Abstract
Background:
The rates of anterior cruciate ligament (ACL) graft failure or contralateral ACL rupture range from 17% to 30% in pediatric patients after ACL reconstruction (ACLR). A contributing factor to the high reinjury rate in this population may be the limited evidence regarding appropriate criteria for allowing unrestricted return to activity (RTA) postoperatively.
Purpose:
To review the literature and identify the most commonly used criteria when determining unrestricted RTA after ACLR in pediatric patients.
Study Design:
Systematic review; Level of evidence, 4.
Methods:
A search was performed of the Medline/PubMed, Cochrane Central Register of Controlled Trials, Embase, CINAHL, and SPORTDiscus databases using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The authors identified studies that included pediatric patients (<19 years of age) and specified the criteria used to determine RTA after ACLR.
Results:
A total of 27 articles met all criteria for review, of which 13 studies only used 1 criterion when determining RTA. Objective criteria were the most common type of criteria for RTA (17 studies). Strength tests (15 studies) and hop tests (10 studies) were the most commonly used tasks when deriving RTA criteria. Only 2 studies used validated questionnaires to assess the patient’s physiological readiness for RTA, and only 2 studies used an objective assessment of movement quality before RTA.
Conclusion:
Only 14 of the 27 reviewed studies reported using >1 criterion when determining RTA. Furthermore, few studies used patient-reported outcome measures or lower limb kinematics as RTA criteria, indicating that more research is needed to validate these metrics in the pediatric population.
Keywords: anterior cruciate ligament, pediatric sports medicine, physical therapy/rehabilitation, return to activity
Injuries to the anterior cruciate ligament (ACL) are increasing in prevalence in the pediatric and adolescent populations (<19 years of age), 9,28 with girls aged 13 to 17 years possessing the highest injury incidence of any sex-age strata. 34 After an ACL injury, a surgical reconstruction (ACLR) is a common treatment option, aimed at facilitating the resumption of preinjury activities. 21 However, only two-thirds of pediatric patients will return to their preinjury levels of activity. 58 Furthermore, once an athlete has returned to activity, the risk for a subsequent ACL injury is considerably higher compared with the initial injury. 65,66,74 Approximately 17% to 19% of adolescent athletes will retear their ACL within 2 years after an ACLR, 18,47,66 with >30% of second ACL injuries occurring within the first 20 sport exposures after return to sports. 66 There is also a discrepancy between reinjury rates in adult and pediatric patients, with 1 study reporting that 17% of patients <18 years of age at the time of their ACLR went on to sustain a second ACL injury, compared with only 4% for patients >25 years. 74
Return to activity (RTA) criteria typically refer to the results of a set of tests, or test batteries, designed to incorporate a number of risk factors, which can be used to clear athletes for RTA at the final stage of rehabilitation. 19 Surprisingly, there are no standardized or widely accepted measures for assessing RTA readiness, 30 which may contribute to the high reinjury rates in pediatric populations. Despite the continuing development of milestone-based postoperative rehabilitation programs for young athletes, 86 there is considerable debate regarding the optimal criteria for RTA clearance in this population. Notably, a recent survey of pediatric orthopaedic surgeons 30 and a review of children hospital’s rehabilitation programs 22 found that the mode of testing and criteria thresholds for activity advancement varied considerably across hospitals and surgeons.
Several systematic reviews have sought to develop a consensus on what criteria should be utilized when releasing patients to unrestricted sports activities postoperatively 1,7,8 as well as determining whether passing an RTA test battery indeed reduces the risk for subsequent ACL injury. 52,83 However, these reviews have not isolated or independently examined the pediatric population in their findings. We postulate that management of pediatric patients with ACL injuries must be considered separately from adults. First, musculoskeletal immaturity dictates that alternative surgical techniques may be required in some patients, and some surgical techniques traditionally used for adults are not performed in children and adolescents. 42,54 Alternative, physeal-respecting and physeal-sparing ACLR techniques have been developed for this population, to help prevent physeal injury and premature growth arrest. 42,54 Furthermore, numerous anatomic and hormonal changes occur during puberty that influence a person’s locomotion. 35,40 This has led to the identification of age-specific risk factors for ACL injury 59,64 and further emphasizes the need for age-specific criteria when evaluating RTA.
The purpose of this systematic review was to identify the most commonly used criteria when assessing RTA readiness post-ACLR in pediatric patients. Our findings can be used to validate or adapt the most commonly used RTA criteria as well as to identify new areas for RTA development according to the identified literature gaps.
Methods
Search Strategy
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed when preparing, conducting, and reporting this review. 57 The protocol for this systematic review was then further finalized, peer reviewed, and published. 69 The electronic databases Medline (PubMed), Embase, CINAHL, SPORTDiscus, and Cochrane Central Register of Controlled Trials were searched from January 1, 2000, through May 31, 2021, to identify studies reporting RTA criteria in pediatric patients recovering from an ACLR. The combination of these databases produces an estimated 97% recall of all primary studies involving orthopaedic surgical interventions. 78 A university librarian assisted with the creation and execution of the search strategy (see Supplemental Material, Table S1). Where possible, terms were mapped to Medical Subject Headings and searched using keywords. Publication details from all identified studies were exported to Covidence systematic review software (Veritas Health Innovation), and duplicates were removed.
Study Identification
Three authors (N.J.R, H.L., and K.J.L.) independently reviewed the title and abstract of each article identified through the literature search. The full text of an article was obtained and evaluated when eligibility could not be assessed from the first screening, and full-text screening was performed independently by 2 authors (NJ.R. and H.L.). Any discrepancies were resolved through a consensus discussion between reviewers, and a senior author (S.C.) was consulted if a disagreement could not be resolved. To supplement the electronic database search, citations within all included studies were manually reviewed to identify any additional studies omitted during the initial database searches.
Studies were considered if they (1) included participants who had undergone a primary ACL reconstructive surgery (any graft type), (2) included a cohort of pediatric participants (<19 years of age at the time of surgery), (3) specified the criteria used to determine unrestricted RTA after ACLR surgery (with enough detail to determine if the criteria were subjective or objective), and (4) published in either English or French. Conference proceedings, surgical techniques, technical notes, letters to the editor, case reports, clinical commentaries, and review articles were excluded. In addition, several studies with potential duplicate participants were identified (eg, same institution, overlapping patient enrollment dates). The authors of these articles were contacted to determine any patient overlap; however, no new information was gathered from these efforts. Thus, for these groups of studies, only the study with the largest patient population was included.
Assessment of Study Quality
The quality of each study, including the risk of bias, was assessed using the validated methodological index for non-randomized studies. 5 Each study was independently assessed by 2 authors (N.J.R. and H.L.), and any disagreements were discussed until a consensus was reached. Articles were not excluded on the basis of the assessment; instead, the results of the quality scoring system for all studies are provided (Supplemental Table S2).
