflecting the small daily doses that try to avoid severe changes. But subtle changes rarely result in meaningful opportunity to adapt. Likewise, even the more aggressive dose intensity associated with stereotactic ablative radiotherapy rarely results in significant changes in anatomical form, especially if given daily or even every other day as is done in most routine practice. In general, therapies that are delivered uninterrupted over a short period of time, particularly if they react only to anatomical form without regard to biological function, may fall short of achieving an adaptive, personalized radiotherapy.
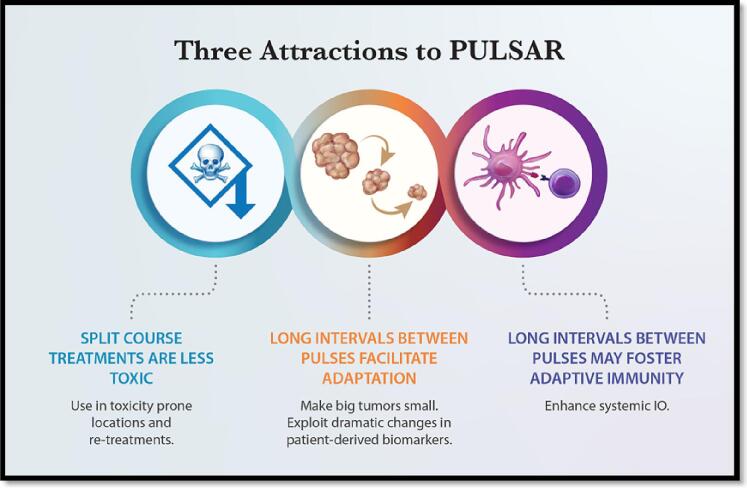
To address this dilemma, PULSAR was developed to insert an intentional pause into a course of radiotherapy [13]. This pause could be counterproductive when giving conventional radiotherapy, but daily doses in the range of stereotactic ablative therapy (SAbR) have been shown to provide durable control without accelerated proliferation in randomized trials [14], [15]. Spacing SAbR doses (called pulses) by a week or months allows more dramatic biological change to ensue. This biology is measured with various assays and correlated to outcome, eventually allowing PULSAR to be steered in a personalized fashion for each individual patient. With the break between pulses, PULSAR is less toxic than routine SAbR (Fig. 1). Preclinical evidence shows that among the biological changes associated with PULSAR is a complimentary relationship with common immunotherapy agents like checkpoint inhibitors, again allowing an enhanced, personalized opportunity to improve adaptive radiotherapy [16].
Fig. 1.
Three important advantages of PULSAR (Personalized, Ultra-fractionated, Stereotactic, Adaptive Radiotherapy).
2.4. Single-dose radiotherapy (SDRT)
Ultra-high dose single fraction radiotherapy has been shown to operate above the threshold of 12 Gy, a dual target model of tumor tissue injury consisting of DNA DSBs and microvascular dysfunction [17]. A transient endothelial acid sphingomyelinase (ASMase)/ceramide-mediated ischemia/reperfusion phenomenon impairs DSB homology-directed repair in tumor cells rendering synthetic tumor cell lethality [17]. Single dose radiotherapy (SDRT) biology, thus, fundamentally differs from the classical tumor cell autonomous single target model of fractionated radiotherapy.
Recent phase II-III clinical trials reported that 24 Gy SDRT renders approximately 90% actuarial 5-year local relapse-free survival (LRFS) in a wide range of human oligometastatic tumors, regardless of tumor type, size or oligometastatic target organ [18], [19], [20]. SDRT delivery may not be feasible in ∼30% of oligometastatic lesions due to interference by dose/volume constraints of a serial organ at risk (OAR) [19]. However, a bystander radiosensitization of a minor sub-volume of the target exposed to a ≥25% dose-sculpted reduction to meet a serial OAR dose/volume constraint has been shown to produce similar tumor control rates to full PTV coverage, provided that ≥60% of the PTV is exposed to ≥24 Gy to trigger the ischemia/reperfusion vascular phenomena equilibrating SDRT lethal signaling throughout the tumor interstitial space [21]. Future studies will also need to address changes in the tumor immune microenvironment (TIME) following ultra-high dose SDRT as a potential contributor to favorable tumor local control rates and improvement in distant metastasis-free survival in oligometastatic patients receiving SDRT metastasis-directed radioablation to all detectable disease deposits [20].
Recently, the results of a proof-of-concept phase II randomized study determining the feasibility and safety of 24 Gy SDRT and its iso-effectiveness to extreme hypofractionated SBRT (5x9 Gy) in the treatment of intermediate risk localized prostate cancer has been reported [22]. At a median of 48 months there were no significant differences in prostate-specific antigen (PSA) relapse-free survival and in late toxicities between the two arms, underscoring the importance of larger studies to establish the indications of SDRT as a potential new standard of care in localized prostate cancer [22].
2.5. Low-dose radiotherapy (LDRT)
Low-dose hyper-radiosensitivity (HRS), i.e. an increased cell killing (per unit dose) than predicted by conventional radiobiological modelling, has been observed in several mammalian cell lines after acute radiation exposure to doses <0.5 Gy. Conversely, a relative increased radioresistance (IRR) has been seen in a dose range of 0.5–1 Gy. The main mechanism regulating the HRS/IRR transition seems to involve checkpoint events in the G2 phase of the cell cycle, which prevent cells damaged by radiation from entering mitosis with unrepaired DNA lesions, so that genome integrity is preserved. Therefore, the low-dose HRS phenomenon may be due to the apoptotic death of a subset of cells that have failed to undergo this early G2-phase checkpoint arrest [23]. The activation of the ATM kinase seems to play a key role in promoting DNA repair and triggering the induced radiation resistance. It seems that in most cases the dose to activate the ATM pathways is higher in tumor than in normal cells, thus creating a therapeutic window to overcome radio-resistance of tumor cells while sparing normal tissues from toxicity.
To exploit this phenomenon in the clinic, low-dose fractionated radiotherapy (LD-FRT), i.e. the use of multiple small dose per fraction (<1.0 Gy), has been proposed as a chemo-sensitizer by synergizing with drugs that enrich the G2-phase cell fraction. Some phase I-II studies and retrospective studies including patients with either advanced or recurrent tumors, reported encouraging therapeutic outcomes and similar tolerability of combined LD-FRT and chemotherapy, as compared with chemotherapy alone [24]. However, the only one randomized phase III trial published so far showed no benefit from adding LD-FRT to induction chemotherapy in patients with locally advanced nasopharyngeal cancer [25].
Similarly, pulsed low dose-rate radiotherapy (PLDR) has been developed to exploit HRS by delivering the daily radiation dose of 2 Gy in 10 sub-fractions (pulses), each of 0.2 Gy, with a 3 min time interval. Several retrospective studies have reported favourable outcomes and toxicity profiles, suggesting that PLDR could be so effective as to overcome radioresistance of recurrent tumors, and also have increased capacity of normal tissue sparing [26], [27]. Recently a single-arm, prospective study showed promising effectiveness and no impairment of neurocognitive function and quality of life by using PLDR in newly diagnosed glioblastoma [28]. Nevertheless, the PLDR technique has never been directly compared with standard reirradiation techniques yet.
In summary, although several experimental results have laid a strong radiobiological foundation for the clinical use of low dose radiotherapy, only a few prospective clinical trials have been published so far. Phase III clinical trials are warranted to evaluate the benefit of combined chemo-LDRT or PLDR over conventional treatments for different types of cancer.
2.6. PATHY radiotherapy
Radiation-induced immunomodulatory effects, such as bystander or abscopal effects, are very rare when conventional radiotherapy is concerned. The reason for this is because traditional radiotherapy was designed primarily to be tumoricidal and not to be immunostimulative. Whole-tumor irradiation, especially including the elective treatment volumes, usually induces lymphopenia due to killing of circulating immune cells and those located in the tumor microenvironment, resulting in immunosuppression and weak immunogenic potential [29]. This radiation-induced immunosuppression might be overcome and shifted to immunostimulation by changing the form of radiotherapy, by, for example, delivering an ablative radiation dose to the partial tumor volume sparing the peritumoral immune-environment. This novel approach can improve the therapeutic ratio adding a component of immune-mediated killing to the radiation-directed tumor cell killing, [29]. The preclinical findings that preceded the development of this new method, showed that the high-single-dose irradiation of hypoxic tumor cells generated a more intense bystander effect than the normoxic cells, suggesting their important role in potentiation of non-targeted radiation effects [30]. This led to the development of a novel concept for PArtial Tumor irradiation targeting HYpoxic segment (PATHY) for induction of the immune-mediated bystander and abscopal effects. Encouraging clinical outcomes in terms of local bulky tumor control, neoadjuvant potential, symptom relief and immunomodulatory effects have been seen [29], [31]. The immunohistochemical and gene-expression analyses of surgically removed abscopal-tumor sites, suggested that delivery of an ablative radiation dose to the partial (hypoxic) tumor volume, delivered at precise time considering the homeostatic fluctuation of the immune response, with sparing the peritumoral immune-environment, would significantly enhance the immune-mediated anti-tumor radiation effects. This unique unconventional radiotherapy technique due to its higher immunogenic potential may improve the prognosis of patients affected by highly complex malignancies, providing additional opportunities for future research in terms of combining novel immuno-modulating agents with more modern radiotherapy approaches.
