Abstract
Metallothionein I (MT-I) and MT-II have been implicated in the protection of cells against reactive oxygen species (ROS), heavy metals, and a variety of pathological and environmental stressors. Here, we show a robust increase in MT-I/MT-II mRNA level and MT proteins in the livers and lungs of C57BL/6 mice exposed to the influenza A/PR8 virus that infects the upper respiratory tract and lungs. Interleukin-6 (IL-6) had a pronounced effect on the induction of these genes in the liver but not the lung. Treatment of the animals with RU-486, a glucocorticoid receptor antagonist, inhibited induction of MT-I/MT-II in both liver and lung, revealing a direct role of glucocorticoid that is increased upon infection in this induction process. In vivo genomic footprinting (IVGF) analysis demonstrated involvement of almost all metal response elements, major late transcription factor/antioxidant response element (MLTF/ARE), the STAT3 binding site on the MT-I upstream promoter, and the glucocorticoid responsive element (GRE1), located upstream of the MT-II gene, in the induction process in the liver and lung. In the lung, inducible footprinting was also identified at a unique gamma interferon (IFN-γ) response element (γ-IRE) and at Sp1 sites. The mobility shift analysis showed activation of STAT3 and the glucocorticoid receptor in the liver and lung nuclear extracts, which was consistent with the IVGF data. Analysis of the newly synthesized mRNA for cytokines in the infected lung by real-time PCR showed a robust increase in the levels of IL-10 and IFN-γ mRNA that can activate STAT3 and STAT1, respectively. A STAT1-containing complex that binds to the γ-IRE in vitro was activated in the infected lung. No major change in MLTF/ARE DNA binding activity in the liver and lung occurred after infection. These results have demonstrated that MT-I and MT-II can be induced robustly in the liver and lung following experimental influenza virus infection by overlapping but distinct molecular mechanisms.
Viral infection of the respiratory tract remains a leading cause of morbidity and mortality worldwide. Influenza virus infection causes approximately 20,000 deaths and 110,000 hospitalizations per year in the United States (13). Influenza virus A is a member of the orthomyxovirus family of enveloped, segmented, negative-strand RNA viruses. This virus replicates in the epithelial cells lining the upper respiratory tract of humans and in both the upper and lower respiratory tract of mice. The infection and initial replication cycle stimulate the production and release of antiviral and proinflammatory cytokines such as alpha, beta, and gamma interferon (IFN) and interleukin-6 (IL-6) (32, 38). The cytokines limit viral replication as well as stimulate the innate immune response, leading to recruitment of activated monocytes/macrophages.
These immune cells use a variety of mechanisms to limit viral replication until the host can generate a cell-mediated, antigen-specific response. One such mechanism involves macrophage phagocytosis, which generates reactive oxygen species. These oxygen species contribute to the immune-mediated pathology associated with the infection. Successful resolution of the infection requires viral clearance as well as restriction of immune-mediated damage. Experimental influenza virus infection also induces expression of a set of cellular genes that include acute-phase proteins in the liver.
Metallothionein I (MT-I) and MT-II are stress response proteins that are coordinately induced at a very high level in response to variety of pathological conditions, including inflammation, bacterial infection, restraint stress, anticancer drugs, heavy metals, and agents that generate reactive oxygen species (for reviews, see references 5 and 21). The unique metal-thiolate bonds of these cysteine-rich, heavy-metal-binding proteins can scavenge most potent hydroxyl and other free radicals very efficiently (60, 64). MT-I and MT-II are expressed in all eukaryotes and are conserved throughout evolution, whereas the isoforms MT-III and MT-IV are expressed only in mammals (58). Unlike MT-I and MT-II, which are ubiquitous (21, 53), MT-III and MT-IV are expressed primarily in the brain and stratified squamous epithelium (58), respectively.
MT-I and MT-II have been implicated in the scavenging of toxic metals, such as cadmium and mercury, as well as in maintaining homeostasis of biologically essential metals, e.g., zinc and copper (42, 43). Recent studies, however, suggest a significant role for MT-I and MT-II in the maintenance of redox balance (51), controlling the activity of zinc-containing enzymes (37, 52), modulating mitochondrial respiration (67), and scavenging free radicals (64). Studies have demonstrated a protective role of MT-I and MT-II against agents that generate free radicals, e.g., NO, UV radiation, and cadmium (45, 46). Recent investigations with transgenic mice overexpressing MT selectively in the heart have shown that MT can protect cardiac tissues from injuries caused by the potent anticancer drug doxorubicin (39, 40).
In general, cells refractory to heavy metals and reactive oxygen species appear to tolerate these insults by producing relatively high levels of MT. The genetic evidence that MT is a free radical scavenger was demonstrated in the yeast Saccharomyces cerevisiae in which Cu-Zn superoxide dismutase (SOD) mutant cells are very sensitive to free-radical generators, (e.g., H2O2 and paraquat), and mammalian or yeast MT could replace the function of SOD in these cells (63). Similarly, we have recently shown that the MT level is significantly elevated in the livers of Cu-Zn SOD-null mice (24).
Most of the agents with which MT-I and MT-II interact (e.g., heavy metals and ROS) are also potent inducers of these genes. The key transcription factor MTF-1 mediates activation of these genes in response to these agents (5, 59). Studies with MTF-1-null embryonic stem (ES) cells have shown that this transcription factor is essential for the basal as well as induced expression of MT-I in response to heavy metals and ROS (28, 35). In vivo genomic footprinting (IVGF) of the MT-I promoter showed occupancy of the metal response elements (MREs) after induction with zinc or cadmium, indicating the involvement of the same DNA-binding protein (25, 49, 50). The mechanism of activation of MTF-1 by heavy metals other than zinc and free radicals has yet to be explored. Apart from MTF-1, glucocorticoid receptor and STAT3 play important roles in the expression of MT genes in response to restraint stress (26, 34) and lipopolysaccharides (47), respectively.
In recent years, our laboratory has been involved in the identification and characterization of factors that upregulate (6, 7) and downregulate (23, 48) MT expression. The molecular mechanisms of activation of MT genes in response to different physiological and pathological stimuli have not yet been fully explored.
Oxidative stress has been implicated in the pathogenesis of certain diseases that include atherosclerosis, postischemic reoxygenation injury, chronic inflammation, and certain viral and parasitic infections (12, 14, 31). In particular, potentially fatal acute influenza virus infection causes edema, hemorrhage, and massive infiltration of inflammatory cells in the upper respiratory tract and the lungs. The molecular mechanisms of viral pathogenesis are not fully understood. Based on the activation of immune cells to produce reactive oxygen species and the known involvement of activated phagocytes in the initiation and promotion of tissue damage via oxidative and proteolytic processes, a role for reactive oxygen species has been proposed in the pathogenesis of influenza virus infection (57). The role of oxidative stress in influenza virus pathogenesis and dietary intervention with selenium have been demonstrated by Beck et al. (8). Because agents that produce reactive oxygen intermediates are known to induce metallothionein, one should anticipate a marked induction of MT during influenza virus infection, particularly in the lung. In the present study, we have demonstrated a robust induction of MT-I and MT-II in the liver and lung of mice infected with the influenza A virus and have further explored the molecular mechanisms of this process.
MATERIALS AND METHODS
Animals.
C57BL/6 male mice 4 to 8 weeks of age and free of virus antibodies were obtained from Charles River, Inc. IL-6 knockout mice generated on C57/BL6 background were purchased from Jackson Laboratories. The animals were housed five per cage at 22 ± 2°C on a 12-h light–12-h dark cycle (lights on at 06:00) and were allowed to acclimatize for at least 1 week before experimentation.
Infection of mice and treatment with RU-486.
Mice were infected intranasally with 16 hemagglutinating units (HAU) in 50 μl of influenza A/PR8 virus diluted in phosphate-buffered saline (PBS). Prior to infection, mice were anesthetized with an intramuscular injection (200 μl) of xylazine diluted in ketamine (Vedco, St. Joseph's, Mo.). Infection was verified by seroconversion (by enzyme-linked immunosorbent assay [ELISA]) as previously published (13, 53). For RU-486 treatment, animals were injected subcutaneously with the vehicle (polyethylene glycol) or the drug (25 mg/kg of body weight) every day, starting 1 day prior to infection. Mice were sacrificed at different days postinfection by cervical dislocation, and tissues were frozen in liquid N2 for RNA isolation or processed immediately for the isolation of nuclei.
Isolation of RNA and Northern blot analysis.
Total RNA was isolated using the guanidinium thiocyanate-acid phenol method (15) and subjected to Northern blot analysis following a published protocol (22).
Estimation of metallothionein proteins in tissue extract and serum.
A cadmium hemoglobin-binding assay was used to estimate MT proteins (19). Frozen tissues were pulverized in liquid N2 by using a tissue meizer and extracted with the homogenization buffer (50 mM Tris [pH 7.4], 1 mM EDTA, protease inhibitor cocktail, and 1 mM dithioerythritol) (4 ml of buffer/g of tissue). The homogenate was centrifuged at 40,000 × g for 30 min at 4°C, and the supernatant was snap-frozen in liquid N2 in small aliquots and stored at −80°C.
Isolation of nuclei and preparation of nuclear extract.
Nuclei were isolated from the liver and the lung by homogenizing in high-density sucrose buffer (2 M sucrose, 25 mM HEPES [pH 7.9], 25 mM KCl, 1 mM EDTA, 10% glycerol, 0.15 mM spermine, 0.5 mM spermidine, and protease and phosphatase inhibitor cocktails [Sigma]) and isolated by sucrose density gradient centrifugation following Gorski et al. (27). The nuclei were resuspended in 3 volumes of buffer A (25 mM HEPES [pH 7.9], 1.5 mM MgCl2, 100 mM KCl, 25% glycerol, 1 mM dithiothreitol [DTT] along with protease and phosphatase inhibitor cocktails) and lysed by adding 1.2 M KCl dropwise to a final concentration of 0.4 M (65). After 30 min on ice, the soluble proteins were collected by centrifuging at 100,000 × g for 30 min at 4°C. The extracts were snap-frozen in small aliquots in liquid N2 and stored at −80°C. These extracts were used for detection of DNA-binding proteins by electrophoretic mobility shift assay (EMSA).
EMSA.
Nuclear extracts were prepared from the livers of control and infected mice as described earlier (26) and incubated with α-32P-labeled specific oligonucleotides in the binding buffer containing 20 mM HEPES (pH 7.9), 75 to 90 mM KCl, 5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 0.2 μg of poly(dI-dC)/μg of protein, and 10 to 12% glycerol. For the supershift assay, the nuclear extract was incubated in binding buffer on ice with antibodies before addition of the labeled oligonucleotide. The DNA-protein complexes were separated by polyacrylamide (4 to 6% acrylamide) gel electrophoresis with 0.25× to 0.5× TBE (Tris-borate-EDTA) as running buffer (depending on the nature of the complex), and analyzed by autoradiography or in a Storm system (Molecular Dynamics). The sequences of the deoxyoligonucleotides used in EMSA were as follows: 1, mMT-I STAT 3, 5′-GATCGAGTTCTCGTAAACTC; 2, MRE-s, 5′-GATCCAGGGAGCTCTGCACACGGCCCGAAAAGTA; 3, MLTF/ARE, 5′-GATCCGCGGGGCGCGTGACTATGCGTGGGCTGGA; 4, mutMLTF/ARE, 5′-GATCCGCGGGGGCCGTGACTATGCGTGGGCTGGA; 5, mMT-I GRE-1, 5′-GATCAAGTGGGGAAACACCATGTACCTCCAG; 6, mMT-I-γ-IRE, 5′-CGGCTCTGCCAGGACGCGGGGCG; and 7, Sp1, 5′-GATCATTCGATCGGGGCGGGGCGAG.
IVGF.
