Cell death is executed along several pathways. Apart from necrotic cell death occurring upon tissue injury, several distinct types of apoptosis have been observed. Apoptosis, or programmed cell death, is critical for tissue homeostasis in multicellular organisms. It plays an important role in many physiological processes, especially in development and in the immune system (39, 80). Many diseases are associated with either too much or too little apoptosis, such as AIDS, cancer, and autoimmunity (39).
On the molecular level, the cell death program can be divided into three parts: initiation, execution, and termination of apoptosis. Apoptosis is initiated by a variety of stimuli, including growth factor withdrawal (“death by neglect”), UV or γ-irradiation, chemotherapeutic drugs, and death receptor signals. In most cases the execution phase is characterized by membrane inversion and exposure of phosphatidylserine, blebbing (zeiosis), fragmentation of the nucleus, chromatin condensation, and DNA degradation. In the termination phase, “apoptotic bodies” are engulfed by phagocytes (39).
The growing subfamily of death receptors is part of the tumor necrosis factor (TNF)/nerve growth factor receptor superfamily. This superfamily is characterized by a sequence of two to five cysteine-rich extracellular repeats. The death receptors contain an intracellular death domain (DD), which is essential for transduction of the apoptotic signal. Six members of this subfamily are known so far, TNF-R1 (also called CD120a), CD95 (APO-1/Fas), DR3 (APO-3, LARD, TRAMP, and WSL1), TRAIL-R1 (APO-2 and DR4), TRAIL-R2 (DR5, KILLER, and TRICK2), and DR6 (67). Among these, CD95 is the best-characterized death receptor (65).
Death receptors are activated by their natural ligands, which have coevolved as a death ligand family, called the TNF family. Except for lymphotoxin α, the death ligands are type II transmembrane proteins which can be converted into a soluble form by the activity of metalloproteases. Several groups reported activity of the soluble CD95 ligand (CD95L) (38), whereas others ascribe the capacity to induce apoptosis to the membrane-bound form (66, 74). Recently, it has been proposed that the activity of soluble CD95L is enhanced by interaction with the extracellular matrix (4). The roles of soluble and membrane-bound CD95L remain to be shown in vivo.
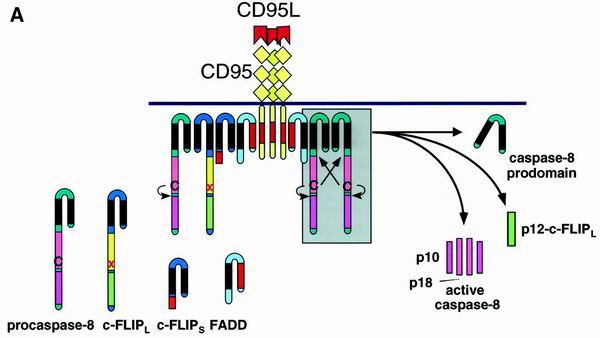
The salient point of death receptor signaling is the formation of a multimolecular complex of proteins triggered by receptor cross-linking either with agonistic antibodies (77) or with death ligands. The structure formed is called the death-inducing signaling complex (DISC) (see Fig. 2A) (36, 37, 71, 82). Among the death receptors, the CD95 DISC has been characterized most extensively. It consists of oligomerized, most probably trimerized, CD95, the serine-phosphorylated adapter Fas-associated death domain protein (FADD)/Mort 1, two isoforms of caspase 8 (caspase 8/a [FLICE, Mach-α1, and Mch5β] and caspase 8/b [Mach-α2]) (50), and CAP3 (a molecule that contains the N-terminal death effector domains [DED] of caspase 8 and an as yet uncharacterized C terminus) (36). According to recent findings, FADD and caspase 8 are also recruited to the DISC of TNF-related apoptosis-inducing ligand R1 (TRAIL-R1) and TRAIL-R2 (7, 37, 71) and are essential for death induction via TRAIL-R2 (71).
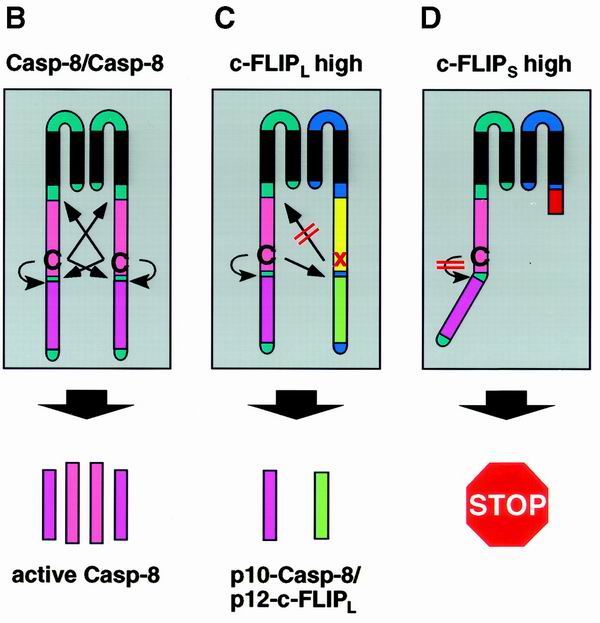
FIG. 2.
Model for c-FLIP-mediated inhibition of procaspase 8 processing at the DISC. (A) The CD95 DISC. For details, refer to the text. (B to D) Depending on the ratio of procaspase 8 and c-FLIP proteins at the DISC (A, grey box), different products are released from the DISC upon receptor triggering. (B) Small amounts of c-FLIP proteins allow processing of procaspase 8, leading to the formation of the active caspase 8 heterotetramer composed of the p18 and p10 subunits. (C) In the presence of large amounts of c-FLIPL procaspase 8 is recruited into the DISC, and cleavage is blocked after generation of the p43 cleavage products of both caspase 8 and c-FLIPL. (D) In the presence of large amounts of c-FLIPS procaspase 8 is recruited into the DISC but remains unprocessed. In each case, modulation of caspase 8 cleavage renders cells resistant to CD95-mediated cell death.
Caspases are a family of aspartate-specific cysteine proteases that are necessary for execution of apoptosis. Caspases are synthesized as proenzymes (zymogens) that are activated by proteolytic cleavage. The active enzyme is a heterotetrameric complex of two large subunits containing the active site and two small subunits. Activation of caspases has been reported to occur after a variety of apoptotic stimuli, including death receptor signals (13).
