Abstract
Background
Recent trials are conflicting as to whether titration of anaesthetic dose using electroencephalography monitoring reduces postoperative delirium. Titration to anaesthetic dose itself might yield clearer conclusions. We analysed our observational cohort to clarify both dose ranges for trials of anaesthetic dose and biological plausibility of anaesthetic dose influencing delirium.
Methods
We analysed the use of sevoflurane in an ongoing prospective cohort of non-intracranial surgery. Of 167 participants, 118 received sevoflurane and were aged >65 yr. We tested associations between age-adjusted median sevoflurane (AMS) minimum alveolar concentration fraction or area under the sevoflurane time×dose curve (AUC-S) and delirium severity (Delirium Rating Scale-98). Delirium incidence was measured with 3-minute Diagnostic Confusion Assessment Method (3D-CAM) or CAM-ICU. Associations with previously identified delirium biomarkers (interleukin-8, neurofilament light, total tau, or S100B) were tested.
Results
Delirium severity did not correlate with AMS (Spearman's ρ=–0.014, P=0.89) or AUC-S (ρ=0.093, P=0.35), nor did delirium incidence (AMS Wilcoxon P=0.86, AUC-S P=0.78). Further sensitivity analyses including propofol dose also demonstrated no relationship. Linear regression confirmed no association for AMS in unadjusted (log (IRR)=–0.06 P=0.645) or adjusted models (log (IRR)=–0.0454, P=0.735). No association was observed for AUC-S in unadjusted (log (IRR)=0.00, P=0.054) or adjusted models (log (IRR)=0.00, P=0.832). No association of anaesthetic dose with delirium biomarkers was identified (P>0.05).
Conclusion
Sevoflurane dose was not associated with delirium severity or incidence. Other biological mechanisms of delirium, such as inflammation and neuronal injury, appear more plausible than dose of sevoflurane.
Clinical trial registration
Keywords: anaesthesia, biomarker, cognitive dysfunction, delirium, dose dependency, postoperative, sevoflurane, surgery
Editor's key points.
-
•
Whether titration of anaesthetic dose using processed electroencephalography can impact postoperative delirium incidence or severity is currently unresolved due in part to limited dose separation in randomised controlled trials.
-
•
Prospectively collected data from a cohort with postoperative delirium testing were analysed for associations between anaesthetic dosing, delirium incidence and severity, and biomarkers of neuronal damage.
-
•
No associations of sevoflurane dose with the incidence or severity of postoperative delirium, or with increases in plasma biomarkers shown to be associated with delirium, were observed.
-
•
Biologically plausible mechanisms other than anaesthetic dose and neuronal damage, such as neuroinflammation and burst suppression, should be investigated further to explain the risk for postoperative delirium.
There is current debate regarding the hypothesis that avoidance of ‘deep anaesthesia’, achieved through titration of depth of anaesthesia using processed electroencephalography (pEEG) monitors, is associated with less delirium.1, 2, 3 We are concerned by the limited biological rationale for why ‘deep anaesthesia’ should be a risk factor for delirium, particularly given that postoperative delirium may occur days after exposure. Gaskell and Sleigh1 propose that the critical feature of titrating to bispectral index (BIS) is to facilitate anaesthetic dose modulation, and express the hypothesis that dose of anaesthetic is linked to delirium in terms of causal diagrams, and explain that the underlying hypothesis tested by studies such as that conducted by Evered and colleagues3 is that relatively small differences in anaesthetic dosing produce serious cognitive changes that may persist for days. Although the exact mechanism for this is unknown, the burst suppression EEG pattern has been posited as a critical mediator of the putative additional delirium risk. Notably, Chan and colleagues4 observed a significant protective effect of BIS guidance compared with usual care in the frequency of postoperative delirium, with a reduction in median end-tidal anaesthetic concentration (ETAC) of 29.7%. Wildes and colleagues5 compared BIS-guided with ETAC-guided anaesthesia and established a small degree of dose separation (difference of 0.11 minimum alveolar concentration [MAC]) but found no significant benefit of pEEG monitoring in preventing postoperative delirium. Evered and colleagues3 randomised participants to a BIS target value of 35 or 50 to achieve a larger dose separation (median difference of 0.2 MAC) than Wildes and colleagues,5 and observed a reduced risk of delirium with the higher BIS target. These data suggest that a minimum difference of 0.2 MAC could produce differences in odds of postoperative delirium.