We followed our published protocol for IVGF in tissue nuclei (25). The nuclei (5 × 106 to 10 × 106 in 500 μl of PBS) were mixed with 500 μl of PBS containing 0.4% dimethyl sulfate for 2 min at room temperature, and the nuclear suspension was immediately diluted to 50 ml with ice-cold PBS and sedimented by centrifugation at 1,500 × g for 2 min. The pellet was washed twice with the same buffer. Next, the nuclei were subjected to proteinase K digestion, and DNA was purified by phenol-chloroform extraction and ethanol precipitation. The resultant DNA was dissolved in Tris-EDTA (TE) and treated with piperidine (1 M) for 30 min at 95°C to cleave the methylated guanosines. The DNA fragments were collected by ethanol precipitation and analyzed on agarose gels to ensure fragment sizes of <500 nucleotides. Next, equal amounts of DNA (1 to 2 μg) from each sample were subjected to LM-PCR with gene-specific primers.
To detect LM-PCR products, a primer extension reaction was performed with 32P-labeled gene-specific primer. The reaction products were separated in a sequencing gel and analyzed by autoradiography. Several primers were used for LM-PCR of the mouse MT-I promoter. To detect footprinting within the first 200 bp of the promoter (that encompasses MREs, MLTF/ARE, and Sp1), we used the primers described earlier (50, 55). To detect footprinting in the upstream region (that spans STAT3 sites), we used the following primers: mMTI-STAT3-3′-1, 5′- AGCCCACGCATAGTCACG; mMTI-STAT3-3′-2, 5′-GACTGGCTCGGATAGGACGAC; mMTI-STAT3-3′-3, 5′-CTGGCTCGGATAGGACGACCC; mMTI-STAT3-5′-1, 5′-TCGATCCAGAGAGAGACCTG; mMTI-STAT3-5′-2, 5′-CTCAGGAACTCCAGGAAAGGAG; and mMTI-STAT3-5′-3, 5′-CAGGAACTCCAGGAAAGGAGAAGC. The annealing temperatures for 3′- and 5′-STAT3 primers were 58.5, 61.4, and 65.4°C and 56, 59.6, and 63.8°C, respectively.
To detect footprinting in the two glucocorticoid response elements (GREs), located 1 kb upstream of mouse MT-II promoter, which controls glucocorticoid-mediated expression of both the MT-I and MT-II genes (44), we designed the following sets of primers for both strands: mMT-I-GRE-3′-1, 5′-GGGTGTTTGCCAAGTAGG; mMT-I-GRE-3′-2, 5′-GGAAAGCCCCACGTAACC; mMT-I-GRE-3′-3, 5′-CCCCACGTAACCCGACCC; mMT-I-GRE-5′-1, 5′-CCAGTAATGCCTGGAATGAG; mMT-I-GRE-5′-2, 5′-CCTGGAATGAGTCAAGAAGGC; and mMT-I-GRE-5′-3, 5′-TGGAATGAGTCAAGAAGGCATTG. The annealing temperatures for 3′- and 5′-GRE primers were 54.5, 58.5, and 63.5°C and 57, 60, and 62.5°C, respectively.
The linker primers were the same as designed by Muller and Wold (88).
Reverse transcription.
Total RNA was isolated from the pooled samples as described above. RNA was reverse transcribed using a commercially available kit (Promega, Madison, Wis.) Briefly, 2 μg of RNA was combined with 5 mM MgCl2, 1 mM each of the for deoxynucleoside triphosphates, reverse transcription buffer, 1 U of RNasin, 15 U of avian myeloblastosis virus reverse transcriptase, and primed with 0.5 μg of random hexamers per μg. After a 10-min incubation at room temperature, samples were heated to 42°C for 1 h, placed in boiling water for 5 min, and then put in ice for another 5 mins. Diethyl pyrocarbonate-treated water was added to the samples to bring the final volume to 100 μl. The resulting cDNA was then stored at −70°C until use in the real-time PCR.
Quantitation of cytokine gene expression using real-time PCR.
Increased expressions of IFN-α,-β, and -γ and IL-6, -10, -12, and -15 due to infection was assessed with total RNA isolated from lung using the real-time PCR technique. The primer and probe sequences for each cytokine are given below. The PCR was run in sealed 96-well microtiter plates. The reaction consisted of cDNA, primers, and fluorescently labeled probes for the cytokine of interest, 18S RNA (internal positive control), and Taqman Universal Master Mix (PE Applied Biosystems, Foster City, Calif.) for a final volume of 25 μl. The final concentrations of primer and probe were 900 nM and 100 nM, respectively, for each of the cytokines and the 18S control.
Following a 2-min incubation at 50°C and a 10-min incubation at 95°C, the reaction ran for 40 cycles. Each cycle consisted of a 15-s denaturing phase at 90°C and a 1-min annealing-extension phase at 60°C. The resulting change in fluorescence was measured using a Applied Biosystems 7700 Prism Sequence Detector (PE Applied Biosystems). Data were analyzed using Sequence Detector version 1.6, which is included with the 7700 Detector.
The relative amount of transcript was determined using the comparative Ct method as described by the manufacturer (User Bulletin 2). The cytokine mRNA levels are expressed as the fold increase over noninfected controls.
The IFN-α primers were forward (5′-TGCAACCCTCCTAGACTCATTCT), reverse, (5′-CCAGCAGGGCGTCTTCCT), and probe (5′-CTGCATCAGACAGCCTTGCAGGTCATT). The IFN-β primers were forward (5′-TGAATGGAAAGATCAACCTCACCTA), reverse (5′-CTCTTCTGCATCTTCTCCGTCA), and probe (5′-AGGGCGGACTTCAAGATCCCTATGGA). The IFN-γ primers were forward (5′-AGCAACAGCAAGGCGAAAA), reverse (5′-CTGGACCTGTGGGTTGTTGA), and probe (5′-CCTCAAACTTGGCAATACTCATGAATGCATCC). The IL-6 primers were forward (5′-TATGAAGTTCCTCTCTGCAAGAGA), reverse (5′-TAGGGAAGGCCGTGGTT), and probe (5′-CCAGCATCAGTCCCAAGAAGGCAACT). The IL-10 primers were forward (5′-TTTGAATTCCCTGGGTGAGAA), reverse (5′-ACAGGGGAGAAATCGATGACA), and probe (5′-TGAAGACCCTCAGGATGCGGCTG). The IL-12 primers were forward (5′-AGCTAACCATCTCCTGGTTTGC), reverse (5′-CCACCTCTACAACATAAACGTCTTTC), and probe (5′-TGCTGGTGTCTCCACTCATGGCCA). The IL-15 primers were forward (5′-TCATATTGACACCACTTTATACACTGACA), reverse (5′-GCAATTCCAGGAGAAAGCAGTT), and probe (5′-CTTTCATCCCAGTTGCAAAGTTACTGCAATG).
RESULTS
Expression of MT-I and MT-II genes is upregulated in livers and lungs of mice infected with influenza A virus.
A mouse model for influenza A virus infection has been used to study the role of the hypothalamus-pituitary-adrenal axis in the pathophysiology of infection and the effect of restraint stress on immunomodulation during the course of infection (18, 33). In this model, mice are infected intranasally with the strain of virus (A/PR8) that selectively infects the upper respiratory tract and the lung. The virus infection caused induction of an array of cytokines, e.g., IL-1α, IL-1β, IL-6, IL-10, IL-12, IL-15, tumor necrosis factor alpha, IFN-α, IFN-β, and IFN-γ. Some of these cytokines, e.g., IL-6, transport to the brain and activate the hypothalamus-pituitary-adrenal axis to secrete glucocorticoids into the circulation. IL-6 also causes activation of genes for the acute-phase response proteins in the liver such as α2-macroglobulin, serum amyloid A, and C-reactive proteins. This lytic virus also induces the formation of reactive oxygen species in the infected cells that leads to cytopathic effect. The host cells, e.g., macrophages, also generate reactive oxygen-species as an inflammatory response against the virus.
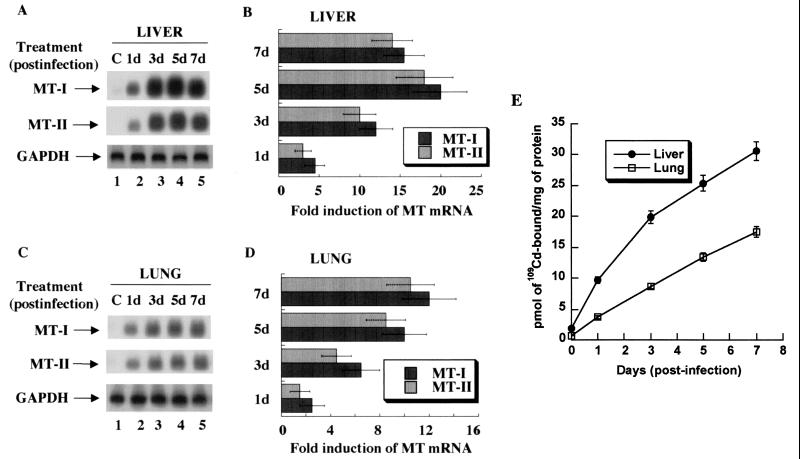
As MTs are potent scavengers of free radicals, we investigated whether MTs could be induced during the course of infection in two tissues, the lung (the site of infection) and the liver (activation of the acute-phase response). MT-I and MT-II mRNA levels in C57BL/6 mice were measured at different days postinfection by Northern blot analysis with 32P-labeled antisense oligonucleotides (Fig. 1A). The expression of both isoforms of MT in the liver was induced as early as 1 day postinfection, and MT-I mRNA reached its maximum level on day 5, after which it started to decline (lanes 1 to 5). The induced expression of the MT-I isoform was slightly higher than that of MT-II (Fig. 1B). The expression profiles of both isoforms in the lung were quite similar to those in the liver (Fig. 1C), but levels continued to increase even on day 7 (lane 5).
FIG. 1.
Time-dependent expression of MT-I and MT-II mRNA and proteins in the liver and lung of C57/BL6 mice infected with the influenza A virus. Total RNA (15 μg) was separated on a denaturing agarose (1.2%) gel, transferred to Hybond N+ membrane, and hybridized to 32P-labeled oligonucleotide antisense to mouse MT-I and MT-II mRNAs or rat glyceroldehyde-3-phosphate dehydrogenase (GAPDH) cDNA. (A) Northern blot analysis of MT-I and MT-II expression in the liver. (B) Induction level of MT-I and MT-II mRNAs. (C) Northern blot and (D) quantitative analyses of MT expression in the lung. The fold induction in the liver or lung is estimated by taking the basal value in the control tissue as 1. Results are the means of three independent experiments ± standard error of the mean (SEM). (E) 109Cadmium binding activity of MTs. One hundred micrograms of protein of the liver and lung extracts from the control and infected animals was heated in a boiling water bath, and the denatured proteins were removed by centrifugation. The supernatant was incubated with 109CdCl2 under optimum binding conditions, excess free cadmium was removed by adding hemoglobin and heat denaturation, and heat-stable Cd-MT was measured by gamma counter. The data are the mean values from the extracts obtained from five animals ± SEM. Each extract was assayed in triplicate. The plot was generated using the Kaleidagraph program.
Quantitation of the 32P signal using the Volume Analysis Program (Molecular Dynamics) also showed that maximal MT-I induction in the liver (15- to 20-fold) (Fig. 1B) was higher than that in the lung (8- to 12-fold) (Fig. 1D). We repeated this experiment three times with five animals in each group, and the results were highly reproducible. Reprobing the blots with antisense oligonucleotides specific for MT-III and MT-IV showed that neither of these isoforms was induced in response to infection in the two tissues (data not shown). These results demonstrate significant elevation of MT-I and MT-II mRNA levels at the site of infection (lung) and the target of the acute-phase response (liver) and similar kinetics of MT-I mRNA induction in the two tissues.