The stoichiometry of the DISC components is not clear, but the necessity of their interactions has been elucidated and lies in the nature of their corresponding subdomains. Oligomerization of CD95 creates a conformation of the receptor DD which, by homophilic interaction, binds the adapter FADD/Mort1 via its DD. In addition to a DD, FADD possesses an N-terminal DED with which it binds procaspase 8 and CAP3. Procaspase 8 is then cleaved at the DISC in three consecutive steps, which lead to the formation of active caspase 8, consisting of a heterotetramer of two p10 and two p18 subunits (see Fig. 2B). The prodomain of caspase 8 remains at the DISC, while active caspase 8 dissociates from the DISC to start the cascade of caspase activation which constitutes the execution phase of apoptosis (47).
Recently, it was reported that death receptors are assembled prior to triggering via so-called pre-ligand binding assembly domains (8, 69). In this case signaling would be induced either by conformational changes of preformed death receptor trimers or, alternatively, by formation of multimeric complexes upon ligand binding.
Several knockout and transgenic mice underscore the central role of the DISC-associated molecules FADD and caspase 8 in signaling via death receptors (79, 88). FADD and caspase 8 knockout mice die at embryonic day 11. They show cardiac failure and abdominal hemorrhage. To study embryonic lethality, FADD−/− chimeric mice were constructed. In thymocytes of these mice, CD95-mediated apoptosis was completely blocked. The same applied to FADD−/− fibroblasts.
Death receptor-mediated apoptosis can be modulated at both the receptor level, e.g., by glycosylation (33, 56), and further downstream by interfering with the apoptotic signaling cascade. For example, inhibitor-of-apoptosis proteins directly inhibit caspases (for a review, see reference 11), and antiapoptotic members of the Bcl-2 family inhibit apoptosis of so-called CD95 type II cells, in which apoptosis is dependent on a mitochondrial pathway (62).
In this review, we discuss the role and mechanism of apoptosis modulation by FLICE-inhibitory proteins (FLIPs).
V-FLIPS
Database mining led to the identification of an entire family of DED-containing proteins (6, 25, 76). Some of these proteins are components of viruses of the gammaherpesvirus class, such as herpesvirus saimiri, human herpesvirus 8, a Kaposi's sarcoma-associated herpesvirus, and moluscum contagiosum virus. These proteins were called viral FLICE-inhibitory proteins (v-FLIPs) (76). v-FLIPs consist of two DEDs (Fig. 1). They were shown to bind to the CD95 DISC and thus inhibit activation of caspase 8. v-FLIPs were capable of inhibiting apoptosis induced via several death receptors (CD95, TNF-R1, DR3, and DR4), suggesting that these receptors use similar signaling pathways (48). In human herpesvirus 8, the v-FLIP coding region is represented by a bicistronic mRNA following the coding region of v-cyclin (19). These coding regions are separated by an internal ribosome entry site (44). v-FLIP is expressed at low levels in latently infected cells (44, 60, 73), but its expression is increased in late Kaposi's sarcoma lesions or upon serum withdrawal from lymphoma cells (44, 73). Deletion of the v-FLIP gene from the genome of herpesvirus saimiri confirmed the antiapoptotic effect of v-FLIP but revealed that it is not essential for replication, transformation, or pathogenicity of herpesvirus saimiri (16).
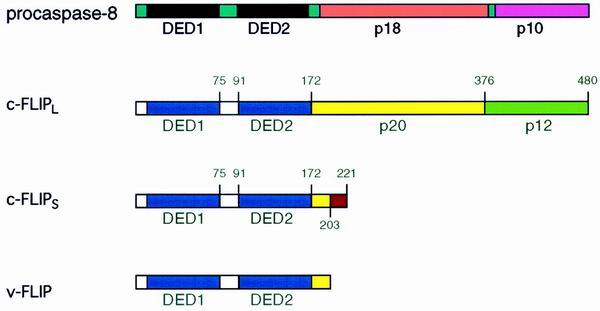
FIG. 1.
Structural similarities between caspase 8 and FLIP. For details, refer to the text.
Mice carrying a T-cell-specific v-FLIP-E8 transgene show strongly reduced thymocyte numbers, although thymocytes of these mice are resistant to CD95-mediated apoptosis (53). The reduction in thymocyte numbers seems to be independent of the CD95 system, since it was also observed in a CD95−/− background. Interestingly, the thymic phenotype resembles that of T cells from FADD dominant-negative transgenic mice, suggesting that another death receptor system distinct from the CD95 system is critically involved in thymocyte selection (52, 84).
C-FLIPS
A human cellular homolog of v-FLIPs was found and termed cellular FLICE-inhibitory protein (c-FLIP; also called FLAME-1, I-FLICE, Casper, CASH, MRIT, CLARP, and usurpin) (17, 20, 26, 29, 30, 57, 68, 72). The c-FLIP gene is located on chromosome 2q33-34 in a cluster of 200 kb together with caspase 8 and caspase 10, suggesting that these genes evolved by duplication (57). Multiple splice variants of c-FLIP have been reported, but so far only two, designated c-FLIPS and c-FLIPL, could be detected on the protein level (63).
c-FLIPL contains tandem DEDs and a caspase-like domain (Fig. 1). However, it lacks amino acid residues that are critical for caspase activity, most notably the cysteine of the catalytic center. c-FLIPS resembles its viral counterparts, only consisting of two DEDs and a short C-terminal part that differs from c-FLIPL (30).
Initially, both proapoptotic (17, 20, 29, 68) and antiapoptotic (17, 26, 30, 57, 72) effects were proposed. Enhanced cell death occurred mainly in experiments using transient overexpression and may have been due to excessive loads of DED-containing proteins that form death effector filaments (70). Data obtained from cells stably overexpressing c-FLIP and from mice deficient in c-FLIP clearly support an antiapoptotic function (30, 32, 41, 57, 63, 87)
MECHANISM OF ACTION
Conflicting data exist about the direct interaction partners of c-FLIP. Among them are FADD (17, 30, 68, 72), caspase-3 (20, 68), caspase 8 (20, 26, 30, 57, 68, 72), caspase 10 (17, 26, 72), TRAF1 (68), TRAF2 (68), and Bcl-xL (20). However, it was also reported that c-FLIP does not interact with FADD (26, 29, 63) or caspase 8 (63) prior to triggering of death receptors.