3. Radiobiological mechanisms of the novel unconventional radiotherapy approaches
3.1. Radiation-induced bystander effect
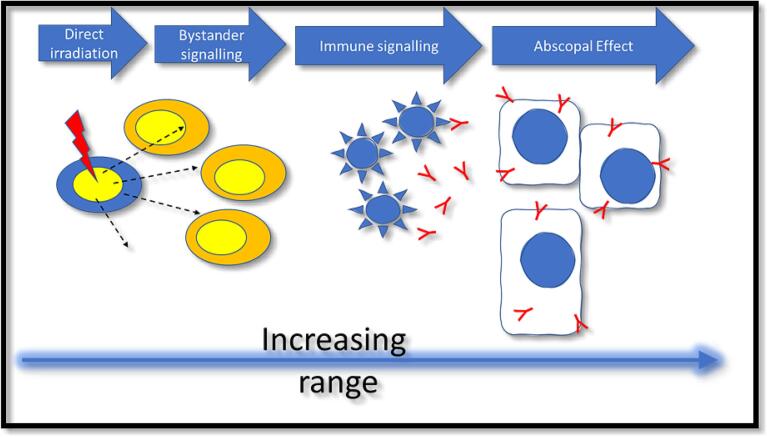
For advanced SFRT radiotherapies, including GRID, LATTICE and Microbeam approaches, our knowledge of the biological drivers of these responses is still limited but it is likely that bystander/abscopal effects play a role. A similar scenario exists for high dose-rate FLASH exposures [32]. The radiation-induced bystander effect is defined as the response of cells to the consequences of their neighbors being irradiated and has been well characterized in vitro [33]. There is a close interrelationship between bystander responses and the abscopal responses, which occur in tissues remote from the irradiation field and this is driven by the immune response (Fig. 2) [34]. A simple approach to simulate a SFRT exposure is to mimic the steep dose-gradients that are inherent in all spatially modulated beams by using a partially shielded exposure of a cell culture to evaluate both direct and bystander responses [35], [36]. These approaches clearly show that signaling occurs away from the irradiated area, leading to increased cell killing and the effect of this cannot be predicted from the delivered dose.
Fig. 2.
Schematic of the interrelationship of bystander and abscopal effects.
One recent work has studied the consequences of different bystander ranges on overall clinical response by simulating these processes and their impact on the effective uniform dose (EUD) delivered to a tumor. For lung and prostate tumors, an increased EUD is observed when signaling plays a role leading to enhanced cell killing but with minimal impact on normal tissue response [37]. The challenge now is to validate this pre-clinically and clinically. Preclinical delivery of SFRT beams is now possible with small animal irradiation platforms, as image-guided beams can be delivered using these systems into tumors and normal tissues [38].
For clinical translation, an elegant example of this is the PATHY project [39], [40]. Here, partial irradiation of bulky tumors has been advocated to target radioresistant regions of a tumor whilst at the same time allowing an immune response to be activated by defining a Bystander Tumor Volume (BTV) within the GTV leading to abscopal effects.
3.2. Radiation-induced abscopal effect
Conventionally fractionated radiotherapy (i.e., 2–3 Gy) mostly induces cell death by DNA DSBs that mainly leads to cell apoptosis. When doses per fraction over 8 Gy are used, other non-targeted effects of radiotherapy appear. These effects are mainly related to the vascular system (endothelial apoptosis) leading to tumor cell necrosis and increased expression of tumor antigens and “eat-me” signals (HGMB1, Calreticulin, ATP), increasing the immune-related cell death [41].
High dose per fraction treatments (SBRT/SRS (stereotactic radiosurgery)) seem to also induce a change in the TIME, reducing the immunosuppressive myeloid-derived suppressor cells (MDSC) and regulatory T cells (Tregs), and increasing polarization of the tumor-associated macrophages (TAMs) to the M1 anti-tumor phenotype [42].
This radiation-induced activation of the immune system is the rationale for the sparsely clinically observed abscopal responses, distant tumor responses observed out of the radiation fields [43]. Unfortunately, tumors inhibit the radiation-induced immune responses through the expression of PD-L1 and other factors that suppress the antitumor effect of T cells, and often, the abscopal response do not appear clinically [44].
Immune Check-point Inhibitors (ICI) are molecules able to block the PD-1/PD-L1 axis increasing T-lymphocyte activity against the tumor. Tumor responses varying from 20 to 50% are related to the development of ICI resistance, mainly regarding PD-L1 expression, T cell infiltration and mutation profile. SBRT/SRS could be an alternative to re-invigorate the immune system in combination with ICI treatment, without the clinical limitations of systemic treatments and with sound basis of its effect on tumor-suppressive microenvironment [45], [46]. Abscopal responses are more common when high SBRT/SRS BED-doses are used, the patients are already in ICI treatment, and radiotherapy is administered to nodal/visceral metastases. Those patients obtaining an abscopal response showed increased survival compared to those not achieving abscopal response [47].
As a conclusion SBRT/SRS is able to reinvigorate the exhausted immune system after ICI tumor resistance, with very limited toxicity most likely through generation of abscopal response.
3.3. Immunomodulation by low-doses
Although radiotherapy has been combined with immunotherapy, the dose and fractionation of radiation that is optimal for such combination therapy is not settled. A consensus review on this subject was recently published [48]. Briefly, radiotherapy can be divided into three immunomodulatory applications, based upon dose and fractionation. Ablative high-dose radiotherapy fractionation, usually delivered by SABR or SRS, induces immunogenic cell death and may be used for in situ vaccination protocols. Sub-ablative fractionation schedules, such as the commonly used 8 Gy × 3, activates the cytosolic DNA-induced STING-dependent type I interferon response [49] and increases the expression of cell surface death receptors, thereby sensitizing irradiated tumor cells to immune cytolysis [50]. Finally, TIME-modulating LDRT (0.5–5 Gy) can be used to reprogram macrophages to the inflammatory M1 phenotype and increase the infiltration of cytotoxic T lymphocytes (CTLs), while reducing Tregs [51]. In contrast to high-dose immunogenic ablative radiotherapy fractionation that induces primarily a CD8 + CTL-mediated anti-tumoral immunity, LDRT was shown to elicit predominantly a CD4(+) Th1 cell-mediated immunity with features of exhausted effector cytotoxic cells, with a subset expressing NKG2D and exhibiting proliferative capacity [52]. In addition, there was activation of dendritic cells expressing the NKG2D ligand RAE1 [52]. Since distant unirradiated tumors can exert a tumor-specific, Treg-dependent suppressive effect on the local and systemic anti-tumoral immune response to in situ vaccination [53], LDRT, using either external beam radiotherapy or theragnostic radio-immunotherapy, can be used to promote inflammation and reprogram the TIME for sensitizing immunologically “cold” resistant tumors to immunotherapy [54], [55]. Traditionally, fractionated radiotherapy has been designed using equal fraction sizes of radiotherapy because of DNA repair and toxicity considerations. This convention was challenged by testing whether low dose fractionation (0.5 Gy × 4) given before or after a high single dose (22 Gy) radiotherapy can increase the infiltration of immune effector cells and improve the tumoricidal effects of high dose ablative radiotherapy fractionation [56]. In a model of metastatic breast cancer, we demonstrated that a novel hybrid radiation dose fractionation regimen, consisting of a single high-dose radiotherapy, followed by post-ablation modulation with four daily low-dose fractions (22 Gy + 0.5 Gy × 4) can reprogram the TIME by diminishing Treg-mediated immune suppression while increasing infiltration of CD8+ effector CTLs, thereby increasing the efficacy of both primary and metastatic tumor control [56]. Similar results have been obtained where high dose radiotherapy to one site, combined with LDRT to multiple sites have shown benefits in inducing “radscopal” tumor response in the LDRT-treated tumors [57], [58]. In summary, LDRT can reprogram the TIME to an inflammatory environment that supports in situ vaccination of hypofractionated radiotherapy.
3.4. Combined particle therapy and immunotherapy
Immunotherapy is considered the most important breakthrough in cancer therapy of the 21st century. However, despite the successes of ICIs monotherapy for malignant melanoma [59], immunotherapy is generally delivered in combination with other cancer treatments such as targeted therapy, chemotherapy, and radiotherapy [60]. The combination of radiotherapy and ICIs is particularly attractive because ionizing radiation is able to elicit an immune response in the irradiated organism thus making it an ideal partner for immunotherapy drugs [61], [62]. However, while some clinical trials demonstrated increased patients’ survival when ICIs are delivered after chemoradiotherapy [63], [64], other trials gave disappointing outcomes [65], [66].
A promising approach to improve the clinical results is to replace conventional X-ray therapy with particle therapy [67], [68]. This is due to both physical and biological characteristics of radiotherapy with accelerated charged particles. Thanks to the Bragg peak, charged particles recue the integral dose to the patient and therefore spare more immune cells compared to conventional radiotherapy, resulting in reduced lymphopenia [69], [70]. On the other hand, the peculiar biological effects of high-linear energy transfer (LET) heavy ions can lead to antigenicity and adjuvanticity, thus resulting in a synergistic effect when combined to immune-stimulating drugs [71].