To determine whether the enhanced expression of MT-I and MT-II mRNAs in the lung and the liver resulted in increased synthesis of MT proteins, we measured total metallothionein content in these tissues by the cadmium-hemoglobin binding assay (Fig. 1E). MTs are unique heat-stable proteins that can bind cadmium (109Cd) with high avidity. Free 109Cd was removed by adding excess hemoglobin, heat denaturation, and precipitating out the denatured proteins (19). The results demonstrate that the accumulation of MT proteins started as early as 1 day postinfection in both the liver and lung and the level continued to increase up to day 7. The protein level in the liver was three- to fourfold higher than that in the lung, which is consistent with the MT mRNA level (Fig. 1). These results suggest that the levels of MT proteins during the course of virus infection were correlated with expression of their genes. The MT protein level in the liver increased further, although the MT mRNA levels had just started to decline (Fig. 1A, lane 5), which is probably due to the higher stability of the MT proteins.
MT induction by influenza A virus infection is significantly inhibited in liver but not in lung of IL-6 null mice.
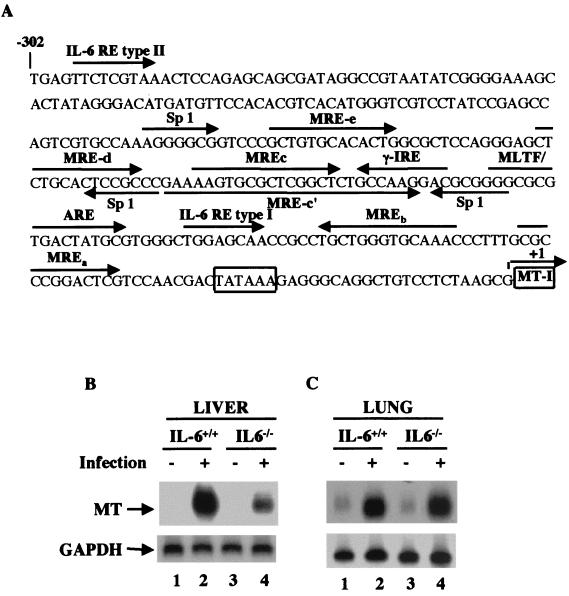
Many cytokine genes are upregulated during influenza virus infection. Among these genes, the proinflammatory cytokine IL-6 mediates induction of acute-phase proteins, including MTs, in the liver (30, 38). Analysis of the MT-I promoter (Fig. 2A) revealed the existence of potential type I and type IL-6 response elements (REs) (41, 47). To explore the role of IL-6 in influenza virus-induced MT-I and MT-II expression, we used mice with a targeted disruption of the IL-6 gene (IL-6−/−) along with wild-type C57BL/6 (IL-6+/+) mice. Despite normal development, IL-6 null mice are unable to activate the acute-phase response in the liver, and their innate immune function is compromised to some extent (2, 10, 56). Five null and five wild-type mice were infected with the virus and sacrificed on day 5 postinfection, and the level of MT-I and MT-II mRNA in the livers and lungs of individual animals was measured using 32P-labeled MT-I cDNA (this probe hybridizes to both isoforms of MT).
FIG. 2.
Induction of MT mRNA in response to influenza A virus infection is significantly inhibited in the livers but not in the lungs of IL-6 knockout mice. (A) Sequence of immediate upstream promoter of mouse MT-I denoting different regulatory elements. (B) IL-6 null mice (IL-6−/−) and C57BL/6 mice (IL-6+/+) were infected with influenza A virus. The animals were sacrificed after 5 days, and total RNA from the liver and the lung was hybridized to 32P-labeled mouse MT-I minigene (pMT-IΔi) (that detects both isoforms) and GAPDH cDNA.
Representative Northern blots from the pooled RNA from livers and lungs of five animals are shown in Fig. 2B and 2C, respectively. The higher induction level in this experiment was due to use of a probe that hybridizes to both MT-I and MT-II isoforms. In the wild-type (IL-6+/+) mice, the MT mRNA level was significantly elevated in both the liver and lung. Quantitative analysis of the data indicated a dramatic inhibition of MT expression (40 to 60% of the control) in response to influenza virus infection in the livers of IL-6−/− mice (Fig. 2B) but no significant inhibition in the lung (Fig. 2C). Reprobing the blot with the antisense MT-II oligonucleotide showed that expression of this isoform was also inhibited in the liver but not in the lung of IL-6 null mice (data not shown). These results indicate that the signaling mechanism activated by virus infection leading to MT induction in the liver is distinct from that in the lung. This is consistent with the report that the primary target site for IL-6 is the liver, where it mediates acute-phase responses by activating certain genes, including that for MT-I, at a very high level.
Glucocorticoid receptor antagonist RU-486 inhibits MT induction in liver and lung of both wild-type and IL-6 knockout mice in response to influenza A virus infection.
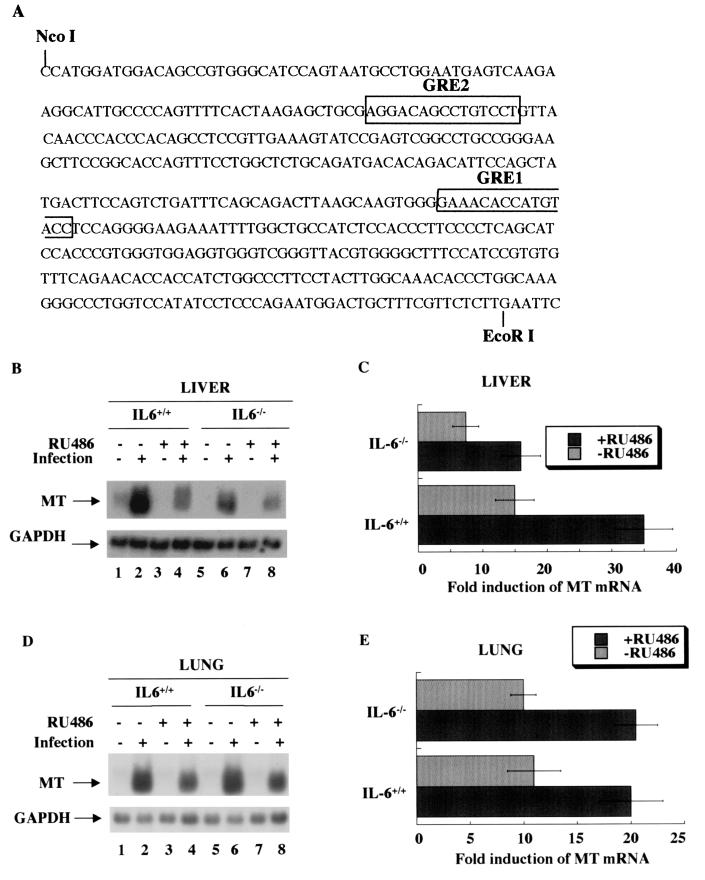
The unabated expression of MT-I in the lung of IL-6 null mice in response to influenza virus infection indicates the involvement of a different physiological mediator(s) in this tissue. Furthermore, MT-I expression in the livers of IL-6 null mice was not completely abolished, indicating interplay of multiple pathways for MT-I gene activation in the liver. One potential candidate is the glucocorticoid hormone, whose level is increased dramatically in a biphasic manner after virus infection (33). Glucocorticoids are known to upregulate MT-I gene expression, and GREs are located 7 kb upstream of the MT-I gene and 1 kb upstream of the MT-II gene (Fig. 3A). These two GREs can independently impart glucocorticoid responsiveness to both the MT-I and MT-II downstream promoters (44).
FIG. 3.
Glucocorticoid receptor antagonist RU-486 blocks MT expression in both the liver and lungs of wild-type and IL-6 null mice. (A) Nucleotide sequence upstream of the MT-II promoter that spans two GREs. RU-486 or vehicle was injected subcutaneously into C57BL/6 and IL-6 knockout mice every day starting 1 day before infection. The animals were sacrificed on day 5, and RNA from the liver and lung was analyzed for MT-I, MT-II, and GAPDH levels as described for Fig. 2. (B and D) Northern blot analysis of MT in wild-type and null IL-6 mice with and without RU-486 treatment. (C and E) Quantitative analysis of Northern blot data. The data presented are the means of three independent experiments ± SEM.
Recently we have shown that restraint stress induces MT-I and MT-II gene expression in the livers of mice by activating the glucocorticoid receptor (GR) (26). To explore the potential role of glucocorticoid in this process, we treated the animals with RU-486, a type II GR antagonist that binds to the ligand-binding domain of GR and promotes its binding to GRE (9) but blocks its transactivation function.
The animals (both wild type and IL-6 null) were injected with the inhibitor or the vehicle every day starting 1 day prior to infection. The animals were sacrificed on day 5 postinfection, and total RNA isolated from five animals was pooled and analyzed by Northern blotting (Fig. 3B to E). As expected, virus-induced MT-I expression was significantly inhibited in the livers of IL-6−/− mice (Fig. 3B, lanes 2 and 6) but not in the lung (Fig. 3D, lanes 2 and 6). Treatment of the animals with RU-486 further inhibited MT-I expression in the livers of both wild-type and IL-6−/− mice by 50 to 60% (Fig. 3B, lanes 4 and 8). The GR antagonist also inhibited MT-I induction in the lung of both wild-type and IL-6 null mice by 40 to 50% (Fig. 3D, lanes 4 and 8). Quantitative analyses of the three such Northern blots are presented in Fig. 3C and 3E, respectively. These results clearly indicate that in addition to IL-6, glucocorticoids play a critical role in MT induction in the liver, whereas in the lung they appear to be a key mediator of MT induction in response to an influenza virus infection.
IVGF studies demonstrate that potential IL-6 type II response element and some MREs on the immediate upstream promoter of MT-I as well as GRE1 located upstream of the MT-II promoter are occupied in liver in response to infection.
Experiments with IL-6 null mice and GR antagonists have indicated the involvement of multiple signaling mechanisms in influenza virus-induced MT-I expression (Fig. 2 and 3). IL-6 can activate target genes by two distinct mechanisms. Transcriptional activation of genes is mediated through the C/EBP family of proteins that bind to type I IL-6 RE located in the promoter regions of proteins that include haptoglobulin, C-reactive protein, and serum amyloid A. Type II IL-6 RE is the cis element through which STAT3 induces expression of a different group of acute-phase proteins, such as α2-macroglobulin and fibrinogens (4). Infection also causes activation of the hypothalamus-pituitary-adrenal axis, leading to an increase in serum glucocorticoid level. Glucocorticoid then binds to the cytoplasmic receptor in target cells, and the activated receptor then translocates to the nucleus and binds to the GRE of target genes, resulting in transcriptional induction. In target cells as well as in residential macrophages virus infection also generates reactive oxygen species that in turn activate redox-sensitive transcription factors, e.g., NF-κB and MTF-1. To identify the cis elements that are involved in upregulating MT-I gene expression by interacting with transcription factors in the liver and lung during virus infection, we performed IVGF.
For IVGF analysis, we focused on the region from bp +1 to −300 of the mouse MT-I promoter (which harbors most of the elements required for basal as well as inducible expression of the gene) and the region upstream of the MT-II gene that harbors two GREs. Sp1 and MLTF stimulate basal expression of the MT-I gene (21, 29). Among the six MREs, MRE-c and MRE-d are occupied for the basal expression of the gene (Fig. 2A), and all MREs are bound by the essential transcription factor MTF-1 in response to heavy metals and oxidative stress (11, 59). Analysis of the MT-I promoter reveals the existence of both type I and type II IL-6 REs (see Fig. 2A) (41, 47).
We designed a series of LM-PCR primers to analyze in vivo footprinting at these cis elements in liver and lung in response to virus infection. IVGF was performed using tissue nuclei from the control and infected animals as described in Materials and Methods. The naked genomic DNA from both the liver and lung was treated with dimethyl sulfate (DMS) and piperidine and amplified to provide the corresponding genomic G- ladder. The differential availability of guanine residues to the methylating reagent in the tissue nuclei from the control or infected mice, under similar conditions, will reflect the presence or absence of DNA-protein interaction at a specific site within the promoter. DNA-protein interaction can result in either protection or hypersensitivity of a G residue towards methylation and subsequent cleavage compared to the naked DNA.