Observational studies of the correlation between anaesthetic dose and delirium have also reported mixed findings. In a post hoc analysis of a randomised trial of BIS monitoring, Chan and colleagues4 noted that mean MAC was 0.25 higher in patients with postoperative delirium compared with those who did not develop delirium. This is in contrast to the findings of Whitlock and colleagues,6 who observed an average ETAC of 0.06 less in delirious compared with non-delirious patients. If anaesthetic dose was driving postoperative delirium, we would expect general anaesthesia to be associated with higher rates of delirium than spinal anaesthesia; however, the only large trial to randomise patients to spinal or general anaesthesia found no difference in rates of postoperative delirium.7
Gaskell and Sleigh1 argue that a trial randomising anaesthetic dose rather than an EEG target may facilitate interpretation of the findings. We therefore investigated an ongoing prospective cohort study looking for a dose-response relationship between anaesthetic exposure and subsequent peak delirium severity or delirium incidence. Given that anaesthetic exposure is the proposed harm,1 a dose–response relationship would support a causal relationship. We have shown similar dose-response effects of perioperative inflammation and neurotoxicity (notably neurofilament light, tau, S100B, and interleukin-8)8, 9, 10, 11, 12 on delirium showing that potentially modifiable mechanisms can be identified with our dataset. We probed if anaesthetic exposure showed a similar relationship with delirium severity, which would support biological plausibility for a link and provide information on what separation of dose would inform design of a large randomised control trial. Our primary hypothesis was that median dose exposure to sevoflurane is associated with delirium severity. Secondary hypotheses were that cumulative exposure to sevoflurane is associated with delirium severity and that sevoflurane exposure is associated with increases in pathological biomarkers of delirium.
Methods
Our data were obtained from the ongoing Interventions for Postoperative Delirium Biomarker-3 (IPOD-B3) prospective longitudinal cohort study enrolling patients >65 yr old undergoing non-intracranial surgery (NCT03124303 and NCT01980511), with cohorts as described.8, 9, 10 Ethical approval was obtained from the University of Wisconsin-Madison (UWM) Institutional Review Board (2015–0374). All patients gave written informed consent for the study. Data are reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.13
Exposures
We investigated two anaesthetic exposure metrics: age-adjusted median sevoflurane MAC fraction (AMS) and area under the curve of cumulative dose by time for sevoflurane (AUC-S). General anaesthesia was administered by the patients' physician anaesthetist and was not standardised by the trial protocol. Sevoflurane was the anaesthetic used in 111 patients. Other volatile anaesthetics were not administered. Propofol was administered for induction, as an intraoperative infusion, or both in some subjects, therefore we conducted additional sensitivity analysis including the total propofol dose as a factor. Nitrous oxide was used infrequently in the cohort, largely as an adjunct to emergence, and the median concentration of nitrous oxide was 0% in every subject, so we did not include nitrous oxide in models. Other adjuncts, such as opioids, were not considered, as these will co-vary with anaesthetic dose and are often modulated (uncontrolled) in randomised controlled trials to facilitate control of anaesthetic dose. As we have limited intraoperative EEG depth of anaesthesia monitoring data available in this cohort (n=13), we cannot address whether EEG changes are associated with harm. We excluded patients with a sevoflurane of AMS <0.2 as this is likely insufficient for a state of general anaesthesia.
Outcomes
Our primary outcome was peak delirium severity using the Delirium Rating Scale Revised-98.14 Our secondary outcome was delirium incidence using 3-minute Diagnostic Confusion Assessment Method (3D-CAM)15 or CAM-ICU (if intubated) score from twice daily assessments over up to 4 days postoperatively. We also collected procedure details and other intraoperative data from the medical record, including the American College of Surgeons National Quality Improvement Program surgical risk of death (NSQIP-D) and serious complications (NSQIP-SC). Baseline cognition was assessed with the Trail Making Test B (TMTB) and the Montreal Cognitive Assessment (MoCA).
Power analysis
Data analysis was conducted in R, a language and environment for statistical computing and R Studio (Base R Version 4.2.0, R Studio Version 2022.7.1.554). Using linear regression and a moderate Cohen's effect size (0.4) with seven predictors for sample size analysis (80% power, P<0.05), we required 97 participants to identify an effect using peak delirium severity as a continuous outcome (using R package ‘pwr’ version 1.3-0).16 Our primary outcome utilised a regression approach to adjust for potential confounders associated with delirium and anaesthetic exposure. We did not adjust for multiple comparisons for the number of models or outcomes tested. The regression models presented showed the optimal model selected by fit, interactions, and explained variability.
Statistical analysis
AMS was calculated by dividing median sevoflurane by the formula for age-adjusted MAC (1.8×10(−0.00269×(age−40)).17 AUC-S was determined by multiplying volatile agent dose by time in seconds and normalised by logarithm (base 10). All patients were included in DRS-R-98 analysis regardless of whether they met the CAM criteria for delirium, given the threshold for diagnosis is arbitrary and delirium severity exists on a wider spectrum. We then calculated descriptive statistics, assessed data distribution by plotting, and used the Shapiro–Wilk test for normality. Outliers were validated by Cook's distance, using a conservative (4 μ) threshold and plotting standardised residuals with a plus or minus 2 threshold. We used Spearman's method correlation given data were not normally distributed and compared delirium group means with a Mann–Whitney U-test/Wilcoxon rank sum test with continuity correction.
