Abstract
Sleep plays a major role in brain health, and cognition. Disrupted sleep is a well-described symptom of Alzheimer’s disease (AD). However, accumulating evidence suggests suboptimal sleep also increases AD risk. The deacetylase Sirtuin 1 (Sirt 1), encoded by the SIRT1 gene, impacts sleep via its relationship to wake-sleep neurotransmitters and somnogens. Evidence from animal and human studies supports a significant and complex relationship between sleep, Sirt 1/ SIRT1 and AD. Numerous hypotheses attempt to explain the critical impact of Sirt 1/ SIRT1 on wake- and sleep- promoting neurons, their related mechanisms and neurotransmitters. However, there is a paucity of studies assessing the interaction between sleep and Sirt 1/ SIRT1, as a principal component of sleep regulation, on AD pathology. In this review, we explore the potential association between Sirt 1/ SIRT1, sleep, and AD aetiology. Given sleep is a likely modifiable risk factor for AD, and recent studies suggest Sirt 1/ SIRT1 activation can be modulated by lifestyle or dietary approaches, further research in this area is required to explore its potential as a target for AD prevention and treatment.
Keywords: Sleep, Sirtuin 1, SIRT1, Circadian clock, Homeostasis, Neurotransmitter, Alzheimer’s disease, Dementia, Aβ-amyloid, Tau
Introduction
Poor quality, or insufficient sleep is associated with a wide range of adverse health outcomes, and subsequent economic burden [1]. Evidence demonstrates a major role for sleep in brain health, as well as maintenance of cognitive abilities by regulating neuroplasticity, processing of information acquired during wakefulness, and memory consolidation [2].
Suboptimal sleep, characterised by difficulty falling and staying asleep, multiple and long awakenings and/or reductions in restorative slow-wave sleep and rapid eye movement (REM) sleep, is common in older adults [3], with prevalence increasing with age. Potential underlying reasons include alterations in sleep behaviour due to aging, stress, anxiety, medication usage, disease, or a combination of these factors [4].
An emerging body of literature has focussed on the relationship of sleep disturbance to neurodegenerative disease. Alzheimer’s disease (AD) patients show clinical symptoms of sleep abnormalities, with increased fragmentation frequentlyone of the first reported symptoms or complaints [5]. Electroencephalogram (EEG) recordings in AD patients demonstrate a significant reduction of slow-wave sleep and REM sleep, and disruption of arousal in response to external factors [6] Moreover, the sleep-wake cycle in AD is usually disrupted due to nocturnal awakenings and reduced daytime wakefulness following naps [7], [8], [9]. The sleep architecture alterations are hypothesized to exacerbate cerebral AD pathology; particularly, enhanced deposition of Aβ-amyloid in the brain due to reduced clearance during deep sleep, which in turn is associated with a worsening of cognitive symptoms [10].
However, accumulating evidence suggests that a bidirectional relationship between sleep and AD exists. Specifically, rather than simply manifesting as a symptom of AD, poor quality, insufficient sleep, or excessive daytime napping [11], are also proposed to increase future risk of AD. Indeed, a 2017 meta-analysis of sleep studies suggests that individuals with sleep disturbances have an estimated risk for AD that is 1.55 times higher than those without sleep disturbances, and the population attributable risk for AD due to poor sleep is as high as 15 % [12]. This report likely underestimates the true problem given that many of the included studies relied on self-report rather than objective sleep measures.
Sleep is regulated by a homeostatic and a circadian process and these two processes control various aspects of sleep behaviour and associated variables. The protein Sirtuin 1 (Sirt 1), is associated with both circadian rhythm and the homeostatic process of sleep via its relationship to wake-sleep neurotransmitters and somnogens [13], [14]. Accordingly, Sirt 1 plays an important role in cell survival and is purported to be protective against Aβ-amyloid deposition and AD-related tau pathology [15], [16]. Here, we review the potential associations between Sirt 1, its encoding gene (SIRT1), sleep regulation and AD. We also discuss some activators of Sirt 1 in the context of AD prevention, and make recommendations for future research which may aid in further understanding the complex and multi-faceted relationship between Sirt 1/ SIRT1, sleep, and AD aetiology.
Sirtuins
Sirtuins, also known as Silent information regulator 2 (Sir2) proteins, were first identified in yeast (Saccharomyces cerevisiae); they are a subtype of a conserved family of nicotinamide adenine dinucleotide (NAD)-dependent protein deacetylases, and are categorized as class III histone deacetylases (HDACs). Sirtuins remove the acetyl group from both histones and non-histone proteins, including transcription factors and enzymes. In humans and other mammals, there are seven sirtuins - Sirt 1 to Sirt 7 - of which, Sirt 1 is the most well-described [17], and is the focus of this review. In humans, Sirt 1 is encoded by the SIRT1 gene on chromosome 10 (Chr10q21.3).
Sirtuins are purported to confer beneficial anti-aging effects, however, levels of these enzymes decrease with age [18]. For this reason, these enzymes may be a good candidate for the study of age-related diseases such as AD [18], [19]. In mammals, Sirt 1 has been shown to be associated with aging, calorie restriction, metabolism, cancer, stress responses, chromosomal stability, cell differentiation, and the circadian clock [20], [21]. The mechanisms by which Sirt 1 declines with age are not known, but its reduction in both human and animal models is evident during midlife when age-related changes in wakefulness and sleep disorders are expected to occur [22], [23].
Sirt 1 and Alzheimer’s disease
Sirt 1 has been suggested to potentially have a protective effect against AD by modulating the acetylation homeostasis of AD-related proteins and enzymes [19], [24]. A hallmark of AD is the accumulation of Aβ-amyloid peptides in the brain due to the sequential cleavage of the amyloid precursor protein (APP), initiated by the enzyme β-secretase. In contrast, α-secretase activation suppresses Aβ-amyloid production [16]. Specifically, Sirt 1, by deacetylation of transcriptional factors related to α-secretase and β-secretase enzymes, could reduce Aβ-amyloid burden in the brain [15], [16]. For example, Sirt 1 directly induces the transcription of the gene encoding α-secretase. Through this mechanism, Sirt 1 removes acetyl groups from the retinoic acid receptor beta, which is a main modulator of α-secretase transcription. Furthermore, induction of α-secretase by Sirt 1 activates the Notch pathway which involves repairing damaged neurons [15]. Sirt 1 can also deacetylate tau to provide sites for ubiquitination of hyperphosphorylated tau, thereby facilitating its proteasomal degradation and preventing the formation of tau tangles [25], another pathological hallmark of AD.
Research has shown that Sirt 1 is expressed in neurons of the hippocampus; a brain region critical for memory and learning functions, which are impaired in AD [21], [26]. Indeed, in AD, Sirt 1 expression in the brain is reduced and this reduction parallels the accumulation of AD pathology and disease progression [27], [28]. Moreover, SIRT1 knockout mice demonstrate cognitive disorders [20], and Sirt 1 pathway dysregulation has been implicated as a critical mediator of pathogenesis in animal models of AD [29]. Importantly however, animal studies have shown that SIRT1 gene expression in the hippocampus and other brain regions can be increased through intervention approaches such as dietary restriction or physical exercise; suggesting potential approaches for augmenting Sirt 1-mediated neuroprotection [18], [30]. This topic will be considered in greater detail, later. The following sections discuss the relationship between Sirt 1 and sleep.
Sleep
In mammals, sleep is divided into stages determined by cortical electroencephalography (EEG) activity: REM sleep, and non-REM (NREM) sleep which includes the 3 sub-stages of N1, N2 and N3 (also referred to as slow-wave sleep). As the most conventional sleep models suggest, wake-promoting neurons (WPNs) and sleep-promoting neurons (SPNs) create a “switch” system in which they compete for network dominance. In the human brain, WPNs and SPNs are found in the brainstem and diencephalon, which accounts for < 1 % of neurons. The majority of WPNs and SPNs are in association with different neurotransmitters, usually with opposing modulatory effects [31].
WPNs control wakefulness via two pathways. Both pathways ascend through the midbrain’s paramedian region before splitting into dorsal and ventral pathways. The dorsal pathway, which targets the thalamus, arises from cholinergic neurons. These neurons are most active during wakefulness and REM sleep, while in NREM sleep they have little activity [32]. The larger ventral pathway which innervates the hypothalamus, basal forebrain, and cortex, originates from monoaminergic cells, including noradrenergic neurons, neurons producing orexin (also known as hypocretin) and serotonergic neurons. These neurons have little activity during REM sleep [31]. SPNs are thought to control sleep through inhibiting wake-promoting centres, including GABAergic neurons in the ventrolateral preoptic nuclei and melanin-concentrating hormone (MCH)-producing neurons in the diencephalon [33], [34]. It is apparent, therefore, that the sleep process is not regulated by a singular molecular mechanism or gene.