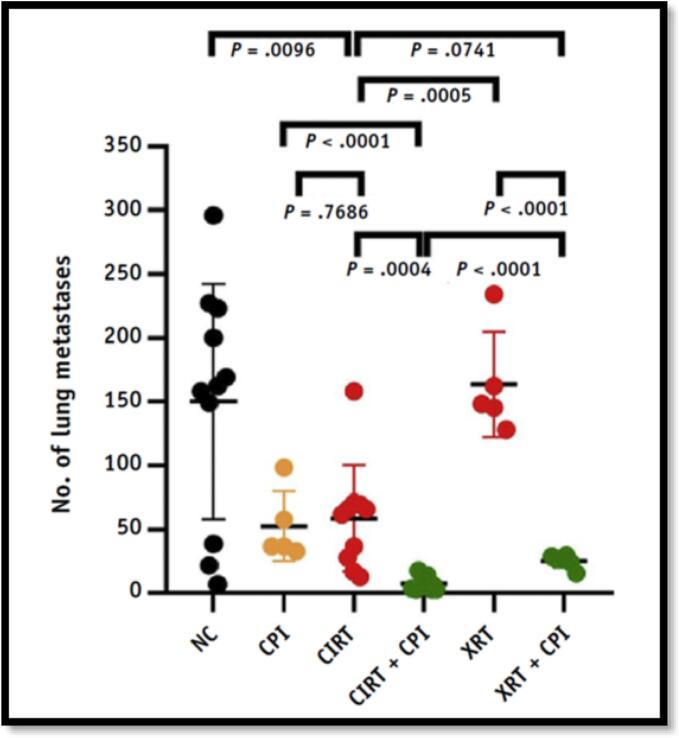
Pre-clinical studies conducted on the combination of immunotherapy and charged particle irradiation have shown promising results so far. An increased second tumor rejection was observed following injection of pre-treated dendritic cells (DCs) and resulted in increased specific lysis activity of CD8+ T cells [72], [73]. In an osteosarcoma mouse model, reduced lung metastases were measured after combination of radiation with ICIs (anti-CTLA4 and anti-PD-1) [74], and the effect was stronger when using 12C-ions than X-rays (75) (Fig. 3). A combination of 12C-ions and anti-PD-1 resulted in more tumor infiltration of CD8+ T cells than any other single or combined treatment investigated and significantly improved the survival of mice in two different melanoma models when iso-effective doses were compared [76]. In an abscopal tumor mouse model, unirradiated tumors showed higher frequencies of naïve T cells activated when 12C-ions were combined with anti-CTLA4 [77].
Fig. 3.
Potential of metastatic suppression of carbon-ion radiotherapy (CIRT). As compared to X-ray radiotherapy (XRT), CIRT alone already results in a significant reduction of the number of superficial lung metastases in an abscopal osteosarcoma mouse model. The effect is enhanced when combined with checkpoint inhibitors (CPI) Reproduction from Helm et al. [75].
In conclusion, combination of radiotherapy and immunotherapy is likely to be widely exploited in the management of advanced solid cancers. Pre-clinical results support the rationale that particle therapy can be the ideal match of immunotherapy.
4. Development of other promising unconventional radiation delivery approaches
4.1. Flash
FLASH has become a fascinating and novel research field attracting the attention of the scientific community and the radiotherapy industry. It is based on irradiation at ultra-high dose rates and has been shown to differentially modify normal tissue and tumor response to irradiation. With more than 30 articles published in preclinical models, the interest of FLASH is convincing (Fig. 4) and show preservation of normal tissue and maintenance anti-tumor efficacy in tumor-free animals, tumors induced subcutaneously, orthotopically, in genetically engineered mouse models (GEMMs), in immunocompetent and immunocompromised animals [78].
Fig. 4.
Preclinical and clinical investigations of FLASH updated from Limoli and Vozenin, Ann Rev Cancer Bio (in press).
This “in vivo” differential effect has been named “the FLASH effect“ and has been reported to occur with electron, photon, and proton beams using single dose, hypofractionated regimen and very recently standard fractionation (Limoli and Vozenin et al. in press). However, today only a few beams have been validated as inducing the FLASH effect and most of the FLASH studies have been performed with the Kinetron at Institut Curie and the Oriatron/eRT6 at the CHUV [78]. These electron irradiators have low energy (4–6 MeV); consequently, their applicability is limited to small animals and superficial tumors (dose depth profile around 1.5 cm). Several groups have modified clinical LINACs into UHDR FLASH-irradiators (17–20 MeV) [79]; likewise, Varian Inc is now offering FLASH-enabled electron LINACs modified from their commercial CLINACs and TRUEBEAM machines. Although the FLASH effect has been primarily observed with electron beams, similar findings have been qualitatively validated in preclinical experiments with photon [80], [81] and proton beams operating at a mean dose rate above 40 Gy/s [82]. The FLASH effect has also been reported recently with the use of proton-FLASH-PBS and Bragg peak proton-FLASH beams. Remarkably, although the structure of the electron, photon, and proton FLASH beams is different, they have all been shown to produce the FLASH effect.
In contrast and although performed at a high-dose rate, some studies have reported the absence of the FLASH effect using synchrotron photon [83], proton [84], and electron [85], [86] beams. These studies might have been carried out with different beam parameters or beyond those required to elicit a significant FLASH effect and emphasize the fact that critical physical parameters required to trigger this biological effect are still not fully understood. In addition, recently trials conducted in domestic and large animals showed that a high single dose could cause late toxicity even when FLASH is used [87]. These studies suggest that the parameters and conditions required to robustly produce the FLASH effect are not sufficiently understood and characterized. Despite this gap of knowledge, FLASH is moving in the clinic. Studies with electron [88] including the first side-by-side comparison of FLASH vs conventional dose rate [89], and proton-FLASH [90] have been performed and show no difference in the toxicity profile triggered by FLASH and conventional dose rate. This type of studies might cast the doubt on the benefit of FLASH and might enhance skepticism whereas more work is needed to optimize FLASH from technological and biological angles.
4.2. Clinical implementation of LET-painting in proton therapy treatment planning
Treatment planning of Intensity modulated proton therapy (IMPT) is performed using a constant relative biological effectiveness (RBE) of 1.1, even if RBE for normal tissue depends on endpoint, α/β ratio, LET and fractionation. Differences in LET definitions in biological experiments and in Monte Carlo (MC) calculations have been observed and accordingly steps towards harmonization of LET-calculations have been taken [91], [92].
Some centers have the capability to calculate LET and RBE in the treatment planning system (TPS), whereas many centers use MC-calculations of LET outside of the clinical TPS, as currently no commercial proton TPS is able to perform LET/RBE-optimization for use in clinical treatment plans. RaySearch Laboratories provide a research proton-TPS, where it is possible to optimize using different options for decreasing LET/RBE in OAR.
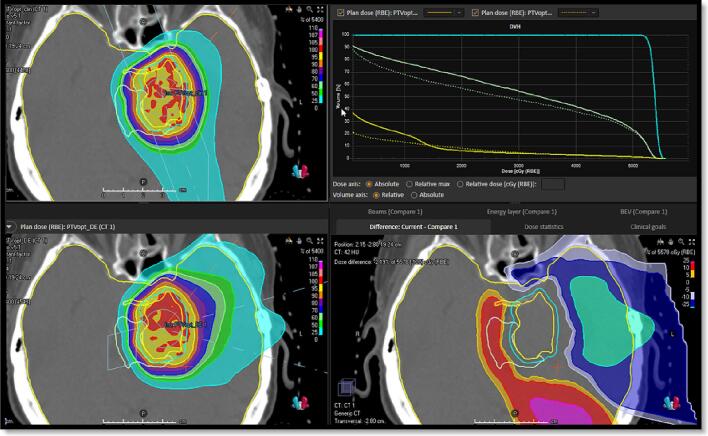
The concept of LET-painting utilizes delivery of high LET radiation only to the radioresistant compartments within the tumor volume so that normal tissues are minimally exposed to this high LET radiation. This results in significant reduction in the radiation-induced toxicity as the normal cells retain their ability to repair. Based on this, we developed a study that focused on decreasing the dose component delivered with protons having an LET above a certain threshold. Unpublished findings from this study showed that the decreased high-LET dose component was associated with a decrease in RBE to the brainstem in treatment of meningioma patients and that a reduction in normal brain doses of 22% was obtained, omitting the limitations of beams with distal edge in the brainstem (Table 1, Fig. 5). To make optimization criteria solely based on LET is questionable, as the combination of intermediate LET values and high dose results in increased RBE. Yet, LET-painting has the potential to reduce the normal tissue complication probability (NTCP) for brainstem necrosis and optical damage [93].
Table 1.
The study included 5 meningioma patients treated to 54 Gy RBE. D2cc of the brainstem for the plans with clinical beam configuration (Clin) and optimized with penalty on the high LET component to the brainstem (Clin opt). Column 3 and 4 show the same values for the conformal plan (DE) and the corresponding optimized plan using an objective on the high LET component of the dose (DE_opt).
| D2cc in GyRBE | Clin | Clin_opt | DE | DE_opt |
|---|---|---|---|---|
| Pt1 DhighLET | 13.1 | 9.0 | 13.7 | 9.4 |
| Pt1 VarRBE | 56.3 | 54.9 | 56.2 | 55.1 |
| Pt2 DhighLET | 11.5 | 8.5 | 17.0 | 9.6 |
| Pt2 VarRBE | 55.6 | 54.9 | 56.9 | 54.9 |
| Pt3 DhighLET | 18.8 | 9.8 | 26.5 | 14.6 |
| Pt3 VarRBE | 59.7 | 57.3 | 61.2 | 56.7 |
| Pt4 DhighLET | 11.6 | 8.7 | 20.7 | 11.3 |
| Pt4 VarRBE | 51.3 | 48.6 | 50.0 | 38.6 |
| Pt5 DhighLET | 16.1 | 9.7 | 18.8 | 10.2 |
| Pt5 VarRBE | 58.8 | 57.5 | 59.4 | 56.9 |
| Average DhighLET | 14.2 | 9.1 | 19.3 | 11.0 |
| Average VarRBE | 56.3 | 54.6 | 56.8 | 52.4 |
Fig. 5.