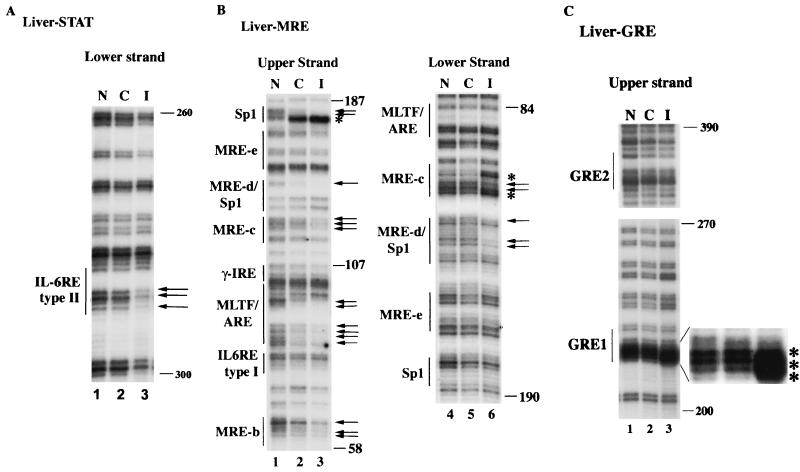
To detect footprinting at the potential IL-6-responsive element located at the MT-I promoter, we used different sets of primers (mMT1-STAT3) that allowed us to read regions upstream of MREs (Fig. 2A). Significant protection was detected at the potential IL-6 type II RE located at −297 bp in the lower strand of the MT-I promoter amplified from the infected liver DNA (Fig. 4A, lane 3). No footprinting was observed in the upper strand spanning this site in the liver nuclei from the infected mice (data not shown). This is most likely because the transcription factor contacts the cis element in a way that leads to hypersensitivity or protection of G residues only in one strand. No constitutive footprinting was detected at the corresponding sites in either strand of the MT-I promoter in the control liver nuclei (lane 2). These results indicate that the inducible binding of transcription factors, probably STATs, occurred at the IL-6 type II RE located at −297 bp in the liver in response to virus infection. No constitutive or inducible footprinting was observed at the putative type I IL-6 RE (Fig. 4B).
FIG. 4.
IVGF studies reveal protection of IL-6 type II RE, MREs, and GRE1 of the MT-I promoter in the livers of infected C57BL/6 mice. The nuclei isolated from the livers of control and infected mice suspended in PBS, as well as naked DNA, were treated with DMS. DNA was then purified, and methylated guanines were cleaved with piperidine. An identical amount of DNA (2 μg) from each sample was then subjected to LM-PCR with specific sets of primers (described in Materials and Methods). The G ladder generated was separated on a sequencing gel and analyzed by autoradiography. (A) Footprinting of the upper and lower strands of MT-I promoter spanning IL-6 RE with 5′ STAT3 primers. (B) Footprinting of the promoter region spanning MREs with 3′ and 5′ mMT-I primers. (C) Footprinting of the promoter region upstream of MT-II spanning GREs with 3′ GRE primers. Lanes N, C, and I denote LM-PCR with naked DNA and DNA isolated from the liver nuclei of uninfected and infected animals, respectively. Numbers on the right denote positions of G residues upstream of the +1 site.
IVGF analysis in the liver revealed footprinting at the Sp1 binding site and MLTF/ARE in the upper strand of both control and infected liver nuclei (Fig. 4B). The footprinting was more pronounced at MRE-b, MRE-c, MRE-d, and the composite MLTF/ARE in the infected liver compared to the control (lane 3). In the lower strand, significant occupancy was detected at MRE-d and MRE-c only in the infected liver nuclei. No constitutive footprint was detected in the lower strand between −84 and −190. These results demonstrate that virus infection resulted in inducible binding of MTF-1 to different MREs. We have observed constitutive occupancy of Sp1 and MLTF/ARE under noninduced condition in both cells in culture as well as in control rat liver (50).
Next, we analyzed footprinting at two GREs as RU-486 blocked virus-mediated MT-I induction (Fig. 3). Each of these GREs can activate a reporter gene in response to glucocorticoids (44). The IVGF data demonstrated the hypersensitivity of three consecutive guanines spanning and flanking the GRE1 sequence in the upper strand in the infected liver (Fig. 4C, lane 3), indicating the involvement of this site in infection-induced MT-I gene expression in the liver. No footprinting was, however, detected at GRE2 in either strand (lane 3) in control or infected liver. These results indicate that although there are two potential GREs upstream of the MT-I and -II promoters, there is preferential in vivo occupancy of GRE1 in the liver in response to virus infection.
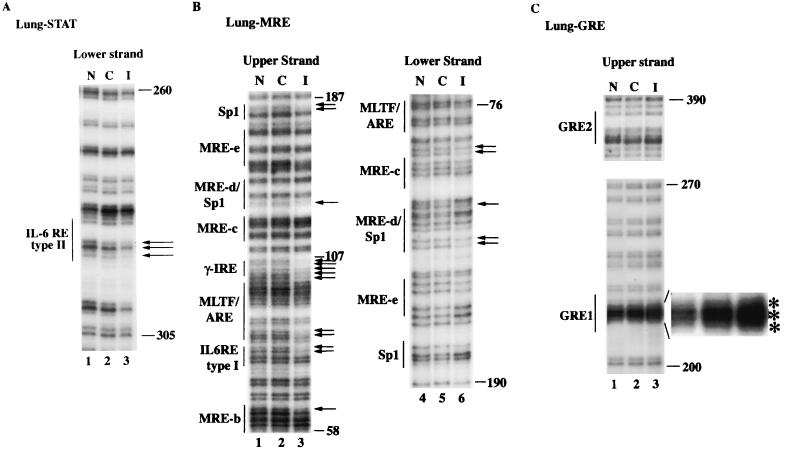
IVGF analysis of MT-I promoter in the lung reveals induced occupancy at a potential IL-6 type II RE, Sp1 sites, MREs, GRE1 and a unique footprinting at γ-IRE in response to infection.
We also analyzed the IVGF profile of the MT-I promoter in the nuclei from the control and infected mouse lung. Northern blot analysis has shown that MT-I was induced in the lung in response to viral infection in both wild-type and IL-6 null mice, which was blocked by RU-486 treatment of the animals (Fig. 2 and 3). We explored the possibility of differential footprinting on the MT-I promoter, particularly at the IL-6 type II RE located at −297 bp in the infected lung. To our surprise, we observed footprinting at this site (Fig. 5A, lane 3) on the MT-I promoter, the pattern of which is identical to that in the liver. These results indicate that in the lung of infected mice, STAT3 is activated via an IL-6-independent pathway, as MT-I was activated in the lung of IL-6 null mice (see Fig. 2 and 3). Indeed, STAT3 can be activated by cytokines other than IL-6, e.g., IL-10, IFN-α, IFN-γ, and a variety of growth factors (1).
FIG. 5.
IVGF studies revealed protection of IL-6 type II RE, MREs, GRE1, and a unique γ-IRE site of the MT-I promoter in the lung of infected C57BL/6 mice. This experiment was done following the same protocol as described for the liver in Fig. 4 with the exception that nuclei and naked DNA from the lung were used. (A) Footprinting with 5′ STAT3 primers. (B) Footprinting with 3′ and 5′ mMT-I primers. (C) Footprinting with 3′ GRE primers.
A distinct footprint was also observed in the infected lung between the MRE-c and MLTF/ARE sites in the upper strand (Fig. 5B, lane 3). Analysis of this intervening sequence revealed the presence of a tentative IFN-γ response element (γ-IRE) located between MRE-c and MLTF/ARE. This site was identified as the IFN-γ-responsive element in the major histocompatibility complex class II gene DPA (66). This unique footprinting is not observed in the liver from the infected animals (Fig. 4B, lane 3), which might be due to activation of an IFN-γ signaling pathway exclusively at the site of infection. Alternatively, the mononuclear cells migrating to the lungs in response to infection, such as natural killer (NK) cells, could be responsible for IFN-γ production.
Constitutive footprinting was not observed at the MREs and Sp1 sites in the lung (Fig. 5B). However, in contrast to the liver, we observed induced binding of Sp1 and occupancy of the ARE site in the upper strand of the MT-I promoter in the infected lung (lane 3). The infection-induced occupancy of MRE-b and MRE-d was observed in the upper strand of the MT-I promoter in the lung (Fig. 5B, lane 3). In the lower strand, we detected virus infection-induced footprinting at MRE-d and MRE-c in the nuclei from infected animals (lane 6). Analysis of the footprinting at the two GREs in the lung revealed inducible footprinting at GRE1, which showed hypersensitivity to DMS treatment, as observed in the infected liver (Fig. 5C). These results indicate that in the lung, virus infection causes inducible occupancy at STAT3, MREs, GRE1, ARE, and γ-IRE sites, implicating activation of multiple transcription factors.
DNA-binding activities of STAT3 and GR are significantly elevated in liver and lung nuclear extracts from infected mice.
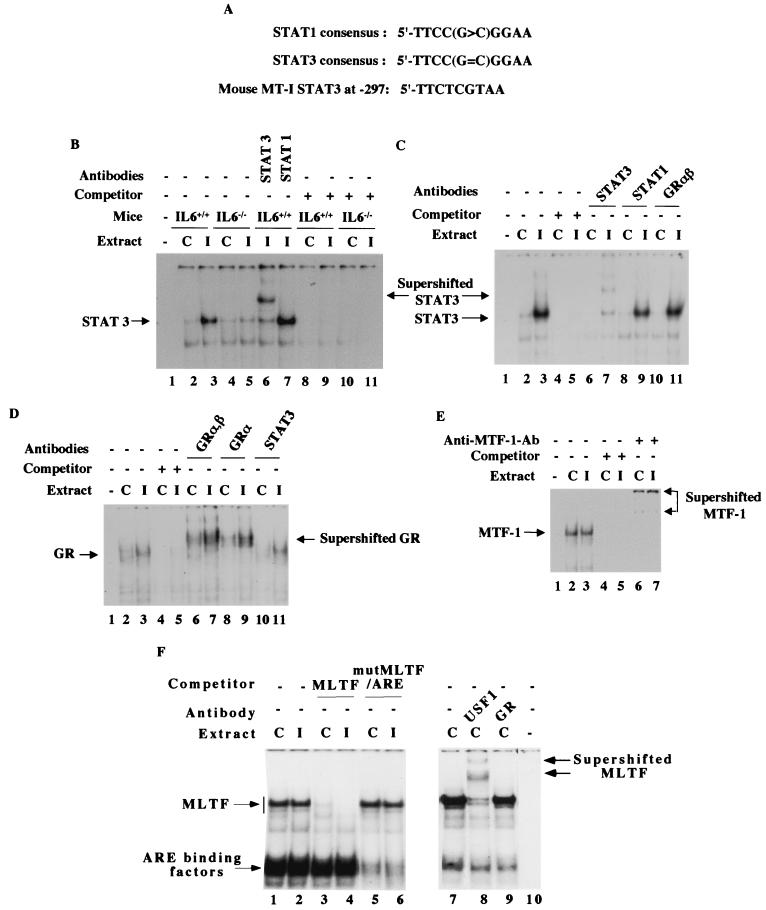
To identify the transcription factors that bind to the cis elements of the MT-I promoter in response to virus infection, we performed EMSA with nuclear extracts prepared from both control and infected tissues. We compared the DNA-binding activities of several transcription factors that included STAT3/STAT1, GR, MTF-1, and MLTF/ARE binding proteins. To identify the transcription factor that binds to IL-6 RE type II, the liver nuclear extract was incubated with an α-32P-labeled oligonucleotide spanning from bp −300 to −277 of the MT-I promoter, where protection was detected in IVGF analysis (Fig. 5A). This oligonucleotide differs from the consensus STAT site at two bases (Fig. 6A). A specific complex was detected in the nuclear extract from the wild-type mice that increased three- to fivefold in the extract from infected animals (Fig. 6B, compare lanes 2 and 3). The upper complex appeared to be the specific one, as its formation was inhibited by a molar excess of unlabeled oligonucleotide (lanes 8 to 11). That STAT3 but not STAT1 was a component of this complex was revealed by supershift of this complex with only anti-STAT3 antibodies (lanes 6 and 7). Minimal activation of the STAT3 complex was observed in the nuclear extract from IL-6 null mice (lanes 4 and 5), which is consistent with the inability of these null mice to express MT-I (Fig. 2).
