Abstract
Background:
Preclinical data demonstrate that opioids modulate brain reward signaling through an inflammatory cascade, but this relationship has yet to be studied in opioid-exposed neonates.
Methods:
Saliva samples of 54 opioid-exposed and sex- and age-matched non-exposed neonates underwent transcriptomic analysis of inflammatory and reward genes. A subset of 22 neonates underwent brain magnetic resonance imaging (MRI) to evaluate white matter injury commonly associated with inflammatory response. Gene expression and brain MRI were compared between opioid- and non-exposed neonates and further stratified by sex and pharmacotherapy need.
Results:
Opioid-exposed females regardless of pharmacotherapy need had higher expression of inflammatory genes than their male counterparts, with notable differences in the expression of CCL2 and CXCL1 in females requiring pharmacotherapy (p=0.01 and 0.06, respectively). Opioid-exposed males requiring pharmacotherapy had higher expression of DRD2 than exposed females (p=0.07), validating our prior research. Higher expression of IL1β, IL6, TNFα, and IL10 was seen in opioid-exposed neonates with T1 white matter hyperintensity (WMH) compared to exposed neonates without WMH (p<0.05).
Conclusion:
Prenatal opioid exposure may promote inflammation resulting in changes in reward signaling and white matter injury in the developing brain, with unique sex-specific effects. The actions of opioids through non-neuronal pathways need further investigation.
INTRODUCTION:
Neonatal abstinence syndrome (NAS) or neonatal opioid withdrawal syndrome (NOWS) is a public health crisis with a high socioeconomic burden.1 Determining which opioid-exposed neonates will develop NAS requiring treatment and prolonged hospitalization remains extremely challenging, limited by the heterogeneity of predictive factors and available scoring systems to assess the need for pharmacotherapy.2,3 Further, genetic variations in neuronal genes involved in opioid metabolism have been shown to modulate withdrawal risk and severity of NAS.4,5
While it is known that opioids act through neuronal pathways, emerging animal data demonstrate that opioids also act via non-neuronal pathways by binding to toll-like receptor type 4 (TLR4) on microglial cells, similar to the natural ligand lipopolysaccharide (LPS).6 Binding to microglia—the resident immune cells in the brain—leads to a subsequent release of cytokines and chemokines7,8 as well as modulation of reward behaviors.9–11 Jantzie and colleagues demonstrated that pups exposed to methadone showed a hyperactive peripheral inflammatory response with accompanying alterations in microglia morphology and behavioral changes. These findings highlight the pro-inflammatory role of opioid exposure in utero with long-term neural injury and cognitive deficits.12 However, no study to date has examined the effects of prenatal opioid exposure on inflammatory and reward modulation in neonates.
Although white matter abnormalities in opioid-exposed neonates have been reported, the etiologies are unclear.13,14 Based on the pro-inflammatory effects of prenatal opioids in preclinical studies, along with the origin of microglia in yolk sac macrophages and their migration to the brain during early fetal development, we postulate that white matter changes seen in opioid-exposed neonates may be pro-inflammatory in origin.15,16 Indeed, the association between inflammation and white matter injury has been widely studied in preterm neonates.17–19
Based upon our previous published data that showed opioid-exposed males have a greater expression of a key reward gene, dopamine receptor type 2 (DRD2),20 we conducted this current pilot, observational study to better understand: 1) the sex-specific inflammatory effects of prenatal opioid exposure and its impact on reward signaling, and 2) the inflammatory role of prenatal opioid exposure on white matter changes.
METHODS:
Salivary Gene Expression Analyses
Saliva samples from 54 neonates born between 34 and 42 weeks’ gestation at Tufts Medical Center (TMC), Boston, MA and Melrose-Wakefield Hospital (MWH), Melrose, MA were collected, following approval by the Institutional Review Board and parental informed consent. Opioid-exposed neonates were defined as being born following a known history of maternal opioid use and/or positive maternal toxicology testing for opioids during the third trimester of pregnancy. The non-exposed group consisted of sex- and gestational age (GA)-matched healthy neonates (within one week) born to mothers without a history of opioid use during gestation. We excluded neonates of diabetic mothers and those born in a setting of chorioamnionitis due to the known pro-inflammatory states associated with these conditions, as well as those with chromosomal or congenital anomalies.21,22 Opioid-exposed neonates were further categorized by the need for pharmacotherapy based on the Modified Finnegan Scoring System.23 Demographic data collected from medical records included gestational age (GA), anthropometric data [birth weight (BW), length (L), head circumference (HC)], sex, mode of delivery, Apgar scores (1, 5 mins), race, ethnicity, maternal smoking status, group B streptococcus (GBS) and Hepatitis C (Hep C) status. In the opioid-exposed group, information on maternal medications, maternal urine toxicology, neonatal toxicology (urine/meconium), and the need for pharmacotherapy were also collected.
All saliva samples were collected within 48 hours of birth. For opioid-exposed neonates, saliva collection occurred prior to the start of pharmacotherapy to exclude any effect of postnatal medication(s). Saliva was collected at least 1 hour from feeding to minimize milk contamination using previously described techniques.24,25 Briefly, neonates’ mouths were gently suctioned using a 1 mL insulin syringe (Becton, Dickinson and Company, Franklin Lakes, NJ) attached to the low-pressure wall suction for approximately 15 to 30 seconds. Whole saliva was immediately placed in Eppendorf tubes pre-filled with 250 μL RNAprotect Saliva Reagent (Qiagen, Hilden, Germany) to minimize RNA degradation, then vortexed and stored at 4°C for at least 48 hours but no more than 28 days pending RNA extraction. RNA was extracted using RNeasy Micro Kit (Qiagen) per the manufacturer’s instructions. On-column DNase treatment was performed for each sample using RNase-free DNase I Set (Qiagen) to minimize DNA contamination. Extracted RNA was stored at −80°C pending reverse transcription.
Samples were converted to cDNA using SuperSript™ VILO cDNA Synthesis Kit and pre-amplified using TaqMan® PreAmp Master Mix Kit (Applied Biosystems) based upon the inventoried amplicons of the seven targeted genes of interest, including key reward gene (dopamine receptor type 2/DRD2) and inflammatory genes (interleukin-1-beta/IL1β, interleukin-6/IL6, interleukin-10/IL10, tumor necrosis factor alpha/TNFα, C-X-C motif chemokine ligand-1/CXCL1, and C-C motif chemokine ligand-2/CCL2). These pro- and anti-inflammatory genes of interest were selected based on opioid-induced inflammatory cascade reported in preclinical studies.12,26–28 Pre-amplification of samples was performed on the Mastercycler® pro S Thermal Cycler (Eppendorf, Hamburg, Germany) at 95°C for 10 minutes (min) for enzyme activation, followed by 14 cycles of preamplification at 95°C for 15 seconds (s) and 60°C for 4 min, then 99°C for 10 minutes for the enzyme inactivation. Pre-amplified cDNA samples were stored at −20°C until qPCR amplification.