Upper left clinical beam configuration avoiding more than one field having distal edge in the brainstem. Lower left the conformal plan. Upper right DVH showing the sparing of Brain-CTV (Yellow), brainstem (Green) with same coverage of CTV (Cyan). Dotted line the conformal plan full line the clinical plan. Lower right panel shows the dose difference map with red colors showing the spared area of the brain. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To make LET-painting available for clinical use, certain issues have to be considered, such as the availability of new optimization capabilities in the TPS in trade off to available resources for implementing a new TPS in the clinic. Methods for including LET/RBE in evaluation of uncertainties have to be developed. In addition, quality assurance (QA) of treatment plans has to be performed not only for dose, but also for LET. Measuring LET is not trivial, and is typically only possible by indirect means. Therefore, there is also a need to compare different methods for experimentally obtaining the average LET in clinical relevant settings that will require discussion amongst various investigators/medical physicists with the primary aim of preparing for commissioning of LET-optimization and patient specific QA of LET-optimized plans.
4.3. Microbeam and minibeam radiation therapy
Among the different forms of SFRT, microbeam (MRT) [94] and minibeam radiation therapy (MBRT) [95] use very narrow (submillimetric) beams. The irradiation in MRT and MBRT is performed with arrays of parallel 50 µm and 0.5 to 1.0 mm wide beams, respectively. This enables the alliance between the advantages of SFRT with the exploitation of the dose volume effects [96]. The beam spacing ranges from 200 to 400 µm in MRT and from 1 to 4 mm in MBRT. MRT and MBRT are still at the preclinical stage but they hold great potential to achieve radical treatments of radioresistant tumors, such as gliomas [97], [98], [99], [100], [101].
MRT and MBRT originated at large 3rd generation synchrotrons [93], facilities offering ideal beam features for these techniques: negligible divergence, kilovoltage X-rays beams and enormous dose rates. MRT and MBRT have shown a remarkable normal tissue sparing at very high peak doses (up to 100 Gy in MBRT and 600 Gy in MRT) [95], [102], [103], [104], [105], [106], [107], [108], and tumor control in aggressive tumor models [109], [110], [111], [112], such as gliomas. It should be highlighted that each technique should be used and compared in its respective therapeutic range (20–30 Gy integral dose in MBRT and 100 Gy in MRT).
The advancement of MRT has been slowed down due to the need for very high dose rates and kilovoltage X-rays beams to avoid blurring of the characteristic patterns of peak-and-valleys [93]. This has confined the exploration of the technique to a few large synchrotron facilities. Novel and recent concepts [113], [114] offer promise of enabling the MRT exploration outside synchrotrons sources, although MRT would still be limited to low energy X-rays beams [115]. The first MRT in trial has been recently performed in dogs [116]. The results of the short follow up (3 months) in treated dogs are promising, however, insufficient for solid conclusions yet.
The wider beams employed in MBRT provide several benefits. Firstly, the patterns of peak-and-valleys are not dependent on the cardio synchronous pulsations, and the technique could be implemented into conventional and low-cost equipment [117]. In addition, MBRT is not limited to the use of low energy X-rays beams but it can be implemented with higher energy X-rays [118], protons [119], carbon [120] and some other heavier ions [121]. The excellent results (71 % pathological complete remission without toxicity) of the first de novo brain tumor dog MBRT trial [122] opens the door to the transition towards clinical trials.
The full picture of the biological mechanisms induced by MRT and MBRT is still missing. Main potential players with some experimental evidence reported in the literature include differential vascular effects [123], cell signaling effects (bystander-like effects) [124], [125], abscopal effects [126], inflammation and immunomodulatory effects [127], [128], and stem cell migration [129].
Currently, the relative weight and interrelation of those possible contributors and how these effects are translated when the spatial dose distribution is modified are still unclear.
Further biological experiments are urgently needed to parameterize the relationship between the irradiation parameters (beam width, spacing, PVDR, peak and valley doses) and the radiobiology.
5. Conclusions
Despite all the advances of modern radiation oncology, cancer remain non-curable for a large proportion of patients. In its current, traditional form, conventional radiotherapy is not able to deliver an ablative radiation dose to these tumors without exceeding the dose-constraints of nearby critical structures. In most of these cases, standard radiotherapy may neither improve survival nor offer an improvement in quality of life, usually resulting in treatment failure. Obviously, these situations require different ways of delivering radiation, exploiting different mechanisms of action. Indeed, the emerging, novel, unconventional radiotherapy approaches appear to be effective in these complex tumors. These unconventional approaches might be subdivided into two subgroups, the one used in the clinic by few clinicians lacking strong biological rational and systematic investigations (GRID, LATTICE, PATHY), and another developed from preclinical studies lacking technological robustness and significant clinical applicability (FLASH, MRT). Merging these two approaches in a top-down and bottom-up manner and shared models and expertise might enhance to their translation. These approaches adopt different radiobiological mechanisms, especially when SFRT are concerned, boosting the radiation-mediated tumor cell killing by adding an immune-mediated killing component resulting in improved radiotherapy therapeutic ratio. The prospective trials utilizing multiple SFRT approaches are ongoing and available data suggest their safety and effectiveness associated with high immunomodulatory and neoadjuvant potential (Table 2). Therefore, the possible combination of SFRT with conventional radiotherapy may become a new standard of care treatment for these complex tumors. This will however require high-level evidence to be built. We believe that these approaches deserve more support and systematic investigations, as they might become the future of radiation oncology, at least for some specific patient subpopulations that cannot benefit from conventional radiotherapy.
Table 2.
Ongoing clinical trials utilizing novel unconventional radiotherapy approaches.
| Clinicaltrials.gov identifier | Study | Type | Study start date | Recruitment status | Planned number of participants | RT technique | RT fx | Condition/disease | Primary endpoint |
|---|---|---|---|---|---|---|---|---|---|
| NCT04168320 | SBRT-PATHY | Phase 1 | October 30, 2018 | Recruiting | 30 | PATHY | 10 or 12 Gy × 3 | Unresectable bulky tumors | Bystander and abscopal effect |
| NCT04875871 | PARTICLE-PATHY | Phase 1–2 | May 6, 2021 | Recruiting | 23 | PATHY | 8, 10, 12 or 15 Gy × 3 | Unresectable bulky tumors | Bystander (local) tumor response rate |
| NCT02333110 | GRID Therapy as Palliative Radiation for Patients With Advanced and Symptomatic Tumors | NA | January 7, 2015 | Recruiting | 50 | GRID | 15–20 Gy × 1 | Advanced and Symptomatic Tumors | Symptom relief |
| NCT04549246 | GRID Therapy for Tumors of the Head, Neck, Thorax, Abdomen, Pelvis and Extremities | Observational | June 18, 2020 | Active, not recruiting | 120 | GRID | 20 Gy × 1 | Head, Neck, Thorax, Abdomen, Pelvis and Extremities | Local/radiographic control rate |
| NCT05121545 | ProGRID | Phase 1 | April 6, 2022 | Recruiting | 12 | GRID | 2–18 Gy × 1 | Sarcoma, Melanoma | Feasibility of the Pro-GRID |
| NCT05443971 | Durvalumab and Grid Therapy for the Treatment of Non-small Cell Lung Cancer in Patients Who Progressed During or After Treatment With the PACIFIC Regimen | Phase 2 | January 30, 2023 | Not yet recruiting | 10 | GRID | 20 Gy × 1 | Stage III NSCLC | Safety of GRID + Durvalumab |
| NCT04553471 | Palliative Lattice Stereotactic Body Radiotherapy (SBRT) for Patients With Sarcoma, Thoracic, Abdominal, and Pelvic Cancers | Phase 1 | September 22, 2020 | Active, not recruiting | 70 | LATTICE | 5-fraction Lattice SBRT delivered to 20 Gy with a simultaneous integrated boost (SIB) to 66.7 Gy | Large Sarcoma, Thoracic, Abdominal, and Pelvic Cancers | Rate of local control |
| NCT05524064 | FAST-02 | Phase 1 | September 20, 2022 | Active, not recruiting | 10 | FLASH | 8 Gy × 1 | Bone Metastases in Thorax | Toxicity, Pain Relief |
| NCT04592887 | FAST-01 | Phase 1 | November 3, 2020 | Active, not recruiting | 10 | FLASH | 8 Gy × 1 | Bone Metastases in Thorax | Workflow Feasibility, Toxicity |
| NCT04986696 | IMPulse | Phase 1 | July 1, 2021 | Recruiting | 46 | FLASH | 7 dose levels: 22 Gy; 24 Gy; 26 Gy; 28 Gy, 30 Gy, 32 Gy and 34 Gy, single fraction | Melanoma | Determination of maximum tolerated dose, Toxicity |
| NCT04779489 | CIRTiN-BC | Phase 1 | September 6, 2021 | Recruiting | 27 | PULSAR | 12 Gy × 1 every 12–16 days | Bladder Cancer | Protocol Completion |
| NCT04786093 | Durvalumab and Stereotactic Radiotherapy for Advanced NSCLC | Phase 2 | August 10, 2021 | Recruiting | 52 | PULSAR | 12–15.5 Gy × 1 every 4 weeks | NSCLC | Quality of Life Scores |
| NCT04889066 | Durvalumab (MEDI4736) and Radiosurgery (fSRT vs. PULSAR) for the Treatment of Non-Small Cell Lung Cancer Brain Metastases | Phase 2 | June 1, 2023 | Not yet recruiting | 46 | PULSAR | 8–9 Gy × 1 every 4 weeks | Brain Metastases of NSCLC | Intracranial clinical benefit |
| NCT05021237 | Ultrafractionated Radiation Therapy for Metastatic Cervical Cancer | Phase 2 | February 1, 2022 | Recruiting | 30 | PULSAR | 8–8.5 Gy × 1 every 3–5 weeks | Cervical Cancer | Overall Survival |
| NCT04035642 | PROSINT II | Phase 2 | June 1, 2019 | Recruiting | 200 | Single Dose RT | 24 Gy × 1 | Prostate | Treatment-related adverse events |
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Summary Declaration of Interest Statement
Chandan Guha reported the following COI: Advisor/Consultant with Focused Ultrasound Foundation, Janssen and Siemens.