Sleep physiology can be studied from two distinct perspectives: 1) timing of sleep, regulated through the circadian process (process C) in the brain, which is a sleep-independent process, and 2) length of sleep, which is governed by the homeostatic, sleep-dependent, process (process S) [35], [36]. There is potential interaction between process C and process S in sleep regulation [37]. Process C in humans is demonstrated by REM sleep, and has a circadian rhythm which closely associates with body temperature. These processes are rarely influenced by sleep and wake during the last 24 h [38]. By contrast, external stimuli such as prior wake or sleep can affect process S which determines N3, and slow-wave activity in EEG [35].
Sirt 1 and sleep - circadian rhythm
As mentioned above, the circadian system is critical for regulating the timing of sleep. Circadian clock regulators are categorized as positive and negative. Brain and Muscle ARNT-Like 1 (BMAL1; also known as Aryl hydrocarbon receptor nuclear translocator-like protein 1, ARNTL) and Circadian Locomotor Output Cycles Kaput (CLOCK, and its paralogue Neuronal Per-Arnt-Sim domain protein 2, NPAS2) are positive regulators in mammals. These so called ‘master genes’ drive rhythmic gene expression and regulate biological functions under circadian control. BMAL1:CLOCK protein heterodimers initiate the transcription of target genes, including genes that encode periods (Pers) and cryptochromes (Crys), which are negative circadian clock regulators. The resulting proteins form dimers which inhibit further transcription of BMAL1 and CLOCK. This negative transcriptional feedback loop allows the cycle to repeat via a low level of transcriptional activity, thereby generating a 24 h rhythm in mammals [39].
CLOCK protein has histone acetyltransferase (HAT) activity. Animal studies have shown that Sirt 1, with its deacetylase function, acts against the HAT activity of CLOCK, subsequently influencing the expression of Cry1, Per1 and Per2 in mice [40], [41], [42]. Deletion of Sirt1 in mouse models leads to disturbance of circadian rhythm as well as inability to adjust to a new light–dark cycle. Reduction of both SIRT1 expression and NAD+ levels with aging could be a possible mechanism for attenuation of circadian control among older adults [13].
Sirt 1 and sleep - the homeostatic process
As mentioned above, whilst the circadian system is critical for regulating the timing of sleep, the homeostatic process is responsible for regulating length of sleep. Homeostatic sleep drive (pressure to sleep) increases as time awake increases, and decreases during sleep, reaching ‘baseline’ after a night of good quality sleep. After sleep deprivation, lost sleep can be compensated for by increasing sleep time. By contrast, excessive sleep is followed by decreased sleep propensity. The homeostatic process is considered an essential regulatory mechanism for sleep.
Delta waves (frequency 0.5–4 Hz) recorded via EEG, are usually associated with N3 (slow-wave) sleep, characterise deep sleep, and are regulated by the homeostatic process [43]. Animal studies suggest a potential role of Sirt 1 in maintaining delta waves during NREM sleep [23], [44]. Consistent with this notion, and with reduced Sirt 1 levels in aging, human research has revealed that delta power in EEG shows age-related decline. Importantly, in addition to sleep quality, delta power has also been linked to longevity, and metabolic complications [45], [46]. It is therefore plausible that Sirt 1 contributes to sleep homeostasis, although the underlying mechanism requires further elucidation.
Satoh et al., showed that aged brain-specific Sirt 1-overexpressing (BRASTO) transgenic mice have higher delta power in NREM sleep compared to control mice, with no difference in delta power during wakefulness, suggesting that aged BRASTO mice have higher quality, or deeper sleep [44]. Similarly, a study of mice with knockdown of SIRT1 in the dorsomedial and lateral hypothalamic nuclei, revealed decreased sleep quality [23]. A recent study in mice proposed that Sirt 1 appears to mediate sleep quality through the Sirt 1/ Nk2 homeobox 1 (Nkx2-1)/ orexin type 2 receptor (Ox2r) pathway, which is a major pathway for maintaining delta power during NREM sleep [44].
Sleep and Alzheimer’s disease
The presence of sleep-wake disturbances in dementia due to AD is well-established [47]. Crucially, rather than presenting only as a co-morbidity, mounting evidence indicates that sleep disturbances increase risk of cognitive decline and dementia [11], [48], [49], by modulating neurobiological brain changes. These brain changes include increased atrophy [50], [51], brain Aβ-amyloid [52] and tau pathology [53] (hallmarks of AD), as well as reduced brain glucose metabolism [54].
Recent studies show the importance of sleep quality for glymphatic system function in the brain [10]. Increased delta power and decreased heart rate during sleep have both been shown to be related to improved glymphatic flow [55]. Like the lymphatic system in other organs, the glymphatic system is a ‘housekeeping’ system for the brain [56], removing metabolic waste products, including tau oligomers and Aβ-amyloid protein which are associated with AD and cognitive function [57]. Moreover, data from mice have shown that increased delta power during recovery sleep specifically promotes cognitive performance that relies on prefrontal brain regions [58].
Circadian changes have been reported in both healthy aging and age-related diseases such as AD. A recent review summarized evidence for age-related alterations in various aspects of the circadian system, including; 1) reduction of circadian-related gene expression including CLOCK and BMAL1, 2) changes in structures responsible for light transmission and processing, including the pupil and retina, and 3) decreased amplitude of rhythmic behaviours, disturbance of circadian timing and increased prevalence of disordered sleep [59]. Even in healthy older adults without clinical symptoms of sleep disorders, the aging process is associated with reduced sleep quality and quantity, reduction in sleep depth and intensity, impairment of sleep integrity and reduced daytime activity frequently with diurnal napping [60].
Accumulating evidence demonstrates that circadian rhythm disturbance in age-related diseases, such as AD, is even more pronounced, with this manifestation proposed as a biomarker of AD pathology presence and severity [61]. Indeed, some key processes that are implicated in AD pathogenesis are supposed to follow a circadian rhythm, including cerebral blood flow, glymphatic system function, Aβ-amyloid clearance, melatonin production and metabolism [61].
As mentioned earlier, in humans, many neuropeptides, neurotransmitters and their receptors are proposed to contribute to sleep physiology. In neurodegenerative diseases such as AD, multiple neurotransmitter systems are impacted [62], [63]. Moreover, impairments of sleep-related neurotransmitters such as the monoaminergic and cholinergic systems have been broadly reported in AD [64], [65], highlighting a mechanism through which sleep disturbance in AD patients can manifest. Furthermore, Sirt 1 has been shown to impact some of these neuropeptides, neurotransmitters and their receptors, suggesting complex interactions via which Sirt 1 can potentially affect sleep, AD neurobiology and the relationship between the two: this subject will be discussed in the next sections.
Sirt 1, sleep and Alzheimer’s disease
Orexin
By influencing both WPNs and SPNs within the, brain, orexin has an essential role in stabilizing sleep and wake cycles [66]. However, the primary role of orexin appears to be in the generation of a wake-promoting signal. Studies suggest that orexin and its receptors also play a role in the pathogenesis and extent of pathology of neurodegenerative diseases such as AD [67]. Dysregulation of the orexin system in the presence of AD pathology, and related changes to sleep disturbance, have been reported [68], [69]. In mild cognitive impairment (a stage which often but not always precedes AD), increased orexin levels in cerebrospinal fluid have been associated with REM sleep impairment [70], [71]. However, the precise mechanism through which changes in the orexin system might deteriorate sleep in AD requires further elucidation. Nevertheless, it is important to note that orexin also plays a critical role as a stress regulator. Stress and stress hormones have been linked to both AD, and reduced sleep quality. Consequently, this additional role of orexin requires consideration when understanding its relationship to sleep and AD.
The orexin type 2 receptor gene (OX2R) is one of the main Sirt 1 target genes in the hypothalamus. Sirt 1 has been shown to up-regulate OX2R, particularly in the dorsomedial and lateral hypothalamus. The OX2R pathway (which also involves orexin type 1 receptor) appears to have critical effects on arousal, physical activity motivation, and metabolism [72]. Consistent with this notion, transgenic mice which overexpress Sirt 1 in the brain demonstrate improved longevity and delayed aging which has been attributed to dose-dependent upregulation of OX2R [72]. Collectively, these findings provide a plausible mechanism(s) through which Sirt 1 can impact sleep and AD via the orexin system.