To properly normalize for varying starting total mRNA input across samples, two reference genes that have been previously shown in our laboratory to remain stable across sex and post-conceptional age were used, i.e., glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and tyrosine 3-mono-oxygenase/tryptophan 5-mono-oxygenase activation protein, zeta polypeptide (YWHAZ).29 Threshold cycle (Ct) values for genes of interest and reference genes were detected using QuantStudio™ 7 Flex Real-Time PCR (Applied Biosystems) machine. The following thermal cycle profile was utilized: incubation at 50°C for 2 min, denaturation at 95°C for 10 min, then 40 cycles of denaturation at 95°C for 15 s, and annealing and extension at 60°C for 60 s. All samples were run in duplicate. A calibrator sample was acquired from a healthy, non-exposed term neonate who was not part of either study group. This calibrator sample was included in every plate to assess plate-to-plate variability. The expression of pro- and anti-inflammatory genes was correlated with DRD2 expression, previously quantified,25 to evaluate the relationship between inflammation and reward signaling.
Brain MRI
Of the 54 neonates, 22 also had structural brain MRIs performed, consisting of 11 opioid-exposed and 11 sex- and GA-matched non-exposed neonates. Brain MRI was acquired within 48 hours of birth using the feed-and-swaddle technique, eliminating the need for sedative medications. No opioid-exposed neonates received pharmacotherapy at the time of the MRI acquisition. Images were acquired on a Phillips Achieva 3.0T (TX) MRI scanner (Philips Healthcare, The Netherlands) with an 8-channel head coil. Imaging sequences included a sagittal 3D T1-weighted turbo field echo (TFE; a magnetization prepared gradient-echo sequence) (field of view (FOV) 17 × 17 cm, matrix 170 × 170, slice thickness 1 mm, interslice gap 0 mm, repetition time (TR) 8.33, echo time (TE) 3.83, flip angle (FA) 10.00, echo train length (ETL) 100, number of excitations (Nex) 2, Bandwidth 189), an axial T2-weighted fast spin-echo sequence (FSE) (FOV 18 × 18 cm, matrix 400 × 277, slice thickness 3 mm, interslice gap 0 mm, TR 5829.27, TE 170, ETL 11, Nex 2, Bandwidth 56), axial diffusion tensor imaging (b-value 1000, directions 16, FOV 20 × 20 cm, matrix 100 × 100, slice thickness 2 mm, interslice gap 0 mm, TR 7820.42, TE 55, FA 90.00, ETL 53, Nex 2, Bandwidth 2969), axial T2*/gradient echo (FOV 18 × 18 cm, matrix 200 × 160, slice thickness 3 mm, interslice gap 0 mm, TR 997.69, TE 16.12, FA 18, ETL 1, Nex 1, Bandwidth 217), a sagittal T2 3D (FOV 17 × 17 cm, matrix 156 × 156, slice thickness 1.10 mm, interslice gap 0 mm, TR 2500, TE 240.33, FA 90, ETL 133, Nex 3, Bandwidth 967), and Sagittal T2 DRIVE (FOV 17 × 17 cm, matrix 296 × 215, slice thickness 2 mm, interslice gap 0 mm, TR 1550, TE 200, FA 90, ETL 57, Nex 1, Bandwidth 321) sequences. Brain MRIs were read and interpreted by a pediatric neuroradiologist blinded to the clinical history or exposure status of the neonates. Given the relatively decreased myelination in this early postnatal age group, T2-weighted images were used to delineate gray and white matter differentiation.30 White matter hyperintensity (WMH) was defined as any white matter region with increased brightness when visualized on a T1-weighted MRI sequence (relative to the cortex). The increased brightness was easily identified given the background hypointensity due to the relatively unmyelinated white matter in neonates.31
Statistical analysis
The data analysis for this study was conducted using Stata 17 (StataCorp, College Station, TX), and figures were rendered using GraphPad Prism 9.2.0 (GraphPad Software, San Diego, CA). Normalized delta Ct (ΔCt) values were obtained by subtracting geometrical mean Ct values of the reference genes from the mean Ct values of each target gene. Gene expression was compared between opioid-exposed and non-exposed neonates. Data were further stratified by sex and the need for pharmacotherapy. For brain MRI, the proportion of WMH was derived by calculating the ratio between the number of WMH and the total number of subjects undergoing MRI. WMH was further analyzed by opioid exposure and salivary gene expression. All statistical testing was two-tailed with alpha=0.05. Categorical data were analyzed using Fisher’s exact test, and continuous data using either two-sample t-test (parametric) or Wilcoxon sum-rank (non-parametric).
RESULTS
Salivary Gene Expression
Demographic data of the 54 neonates undergoing salivary gene expression analyses are provided in Table 1. Compared to the non-exposed neonates, opioid-exposed neonates had significantly fewer non-white and Hispanic participants, smaller HC percentiles, less exposure to maternal GBS, and higher exposure to maternal Hep C and cigarette smoking. Opioid- and non-exposed neonates did not differ significantly in the expression of inflammatory and reward genes (Figure 1a). However, when stratified by sex, opioid-exposed females showed higher expression of inflammatory genes compared to opioid-exposed males, most notably for CXCL1 (p=0.06), followed by IL1β and CCL2 (p=0.15) (Figure 1b). Within the exposed group requiring pharmacotherapy, stratification by sex also showed that females compared to males had higher expression of CCL2 (p=0.01) and CXCL1 and IL1β (p=0.07 and 0.15, respectively) (Figure 1c). The expression of DRD2 was higher in opioid-exposed males requiring pharmacotherapy compared to their female counterparts (p=0.07).
Table 1.