Slavisa Tubin reported the following COI: pending patent(s)/international patent application PCT/EP2019/052164 published as WO 2019/162050.
Xiaodong Wu is the primary inventor of US Patent No. 8,395,131: Method of 3D Lattice Radiotherapy.
Yolanda Prezado holds a patent on the implementation of MBRT at a small animal irradiator USBR16CNRRAD/sl (USA) and PCT/EP2017/078096 (international).
Robert Timmerman is PI of grants – Varian Medical Systems, Elekta Oncology, and Accuray, Inc.
References
- 1.Billena C., Khan A.J. A Current Review of Spatial Fractionation: Back to the Future? Int J Radiat Oncol Biol Phys. 2019;104(1):177–187. doi: 10.1016/j.ijrobp.2019.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan W., et al. Spatially fractionated radiation therapy: History, present and the future. Clin Transl Radiat Oncol. 2020;20:30–38. doi: 10.1016/j.ctro.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prezado Y. Divide and conquer: spatially fractionated radiation therapy. Expert Rev Mol Med. 2022;24:12. [Google Scholar]
- 4.Moghaddasi L., Reid P., Bezak E., Marcu L.G. Radiobiological and Treatment-Related Aspects of Spatially Fractionated Radiotherapy. Int J Mol Sci. 2022;23:3366. doi: 10.3390/ijms23063366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohiuddin M., Park H., Hallmeyer S., Richards J. High-Dose Radiation as a Dramatic, Immunological Primer in Locally Advanced Melanoma. Cureus. 2015;7(12):e417. doi: 10.7759/cureus.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neuner G, Mohiuddin MM, Vander Walde N, Goloubeva O, Ha J, Yu CX, Regine WF. High-dose spatially fractionated GRID radiation therapy (SFGRT): a comparison of treatment outcomes with Cerrobend vs. MLC SFGRT. Int J Radiat Oncol Biol Phys. 2012 Apr 1;82(5):1642-9. doi: 10.1016/j.ijrobp.2011.01.065. Epub 2011 Apr 29. PMID: 21531514. [DOI] [PubMed]
- 7.Huhn J.L., Regine W.F., Valentino J.P., Meigooni A.S., Kudrimoti M., Mohiuddin M. Spatially fractionated GRID radiation treatment of advanced neck disease associated with head and neck cancer. Technol Cancer Res Treat. 2006;5(6):607–612. doi: 10.1177/153303460600500608. PMID: 17121437. [DOI] [PubMed] [Google Scholar]
- 8.Wu X, Ahmed M M., Wright J, et al. (March 05, 2010) On Modern Technical Approaches of Three-Dimensional High-Dose Lattice Radiotherapy (LRT). Cureus 2(3): e9. doi:10.7759/cureus.9.
- 9.Amendola B, Perez N, Amendola M A., et al. (September 27, 2010) Lattice Radiotherapy with RapidArc for Treatment of Gynecological Tumors: Dosimetric and Early Clinical Evaluations. Cureus 2(9): e15. doi:10.7759/cureus.15.
- 10.Wu X., Perez N.C., Zheng Y., Li X., Jiang L., Amendola B.E., et al. The Technical and Clinical Implementation of LATTICE Radiation Therapy (LRT) Radiat Res. 2020 Dec 1;194(6):737–746. doi: 10.1667/RADE-20-00066.1. PMID: 33064814. [DOI] [PubMed] [Google Scholar]
- 11.Kanagavelu S, Gupta S, Wu X, Philip S, Wattenberg MM, Hodge JW, Couto MD, Chung KD, Ahmed MM. In vivo effects of lattice radiation therapy on local and distant lung cancer: potential role of immunomodulation. Radiat Res. 2014 Aug;182(2):149-62. doi: 10.1667/RR3819.1. Epub 2014 Jul 18. PMID: 25036982; PMCID: PMC7670883. [DOI] [PMC free article] [PubMed]
- 12.Jiang L., Li X., Zhang J., Li W., Dong F., Chen C., et al. Combined High-Dose LATTICE Radiation Therapy and Immune Checkpoint Blockade for Advanced Bulky Tumors: The Concept and a Case Report. Front Oncol. 2021 Feb;12(10) doi: 10.3389/fonc.2020.548132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris Z., Dohopolski M., Rahimi A., Timmerman R. Future Directions in the Use of SAbR for the Treatment of Oligometastatic Cancers. Semin Radiat Oncol. 2021;31(3):253–262. doi: 10.1016/j.semradonc.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Hartsell W.F., Scott C.B., Bruner D.W., et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798–804. doi: 10.1093/jnci/dji139. [DOI] [PubMed] [Google Scholar]
- 15.Foro Arnalot P., Fontanals A.V., Galceran J.C., et al. Randomized clinical trial with two palliative radiotherapy regimens in painful bone metastases: 30 Gy in 10 fractions compared with 8 Gy in single fraction. Radiother Oncol. 2008;89(2):150–155. doi: 10.1016/j.radonc.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Moore C., Hsu C.C., Chen W.M., et al. Personalized Ultrafractionated Stereotactic Adaptive Radiotherapy (PULSAR) in Preclinical Models Enhances Single-Agent Immune Checkpoint Blockade. Int J Radiat Oncol Biol Phys. 2021;110(5):1306–1316. doi: 10.1016/j.ijrobp.2021.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodo S., Campagne C., Thin T.H., et al. Single-dose radiotherapy disables tumor cell homologous recombination via ischemia/reperfusion injury. J Clin Invest. 2019;129(2):786–801. doi: 10.1172/JCI97631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greco C., Zelefsky M.J., Lovelock M., et al. Predictors of local control after single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases. Int J Radiat Oncol Biol Phys. 2011;79(4):1151–1157. doi: 10.1016/j.ijrobp.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 19.Greco C., Pares O., Pimentel N., et al. Phenotype-Oriented Ablation of Oligometastatic Cancer with Single Dose Radiation Therapy. Int J Radiat Oncol Biol Phys. 2019;104(3):593–603. doi: 10.1016/j.ijrobp.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 20.Zelefsky M., Yamada J., Greco C., et al. Phase 3 Multi-Center, Prospective, Randomized Trial Comparing Single-Dose 24 Gy Radiation Therapy to a 3-Fraction SBRT Regimen in the Treatment of Oligometastatic Cancer. Int J Radiat Oncol Biol Phys. 2021;110(3):672–679. doi: 10.1016/j.ijrobp.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greco C., Kolesnick R., Fuks Z. Conformal Avoidance of Normal Organs at Risk by Perfusion-Modulated Dose Sculpting in Tumor Single-Dose Radiation Therapy. Int J Radiat Oncol Biol Phys. 2021;109(1):288–297. doi: 10.1016/j.ijrobp.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greco C., Pares O., Pimentel N., et al. Safety and Efficacy of Virtual Prostatectomy with Single-Dose Radiotherapy in Patients with Intermediate-Risk Prostate Cancer: Results from the PROSINT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2021 doi: 10.1001/jamaoncol.2021.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marples B., Collis S.J. Low-dose hyper-radiosensitivity: past, present, and future. Int J Radiat Oncol Biol Phys. 2008;70(5):1310–1318. doi: 10.1016/j.ijrobp.2007.11.071. PMID: 18374221. [DOI] [PubMed] [Google Scholar]
- 24.Scirocco E., Cellini F., Zamagni A., Macchia G., Deodato F., Cilla S., et al. Clinical Studies on Ultrafractionated Chemoradiation: A Systematic Review. Front Oncol. 2021;16(11) doi: 10.3389/fonc.2021.748200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Rajhi N.M., Khalil E.M., Ahmad S., Soudy H., AlGhazi M., Fatani D.M., et al. Low-dose fractionated radiation with induction docetaxel and cisplatin followed by concurrent cisplatin and radiation therapy in locally advanced nasopharyngeal cancer: A randomized phase II-III trial. Hematol Oncol Stem Cell Ther. 2021;14(3):199–205. doi: 10.1016/j.hemonc.2020.05.005. Epub 2020 May 21 PMID: 32504593. [DOI] [PubMed] [Google Scholar]
- 26.Bovi J.A., Prah M.A., Retzlaff A.A., Schmainda K.M., Connelly J.M., Rand S.D., et al. Pulsed Reduced Dose Rate Radiotherapy in Conjunction With Bevacizumab or Bevacizumab Alone in Recurrent High-grade Glioma: Survival Outcomes. Int J Radiat Oncol Biol Phys. 2020;108(4):979–986. doi: 10.1016/j.ijrobp.2020.06.020. Epub 2020 Jun 27. PMID: 32599030; PMCID: PMC8655709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burr A.R., Robins H.I., Bayliss R.A., Howard S.P. Pulsed reduced dose rate for reirradiation of recurrent breast cancer. Pract Radiat Oncol. 2020;10(2):e61–e70. doi: 10.1016/j.prro.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Almahariq M.F., Quinn T.J., Arden J.D., Roskos P.T., Wilson G.D., Marples B., et al. Pulsed radiation therapy for the treatment of newly diagnosed glioblastoma. Neuro Oncol. 2021 Mar 25;23(3):447–456. doi: 10.1093/neuonc/noaa165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tubin S., Gupta S., Grusch M., Popper H.H., Brcic L., et al. Shifting the Immune-Suppressive to Predominant Immune-Stimulatory Radiation Effects by SBRT-PArtial Tumor Irradiation Targeting HYpoxic Segment (SBRT-PATHY) Cancers. 2021;13:50. doi: 10.3390/cancers13010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tubin S., Ahmed M.M., Gupta S. Radiation and hypoxia-induced non-targeted effects in normoxic and hypoxic conditions in human lung cancer cells. Int J Radiat Biol. 2018;94:199–211. doi: 10.1080/09553002.2018.1422085. [DOI] [PubMed] [Google Scholar]