Acetylcholine
As part of the arousal system, acetylcholine (ACh) is critical for waking and for REM sleep. However, direct analysis of ACh has been problematic. Consequently, some believe that ACh does not regulate sleep, but it is sleep stages which govern Ach [73]. In AD, basal forebrain cholinergic nuclei degenerate. Accordingly, choline acetyltransferase and acetylcholinesterase activity decrease, which appears to be the major reason for cholinergic dysregulation in AD patients [5]. It is possible that the loss of cholinergic tone is responsible for both cognitive and arousal dysfunction.
Sirt 1 appears to modulate both ACh receptor expression in the brain [14], and choline’s expression [74] suggesting another mechanism through which Sirt 1 can potentially impact sleep and AD. Cytidine-5′-diphosphate- (CDP) choline is an endogenous compound produced by the body which serves as a choline source in metabolic pathways for the biosynthesis of ACh. CDP-choline is a neuroprotective substance which exerts significant beneficial effects on memory function and behaviour [74]. A recent study showed that CDP-choline increases Sirt 1 protein expression in rat brain, conferring neuroprotection [75]. It has also been suggested that alpha 7 nicotinic ACh receptors can improve Sirt 1 activity, likely by elevating intracellular levels of its cofactor NAD+. Moreover, the anti-senescence properties of these receptors appear to be mediated by Sirt 1 [76]. Importantly, in an AD animal model, treatment with an alpha 7 nicotinic ACh receptor agonist produced neuroprotective effects coupled with an improvement in learning and memory ability [77]. It is conceivable that these beneficial effects were mediated by Sirt 1.
Monoaminergic neurotransmitters
The main roles of monoaminergic neurotransmitter systems in the sleep-wake cycle have been reported broadly [5], [31], [32]. These systems are usually most active during wakefulness, are slow in NREM sleep, cease function before and after REM sleep, and start firing again before wakefulness begins [78]. Monoaminergic system impairment is common in AD. Generally, due to the low number of monoaminergic fibres in the brain, and given these neurons have long, unmyelinated axons, they are more vulnerable to neurological abnormalities such as the presence of AD pathology. Indeed, monoaminergic neurons project into the hippocampus and regions of the cortex which are significantly affected by both hyperphosphorylated tau and Aβ-amyloid accumulation [79].
SIRT1 is expressed in monoaminergic neurons, where its presence is critical to normal wakefulness and the integrity of wake-active neurons. Transgenic whole animal, and conditional loss of brain SIRT1 in adult mice results in significant wake disturbance and reduction in wake time, without impairment in sleep consolidation [23]. Other aspects of this study also revealed an age-related reduction in SIRT1 in wake-active neurons, excluding serotoninergic wake-active neurons [23]. Another mouse study revealed that SIRT1 can modulate monoamine levels by deacetylating Nescient Helix-Loop-Helix 2 (NHLH2), a neuronal transcription factor for the gene (MAO-A) which encodes the catabolizing enzyme monoamine oxidase A [80]. Moreover, in vitro research demonstrated that overexpression of microRNA (miR-142) decreased neuronal expression and subsequent enzymatic activity of MAO-A through downregulation of SIRT1 [81]. Abnormal microRNA expression has been implicated in the pathogenesis of several neurodegenerative disorders, potentially via SIRT1-mediated altered neurotransmission. Thus, monoaminergic neurotransmitter systems represent another mechanism through which Sirt 1 can potentially impact sleep and AD.
Melanin-concentrating hormone
The neuropeptide melanin-concentrating hormone (MCH) contributes to locomotor activity reduction, energy conservation, and enhancing sleep when energy balance is positive [82]. It likely also has a critical role in negative energy balance by reducing activity and REM sleep [83]. Depletion of MCH either by gene knockout or antagonist is proposed to reduce NREM and REM sleep, increase sleep fragmentation and heighten alertness [84].
Studies of animal models have shown that increased MCH results in improved memory, learning and performance [85]. Emerging evidence has also shown that MCH function is disturbed in AD, and this disturbance correlates with AD-related phenotypes such as neurofibrillary tangles of hyperphosphorylated tau. MCH receptors are widely distributed in the hippocampus and cortex; regions which are vulnerable to AD-related neuropathology and neurodegeneration. Thus, disturbed MCH function in AD and its potential impact on learning and memory performance could be mediated by a reduction of MCH receptors.
A recent study demonstrated that Sirt 1 in pro-opiomelanocortin (POMC)-expressing neurons, which promote sleep, is involved in MCH regulation. Therefore, SIRT1 inhibition in these regions might impair MCH functions. Further, the Sirt 1 (SIRT1)/ Forkhead Box O1 (FoxO1)/ POMC signalling pathway is proposed as a potential regulatory mechanism for MCH action [86]. This pathway appears to contribute to regulation of energy balance and food intake, and can influence the sleep-wake cycle [87]. Collectively, this evidence suggests MCH as another target through which Sirt 1 can potentially impact sleep and AD.
Adenosine
The neuromodulator adenosine, and its receptors, appear to be critical for both circadian rhythm and homeostatic sleep drive. Adenosine itself has somnogenic properties and is considered a ‘sleep substance’, modulating the sleep-wake cycle via its A1 and A2A receptor subtypes (the most common receptor subtypes in the mammalian brain). Research has shown that depletion of its derivative adenosine triphosphate (ATP), and an increase of extracellular adenosine levels, are both positively associated with sleep patterns [88], [89]. Activation of A1 receptors enhances slow-wave activity [90], the main indicator of homeostatic sleep regulation. Moreover, caffeine has been postulated to mediate the sleep-wake pattern through A2A receptors [91]. The adenosine-derived signalling molecule, cyclic AMP, is itself a circadian clock component that indirectly induces transcription of many circadian genes, as well as influencing cell cycle timing. AMP kinase, a cellular energy sensor dependent upon AMP, can phosphorylate multiple clock proteins, including Sirt 1, thereby upregulating this enzyme, and influencing downstream pathways. As stated previously, Sirt 1 function is also modulated by NAD+ cofactor levels: importantly, however, these levels are influenced by both circadian and metabolic regulation [92].
The neuromodulatory role of adenosine and its receptors has been reported in various neurodegenerative conditions, including AD [93]. Epilepsy is reported in 10 % to 22 % of AD patients, frequently occurring during the early disease stages, or even before the formation of cerebral Aβ-amyloid plaques [94]. It is believed that global DNA hypermethylation is associated with chronic epilepsy [95], and impairment of adenosine homeostasis is involved in this process. Accordingly, therapeutic adenosine may improve DNA methylation profiles and thereby ameliorate epilepsy progression [94]. A recent study demonstrated that Sirt 1 in the brain is essential for managing epilepsy, and adenosine improves epigenetic modifications, neuron survival and synaptic plasticity [93]. However, the influence of adenosine impairment in AD pathophysiology is complex. In the short term, increased adenosine tone could have therapeutic properties by suppressing methyltransferase and consequently reducing changes in DNA methylation, which are common in the brain of AD patients [96]. Moreover, higher adenosine levels, and subsequent increased A1 receptor activity improves the hyperexcitability and excitotoxicity network in AD parenchyma. By contrast, increased A2 receptor activity due to increased adenosine tone results in memory deficit and AD pathology. Collectively, these findings suggest that the positive effect of elevated adenosine levels are likely receptor subtype-dependent [96].
Melatonin
Melatonin (N-acetyl-5-methoxytryptamine), a metabolite of the amino acid tryptophan, is produced in the pineal gland. In humans, melatonin contributes to a number of physiological processes including regulating circadian rhythm and sleep physiology. After two hours of endogenous secretion of melatonin at night, sleep propensity sharply increases [97]. In diurnal species, melatonin reduces the wake-promoting signal of the circadian clock, thereby promoting sleep [98].
Nocturnal secretion of melatonin is disrupted with advancing age and in neurodegenerative disorders such as AD, furthering abnormal sleep [99]. Melatonin secretion declines in mild cognitive impairment with the earliest manifestations of AD neuropathology [100], [101], and continues to decline with disease progression [100]. A recent study reported that mild cognitive impairment patients with changes in melatonin production show disturbance in the circadian clock, causing an elevation in wakefulness at night, and enhanced REM latency [102]. One candidate mechanism potentially underlying these effects could be the overregulation of monoamine oxidase which occurs in AD, resulting in depletion of melatonin’s precursor, serotonin [103].