The mechanism of cell death attenuation by c-FLIP has not been completely elucidated. It was suggested that c-FLIP, as a potential competitive inhibitor, precludes recruitment of caspase 8 to the DISC and thereby prevents its activation (57). This idea was supported by the fact that upon overexpression of the viral homolog v-FLIP-E8, it was recruited to the DISC, thereby interfering with caspase 8 recruitment and activation (76).
Scaffidi et al. demonstrated that the cellular FLIP proteins c-FLIPS and c-FLIPL are also recruited to the DISC. However, this does not preclude caspase 8 from recruitment to the DISC (63). It could be shown that c-FLIPL is cleaved into a p43 subunit that remains at the DISC and a p12 subunit which is released. In the presence of c-FLIPL caspase 8 is still cleaved upon recruitment to the DISC (Fig. 2C) (63). However, its cleavage is incomplete, leading to the generation of the p43 and p41 subunits and concomitant release of the p10 subunit (41). Recently, we were able to show that large amounts of c-FLIPS completely prevent caspase 8 processing at the DISC (Fig. 2D) (41). Assuming that caspase 8 processing at the DISC occurs via trans- and autocatalytic cleavage of dimers according to the induced-proximity model (51), these results suggest that the initial step of caspase 8 cleavage proceeds autocatalytically, but requires a caspase domain as a counterpart in order to achieve an active conformation. This caspase domain can even be inactive, as in the case of c-FLIPL. In contrast, the second step of caspase 8 processing occurs transcatalytically and therefore requires functional caspase 8 as a counterpart of the dimer. These results explain the fact that in the presence of c-FLIPS procaspase 8 is not cleaved at all. However, it remains to be clarified in detail whether the differences in the mechanisms of apoptosis inhibition also reflect different functional roles of c-FLIPS and c-FLIPL and by which downstream mechanisms they are mediated.
REGULATION OF C-FLIP EXPRESSION
The signaling pathways by which c-FLIP expression is modulated are not well understood. It was reported that mitogen-activated protein (MAP) kinase kinase 1 (MKK1) was able to rescue concanavalin A-stimulated Jurkat cells from CD95-induced apoptosis, which correlated with an increase in c-FLIP mRNA expression (86). Moreover, inhibition of MAP kinase activity led to decreased c-FLIP expression on the transcriptional level. Upregulation of c-FLIP induced by transforming growth factor beta (TGF-β) in microglia was also reported to be blocked by inhibition of MAP kinases (64). However, modulation of c-FLIP levels was not observed upon CD3 triggering of Jurkat cells, although apoptosis could be augmented by inhibition of MAP kinases (23). It was suggested that MAP kinases rather modulate phosphorylation of the proapoptotic Bcl-2 family member Bad than influence the DISC. Recently, it was demonstrated that within a panel of tumor cell lines, only in a certain subset was c-FLIP expression dependent on MAP kinases (54). In contrast, c-FLIP expression seemed to be dependent on the activity of the phosphatidylinositol 3-kinase/Akt pathway in all cells tested. Thus, the contribution of kinase signaling pathways to modulation of c-FLIP remains elusive and might be cell type dependent. Two recent studies showed that c-FLIP is upregulated upon activation of NF-κB (40, 49).
Metabolic inhibitors acting on either transcription or translation were shown to rapidly abolish c-FLIP expression, indicating that c-FLIP mRNA is unstable (15). Modulation of mRNA stability has been shown to be a potent mechanism of regulating protein levels in other systems (24). It might well be that modulation of c-FLIP expression is achieved similarly.
PHYSIOLOGICAL ROLES FOR C-FLIP
A number of potential physiological stimuli responsible for c-FLIP-mediated rescue from death receptor-induced apoptosis have been suggested. In this respect the immune system has been at the center of investigation.
One of the initial studies described the downregulation of c-FLIPL in activated primary T cells, suggesting that downregulation of c-FLIP renders T cells susceptible to activation-induced cell death (AICD) (30). Moreover, it was reported that interleukin-2 (IL-2) enhances AICD in CD4+ T cells by upregulation of CD95L and concomitant downregulation of c-FLIP mRNA (59). Downregulation of c-FLIP upon IL-2 administration was later linked to the G1/S transition of activated T cells because T cells treated with cell cycle-blocking reagents do not downregulate c-FLIP (3). Retrovirus-mediated reconstitution of c-FLIP in activated murine T and B cells rescued these cells from CD95-mediated AICD (78).
In vivo, increased c-FLIP expression led to the production of autoantibodies and to the development of autoimmune diseases, suggesting that modulation of c-FLIP is necessary in order to maintain self-tolerance (78). In contrast to the studies described above, Scaffidi et al. did not detect any modulation of c-FLIP proteins upon stimulation of primary human T cells (63). Instead, the transition from resistance to sensitivity correlated with an increase in the capacity to form a DISC despite comparable amounts of CD95 surface expression. Thus, freshly activated T cells resemble CD95 type II cells, whereas long-term activated T cells resemble type I cells. In parallel, T cells switch from a Bcl-xL-high to a Bcl-xL-low state, allowing apoptotic activation of mitochondria. Since DISC formation itself is affected in this switch, it seems unlikely that c-FLIP plays a major role in rendering T cells sensitive to AICD. Until now, there has been no conclusive explanation for the differences observed. However, one might speculate that species differences between humans and mice have to be faced here.
So far, most of the studies concerning c-FLIP have focused on the long form, c-FLIPL, most likely because it is generally more abundant in cells. However, c-FLIPS was recently identified as a new effector of CD28-mediated costimulation (35) and as a mediator of resistance to AICD after T-cell receptor (TCR) restimulation (34). CD28 costimulatory signals led to protection from anti-CD3-triggered apoptosis by interfering at three different levels in the signaling cascade: (i) by prevention of upregulation of CD95L, (ii) at the mitochondria by induction of Bcl-xL, and (iii) at the DISC by upregulation of c-FLIPS. This last level of protection might prove to be the most important one because it prevents cells not only from suicide, but also from fratricide, and is far more upstream in the apoptotic signaling cascade than Bcl-xL. Restimulation of activated primary human T cells via the TCR also led to massive upregulation of c-FLIPS, which prevented DISC activity after triggering of the CD95 pathway and therefore protected from apoptosis. An upregulation of murine c-FLIP upon repeated antigen exposure was also observed in a TCR-transgenic model (28). Repeated stimulation by antigen leads to the generation of memory T cells (42, 45). Therefore, upregulation of c-FLIPS levels might be one of the molecular mechanisms that determines T-cell memory.