- 31.Tubin S., Fossati P., Carlino A., Martino G., Gora J., et al. Novel Carbon Ion and Proton Partial Irradiation of Recurrent Unresectable Bulky Tumors (Particle-PATHY): Early Indication of Effectiveness and Safety. Cancers (Basel) 2022;14(9):2232. doi: 10.3390/cancers14092232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedl A.A., Prise K.M., Butterworth K.T., Montay-Gruel P., Favaudon V. Radiobiology of the FLASH effect. Med Phys. 2022;49(3):1993–2013. doi: 10.1002/mp.15184. [DOI] [PubMed] [Google Scholar]
- 33.Prise K.M., O'Sullivan J.M. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. 2009;9(5):351–360. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daguenet E., Louati S., Wozny A.S., Vial N., Gras M., Guy J.B., et al. Radiation-induced bystander and abscopal effects: important lessons from preclinical models. Br J Cancer. 2020;123(3):339–348. doi: 10.1038/s41416-020-0942-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butterworth K.T., McGarry C.K., Trainor C., McMahon S.J., O'Sullivan J.M., Schettino G., et al. Dose, dose-rate and field size effects on cell survival following exposure to non-uniform radiation fields. Phys Med Biol. 2012;57(10):3197–3206. doi: 10.1088/0031-9155/57/10/3197. [DOI] [PubMed] [Google Scholar]
- 36.Butterworth K.T., McGarry C.K., Trainor C., O'Sullivan J.M., Hounsell A.R., Prise K.M. Out-of-field cell survival following exposure to intensity-modulated radiation fields. Int J Radiat Oncol Biol Phys. 2011;79(5):1516–1522. doi: 10.1016/j.ijrobp.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMahon S.J., Butterworth K.T., McGarry C.K., Trainor C., O'Sullivan J.M., Hounsell A.R., et al. A computational model of cellular response to modulated radiation fields. Int J Radiat Oncol Biol Phys. 2012;84(1):250–256. doi: 10.1016/j.ijrobp.2011.10.058. [DOI] [PubMed] [Google Scholar]
- 38.Brown K.H., Ghita M., Dubois L.J., de Ruysscher D., Prise K.M., Verhaegen F., et al. A scoping review of small animal image-guided radiotherapy research: Advances, impact and future opportunities in translational radiobiology. Clin Transl Radiat Oncol. 2022;34:112–119. doi: 10.1016/j.ctro.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tubin S., Fossati P., Carlino A., Martino G., Gora J., Stock M., et al. Novel Carbon Ion and Proton Partial Irradiation of Recurrent Unresectable Bulky Tumors (Particle-PATHY): Early Indication of Effectiveness and Safety. Cancers. 2022;14(9) doi: 10.3390/cancers14092232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tubin S., Gupta S., Grusch M., Popper H.H., Brcic L., Ashdown M.L., et al. Shifting the Immune-Suppressive to Predominant Immune-Stimulatory Radiation Effects by SBRT-PArtial Tumor Irradiation Targeting HYpoxic Segment (SBRT-PATHY) Cancers. 2020;13(1) doi: 10.3390/cancers13010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sologuren I., Rodríguez-Gallego C., Lara P.C. Immune effects of high dose radiation treatment: implications of ionizing radiation on the development of bystander and abscopal effects. Transl Cancer Res. 2014;3(1):18–31. [Google Scholar]
- 42.Lara P.C., López-Peñalver J.J., Farias Vde A., Ruiz-Ruiz M.C., Oliver F.J., Ruiz de Almodóvar J.M. Direct and bystander radiation effects: a biophysical model and clinical perspectives. Cancer Lett. 2015;356(1):5–16. doi: 10.1016/j.canlet.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Park S.S., Dong H., Liu X., Harrington S.M., Krco C.J., Grams M.P., et al. PD-1 Restrains Radiotherapy-Induced Abscopal Effect. Cancer Immunol Res. 2015;3(6):610–619. doi: 10.1158/2326-6066.CIR-14-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Topalian S.L., Taube J.M., Anders R.A., Pardoll D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chicas-Sett R., Morales-Orue I., Rodriguez-Abreu D., Lara-Jimenez P. Combining radiotherapy and ipilimumab induces clinically relevant radiation-induced abscopal effects in metastatic melanoma patients: A systematic review.Clin Transl. Radiat Oncol. 2017;23(9):5–11. doi: 10.1016/j.ctro.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chicas-Sett R., Zafra J., Rodriguez-Abreu D., Castilla-Martinez J., Benitez G., Salas B., et al. Combination of SABR With Anti-PD-1 in Oligoprogressive Non-Small Cell Lung Cancer and Melanoma: Results of a Prospective Multicenter Observational Study. Int J Radiat Oncol Biol Phys. 2022;S0360–3016(22):00421–00427. doi: 10.1016/j.ijrobp.2022.05.013. [DOI] [PubMed] [Google Scholar]
- 47.Chicas-Sett R., Morales-Orue I., Castilla-Martinez J., Zafra-Martin J., Kannemann A., Blanco J., et al. Stereotactic Ablative Radiotherapy Combined with Immune Checkpoint Inhibitors Reboots the Immune Response Assisted by Immunotherapy in Metastatic Lung Cancer: A Systematic Review. Int J Mol Sci. 2019;20(9):2173. doi: 10.3390/ijms20092173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Demaria S, Guha C, Schoenfeld J, Morris Z, Monjazeb A, Sikora A, et al. Radiation dose and fraction in immunotherapy: one-size regimen does not fit all settings, so how does one choose? J Immunother Cancer. 2021;9(4). Epub 2021/04/09. doi: 10.1136/jitc-2020-002038. PubMed PMID: 33827904; PubMed Central PMCID: PMCPMC8031689. [DOI] [PMC free article] [PubMed]
- 49.Vanpouille-Box C., Alard A., Aryankalayil M.J., Sarfraz Y., Diamond J.M., Schneider R.J., et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chakraborty M., Abrams S.I., Camphausen K., Liu K., Scott T., Coleman C.N., et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170(12):6338–6347. doi: 10.4049/jimmunol.170.12.6338. PubMed PMID: 12794167. [DOI] [PubMed] [Google Scholar]
- 51.Klug F., Prakash H., Huber P.E., Seibel T., Bender N., Halama N., et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24(5):589–602. doi: 10.1016/j.ccr.2013.09.014. PubMed PMID: 24209604. [DOI] [PubMed] [Google Scholar]
- 52.Herrera FG, Ronet C, Ochoa de Olza M, Barras D, Crespo I, Andreatta M, et al. Low-Dose Radiotherapy Reverses Tumor Immune Desertification and Resistance to Immunotherapy. Cancer Discov. 2022;12(1):108-33. Epub 2021/09/05. doi: 10.1158/2159-8290.CD-21-0003. PubMed PMID: 34479871. [DOI] [PMC free article] [PubMed]
- 53.Morris ZS, Guy EI, Werner LR, Carlson PM, Heinze CM, Kler JS, et al. Tumor-Specific Inhibition of In Situ Vaccination by Distant Untreated Tumor Sites. Cancer Immunol Res. 2018;6(7):825-34. Epub 2018/05/12. doi: 10.1158/2326-6066.CIR-17-0353. PubMed PMID: 29748391; PubMed Central PMCID: PMCPMC6030484. [DOI] [PMC free article] [PubMed]
- 54.Herrera FG, Romero P, Coukos G. Lighting up the tumor fire with low-dose irradiation. Trends Immunol. 2022;43(3):173-9. Epub 2022/02/03. doi: 10.1016/j.it.2022.01.006. PubMed PMID: 35105519. [DOI] [PubMed]
- 55.Ochoa-de-Olza M, Bourhis J, Coukos G, Herrera FG. Low-dose irradiation for reversing immunotherapy resistance: how to translate? J Immunother Cancer. 2022;10(7). Epub 2022/07/15. doi: 10.1136/jitc-2022-004939. PubMed PMID: 35835490; PubMed Central PMCID: PMCPMC9289035. [DOI] [PMC free article] [PubMed]
- 56.Savage T, Pandey S, Guha C. Postablation Modulation after Single High-Dose Radiation Therapy Improves Tumor Control via Enhanced Immunomodulation. Clin Cancer Res. 2020;26(4):910-21. Epub 2019/11/24. doi: 10.1158/1078-0432.CCR-18-3518. PubMed PMID: 31757878. [DOI] [PubMed]
- 57.Menon H, Ramapriyan R, Cushman TR, Verma V, Kim HH, Schoenhals JE, et al. Role of Radiation Therapy in Modulation of the Tumor Stroma and Microenvironment. Front Immunol. 2019;10:193. Epub 2019/03/05. doi: 10.3389/fimmu.2019.00193. PubMed PMID: 30828330; PubMed Central PMCID: PMCPMC6384252. [DOI] [PMC free article] [PubMed]
- 58.Patel RR, Verma V, Barsoumian HB, Ning MS, Chun SG, Tang C, et al. Use of Multi-Site Radiation Therapy for Systemic Disease Control. Int J Radiat Oncol Biol Phys. 2021;109(2):352-64. Epub 2020/08/18. doi: 10.1016/j.ijrobp.2020.08.025. PubMed PMID: 32798606. [DOI] [PMC free article] [PubMed]
- 59.Robert C., Schachter J., Long G.V., Arance A., Grob J.J., Mortier L., et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 60.Upadhaya S., Neftelinov S.T., Hodge J., Campbell J. Challenges and opportunities in the PD1/PDL1 inhibitor clinical trial landscape. Nat Rev Drug Discov. 2022;21:482–483. doi: 10.1038/d41573-022-00030-4. [DOI] [PubMed] [Google Scholar]