Animal and in vitro studies also suggest melatonin administration improves AD pathology; potentially protecting against Aβ-amyloid production via increased α-secretase activity and decreased β- and γ-secretases [104], [105], [106]. Moreover, melatonin has been shown to up-regulate ADAM10 (A Disintegrin and Metalloproteinase 10) in vitro, through SIRT1 pathway activation [104]. ADAM10 is the major physiological α-secretase in neurons, responsible for cleaving APP in a manner that suppresses Aβ-amyloid production. These in vitro findings are further supported by an animal study where the long-term administration of melatonin to aged mice induced beneficial changes to secretase activity in the hippocampus that were accompanied by reduction of phosphorylated NF-κB and increased Sirt 1 [106]. Attenuated spatial memory impairment was also evident following melatonin treatment. These findings led the authors to suggest dietary supplementation, to mitigate age-related loss of melatonin, as a potential therapeutic strategy for AD prevention and progression [106].
The impact of melatonin treatment has also been examined in total sleep-deprived rats [107]. Following total sleep-deprivation, significantly impaired spatial memory was evident, coupled with drastically reduced Sirt 1 levels in hippocampal pyramidal and granular cell layers. However, in total sleep-deprived animals receiving melatonin doses of 5, 25, 50 or 100 mg/kg/day, hippocampal Sirt 1 expression was preserved. These neurobiological benefits of melatonin treatment were accompanied by considerably better performance on behavioural testing. Consequently, the authors proposed melatonin as a potential therapeutic strategy aimed at preventing memory deficits caused by total sleep-deprivation, and suggested Sirt 1 may partially modulate these beneficial effects [107].
Several studies also highlight a major role for Sirt 1 in the modulation of melatonin function in improving insulin resistance, aging, and anti-inflammatory effects; [108], [109], [110] factors which impact AD risk and progression. Collectively, the evidence presented in this section suggests melatonin as another target through which Sirt 1 can potentially impact sleep and AD.
The role of the retina
In mammals, the functional photopigment melanopsin is localised within intrinsically photosensitive retinal ganglion cells (ipRGCs), and is involved in the mediation of non-visual photoreceptive tasks. Melanopsin’s functions demonstrate that light not only relays information to the circadian clock but also has a complex interaction with several neurological and pathological processes [111], [112]. The body’s reaction to light is critical for regulation of rhythmic physiologic functions such as hormonal cycles, and expression of the negative circadian clock regulator genes Per and Cry. Thus, ipRGCs play a major role in adjusting the circadian clock.
Acquired, or inherited depletion of ipRGCs, in neurodegenerative diseases, leads to impairment of dopaminergic neurons in the retina. Similarly, disturbed light detection, or transmission onto the retina due to advancing age or conditions such as cataract, leads to deregulation of circadian synchrony, and subsequent impairment of a wide range of physiological processes, including sleep. Consistent with these findings, reduction of melanopsin appears to be involved in insomnia, depression and cognitive decline [112], [113]. Blue light (460 nm), however, has been proposed to improve cognitive abilities during both daytime and night-time [114], [115]. Yet, circadian clock desynchrony could influence exposure, and response, to the blue light spectrum [116], which in older adults may cause reduced melatonin levels and impaired alertness [115].
Cohort studies have demonstrated retinal thinning and retinal vascular disturbance in AD [117]. Moreover, the presence of AD hallmarks including Aβ-amyloid plaques, hyperphosphorylated tau, and neurodegeneration have all been reported in the retina of prodromal and symptomatic AD patients [118]. Indeed, with evolving imaging techniques, recent studies have suggested that an inexpensive and non-invasive retinal scan might help diagnose AD in its early stages (reviewed in [118]).
Sirt 1 is present in the retinal pigment epithelium, lens, cornea, ciliary body, and neuroretina in both animals and humans [119], [120]. In SIRT1‑deficient mice, significantly thinner retinal cell layers and disordered inner and outer nuclear layers are evident [121], as well as elevated apoptosis of retinal progenitor cells [122]. Sirt 1 plays a critical role in retinal and ocular systems, and their pathology, through influencing several mechanisms including aging, inflammation, oxidative stress, angiogenesis and neuroprotection [123], [124]. The expression of SIRT1 in retinal tissue depends on light, increasing at night-time. This expression pattern has not been reported in the liver or brain, highlighting tissue or organ-specific regulation of SIRT1 [125]. It is likely that the presence and severity of Alzheimer’s retinopathy negatively impacts the quantity and quality of light transmitted to the retina, thereby influencing physiological functions such as sleep, SIRT1 expression, and their interrelationship.
Activators of Sirt 1 - dietary factors and other putative modifying strategies
Multiple dietary factors, other agents and strategies have been proposed to activate Sirt 1/ SIRT1. For example, intermittent fasting, calorie restriction, and calorie restriction combined with exercise have all been shown to increase levels of Sirt 1 mRNA in human muscle tissue [126], [127]. Animal studies have also shown that calorie restriction increases Sirt 1 levels in the brain; specifically in the hypothalamus [72], a brain region that is particularly vulnerable to AD neuropathology. The anthraquinone compound rhein (4,5-dihydroxyanthraquinone-2-carboxylic acid), derived from rhubarb, has been shown to reduce brain Aβ-amyloid deposits, neuroinflammation, and ameliorate cognitive impairment in a transgenic mouse model of AD [128]. The authors attributed the beneficial effects of rhein treatment seen in these animals to activation of the Sirt 1/ peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) pathway, which improved mitochondrial biogenesis [128]. However, the therapeutic efficacy of rhein treatment in humans remains to be determined. The biophenol DOPET (3,4-Dihydroxyphenylethanol) found in olive oil, grape juice, and wine, has also been evaluated for its effects in an AD mouse model. DOPET treatment reversed SIRT1 dysregulation and attenuated spatio-cognitive deficits in these animals [129]. The observed increase in SIRT1 activity was accompanied by α-secretase-mediated enhanced clearance of neurotoxic Aβ-amyloid, thereby conferring neuroprotection. The polyphenol resveratrol, found in grapes and grape products such as red wine, is well established as an activator of Sirt 1 both in vitro and in vivo. Moreover, in studies of AD patients, high doses of resveratrol have been shown to reduce AD biomarker levels in cerebrospinal fluid [130], confer neuroprotection in brain regions affected in early AD [131], as well as slow cognitive decline and maintain function in mild to moderate AD [132]. It should, however, be acknowledged that the beneficial effects of resveratrol are unlikely to be solely dependent on Sirt 1 activation as this polyphenolic compound has been shown to modulate multiple intracellular signalling pathways [133]. However, whether such sirt 1/ SIRT1-activating strategies as those described above modulate AD risk, potentially via impacting sleep, remains to be determined, as does their possible therapeutic role in the treatment of AD.
Future research directions
A growing body of evidence from both animal and human studies supports the existence of a complex and multi-faceted relationship between Sirt 1/ SIRT1, sleep, and AD aetiology. However, further research is required to fully elucidate this relationship. One recommendation for future research is the simultaneous measurement of Sirt 1 and sleep in well-characterised longitudinal cohorts of aging and AD. Specifically, by measuring Sirt 1 levels in plasma, serum or cerebrospinal fluid, and sleep parameters such as efficiency, duration and time spent in sleep stages, across the AD continuum of preclinical, prodromal and dementia stages, the relationship between Sirt 1, sleep, and AD progression could be further elucidated. Moreover, by including individuals who remain cognitively unimpaired over time (both with and without the presence of AD biomarkers) in such a study, the effect of healthy versus pathological aging could also be considered. Such analysis is important for understanding the impact of age as a mediator of the Sirt 1/ SIRT1, sleep, and AD relationship, given that advancing age is the greatest risk factor for AD, sleep changes with age, and Sirt 1 is associated with anti-aging effects. Once the relationship between Sirt 1/ SIRT1, sleep, and AD aetiology has been well characterised, the logical next step would be to investigate the impact of Sirt 1 activators (e.g., resveratrol, dietary approaches including intermittent fasting and calorie restriction, etc.) on sleep parameters and AD biomarkers measured simultaneously. This may inform how such approaches could potentially be employed in the development of strategies aimed at delaying or ideally preventing the onset of dementia due to AD.