Data Extraction and Outcome Measures
A standardized data sheet was used to record the following information and outcome data: study type, number of patients, patient sex, patient age at surgery, surgical technique and graft type, concomitant injuries, length of follow-up, criteria used to determine RTA, number of patients who returned to sports activities, number of failed ACLRs (post-RTA), and number of contralateral ACL ruptures (post-RTA). Continuous variables were recorded as the mean ± standard deviation (SD). If the mean or SD was not reported, it was estimated according to a previously validated formula: (higher range value – lower range value)/4 or interquartile range/1.35. 36,37 Categorical variables (eg, reinjury rate) were recorded as frequencies with percentages. If results were reported separately for multiple cohorts within the same paper, the cohorts were combined and recorded together. 38 The primary outcome of interest was the RTA assessment used by each study when determining clearance to unrestricted RTA, recorded according to (1) how many criteria were used; (2) whether the criteria were time based, subjective, or objective; and (3) the specific test or benchmark used.
Results
Search Strategy
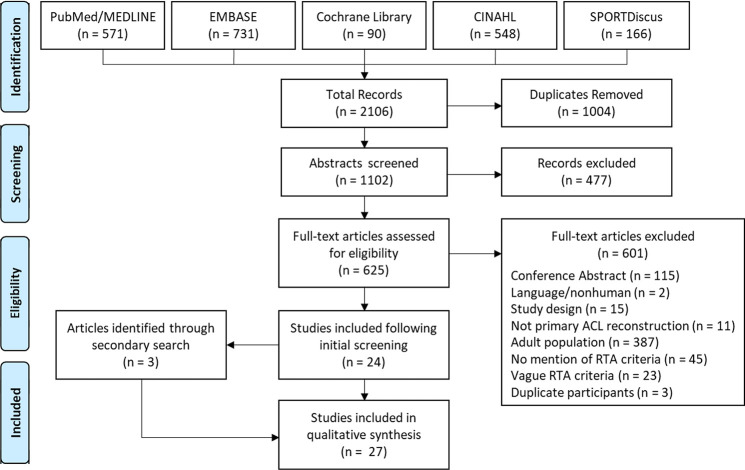
The electronic database search identified 2106 studies; after 1004 duplicates were removed, 1102 articles remained as the total yield. After the review of titles and abstracts, 477 studies were excluded, and the full texts of the remaining 625 were procured for detailed assessment. Of these, 601 were excluded, leaving 24 studies from the initial search. An additional 3 studies were then identified from the manual search of the reference lists. A qualitative analysis was therefore performed on 27 studies (Figure 1). Of the 601 excluded articles, 23 met the inclusion criteria but did not describe the RTA criteria with enough detail to be included in this review; these studies are listed in Supplemental Table S3.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart for study inclusion. ACL, anterior cruciate ligament; RTA, return to activity.
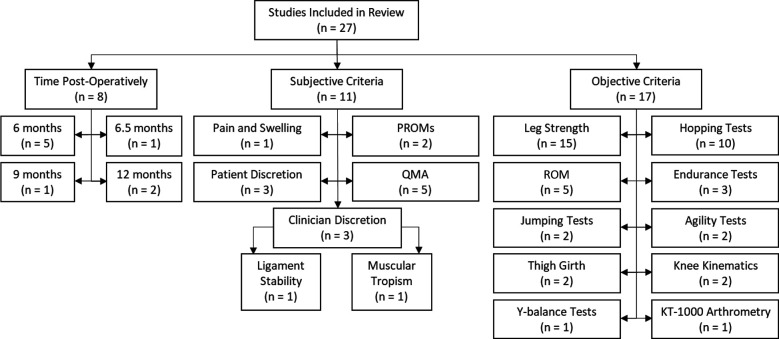
A total of 2289 patients with an estimated mean patient age of 14.1 ± 2.3 years were included in this review. Of the 27 studies included, 13 studies ¶ only used 1 criterion when determining RTA, 2 studies 70,74 used 2 different criteria, and 12 studies # used ≥3 criteria. In addition, 8 studies 3,23,25,60,70,73,80,85 used elapsed time postoperatively, 11 studies ** used subjective criteria, and 17 studies †† used objective criteria as one of the components when determining RTA (Figure 2). The graft failure rates for the ACLR ranged from 0% 25 to 39%, 70 and injuries to the contralateral ACL ranged from 0% 6 to 19.2%. 41 A summary of all study characteristics can be found in Appendix Table A1.
Figure 2.
Flowchart of factors used to determine unrestricted return to activity after an anterior cruciate ligament reconstruction in pediatric patients. Studies that reported >1 return-to-activity criterion were tallied in multiple categories. PROM, patient-reported outcome measure; QMA, Quality Movement Assessment; ROM, range of motion.
Time Postoperatively
Time after ACLR surgery was the only criterion for determining RTA in 6 studies, 3,23,25,60,73,80 while 1 study 70 used time postoperatively in combination with a subjective criterion and 1 study 85 used time postoperatively in combination with subjective and objective criteria. The time points used to release patients to full activity ranged from 6 months to 12 months post-ACLR, with the most common time point being 6 months (5 studies 23,60,70,73,85 ).
Subjective Criteria
The second most commonly used category for RTA (11 studies) was subjective criteria. Of these, 3 studies 44,45,76 used only subjective factors when determining RTA, 1 study 70 used subjective criteria in combination with time, 1 study 85 used subjective criteria in combination with time and objective criteria, and 6 studies 29,41,46,67,68,75 used subjective and objective criteria. The most common subjective criterion was a movement quality assessment, typically evaluated based on movement mechanics, balance, and form. 29,46,67,68,85 Of note, only 5 studies 41,44,75,76,85 considered the patient’s psychological status before RTA, with only 2 studies 41,85 using validated questionnaires (eg, Pediatric International Knee Documentation Committee Questionnaire [Pedi-IKDC] and Anterior Cruciate Ligament–Return to Sport after Injury scale [ACL-RSI]).
Objective Criteria
Most of the reviewed studies (n = 17) used objective criteria to determine RTA. Objective criteria were the only reported criteria used to determine RTA in 10 studies, ‡‡ whereas 6 studies 29,41,46,67,68,75 used both subjective and objective criteria, and 1 study 85 used objective, subjective, and time-based criteria. The most commonly used objective criterion was leg strength (15 studies §§ ). Only 1 study 29 reported knee laxity as a factor for RTA, measured through KT-1000 arthrometry. In addition, only 2 studies 67,68 provided an objective measurement of movement quality by comparing knee flexion angles during a single-leg dip.
Strength and Functional Tasks
The most commonly reported strength or functional tasks for deriving RTA criteria were isokinetic strength (9 studies ∥ ∥ ) and anterior hop (8 studies 29,41,46,53,67,68,81,84 ) (Table 1). Strength and functional tasks were typically evaluated using a limb symmetry index (LSI), with an LSI >90% as the most common threshold for RTA (Table 2). ¶¶
Table 1.