Demographic data of subjects included in the salivary gene expression analysis
| Non-Exposed (N=27) | Opioid-Exposed (N=27) | P | |
|---|---|---|---|
| Male sex | 13 (48.1) | 13 (48.1) | NA |
| Non-white race | 11/26 (42.3) | 3/27 (11.1) | 0.01 |
| Hispanic ethnicity | 8/26 (30.8) | 1/27 (3.7) | 0.01 |
| GA (weeks) | 38.1 (37.0, 38.6) | 38.1 (37.0, 39.0) | 0.97 |
| BW (grams) | 3079.0 (426.7) | 2944.5 (659.3) | 0.38 |
| BW percentile | 51.8 (22.6) | 41.5 (29.4) | 0.15 |
| Length (cm) | 48.9 (2.1) | 47.0 (4.8) | 0.07 |
| Length percentile | 53.6 (21.0) | 43.2 (32.6) | 0.17 |
| HC (cm) | 34.4 (2.9) | 33.5 (4.2) | 0.37 |
| HC percentile | 55.8 (24.9) | 38.8 (33.0) | 0.04 |
| SGA | 0 (0.0) | 7 (25.9) | 0.01 |
| Cesarean section | 14 (51.9) | 13 (48.2) | 1.00 |
| 1-minute Apgar | 9.0 (8.0, 9.0) | 9.0 (7.0, 9.0) | 0.44 |
| 5-minute Apgar | 9.0 (9.0, 9.0) | 9.0 (9.0, 9.0) | 0.37 |
| Positive GBS status | 10/24 (41.7) | 3/23 (13.0) | 0.05 |
| Positive Hepatitis C status | 0/16 (0.0) | 13/27 (50.0) | <0.01 |
| Maternal Smoking (N, %) | 2 (7.4) | 16 (59.3) | <0.01 |
| Methadone | 15 (55.6) | ||
| Polysubstance | NA | 12 (44) | NA |
Matched, NA=not available/applicable, GA=gestational age, BW=birth weight, HC=head circumference, SGA=small for gestational age, GBS=group B streptococcus.
Polysubstance indicates presence of prenatal opioids and other substances, including marijuana, benzodiazepines, barbiturates, gabapentin, amphetamine/dextroamphetamine, cocaine, heroin.
Data are presented as mean (standard deviation) or median (interquartile ranges) for continuous measures, and N (%) for categorical measures. Bolded p values represent significant differences
Figure 1.
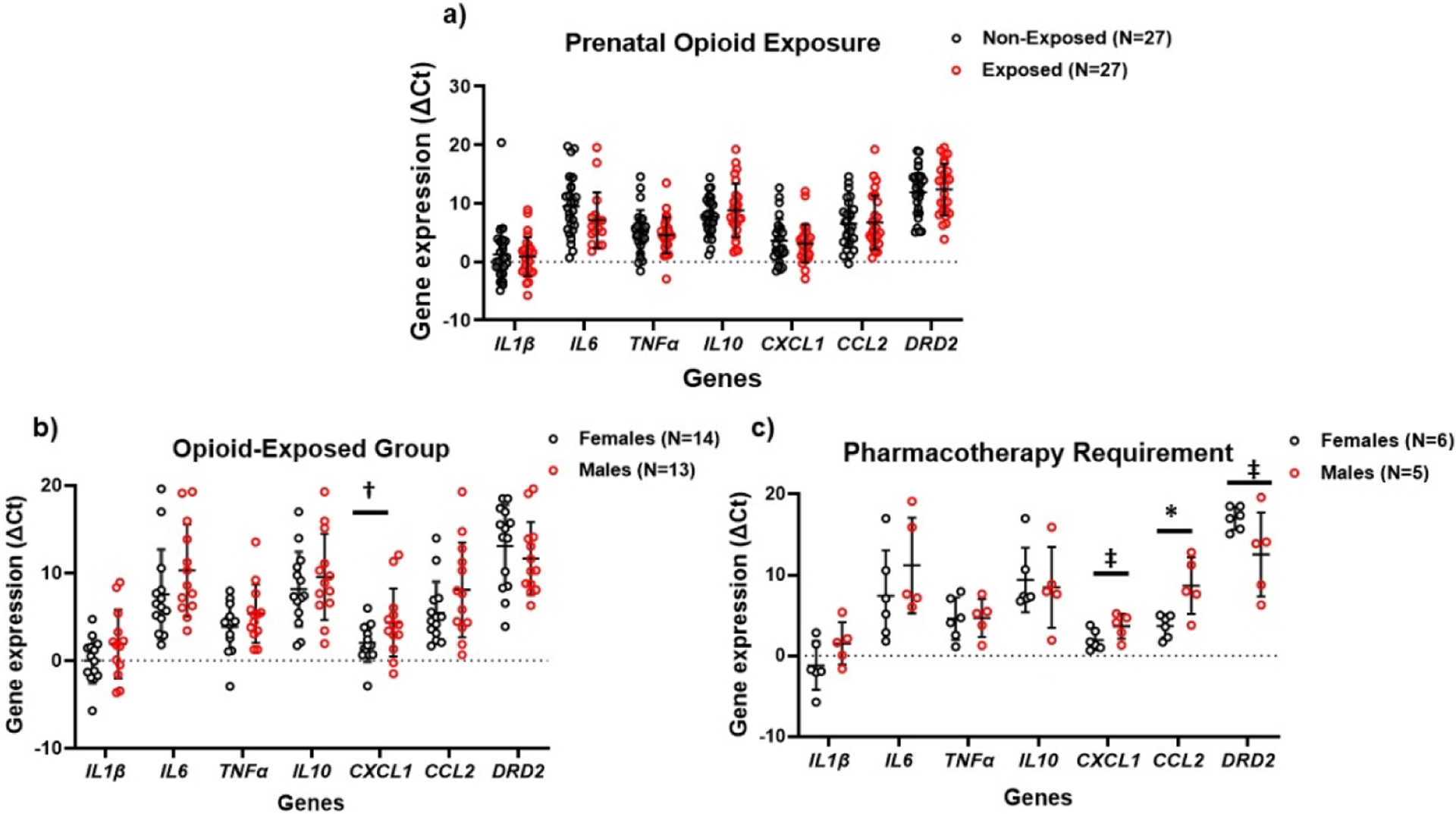
Differential gene expression stratified by presence of prenatal opioid exposure (a); by sex in opioid-exposed neonates (b); and in exposed neonates requiring pharmacotherapy (c). Greater expression of inflammatory genes is observed in opioid-exposed females and in females requiring pharmacotherapy, with greater expression of DRD2 in opioid-exposed males and in males requiring pharmacotherapy.
ΔCt values are inversely proportional to the gene expression levels.
* p<0.05, †p=0.06, ‡p=0.07
Brain MRI
Within the cohort of 22 neonates who underwent brain MRI, opioid-exposed neonates had significantly fewer Hispanic participants, smaller BW and HC, higher incidence of small for gestational age (SGA), and higher exposure to maternal smoking compared to non-exposed neonates (Table 2). Detailed MRI reporting is provided in Table 3. Punctate T1 WMH was seen in six opioid-exposed neonates compared to two in the non-exposed neonates (p=0.18) (Figure 2). Of the six opioid-exposed neonates with T1 WMH, four were females, and two were males. More neonates were exposed to buprenorphine than methadone (66.7% vs 33.3%). Only one neonate with T1 WMH needed pharmacotherapy. Figure 3 shows salivary gene expression of the 11 opioid-exposed neonates who underwent brain MRI, with significantly higher expression of IL1β, IL6, TNFα, and IL10 in neonates with T1 WMH than those without T1 WMH (p <0.01 to 0.04). The expression of CXCL1, CCL2, and DRD2 was also higher in the group with than without T1 WMH, although these differences were not significant.