Conclusion
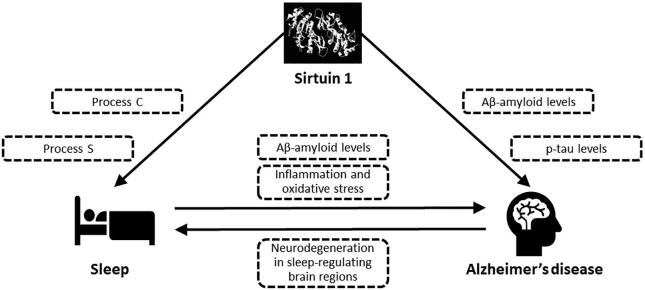
To conclude, a growing body of evidence from both animal and human studies supports a significant and complex relationship between Sirt 1/ SIRT1, sleep and AD (summarized in Fig. 1). Numerous hypotheses have been generated in order to explain the critical impact of Sirt 1/ SIRT1 on WPNs, SPNs and their related mechanisms and neurotransmitters. However, there is a relative lack of studies assessing the interaction between sleep and Sirt 1/ SIRT1, as a principal component of the circadian clock, on AD pathology. In this review, we have explored the potential association between Sirt 1/ SIRT1, sleep, and AD aetiology. Given that sleep is a likely modifiable risk factor for AD, and that recent studies suggest Sirt 1/ SIRT1 activation can be modulated by lifestyle or dietary approaches, further research in this area is required to explore its potential as a target for AD prevention and treatment.
Fig. 1.
Potential interaction between sirtuin 1, sleep, and Alzheimer’s disease.Sirtuin 1 can modulate levels of pathological hallmarks of Alzheimer’s disease (Aβ-amyloid and hyperphosphorylated tau; p-tau). This pathology, via widespread neurodegeneration that impacts sleep-regulating brain regions, is associated with disrupted sleep; one of the most common symptoms of Alzheimer’s disease. Sirtuin 1 can also directly impact sleep via effects on both circadian rhythm (Process C) and homeostatic sleep drive (Process S). In addition to being a symptom of Alzheimer’s disease, suboptimal sleep has also been linked to increased risk of Alzheimer’s disease via reduced brain Aβ-amyloid clearance, oxidative stress and inflammation. Sirtuin 1 has been shown to impact multiple neuropeptides, neurotransmitters and their receptors, suggesting complex interactions via which Sirtuin 1 can potentially affect sleep, Alzheimer’s disease neurobiology, and the relationship between the two. These neuropeptides and neurotransmitters include orexin, acetylcholine, monoaminergic neurotransmitters, melanin-concentrating hormone, adenosine, and melatonin.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowlegement
MM is the recipient of an Australian Government Research Training Program Scholarship. TP is supported by an Edith Cowan University Strategic Research Fellowship. SML has previously been a paid consultant to Alzhyme. SRRS is supported by a National Health and Medical Research Council (NHMRC) Investigator Grant (GNT1197315).
References:
- 1.Daley M., Morin C.M., LeBlanc M., Grégoire J.-P., Savard J., Baillargeon L. Insomnia and its relationship to health-care utilization, work absenteeism, productivity and accidents. Sleep Med. 2009;10(4):427–438. doi: 10.1016/j.sleep.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Musiek E.S., Holtzman D.M. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science. 2016;354(6315):1004–1008. doi: 10.1126/science.aah4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ancoli-Israel S. Sleep and aging: prevalence of disturbed sleep and treatment considerations in older adults. J Clin Psychiatry. 2005;66 Suppl 9:24-30; quiz 42-23. [PubMed]
- 4.Cooke J.R., Ancoli-Israel S. Sleep and its disorders in older adults. Psychiatric Clinics. 2006;29(4):1077–1093. doi: 10.1016/j.psc.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Van Erum J, Van Dam D, De Deyn PP. Alzheimer’s disease: neurotransmitters of the sleep-wake cycle. Neuroscience & Biobehavioral Reviews. 2019. [DOI] [PubMed]
- 6.Prinz P.N., Peskind er, vitaliano pp,, et al. Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. J Am Geriatr Soc. 1982;30(2):86–92. doi: 10.1111/j.1532-5415.1982.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 7.Bliwise D.L., Watts R.L., Watts N., Rye D.B., Irbe D., Hughes M. Disruptive nocturnal behavior in Parkinson's disease and Alzheimer's disease. J Geriatr Psychiatry Neurol. 1995;8(2):107–110. doi: 10.1177/089198879500800206. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y.-L., Liu R.-Y., Wang Q.-S., Van Someren E.J., Xu H., Zhou J.-N. Age-associated difference in circadian sleep–wake and rest–activity rhythms. Physiol Behav. 2002;76(4–5):597–603. doi: 10.1016/s0031-9384(02)00733-3. [DOI] [PubMed] [Google Scholar]
- 9.Prinz P.N., Vitiello M.V., Raskind M.A., Thorpy M.J. Sleep disorders and aging. N Engl J Med. 1990;323(8):520–526. doi: 10.1056/NEJM199008233230805. [DOI] [PubMed] [Google Scholar]
- 10.Xie L., Kang H., Xu Q., et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li P., Gao L., Yu L., et al. Daytime napping and Alzheimer's dementia: A potential bidirectional relationship. Alzheimers Dement. 2022 doi: 10.1002/alz.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bubu O.M., Brannick M., Mortimer J., et al. Sleep, Cognitive impairment, and Alzheimer's disease: A Systematic Review and Meta-Analysis. Sleep. 2017;40(1) doi: 10.1093/sleep/zsw032. [DOI] [PubMed] [Google Scholar]
- 13.Chang H.-C., Guarente L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell. 2013;153(7):1448–1460. doi: 10.1016/j.cell.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang P.-S., Son J.-H., Abbott L., Winzer-Serhan U. Regulated expression of neuronal SIRT1 and related genes by aging and neuronal β2-containing nicotinic cholinergic receptors. Neuroscience. 2011;196:189–202. doi: 10.1016/j.neuroscience.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Saftig P., Lichtenthaler S.F. The alpha secretase ADAM10: A metalloprotease with multiple functions in the brain. Prog Neurobiol. 2015;135:1–20. doi: 10.1016/j.pneurobio.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Lee H.R., Shin H.K., Park S.Y., et al. Cilostazol suppresses β-amyloid production by activating a disintegrin and metalloproteinase 10 via the upregulation of SIRT1-coupled retinoic acid receptor-β. J Neurosci Res. 2014;92(11):1581–1590. doi: 10.1002/jnr.23421. [DOI] [PubMed] [Google Scholar]
- 17.Finkel T., Deng C.X., Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460(7255):587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quintas A., de Solís A.J., Díez-Guerra F.J., Carrascosa J.M., Bogónez E. Age-associated decrease of SIRT1 expression in rat hippocampus: prevention by late onset caloric restriction. Exp Gerontol. 2012;47(2):198–201. doi: 10.1016/j.exger.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Donmez G. The neurobiology of sirtuins and their role in neurodegeneration. Trends Pharmacol Sci. 2012;33(9):494–501. doi: 10.1016/j.tips.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Michán S., Li Y., Chou M.-M.-H., et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30(29):9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bordone L., Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6(4):298. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 22.Colas D., Cespuglio R., Sarda N. Sleep wake profile and EEG spectral power in young or old senescence accelerated mice. Neurobiol Aging. 2005;26(2):265–273. doi: 10.1016/j.neurobiolaging.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Panossian L., Fenik P., Zhu Y., Zhan G., McBurney M.W., Veasey S. SIRT1 regulation of wakefulness and senescence-like phenotype in wake neurons. J Neurosci. 2011;31(11):4025–4036. doi: 10.1523/JNEUROSCI.5166-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herskovits A.Z., Guarente L. SIRT1 in neurodevelopment and brain senescence. Neuron. 2014;81(3):471–483. doi: 10.1016/j.neuron.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Min S.-W., Cho S.-H., Zhou Y., et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67(6):953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mu Y., Gage F.H. Adult hippocampal neurogenesis and its role in Alzheimer's disease. Mol Neurodegener. 2011;6(1):85. doi: 10.1186/1750-1326-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Julien C., Tremblay C., Émond V., et al. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol. 2009;68(1):48–58. doi: 10.1097/NEN.0b013e3181922348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutz M.I., Milenkovic I., Regelsberger G., Kovacs G.G. Distinct patterns of sirtuin expression during progression of Alzheimer’s disease. NeuroMol Med. 2014;16(2):405–414. doi: 10.1007/s12017-014-8288-8. [DOI] [PubMed] [Google Scholar]