The homeostasis not only of T cells but also of B cells seems to involve modulation of c-FLIPL levels. Recently, it was reported that c-FLIPL is upregulated and recruited to the DISC in human tonsillar B cells upon ligation of CD40 or the B-cell receptor for antigen (BCR) (21, 85). Moreover, c-FLIPL seems to be part of the regulatory mechanism that determines survival of germinal center B cells after successful affinity maturation of the BCR (22). Upon receipt of the survival signal via CD40, c-FLIPL persists at the CD95 DISC and thus protects germinal center B cells from undergoing apoptosis.
Dendritic cells (DCs) and macrophages form another important part of the immune system, being responsible for antigen presentation to T and B cells, but also for a number of autoimmune diseases like rheumatoid arthritis and atherosclerosis. Macrophages differentiate from circulating blood monocytes and thereby switch from a CD95-sensitive to a CD95-resistant phenotype, which correlates with increased expression of c-FLIP (55). Immature DCs capture antigen in peripheral tissues and deliver it to lymphoid organs. During migration, the DCs mature, i.e., they reduce their capacity to capture antigen and increase expression of costimulatory molecules. After 2 days, mature DCs disappear from lymphoid organs, presumably by undergoing apoptosis. Recently, it was reported that immature but not mature DCs are susceptible to death receptor-mediated apoptosis, whereas mature but not immature DCs highly express c-FLIPL (43). Therefore, the disappearance of mature DCs from the lymphoid organs seems to be independent of the death receptor system, which might be more important to maintain homeostasis of immature DCs.
c-FLIP is prominently expressed in cardiac tissue. It has been reported that in infarcted cardiac tissue, c-FLIP expression is reduced (57). Cardiac myocytes that underwent apoptosis upon ischemia and reperfusion lacked c-FLIP expression, in contrast to surrounding healthy tissue. Interestingly, mice deficient in c-FLIP die at embryonic day 10.5 most probably due to cardiac failure (87). These results suggest that c-FLIP plays an essential role in heart development. The cardiac phenotype of c-FLIP−/− mice strongly resembled that of caspase 8−/− and FADD−/− mice (79, 88). These similarities suggest that for heart development, a functional interplay between the three DISC components FADD, caspase 8, and c-FLIP is absolutely required. However, the question arises whether this interplay requires a signal from a known or unknown death receptor or a different type of receptor. Moreover, it remains elusive whether the signal required for heart development is associated with regulation of apoptosis or opens up a novel role for the three molecules involved. Since the phenotypes of the three types of deficient mice are similar, although c-FLIP is antiapoptotic whereas FADD and caspase 8 are proapoptotic, it is very suggestive that apoptosis-independent signaling defects give rise to the hemorrhagic phenotype. However, assuming that apoptosis needs to be tightly regulated for tissue formation during embryonic development, dysregulation of apoptosis might still be the cause of hemorrhagic failure.
It has also been reported that anoikis (matrix detachment) (5) and apoptosis of endothelial cells induced by oxidized low-density lipoprotein (LDL) (61) correlated with modulations of c-FLIP expression.
Dysregulation of apoptosis signaling is often associated with disease formation. Alterations in sensitivity and resistance to death receptor-mediated apoptosis have been reported to be involved in autoimmune diseases (38) and cancer (14). As described above, c-FLIP is an important regulator of these apoptosis signaling pathways and might therefore account for the development of such diseases. In human melanoma cells, the expression level of c-FLIP correlated with resistance to TRAIL-induced apoptosis (18). However, this correlation was questioned after analysis of a broader panel of melanoma cell lines (89). Recently, it was reported that in Epstein-Barr virus (EBV)-transformed cells, resistance to CD95-mediated apoptosis correlated with an increased c-FLIP/caspase 8 ratio (75). Therefore, high levels of c-FLIP might contribute to EBV-induced tumorigenesis in Burkitt's lymphoma.
It has also been reported that both v-FLIP and c-FLIP mediate the immune escape of tumors (12, 46). Tumors with high expression levels of c-FLIP were shown to escape from T-cell-mediated immunity in vivo, although the perforin-granzyme pathway was not impaired. In addition, it was demonstrated that in vivo tumor cells were selected for elevated c-FLIP levels (46). v-FLIP promoted tumor establishment and progression in vivo by prevention of death receptor-mediated cytotoxicity (12).
As described above, there is a broad line of evidence for c-FLIP's being one of the central regulators of death receptor-mediated apoptosis. However, it should be kept in mind that both function and potential physiological roles of c-FLIP have been controversial, e.g., the role of c-FLIP in AICD of T cells and in melanoma cells. It is important to mention that at present it is not clear what amounts of c-FLIP are required to protect cells from apoptosis or whether it is the concentration of c-FLIP or the ratio between c-FLIP and caspase 8 or CD95 that determines sensitivity or resistance. Most reports on the involvement of c-FLIP in regulation of physiological processes are based on correlations that still wait for substantiation by careful mechanistic analysis or interference with c-FLIP expression. Therefore, mice with tissue-restricted deficiency of c-FLIP would give the deepest insight into its physiological role.
TRANSCRIPTIONAL ACTIVATION THROUGH C-FLIP
One of the death receptors, TNF-R1, has a dual role and transmits both death signals, similar to CD95, and survival signals via activation of NF-κB (83). CD95 is generally described as a pure death receptor. However, several studies suggest that proliferative signals also emanate from CD95 (1, 2, 58). Recently, it was reported that c-FLIP enhances the proliferative signaling pathway of CD95 after TCR triggering in Jurkat cells by increased recruitment of RIP, TRAF1, and TRAF2 to the CD95 DISC, which then leads to activation of NF-κB and ERK signaling pathways (31). Similar observations have been made in mice transgenic for human c-FLIPL (31).
The modulation of NF-κB signaling pathways was also demonstrated for other viral and human DED-containing proteins, such as several v-FLIP proteins, FADD, caspase 8, and caspase 10 (9, 10, 27). For c-FLIP, caspase 8, and caspase 10 the activating effect was assigned to the tandem DED. Furthermore, it was shown that caspase activity was dispensable for activation of NF-κB. In contrast to the studies described above, Wajant et al. showed that NF-κB activation upon death receptor triggering is dependent on a factor that is sensitive to metabolic inhibitors (81). They reported that overexpression of c-FLIP or a deficiency in FADD inhibited signaling to NF-κB. Finally, it is important to mention that NF-κB activation was not altered in c-FLIP−/− mice (87). Thus, the role of c-FLIP and its homologs and the role of death receptors as such in activation of proliferation signals require further investigation, especially with respect to in vivo conditions.