FIG. 6.
Analysis of DNA-binding activities of different transcription factors that bind to the MT-I promoter in the liver nuclear extract from virus-infected and control mice. (A) Homology of the IL-6 type II RE of mouse MT-I promoter to STAT3 and STAT1 consensus sites. (B) STAT3 but not STAT1 binds to IL-6 type II RE of the mouse MT-I promoter, which is significantly increased in the liver nuclear extract of influenza A virus-infected wild-type mice but not that of IL-6 knockout mice. Nuclear extracts (10 μg of protein) from each group of mice were incubated on ice for 30 min with 32P-labeled STAT3 oligonucleotide (0.1 to 0.2 ng) in the binding buffer [12 mM HEPES (pH 7.9), 75 mM KCl, 5 mM MgCl2, 0.5 mM DTT, and 2 μg of poly(dI-dC)]. The DNA-protein complexes were separated at 150 V for 4 h at room temperature on a polyacrylamide (6% acrylamide) gel with 0.5× TBE as the running buffer. The dried gel was subjected to autoradiography as well as the PhosphorImager. For supershift assay, the nuclear extract was incubated on ice with 0.4 ng of anti-STAT1 (sc-346) or anti-STAT3 (sc-483) antibodies (Santa Cruz Biotechnology) before addition of the labeled oligonucleotide. For competitive EMSA, the reaction mixture was incubated with a 100-fold molar excess of the unlabeled oligonucleotide before addition of the labeled probe. Lane 1 represents the reaction mixture without the extract. (C) Glucocorticoid receptor does not physically interact with STAT3–IL-6 type II RE complex formed on MT-I promoter. The liver nuclear extracts from the control (lanes C) and infected (lanes I) IL-6+/+ mice were incubated with either anti-STAT3, anti-STAT1, or anti-GRαβ (sc-1004) antibodies. For competitive EMSA, the reaction mixture was incubated with a 100-fold molar excess of the unlabeled oligonucleotide before addition of the labeled probe. (D) The DNA-binding activity of the glucocorticoid receptor (GR) is increased in the liver nuclear extract from infected mice. Nuclear extracts (20 μg of protein) were incubated with 32P-labeled GRE1 oligonucleotide (0.2 to 0.4 ng) in the binding buffer containing 12 mM HEPES (pH 7.9), 60 mM KCl, 5 mM MgCl2, 0.5 mM DTT, and 4 μg of poly(dI-dC) on ice for 30 min at 4°C. DNA-protein complexes were separated on a polyacrylamide gel (4% acrylamide) with 0.5× TBE as the running buffer. For supershift assay, the nuclear extract was incubated for 1 h with 0.2 μg of anti-GRαβ, GRα (sc-1002), or STAT3 IgG (Santa Cruz Biotechnology) before addition of the labeled oligonucleotide. Lane 1 indicates the labeled oligonucleotide incubated without any extract. For the competition experiment, the reaction mixture was incubated with a 100-fold molar excess of the unlabeled oligonucleotide before addition of the corresponding labeled oligonucleotide. (E) Constitutive DNA-binding activity of MTF-1 is high in the liver nuclear extract of wild-type and IL-6 null mice and is not increased in the extract from infected animals. Nuclear extracts (10 μg of protein) were incubated at 4°C for 20 min with 32P-labeled MRE-s oligonucleotide (0.2 to 0.4 ng), to which MTF-1 binds specifically, in the same binding buffer as that of STAT3. The DNA-protein complexes were separated on a polyacrylamide gel (4% acrylamide) with 0.25× TBE as the running buffer. For supershift assay, the reaction mixture after the binding reaction was incubated for 1 h with 1 μl of anti-MTF-1 antiserum. For competitive EMSA, the reaction mixture was incubated with a 100-fold molar excess of the unlabeled oligonucleotide before addition of the labeled probe. Lane 1 denotes the labeled oligonucleotide incubated in the absence of extract. (F) The DNA-binding activities of factors that bind to MLTF/ARE composite site are not altered in the liver nuclear extract from infected animals. Nuclear extracts (10 μg of protein) were incubated with 32P-labeled MLTF/ARE oligonucleotide following the same conditions as described for STAT3. The DNA-protein complexes were resolved by electrophoresis on a polyacrylamide gel (6% acrylamide) with 0.5× TBE as the running buffer. For supershift assay, the extract was incubated with anti-USF1 (sc-229) antibodies (Santa Cruz Biotechnology) in the binding buffer before the reaction. The DNA-protein complexes were analyzed by autoradiography and quantitated by Storm system (Amersham).
Glucocorticoids and IL-6 can synergistically activate certain promoters through either IL-6 RE or GRE (62, 68). To determine whether GR is a component of the complex that binds to the STAT3 site of the MT-I promoter, the reaction mixture was incubated with antibodies that could supershift both the α and β isoforms of GR (Fig. 6C). The specific complex was supershifted with anti-STAT3 antibodies (lane 7) but not with anti-GR (lane 11) or anti-STAT1 (lane 9) antibodies, indicating that neither GR nor STAT1 is a component of the complex. These results demonstrate that STAT3 can indeed bind to the potential IL-6 type II RE on the mouse MT-I promoter and that its activity was significantly increased in the liver of infected wild-type mice, leading to inducible binding and gene activation.
Next, we measured the DNA-binding activity of GR, as IVGF analysis demonstrated occupancy at GRE1 located far upstream of the MT-I promoter. A specific complex was detected in the liver nuclear extract, the activity of which increased two- to threefold in the extract from infected mice (Fig. 6D, lanes 2 and 3). The specificity of the complex was evident by competition with unlabeled oligonucleotide (lanes 4 and 5) and supershift with anti-GR antibodies (lanes 6 to 9) but not with STAT3 antibodies (lanes 10 and 11). GRα and probably GRβ are part of this complex, as antibodies against both isoforms could specifically supershift the complex. Activation of GR in the extract from infected animals was more evident from the quantitation of the signal from the supershifted complex. These results clearly show that virus infection causes activation of the glucocorticoid receptors that then occupy GRE1 of the MT promoter, leading to transcriptional activation of the gene in the liver nuclei.
We also measured the DNA-binding activity of MTF-1. This key transcription factor is required for basal as well as induced expression of the MT-I gene in response to heavy metals and reactive oxygen species (28, 35). Among these agents, only zinc can directly activate MTF-1. The mechanism of its activation by inducers other than zinc is still an enigma. To determine whether influenza virus infection has any effect on MTF-1 activity, we performed EMSA with a 32P-labeled MRE-s oligonucleotide, to which MTF-1 specifically binds (59). Liver nuclear extracts from the control mice showed high MTF-1 activity (Fig. 6E, lane 2) that did not increase significantly after infection (lane 3). The complexes formed with the extracts of the control and infected mice were competed with unlabeled MRE-s oligonucleotide (lanes 4 and 5) and supershifted with antibodies against MTF-1 (lanes 6 and 7). These results indicate that, unlike STAT3 and GR, the constitutive DNA-binding activity of MTF-1 was significantly higher in the liver nuclear extract because of its role in basal MT expression and was not further elevated during infection.
The complexes formed at the composite major late transcription factor/antioxidant response element (MLTF/ARE) site were measured in the liver nuclear extract from control and infected mice (Fig. 6F). Among the two specific complexes formed, the slower-migrating one was MLTF/upstream stimulatory factor (USF), as its formation could be competed by MLTF consensus oligonucleotide (lanes 3 and 4) and the complex could be supershifted with anti-USF1 antibodies (lane 8). The faster-migrating complex appears to be ARE binding protein, as its formation could be competed by a mutant MLTF/ARE oligonucleotide (lanes 5 and 6). The formation of both of these complexes was unaltered in the liver nuclear extract from the infected mice (lanes 1 and 2). These results indicate that the basal activity of the proteins that bind to MLTF/ARE was high in the liver nuclear extract and these activities were not changed to any significant level after viral infection.
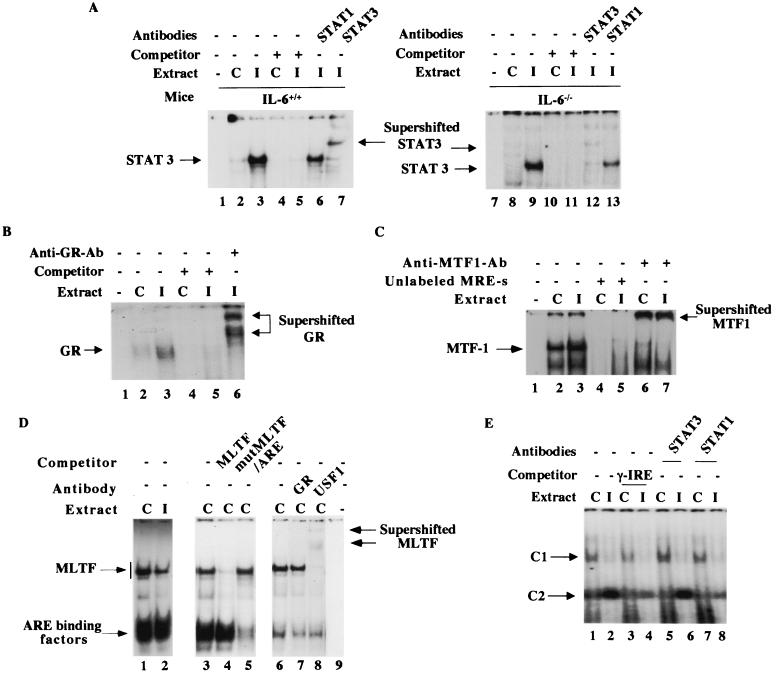
We also measured the DNA-binding activities of the trans-acting factors in the lung nuclear extract (Fig. 7). EMSA results revealed formation of a specific complex at the potential site located between −297 and −277 that was indeed activated three- to fourfold in the lung nuclear extract (Fig. 7A, lanes 2 and 3). Supershift of this complex with anti-STAT3 but not with anti-STAT1 antibodies confirmed its identity as STAT3. These results demonstrate that the nature of the complex formed at this site was identical to that formed in the liver. Comparable activation of STAT3 was observed after infection when nuclear extracts from IL-6 null mice were analyzed (compare lanes 8 and 9 with lanes 2 and 3, respectively). This data corroborated the IVGF results. Similarly, the binding of GR at GRE1 was activated two- to threefold in the lung nuclear extract from the infected mice (Fig. 7B, lanes 2 and 3). The specificity of the complex was determined by supershift with anti-GR antibodies (Fig. 7B, lane 6). As observed in the liver, the constitutive activity of MTF-1 in the lung nuclear extract was high (Fig. 7C, lane 2) and did not increase significantly after infection (lane 3). EMSA studies showed that MLTF activity decreased in the lung nuclear extract of infected mice compared to the control, whereas ARE binding activity remained unchanged (Fig. 7D, lanes 1 and 2). The reduced activity of MLTF was probably due to its instability during isolation of nuclei and extraction of proteins from the lung of infected mice.
FIG. 7.
DNA-binding activities of STAT3, GR, and γ-IRE binding protein are increased whereas those of MTF-1 and proteins binding to MLTF/ARE remain unaltered in lung nuclear extract from infected animals. The lung nuclear extracts were incubated with 32P-labeled (A) STAT3, (B) GRE1, (C) MRE-8, and (D) MLTF/ARE oligonucleotides under the conditions described for the liver nuclear extracts. (E) γ-IRE binding activity was assayed with 10 μg of protein of each nuclear extract in the binding buffer used for STAT3.