- 29.Sun T., Zhao K., Liu M., et al. miR-30a-5p induces Abeta production via inhibiting the nonamyloidogenic pathway in Alzheimer's disease. Pharmacol Res. 2022;178 doi: 10.1016/j.phrs.2022.106153. [DOI] [PubMed] [Google Scholar]
- 30.Revilla S., Suñol C., García-Mesa Y., Giménez-Llort L., Sanfeliu C., Cristòfol R. Physical exercise improves synaptic dysfunction and recovers the loss of survival factors in 3xTg-AD mouse brain. Neuropharmacology. 2014;81:55–63. doi: 10.1016/j.neuropharm.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 31.Oh J., Petersen C., Walsh C.M., Bittencourt J.C., Neylan T.C., Grinberg L.T. The role of co-neurotransmitters in sleep and wake regulation. Mol Psychiatry. 2019;24(9):1284–1295. doi: 10.1038/s41380-018-0291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saper C.B., Scammell T.E., Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 33.Alam M.A., Kumar S., McGinty D., Alam M.N., Szymusiak R. Neuronal activity in the preoptic hypothalamus during sleep deprivation and recovery sleep. J Neurophysiol. 2013;111(2):287–299. doi: 10.1152/jn.00504.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallopin T., Fort P., Eggermann E., et al. Identification of sleep-promoting neurons in vitro. Nature. 2000;404(6781):992. doi: 10.1038/35010109. [DOI] [PubMed] [Google Scholar]
- 35.Borbély A.A. A two process model of sleep regulation. Hum neurobiol. 1982;1(3):195–204. [PubMed] [Google Scholar]
- 36.Dauvilliers Y., Maret S., Tafti M. Genetics of normal and pathological sleep in humans. Sleep Med Rev. 2005;9(2):91–100. doi: 10.1016/j.smrv.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Tobler I., Achermann P. Sleep homeostasis Scholarpedia. 2007;2(10):2432. [Google Scholar]
- 38.Czeisler C.A., Zimmerman J.C., Ronda J.M., Moore-Ede M.C., Weitzman E.D. Timing of REM sleep is coupled to the circadian rhythm of body temperature in man. Sleep. 1980;2(3):329–346. [PubMed] [Google Scholar]
- 39.Hirayama J., Sahar S., Grimaldi B., et al. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature. 2007;450(7172):1086. doi: 10.1038/nature06394. [DOI] [PubMed] [Google Scholar]
- 40.Etchegaray J.-P., Lee C., Wade P.A., Reppert S.M. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature. 2003;421(6919):177. doi: 10.1038/nature01314. [DOI] [PubMed] [Google Scholar]
- 41.Nakahata Y., Kaluzova M., Grimaldi B., et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134(2):329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang R.-H., Zhao T., Cui K., et al. Negative reciprocal regulation between Sirt1 and Per2 modulates the circadian clock and aging. Sci Rep. 2016;6(1):1–15. doi: 10.1038/srep28633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lehmann D., Ozaki H., Pal I. EEG alpha map series: brain micro-states by space-oriented adaptive segmentation. Electroencephalogr Clin Neurophysiol. 1987;67(3):271–288. doi: 10.1016/0013-4694(87)90025-3. [DOI] [PubMed] [Google Scholar]
- 44.Satoh A., Brace C.S., Rensing N., et al. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18(3):416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Landolt H.-P., Dijk D.-J., Achermann P., Borbely A.A. Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res. 1996;738(2):205–212. doi: 10.1016/s0006-8993(96)00770-6. [DOI] [PubMed] [Google Scholar]
- 46.Dew M.A., Hoch C.C., Buysse D.J., et al. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65(1):63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 47.Hatfield C.F., Herbert J., van Someren E.J., Hodges J.R., Hastings M.H. Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer's dementia. Brain. 2004;127(Pt 5):1061–1074. doi: 10.1093/brain/awh129. [DOI] [PubMed] [Google Scholar]
- 48.Potvin O., Lorrain D., Forget H., et al. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep. 2012;35(4):491–499. doi: 10.5665/sleep.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sterniczuk R., Theou O., Rusak B., Rockwood K. Sleep disturbance is associated with incident dementia and mortality. Curr Alzheimer Res. 2013;10(7):767–775. doi: 10.2174/15672050113109990134. [DOI] [PubMed] [Google Scholar]
- 50.Sexton C.E., Storsve A.B., Walhovd K.B., Johansen-Berg H., Fjell A.M. Poor sleep quality is associated with increased cortical atrophy in community-dwelling adults. Neurology. 2014;83(11):967–973. doi: 10.1212/WNL.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lo J.C., Loh K.K., Zheng H., Sim S.K., Chee M.W. Sleep duration and age-related changes in brain structure and cognitive performance. Sleep. 2014;37(7):1171–1178. doi: 10.5665/sleep.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown B.M., Rainey-Smith S.R., Villemagne V.L., et al. The Relationship between Sleep Quality and Brain Amyloid Burden. Sleep. 2016;39(5):1063–1068. doi: 10.5665/sleep.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winer J.R., Mander B.A., Helfrich R.F., et al. Sleep as a Potential Biomarker of Tau and beta-Amyloid Burden in the Human Brain. J Neurosci. 2019;39(32):6315–6324. doi: 10.1523/JNEUROSCI.0503-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Osorio R.S., Pirraglia E., Gumb T., et al. Imaging and cerebrospinal fluid biomarkers in the search for Alzheimer's disease mechanisms. Neurodegener Dis. 2014;13(2–3):163–165. doi: 10.1159/000355063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hablitz L.M., Vinitsky H.S., Sun Q., et al. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci Adv. 2019;5(2):eaav5447. doi: 10.1126/sciadv.aav5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iliff J.J., Wang M., Liao Y., et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111-147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benveniste H., Liu X., Koundal S., Sanggaard S., Lee H., Wardlaw J. The glymphatic system and waste clearance with brain aging: a review. Gerontology. 2019;65(2):106–119. doi: 10.1159/000490349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bjorness T.E., Kelly C.L., Gao T., Poffenberger V., Greene R.W. Control and function of the homeostatic sleep response by adenosine A1 receptors. J Neurosci. 2009;29(5):1267–1276. doi: 10.1523/JNEUROSCI.2942-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duffy J.F., Zitting K.-M., Chinoy E.D. Aging and circadian rhythms. Sleep medicine clinics. 2015;10(4):423–434. doi: 10.1016/j.jsmc.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bliwise D.L., Ansari F.P., Straight L.-B., Parker K.P. Age changes in timing and 24-hour distribution of self-reported sleep. The American journal of geriatric psychiatry. 2005;13(12):1077–1082. doi: 10.1176/appi.ajgp.13.12.1077. [DOI] [PubMed] [Google Scholar]