CONCLUSIONS
Signaling via the CD95 system has been under intense investigation in recent years, and considerable progress has been made in the elucidation of death induction (for a review, see reference 65). However, regulation of the death receptor systems is not well understood yet.
The c-FLIP proteins seem to be major players in modulation of the death signal. This statement is likely to hold despite a number of controversies. Several physiological and pathological conditions were proposed to be dependent on blocking of apoptosis by c-FLIP. However, it has to be kept in mind that regulatory processes are often complex, and thus, correlation of expression levels with biological effects is often not convincing. In addition, the question of the functional difference between the two splice variants of c-FLIP remains. So far, they have proved to be comparably efficient in inhibiting death receptor-mediated apoptosis (30) but seem to interfere at different levels (41). With respect to NF-κB activation, no major differences have been observed (31). Finally, the existence of more than the two splice variants on the protein level remains to be shown.
REFERENCES
- 1.Aggarwal S, Gupta A, Nagata S, Gupta S. Programmed cell death (apoptosis) in cord blood lymphocytes. J Clin Immunol. 1997;17:63–73. doi: 10.1023/a:1027340529644. [DOI] [PubMed] [Google Scholar]
- 2.Alderson M R, Tough T W, Davis-Smith T, Braddy S, Falk B, Schooley K A, Goodwin R G, Smith C A, Ramsdell F, Lynch D H. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Algeciras-Schimnich A, Griffith T S, Lynch D H, Paya C V. Cell cycle-dependent regulation of FLIP levels and susceptibility to Fas-mediated apoptosis. J Immunol. 1999;162:5205–5211. [PubMed] [Google Scholar]
- 4.Aoki K, Kurooka M, Chen J J, Petryniak J, Nabel E G, Nabel G J. Extracellular matrix interacts with soluble CD95L: retention and enhancement of cytotoxicity. Nat Immunol. 2001;2:333–337. doi: 10.1038/86336. [DOI] [PubMed] [Google Scholar]
- 5.Aoudjit F, Vuori K. Matrix attachment regulates Fas-induced apoptosis in endothelial cells: a role for c-flip and implications for anoikis. J Cell Biol. 2001;152:633–643. doi: 10.1083/jcb.152.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertin J, Armstrong R C, Ottilie S, Martin D A, Wang Y, Banks S, Wang G H, Senkevich T G, Alnemri E S, Moss B, Lenardo M J, Tomaselli K J, Cohen J I. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:1172–1176. doi: 10.1073/pnas.94.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodmer J L, Holler N, Reynard S, Vinciguerra P, Schneider P, Juo P, Blenis J, Tschopp J. TRAIL receptor-2 signals apoptosis through FADD and caspase 8. Nat Cell Biol. 2000;2:241–243. doi: 10.1038/35008667. [DOI] [PubMed] [Google Scholar]
- 8.Chan F K, Chun H J, Zheng L, Siegel R M, Bui K L, Lenardo M J. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 2000;288:2351–2354. doi: 10.1126/science.288.5475.2351. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhary P M, Eby M T, Jasmin A, Kumar A, Liu L, Hood L. Activation of the NF-κB pathway by caspase 8 and its homologs. Oncogene. 2000;19:4451–4460. doi: 10.1038/sj.onc.1203812. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhary P M, Jasmin A, Eby M T, Hood L. Modulation of the NF-κB pathway by virally encoded death effector domains-containing proteins. Oncogene. 1999;18:5738–5746. doi: 10.1038/sj.onc.1202976. [DOI] [PubMed] [Google Scholar]
- 11.Deveraux Q L, Reed J C. IAP family proteins—suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 12.Djerbi M, Screpanti V, Catrina A I, Bogen B, Biberfeld P, Grandien A. The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J Exp Med. 1999;190:1025–1032. doi: 10.1084/jem.190.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Earnshaw W C, Martins L M, Kaufmann S H. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 14.Friesen C, Fulda S, Debatin K M. Cytotoxic drugs and the CD95 pathway. Leukemia. 1999;13:1854–1858. doi: 10.1038/sj.leu.2401333. [DOI] [PubMed] [Google Scholar]
- 15.Fulda S, Meyer E, Debatin K M. Metabolic inhibitors sensitize for CD95 (APO-1/Fas)-induced apoptosis by downregulating Fas-associated death domain-like interleukin 1-converting enzyme inhibitory protein expression. Cancer Res. 2000;60:3947–3956. [PubMed] [Google Scholar]
- 16.Glykofrydes D, Niphuis H, Kuhn E M, Rosenwirth B, Heeney J L, Bruder J, Niedobitek G, Müller-Fleckenstein I, Fleckenstein B, Ensser A. Herpesvirus saimiri vFLIP provides an antiapoptotic function but is not essential for viral replication, transformation, or pathogenicity. J Virol. 2000;74:11919–11927. doi: 10.1128/jvi.74.24.11919-11927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goltsev Y V, Kovalenko A V, Arnold E, Varfolomeev E E, Brodianskii V M, Wallach D. CASH, a novel caspase homologue with death effector domains. J Biol Chem. 1997;272:19641–19644. doi: 10.1074/jbc.272.32.19641. [DOI] [PubMed] [Google Scholar]
- 18.Griffith T S, Lynch D H. TRAIL: a molecule with multiple receptors and control mechanisms. Curr Opin Immunol. 1998;10:559–563. doi: 10.1016/s0952-7915(98)80224-0. [DOI] [PubMed] [Google Scholar]
- 19.Grundhoff A, Ganem D. Mechanisms governing expression of the v-FLIP gene of Kaposi's sarcoma-associated herpesvirus. J Virol. 2001;75:1857–1863. doi: 10.1128/JVI.75.4.1857-1863.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han D K M, Chaudhary P M, Wright M E, Friedman C, Trask B J, Riedel R T, Baskin D G, Schwartz S M, Hood L. MRIT, a novel death-effector domain-containing protein, interacts with caspases and BclXL and initiates cell death. Proc Natl Acad Sci USA. 1997;94:11333–11338. doi: 10.1073/pnas.94.21.11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hennino A, Berard M, Casamayor-Palleja M, Krammer P H, Defrance T. Regulation of the Fas death pathway by FLICE-inhibitory protein in primary human B cells. J Immunol. 2000;165:3023–3030. doi: 10.4049/jimmunol.165.6.3023. [DOI] [PubMed] [Google Scholar]