Results
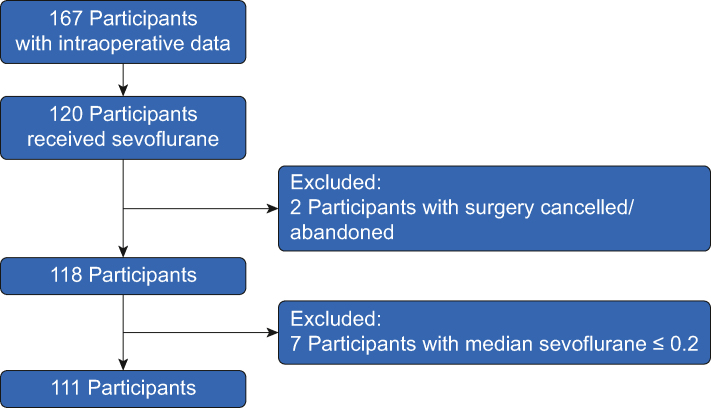
We analysed all available data with a sample size of 111 eligible participants receiving sevoflurane with an AMS >0.2, (Figure 1, delirium n=28 [25.2%], no-delirium n=83). Of these, 109 participants also received propofol. Median AMS was 1.06 (range: 0.20–1.54) in delirious participants and 1.05 (range: 0.24–1.39) in non-delirious participants. The delirium group had higher surgical risk of death (NSQIP-D, Wilcoxon P=0.012), serious complications (NSQIP-SC, Wilcoxon P=0.003) and longer operating times (P=0.006, Table 1) than those without delirium.
Fig 1.
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) diagram.
Table 1.
Patient characteristics. MoCA, Montreal Cognitive Assessment score; NSQIP, American College of Surgeons National Quality Improvement Program risk of death (NSQIP-D) or serious complications (NSQIP-SC); sd, standard deviation; TMTB, Trail Making Test B. ∗Mean (sd), range; n/N (%). †Wilcoxon rank sum test ‡P<0.05; ¶P<0.01; ∗∗∗P<0.001.
| Characteristic | N | Overall, N=111∗ | Delirium incidence |
P-value† | |
|---|---|---|---|---|---|
| Yes, N=28∗ | No, N=83∗ | ||||
| Participant age (yr) | 111 | 72 (5), 65–84 | 72 (4), 66–79 | 72 (5), 65–84 | >0.9 |
| Sex | 111 | 0.067 | |||
| Female | 47/111 (42%) | 16/28 (57%) | 31/83 (37%) | ||
| Male | 64/111 (58%) | 12/28 (43%) | 52/83 (63%) | ||
| NSQIP-D | 111 | 1.82 (2.62), 0.00–14.00 | 2.99 (3.39), 0.10–14.00 | 1.43 (2.19), 0.00–13.10 | 0.012‡ |
| NSQIP-SC | 111 | 15 (10), 0–45 | 21 (13), 4–45 | 13 (8), 0–39 | 0.003¶ |
| Blood loss (ml) | 111 | 907 (2023), 0–15 000 | 1785 (3489), 0–15 000 | 611 (1064), 0–7000 | 0.067 |
| Operating time | 111 | 297 (151), 90–824 | 384 (196), 125–824 | 268 (121), 90–587 | 0.006∗∗ |
| TMTB baseline (s) | 110 | 92 (49), 31–300 | 107 (57), 44–283 | 87 (44), 31–300 | 0.056 |
| MoCA baseline | 105 | 24 (3), 13–30 | 23 (3), 18–29 | 24 (3), 13–30 | 0.4 |
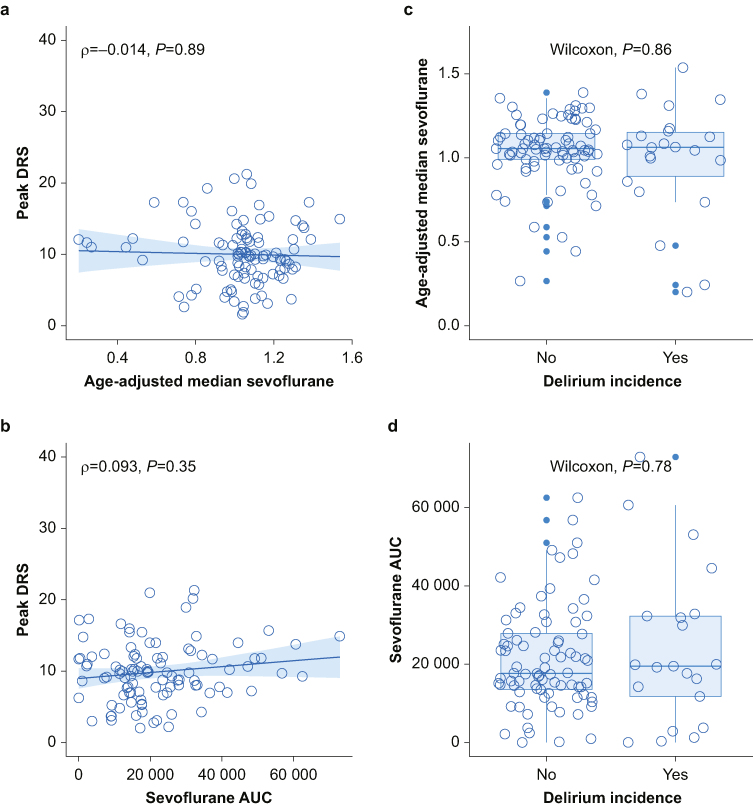
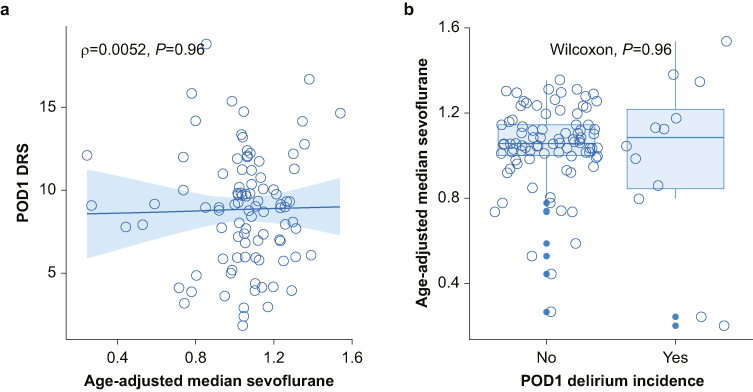
We performed analyses on a dataset excluding formal outliers (eight for DRS∼AUC-S, seven for DRS∼AMS). Figure 2 shows that peak delirium severity did not correlate with either measure of sevoflurane, AMS (ρ=–0.014, P=0.89, Fig 2a) or AUC-S (ρ=0.093, P=0.35, Fig 2b). There was also no statistical difference in means for the binary outcome of delirium incidence (AMS Wilcoxon P=0.86 Fig 2c, AUC-S Wilcoxon P=0.78, Fig 2d). When analysis was further limited to peak delirium severity on postoperative day 1 (as anaesthesia appears more likely to influence events on postoperative day 1 than later), there was no correlation with AMS (ρ=0.0052, P=0.96, Fig 3a) and no difference between delirium group means (Wilcoxon, P=0.96, Fig 3b).