Frequency of Dynamic Tasks Reported in the Literature for Accessing Return to Activity
| Task | No. of Studies |
|---|---|
| Isokinetic strength | 9 |
| Anterior hop | 8 |
| Triple hop | 7 |
| Cross hop | 5 |
| Timed hop | 3 |
| Isometric strength | 2 |
| Single-heel raise | 2 |
| Single-leg dip | 2 |
| Tuck jump | 2 |
| Shuffle t test | 2 |
| Sprint/cutting t test | 2 |
| Vertical jump | 1 |
| Y-balance test | 1 |
| Shuttle run | 1 |
| Sprint and back peddle | 1 |
| Sprint stop and go | 1 |
| Cutting 90° | 1 |
| Side shuffle 90° | 1 |
Table 2.
Frequency of LSI Cutoff Thresholds Reported in the Literature for Each Dynamic Task a
| LSI Cutoff Threshold Reported, No. of Studies | ||||
|---|---|---|---|---|
| Criterion | 100% Symmetry | ≥90% Symmetry | ≥88% Symmetry | ≥85% Symmetry |
| Leg strength | — | 7 | — | 5 |
| Hop tests | — | 6 | — | 1 |
| Jumping tests | — | 1 | — | — |
| Endurance tests | — | 3 | 2 | — |
| Agility tests | — | 1 | — | 1 |
| Y-balance test | — | 1 | — | — |
| Knee kinematics | 2 | — | — | — |
| KOS-ADLS | — | 1 | — | — |
| GRS | — | 1 | — | — |
| Total | 2 | 21 | 2 | 7 |
a Dashes indicate no studies reporting that cutoff threshold. GRS, Global Rating Scale of perceived knee function; KOS-ADLS, Knee Outcome Survey–Activities of Daily Living Scale; LSI, limb symmetry index.
Discussion
This systematic review identified the most commonly used variables reported in the literature when determining unrestricted RTA after an ACLR in pediatric patients. There was substantial variation in the criteria used to determine RTA across studies, with no clear “most common” criteria. These findings are consistent with systematic reviews looking at RTA criteria in adults, 7 as well as studies showing significant variability in the RTA criteria used by children’s hospitals and pediatric orthopaedic surgeons. 22,30 One possible explanation for this variation in RTA criteria is that progression of activity after an ACLR is typically a collaborative process, with shared decision making between the orthopaedic surgeon and the rehabilitation specialist(s). 30
In contrast to a systematic review of RTA in adults, which found that time-based measures dominated RTA criteria (time was the sole RTA criterion in 42% of studies), 13 our findings showed that the most common types of RTA criteria in pediatric and adolescent patients were objective-based (17/27 studies). There are several potential explanations for the higher rate of objective criteria in pediatric studies. First, there are clear methodological differences between the 2 studies, as this review excluded research that (1) did not specify any RTA criteria and (2) did not specify the criteria with enough detail to assess if they were subjective or objective. Second, since this review was specific to pediatric participants, this could suggest that RTA criteria used in pediatric patients are more objective based relative to the criteria used in adult populations. However, it is not clear why this difference may exist. Orthopaedic surgeons could adjust their RTA criteria based on patient’s age/maturation, or there may be environmental factors (increased access to multidisciplinary teams, higher prevalence of pediatric teams located in academic centers, etc) contributing to the difference between patient populations. Finally, this review identified that almost half of the studies (13/27) only used 1 criterion when determining RTA. Considering the numerous psychological, 12,15 biomechanical, 11,56,77 and biological 33 changes that occur after an ACLR, it is unlikely that 1 metric is sufficient when assessing RTA readiness. To include a combination of RTA measures, a collaborative process, including access to the required personnel and equipment, is needed.
With regard to time-based RTA criteria, pediatric patients were typically cleared for RTA between 6 and 12 months after ACLR (8/27 studies). Interestingly, a 2011 study of the Canadian Orthopaedic Association showed that the majority of surgeons allowed RTA between 6 and 9 months postoperatively. 55 However, the inconsistencies in RTA timing should be considered in light of research showing that the rate of secondary knee injuries decreased by approximately 51% for each month that RTA was delayed until 9 months after ACLR. 32 In addition to prolonging the postoperative time before RTA, recent literature has advocated for the use of patient-reported outcome measures (PROMs) to quantify both functional deficits and psychological readiness before RTA. 2,19,48 Despite evidence that the scores from these assessments are associated with interlimb functional asymmetries 87 and an increased risk of second ACL injuries, 63 only 2 studies measured PROMs using validated questionnaires (Pedi-IKDC, ACL-RSI, etc). 41,85 Considering that patient psychological factors are predictive of ACLR outcomes, 4,20 validated and age-appropriate PROMs seem warranted as RTA criteria. This underscores the importance of developing and validating pediatric-specific PROMs questionnaires, as many PROMs in adults are not transferable to the younger population. 39
Our review demonstrates that very few studies (2/27) used an objective assessment of movement biomechanics when determining RTA. 67,68 Despite advances in modern operative techniques and rehabilitation programs, there is strong evidence that deficits in balance, proprioception, muscle strength, and neuromuscular control exist for many months postoperatively. 11,43,56,77 Numerous studies have shown that even when a patient’s rehabilitation is deemed successful, differences in joint kinematics and gait patterns (eg, anteroposterior translation, hamstring muscle activation) can be observed for up to 5 to 10 years after an ACLR. 11,43,56,77 These findings indicate that RTA criteria that solely focus on spatiotemporal variables (eg, distance and time) or subjective evaluations of movement quality are not adequate to identify individuals with dysfunctional movement patterns. Future research should standardize, validate, and make uniformly available a clear and usable battery of objective, in-depth biomechanical assessments.