Table 2.
Demographic characteristics of the MRI cohort
| Non-Exposed (N=11) | Opioid-Exposed (N=11) | P | |
|---|---|---|---|
| Male sex | 5 (45.5) | 5 (45.5) | NA* |
| Non-white race | 5 (45.5) | 3 (27.3) | 0.66 |
| Hispanic ethnicity | 6 (54.5) | 0 (0.0) | 0.01 |
| GA (weeks) | 37.1 (36.6, 38.6) | 37.2 (36.2, 39.1) | 0.87 |
| BW (grams) | 3146.4 (337.9) | 2631.2 (653.7) | 0.03 |
| BW percentile | 65.2 (10.4) | 28.8 (26.0) | <0.01 |
| Length (cm) | 48.3 (2.1) | 46.4 (4.4) | 0.22 |
| Length percentile | 56.9 (23.7) | 33.5 (35.6) | 0.09 |
| HC (cm) | 35.4 (4.3) | 32.3 (2.8) | 0.06 |
| HC percentile | 65.1 (24.1) | 34.5 (34.6) | 0.03 |
| SGA | 0 (0.0) | 5 (45.5) | 0.04 |
| Cesarean section | 7 (63.6) | 4 (36.4) | 0.39 |
| 1-minute Apgar | 8.0 (8.0, 9.0) | 8.0 (6.0, 9.0) | 0.55 |
| 5-minute Apgar | 9.0 (8.5, 9.0) | 9.0 (8.5, 9.0) | 0.90 |
| Positive GBS status | 5/9 (55.6) | 2/10 (20.0) | 0.17 |
| Positive Hepatitis C status | 0/10 (0.0) | 4/10 (40.0) | 0.09 |
| Maternal Smoking | 2 (18.2) | 7 (63.6) | 0.03 |
| Methadone | 3 (27.3) | ||
| Polysubstance | NA | 4 (36.4) | NA |
Matched, NA=not available/applicable, GA=gestational age, BW=birth weight, HC=head circumference, SGA=small for gestational age.
Data are presented as mean (standard deviation) or median (interquartile ranges) for continuous measures, and N (%) for categorical measures. Bolded p values represent significant differences.
Table 3.
MRI findings in neonates with and without prenatal opioid exposure
| Sex | Non-Exposed (N=11) | Opioid-Exposed (N=11) | |||
|---|---|---|---|---|---|
| GA | Result | GA | Exposure | Result | |
| M | 35 1/7 | Normal | 34 0/7 | Meth | Parturitional bleed, otherwise normal |
| 34 6/7 | Normal | 34 0/7 | Bup + Naloxone | Punctate WMH | |
| 38 4/7 | Parturitional bleed, otherwise normal | 37 4/7 | Meth | Punctate WMH | |
| 38 6/7 | Punctate WMH | 39 1/7 | Bup | Normal | |
| 39 1/7 | Punctate WMH | 39 4/7 * | Bup | Normal | |
| F | 37 1/7 | Normal | 36 2/7 * | Meth | Punctate WMH |
| 36 6/7 | Normal | 37 0/7 | Bup + Naloxone | Normal | |
| 37 0/7 | Normal | 37 1/7 | Bup + Naloxone | Punctate WMH | |
| 37 1/7 | Normal | 37 2/7 | Bup | Punctate WMH | |
| 38 1/7 | Parturitional bleed, otherwise normal | 38 2/7* | Bup | Normal | |
| 39 2/7 | Incidental connatal cyst, otherwise normal | 39 6/7 | Bup + Naloxone | Punctate WMH | |
| Proportion of WMH | P value | |
|---|---|---|
| Opioid-Exposed (N=11) | Non-Exposed (N=11) | |
| 6 (54.5%) | 2 (18.2%) | |
| 66.7% Females | 0.0% Females | 0.10 |
receiving pharmacotherapy.
GA=gestational age, M=males, F=females, Bup=buprenorphine, Meth=methadone, WMH=white matter hyperintensity. Data are presented as N (%) for categorical measures.
Figure 2.
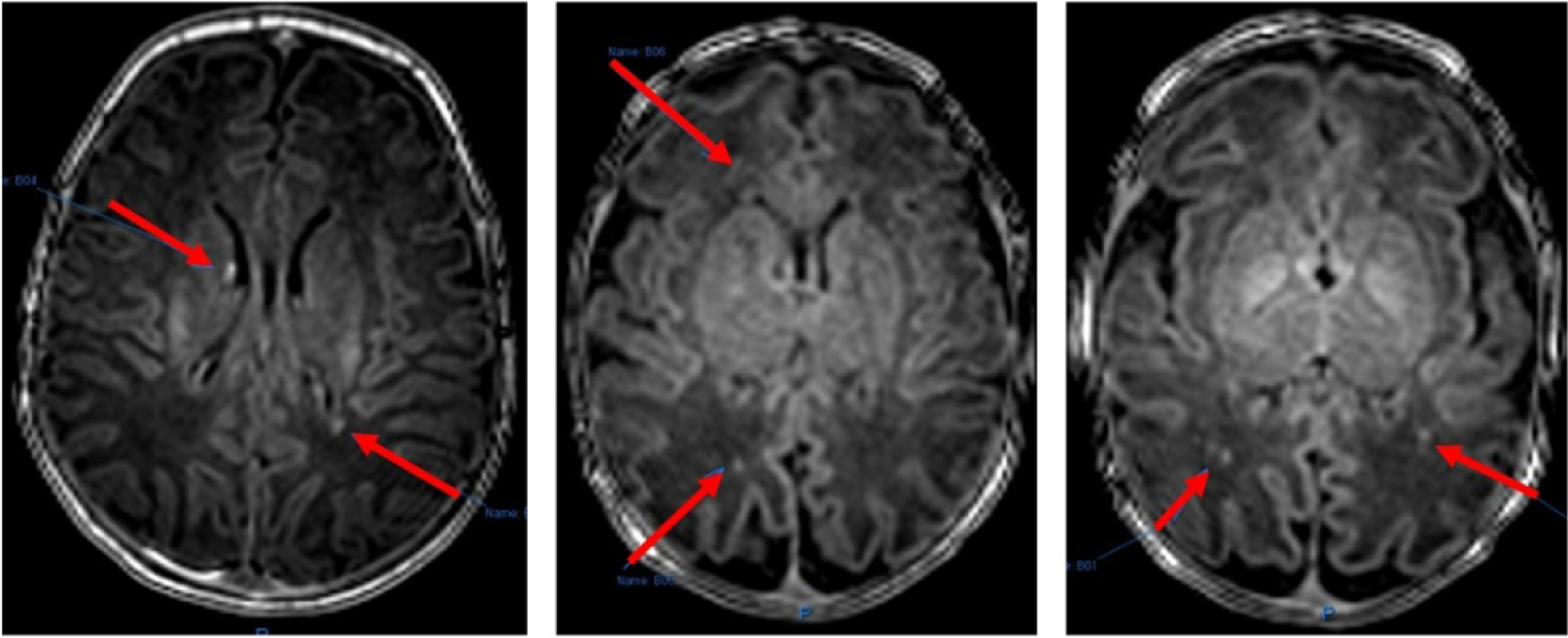
Punctate T1 white matter hyperintensities seen on the brain MRI of neonates in this study (red arrows). Images courtesy of Dr. Neel Madan.