In the infected lung, we observed a distinct and unique footprinting at a tentative γ-IRE site in the proximal promoter. This site is involved in IFN-γ-mediated gene expression, as determined by transient-transfection studies (66). To the best of our knowledge, the trans-acting factor binding to this site has not been identified. We attempted to identify the complex that binds to γ-IRE by EMSA and subsequent supershift with relevant antibodies. Nuclear extract was prepared from both control and infected lung and incubated with α-32P-labeled γ-IRE (bp −123 to −100) of the mouse MT-I gene. A specific complex, C2, was detected in the nuclear extract from the infected mice (Fig. 7E, lane 2), which was barely detectable in the control lung nuclear extract (lane 1). This complex appeared to be specific, as it was competed out by a molar excess of the unlabeled oligonucleotide. Supershift analysis showed that STAT1 but not STAT3 might be involved in the formation of C2 complex, as only the antibodies against STAT1 were able to destabilize the C2 complex (lanes 7 and 8). Further investigation is necessary to decipher the exact nature of the C2 complex that plays an important role in activating the MT-I gene in the infected lung. Another complex, C1, was detected in the control lung, which was significantly reduced in the infected lung (lanes 1 and 2). This complex was partially competed out by unlabeled γ-IRE oligonucleotide and was not supershifted by either STAT1 or STAT3 antibody. The nature and role of this complex in MT-I induction remain to be studied.
Real-time PCR analysis of lung tissue reveals increased expression of cytokines that signal through STAT1 or STAT3 pathway.
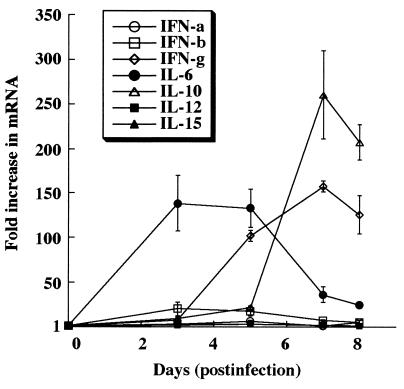
As the IVGF and EMSA data from the lung demonstrated, MT-I expression is increased in response to viral infection via STAT1 and STAT3, so we assessed the expression of cytokines that signal through these pathways. Total RNA from the pooled lungs was reverse transcribed, and the resulting cDNA was used in the real-time PCR (see Materials and Methods for details). The relative increase in the expression (mRNA) of each of the cytokines in the infected tissues was determined using the comparative Ct method, with corresponding tissue from the noninfected animals as the baseline (Fig. 8). The data (representative of three different experiments) show increased expression of the cytokines at different days postinfection. Although the fold increase in cytokine expression varied among experiments due to variability of the infection, the pattern of expression remained similar in each case.
FIG. 8.
Influenza virus infection induces expression of cytokines that signal via the STAT1 or STAT3 pathway. Pooled RNA from the lungs of five animals (control and infected) was reverse transcribed into cDNA to assess cytokine gene expression in the lung during influenza virus infection. The mRNA levels for cytokines were measured using the 7700 Sequence Detector (see Materials and Methods for details). The y axis indicates the fold increase in mRNA of the cytokines in infected lung compared to that of the uninfected control, and the x axis denotes days of infection. The data presented are the mean of three independent experiment ± SEM.
Since the type I and II interferons can signal through the STAT1 pathway, we examined the expression of these cytokines in the lungs of the infected animals. IFN-α/β are important in limiting the early replication and spread of the virus. In particular, RNA viruses are strong inducers of IFN-α and IFN-β due to formation of double-stranded RNA during the replication cycle (17). During early stages of infection (on day 3), the level of IFN-β increased 20-fold, after which it started to decline (Fig. 8). The level of IFN-γ, a Th1 cytokine vital for the development of cell-mediated immunity, started to increase on day 3 and attained a level greater than 150-fold on day 7 postinfection. The observed increase in IFN-γ expression offers a potential explanation for the footprinting observed at the γ-IRE in the lung (Fig. 5C) and suggests its role in the increase in STAT1 activity observed in the lungs.
The next set of cytokines that we examined signal through the STAT3 pathway. The IL-6 mRNA level increased in the lungs of infected animals during early stages of infection (on day 3), after which it started to decline. Its expression was, however, not critical for the induction of MT expression in the lung, as demonstrated by the IL-6 knockout experiment (Fig. 2). Because IL-6 is not critical for the induction of MT in the lungs of infected mice, other cytokines (IL-10, IL-12, and IL-15) that utilize the STAT3 pathway are likely to promote MT gene expression. The level of IL-10, a Th2 cytokine important for humoral immunity, started to increase on day 3 postinfection, and its level increased more than 260-fold compared to the uninfected control lung on day 7 postinfection. On the contrary, the levels of IL-12 and IL-15, produced by macrophages to activate NK cells, were not markedly elevated following viral infection. The large increase in IL-10 expression suggests that IL-10 is responsible for the observed increase in lung STAT3 activity in response to viral infection.
The real-time PCR analysis of lung RNA demonstrated a significant increase in the levels of cytokines that signal through the STAT1 and STAT3 pathways. These results reinforce the IVGF and EMSA data (Fig. 5 and 7) from the lung by demonstrating robust expression of IFN-γ and IL-10, which signal via STAT1 and STAT3, respectively. Although the IL-6 level increased as high as 130-fold on day 3 postinfection, its effect on MT-I induction was minimal, as IL-6 null mice could induce lung MT-I to the same extent as the wild-type mice (Fig. 2 and 3).
DISCUSSION
Infection by influenza A virus causes an acute infection of the upper respiratory tract (in humans) and the lung (in rodents). This lytic infection activates resident phagocytes in the tissues to generate reactive oxygen species as part of the host's defense mechanism against virus replication. In order to protect the cells from virus-induced oxidative stress, host cells must have evolved some antioxidant mechanism that effectively scavanges the reactive oxygen species. Because MT has been implicated in such scavenging, it was of interest to investigate whether this gene was activated following an influenza virus infection. The present study clearly demonstrates that this is indeed the case.
Both MT genes (MT-I and MT-II) were coordinately induced in a time-dependent manner in the lung and liver, with maximal expression attained within 5 days following infection. To our knowledge, the present study is the first demonstration of the activation of MT genes in response to an experimental viral infection in an animal model and elucidation of the molecular mechanisms for the induction process. One recent study using DNA microarray technology has identified MT-IIA as one of the genes induced in HeLa cells infected with influenza A virus (20). This study does not, however, address the molecular mechanisms of MT induction in specific tissues of organisms.
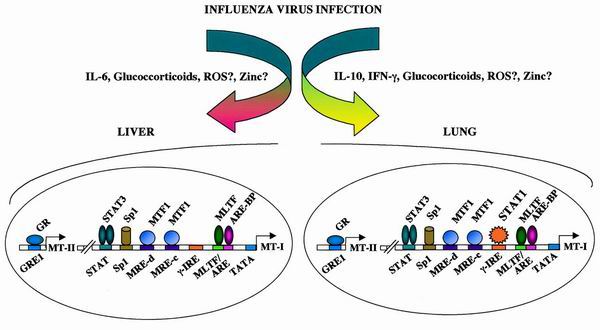
Although induction of MT-I in the liver and lung involves overlapping mechanisms, there are unique differences in the signaling mechanisms in the induction of the same gene in the two tissues. It is known that infection of mice with influenza A virus evokes an acute-phase response in the liver and activates the hypothalamus-pituitary-adrenal axis, leading to increased serum glucocorticoid levels (32, 36). Viral infection also induces the expression of proinflammatory cytokines, e.g., IL-1, IL-6, and IL-10, some of which activate the genes involved in the generation of reactive oxygen species. In the present study, we have observed that the expression of several cytokines, IL-6, IL-10, IL-12, and IL-15 as well as IFN-α, IFN-β, and IFN-γ, was elevated in the lung during viral infection (Fig. 8). We have shown the involvement of multifarious signaling mechanisms that include cytokines, glucocorticoids, and perhaps zinc and reactive oxygen species (Fig. 9) in the induction of MT in response to virus infection. It appears that simultaneous participation of several transcription factors, such as STAT3, STAT1, GR, MTF -1, Sp1, MLTF, and ARE binding proteins, leads to robust induction of MT gene expression in the liver and lung.
FIG. 9.
Schematic model representing probable signaling pathways and transcription factors (based on our results) involved in induction of the MT-I and MT-II genes in response to infection with influenza virus.
An important difference in the profile of MT induction in the liver versus lung is that MT expression in the lung continued to rise through day 7 postinfection, in contrast to the peak induction on day 5 in the liver (Fig. 1). This difference in the kinetics of MT induction may be due to trafficking of immune cells to the site of infection. These incoming cells (e.g., macrophages, NK cells, and T cells) will produce cytokines that are not necessarily formed in the resident cells. The prolonged expression of the MT genes in the lung relative to liver may therefore arise from the trafficking cells and the higher levels of cytokines that they produce.
Another noteworthy observation is the ability of IL-6 null mice to express the MT-I gene in the lung but not in the liver. Among the proinflammatory cytokines, IL-6 can directly activate the MT-I promoter in hepatocytes (54, 61). IL-6 had a more profound effect on the activation of MT genes in the liver relative to the lung following infection by influenza virus. This differential action of IL-6 is probably due to a higher concentration of the IL-6 receptor complex on the surface of liver cells compared to lung cells. Indeed, administration of radiolabeled-IL-6 to mice led to its maximal accumulation in the liver (1). Alternatively, IL-6 deficiency in the lung of null mice may be compensated for by other cytokines with overlapping signaling mechanisms. The cytokines that are expressed at a significantly high level following viral infection—IL-10, IL-12, and IL-15—can activate STAT3 (1, 30, 36). In addition to this, some growth factors, e.g., granulocyte colony-stimulating factor, produced during viral infection can also activate STAT3 (28, 33).
Quantitative analysis of the expression profiles of various cytokines by real-time PCR in the lung revealed an extremely high level of IL-10 during infection (Fig. 8). This is probably responsible for STAT3 activation and MT induction in the lung. Viral infection caused a significant increase in IL-6 mRNA in the lung, particularly at the early stage of infection (day 3). Interestingly, the IL-10 level started to rise on day 3, when the IL-6 level began to decline (Fig. 8). We cannot rule out the potential role of IL-6 in MT induction during early stages of infection (days 1 to 3), when the IL-10 level is significantly low.
To decipher the mechanism of activation of the MT-I gene in liver and lung, we analyzed the MT-I-proximal promoter as well as the region upstream of the MT-II gene that harbors relevant cis elements (see Fig. 2 and 3). Because IL-6 plays a key role in MT-I gene induction in the liver but not in the lung, we explored the characteristics of footprinting at the IL-6 response elements in the liver and lung. IVGF analysis revealed occupancy of the same STAT3 site in both tissues. This observation validates our assumption that STAT3 was indeed activated in the lung by a cytokine(s) other than IL-6.
IL-6 activates acute-phase response genes in the liver by two distinct mechanisms mediated through different cis elements (3). Analysis of the MT-I promoter reveals the existence of putative type I and type II IL-6 REs. IVGF analysis demonstrated occupancy of only type II IL-6 RE located between −297 and −277 bp in response to viral infection (Fig. 4A and 5A). This observation correlates well with a significant increase in the DNA-binding activity of STAT3 in the liver nuclear extract from the infected wild-type mice but not from IL-6 null mice. On the contrary, IVGF analysis did not show inducible occupancy of IL-6 type I RE in either liver or lung, which is consistent with the lack of inducible binding of any factor to the oligonucleotide spanning this site in EMSA using tissue nuclear extract from the infected mice (data not shown).