This is of particular note considering that the most commonly used strength and functional tasks were evaluated by comparing between-limb spatiotemporal differences (eg, LSI). Specifically, LSI during a lower limb strength test was the most commonly used objective criterion for RTA (15/27 studies). Although >90% was the most commonly used threshold, a recent systematic review showed that mean LSI values for isokinetic knee extension were frequently in the 70% range at 6 months and remained below 90% in almost all categories at the 1-year mark postoperatively. 1 These findings may explain why several studies used a lower LSI of 85% when evaluating strength testing before RTA. The most frequently used functional tasks were the 4 standard hop tests (single-leg, cross hop, triple, and 6-m timed hop tests), 61 also evaluated with an LSI threshold of >90% for RTA. Despite the widespread use of these tests in pediatric and adult patients, the results of hop testing have not been correlated with a reduced risk for reinjury. 52,83 They have, however, been correlated with knee function as measured with self-reported questionnaires. 31,50 Previous research suggests that the 6-m timed and cross hop tests are the best predictors of normal subjective knee function as measured by the 2000 IKDC Subjective Knee Evaluation form. 31,50 This is likely because the cross hop test requires coordination and strength in the frontal, sagittal, and coronal planes. Interestingly, according to our review, the cross hop and 6-m timed hop tests were the least frequently used of the 4 common hopping tests. This is consistent with previous research showing that only 30% of orthopaedic surgeons used the full complement of hop tests described by Noyes et al, 61 despite experts advocating for the entire hop battery. 2,19,50,51
Despite the widespread use of LSI in the literature, the support for LSIs as an RTA criterion is mixed. Some studies have shown that patients who achieved an LSI >90% during lower limb strength and functional testing 6 months after ACLR had superior knee function and higher activity levels 2 years after surgery. 79 However, it should be noted that comparing between-limb differences after ACLR surgery has limitations. As activity levels decline after injury, strength and functional deficits occur in both the involved and the contralateral limb. 16 This is supported in the literature, with some research showing that patients with excellent performance on their isokinetic strength testing at 6 months are at a greater risk for sustaining a contralateral ACL injury. 79 This is likely a consequence of reduced functional status in both limbs after ACLR and may indicate that LSIs are not sufficient as a stand-alone criterion for RTA. 79
Finally, it should be noted that many of the RTA assessments identified in this review, such as hop test LSIs, were derived from research conducted on adult populations. 2,26,27 Although these RTA assessments reflect the best available evidence, it must be acknowledged that these assessments do not always translate to the pediatric population. In particular, pediatric RTA assessments should take into consideration maturational changes, as pediatric patients are expected to RTA with their peers who have the advantage of developing from a musculoskeletal perspective without the dysfunction associated with an ACL injury. Furthermore, sex-specific changes during puberty, such as pelvic-width growth 17 and increased estrogen production, 49 have been linked to an increased risk for ACL injury in females. 10 Thus, considering the known sex- and age-specific risk factors for ACL injury, 59,64 and the unique challenges of treating a pediatric population, the creation and validation of sex- and age-specific RTA assessment criteria are needed. It is also worth noting that RTA criteria are typically derived by retrospectively identifying associations between routine postoperative testing at 6 to 12 months and ACL reinjury rates. 13 However, to effectively develop sex- and age-specific RTA criteria, RTA testing should be performed immediately before RTA and retrospectively investigated for predictive factors for reinjury.
This review only included studies that reported RTA criteria with sufficient detail to assess whether the criteria were subjective or objective. Thus, there is potential that some of the excluded investigations did in fact measure RTA criteria but did not include sufficient detail to make this information usable and were therefore excluded. To enhance the transparency of our review, we have included the list of articles excluded for having insufficiently detailed RTA criteria (Supplemental Table S3). In addition, only 3 studies 29,75,76 included the participant’s sport and only 10 studies ## included the sport level. Thus, while activity type and level may influence the RTA criteria used by clinicians, we were not able to incorporate this information in our analysis. Furthermore, from this review, we were not able to compare the ACL failure rates associated with each RTA criterion. This type of analysis would require a separate investigation in which cohorts are carefully matched for graft type, sex ratio, chronicity of injury, concomitant injuries, articular cartilage deterioration, postoperative sports activity level, and time of follow-up.
Conclusion
With limited available evidence to support pediatric RTA decision making, 62 it is not surprising that we found significant variability in the field, with no consensus on the appropriate criteria for RTA after a pediatric ACLR. Furthermore, almost half of the studies included in the review only used 1 criterion when determining RTA timing, despite the multifactorial nature of ACLR. According to our findings, current criteria have also focused on more easily collected metrics, such as time from surgery or hopping LSIs. Criteria such as PROMs and objective lower limb biomechanics are seldom used as RTA criteria despite some evidence suggesting their association with ACLR outcomes. Given the lack of consensus regarding RTA metrics in pediatric patients, there is a clear need for future research to validate and support evidence-based RTA criteria in this high-risk population. This review provides the foundation for future research to build effective and standardized RTA assessments for pediatric patients. Researchers can use our findings to validate and adapt current RTA criteria or target new areas for RTA development according to the identified literature gaps. These methods must be accessible and compatible with clinical practice to facilitate adaptation in the clinical environment, while also being age-- and sexspecific.
Supplemental Material for this article is available at http://journals.sagepub.com/doi/suppl/full/10.1177/23259671231154540#supplementarymaterials
Supplemental Material
Supplemental Material, sj-pdf-1-ojs-10.1177_23259671231154540 for Criteria Used to Determine Unrestricted Return to Activity After ACL Reconstruction in Pediatric and Adolescent Patients: A Systematic Review by Nicholas J. Romanchuk, Holly Livock, Kenneth J. Lukas, Michael J. Del Bel, Daniel L. Benoit and Sasha Carsen in Orthopaedic Journal of Sports Medicine
Acknowledgment
The authors acknowledge Nigèle Langois for her help in creating the literature search strategy and Patrick Sachsalber for his contribution to the data collection. The authors would also like to thank the Ontario Graduate Scholarship, Natural Sciences and Engineering Research Council of Canada, Arthroscopy Association of North America, CHEO Research Institute, and University of Ottawa Department of Surgery for their support.
APPENDIX
Appendix Table A1.