Fig 2.
Correlations between sevoflurane dose, delirium severity, and delirium incidence in 111 participants. Spearman's correlation and Wilcoxon tests were used: (a) and (c) excludes seven outliers for DRS∼AMS, and (b) and (d) excludes eight outliers for DRS∼raw AUC-S. AMS, age-adjusted median sevoflurane minimum alveolar concentration fraction; AUC, area under the curve for sevoflurane (time×dose); DRS, Delirium Rating Scale Revised-98; ρ, rho, Spearman's correlation.
Fig 3.
Correlations between sevoflurane dose, delirium severity, and delirium incidence on postoperative day 1. Spearman's correlation and Wilcoxon tests were used. Seven outliers were excluded for DRS∼AMS. POD1 peak delirium severity includes complete cases only (n=101). AMS, age-adjusted median sevoflurane minimum alveolar concentration fraction; DRS, Delirium Rating Scale Revised-98; POD, postoperative day; ρ, rho, Spearman's correlation.
For our multivariable analyses, generalised linear regressions were adjusted to a Poisson family distribution given Shapiro P=0.0481. In Table 2, the Poisson regression model regression showed no association for peak delirium severity with AMS in unadjusted (log (IRR)=–0.06, P=0.645) or adjusted (log (IRR)=–0.0454, P=0.735) models. Operating time, baseline TMTB, age, female sex, and an interaction between age and TMTB were significant positive predictors of delirium severity in the adjusted model (Table 2). Similarly, AUC-S did not predict delirium severity in an adjusted model (log (IRR)=0.000, P=0.832) (Supplementary Table S1).
Table 2.
Poisson regression peak Delirium Rating Scale vs age-adjusted median sevoflurane dose. Seven outliers were excluded for DRS∼AMS. Models run with Poisson family distribution (dataset after outlier exclusion Shapiro P=0.0481). AMS×operating time (P=0.658), NSQIP-D×operating time (P=0.863), and AMS×NSQIP-D (P=0.171) were all non-significant and were removed from the model. AIC, Akaike's Information Criteria; BIC, Bayesian Information Criteria; CI, confidence interval, df, degrees of freedom; DRS, Delirium Rating Scale Revised-98; IRR, incidence rate ratio; NSQIP, American College of Surgeons National Quality Improvement Program, surgical risk of death (NSQIP-D); SE, standard error; TMTB, Trail Making Test B.