- 61.Homolak J., Mudrovčić M., Vukić B., Toljan K. Circadian rhythm and Alzheimer’s disease. Medical Sciences. 2018;6(3):52. doi: 10.3390/medsci6030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Francis P.T. The interplay of neurotransmitters in Alzheimer's disease. CNS Spectr. 2005;10(S18):6–9. doi: 10.1017/s1092852900014164. [DOI] [PubMed] [Google Scholar]
- 63.Lanari A., Amenta F., Silvestrelli G., Tomassoni D., Parnetti L. Neurotransmitter deficits in behavioural and psychological symptoms of Alzheimer's disease. Mech Ageing Dev. 2006;127(2):158–165. doi: 10.1016/j.mad.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 64.H Ferreira-Vieira T, M Guimaraes I, R Silva F, M Ribeiro F. Alzheimer's disease: targeting the cholinergic system. Current neuropharmacology. 2016;14(1):101-115. [DOI] [PMC free article] [PubMed]
- 65.Vermeiren Y., Janssens J., Aerts T., et al. Brain serotonergic and noradrenergic deficiencies in behavioral variant frontotemporal dementia compared to early-onset Alzheimer’s disease. J Alzheimers Dis. 2016;53(3):1079–1096. doi: 10.3233/JAD-160320. [DOI] [PubMed] [Google Scholar]
- 66.Mochizuki T., Crocker A., McCormack S., Yanagisawa M., Sakurai T., Scammell T.E. Behavioral state instability in orexin knock-out mice. J Neurosci. 2004;24(28):6291–6300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang C., Wang Q., Ji B., et al. The orexin/receptor system: molecular mechanism and therapeutic potential for neurological diseases. Front Mol Neurosci. 2018;11:220. doi: 10.3389/fnmol.2018.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fronczek R., van Geest S., Frölich M., et al. Hypocretin (orexin) loss in Alzheimer's disease. Neurobiol Aging. 2012;33(8):1642–1650. doi: 10.1016/j.neurobiolaging.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 69.Schmidt F.M., Kratzsch J., Gertz H.-J., et al. Cerebrospinal fluid melanin-concentrating hormone (MCH) and hypocretin-1 (HCRT-1, orexin-A) in Alzheimer’s disease. PLoS ONE. 2013;8(5):e63136. doi: 10.1371/journal.pone.0063136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maestri M., Carnicelli L., Tognoni G., et al. Non-rapid eye movement sleep instability in mild cognitive impairment: a pilot study. Sleep Med. 2015;16(9):1139–1145. doi: 10.1016/j.sleep.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 71.Peter-Derex L., Yammine P., Bastuji H., Croisile B. Sleep and Alzheimer's disease. Sleep Med Rev. 2015;19:29–38. doi: 10.1016/j.smrv.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 72.Satoh A., Brace C.S., Ben-Josef G., et al. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J Neurosci. 2010;30(30):10220–10232. doi: 10.1523/JNEUROSCI.1385-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gais S., Born J. Declarative memory consolidation: mechanisms acting during human sleep. Learning & Memory. 2004;11(6):679–685. doi: 10.1101/lm.80504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gareri P., Castagna A., Cotroneo A.M., Putignano S., De Sarro G., Bruni A.C. The role of citicoline in cognitive impairment: pharmacological characteristics, possible advantages, and doubts for an old drug with new perspectives. Clin Interv Aging. 2015;10:1421. doi: 10.2147/CIA.S87886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hurtado O., Hernández-Jiménez M., Zarruk J.G., et al. Citicoline (CDP-choline) increases S irtuin1 expression concomitant to neuroprotection in experimental stroke. J Neurochem. 2013;126(6):819–826. doi: 10.1111/jnc.12269. [DOI] [PubMed] [Google Scholar]
- 76.Li D.-J., Huang F., Ni M., Fu H., Zhang L.-S., Shen F.-M. α7 Nicotinic Acetylcholine Receptor Relieves Angiotensin II–Induced Senescence in Vascular Smooth Muscle Cells by Raising Nicotinamide Adenine Dinucleotide-Dependent SIRT1 Activity. Arterioscler Thromb Vasc Biol. 2016;36(8):1566–1576. doi: 10.1161/ATVBAHA.116.307157. [DOI] [PubMed] [Google Scholar]
- 77.Medeiros R., Castello N.A., Cheng D., et al. α7 Nicotinic receptor agonist enhances cognition in aged 3xTg-AD mice with robust plaques and tangles. The American journal of pathology. 2014;184(2):520–529. doi: 10.1016/j.ajpath.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 78.Steriade M.M., McCarley R.W. Brainstem control of wakefulness and sleep. Springer Science & Business. Media. 2013 [Google Scholar]
- 79.Trillo L., Das D., Hsieh W., et al. Ascending monoaminergic systems alterations in Alzheimer's disease. Translating basic science into clinical care. Neurosci Biobehav Rev. 2013;37(8):1363–1379. doi: 10.1016/j.neubiorev.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 80.Libert S., Pointer K., Bell E.L., et al. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell. 2011;147(7):1459–1472. doi: 10.1016/j.cell.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chaudhuri A.D., Yelamanchili S.V., Fox H.S. MicroRNA-142 reduces monoamine oxidase A expression and activity in neuronal cells by downregulating SIRT1. PLoS ONE. 2013;8(11) doi: 10.1371/journal.pone.0079579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burdakov D., Karnani M.M., Gonzalez A. Lateral hypothalamus as a sensor-regulator in respiratory and metabolic control. Physiol Behav. 2013;121:117–124. doi: 10.1016/j.physbeh.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Willie J.T., Sinton C.M., Maratos-Flier E., Yanagisawa M. Abnormal response of melanin-concentrating hormone deficient mice to fasting: hyperactivity and rapid eye movement sleep suppression. Neuroscience. 2008;156(4):819–829. doi: 10.1016/j.neuroscience.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shimada M., Tritos N.A., Lowell B.B., Flier J.S., Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396(6712):670. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 85.Adamantidis A., de Lecea L. A role for Melanin-Concentrating Hormone in learning and memory. Peptides. 2009;30(11):2066–2070. doi: 10.1016/j.peptides.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Al-Massadi O., Quiñones M., Clasadonte J., et al. MCH Regulates SIRT1/FoxO1 and Reduces POMC Neuronal Activity to Induce Hyperphagia, Adiposity, and Glucose Intolerance. Diabetes. 2019;68(12):2210–2222. doi: 10.2337/db19-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goldstein N, Levine BJ, Loy KA, et al. Hypothalamic neurons that regulate feeding can influence sleep/wake states based on homeostatic need. Current Biology. 2018;28(23):3736-3747. e3733. [DOI] [PMC free article] [PubMed]
- 88.Porkka-Heiskanen T., Strecker R.E., Thakkar M., Bjørkum A.A., Greene R.W., McCarley R.W. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276(5316):1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Porkka-Heiskanen T., Kalinchuk A., Alanko L., Urrila A., Stenberg D. Adenosine, energy metabolism, and sleep. The Scientific World Journal. 2003;3:790–798. doi: 10.1100/tsw.2003.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Benington J.H., Kodali S.K., Heller H.C. Stimulation of A1 adenosine receptors mimics the electroencephalographic effects of sleep deprivation. Brain Res. 1995;692(1–2):79–85. doi: 10.1016/0006-8993(95)00590-m. [DOI] [PubMed] [Google Scholar]
- 91.Huang Z.-L., Qu W.-M., Eguchi N., et al. Adenosine A 2A, but not A 1, receptors mediate the arousal effect of caffeine. Nat Neurosci. 2005;8(7):858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- 92.Muheim C., Brown S.A. Adenosine and other purinergic products in circadian timing. In: Adenosine Springer. 2013:213–232. [Google Scholar]
- 93.Martins I. Sirtuin 1 and adenosine in brain disorder therapy. J Clin Epigenet. 2017;3(1):2472-1158.100045.