Characteristics of the Included Studies a
| Lead Author (Year) | Sample Size, n | Age at Surgery, y | RTA Criteria | Follow-up, y | No. (%) of ACL Graft Failures | No. (%) of Contralateral ACL Injuries |
|---|---|---|---|---|---|---|
| Anderson (2003) 3 | 12 | 13.3 ± 1.4 | Time (∼6.5 mo) | 4.1 ± 1.9 | 0 (0) | 0 (0) |
| Aronowitz (2000) 6 | 19 | 13.3 ± 1 | Quad strength (LSI >90%) | 2.1 ± 1.1 | 0 (0) | 0 (0) |
| Calvo (2015) 14 | 27 | 13 ± 1 | Isokinetic strength (LSI >90%) | 10.6 ± 0.8 | 4 (15) | NR |
| Fourman (2021) 23 | 43 | 11.4 ± 1.8 | 6 mo noncontact sports; 9 mo contact sports | 5.5 ± 2.4 | 4 (11) | NR |
| Gagliardi (2020) 24 | 81 | 15.9 ± 1.7 | Endurance, jumping, strength and agility single-leg tests (LSI >90%) | 3.1 ± 0.2 | 1 (1) | 8 (10) |
| Goddard (2013) 25 | 32 | 12.4 ± 2.5 | Time (12 mo) | 2 ± 0 | 0 (0) | NR |
| Graziano (2017) 29 | 42 | 12 ± 1.3 | KT-1000 arthrometry (LSI), isokinetic leg strength (LSI), single-leg hop (LSI), sport-specific movements (control and quality) | NR | 3 (7) | 2 (5) |
| Johnson (2020) 41 | 26 | NR | Quadriceps index >90%, single-leg hop test >90%, KOS-ADLS >90%, single-item GRS >90%, surgeon approval | 2 ± 0 | 3 (12) | 5 (19) |
| Kocher (2018) 44 | 237 | 11.2 ± 1.7 | Patient tolerance | 6.2 ± 5.7 | 9 (7) | NR |
| Lanzetti (2020) 45 | 42 | 12.5 ± 0.8 | Muscular tropism judged by surgeon | 8 ± 12.5 | 2 (5) | 1 (2) |
| Larson (2016) 46 | 29 | 13.9 ± 1.6 | Anterior and triple hop (LSI >90%, landing and pivoting mechanics) | 4 ± 1.3 | 5 (17) | 5 (17) |
| Luo (2015) 53 | 124 | 16.1 ± 1.4 | Satisfactory score on 6 of the 7 isokinetic knee extension and flexion strength (LSI >85%), vertical jump, single- and triple-hop tests (LSI >90%) | 0.5 ± 0 | NR | NR |
| Nikolaou (2011) 60 | 8 | 13.7 ± 1.1 | 6 mo | NR | 4 (4) | NR |
| Pennock (2018) 67 | 30 | 11.8 ± 1.6 | ROM (LSI 100%), girth (0-1 cm), isokinetic leg strength (LSI), single-leg heel raise (LSI >90%), single-leg dip angle (LSI 100%), single-leg dip endurance (LSI >88%), single-leg anterior, cross, and triple hop (LSI >90%), tuck jump (no deviations), t test shuffle and sprint (no hesitation or valgus) | 3.2 ± 0.8 | 4 (15) | 3 (12) |
| Pennock (2019) 68 | 90 | 14.8 ± 1.4 | Same as for Pennock (2018) | 2.7 ± 0.9 | 13 (16) | 10 (12) |
| Salmon (2018) 70 | 39 | 16 ± 1 | Time (6 mo), ligamentous stability judged by surgeon | NR | 15 (39) | 5 (13) |
| Sankar (2006) 72 | 12 | 15.6 ± 1.1 | Isokinetic quadriceps strength (LSI >85%) | 5.3 ± 1.4 | 0 (0) | 0 (0) |
| Sankar (2008) 71 | 247 | 15.4 ± 1.3 | Quadriceps strength (LSI >85%) | 6.3 ± 2 | 17 (7) | NR |
| Severyns (2016) 73 | 11 | 13.5 ± 1.2 | Time (6 mo) | 2.08 ± 1.3 | NR | NR |
| Shelbourne (2004) 75 | 16 | 14.8 ± 0.7 | ROM (LSI 100%), patient confidence, isokinetic quadriceps strength (LSI >90%) | 3.4 ± 1.1 | 1 (6) | 2 (13) |
| Shelbourne (2009) 74 | 528 | NR | ROM (LSI 100%), isokinetic quadriceps strength (LSI >85%) | >5 | 46 (9) | 46 (9) |
| Shelbourne (2009) 76 | 402 | 15.6 ± 1 | Patients felt comfortable | 9.8 ± 5.2 | 43 (11) | 63 (16) |
| Streich (2010) 80 | 16 | 11 ± 0.8 | Time (12 mo) | 5.8 ± 0.9 | 0 (0) | NR |
| Sugimoto (2020) 81 | 105 | 13.4 ± 1.4 | Isometric leg strength (LSI >90%); anterior, cross, triple, and timed hop tests (LSI >90%); Y-balance test (LSI >90%) | 0.5-0.75 | NR | NR |
| Wall (2017) 82 | 27 | 11.4 ± 1.9 | Leg strength, hop, agility (LSI >85%) | 3.6 ± 1.4 | 4 (15) | 2 (7) |
| Willimon (2015) 84 | 21 | 11.8 ± 1 | Anterior, cross, triple, and timed hops (LSI) | 3 ± 1.5 | 2 (10) | NR |
| Willson (2018) 85 | 23 | 13 ± 1.4 | Time (6 mo), no pain or swelling; ROM (LSI 100%), Pedi-IKDC and ACL-RSI score, isometric and isokinetic leg strength (LSI >90%), hopping tests (distance and form) | 1.8 ± 1 | 0 (0) | 3 (13) |
a Data are reported as n (%) or mean ± SD unless otherwise indicated. ACL, anterior cruciate ligament; ACL-RSI, Anterior Cruciate Ligament–Return to Sport after Injury scale; GRS, Global Rating Scale of perceived knee function; KOS-ADLS, Knee Outcome Survey–Activities of Daily Living Scale; LSI, limb symmetry index; NR, not reported; Pedi-IKDC, Pediatric International Knee Documentation Committee questionnaire; ROM, range of motion; RTA, return to activity.
Final revision submitted September 22, 2022; accepted October 11, 2022.
One or more of the authors has declared the following potential conflict of interest or source of funding: S.C. has received research support from ConMed Linvatec and consulting fees from Smith & Nephew and Stryker. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Abrams GD, Harris JD, Gupta AK, et al. Functional performance testing after anterior cruciate ligament reconstruction. Orthop J Sports Med. 2014;2(1):2325967113518305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adams D, Logerstedt D, Hunter-Giordano A, Axe MJ, Snyder-Mackler L. Current concepts for anterior cruciate ligament reconstruction: a criterion-based rehabilitation progression. J Orthop Sports Phys Ther. 2012;42(7):601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson AF. Transepiphyseal replacement of the anterior cruciate ligament in skeletally immature patients. A preliminary report. J Bone Joint Surg Am. 2003;85(7):1255–1263. [DOI] [PubMed] [Google Scholar]
- 4. Ardern CL, Taylor NF, Feller JA, Webster KE. A systematic review of the psychological factors associated with returning to sport following injury. Br J Sports Med. 2013;47(17):1120–1126. [DOI] [PubMed] [Google Scholar]
- 5. Arem Lim KS, Mile Ini EN, Amien Orestier DF, et al. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. [DOI] [PubMed] [Google Scholar]
- 6. Aronowitz ER, Ganley TJ, Goode JR, Gregg JR, Meyer JS. Anterior cruciate ligament reconstruction in adolescents with open physes. Am J Sports Med. 2000;28(2):168–175. [DOI] [PubMed] [Google Scholar]
- 7. Barber-Westin SD, Noyes FR. Factors used to determine return to unrestricted sports activities after anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(12):1697–1705. [DOI] [PubMed] [Google Scholar]
- 8. Barber-Westin SD, Noyes FR. Objective criteria for return to athletics after anterior cruciate ligament reconstruction and subsequent reinjury rates: a systematic review. Phys Sportsmed. 2011;39(3):100–110. [DOI] [PubMed] [Google Scholar]
- 9. Beck N, Lawrence J, Nordin J, DeFor TA, Tompkins M. ACL tears in school-aged children and adolescents: has there been an increased incidence over the last 20 years? Pediatrics. 2017;139(3):e20161877. [DOI] [PubMed] [Google Scholar]