- 22.Hennino A, Berard M, Krammer P H, Defrance T. FLICE-inhibitory protein is a key regulator of germinal center B cell apoptosis. J Exp Med. 2001;193:447–458. doi: 10.1084/jem.193.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmström T H, Schmitz I, Söderström T S, Poukkula M, Johnson V L, Chow S C, Krammer P H, Eriksson J E. MAPK/ERK signaling in activated T cells inhibits CD95/Fas-mediated apoptosis downstream of DISC assembly. EMBO J. 2000;19:5418–5428. doi: 10.1093/emboj/19.20.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holtmann H, Winzen R, Holland P, Eickemeier S, Hoffmann E, Wallach D, Malinin N L, Cooper J A, Resch K, Kracht M. Induction of interleukin-8 synthesis integrates effects on transcription and mRNA degradation from at least three different cytokine- or stress-activated signal transduction pathways. Mol Cell Biol. 1999;19:6742–6753. doi: 10.1128/mcb.19.10.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu S, Vincenz C, Buller M, Dixit V M. A novel family of viral death effector domain-containing molecules that inhibit both CD-95- and tumor necrosis factor receptor-1-induced apoptosis. J Biol Chem. 1997;272:9621–9624. doi: 10.1074/jbc.272.15.9621. [DOI] [PubMed] [Google Scholar]
- 26.Hu S, Vincenz C, Ni J, Gentz R, Dixit V M. I-FLICE, a novel inhibitor of tumor necrosis factor receptor-1- and CD-95-induced apoptosis. J Biol Chem. 1997;272:17255–17257. doi: 10.1074/jbc.272.28.17255. [DOI] [PubMed] [Google Scholar]
- 27.Hu W H, Johnson H, Shu H B. Activation of NF-kappaB by FADD, Casper, and caspase 8. J Biol Chem. 2000;275:10838–10844. doi: 10.1074/jbc.275.15.10838. [DOI] [PubMed] [Google Scholar]
- 28.Inaba M, Kurasawa K, Mamura M, Kumano K, Saito Y, Iwamoto I. Primed T cells are more resistant to Fas-mediated activation-induced cell death than naive T cells. J Immunol. 1999;163:1315–1320. [PubMed] [Google Scholar]
- 29.Inohara N, Koseki T, Hu Y, Chen S, Nunez G. CLARP, a death effector domain-containing protein interacts with caspase 8 and regulates apoptosis. Proc Natl Acad Sci USA. 1997;94:10717–10722. doi: 10.1073/pnas.94.20.10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer J L, Schröter M, Burns K, Mattmann C, Rimoldi D, French L E, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 31.Kataoka T, Budd R C, Holler N, Thome M, Martinon F, Irmler M, Burns K, Hahne M, Kennedy N, Kovacsovics M, Tschopp J. The caspase 8 inhibitor FLIP promotes activation of NF-κB and Erk signaling pathways. Curr Biol. 2000;10:640–648. doi: 10.1016/s0960-9822(00)00512-1. [DOI] [PubMed] [Google Scholar]
- 32.Kataoka T, Schröter M, Hahne M, Schneider P, Irmler M, Thome M, Froelich C J, Tschopp J. FLIP prevents apoptosis induced by death receptors but not by perforin/granzyme B, chemotherapeutic drugs, and gamma irradiation. J Immunol. 1998;161:3936–3942. [PubMed] [Google Scholar]
- 33.Keppler O T, Peter M E, Hinderlich S, Moldenhauer G, Stehling P, Schmitz I, Schwartz-Albiez R, Reutter W, Pawlita M. Differential sialylation of cell surface glycoconjugates in a human B lymphoma cell line regulates susceptibility for CD95 (APO-1/Fas)-mediated apoptosis and for infection by a lymphotropic virus. Glycobiology. 1999;9:557–569. doi: 10.1093/glycob/9.6.557. [DOI] [PubMed] [Google Scholar]
- 34.Kirchhoff S, Müller W W, Krueger A, Schmitz I, Krammer P H. TCR-mediated up-regulation of c-FLIPshort correlates with resistance toward CD95-mediated apoptosis by blocking death-inducing signaling complex activity. J Immunol. 2000;165:6293–6300. doi: 10.4049/jimmunol.165.11.6293. [DOI] [PubMed] [Google Scholar]
- 35.Kirchhoff S, Müller W W, Li-Weber M, Krammer P H. Up-regulation of c-FLIPshort and reduction of activation-induced cell death in CD28-costimulated human T cells. Eur J Immunol. 2000;30:2765–2774. doi: 10.1002/1521-4141(200010)30:10<2765::AID-IMMU2765>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 36.Kischkel F C, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer P H, Peter M E. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kischkel F C, Lawrence D A, Chuntharapai A, Schow P, Kim K J, Ashkenazi A. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase 8 to death receptors 4 and 5. Immunity. 2000;12:611–620. doi: 10.1016/s1074-7613(00)80212-5. [DOI] [PubMed] [Google Scholar]
- 38.Krammer P H. CD95(APO-1/Fas)-mediated apoptosis: live and let die. Adv Immunol. 1999;71:163–210. doi: 10.1016/s0065-2776(08)60402-2. [DOI] [PubMed] [Google Scholar]
- 39.Krammer P H. CD95's deadly mission in the immune system. Nature. 2000;407:789–795. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 40.Kreuz S, Siegmund D, Scheurich P, Wajant H. NF-κB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol. 2001;21:3964–3973. doi: 10.1128/MCB.21.12.3964-3973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krueger A, Schmitz I, Baumann S, Krammer P H, Kirchhoff S. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase 8 activation at the CD95 death-inducing signaling complex. J Biol Chem. 2001;276:20633–20640. doi: 10.1074/jbc.M101780200. [DOI] [PubMed] [Google Scholar]