Studies with RU-486, the type II glucocorticoid receptor antagonist, indicate a critical role of glucocorticoids in MT-I induction. It is known that serum glucocorticoid level is elevated upon viral infection (33). Interestingly, IVGF analysis demonstrated footprinting at one (GRE1) of the two GREs (44) in both the liver and lung in response to viral infection. To our knowledge, this is the first report of genomic footprinting at a GRE site that allowed us to functionally distinguish the two GREs in vivo during viral infection. The reason for the preferential occupancy of GRE1 in both tissues in response to infection is not evident. Although both GREs can impart glucocorticoid sensitivity to a reporter gene in a transient-transfection assay, GRE1 may be the functional element in the chromatin context. Alternatively, it is conceivable that the corticosterone level induced in response to viral infection is optimal for binding to GRE1. It is also known that the glucocorticoid receptor and STAT3 can activate a gene synergistically using either the STAT3 binding site or GRE as the cis element (62, 68). Such a mechanism does not appear to be involved in MT induction following viral infection, as the DNA-protein complex formed between STAT3 and IL-6 type II RE could not be supershifted by anti-GR antibodies. Similarly, the complex formed with GRE1 and GR could not be supershifted with anti-STAT3 antibodies. These results imply that STAT3 and GR transactivate the MT-I promoter in response to infection by influenza virus by binding to the individual cognate sites rather than by protein-protein interaction.
Another significant finding is the unique footprinting in the lung at a potential IFN-γ RE (γ-IRE) located between −105 and −115 of the MT-I promoter (Fig. 5B). This is the first demonstration of such a cis-acting element on the MT-I promoter. The occupancy of this site in vivo has never been observed when the MT-I gene is induced in cells in culture or in the liver by other agents that include heavy metals (50), hydrogen peroxide (16), and lipopolysaccharides (47). This specific footprinting of the MT-I promoter clearly demonstrates that MT-I induction by different agents follows distinct signaling pathways. The lack of γ-IRE footprinting in the liver in response to infection suggests that the expression and activity of the interferons are specific to the lung, the site of infection, and that a different signaling pathway is activated in this tissue. The transcription factors that are activated upon interferon signaling include STAT1, STAT3, and interferon response factor, capable of binding to this site. An in vitro binding assay demonstrated the function of a distinct complex containing STAT1 in the infected lung nuclear extract and the γ-IRE oligonucleotide. Further study is necessary to determine the exact nature of the complex.
Although we considered cytokines and glucocorticoids as the major players in MT-I induction during viral infection, the importance of MTF-1, the key and indispensable transcription factor, cannot be overlooked. IVGF analysis of the liver and lung nuclei from the infected mice demonstrated characteristic footprinting of a majority of the MREs in both tissues. These results suggest that MREs mediate not only heavy-metal-induced expression of MT-I, but also influenza virus-induced expression. In the lung, free radicals are generated following viral replication and the host's immune response to the infection that results in the activation of MTF-1 and its occupancy of MREs. It is conceivable that the systemic release of proinflammatory cytokines produced in response to viral infection leads to oxidative stress in the host liver as well. Alternatively, the footprinting at MREs in the liver might be due to mobilization of zinc following infection. Indeed, preliminary analysis of the zinc level in the liver during infection has shown a 25 to 40% increase in the level compared to that in the uninfected control mice of the same age (W. Erdahl and K. Ghoshal, unpublished data). Another possibility is the activation of MTF-1 during the acute-phase response. EMSA results showed that the constitutive DNA-binding activity of MTF-1 is quite high in these tissues and is not activated further following infection. MTF-1 shows similar behavior in response to almost all inducers, except zinc, that directly activate MTF-1 by binding to its six zinc fingers (59). In MTF-1 null cells, the basal MT-I level is significantly low and it cannot be induced (35), pointing to an absolute requirement of MTF-1 for MT-I expression.
Our recent studies have shown that IVGF profiles of the MT-I promoter after cadmium and zinc treatment are similar, and cadmium is a more potent inducer than zinc (50). The MTF-1 activity in the nuclear extract from the cadmium-treated cells is, however, very similar to that of the control cells. These results strongly argue for MTF-1 activation in response to various inducers in vivo and its loss with the exception of zinc in the cell-free system. Elucidation of the signaling mechanism of MTF-1 activation in the liver and lung is of immense importance.
It is possible that the activation of MTF-1 in lung and liver follows distinct pathways. The tissue damage at the site of infection (the lung) caused by viral replication and host immune response will generate a battery of free radicals locally that can activate the MT-I gene. Alternatively, proinflammatory cytokines may activate MTF-1 by an as yet unknown mechanism. Similarly, EMSA results demonstrate that more than one protein binds to the composite MLTF/ARE, none of which are increased in the extract prepared from the livers and lungs of infected animals (Fig. 6F and 7D). It is possible that in vivo binding of MTF-1 to the MREs can facilitate occupancy of MLTF/ARE by USF and ARE binding proteins. Alternatively, infection results in posttranslational modifications of these factors outside the DNA-binding domain, resulting in their recruitment to MLTF/ARE. Identification of the protein(s) that binds to ARE will be of considerable interest.
We propose that MT is a part of the cellular antioxidant mechanism that protects the host from the cytopathic effect of free radicals generated upon viral infection and that the balance between the host cell pro- and antioxidant activity and removal of free radicals determines the severity of the infection. It is conceivable that the therapeutic use of zinc lozenges, which significantly reduce the manifestation of the influenza virus infection, is probably due to MT induction. It is therefore logical to expect that MT-I and MT-II knockout mice will be more susceptible to influenza virus infection than genetically matched wild-type animals. It would be of interest to explore whether the morbidity and mortality of the null mice are higher than those of the wild-type mice. Studies along these lines are in progress.
ACKNOWLEDGMENTS
We thank Richard Palmiter and Walter Schaffner for mouse MT-I minigene and anti-MTF-1 antibodies, respectively; Jaharul Haque for valuable suggestions in assaying STAT3 activity; and Aaron Fawn for critically reading the manuscript.
This research was supported, in part, by grants ES10874 and CA81024 (S.T.J.) and MH46801-09 (J.F.S).
Kalpana Ghoshal and Sarmila Majumder contributed equally to this work.
REFERENCES
- 1.Akira S. Roles of STAT3 defined by tissue-specific gene targeting. Oncogene. 2000;19:2607–2611. doi: 10.1038/sj.onc.1203478. [DOI] [PubMed] [Google Scholar]
- 2.Alonzi T, Fattori E, Cappelletti M, Ciliberto G, Poli V. Impaired Stat3 activation following localized inflammatory stimulus in IL-6-deficient mice. Cytokine. 1998;10:13–18. doi: 10.1006/cyto.1997.0250. [DOI] [PubMed] [Google Scholar]
- 3.Alonzi T, Gorgoni B, Screpanti I, Gulino A, Poli V. Interleukin-6 and CAAT/enhancer binding protein beta-deficient mice act as tools to dissect the IL-6 signalling pathway and IL-6 regulation. Immunobiology. 1997;198:144–156. doi: 10.1016/s0171-2985(97)80035-6. [DOI] [PubMed] [Google Scholar]
- 4.Alonzi T, Maritano D, Gorgoni B, Rizzuto G, Libert C, Poli V. Essential role of STAT3 in the control of the acute-phase response as revealed by inducible gene activation in the liver. Mol Cell Biol. 2001;21:1621–1632. doi: 10.1128/MCB.21.5.1621-1632.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews G K. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol. 2000;59:95–104. doi: 10.1016/s0006-2952(99)00301-9. [DOI] [PubMed] [Google Scholar]
- 6.Aniskovitch L P, Jacob S T. Purification and characterization of a rat liver protein that recognizes CCAAT-homologous sequences of the metallothionein promoter and trans-activates this promoter. Arch Biochem Biophys. 1997;341:337–346. doi: 10.1006/abbi.1997.9976. [DOI] [PubMed] [Google Scholar]
- 7.Aniskovitch L P, Jacob S T. Distinct rat proteins can recognize CCAAT-homologous sequences of the metallothionein promoter and trans-activate this promoter. Oncogene. 1998;16:1475–1486. doi: 10.1038/sj.onc.1201659. [DOI] [PubMed] [Google Scholar]
- 8.Beck M A, Handy J, Levander O A. The role of oxidative stress in viral infections. Ann N Y Acad Sci. 2000;917:906–912. doi: 10.1111/j.1749-6632.2000.tb05456.x. [DOI] [PubMed] [Google Scholar]
- 9.Belikov S, Gelius B, Wrange O. Hormone-induced nucleosome positioning in the MMTV promoter is reversible. EMBO J. 2001;20:2802–2811. doi: 10.1093/emboj/20.11.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benihoud K, Salone B, Esselin S, Opolon P, Poli V, Di Giovine M, Perricaudet M, Saggio I. The role of IL-6 in the inflammatory and humoral response to adenoviral vectors. J Genet Med. 2000;2:194–203. doi: 10.1002/(SICI)1521-2254(200005/06)2:3<194::AID-JGM102>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Brugnera E, Georgiev O, Radtke F, Heuchel R, Baker E, Sutherland G R, Schaffner W. Cloning, chromosomal mapping and characterization of the human metal-regulatory transcription factor MTF-1. Nucleic Acids Res. 1994;22:3167–3173. doi: 10.1093/nar/22.15.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buffinton G D, Christen S, Peterhans E, Stocker R. Oxidative stress in lungs of mice infected with influenza A virus. Free Radic Res Commun. 1992;16:99–110. doi: 10.3109/10715769209049163. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control. 2000. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 49.