- 10. Boden BP, Sheehan FT, Torg JS, Hewett TE. Noncontact anterior cruciate ligament injuries: mechanisms and risk factors. J Am Acad Orthop Surg. 2010;18(9):520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bourne MN, Bruder AM, Mentiplay BF, Carey DL, Patterson BE, Crossley KM. Eccentric knee flexor weakness in elite female footballers 1–10 years following anterior cruciate ligament reconstruction. Phys Ther Sport. 2019;37:144–149. [DOI] [PubMed] [Google Scholar]
- 12. Brewer BW, Cornelius AE, Stephan Y, Van Raalte J. Self-protective changes in athletic identity following anterior cruciate ligament reconstruction. Psychol Sport Exerc. 2010;11(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burgi CR, Peters S, Ardern CL, et al. Which criteria are used to clear patients to return to sport after primary ACL reconstruction? A scoping review. Br J Sports Med. 2019;53(18):1154–1161. [DOI] [PubMed] [Google Scholar]
- 14. Calvo R, Figueroa D, Gili F, et al. Transphyseal anterior cruciate ligament reconstruction in patients with open physes: 10-year follow-up study. Am J Sports Med. 2015;43(2):289–294. [DOI] [PubMed] [Google Scholar]
- 15. Christino MA, Fantry AJ, Vopat BG. Psychological aspects of recovery following anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2015;23(8):501–509. [DOI] [PubMed] [Google Scholar]
- 16. Chung KS, Ha JK, Yeom CH, et al. Are muscle strength and function of the uninjured lower limb weakened after anterior cruciate ligament injury? Am J Sports Med. 2015;43(12):3013–3021. [DOI] [PubMed] [Google Scholar]
- 17. Coleman WH. Sex differences in the growth of the human bony pelvis. Am J Phys Anthropol. 1969;31(2):125–151. [DOI] [PubMed] [Google Scholar]
- 18. Dekker TJ, Godin JA, Dale KM, Garrett WE, Taylor DC, Riboh JC. Return to sport after pediatric anterior cruciate ligament reconstruction and its effect on subsequent anterior cruciate ligament injury. J Bone Joint Surg Am. 2017;99(11):897–904. [DOI] [PubMed] [Google Scholar]
- 19. Dingenen B, Gokeler A. Optimization of the return-to-sport paradigm after anterior cruciate ligament reconstruction: a critical step back to move forward. Sport Med. 2017;47(8):1487–1500. [DOI] [PubMed] [Google Scholar]
- 20. Everhart JS, Best TM, Flanigan DC. Psychological predictors of anterior cruciate ligament reconstruction outcomes: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2015;23(3):752–762. [DOI] [PubMed] [Google Scholar]
- 21. Feucht MJ, Cotic M, Saier T, et al. Patient expectations of primary and revision anterior cruciate ligament reconstruction. Knee Surg Sport Traumatol Arthrosc. 2016;24(1):201–207. [DOI] [PubMed] [Google Scholar]
- 22. Forrester LA, Schweppe EA, Popkin CA. Variability in rehabilitation protocols following pediatric anterior cruciate ligament (ACL) reconstruction. Phys Sportsmed. 2019;47(4):448–454. [DOI] [PubMed] [Google Scholar]
- 23. Fourman MS, Hassan SG, Roach JW, Grudziak JS. Anatomic all-epiphyseal ACL reconstruction with “inside-out” femoral tunnel placement in immature patients yields high return to sport rates and functional outcome scores a minimum of 24 months after reconstruction. Knee Surg Sport Traumatol Arthrosc. 2021;29(12):4251–4260. [DOI] [PubMed] [Google Scholar]
- 24. Gagliardi AG, Carry PM, Parikh HB, Albright JC. Outcomes of quadriceps tendon with patellar bone block anterior cruciate ligament reconstruction in adolescent patients with a minimum 2-year follow-up. Am J Sports Med. 2020;48(1):93–98. [DOI] [PubMed] [Google Scholar]
- 25. Goddard M, Bowman N, Salmon LJ, Waller A, Roe JP. Endoscopic anterior cruciate ligament reconstruction in children using living donor hamstring tendon allografts. Am J Sports Med. 2013;41(3):567–574. [DOI] [PubMed] [Google Scholar]
- 26. Gokeler A, Welling W, Benjaminse A, Lemmink K, Seil R, Zaffagnini S. A critical analysis of limb symmetry indices of hop tests in athletes after anterior cruciate ligament reconstruction: a case control study. Orthop Traumatol Surg Res. 2017;103(6):947–951. [DOI] [PubMed] [Google Scholar]
- 27. Gokeler A, Welling W, Zaffagnini S, Seil R, Padua D. Development of a test battery to enhance safe return to sports after anterior cruciate ligament reconstruction. Knee Surg Sport Traumatol Arthrosc. 2017;25(1):192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gornitzky AL, Lott A, Yellin JL, Fabricant PD, Lawrence JT, Ganley TJ. Sport-specific yearly risk and incidence of anterior cruciate ligament tears in high school athletes: a systematic review and meta-analysis. Am J Sports Med. 2016;44(10):2716–2723. [DOI] [PubMed] [Google Scholar]
- 29. Graziano J, Chiaia T, de Mille P, Nawabi DH, Green DW, Cordasco FA. Return to sport for skeletally immature athletes after ACL reconstruction: preventing a second injury using a quality of movement assessment and quantitative measures to address modifiable risk factors. Orthop J Sports Med. 2017;5(4):2325967117700599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greenberg EM, Greenberg ET, Albaugh J, Storey E, Ganley TJ. Anterior cruciate ligament reconstruction rehabilitation clinical practice patterns: a survey of the PRiSM Society. Orthop J Sports Med. 2019;7(4):2325967119839041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grindem H, Logerstedt D, Eitzen I, et al. Single-legged hop tests as predictors of self-reported knee function in nonoperatively treated individuals with anterior cruciate ligament injury. Am J Sports Med. 2011;39(11):2347–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grindem H, Snyder-Mackler L, Moksnes H, Engebretsen L, Risberg MA. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware-Oslo ACL cohort study. Br J Sports Med. 2016;50(13):804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harkey MS, Luc BA, Golightly YM, et al. Osteoarthritis-related biomarkers following anterior cruciate ligament injury and reconstruction: a systematic review. Osteoarthritis Cartilage. 2015;23(1):1–12. [DOI] [PubMed] [Google Scholar]
- 34. Herzog MM, Marshall SW, Lund JL, Pate V, Mack CD, Spang JT. Incidence of anterior cruciate ligament reconstruction among adolescent females in the United States, 2002 through 2014. JAMA Pediatr. 2017;171(8):808–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hewett TE, Myer GD, Ford KR. Decrease in neuromuscular control about the knee with maturation in female athletes. J Bone Joint Surg Am. 2004;86(8):1601–1608. [DOI] [PubMed] [Google Scholar]