- 61.Demaria S., Golden E.B., Formenti S.C. Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol. 2015;1:1325–1332. doi: 10.1001/jamaoncol.2015.2756. [DOI] [PubMed] [Google Scholar]
- 62.Ngwa W., Irabor O.C., Schoenfeld J.D., Hesser J., Demaria S., Formenti S.C. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18:313–322. doi: 10.1038/nrc.2018.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Antonia S.J., Villegas A., Daniel D., Vicente D., Murakami S., Hui R., et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 64.Theelen W.S.M.E., Chen D., Verma V., Hobbs B.P., Peulen H.M.U., Aerts J.G.J.V., et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30391-X. [DOI] [PubMed] [Google Scholar]
- 65.Lee N.Y., Ferris R.L., Psyrri A., Haddad R.I., Tahara M., Bourhis J., et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021;22:450–462. doi: 10.1016/S1470-2045(20)30737-3. [DOI] [PubMed] [Google Scholar]
- 66.Schoenfeld J.D., Giobbie-Hurder A., Ranasinghe S., Kao K.Z., Lako A., Tsuji J., et al. Durvalumab plus tremelimumab alone or in combination with low-dose or hypofractionated radiotherapy in metastatic non-small-cell lung cancer refractory to previous PD(L)-1 therapy: an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2022;23:279–291. doi: 10.1016/S1470-2045(21)00658-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Durante M., Brenner D.J., Formenti S.C. Does heavy ion therapy work through the immune system? Int J Radiat Oncol Biol Phys. 2016;96:934–936. doi: 10.1016/j.ijrobp.2016.08.037. [DOI] [PubMed] [Google Scholar]
- 68.Durante M., Formenti S. Harnessing radiation to improve immunotherapy: better with particles? Br J Radiol. 2020;93:20190224. doi: 10.1259/bjr.20190224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim N., Myoung Noh J., Lee W., Park B., Park H., Young Park J., et al. Proton beam therapy reduces the risk of severe radiation-induced lymphopenia during chemoradiotherapy for locally advanced non-small cell lung cancer: A comparative analysis of proton versus photon therapy. Radiother Oncol. 2021;156:166–173. doi: 10.1016/j.radonc.2020.12.019. [DOI] [PubMed] [Google Scholar]
- 70.Mohan R., Liu A.Y., Brown P.D., Mahajan A., Dinh J., Chung C., et al. Proton therapy reduces the likelihood of high-grade radiation–induced lymphopenia in glioblastoma patients: phase II randomized study of protons vs photons. Neuro Oncol. 2020 doi: 10.1093/neuonc/noaa182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Helm A., Fournier C., Durante M. Particle radiotherapy and molecular therapies: mechanisms and strategies towards clinical applications. Expert Rev Mol Med. 2022;24:e8. doi: 10.1017/erm.2022.2. [DOI] [PubMed] [Google Scholar]
- 72.Ohkubo Y., Iwakawa M., Seino K.-I., Nakawatari M., Wada H., Kamijuku H., et al. Combining Carbon Ion Radiotherapy and Local Injection of α-Galactosylceramide–Pulsed Dendritic Cells Inhibits Lung Metastases in an In Vivo Murine Model. Int J Radiat Oncol. 2010;78:1524–1531. doi: 10.1016/j.ijrobp.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 73.Matsunaga A., Ueda Y., Yamada S., Harada Y., Shimada H., Hasegawa M., et al. Carbon-ion beam treatment induces systemic antitumor immunity against murine squamous cell carcinoma. Cancer. 2010;116:3740–3748. doi: 10.1002/cncr.25134. [DOI] [PubMed] [Google Scholar]
- 74.Takahashi Y., Yasui T., Minami K., Tamari K., Hayashi K., Otani K., et al. Carbon ion irradiation enhances the antitumor efficacy of dual immune checkpoint blockade therapy both for local and distant sites in murine osteosarcoma. Oncotarget. 2019;10:633–646. doi: 10.18632/oncotarget.26551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Helm A., Tinganelli W., Simoniello P., Kurosawa F., Fournier C., Shimokawa T., et al. Reduction of Lung Metastases in a Mouse Osteosarcoma Model Treated With Carbon Ions and Immune Checkpoint Inhibitors. Int J Radiat Oncol. 2021;109:594–602. doi: 10.1016/j.ijrobp.2020.09.041. [DOI] [PubMed] [Google Scholar]
- 76.Zhou H., Tu C., Yang P., Li J., Kepp O., Li H., et al. Carbon ion radiotherapy triggers immunogenic cell death and sensitizes melanoma to anti-PD-1 therapy in mice. Oncoimmunology. 2022;11:1–11. doi: 10.1080/2162402X.2022.2057892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hartmann L., Osen W., Eichmüller O.L., Kordaß T., Furkel J., Dickes E., et al. Carbon ion irradiation plus CTLA4 blockade elicits therapeutic immune responses in a murine tumor model. Cancer Lett. 2022: doi: 10.1016/j.canlet.2022.215928. [DOI] [PubMed] [Google Scholar]
- 78.Vozenin M.C., Bourhis J., Durante M. Towards clinical translation of FLASH radiotherapy. Nat Rev Clin Oncol. 2022 Dec;19(12):791–803. doi: 10.1038/s41571-022-00697-z. Epub 2022 Oct 27. PMID: 36303024. [DOI] [PubMed] [Google Scholar]
- 79.Schuler E., Acharya M., Montay-Gruel P., et al. Ultra-high dose rate electron beams and the FLASH effect: From preclinical evidence to a new radiotherapy paradigm. Med Phys. 2022;49:2082–2095. doi: 10.1002/mp.15442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Montay-Gruel P., Bouchet A., Jaccard M., et al. X-rays can trigger the FLASH effect: Ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother Oncol. 2018;129:582–588. doi: 10.1016/j.radonc.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 81.Gao F., Yang Y., Zhu H., et al. First demonstration of the FLASH effect with ultrahigh dose rate high-energy X-rays. Radiother Oncol. 2022;166:44–50. doi: 10.1016/j.radonc.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 82.Diffenderfer E.S., Sorensen B.S., Mazal A., et al. The current status of preclinical proton FLASH radiation and future directions. Med Phys. 2022;49:2039–2054. doi: 10.1002/mp.15276. [DOI] [PubMed] [Google Scholar]
- 83.Smyth L.M.L., Donoghue J.F., Ventura J.A., et al. Comparative toxicity of synchrotron and conventional radiation therapy based on total and partial body irradiation in a murine model. Sci Rep. 2018;8:12044. doi: 10.1038/s41598-018-30543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beyreuther E., Brand M., Hans S., et al. Feasibility of proton FLASH effect tested by zebrafish embryo irradiation. Radiother Oncol. 2019 doi: 10.1016/j.radonc.2019.06.024. [DOI] [PubMed] [Google Scholar]
- 85.Venkatesulu B.P., Sharma A., Pollard-Larkin J.M., et al. Ultra high dose rate (35 Gy/sec) radiation does not spare the normal tissue in cardiac and splenic models of lymphopenia and gastrointestinal syndrome. Sci Rep. 2019;9:17180. doi: 10.1038/s41598-019-53562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Venkatesulu B.P., Sharma A., Pollard-Larkin J.M., et al. Author Correction: Ultra high dose rate (35 Gy/sec) radiation does not spare the normal tissue in cardiac and splenic models of lymphopenia and gastrointestinal syndrome. Sci Rep. 2020;10:11018. doi: 10.1038/s41598-020-67913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rohrer Bley C., Wolf F., Goncalves Jorge P., et al. Dose and volume limiting late toxicity of FLASH radiotherapy in cats with squamous cell carcinoma of the nasal planum and in mini-pigs. Clin Cancer Res. 2022 doi: 10.1158/1078-0432.CCR-22-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bourhis J., Sozzi W.J., Jorge P.G., et al. Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol. 2019;139:18–22. doi: 10.1016/j.radonc.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 89.Gaide O., Herrera F., Sozzi W.J., et al. Comparison of ultra-high versus conventional dose rate radiotherapy in a patient with cutaneous lymphoma. Radiother Oncol. 2022 doi: 10.1016/j.radonc.2021.12.045. [DOI] [PubMed] [Google Scholar]
- 90.Mascia A.E., Daugherty E.C., Zhang Y., Lee E., Xiao Z., Sertorio M., et al. Proton FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases: The FAST-01 Nonrandomized Trial. JAMA Oncol. 2023 Jan 1;9(1):62–69. doi: 10.1001/jamaoncol.2022.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kalholm F., Grzanka L., Traneus E., Bassler N. A systematic review on the usage of averaged LET in radiation biology for particle therapy. Radiother Oncol. 2021;161:211–221. doi: 10.1016/j.radonc.2021.04.007. [DOI] [PubMed] [Google Scholar]
- 92.Hahn C., Oden J., Dasu A., Vestergaard A., Fuglsang Jensen M., Sokol O., et al. Towards harmonizing clinical linear energy transfer (LET) reporting in proton radiotherapy: a European multi-centric study. Acta Oncol. 2021;1–9 doi: 10.1080/0284186X.2021.1992007. [DOI] [PubMed] [Google Scholar]