| Characteristic | log (IRR) | SE | 95% CI | P-value |
|---|---|---|---|---|
| Unadjusted analysis | ||||
| (Intercept) | 2.4 | 0.140 | 2.1–2.6 | <0.001∗∗∗ |
| Age-adjusted median sevoflurane dose | –0.06 | 0.133 | –0.32 to 0.20 | 0.645 |
| Adjusted analysis | ||||
| (Intercept) | –0.5089 | 1.18 | –2.8152 to 1.8051 | 0.666 |
| Age-adjusted median sevoflurane dose | –0.0454 | 0.134 | –0.3057 to 0.2210 | 0.735 |
| Age | 0.0347 | 0.016 | 0.0032–0.0660 | 0.031∗ |
| Sex (male) | –0.1487 | 0.064 | –0.2732 to –0.0237 | 0.020∗ |
| NSQIP-D | 0.0129 | 0.013 | –0.0133 to 0.0382 | 0.325 |
| Operating time | 0.0010 | 0.000 | 0.0006, 0.0014 | <0.001∗∗∗ |
| TMTB baseline | 0.0247 | 0.012 | 0.0009–0.0485 | 0.042∗ |
| Age ∗ TMTB baseline | –0.0003 | 0.000 | –0.0006 to 0.0000 | 0.049∗ |
As our data could be confounded by administration of other anaesthetics, we also tested whether propofol dose influenced the relationship with AMS and delirium in a Poisson family regression model (Supplementary Table S2). Neither AMS (log (IRR)=–0.0918, P=0.541) nor intraoperative propofol dose (log (IRR)=–0.0752, P=0.514) predicted peak delirium severity. The only covariates of significance were operating time (log (IRR)=0.0011, P<0.001) and male sex (log (IRR)=−0.1483, P=0.026). The interaction between AMS and propofol dose (P=0.486) was not significant and hence was excluded.
To assess the influence of sevoflurane dose on biomarkers of inflammation and neuronal injury, we explored the relationship of AUC-S and AMS with peak preoperative to postoperative change in each biomarker. We observed no correlation between AMS and plasma interleukin-8 (ρ=–0.06, P=0.55), neurofilament light (ρ=–0.081, P=0.49), tau (ρ=0.017, P=0.89), or S100B (ρ=0.14, P=0.41) (Supplementary Fig. S1). There was also no correlation between peak change in any biomarker and AUC-S.
Discussion
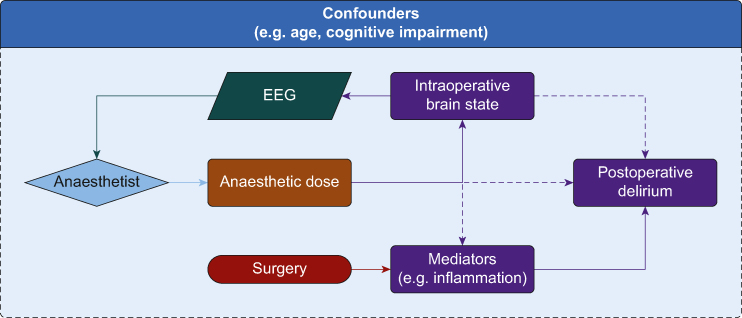
Our data provide additional data regarding the controversy around the relationship of depth of anaesthesia and postoperative delirium incidence and severity. We observed no correlation between sevoflurane dose and delirium incidence or severity. We similarly noted no correlation between sevoflurane dose and plasma interleukin-8, neurofilament light, tau, or S100B. The lack of association of anaesthetic dose with key parameters (inflammation, blood–brain barrier permeability, and neuronal injury) that have been implicated in the pathophysiology of postoperative delirium does not support a causal chain involving anaesthetic exposure (Fig 4). Deiner and colleagues18 recently showed that anaesthetic exposure was not associated with inflammation or neuronal injury in older volunteers, supporting our biomarker observations.
Fig 4.
Causal diagram. Figure 4 presents a causal diagram depicting possible mechanisms for postoperative delirium. Hypothetical pathways whereby anaesthetic dose, as controlled by the anaesthetist, could play a causal role in postoperative delirium incidence are depicted by dashed lines. The hypothesis that risk of delirium is reduced by titration of anaesthetic dose to achieve a particular brain state (as reflected by processed EEG) is depicted as a feedback loop including the anaesthetist. Solid lines show effects with strong evidence, while dashed lines suggest unproved links. We have not drawn all the confounders and their interactions in this causal diagram for simplicity, but there are multiple confounders that should be considered for different interactions.
In our observational cohort, no link between anaesthetic dose and delirium, or known pathological biomarkers of delirium, was observed. Randomised trials of anaesthetic dose currently provide the main means for comparison of this finding, as pEEG monitoring trials offer an indirect proxy for dose. Nonetheless we are unaware of any trials directly randomising anaesthetic dose with an outcome of delirium.