- 94.Vossel K.A., Tartaglia M.C., Nygaard H.B., Zeman A.Z., Miller B.L. Epileptic activity in Alzheimer's disease: causes and clinical relevance. The Lancet Neurology. 2017;16(4):311–322. doi: 10.1016/S1474-4422(17)30044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Williams-Karnesky R.L., Sandau U.S., Lusardi T.A., et al. Epigenetic changes induced by adenosine augmentation therapy prevent epileptogenesis. J Clin Investig. 2013;123(8):3552–3563. doi: 10.1172/JCI65636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cellai L., Carvalho K., Faivre E., et al. The Adenosinergic Signaling: A Complex but Promising Therapeutic Target for Alzheimer’s Disease. Front Neurosci. 2018;12:520. doi: 10.3389/fnins.2018.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zisapel N. Sleep and sleep disturbances: biological basis and clinical implications. Cell Mol Life Sci. 2007;64(10):1174. doi: 10.1007/s00018-007-6529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zisapel N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br J Pharmacol. 2018;175(16):3190–3199. doi: 10.1111/bph.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tresguerres J.A., Kireev R., Tresguerres A.F., Borras C., Vara E., Ariznavarreta C. Molecular mechanisms involved in the hormonal prevention of aging in the rat. The Journal of steroid biochemistry and molecular biology. 2008;108(3–5):318–326. doi: 10.1016/j.jsbmb.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 100.Zhou J.N., Liu R.Y., Kamphorst W., Hofman M.A., Swaab D.F. Early neuropathological Alzheimer's changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J Pineal Res. 2003;35(2):125–130. doi: 10.1034/j.1600-079x.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 101.Wu Y.-H., Feenstra M.G., Zhou J.-N., et al. Molecular changes underlying reduced pineal melatonin levels in Alzheimer disease: alterations in preclinical and clinical stages. The Journal of clinical endocrinology & metabolism. 2003;88(12):5898–5906. doi: 10.1210/jc.2003-030833. [DOI] [PubMed] [Google Scholar]
- 102.Naismith S.L., Hickie I.B., Terpening Z., et al. Circadian misalignment and sleep disruption in mild cognitive impairment. J Alzheimers Dis. 2014;38(4):857–866. doi: 10.3233/JAD-131217. [DOI] [PubMed] [Google Scholar]
- 103.Wu Y.-H., Fischer D.F., Swaab D.F. A promoter polymorphism in the monoamine oxidase A gene is associated with the pineal MAOA activity in Alzheimer's disease patients. Brain Res. 2007;1167:13–19. doi: 10.1016/j.brainres.2007.06.053. [DOI] [PubMed] [Google Scholar]
- 104.Tajes M., Gutierrez-Cuesta J., Ortuno-Sahagun D., Camins A., Pallas M. Anti-aging properties of melatonin in an in vitro murine senescence model: involvement of the sirtuin 1 pathway. J Pineal Res. 2009;47(3):228–237. doi: 10.1111/j.1600-079X.2009.00706.x. [DOI] [PubMed] [Google Scholar]
- 105.Panmanee J., Nopparat C., Chavanich N., et al. Melatonin regulates the transcription of betaAPP-cleaving secretases mediated through melatonin receptors in human neuroblastoma SH-SY5Y cells. J Pineal Res. 2015;59(3):308–320. doi: 10.1111/jpi.12260. [DOI] [PubMed] [Google Scholar]
- 106.Mukda S., Panmanee J., Boontem P., Govitrapong P. Melatonin administration reverses the alteration of amyloid precursor protein-cleaving secretases expression in aged mouse hippocampus. Neurosci Lett. 2016;621:39–46. doi: 10.1016/j.neulet.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 107.Chang H.M., Wu U.I., Lan C.T. Melatonin preserves longevity protein (sirtuin 1) expression in the hippocampus of total sleep-deprived rats. J Pineal Res. 2009;47(3):211–220. doi: 10.1111/j.1600-079X.2009.00704.x. [DOI] [PubMed] [Google Scholar]
- 108.Tresguerres J.A., Cuesta S., Kireev R.A., Garcia C., Acuña-Castroviejo D., Vara E. Beneficial effect of melatonin treatment on age-related insulin resistance and on the development of type 2 diabetes. Hormone molecular biology and clinical investigation. 2013;16(2):47–54. doi: 10.1515/hmbci-2013-0041. [DOI] [PubMed] [Google Scholar]
- 109.Cuesta S., Kireev R., García C., Rancan L., Vara E., Tresguerres J.A. Melatonin can improve insulin resistance and aging-induced pancreas alterations in senescence-accelerated prone male mice (SAMP8) Age. 2013;35(3):659–671. doi: 10.1007/s11357-012-9397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kireev R.A., Vara E., Viña J., Tresguerres J.A. Melatonin and oestrogen treatments were able to improve neuroinflammation and apoptotic processes in dentate gyrus of old ovariectomized female rats. Age. 2014;36(5):9707. doi: 10.1007/s11357-014-9707-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Berson D.M. Strange vision: ganglion cells as circadian photoreceptors. Trends Neurosci. 2003;26(6):314–320. doi: 10.1016/S0166-2236(03)00130-9. [DOI] [PubMed] [Google Scholar]
- 112.Turner P.L., Mainster M.A. Circadian photoreception: ageing and the eye’s important role in systemic health. Br J Ophthalmol. 2008;92(11):1439–1444. doi: 10.1136/bjo.2008.141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Turner P.L., Van Someren E.J., Mainster M.A. The role of environmental light in sleep and health: effects of ocular aging and cataract surgery. Sleep Med Rev. 2010;14(4):269–280. doi: 10.1016/j.smrv.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 114.Vandewalle G., Maquet P., Dijk D.-J. Light as a modulator of cognitive brain function. Trends in cognitive sciences. 2009;13(10):429–438. doi: 10.1016/j.tics.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 115.Sletten T.L., Revell V.L., Middleton B., Lederle K.A., Skene D.J. Age-related changes in acute and phase-advancing responses to monochromatic light. J Biol Rhythms. 2009;24(1):73–84. doi: 10.1177/0748730408328973. [DOI] [PubMed] [Google Scholar]
- 116.Revell V.L., Debra J. Impact of age on human non-visual responses to light. Sleep and biological rhythms. 2010;8(2):84–94. [Google Scholar]
- 117.Cheung CY-l, Ong YT, Ikram MK, et al. Microvascular network alterations in the retina of patients with Alzheimer's disease. Alzheimer's & Dementia. 2014;10(2):135-142. [DOI] [PubMed]
- 118.Mirzaei N., Shi H., Oviatt M., et al. Alzheimer's Retinopathy: Seeing Disease in the Eyes. Front Neurosci. 2020;14:921. doi: 10.3389/fnins.2020.00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jaliffa C., Ameqrane I., Dansault A., et al. Sirt1 involvement in rd10 mouse retinal degeneration. Invest Ophthalmol Vis Sci. 2009;50(8):3562–3572. doi: 10.1167/iovs.08-2817. [DOI] [PubMed] [Google Scholar]
- 120.Maloney S.C., Antecka E., Odashiro A.N., et al. Expression of SIRT1 and DBC1 in developing and adult retinas. Stem cells international. 2012;2012 doi: 10.1155/2012/908183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cheng H-L, Mostoslavsky R, Saito Si, et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proceedings of the National Academy of Sciences. 2003;100(19):10794-10799. [DOI] [PMC free article] [PubMed]
- 122.Chen D., Pacal M., Wenzel P., Knoepfler P.S., Leone G., Bremner R. Division and apoptosis of E2f-deficient retinal progenitors. Nature. 2009;462(7275):925–929. doi: 10.1038/nature08544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yi J., Luo J. SIRT1 and p53, effect on cancer, senescence and beyond. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics. 2010;1804(8):1684–1689. doi: 10.1016/j.bbapap.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang H., Park S.-H., Pantazides B.G., et al. SIRT2 directs the replication stress response through CDK9 deacetylation. Proc Natl Acad Sci. 2013;110(33):13546–13551. doi: 10.1073/pnas.1301463110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ban N., Ozawa Y., Inaba T., et al. Light–dark condition regulates sirtuin mRNA levels in the retina. Exp Gerontol. 2013;48(11):1212–1217. doi: 10.1016/j.exger.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 126.Civitarese A.E., Carling S., Heilbronn L.K., et al. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4(3):e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Heilbronn L.K., Civitarese A.E., Bogacka I., Smith S.R., Hulver M., Ravussin E. Glucose tolerance and skeletal muscle gene expression in response to alternate day fasting. Obes Res. 2005;13(3):574–581. doi: 10.1038/oby.2005.61. [DOI] [PubMed] [Google Scholar]
- 128.Yin Z., Gao D., Du K., et al. Rhein Ameliorates Cognitive Impairment in an APP/PS1 Transgenic Mouse Model of Alzheimer's Disease by Relieving Oxidative Stress through Activating the SIRT1/PGC-1alpha Pathway. Oxid Med Cell Longev. 2022;2022:2524832. doi: 10.1155/2022/2524832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Arunsundar M., Shanmugarajan T.S., Ravichandran V. 3,4-dihydroxyphenylethanol attenuates spatio-cognitive deficits in an Alzheimer's disease mouse model: modulation of the molecular signals in neuronal survival-apoptotic programs. Neurotox Res. 2015;27(2):143–155. doi: 10.1007/s12640-014-9492-x. [DOI] [PubMed] [Google Scholar]
- 130.Turner R.S., Thomas R.G., Craft S., et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85(16):1383–1391. doi: 10.1212/WNL.0000000000002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee J., Torosyan N., Silverman D.H. Examining the impact of grape consumption on brain metabolism and cognitive function in patients with mild decline in cognition: A double-blinded placebo controlled pilot study. Exp Gerontol. 2017;87(Pt A):121–128. doi: 10.1016/j.exger.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 132.Moussa C., Hebron M., Huang X., et al. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer's disease. J Neuroinflammation. 2017;14(1):1. doi: 10.1186/s12974-016-0779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Abozaid O.A.R., Sallam M.W., El-Sonbaty S., Aziza S., Emad B., Ahmed E.S.A. Resveratrol-Selenium Nanoparticles Alleviate Neuroinflammation and Neurotoxicity in a Rat Model of Alzheimer's Disease by Regulating Sirt1/miRNA-134/GSK3beta Expression. Biol Trace Elem Res. 2022 doi: 10.1007/s12011-021-03073-7. [DOI] [PubMed] [Google Scholar]