- 42.Kundig T M, Bachmann M F, Oehen S, Hoffmann U W, Simard J J, Kalberer C P, Pircher H, Ohashi P S, Hengartner H, Zinkernagel R M. On the role of antigen in maintaining cytotoxic T-cell memory. Proc Natl Acad Sci USA. 1996;93:9716–9723. doi: 10.1073/pnas.93.18.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leverkus M, Walczak H, McLellan A, Fries H W, Terbeck G, Brocker E B, Kampgen E. Maturation of dendritic cells leads to up-regulation of cellular FLICE-inhibitory protein and concomitant down-regulation of death ligand-mediated apoptosis. Blood. 2000;96:2628–2631. [PubMed] [Google Scholar]
- 44.Low W, Harries M, Ye H, Du M Q, Boshoff C, Collins M. Internal ribosome entry site regulates translation of Kaposi's sarcoma-associated herpesvirus FLICE-inhibitory protein. J Virol. 2001;75:2938–2945. doi: 10.1128/JVI.75.6.2938-2945.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ludewig B, Oehen S, Barchiesi F, Schwendener R A, Hengartner H, Zinkernagel R M. Protective antiviral cytotoxic T cell memory is most efficiently maintained by restimulation via dendritic cells. J Immunol. 1999;163:1839–1844. [PubMed] [Google Scholar]
- 46.Medema J P, de Jong J, van Hall T, Melief C J, Offringa R. Immune escape of tumors in vivo by expression of cellular FLICE-inhibitory protein. J Exp Med. 1999;190:1033–1038. doi: 10.1084/jem.190.7.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medema J P, Scaffidi C, Kischkel F C, Shevchenko A, Mann M, Krammer P H, Peter M E. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meinl E, Fickenscher H, Thome M, Tschopp J, Fleckenstein B. Antiapoptotic strategies of lymphotropic viruses. Immunol Today. 1998;19:474–479. doi: 10.1016/s0167-5699(98)01309-7. [DOI] [PubMed] [Google Scholar]
- 49.Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-κB signals induce the expression of c-FLIP. Mol Cell Biol. 2001;21:5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muzio M, Chinnaiyan A M, Kischkel F C, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 51.Muzio M, Stockwell B R, Stennicke H R, Salvesen G S, Dixit V M. An induced proximity model for caspase 8 activation. J Biol Chem. 1998;273:2926–2930. doi: 10.1074/jbc.273.5.2926. [DOI] [PubMed] [Google Scholar]
- 52.Newton K, Harris A W, Strasser A. FADD/MORT1 regulates the pre-TCR checkpoint and can function as a tumour suppressor. EMBO J. 2000;19:931–941. doi: 10.1093/emboj/19.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.OhYama T, Tsukumo S, Yajima N, Sakamaki K, Yonehara S. Reduction of thymocyte numbers in transgenic mice expressing viral FLICE-inhibitory protein in a Fas-independent manner. Microbiol Immunol. 2000;44:289–297. doi: 10.1111/j.1348-0421.2000.tb02498.x. [DOI] [PubMed] [Google Scholar]
- 54.Panka D J, Mano T, Suhara T, Walsh K, Mier J W. Phosphatidylinositol 3-kinase/Akt activity regulates c-FLIP expression in tumor cells. J Biol Chem. 2001;276:6893–6896. doi: 10.1074/jbc.C000569200. [DOI] [PubMed] [Google Scholar]
- 55.Perlman H, Pagliari L J, Georganas C, Mano T, Walsh K, Pope R M. FLICE-inhibitory protein expression during macrophage differentiation confers resistance to Fas-mediated apoptosis. J Exp Med. 1999;190:1679–1688. doi: 10.1084/jem.190.11.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peter M, Hellbardt S, Schwartz-Albiez A, Westendorp M, Walczak H, Moldenhauer G, Grell M, Krammer P. Cell surface sialylation plays a role in modulating sensitivity to APO-1-mediated apoptotic cell death. Cell Death Differ. 1995;2:163–171. [PubMed] [Google Scholar]
- 57.Rasper D M, Vaillancourt J P, Hadano S, Houtzager V M, Seiden I, Keen S L, Tawa P, Xanthoudakis S, Nasir J, Martindale D, Koop B F, Peterson E P, Thornberry N A, Huang J, MacPherson D P, Black S C, Hornung F, Lenardo M J, Hayden M R, Roy S, Nicholson D W. Cell death attenuation by 'Usurpin', a mammalian DED-caspase homologue that precludes caspase 8 recruitment and activation by the CD-95 (Fas, APO-1) receptor complex. Cell Death Differ. 1998;5:271–288. doi: 10.1038/sj.cdd.4400370. [DOI] [PubMed] [Google Scholar]
- 58.Rathmell J C, Townsend S E, Xu J C, Flavell R A, Goodnow C C. Expansion or elimination of B cells in vivo: dual roles for CD40- and Fas (CD95)-ligands modulated by the B cell antigen receptor. Cell. 1996;87:319–329. doi: 10.1016/s0092-8674(00)81349-5. [DOI] [PubMed] [Google Scholar]
- 59.Refaeli Y, Van Parijs L, London C A, Tschopp J, Abbas A K. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 1998;8:615–623. doi: 10.1016/s1074-7613(00)80566-x. [DOI] [PubMed] [Google Scholar]
- 60.Sarid R, Flore O, Bohenzky R A, Chang Y, Moore P S. Transcription mapping of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1) J Virol. 1998;72:1005–1012. doi: 10.1128/jvi.72.2.1005-1012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sata M, Walsh K. Endothelial cell apoptosis induced by oxidized LDL is associated with the down-regulation of the cellular caspase inhibitor FLIP. J Biol Chem. 1998;273:33103–33106. doi: 10.1074/jbc.273.50.33103. [DOI] [PubMed] [Google Scholar]
- 62.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K M, Krammer P H, Peter M E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scaffidi C, Schmitz I, Krammer P H, Peter M E. The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem. 1999;274:1541–1548. doi: 10.1074/jbc.274.3.1541. [DOI] [PubMed] [Google Scholar]
- 64.Schlapbach R, Spanaus K S, Malipiero U, Lens S, Tasinato A, Tschopp J, Fontana A. TGF-beta induces the expression of the FLICE-inhibitory protein and inhibits Fas-mediated apoptosis of microglia. Eur J Immunol. 2000;30:3680–3688. doi: 10.1002/1521-4141(200012)30:12<3680::AID-IMMU3680>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 65.Schmitz I, Kirchhoff S, Krammer P H. Regulation of death receptor-mediated apoptosis pathways. Int J Biochem Cell Biol. 2000;32:1123–1136. doi: 10.1016/s1357-2725(00)00048-0. [DOI] [PubMed] [Google Scholar]