Two trials have failed to show anaesthetic dose separation with pEEG monitoring vs usual care and observed no effect on postoperative delirium.19,20 Of studies that did establish a difference in dose, two observed a significant protective effect of intraoperative EEG monitoring in developing delirium with differences in MAC of 0.2–0.36 between trial groups.3,4 Wildes and colleagues5 found a smaller degree of dose separation (0.11 MAC) and no effect on preventing postoperative delirium. The reasons for the discordance in findings are discussed by Whitlock and colleagues,2 however, one interpretation pertinent to our findings is that the two positive trials were done in East Asian populations, whereas the negative trial was done in a US population similar to our study. The possibility of genotype being an explanatory variable for anaesthetic dose-mediated delirium is intriguing. However, the stark differences in methodology between these trials and a long list of potential confounders in the outcome make it difficult to conclude that a single factor such as genetics explains the differences between these trials and with our findings.
We observed that a lower anaesthetic dose correlated with a higher likelihood of delirium in a binary logistic regression model,6 suggesting that specific patients are vulnerable to a ‘relative anaesthetic overdose’. The results of the interactions in our multivariable regression model suggest baseline TMTB and age do not predict vulnerability to putative anaesthetic-mediated delirium. Whether other cognitive assessment batteries, imaging markers, or blood tests can identify vulnerable populations remains to be seen. Identifying those who might benefit from anaesthetic dose reduction has proved difficult; apart from acute emergence delirium, there is currently no known biological mechanism linking anaesthetic exposure to postoperative delirium, in particular delirium occurring from postoperative Day 1 onwards. There is no clear evidence that anaesthetics are directly neurotoxic in adults,21,22 which is consistent with our data that showed no correlation between sevoflurane dose and plasma neurofilament light or total tau. One inference is that if anaesthetics do play a causative role in delirium, they might do so through systemic side-effects, such as intraoperative hypotension.
The hypothesised causative link between anaesthetic dose and delirium could be greater than the sum of indirect systemic effects. The ‘neuroinflammatory hypothesis of delirium’ contends that delirium is a maladaptive response to systemic inflammation, such as that induced by surgery.23, 24, 25 Assuming a causative role of neuroinflammation in delirium, direct effects of anaesthetics are unlikely as most data suggest that they are not inherently proinflammatory.18,26 Indeed, we observed no correlation between plasma interleukin-8 and sevoflurane dose. An alternative mechanism has been uncovered in rodents, in which anaesthetics have been associated with breakdown of the blood–brain barrier.27, 28, 29, 30, 31 An association between blood–brain barrier breakdown and delirium has been shown in an overlapping group of patients as in this study.12 However, we observed no correlation between sevoflurane dose and peak change in plasma S100B, suggesting anaesthetic exposure does not modulate blood–brain barrier permeability. Overall for the biomarker studies, we found no significant relationship over a clinically usable concentration range. Our data show that the putative mechanistic link between anaesthetic dosing and delirium needs to be clarified. Given that inflammation, blood–brain barrier permeability and neurotoxicity show clear dose-response relationships with delirium severity, and that anaesthetic exposure does not modulate these critical mechanisms in older patients, the basis for this proposed causal chain appears fragile.
There are a number of potential limitations that might be levelled at our analysis. Firstly, the anaesthetists in our study were already expert at identifying participants at risk of delirium and titrated the anaesthetic accordingly, thus eliminating anaesthetic dose as a cause. Although this is possible, we consider it unlikely. For example, anaesthetists were blinded from the cognitive data in our cohort which likely limits delirium risk prediction. Secondly, patients in this cohort fall into a ‘deep anaesthesia’ category as our median MAC ratio was 1.07. However, we focus on the dose–response relationship and not on median dose values. Indeed, our range of MAC values was very wide in the cohort, and certainly in excess of the 0.2 MAC difference suggested as important in clinical trials. Similar approaches have yielded several dose–response relationships in the past for inflammation, blood–brain barrier permeability, and neuronal injury. Nonetheless, one could argue there is an inflection point in the curve at doses below those administered in our cohort. To address this, data from other sources (e.g. Evered and colleagues3) could be used to conduct similar analyses to identify potential relationships with delirium severity or delirium incidence. Thirdly, the hierarchy of evidence suggests that we should dismiss cohort studies that are not concordant with randomised controlled trials. Although we agree randomisation is helpful in defining causality, there are issues with randomised controlled trials in this area as described by Gaskell and Sleigh.1 Biological plausibility remains a central tenet, and ultimately a biological rationale that can explain the proposed link between anaesthetic dose and delirium would further this research area significantly. For small differences in anaesthetic concentrations to mediate an effect on delirium the relationship would need to be strong. We were unable to identify any relationship in our data. Hence, we were unable to identify anaesthetic doses associated with differences in outcomes across our study population.