- 93.Hahn C., Heuchel L., Oden J., Traneus E., Wulff J., Plaude S., et al. Comparing biological effectiveness guided plan optimization strategies for cranial proton therapy: potential and challenges. Radiat Oncol. 2022;17(1):169. doi: 10.1186/s13014-022-02143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Slatkin D.N., et al. Microbeam radiation therapy. Med Phys. 1992;19(6):1395–1400. doi: 10.1118/1.596771. [DOI] [PubMed] [Google Scholar]
- 95.Dilmanian F.A., et al. Interlaced x-ray microplanar beams: a radiosurgery approach with clinical potential. Proc Natl Acad Sci U S A. 2006;103(25):9709–9714. doi: 10.1073/pnas.0603567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.ZEMAN, W., H.J. CURTIS, and C.P. BAKER, Histopathologic effect of high-energy-particle microbeams on the visual cortex of the mouse brain. Radiat Res, 1961. 15: p. 496-514. [PubMed]
- 97.Bouchet A., et al. Better Efficacy of Synchrotron Spatially Microfractionated Radiation Therapy Than Uniform Radiation Therapy on Glioma. Int J Radiat Oncol Biol Phys. 2016;95(5):1485–1494. doi: 10.1016/j.ijrobp.2016.03.040. [DOI] [PubMed] [Google Scholar]
- 98.Dilmanian F.A., et al. Response of rat intracranial 9L gliosarcoma to microbeam radiation therapy. Neuro Oncol. 2002;4(1):26–38. doi: 10.1215/15228517-4-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fernandez-Palomo, C., et al., Erratum: Fernandez-Palomo, C., et al. Animal Models in Microbeam Radiation Therapy: A Scoping Review. Cancers (Basel), 2020. 12(11). [DOI] [PMC free article] [PubMed]
- 100.Lamirault C., et al. Spatially Modulated Proton Minibeams Results in the Same Increase of Lifespan as a Uniform Target Dose Coverage in F98-Glioma-Bearing Rats. Radiat Res. 2020;194(6):715–723. doi: 10.1667/RADE-19-00013.1. [DOI] [PubMed] [Google Scholar]
- 101.Prezado Y., et al. Tumor Control in RG2 Glioma-Bearing Rats: A Comparison Between Proton Minibeam Therapy and Standard Proton Therapy. Int J Radiat Oncol Biol Phys. 2019;104(2):266–271. doi: 10.1016/j.ijrobp.2019.01.080. [DOI] [PubMed] [Google Scholar]
- 102.Bräuer-Krisch E., et al. Effects of pulsed, spatially fractionated, microscopic synchrotron X-ray beams on normal and tumoral brain tissue. Mutat Res. 2010;704(1–3):160–166. doi: 10.1016/j.mrrev.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 103.Bouchet A., et al. Effects of microbeam radiation therapy on normal and tumoral blood vessels. Phys Med. 2015;31(6):634–641. doi: 10.1016/j.ejmp.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 104.Deman P., et al. Monochromatic minibeams radiotherapy: from healthy tissue-sparing effect studies toward first experimental glioma bearing rats therapy. Int J Radiat Oncol Biol Phys. 2012;82(4):e693–e700. doi: 10.1016/j.ijrobp.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 105.Prezado Y., et al. Tolerance to Dose Escalation in Minibeam Radiation Therapy Applied to Normal Rat Brain: Long-Term Clinical. Radiological and Histopathological Analysis Radiat Res. 2015;184(3):314–321. doi: 10.1667/RR14018.1. [DOI] [PubMed] [Google Scholar]
- 106.Prezado Y., et al. Proton minibeam radiation therapy spares normal rat brain: Long-Term Clinical, Radiological and Histopathological Analysis. Sci Rep. 2017;7(1):14403. doi: 10.1038/s41598-017-14786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Prezado Y., et al. Transfer of Minibeam Radiation Therapy into a cost-effective equipment for radiobiological studies: a proof of concept. Sci Rep. 2017;7(1):17295. doi: 10.1038/s41598-017-17543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lamirault C., et al. Short and long-term evaluation of the impact of proton minibeam radiation therapy on motor, emotional and cognitive functions. Sci Rep. 2020;10(1):13511. doi: 10.1038/s41598-020-70371-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fernandez-Palomo C., et al. Complete Remission of Mouse Melanoma after Temporally Fractionated Microbeam Radiotherapy. Cancers (Basel) 2020;12(9) doi: 10.3390/cancers12092656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Prezado Y., et al. Proton minibeam radiation therapy widens the therapeutic index for high-grade gliomas. Sci Rep. 2018;8(1):16479. doi: 10.1038/s41598-018-34796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prezado Y., et al. Increase of lifespan for glioma-bearing rats by using minibeam radiation therapy. J Synchrotron Radiat. 2012;19(Pt 1):60–65. doi: 10.1107/S0909049511047042. [DOI] [PubMed] [Google Scholar]
- 112.Bertho A., et al. First Evaluation of Temporal and Spatial Fractionation in Proton Minibeam Radiation Therapy of Glioma-Bearing Rats. Cancers (Basel) 2021;13(19) doi: 10.3390/cancers13194865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hadsell M., et al. A first generation compact microbeam radiation therapy system based on carbon nanotube X-ray technology. Appl Phys Lett. 2013;103(18) doi: 10.1063/1.4826587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bartzsch S., Oelfke U. Line focus x-ray tubes-a new concept to produce high brilliance x-rays. Phys Med Biol. 2017;62(22):8600–8615. doi: 10.1088/1361-6560/aa910b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Prezado Y., et al. X-ray energy optimization in minibeam radiation therapy. Med Phys. 2009;36(11):4897–4902. doi: 10.1118/1.3232000. [DOI] [PubMed] [Google Scholar]
- 116.Adam J.F., et al. Toward Neuro-Oncologic Clinical Trials of High-Dose-Rate Synchrotron Microbeam Radiation Therapy: First Treatment of a Spontaneous Canine Brain Tumor. Int J Radiat Oncol Biol Phys. 2022;113(5):967–973. doi: 10.1016/j.ijrobp.2022.04.022. [DOI] [PubMed] [Google Scholar]
- 117.Bazyar S., et al. Minibeam radiotherapy with small animal irradiators; in vitro and in vivo feasibility studies. Phys Med Biol. 2017;62(23):8924–8942. doi: 10.1088/1361-6560/aa926b. [DOI] [PubMed] [Google Scholar]
- 118.Kundapur V., et al. Is Mini Beam Ready for Human Trials? Results of Randomized Study of Treating De-Novo Brain Tumors in Canines Using Linear Accelerator Generated Mini Beams. Radiat Res. 2022;198(2):162–171. doi: 10.1667/RADE-21-00093.1. [DOI] [PubMed] [Google Scholar]
- 119.Prezado Y., Fois G.R. Proton-minibeam radiation therapy: a proof of concept. Med Phys. 2013;40(3) doi: 10.1118/1.4791648. [DOI] [PubMed] [Google Scholar]
- 120.Dilmanian F.A., et al. Interleaved carbon minibeams: an experimental radiosurgery method with clinical potential. Int J Radiat Oncol Biol Phys. 2012;84(2):514–519. doi: 10.1016/j.ijrobp.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 121.Prezado Y., et al. A Potential Renewed Use of Very Heavy Ions for Therapy: Neon Minibeam Radiation Therapy. Cancers (Basel) 2021;13(6) doi: 10.3390/cancers13061356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sabatasso S., et al. Microbeam radiation-induced tissue damage depends on the stage of vascular maturation. Int J Radiat Oncol Biol Phys. 2011;80(5):1522–1532. doi: 10.1016/j.ijrobp.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 123.Asur R.S., et al. Spatially fractionated radiation induces cytotoxicity and changes in gene expression in bystander and radiation adjacent murine carcinoma cells. Radiat Res. 2012;177(6):751–765. doi: 10.1667/rr2780.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Asur R., et al. High dose bystander effects in spatially fractionated radiation therapy. Cancer Lett. 2015;356(1):52–57. doi: 10.1016/j.canlet.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Forrester H.B., et al. Abscopal Gene Expression in Response to Synchrotron Radiation Indicates a Role for Immunological and DNA Damage Response Genes. Radiat Res. 2020;194(6):678–687. doi: 10.1667/RADE-19-00014.1. [DOI] [PubMed] [Google Scholar]
- 126.Johnsrud A.J., et al. Evidence for Early Stage Anti-Tumor Immunity Elicited by Spatially Fractionated Radiotherapy-Immunotherapy Combinations. Radiat Res. 2020;194(6):688–697. doi: 10.1667/RADE-20-00065.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bertho A., et al. Evaluation of the role of the immune system response following minibeam radiation therapy. Int J Radiat Oncol Biol Phys. 2022 doi: 10.1016/j.ijrobp.2022.08.011. [DOI] [PubMed] [Google Scholar]
- 128.Fukunaga H., et al. Spatially Fractionated Microbeam Analysis of Tissue-sparing Effect for Spermatogenesis. Radiat Res. 2020;194(6):698–706. doi: 10.1667/RADE-19-00018.1. [DOI] [PubMed] [Google Scholar]
- 129.Fukunaga H. Stem Cell Migration: A Possible Mechanism for the Tissue-Sparing Effect of Spatially Fractionated Radiation. Radiat Res. 2021;196(6):680–685. doi: 10.1667/RADE-21-00134.1. [DOI] [PubMed] [Google Scholar]