Figure 3.
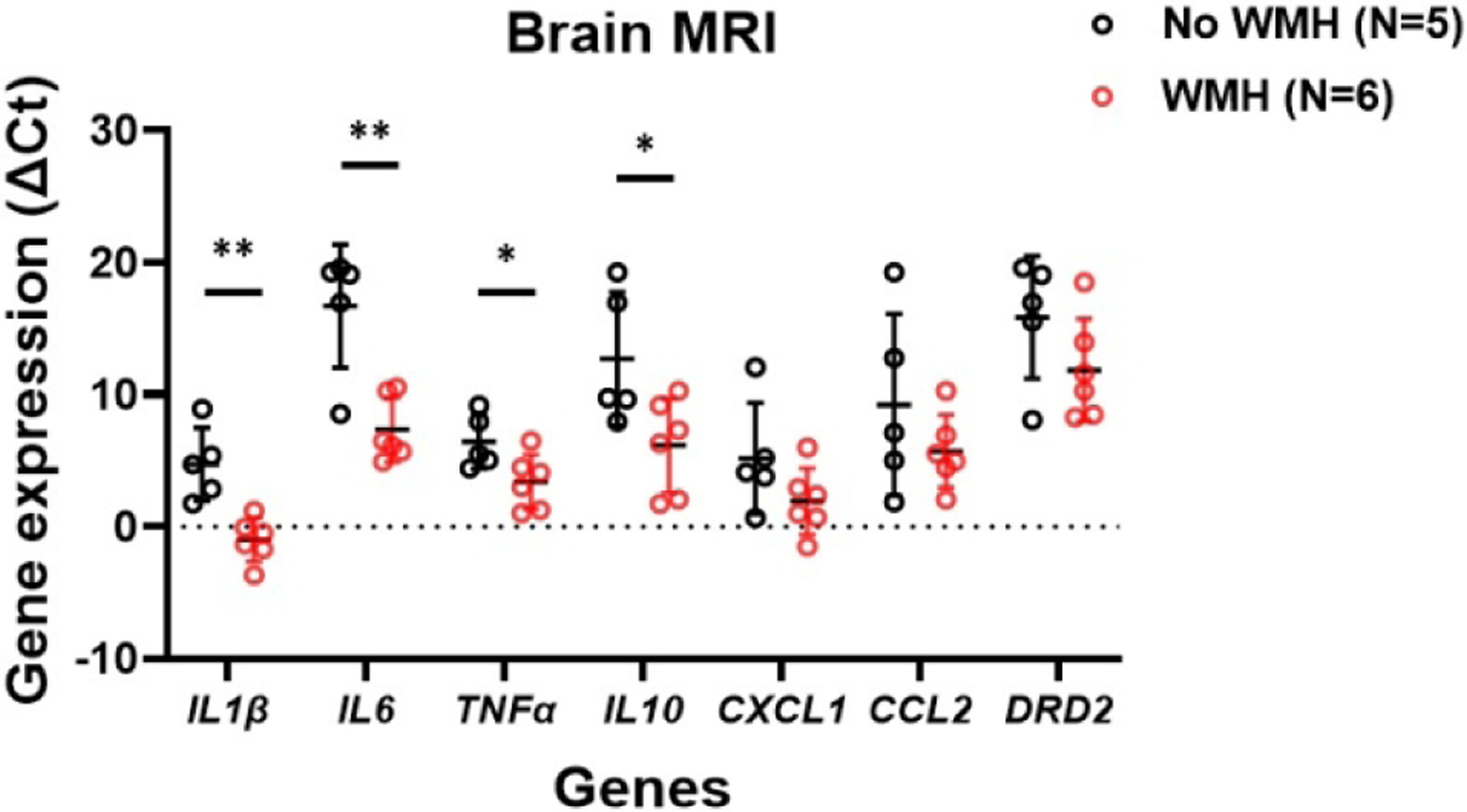
Differential gene expression in opioid-exposed neonates stratified by presence of white matter hyperintensity (WMH) on brain MRI. Greater pro-inflammatory and anti-inflammatory genes are seen with WMH. Greater expression of reward gene is also seen with WMH.
ΔCt values are inversely proportional to the gene expression levels.
** p<0.01, * p<0.05
Discussion:
The sex-specific inflammatory effects of prenatal opioid exposure:
While the impact of prenatal opioid exposure on the developing brain has become the subject of intense interest in recent years, this pilot study appears to be the first to demonstrate that inflammation may mediate the adverse effects of prenatal opioids on the neonatal brain in a sex-specific manner. Animal studies have shown that opioids bind to TLR4 via the MyD88-dependent pathway similar to LPS, an outer surface component of Gram-negative bacteria and a natural ligand for TLR4.12,32,33 Binding of the ligands to TLR4 induces downstream pro-inflammatory, neuroexcitatory cascades via the MyD88-dependent intracellular pathways and the release of cytokines and chemokines.34–36 Such interaction between opioids and TLR4 is mainly observed in the neuroglial cells, e.g., astrocytes and microglia, leading to neuroinflammation.34,36 In vivo studies using peripheral blood mononuclear cells have demonstrated that naltrexone, an opioid antagonist, blocks TLR binding and inhibits the production of TNFα and IL6.36 Administration of naloxone in rats significantly suppresses the self-administration of remifentanil (a μ-opioid agonist) by blocking the TLR4 pathway.7 Similarly, mice deficient in TLR4 or MyD88 were unable to signal through the TLR4-mediated signaling cascade and displayed significantly reduced opioid-induced conditioned place preference,7 a behavioral paradigm used to mimic the positive reinforcing effect of drugs or to measure reward cues.37 Combined, these data indicate that opioids act through non-opioid receptor pathways with molecular and behavioral effects.
Our pilot study supports preclinical data on the pro-inflammatory impact of opioids. While we observed no significant differences in gene expression between opioid- and non-exposed neonates, stratification by sex suggests that opioid exposure enhances the expression of pro-inflammatory genes in opioid-exposed females, especially those requiring pharmacotherapy, e.g., CXCL1 and CCL2. These findings support the potential linkage between inflammation and opioid exposure in utero and the severity of NAS in the female cohort and highlight the need to further follow this cohort to understand the long-term effects of this pro-inflammatory process.