These data do not support the link between anaesthetic dose and delirium in the causal diagram proposed for testing by Gaskell and Sleigh.1 Rather, if there is an effect, there is some specific pathological brain state that, in response to anaesthesia (perhaps in vulnerable individuals), predisposes to delirium. The descriptors of this pathological brain state remain unclear, with the most likely candidate being burst suppression (as this rarely occurs physiologically). However further research is required to establish the mechanisms of harm in burst suppression and the critical amounts required to produce this potential harm (if any). Indeed, burst suppression does not appear harmful in the young32 and is induced as a neuroprotective therapy in certain clinical situations. We are unable to advise on a suitable dose separation for a future randomised controlled trial and suggest that other datasets, including published randomised pEEG trials themselves, are explored more thoroughly to define critical characteristics of such a trial.
Conclusions
Anaesthetic dose was not associated with delirium severity or incidence or pathological biomarkers of delirium. Further research is required to identify vulnerable populations who might benefit from intraoperative EEG monitoring (if any) to reduce the severity of delirium and the critical features of the intraoperative EEG state (if any) that are linked to delirium.
Authors' contributions
Designed the study: RDS, RCL, RAP
Consultation in design of study: AG, TM
Recruited participants, and collected and processed data: MP, CC, DK, CR
Supplied the assays and managed biofluids analysis: HZ, KB
Analysed data and drafted the manuscript: HZ, JT
Provided input into data analysis and drafting the manuscript: TP
Provided critical feedback on the manuscript: all authors
Declarations of interest
All authors declare no competing interests that may be relevant to the submitted work. HZ has served at scientific advisory boards, as a consultant, or both for AbbVie, Alector, Eisai, Denali, Roche, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, NervGen, AZTherapies, CogRx, and Red Abbey Labs, has given lectures in symposia sponsored by Cellectricon, Fujirebio, AlzeCure, and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS). KB has served as a consultant or at advisory boards for Alector, Alzheon, CogRx, Biogen, Lilly, Novartis, and Roche Diagnostics, and is a co-founder of BBS, all unrelated to the work presented in this paper. RDS is an editor of the British Journal of Anaesthesia.
Funding
US National Institutes of Health (NIH) (R01 AG063849-01 to RDS, RL, RAP); NIH (K23 AG055700 to RDS).
Data availability
Study data are available on reasonable request to qualified investigators with appropriate approvals
Handling editor: Hugh C Hemmings Jr
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2022.08.022.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Gaskell A., Sleigh J. The quagmire of postoperative delirium: does dose matter? Br J Anaesth. 2021;127:664–666. doi: 10.1016/j.bja.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Whitlock E.L., Gross E.R., King C.R., Avidan M.S. Anaesthetic depth and delirium: a challenging balancing act. Br J Anaesth. 2021;127:667–671. doi: 10.1016/j.bja.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Evered L.A., Chan M.T.V., Han R., et al. Anaesthetic depth and delirium after major surgery: a randomised clinical trial. Br J Anaesth. 2021;127:704–712. doi: 10.1016/j.bja.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan M.T., Cheng B.C., Lee T.M., Gin T., Group C.T. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25:33–42. doi: 10.1097/ANA.0b013e3182712fba. [DOI] [PubMed] [Google Scholar]
- 5.Wildes T.S., Mickle A.M., Ben Abdallah A., et al. Effect of electroencephalography-guided anesthetic administration on postoperative delirium among older adults undergoing major surgery: the ENGAGES randomized clinical trial. JAMA. 2019;321:473–483. doi: 10.1001/jama.2018.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitlock E.L., Torres B.A., Lin N., et al. Postoperative delirium in a substudy of cardiothoracic surgical patients in the BAG-RECALL clinical trial. Anesth Analg. 2014;118:809–817. doi: 10.1213/ANE.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neuman M.D., Feng R., Carson J.L., et al. Spinal anesthesia or general anesthesia for hip surgery in older adults. N Engl J Med. 2021;385:2025–2035. doi: 10.1056/NEJMoa2113514. [DOI] [PubMed] [Google Scholar]
- 8.Tanabe S., Mohanty R., Lindroth H., et al. Cohort study into the neural correlates of postoperative delirium: the role of connectivity and slow-wave activity. Br J Anaesth. 2020;125:55–66. doi: 10.1016/j.bja.2020.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballweg T., White M., Parker M., et al. Association between plasma tau and postoperative delirium incidence and severity: a prospective observational study. Br J Anaesth. 2021;126(2):458–466. doi: 10.1016/j.bja.2020.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casey C.P., Lindroth H., Mohanty R., et al. Postoperative delirium is associated with increased plasma neurofilament light. Brain. 