- 36. Higgins JP, Deeks JJ, Altman DG. Special topics in statistics. In: Higgins JP, Green S. (eds). Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. John Wiley & Sons; 2008:481–529. [Google Scholar]
- 37. Higgins JP, Deeks JJ. Selecting studies and collecting data. In: Higgins JP, Deeks JJ. (eds). Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. John Wiley & Sons; 2008:151–185. [Google Scholar]
- 38. Higgins JP, Li T, Deeks JJ. Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. (eds). Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Wiley; 2019:143–176. [Google Scholar]
- 39. Iversen MD, Lee B, Connell P, Andersen J, Anderson AF, Kocher MS. Validity and comprehensibility of the International Knee Documentation Committee Subjective Knee Evaluation form in children. Scand J Med Sci Sports. 2010;20(1):e87–e95. [DOI] [PubMed] [Google Scholar]
- 40. Jensen RK, Nassas G. Growth of segment principal moments of inertia between four and twenty years. Med Sci Sports Exerc. 1988;20(6):594–604. [PubMed] [Google Scholar]
- 41. Johnson J, Capin J, Arundale A, Zarzycki R, Smith A, Snyder-Mackler L. A secondary injury prevention program may decrease contralateral anterior cruciate ligament injuries in female athletes: 2-year injury rates in the ACL-SPORTS randomized controlled trial. J Orthop Sports Phys Ther. 2020;50(9):523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kaeding CC, Flanigan D, Donaldson C. Surgical techniques and outcomes after anterior cruciate ligament reconstruction in preadolescent patients. Arthroscopy. 2010;26(11):1530–1538. [DOI] [PubMed] [Google Scholar]
- 43. Kaur M, Cury Ribeire D, Theis JC, Webster KE, Sole G. Movement patterns of the knee during gait following ACL reconstruction: a systematic review and meta-analysis. Sports Med. 2016;46(12):1869–1895. [DOI] [PubMed] [Google Scholar]
- 44. Kocher MS, Heyworth BE, Fabricant PD, Tepolt FA, Micheli LJ. Outcomes of physeal-sparing ACL reconstruction with iliotibial band autograft in skeletally immature prepubescent children. J Bone Joint Surg Am. 2018;100(13):1087–1094. [DOI] [PubMed] [Google Scholar]
- 45. Lanzetti RM, Pace V, Ciompi A, Perugia D, Spoliti M, Falez F. Over the top anterior cruciate ligament reconstruction in patients with open physes: a long-term follow-up study. Int Orthop. 2020;44(4):771–778. [DOI] [PubMed] [Google Scholar]
- 46. Larson CM, Heikes CS, Ellingson CI, et al. Allograft and autograft transphyseal anterior cruciate ligament reconstruction in skeletally immature patients: outcomes and complications. Arthrosc J Arthrosc Relat Surg. 2016;32(5):860–867. [DOI] [PubMed] [Google Scholar]
- 47. Law MA, Ko YA, Miller AL, et al. Age, rehabilitation and surgery characteristics are re-injury risk factors for adolescents following anterior cruciate ligament reconstruction. Phys Ther Sport. 2021;49:196–203. [DOI] [PubMed] [Google Scholar]
- 48. Lentz TA, Zeppieri G, Tillman SM, et al. Comparison of physical impairment, functional, and psychosocial measures based on fear of reinjury/lack of confidence and return-to-sport status after ACL reconstruction. Am J Sports Med. 2015;43(2):345–353. [DOI] [PubMed] [Google Scholar]
- 49. Liu SH, Al-Shaikh R, Panossian V, et al. Primary immunolocalization of estrogen and progesterone target cells in the human anterior cruciate ligament. J Orthop Res. 1996;14(4):526–533. [DOI] [PubMed] [Google Scholar]
- 50. Logerstedt D, Grindem H, Lynch A, et al. Single-legged hop tests as predictors of self-reported knee function after anterior cruciate ligament reconstruction: the Delaware-Oslo ACL cohort study. Am J Sports Med. 2012;40(10):2348–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Logerstedt DS, Snyder-Mackler L, Ritter RC, Axe MJ, Godges JJ. Knee stability and movement coordination impairments: knee ligament sprain. J Orthop Sports Phys Ther. 2010;40(4):A1–A37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Losciale JM, Zdeb RM, Ledbetter L, Reiman MP, Sell TC. The association between passing return-to-sport criteria and second anterior cruciate ligament injury risk: a systematic review with meta-analysis. J Orthop Sports Phys Ther. 2019;49(2):43–54. [DOI] [PubMed] [Google Scholar]
- 53. Luo DT, Ashraf A, Dahm DL, Stuart MJ, McIntosh AL. Femoral nerve block is associated with persistent strength deficits at 6 months after anterior cruciate ligament reconstruction in pediatric and adolescent patients. Am J Sports Med. 2015;43(2):331–336. [DOI] [PubMed] [Google Scholar]
- 54. McConkey MO, Bonasia DE, Amendola A. Pediatric anterior cruciate ligament reconstruction. Curr Rev Musculoskelet Med. 2011;4(2):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McRae SM, Chahal J, Leiter JR, Marx RG, MacDonald PB. Survey study of members of the Canadian Orthopaedic Association on the natural history and treatment of anterior cruciate ligament injury. Clin J Sport Med. 2011;21(3):249–258. [DOI] [PubMed] [Google Scholar]
- 56. Messer DJ, Shield AJ, Williams MD, Timmins RG, Bourne MN. Hamstring muscle activation and morphology are significantly altered 1–6 years after anterior cruciate ligament reconstruction with semitendinosus graft. Knee Surg Sport Traumatol Arthrosc. 2020;28(3):733–741. [DOI] [PubMed] [Google Scholar]
- 57. Moher D, Liberati A, Tetzlaff J, Altman DG; the PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Morgan MD, Salmon LJ, Waller A, Roe JP, Pinczewski LA. Fifteen-year survival of endoscopic anterior cruciate ligament reconstruction in patients aged 18 years and younger. Am J Sports Med. 2016;44(2):384–392. [DOI] [PubMed] [Google Scholar]
- 59. Myer GD, Sugimoto D, Thomas S, Hewett TE. The influence of age on the effectiveness of neuromuscular training to reduce anterior cruciate ligament injury in female athletes: a meta-analysis. Am J Sports Med. 2013;41(1):203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nikolaou P, Kalliakmanis A, Bousgas D, et al. Intraarticular stabilization following anterior cruciate ligament injury in children and adolescents. Knee Surg Sport Traumatol Arthrosc. 2011;19:801–805. [DOI] [PubMed] [Google Scholar]
- 61. Noyes FR, Barber SD, Mangine RE. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am J Sports Med. 1991;19(5):513–518. [DOI] [PubMed] [Google Scholar]
- 62. Otsuki R, Del Bel MJ, Benoit DL. Sex differences in muscle activation patterns associated with anterior cruciate ligament injury during landing and cutting tasks: a systematic review. J Electromyogr Kinesiol. 2021;60:102583. [DOI] [PubMed] [Google Scholar]