Prenatal opioid effect on reward signaling may also be sex-specific:
In the current study, we demonstrated a trend towards greater DRD2 expression in males with prenatal opioid exposure compared to opioid-exposed females and in those requiring pharmacotherapy compared to those not requiring pharmacotherapy. These findings replicate our prior research and further support the hypothesis that prenatal opioid exerts a sex-specific impact of prenatal opioid exposure on the expression of DRD2.20 Previously, we demonstrated that higher expression of DRD2 in opioid-exposed males compared to their female counterparts might be linked to NAS severity, the need for pharmacotherapy, and hyperphagia. We postulated that the higher expression of DRD2 might also predispose males to heightened reward-seeking behavior. Therefore, our current results highlight the potential sex-specific and unique mechanisms by which prenatal opioids adversely impact males and females. While the expression of DRD2 is lower in opioid-exposed females than opioid-exposed males, their increased inflammatory profiles may predispose females to a completely different risk profile. Studies by Volkow et al. demonstrated that adults with drug addiction and those with obesity had decreased DRD2 availability on their positron emission tomography imaging, postulated to underlie the drive for compulsive drug- and food-taking behavior.38,39 Furthermore, while male sex is associated with worse NAS severity in the neonatal period and a higher incidence of opioid use disorder (OUD) in adulthood, studies have shown that females with OUD are known to develop dependence more rapidly than males and are at higher risk of developing depression and comorbid psychiatric conditions.40–44 Further investigation on the differential expression of DRD2 in males and females may elucidate the unique behavioral and psychiatric manifestations across sex.
Inflammation may modulate white matter injury in opioid-exposed neonates:
Our opioid-exposed group undergoing brain MRI also had smaller HC, BW, and a higher incidence of SGA compared to unexposed neonates, replicating previous studies in the literature.45–47 In addition to the structural brain differences, the punctate WMH findings corroborate the report by Merhar et al., where 8/20 opioid-exposed neonates also had punctate white matter lesions on the structural brain MRI compared to none in the non-exposed group.14 Unlike preterm neonates who are at risk for white matter injury due to the arrest in oligodendrocyte lineage maturation and disruption of myelination during critical developmental stages,48,49 punctate white matter lesions seen in term neonates may stem from ischemic changes, small hemorrhages, or gliosis.50 White matter abnormalities have also been reported in adult methadone users, ranging from punctate to large confluent lesions.51 The significance of WMH on long-term neurodevelopmental outcomes is unclear. Some studies have demonstrated diffuse and extensive lesions that were not predictive of short-term neurodevelopmental outcomes.52 In contrast, others have shown that even punctate lesions were associated with compromised cognitive and developmental outcomes.53 Additionally, location rather than lesion load is often the major determining factor for long-term outcomes.53 Hayman et al. demonstrated that punctate white matter lesions in early brain MRI might no longer be evident in later imaging. Alternatively, they may also lead to further gliosis and/or white matter loss.50 Therefore, it is vital to perform longitudinal imaging in infants with abnormal brain MRI, especially in conjunction with neurodevelopmental assessments. Higher expression of DRD2 seen with WMH corroborates preclinical data demonstrating the modulatory effects of opioids on brain reward signaling and highlights the need to further investigate the linkage between inflammation, reward signaling, and white matter injury in opioid-exposed neonates.
The significantly greater expression of inflammatory genes in opioid-exposed neonates with WMH compared to those without WMH suggests that opioid exposure in utero may play an important role in the pathogenesis of WMH. Our findings are consistent with animal data where prenatal opioid exposure leads to brain inflammation accompanied by impaired myelination, glial injuries, white matter gliosis, as well as decreased fractional anisotropy on diffusion sequences on brain MRI.12,54 Opioids, through their action on TLR4 on microglia and the release of inflammatory mediators, may promote white matter injuries through direct cytotoxic effects on the oligodendrocyte lineage and subsequent disruption in the myelination process.49 Indeed, such a mechanism has been proposed in premature neonates, where the immature and permeable blood-brain barrier allows the transfer of peripheral inflammatory cytokines and the disruption of brain myelination.55,56 To the best of our knowledge, our study is the first to show the role of inflammation in white matter injuries in neonates with opioid exposure in utero.
Opioid effects in preterm infants and irrespective of the need for pharmacotherapy:
While it is widely recognized that preterm neonates exposed to in utero opioids experience fewer signs of withdrawal,2,57 two of the six neonates with WMH findings in our study were <37 weeks’ GA. This highlights that prenatal opioid exposure may affect the brain of preterm neonates. Additionally, while our study was not longitudinal, the WMH at varying ages—34 to 39 6/7 weeks’ GA—may indicate the impact of in utero opioid exposure on brain myelination and white matter integrity across gestation. Importantly, the discordance between the need for pharmacotherapy and the presence of WMH is a novel observation. Among six opioid-exposed neonates with WMH findings, only one needed pharmacotherapy; among three opioid-exposed neonates receiving pharmacotherapy, only one had WMH. Our results, therefore, further emphasize the need for longitudinal monitoring and long-term follow-up for all opioid-exposed neonates, irrespective of gestational age and the need for pharmacotherapy.
A limitation of our study is the relatively small sample size, which likely renders our results difficult to generalize58 and reduces the statistical power of our comparisons, particularly in subgroup analyses where the subject numbers become smaller. Due to the small sample size and pilot nature of the study, we could not adjust for multiple comparisons; so the significance of findings should be interpreted cautiously given the potential for Type I errors. Furthermore, while we matched our exposed and non-exposed groups on the important confounders of sex and GA, these groups differed on several important potential confounders as listed on Table 1 (e.g., race, ethnicity, Hep C, SGA, and smoking status), all of which may be related to the gene expression and brain MRI findings. For instance, SGA has been associated with greater inflammation which may have contributed to the presence of WMH in our study.59 Similarly, it is possible that prenatal opioid exposure may in part explain why these neonates are born smaller.45,46 We also were unable to evaluate all of the multiple factors that may lead to heightened inflammation in the postnatal and neonatal periods, such as maternal obesity, diet, and socioeconomic status. Future work with much larger sample sizes should incorporate adjusted analyses to control for these differences.
Our study demonstrated the WMH findings in two non-exposed neonates. While several etiologies may cause T1 WMH, it is unclear why two healthy neonates in our study would develop WMH; could this be a non-specific finding, and does it correlate with long-term outcomes? Future studies will continue to enroll non-exposed neonates to examine the prevalence of T1 WMH in the presence or absence of prenatal opioid exposure.