- 14.Choi A M, Knobil K, Otterbein S L, Eastman D A, Jacoby D B. Oxidant stress responses in influenza virus pneumonia: gene expression and transcription factor activation. Am J Physiol. 1996;271:L383–391. doi: 10.1152/ajplung.1996.271.3.L383. [DOI] [PubMed] [Google Scholar]
- 15.Chomczynski P, Sacchi N. single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 16.Dalton T P, Li Q, Bittel D, Liang L, Andrews G K. Oxidative stress activates metal-responsive transcription factor-1 binding activity: occupancy in vivo of metal response elements in the metallothionein-I gene promoter. J Biol Chem. 1996;271:26233–26241. doi: 10.1074/jbc.271.42.26233. [DOI] [PubMed] [Google Scholar]
- 17.De Maeyer E, De Maeyer-Guignard J. Type I interferons. Int Rev Immunol. 1998;17:53–73. doi: 10.3109/08830189809084487. [DOI] [PubMed] [Google Scholar]
- 18.Dobbs C M, Feng N, Beck F M, Sheridan J F. Neuroendocrine regulation of cytokine production during experimental influenza viral infection: effects of restraint stress-induced elevation in endogenous corticosterone. J Immunol. 1996;157:1870–1877. [PubMed] [Google Scholar]
- 19.Eaton D L, Cherian M G. Determination of metallothionein in tissues by cadmium-hemoglobin affinity assay. Methods Enzymol. 1991;205:83–88. doi: 10.1016/0076-6879(91)05089-e. [DOI] [PubMed] [Google Scholar]
- 20.Geiss G K, An M C, Bumgarner R E, Hammersmark E, Cunningham D, Katze M G. Global impact of influenza virus on cellular pathways is mediated by both replication-dependent and -independent events. J Virol. 2001;75:4321–4331. doi: 10.1128/JVI.75.9.4321-4331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghoshal K, Jacob S T. Regulation of metallothionein gene expression. Prog Nucleic Acid Res Mol Biol. 2000;66:357–384. doi: 10.1016/s0079-6603(00)66034-8. [DOI] [PubMed] [Google Scholar]
- 22.Ghoshal K, Li Z, Jacob S T. Overexpression of the large subunit of the protein Ku suppresses metallothionein-I induction by heavy metals. Proc Natl Acad Sci USA. 1998;95:10390–10395. doi: 10.1073/pnas.95.18.10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghoshal, K., S. Majumder, and S. T. Jacob. Analysis of promoter methylation and its role in silencing metallothionein-I gene expression in some tumor cells. Methods Enzymol., in press. [DOI] [PubMed]
- 24.Ghoshal K, Majumder S, Li Z, Bray T M, Jacob S T. Transcriptional induction of metallothionein-I and -II genes in the livers of Cu,Zn-superoxide dismutase knockout mice. Biochem Biophys Res Commun. 1999;264:735–742. doi: 10.1006/bbrc.1999.1563. [DOI] [PubMed] [Google Scholar]
- 25.Ghoshal K, Majumder S, Li Z, Dong X, Jacob S T. Suppression of metallothionein gene expression in a rat hepatoma because of promoter-specific DNA methylation. J Biol Chem. 2000;275:539–547. doi: 10.1074/jbc.275.1.539. [DOI] [PubMed] [Google Scholar]
- 26.Ghoshal K, Wang Y, Sheridan J F, Jacob S T. Metallothionein induction in response to restraint stress: transcriptional control, adaptation to stress, and role of glucocorticoid. J Biol Chem. 1998;273:27904–27910. doi: 10.1074/jbc.273.43.27904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorski K, Carneiro M, Schibler U. Tissue specific in vitro transcription from the mouse albumin promoter. Cell. 1986;47:767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- 28.Gunes C, Heuchel R, Georgiev O, Muller K H, Lichtlen P, Bluthmann H, Marino S, Aguzzi A, Schaffner W. Embryonic lethality and liver degeneration in mice lacking the metal- responsive transcriptional activator MTF-1. EMBO J. 1998;17:2846–2854. doi: 10.1093/emboj/17.10.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamer D H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- 30.Heinrich P C, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hennet T, Peterhans E, Stocker R. Alterations in antioxidant defences in lung and liver of mice infected with influenza A virus. J Gen Virol. 1992;73:39–46. doi: 10.1099/0022-1317-73-1-39. [DOI] [PubMed] [Google Scholar]
- 32.Hennet T, Ziltener H J, Frei K, Peterhans E. A kinetic study of immune mediators in the lungs of mice infected with influenza A virus. J Immunol. 1992;149:932–939. [PubMed] [Google Scholar]
- 33.Hermann G, Tovar C A, Beck F M, Sheridan J F. Kinetics of glucocorticoid response to restraint stress and/or experimental influenza viral infection in two inbred strains of mice. modulates mononuclear cell trafficking during an experimental influenza viral infection. J Neuroimmunol. 1994;49:25–33. doi: 10.1016/0165-5728(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez J, Carrasco J, Belloso E, Giralt M, Bluethmann H, Kee Lee D, Andrews G K, Hidalgo J. Metallothionein induction by restraint stress: role of glucocorticoids and IL-6. Cytokine. 2000;12:791–796. doi: 10.1006/cyto.1999.0629. [DOI] [PubMed] [Google Scholar]
- 35.Heuchel R, Radtke F, Georgiev O, Stark G, Aguet M, Schaffner W. The transcription factor MTF-1 is essential for basal and heavy metal-induced metallothionein gene expression. EMBO J. 1994;13:2870–2875. doi: 10.1002/j.1460-2075.1994.tb06581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ihle J N. STATs: signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- 37.Jacob C, Maret W, Vallee B L. Control of zinc transfer between thionein, metallothionein, and zinc proteins. Proc Natl Acad Sci USA. 1998;95:3489–3494. doi: 10.1073/pnas.95.7.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Julkunen I, Sareneva T, Pirhonen J, Ronni T, Melen K, Matikainen S. Molecular pathogenesis of influenza A virus infection and virus-induced regulation of cytokine gene expression. Cytokine Growth Factor Rev. 2001;12:171–180. doi: 10.1016/s1359-6101(00)00026-5. [DOI] [PubMed] [Google Scholar]
- 39.Kang Y J. The antioxidant function of metallothionein in the heart. Proc Soc Exp Biol Med. 1999;222:263–273. doi: 10.1046/j.1525-1373.1999.d01-143.x. [DOI] [PubMed] [Google Scholar]
- 40.Kang Y J, Zhou Z X, Wang G W, Buridi A, Klein J B. Suppression by metallothionein of doxorubicin-induced cardiomyocyte apoptosis through inhibition of p38 mitogen-activated protein kinases. J Biol Chem. 2000;275:13690–13698. doi: 10.1074/jbc.275.18.13690. [DOI] [PubMed] [Google Scholar]
- 41.Kasutani K, Itoh N, Kanekiyo M, Muto N, Tanaka K. Requirement for cooperative interaction of interleukin-6 responsive element type 2 and glucocorticoid responsive element in the synergistic activation of mouse metallothionein-I gene by interleukin-6 and glucocorticoid. Toxicol Appl Pharmacol. 1998;151:143–151. doi: 10.1006/taap.1998.8452. [DOI] [PubMed] [Google Scholar]
- 42.Kelly E J, Palmiter R D. A murine model of Menkes disease reveals a physiological function of. Nat Genet. 1996;13:219–222. doi: 10.1038/ng0696-219. [DOI] [PubMed] [Google Scholar]
- 43.Kelly E J, Quaife C J, Froelick G J, Palmiter R D. Metallothionein I and II protect against zinc deficiency and zinc. J Nutr. 1996;126:1782–1790. doi: 10.1093/jn/126.7.1782. [DOI] [PubMed] [Google Scholar]
- 44.Kelly E J, Sandgren E P, Brinster R L, Palmiter R D. A pair of adjacent glucocorticoid response elements regulate expression of two mouse metallothionein genes. Proc Natl Acad Sci USA. 1997;94:10045–10050. doi: 10.1073/pnas.94.19.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kondo Y, Rusnak J M, Hoyt D G, Settineri C E, Pitt B R, Lazo J S. Enhanced apoptosis in metallothionein null cells. Mol Pharmacol. 1997;52:195–201. doi: 10.1124/mol.52.2.195. [DOI] [PubMed] [Google Scholar]
- 46.Lazo J S, Kondo Y, Dellapiazza D, Michalska A E, Choo K H, Pitt B R. Enhanced sensitivity to oxidative stress in cultured embryonic cells from transgenic mice deficient in metallothionein I and II genes. J Biol Chem. 1995;270:5506–5510. doi: 10.1074/jbc.270.10.5506. [DOI] [PubMed] [Google Scholar]
- 47.Lee D K, Carrasco J, Hidalgo J, Andrews G K. Identification of a signal transducer and activator of transcription (STAT) binding site in the mouse metallothionein-I promoter involved in interleukin-6-induced gene expression. Biochem J. 1999;337:59–65. [PMC free article] [PubMed] [Google Scholar]
- 48.Majumder S, Ghoshal K, Gronostajski R M, Jacob S T. Downregulation of constitutive and heavy metal-induced metallothionein-I expression by nuclear factor I. Gene Expr. 2001;9:203–215. doi: 10.3727/000000001783992588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Majumder S, Ghoshal K, Li Z, Bo Y, Jacob S T. Silencing of metallothionein-I gene in mouse lymphosarcoma cells by methylation. Oncogene. 1999;18:6287–6295. doi: 10.1038/sj.onc.1203004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Majumder S, Ghoshal K, Li Z, Jacob S T. Hypermethylation of metallothionein-I promoter and suppression of its induction in cell lines overexpressing the large subunit of Ku protein. J Biol Chem. 1999;274:28584–28589. doi: 10.1074/jbc.274.40.28584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maret W. The function of zinc metallothionein: a link between cellular zinc and redox state. J Nutr. 2000;130:1455S–1458S. doi: 10.1093/jn/130.5.1455S. [DOI] [PubMed] [Google Scholar]
- 52.Maret W, Jacob C, Vallee B L, Fischer E H. Inhibitory sites in enzymes: zinc removal and reactivation by thionein. Proc Natl Acad Sci USA. 1999;96:1936–1940. doi: 10.1073/pnas.96.5.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moffatt, P. and Denizeau, F. 1997. Metallothionein in physiological and pathphysiological processes. Drug Metab. Rev. 29:261–307. [DOI] [PubMed]
- 54.Molotkov A, Nishimura N, Satoh M, Tohyama C. Role of IL-6 in the induction of hepatic metallothionein in mice after partial hepatectomy. Life Sci. 2000;66:963–970. doi: 10.1016/s0024-3205(99)00661-x. [DOI] [PubMed] [Google Scholar]
- 55.Mueller P R, Salser S J, Wold B. Constitutive and metal-inducible protein:DNA interactions at the mouse metallothionein I promoter examined by in vivo and in vitro footprinting. Genes Dev. 1988;2:412–427. doi: 10.1101/gad.2.4.412. [DOI] [PubMed] [Google Scholar]
- 56.Penkowa M, Giralt M, Carrasco J, Hadberg H, Hidalgo J. Impaired inflammatory response and increased oxidative stress and neurodegeneration after brain injury in interleukin-6-deficient mice. Glia. 2000;32:271–285. doi: 10.1002/1098-1136(200012)32:3<271::aid-glia70>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 57.Peterhans E. Oxidants and antioxidants in viral diseases: disease mechanisms and metabolic regulation. J Nutr. 1997;127:962S–965S. doi: 10.1093/jn/127.5.962S. [DOI] [PubMed] [Google Scholar]
- 58.Quaife C J, Findley S D, Erickson J C, Froelic G J, Kelly E J, Zambrowicz B P, Palmiter R D. Induction of a new metallothionein isoform (MT-IV) occurs during differentiation of stratified squamous epithelia. Biochemistry. 1994;33:7250–7259. doi: 10.1021/bi00189a029. [DOI] [PubMed] [Google Scholar]
- 59.Radtke F, Heuchel R, Georgiev O, Hergersberg M, Gariglio M, Dembic Z, Schaffner W. Cloned transcription factor MTF-1 activates the mouse metallothionein I promoter. EMBO J. 1993;12:1355–1362. doi: 10.1002/j.1460-2075.1993.tb05780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sato M, Bremner I. Oxygen free radicals and metallothionein. Free Radic Biol Med. 1993;14:325–337. doi: 10.1016/0891-5849(93)90029-t. [DOI] [PubMed] [Google Scholar]
- 61.Schroeder J J, Cousins R J. Interleukin 6 regulates metallothionein gene expression and zinc metabolism in hepatocyte monolayer cultures. Proc Natl Acad Sci USA. 1990;87:3137–3141. doi: 10.1073/pnas.87.8.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takeda T, Kurachi H, Yamamoto T, Nishio Y, Nakatsuji Y, Morishige K, Miyake A, Murata Y. Crosstalk between the interleukin-6 (IL-6)-JAK-STAT and the glucocorticoid-nuclear receptor pathway: synergistic activation of IL-6 response element by IL-6 and glucocorticoid. J Endocrinol. 1998;159:323–330. doi: 10.1677/joe.0.1590323. [DOI] [PubMed] [Google Scholar]
- 63.Tamai K T, Gralla E B, Ellerby L M, Valentine J S, Thiele D J. Yeast and mammalian metallothioneins functionally substitute for yeast copper-zinc superoxide dismutase. Proc Natl Acad Sci USA. 1993;90:8013–8017. doi: 10.1073/pnas.90.17.8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thornalley P J, Vasak M. Possible role for metallothionein in protection against radiation-induced oxidative stress: kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim Biophys Acta. 1985;827:36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- 65.Wadzinski B E, Wheat W H, Jaspers S, Peruski L F, Jr, Lickteig R L, Johnson G L, Klemm D J. Nuclear protein phosphatase 2A dephosphorylates protein kinase A-phosphorylated CREB and regulates CREB transcriptional stimulation. Mol Cell Biol. 1993;13:2822–2834. doi: 10.1128/mcb.13.5.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Z, Sugawara M, Ponath P D, Wessendorf L, Banerji J, Li Y, Strominger J L. Interferon gamma response region in the promoter of the human DPA gene. Proc Natl Acad Sci USA. 1990;87:9226–9230. doi: 10.1073/pnas.87.23.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ye B, Maret W, Vallee B L. Zinc metallothionein imported into liver mitochondria modulates respiration. Proc Natl Acad Sci USA. 2001;98:2317–2322. doi: 10.1073/pnas.041619198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Z, Jones S, Hagood J S, Fuentes N L, Fuller G M. STAT3 acts as a coactivator of glucocorticoid receptor signaling. J Biol Chem. 1997;272:30607–30610. doi: 10.1074/jbc.272.49.30607. [DOI] [PubMed] [Google Scholar]