2020;143:47–54. doi: 10.1093/brain/awz354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker M., White M., Casey C., et al. Cohort analysis of the association of delirium severity with cerebrospinal fluid amyloid-tau-neurodegeneration pathologies. J Gerontol A Biol Sci Med Sci. 2022;77:494–501. doi: 10.1093/gerona/glab203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor J., Parker M., Casey C., et al. Postoperative delirium and changes in the blood -brain barrier, neuroinflammation, and cerebrospinal fluid lactate: a prospective cohort study. Br J Anaesth. 2022;129(2):219–230. doi: 10.1016/j.bja.2022.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 14.Trzepacz P.T., Mittal D., Torres R., Kanary K., Norton J., Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosciences. 2001;13:229–242. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- 15.Marcantonio E.R., Ngo L.H., O'Connor M., et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161:554–561. doi: 10.7326/M14-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Champely S. pwr: basic functions for power analysis. R Package Version 1.3-0. 2020 https://CRAN.R-project.org/package=pwr [Google Scholar]
- 17.Mapleson W.W. Effect of age on MAC in humans: a meta-analysis. Br J Anaesth. 1996;76:179–185. doi: 10.1093/bja/76.2.179. [DOI] [PubMed] [Google Scholar]
- 18.Deiner S., Baxter M.G., Mincer J.S., et al. Human plasma biomarker responses to inhalational general anaesthesia without surgery. Br J Anaesth. 2020;125:282–290. doi: 10.1016/j.bja.2020.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang C.J., Jin Z., Sands L.P., et al. ADAPT-2: a randomized clinical trial to reduce intraoperative EEG suppression in older surgical patients undergoing major noncardiac surgery. Anesth Analg. 2020;131:1228–1236. doi: 10.1213/ANE.0000000000004713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang E., Wang L., Ye C., et al. Effect of electroencephalography spectral edge frequency (SEF) and patient state index (PSI)-guided propofol-remifentanil anesthesia on delirium after laparoscopic surgery: the eMODIPOD randomized controlled trial. J Neurosurg Anesthesiol. 2022;34:183–192. doi: 10.1097/ANA.0000000000000823. [DOI] [PubMed] [Google Scholar]
- 21.Jevtovic-Todorovic V., Absalom A.R., Blomgren K., et al. Anaesthetic neurotoxicity and neuroplasticity: an expert group report and statement based on the BJA Salzburg Seminar. Br J Anaesth. 2013;111:143–151. doi: 10.1093/bja/aet177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perouansky M., Hemmings H.C., Jr. Neurotoxicity of general anesthetics: cause for concern? Anesthesiology. 2009;111:1365–1371. doi: 10.1097/ALN.0b013e3181bf1d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark I.A., Vissel B. The inflammatory nature of post-surgical delirium predicts benefit of agents with anti-TNF effects, such as dexmedetomidine. Front Neurosci. 2018;12:257. doi: 10.3389/fnins.2018.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maldonado J.R. Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry. 2018;33:1428–1457. doi: 10.1002/gps.4823. [DOI] [PubMed] [Google Scholar]
- 25.Cerejeira J., Firmino H., Vaz-Serra A., Mukaetova-Ladinska E.B. The neuroinflammatory hypothesis of delirium. Acta Neuropathol. 2010;119:737–754. doi: 10.1007/s00401-010-0674-1. [DOI] [PubMed] [Google Scholar]
- 26.Hadimioglu N., Ulugol H., Akbas H., Coskunfirat N., Ertug Z., Dinckan A. Combination of epidural anesthesia and general anesthesia attenuates stress response to renal transplantation surgery. Transplant Proc. 2012;44:2949–2954. doi: 10.1016/j.transproceed.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Acharya N.K., Goldwaser E.L., Forsberg M.M., et al. Sevoflurane and Isoflurane induce structural changes in brain vascular endothelial cells and increase blood-brain barrier permeability: possible link to postoperative delirium and cognitive decline. Brain Res. 2015;1620:29–41. doi: 10.1016/j.brainres.2015.04.054. [DOI] [PubMed] [Google Scholar]
- 28.Yang S., Gu C., Mandeville E.T., et al. Anesthesia and surgery impair blood-brain barrier and cognitive function in mice. Front Immunol. 2017;8:902. doi: 10.3389/fimmu.2017.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Z., Satomoto M., Adachi Y.U., Makita K. Blood-brain barrier disruption caused by neonatal sevoflurane-induced depends on exposure time and is reversible in mice. Korean J Anesthesiol. 2019;72:389–391. doi: 10.4097/kja.d.19.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thal S.C., Luh C., Schaible E.V., et al. Volatile anesthetics influence blood-brain barrier integrity by modulation of tight junction protein expression in traumatic brain injury. PLoS One. 2012;7 doi: 10.1371/journal.pone.0050752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu N., Guo D., Wang H., et al. Involvement of the blood-brain barrier opening in cognitive decline in aged rats following orthopedic surgery and high concentration of sevoflurane inhalation. Brain Res. 2014;1551:13–24. doi: 10.1016/j.brainres.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Shortal B.P., Hickman L.B., Mak-McCully R.A., et al. Duration of EEG suppression does not predict recovery time or degree of cognitive impairment after general anaesthesia in human volunteers. Br J Anaesth. 2019;123:206–218. doi: 10.1016/j.bja.2019.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Study data are available on reasonable request to qualified investigators with appropriate approvals