Another limitation is in our ability to extrapolate changes in salivary gene expression to actual changes in the brain. Although DRD2 is primarily expressed in the brain (https://www.proteinatlas.org/ENSG00000149295-DRD2/tissue), this gene may be expressed elsewhere in the body. Therefore, our results could not specifically ascertain if salivary expression of DRD2 represented brain reward signaling. While brain imaging and functional/brain connectivity analyses may elucidate brain reward signaling changes resulting from prenatal opioid exposure, a confirmative way to link the peripheral and central expression of DRD2 is through a transcriptomic analysis of DRD2 in the brain. Such an undertaking would only be possible through preclinical studies and highlights the importance of collaborative efforts between clinical and basic researchers. Finally, our study only includes salivary and brain MRI data at a one-time point, thereby limiting serial or longitudinal understanding of the long-term impact of prenatal opioid exposure. Future studies will include serial data points, as well as the addition of diffusion tensor imaging, which estimates white matter microarchitecture and myelination effects of prenatal opioid exposure.60
The strengths of these pilot data lie in the non-invasive methods to understand the impact of prenatal opioid exposure on the developing brain. Neonatal saliva and brain MRI platforms are safe, relatively easy to obtain, and may be performed repetitively, allowing for serial data points and longitudinal studies. Obtained immediately after birth and prior to pharmacotherapy, our brain imaging and salivary transcriptomic data reflect in utero opioid effects rather than the postnatal, pharmacologic, or environmental effects. While other studies have demonstrated white matter abnormalities in the context of opioid exposure or use, these studies were done later in life and likely reflected confounding factors that may result in white matter changes.13,14 Despite the small size, our pilot study is proof of concept and demonstrates the feasibility of studying serial inflammatory gene expression in neonatal saliva and white matter changes in brain MRI starting immediately after birth. These pilot data also provide estimates of effect size and power calculation for future hypothesis-testing studies. Finally, our study demonstrates the importance of studying sex as an important variable contributing to differential outcomes in opioid-exposed neonates. The differential gene expression in the male and female cohorts demonstrate the importance of sex as a biological variable in all types of research, especially in pediatric research, where an early understanding of sex differences serves as a crucial foundation for personalized medicine and individualized interventions.
In conclusion, our pilot study is the first to examine the linkage between inflammation, reward signaling, and white matter injury in opioid-exposed neonates. Salivary gene expression highlights the potential sex-specific inflammatory effects of prenatal opioids, with a greater effect in females than males. The sex-specific effect of prenatal opioids on the expression of reward gene also underlines the need to further examine such effects on future reward-seeking behaviors and consequences. The postnatal T1 WMH findings and evidence of heightened inflammation in T1 WMH also highlight the potential need to examine the effects of prenatal opioids on the opioid- and non-opioid receptor pathways and the need to follow up this population longitudinally. Adding to the field that has begun to recognize the long-term consequences of prenatal opioid exposure, our pilot study provides early evidence at the molecular and structural levels that may justify the urgent need to implement robust anticipatory guidance and follow-up regimens post-hospital discharge. Larger studies are urgently needed to better understand this critical public health topic.
Key points/Impact:
Opioid-exposed neonates are at risk for punctate T1 white matter hyperintensity (WMH).
Females carry a greater propensity for WMH.
Salivary transcriptomic data showed significantly higher expression of inflammatory genes in opioid-exposed neonates with WMH than those without WMH, irrespective of pharmacotherapy need.
Adding to prior studies, our findings suggest that prenatal opioid exposure may modulate white matter injury and reward signaling through a pro-inflammatory process that is sex specific.
This novel study highlights the short-term molecular and structural effects of prenatal opioids and the need to elucidate the long-term impact of prenatal opioid exposure.
Acknowledgment
We would like to thank all the families for participating in this study. We also thank the entire staff at the Tufts Shields MRI for their assistance in organizing and acquiring the images.
Funding
This study received funding from K12 Building Interdisciplinary Research for Careers in Women’s Health (BIRCWH) Grant #5K12HD092535-05, Charles H. Hood Foundation Child Health Research Grant, and Tufts Tiny Feet Funds. The project was supported by the National Center for Advancing Translational Sciences, NIH, #UL1TR002544. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Competing Interests
All authors declare no conflicts of interest.
Consent Statement
Informed consents were obtained from all subjects enrolled in this study.
Data Availability
Materials described in the manuscript, including all relevant raw data, are available for research and non-commercial purposes upon reasonable request, without breaching participant confidentiality.
REFERENCES:
- 1.Honein MA, Boyle C & Redfield RR Public health surveillance of prenatal opioid exposure in mothers and neonates. Pediatrics 143, e20183801 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelty E & Preen DB Risk factors associated with the occurrence of neonatal opioid withdrawal syndrome: a review. CNS Drugs 33, 1113–20 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Isemann BT, Stoeckle EC, Taleghani AA & Mueller EW Early prediction tool to identify the need for pharmacotherapy in neonates at risk for neonatal abstinence syndrome. Pharmacother 37, 840–8 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Wachman EM, et al. Association of OPRM1 and COMT single-nucleotide polymorphisms with hospital length of stay and treatment of neonatal abstinence syndrome. JAMA 309, 1821–7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole FS, Wegner DJ, Davis JM The genomics of neonatal abstinence syndrome. Front. Pediatr 5, 176 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, et al. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc. Natl. Acad. Sci 109, 6325–30 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bland ST, Hutchinson MR, Maier SF, Watkins LR & Johnson KW The glial activation inhibitor AV411 reduces morphine-induced nucleus accumbens dopamine release. Brain Behav. Immun 23, 492–7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutchinson MR, et al. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J. Neurosci 32, 11187–200 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacagnina MJ, Rivera PD & Bilbo SD Glial and neuroimmune mechanisms as critical modulators of drug use and abuse. Neuropsychopharm 42,156–77 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kashima DT & Grueter BA Toll-like receptor 4 deficiency alters nucleus accumbens synaptic physiology and drug reward behavior. Proc. Natl. Acad. Sci 114, 8865–70 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H & Largent-Milnes TM & Vanderal TW Glial neuroimmune signaling in opioid reward. Brain. Res. Bulletin 155, 102–10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jantzie LL, et al. Prenatal opioid exposure: the next neuroinflammatory disease. Brain Behav. Immun 84, 45–58 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monnelly VJ, et al. Prenatal methadone exposure is associated with altered neonatal brain development. Neuroimage Clin 18, 9–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merhar SL, et al. White matter injury and structural anomalies in infants with prenatal opioid exposure. AJNR Am. J. Neuroradiol 40, 2161–2165 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saijo K & Glass CK Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol 11, 775–87 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Ginhoux F & Prinz M Origin of microglia: current concepts and past controversies. Cold Spring Harb Perspect. Biol 7, a020537 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin C, Londono I, Mallard C & Lodygensky GA New means to assess neonatal inflammatory injury. J. Neuroinflammation 