- 66.Schneider P, Holler N, Bodmer J L, Hahne M, Frei K, Fontana A, Tschopp J. Conversion of membrane-bound Fas (CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med. 1998;187:1205–1213. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter M E. Apoptosis signaling by death receptors. Eur J Biochem. 1998;254:439–459. doi: 10.1046/j.1432-1327.1998.2540439.x. [DOI] [PubMed] [Google Scholar]
- 68.Shu H B, Halpin D R, Goeddel D V. Casper is a FADD- and caspase-related inducer of apoptosis. Immunity. 1997;6:751–763. doi: 10.1016/s1074-7613(00)80450-1. [DOI] [PubMed] [Google Scholar]
- 69.Siegel R M, Frederiksen J K, Zacharias D A, Chan F K, Johnson M, Lynch D, Tsien R Y, Lenardo M J. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science. 2000;288:2354–2357. doi: 10.1126/science.288.5475.2354. [DOI] [PubMed] [Google Scholar]
- 70.Siegel R M, Martin D A, Zheng L, Ng S Y, Bertin J, Cohen J, Lenardo M J. Death-effector filaments: novel cytoplasmic structures that recruit caspases and trigger apoptosis. J Cell Biol. 1998;141:1243–1253. doi: 10.1083/jcb.141.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sprick M R, Weigand M A, Rieser E, Rauch C T, Juo P, Blenis J, Krammer P H, Walczak H. FADD/MORT1 and caspase 8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity. 2000;12:599–609. doi: 10.1016/s1074-7613(00)80211-3. [DOI] [PubMed] [Google Scholar]
- 72.Srinivasula S M, Ahmad M, Ottilie S, Bullrich F, Banks S, Wang Y, Fernandes-Alnemri T, Croce C M, Litwack G, Tomaselli K J, Armstrong R C, Alnemri E S. FLAME-1, a novel FADD-like antiapoptotic molecule that regulates Fas/TNFR1-induced apoptosis. J Biol Chem. 1997;272:18542–18545. doi: 10.1074/jbc.272.30.18542. [DOI] [PubMed] [Google Scholar]
- 73.Stürzl M, Hohenadl C, Zietz C, Castanos-Velez E, Wunderlich A, Ascherl G, Biberfeld P, Monini P, Browning P J, Ensoli B. Expression of K13/v-FLIP gene of human herpesvirus 8 and apoptosis in Kaposi's sarcoma spindle cells. J Natl Cancer Inst. 1999;91:1725–1733. doi: 10.1093/jnci/91.20.1725. [DOI] [PubMed] [Google Scholar]
- 74.Suda T, Hashimoto H, Tanaka M, Ochi T, Nagata S. Membrane Fas ligand kills human peripheral blood T lymphocytes, and soluble Fas ligand blocks the killing. J Exp Med. 1997;186:2045–2050. doi: 10.1084/jem.186.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tepper C G, Seldin M F. Modulation of caspase 8 and FLICE-inhibitory protein expression as a potential mechanism of Epstein-Barr virus tumorigenesis in Burkitt's lymphoma. Blood. 1999;94:1727–1737. [PubMed] [Google Scholar]
- 76.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J L, Schröter M, Scaffidi C, Krammer P H, Peter M E, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 77.Trauth B C, Klas C, Peters A M, Matzku S, Moller P, Falk W, Debatin K M, Krammer P H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989;245:301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- 78.Van Parijs L, Refaeli Y, Abbas A K, Baltimore D. Autoimmunity as a consequence of retrovirus-mediated expression of C-FLIP in lymphocytes. Immunity. 1999;11:763–770. doi: 10.1016/s1074-7613(00)80150-8. [DOI] [PubMed] [Google Scholar]
- 79.Varfolomeev E E, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann J S, Mett I L, Rebrikov D, Brodianski V M, Kemper O C, Kollet O, Lapidot T, Soffer D, Sobe T, Avraham K B, Goncharov T, Holtmann H, Lonai P, Wallach D. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 80.Vaux D L, Korsmeyer S J. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 81.Wajant H, Haas E, Schwenzer R, Muhlenbeck F, Kreuz S, Schubert G, Grell M, Smith C, Scheurich P. Inhibition of death receptor-mediated gene induction by a cycloheximide-sensitive factor occurs at the level of or upstream of Fas-associated death domain protein (FADD) J Biol Chem. 2000;275:24357–24366. doi: 10.1074/jbc.M000811200. [DOI] [PubMed] [Google Scholar]
- 82.Walczak H, Sprick M R. Biochemistry and function of the DISC. Trends Biochem Sci. 2001;26:452–453. doi: 10.1016/s0968-0004(01)01895-3. [DOI] [PubMed] [Google Scholar]
- 83.Wallach D, Varfolomeev E E, Malinin N L, Goltsev Y V, Kovalenko A V, Boldin M P. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 84.Walsh C M, Wen B G, Chinnaiyan A M, O'Rourke K, Dixit V M, Hedrick S M. A role for FADD in T cell activation and development. Immunity. 1998;8:439–449. doi: 10.1016/s1074-7613(00)80549-x. [DOI] [PubMed] [Google Scholar]
- 85.Wang J, Lobito A A, Shen F, Hornung F, Winoto A, Lenardo M J. Inhibition of Fas-mediated apoptosis by the B cell antigen receptor through c-FLIP. Eur J Immunol. 2000;30:155–163. doi: 10.1002/1521-4141(200001)30:1<155::AID-IMMU155>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 86.Yeh J H, Hsu S C, Han S H, Lai M Z. Mitogen-activated protein kinase kinase antagonized fas-associated death domain protein-mediated apoptosis by induced FLICE-inhibitory protein expression. J Exp Med. 1998;188:1795–1802. doi: 10.1084/jem.188.10.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yeh W C, Itie A, Elia A J, Ng M, Shu H B, Wakeham A, Mirtsos C, Suzuki N, Bonnard M, Goeddel D V, Mak T W. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12:633–642. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- 88.Yeh W C, Pompa J L, McCurrach M E, Shu H B, Elia A J, Shahinian A, Ng M, Wakeham A, Khoo W, Mitchell K, El-Deiry W S, Lowe S W, Goeddel D V, Mak T W. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 89.Zhang X D, Franco A, Myers K, Gray C, Nguyen T, Hersey P. Relation of TNF-related apoptosis-inducing ligand (TRAIL) receptor and FLICE-inhibitory protein expression to TRAIL-induced apoptosis of melanoma. Cancer Res. 1999;59:2747–2753. [PubMed] [Google Scholar]