- 63. Paterno MV, Flynn K, Thomas S, Schmitt LC. Self-reported fear predicts functional performance and second ACL injury after ACL reconstruction and return to sport: a pilot study. Sports Health. 2018;10(3):228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Paterno MV, Huang B, Thomas S, Hewett TE, Schmitt LC. Clinical factors that predict a second ACL injury after ACL reconstruction and return to sport: preliminary development of a clinical decision algorithm. Orthop J Sports Med. 2017;5(12):2325967117745279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of contralateral and ipsilateral anterior cruciate ligament (ACL) injury after primary ACL reconstruction and return to sport. Clin J Sport Med. 2012;22(2):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of second ACL injuries 2 years after primary ACL reconstruction and return to sport. Am J Sports Med. 2014;42(7):1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pennock AT, Chambers HG, Turk RD, Parvanta KM, Dennis MM, Edmonds EW. Use of a modified all-epiphyseal technique for anterior cruciate ligament reconstruction in the skeletally immature patient. Orthop J Sports Med. 2018;6(7):2325967118781769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pennock AT, Johnson KP, Turk RD, et al. Transphyseal anterior cruciate ligament reconstruction in the skeletally immature: quadriceps tendon autograft versus hamstring tendon autograft. Orthop J Sports Med. 2019;7(9):2325967119872450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Romanchuk NJ, Livock H, Lukas KJ, Del Bel MJ, Benoit DL, Carsen S. Protocol for the systematic review of return-to-activity criteria in adolescent patients following an anterior cruciate ligament reconstruction. Syst Rev. 2022;11(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Salmon LJ, Heath E, Akrawi H, Roe JP, Linklater J, Pinczewski LA. 20-year outcomes of anterior cruciate ligament reconstruction with hamstring tendon autograft: the catastrophic effect of age and posterior tibial slope. Am J Sports Med. 2018;46(3):531–543. [DOI] [PubMed] [Google Scholar]
- 71. Sankar W, Carrigan R, Gregg J, Ganley T. Anterior cruciate ligament reconstruction in adolescents: a survivorship analysis. Am J Orthop. 2008;37(1):47–49. [PubMed] [Google Scholar]
- 72. Sankar W, Wells L, Sennett BJ, Wiesel BB, Ganley TJ. Combined anterior cruciate ligament and medial collateral ligament injuries in adolescents. J Pediatr Orthop. 2006;26(6):733–736. [DOI] [PubMed] [Google Scholar]
- 73. Severyns M, Lucas G, Jallageas R, et al. ACL reconstruction in 11 children using the Clocheville surgical technique: objective and subjective evaluation. Orthop Traumatol Surg Res. 2016;102(4):205–208. [DOI] [PubMed] [Google Scholar]
- 74. Shelbourne KD, Gray T, Haro M. Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med. 2009;37(2):246–251. [DOI] [PubMed] [Google Scholar]
- 75. Shelbourne KD, Gray T, Wiley BV. Results of transphyseal anterior cruciate ligament reconstruction using patellar tendon autograft in Tanner stage 3 or 4 adolescents with clearly open growth plates. Am J Sports Med. 2004;32(5):1218–1222. [DOI] [PubMed] [Google Scholar]
- 76. Shelbourne KD, Sullivan AN, Bohard K, Gray T, Urch SE. Return to basketball and soccer after anterior cruciate ligament reconstruction in competitive school-aged athletes. Sports Health. 2009;1(3):236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shimizu T, Cheng Z, Samaan MA, et al. Increases in joint laxity after anterior cruciate ligament reconstruction are associated with sagittal biomechanical asymmetry. Arthrosc J Arthrosc Relat Surg. 2019;35(7):2072–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Slobogean GP, Verma A, Giustini D, Slobogean BL, Mulpuri K. MEDLINE, EMBASE, and Cochrane index most primary studies but not abstracts included in orthopedic meta-analyses. J Clin Epidemiol. 2009;62(12):1261–1267. [DOI] [PubMed] [Google Scholar]
- 79. Sousa PL, Krych AJ, Cates RA, Levy BA, Stuart MJ, Dahm DL. Return to sport: does excellent 6-month strength and function following ACL reconstruction predict midterm outcomes? Knee Surg Sport Traumatol Arthrosc. 2017;25(5):1356–1363. [DOI] [PubMed] [Google Scholar]
- 80. Streich NA, Barié A, Gotterbarm T, Keil M, Schmitt H. Transphyseal reconstruction of the anterior cruciate ligament in prepubescent athletes. Knee Surg Sport Traumatol Arthrosc. 2010;18(11):1481–1486. [DOI] [PubMed] [Google Scholar]
- 81. Sugimoto D, Heyworth BE, Carpenito SC, Davis FW, Kocher MS. Low proportion of skeletally immature patients met return-to-sports criteria at 7 months following ACL reconstruction. Phys Ther Sport. 2020;44:143–150. [DOI] [PubMed] [Google Scholar]
- 82. Wall EJ, Ghattas PJ, Eismann EA, Myer GD, Carr P. Outcomes and complications after all-epiphyseal anterior cruciate ligament reconstruction in skeletally immature patients. Orthop J Sports Med. 2017;5(3):2325967117693604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Webster KE, Hewett TE. What is the evidence for and validity of return-to-sport testing after anterior cruciate ligament reconstruction surgery? A systematic review and meta-analysis. Sports Med. 2019;49(6):917–929. [DOI] [PubMed] [Google Scholar]
- 84. Willimon SC, Jones CR, Herzog MM, May KH, Leake MJ, Busch MT. Micheli anterior cruciate ligament reconstruction in skeletally immature youths: a retrospective case series with a mean 3-year follow-up. Am J Sports Med. 2015;43(12):2974–2981. [DOI] [PubMed] [Google Scholar]
- 85. Willson RG, Kostyun RO, Milewski MD, Nissen CW. Anterior cruciate ligament reconstruction in skeletally immature patients: early results using a hybrid physeal-sparing technique. Orthop J Sports Med. 2018;6(2):2325967118755330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yellin JL, Fabricant PD, Gornitzky A, et al. Rehabilitation following anterior cruciate ligament tears in children: a systematic review. JBJS Rev. 2016;4(1):e4. [DOI] [PubMed] [Google Scholar]
- 87. Zarzycki R, Failla M, Capin JJ, Snyder-Mackler L. Psychological readiness to return to sport is associated with knee kinematic asymmetry during gait following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2018;48(12):968–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-ojs-10.1177_23259671231154540 for Criteria Used to Determine Unrestricted Return to Activity After ACL Reconstruction in Pediatric and Adolescent Patients: A Systematic Review by Nicholas J. Romanchuk, Holly Livock, Kenneth J. Lukas, Michael J. Del Bel, Daniel L. Benoit and Sasha Carsen in Orthopaedic Journal of Sports Medicine