12, 180 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leviton A, et al. The relationship between early concentrations of 25 blood proteins and cerebral white matter injury in preterm newborns: the ELGAN study. J. Pediatr 158, 897–903 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Zhan D, et al. Intrauterine inflammation induced white matter injury protection by fibrinogen-like protein 2 deficiency in perinatal mice. Pediatr. Res 89, 1706–14 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yen E, et al. Sex-dependent gene expression in infants with neonatal opioid withdrawal syndrome. J. Pediatr 214, 60–5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pace NP & Vassallo J Association between neutrophil-lymphocyte ratio and gestational diabetes—a systematic review and meta analysis. J. Endo. Soc 5, 1–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cappelletti M, Presicce P & Kallapur SG Immunobiology of acute chorioamnionitis. Front. Immunol 11, 649 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baloch RQ, Pinto JM, Greenberg P, Kuo Y-H & Siu A Evaluation and analysis of modified Finnegan scoring system for assessment of neonatal abstinence syndrome. J. Opioid. Manag 16,189–196 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Dietz JA, Johnson KL, Wick HC, Bianchi DW & Maron JL Optimal techniques for mRNA extraction from neonatal salivary supernatant. Neonatology 101, 55–60 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yen E, Kaneko-Tarui T & Maron JL Technical considerations and protocol optimization for neonatal salivary biomarker discovery and analysis. Front. Pediatr 8, 618553 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramesh G, MacLean AG & Philipp MT Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm 2013, 480739 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bachtell R, et al. Targeting the toll of drug abuse: the translational potential of toll-like receptor 4. CNS Neurol. Disord. Drug Targets 14, 692–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui C, Shurtleff D & Harris RA Neuroimmune mechanisms of alcohol and drug addiction. Int. Rev. Neurobiol 118, 1–12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khanna P, Johnson KL & Maron JL Optimal reference genes for RT-qPCR normalization in the newborn. Biotech. Histochem 92, 459–66 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Korom M, et al. Dear reviewers: responses to common reviewer critiques about infant neuroimaging studies. Dev. Cogn. Neurosci 53, 101055 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buyanova IS & Arsalidou M Cerebral white matter myelination and relations to age, gender, and cognition: a selective review. Front. Hum. Neurosci 15, 662031 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens CW, Aravind S, Das S & Davis RL Pharmacological characterization of LPS and opioid interactions at the toll-like receptor 4. British J. Pharmacol 168, 1421–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang P, et al. Toll-like receptor 4 (TLR4)/opioid receptor pathway crosstalk and impact on opioid analgesia, immune function, and gastrointestinal motility. Front. Immunol 11, 1456 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutchinson MR, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav. Immun 24, 83–95 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yirmiya R & Goshen I Immune modulation of learning, memory, neural plasticity, and neurogenesis. Brain Behav. Immun 25, 181–213 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Cant R, Dalgleish AG & Allen RL Naltrexone inhibits IL-6 and TNFα production in human immune cell subsets following stimulation with ligands for intracellular toll-like receptors. Front. Immunol 8, 809 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun Y, Chen G, Zhou K & Zhu Y A conditioned place preference protocol for measuring incubation of craving in rats. J. Vis. Exp 141, e58384 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Volkow ND, Fowler JS, Wang G-J & Swanson JM Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol. Psychiatry 9, 557–69 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Volkow ND, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible confounding factors. NeuroImage 42, 1537–43 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charles MK, et al. Male sex associated with increased risk of neonatal abstinence syndrome. Hosp. Pediatr 7, 328–34 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silver ER & Hur C Gender differences in prescription opioid use and misuse: implications for men’s health and the opioid epidemic. Prev. Med 131, 105946 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Jeanne M, et al. Sex differences among opioid-abusing patients with chronic pain in a clinical trial. J. Addict. Med 9, 46–52 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beagley SM, et al. Incidence and characteristics of non-fatal opioid overdose among youths aged 11 to 24 years by sex. JAMA Netw. Open 3, e2030201 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kennedy AP, Epstein DH, Phillips KA & Preston KL Sex differences in cocaine/heroin users: drug-use triggers and craving in daily life. Drug Alcohol Depend 132, 29–37 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graeve R, et al. Infants’ prenatal exposure to opioids and the association with birth outcomes: a systematic review and meta-analysis. Paediatr. Perinat. Epidemiol 36, 125–43 (2022). [DOI] [PubMed] [Google Scholar]
- 46.Azuine RE, et al. Prenatal risk factors and perinatal and postnatal outcomes associated with maternal opioid exposure in an urban, low-income, multiethnic US population. JAMA Netw Open 2, e196405 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leyenaar JK, et al. Infant mortality associated with prenatal opioid exposure. JAMA Pediatr 175, 706–14 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Back SA White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol 134, 331–49 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Back SA & Rosenberg PA Pathophysiology of glia in perinatal white matter injury. Glia 62, 1790–1815 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayman M, et al. Punctate white-matter lesions in the full-term newborn: underlying aetiology and outcome. Eur. J. Paed. Neurol 23, 280–7 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Alaee A, Zarghami M, Farnia S, Khademloo M & Khoddad T Comparison of brain white matter hyperintensities in methamphetamine and methadone dependent patients and healthy controls. Iran J. Radiol 11, e14275 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kidokoro H, Anderson PJ, Doyle LW, Neil JJ & Inder TE High signal intensity on T2-weighted MR imaging at term-equivalent age in preterm infants does not predict 2-year neurodevelopmental outcomes. AJNR Am. J. Neuroradiol 32, 2005–10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo T, et al. Quantitative assessment of white matter injury in preterm neonates: association with outcomes. Neurol 88, 614–22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galinsky R, et al. Magnetic resonance imaging correlates of white matter gliosis and injury in preterm fetal sheep exposed to progressive systemic inflammation. Int. J. Mol. Sci 21, 8891 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leviton A & Dammann O Coagulation, inflammation, and the risk of neonatal white matter damage. Pediatr. Res 55, 542–5 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Gilles FH & Leviton A Neonatal white matter damage and the fetal inflammatory response. Semin. Fetal Neonatal Med 25, 101111 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Ruwanpathirana R, et al. Prematurity reduces the severity and need for treatment of neonatal abstinence syndrome. Acta Paediatr 104, e188–94 (2015). [DOI] [PubMed] [Google Scholar]
- 58.Oakes LM Sample size, statistical power, and false conclusions in infant looking-time research. Infancy 22, 436–9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lausten-Thomsen U, Olsen M, Greisen G, & Schmiegelow K Inflammatory markers in umbilical cord blood from small-for-gestational-age newborns. Fetal Pediatr. Pathol 33, 114–8 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Yepes-Calderon F, et al. Tractography in the clinics: implementing a pipeline to characterize early brain development. NeuroImage Clin 14, 629–40 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Materials described in the manuscript, including all relevant raw data, are available for research and non-commercial purposes upon reasonable request, without breaching participant confidentiality.


